- 1Department of Pharmacy, Hong Kong Children's Hospital, Hong Kong, China
- 2Department of Paediatrics, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, China
- 3School of Pharmacy, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, China
- 4Department of Pharmacy, Prince of Wales Hospital, Hong Kong, China
Background: Deviations from the optimal vancomycin dosing may occur in the neonatal and pediatric population due to inconsistencies in the recommended dosing algorithms. This study aims to collect the expert opinions of clinicians who practice in the neonatal or pediatric intensive care units (NICU/PICUs) of 12 major medical centers in Hong Kong.
Methods: This was a multicenter, cross-sectional study. Eligible physicians and pharmacists completed a structured questionnaire to identify the challenges they encountered when selecting the initial intermittent vancomycin dosing. They also answered questions concerning therapeutic monitoring services (TDM) for vancomycin, including the targeted trough levels for empirical vancomycin regimens administered for complicated and uncomplicated infections.
Results: A total of 23 physicians and 43 pharmacists completed the survey. The top clinical parameters reported as most important for determining the initial vancomycin dosing were renal function (90.9%), post-menstrual/postnatal age (81.8%), body weight (66.7%), and suspected/documented pathogen (53.0%). Respondents reported challenges such as difficulties in determining the optimal initial dose for a targeted level (53.0%), inconsistencies between dosing references (43.9%) and a lack of clear hospital guidelines (27.3%). Half of the pharmacists (48.8%) reported that they had helped to interpret the TDM results and recommend vancomycin dose adjustments in >75% of cases. For methicillin-resistant Staphylococcus aureus infection, physicians, and pharmacists reported target trough levels of ~10–15 and 15–20 mg/L, respectively. For suspected moderate/uncomplicated Gram-positive infections physicians tended to prefer a lower trough range of 5–10 mg/L, while pharmacists preferred a range of 10–15 mg/L.
Conclusions: Our results demonstrate that clinicians used varying vancomycin dosing guidelines in their practices. The multidisciplinary TDM service in Hong Kong can be improved further by establishing a standardized dosing guideline and implementing a well-structured, evidence-based service protocol. Future work includes conducting drug utilization studies to evaluate real-world antimicrobial usage patterns and the impact on tangible clinical outcomes, and developing pharmacokinetic-guided dose calculator for antimicrobials in critically ill neonates and pediatric patients.
Key Points
• Clinicians used varying dosing guidelines in their selection of initial IV vancomycin dosing for patients of NICU/PICUs in Hong Kong.
• Despite the purported benefits of therapeutic drug monitoring (TDM) services, the availability and scope of this clinical service varies among local public hospitals.
• A standardized dosing guideline is needed to decrease variability and improve local practice.
• Future work includes conducting drug utilization studies and developing pharmacokinetic-guided dose calculator for antimicrobials in critically ill neonates and pediatric patients.
Introduction
Critically ill neonatal and pediatric patients face a higher risk of nosocomial infection due to a combination of immature humoral and cellular immunity and the use of invasive techniques such as central venous catheterization and endotracheal intubation. Neonatal bacterial sepsis causes death in 10–20% of cases and morbidity in 25–30% of survivors. Therefore, effective and timely antimicrobial treatment regimens are often life-saving (1).
Selection of antimicrobial therapy for a critically ill patient may vary based on the nature of treatment (empirical or directed), source of infection and/or likely pathogen(s) and susceptibility. Vancomycin, a glycopeptide antibiotic, is often prescribed to cover resistant Gram-positive organisms such as methicillin-resistant Staphylococcus aureus (MRSA) and coagulase-negative Staphylococci (CoNS), particularly methicillin-resistant coagulase-negative Staphylococci (MRCoNS). MRSA is among the most threatening pathogens and the principal indicator of nosocomial risk. The incidence of neonatal sepsis has also increased over time in developed countries among very low-birthweight (VLBW) infants, and CoNS has emerged as a major pathogen in this context (2, 3). In a NICU/PICU setting, empirical vancomycin therapy is commonly initiated in a patient with suspected staphylococcal infections until the susceptibility pattern of the pathogen is confirmed.
The optimization of the initial vancomycin dosing required to achieve a therapeutic plasma level, and reducing the time taken to reach this level, have been proven to improve clinical outcomes in adults (4). However, deviations from the optimal vancomycin dosing may occur in the neonatal and pediatric population due to inconsistencies in the recommended dosing algorithms (5–8). This lack of consensus is illustrated by the wide variation in dosing regimens recommended in reference guidelines and the targeted therapeutic levels (Supplementary Materials 1, 2) (9–14). One study conducted at a pediatric tertiary care medical center in the United States observed that 35% of initial vancomycin courses were inappropriate (15). Another study observed that 74% of vancomycin dosing regimens administered according to the British National Formulary for Children (BNFc) recommendations did not achieve the target trough level (16). This issue is concerning because the inappropriate or suboptimal use of vancomycin can lead to treatment failure and enable the emergence of antibiotic resistance. Many of the above recommendations are supported by a relatively weak level of evidence, and the optimal dosing and monitoring of vancomycin remain controversial. Recent guidelines for vancomycin dosing suggest an area under the curve (AUC)/minimum inhibitory concentration (MIC) ratio >400 as the primary targeted therapeutic parameter to treat MRSA infections (17). The considerable variability in dosing recommendations of vancomycin is an ongoing challenge.
