- 1Laboratory of Hematology-Oncology, Department of Woman's and Child's Health, University of Padova, Padova, Italy
- 2Children's Cancer Research Institute (CCRI), St. Anna Kinderkrebsforschung, Vienna, Austria
Minimal residual disease (MRD) by multiparametric flow cytometry (MFC) has been recently shown as a strong and independent prognostic marker of relapse in pediatric AML (pedAML) when measured at specific time points during Induction and/or Consolidation therapy. Hence, MFC-MRD has the potential to refine the current strategies of pedAML risk stratification, traditionally based on the cytogenetic and molecular genetic aberrations at diagnosis. Consequently, it may guide the modulation of therapy intensity and clinical decision making. However, the use of non-standardized protocols, including different staining panels, analysis, and gating strategies, may hamper a broad implementation of MFC-MRD monitoring in clinical routine. Besides, the thresholds of MRD positivity still need to be validated in large, prospective and multi-center clinical studies, as well as optimal time points of MRD assessment during therapy, to better discriminate patients with different prognosis. In the present review, we summarize the most relevant findings on MFC-MRD testing in pedAML. We examine the clinical significance of MFC-MRD and the recent advances in its standardization, including innovative approaches with an automated analysis of MFC-MRD data. We also touch upon other technologies for MRD assessment in AML, such as quantitative genomic breakpoint PCR, current challenges and future strategies to enable full incorporation of MFC-MRD into clinical practice.
Introduction
Pediatric acute myeloid leukemia (pedAML) is a heterogeneous group of hematological malignancies classified according to morphology, immunophenotyping, and genomics (1). In the last few decades, significant progress has been obtained in the outcome of pedAML, with an increase of survival rate up to 70% (2). The results depend on the improvement in risk stratification including genetic features at diagnosis, intensification of chemotherapy together with the amelioration of supportive care, and application of hematopoietic stem cell transplantation (HSCT) to specific subgroups of patients (3, 4). Nevertheless a large amount of patients (20–41%) encounters relapse, with non-acceptable final outcome in about 50–70% of them (5–13). Consequently, ever better definition of the factors predictive for relapse may improve the outcome. Among those, the residual disease assessment has been emerging as an ever more essential tool for patients' management and risk classification (6, 14–21).
Minimal or, more appropriately, measurable residual disease (MRD) detection allows the identification of 0.1–0.001% leukemic cells according to the adopted technique. MRD assessment in AML may establish a more in-depth remission status compared with the morphology-based evaluation, refining outcome prediction. A proper MRD approach should be sensitive and highly specific, reproducible and standardized with ideally an extended inter-institutional validation. Different techniques are currently available for MRD detection in pedAML, each one showing advantages and limitations: quantitative RNA-based polymerase chain reaction (RT-PCR) analysis of specific gene fusions, next-generation sequencing (NGS), gene expression profiling (GEP) and multiparametric flow cytometry detection (MFC) of aberrant immunophenotypes (22). Nowadays, RT-PCR and MFC-MRD are applied in the clinical setting (23, 24).
RT-PCR of fusion transcripts allows MRD detection with a sensitivity level of up to 0.001%. Regardless, it is applicable only in about 50–60% of pedAML with a detectable fusion gene or mutations. Moreover, a precise quantification of MRD may be difficult due to the unpredictable number of transcripts per leukemic cell (23). Among AML molecular alterations, the persistence of RUNX1-RUNX1T1 and CBFB-MYH11 fusion transcripts in continuous long-term remission have been already described, hampering the clinical relevance of MRD detection (25–27). Conversely, a slow molecular response at the end of Induction in t(8;21)-rearranged AML is associated with a higher risk of relapse when compared to an MRD reduction of at least 2 logs (28). Finally, different studies showed uncertain results on the role of FLT3-internal tandem duplication (ITD) and NPM1 mutations as MRD markers, with a potential instability of those lesions between diagnosis and relapse (14, 19). ITD-Allelic Ratio levels and molecular MRD should be included in the clinical management of FLT3-ITD AML patients since children with high levels of RT-PCR-MRD after the first Induction course had worse event free survival (EFS) (29).
NGS allows the identification of molecular anomalies, particularly relevant in AML with normal cytogenetics (30). In principle, it may be applied to all patients but requires highly specialized bioinformatics analysis. Moreover, the role of NGS in MRD detection is still controversial. While it may detect subclonal changes during therapy, potentially crucial for patients' outcome, NGS-MRD can be affected by mutations belonging to clonal hematopoiesis and ancestral clones (31).
MFC-MRD has a lower sensitivity than RT-PCR (up to 0.01%), but it is applicable in more than 90% of patients. Therefore, MFC-MRD is generally the method of choice for MRD detection in clinical AML studies. MFC-MRD may be assessed through two different techniques: (i) Leukemia-Associated Immunophenotype (LAIP) approach allows to identify leukemic blasts immunophenotype at diagnosis and track it at re-evaluation points; (ii) Different-from-Normal approach relies on the discrimination of aberrant immunophenotypes from normal cells during follow-up (32, 33).
This review focuses on the most relevant findings on MFC-MRD in pedAML and examines recent advances and new approaches for its standardization, including novel concepts for automated analysis of MFC-MRD data.
MFC-MRD: From Past to Present
Even if MRD monitoring has become the standard of care in ALL, only recently it has been acquiring an even more important role in AML management (34).
In the last three decades, different retrospective studies on MFC-MRD monitoring have been performed in adult and pedAML. They suggested its strategic role in AML risk stratification. Regardless, to date, no guidelines or recommendations are available on methods, time points, and clinical application of MFC-MRD in AML. That depends on the heterogeneity of MRD assessment in AML, due to different available techniques, difficulties in comparing MRD data among laboratories and clinical trials, and the impact of sample quality on MRD level (34–38).
