- 1Wellcome Trust-Medical Research Council Institute of Metabolic Science, University of Cambridge, Cambridge, United Kingdom
- 2Department of Paediatrics, University of Cambridge, Cambridge, United Kingdom
Recent advances in diabetes technology have led to the development of closed-loop insulin delivery systems for the management of type 1 diabetes. Several such systems are now commercially available for children and young people. While all available systems have been shown to improve glycaemic control and quality of life in this population, qualitative data also highlights the challenges in using closed-loop systems, which vary among different pediatric age-groups. Very young children require systems that are able to cope with low insulin doses and significant glycaemic variability due to their high insulin sensitivity and unpredictable eating and exercise patterns. Adolescents' compliance is often related to size and number of devices, usability of the systems, need for calibrations, and their ability to interact with the system. Given the speed of innovations, understanding the capabilities and key similarities and differences of current systems can be challenging for healthcare professionals, caregivers and young people with type 1 diabetes alike. The aim of this review is to summarize the key evidence on currently available closed-loop systems for children and young people with type 1 diabetes, as well as commenting on user experience, where real-world data are available. We present findings on a system-basis, as well as identifying specific challenges in different pediatric age-groups and commenting on how current systems might address these. Finally, we identify areas for future research with regards to closed-loop technology tailored for pediatric use and how these might inform reimbursement and alleviate disease burden.
Type 1 diabetes is a lifelong, incurable condition characterized by a deficiency of insulin caused by immune-mediated destruction of pancreatic beta-cells in genetically predisposed individuals (1). Its incidence in the pediatric population is increasing by around 3% per year (2), and more than one million children and young people under the age of 20 years are living with the condition worldwide (3). Tight glycaemic control is challenging to achieve, and the majority of children and young people with type 1 diabetes do not meet treatment guidelines for target glycated hemoglobin (HbA1c) (4–7), or only achieve good glycaemic control at the expense of high management burden (8–10). Meeting glycaemic targets is vital, as higher HbA1c levels are associated with an increased risk of premature morbidity and mortality (11–13).
Over the past decades, several new technologies have been developed to improve management of type 1 diabetes, including insulin pumps and real-time continuous glucose monitoring (CGM) devices (14). However, rather than improving, HbA1c levels have worsened in the pediatric age group over the last 10 years (5). While insulin pumps and CGM devices have been shown to lower HbA1c levels and reduce the risk of diabetic ketoacidosis (15–17), they require significant user-input and frequent insulin dosing adjustments to achieve good glycaemic control (10). In recent years, the development of closed-loop systems, which link insulin delivery to sensor glucose levels, have started to transform management of type 1 diabetes (18, 19). These closed-loop systems utilize an algorithm that automatically adjusts insulin delivery via an insulin pump based on real-time sensor glucose levels. This glucose-responsive automated insulin delivery more closely replicates normal physiology. Current hybrid closed-loop systems continue to require user-initiated prandial insulin boluses.
This review summarizes key evidence on current closed-loop systems for children and young people with type 1 diabetes, as well as commenting on user experience and specific challenges in different pediatric age-groups.
Closed-Loop Systems
Four hybrid closed-loop systems are currently commercially available and licensed for use in children and young people, with varying minimum age for use. These systems are: 670G hybrid closed-loop (HCL) system and 780G advanced HCL (AHCL) (Medtronic, Northridge, California); CamAPS FX interoperable app (CamDiab, Cambridge, UK); and the Control IQ system (Tandem Inc., San Diego, California). Further hybrid closed-loop systems are in development, with pivotal trials under way (20). Table 1 summarizes the key clinical trial evidence of commercialized systems in children and young people.