Addressing antibiotic resistance has been a global health agenda over the recent years, and is currently one of the top research priorities in Hong Kong. In 2019, a multidisciplinary team consisting neonatologist, pediatricians, and clinical pharmacists initiated a mixed-methods approach to address appropriate antimicrobial use in the pediatric population. The overarching aim is to implement personalized, pharmacokinetic-guided dosing of antimicrobials in critically ill patients in the NICU/PICU through a mixed-methods research approach (Supplementary Material 3). At the preliminary stage, this study was developed to collect the expert opinions of a representative sample of physicians and pharmacists who practice in the NICU/PICUs of 12 major medical institutions in Hong Kong. This survey aimed to describe the initial vancomycin dose selection practices used in these NICU/PICUs while focusing on the key factors influencing dose selection, preferred references, and target trough level. We also aimed to identify the challenges associated with initial vancomycin dosing and the corresponding therapeutic drug monitoring (TDM) practices.
Methods
This multicenter cross-sectional survey was conducted between January and December 2018. Approval was obtained from The Chinese University of Hong Kong (CUHK) Survey and Behavioral Research Ethics Committee prior to study initiation.
Respondents
Physicians and pharmacists were recruited from 12 major medical centers within the Hospital Authority (HA; Supplementary Material 4), the statutory body that manages all public hospitals in Hong Kong, using a convenience sampling approach. Eligible respondents met the following criteria: (1) status as a practicing physician or pharmacist; (2) active practice in a participating NICU or/and PICU or general pediatric ward; and (3) prescribing of vancomycin (for physicians) or processing or dispensation of vancomycin prescriptions (for pharmacists) for at least one NICU or PICU patients per month. These criteria ensured that we targeted a homogenous sample of practitioners who were directly involved in the management of vancomycin for critically ill pediatric or/and neonatal patients.
Survey Design
The investigators designed the survey based on the existing literature (18–21). The questionnaire comprised three sections (Table 1). The first section collected information about the respondents' clinical experiences, including their primary area(s) of practice and duration of healthcare practice (years). The second section comprised six questions designed to identify the references and clinical factors that respondents considered relevant when selecting the initial vancomycin dosing, as well as the encountered challenges. The third section comprised questions concerning TDM for vancomycin for different scenarios based on the type of infection and pathogens. This is because clinicians typically attempt to predict and narrow down the likely diagnosis and pathogenic bacteria based on the patient's signs/symptoms and clinical assessments when deciding empirical treatment. They would then make modifications to the treatment plan when the culture result is completed. The pre-identified scenarios include the targeted trough levels for empirical vancomycin regimens administered to a case of suspected uncomplicated and complicated Gram-positive infection and to a confirmed case of MRSA or CoNS infection. Examples of complicated infections included bacteremia, osteomyelitis, meningitis, severe pneumonia, endocarditis, and deep-seated infections. Intermittent infusion of vancomycin is assumed for all scenarios as continuous infusion is rarely administered in Hong Kong. Respondents were also asked to identify the reasons for not adjusting a patient's vancomycin dosing regimen in the event of a suboptimal trough level and the challenges of the current TDM services in their respective institutions. All questions were closed-ended, and the respondents were presented with choices as outlined in Table 1. A space for open-ended responses was provided at the end of the questionnaire for respondents to provide additional comments on any of the questions in the survey. The self-administered English-language survey required ~10 min for completion. The final versions of the surveys were revised through a pilot-test among seven physicians and pharmacists.
Statistical Analysis
We did not conduct a powered sample size calculation because of the descriptive nature of this study. Furthermore, we expected the sample size to be limited by the small cohort of healthcare professionals who specialize in pediatric and neonatal critical care medicine in Hong Kong. To avoid under representation of smaller institutions, we distributed a standard number of five copies of the survey to each institution to solicit their participation, as it is reasonable to assume that each institution should have at least five physicians and pharmacists providing service to the PICU/NICU. Department managers were asked to make additional copies when needed and track the distribution of the surveys.
Descriptive statistics were used to analyze the practitioners' responses to each question. Heat maps were used to depict graphically the different ranges of targeted trough values reported by the respondents. An exploratory analysis based on the Kolmogorov–Smirnov-test was conducted to examine differences in the distribution patterns of the targeted trough level ranges reported by physicians and pharmacists. All analyses were conducted using the Statistics Package for Social Science, version 25 (IBM, Armonk, NY: IBM Corp.).