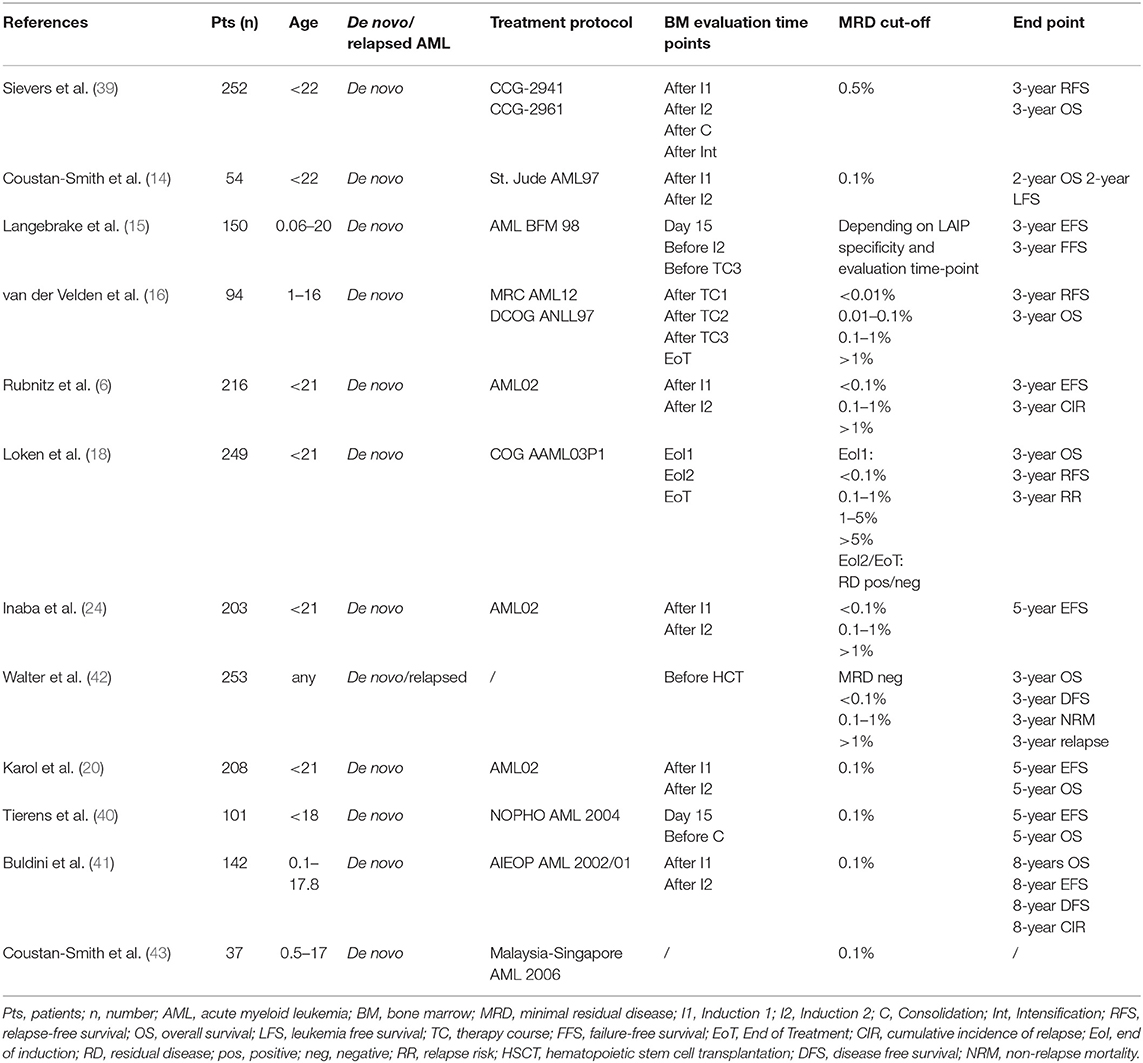
Regarding pediatric cohorts, in 2003, the COG group published the results of a prospective study on 252 de novo pediatric AMLs. MFC-MRD emerged as the most influential independent prognostic factor associated with poor outcome (39).
In the same year, Coustan-Smith et al. (14) applied to AML-MRD a four-color MFC approach usually adopted in pediatric acute lymphoblastic leukemia. That allowed to reach a sensitivity level of 0.1–0.01% of leukemic cells. MFC-MRD resulted as an independent predictor of outcome.
The described technique was subsequently applied to a cohort of 232 children consecutively enrolled in the AML02 multicenter trial. MFC-MRD was adopted as risk-stratification criteria together with the genetic features. MRD positivity was defined as 1 or more leukemic cells per 1,000 mononuclear bone marrow (BM) cells (≥0.1%). MRD positivity after Induction I was associated with an unfavorable outcome in high-risk AML (P = 0.01). Moreover, any MRD positivity after Induction II was predictive of an adverse outcome. The authors were able to monitor MRD in more than 90% of patients after each therapeutic course. The combined approach showed an improvement in patients' outcome (6).
In support to the St. Jude study, MFC-MRD was an independent prognostic variable in the Dutch Childhood Oncology Group ANLL 97/Medical Research Council of the UK AML12 experience, as well as in the COG AAML03P1 AML study (16, 18).
Regardless, the AML-BFM study published in 2006 did not find any significant role of MFC-MRD based on a standardized panel for four-color immunophenotyping in outcome prediction when compared to other known risk factors. A significant difference in 3-years EFS was demonstrated in the presence of positive MFC-MRD before the second Induction, and third therapy course but those data were not confirmed by a multivariable analysis including FAB subtype, cytogenetics, and morphologically determined blasts on day 15 (15).
Finally, two recent European studies strengthened the prognostic role of MFC-MRD monitoring in pedAML. In 2016, Tierens et al. (40) retrospectively analyzed MFC-MRD prognostic impact in a cohort of 201 children enrolled in the NOPHO-AML 2004 trial. MRD was detected by LAIP technique at two different time points (day 15 of Induction therapy and before Consolidation therapy). Samples with at least 0.1% leukemic events were considered MRD positive. In a univariate analysis, MFC-MRD positivity on day 15 and before Consolidation therapy was associated with a statistically significant adverse 5-years EFS and overall survival (OS). In a multivariate analysis including age, sex, leucocyte count, FLT3-ITD mutations, core-binding factor mutations, residual disease and BM morphology at both time points, only MFC-MRD positivity before Consolidation therapy still was associated with an unfavorable outcome, with a strong impact both on EFS and OS (40).