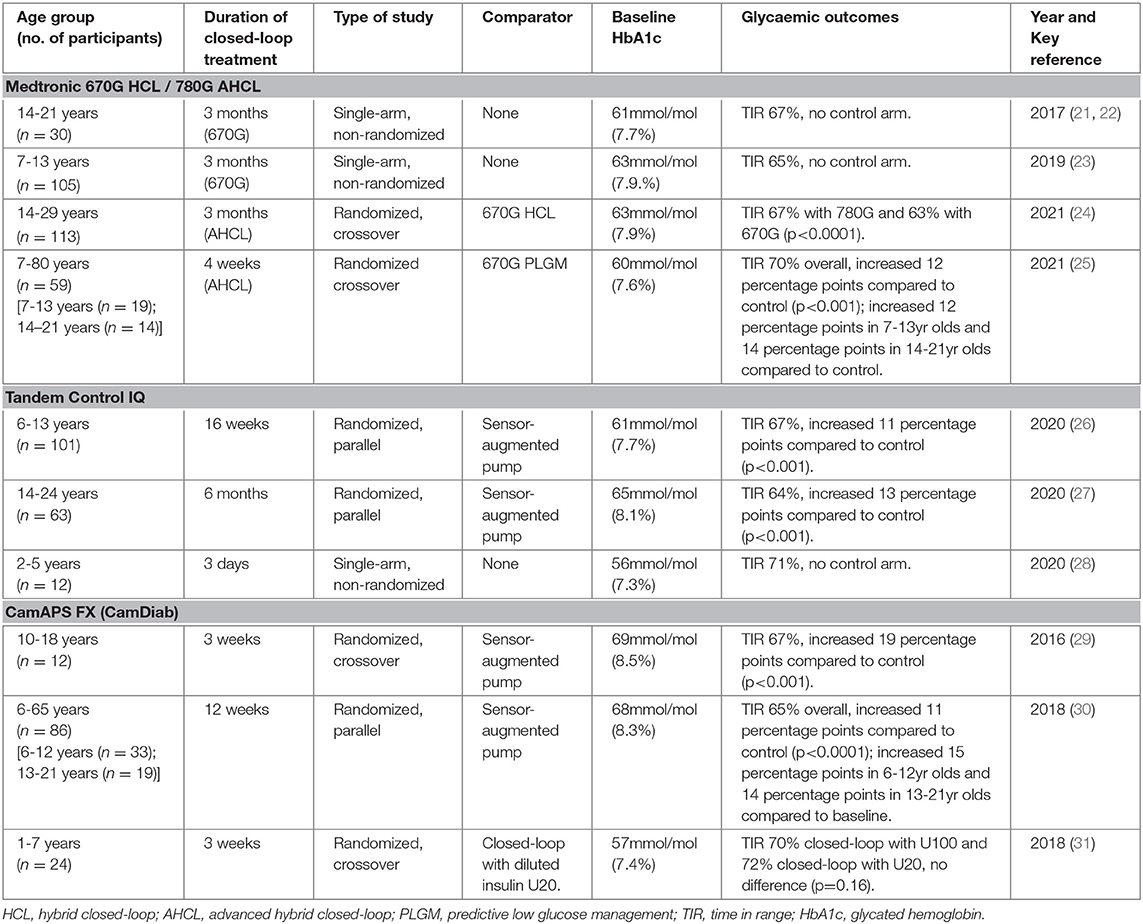
Table 1. Key clinical trials of closed-loop insulin delivery in children and young people using commercialized systems.
The closed-loop algorithm is embedded in the software of the tethered insulin pump and communicates wirelessly with the compatible glucose sensor for the Medtronic and Tandem systems (14). For CamAPS FX, the algorithm is embedded in an app, classed as a medical device, residing on an unlocked smartphone that communicates wirelessly with a compatible insulin pump and glucose sensor (14). As more hybrid closed-loop systems become commercially available, it is becoming increasingly complex for people with type 1 diabetes, their families and healthcare professionals to navigate different technologies. While similar in basic principle, there are important differences between each hybrid closed-loop system, and clinicians need to understand key device characteristics in order to provide appropriate clinical support and guidance to children, young people and their families. Several available closed-loop systems now provide online training and education modules, both for healthcare professionals and users, and increasingly module completion is a requirement to allow initiation of closed-loop. Table 2 outlines the capabilities and key similarities and differences of current systems using the CARES paradigm, an educational tool developed by Messer et al. (32), that provides a practical framework to identify key concepts for each closed-loop system. Understanding these key concepts will allow healthcare professionals to set appropriate expectations of hybrid closed-loop system capabilities, and to adjust settings for treatment optimisation to maximize the benefits of this novel therapy.

Table 2. Comparison of commercially available closed-loop systems using the CARES paradigm (32).
Clinical trials show that hybrid closed-loop insulin delivery is safe and improves glycaemic outcomes in children of all ages (10, 33), but high quality real-world data remains scarce, particularly for newer systems licensed in the last 12–18 months. Considering the evidence available in terms of system capabilities, qualitative research and observational real-world data, we discuss age-specific challenges in children and young people and how closed-loop systems might address these.
Toddlers and Young Children
Type 1 diabetes is challenging to manage at any age, but management is further complicated in young children under the age of 7 by a variety of unique physiological, behavioral and developmental factors. Young children have higher variability in insulin requirements and higher insulin sensitivity than older children and adults (34), as well as more unpredictable eating and activity patterns. Furthermore, hypoglycaemia is frequently asymptomatic and can be prolonged, particularly at night-time (35, 36). These factors and resulting parental worry lead to high management burden for parents and caregivers with negative impact on family quality of life (9).
Closed-loop studies in this age-group have been of short duration in small cohorts (Table 1). One of the main benefits of hybrid closed-loop insulin delivery in young children is improved glycaemic control at night-time. In a 3-week closed-loop study in 24 very young children, time in the target range 3.9–10.0 mmol/L was highest overnight with reduction in hypoglycaemia compared to daytime (31) (Table 1). Variability in insulin requirements in young children is highest overnight (34), and closed-loop systems are uniquely positioned to address this by delivering insulin in a glucose-responsive manner. Qualitative data shows that parents noted improvements in quality of sleep with closed-loop, both for themselves and their child (37, 38).
