- Division of Nephrology, Phoenix Children's Hospital, Phoenix, AZ, United States
Patients with chronic kidney disease are at substantial risk for malnutrition, characterized by protein energy wasting and micronutrient deficiency. Studies show a high prevalence rate of malnutrition in both children and adults with chronic kidney disease. Apart from abnormalities in growth hormone-insulin like growth factor axis, malnutrition also plays a role in the development of stunted growth, commonly observed in children with chronic kidney disease. The pathogenic mechanisms of malnutrition in chronic kidney disease are complex and involve an interplay of multiple pathophysiologic alterations including decreased appetite and nutrient intake, hormonal derangements, metabolic imbalances, inflammation, increased catabolism, and dialysis related abnormalities. Malnutrition increases the risk of morbidity, mortality and overall disease burden in these patients. The simple provision of adequate calorie and protein intake does not effectively treat malnutrition in patients with chronic kidney disease owing to the intricate and multifaceted derangements affecting nutritional status in these patients. A clear understanding of the pathophysiologic mechanisms involved in the development of malnutrition in chronic kidney disease is necessary for developing strategies and interventions that are effective, and capable of restoring normal development and mitigating negative clinical outcomes. In this article, a review of the pathophysiologic mechanisms of malnutrition in chronic kidney disease is presented.
Introduction
The American Society for Parenteral and Enteral Nutrition defines malnutrition as “an imbalance between nutrient requirement and intake resulting in cumulative deficits of energy, protein or micronutrients that may negatively affect growth, development and other relevant outcomes” (1). This definition assumes a state of undernutrition, which constitutes protein energy wasting and micronutrient deficiency. For the purposes of this review, the term malnutrition refers to nutrient deficiency and undernutrition.
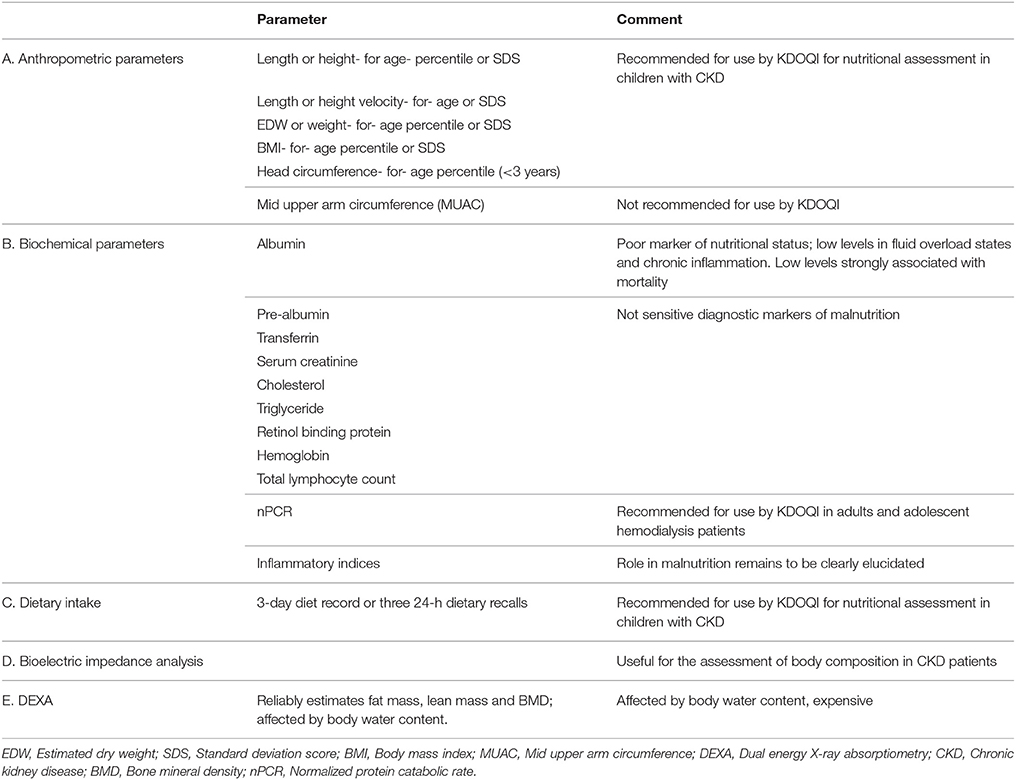
Malnutrition is prevalent in both developing and developed countries, and is an important risk factor for morbidity and mortality. Unlike in developing countries where malnutrition is linked to poor socioeconomic conditions, malnutrition in the developed countries typically occurs in the context of acute or chronic illness (1, 2). While acute illness primarily affects weight, chronic illness impacts linear growth (1). Children with chronic kidney disease (CKD) are often stunted and malnutrition is recognized as a key player in the development of growth failure in this patient population (3–6). Depending on the clinical parameters used to define malnutrition, a prevalence of 20–45% has been reported in various studies in children with CKD (6–8). Using the subjective global assessment scale (SGA), a recent study found a prevalence of protein energy wasting in 31% of adults with CKD, including dialysis and non-dialysis patients (9). The clinical parameters used variously to assess nutritional status in patients with CKD are shown in Table 1. In addition to growth impairment, malnutrition has also been shown to increase the risk of morbidity and mortality in both adult and pediatric patients with CKD (10–12). While malnutrition in the general population might result from decreased intake, malnutrition in CKD is not entirely explained by reduced nutritional intake. A delicate interplay of multiple factors, including hormonal imbalances, decreased appetite and food intake, inflammation, increased catabolism, nutrient losses in dialysate and metabolic derangements predispose chronic kidney disease patients to malnutrition (13–16). A clear understanding of the pathophysiologic mechanisms of malnutrition in patients with CKD is essential to planning strategic interventions to improve growth and development and mitigate negative clinical outcomes. The purpose of this review is to elucidate the underlying complex mechanisms involved in the development of malnutrition in CKD and highlight the associated poor clinical outcomes. Other aspects of malnutrition including assessment and management are reviewed separately in this journal issue.
