- 1Division of Neonatology, Department of Pediatrics, University of Kentucky, Lexington, KY, United States
- 2Department of Pediatrics, Johns Hopkins All Children's Hospital in Florida, St. Petersburg, FL, United States
- 3Department of Biostatistics, College of Public Health, University of Kentucky, Lexington, KY, United States
Objectives: To compare the Shennan's and the consensus definition of Bronchopulmonary Dysplasia (BPD) from the National Institutes of Health (NIH) workshop and analyze specific risk factors associated with each definition.
Study design: Retrospective analysis of records of 274 infants admitted to a level IV intensive care unit. Infants were classified as having BPD or no BPD by both definitions. Differences in incidence and risk factors were analyzed. Statistical methods included descriptive statistics, comparative tests, and marginal logistic regression modeling.
Results: The estimated difference in prevalence was 32% [95% CI: (26%, 37%), (p < 0.0001)] between both criteria. The prevalence of BPD was 80% higher based on the NIH criteria [RR = 1.80; 95% CI: (1.58, 2.06)]. Infants with no BPD by the Shennan definition were breathing room air with or without positive or continuous pressure support and were most likely to be discharged home on oxygen [OR = 4.47, 95% CI: (1.20, 16.61), p = 0.03]. Gestational age, birth weight, and 1-min Apgar score predicted BPD by both definitions. Chorioamnionitis increased the risk of BPD by the Shennan definition but was associated with lower risk by the NIH criteria. IUGR was associated with BPD by the Shennan definition and with severe BPD by the NIH criteria.
Conclusion: Compared to the Shennan's definition, the NIH consensus identified 80% more infants with BPD and is a better predictor of oxygen requirement at discharge. Until a new better criteria is develop, the NIH consensus definition should be used across centers.
Introduction
Bronchopulmonary dysplasia (BPD) is a term coined in 1967 to describe the clinical, pathologic and radiographic features of preterm infants that require prolonged mechanical ventilation and oxygen support (1). Preterm infants initially developed hyaline membrane disease and, despite the high mortality rate, survived, but with severe mucosal, alveolar, and vascular changes due to the prolonged exposure to high ventilator pressures and oxygen (1). As survival improved and new advances in neonatal care were introduced, infants developed a different BPD, sometimes described as a “new BPD,” which was characterized by an arrest in alveolar development, less fibrosis, and a more uniform inflammation (2, 3). In spite of the advances in the identification of factors that are related to the development of BPD (4–10) and its prevention (11, 12), BPD continues to be the most prevalent sequelae among survivors following preterm birth (13, 14).
Defining BPD has been a topic of debate ever since the initial criteria was proposed (3, 15–17). The original definition included infants with oxygen requirement for 28 days with associated radiographic changes (3, 17). Shennan et al. found that oxygen requirement for the first 28 days provided a poor positive predictive value for abnormal pulmonary findings as the gestational age decreases. However, oxygen need at 36 weeks Post-Menstrual Age (PMA) predicted abnormal pulmonary outcomes better (3, 18). Therefore, oxygen requirement at 36 weeks PMA became the criteria for BPD definition in infants born with birth weight (BW) < 1,500 g (18). This is the definition adopted by the Vermont-Oxford Network (19). However, the continued use of oxygen for the first 28 days is influenced by variation in clinical practices or protocols established at different centers (3, 14).
In lieu of the original definition, a new definition was proposed in 2001 as summarized by Jobe and Bancalari (3) from the discussions in a workshop organized by the National Institutes of Health (NIH). The definition of BPD differed according to gestational age (GA). For those born at GA < 32 weeks, BPD referred to requirement of oxygen support (>21%) for at least 28 days and a subsequent assessment at 36 weeks PMA or discharge, whichever comes first. In those born with GA > 32 weeks, BPD referred to the requirement of supplemental oxygen < 21% for at least 28 days and a subsequent assessment at 56 days post-natal age or discharge, whichever comes first. At the time of this assessment, those infants with no oxygen requirement were classified as having mild BPD. Moderate BPD was diagnosed in those requiring < 30% oxygen and severe BPD in those with a need for positive pressure ventilation/continuous positive pressure (PPV/CPAP) and/or oxygen requirement ≥30% (3). With the use of the new severity-based definition, 68% of infants admitted to centers from the Neonatal Research Network were diagnosed to have BPD (27% mild, 23% moderate and 18% severe) during the years 2003–2007 (17). This definition has since been adopted by the Eunice Kennedy Shriver National Institute of Child Health and Development Neonatal Research Network (3).
