- 1Department of Pharmacy, Aga Khan University Hospital, Karachi, Pakistan
- 2Department of Pediatrics & Child Health, Aga Khan University, Karachi, Pakistan
Background: Multiple-drug-resistant Gram-negative bacteria (MDR-GNB)-associated neonatal ventriculitis is a life-threatening complication that needs timely diagnosis and effective treatment with broad-spectrum antimicrobials in critical-care settings. Inadequate penetration of antibiotics through the blood–brain barrier also demands an intraventricular (IVT) route of administration. This study reports mortality and neurodevelopmental sequelae of neonates till 18 months of age, who received IVT-colistin for treating MDR-GNB associated ventriculitis.
Methods: In a case series of seven neonates with ventriculitis due to MDR-GNB at NICU of Aga Khan University Hospital, Pakistan, between June 2015 and 2018, we reviewed IVT-colistin therapy in critically ill neonates. Treatment outcomes were assessed based on clinical sign's resolution and MDR-GNB eradication in subsequent CSF cultures. Neurodevelopmental outcomes were evaluated at 18 months after discharge.
Results: The average birth weight was 1.38 kg (range: 1.02–1.5 kg), and the average gestational age was 30.7 weeks (ranged: 26–34 weeks). All neonates reported colistin-sensitive MDR-GNB in CSF, five with Acinetobacter baumannii, and polymicrobial CNS infection was found in two patients (one due to Klebsiella pneumonia and A. baumannii and one due to K. pneumonia and Escherichia coli). All neonates received IVT colistin and concomitant intravenous meropenem, and five of them also received intravenous colistin. One neonate died. At the 18-month assessment, only one neonate had cerebral palsy and hydrocephaly and 50% had seizure disorders.
Conclusion: Practicing intraventricular antibiotics in the neonatal population is challenging but may be used successfully, especially to overcome the limitation of poor penetration through the blood–brain barrier.
Background
Central nervous system (CNS) infections are caused by several organisms, clinically presented as meningitis, ventriculitis, and brain abscess. The type of organisms involved and their susceptibility to available antimicrobials are the important factors in the clinical outcome and survival of these patients. It is crucial to timely recognize and manage these patients to reduce morbidity and mortality rates.
Nosocomial CNS infections are mostly a significant complication in patients undergoing neurosurgery (1), ranging from 0.3 to 6.5% in these patients (2–4). Its occurrence is about 8% in patients with external ventricular drain (EVD) (5, 6). In addition, one-third of the ventriculoperitoneal (VP) shunts implanted may lead to CNS infection (7). Neonatal CNS infections may occur concomitantly with the onset of Gram-negative pathogen-associated bacteremia (8–10). The overall neonatal mortality due to infections have reduced over the last few decades, but neonatal CNS infection-related morbidity remains almost unchanged (11, 12). Recent research has been focusing on improved diagnostic and preventive techniques and adjunctive therapies for improved neonatal outcomes (13).
The type of causative organisms of neonatal CNS infections depends on the gestational/postnatal age and facility setting (11, 14, 15). The treatment target for the nosocomial infections mostly involves multiple-drug-resistant (MDR) Gram-negative bacteria (GNB) such as Acinetobacter baumannii and Klebsiella pneumoniae (16). Studies have reported higher mortality ranges from 71.3 to 72.6% in patients with MDR-GNB-associated ventriculitis after neurosurgical procedures, mostly because of microbial resistance and inadequate antimicrobial penetration through the blood–brain barrier (BBB) (17, 18). Similarly, Chen et al. have reported up to 30% mortality rates in patients with carbapenem-resistant organism-associated CNS infections (19).
Fortunately, A. baumannii and K. pneumoniae are found susceptible to colistin, but limited penetration of colistin to cerebrospinal fluid (CSF) remains another challenge. These subtherapeutic levels require very high intravenous (IV) colistin doses. Consequently, the intrathecal (IT) or intraventricular (IVT) route is more appropriate to achieve the obligatory therapeutic colistin concentration to combat the MDR-GNB (20–24).
In adults and pediatric patients, neurological complications and sequelae of CNS infection have been studied. More severe illness, specific pathogens, earlier onset of paresis, and seizure have been reported as associated risk factors (25–27). Ventriculitis secondary to meningitis may occur in >20% of neonates (28). Survivors of neonatal meningitis and ventriculitis are at a much higher risk for neurodevelopmental impairments including motor function, hearing, vision, hydrocephalus, and seizure disorder.
Until today, very limited data is there about the usage of IVT/IT colistin in neonates, mostly in the form of case reports (29–31). That is not enough for the development of an exact guideline for IT/IVT colistin use in neonates for MDR-GNB-associated CNS infection. We encountered seven neonates in our neonatal intensive care unit (NICU) in 3 years, who had MDR-GNB-associated ventriculitis and treated with IVT colistin. Here, we are sharing the onset of CNS infection, treatment, and outcome of neonates in terms of survival and neurological complications.
Method
Study Design and Data Collection
In this retrospective case series, we reviewed the medical record of seven neonates at our 24-bedded NICU and the step-down unit of the Aga Khan University Hospital, Karachi, Pakistan, between June 2015 and June 2018. All the neonates included for the study were reported with the isolation of MDR-GNB from the CSF, developed ventriculitis during the NICU stay, and received colistin via the IVT route for >72 h.
