- 1Pediatrics Clinic, “Angelo Nocivelli” Institute of Molecular Medicine, Azienda Socio Sanitaria Territoriale Spedali Civili of Brescia, Brescia University, Brescia, Italy
- 2Flow Cytometry, Clinical Chemistry Laboratory, Azienda Socio Sanitaria Territoriale Spedali Civili of Brescia, Brescia, Italy
- 3Cystic Fibrosis Regional Support Center, Azienda Socio Sanitaria Territoriale Spedali Civili of Brescia, Brescia, Italy
Since the beginning of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, data registered in the European countries revealed increasing cases of infection in cystic fibrosis (CF) patients. In the course of this pandemic, we enrolled 17 CF patients for a study evaluating inflammatory markers. One of them developed COVID-19, giving us the possibility to analyze inflammatory markers in the acute phase as compared to levels detected before and after the infectious episode and to levels measured in the other CF patients enrolled to the study who did not experience COVID-19 and 23 patients referred to our center for SARS-CoV-2 infection.
Introduction
Since the beginning of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, data registered in the European countries revealed increasing cases of infection in cystic fibrosis (CF) patients with mild clinical manifestations of coronavirus disease 2019 (COVID-19) and usually favorable outcomes (1–4). This is surprising since due to their pulmonary impairment, CF patients are supposed to be considered subject to an increased risk of developing severe manifestations in case of SARS-CoV-2 infection. During the first wave of the SARS-CoV-2 pandemic (March to April 2020), we enrolled 17 CF subjects to evaluate the plasma levels of cytokines and chemokines. One of them developed COVID-19 during the study, giving us the opportunity to evaluate her immune and inflammatory response in the acute phase of SARS-CoV-2 infection.
Case Report
We report on a 14-year-old girl affected by CF with pancreatic insufficiency followed at the Cystic Fibrosis Regional Support Center of the Pediatrics Clinic, University of Brescia, ASST Spedali Civili di Brescia (Brescia, Italy). She was diagnosed in the first days of her life, presenting with meconium ileus requiring surgery and CF newborn screening positive, being homozygous for the c.1521_1523delCTT (p.F508del) CF-causing mutation of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) gene. During her follow-up, she developed chronic lung disease with bronchiectasis complicated by Pseudomonas aeruginosa intermittent respiratory infection. She was under treatment with pancreatic enzyme replacement therapy and multivitamin supplementation and received CFTR modulator therapy with lumacaftor/ivacaftor from July 2019. In March 2020, she was referred to our Emergency Department for persisting cough with purulent expectoration and dyspnea. At hospital admission, she presented good general conditions, apyretic (T 36.8°C) and tachypneic with oxygen desaturation (SpO2 93%). Physical examination revealed diffuse pulmonary crackles. Chest X-ray showed diffuse interlobular septal thickening with parenchymal infiltrates in the right hilus and the nasopharyngeal swab tested positive for SARS-CoV-2. The patient was hospitalized at the Pediatric COVID-19 Unit of the Pediatrics Clinic of Spedali Civili where she was treated with hydroxychloroquine 200 mg twice a day, according to our internal COVID-19 diagnostic and therapeutic protocol, broad-spectrum antibiotic therapy with cefotaxime, chest physiotherapy with positive expiratory pressure mask, and oxygen supplementation. Two days after the beginning of this therapy, the patient reported an improvement of pulmonary symptoms no longer requiring oxygen supplementation. During the hospitalization, she did not present fever or any other symptoms related to COVID-19. She was discharged after 10 days when two distinct nasopharyngeal swabs tested negative for SARS-CoV-2. After discharge, the patient continued the scheduled follow-up visits at the Cystic Fibrosis Regional Support Center. At the latest visit, she exhibited a FEV1 of 91% without presenting any complications related to the previous SARS-CoV-2 infection.