Using one definition or another can cause differences in the incidence of BPD. In 2005, a study by Ehrenkranz et al. found that 44% of infants were diagnosed with BPD as defined by oxygen use at 36 weeks PMA compared to 77% by using the NIH definition (20). These findings were confirmed by Poindexter et al. who found a 10% difference in the no BPD diagnosis between both definitions (21). Despite this, different centers across the country, as well as several multicenter trials and quality improvement initiatives, use either definition in characterizing infants with or without BPD (13, 20, 22–25). This study aimed to compare the BPD definitions by NIH and Shennan in an effort to understand how the differences between these two definitions would have an impact in clinical practice and follow up after discharge. As a secondary objective, we evaluated the factors that would predict BPD by each definition.
Patients and Methods
This retrospective study was conducted with a cohort of premature infants admitted to a level IV Neonatal Intensive Care Unit (NICU) during 2013, 2014, and 2015. This study was approved by the Institutional Review Board. Electronic medical records of the study infants were reviewed and pertinent variables were entered into the password protected data base (REDCap software, Version 6.13.2 @ 2016 Vanderbilt University, Nashville TN) (26). Inclusion criteria were admission to the NICU, GA ≤ 30 weeks, and survival up to 36 weeks PMA. Exclusion criteria included GA > 30 weeks, presence of significant congenital anomalies, or death before 36 weeks PMA.
Prenatal factors, such as use of antenatal steroids, maternal chorioamnionitis, intrauterine growth restriction, preeclampsia and prolonged rupture of membranes were noted. Other variables recorded were BW, GA, inborn/outborn status, gender, Apgar scores at 1 and 5 min, mode of delivery, surfactant administration and length of stay in days. Infants who survived at 36 weeks PMA were categorized as BPD or No BPD based on the Shennan and NIH criteria. The BPD outcome of interest was need for oxygen at discharge or death after 36 weeks PMA, whichever comes first.
Statistical Analysis
Descriptive statistics included determination of either means ± standard deviations or medians with interquartile ranges (IQR) for continuous variables and frequencies and percentages for categorical variables. McNemar's test was used to compare incidences between the two different BPD criteria, and generalized estimating equations (GEE) (27) were used to estimate the difference and ratio of these incidences. Comparisons of BPD and No BPD classifications for each definition separately were conducted utilizing two-sample t-tests, Wilcoxon tests, and either chi-square or Fisher's exact tests. Comparisons of Shennan vs. NIH criteria with respect to the impacts of potential predictors on the odds of BPD were made using GEE and marginal logistic regression modeling. Furthermore, with respect to oxygen requirement at 28 days, we calculated the C statistic, an estimate for the area under the receiver-operating characteristic curve and thus the predictive accuracy of this variable, and the optimal cutoff value based on the minimum Euclidean distance and maximum sum of sensitivity and specificity. Additionally, we determined the sensitivities, specificities, positive predictive values, negative predictive values, and the proportion correctly classified based on these optimal cutoffs. Tests were two-sided, with statistical significance defined as p < 0.05. Analyses were conducted in SAS version 9.4 (SAS Institute, Cary, N.C.).
Results
A total of 247 infants fulfilled our inclusion criteria. Of these, 38 infants were evaluated at discharge and 209 at 36 weeks GA. Mean GA was 27.0 ± 1.7 weeks, mean BW was 975 ± 268 g, 58% were males and 29% of the infants were transferred to our facility. Sixty-eight percent and 12% of the infants were born after a full course and partial course of antenatal steroids, respectively. Delivery was by cesarean section in 72% of the infants. The median (range) Apgar scores were 4 (2, 6) and 6 (4, 8) at 1 and 5 min, respectively. Surfactant in the delivery room was given to 88% of the infants; of these, 31% received a second dose and 7% received a third dose. The average length of stay was 87 ± 43 days.