Due to the high mortality rate with the emergence of MDR-GNB infections and the concern about poor CNS penetration of IV colistin, we offered adjunctive IVT colistin treatment to these neonates who had CNS infection. Infection control and preventive measures were strictly followed by the healthcare providers, such as contact isolation and hand hygiene.
For each case, data were collected regarding the demographic characteristics (gestational and postnatal age, sex, birth weight); primary and current diagnosis; the clinical and laboratory information about the infections; onset of CNS infection; hematological parameters (hemoglobin, neutrophil count, platelet counts, white blood cell (WBC) count, and C-reactive protein); presence of medical devices; concomitant antibiotic use along with susceptibility pattern, dose, and duration of IV; IVT colistin therapy; days to sterilized CSF; and IVT colistin-associated adverse effects and the outcome. After discharge, all surviving patients underwent a neurological examination performed by a pediatric neurologist, and the record was reviewed for the neurodevelopmental assessment reports, based on the broad classification of status at 18 months of age. It comprised of documentations of abnormal neurodevelopmental outcomes like motor dysfunction (cerebral palsy or monoplegia) and visual and hearing deficit with standardized criterion.
Treatment Regimen of Intraventricular Colistin
Colistimethate sodium (CMS) is available in a vial containing 80 mg dry powder equivalent to 33.3 mg colistin base and further diluted in 2 ml of sterile preservative-free normal saline (NS). Colistin (base) was administered at a dose of 0.16–0.24 mg/kg as a single daily dose via the intraventricular route (21, 32, 33). All IVT doses were dispensed to nursing units in prefilled ready-to-administer form. The dose was administered through EVD by the neurosurgery team. Neonates were monitored for any hemodynamic changes during and after each dose (34).
Operational Definition
Nosocomial infections were defined according to the standard definition by the Centers for Disease Control and Prevention (35). The IVT colistin-associated most common and life-threatening adverse effect is reversible chemical ventriculitis, which presents with sterile CSF cultures and signs of meningeal irritation, that is, altered mental state, fever, increased WBC, and lower glucose concentration in the CSF, and the presentation is like bacterial meningitis (21). MDRO was defined as an isolated pathogen non-susceptible to at least one agent of ≥3 antimicrobial categories (36). MDR-GNB-associated meningitis was defined as the presence of MDR-GNB in CSF cultures, and clinical presentation of neonatal meningitis is defined as the presence of signs like hyper or hypothermia, respiratory distress, hypotonia, lethargy or irritability, feed intolerance, apnea, lower heart rate and blood pressures, bulging anterior fontanel, seizures, and hypo or hyperglycemia (37). Choroid plexitis and inflammation of the ependymal lining of ventricles is called ventriculitis. Clinical symptoms of meningitis, failure to respond to appropriate antibiotic therapy, and signs of elevated intracranial pressure (ICP) suggest the diagnosis of ventriculitis. Further magnetic resonance imaging (MRI), lumbar puncture (LP), and fontanel tape aid in the diagnosis of ventriculitis (38, 39).
Microbiology
For assessing the treatment response, the CSF of each neonate was collected and sent for chemical and microscopic analysis prior to IVT colistin administration. GNB were identified and checked for antibiotic susceptibility through the Vitek-2 compact system. Furthermore, colistin susceptibility of isolated GNB was confirmed by using the broth microdilution method. These isolated pathogens with minimum inhibitory concentration (MIC) of ≤2 mg/dl were documented as colistin-sensitive using Clinical Laboratory Standards Institute (CLSI) guidelines (40).
Outcome Measures and Data Analysis
There were two primary outcomes of this study: (i) the cure of the patient fulfilling the following criteria: (a) MDR-GNB eradication in subsequent CSF cultures and (b) resolution of clinical signs of neonatal meningitis and with no further requirement of antimicrobial therapy; and (ii) neurodevelopmental outcomes till 18 months follow up after discharge. In the tables, we incorporated the demographic, clinical, treatment, and neurodevelopmental outcome details of each case.
Results
During the study period, 3,264 neonates were admitted to our NICU. A total of 153 neonates received colistin therapy through any route. Of 153 neonates, 15 patients had culture-proven meningitis. Seven neonates developed ventriculitis and reported the isolation of colistin-sensitive MDR-GNB in CSF culture, thus treated with IVT colistin. Five of which were due to A. baumannii, and polymicrobial CNS infection was found in two patients (one of which was due to K. pneumonia and A. baumannii and one of which was due to K. pneumonia and Escherichia coli). All these critically ill neonates with MDR-GNB infections had received empirical antimicrobial therapy as per our institutional guidelines (41).
