- 1Department of Pediatrics, Chang Gung Memorial Hospital, Keelung, Taiwan
- 2Chang Gung University College of Medicine, Taoyuan, Taiwan
- 3Department of Physiology, National Taiwan University College of Medicine, Taipei, Taiwan
- 4Division of Gastroenterology, Department of Pediatrics, Chang Gung Memorial Hospital, Taoyuan, Taiwan
- 5Division of Allergy, Asthma and Rheumatology, Department of Pediatrics, Chang Gung Memorial Hospital, Taoyuan, Taiwan
- 6Division of Chest, Department of Pediatrics, Chang Gung Memorial Hospital, Taoyuan, Taiwan
- 7Department of Pediatrics, Municipal TuCheng Hospital, Chang Gung Memorial Hospital, New Taipei City, Taiwan
Background: The impact of abdominal obesity (AO) on plasma fatty acid changes and cardiometabolic risk in children who are obese and overweight has rarely been investigated. This study determined whether plasma fatty acid composition differed between children with AO and those without AO and its relationship with metabolic risk, particularly in the obese and overweight groups.
Methods: A total of 181 schoolchildren (aged 7–18 years) were included. Anthropometric and biochemical data and plasma fatty acid profiles were analyzed, and the indices of desaturase activity were estimated. Children were categorized based on their body weight and AO status. A continuous metabolic risk score was calculated using the sum of the z-scores of metabolic variables. A one-way analysis of variance test was used to compare the composition ratio of fatty acids between children with and without AO in the obese and overweight groups and normal-weight controls. Pearson analysis was also used to explore significant fatty acid and desaturase indicators associated with metabolic abnormalities.
Results: Children who were obese and overweight (N = 126) displayed higher dihomo-γ-linolenic acid (20:3n-6) and γ-linolenic acid (18:3n-6) proportions than normal-weight controls (N = 55), but lower heptadecanoic acid (17:0) proportion, regardless of the AO status of each individual. Obese and overweight children with AO (N = 89), but not their non-AO counterparts (N = 37), exhibited a significantly higher proportion of palmitoleic acid (16:1n-7) than the remaining study groups. Pearson analysis showed that high proportions of palmitoleic acid and dihomo-γ-linolenic acid, as well as increased stearoyl-coenzyme A desaturase-1(16) and delta-6 desaturase and decreased delta-5 desaturase activities, are strongly correlated with weight-height ratio, homeostasis model of assessment values for insulin resistance, hypertriglyceridemia, and continuous metabolic risk scores.
Conclusion: Higher palmitoleic acid and dihomo-γ-linolenic acid proportions, as well as increased stearoyl-coenzyme A desaturase-1(16) and delta-6 desaturase and decreased delta-5 desaturase activities are associated with AO and increased metabolic risk in children who are obese and overweight.
Introduction
Childhood obesity has been increasing at an alarming rate in recent decades (1). In obesity, hypertrophic adipocytes and adipose tissue-resident immune cells display a chronic proinflammatory profile by altering the secretion of adipokines and lipokines, which exacerbate cardiometabolic disease (2–4). Evidence also indicates that lipoprotein and fatty acid (FA) change in relation to abdominal obesity (AO) and overweight status (5, 6). In a birth cohort study, Kjellberg et al. reported that 26% of 6-year-old children already had one or more risk factors of metabolic syndrome, including increased waist circumference, dyslipidemia, insulin resistance (IR), and elevated blood pressure (7). Metabolic dysregulation during childhood has been shown to increase the risk of metabolic syndrome, type 2 diabetes, and cardiovascular diseases in adulthood (1, 8). Hence, it is crucial to detect and intervene in health threats from childhood.
Desaturase is involved in the biosynthesis of monounsaturated FA (MUFA) and polyunsaturated FA (PUFA) (9, 10). There is mounting evidence that changes in FA composition and desaturase activity are associated with metabolic diseases (11–14). Previous studies involving adults have identified the association between higher levels of palmitoleic acid (16:1n-7), dihomo-γ-linolenic acid (DGLA) (20:3n-6), oleic acid (18:1n-9), and γ-linolenic acid (18:3n-6), but a lower level of linoleic acid (18:2n-6); a higher stearoyl-coenzyme desaturase-1 (SCD1), delta-6 desaturase (D6D), but a lower delta-5 desaturase (D5D) activity for AO (11), metabolic syndrome (11, 15), and type 2 diabetes (13, 14). In pediatric populations, although a similar relationship was observed between changes in FA composition and hypertriglyceridemia (16) and higher cardiometabolic risk (17) in obese individuals (16, 18), whether children with AO display different FA characteristics and metabolic risk from children who are simply obese has rarely been investigated (19) and is of interest to physicians. It is reasonable to propose that AO, rather than weight status, may be a more important factor that influences plasma FA composition and metabolic risk in children.
In the present study, we first compared the differences in anthropometric, biochemical, and FA composition data in relation to body weight and AO status. Thereafter, we examined plasma FA profiles and how the indices of desaturase activity [SCD1(16), SCD1(18), D5D, and D6D] are associated with individual and combined metabolic risk factors. We aimed to investigate any significant association between plasma FA and metabolic risk factors in children who are obese and overweight, particularly among those with AO.
Materials and Methods
Study Participants
From March 2015 to October 2018, we recruited obese and overweight children aged 5–18 years who were willing to undergo anthropometric and body composition measurements from the outpatient clinic in Chang Gung Memorial Hospital, Keelung, in addition to normal-weight controls from the Prediction of Allergies in Taiwanese Children (PATCH) cohort study. Detailed information on demographic data and medical and general health conditions were obtained using questionnaires. Participants with chronic illness or receiving medications that could affect glucose or lipid metabolism were excluded from the study. This study was approved by the Ethics Committee of Chang Gung Memory Hospital (103-6519A3, 104-7100C, 106-3610C, and 201901820A3) and conducted in accordance with the Declaration of Helsinki as revised in 1983. Written informed consent was obtained from all children and their parents after they received detailed information on the objectives and design of the study.
