- 1Department of Pediatric Cardiology, Heart Center, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China
- 2Department of Pediatrics, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China
Introduction: Amiodarone is an effective anti-arrhythmic drug, but there are many clinical side effects that limit its application. There are no case reports of amiodarone-related pure red cell aplastic anemia (PRCA).
Case Presentation: Here, we present a case of amiodarone-related PRCA and hypothyroidism in a 7-month-old boy. The patient had a total anomalous pulmonary venous connection (the cardiac type) and had undergone cardiac surgery at the age of 2 months. Eleven days after the operation, atrial tachycardia was observed. Amiodarone was administered orally (15 mg/kg.d), following which the arrhythmia was under control. Subsequently, the patient was prescribed amiodarone (5 mg/kg.d) and discharged. Regular medical consultations were not conducted as required. At 7 months of age (5 months after the operation), the patient returned to the hospital for re-examination. The electrocardiogram showed intermittent sinus bradycardia, occasional junctional escape beats, hemoglobin 7.9 g/DL, and thyroid function—TSH 9.660 uIU/mL.
Results: Amiodarone was discontinued. Thyroxine was administered orally. Subsequently, the heart rate improved and TSH returned to normal levels. Nutritional therapy was recommended based on a diagnosis of nutrition-related anemia. A re-visit at 9 months of age showed that the weight was 6 kg, but the routine blood test indicated that hemoglobin was 5.9 g/DL with positive cell anemia and low reticulocyte count. Bone marrow cytology examination suggested PRCA. The hemoglobin level was gradually restored after treatment with prednisone.
Conclusion: The use of amiodarone in small infants and young children and its side effects should be carefully monitored. The potential mechanism of amiodarone-related PRCA needs further study.
Introduction
Amiodarone is a class III, broad-spectrum, anti-arrhythmic drug that is highly effective in treating both atrial and ventricular arrhythmias (1). However, it is associated with a wide variety of side effects that limit its clinical application. Adverse effects include thyroid dysfunction, pulmonary fibrosis, optic neuritis, ataxia, and hepatitis (2–4). Hematologic side effects include bone marrow granulomas, pancytopenia, hemolytic anemia, neutropenia, and thrombocytopenia (5–8). Amiodarone-related aplastic anemia is very rare, and to the best of our knowledge, only one such case has been reported in an adult (9). We describe a pediatric patient who developed pure red cell aplastic anemia (PRCA) and hypothyroidism during amiodarone therapy.
Case Report
A 7-month-old, Chinese, male patient was referred to our center for post-operative evaluation of total anomalous pulmonary venous connection (the cardiac type, with anomalous connections to the coronary sinus), which was diagnosed and operated at the age of 2 months. The patient was born after a full-term gestation, from non-consanguineous parents and the weight at birth was 3.7 kg.
The pre-operation body weight was 4.1 kg. Eleven days after the operation, atrial tachycardia was observed. Maximum heart rate was about 200 beats per minute. Amiodarone was administered orally (15 mg/kg.d), and subsequently, the arrhythmia was under control. Amiodarone was reduced to 10 mg/kg.d after 4 days and to 5 mg/kg.d after 1 week. The patient was discharged with a prescription for amiodarone (5 mg/kg.d). Regular medical consultations were not conducted as required.
Physical examination of the child at 7 months of age showed that his weight was 4 kg (3 standard deviations below the mean) and height, was 62 cm (3 standard deviations below the mean). At rest, his heart rate was slow−80 beats per minute. Blood exams showed that hemoglobin was 7.9 g/DL with positive cell anemia, and thyroid function: TSH 9.660 uIU/mL(normal reference range: 0.5–5 uIU/mL). Serum ferritin, serum iron, folic acid, and vitamin B12 were all detected at normal levels. The serum bilirubin was not high, and the urobilinogen and hemolytic tests were all negative. The electrocardiogram showed intermittent sinus bradycardia with occasional junctional escape beats. These symptoms were diagnosed as the side effects of excess amiodarone. As a result, it was discontinued. Thyroxine was administered orally. Subsequently, the heart rate improved, and TSH level returned to normal.
Re-examination at 8 months of age showed that the weight had increased by 1.3 kg; TSH was normal but the child was anemic and hemoglobin was 7.0 g/DL. Since the patient was underweight, nutritional therapy was recommended. A re-visit at 9 months of age showed that the weight was 6 kg, but a routine blood test indicated that hemoglobin was 5.9 g/DL with positive cell anemia and low reticulocyte count. Bone marrow cytology examination suggested PRCA (Figure 1). The parents denied that the child had been exposed to drugs such as chloramphenicol and ampicillin that could cause aplastic anemia. All tests were negative, including cytomegalovirus, Epstein-Barr virus, and parvovirus B19. There was no family history of anemia. Prednisone was administered orally (2 mg/kg.d). Regular follow-up in pediatric clinics, every 2–4 weeks, was recommended. Two weeks after treatment with prednisone, the hemoglobin increased to 8.2 g/DL. After 4 weeks, the hemoglobin further increased to 11.2 g/DL (Figure 2). Two months after prednisone treatment, prednisone dosage was reduced to 0.5 mg/kg.d.