Improvements in glycaemic control are less marked in the daytime, which is likely related to unpredictable eating and activity patterns. Bolus timing is challenging in this age group, as children frequently graze or do not complete meals. Many parents choose to bolus with or shortly after the start of the meal, leading to post-prandial hyperglycaemia (39, 40). The pharmacokinetics and pharmacodynamics of current rapid-acting insulins limit the closed-loop system's ability to mitigate immediate post-prandial hyperglycaemia (41), and the resultant increase in algorithm-driven insulin delivery increases the risk of delayed hypoglycaemia. New ultra-rapid acting insulins, which have faster onset and offset of action, have the potential to address this issue, but these have not been trialed in closed-loop systems in the pediatric age-group.
Another limitation of current closed-loop systems is the minimum total daily insulin dose required for optimal system performance. While this does not preclude use in those young children with a very low total daily dose, it can limit the benefit of closed-loop therapy due to the high variability of absorption with such small volumes (42). A randomized trial comparing closed-loop insulin delivery using diluted and standard strength insulin showed no difference in glycaemic outcomes between the two groups (31). However, only a small number of participants had a total daily insulin dose of <10 units in this cohort. Previous shorter closed-loop studies using diluted insulin in this age group showed reduced inter-individual variability in time to peak insulin action with diluted insulin (42), suggesting that insulin dilution may be beneficial on a case-by-case basis in those with a very low total daily insulin dose.
In spite of these limitations, qualitative studies reported parents spending less time performing diabetes-related activities and feeling less stressed when their child was using closed-loop, resulting in reduced management burden overall (37, 38).
There is limited real-world closed-loop data available for very young children. This is in part due to the fact that closed-loop insulin delivery is only licensed for one commercialized system (CamAPS FX) in this age group, with other systems being used off-license in some centers. A retrospective case series of the 670G HCL system in 16 children under the age of 7 years showed improvements in glycaemic control compared to baseline (43). There was an increase in time in hypoglycaemia, however this was still low at 2.4% (43). Importantly, the results of further clinical closed-loop trials in very young children are expected to be reported in the near future, and should result in licensing of a wider variety of systems in this age group.
Children
There is significantly more evidence of closed-loop safety and efficacy in school-aged children, compared to those below the age of 7 years. Studies of longer duration in larger cohorts show significant improvements in glycaemic control (26, 30), with no difference in time in hypoglycaemia (Table 1).
Despite their young age, school-aged children often independently manage their diabetes to a significant extent (10). This is in part due to a high turnover of caregiving adults, whose diabetes management knowledge is often minimal (44). This leads parents and children to tolerate higher glucose levels to avoid hypoglycaemia (10, 45), and may limit children's ability to participate in certain activities or events without parental supervision.
Closed-loop systems address this issue in two ways. The automation of insulin delivery in response to real-time sensor glucose levels reduces the need for user input, and events such as post-prandial hyperglycaemia or exercise-induced hypoglycaemia may be prevented or attenuated by the closed-loop system itself. This system-innate responsiveness has the potential to give children more freedom in their activities by increasing parents' confidence in the child's safety. In a qualitative study interviewing parents of children using a closed-loop system, they reported being more willing to allow their child to participate in activities such as school trips or sleepovers than before (21). Secondly, the remote monitoring capabilities of some closed-loop systems give reassurance to parents and children, by allowing parents to adopt a watchful waiting approach, and to intervene and support their child's decision making if required (46). Both parents and children reported closed-loop insulin delivery improving their quality of life and reducing diabetes management burden (21, 38).
While clinical trial evidence shows significant benefits with closed-loop insulin delivery in terms of glycaemic control, parents noted the importance of trusting the closed-loop system for optimum benefit (21). They noted that an initial adjustment period was required, during which they realized that taking action to address low or high glucose levels could be counter-productive to the system's ability to manage glucose levels (21). Additional education around minimizing interventions when using closed-loop insulin delivery could be beneficial when commencing this therapy.
Similar to younger children, real-world closed-loop data is limited. All commercially available closed-loop systems, apart from the 670G HCL system, were only licensed for children in the last 12–18 months. A prospective observational study of people aged 9–61 years using the 670G HCL system for 1 year found that closed-loop use declined significantly over time with a high proportion of closed-loop discontinuation (47). Children and adolescents were more likely to discontinue closed-loop. The main reasons for discontinuation were frequent sensor calibration requirements and a high number of closed-loop exits (47). Another prospective observational study of youth aged 2–25 years using the 670G HCL system showed similar results, with a steady decline of closed-loop use over time (48). Newer generation systems, such as Control IQ and CamAPS FX, using a factory-calibrated sensor alleviate a key reason for closed-loop discontinuation. While the 780G AHCL system still requires sensor calibration, clinical trials show a significant reduction in closed-loop exits (24), suggesting improved usability in this newer iteration.