Anthropometric, Blood Pressure, and Body Composition Measurements
Each participant underwent comprehensive outpatient evaluation. All anthropometric indicators and body composition measurements were performed by two well-trained research assistants, following standardized international recommendations (12). Briefly, body weight (BW) and body height (BH) were measured using an electronic scale (Super-view-HW3050, Hualien, Taiwan; precision of 0.1 kg and 0.1 cm, respectively). BH and BW were used to calculate the body mass index (BMI) in kg/m2. Waist circumference was measured at the level of the umbilicus. Hip circumference was measured at the point of maximal buttock protrusion. Waist-to-hip (WHR) and waist-to-height (WHtR) ratios were calculated from these measurements. Blood pressure (BP) was measured using a vital signs digital monitor (Chang Gung Medical Technology, Taipei, Taiwan) on the upper right arm after a 10-min seated rest. Detailed body composition data, including body fat percentage, trunk fat percentage, total body fat, fat-free mass, total body water, and total body muscle (20) were obtained using a multi-frequency (20 and 100 kHz) bioelectrical impedance analysis device that uses an 8-point tactile electrode system (InBody 230, Seoul, Korea).
Assessment of Dietary Intake
Starting April 2017, on the day of blood sampling and anthropometric measurements, a 1-month recall of the PATCH study-designed food frequency questionnaire (FFQ) was completed for each participant to estimate the usual frequency of consumption of the following food items: milk, fruits, vegetables, meat, shellfish, fish, nuts, juices, beverages, snacks, and fast foods. Each FFQ was filled with parental assistance. Each subject was asked how often they consumed individual food items using a 4-point scale (1: none; 2: 1–2 days per week; 3: 3–4 days per week; and 4: ≥5 days per week).
Collection of Biochemical Data
Blood samples (10 mL) were collected from study participants after overnight fasting, and biochemical markers including glucose, insulin, triglyceride (TG), total cholesterol, low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) were determined using the electrochemiluminescence method (Roche cobas P512, Mannheim, Germany). The homeostasis model of assessment values for insulin resistance (HOMA-IR) was also obtained by using the formula proposed by Matthews et al.: insulin (μU/mL) × glucose (mg/dL)/405 (21).
Plasma FA Analysis
Total lipids were extracted from the plasma samples and converted to their methyl ester equivalents as described previously (12, 22), and analyzed using an Agilent 7820A GC using flame ionization detection on a SP-2560 polar fused silica capillary column (100 m × 0.25 mm × 0.2 μm; Supelco Inc., PA, USA) with nitrogen as the carrier gas (12). The FA peaks were identified by comparing retention times with those of a standard mixture of GLC-68A, GLC-481B, GLC-532, GLC-744 (Nu-Chek Prep), 37 FAME, trans 16:1n-7, trans 18:1n-7, trans 18:1n-9, and cis/trans 18:2n-6 (all obtained from Supelco Inc., or Sigma). 13:0 free fatty acid (10 μg) was added as an internal standard. FA composition was expressed as the weight of a percentage of the total weight of carbon-12 to carbon-24 FAs (wt%). The estimated desaturase activity was calculated using the ratio of FA in plasma (11, 17, 23); SCD1(16) activity = (16:1n-7/16:0), SCD1(18) activity = (18:1n-9/18:0), D5D activity = (20:4n-6/20:3n-6), and D6D activity = (20:3n-6/18:2n-6).
Definitions Used in This Study
Abdominal obesity (AO) group: Obese (BMI ≥ 95th percentile) or overweight (BMI ≥ 85–95th percentile) children (24) with WHtR ≥ 0.5 (25, 26).
Non-AO group: Obese or overweight children (24) with WHtR < 0.5.
Normal-weight healthy controls (HC): Normal-weight (BMI = 10–85th percentile) children (24) with a normal cardiometabolic profile, with WHtR < 0.5.
Combined metabolic risk assessment: Five risk factors were assessed in the study: (1) AO (WHtR ≥ 0.5) (25, 26); (2) high TG ≥ 150 mg/dL (27); (3) low HDL-C (boys < 40 mg/dL; girls < 50 mg/dL) (27); and (4) HOMA-IR ≥ 4.5 (currently, no universal definition of IR is applicable in children. Therefore, we used the 75th percentile of HOMA-IR of all participants as the threshold of IR); (5) hypertension: systolic or diastolic BP ≥ 95th percentile for children between 1 and 12 years of age, and BP ≥ 130/85 mmHg for 13-year-old and older individuals (28).
Continuous metabolic risk (CMR) scores: A CMR score was calculated using five metabolic variables, including WHtR, TG, HDL-C, HOMA-IR, and systolic BP. Each CMR score component was internally standardized using z-scores. The z-score values of each metabolic variable were then summed using the following equation: WHtR + TG – HDL-C + HOMA-IR + systolic BP. The z-score of HDL-C was multiplied by−1 because it was inversely associated with cardiometabolic risk.
Statistical Analysis
Numerical variables were summarized as mean ± standard deviation (SD) or as frequencies and percentages. The normality of data distribution was checked. Associations between categorical variables were analyzed using the chi-square test. One-way analysis of variance (ANOVA) was used to test for differences in anthropometric and biochemical data, plasma FA composition, and estimated desaturase indices between the AO, non-AO, and HC groups. The correlation coefficients between selected FA proportions, estimated desaturase indices, obesity indices, metabolic risk factors, and CMR scores were determined using Pearson analyses. Furthermore, a multivariate regression analysis with a stepwise procedure adjusted for age, sex, and total saturated FA (SFA), MUFA, n3- and n6-PUFA concentrations, was performed to examine the associations between CMR scores, significant FA proportions, and indices of desaturase activity. Histograms were prepared using mean values and standard deviations. Statistical significance was set at p < 0.05. All statistical analyses were performed using IBM SPSS Statistics version 20 (Armonk, NY, USA).