Protein Energy Wasting in Chronic Kidney Disease
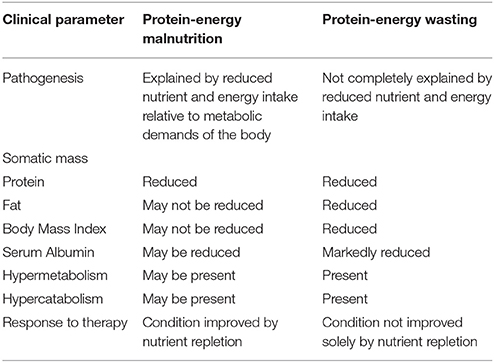
The International Society of Renal Nutrition and Metabolism (ISRNM) defines protein energy wasting as a “the state of decreased body stores of protein and energy fuels (that is, body protein and fat masses)” (17). This term was proposed by ISRNM in 2008 to specifically refer to a state of decreased body stores of protein and fat (wasting). This is to be distinguished from protein energy malnutrition, a form of protein energy wasting characterized purely by inadequate dietary intake. Moreover, unlike protein energy malnutrition, protein energy wasting cannot be corrected solely by increasing energy intake (17). A comparison between protein energy wasting and protein energy malnutrition can be found in Table 2. Protein energy wasting is prevalent in patients with CKD, and is associated with impaired growth and development in children, increased risk of cardiovascular disease, infection and death (18). In children with protein energy wasting, anthropometric measurements fall 2 standard deviations below the normal weight for age (underweight), height for age (stunting) and weight for height (wasting) (19).
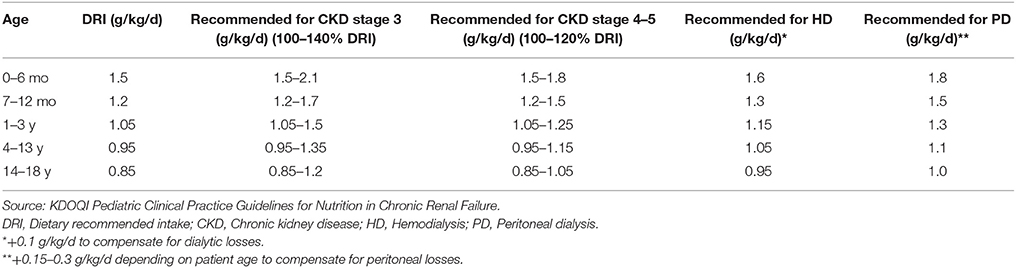
The pathogenesis of protein energy wasting is complex and multifactorial. Decreased protein and energy intake due to anorexia, increased protein catabolism, decreased anabolism, chronic inflammation, metabolic acidosis and hormonal imbalances have all been linked to protein energy wasting as etiological factors (14, 17, 20, 21) (Figure 1). Anorexia is common in patients with CKD and may result from alterations in orexigenic (appetite stimulating) and anorexigenic (appetite inhibiting) hormones, accumulation of metabolic waste products in the body in kidney failure, abnormal taste and the effect of medications on taste buds. Accumulative impact of these factors results in decreased nutrient intake. The result of a chronic inflammatory state in CKD is an increase in resting energy expenditure, which promotes protein catabolism and decreased anabolism. Studies have shown an increase in resting energy expenditure ranging from 12 to 20% during dialysis (22), suggesting an increased need for protein and energy intake in dialysis patients. Protein catabolism, in addition to increased protein losses (mostly amino acids) through dialysis techniques (both hemodialysis and peritoneal dialysis) and decreased synthesis of albumin leads to a state of negative nitrogen balance and muscle wasting (14, 23). In children on peritoneal dialysis, peritoneal protein losses can be significant and contribute to the development of protein malnutrition and growth impairment. An inverse correlation between body surface area and peritoneal losses of protein in children on continuous cycling peritoneal dialysis is well recognized, with younger children experiencing greater protein losses (24, 25). These losses are especially high during episodes of peritonitis (25). Although adequate protein intake is essential in children with CKD to avoid negative nitrogen balance and help preserve muscle mass, this in itself may not be adequate in preventing protein energy wasting (21, 26). Control of co-morbidities and the chronic inflammatory state is also necessary. The National Kidney Foundation Kidney Disease Outcomes Quality Initiative (KDOQI) recommends maintaining “dietary protein intake (DPI) at 100–140% of the Dietary Reference Intake (DRI) for ideal body weight in children with CKD stage 3 and at 100–120% of the DRI in children with CKD stages 4–5. In children with CKD stage 5D, it suggests to maintain DPI at 100% of the DRI for ideal body weight plus an allowance for dialytic protein and amino acid losses.” The KDOQI recommendations for protein intake in children with CKD is presented in Table 3. Energy requirements for children with CKD stages 2–5 and those on dialysis are recommended to be at 100% of the estimated energy requirements for chronological age (27).
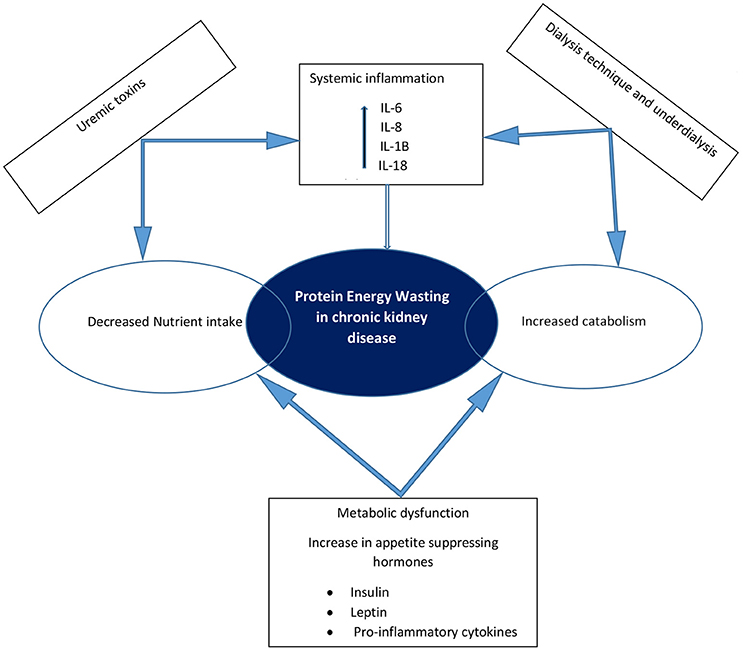
Figure 1. Schematic representation of the causes of protein energy wasting and pathophysiologic interactions in chronic kidney disease.
