- 1Department of Rehabilitation Medicine, Seoul National University College of Medicine, Seoul, South Korea
- 2Department of Rehabilitation Medicine, Seoul National University Hospital, Seoul, South Korea
- 3Department of Rehabilitation Medicine, Ewha Women's University Seoul Hospital, Ewha Women's University School of Medicine, Seoul, South Korea
It has been suggested that oral feeding trial has therapeutic implications for improving oral-motor and swallowing function in infants and young children fed via an enteral tube or gastrostomy. This study aimed to investigate whether oral feeding challenges in children with tracheostomy could improve feeding outcomes, even with the finding of aspiration compared to those who did not receive oral feeding at all. Children (age <7 years) with tracheostomy who had thin fluid aspiration on videofluoroscopic swallowing study (VFSS) were included in this retrospective study. Enrolled children were then divided into two feeding method groups according to the physician's decision at the time of VFSS: oral feeding (OF) group and non-oral feeding (NOF) group. Data were obtained from 47 children (median age: 49.75 months, interquartile range [IQR]: 24.08–79.42). The incidence of pneumonia within 1 year after the VFSS was not different between NOF (n = 17) and OF (n = 30) groups. In OF group, 11 subjects achieved full oral feeding and 16 subjects were in partial oral feeding status 1 year after the VFSS. On the contrary, only one subject achieved full oral feeding and 5 subjects were in partial oral feeding status in NOF group (p < 0.001). Initial and follow-up penetration-aspiration scale on VFSS were different only in the OF group (p = 0.003). These results suggest that oral feeding challenges might be attempted even with the findings of aspiration in infants or young children with tracheostomy.
Introduction
In children with swallowing difficulties, aspiration of food and fluid is commonly observed and is associated with a wide range of diseases. Current management decisions for aspiration generally include tube feeding, restricting aspirated diets, and providing texture-modified foods and thickened fluids (1–3). Young children usually refuse thickened fluids (3), resulting in a management dilemma for both medical professionals and families (2).
With regard to starting tube feeding during the first few years of life, it has been reported that the feeding outcome could be poor although the pharyngeal phase of swallowing is well preserved (4). Therefore, oral feeding during tube feeding is recommended and encouraged (4). It is reported that providing a taste or texture experience in early childhood, not only for nutritional purposes, can also help facilitate chewing skills in tube feeding children (5). Children who are exclusively tube fed would not have the opportunity to experience the sensations of food in the mouth and might be deprived of developing the oral-motor skills to manage different food consistencies and textures (6).
In 2016, McSweeney et al. reported that oral feeding of nectar or honey-thickened liquids instead of tube feeding via gastrostomy reduced the hospitalization frequency in children with aspiration or penetration findings on their videofluoroscopic swallow study (VFSS) (3). Prior to this report, we recommended exclusive tube feeding without oral feeding trial for children who showed aspiration findings on VFSS. However, based on the report and our clinical experience, we changed the strategy and began to try oral feeding challenges in infants or young children with aspiration instead of exclusive tube feeding. Especially in infants and young children with tracheostomy, oral feeding was tried more aggressively than before because the removal of aspirates could be possible through the tracheostomy (6). The amount of oral feeding challenges ranged from minimal to full nutrition according to the amount of aspiration, pharyngeal wall motion, epiglottis closure, and caregiver skills. A physician (one of the authors) decided whether to start oral feeding trial and the amount of the challenge.
In this study, we hypothesized that swallowing function could improve over time if infants or young children attempted oral feeding before the aero-digestive centers are fully established.
Thus, this study aimed to investigate whether oral feeding challenges in children with tracheostomy can improve feeding outcomes, even with the finding of aspiration compared to group that did not receive oral diets at all.
Materials and Methods
All study-related procedures were performed in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Declaration of Helsinki. Ethical approval for the study was obtained from our institutional review board (Approval No. 1803-115-932), which waived the requirement for informed consent due to the retrospective nature of the study. The following inclusion criteria were applied to potential subjects: (1) confirmed aspiration of fluid in a VFSS at <7 years of age between 2011 and 2017, (2) had a tracheostomy at the time of VFSS, and (3) medical records were available at least 1 year after the initial VFSS. When performing VFSS, foods with fluid consistency (e.g., water, juice) were always used, but foods with different consistencies (e.g., Yoplait, puree, cookies) were also used depending on individual eating habits. In many cases, parents brought foods that the children commonly ate at home and we tested these diets. VFSS results when using the diets other than fluid ones were not included in the inclusion or exclusion criteria. Patients with fluid swallowing of <2 times on VFSS records and those who refused food during VFSS were excluded from the analysis.