Results
Study Participants
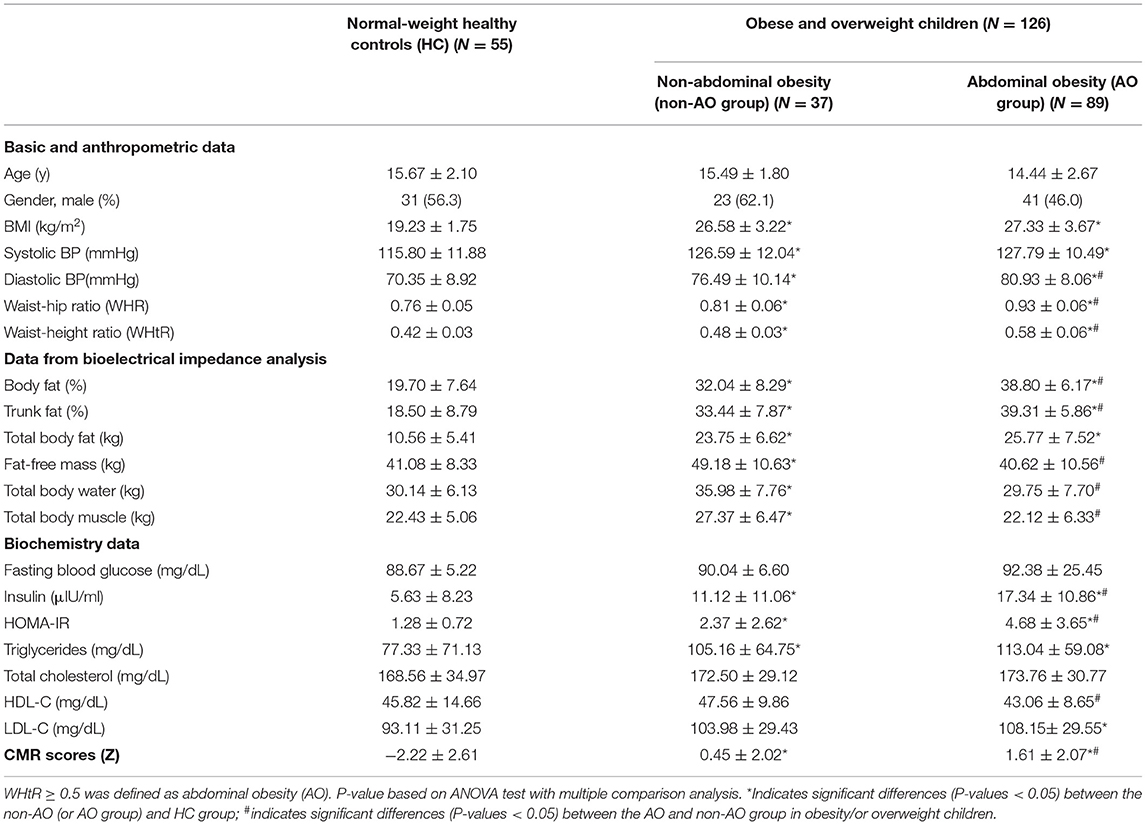
At the initial evaluation, 228 children ranging from 5 to 18 years of age were enrolled. To avoid potential confounding factors, we excluded 12 children with incomplete FA analysis, 34 normal-weight children with either WHtR ≥ 0.5 or cardiometabolic abnormalities, and one obese child with type 2 diabetes. Our final sample included 181 schoolchildren (96 males and 85 females, aged 7–18 years, with an average age of 15.01 ± 2.17 years). The characteristics of the children participating in this study are summarized in Table 1. In total, 99 (54.7%) study participants were obese, 27 (14.9%) were overweight, and 55 (30.4%) were normal-weight and served as healthy controls (HCs). Of the 126 children who were obese and overweight, 89 (70.6%) showed AO and the remaining 37 (29.4%) did not (Table 1). There were no intergroup differences in the mean age or sex. Based on the 1-month FFQ recall, the usual consumption of food items, including milk, fruits, vegetables, meat, shellfish, fish, nuts, juices, beverages, snacks, and fast foods did not differ significantly between the non-AO and AO groups (data not shown).
Intergroup Comparison of Obesity Indices and Biochemical Data
As shown in Table 1, a significant and progressive increase in diastolic BP, WHR, WHtR, body fat percentage, and trunk fat percentage were observed in the HC, non-AO, and AO groups. Based on the metabolic profile, insulin, HOMA-IR, and CMR scores were significantly different among the three groups.
Intergroup Comparison of Plasma FA Profile
We compared the plasma FA profiles of the HC, AO, and non-AO groups (Table 2). In general, neither total SFA nor MUFA or the n-6 or n-3 series of PUFAs showed any differences between the HC and obese children in the non-AO groups. Participants in the AO group exhibited a significantly higher proportion of MUFAs than the HC group (P = 0.001), and a lower proportion of n6-PUFA than the non-AO group (P = 0.048). Analysis of FA composition showed that children who were obese and overweight exhibited higher DGLA (20:3n-6), α-linolenic acid (18:3n-3), γ-linolenic acid (18:3n-6), docosapentaenoic acid (22:5n-6), and clupanodonic acid (22: 5n-3) proportions, whereas lower heptadecanoic acid (17:0) and stearic acid (18:0) proportions than those in the normal-weight controls, regardless of whether participants had AO. Participants in the AO group exhibited higher proportions of palmitoleic acid (16:1n-7), DGLA (20:3n-6), and docosapentaenoic acid (22:5n-6), but lower proportions of myristoleic acid (14:1), vaccenic acid (18:1n-7), linoleic acid (18:2n-6), and nervonic acid (24:1n-9) than did participants in the non-AO group. The proportions of other FAs between the study groups are shown in Table 2.
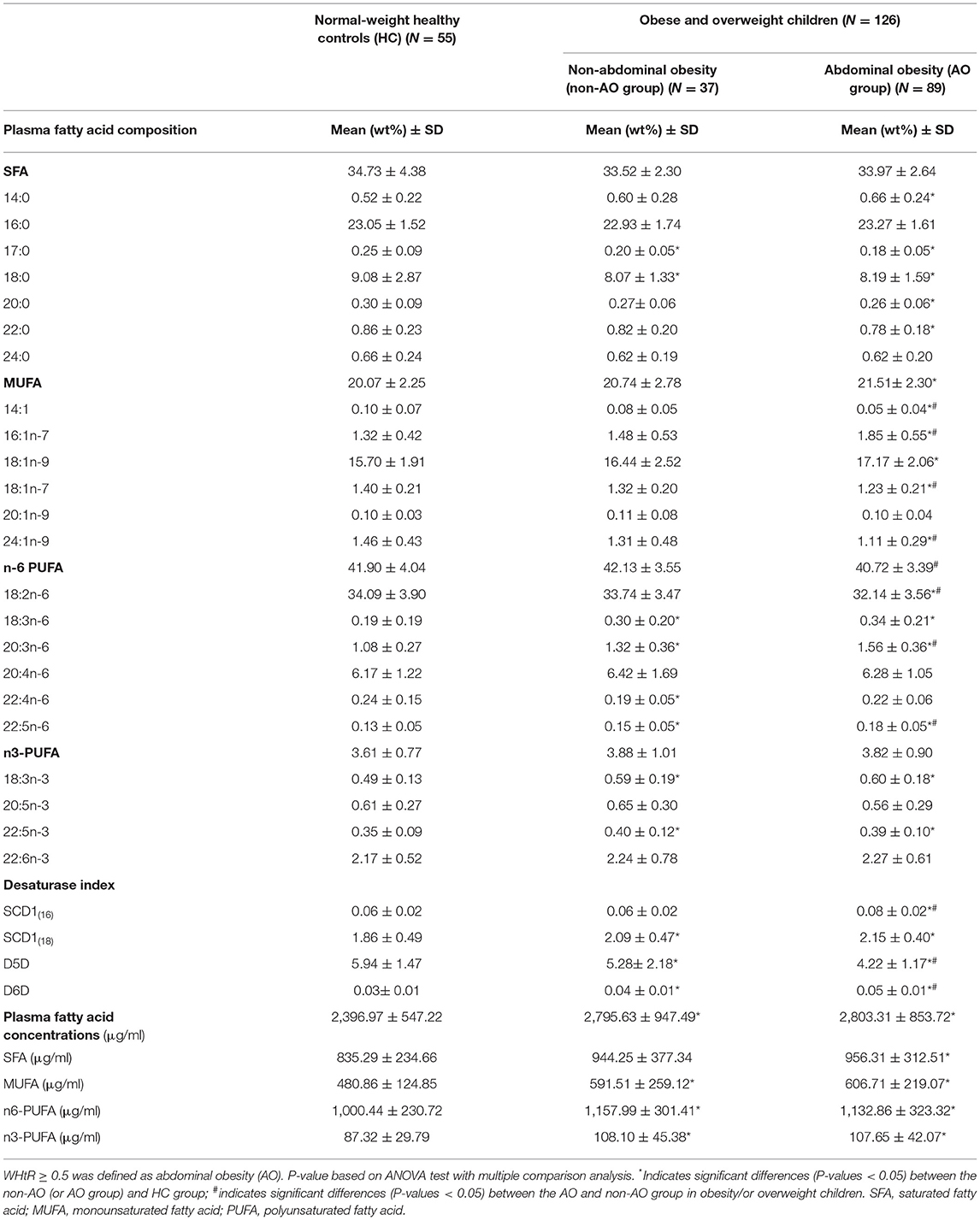
Table 2. Plasma fatty acid profile and estimated desaturase activities with regard to body weight status and abdominal obesity.