Young People
HbA1c levels are highest in young people aged 13–17 years (5). Diabetes self-management is particularly challenging in this age group due to a variety of factors, including peer group influences, importance of body image, less parental oversight, greater risk-taking, and fear of hypoglycaemia, leading to higher levels of diabetes distress (49, 50). Closed-loop insulin delivery offers a novel way to address these issues, although important considerations remain with regards to choice of system for individual users.
Clinical studies have shown that closed-loop insulin delivery significantly improves glycaemic control in this age-group (25, 51), including in those with sub-optimal glycaemic control (30), and that improvements are sustained over time (27, 52). Importantly, qualitative studies of young people using closed-loop and their parents have reported significant improvements in quality of life and reduced diabetes management burden (21, 46, 49, 50).
Fitting in with peers and taking part in normal activities is very important to young people (50), which can lead them to neglect diabetes self-management tasks such as finger prick blood glucose checks and pre-meal bolusing (53). Glucose sensors reduce burden and allow young people to discreetly check glucose levels, as well as allowing glucose-responsive insulin delivery in closed-loop. While sensors requiring calibration can be a significant factor in low closed-loop usage (38, 48, 54), several systems now use factory-calibrated glucose sensors, with high sensor wear reported in clinical trials (52) and reduced device-burden reported in qualitative studies (49). In a qualitative study of young people using closed-loop from onset of diabetes, participants reported that the closed-loop system had helped them continue to lead normal lives despite having diabetes (49) by alleviating the need for disruptive finger pricking and automatically adjusting insulin delivery in response to high or low glucose levels (50, 55).
Current closed-loop insulin delivery systems are all hybrid systems, which require user-initiated prandial boluses for optimal efficacy. However, studies have shown that systems have the ability to cope with missed boluses, while still providing improvements in short-term glycaemic control without an increase in hypoglycaemia (53). Data from a recent 6-month closed-loop study using Control IQ in young people aged 14–24 years showed sustained glycaemic improvements (27), suggesting that closed-loop remains efficacious in a group where there is higher likelihood of sub-optimal compliance (53).
Common barriers to closed-loop insulin delivery in this age group are device burden and alarm frequency (49). Young people preferentially wear devices in non-visible locations (21) and at times avoid activities where devices may be visible to others, such as swimming (49). A system with remote data viewing and bolusing capability via a mobile phone was positively received by young people, as this offered maximum discretion in peer environments (50) while enabling them to make management decisions (21, 49). Audible alarms can negatively impact quality of life, and in a qualitative study of closed-loop in this age group parents reported some young people opting to disconnect from the system when socializing with peers to limit alarms sounding in public (50). Most systems now feature personalisable alarm settings and healthcare providers should support young people in choosing settings that minimize interruptions while providing an adequate safety net.
Real-world data of the first commercially available hybrid closed-loop system, the 670G HCL system, showed high rates of closed-loop discontinuation, due to frequent sensor calibration requirements and a high number of closed-loop exits (47, 48). As described above, newer generation systems have shown much higher closed-loop use and fewer closed-loop exits in clinical trials (24, 27). A recently published real-world study assessed glycaemic control and quality of life by administering questionnaires to more than 1,000 Control-IQ users aged 14 years and over (21). Users reported a positive impact on their quality of life and sleep quality over a 2-month period after starting closed-loop therapy. Minimizing device and alarm burden and enabling easy and discreet user interaction, while maintaining glycaemic benefits should be the main goals of further closed-loop system developments in this age group.
Future Research
Across all pediatric age groups device burden and connectivity problems remain an issue with regards to closed-loop insulin delivery (49, 50, 55). System-integration with factory-calibrated sensors is paramount to ensure high and sustained closed-loop usage. Connectivity issues resulting in closed-loop exits need to be improved, for example by increasing allowable distance between devices or integrating the algorithm with smart devices, such as watches. Remote data viewing and bolusing capabilities are highly valued by parents of young children, as this minimizes disturbance during sleep or play, and also by young people, where it allows discreet interaction with the closed-loop system, and this should become standard to all commercial closed-loop systems (21). Furthermore, automatic cloud storage and data sharing facilitates interaction with healthcare providers and improves remote consultations, reducing burden (56). Currently, most systems are limited to one pump and CGM model, with specific devices having user-dependent pros and cons. Inter-operable systems, where users can mix and match devices that suit their individual needs, should be the focus of future developments.
The majority of systems are not licensed for very young children, and there is lack of clinical trial evidence with regards to efficacy and safety over longer periods. Clinical guidance is required for those whose total daily insulin dose is below the required threshold for closed-loop operation. Particularly for young people, new faster insulins could provide an increasingly realistic pathway to a more fully closed-loop system, where accurate carbohydrate counting and prandial bolusing is no longer required. Closed-loop studies in the pediatric age-group with ultra-rapid acting insulins are required to assess feasibility and safety.