Enrolled children were then divided into two groups according to the feeding method received, per physician's decision at the time of VFSS: (1) oral feeding group (OF group), who were recommended partial oral feeding (POF) or full oral feeding (FOF) and (2) non-oral feeding (NOF) group, who were recommended exclusive tube feeding.
Oral Feeding Trial
The caregiver played an important role in the oral feeding challenge. Oral feeding trial was conducted only when the parents were skillful and cooperative in suctioning during and immediately after the oral feeding. The oral feeding trial tended to be not applicable in school-age children, because parents were not able to perform suction while the children were in school. Therefore, we only included preschool children (<7 years of age) for the analysis. The oral diet was started at a low dose of 0.1–0.2 cc for the first time and gradually increased. The physician recommended the rate of increase considering the results of VFSS. Suction was carried out using portable equipment for home use. The depth and frequency of suctioning were not specified, but caregivers were instructed to perform shallow and gentle suctioning.
Outcome Assessment
Primary outcome was defined as the feeding status (FOF, POF, or NOF) 1 year after the VFSS. The feeding status at the time and 1 year after the VFSS was recorded for all children through a medical record survey. Secondary outcomes included the occurrence of pneumonia and days of hospitalization related to pulmonary complications within 1 year after the VFSS.
Patient Characteristics Assessed at the Initial VFSS
Patient records were reviewed for the sex and age of the patients at the time of their first VFSS. Initial feeding status before the VFSS was recorded for all children. The penetration-aspiration scale (PAS) on VFSS was rated by one of the authors. PAS scale is an 8-point scale developed to characterize the severity of airway invasion events viewed during VFSS, capturing the location to which material is observed to travel and then qualifying that information based on whether the material remains there at the end of the swallow or is ejected to safer (anatomically higher) locations (7). A score of 1 reflects no entry of material into the airway, scores of 2–5 reflect penetration of material past the laryngeal aditus into the supraglottic space and traveling as far as the true vocal folds, while scores of 6–8 reflect tracheal aspiration of material below the true vocal folds (7, 8).
Follow-Up VFSS
For children who underwent both initial VFSS and 1 year follow-up VFSS, the pharyngeal transit time (PTT) as well as the PAS scale (7, 8) were compared to verify improvement or deterioration of swallowing function. PTT was defined as the total time of the bolus passage through the pharynx (9), from when the bolus head passed the ramus of the mandible to the time the bolus tail completely cleared the pharyngoesophageal segment (10). The PAS and PTT were rated retrospectively by one of the authors who were blinded to the group assignment.
Statistics
Differences in continuous variables between the two groups were analyzed using the Wilcoxon rank sum test. In case of categorical variable, statistical significance was calculated by using Chi-square or Fisher's exact test. Main diagnoses were categorized as brain lesion as well as neuromuscular, cardiac, gastrointestinal, pulmonary, and otolaryngeal comorbidities for univariate and multivariate analyses; the comorbidities were not considered to be mutually exclusive. Given that the primary outcome comprised three categorical nominal variables (POF, FOF, and NOF), multinomial logistic regression was used to estimate the odds ratio (OR). Mann-Whitney U-test was used to compare initial PTT and follow-up PTT between groups. Analyses were performed using SAS statistical software (SAS system for Windows, version 9.4; SAS institute, Cary, NC). p < 0.05 was considered statistically significant.