Micronutrient Deficiency in Chronic Kidney Disease
CKD predisposes patients to vitamin and mineral deficiencies, which may contribute to comorbidities such as anemia, cardiovascular disease, and metabolic imbalances. The overall decrease in nutritional intake, dietary restrictions, poor intestinal absorption, inflammatory state, metabolic acidosis, and dialysate losses all put the CKD patient at risk for micronutrient deficiencies (28). Studies in CKD patients including dialysis and non-dialysis patients shows a decrease in the intake of micronutrients such as vitamins, folate, iron, and pantothenic acid (29). A recent examination of a cohort of children with CKD showed that 28% were deficient in 25 hydroxyvitamin D (30). Losses of zinc, selenium, folic acid, pyridoxine and ascorbic acid during hemodialysis are well documented. Despite the limited intake and dialysate losses of micronutrients, appropriate levels of supplementation of these micronutrients in CKD patients are yet to be clearly determined. For instance, multivitamin supplementation in children on both hemodialysis and peritoneal dialysis have been shown to result in intakes exceeding the recommended daily allowance for these vitamins (28, 29, 31). The 2009 KDOQI pediatric nutrition guidelines “suggested that supplementation of vitamins and trace elements be provided to children with CKD stages 2–5 if dietary intake alone does not meet 100% of the DRI or if clinical evidence of a deficiency, possibly confirmed by low blood levels of the vitamin or trace element, is present. It is suggested that children with CKD stage 5D receive a water-soluble vitamin supplement” (27). However, these recommendations are based mostly on limited and low quality evidence and expert opinion. A recent systematic analysis has concluded that there is insufficient evidence to support the routine supplementation of vitamins in patients on hemodialysis and an individualized approach to supplementation is recommended (32).
Pathophysiologic Mechanisms of Protein Energy Wasting in Chronic Kidney Disease
Multiple risk factors underlie the pathogenesis of malnutrition in patients with CKD. The following is an overview of the various mechanisms involved.
Metabolic Dysfunction in Chronic Kidney Disease
The metabolic milieu in CKD is significantly altered due to the progressive accumulation of metabolic by-products that are naturally cleared by the kidneys. Metabolic derangements such as metabolic acidosis, hyperparathyroidism, insulin resistance, upregulation of the renin angiotensin aldosterone system and dyslipidemia are common in CKD (33). Metabolic acidosis occurs early in CKD because of reduced excretion of the acid load generated by metabolic activity. Multiple studies have shown an association between metabolic acidosis and increased protein catabolism in patients with CKD (34–36). Proteolysis induced by an up regulation of the ubiquitin-proteasome system, also facilitates the degradation of whole body protein. Metabolic acidosis, chronic inflammation, insulin resistance and increased angiotensin II levels, all of which are seen in CKD, also stimulate the ubiquitin-proteasome system of enzymes (36, 37). As a result, muscle wasting and malnutrition ensue. Uremic toxins, which are progressively retained in CKD, are also known to inhibit lipoprotein lipase and hepatic lipase resulting in lack of degradation of lipids and dyslipidemia (38). Correction of metabolic derangements through the provision of adequate dialysis and use of available medical therapies is an important treatment strategy in the management of malnutrition in CKD (36). Although several reports have suggested an increase in resting energy expenditure in patients with CKD, others have suggested that non dialyzed CKD patients' resting energy expenditure is similar or even lower than their healthy age matched controls (39, 40).
Hormonal Imbalance and Appetite Regulation in Chronic Kidney Disease
The kidneys play a major role in the synthesis and regulation of a wide variety of hormones in the body. As kidney function declines, hormonal imbalance becomes a characteristic feature and this has been implicated in the suppression of appetite, muscle wasting and growth impairment in CKD. Insulin resistance occurs early in CKD. Using the euglycemic insulin clamp technique, DeFronzo and colleagues were able to demonstrate reduced glucose metabolism and sensitivity to insulin in uremic patients (41). This technique allows for assessment of sensitivity of skeletal muscle to insulin, which is the dominant site of insulin resistance in CKD. Insulin resistance in these patients leads to increased protein catabolism and muscle wasting. Additionally, insulin resistance has been implicated in the progressive deterioration of kidney function in CKD (14, 42, 43).
Growth impairment, defined by height standard deviation score < −1.88 (less than the 3rd percentile), is common in children with CKD and is associated with poor outcomes. The growth hormone (GH), insulin-like growth factor-1 (IGF-1) and insulin-like growth factor binding protein (IGF-1BP) axis is significantly altered in CKD. Growth impairment occurs as a result of tissue resistance to the effects of GH and not deficiency per se, since levels of these hormones are known to be normal or elevated in CKD (14, 26, 44). Excessive water and salt loss, especially in children with congenital renal disorders, contribute to growth impairment in chronic kidney disease (45).