Due to the novelty of closed-loop insulin delivery technology there is little real-world evidence to guide clinicians and users. Longer-term real-world studies are required to assess whether glycaemic and quality of life benefits are sustained long-term and what features are most desired by users to improve ease-of-use. This will inform system reimbursement, facilitating wider access across the diabetes population. Furthermore, several systems now incorporate personalisable glucose targets, as well as user-initiated modes that reduce or intensify insulin delivery. While users have expressed a wish for more collaboration with closed-loop systems in qualitative studies (49), safety and efficacy of these features needs to be evaluated to help optimize their use.
One of the most important issues facing healthcare providers and the diabetes community is access to closed-loop therapy. Insurance coverage of closed-loop therapy is currently poorly established, and the high cost of CGM and insulin pumps is a significant barrier to uptake for those who cannot afford to self-fund the technology (57). This may lead to growing disparities in those from lower socio-economic backgrounds (57). Future research needs to incorporate robust health economic analysis and should aim to show long-term cost-effectiveness to aid reimbursement for closed-loop therapy.
Conclusion
Closed-loop insulin delivery improves glycaemic control in all pediatric age groups, while crucially reducing the high management burden associated with this chronic disease, thus improving quality of life for the whole family. Children, young people, and their families now have a variety of commercially available closed-loop systems to choose from, with further systems in development. Future research should focus on improving systems to further reduce diabetes management burden and optimize efficacy, ultimately informing system reimbursement and facilitating wider access across the diabetes population.
Author Contributions
JF wrote the manuscript. RH edited, critically reviewed, and approved the final submitted version of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Supported by the Efficacy and Mechanism Evaluation National Institute for Health Research (14/23/09); National Institute of Diabetes and Digestive and Kidney Diseases (UC4DK108520); JDRF; The Leona M. and Harry B. Helmsley Trust (#2016PG-T1D046); National Institute for Health Research Cambridge Biomedical Research Centre; European Union Horizon 2020 research and innovation programme (grant agreement no. 731560); and Wellcome Trust Strategic Award (100574/Z/12/Z).
Disclaimer
The views expressed are those of the authors and not necessarily those of the funders. No sponsor had any role in the study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Conflict of Interest
RH reports having received speaker honoraria from Eli Lilly and Novo Nordisk, serving on advisory panel for Eli Lilly and Novo Nordisk; receiving personal fees from BBraun, Medtronic and Abbott Diabetes Care; patents related to closed-loop insulin delivery, shareholder and director at CamDiab Ltd.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Todd JA. Etiology of type 1 diabetes. Immunity. (2010) 32:457–67. doi: 10.1016/j.immuni.2010.04.001
2. Patterson CC, Harjutsalo V, Rosenbauer J, Neu A, Cinek O, Skrivarhaug T, et al. Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centers in the 25 year period 1989-2013: a multicentre prospective registration study. Diabetologia. (2019) 62:408–17. doi: 10.1007/s00125-018-4763-3
3. Patterson CC, Karuranga S, Salpea P, Saeedi P, Dahlquist G, Soltesz G, et al. Worldwide estimates of incidence, prevalence and mortality of type 1 diabetes in children and adolescents: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. (2019) 157:107842. doi: 10.1016/j.diabres.2019.107842
4. Mair C, Wulaningsih W, Jeyam A, McGurnaghan S, Blackbourn L, Kennon B, et al. Glycaemic control trends in people with type 1 diabetes in Scotland 2004-2016. Diabetologia. (2019) 62:1375–84. doi: 10.1007/s00125-019-4900-7
5. Foster NC, Beck RW, Miller KM, Clements MA, Rickels MR, DiMeglio LA, et al. State of type 1 diabetes management and outcomes from the T1D Exchange in 2016-2018. Diabetes Technol Ther. (2019) 21:66–72. doi: 10.1089/dia.2018.0384
6. Wood JR, Miller KM, Maahs DM, Beck RW, DiMeglio LA, Libman IM, et al. Most youth with type 1 diabetes in the T1D Exchange clinic registry do not meet American Diabetes Association or International Society for Pediatric and Adolescent Diabetes clinical guidelines. Diabetes Care. (2013) 36:2035–7. doi: 10.2337/dc12-1959
7. DiMeglio LA, Acerini CL, Codner E, Craig ME, Hofer SE, Pillay K, et al. ISPAD Clinical Practice Consensus Guidelines 2018: glycemic control targets and glucose monitoring for children, adolescents, and young adults with diabetes. Pediatr Diabetes. (2018) 19(Suppl. 27):105–14. doi: 10.1111/pedi.12737