In 2017, Buldini et al. (41) published a retrospective study on the prognostic role of MFC-MRD in a cohort of 142 pedAML patients treated according to the Associazione Italiana di Emato-Oncologia Pediatrica (AIEOP)-AML 2002/01 trial. LAIP-MRD was assessed by 5-color MFC after the first and the second Induction courses, respectively, with a sensitivity cut-off of 0.1%. After the first Induction course, different MRD level (<0.1% vs. ≥ 0.1%) correlated with different 8-year disease-free survival (DFS) (73.1 ± 5.6% vs. 35.2 ± 7.2%, respectively, P < 0.01), as well as 8-years OS (82.2% vs. 51.6%, respectively, P < 0.01). Similar results were observed for MRD levels after the second Induction therapy course (8-years DFS: 68.4 ± 7·9% for MRD < 0.1% vs. 21.9 ± 9·4% for MRD ≥ 0.1%, P < 0.01; 8-years OS: 77.1% for MRD < 0.1% vs. 55.5% for MRD ≥0.1%). In a multivariate analysis, MRD ≥ 0.1% after the first Induction course was still associated with an adverse outcome (41).
Table 1 includes a complete list of the studies on MFC-MRD monitoring in AML.
Challenges of MFC-MRD Assessment in AML
One of the most significant challenges of reliable MFC-MRD monitoring in AML is the requirement of well-trained experts in data interpretation. It is well recognized that intra- and inter-leukemic immunophenotypic heterogeneity is a common finding in AML (44). Leukemic blasts also need to be discriminated from normal myeloid progenitors during hematopoietic regeneration. Furthermore, immunophenotypic changes may occur during therapy, making MRD data interpretation even more challenging. Hence, a profound knowledge of myeloid cell compartments at various differentiation stages in normal and regenerating BM is required. To this aim, multicenter trials should contemplate an extensive training of MFC-MRD operators, including face-to-face activities with experts and internet-based data reviewing. Furthermore, a program for continuous quality control through recurrent ring trials is warranted. A ring trial is a proficiency testing in which identical samples are sent to the participating laboratories. The laboratories are expected to analyze the samples within an agreed period and send the results back to the coordinator center. That allows monitoring the performance of participating laboratories during time.
Besides, an extensive database of “normal” and regenerating BM at different time points aids in establishing a “range of normality” for each marker and immunophenotype. That can be achieved only by building up international networks among centralized reference laboratories to share resources and improve the applicability and accuracy of MFC-MRD (45).
Standardization Efforts for MFC-MRD in AML
To ensure comparability of results among different laboratories, and facilitate the clinical use of MFC-MRD as a surrogate for OS and EFS, a standardized and reproducible assay is required (46, 47). Currently, multiple approaches for MFC-MRD detection and quantification are adopted: those refer to methodologies of sample processing, as well as antibody panels. There is no consensus on instrument set-up, data acquisition, analysis (e.g., gating strategies), and interpretation. The calculation of MRD load is not standardized yet, since different laboratories use different denominators for MRD enumeration (e.g., percentage of total nucleated cells, CD45+ cells, or mononuclear cells).
Several national and international networks have been established to optimize and standardize MFC-MRD detection and quantification (46–51). In this context, the European Leukemia Network recently published an extensive consensus document (52) on MFC-MRD measurement in adult AML with recommendations for common approaches including definition of time-points, thresholds, technical requirements, marker panels, and results reporting (32). Consistent adherence to such a standardized approach will likely overcome many of the current MFC-MRD limitations. Regardless, differences between pediatric and adult AML need to be considered, especially concerning LAIPs specificity (11, 53–55).
Conventional approaches for analysis and interpretation of MFC-MRD data, lag behind standardization of the wet-lab issues because of the many limitations of manual gating and data analysis strategies, especially when dealing with higher-dimensional complex datasets. Hence, data analysis and interpretation currently is kind of a bottleneck for safely applying MFC-MRD methodology in AML since it strongly relies on the operator's skills and is highly subjective and time-consuming (56). This is especially true for increasingly extensive marker combinations used for MRD detection. Several approaches for automated MFC data analysis have been proposed (57–64) to overcome this bottleneck by (i) providing a superior resolution compared to conventional manual gating with the whole multi-parameter MFC data space at once (instead of 2-D plot-based visualization), (ii) increasing results comparability and reproducibility through reduction of subjectivity caused by manual operator gating and (iii) substantially reducing the workload (e.g., extensive staff trainings) and laboratory costs. Recently, several national reference laboratories of the iBFM-FLOW network and the AIEOP-BFM AML FLOW-MRD study group from across Europe [Austria, Germany, Italy, Poland, Russia (Moscow) and South America (Argentina)] have joined forces in a project called flowCLUSTER, dedicated to foster standardization and automation of MFC-MRD analysis in pedAML. They used machine learning technologies (61, 65), similarly to what already pursued in automated MFC-MRD data analysis of acute lymphoblastic leukemiasamples (57, 60, 64). It can be assumed that such an automated tool, together with central review and a program of continuous quality assessment, will provide standardization and high resolution in MFC-MRD assessment.