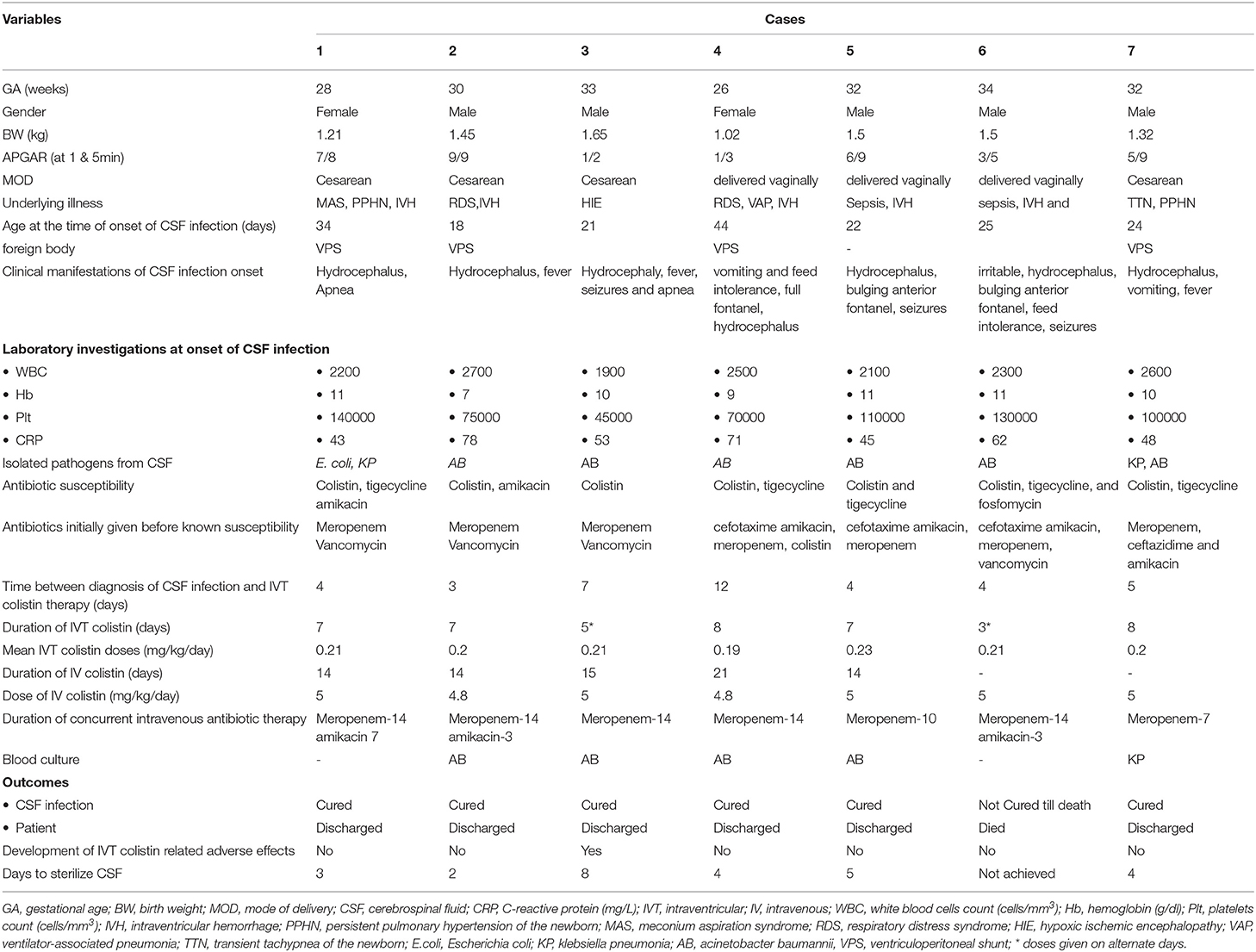
Neonatal characteristics, isolated MDR-GNB species, and treatment detail of each case are shown in Table 1. Out of seven neonates with MDR-GNB infections, five were male, the average birth weight was 1.38 kg (range: 1.02 to 1.5 kg), and the average gestational age was 30.7 weeks (range: 26 to 34 weeks). At the time of onset of ventriculitis, the average age of neonates was 26.9 days (range: 18 to 44 days). Five neonates had intraventricular hemorrhage (IVH), and one neonate had hypoxic–ischemic encephalopathy as associated diagnosis with ventriculitis. Two neonates had a history of pneumonia, and two had persistent pulmonary hypertension of the newborn (PPHN) before the onset of CNS infection. Four neonates had a VP shunt while three patients had no drainage system.
Based on the previous culture reports, susceptibility pattern, and clinical grounds, to treat the ongoing infections neonates were given other IV antibiotics. Three neonates were treated with cefotaxime and amikacin initially, then switched to meropenem, either alone or in combination with colistin or vancomycin. Three neonates were treated with meropenem plus vancomycin. One neonate received ceftazidime and amikacin after discontinuation of meropenem. Four neonates developed concomitant pneumonia (three with A. baumannii and one with K. pneumoniae). The blood cultures of five neonates were also positive with MDR-GNB (four with A. baumannii and one with K. pneumoniae).
From all the cases, five neonates were treated with concomitant IV colistin in the dose of 5 mg/kg/day in daily divided doses for an average duration of 15.6 days (range: 14 to 21 days), whereas all of seven neonates received concomitant meropenem and three of them were given amikacin. The average duration between the diagnosis of CSF infection and IVT colistin therapy was 5.6 days (range: 3 to 12 days), and the average period to attain sterile CSF among six survived neonates was 3.72 days (range: 2 to 8 days). Among all, one neonate died during the treatment, due to A. baumannii. Till the last examination, CSF was unsterile in this patient and had received three doses of IVT colistin on alternate days.