Inflammatory Marker Analysis
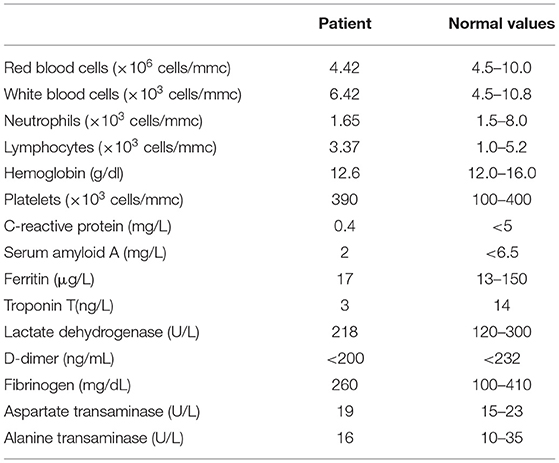
At her hospital admission, the patient underwent laboratory tests, including blood counts, biochemical markers of inflammation (Table 1), plasmatic levels of cytokines [interferon-α (IFN-α), interferon-γ (IFN-γ), IL-1β, IL-4, IL-5, IL-6, IL-10, IL-12/p70, IL-17A, and tumor necrosis factor-α (TNF-α)] and chemokines (CXCL10/IP10, CXCL9/MIG, CCL5/RANTES, CCL2/MCP-1, and CXCL8/IL-8) (Table 2), and assessment of lymphocyte subsets, after informed consent registration obtained from both her parents (Study SARS-CoV-2-PED - NP4047 approved by Ethical Committee Spedali Civili, Brescia). Cytokine and chemokine plasma dosage has been performed with assay on plasma isolated from whole blood of each patient collected in EDTA, immediately frozen at −80°C in various aliquots and defrosted at the time of the analysis.
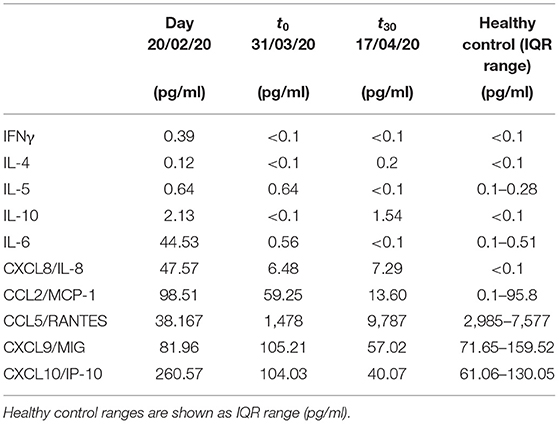
Table 2. Chemokine and cytokine levels of the CF patient were analyzed before SARS-CoV-2 infection, at the time of hospital admission (t0) and 30 days after discharge (t30).
Plasma levels of CXCL10 (IP-10), CXCL8 (IL-8), CXCL9 (MIG), CCL5 (RANTES), and CCL2 (MCP-1) have been analyzed with flow cytometric bead array method, using the Human Chemokine Kit (Becton Dickinson, San Jose CA), following the manufacturer's instructions. IL-1β, IL-2, IL4, IL-5, IL-6, IL-10, TNF-α, IL-17A, IFN,-α, and IFN-γ have been dosed by Flex set custom cytometric bead array technique (Becton Dickinson). Afterward, data have been acquired on BD FACSCanto II flow cytometer and analyzed by FCAP v3 software.
Cytokine and chemokine values were compared with the levels measured in 23 children/young adults affected by COVID-19 (Study NP4000 approved by Ethical Committee Spedali Civili, Brescia) and with the levels measured in 16 subjects with CF (Study NP3087 approved by Ethical Committee Spedali Civili, Brescia evaluating cytokines and chemokines in CF patients) who were not affected by SARS-CoV-2 infection (Figure 1). These patients were previously enrolled in an ongoing study aimed to define inflammatory response in patients with CF.