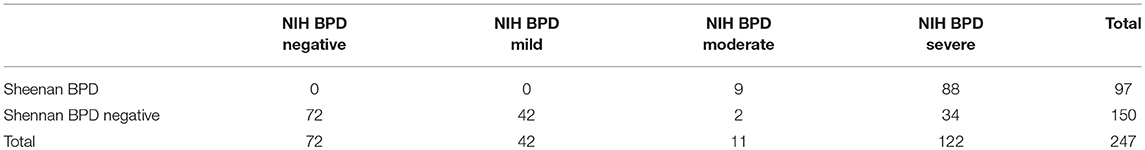
Regarding BPD classification in our cohort, 39% (97 infants) were found to require oxygen at 36 weeks and were classified to have BPD by the Shennan definition, compared to 71% (175 infants) using the NIH definition. Of those with BPD by NIH criteria, 17% (42), 4% (11), and 49% (122 infants) were classified as mild, moderate and severe BPD, respectively. Additionally, 53.9% of those with mild BPD by the NIH definition were classified as not having BPD using the Shennan definition. Table 1 depicts the classification of infants by both definitions. The difference in BPD incidence between criteria was significant (p < 0.0001). Specifically, the estimated difference in incidence was 32% [95% CI: (26%, 37%)], such that the incidence was estimated to be 80% higher based on the NIH criteria [RR = 1.80; 95% CI: (1.58, 2.06)].
Of those classified as no BPD by the Shennan's definition, 78 infants were identified as having BPD by the NIH definition. Of these, 42, 2, and 34 infants were classified as mild, moderate and severe BPD, respectively. Further analysis showed that 34/78 (43.6%) were receiving some form of positive pressure ventilation (41% on CPAP, 1.3% on NIPPV, and 1.3% on a mechanical ventilator) and the remaining 2/78 (2.6%) were on nasal cannula.
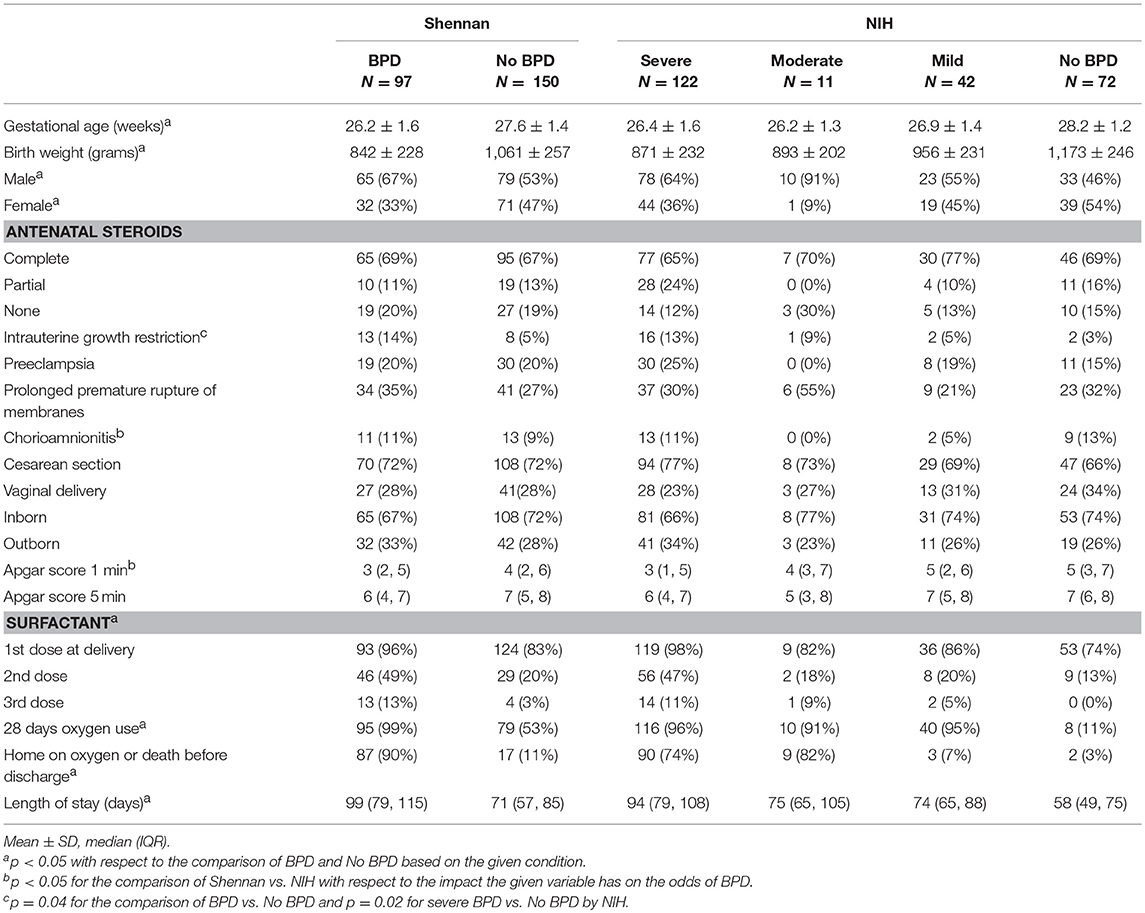
In order to evaluate how maternal, perinatal, and infant factors are predictive of BPD, we analyzed their association with each BPD definition (Table 2). An increase of 1 week in GA [Shennan-OR = 0.56, 95% CI: (0.46, 0.69), p < 0.0001; NIH-OR = 0.44, 95% CI: (0.34, 0.56), p < 0.0001], an increase of 1 in Apgar score at 1-min [Shennan-OR = 0.88, 95% CI: (0.79, 0.99), p = 0.04; NIH-OR = 0.78, 95% CI: (0.69, 0.89), p = 0.0001], and an increase of 100 gm in BW [Shennan-OR = 0.69, 95% CI: (0.60, 0.78), p < 0.0001; NIH-OR = 0.63, 95% CI: (0.55, 0.71), p < 0.0001] were associated with significant decreases in odds of BPD by either definition; however, the decrease is significantly greater when using the NIH definition (p ≤ 0.04).
Furthermore, having chorioamnionitis was estimated to increase the odds of BPD based on the Shennan's definition [OR = 1.36, 95% CI: (0.58, 3.19), p = 0.47], but decreased the odds based on the NIH definition [OR = 0.66, 95% CI: (0.28, 1.58), p = 0.35]. These disparate results between definitions (p = 0.04) correlates with the controversy that chorioamnionitis is a risk factor for BPD for some authors but not all (10, 17). Of note, IUGR was significantly associated with BPD by the Shennan definition and with severe BPD by NIH criteria.
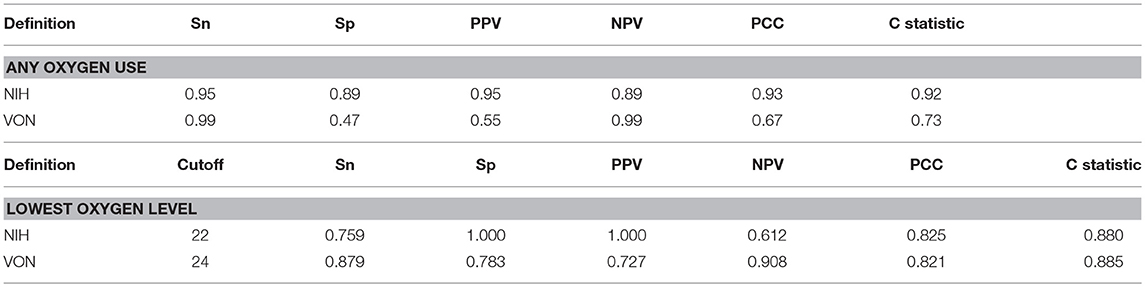
Of all the factors that were associated with BPD, oxygen use at 28 days post-natal age was the best predictor for the development of BPD by either definition. Oxygen use at 28 days had a comparable sensitivity, 95 and 99%, respectively, as predictor of BPD by NIH and the Shennan criteria. However, the use of oxygen at 28 days had a better specificity, positive predictive value and higher proportion of correctly classified when defining BPD using NIH criteria. Oxygen use at 28 days had the best negative predictive value (99%) for BPD by Shennan's definition. Further, we estimated that the optimal cutoff for oxygen requirement at 28 days were FiO2 of 0.22 and 0.24, respectively, as predictor of BPD by NIH and Shennan's criteria (Table 3).