8. Anderzen J, Hermann JM, Samuelsson U, Charalampopoulos D, Svensson J, Skrivarhaug T, et al. International benchmarking in type 1 diabetes: large difference in childhood HbA1c between eight high-income countries but similar rise during adolescence-A quality registry study. Pediatr Diabetes. (2020) 21:621–7. doi: 10.1111/pedi.13014
9. Harrington KR, Boyle CT, Miller KM, Hilliard ME, Anderson BJ, Van Name M, et al. Management and family burdens endorsed by parents of youth <7 years old with type 1 diabetes. J Diabetes Sci Technol. (2017) 11:980–7. doi: 10.1177/1932296817721938
10. Sherr JL. Closing the loop on managing youth with type 1 diabetes: children are not just small adults. Diabetes Care. (2018) 41:1572–8. doi: 10.2337/dci18-0003
11. Diabetes Control Complications Trial/Epidemiology of Diabetes Interventions Complications Study Research Group. Mortality in type 1 diabetes in the DCCT/EDIC versus the general population. Diabetes Care. (2016) 39:1378–83. doi: 10.2337/dc15-2399
12. Ehrmann D, Kulzer B, Roos T, Haak T, Al-Khatib M, Hermanns N. Risk factors and prevention strategies for diabetic ketoacidosis in people with established type 1 diabetes. Lancet Diabetes Endocrinol. (2020) 8:436–46. doi: 10.1016/S2213-8587(20)30042-5
13. Basina M, Maahs DM. Age at type 1 diabetes onset: a new risk factor and call for focused treatment. Lancet. (2018) 392:453–4. doi: 10.1016/S0140-6736(18)31811-7
14. Leelarathna L, Choudhary P, Wilmot EG, Lumb A, Street T, Kar P, et al. Hybrid closed-loop therapy: where are we in 2021? Diabetes Obes Metab. (2020) 23:655–60. doi: 10.1111/dom.14273
15. Tauschmann M, Hermann JM, Freiberg C, Papsch M, Thon A, Heidtmann B, et al. Reduction in diabetic ketoacidosis and severe hypoglycemia in pediatric type 1 diabetes during the first year of continuous glucose monitoring: a multicenter analysis of 3,553 subjects from the DPV Registry. Diabetes Care. (2020) 43:e40–2. doi: 10.2337/dc19-1358
16. Misso ML, Egberts KJ, Page M, O'Connor D, Shaw J. Continuous subcutaneous insulin infusion (CSII) versus multiple insulin injections for type 1 diabetes mellitus. Cochrane Database Syst Rev. (2010). CD005103. doi: 10.1002/14651858.CD005103.pub2
17. Johnson SR, Cooper MN, Jones TW, Davis EA. Long-term outcome of insulin pump therapy in children with type 1 diabetes assessed in a large population-based case-control study. Diabetologia. (2013) 56:2392–400. doi: 10.1007/s00125-013-3007-9
18. Bekiari E, Kitsios K, Thabit H, Tauschmann M, Athanasiadou E, Karagiannis T, et al. Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. BMJ. (2018) 361:k1310. doi: 10.1136/bmj.k1310
19. Weisman A, Bai J-W, Cardinez M, Kramer CK, Perkins BA. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomized controlled trials. Lancet Diabetes Endocrinol. (2017) 5:501–12. doi: 10.1016/S2213-8587(17)30167-5
20. Boughton CK, Hovorka R. New closed-loop insulin systems. Diabetologia. (2021) 64:1007–15. doi: 10.1007/s00125-021-05443-1
21. Lawton J, Blackburn M, Rankin D, Allen JM, Campbell FM, Leelarathna L, et al. Participants' experiences of, and views about, daytime use of a day-and-night hybrid closed-loop system in real life settings: longitudinal qualitative study. Diabetes Technol Ther. (2019) 21:119–27. doi: 10.1089/dia.2018.0306
22. Garg SK, Weinzimer SA, Tamborlane WV, Buckingham BA, Bode BW, Bailey TS, et al. Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol. Ther. (2017) 19:155–63. doi: 10.1089/dia.2016.0421
23. Forlenza GP, Pinhas-Hamiel O, Liljenquist DR, Shulman DI, Bailey TS, Bode BW, et al. Safety evaluation of the MiniMed 670G System in children 7-13 Years of age with type 1 diabetes. Diabetes Technol Ther. (2019) 21:11–9. doi: 10.1089/dia.2018.0264
24. Bergenstal RM, Nimri R, Beck RW, Criego A, Laffel L, Schatz D, et al. A comparison of two hybrid closed-loop systems in adolescents and young adults with type 1 diabetes (FLAIR): a multicentre, randomized, crossover trial. Lancet. (2021) 397:208–19. doi: 10.1016/S0140-6736(20)32514-9
25. Collyns OJ, Meier RA, Betts ZL, Chan DSH, Frampton C, Frewen CM, et al. Improved glycemic outcomes with Medtronic MiniMed Advanced Hybrid Closed-Loop delivery: results from a randomized crossover trial comparing automated insulin delivery with predictive low glucose suspend in people with type 1 diabetes. Diabetes Care. (2021) 44:969–75. doi: 10.2337/figshare.13618910
26. Breton MD, Kanapka LG, Beck RW, Ekhlaspour L, Forlenza GP, Cengiz E, et al. A randomized trial of closed-loop control in children with type 1 diabetes. N Engl J Med. (2020) 383:836–45. doi: 10.1056/NEJMoa2004736
27. Isganaitis E, Raghinaru D, Ambler-Osborn L, Pinsker JE, Buckingham BA, Wadwa RP, et al. Closed-loop insulin therapy improves glycemic control in adolescents and young adults: outcomes from the international Diabetes Closed-Loop (iDCL) trial. Diabetes Technol Ther. (2021). doi: 10.1089/dia.2020.0572. [Epub ahead of print].