Results
Overview
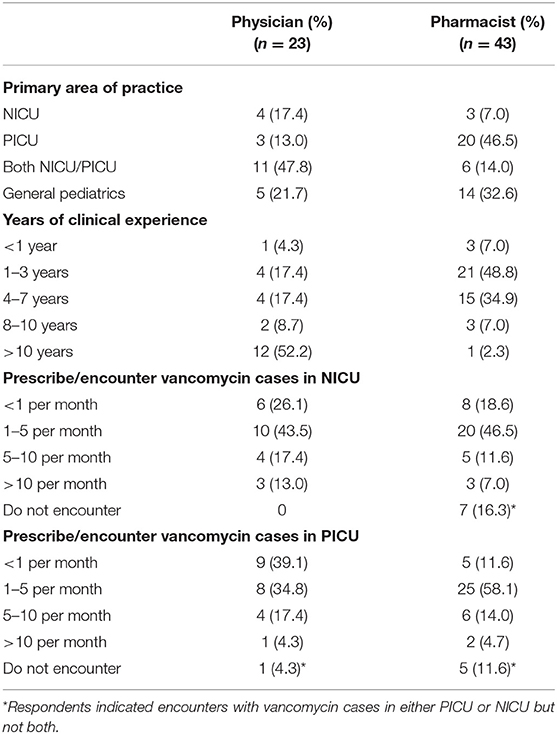
A total of 23 physicians and 43 pharmacists completed the survey, yielding response rates of 67.6 and 61.4%, respectively. The respondents' demographics are summarized in Table 2. Respondents provided service in 12 medical institutions which are distributed across the three main districts in Hong Kong (Supplementary Material 5). The majority of physicians (78.3%) and pharmacists (67.4%) indicated the NICU and/or PICU as their primary area of practice. More than 50% of physicians had more than 10 years of clinical experience, while most pharmacists had 1–7 years of experience in pediatrics. The respondents reported that they handled a median of 1–5 vancomycin cases per month in the NICU/PICU.
Vancomycin Initial Dosing Regimen
Regarding the drug references used to assess vancomycin dosing in the NICU (Supplementary Material 6), ~60% of the respondents selected the Micromedex Neofax®, while 37.8 and 33.3% selected the UpToDate®/Lexicomp® and local hospital guidelines, respectively. Among respondents in the PICU, 59% selected the UpToDate®/Lexicomp®, while 39.4 and 33.3% selected the British National Formulary for Children (BNFc) and Drug Doses (Frank Shann), respectively (Supplementary Material 6).
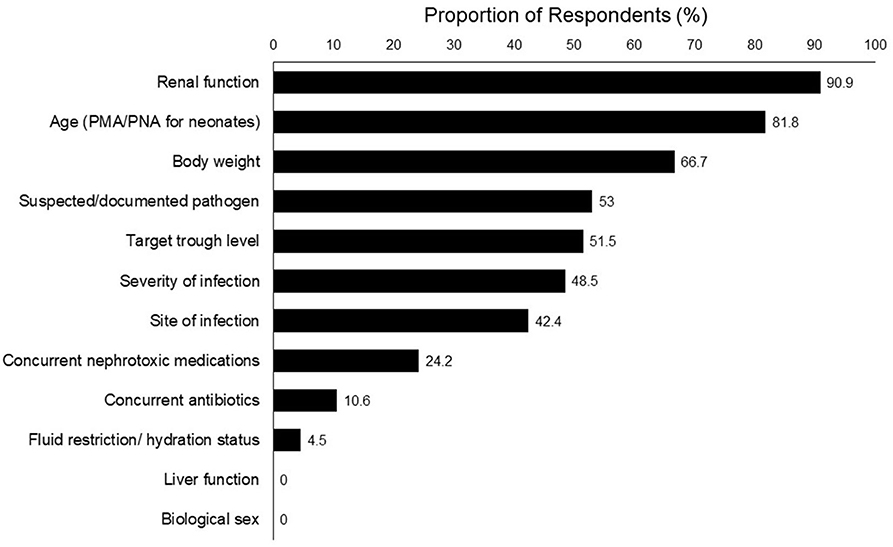
The top 5 clinical parameters reported as most important for determining the initial vancomycin dosing were renal function (90.9%), age [post-menstrual age (PMA)/postnatal age (PNA) for neonates] (81.8%), body weight (66.7%), suspected/documented pathogen (53.0%), and target trough level (51.5%) (Figure 1).
A number of respondents provided open-ended responses on the rationale behind their dose selection. They expressed confusion regarding precise dosing due to varying dosing recommendations. One physician mentioned that in his/her opinion, the renal function is the most important factor to consider especially for a hemodynamically unstable patient, as it would directly affect the clearance of vancomycin. Pharmacists also indicated that they would consider the pharmacokinetics and pharmacodynamics profile of the concurrent medications because most critically ill patients are on multiple drugs, of which some may have a narrow therapeutic range.
Challenges Concerning Empirical Dosing
The respondents identified several challenges regarding the empirical vancomycin dosing (Figure 2). Physicians' challenges included uncertainty regarding the target serum level (37.2%), inconsistencies among different dosing references (30.4%), difficulty in determining the optimal initial dose for a targeted level (26.1%). There seemed to be a higher proportion of pharmacists who identified challenges with empirical dosing. More than two-thirds of the pharmacists (67.4%) considered it difficult to determine the optimal initial dose required to achieve a target serum level. Other challenges encountered by the pharmacists included inconsistencies between dosing references (51.2%) and a lack of clear hospital guidelines (37.2%).