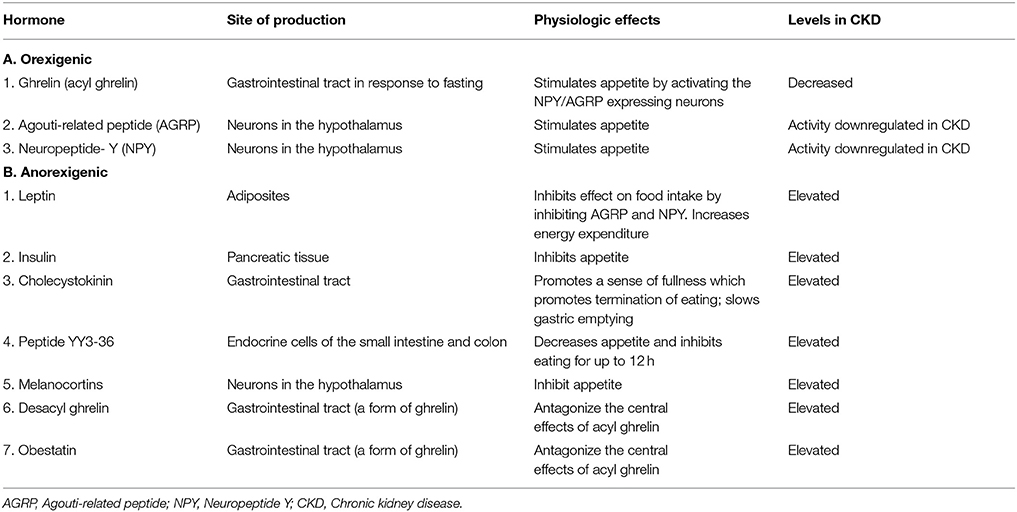
Another common feature of CKD is anorexia, which worsens with deterioration of kidney function. Anorexia leads to decreased nutritional intake, which predisposes to protein energy wasting and micronutrient deficiency in patients with CKD. The mechanism of appetite suppression in these patients is attributed to complex dysregulation of neuroendocrine pathways involving orexigenic (appetite stimulating) and anorexigenic (appetite inhibiting) substances (Table 4). The role of insulin (produced by the pancreas), leptin (produced by adipose tissue), ghrelin (produced in the gastrointestinal tract), and pro-inflammatory cytokines including IL-6 and TNF-alpha in CKD related anorexia have been investigated in recent years. Insulin and leptin are hormones whose levels are known to be elevated in CKD. Elevated levels of insulin and leptin have been implicated in the suppression of appetite and stimulation of energy expenditure in patients with CKD (46, 47). Leptin belongs to the family of IL-6 cytokines and also plays a pro-inflammatory role in CKD (48). Interleukin-6 and TNF-alpha are also involved in appetite suppression in CKD (46, 47, 49, 50). Ghrelin is an appetite-stimulating hormone secreted by the stomach. Its role in anorexia in CKD has been extensively investigated. It is typically released in response to fasting and has potent appetite stimulatory properties (51). Ghrelin exists in 3 different forms including acyl ghrelin, desacyl ghrelin and obestatin, each of which has a different effect on appetite (Table 4). While some studies have demonstrated elevated levels of ghrelin in patients with CKD, others have found no difference in the levels when compared to healthy control subjects (48, 52–55). Administration of ghrelin in CKD subjects has been shown to improve food intake and lean body mass and could represent a therapeutic approach to the treatment of protein energy malnutrition in these patients (56–58). It is important to remember that medication use in CKD can also affect taste and appetite, decreasing nutritional intake.
Inflammation and Protein Energy Wasting in Chronic Kidney Disease
Chronic systemic inflammation is highly prevalent in patients with CKD and is associated with increased disease burden. Markers of inflammation including pro-inflammatory cytokines IL-6, IL-1β, IL-18, IL-6, TNFα, IL-8 and C - reactive protein (CRP) levels are elevated in patients with CKD and have been linked to increased mortality rates in these patients. Hypoalbuminemia and elevated ferritin levels are other markers of inflammation, with hypoalbuminemia being a strong predictor of mortality in these patient (11, 23, 59). Increased hospitalization rates, resistance to erythropoietin stimulating agents, endothelial vascular injury, coronary calcification, increased resting energy expenditure and catabolism leading to protein energy wasting are all known consequences of chronic inflammation in CKD in both children and adults (12, 23, 60–62). Chronic inflammatory state induces anorexia, decreases protein and caloric intake and reduces synthesis of albumin leading to protein energy wasting and hypoalbuminemia (63). Recent studies have proposed the use of the malnutrition-inflammation score (MIS) to more accurately define the malnutrition and inflammatory burden in patients with CKD, rather than the use of individual indices. This score has shown a strong correlation with CRP and superiority in predicting poor outcomes in dialysis patients (64–66). A comprehensive and multifaceted approach to the treatment of inflammation in patients with CKD might mitigate malnutrition and lead to more favorable outcomes.
Altered Bowel Flora in Chronic Kidney Disease
The intestinal microbial flora is significantly altered in patients with CKD and this has been thought to play a pathogenic role in the chronic inflammatory state seen in CKD. Quantitative studies have shown a reduction in the total number and composition of bacteria in patients with end stage kidney disease (67). These changes lead to the generation and systemic accumulation of pro-inflammatory uremic toxins including indoxyl sulfate, p-cresyl sulfate, amines, ammonia and rimethylamine-N-oxide. Moreover, disruption of the intestinal epithelial barrier in patients with CKD facilitates the systemic absorption of these toxins. These uremic toxins induce inflammation, endothelial injury, cardiovascular disease and protein energy wasting (68, 69). Therapeutic strategies aimed at normalizing intestinal microbiota in patients with CKD might help ameliorate chronic inflammation, malnutrition and other comorbidities.
Effect of Gastroparesis on Nutritional Status in Chronic Kidney Disease
Delayed gastric emptying (gastroparesis) is common in patients with CKD, as demonstrated in multiple gastric emptying studies (70–72). The elevated levels of gastrointestinal hormones including gastrin, cholecystokinin and gastric inhibitory polypeptide may alter gastric motor function in CKD patients (73). Gastroparesis is associated with gastrointestinal symptoms such as dyspepsia, early satiety, bloating, vomiting, and gastroesophageal reflux. These symptoms contribute to decreased nutritional intake in no small measure and are an important cause of malnutrition in patients with chronic kidney disease (71). Dialysis therapy and the use of prokinetic agents such as erythromycin or metoclopramide have been shown to improve gastrointestinal motor function in these patients (70, 74).