We also compared the plasma FA concentrations in the HC, AO, and non-AO groups (Table 2). Our data revealed that children who were obese and overweight, regardless of AO status, exhibited significantly higher levels of total plasma FAs (μg/mL), as well as MUFA (μg/mL), n6-polyunsaturated FA (PUFA) (μg/mL), and n3-PUFA concentrations (μg/mL) than the HC group.
Intergroup Comparison of Desaturase Activities
The estimated levels of desaturase activity are presented in Table 2. Participants in the AO group displayed significantly higher SCD1(16) and D6D activities, but lower D5D activity, than the HC and non-AO groups (all P < 0.05). Nonetheless, the SCD1(18) activity was higher in the children who were obese and overweight but showed no differences between the AO and non-AO groups (P > 0.05) (Table 2).
Correlation Between FA Compositions, Desaturase Activities, and Cardio-Metabolic Risk Markers
We detected positive correlations between metabolic variables (insulin, HOMA-IR, and TG levels), CMR scores, and anthropometric indices (BMI, WHR, WHtR, and trunk fat percentage) and the proportions of palmitoleic acid (16:1n-7) (r = 0.41–0.51), DGLA (20:3n-6) (r = 0.22–0.55), γ-linolenic (18:3n-6) (r = 0.24–0.35), and activity of SCD1(16) (r = 0.38–0.50) and D6D (r = 0.27–0.53); an inverse correlation with heptadecanoic acid (C17:0) (r = −0.31 to −0.52) and D5D expression (r = −0.42 to −0.51) was also observed (Table 3). Nonetheless, these parameters were weakly correlated with SCD1(18) activity (r = 0.20–0.55). Associations of systolic BP, glucose, total cholesterol, and HDL-C are shown in Table 3. In the stepwise logistic regression model adjusted for age, sex, and total SFA, MUFA, n3- and n6-PUFA concentrations, D5D activity was selected as an independent indicator inversely associated with CMR scores (adjusted beta = −0.21, P = 0.019).

Table 3. Correlation coefficient between single fatty acid proportions, estimated desaturase activities, and selected metabolic risk factors.
Furthermore, our data revealed that WHtR presented a stronger correlation with trunk fat percentage (r = 0.76 vs. 0.71), insulin (r = 0.62 vs. 0.58), triglyceride (r = 0.34 vs. 0.31) levels, and total plasma FA concentrations (r = 0.21 vs. 0.16) than with BMI (data not shown).
Association Between FA Compositions, Desaturase Activities, and Estimated Metabolic Risk
Hypertension was observed in 90 (49.7%), AO in 89 (49.2%), low HDL-C in 88 (48.6%), IR in 45 (24.9%), and hypertriglyceridemia in 28 (15.5%) children. Figure 1 shows the metabolic risk clustering based on the assessment of the above-mentioned five risk factors. In total, 51 (28.2%) children had three or more risk factors, 75 (41.4%) had one or two risk factors, and 55 (30.4%) had none of the risk factors. Likewise, the proportions of palmitoleic acid (16:1n-7) and DGLA (20:3n-6) (Figures 1A,B), and the activities of SCD1(16), SCD1(18), and D6D (Figures 1C,D,F) showed an upward trend, whereas the activity of D5D showed a downward trend as metabolic alterations increased (Figure 1E). Of note, we observed that the proportion of DGLA (20:3n-6) (1.08 ± 0.27% vs. 1.39 ± 0.38% vs. 1.55 ± 0.39%, P < 0.001) and D5D activity (5.94 ± 1.47 vs. 4.82 ± 1.23 vs. 4.19 ± 1.63, P < 0.001) changed significantly from zero, to one or two, in the ≥ 3 metabolic risk factors groups (Figures 1B,E).