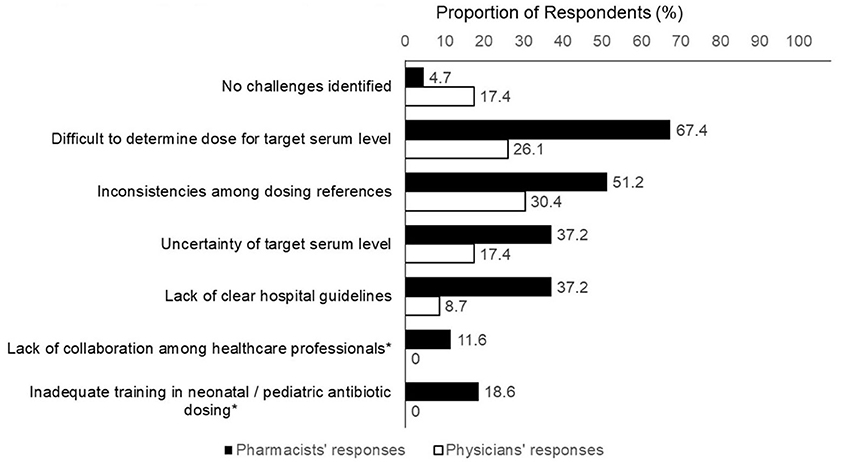
Figure 2. Challenges concerning empirical vancomycin dosing. *Item was not included in physician's version of questionnaire.
Vancomycin Therapeutic Drug Monitoring
The majority of respondents indicated that TDM was ordered for all vancomycin cases in the NICU (80.3%) and PICU (69.7%), and these respondents were from half of the represented institutions. Vancomycin TDM was not performed in certain situations where the drug was prescribed for surgical prophylaxis (65.2%), for short-term use (<72 h; 57.6%), and for empirical use while culture results are pending (18.2%) (Supplementary Material 7).
Half of the pharmacists (48.8%) reported that they had helped to interpret the TDM results and recommend vancomycin dose adjustments in >75% of cases. These pharmacists provided recommendations to improve the TDM service (Figure 3), including the need for a more rapid turnaround time for serum level (76.2%), the development of a standardized guideline for dosing adjustment based on TDM results (73.8%), and more collaboration with physicians (11.9%). The pharmacists also suggested other means of support, such as granting pharmacists the right to order a TDM level and the provision of weekend clinical TDM services from pharmacists.

Figure 3. Challenges faced by pharmacists in establishing therapeutic drug monitoring service (n = 43).
Targeted Trough Ranges for Specific Types of Infections
The color-coded heat graphs revealed that both physicians and pharmacists reported targeted vancomycin trough levels of 15–20 mg/L for a suspected severe/complicated Gram-positive infection when intermittent infusion of vancomycin is administered (Figures 4A,B, respectively). For MRSA infection, physicians and pharmacists reported target trough levels of ~10–15 and 15–20 mg/L, respectively. Both groups of respondents reported targeted trough levels of 10–15 mg/L for confirmed cases of MRCoNS. The respondents' targeted trough levels for suspected moderate/uncomplicated Gram-positive infections ranged between 5 and 15 mg/L. However, physicians tended to prefer a lower trough range of 5–10 mg/L, while pharmacists preferred a range of 10–15 mg/L. Only one physician indicated that their institution adopted an AUC/MIC ≥400 as a therapeutic indicator. Graphical inspection revealed that though responses differ among each respondent, the overall pattern did not differ much across districts for MRSA, MRCoNS, and suspected moderate/uncomplicated Gram-positive infections (Supplementary Material 8).
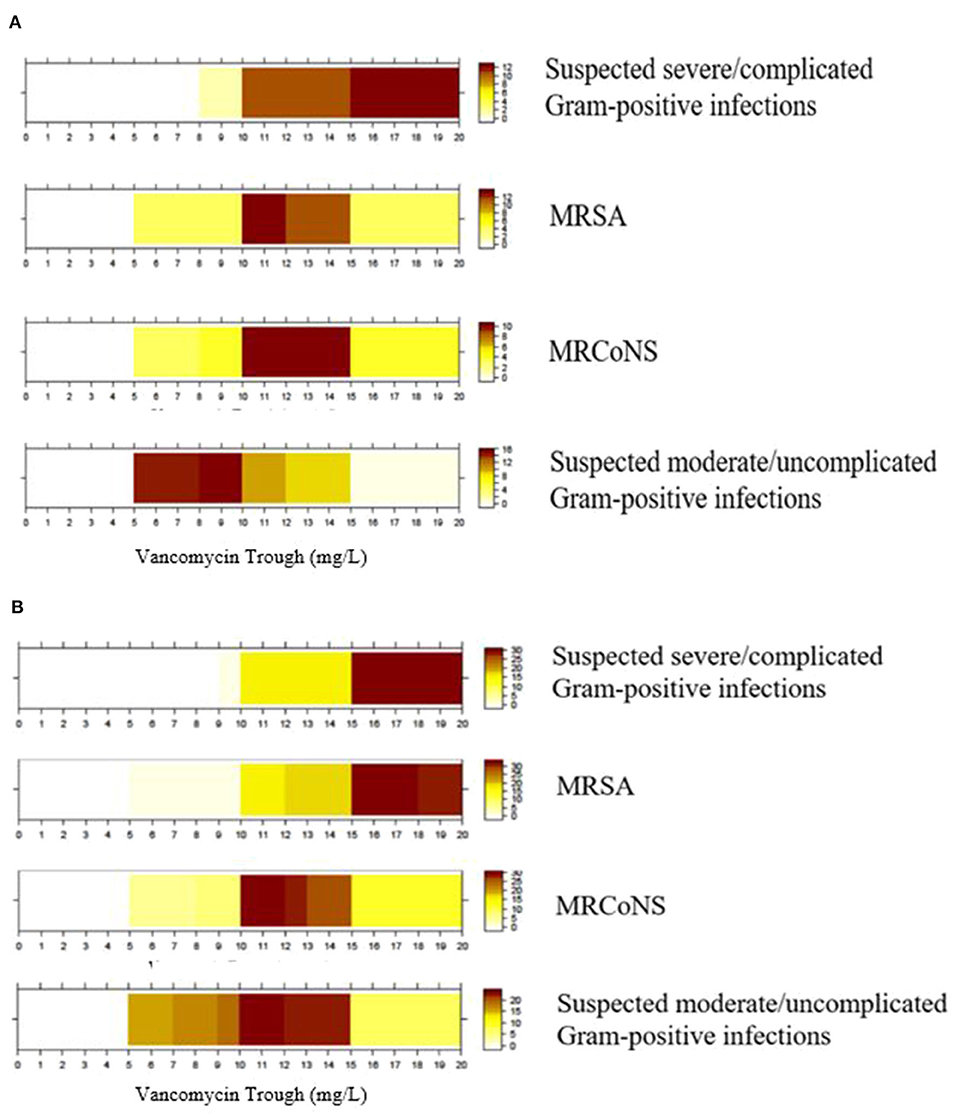
Figure 4. Targeted trough range (mg/L) for vancomycin prescribed for different indications. (A) Physicians' target trough range (n = 23). (B) Pharmacists' target trough range (n = 43). Color intensity is directly proportional to the proportion of respondents who selected that though range. MRSA, Methicillin-resistant Staphylococcus aureus; MRCoNS, Methicillin-resistant coagulase-negative Staphylococci; Examples for complicated infections include bacteraemia, osteomyelitis, meningitis, severe pneumonia, endocarditis, and deep-seated infections.