Identification and Integration of Suitable LAIPs to Monitor MRD in AML
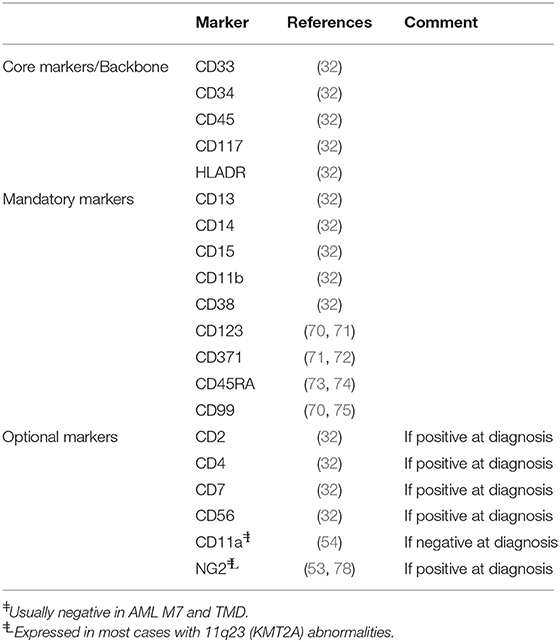
Diagnostic laboratories have shifted from 4 to 6 colors upto 12 colors MFC, with a substantial improvement of LAIP detection and discrimination between aberrant and normal cells (32, 66). Integration of computational methods into the diagnostic workflow will further facilitate the use of even more complex staining panels. However, suitable markers or patterns of antigen co-expression unequivocally distinct from those of normal hematopoietic cells should be available.
Aberrant LAIPs can be identified at diagnosis in the vast majority of childhood AML cases (16). Nevertheless, LAIPs are not always reliable and sensitive for MRD monitoring due to several reasons (49, 67, 68). First, LAIPs can be expressed only by a subpopulation of AML blast cells, potentially hampering MRD detection in follow-up samples. Second, not all LAIPs are stable during the follow-up, possibly resulting in false-negative MRD estimation (69). Besides, the suitability of an antigen for MRD assessment strongly depends on both the degree of its background expression on normal cells and its discriminative expression pattern.
Current marker panel recommendations, including those published by the ELN (32) contain broadly useful markers like the core/backbone markers CD33, CD34, CD45, CD117, and HLADR, the mandatory markers CD13, CD14, CD15, CD11b, CD38 (32), CD123 (70, 71), CD371 (71, 72), CD45RA (73, 74), and CD99 (70, 75), as well as optional markers as per diagnosis, e.g., CD56. In the recent years several new, promising markers have been identified. CD11a is consistently expressed on normal leukocytes and CD34+ progenitors in BM (76, 77). Boztug et al. (54) reported that CD11a deficiency is highly specific for AML-M7, Down Syndrome (DS) AML and transient myeloproliferative disease, making it an ideal candidate for MRD monitoring in these subtypes (Table 2). Advanced technologies including GEP (43) or mass spectrometry based on cell surface capture technology (79) are increasingly used to discover novel markers for MFC-MRD. Recently, Coustan-Smith et al. (43) used genome-wide gene expression analysis to identify different marker profiles specific for AML and normal hematopoietic cells. The authors identified twenty-two markers able to improve the discrimination between leukemic and normal cells. Notably, their expression was stable during chemotherapy, as well as upon relapse (43). In an attempt to identify targets for CAR-T cell therapy, Perna et al. (80) generated an extensive dataset of AML surface proteins using proteomics and transcriptomics. They identified several antigens highly expressed in AML bulk and leukemic stem cells but expressed only at very low levels in normal hematopoietic cells.
May MFC-MRD Represent a Surrogate Endpoint for Survival in Pediatric AML?
In the majority of late-phase clinical trials, the primary endpoints are OS and EFS (81). Hence, a long follow-up period is required before drawing any conclusions on the efficacy of new therapy regimen and new anti-AML-drugs. To accelerate the discovery and approval of new drugs for pedAML, the call for a suitable surrogate for OS and EFS is steadily growing louder. Recently, the Food and Drug Administration (FDA) published a guidance document on the role of MRD in the development of drug products (FDA website, 2018). The use of MFC-MRD as a new early endpoint for the assessment of therapeutic response in clinical trials is intriguing. MFC-MRD detection in AML may expedite drug approval or prevent the adverse continuation of suboptimal treatment strategies (82). Several studies have already shown the potentialities of MRD as surrogate endpoints in AML (83–85).
Conclusions
With continuous efforts in standardization, MFC-MRD response may guide treatment intensity, and become a useful surrogate endpoint for clinical drug development in pedAML. Hence it is time to perform prospective, multicenter randomized trials to evaluate the impact of therapeutic interventions driven by MFC-MRD, together with its role as an early marker of response-to-therapy and a potential surrogate survival endpoint.
Author Contributions
BB and MD designed the study. BB, MM-G, EV, and MD wrote the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Swerdlow SH, Campo E, Lee Harris N, Jaffe ES, Pileri SA, Stein H, et al. WHO classification of Tumors of Haematopoietic and Lymphoid Tissues. Lyon: IARC (2008).