All six survived neonates who achieved the sterile CSF phase were cured without relapse after completion of the treatment course. On average, neonates received IVT colistin for 7.4 days (range: 7 to 10 days). Intraventricular colistin-associated adverse effect was developed in one neonate and thus received IVT colistin on alternate days.
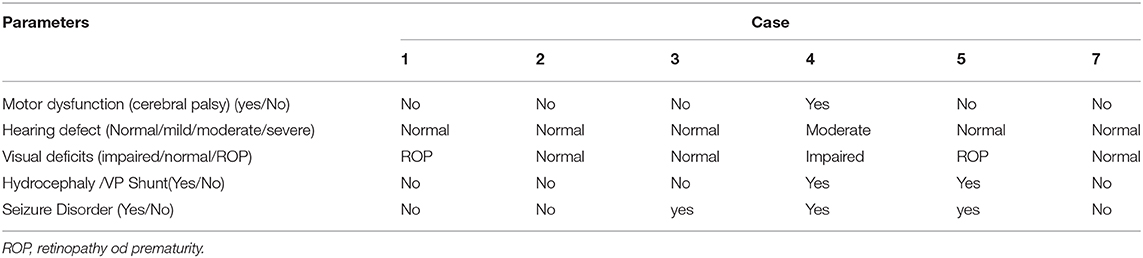
Table 2 shows the results of the neurodevelopment assessment of survived infants at 18 months of age. One of the neonates who followed in the neurology clinic had cerebral palsy (CP), seizure disorder (on anticonvulsant therapy), and impaired hearing and vision. One neonate had hydrocephalus, epilepsy, and developed retinopathy of prematurity (ROP). Both cases were on follow-up with a neurologist. From the remaining four, one baby received ROP treatment and one was on antiepileptic; otherwise, these four babies were found normal at the 18-month assessment.
Discussion
Ventriculitis secondary to meningitis is more common in neonates (42) and may occur in >20% of neonates with meningitis (28). Moreover, significantly higher morbidity and mortality due to MDR-GNB-associated meningitis and ventriculitis need extra precautionary measures and further therapeutic considerations to reduce the consequences of such life-threatening complications (17–19). Increased pathogen resistance to most of the antimicrobial agents and their poor CNS penetration are the contributing factors. Therefore, several studies have chosen the IVT/IT route of administration to directly deliver the antibiotic, to achieve the desired therapeutic steady state to fight against these pathogens (20, 21, 23, 43). Though meropenem is used empirically for nosocomial MDR-GNB-associated meningitis and ventriculitis (44, 45), unfortunately, it is becoming progressively ineffective due to rapidly increased resistance (more than 40%) among these pathogens (46). Fortunately, these MDR strains remain sensitive to polymyxins, an old class of antimicrobial agents from the 1950s (41). However, due to the polycationic structure and the higher molecular weight polymyxins have a poor capacity to cross the BBB when administered through the IV route (47). Consequently, IV colistin (polymyxin E) remains unable to achieve therapeutic CSF concentration to fight against deadly MDR-GNB (48).
For treating IVH and posthemorrhagic hydrocephalus in neonates along with the pharmacological approach, other modalities, such as EVD, serial lumbar or ventricular taps, and ventriculoperitoneal (VP) shunts, are used (49–51). In addition, for administering IVT/IT medications special tools are needed, such as EVD, but these foreign bodies serve as a potential infection source (52). In adult studies, the IT route of administration is used by a repeated lumbar puncture on daily basis; thus, removal of lumbar drain and EVD is preferred to prevent foreign bodies' systemic involvement (20). However, the IT route of administration is comparatively not feasible and difficult in neonates; therefore, IVT is preferred (53, 54).
The Infectious Diseases Society of America (IDSA) guidelines recommend using IV and IT/IVT polymyxins (polymyxin B and colistin) with meropenem in carbapenem-resistance strain-associated infections (52). The use of colistin through IVT/IT routes is advocated in adults' clinical practices, but neonatal studies are very limited (21). Karaiskos et al. reviewed IVT/IT colistin usage in multidrug-resistant gram-negative strain-associated meningitis and ventriculitis (21), involving 81 patients (71 adult and 10 children/neonates). The clinical and microbiological outcomes were achieved in 89% of patients. In addition, nine cases (11%) experienced an adverse effect of chemical ventriculitis. In our study, only one patient experienced this adverse effect. A comparatively shorter duration of IVT antibiotic use might explain this difference. The CSF cultures of our patients were persistently sterile, but chances of reinfection need to be considered. In another review, Katragkou et al. discussed the treatment of MDR A. baumannii-associated CNS infection by using IVT/IT colistin alone or as an adjunctive to systemic antimicrobials (55). In our study, neonatal CSF became sterile within an average of 3.72 days (range 2–8 days), which is similar to earlier literature (56, 57). Previous adult and pediatric studies have concluded that the use of IT/IVT colistin was found safe and effective (56, 57).