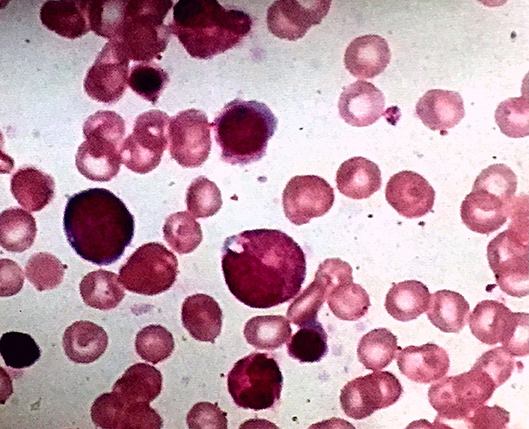
Figure 1. Bone marrow smear (×100 magnification) showing normal trilineage hematopoiesis with the presence of erythroid precursors.
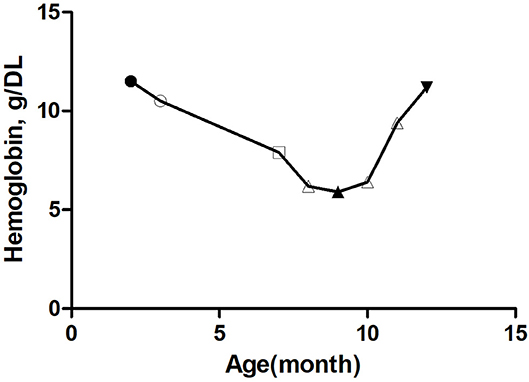
Figure 2. Trends of hemoglobin with age in this patient.  Preoperative hemoglobin,
Preoperative hemoglobin,  Hemoglobin before taking amiodarone,
Hemoglobin before taking amiodarone,  Hemoglobin after 4 months of amiodarone administration,
Hemoglobin after 4 months of amiodarone administration,  Hemoglobin before treatment with prednisone,
Hemoglobin before treatment with prednisone,  Hemoglobin after 3 months of prednisone administration. △ The first one: Hemoglobin 1 month before amiodarone administration, The second one: Hemoglobin 1 month after amiodarone administration, The third one: Hemoglobin 2 months after amiodarone administration.
Hemoglobin after 3 months of prednisone administration. △ The first one: Hemoglobin 1 month before amiodarone administration, The second one: Hemoglobin 1 month after amiodarone administration, The third one: Hemoglobin 2 months after amiodarone administration.
At present, the child is 1 year old, 7 kg in weight, and 67 cm in height. Five months after treatment with prednisone), the hemoglobin was 11.3 g/DL (Figure 2). Thyroid function was normal. The current dose of prednisone is 2.5 mg per day.
Discussion
PRCA syndrome is defined by severe reticular cell reduction, a significant reduction in or lack of bone marrow erythrocyte precursors, and cell-less normal anemia (10). PRCA includes congenital and acquired pure erythrocytes. Acquired PRCA may be related to lymphoblastic diseases, especially chronic lymphoblastic leukemia; autoimmune disorders; infections, especially the B19 viral infection; pregnancy; hematologic malignancies; non-hematologic neoplasms; and drugs and toxins (10). It is reported that there are many drugs that cause PRCA such as ampicillin, carbamazepine, etc (11, 12). However, there are no reports of amiodarone-related PRCA.
Amiodarone-related blood system damage is reported more often but reports of anemia are rare. Our patient had no anemia before and immediately after the operation. After the use of amiodarone, positive cell anemia occurred, white blood cells and platelets were normal, reticular red blood cells were reduced, and bone marrow examination suggested PRCA. Although amiodarone was discontinued, the anemia continued to worsen in our patient for 2 months, and this may be due to the extremely large volume of distribution and long half-life of amiodarone. Anemia improved after the patient was treated with prednisone. This is consistent with the assumption that acquired PRCA is associated with immune disorders (10). Major factors that lead to amiodarone-related side effects include long-term high-dose usage and low body weight (13). This patient had complex, congenital heart disease, low weight, and long-term usage of the drug due to irregular follow-up. Therefore, the use of amiodarone in low-weight children, especially for long periods, should be carefully monitored.
In addition to PRCA, our patient had hypothyroidism. There have been several reports of side effects of amiodarone-related thyroid function impairment (14–16). Thus, it is consistent with the amiodarone-related side effects. Moreover, the child showed bradycardia in the electrocardiogram. After discontinuation of amiodarone therapy, the electrocardiogram became normal. There was a significant increase in weight after thyroid hormone replacement therapy. At present, the mechanism of amiodarone-related PRCA is unclear and needs to be further studied.