Results
Subjects
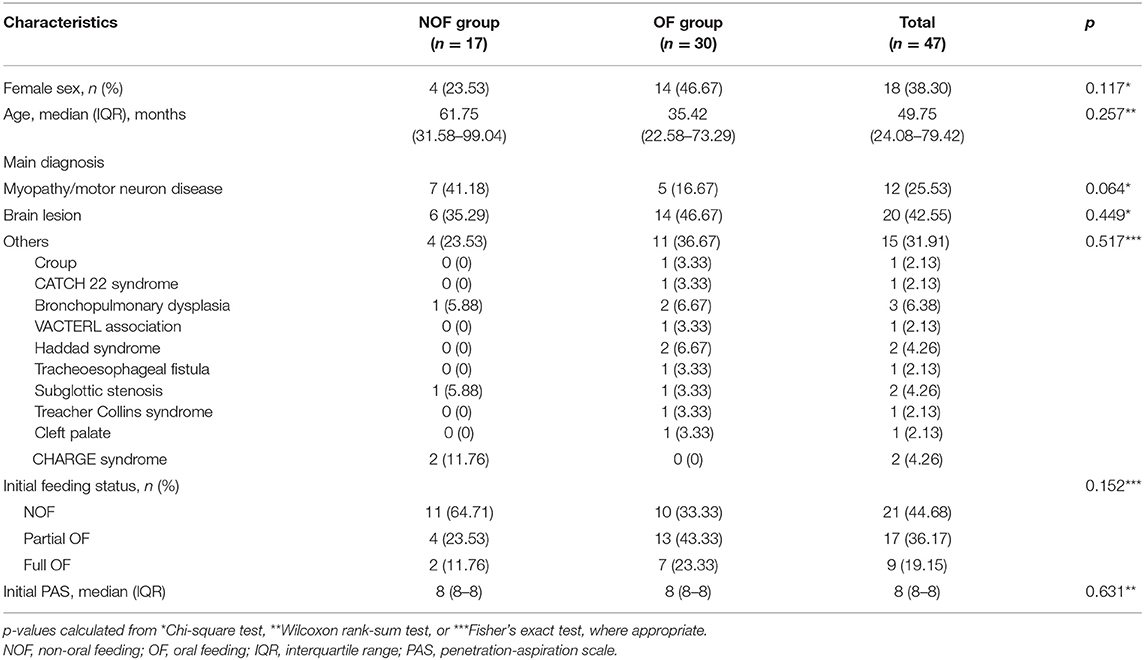
Data were obtained for 47 children (median age: 49.75 months, interquartile range [IQR]: 24.08–79.42) who had tracheostomies and confirmed aspiration of fluid on initial VFSS between 2011 and 2017 (Figure 1). Seventeen children were assigned to the NOF group (median age: 61.75 months, IQR: 31.58–99.04), and 30 children to the OF group (median age: 35.42 months, IQR: 22.58–73.29). The characteristics of each group at the time of the VFSS are presented in Table 1. Initial feeding status before VFSS was not different between the OF and NOF groups (p = 0.152).
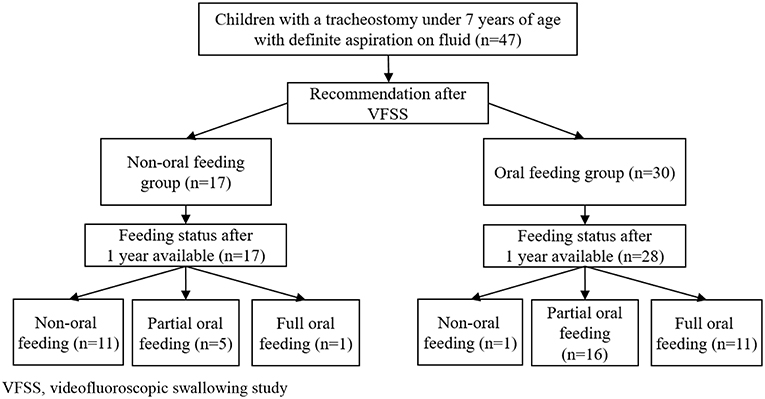
Figure 1. Flowchart of children with tracheostomy younger than 7 years of age with definite fluid aspiration confirmed via VFSS. VFSS, videofluoroscopic swallowing study.