Effect of Dialysis Procedure and Dialysis Dose on Nutrition in Chronic Kidney Disease
While malnutrition is common in dialysis patients in general, the dialysis technique itself might contribute to nutritional deficits in unique ways. For instance, hemodialysis patients have higher levels of CRP, inflammation, oxidative stress and increased protein muscle breakdown when compared to other CKD patients. This has been attributed to the induction of a cascade of inflammatory pathways when blood comes in contact with the dialyzer membrane (61, 75). As previously discussed, losses of albumin are enhanced in peritoneal dialysis, putting these patients at greater risk for protein energy malnutrition (7, 24). Conversely, less than optimal or under-dialysis might lead to decreased clearance of uremic toxins, predisposing patients to the development of malnutrition. Multiple studies have investigated the impact of dialysis dose on the nutritional status of dialysis patients and found an improvement in the nutritional status of those patients receiving more dialysis (76–80). At a minimum, patients receiving dialysis should be provided the KDOQI recommended dialysis dose for both hemodialysis and peritoneal dialysis to avoid under-dialysis.
Therapeutic Considerations
Treatment of malnutrition in patients with CKD requires a multidisciplinary, multifaceted and individualized approach. The renal dietitian is central to the assessment and monitoring of the nutritional status of the child with CKD. Other team members include the nephrologist, nurses, social workers, caregivers and therapists. The KDOQI recommended clinical parameters for the assessment of nutritional status in children with chronic kidney disease are depicted in Table 1. These recommendations emphasize the need to integrate these parameters as no single marker can give an accurate assessment of nutritional status. Once a comprehensive assessment has been made, an individualized dietary prescription should ensure adequate intake of calories, micronutrients and protein to promote growth and development of the child (Table 2). Supplemental feeding through nasogastric and gastric tubes might be necessary to provide adequate intake. Treatment of comorbidities such as metabolic acidosis, gastroesophageal reflux disease, and constipation are necessary for effective management of malnutrition. The provision of adequate dialysis cannot be overemphasized in patients on chronic dialysis therapy. Finally, careful and continuous monitoring of nutritional status is essential for sustaining growth and development and the recommended intervals can be found in the KDOQI clinical practice guidelines (27).
Summary and Future Directions
Chronic kidney disease creates a complex pathologic environment characterized by metabolic alterations that affect nutrient intake, metabolism and energy expenditure, predisposing patients to the development of malnutrition and an increased risk for morbidity and mortality. Inflammation, metabolic acidosis, uremic toxins and hormonal dysregulation all play significant roles in the pathogenesis of protein energy wasting and growth failure in children with CKD. Supplementation of protein and calories alone may not adequately control malnutrition. A multifaceted approach including the treatment of comorbidities is necessary. Therapeutic interventions that specifically target inflammation and other metabolic derangements are essential for the successful treatment of malnutrition in these patients. A better understanding of the mechanisms involved in hormonal regulation of appetite in patients with CKD might lead to the development of novel and effective therapies for malnutrition.
Author Contributions
The author confirms being the sole contributor of this work and approved it for publication.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
I wish to thank Dr. Kanwal Kher for reviewing this article prior to submission.
References
1. Becker PJ, Nieman Carney L, Corkins MR, Monczka J, Smith E, Smith SE, et al. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: indicators recommended for the identification and documentation of pediatric malnutrition (undernutrition). J Acad Nutr Diet. (2014) 114:1988–2000. doi: 10.1016/j.jand.2014.08.026
2. Grover Z, Ee LC. Protein energy malnutrition. Pediatr Clin North Am. (2009) 56:1055–68. doi: 10.1016/j.pcl.2009.07.001
3. Holliday MA. Calorie deficiency in children with uremia: effect upon growth. Pediatrics (1972) 50:590–7.
4. Rigden SP, Start KM, Rees L. Nutritional management of infants and toddlers with chronic renal failure. Nutr Health (1987) 5:163–74. doi: 10.1177/026010608700500407
5. Foreman JW, Abitbol CL, Trachtman H, Garin EH, Feld LG, Strife CF, et al. Nutritional intake in children with renal insufficiency: a report of the growth failure in children with renal diseases study. J Am Coll Nutr. (1996) 15:579–85. doi: 10.1080/07315724.1996.10718633
6. Sozeri B, Mir S, Kara OD, Dincel N. Growth impairment and nutritional status in children with chronic kidney disease. Iran J Pediatr. (2011) 21:271–7.
7. Brem AS, Lambert C, Hill C, Kitsen J, Shemin DG. Prevalence of protein malnutrition in children maintained on peritoneal dialysis. Pediatr Nephrol. (2002) 17:527–30. doi: 10.1007/s00467-002-0886-2
8. Apostolou A, Printza N, Karagiozoglou-Lampoudi T, Dotis J, Papachristou F. Nutrition assessment of children with advanced stages of chronic kidney disease-A single center study. Hippokratia (2014) 18:212–6.