We also assessed the need for oxygen at discharge or death before discharge after 36 weeks PMA. Of those with no BPD, 11 and 3% of the infants by Shennan and NIH criteria, respectively, were discharged home on oxygen or died before discharge. Infants who were classified as having no BPD by Shennan were more likely to be discharged home on oxygen compared to those classified as not having BPD by the NIH definition [OR = 4.47, 95% CI: (1.20, 16.61), p = 0.03].
Discussion
The incidence of BPD using the NIH criteria is 80% higher when compared to the Shennan's definition with an estimated difference of 32%. This difference is consistent with other publications (20, 21). Associated with either definition are the risk factors: BW, GA, male sex, IUGR, low Apgar scores, intubation in the delivery room, and additional surfactant doses. Infants classified as not having BPD by the Shennan criteria are more likely to go home on oxygen compared to those classified as no BPD by the NIH criteria.
Throughout the years, the characteristics and treatment of infants who developed BPD have remarkably changed since it was initially described by Northway (1). The widespread uses of antenatal steroids and surfactant administration have reduced the risk of BPD in the at-risk population (28). Nevertheless, BPD continues to be a major cause of morbidity and mortality in the extremely low birth weight (ELBW) infants (24, 28, 29). Because studies in the past several years have drawn attention to the associated unfavorable pulmonary or functional outcomes related to BPD, prediction models or scoring systems have been proposed to identify those at risk (4, 6–9, 15, 30)1. Most prediction models have included similar perinatal risk factors that we noted in our study (8, 31). In our cohort as well as in other studies, oxygen use at 28 days post-natal age was the best predictor of BPD by either definition (18), more specifically any oxygen use when using the NIH criteria. This observation has the potential to be included when developing BPD predictor models (18, 32).
The definition of BPD in a preterm infant carries an important influence in predicting future health issues or interventions (24, 29, 33–36). By Shennan's criteria 50% of infants receiving oxygen at 36 weeks PMA were destined to have abnormal pulmonary outcomes as measured by re-hospitalization secondary to respiratory infection or disease like asthma or the need for pulmonary medications, such as bronchodilators or steroids (18). Similarly, by using the NIH criteria, Ehrenkranz et al. reported an increasing incidence of adverse pulmonary outcome as BPD becomes more severe (20).
The prevalence of BPD has been used as an index of quality of care when comparing different centers (23). Specifically, it is used as an estimate of center-specific therapeutic strategy-effectiveness as well as a gauge of when management protocols need to be updated (37–39). Of concern is that in our cohort, 80% more infants were identified as having BPD by NIH compared to Shennan's definition which fails to identify infants on respiratory support at 36 weeks GA as having BPD. Contrary to expectation, the inclusion of need for respiratory support in the definition has not been widely accepted across major centers (23, 32, 40). By not correctly classifying these infants, it becomes harder for clinicians and researchers to evaluate the real impact of BPD on long term pulmonary outcomes (11, 25, 37). Those with no BPD by the Shennan definition were four times more likely to go home on oxygen compared to those with no BPD by NIH criteria. A measure that is highly predictive at 36 weeks PMA of the need for oxygen on discharge is relevant to assist families, even prior to discharge, to access resources and medical services that will be needed for weeks or months after NICU hospitalization (24, 34, 36, 39).
Bronchopulmonary dysplasia has also been associated with changes in the pulmonary vasculature of affected infants like, decreased in the cross-sectional vascular bed, increased pulmonary vascular tone and reactivity (41). Therefore, infants that are diagnosed with BPD have an increased risk of developing pulmonary artery hypertension (PH) which adds significantly to the morbidity and mortality of these infants (42). The presence of IUGR and SGA have been identify as an additional risk factor for the development of PH (31, 41, 43). However, the evaluation of PH in infants with BPD is highly variable as well as its management (31, 42). Even though none of the current definitions assist with stratification of risk for BPD/PH, a standardize use of a definition would aid in the identification of infants at risk for PH.