Aminoglycosides, tigecycline, rifampin, and sulbactam have also been used effectively as alternative therapeutic options (21). However, in our study meropenem was the only antibiotic used in all the seven patients concomitant with IVT colistin and IV colistin was used in 5/7 patients, which could be justified due to the presence of blood and pulmonary infections. In addition, combination therapy with colistin, meropenem, and ampicillin-sulbactam has been reported to reduce the mortality rate (21). Colistin resistance among A. baumannii and K. pneumonia are increasingly reported and a case report from South Africa identified the presence of colistin-resistant A baumannii-associated ventriculitis in a neonate (56). Another case report by Mehar et al. reported septicemia with meningitis due to carbapenem-resistant (susceptible to colistin) A. baumannii in a preterm neonate. The infant was successfully treated with IVT colistin and IV netilmicin (58). MDR-GNB-associated neonatal ventriculitis and meningitis is a challenge to manage using conventional IV antibiotics and found inadequate to eradicate these life-threatening pathogens. Fortunately, all the seven cases in our study had colistin-susceptible MDR-GNB-associated CNS infection and the MDR strain did not acquire resistance against colistin in any patient which resulted in 86% success with IVT colistin treatment.
A neonatal study for evaluating the neurodevelopmental defects had reported that from the neonates who experienced bacterial CNS infection and survived, 67% developed neurologic sequelae (59). In our study, one neonate died with uncontrolled seizures, and from survivors, only one neonate who had initial seizures presented with unfavorable neurological effects at 18 months. From six survived neonates, three developed seizures. These findings are also supported by a previous study, which shared that the presence of initial seizure is a significant predictor of unfavorable outcome (60). To the best of our knowledge, no study has been conducted to evaluate the neurodevelopmental effects at 18 months of age, in preterm neonates, who received IVT antibiotics for treating MDR-GNB-associated ventriculitis.
The increasing prevalence of MDRO causing neonatal sepsis including meningitis and ventriculitis in some middle- and low-income countries is a matter of great concern (41, 56). Also, the implementation of infection control and prevention strategies is a global challenge in these regions to control the spread of MDR-GNB. Furthermore, in our region with limited advanced NICU settings, the transfer of ill neonates from other facilities is an additional factor in the spread of MDR pathogens. Implementation of more firm screening measures may help to prevent the spread by detecting colonized patients earlier (61). Fortunately, in our NICU patient cohorting is done to keep the externally transferred and infected infants in the isolation room provided negative pressure. Our study had limitations of a single-centered retrospective case series study design, the association of confounders, and a small sample size.
Conclusion
Multidrug-resistant pathogen-associated meningitis and ventriculitis are evolving in neonates, and currently available antimicrobials have deprived outcomes in CNS infection due to poor penetration through the blood–brain barrier. However, practicing intraventricular antibiotics in the neonatal population is challenging but may be used successfully. The efficient treatment approach with suitable antibiotic usage through an appropriate route of administration may enhance survival and improve neurodevelopmental outcomes. The spread of multidrug-resistant pathogens can be reduced through implementing and continuing practicing simple infection control and prevention measures. Further prospective, controlled studies are warranted.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethical Review Committee, Aga Khan University Karachi, Pakistan. Written informed consent to participate in this study was waived as the data was collected retroactively.
Author Contributions
GA, MS, and KH conceptualized the design. GA and JI carried out data collection. KH and GA contributed to pharmacy data retrieval. GA wrote the draft. MS, KH, and JI carried out the analysis. MS supervised the study and critically revised the subsequent drafts. All authors contributed to the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to acknowledge the pharmacy team, nursing staff, and residents in NICU who facilitated data collection. We are grateful to Abdul Moiz Hussain for facilitating in edits.
References
1. Metersky ML, Williams A, Rafanan AL. Retrospective analysis: are fever and altered mental status indications for lumbar puncture in a hospitalized patient who has not undergone neurosurgery? Clin Infect Dis. (1997) 25:285–8. doi: 10.1086/514531
2. McClelland S III, Hall WA. Postoperative central nervous system infection: incidence and associated factors in 2111 neurosurgical procedures. Clin Infect Dis. (2007) 45:55–9. doi: 10.1086/518580
3. Korinek A-M, Baugnon T, Golmard J-L, van Effenterre R, Coriat P, Puybasset L. Risk factors for adult nosocomial meningitis after craniotomy roleof antibiotic prophylaxis. Neurosurgery. (2006) 59:126–33. doi: 10.1227/01.NEU.0000220477.47323.92
4. Lin C, Zhao X, Sun H. Analysis on the risk factors of intracranial infection secondary to traumatic brain injury. Chin J Traumatol. (2015) 18:81–3. doi: 10.1016/j.cjtee.2014.10.007
5. Laxmi S, Tunkel AR. Healthcare-associated bacterial meningitis. Curr Infect Dis Rep. (2011) 13:367–73. doi: 10.1007/s11908-011-0190-z
6. Lozier AP, Sciacca RR, Romagnoli MF, Connolly ES Jr. Ventriculostomy-related infections: a critical review of the literature. Neurosurgery. (2002) 51:170–82. doi: 10.1097/00006123-200207000-00024
7. Eymann R, Chehab S, Strowitzki M, Steudel W-I, Kiefer M. Clinical and economic consequences of antibiotic-impregnated cerebrospinal fluid shunt catheters. J Neurosur Pediatr. (2008) 1:444–50. doi: 10.3171/PED/2008/1/6/444
8. Gaschignard J, Levy C, Romain O, Cohen R, Bingen E, Aujard Y, et al. Neonatal bacterial meningitis: 444 cases in 7 years. Pediatr Infect Dis J. (2011) 30:212–7. doi: 10.1097/INF.0b013e3181fab1e7
9. Trijbels-Smeulders MA, Kimpen JL, Kollée LA, Bakkers J, Melchers W, Spanjaard L, et al. Serotypes, genotypes, and antibiotic susceptibility profiles of group B streptococci causing neonatal sepsis and meningitis before and after introduction of antibiotic prophylaxis. Pediatr Infect Dis J. (2006) 25:945–8. doi: 10.1097/01.inf.0000237821.65559.08
10. May M, Daley AJ, Donath S, Isaacs D. Early onset neonatal meningitis in Australia and New Zealand, 1992–2002. Arch Dis Child Fetal Neonatal Ed. (2005) 90:F324–7. doi: 10.1136/adc.2004.066134
11. Polin RA, Harris MC, editors. Neonatal bacterial meningitis. Semin Neonatol. (2001) 6:157–62. doi: 10.1053/siny.2001.0045
12. Heath PT, Okike IO, Oeser C. Neonatal meningitis: can we do better? In: Curtis N, Finn A, Pollard A, editors. Hot Topics in Infection and Immunity in Children VIII. New York, NY: Springer (2012). p. 11–24.