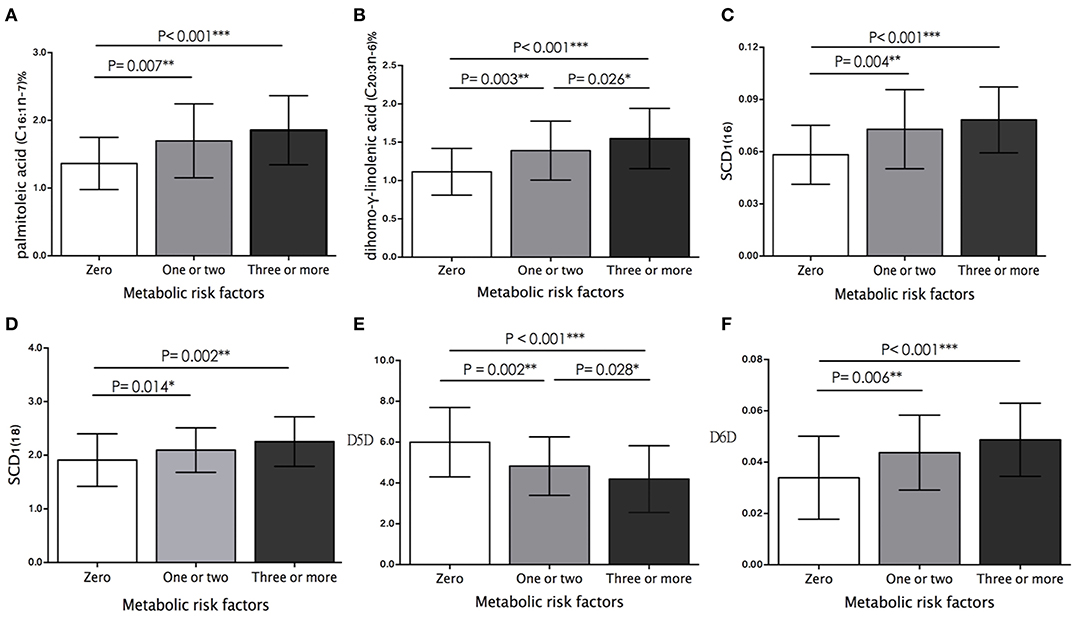
Figure 1. Comparison of plasma (A) palmitoleic acid proportion, (B) dihomo-γ-linolenic acid proportion (DGLA), and estimated activity of (C) SCD1(16), (D) SCD1(18), (E) D5D, and (F) D6D between study participants who had zero, one or two, and more than three metabolic risk factors. Histograms were prepared using mean values and SD. The differences between study groups are based on ANOVA test with multiple comparison analysis. Significance levels: *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
In the present study, we first explored the impact of obesity on plasma FA changes and metabolic risk in children. The results showed that higher DGLA (20:3n-6) and γ-linolenic acid (18:3n-6) proportions, but a lower proportion of heptadecanoic acid (17:0) was observed in children who were obese and overweight than in normal-weight healthy controls. In addition, the proportions of DGLA (20:3n-6) and γ-linolenic acid (18:3n-6) showed a positive correlation, whereas the proportion of heptadecanoic acid (17:0) was inversely correlated with IR, hypertriglyceridemia, and CMR scores. Taken together, our data suggest that DGLA (20:3n-6) and γ-linolenic acid (18:3n-6) proportions tended to increase, but the proportion of heptadecanoic acid (17:0) tended to decrease in children who were either obese or overweight with increased metabolic risk. Adipose tissue is an endocrine organ secreting factor that can both improve and impair insulin sensitivity (4). In obesity, elevated FA levels are associated with reduced glucose uptake by peripheral tissues and increased IR (4). Furthermore, elevated FA levels may drive lipid accumulation in visceral adipose tissue and very-low-density lipoprotein triglyceride synthesis and release, contributing to the dyslipidemic phenotype of metabolic syndrome (2–4). Consistent with our findings, the literature reveals that higher proportions of DGLA (20:3n-6) (11, 13, 15, 18, 29) and γ-linolenic acid (18:3n-6) (11, 13, 29) in plasma are linked to obesity and metabolic syndrome components. Heptadecanoic acid (17:0), a minor SFA in ruminant fat, has been reported to be an objective biomarker of milk fat intake (30). Warensjö et al. reported a relationship between heptadecanoic acid (17:0) and a lower risk of myocardial infarction in women (30). Sawh et al. also reported that plasma iso- C17:0 was inversely correlated with hepatic steatosis in children (31). More studies are required to validate the potential metabolic benefits of heptadecanoic acid in cardiometabolic health in humans (32, 33).
Next, we explored the impact of AO on plasma FA changes and metabolic risk in children. In comparison to peripheral fat, the accumulation of intra-abdominal fat has been strongly associated with metabolic disorders (34). A WHtR ≥ 0.5 has been reported as an appropriate cut-off point for classifying cardio-metabolic risk in children and adolescents, regardless of sex, age, and ethnicity (22, 35–37). Although we observed a significant and progressive increase in DGLA (20:3n-6) proportions from the HC to the non-AO and AO groups, whether abdominal or body fat made a greater contribution to the result was uncertain. Our data revealed that children who were obese and overweight and with AO, but not their non-AO counterparts, exhibited a significantly higher proportion of palmitoleic acid (16:1n-7) than the remaining study groups. Furthermore, we demonstrated that elevated palmitoleic acid (16:1n-7) proportion, in conjunction with increased SCD1(16) activity, are positively associated with IR, hypertriglyceridemia, and CMR scores. Notably, palmitoleic acid (16:1n-7) is exclusively regulated by SCD1(16) activity and is regarded as an important product of de novo lipogenesis (38). Our findings imply that increased palmitoleic acid (16:1n-7) proportion, a product related to SCD1(16) activity, not only increases the likelihood of abdominal fat accumulation, but also obesity-related metabolic abnormalities. The question of whether elevated palmitoleic acid (16:1n-7) has adverse effects on metabolic disorders remains controversial (9). Our findings are consistent with those of other reports (15, 18, 23, 38). Nonetheless, several animal and human studies have reported that palmitoleic acid (16:1n-7) may enhance whole-body insulin sensitivity, increase hepatic FA oxidation, and improve blood lipid profile (39), and are associated with a low prevalence of diabetes and cardiovascular risk (9, 40, 41). The discrepancies in the effects of palmitoleic acid (16:1n-7) on insulin actions and metabolic outcomes may be explained by the different ethnicities, ages, underlying health conditions, and the duration of such conditions (9).
Finally, consistent with previous reports, the present study showed that high D6D (16–18) and low D5D (11, 15, 18, 19) activities were significantly associated with metabolic risk. Furthermore, D5D was the best indicator of CMR scores in our study. D6D and D5D are critical for PUFA metabolism, Linoleic acid (18:2n-6) is metabolized to γ-linolenic acid (18:3n-6) by D6D, and then progressively elongated to DGLA (20:3n-6). D5D is the rate-limiting enzyme that converts DGLA to arachidonic acid (20:4n-6) (42, 43). Previous studies have revealed that single nucleotide polymorphisms located within or near the FADS1- FADS2 gene cluster on chromosome 11 may alter desaturation rates of both D6D and D5D, as well as the associated PUFA levels (44, 45). Minor allele carriers of rs174546, rs174547, and rs174566 were identified to be associated with increased DGLA, reduced arachidonic acid levels, and lower D5D activity (44–46). In addition, rs968567 was linked to higher D6D activity (45). Further longitudinal studies to explore the genetic variability associated with PUFA metabolism for those individuals suffering metabolic abnormalities since childhood would be potentially interesting.