A third definition of BPD, the “physiologic definition,” was proposed in 2003 (44). Infants that required positive pressure and oxygen ≥0.3 at 36 weeks PMA were classified as BPD, those requiring FiO2 < 0.3 required an oxygen reduction test for up to 2 weeks based on oxygen saturations (44). However, this definition has not gained popularity. Compared to the definition of oxygen requirements at 36 weeks PMA, the “physiologic definition” took in consideration the use of positive pressure and showed a 10% reduction in the incidence of BPD with less variability among centers (45).
In summary, BPD incidence differs depending upon the definition used. Furthermore, if the definition is based on oxygen requirement alone, being in room air at 36 weeks is not necessarily a predictor of not requiring oxygen at discharge. Lacking the capability into determination of alveolar and pulmonary vascular pathology, we strongly urge for a consensus in defining BPD from a clinical perspective, if BPD is to serve as one of clinical outcome measures across centers. Indeed, a sound recommendation on which definition to use will standardized the incidence of BPD as alluded to by Bancalari et al. (25). Also the definition is to be such that it will not only identify those infants with BPD but will also be a predictor of the pulmonary care needs at discharge and follow-up.
Author Contributions
EG had primary responsibility for protocol development, outcome assessment, preliminary data analysis, and writing the manuscript. VC and AS participated in the development of the protocol, patient screening, enrollment, and writing of the manuscript. PW participated in the protocol design, was responsible for all the statistical analysis and contributed to the writing of the manuscript. HB supervised the design and execution of the study, performed the final data analyses and contributed to the writing of the manuscript.
Funding
The project described was supported by the National Center for Advancing Translational Sciences, UL1TR00017, and the Dean of the College of Medicine, University of Kentucky. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the University of Kentucky.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
1. ^Palta 1998 Evaluation of Criteria for CLD.pdf.
References
1. Northway WH Jr., Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med. (1967) 276:357–68. doi: 10.1056/NEJM196702162760701
3. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. (2001) 163:1723–9. doi: 10.1164/ajrccm.163.7.2011060
4. Sinkin RA, Cox C, Phelps DL. Predicting risk for bronchopulmonary dysplasia: selection criteria for clinical trials. Pediatrics (1990) 86:728–36.
5. Rozycki HJ, Narla L. Early versus late identification of infants at high risk of developing moderate to severe bronchopulmonary dysplasia. Pediatr Pulmonol. (1996) 21:345–52. doi: 10.1002/(SICI)1099-0496(199606)21:6<345::AID-PPUL1>3.0.CO;2-K
6. Ryan SW, Nycyk J, Shaw BN. Prediction of chronic neonatal lung disease on day 4 of life. Eur J Pediatr. (1996) 155:668–71.
7. Kim YD, Kim EA, Kim KS, Pi SY, Kang W. Scoring method for early prediction of neonatal chronic lung disease using modified respiratory parameters. J Korean Med Sci. (2005) 20:397–401. doi: 10.3346/jkms.2005.20.3.397
8. Ambalavanan N, Van Meurs KP, Perritt R, Carlo WA, Ehrenkranz RA, Stevenson DK, et al. Predictors of death or bronchopulmonary dysplasia in preterm infants with respiratory failure. J Perinatol. (2008) 28:420–6. doi: 10.1038/jp.2008.18
9. Laughon MM, Langer JC, Bose CL, Smith PB, Ambalavanan N, Kennedy KA, et al. Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. Am J Respir Crit Care Med. (2011) 183:1715–22. doi: 10.1164/rccm.201101-0055OC
10. Trembath A, Laughon MM. Predictors of bronchopulmonary dysplasia. Clin Perinatol. (2012) 39:585–601. doi: 10.1016/j.clp.2012.06.014
11. Beam KS, Aliaga S, Ahlfeld SK, Cohen-Wolkowiez M, Smith PB, Laughon MM. A systematic review of randomized controlled trials for the prevention of bronchopulmonary dysplasia in infants. J Perinatol. (2014) 34:705–10. doi: 10.1038/jp.2014.126
12. Isayama T, Iwami H, McDonald S, Beyene J. Association of noninvasive ventilation strategies with mortality and bronchopulmonary dysplasia among preterm infants: a systematic review and meta-analysis. JAMA (2016) 316:611–24. doi: 10.1001/jama.2016.10708
13. Jensen EA, Schmidt B. Epidemiology of bronchopulmonary dysplasia. Birth Defects Res A Clin Mol Teratol. (2014) 100:145–57. doi: 10.1002/bdra.23235
14. Day CL, Ryan RM. Bronchopulmonary dysplasia: new becomes old again! Pediatr Res. (2017) 81:210–3. doi: 10.1038/pr.2016.201
15. Toce SS, Farrell PM, Leavitt LA, Samuels DP, Edwards DK. Clinical and roentgenographic scoring systems for assessing bronchopulmonary dysplasia. Am J Dis Child. (1984) 138:581–5.