Changes in Feeding Status
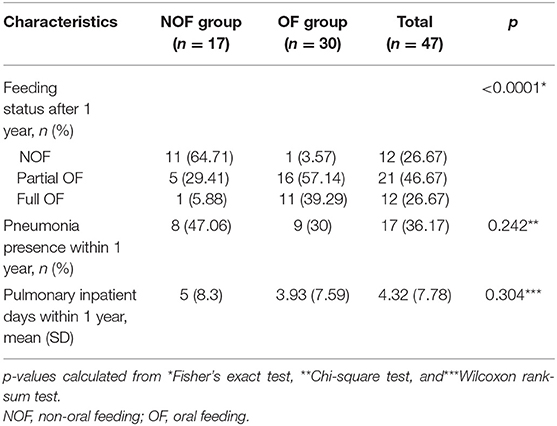
In children with tracheostomies, there was a significant difference in feeding status after 1 year between the OF and NOF groups (Table 2, Figure 1). In the OF group, 11 subjects achieved FOF and 16 subjects were in POF status. The incidence of pneumonia and pulmonary inpatient days within 1 year after the VFSS was not different between the OF and NOF groups.
The presence of neuromuscular disease was different between the two groups, with 8 (47.06%) in the NOF group and 5 (16.67%) in the OF group having a neuromuscular disease (p = 0.041). Brain lesions were present in 8 (47.06%) children in the NOF group and 15 (50%) in the OF group. Gastrointestinal, cardiac, otolaryngology, and pulmonary comorbidities were present in 2 (11.76%), 5 (29.41%), 3 (17.65%), and 11 (64.71%) children in the NOF group and 6 (20%), 10 (33.33%), 11 (36.67%), and 15 (50%) in the OF group, respectively. There were no differences in the main diagnosis categories, except for the neuromuscular disease category, between the OF and NOF groups.
Univariate and multinomial logistic regression analysis was conducted to calculate the OR of each factor affecting the feeding status after 1 year. Compared with the NOF group, the OF group showed an OR of 35.714 (p = 0.002) for feeding status 1 year after VFSS for POF compared with NOF (reference group), and an OR of 125 (p = 0.001) for FOF compared with NOF (Table 3). Various comorbidities including neuromuscular comorbidity and initial feeding status before the VFSS were not found to be significant predictors of dietary status 1 year after VFSS. To control multi-collinearity between factors with group variable (OF group vs. NOF group) included in the model, stepwise selection was performed (entry condition p < 0.05, removal condition p > 0.05). However, there was no factor at the significance level of 5% with group variable (OF group vs. NOF group) included in the model.