13. Shane AL, Stoll BJ. Recent developments and current issues in the epidemiology, diagnosis, and management of bacterial and fungal neonatal sepsis. Am J Perinatol. (2013) 30:131–42. doi: 10.1055/s-0032-1333413
14. Stoll BJ, Hansen NI, Sánchez PJ, Faix RG, Poindexter BB, Van Meurs KP, et al. Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics. (2011) 127:817–26. doi: 10.1542/peds.2010-2217
15. Martin RJ, Fanaroff AA, Walsh MC. Fanaroff and Martin's Neonatal-Perinatal Medicine e-book: Diseases of the Fetus and Infant. London: Elsevier Health Sciences; 2014 Aug 20. city London
16. Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, et al. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis. (2006) 6:589–601. doi: 10.1016/S1473-3099(06)70580-1
17. Cascio A, Conti A, Sinardi L, Iaria C, Angileri FF, Stassi G, et al. Post-neurosurgical multidrug-resistant Acinetobacter baumannii meningitis successfully treated with intrathecal colistin. A new case and a systematic review of the literature. Int J Infect Dis. (2010) 14:e572–9. doi: 10.1016/j.ijid.2009.06.032
18. Tuon FF, Penteado-Filho SR, Amarante D, Andrade MA, Borba LA. Mortality rate in patients with nosocomial Acinetobacter meningitis from a Brazilian hospital. Braz J Infect Dis. (2010) 14:437–40. doi: 10.1016/S1413-8670(10)70090-8
19. Chen S, Chang W, Lu C, Chuang Y, Tsai H, Tsai N, et al. Culture-proven bacterial meningitis in elderly patients in southern Taiwan: clinical characteristics and prognostic factors. Acta Neurol Taiwan. (2006) 15:84–91.
20. Razmkon A, Mehrafshan A, Bakhtazad A. Management of post-neurosurgical Acinetobacter infections: experiences obtained during an outbreak. Acta Neurochir. (2011) 153:435. doi: 10.1007/s00701-010-0857-5
21. Karaiskos I, Galani L, Baziaka F, Giamarellou H. Intraventricular and intrathecal colistin as the last therapeutic resort for the treatment of multidrug-resistant and extensively drug-resistant Acinetobacter baumannii ventriculitis and meningitis: a literature review. Int J Antimicrob Agents. (2013) 41:499–508. doi: 10.1016/j.ijantimicag.2013.02.006
22. Imberti R, Cusato M, Accetta G, Marinò V, Procaccio F, Del Gaudio A, et al. Pharmacokinetics of colistin in cerebrospinal fluid after intraventricular administration of colistin methanesulfonate. Antimicrob Agents Chemother. (2012) 56:4416–21. doi: 10.1128/AAC.00231-12
23. Falagas ME, Rafailidis PI, Matthaiou DK, Virtzili S, Nikita D, Michalopoulos A. Pandrug-resistant Klebsiella pneumoniae, Pseudomonas aeruginosa and Acinetobacter baumannii infections: characteristics and outcome in a series of 28 patients. Int J Antimicrob Agents. (2008) 32:450–4. doi: 10.1016/j.ijantimicag.2008.05.016
24. De Bonis P, Lofrese G, Scoppettuolo G, Spanu T, Cultrera R, Labonia M, et al. Intraventricular versus intravenous colistin for the treatment of extensively drug resistant Acinetobacter baumannii meningitis. Eur J Neurol. (2016) 23:68–75. doi: 10.1111/ene.12789
25. Jim KK, Brouwer MC, van der Ende A, van de Beek D. Subdural empyema in bacterial meningitis. Neurology. (2012) 79:2133–9. doi: 10.1212/WNL.0b013e3182752d0e
26. Theodoridou K, Vasilopoulou VA, Katsiaflaka A, Theodoridou MN, Roka V, Rachiotis G, et al. Association of treatment for bacterial meningitis with the development of sequelae. Int J Infect Dis. (2013) 17:e707–13. doi: 10.1016/j.ijid.2013.02.009
27. Hénaff F, Levy C, Cohen R, Picard C, Varon E, Le Guen CG, et al. Risk factors in children older than 5 years with pneumococcal meningitis: data from a national network. Pediatr Infect Dis J. (2017) 36:457–61. doi: 10.1097/INF.0000000000001470
28. Kline MW. Infectious causes of neonatal seizures. In: The Epilepsies. Cambridge, MA: Academic Press (1999). p. 491–493.