9. Dai L, Mukai H, Lindholm B, Heimbürger O, Barany P, Stenvinkel P, et al. Clinical global assessment of nutritional status as predictor of mortality in chronic kidney disease patients (2017) 12:e0186659. doi: 10.1371/journal.pone.0186659
10. Kang SS, Chang JW, Park Y. Nutritional status predicts 10-year mortality in patients with end-stage renal disease on hemodialysis. Nutrients (2017) 9:E399. doi: 10.3390/nu9040399
11. Wong CS, Hingorani S, Gillen DL, Sherrard DJ, Watkins SL, Brandt JR, et al. Hypoalbuminemia and risk of death in pediatric patients with end-stage renal disease. Kidney Int. (2002) 61:630–7. doi: 10.1046/j.1523-1755.2002.00169.x
12. Canpolat N, Caliskan S, Sever L, Tasdemir M, Ekmekci OB, Pehlivan G, et al. Malnutrition and its association with inflammation and vascular disease in children on maintenance dialysis. Pediatr Nephrol. (2013) 28:2149–56. doi: 10.1007/s00467-013-2527-3
13. Mastrangelo A, Paglialonga F, Edefonti A. Assessment of nutritional status in children with chronic kidney disease and on dialysis. Pediatr Nephrol. (2014) 29:1349–58. doi: 10.1007/s00467-013-2612-7
14. Zha Y, Qian Q. Protein nutrition and malnutrition in CKD and ESRD. Nutrients (2017) 9:E208. doi: 10.3390/nu9030208
15. Kohaut EC. Chronic renal disease and growth in childhood. Curr Opin Pediatr. (1995) 7:171–5. doi: 10.1097/00008480-199504000-00010
16. Mak RH. Cachexia in children with chronic kidney disease: challenges in diagnosis and treatment. Curr Opin Support Palliat Care (2016) 10:293–7. doi: 10.1097/SPC.0000000000000217
17. Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. (2008) 73:391–8. doi: 10.1038/sj.ki.5002585
18. Ingulli EG, Mak RH. Growth in children with chronic kidney disease: Role of nutrition, growth hormone, dialysis, and steroids. Curr Opin Pediatr. (2014) 26:187–92. doi: 10.1097/MOP.0000000000000070
19. Müller O, Krawinkel M. Malnutrition and health in developing countries. CMAJ (2005) 173:279–86. doi: 10.1503/cmaj.050342
20. Ikizler TA, Cano NJ, Franch H, Fouque D, Himmelfarb J, Kalantar-Zadeh K, et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: a consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. (2013) 84:1096–107. doi: 10.1038/ki.2013.147
21. Carrero JJ, Stenvinkel P, Cuppari L, Ikizler TA, Kalantar-Zadeh K, Kaysen G, et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: a consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J Ren Nutr. (2013) 23:77–90. doi: 10.1053/j.jrn.2013.01.001
22. Kaysen GA, Greene T, Daugirdas JT, Kimmel PL, Schulman GW, Toto RD, et al. Longitudinal and cross-sectional effects of C-reactive protein, equilibrated normalized protein catabolic rate, and serum bicarbonate on creatinine and albumin levels in dialysis patients. Am J Kidney Dis. (2003) 42:1200–11. doi: 10.1053/j.ajkd.2003.08.021
23. Utaka S, Avesani CM, Draibe SA, Kamimura MA, Andreoni S, Cuppari L. Inflammation is associated with increased energy expenditure in patients with chronic kidney disease. Am J Clin Nutr. (2005) 82:801–5. doi: 10.1093/ajcn/82.4.801
24. Quan A, Baum M. Protein losses in children on continuous cycler peritoneal dialysis. Pediatr Nephrol. (1996) 10:728–31. doi: 10.1007/s004670050200
25. Canepa A, Perfumo F, Carrea A, Menoni S, Trivelli A, Delucchi P, et al. Nutritional status in children receiving chronic peritoneal dialysis. Perit Dial Int. (1996) 16 (Suppl.1):S526–31.
26. Rees L, Shaw V. Nutrition in children with CRF and on dialysis. Pediatr Nephrol. (2007) 22:1689–702. doi: 10.1007/s00467-006-0279-z
27. KDOQI Work Group. KDOQI Clinical Practice Guideline for Nutrition in Children with CKD: 2008 update. Executive summary. Am J Kidney Dis. (2009) 53(3 Suppl. 2):S11–S104. doi: 10.1053/j.ajkd.2008.11.017
28. Jankowska M, Rutkowski B, Debska-Slizien A. Vitamins and microelement bioavailability in different stages of chronic kidney disease. Nutrients (2017) 9:E282. doi: 10.3390/nu9030282
29. Pereira AM, Hamani N, Nogueira PC, Carvalhaes JT. Oral vitamin intake in children receiving long-term dialysis. J Ren Nutr. (2000) 10:24–9. doi: 10.1016/S1051-2276(00)90019-0
30. Kumar J, McDermott K, Abraham AG, Friedman LA, Johnson VL, Kaskel FJ, et al. Prevalence and correlates of 25-hydroxyvitamin D deficiency in the Chronic Kidney Disease in Children (CKiD) cohort. Pediatr Nephrol. (2016) 31:121–9. doi: 10.1007/s00467-015-3190-7
31. Steiber AL, Kopple JD. Vitamin status and needs for people with stages 3-5 chronic kidney disease. J Ren Nutr. (2011) 21:355–68. doi: 10.1053/j.jrn.2010.12.004
32. Tucker BM, Safadi S, Friedman AN. Is routine multivitamin supplementation necessary in US chronic adult hemodialysis patients? A systematic review. J Ren Nutr. (2015) 25:257–64. doi: 10.1053/j.jrn.2014.09.003
33. Slee AD. Exploring metabolic dysfunction in chronic kidney disease. Nutr Metab (Lond) (2012) 9:36. doi: 10.1186/1743-7075-9-36
34. Boirie Y, Broyer M, Gagnadoux MF, Niaudet P, Bresson JL. Alterations of protein metabolism by metabolic acidosis in children with chronic renal failure. Kidney Int. (2000) 58:236–41. doi: 10.1046/j.1523-1755.2000.00158.x