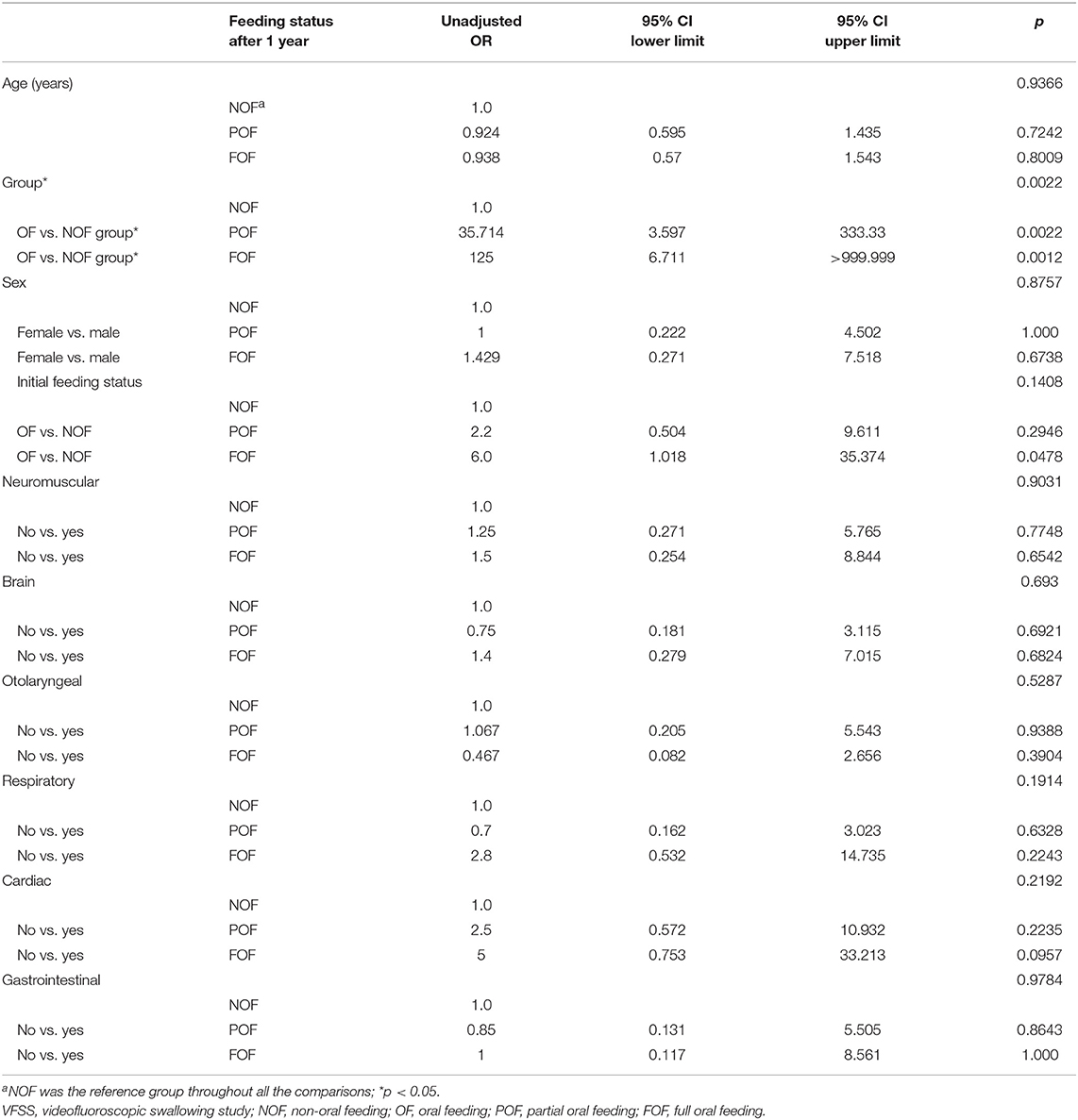
Table 3. Univariate analysis via multinomial logistic regression for feeding status (full oral feeding, partial oral feeding, or non-oral feeding) 1 year after the initial VFSS.
Changes in VFSS Findings
For 29 children with follow-up VFSS (n = 21, OF group; n = 8, NOF group), changes in PAS and PTT were analyzed. The change in PAS between the first and follow-up VFSS according to each group is shown in Figure 2. The OF group showed a significant difference between the initial PAS (median 8, IQR: 8–8) and follow-up PAS (median 6, IQR: 1–8) (p = 0.003 for Wilcoxon signed-rank test, Figure 2A). However, there was no difference between the initial PAS (median PAS, IQR: 8–8) and follow-up PAS (median 8, IQR: 3.75–8) in the NOF group (p = 0.180 for Wilcoxon signed-rank test, Figure 2B).
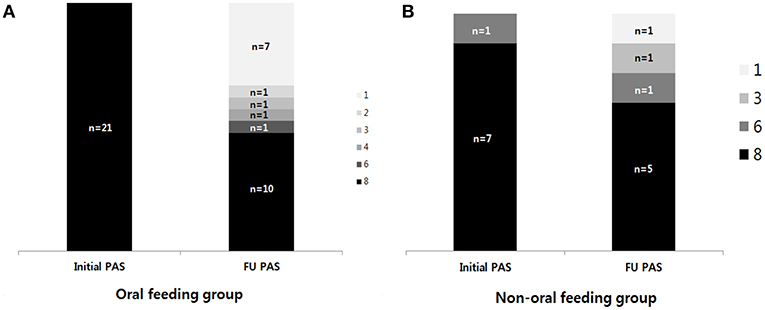
Figure 2. The change in the penetration-aspiration scale (PAS) between the first VFSS and follow-up VFSS. (A) Oral feeding group; (B) non-oral feeding group. (1) Material does not enter the airway. (2) Material enters the airway, remains above the vocal folds, and is ejected from the airway. (3) Material enters the airway, remains above the vocal folds, and is not ejected from the airway. (4) Material enters the airway, contacts the vocal folds, and is ejected from the airway. (5) Material enters the airway, contacts the vocal folds, and is not ejected from the airway. (6) Material enters the airway, passes below the vocal folds, and is ejected into the larynx or out of the airway. (7) Material enters the airway, passes below the vocal folds, and is not ejected from the trachea despite effort. (8) Material enters the airway, passes below the vocal folds, and no effort is made to eject.