29. Alaoui S, Nejmi S, Chakir A, Hmamouchi B, Chlilek A, editors. Intraventricular colistin use in neonatal meningitis caused by Acinetobacter baumanii. Ann Fr Anesth Reanim. (2011) 30:854–5. doi: 10.1016/j.annfar.2011.07.008
30. Tekgündüz KS, Demirelli Y, Caner I, Kara M. Intraventricular colistin use in a premature infant with cerebral abscess and ventriculitis. J Clin Neonatol. (2015) 4:132. doi: 10.4103/2249-4847.154112
31. Piparsania S, Rajput N, Bhatambare G. Intraventricular polymyxin B for the treatment of neonatal meningo-ventriculitis caused by multi-resistant Acinetobacter baumannii-case report and review of literature. Turk J Pediatr. (2012) 54:548–54.
32. Markantonis S, Markou N, Fousteri M, Sakellaridis N, Karatzas S, Alamanos I, et al. Penetration of colistin into cerebrospinal fluid. Antimicrob Agents Chemother. (2009) 53:4907–10. doi: 10.1128/AAC.00345-09
33. Bargiacchi O, De Rosa FG. Intrathecal or intraventricular colistin: a review. Infez Med. (2016) 24:3–11.
34. Nakwan N, Wannaro J, Thongmak T, Pornladnum P, Saksawad R, Nakwan N, et al. Safety in treatment of ventilator-associated pneumonia due to extensive drug-resistant Acinetobacter baumannii with aerosolized colistin in neonates: a preliminary report. Pediatr Pulmonol. (2011) 46:60–6. doi: 10.1002/ppul.21324
35. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care–associated infection and criteria for specific types of infections in the acute care setting. American J Infect Control. (2008) 36:309–32. doi: 10.1016/j.ajic.2008.03.002
36. Magiorakos AP, Srinivasan A, Carey R, Carmeli Y, Falagas M, Giske C, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin microbiol Infect. (2012) 18:268–81. doi: 10.1111/j.1469-0691.2011.03570.x
37. Ku LC, Boggess KA, Cohen-Wolkowiez M. Bacterial meningitis in infants. Clin Perinatol. New York, NY (2015) 42:29–45. doi: 10.1016/j.clp.2014.10.004
38. Orman G, Rossi A, Meoded A, Huisman TA. Children With Acute Neurological Emergency. Diseases of the Brain, Head and Neck, Spine 2020–2023. New York, NY: Springer (2020). p. 179–90.
39. Maunu J, Parkkola R, Rikalainen H, Lehtonen L, Haataja L, Lapinleimu H. Brain and ventricles in very low birth weight infants at term: a comparison among head circumference, ultrasound, and magnetic resonance imaging. Pediatrics. (2009) 123:617–26. doi: 10.1542/peds.2007-3264
40. Wayne P. Performance Standards for Antimicrobial Susceptibility Testing. Clinical and Laboratory Standards Institute. Maryland, MD (2011).
41. Ambreen G, Salat MS, Hussain K, Raza SS, Ali U, Azam I, et al. Efficacy of colistin in multidrug-resistant neonatal sepsis: experience from a tertiary care center in Karachi, Pakistan. Arch Dis Child. (2020) 105:830–6. doi: 10.1136/archdischild-2019-318067
42. Harris L, Munakomi S. Ventriculitis. StatPearls [Internet]: StatPearls Publishing. Treasure Island, FL (2019).
43. Snoeck M, Van Engelen B, Küsters B, Lammens M, Meijer R, Molenaar J, et al. RYR 1-related myopathies: a wide spectrum of phenotypes throughout life. Euro J Neurol. (2015) 22:1094–112. doi: 10.1111/ene.12713
44. Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. (2004) 39:1267–84. doi: 10.1086/425368
45. Garnacho-Montero J, Amaya-Villar R. Multiresistant Acinetobacter baumannii infections: epidemiology and management. Curr Opin Infect Dis. (2010) 23:332–9. doi: 10.1097/QCO.0b013e32833ae38b
46. Metan G, Alp E, Aygen B, Sumerkan B. Carbapenem-resistant Acinetobacter baumannii: an emerging threat for patients with post-neurosurgical meningitis. Int J Antimicrob Agents. (2007) 29:112. doi: 10.1016/j.ijantimicag.2006.08.035
47. Lopez-Alvarez B, Martin-Laez R, Farinas M, Paternina-Vidal B, García-Palomo J, Vázquez-Barquero A. Multidrug-resistant Acinetobacter baumannii ventriculitis: successful treatment with intraventricular colistin. Acta Neurochirurgica. (2009) 151:1465–72. doi: 10.1007/s00701-009-0382-6
48. Peirano G, van der Bij AK, Freeman JL, Poirel L, Nordmann P, Costello M, et al. Characteristics of Escherichia coli sequence type 131 isolates that produce extended-spectrum β-lactamases: global distribution of the H30-Rx sublineage. Antimicrob Agents Chemother. (2014) 58:3762–7. doi: 10.1128/AAC.02428-14
49. Harbaugh RE, Saunders RL, Edwards WH. External ventricular drainage for control of posthemorrhagic hydrocephalus in premature infants. J Neurosurg. (1981) 55:766–70. doi: 10.3171/jns.1981.55.5.0766
50. Kreusser KL, Tarby TJ, Kovnar E, Taylor DA, Hill A, Volpe JJ. Serial lumbar punctures for at least temporary amelioration of neonatal posthemorrhagic hydrocephalus. Pediatrics. (1985) 75:719–24.