35. Wassner SJ, Holliday MA. Protein metabolism in chronic renal failure. Semin Nephrol. (1989) 9:19–23.
36. Pickering WP, Price SR, Bircher G, Marinovic AC, Mitch WE, Walls J. Nutrition in CAPD: serum bicarbonate and the ubiquitin-proteasome system in muscle. Kidney Int. (2002) 61:1286–92. doi: 10.1046/j.1523-1755.2002.00276.x
37. Rajan V, Mitch WE. Ubiquitin, proteasomes and proteolytic mechanisms activated by kidney disease. Biochim Biophys Acta (2008) 1782:795–9. doi: 10.1016/j.bbadis.2008.07.007
38. Stegmayr B. Uremic toxins and lipases in haemodialysis: a process of repeated metabolic starvation. Toxins (Basel) (2014) 6:1505–11. doi: 10.3390/toxins6051505
39. Avesani CM, Kamimura MA, Cuppari L. Energy expenditure in chronic kidney disease patients. J Ren Nutr. (2011) 21:27–30. doi: 10.1053/j.jrn.2010.10.013
40. Mak RH, Cheung W. Energy homeostasis and cachexia in chronic kidney disease. Pediatr Nephrol. (2006) 21:1807–14. doi: 10.1007/s00467-006-0194-3
41. DeFronzo RA, Alvestrand A, Smith D, Hendler R, Hendler E, Wahren J. Insulin resistance in uremia. J Clin Invest. (1981) 67:563–8. doi: 10.1172/JCI110067
42. Spoto B, Pisano A, Zoccali C. Insulin resistance in chronic kidney disease: a systematic review. Am J Physiol Renal Physiol. (2016) 311:F1087–108. doi: 10.1152/ajprenal.00340.2016
43. Siew ED, Pupim LB, Majchrzak KM, Shintani A, Flakoll PJ, Ikizler TA. Insulin resistance is associated with skeletal muscle protein breakdown in non-diabetic chronic hemodialysis patients. Kidney Int. (2007) 71:146–52. doi: 10.1038/sj.ki.5001984
44. Roelfsema V, Clark RG. The growth hormone and insulin-like growth factor axis: its manipulation for the benefit of growth disorders in renal failure. J Am Soc Nephrol. (2001) 12:1297–306.
45. Salas P, Pinto V, Rodriguez J, Zambrano MJ, Mericq V. Growth retardation in children with kidney disease. Int J Endocrinol. (2013) 2013:970946. doi: 10.1155/2013/970946
46. Mitch WE. Cachexia in chronic kidney disease: a link to defective central nervous system control of appetite. J Clin Invest (2005) 115:1476–8. doi: 10.1172/JCI25255
47. Oner-Iyidogan Y, Gurdol F, Kocak H, Oner P, Cetinalp-Demircan P, Caliskan Y, et al. Appetite-regulating hormones in chronic kidney disease patients. J Ren Nutr. (2011) 21:316–21. doi: 10.1053/j.jrn.2010.07.005
48. Stenvinkel P, Pecoits-Filho R, Lindholm B. Leptin, ghrelin, and proinflammatory cytokines: compounds with nutritional impact in chronic kidney disease? Adv Ren Replace Ther. (2003) 10:332–45. doi: 10.1053/j.arrt.2003.08.009
49. Mak RH, Cheung W, Cone RD, Marks DL. Orexigenic and anorexigenic mechanisms in the control of nutrition in chronic kidney disease. Pediatr Nephrol. (2005) 20:427–31. doi: 10.1007/s00467-004-1789-1
50. Mafra D, Jolivot A, Chauveau P, Drai J, Azar R, Michel C, et al. Are ghrelin and leptin involved in food intake and body mass index in maintenance hemodialysis? J Ren Nutr. (2010) 20:151–7. doi: 10.1053/j.jrn.2009.08.007
51. Inui A. Ghrelin: an orexigenic and somatotrophic signal from the stomach. Nat Rev Neurosci. (2001) 2:551–60. doi: 10.1038/35086018
52. Szczepanska M, Szprynger K, Mazur B, Zwolinska D, Kiliś-Pstrusinska K, Makulska I. Plasma ghrelin levels in children with chronic renal failure on peritoneal dialysis. Perit Dial Int. (2007) 27:61–6.
53. Caliskan Y, Yelken B, Gorgulu N, Ozkok A, Yazici H, Telci A, et al. Comparison of markers of appetite and inflammation between hemodialysis patients with and without failed renal transplants. J Ren Nutr. (2012) 22:258–67. doi: 10.1053/j.jrn.2011.07.005
54. Arbeiter AK, Büscher R, Petersenn S, Hauffa BP, Mann K, Hoyer PF. Ghrelin and other appetite-regulating hormones in paediatric patients with chronic renal failure during dialysis and following kidney transplantation. Nephrol Dial Transplant (2009) 24:643–6. doi: 10.1093/ndt/gfn529
55. Eftekhari MH, Ranjbar-Zahedani M, Basiratnia M, Rezaianzadeh A, Faghih S. Comparison of appetite-regulating hormones and body composition in pediatric patients in predialysis stage of chronic kidney disease and healthy control group. Iran J Med Sci. (2015) 40:27–33.