Our exploratory analysis revealed no statistical difference between the targeted trough ranges reported by physicians and pharmacists for suspected severe/complicated Gram-positive infections (P = 1.00), MRSA infections (P = 0.49), MRCoNS infections (P = 1.00), and suspected moderate/uncomplicated Gram-positive infections (P = 0.74).
Discussion
This study aimed to describe the prescribing preferences of physicians and pharmacists regarding the initial IV intermittent vancomycin dosing and TDM in multiple NICU/PICUs of Hong Kong. Our results demonstrate that these clinicians used varying drug references and vancomycin dosing guidelines in their practices. Generally, the trends in vancomycin target trough levels were similar between the physicians and pharmacists. The highest target trough level was reported for suspected severe/complicated Gram-positive and MRSA infections, followed by MRCoNS and suspected moderate/uncomplicated Gram-positive infections.
Although the AUC/MIC ratio is the primary predictive therapeutic parameter during a vancomycin regimen, the monitoring of trough concentrations as a surrogate parameter is a common practice in clinical settings. Current guidelines regarding vancomycin TDM in adult patients with MRSA infection recommend aggressive dosing when the MIC of the organism is ≤1 mg/L, with a target AUC/MIC ratio >400. This goal is to increase the vancomycin dose exposure and ensure adequate serum and tissue concentrations, particularly in critically ill patients such as those with sepsis, infective endocarditis, meningitis, and pneumonia. Extrapolating adult data to the pediatric population, an expert consensus indicated that an AUC/MIC ratio >400 corresponds to a targeted trough concentration of 15–20 mg/L at a MIC >1 mg/L (22–24). However, our study revealed that the majority of physicians tended to select a target trough level of 10–15 mg/L for MRSA infection, which is lower than the level of 15–20 mg/L suggested by clinical guidelines. This finding has a few implications. First, a clinical consensus on the targeted trough among physicians and pharmacists is needed. Second, with a set of pre-determined trough levels for different clinical scenarios, our future work is to conduct in-house drug utilization studies to evaluate whether the targets are achieved with current dosing strategies. This will shed light on the appropriateness and adequacy of existing dosing approaches. Third, to address concerns about an increase in vancomycin treatment failures, we recommend that clinicians should actively monitor MIC trends within each institution and review the targeted trough ranges. This information should be used to advise the appropriate empirical vancomycin dosing in this special population.
Our results also demonstrated that physicians and pharmacists used different drug references. This practice may lead to a variety of initial dosing regimens. This lack of consensus regarding dosing is illustrated by the wide variations in dosing regimens and targeted therapeutic levels across the reference guidelines (Supplementary Materials 1, 2). Consequently, two studies reported that ~75% of vancomycin dosing regimens designed with respect to the British National Formulary for Children (BNFc) and Neofax recommendations do not achieve the target trough levels (5, 6). Although few studies have involved the pediatric population, the optimization of initial vancomycin dosing to reduce the time required to reach a therapeutic serum level has been proven to improve clinical outcomes. Accordingly, this process should be a goal of therapy (4). However, it is difficult to achieve dose optimization in the neonatal and pediatric population in the absence of an evidence-based empirical dosing recommendation. Our results obtained from a small population of pediatric physicians and pharmacists in Hong Kong suggest a lack of standardization regarding the administration of vancomycin to NICU/PICU patients. As the pediatric critical care service in Hong Kong moves toward centralization at a new pediatric hospital, this issue may become problematic at the early stage when physicians of different institutions adopt varying prescribing preferences. However, we foresee that the gradual development of a standardized dosing guideline for empirical and targeted vancomycin use and a centralized TDM service may potentially improve the overall care for NICU/PICU patients.