51. Marlin A. Treatment of posthemorrhagic ventriculomegaly in the preterm infant: use of the subcutaneous ventricular reservoir. Concepts Pediat Neurosurg. (1988) 8:15–22.
52. Tunkel AR, Hasbun R, Bhimraj A, Byers K, Kaplan SL, Scheld WM, et al. Infectious diseases society of America's clinical practice guidelines for healthcare-associated ventriculitis and meningitis. Clin Infect Dis. (2017) 64:e34–65. doi: 10.1093/cid/ciw861
53. Greenfield JP, Bilsky MH. Neurosurgical interventions for leptomeningeal tumor. In: Leptomeningeal Metastases. New York, NY: Springer (2005). p. 107–19.
54. Patel MM, Goyal BR, Bhadada SV, Bhatt JS, Amin AF. Getting into the brain. CNS Drugs. (2009) 23:35–58. doi: 10.2165/0023210-200923010-00003
55. Katragkou A, Roilides E. Successful treatment of multidrug-resistant Acinetobacter baumannii central nervous system infections with colistin. J Clin Microbiol. (2005) 43:4916–7. doi: 10.1128/JCM.43.9.4916-4917.2005
56. Mahabeer P, Mzimela BW, Lawler MA, Singh-Moodley A, Singh R, Mlisana KP. Colistin-resistant Acinetobacter baumannii as a cause of neonatal ventriculitis. S Afr J Infect Dis. (2018) 33:1–3. doi: 10.1080/23120053.2018.1509183
57. Pandey S, Li L, Deng XY, Cui DM, Gao LJFiN. Outcome following the treatment of ventriculitis caused by multi/extensive drug resistance gram negative bacilli: Acinetobacter Baumannii and klebsiella pneumonia. Front Neurol. (2019) 9:1174. doi: 10.3389/fneur.2018.01174
58. Mehar V, Zade P, Joshi M, Rajput N, Bhatambar G. Neonatal ventriculitis with multi drug resistant Acinetobactor baumanii: a case report and review of literature. Pediat Therapeut. (2012) 2:131. doi: 10.4172/2161-0665.1000131
59. Di Mauro A, Cortese F, Laforgia N, Pantaleo B, Giuliani R, Bonifazi D, et al. Neonatal bacterial meningitis: a systematic review of European available data. Minerva Pediatr. (2019) 71:201–8. doi: 10.23736/S0026-4946.17.05124-6
60. Hsu M-H, Hsu J-F, Kuo H-C, Lai M-Y, Chiang M-C, Lin Y-J, et al. Neurological complications in young infants with acute bacterial meningitis. Front Neurol. (2018) 9:903. doi: 10.3389/fneur.2018.00903
61. Tacconelli E, Cataldo M, Dancer S, De Angelis G, Falcone M, Frank U, et al. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clin Microbiol Infect. (2014) 20:1–55. doi: 10.1111/1469-0691.12427
Keywords: colistin, intraventricular antibiotics, neonates, multiple drugs resistant, ventriculitis
Citation: Hussain K, Sohail Salat M, Ambreen G and Iqbal J (2021) Neurodevelopment Outcome of Neonates Treated With Intraventricular Colistin for Ventriculitis Caused by Multiple Drug-Resistant Pathogens—A Case Series. Front. Pediatr. 8:582375. doi: 10.3389/fped.2020.582375
Received: 17 July 2020; Accepted: 16 November 2020;
Published: 20 January 2021.
Edited by:
Fiammetta Piersigilli, Cliniques Universitaires Saint-Luc, BelgiumReviewed by:
Aakash Pandita, Sanjay Gandhi Post Graduate Institute of Medical Sciences (SGPGI), IndiaKhalid Nasirul Haque, The Children's Hospital & The Institute of Child Health, Pakistan
Copyright © 2021 Hussain, Sohail Salat, Ambreen and Iqbal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gul Ambreen, Z3VsLmFtYnJlZW5AYWt1LmVkdQ==
 Kashif Hussain
Kashif Hussain Muhammad Sohail Salat
Muhammad Sohail Salat Gul Ambreen
Gul Ambreen Javaid Iqbal2
Javaid Iqbal2