56. Deboer MD, Zhu X, Levasseur PR, Inui A, Hu Z, Han G, et al. Ghrelin treatment of chronic kidney disease: improvements in lean body mass and cytokine profile. Endocrinology (2008) 149:827–35. doi: 10.1210/en.2007-1046
57. Bossola M, Tazza L, Luciani G. Mechanisms and treatment of anorexia in end-stage renal disease patients on hemodialysis. J Ren Nutr. (2009) 19:2–9. doi: 10.1053/j.jrn.2008.10.003
58. Barazzoni R, Gortan Cappellari G, Zanetti M, Guarnieri G. Ghrelin and muscle metabolism in chronic uremia. J Ren Nutr. (2012) 22:171–5. doi: 10.1053/j.jrn.2011.10.017
59. Castillo-Rodríguez E, Pizarro-Sánchez S, Sanz AB, Ramos AM, Sanchez-Ni-o MD, Martin-Cleary C, et al. Inflammatory Cytokines as Uremic Toxins: “Ni Son Todos Los Que Estan, Ni Estan Todos Los Que Son”. Toxins (Basel) (2017) 9:E114. doi: 10.3390/toxins9040114
60. Srivaths PR, Silverstein DM, Leung J, Krishnamurthy R, Goldstein SL. Malnutrition-inflammation-coronary calcification in pediatric patients receiving chronic hemodialysis. Hemodial Int. (2010) 14:263–9. doi: 10.1111/j.1542-4758.2010.00442.x
61. Sylvestre LC, Fonseca KPD, Stinghen AEM, Pereira AM, Meneses RP, Pecoits-Filho R. The malnutrition and inflammation axis in pediatric patients with chronic kidney disease. Pediatr Nephrol. (2007) 22:864–73. doi: 10.1007/s00467-007-0429-y
62. Meuwese CL, Carrero JJ, Stenvinkel P. Recent insights in inflammation-associated wasting in patients with chronic kidney disease. Contrib Nephrol. (2011) 171:120–6. doi: 10.1159/000327228
63. Jankowska M, Cobo G, Lindholm B, Stenvinkel P. Inflammation and protein-energy wasting in the uremic milieu. Contrib Nephrol. (2017) 191:58–71. doi: 10.1159/000479256
64. Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis. (2001) 38:1251–63. doi: 10.1053/ajkd.2001.29222
65. Iorember FM, Bamgbola OF. Pilot validation of objective malnutrition-inflammation scores in pediatric and adolescent cohort on chronic maintenance dialysis. SAGE Open Med. (2014) 2:2050312114555564. doi: 10.1177/2050312114555564
66. Naeeni AE, Poostiyan N, Teimouri Z, Mortazavi M, Soghrati M, Poostiyan E, et al. Assessment of severity of malnutrition in peritoneal dialysis patients via malnutrition: inflammatory score. Adv Biomed Res. (2017) 6:128. doi: 10.4103/abr.abr_554_13
67. Jiang S, Xie S, Lv D, Wang P, He H, Zhang T, et al. Alteration of the gut microbiota in Chinese population with chronic kidney disease. Sci Rep. (2017) 7:2870. doi: 10.1038/s41598-017-02989-2
68. Mafra D, Lobo JC, Barros AF, Koppe L, Vaziri ND, Fouque D. Role of altered intestinal microbiota in systemic inflammation and cardiovascular disease in chronic kidney disease. Future Microbiol. (2014) 9:399–410. doi: 10.2217/fmb.13.165
69. Vaziri ND, Zhao Y-Y, Pahl MV. Altered intestinal microbial flora and impaired epithelial barrier structure and function in CKD: the nature, mechanisms, consequences and potential treatment. Nephrol Dial Transplant (2016) 31:737–46. doi: 10.1093/ndt/gfv095
70. Salles Junior LD, Santos PR, dos Santos AA, de Souza MHLP. Dyspepsia and gastric emptying in end-stage renal disease patients on hemodialysis. BMC Nephrol. (2013) 14:275. doi: 10.1186/1471-2369-14-275
71. Stompór T, Hubalewska-Hola A, Staszczak A, Sulowicz W, Huszno B, Szybinski Z. Association between gastric emptying rate and nutritional status in patients treated with continuous ambulatory peritoneal dialysis. Perit Dial Int. (2002) 22:500–5.
72. Strid H, Simrén M, Stotzer P-O, Abrahamsson H, Björnsson ES. Delay in gastric emptying in patients with chronic renal failure. Scand J Gastroenterol. (2004) 39:516–20. doi: 10.1080/00365520410004505
73. Owyang C, Miller LJ, DiMagno EP, Brennan LA, Go VL. Gastrointestinal hormone profile in renal insufficiency. Mayo Clin Proc. (1979) 54:769–73.
74. Ross EA, Koo LC. Improved nutrition after the detection and treatment of occult gastroparesis in nondiabetic dialysis patients. Am J Kidney Dis. (1998) 31:62–6. doi: 10.1053/ajkd.1998.v31.pm9428453
75. Liakopoulos V, Roumeliotis S, Gorny X, Dounousi E, Mertens PR. Oxidative stress in hemodialysis patients: a review of the literature. Oxid Med Cell Longev. (2017) 2017:3081856. doi: 10.1155/2017/3081856
76. Rashidi AA, Soleimani AR, Nikoueinejad H, Sarbolouki S. The evaluation of increase in hemodialysis frequency on C-reactive protein levels and nutritional status. Acta Med Iran (2013) 51:119–24.
77. Schulman G. Nutrition in daily hemodialysis. Am J Kidney Dis. (2003) 41(3 Suppl 1):S112–5. doi: 10.1053/ajkd.2003.50098
78. Teixeira Nunes F, de Campos G, Xavier de Paula SM, Merhi VAL, Portero-McLellan KC, da Motta DG, et al. Dialysis adequacy and nutritional status of hemodialysis patients. Hemodial Int. (2008) 12:45–51. doi: 10.1111/j.1542-4758.2008.00239.x
79. van Hoeck KJM, Rusthoven E, Vermeylen L, Vandesompel A, Marescau B, Lilien M, et al. Nutritional effects of increasing dialysis dose by adding an icodextrin daytime dwell to Nocturnal Intermittent Peritoneal Dialysis (NIPD) in children. Nephrol Dial Transplant (2003) 18:1383–7. doi: 10.1093/ndt/gfg120
Keywords: malnutrition, undernutrition, chronic kidney disease, dialysis, protein energy wasting, nutrient deficiency
Citation: Iorember FM (2018) Malnutrition in Chronic Kidney Disease. Front. Pediatr. 6:161. doi: 10.3389/fped.2018.00161
Received: 27 March 2018; Accepted: 15 May 2018;
Published: 20 June 2018.
Edited by:
Sun-Young Ahn, Children's National Health System, United StatesReviewed by:
Dagmara Borzych-Duzalka, Gdańsk Medical University, PolandT. Keefe Davis, Washington University in St. Louis, United States
Copyright © 2018 Iorember. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Franca M. Iorember, ZmlvcmVtYmVyQHBob2VuaXhjaGlsZHJlbnMuY29t
 Franca M. Iorember
Franca M. Iorember