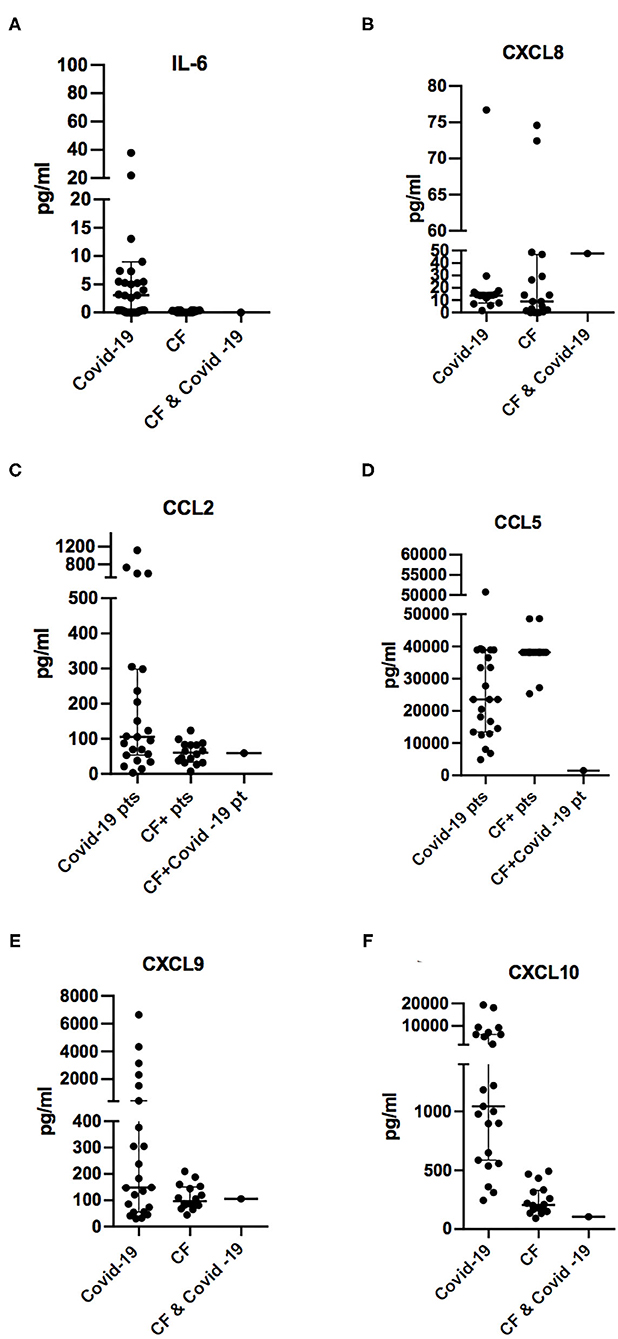
Figure 1. Comparison of plasmatic concentrations of chemokines and cytokines IL-6 (A), CXCL8/IL-8 (B), CCL2/MCP1 (C), CCL5/RANTES (D), CXCL9/MIG (E), and CXCL10/IP-10 (F) from otherwise healthy subjects during SARS-CoV-2 infection (COVID-19), cystic fibrosis patients not infected with SARS-CoV-2 (CF), and the 14-year-old CF patient in the acute phase of viral infections (CF and COVID-19). Concentration levels are reported in the groups as median ± IQR (pg/ml).
CF patients presented a median age of 18.3 years without ongoing exacerbations at the time of blood samples, which were collected from May 2019 to February 2020. Ten out of 16 CF patients were female, and 14 presented pancreatic insufficiency, including three with type 1 diabetes mellitus. The median value of FEV 1 in the cohort was 83.65%. Six of them presented chronic inflammation due to P. aeruginosa. The other two patients showed infection by methicillin-resistant Staphylococcus aureus (MRSA) and one by Achromobacter denitrificans. All patients at the time of blood collection did not display active lung disease and specific infectious events and were not receiving immunomodulatory treatment or corticosteroids. Three CF subjects were under treatment with lumacaftor/ivacaftor at the time of blood draw.