The strength of this study lies in the distinct comparison of plasma FA profiles from all lipid classes and cardiometabolic risk between children with and without AO in the obese and overweight groups, and normal-weight, metabolic healthy controls. To date, several pediatric studies have reported that plasma FA or desaturase activity changes might be linked to obesity (16, 18, 38), whereas some studies have provided limited FA profiles (19, 38, 47), desaturase indicators (16), data obtained from different plasma components (15, 18), or based on the context of Western countries (16, 17). Only a few pediatric studies have investigated the impact of AO on plasma FA changes (19). However, this was a small sample size study (n = 58) that classified AO and non-AO participants by IDFEFICS criteria released for European children (48), and both obese and non-obese children with AO were included in the analysis, which likely led to mixed results (19). In another pediatric study, Choi et al. suggested that waist circumference and D6D levels could predict future IR and metabolic risk in Korean boys; whether WHtR had similar associations was not confirmed in the study (18).
The present study has some limitations. First, the cross-sectional design could not be used to determine causality. Next, although a 1-month recall of FFQ was applied to evaluate the dietary intake and the usual consumption of food frequency in the 1-month recall questionnaires, we did not access detailed information on total food fat, carbohydrate, energy, or habitual dietary intake in the study. Therefore, we were unable to adjust for these important covariates in the logistic regression analysis. Evidence indicates that carbohydrate and fat consumption may influence FA composition and SCD1 and D6D activities (49, 50). Furthermore, it is notable that the composition and incorporation of FA in the plasma and blood cells are the result of distinct processes, such as short- or long-term dietary intake, metabolism, and peripheral utilization (51). This study only measured plasma FA reflecting intake status and de novo lipogenesis over days to weeks; FA in other blood cells reflecting long-term effects was not measured; therefore, the present results on FA composition and its association with metabolic risk may differ from other sources of analyzed samples (51). Finally, although the age and sex distributions were similar between the AO and non-AO groups, we did not evaluate the hormonal changes and puberty stages in our study participants, which may influence body fat distribution and FA profiles (52). Therefore, our data should be interpreted with caution.
In summary, our data suggest that higher DGLA (20:3n-6) and γ-linolenic acid (18:3n-6), but lower heptadecanoic acid (17:0) proportions were more prevalent in children who are obese and overweight with increased metabolic risk. Furthermore, increased palmitoleic acid (16:1n-7) proportion, a product related to SCD1(16) activity, is associated with abdominal fat accumulation and obesity-related metabolic abnormalities. Overall, in our study, low D5D activity was the best indicator of CMR scores.
Data Availability Statement
The datasets generated for this study are available upon reasonable request from the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Research Ethics Committee of Chang Gung Memory Hospital (103-6519A3, 104-7100C, 106-3610C, and 201901820A3). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
M-CH and H-MS involved in the laboratory work, statistical analysis and interpretation of its results. M-CH wrote the first draft of the manuscript, and J-LH and H-MS edited it. All authors were involved in the study design, recruitment of participants, written consent, reviewed the manuscript and approved the final version of the manuscript.
Funding
This study was funded by grants from Chang Gung Memorial Hospital (CMRPG 2E0131-35, 2K0321). The funders had no role in the design of the study and collection, analysis and interpretation of data and in writing the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
All authors are grateful to the study participants and their parents, and to Yi-Ling Hsiao and Yi-Wen Lin for data collection and technical assistance.
Abbreviations
AO, abdominal obesity; CMR scores, continuous metabolic risk scores; DGLA, dihomo-γ-linolenic acid; D5D, Delta-5 desaturase; D6D, Delta-6 desaturase; FA, fatty acids; FFQ, food frequency questionnaire; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; SCD1, stearoyl-coenzyme desaturase-1; SFA, saturated fatty acid; TG, triglyceride; WHR, waist-to-hip ratio; WHtR, waist-to height ratio.
References
1. Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. (2004) 350:2362–74. doi: 10.1056/NEJMoa031049
2. Scheja L, Heeren J. The endocrine function of adipose tissues in health and cardiometabolic disease. Nat Rev Endocrinol. (2019) 15:507–24. doi: 10.1038/s41574-019-0230-6
3. McMorrow AM, Connaughton RM, Lithander FE, Roche HM. Adipose tissue dysregulation and consequences in childhood and adolescent obesity: potential impact of dietary fat quality. Proc Nutr Soc. (2015) 74:67–82. doi: 10.1017/S002966511400158X
4. Smith U, Kahn BB. Adipose tissue regulates insulin sensitivity: role of adipogenesis, de novo lipogenesis and novel lipids. J Intern Med. (2016) 280:465–75. doi: 10.1111/joim.12540
5. Kang M, Lee A, Yoo HJ, Kim M, Kim M, Shin DY, et al. Association between increased visceral fat area and alterations in plasma fatty acid profile in overweight subjects: a cross-sectional study. Lipids Health Dis. (2017) 16:248. doi: 10.1186/s12944-017-0642-z