The surveyed physicians and pharmacists considered several factors to be important determinants of the initial vancomycin dosing. They gave unanimous responses on crucial determinants such as post-menstrual age, body weight, severity of infection, and renal function. These parameters have also been reported as factors of concern in the literature and references regarding the selection of an initial vancomycin dosing regimen. Among critically ill neonatal and pediatric patients, changes in renal function, fluid resuscitation, and tissue perfusion can influence the clearance of vancomycin. Importantly, the existing references and guidelines differ with respect to covariates (25). Standardized dosing regimens for pediatric patients, which includes neonates, infants, and children, remain controversial because of the high pharmacokinetic variability within this diverse category. Compared with adults, neonates have a higher extracellular fluid volume and a limited but rapidly increasing capacity for renal elimination. Consequently, vancomycin pharmacokinetics evolve significantly as neonates mature (25). The current landscape of multiple dose recommendations pose difficulties on the clinicians to determine dose that can achieve target AUC/MIC goals. This has led institutions to adopt their own measures of developing internal dosing recommendations based on clinical variables collected from their own specific patient population and through stimulation of real-world data (26, 27). Recent studies have also demonstrated the value of using population pharmacokinetic data to personalize empirical vancomycin dosing. Researchers have begun to assess the validity, predictive performance, and generalizability of a published vancomycin pharmacokinetic model retrospectively through a model simulation based on a cohort of neonatal and pediatric patients (28, 29). The existing literature contains promising evidence supporting the use of pharmacokinetic parameters and computational strategies to estimate the extent of drug exposure in this special population. Our proposed future work is to examine the contribution of the identified clinical variables on serum levels using real-world data through drug utilization studies, and using these clinical variables as predictors in developing dosing calculators. We believe that this approach can be extended to establishing pharmacokinetic-guided dosing calculators of antimicrobials in children with other diseases or ethnic origins.
The TDM of medications with narrow therapeutic windows, such as vancomycin, has long been a standard of practice worldwide. Studies investigating the effects of pharmacist-managed TDM services have repeatedly demonstrated improvements in clinical outcomes. The benefits include an improvement in clinical efficacy, as indicated by reductions in the duration of antibiotic use, length of hospital stay, and mortality (4, 17). In a locally conducted pilot study, a pharmacist-managed TDM service exhibited a nearly 50% reduction in the time required to reach target therapeutic levels (30). Despite the purported benefits of TDM, the availability and scope of this clinical service varies among local public hospitals. The respondents in our study identified various obstacles to the optimization of TDM services in their institutions, including logistical barriers such as a slow laboratory turnover time and a lack of autonomy of pharmacists to modify dosing regimens. Based on our observations of local practices, we note that vancomycin dosage adjustments are usually based on incrementally derived dose adjustments, rather than individualized calculations based on pharmacokinetics. This practice suggests a misconception of the principles of TDM and a significant gap in service. The establishment of a well-defined pharmacist-led TDM service and the use of population-based pharmacokinetic modeling principles may resolve challenges such as the determination of a dose for a target serum level.
These study findings should be considered in the context of several limitations. We used a convenience sampling method and obtained a modest response rate. However, efforts were made to disseminate the survey through representatives (e.g., pharmacy managers) at the participating institutions. To highlight, these institutions include hospitals with the largest NICUs and PICUs in Hong Kong and highest usage rates of vancomycin. This approach may have helped to establish the sampling frame and likely strengthened the validity and applicability of the study findings in Hong Kong. It is inevitable that the aggregate responses might be dominated by the major acute hospitals. However, we also purposively distributed the survey to the smaller institutions to ensure that their responses are not omitted due to suboptimal sampling. We also found that the distribution of respondents across the main districts did appear reasonable. The clinicians' practices were self-reported and may not accurately reflect their actual practices in a clinical setting. Therefore, we are currently conducting drug utilization studies to evaluate antimicrobial usage patterns and the impacts on tangible clinical outcomes in the pediatric population. As this study is descriptive in nature, we emphasize that the findings should not be considered the primary and sole evidence on any aspect of this subject. Our study should be interpreted as an effort to understand the perceptions and practices of the pediatric critical care medicine community in Hong Kong. More importantly, findings of this survey will be used to direct future studies within this theme-based research. For example, we found that physicians and pharmacists collectively indicated a target trough of 15–20 mg/L for suspected severe/complicated Gram-positive infection; we will apply this target trough as the threshold when we evaluate clinical outcomes in the subsequent drug utilization studies, as well as whether the current dosing approach can effectively achieve this target trough range.
Conclusion
In conclusion, we believe that our findings have important implications. From a practice perspective, a standardized dosing guideline is needed to decrease variability and improve local practice. The clinicians' prescribing practices and the effectiveness of using current dosing guidelines to achieve targeted therapeutic trough levels should be evaluated in future drug utilization studies. The multidisciplinary TDM service in Hong Kong can be improved further by addressing logistic barriers and implementing a well-structured, evidence-based service protocol. From a research perspective, the next phase of this work includes conducting a multi-centered drug utilization studies to review real-world data on vancomycin use in the PICU/NICU. The clinical and outcomes data collected will be used to construct pharmacokinetics model for the PICU and NICU cohorts. Future work will also include the performance of comparative studies to demonstrate the effectiveness of a pharmacokinetics-driven dosing regimen vs. standard practice, particularly within the Chinese pediatric population. Globally, drug-related problems (particularly inappropriate dose selection) are common in NICU/PICU, hence predominating problems of suboptimal treatment. We believe that our systematic approach in identifying and addressing therapeutic gaps may potentially be extended to standardize dosing of antimicrobials and other drugs of narrow therapeutic index in the NICU/PICU. The eventual goal is to achieve personalized, pharmacokinetic-guided dosing of drugs in critically ill neonates and pediatric patients.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by The Chinese University of Hong Kong (CUHK) Survey and Behavioral Research Ethics Committee. Written informed consent for participation was not required for this study in accordance with the institutional requirements.