Children with COVID-19 included 23 subjects (average age of 6.7 years). All patients were tested positive for SARS-CoV-2 at nasopharyngeal swab and presented mild or moderate course of COVID-19, without evidence of previous chronic respiratory diseases. All of them showed fever at the onset of symptoms, while only four patients manifested gastrointestinal symptoms. The other four patients reported influenza-like symptoms (rhinitis and cough). Chest X-ray showed interstitial thickening in 14 out of 23 cases, but only two children needed respiratory support: one with high-flow nasal cannula (HFNC) for a few days, and the other one with free-flow oxygen via facemask. Blood samples for plasma cytokine analysis were collected at the time of hospitalization prior to any treatment.
Because the index patient had been enrolled to study NP3087 before the occurrence of the SARS-CoV-2 pandemic, we had the opportunity to compare IL-6, CXCL8, CCL2, CCL5, CXC9, and CXCL10 plasmatic levels with those measured about 1 month before the viral infection. Because there are no standardized reference values for cytokines and chemokines, we analyzed for comparison the levels of cytokines and chemokines measured in 10 healthy control subjects (mean age: 45 years) and reported as median ± IQR (pg/ml) in Table 1.
Lymphocyte subpopulations, which were analyzed about 1 month before SARS-CoV-2 infection, at the time of acute infection, and 1 month after recovery, did not reveal significant changes of lymphocyte subsets, CD4+/CD8+ ratio, number of activated T cells, distribution of naïve, central memory, effector memory, and terminally differentiated CD4+ and CD8+ cells (data not shown). This is consistent with reports showing that children with SARS-CoV-2 infection do not display an increase of activation markers of T cell in the same extent observed in adults with COVID-19 (5).
Comments
Since December 2019, a global effort is in progress in order to restrain the COVID-19 pandemic and identify the main pathophysiologic patterns of a disease that can drive severe complications. SARS-CoV-2 infection presents a broad spectrum of symptoms, ranging from influenza-like manifestations to severe progression toward pneumonia with acute respiratory distress syndrome, septic shock, cytokine storm syndrome, and coagulation dysfunction (6). CF (OMIM #219700) is a systemic disease caused by homozygous or compound heterozygous mutations in the CF transmembrane conductance regulator gene (OMIM *602421) on chromosome 7q31 (7). Described as a triad of chronic obstructive pulmonary disease, exocrine pancreatic insufficiency, and elevated chloride concentration in sweat, the clinical phenotype can be heterogeneous, ranging from mild to severe forms of multi-organ disorders (8).
Reduced performance of CFTR channels leads to a pathogenic reduction in chloride and bicarbonate secretion and in the volume and pH of the airway surface liquid with subsequent abnormal mucous secretion and decreased mucociliary clearance (9). In addition, altered CFTR function affects the contribution of the epithelium to innate immunity through endogenous activation of nuclear factor κ light-chain enhancer of activated B cells (NF-κB) with subsequent anti-apoptotic and proinflammatory signaling by increasing expressions of IL-8 and granulocyte monocyte colony-stimulating factor (GM-CSF) (10, 11). Moreover, the surface expression of toll-like receptor-4 (TLR4) of epithelial cells in CF patients is reduced, resulting in decreased type I IFN regulation and proinflammatory signaling even in the absence of infectious trigger as well as exaggerated responses to microbial antigens (10–12).
The T-cell function appears to be also affected by defects in CFTR, and several studies have described reduced recruitment by CF epithelial cells of T cells in response to S. aureus, as well as altered T-cell responses to Aspergillus fumigatus in both human and murine models (10, 13, 14).
Recent studies of the host's immune response in SARS-CoV-2 suggest that early steps of the adaptive immune activity are connected with the formation of antigen-specific cytotoxic T cells and the synthesis of neutralizing antibodies (15–18). Although severe SARS-CoV-2 infection is characterized by extensive involvement of the adaptive immune system, the role of dysregulated innate inflammatory cytokine and chemokine responses is still to be confirmed as a contribution to acute COVID-19 severity (19). It has been reported that COVID-19 patients with severe outcome display higher plasma chemokines CXCL8/IL-8, CXCL9/MIG, and CXCL10/IP10, and cytokines IL-6 and IL-10 than patients with the mild disease (20), while children, who usually have a favorable outcome of SARS-CoV-2 infection, present lower levels of T-cell activation and pro-inflammatory cytokines (5). In particular, an increased level of proinflammatory cytokines, such as IL-2R, IL-6, IL-10, and TNF-α, has been associated with extensive lung damage and severe illness (21, 22).