6. Kishino T, Watanabe K, Urata T, Takano M, Uemura T, Nishikawa K, et al. Visceral fat thickness in overweight men correlates with alterations in serum fatty acid composition. Clin Chim Acta. (2008) 398:57–62. doi: 10.1016/j.cca.2008.08.010
7. Kjellberg E, Roswall J, Bergman S, Almqvist-Tangen G, Alm B, Dahlgren J. Longitudinal birth cohort study found that a significant proportion of children had abnormal metabolic profiles and insulin resistance at 6 years of age. Acta Paediatr. (2019) 108:486–92. doi: 10.1111/apa.14599
8. Burke V, Beilin LJ, Simmer K, Oddy WH, Blake KV, Doherty D, et al. Predictors of body mass index and associations with cardiovascular risk factors in Australian children: a prospective cohort study. Int J Obes. (2005) 29:15–23. doi: 10.1038/sj.ijo.0802750
9. Frigolet ME, Gutiérrez-Aguilar R. The role of the novel lipokine palmitoleic acid in health and disease. Adv Nutr. (2017) 8:173S−81S. doi: 10.3945/an.115.011130
10. Zhang JY, Kothapalli KS, Brenna JT. Desaturase and elongase-limiting endogenous long-chain polyunsaturated fatty acid biosynthesis. Curr Opin Clin Nutr Metab Care. (2016) 19:103–10. doi: 10.1097/MCO.0000000000000254
11. Kawashima A, Sugawara S, Okita M, Akahane T, Fukui K, Hashiuchi M, et al. Plasma fatty acid composition, estimated desaturase activities, and intakes of energy and nutrient in Japanese men with abdominal obesity or metabolic syndrome. J Nutr Sci Vitaminol. (2009) 55:400–6. doi: 10.3177/jnsv.55.400
12. Hua MC, Su HM, Yao TC, Kuo ML, Lai MW, Tsai MH, et al. Alternation of plasma fatty acids composition and desaturase activities in children with liver steatosis. PLoS ONE. (2017) 12:e0182277. doi: 10.1371/journal.pone.0182277
13. Lankinen MA, Stančáková A, Uusitupa M, Ågren J, Pihlajamäki J, Kuusisto J, et al. Plasma fatty acids as predictors of glycaemia and type 2 diabetes. Diabetologia. (2015) 58:2533–44. doi: 10.1007/s00125-015-3730-5
14. Kröger J, Schulze MB. Recent insights into the relation of Delta 5 desaturase and Delta 6 desaturase activity to the development of type 2 diabetes. Curr Opin Lipidol. (2012) 23:4–10. doi: 10.1097/MOL.0b013e32834d2dc5
15. Mayneris-Perxachs J, Guerendiain M, Castellote AI, Estruch R, Covas MI, Fitó M, et al. Plasma fatty acid composition, estimated desaturase activities, and their relation with the metabolic syndrome in a population at high risk of cardiovascular disease. Clin Nutr. (2014) 33:90–7. doi: 10.1016/j.clnu.2013.03.001
16. Wolters M, Schlenz H, Börnhorst C, Risé P, Galli C, Moreno LA, et al. IDEFICS Consortium. Desaturase activity is associated with weight status and metabolic risk markers in young children. J Clin Endocrinol Metab. (2015) 100:3760–9. doi: 10.1210/jc.2015-2693
17. Venäläinen T, Ågren J, Schwab U, de Mello VD, Eloranta AM, Laaksonen DE, et al. Cross-sectional associations of plasma fatty acid composition and estimated desaturase and elongase activities with cardiometabolic risk in Finnish children–The PANIC study. J Clin Lipidol. (2016) 10:82–91. doi: 10.1016/j.jacl.2015.09.004
18. Choi YS, Jang HB, Park JY, Lee HJ, Kang JH, Park KH, et al. Associations between estimated desaturase activity and insulin resistance in korean boys. Osong Public Health Res Perspect. (2014) 5:251–7. doi: 10.1016/j.phrp.2014.08.008
19. Aristizabal JC, González-Zapata LI, Estrada-Restrepo A, Monsalve-Alvarez J, Restrepo-Mesa SL, Gaitán D. Concentrations of plasma free palmitoleic and dihomo-gamma linoleic fatty acids are higher in children with abdominal obesity. Nutrients. (2018) 10:31. doi: 10.3390/nu10010031
20. Lee LW, Liao YS, Lu HK, Hsiao PL, Chen YY, Chi CC, et al. Validation of two portable bioelectrical impedance analyses for the assessment of body composition in school age children. PLoS ONE. (2017) 12:e0171568. doi: 10.1371/journal.pone.0171568
21. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and Beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. (1985) 28:412–9. doi: 10.1007/BF00280883
22. Moser AB, Jones DS, Raymond GV, Moser HW. Plasma and red blood cell fatty acids in peroxisomal disorders. Neurochem Res. (1999) 24:187–97. doi: 10.1023/A:1022549618333
23. Paillard F, Catheline D, Duff FL, Bouriel M, Deugnier Y, Pouchard M, et al. Plasma palmitoleic acid, a product of stearoyl-coA desaturase activity, is an independent marker of triglyceridemia and abdominal adiposity. Nutr Metab Cardiovasc Dis. (2008) 18:436–40. doi: 10.1016/j.numecd.2007.02.017
24. Chen W, Chang MH. New growth charts for Taiwanese children and adolescents based on World Health Organization standards and health related physical fitness. Pediatr Neonatol. (2010) 51:69–79. doi: 10.1016/S1875-9572(10)60014-9
25. Nawarycz T, So HK, Choi KC, Sung RY, Li AM, Nelson EA, et al. Waist-to-height ratio as a measure of abdominal obesity in southern Chinese and European children and adolescents. Int J Obes. (2016) 40:1109–18. doi: 10.1038/ijo.2015.251
26. Browning LM, Hsieh SD, Ashwell M. Systematic review of waist-to-height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 0.5 could be a suitable global boundary value. Nutr Res Rev. (2010) 23:247–69. doi: 10.1017/S0954422410000144
27. The Statement of Taiwan Pediatric Association for Child and Adolescent Metabolic Syndrome. Taiwan Pediatric Association (2016). Available online at: https://www.Pediatr.Org.Tw/people/edu_info.Asp?Id=33 (accessed November, 1 2018).
28. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. (2004) 114:555–76. doi: 10.1542/peds.114.2.S2.555
29. Decsi T, Csábi G, Török K, Erhardt E, Minda H, Burus I, et al. Polyunsaturated fatty acids in plasma lipids of obese children with and without metabolic cardiovascular syndrome. Lipids. (2000) 35:1179–84. doi: 10.1007/s11745-000-0634-7
30. Warensjö E, Jansson JH, Cederholm T, Boman K, Eliasson M, Hallmans G, et al. Biomarkers of milk fat and the risk of myocardial infarction in men and women: a prospective, matched case-control study. Am J Clin Nutr. (2010) 92:194–202. doi: 10.3945/ajcn.2009.29054
31. Sawh MC, Wallace M, Shapiro E, Goyal NP, Newton KP, Yu EL, et al. Dairy fat intake, plasma C15: 0 and plasma Iso-C17: 0 are inversely associated with liver fat in children. J Pediatr Gastroenterol Nutr. (2021) 72:e90–6. doi: 10.1097/MPG.0000000000003040
32. Ratnayake WM. Concerns about the use of 15:0, 17:0, and trans-16:1n-7 as biomarkers of dairy fat intake in recent observational studies that suggest beneficial effects of dairy food on incidence of diabetes and stroke. Am J Clin Nutr. (2015) 101:1102–3. doi: 10.3945/ajcn.114.105379
33. Risérus U, Marklund M. Milk fat biomarkers and cardiometabolic disease. Curr Opin Lipidol. (2017) 28:46–51. doi: 10.1097/MOL.0000000000000381
34. Soto González A, Bellido D, Buño MM, Pértega S, De Luis D, Martínez-Olmos M, et al. Predictors of the metabolic syndrome and correlation with computed axial tomography. Nutrition. (2007) 23:36–45. doi: 10.1016/j.nut.2006.08.019
35. Jiang Y, Dou YL, Xiong F, Zhang L, Zhu GH, Wu T, et al. Waist-to-height ratio remains an accurate and practical way of identifying cardiometabolic risks in children and adolescents. Acta Paediatr. (2018). doi: 10.1111/apa.14323
36. Gamboa Delgado EM, Domínguez Urrego CL, Quintero Lesmes DC. Waist-To-Height ratio and its relation with cardiometabolic risk factors in children from Bucaramanga, Colombia. Nutr Hosp. (2017) 34:1338–44. doi: 10.20960/nh.1059
37. Ashwell M, Hsieh SD. Six reasons why the waist to height ratio is a rapid and effective global indicator for health risks of obesity and how its use could simplify the international public health message on obesity. Int J Food Sci Nutr. (2005) 56:303–7. doi: 10.1080/09637480500195066
38. Okada T, Furuhashi N, Kuromori Y, Miyashita M, Iwata F, Harada K. Plasma palmitoleic acid content and obesity in children. Am J Clin Nutr. (2005) 82:747–50. doi: 10.1093/ajcn/82.4.747
39. de Souza CO, Vannice GK, Rosa Neto JC, Calder PC. Is palmitoleic acid a plausible non- pharmacological strategy to prevent or control chronic metabolic and inflammatory disorders? Mol Nutr Food Res. (2018) 62. doi: 10.1002/mnfr.201700504
40. Stefan N, Kantartzis K, Celebi N, Staiger H, Machann J, Schick F, et al. Circulating palmitoleate strongly and independently predicts insulin sensitivity in humans. Diabetes Care. (2010) 33:405–7. doi: 10.2337/dc09-0544
41. Bernstein AM, Roizen MF, Martinez L. Purified palmitoleic acid for the reduction of high-sensitivity C-reactive protein and serum lipids: a double-blinded, randomized, placebo controlled study. J Clin Lipidol. (2014) 8:612–7. doi: 10.1016/j.jacl.2014.08.001
42. Field AE, Willett WC, Lissner L, Colditz GA. Dietary fat and weight gain among women in the Nurses' Health Study. Obesity. (2007) 15:967–76. doi: 10.1038/oby.2007.616
43. Tsurutani Y, Inoue K, Sugisawa C, Saito J, Omura M, Nishikawa T. Increased serum dihomo-γ-linolenic acid levels are associated with obesity, body fat accumulation, and insulin resistance in japanese patients with type 2 diabetes. Intern Med. (2018) 57:2929–35. doi: 10.2169/internalmedicine.0816-18
44. de Toro-Martín J, Guénard F, Rudkowska I, Lemieux S, Couture P, Vohl MC. A common variant in ARHGEF10 alters delta-6 desaturase activity and influence susceptibility to hypertriglyceridemia. J Clin Lipidol. (2018) 12:311–20.e3. doi: 10.1016/j.jacl.2017.10.020
45. Bokor S, Dumont J, Spinneker A, Gonzalez-Gross M, Nova E, Widhalm K, et al. Single nucleotide polymorphisms in the FADS gene cluster are associated with delta-5 and delta-6 desaturase activities estimated by serum fatty acid ratios. J Lipid Res. (2010) 51:2325–33. doi: 10.1194/jlr.M006205
46. Guan W, Steffen BT, Lemaitre RN, Wu JHY, Tanaka T, Manichaikul A, et al. Genome-wide association study of plasma N6 polyunsaturated fatty acids within the cohorts for heart and aging research in genomic epidemiology consortium. Circ Cardiovasc Genet. (2014) 7:321–31. doi: 10.1161/CIRCGENETICS.113.000208
47. Reinehr T, Kiess W, Andler W. Insulin sensitivity indices of glucose and free fatty acid metabolism in obese children and adolescents in relation to serum lipids. Metabolism. (2005) 54:397–402. doi: 10.1016/j.metabol.2004.10.008
48. Nagy P, Kovacs E, Moreno LA, Veidebaum T, Tornaritis M, Kourides Y, et al. IDEFICS consortium. Percentile reference values for anthropometric body composition indices in European children from the IDEFICS study. Int J Obes. (2014) 38 (Suppl. 2):S15–25. doi: 10.1038/ijo.2014.131
49. Mangravite LM, Dawson K, Davis RR, Gregg JP, Krauss RM. Fatty acid desaturase regulation in adipose tissue by dietary composition is independent of weight loss and is correlated with the plasma triacylglycerol response. Am J Clin Nutr. (2007) 86:759–67. doi: 10.1093/ajcn/86.3.759
50. Stawarska A, Białek A, Tokarz A. The type of dietary fat and dietary energy restriction affects the activity of the desaturases in the liver microsomes. Prostaglandins Leukot Essent Fatty Acids. (2018) 128:62–6. doi: 10.1016/j.plefa.2017.12.001
51. Risé P, Eligini S, Ghezzi S, Colli S, Galli C. Fatty acid composition of plasma, blood cells and whole blood: relevance for the assessment of the fatty acid status in humans. Prostaglandins Leukot Essent Fatty Acids. (2007) 76:363–9. doi: 10.1016/j.plefa.2007.05.003
Keywords: abdominal obesity, children, dihomo-γ-linolenic acid, desaturase activities, metabolic risk, palmitoleic acid
Citation: Hua M-C, Su H-M, Lai M-W, Yao T-C, Tsai M-H, Liao S-L, Lai S-H and Huang J-L (2021) Palmitoleic and Dihomo-γ-Linolenic Acids Are Positively Associated With Abdominal Obesity and Increased Metabolic Risk in Children. Front. Pediatr. 9:628496. doi: 10.3389/fped.2021.628496
Received: 12 November 2020; Accepted: 15 March 2021;
Published: 09 April 2021.
Edited by:
Gianluca Tornese, Institute for Maternal and Child Health Burlo Garofolo (IRCCS), ItalyReviewed by:
Júlia Calvo Galhardo, Universidade NOVA de Lisboa, PortugalPamela Arielle Nono Nankam, Helmholtz-Gemeinschaft Deutscher Forschungszentren (HZ), Germany
Copyright © 2021 Hua, Su, Lai, Yao, Tsai, Liao, Lai and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Man-Chin Hua, bWVuY2hpbkBjZ21oLm9yZy50dw==; Jing-Long Huang, bG9uZ0BjZ21oLm9yZy50dw==
 Man-Chin Hua
Man-Chin Hua Hui-Min Su3
Hui-Min Su3