16. Weinstein MR, Peters ME, Sadek M, Palta M. A new radiographic scoring system for bronchopulmonary dysplasia. Newborn Lung Project. Pediatr Pulmonol. (1994) 18:284–9.
17. Kair LR, Leonard DT, Anderson JM. Bronchopulmonary dysplasia. Pediatr Rev. (2012) 33:255–63; quiz 63–4. doi: 10.1542/pir.33-6-255
18. Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics (1988) 82:527–32.
19. Network VO. 2018 Manual of Operations: Part 2. Data Definitions & Infant Data Forms (2017). Available online at: https://public.vtoxford.org/wp-content/uploads/2017/04/Manual_of_Operations_Part2_v22-1.pdf
20. Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics (2005) 116:1353–60. doi: 10.1542/peds.2005-0249
21. Poindexter BB, Feng R, Schmidt B, Aschner JL, Ballard RA, Hamvas A, et al. Comparisons and limitations of current definitions of bronchopulmonary dysplasia for the prematurity and respiratory outcomes program. Ann Am Thorac Soc. (2015) 12:1822–30. doi: 10.1513/AnnalsATS.201504-218OC
22. Kim JK, Chang YS, Sung S, Ahn SY, Yoo HS, Park WS. Trends in survival and incidence of bronchopulmonary dysplasia in extremely preterm infants at 23–26 weeks gestation. J Korean Med Sci. (2016) 31:423–9. doi: 10.3346/jkms.2016.31.3.423
23. Lapcharoensap W, Gage SC, Kan P, Profit J, Shaw GM, Gould JB, et al. Hospital variation and risk factors for bronchopulmonary dysplasia in a population-based cohort. JAMA Pediatr. (2015) 169:e143676. doi: 10.1001/jamapediatrics.2014.3676
24. Doyle LW, Carse E, Adams AM, Ranganathan S, Opie G, Cheong JLY, et al. Ventilation in extremely preterm infants and respiratory function at 8 years. N Engl J Med. (2017) 377:329–37. doi: 10.1056/NEJMoa1700827
25. Bancalari E, Jain D. Bronchopulmonary dysplasia: can we agree on a definition? Am J Perinatol. (2018) 35:537–40. doi: 10.1055/s-0038-1637761
26. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. (2009) 42:377–81. doi: 10.1016/j.jbi.2008.08.010
27. Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika (1986) 73:13–22. doi: 10.1093/biomet/73.1.13
28. Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics (2010) 126:443–56. doi: 10.1542/peds.2009-2959
29. Abman SH, Bancalari E, Jobe A. The evolution of bronchopulmonary dysplasia after 50 years. Am J Respir Crit Care Med. (2017) 195:421–4. doi: 10.1164/rccm.201611-2386ED
30. Zhang ZQ, Huang XM, Lu H. Early biomarkers as predictors for bronchopulmonary dysplasia in preterm infants: a systematic review. Eur J Pediatr. (2014) 173:15–23. doi: 10.1007/s00431-013-2148-7
31. Nagiub M, Kanaan U, Simon D, Guglani L. Risk factors for development of pulmonary hypertension in infants with bronchopulmonary dysplasia: systematic review and meta-analysis. Paediatr Respir Rev. (2017) 23:27–32. doi: 10.1016/j.prrv.2016.11.003
32. Hentschel J, Berger TM, Tschopp A, Muller M, Adams M, Bucher HU, et al. Population-based study of bronchopulmonary dysplasia in very low birth weight infants in Switzerland. Eur J Pediatr. (2005) 164:292–7. doi: 10.1007/s00431-005-1623-1
33. Bhandari A, Bhandari V. Pitfalls, problems, and progress in bronchopulmonary dysplasia. Pediatrics (2009) 123:1562–73. doi: 10.1542/peds.2008-1962