To our knowledge, this is the first preliminary study that reports on the immunological characteristics of a patient affected by CF with laboratory-confirmed SARS-CoV-2 infection. The index patient presented with symptoms such as fever, cough, fatigue, and myalgia, which are reported to be the most common at the onset of the disease (23). Studies about laboratory markers of inflammation in COVID-19 disease support the evidence that leukocytosis (≥10 ×109/L) and lymphopenia (<0.8 ×109/L) were associated with an increase in blood concentrations of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), lactate dehydrogenase (LDH), serum C-reactive protein (CRP), ferritin, and D-dimer, which are markedly higher in severe cases than in moderate ones (23, 24). This is consistent with our findings: the patients presented a mild course of infection, without any severe complication and any remarkable variations of acute-phase reactants and serum biochemistry values with subsequent rapid healing (Table 1). Case reports of CF patients with SARS-CoV-2 infection are increasingly described, and even though the CF population infected differs for age, sex, and variable pulmonary impairment with a wide range of complications and chronic respiratory infections, recent reports describe that the course of COVID-19 disease in CF appears to be milder than expected and less severe than casuistry of patients with other underlying lung diseases (9, 25, 26).
While most of the COVID-19 patients referred to our unit showed high levels of the proinflammatory cytokines and chemokines IL-6, CCL2, CCL5, CXC9, and CXCL10, low levels of these cytokines were detected in the blood of the 14-year-old CF patient during the SARS-CoV-2 infection.
In contrast, the neutrophil chemoattractant CXCL8/IL-8 was detected in the blood of CF patients at a slightly elevated level at the concentration range measured in CF patients without SARS-CoV-2 infection (Figure 1). This is consistent with the high levels of this chemokine, which are often detected in the blood of CF patients, especially in those with P. aeruginosa infection (27–29).
In the CF patient with COVID-19, we detected in the acute phase a lower concentration of CCL5/RANTES in comparison to chemokine levels measured in the same subject before the infection and in other CF patients not affected by SARS-CoV-2 (Figure 1 and Table 2). CCL5/RANTES is a chemokine produced by CD8+ T cells, epithelial cells, fibroblasts, and platelets, which plays a key role in the immune response to respiratory viral infections. Lower expression of CCL5/RANTES during SARS-CoV-2 infection in the CF patient might be related to the low levels of other proinflammatory cytokines or to a possible immunoregulatory role of CFTR channels on the host antiviral response.
Low blood concentrations of CXCL9/MIG and CXCL10/IP-10 might be a consequence of the low levels of T cell activation because the two chemokines are induced by IFN-γ, which is expressed by activated T lymphocytes, especially Type 1 helper (Th1) cells and natural killer (NK) cells during the immune response. Therefore, low levels of CXCL9 and CXCL10 in the CF patient affected by COVID-19 suggest that the antiviral response was not associated with Th1 polarization, which has been associated with an unfavorable outcome of COVID-19 in adult subjects (20).
Interestingly, in the index patient, the main inflammatory markers reported to be upregulated in SARS-CoV-2 infection (21, 30) appear to be even lower than the average measured in the CF patient without COVID-19 infection. This is surprising considering that in CF, the respiratory tracts are more susceptible to upregulated inflammation, particularly when triggered by new infectious agents (9, 31). In addition, because the CF patient was receiving treatment with lumacaftor/ivacaftor during COVID-19 episode, we speculate that treatment with CFTR modulators did not have a detrimental effect on the outcome of the disease and raises the question of the potential role of these drugs on regulating immune response or angiotensin-converting enzyme 2 (ACE2) expression. Although further studies are needed to answer these relevant questions, it is notable that levels of cytokines and chemokines in the other CF patients treated with lumacaftor/ivacaftor were in the same range as those in untreated CF patients.