2. Gamis AS, Alonzo TA, Perentesis JP, Meshinchi S, COG Acute Myeloid Leukemia Committee. Children's Oncology Group's 2013 blueprint for research: acute myeloid leukemia. Pediatr Blood Cancer. (2013) 60:964–71. doi: 10.1002/pbc.24432
3. Rubnitz JE, Inaba H. Childhood acute myeloid leukaemia. Br J Haematol. (2012) 159:259–87. doi: 10.1111/bjh.12040
4. Song TY, Lee SH, Kim G, Baek HJ, Hwang TJ, Kook H. Improvement of treatment outcome over 2 decades in children with acute myeloid leukemia. Blood Res. (2018) 53:25–34. doi: 10.5045/br.2018.53.1.25
5. Tsukimoto I, Tawa A, Horibe K, Tabuchi K, Kigasawa H, Tsuchida M, et al. Risk-stratified therapy and the intensive use of cytarabine improves the outcome in childhood acute myeloid leukemia: the AML99 trial from the Japanese Childhood AML Cooperative Study Group. J Clin Oncol. (2009) 27:4007–13. doi: 10.1200/JCO.2008.18.7948
6. Rubnitz JE, Inaba H, Dahl G, Ribeiro RC, Bowman WP, Taub J, et al. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. Lancet Oncol. (2010) 11:543–52. doi: 10.1016/S1470-2045(10)70090-5
7. Abrahamsson J, Forestier E, Heldrup J, Jahnukainen K, Jonsson OG, Lausen B, et al. Response-guided induction therapy in pediatric acute myeloid leukemia with excellent remission rate. J Clin Oncol. (2011) 29:310–5. doi: 10.1200/JCO.2010.30.6829
8. Gibson BE, Webb DK, Howman AJ, De Graaf SS, Harrison CJ, Wheatley K. Results of a randomized trial in children with Acute Myeloid Leukaemia: medical research council AML12 trial. Br J Haematol. (2011) 155:366–76. doi: 10.1111/j.1365-2141.2011.08851.x
9. Cooper TM, Franklin J, Gerbing RB, Alonzo TA, Hurwitz C, Raimondi SC, et al. AAML03P1, a pilot study of the safety of gemtuzumab ozogamicin in combination with chemotherapy for newly diagnosed childhood acute myeloid leukemia: a report from the Children's Oncology Group. Cancer. (2012) 118:761–9. doi: 10.1002/cncr.26190
10. Hasle H, Abrahamsson J, Forestier E, Ha SY, Heldrup J, Jahnukainen K, et al. Gemtuzumab ozogamicin as postconsolidation therapy does not prevent relapse in children with AML: results from NOPHO-AML 2004. Blood. (2012) 120:978–84. doi: 10.1182/blood-2012-03-416701
11. Creutzig U, Zimmermann M, Bourquin JP, Dworzak MN, Fleischhack G, Graf N, et al. Randomized trial comparing liposomal daunorubicin with idarubicin as induction for pediatric acute myeloid leukemia: results from Study AML-BFM 2004. Blood. (2013) 122:37–43. doi: 10.1182/blood-2013-02-484097
12. Pession A, Masetti R, Rizzari C, Putti MC, Casale F, Fagioli F, et al. Results of the AIEOP AML 2002/01 multicenter prospective trial for the treatment of children with acute myeloid leukemia. Blood. (2013) 122:170–8. doi: 10.1182/blood-2013-03-491621
13. Kaspers G. How I treat paediatric relapsed acute myeloid leukaemia. Br J Haematol. (2014) 166:636–45. doi: 10.1111/bjh.12947
14. Coustan-Smith E, Ribeiro RC, Rubnitz JE, Razzouk BI, Pui CH, Pounds S, et al. Clinical significance of residual disease during treatment in childhood acute myeloid leukaemia. Br J Haematol. (2003) 123:243–52. doi: 10.1046/j.1365-2141.2003.04610.x
15. Langebrake C, Creutzig U, Dworzak M, Hrusak O, Mejstrikova E, Griesinger F, et al. Residual disease monitoring in childhood acute myeloid leukemia by multiparameter flow cytometry: the MRD-AML-BFM Study Group. J Clin Oncol. (2006) 24:3686–92. doi: 10.1200/JCO.2005.05.4312
16. van der Velden VH, van der Sluijs-Geling A, Gibson BE, te Marvelde JG, Hoogeveen PG, Hop WC, et al. Clinical significance of flowcytometric minimal residual disease detection in pediatric acute myeloid leukemia patients treated according to the DCOGANLL97/MRC AML12 protocol. Leukemia. (2010) 24:1599–606. doi: 10.1038/leu.2010.153
17. Buccisano F, Maurillo L, Del Principe MI, Del Poeta G, Sconocchia G, Lo-Coco F, et al. Prognostic and therapeutic implications of minimal residual disease detection in acute myeloid leukemia. Blood. (2012) 119:332–41. doi: 10.1182/blood-2011-08-363291
18. Loken MR, Alonzo TA, Pardo L, Gerbing RB, Raimondi SC, Hirsch BA, et al. Residual disease detected by multidimensional flow cytometry signifies high relapse risk in patients with de novo acute myeloid leukemia: a report from Children's Oncology Group. Blood. (2012) 120:1581–8. doi: 10.1182/blood-2012-02-408336
19. Coustan-Smith E, Campana D. Should evaluation for minimal residual disease be routine in acute myeloid leukemia? Curr Opin Hematol. (2013) 20:86–92. doi: 10.1097/MOH.0b013e32835dd90a
20. Karol SE, Coustan-Smith E, Cao X, Shurtleff SA, Raimondi SC, Choi JK, et al. Prognostic factors in children with acute myeloid leukaemia and excellent response to remission induction therapy. Br J Haematol. (2015) 168:94–101. doi: 10.1111/bjh.13107
21. Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. (2017) 129:424–47. doi: 10.1182/blood-2016-08-733196
22. Jongen-Lavrencic M, Grob T, Hanekamp D, Kavelaars FG, Hinai A, Zeilemaker A, et al. Molecular minimal residual disease in acute myeloid leukemia. N Engl J Med. (2018) 378:1189–245. doi: 10.1056/NEJMoa1716863
23. Campana D. Status of minimal residual disease testing in childhood haematological malignancies. Br J Haematol. (2008) 143:481–9. doi: 10.1111/j.1365-2141.2008.07350.x