34. Carlo WA. Long-term respiratory morbidities after bronchopulmonary dysplasia despite current therapies. J Pediatr. (2014) 164:12–3. doi: 10.1016/j.jpeds.2013.09.046
35. Davis PG, Thorpe K, Roberts R, Schmidt B, Doyle LW, Kirpalani H, et al. Evaluating “old” definitions for the “new” bronchopulmonary dysplasia. J Pediatr. (2002) 140:555–60. doi: 10.1067/mpd.2002.123291
36. El Mazloum D, Moschino L, Bozzetto S, Baraldi E. Chronic lung disease of prematurity: long-term respiratory outcome. Neonatology (2014) 105:352–6. doi: 10.1159/000360651
37. Nelin LD, Bhandari V. How to decrease bronchopulmonary dysplasia in your neonatal intensive care unit today and “tomorrow”. F1000Res (2017) 6:539. doi: 10.12688/f1000research.10832.1
38. Payne NR, LaCorte M, Karna P, Chen S, Finkelstein M, Goldsmith JP, et al. Reduction of bronchopulmonary dysplasia after participation in the Breathsavers Group of the Vermont Oxford Network Neonatal Intensive Care Quality Improvement Collaborative. Pediatrics (2006) 118 Suppl. 2:S73–7. doi: 10.1542/peds.2006-0913C
39. Ronkainen E, Dunder T, Peltoniemi O, Kaukola T, Marttila R, Hallman M. New BPD predicts lung function at school age: follow-up study and meta-analysis. Pediatr Pulmonol. (2015) 50:1090–8. doi: 10.1002/ppul.23153
40. Choi CW, Kim BI, Kim EK, Song ES, Lee JJ. Incidence of bronchopulmonary dysplasia in Korea. J Korean Med Sci. (2012) 27:914–21. doi: 10.3346/jkms.2012.27.8.914
41. Khemani E, McElhinney DB, Rhein L, Andrade O, Lacro RV, Thomas KC, et al. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics (2007) 120:1260–9. doi: 10.1542/peds.2007-0971
42. Slaughter JL, Pakrashi T, Jones DE, South AP, Shah TA. Echocardiographic detection of pulmonary hypertension in extremely low birth weight infants with bronchopulmonary dysplasia requiring prolonged positive pressure ventilation. J Perinatol. (2011) 31:635–40. doi: 10.1038/jp.2010.213
43. Check J, Gotteiner N, Liu X, Su E, Porta N, Steinhorn R, et al. Fetal growth restriction and pulmonary hypertension in premature infants with bronchopulmonary dysplasia. J Perinatol. (2013) 33:553–7. doi: 10.1038/jp.2012.164
44. Walsh MC, Wilson-Costello D, Zadell A, Newman N, Fanaroff A. Safety, reliability, and validity of a physiologic definition of bronchopulmonary dysplasia. J Perinatol. (2003) 23:451–6. doi: 10.1038/sj.jp.7210963
Keywords: chronic lung disease, BPD definition, premature, infants, bronchopulmonary dysplasia
Citation: Gomez Pomar E, Concina VA, Samide A, Westgate PM and Bada HS (2018) Bronchopulmonary Dysplasia: Comparison Between the Two Most Used Diagnostic Criteria. Front. Pediatr. 6:397. doi: 10.3389/fped.2018.00397
Received: 13 September 2018; Accepted: 30 November 2018;
Published: 12 December 2018.
Edited by:
Vineet Bhandari, Drexel University, United StatesReviewed by:
Jegen Kandasamy, University of Alabama at Birmingham, United StatesTrent E. Tipple, University of Alabama at Birmingham, United States
Copyright © 2018 Gomez Pomar, Concina, Samide, Westgate and Bada. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Enrique Gomez Pomar, ZW5yaXF1ZS5nb21lekB1a3kuZWR1
 Enrique Gomez Pomar
Enrique Gomez Pomar Vanessa A. Concina1
Vanessa A. Concina1 Philip M. Westgate
Philip M. Westgate Henrietta S. Bada
Henrietta S. Bada