In conclusion, doctors should be careful regarding the use of amiodarone in young and low-weight children and avoid long-term use. Side effects should be closely monitored during use.
Data Availability
All datasets generated and analyzed in this study are included in the manuscript and the supplementary files.
Ethics Statement
Written informed consent was obtained from patients' parents for publication of this case report and any potentially identifying information. The work was exempt from the ethics committee review/approval.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Vamos M, Hohnloser SH. Amiodarone and dronedarone: an update. Trends Cardiovasc Med. (2016) 26:597–602. doi: 10.1016/j.tcm.2016.03.014
2. Rickard JP, Negrelli J, Olson JL, Dick T. Frequency of adverse event monitoring in ambulatory patients on amiodarone or dofetilide. J Pharm Pract. (2018) 31:457–61. doi: 10.1177/0897190017729523
3. Rankin S, Elder DH, Ogston S, George J, Lang CC, Choy AM. Population-level incidence and monitoring of adverse drug reactions with long-term amiodarone therapy. Cardiovasc Ther. (2017) 35:e12258. doi: 10.1111/1755-5922.12258
4. Ul Rehman S, Siddiqui N, Khan NS, Sobia R, Assaly R. Multisystem side effects of amiodarone. Am J Med Sci. (2015) 349:454. doi: 10.1097/MAJ.0000000000000213
5. Erie AJ, McClure RF, Wolanskyj AP. Amiodarone-induced bone marrow granulomas: an unusual cause of reversible pancytopenia. Hematol Rep. (2010) 2:e6. doi: 10.4081/hr.2010.e6
6. Arpin MP, Alt M, Kheiralla JC, Chabrier G, Welsch M, Imbs JL, Imler M. Hyperthyroidism and immune hemolytic anemia following amiodarone therapy. Rev Med Interne. (1991) 12:309–11. doi: 10.1016/S0248-8663(05)82871-1
7. Groneberg DA, Barkhuizen A. Neutropenia during treatment with amiodarone. Am J Med. (2001) 110:671. doi: 10.1016/S0002-9343(01)00707-0
8. Sahud MA, Caulfield M, Clarke N, Koch R, Bougie D, Aster R. Acute thrombocytopenia in patients treated with amiodarone is caused by antibodies specific for platelet membrane glycoproteins. Br J Haematol. (2013) 163:260–7. doi: 10.1111/bjh.12521
9. Lossos IS, Matzner Y. Aplastic anemia associated with amiodarone therapy. Acta Haematol. (1992) 87:213. doi: 10.1159/000204776
10. Means RT Jr. Pure red cell aplasia. Hematology Am Soc Hematol Educ Program. (2016) 2016:51–6. doi: 10.1182/asheducation-2016.1.51
11. Yokota S, Kawabe K, Yamada H, Nunomura M. Case of subcutaneous abscess caused by Nocardia farcinica in an aplastic anemia patient. Nihon Ishinkin Gakkai Zasshi. (2010) 51:93–7. doi: 10.3314/jjmm.51.93
12. Chandra Sekhar C, Ramana Reddi V, Srinivas B, Naik KR. Pure red cell aplasia associated with carbamazepine. J Assoc Physicians India. (1998) 46:655–6.
13. Yamada Y, Shiga T, Matsuda N, Hagiwara N, Kasanuki H. Incidence and predictors of pulmonary toxicity in Japanese patients receiving low-dose amiodarone. Circ J. (2007) 71:1610–6. doi: 10.1253/circj.71.1610
14. Bogazzi F, Tomisti L, Di Bello V, Martino E. Amiodarone-induced thyrotoxicosis. G Ital Cardiol. (2017) 18:219–29. doi: 10.1714/2674.27399
Keywords: pure red cell aplastic anemia, amiodarone, hypothyroidism, congenital heart disease, prednisone
Citation: Ba H, Xu L, Peng H, Li X, Lin Y, Wang H and Qin Y (2019) Amiodarone-Related Pure Red Cell Aplastic Anemia and Hypothyroidism in a Child With Total Anomalous Pulmonary Venous Connection. Front. Pediatr. 7:361. doi: 10.3389/fped.2019.00361
Received: 29 April 2019; Accepted: 19 August 2019;
Published: 03 September 2019.
Edited by:
Matitiahu Berkovitch, Assaf Harofeh Medical Center, IsraelReviewed by:
Jonathan Burton Wagner, Children's Mercy Hospital, United StatesRyan Sol Funk, University of Kansas Medical Center Research Institute, United States
Copyright © 2019 Ba, Xu, Peng, Li, Lin, Wang and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Youzhen Qin, cXlvdXpoZW5AMTYzLmNvbQ==
†These authors have contributed equally to this work
 Hongjun Ba
Hongjun Ba Lingling Xu2†
Lingling Xu2†