In the OF group, there was a tendency for follow-up PTT (median 0.701 s, IQR: 0.600–0.984) to be shorter than the initial PTT (median 0.901s, IQR: 0.784–1.517), although the difference was not statistically significant (p = 0.077 for paired t-test). In the NOF group, follow-up PTT (median 1.418 s, IQR: 0.985–3.162) was not different from the initial PTT (median 1.437 s, IQR: 0.851–2.386). There was no difference in initial PTT between the OF and NOF groups (p = 0.153 for Mann-Whitney U-test), but the follow-up PTT was shorter in the OF group than in the NOF group (p = 0.003 for Mann-Whitney U-test).
Discussion
There have been few reports regarding the effects of oral feeding trial on the development of swallowing function in children with aspiration. The results of this study suggest that swallowing function might improve over time if infants or young children attempt oral feeding before the aero-digestive centers are fully established despite aspiration findings on VFSS. Moreover, improvement in follow-up PAS was observed only in the group that received oral feeding.
From birth to 3 years of age, children learn to adapt the swallowing mechanism to accommodate different foods, fluids, and textures (11, 12). Ruark et al. (13) investigated pharyngeal muscle activity during swallowing in children, and showed that children established control of swallowing with different consistencies of food until 5 years of age. Even in children with aspiration who had undergone gastrostomy, it was reported that within a year more than half of the children showed improved swallowing function on VFSS when they were allowed oral feeding (3). However, the study primarily investigated the trajectory of deglutition function in children with gastrostomy and did not evaluate the effects of oral feeding challenge.
The Impacts of Tracheostomy on Swallowing Function
In adults with stroke, it was reported that patients with tracheostomies had inferior swallowing function and kinematics compared to those without tracheostomies (14). In children with tracheostomies, it was reported that the time required to close the laryngeal vestibule once the arytenoids initiate anterior movement was longer than in those with no tracheostomy (15). Pre-swallow spillage and deficit in the pharyngeal phase were also frequently observed in children with tracheostomy (15).
Although tracheostomy interferes with swallowing function, it has the potential to promote oral feeding challenge (16). Oral feeding may be less likely to induce aspiration pneumonia because of the high accessibility of suctioning through tracheostomy. In this study, the oral feeding group showed better feeding outcome than the non-oral feeding group, suggesting the possibility of losing this opportunity if the feeding trial was not implemented in the infants or young children with tracheostomy.
The Course of Children With Neuromuscular Disease
Although in the present study there was a difference in the frequency of neuromuscular disease between those who received oral feeding and those who did not, this seemed not to be a factor affecting feeding outcome after 1 year according to the multinomial logistic regression analysis. Among the OF group, 5 children were diagnosed with neuromuscular disease, 3 with congenital myopathy, and 2 with muscular dystrophy. Among them, 3 achieved FOF and 2 achieved POF after 1 year. Two children developed pneumonia within 1 year. Among the NOF group, 8 children were diagnosed with neuromuscular disease, 5 with congenital myopathy, 2 with muscular dystrophy, and 1 with motor neuron disease. Among them, 4 achieved POF and 4 remained at NOF status. Four of them developed pneumonia within 1 year. These results suggest that the swallowing function could be improved at least in the short term by the growth of the pharyngeal muscles (11, 17, 18) and longer duration of pharyngeal muscle activity (13) despite the progressive nature of neuromuscular disease, and oral feeding challenge might help attain the improvement.
Concerns for Oral Feeding Trial in Children With Tracheostomy
Firstly, frequent suctioning may cause laryngeal wall irritation and tracheal wall injury. Injurious prolapse of the tracheal mucosa may occur by suctioning (19). Although there have been no guideline regarding the technical aspects of suctioning for this specific purpose, judicious gentle suctioning could be helpful in preventing those injuries on trachea.