24. Inaba H, Coustan-Smith E, Cao X, Pounds SB, Shurtleff SA, Wang KY, et al. Comparative analysis of different approaches to measure treatment response in acute myeloid leukemia. J Clin Oncol. (2012) 30:3625–32. doi: 10.1200/JCO.2011.41.5323
25. Mosna F, Gottardi M. Stem cell modeling of core binding factor acute myeloid leukemia. Stem Cells Int. (2016) 2016:7625827. doi: 10.1155/2016/7625827
26. Yin JA, O'Brien MA, Hills RK, Daly SB, Wheatley K, Burnett AK. Minimal residual disease monitoring by quantitative RT-PCR in core binding factor AML allows risk stratification and predicts relapse: results of the United Kingdom MRC AML-15 trial. Blood. (2012) 120:2826–36. doi: 10.1182/blood-2012-06-435669
27. Song J, Mercer D, Hu X, Liu H, Li MM. Common leukemia- and lymphoma-associated genetic aberrations in healthy individuals. J Mol Diagn. (2011) 13:213–9. doi: 10.1016/j.jmoldx.2010.10.009
28. Pigazzi M, Manara E, Buldini B, Beqiri V, Bisio V, Tregnago C, et al. Minimal residual disease monitored after induction therapy by RQ-PCR can contribute to tailor treatment of patients with t(8. 21) RUNX1-RUNX1T1 rearrangement. Haematologica. (2015) 100:e99–101. doi: 10.3324/haematol.2014.114579
29. Manara E, Basso G, Zampini M, Buldini B, Tregnago C, Rondelli R, et al. Characterization of children with FLT3-ITD acute myeloid leukemia: a report from the AIEOP AML-2002 study group. Leukemia. (2017) 31:18–25. doi: 10.1038/leu.2016.177
30. Ley TJ, Mardis ER, Ding L, Fulton B, McLellan MD, Chen K, et al. DNA sequencing of a cytogenetically normal acute myeloid leukaemia genome. Nature. (2008) 456:66–72. doi: 10.1038/nature07485
31. Voso MT, Ottone T, Lavorgna S, Venditti A, Maurillo L, Lo-Coco F, et al. MRD in AML: the role of new techiques. Front Oncol. (2019) 9:655. doi: 10.3389/fonc.2019.00655
32. Schuurhuis GJ, Heuser M, Freeman S, Bené MC, Buccisano F, Cloos J, et al. Minimal/measurable residual disease in AML: a consensus document from the European LeukemiaNet MRD Working Party. Blood. (2018) 131:1275–91. doi: 10.1182/blood-2017-09-801498
33. Jacobsohn DA, Loken MR, Fei M, Adams A, Brodersen LE, Logan BR, et al. Outcome of measurable residual disease in pediatric acute myeloid leukemia before and after hematopoietic stem cell transplant: validation of difference from normal flow cytometry with chimerism studies and Wilms Tumor 1 gene expression. Biol Blood marrow Transplant. (2018) 24:2040–6. doi: 10.1016/j.bbmt.2018.06.010
35. San Miguel JF, Martínez A, Macedo A, Vidriales MB, López-Berges C, González M, et al. Immunophenotyping investigation of minimal residual disease is a useful approach for predicting relapse in acute myeloid leukemia patients. Blood. (1997) 90:2465–70.
36. San Miguel JF, Vidriales MB, Lopez-Berges C, Díaz-Mediavilla J, Gutiérrez N, Cañizo C, et al. Early immunophenotypical evaluation of minimal residual disease in acute myeloid leukemia identifies different patient risk groups and may contribute to postinduction treatment stratification. Blood. (2001) 98:1746–51. doi: 10.1182/blood.V98.6.1746
37. Venditti A, Buccisano F, Del Poeta G, Maurillo L, Tamburini A, Cox C, et al. Level of minimal residual disease after consolidation therapy predicts outcome in acute myeloid leukemia. Blood. (2000) 96:3948–52.
38. Kern W, Voskova D, Schoch C, Schnittger S, Hiddemann W, Haferlach T. Prognostic impact of early response to induction therapy as assessed by multiparameter flow cytometry in acute myeloid leukemia. Haematologica. (2004) 89:528–40.
39. Sievers EL, Lange BJ, Alonzo TA, Gerbing RB, Bernstein ID, Smith FO, et al. Immunophenotypic evidence of leukemia after induction therapy predicts relapse: results from a prospective Children's Cancer Group study of 252 patients with acute myeloid leukemia. Blood. (2003) 101:3398–406. doi: 10.1182/blood-2002-10-3064
40. Tierens A, Bjørklund E, Siitonen S, Marquart HV, Wulff-Juergensen G, Pelliniemi TT, et al. Residual disease detected by flow cytometry is an independent predictor of survival in childhood acute myeloid leukaemia. results of the NOPHO-AML 2004 study. Br J Haematol. (2016) 174:600–9. doi: 10.1111/bjh.14093
41. Buldini B, Rizzati F, Masetti R, Fagioli F, Menna G, Micalizzi C, et al. Prognostic significance of flow-cytometry evaluation of minimal residual disease in children with acute myeloid leukaemia treated according to the AIEOP-AML 2002/01 study protocol. Br J Haematol. (2017) 177:116–26. doi: 10.1111/bjh.14523
42. Walter RB, Buckley SA, Pagel JM, Wood BL, Storer BE, Sandmaler BM, et al. Significance of minimal residual disease before myeloablative allogeneic hematopoietic cell transplantation for AML in first and second complete remission. Blood. (2013) 122:1813–21. doi: 10.1182/blood-2013-06-506725
43. Coustan-Smith E, Song G, Shurtleff S, Yeoh AE, Chng WJ, Chen SP, et al. Universal monitoring of minimal residual disease in acute myeloid leukemia. JCI Insight. (2018) 3:e98561. doi: 10.1172/jci.insight.98561
44. Zeijlemaker W, Gratama JW, Schuurhuis GJ. Tumor heterogeneity makes AML a “moving target” for detection of residual disease. Cytometry B Clin Cytom. (2014) 86:3–14. doi: 10.1002/cytob.21134
45. Dworzak MN, Gaipa G, Ratei R, Veltroni M, Schumich A, Maglia O, et al. Standardization of flow cytometric minimal residual disease evaluation in acute lymphoblastic leukemia: multicentric assessment is feasible. Cytometry B Clin Cytom. (2008) 74:331–40. doi: 10.1002/cyto.b.20430