A variety of variables have been considered as protective factors in CF patients affected with SARS-CoV-2 infection, such as the long-term use of antibiotics with anti-inflammatory or antiviral effects like azithromycin (32, 33). Moreover, it has been postulated that mutations in the CFTR gene may alter the protein expression of ACE2 through upregulation of vascular endothelial growth factor A (VEFGA) in such a way to mitigate the effects of infection on lung damage (9, 34). Furthermore, in vitro experiments on CF bronchial epithelial lines (CFBEs) showed no evidence that mutations in CFTR induce a hyperinflammatory response in CF epithelial cells (35), suggesting that other unknown regulatory mechanisms could be involved in the regulation of inflammation in CF cell lines. It has been presumed that epigenetic phenomena, such as DNA methylation or histone tail modification profiles, could modulate the gene expression and that could be cell-line specific, independent from F508 mutation (34).
Conclusion
In conclusion, our report has revealed in a young CF patient with acute COVID-19 lower activation of the immune response as measured by levels of cytokines or chemokines such as IL-6, CCL2, CCL5, CXCL8, CXCL9, and CXCL10 than otherwise healthy pediatric patients, referred to our center for COVID-19. Interestingly, IL-6, CXCL8, CCL2, CCL5, and CXCL10 plasmatic concentrations were lower during the COVID-19 infectious episode as compared to levels measured 1 month earlier. These cytokines persisted low when re-evaluated after 1 month, suggesting that immune response against SARS-CoV-2 infection was not associated with increased inflammatory response.
Despite being a single case report, our experience suggests the possibility that SARS-CoV-2-related inflammatory disorders may express different clinical phenotypes depending on the pre-existing inflammatory and immune conditions.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by NP3087, NP4000, NP4047. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author Contributions
GB and RB: conceptualization and writing—original draft preparation. GB, MG, DM, MC, FC, IC, RP, PP, ST, and RB: data curation. GB, RP, PP, ST, and RB: writing—review and editing. RB: supervision, project administration, and funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from Fondazione Cariplo in collaboration with Regione Lombardia and Fondazione Umberto Veronesi (Rif. 2020-1355 and 2020-1376).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the patients, the patients' families, and the nurses for all their efforts.
References
1. Introduction | European Cystic Fibrosis Society (ECFS). Available at: https://www.ecfs.eu/ecfspr (accessed October 2, 2020).
2. McClenaghan E, Cosgriff R, Brownlee K, Ahern S, Burgel P-R, Byrnes CA, et al. The global impact of SARS-CoV-2 in 181 people with cystic fibrosis. J Cyst Fibros. (2020) 19:868–71. doi: 10.1016/j.jcf.2020.10.003
3. Poli P, Timpano S, Goffredo M, Padoan R, Badolato R. Asymptomatic case of Covid-19 in an infant with cystic fibrosis. J Cyst Fibros. (2020) 19:e18. doi: 10.1016/j.jcf.2020.03.017
4. Pecho-Silva S, Navarro-Solsol AC, Panduro-Correa V, Arteaga-Livias K, Rodriguez-Morales AJ, Dhama K. COVID-19 and cystic fibrosis: diagnostic difficulties or incorrect methods? J Pure Appl Microbiol. (2020) 14:1681–5. doi: 10.22207/JPAM.14.3.06