Secondly, many parents of children who underwent an oral feeding trial reported that food was expelled through the tracheostomy during feeding without suctioning (for example, when the grape juice was fed, the purple liquid came out). Suction should be considered to be an incomplete method of removing all aspirates. If the aspirate not removed by suction or drainage through the tracheostomy exceeds a certain threshold, respiratory complications, such as aspiration pneumonia might occur. However, aspirates below a certain amount may not cause problems. Weiss (20) suggested that infants and children may lack some of the compensatory mechanisms that enable adults to protect the airway. Tutor and Gosa reported that aspiration was observed in children with non-pathologic conditions (21).
Study Limitations
There are several limitations to this study. Firstly, this was a single-center study with a small number of subjects, which may have led to a selection bias. Secondly, because this study retrospectively obtained information from the medical records, it is possible that patients were regarded as having no respiratory complications if they had been previously treated for pneumonia in another hospital. Moreover, we could not clearly distinguish whether pneumonia was related to aspiration or not; thus, we analyzed pulmonary admission days and occurrence of pneumonia, as performed in the previous studies (3, 20, 22). Thirdly, although multinomial logistic regression analysis revealed that neuromuscular disease did not affect the feeding outcome, the proportion of patients with such disease was higher in the NOF group, which may have affected the outcome. Lastly, information regarding weight loss or dehydration that could be induced from oral feeding challenges was not obtained owing to the retrospective nature of the study. When the children experienced these problems, they also received tube feeding in parallel; hence, they were categorized into the POF group.
Conclusions
In infants and young children with tracheostomy, oral feeding challenge improved the feeding outcome without an increased risk of pneumonia, although aspiration was confirmed by VFSS. These results suggest that oral feeding challenges might be attempted if aspirates can be removed through the tracheostomy even with the findings of aspiration.
Data Availability
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Ethical approval was obtained from the Seoul National University Hospital Institutional Review Board (IRB) No. 1803-115-932, which waived the requirement for informed consent due to the retrospective nature of the study.
Author Contributions
YY: acquisition of data, analysis and interpretation of data, writing, and critical revision of manuscript. B-MO: analysis and interpretation of data, and critical revision of manuscript. SY: critical revision of manuscript. H-IS: study concept and design, acquisition of data, analysis and interpretation of data, study supervision, and critical revision of manuscript for intellectual content.
Funding
This research was conducted through contributions donated to the Seoul National University Children's Hospital by Sir. Suhwon Suh (Seoul National University Hospital Assignment No. 3020190030).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We appreciate the statistical advice of the Medical Research Collaborating Center, Seoul National University Hospital.
References
1. Cichero JA. Thickening agents used for dysphagia management: effect on bioavailability of water, medication and feelings of satiety. Nutr J. (2013) 12:54. doi: 10.1186/1475-2891-12-54
2. Weir K, McMahon S, Chang AB. Restriction of oral intake of water for aspiration lung disease in children. Cochrane Database Syst Rev. (2012) 9:CD005303. doi: 10.1002/14651858.CD005303.pub3
3. Mcsweeney ME, Kerr J, Amirault J, Mitchell PD, Larson K, Rosen R. Oral feeding reduces hospitalizations compared with gastrostomy feeding in infants and children who aspirate. J Pediatr. (2016) 170:79–84. doi: 10.1016/j.jpeds.2015.11.028
4. Dello Strologo L, Principato F, Sinibaldi D, Appiani AC, Terzi F, Dartois AM, et al. Feeding dysfunction in infants with severe chronic renal failure after long-term nasogastric tube feeding. Pediatr Nephrol. (1997) 11:84–6. doi: 10.1007/s004670050239
5. Mason SJ, Harris G, Blissett J. Tube feeding in infancy: implications for the development of normal eating and drinking skills. Dysphagia. (2005) 20:46–61. doi: 10.1007/s00455-004-0025-2
6. Morris SE. Development of oral-motor skills in the neurologically impaired child receiving non-oral feedings. Dysphagia. (1989) 3:135–54. doi: 10.1007/BF02407132
7. Steele CM, Grace-Martin K. Reflections on clinical and statistical use of the penetration-aspiration scale. Dysphagia. (2017) 32:601–16. doi: 10.1007/s00455-017-9809-z
8. JC Rosenbek Robbins JA, Roecker EB, Coyle JL. A penetration-aspiration scale. Dysphagia. (1996) 11:93–8. doi: 10.1007/BF00417897