28. Ekhlaspour L, Schoelwer MJ, Forlenza GP, Deboer MD, Norlander L, Hsu L, et al. Safety and performance of the Tandem t:slim X2 with Control-IQ automated insulin delivery system in toddlers and preschoolers. Diabetes Technol Ther. (2020). doi: 10.1089/dia.2020.0507. [Epub ahead of print].
29. Tauschmann M, Allen JM, Wilinska ME, Thabit H, Acerini CL, Dunger DB, et al. Home use of day-and-night hybrid closed-loop insulin delivery in suboptimally controlled adolescents with type 1 diabetes: a 3-week, free-living, randomized crossover trial. Diabetes Care. (2016) 39:2019–25. doi: 10.2337/dc16-1094
30. Tauschmann M, Thabit H, Bally L, Allen JM, Hartnell S, Wilinska ME, et al. Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomized trial. Lancet. (2018) 392:1321–9. doi: 10.1016/S0140-6736(18)31947-0
31. Tauschmann M, Allen JM, Nagl K, Fritsch M, Yong J, Metcalfe E, et al. Home use of day-and-night hybrid closed-loop insulin delivery in very young children: a multi-center, 3-week, randomized trial. Diabetes Care. (2019) 42:594–600. doi: 10.2337/dc18-1881
32. Messer LH, Berget C, Forlenza GP. A clinical guide to advanced diabetes devices and closed-loop systems using the CARES paradigm. Diabetes Technol Ther. (2019) 21:462–9. doi: 10.1089/dia.2019.0105
33. Dovc K, Battelino T. Closed-loop insulin delivery systems in children and adolescents with type 1 diabetes. Expert Opin Drug Deliv. (2020) 17:157–66. doi: 10.1080/17425247.2020.1713747
34. Dovc K, Boughton C, Tauschmann M, Thabit H, Bally L, Allen JM, et al. Young children have higher variability of insulin requirements: observations during hybrid closed-loop insulin delivery. Diabetes Care. (2019) 42:1344–7. doi: 10.2337/dc18-2625
35. Wiltshire EJ, Newton K, McTavish L. Unrecognised hypoglycaemia in children and adolescents with type 1 diabetes using the continuous glucose monitoring system: prevalence and contributors. J Paediatr Child Health. (2006) 42:758–63. doi: 10.1111/j.1440-1754.2006.00973.x
36. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study G. Prolonged nocturnal hypoglycemia is common during 12 months of continuous glucose monitoring in children and adults with type 1 diabetes. Diabetes Care. (2010) 33:1004–8. doi: 10.2337/dc09-2081
37. Musolino G, Dovc K, Boughton CK, Tauschmann M, Allen JM, Nagl K, et al. Reduced burden of diabetes and improved quality of life: experiences from unrestricted day-and-night hybrid closed-loop use in very young children with type 1 diabetes. Pediatr Diabetes. (2019) 20:794–9. doi: 10.1111/pedi.12872
38. Barnard KD, Wysocki T, Ully V, Mader JK, Pieber TR, Thabit H, et al. Closing the loop in adults, children and adolescents with suboptimally controlled type 1 diabetes under free living conditions: a psychosocial substudy. J Diabetes Sci Technol. (2017) 11:1080–8. doi: 10.1177/1932296817702656
39. Streisand R, Monaghan M. Young children with type 1 diabetes: challenges, research, and future directions. Curr Diab Rep. (2014) 14:520. doi: 10.1007/s11892-014-0520-2
40. Peters A, Van Name MA, Thorsted BL, Piltoft JS, Tamborlane WV. Postprandial dosing of bolus insulin in patients with type 1 diabetes: a cross-sectional study using data from the T1d Exchange Registry. Endocr Pract. (2017) 23:1201–9. doi: 10.4158/EP171813.OR
41. Evans M, Wilkinson M, Giannpolou A. Fast-acting insulin aspart: the rationale for a new mealtime insulin. Diabetes Ther. (2019) 10:1793–800. doi: 10.1007/s13300-019-00685-0
42. Ruan Y, Elleri D, Allen JM, Tauschmann M, Wilinska ME, Dunger DB, et al. Pharmacokinetics of diluted (U20) insulin aspart compared with standard (U100) in children aged 3-6 years with type 1 diabetes during closed-loop insulin delivery: a randomized clinical trial. Diabetologia. (2015) 58:687–90. doi: 10.1007/s00125-014-3483-6
43. Salehi P, Roberts AJ, Kim GJ. Efficacy and safety of real-life usage of MiniMed 670G Automode in children with type 1 diabetes less than 7 years old. Diabetes Technol Ther. (2019) 21:448–51. doi: 10.1089/dia.2019.0123
44. Driscoll KA, Volkening LK, Haro H, Ocean G, Wang Y, Jackson CC, et al. Are children with type 1 diabetes safe at school? Examining parent perceptions. Pediatr Diabetes. (2015) 16:613–20. doi: 10.1111/pedi.12204
45. Lawton J, Waugh N, Barnard KD, Noyes K, Harden J, Stephen J, et al. Challenges of optimizing glycaemic control in children with Type 1 diabetes: a qualitative study of parents' experiences and views. Diabet Med. (2015) 32:1063–70. doi: 10.1111/dme.12660
46. Lawton J, Hart R, Kimbell B, Allen J, Beeser R, Boughton C, et al. Data sharing whilst using a closed-loop system: qualitative study of adolescents' and parents' experiences and views. Diabetes Technol Ther. (2021). doi: 10.1089/dia.2020.0637. [Epub ahead of print].