46. Brooimans RA, van der Velden VHJ, Boeckx N, Slomp J, Preijers F, Te Marvelde JG, et al. Immunophenotypic measurable residual disease (MRD) in acute myeloid leukemia: is multicentric MRD assessment feasible? Leuk Res. (2019) 76:39–47. doi: 10.1016/j.leukres.2018.11.014
47. Bruggemann M, Schrauder A, Raff T, Pfeifer H, Dworzak M, Ottmann OG, et al. Standardized MRD quantification in European ALL trials: proceedings of the Second International Symposium on MRD assessment in Kiel, Germany, 18–20 September 2008. Leukemia. (2010) 24:521–35. doi: 10.1038/leu.2009.268
48. Theunissen P, Mejstrikova E, Sedek L, van der Sluijs-Gelling AJ, Gaipa G, Bartels M, et al. Standardized flow cytometry for highly sensitive MRD measurements in B-cell acute lymphoblastic leukemia. Blood. (2017) 129:347–57. doi: 10.1182/blood-2016-07-726307
49. Dworzak MN, Buldini B, Gaipa G, Ratei R, Hrusak O, Luria D, et al. AIEOP-BFM consensus guidelines 2016 for flow cytometric immunophenotyping of Pediatric acute lymphoblastic leukemia. Cytometry B Clin Cytom. (2018) 94:82–93. doi: 10.1002/cyto.b.21518
50. Lacombe F, Bernal E, Bloxham D, Couzens S, Porta MG, Johansson U, et al. Harmonemia: a universal strategy for flow cytometry immunophenotyping-A European LeukemiaNet WP10 study. Leukemia. (2016) 30:1769–72. doi: 10.1038/leu.2016.44
51. Irving J, Jesson J, Virgo P, Case M, Minto L, Eyre L, et al. Establishment and validation of a standard protocol for the detection of minimal residual disease in B lineage childhood acute lymphoblastic leukemia by flow cytometry in a multi-center setting. Haematologica. (2009) 94:870–4. doi: 10.3324/haematol.2008.000414
52. Feller N, van der Velden VH, Brooimans RA, Boeckx N, Preijers F, Kelder A, et al. Defining consensus leukemia-associated immunophenotypes for detection of minimal residual disease in acute myeloid leukemia in a multicenter setting. Blood Cancer J. (2013) 3:e129. doi: 10.1038/bcj.2013.27
53. Mauvieux L, Delabesse E, Bourquelot P, Radford-Weiss I, Bennaceur A, Flandrin G, et al. NG2 expression in MLL rearranged acute myeloid leukaemia is restricted to monoblastic cases. Br J Haematol. (1999) 107:674–6. doi: 10.1046/j.1365-2141.1999.01730.x
54. Boztug H, Schumich A, Potschger U, Muhlegger N, Kolenova A, Reinhardt K, et al. Blast cell deficiency of CD11a as a marker of acute megakaryoblastic leukemia and transient myeloproliferative disease in children with and without Down syndrome. Cytometry B Clin Cytom. (2013) 84:370–8. doi: 10.1002/cyto.b.21082
55. Gruber TA, Downing JR. The biology of pediatric acute megakaryoblastic leukemia. Blood. (2015) 126:943–9. doi: 10.1182/blood-2015-05-567859
56. Mair F, Hartmann FJ, Mrdjen D, Tosevski V, Krieg C, Becher B. The end of gating? An introduction to automated analysis of high dimensional cytometry data. Eur J Immunol. (2016) 46:34–43. doi: 10.1002/eji.201545774
57. Reiter M, Hoffmann J, Kleber F, Schumich A, Peter G, Kromp F, et al. Towards automation of flow cytometric analysis for quality-assured follow-up assessment to guide curative therapy for acute lymphoblastic leukaemia in children. Mag Eur Med Oncol. (2014) 7:219–26. doi: 10.1007/s12254-014-0172-6
58. van Gassen S, Callebaut B, Van Helden MJ, Lambrecht BN, Demeester P, Dhaene T, et al. FlowSOM: using self-organizing maps for visualization and interpretation of cytometry data. Cytometry A. (2015) 87:636–45. doi: 10.1002/cyto.a.22625
59. Ni W, Hu B, Zheng C, Tong Y, Wang L, Li QQ, et al. Automated analysis of acute myeloid leukemia minimal residual disease using a support vector machine. Oncotarget. (2016) 7:71915–21. doi: 10.18632/oncotarget.12430
60. Reiter M, Rota P, Kleber F, Diem M, Groeneveld-Krentz S, Dworzak M. Clustering of cell populations in flow cytometry data using a combination of Gaussian mixtures. Pattern Recogn. (2016) 60:1029–40. doi: 10.1016/j.patcog.2016.04.004
61. Licandro R, Reiter M, Diem M, Dworzak MN, Schumich A, Langs G, et al. WGAN latent space embeddings for blast identification in childhood acute myeloid leukaemia. In: 2018 24th International Conference on Pattern Recognition (ICPR) Beijing, China (2018).
62. Rahim A, Meskas J, Drissler S, Yue A, Lorenc A, Laing A, et al. High throughput automated analysis of big flow cytometry data. Methods. (2018) 134–135:164–76. doi: 10.1016/j.ymeth.2017.12.015
63. Conrad VK, Dubay CJ, Malek M, Brinkman RR, Koguchi Y, Redmond WL. Implementation and validation of an automated flow cytometry analysis pipeline for human immune profiling. Cytometry A. (2019) 95:183–91. doi: 10.1002/cyto.a.23664
64. Reiter M, Diem M, Schumich A, Maurer-Granofszky M, Karawajew L, Rossi JG, et al. Automated flow citometry MRD assessment in childhood acute B-lymphoblastic leukemia using supervised machine learning. Cytometry A. (2019) 95:966–75. doi: 10.1002/cyto.a.23852
65. Licandro R, Reiter M, Diem M, Dworzak MN, Schumich A, Kampel M. Application of machine learning for automatic MRD assessment in paediatric acute myeloid leukaemia. In: Proceedings of the 7th International Conference on Pattern Recognition Applications and Methods (ICPRAM 2018) (Funchal) (2018).