9. Logemann JA. Manual for the Videofluorographic Study of Swallowing. 2nd ed. Nerang, QLD: Pro-Ed (1993). p. 116–17.
10. Weckmueller J, Easterling C, Arvedson J. Preliminary temporal measurement analysis of normal oropharyngeal swallowing in infants and young children. Dysphagia. (2011) 26:135–43. doi: 10.1007/s00455-010-9283-3
11. Hennessey NW, Fisher G, Ciccone N. Developmental changes in pharyngeal swallowing acoustics: a comparison of adults and children. Logoped Phoniatr Vocol. (2018) 43:63–72. doi: 10.1080/14015439.2017.1326526
12. Reynolds EW, Vice FL, Gewolb IH. Variability of swallow-associated sounds in adults and infants. Dysphagia. (2009) 24:13–19. doi: 10.1007/s00455-008-9160-5
13. Ruark JL, McCullough GH, Peters RL, Moore CA. Bolus consistency and swallowing in children and adults. Dysphagia. (2002) 17:24–33. doi: 10.1007/s00455-001-0098-0
14. Seo HG, Kim JG, Nam HS, Lee WH, Han TR, Oh BM. Swallowing function and kinematics in stroke patients with tracheostomies. Dysphagia. (2017) 32:393–400. doi: 10.1007/s00455-016-9767-x
15. Abraham SS, Wolf EL. Swallowing physiology of toddlers with long-term tracheostomies: a preliminary study. Dysphagia. (200) 15:206–12. doi: 10.1007/s004550000029
16. Joseph RA, Evitts P, Bayley EW, Tulenko C. Oral feeding outcome in infants with a tracheostomy. J Pediatr Nurs. (2017) 33:70–5. doi: 10.1016/j.pedn.2016.12.012
17. Rommel N, Bellon E, Hermans R, Smet M, De Meyer AM, Feenstra L, et al. Development of the orohypopharyngeal cavity in normal infants and young children. Cleft Palate Craniofac J. (2003) 40:606–11. doi: 10.1597/1545-1569_2003_040_0606_dotoci_2.0.co_2
18. Park Y, Jun B, Song C, Park C, Kim M, Cho S, et al. Temporal analysis of oropharyngeal swallow in children. Kor J Otolaryngol. (2004) 47:661–5. Available online at: http://www.kjorl.org/journal/view.php?number=2387
19. Suys E, Nieboer K, Stiers W, De Regt J, Huyghens L, Spapen H. Intermittent subglottic secretion drainage may cause tracheal damage in patients with few oropharyngeal secretions. Intens Crit Care Nurs. (2013) 29:317–20. doi: 10.1016/j.iccn.2013.02.007
21. Tutor JD, Gosa MM. Dysphagia and aspiration in children. Pediatr Pulmonol. (2012) 47:321–37. doi: 10.1002/ppul.21576
Keywords: children, deglutition, deglutition disorders, tracheostomy, videofluoroscopic swallowing study, aspiration, oral feeding
Citation: Yi YG, Oh B-M, Yang S and Shin H-I (2019) Oral Feeding Challenges in Children With Tracheostomy Can Improve Feeding Outcomes, Even With the Finding of Aspiration. Front. Pediatr. 7:362. doi: 10.3389/fped.2019.00362
Received: 07 June 2019; Accepted: 20 August 2019;
Published: 04 September 2019.
Edited by:
Andrew S. Day, University of Otago, New ZealandReviewed by:
Selen Serel Arslan, Hacettepe University, TurkeyMatjaž Homan, University Medical Centre Ljubljana, Slovenia
Copyright © 2019 Yi, Oh, Yang and Shin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hyung-Ik Shin, aHl1bmdpazFAc251LmFjLmty
 You Gyoung Yi
You Gyoung Yi Byung-Mo Oh
Byung-Mo Oh Seoyon Yang3
Seoyon Yang3 Hyung-Ik Shin
Hyung-Ik Shin