Author Contributions
TC, JL, TL, CE, and YC: analysis. TC, JL, and YC: drafting the work. All authors: conception or design of the work, acquisition of data, interpretation of data, revising it critically for important intellectual content, and provide approval for publication of the content.
Funding
This study was funded in part by the Student Campus Work Scheme 2017/18 (ref: 17107) of the Chinese University of Hong Kong (CUHK), awarded to YC.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would also like to acknowledge Mr. William Kwan, Mr. Tom Leung, Mr. Brian Chan, and Mr. Pakki Yip from the School of Pharmacy, CUHK for their assistance with data entry and cleaning.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2020.538298/full#supplementary-material
References
1. van den Hoogen A, Gerards LJ, Verboon-Maciolek MA, Fleer A, Krediet TG. Long-term trends in the epidemiology of neonatal sepsis and antibiotic susceptibility of causative agents. Neonatology. (2009) 97:22–8. doi: 10.1159/000226604
2. Heo JS, Shin SH, Jung YH, Kim E, Choi EH, Kim H, et al. Neonatal sepsis in a rapidly growing, tertiary neonatal intensive care unit: trends over 18 years. Pediatr Int. (2015) 57:909–16. doi: 10.1111/ped.12654
3. Shane AL, Stoll BJ. Neonatal sepsis: progress towards improved outcomes. J Infect. (2013) 68:S2–32. doi: 10.1016/j.jinf.2013.09.011
4. Cardile A, Tan C, Lustik M, Stratton A, Madar C, Elegino J, et al. Optimization of time to initial vancomycin target trough improves clinical outcomes. Springer Plus. (2015) 4:1–14. doi: 10.1186/s40064-015-1146-9
5. Hoang J, Dersch-Mills D, Bresee L, Kraft T, Vanderkooi OG. Achieving therapeutic vancomycin levels in pediatric patients. Can J Hosp Pharm. (2014) 67:416. doi: 10.4212/cjhp.v67i6.1403
6. Sinkeler FS, de Haan TR, Hodiamont CJ, Bijleveld YA, Pajkrt D, Mathôt RAA. Inadequate vancomycin therapy in term and preterm neonates: a retrospective analysis of trough serum concentrations in relation to minimal inhibitory concentrations. BMC Pediatrics. (2014) 14:193. doi: 10.1186/1471-2431-14-193
7. Lim LA, Chin MSH, Devan DK, Hui JH, Keowmani K. Evaluation of vancomycin initial dosing and the resultant trough level in paediatric patients. Malays J Pharm Sci. (2014) 12:67.
8. Chhim RF, Arnold SR, Lee KR. Vancomycin dosing practices, trough concentrations, and predicted area under the curve in children with suspected invasive staphylococcal infections. J Pediatr Infect Dis Soc. (2013) 2:259–62. doi: 10.1093/jpids/pis083
9. British MA, Paediatric FC, Royal Pharmaceutical Society of Great Britain, Royal College of Paediatrics and Child Health Neonatal and Paediatric Pharmacists Group. BNF for Children (BNFC) 2016–2017. London: Pharmaceutical Press (2016). Available online at: https://books.google.com.hk/books?id=rbSQrgEACAAJ (accessed August 25, 2020).
10. Patterson C, Australian MH. AMH Children's Dosing Companion 2017. Adelaide, SA: Australian Medicines Handbook Pty. Limited (2017). Available online at: https://books.google.com.hk/books?id=ZYpntAEACAAJ (accessed August 25, 2020).
11. Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. (2011) 52:e1–55. doi: 10.1093/cid/ciq146
12. Shann F. Drug Doses. 17th ed. Melbourne, VIC: Collective Pty. Limited (2017). Available online at: https://books.google.com.hk/books?id=h5D2MAAACAAJ (accessed August 25, 2020).
13. Lexicomp. Pediatric and Neonatal Lexi-Drugs. (2017). Available online at: http://online.lexi.com/lco/action/home (accessed 28 May, 2018).
14. Young TE, Mangum B. Neofax: A Manual of Drugs Used in Neonatal Care. Montvale, NJ: Thomson (2007). Available online at: https://books.google.com.hk/books?id=p4QWGQAACAAJ (accessed August 25, 2020).