47. Lal RA, Basina M, Maahs DM, Hood K, Buckingham B, Wilson DM. One year clinical experience of the first commercial hybrid closed-loop system. Diabetes Care. (2019) 42:2190–6. doi: 10.2337/dc19-0855
48. Berget CM, Laurel H, Vigers Tim Frohnert, Brigitte I, Pyle L, et al. Six months of hybrid closed loop in the real-world: an evaluation of children and young adults using the 670G system. Pediatr Diabetes. (2020) 21:310–8. doi: 10.1111/pedi.12962
49. Rankin D, Kimbell B, Besser R, Boughton C, Campbell F, Elleri D, et al. Adolescents' experiences of using a smartphone application hosting a closed-loop algorithm to manage type 1 diabetes in everyday life: qualitative study. J Diabetes Sci Technol. (2021). In press. doi: 10.17863/CAM.64591
50. Rankin D, Kimbell B, Hovorka R, Lawton J. Adolescents' and their parents' experiences of using a closed-loop system to manage type 1 diabetes in everyday life: qualitative study. Chronic Illn. (2021):1742395320985924. doi: 10.1177/1742395320985924
51. Forlenza GP, Buckingham BA, Brown SA, Bode BW, Levy CJ, Criego AB, et al. First outpatient evaluation of a tubeless automated insulin delivery system with customizable glucose targets in children and adults with type 1 diabetes. Diabetes Technol Ther. (2021). [Epub ahead of print].
52. Brown SA, Kovatchev BP, Raghinaru D, Lum JW, Buckingham BA, Kudva YC, et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med. (2019) 381:1707–17. doi: 10.1056/NEJMoa1907863
53. Chernavvsky DR, DeBoer MD, Keith-Hynes P, Mize B, McElwee M, Demartini S, et al. Use of an artificial pancreas among adolescents for a missed snack bolus and an underestimated meal bolus. Pediatr Diabetes. (2016) 17:28–35. doi: 10.1111/pedi.12230
54. Messer LH, Berget C, Vigers T, Pyle L, Geno C, Wadwa RP, et al. Real world hybrid closed-loop discontinuation: predictors and perceptions of youth discontinuing the 670G system in the first 6 months. Pediatr Diabetes. (2020) 21:319–27. doi: 10.1111/pedi.12971
55. Iturralde E, Tanenbaum ML, Hanes SJ, Suttiratana SC, Ambrosino JM, Ly TT, et al. Expectations and attitudes of individuals with type 1 diabetes after using a hybrid closed loop system. Diabetes Educ. (2017) 43:223–32. doi: 10.1177/0145721717697244
56. Fuchs J, Hovorka R. COVID-19 and diabetes: could diabetes technology research help pave the way for remote healthcare? J Diabetes Sci Technol. (2020) 14:735–6. doi: 10.1177/1932296820929714
Keywords: diabetes technology, young people, type 1 diabetes (or diabetes), closed-loop insulin delivery, artificial pancreas (AP), children
Citation: Fuchs J and Hovorka R (2021) Benefits and Challenges of Current Closed-Loop Technologies in Children and Young People With Type 1 Diabetes. Front. Pediatr. 9:679484. doi: 10.3389/fped.2021.679484
Received: 11 March 2021; Accepted: 01 April 2021;
Published: 30 April 2021.
Edited by:
Marco Marigliano, Integrated University Hospital Verona, ItalyReviewed by:
Valentino Cherubini, Azienda Ospedaliero Universitaria Ospedali Riuniti, ItalyBonfanti Riccardo, San Raffaele Hospital (IRCCS), Italy
Copyright © 2021 Fuchs and Hovorka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Julia Fuchs, amY2NzRAY2FtLmFjLnVr
 Julia Fuchs
Julia Fuchs Roman Hovorka1,2
Roman Hovorka1,2