66. van Dongen JJ, van der Velden VH, Bruggemann M, Orfao A. Minimal residual disease diagnostics in acute lymphoblastic leukemia: need for sensitive, fast, and standardized technologies. Blood. (2015) 125:3996–4009. doi: 10.1182/blood-2015-03-580027
67. Al-Mawali A, Gillis D, Lewis I. The role of multiparameter flow cytometry for detection of minimal residual disease in acute myeloid leukemia. Am J Clin Pathol. (2009) 131:16–26. doi: 10.1309/AJCP5TSD3DZXFLCX
68. Gaipa G, Buracchi C, Biondi A. Flow cytometry for minimal residual disease testing in acute leukemia: opportunities and challenges. Expert Rev Mol Diagn. (2018) 18:775–87. doi: 10.1080/14737159.2018.1504680
69. Baer MR, Stewart CC, Dodge RK, Leget G, Sule N, Mrozek K, et al. High frequency of immunophenotype changes in acute myeloid leukemia at relapse: implications for residual disease detection (Cancer and Leukemia Group B Study 8361). Blood. (2001) 97:3574–80. doi: 10.1182/blood.V97.11.3574
70. Angelini DF, Ottone T, Guerrera G, Lavorgna S, Cittadini M, Buccisano F, et al. A leukemia-associated CD34/CD123/CD25/CD99+ immunophenotype identifies FLT3-mutated clones in acute myeloid leukemia. Clin Cancer Res. (2015) 21:3977–85. doi: 10.1158/1078-0432.CCR-14-3186
71. Roug AS, Larsen HO, Nederby L, Just T, Brown G, Nyvold CG, et al. hMICL and CD123 in combination with a CD45/CD34/CD117 backbone—a universal marker combination for the detection of minimal residual disease in acute myeloid leukaemia. Br J Haematol. (2014) 164:212–22. doi: 10.1111/bjh.12614
72. Larsen HO, Roug AS, Just T, Brown GD, Hokland P. Expression of the hMICL in acute myeloid leukemia-a highly reliable disease marker at diagnosis and during follow-up. Cytometry B Clin Cytom. (2012) 82:3–8. doi: 10.1002/cyto.b.20614
73. Kersten B, Valkering M, Wouters R, van Amerongen R, Hanekamp D, Kwidama Z, et al. CD45RA, a specific marker for leukaemia stem cell sub-populations in acute myeloid leukaemia. Br J Haematol. (2016) 173:219–35. doi: 10.1111/bjh.13941
74. Zeijlemaker W, Kelder A, Oussoren-Brockhoff YJ, Scholten WJ, Snel AN, Veldhuizen D, et al. A simple one-tube assay for immunophenotypical quantification of leukemic stem cells in acute myeloid leukemia. Leukemia. (2016) 30:439–46. doi: 10.1038/leu.2015.252
75. Strehl S, Konig M, Boztug H, Cooper BW, Suzukawa K, Zhang SJ, et al. All-trans retinoic acid and arsenic trioxide resistance of acute promyelocytic leukemia with the variant STAT5B-RARA fusion gene. Leukemia. (2013) 27:1606–10. doi: 10.1038/leu.2012.371
76. Dworzak MN, Fritsch G, Fleischer C, Printz D, Froschl G, Buchinger P, et al. Comparative phenotype mapping of normal vs. malignant pediatric B-lymphopoiesis unveils leukemia-associated aberrations Exp Hematol. (1998) 26:305–13.
77. Kansas GS, Muirhead MJ, Dailey MO. Expression of the CD11/CD18, leukocyte adhesion molecule 1, and CD44 adhesion molecules during normal myeloid and erythroid differentiation in humans. Blood. (1990) 76:2483–92.
78. Petrovici K, Graf M, Hecht K, Reif S, Pfister K, Schmetzer H. Use of NG2 (7.1) in AML as a tumor marker and its association with a poor prognosis. Cancer Genomics Proteomics. (2010) 7:173–80.
79. Mirkowska P, Hofmann A, Sedek L, Slamova L, Mejstrikova E, Szczepanski T, et al. Leukemia surfaceome analysis reveals new disease-associated features. Blood. (2013) 121:e149–159. doi: 10.1182/blood-2012-11-468702
80. Perna F, Berman SH, Soni RK, Mansilla-Soto J, Eyquem J, Hamieh M, et al. Integrating proteomics and transcriptomics for systematic combinatorial chimeric antigen receptor therapy of AML. Cancer Cell. (2017) 32:506–519.e505. doi: 10.1016/j.ccell.2017.09.004
81. Medeiros BC. Interpretation of clinical endpoints in trials of acute myeloid leukemia. Leuk Res. (2018) 68:32–9. doi: 10.1016/j.leukres.2018.02.002
82. Hourigan CS, Gale RP, Gormley NJ, Ossenkoppele GJ, Walter RB. Measurable residual disease testing in acute myeloid leukaemia. Leukemia. (2017) 31:1482–90. doi: 10.1038/leu.2017.113
83. Smith BD, Roberts AW, Roboz GJ, DeWitte M, Ferguson A, Garrett L, et al. Minimal Residual Disease (MRD) as exploratory endpoint in a phase 1 study of the anti-CD123 Mab CSL362 given as post-remission therapy in adult acute myeloid leukemia (AML). Blood. (2015) 126:3819.
84. Prebet T, Bertoli S, Delaunay J, Pigneux A, Delabesse E, Mozziconacci MJ, et al. Anthracycline dose intensification improves molecular response and outcome of patients treated for core binding factor acute myeloid leukemia. Haematologica. (2014) 99:e185–187. doi: 10.3324/haematol.2014.109827
Keywords: acute myeloid leukemia, minimal residual disease, multiparametric flow cytometry, childhood, risk stratification
Citation: Buldini B, Maurer-Granofszky M, Varotto E and Dworzak MN (2019) Flow-Cytometric Monitoring of Minimal Residual Disease in Pediatric Patients With Acute Myeloid Leukemia: Recent Advances and Future Strategies. Front. Pediatr. 7:412. doi: 10.3389/fped.2019.00412
Received: 11 June 2019; Accepted: 25 September 2019;
Published: 11 October 2019.
Edited by:
Maria Ester Bernardo, San Raffaele Hospital (IRCCS), ItalyReviewed by:
Concetta Micalizzi, Giannina Gaslini Institute (IRCCS), ItalyYana Pikman, Dana–Farber Cancer Institute, United States
Copyright © 2019 Buldini, Maurer-Granofszky, Varotto and Dworzak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael N. Dworzak, bWljaGFlbC5kd29yemFrQHN0YW5uYS5hdA==
 Barbara Buldini
Barbara Buldini Margarita Maurer-Granofszky
Margarita Maurer-Granofszky Elena Varotto
Elena Varotto Michael N. Dworzak
Michael N. Dworzak