15. Bolon MK, Arnold AD, Feldman HA, Rehkopf DH, Strong EF, Goldmann DA, et al. Evaluating vancomycin use at a pediatric hospital: new approaches and insights. Infect Control Hosp Epidemiol. (2005) 26:47–55. doi: 10.1086/502486
16. Starkey E, Wignell A. Vancomycin dose and drug monitoring in paediatrics. Arch Dis Child. (2011) 96:A44. doi: 10.1136/adc.2011.212563.98
17. Álvarez R, López Cortés LE, Molina J, Cisneros JM, Pachón J. Optimizing the clinical use of vancomycin. Antimicrob Agents Chemother. (2016) 60:2601–9. doi: 10.1128/AAC.03147-14
18. Tobin CM, Darville JM, Thomson AH, Sweeney G, Wilson JF, MacGowan AP, White LO. Vancomycin therapeutic drug monitoring: is there a consensus view? The results of a UK national external quality assessment scheme (UK NEQAS) for antibiotic assays questionnaire. J Antimicrob Chemother. (2002) 50:713–8. doi: 10.1093/jac/dkf212
19. Kadambari S, Heath PT, Sharland M, Lewis S, Nichols A, Turner MA. Variation in gentamicin and vancomycin dosage and monitoring in UK neonatal units. J Antimicrob Chemother. (2011) 66:2647–50. doi: 10.1093/jac/dkr351
20. Buyle F, Decruyenaere J, De Waele J, Tulkens P, Van Audenrode T, Depuydt P, et al. A survey of beta-lactam antibiotics and vancomycin dosing strategies in intensive care units and general wards in Belgian hospitals. Eur J Clin Microbiol Infect Dis. (2013) 32:763–8. doi: 10.1007/s10096-012-1803-7
21. Paladino JA, Sunderlin JL, Adelman MH, Singer ME, Schentag JJ. Observations on vancomycin use in U.S. hospitals. Am J Health Syst Pharm. (2007) 64:1633–41. doi: 10.2146/ajhp060651
22. Pai MP, Neely M, Rodvold KA, Lodise TP. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev. (2014) 77:50–7. doi: 10.1016/j.addr.2014.05.016
23. Hale CM, Seabury RW, Steele JM, Darko W, Miller CD. Are vancomycin trough concentrations of 15 to 20 mg/L associated with increased attainment of an AUC/MIC ≥ 400 in patients with presumed MRSA infection? J Pharm Pract. (2017) 30:329–35. doi: 10.1177/0897190016642692
24. Kishk OA, Lardieri AB, Heil EL, Morgan JA. Vancomycin AUC/MIC and corresponding troughs in a pediatric population. J Pediatr Pharmacol Ther. (2017) 22:41–7. doi: 10.5863/1551-6776-22.1.41
25. Marsot A, Boulamery A, Bruguerolle B, Simon N. Vancomycin: a review of population pharmacokinetic analyses. Clin Pharmacokinet. (2012) 51:1–13. doi: 10.2165/11596390-000000000-00000
26. Kim J, Walker SA, Iaboni DC, Walker SE, Elligsen M, Dunn MS, et al. Determination of vancomycin pharmacokinetics in neonates to develop practical initial dosing recommendations. Antimicrob Agents Chemother. (2014) 58:2830–40. doi: 10.1128/AAC.01718-13
27. Reilly AM, Ding MX, Rower JE, Kiser T. The effectiveness of a vancomycin dosing guideline in the neonatal intensive care unit achieving goal therapeutic trough concentrations. J Clin Pharmacol. (2019) 59:997–1005. doi: 10.1002/jcph.1392
28. Stockmann C, Hersh A, Roberts J, Bhongsatiern J, Korgenski E, Spigarelli M, et al. Predictive performance of a vancomycin population pharmacokinetic model in neonates. Infect Dis Ther. (2015) 4:187–98. doi: 10.1007/s40121-015-0067-9
29. Chen Y, Wu D, Dong M, Zhu Y, Lu J, Li X, et al. Population pharmacokinetics of vancomycin and AUC-guided dosing in Chinese neonates and young infants. Eur J Clin Pharmacol. (2018) 74:921–30. doi: 10.1007/s00228-018-2454-0
Keywords: critically ill, therapeutic monitoring of antibiotic levels, pediatric ICU, neonatal ICU, vancomycin
Citation: Chow TCH, Li JYS, Wong JCL, Poon FMH, Lam HS, Lam TTN, Lee CP, Ewig CLY and Cheung YT (2020) Vancomycin Prescribing Practices and Therapeutic Drug Monitoring for Critically Ill Neonatal and Pediatric Patients: A Survey of Physicians and Pharmacists in Hong Kong. Front. Pediatr. 8:538298. doi: 10.3389/fped.2020.538298
Received: 27 February 2020; Accepted: 04 November 2020;
Published: 30 November 2020.
Edited by:
Hans Van Rostenberghe, Universiti Sains Malaysia (USM), MalaysiaReviewed by:
Rinawati Rohsiswatmo, RSUPN Dr. Cipto Mangunkusumo, IndonesiaAnne Smits, University Hospitals Leuven, Belgium
Copyright © 2020 Chow, Li, Wong, Poon, Lam, Lam, Lee, Ewig and Cheung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yin Ting Cheung, eWludGluZy5jaGV1bmdAY3Voay5lZHUuaGs=
†These authors have contributed equally to this work
 Twinny Cheuk Hin Chow1†
Twinny Cheuk Hin Chow1† Jasper Chak Ling Wong
Jasper Chak Ling Wong Freddie Man Hong Poon
Freddie Man Hong Poon Hugh Simon Lam
Hugh Simon Lam Teddy Tai-ning Lam
Teddy Tai-ning Lam Chui Ping Lee
Chui Ping Lee Celeste Lom-ying Ewig
Celeste Lom-ying Ewig Yin Ting Cheung
Yin Ting Cheung