5. Moratto D, Giacomelli M, Chiarini M, Savarè L, Saccani B, Motta M, et al. Immune response in children with COVID-19 is characterized by lower levels of T cell activation than infected adults. Eur J Immunol. (2020) 50:1412–14. doi: 10.1002/eji.202048724
6. Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. (2020) 382:1177–79. doi: 10.1056/NEJMc2001737
7. Wang Y, Wrennall JA, Cai Z, Li H, Sheppard DN. Understanding how cystic fibrosis mutations disrupt CFTR function: from single molecules to animal models. Int J Biochem Cell Biol. (2014) 52:47–57. doi: 10.1016/j.biocel.2014.04.001
8. Cutting GR. Cystic fibrosis genetics: from molecular understanding to clinical application. Nat Rev Genet. (2015) 16:45–56. doi: 10.1038/nrg3849
9. Fainardi V, Longo F, Chetta A, Esposito S, Pisi G. Sars-CoV-2 infection in patients with cystic fibrosis. An overview. Acta Biomed. (2020) 91:e2020035. doi: 10.23750/abm.v91i3.10391
10. Cohen TS, Prince A. Cystic fibrosis: a mucosal immunodeficiency syndrome. Nat Med. (2012) 18:509–19. doi: 10.1038/nm.2715
12. Bruscia EM, Bonfield TL. Cystic fibrosis lung immunity: the role of the macrophage. J Innate Immun. (2016) 8:550–63. doi: 10.1159/000446825
13. Kreindler JL, Steele C, Nguyen N, Chan YR, Pilewski JM, Alcorn JF, et al. Vitamin D3 attenuates Th2 responses to Aspergillus fumigatus mounted by CD4+ T cells from cystic fibrosis patients with allergic bronchopulmonary aspergillosis. J Clin Invest. (2010) 120:3242–54. doi: 10.1172/JCI42388
14. Mueller C, Braag SA, Keeler A, Hodges C, Drumm M, Flotte TR. Lack of cystic fibrosis transmembrane conductance regulator in CD3+ lymphocytes leads to aberrant cytokine secretion and hyperinflammatory adaptive immune responses. Am J Respir Cell Mol Biol. (2011) 44:922–9. doi: 10.1165/rcmb.2010-0224OC
15. Azkur AK, Akdis M, Azkur D, Sokolowska M, van de Veen W, Brüggen MC, et al. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy Eur J Allergy Clin Immunol. (2020) 75:1564–81. doi: 10.1111/all.14364
16. Zheng M, Gao Y, Wang G, Song G, Liu S, Sun D, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. (2020) 17:533–5. doi: 10.1038/s41423-020-0402-2
17. Mehul Suthar AS, Zimmerman MG, Kauffman RC, Ahmed R, Roback JD, Wrammert J, et al. Rapid generation of neutralizing antibody responses in COVID-19 patients. Cell Rep Med. (2020) 1:100040. doi: 10.1101/2020.05.03.20084442
18. Stephens DS, McElrath MJ. COVID-19 and the path to immunity. JAMA. (2020) 324:1279–81. doi: 10.1001/jama.2020.16656
19. Sinha P, Matthay M, Calfee C. Is a “cytokine storm” relevant to COVID-19? JAMA Intern Med. (2020) 180:1152–54. doi: 10.1001/jamainternmed.2020.3313
20. Tincati C, Cannizzo ES, Giacomelli M, Badolato R, d'Arminio Monforte A, Marchetti G. Heightened circulating interferon-inducible chemokines, and activated pro-cytolytic th1-cell phenotype features Covid-19 aggravation in the second week of illness. Front Immunol. (2020) 11:580987. doi: 10.3389/fimmu.2020.580987
21. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497. doi: 10.1145/3366423
22. Angioni R, Sánchez-Rodríguez R, Munari F, Bertoldi N, Arcidiacono D, Cavinato S, et al. Age-severity matched cytokine profiling reveals specific signatures in Covid-19 patients. Cell Death Dis. (2020) 11:957. doi: 10.1038/s41419-020-03151-z
23. Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. (2020) 130:2620–9. doi: 10.1172/JCI137244
24. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
25. Cosgriff R, Ahern S, Bell SC, Brownlee K, Burgel PR, Byrnes C, et al. A multinational report to characterise SARS-CoV-2 infection in people with cystic fibrosis. J Cyst Fibros. (2020) 19:355–8. doi: 10.1016/j.jcf.2020.04.012
26. Blanchon S, Fernandez C, Guerin S, Crisinel PA, Rochat I. COVID-19: a message of hope from a young girl with severe cystic fibrosis. Pediatr Pulmonol. (2020) 55:1546–7. doi: 10.1002/ppul.24812
27. Rimessi A, Bezzerri V, Salvatori F, Tamanini A, Nigro F, Dechecchi MC, et al. PLCB3 loss of function reduces Pseudomonas aeruginosa-dependent IL-8 release in cystic fibrosis. Am J Respir Cell Mol Biol. (2018) 59:428–36. doi: 10.1165/rcmb.2017-0267OC
28. McElvaney OJ, McElvaney NG. Targeting IL-8 in cystic fibrosis: enough but not too much. Am J Respir Cell Mol Biol. (2018) 59:401–2. doi: 10.1165/rcmb.2018-0145ED
29. Sznurkowska K, Kazmierska K, Sledziński T, Zagierski M, Liberek A, Szlagatys-Sidorkiewicz A. Serum chemerin level, cytokine profile and nutritional status in children with cystic fibrosis. Acta Biochim Pol. (2019) 66:445–9. doi: 10.18388/abp.2019_2858
30. Chu H, Chan JFW, Wang Y, Yuen TTT, Chai Y, Hou Y, et al. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19. Clin Infect Dis. (2020) 71:1400–9. doi: 10.1093/cid/ciaa410
31. Stenbit AE, Flume PA. Pulmonary exacerbations in cystic fibrosis. Curr Opin Pulm Med. (2011) 17:442–7. doi: 10.1097/MCP.0b013e32834b8c04
32. Menzel M, Akbarshahi H, Bjermer L, Uller L. Azithromycin induces anti-viral effects in cultured bronchial epithelial cells from COPD patients. Sci Rep. (2016) 6:28698. doi: 10.1038/srep28698
33. Cigana C, Nicolis E, Pasetto M, Assael BM, Melotti P. Anti-inflammatory effects of azithromycin in cystic fibrosis airway epithelial cells. Biochem Biophys Res Commun. (2006) 350:977–82. doi: 10.1016/j.bbrc.2006.09.132
34. Voisin G, Bouvet GF, Legendre P, Dagenais A, Massé C, Berthiaume Y. Oxidative stress modulates the expression of genes involved in cell survival in ΔF508 cystic fibrosis airway epithelial cells. Physiol Genomics. (2014) 46:634–46. doi: 10.1152/physiolgenomics.00003.2014
Keywords: SARS-CoV-2 infection, T cell activation, inflammatory activation, cystic fibrosis, cytokines
Citation: Baresi G, Giacomelli M, Moratto D, Chiarini M, Conforti IC, Padoan R, Poli P, Timpano S, Caldarale F and Badolato R (2021) Case Report: Analysis of Inflammatory Cytokines IL-6, CCL2/MCP1, CCL5/RANTES, CXCL9/MIG, and CXCL10/IP10 in a Cystic Fibrosis Patient Cohort During the First Wave of the COVID-19 Pandemic. Front. Pediatr. 9:645063. doi: 10.3389/fped.2021.645063
Received: 22 December 2020; Accepted: 17 May 2021;
Published: 06 July 2021.
Edited by:
Marzia Duse, Sapienza University of Rome, ItalyReviewed by:
Silvia Ricci, Università di Firenze, ItalyAlfonso J. Rodriguez-Morales, Fundacion Universitaria Autónoma de las Américas, Colombia
Copyright © 2021 Baresi, Giacomelli, Moratto, Chiarini, Conforti, Padoan, Poli, Timpano, Caldarale and Badolato. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesca Caldarale, ZnJhbmNlc2NhLmNhbGRhcmFsZUBnbWFpbC5jb20=
 Giulia Baresi
Giulia Baresi Mauro Giacomelli
Mauro Giacomelli Daniele Moratto2
Daniele Moratto2 Rita Padoan
Rita Padoan Piercarlo Poli
Piercarlo Poli Francesca Caldarale
Francesca Caldarale Raffaele Badolato
Raffaele Badolato