- 1Department of Pediatrics, University of Mississippi Medical Center, Jackson, MS, United States
- 2Department of Pediatrics, University of Louisville School of Medicine and Norton Children's Hospital, Louisville, KY, United States
- 3Department of Pediatrics, Tulane University School of Medicine, New Orleans, LA, United States
- 4Department of Pediatrics, University of Vermont, Burlington, VT, United States
- 5Department of Community and Public Health Sciences, University of Montana, Missoula, MT, United States
- 6Fran and Earl Ziegler College of Nursing, University of Oklahoma Health Sciences Center, Oklahoma City, OK, United States
- 7Dartmouth-Hitchcock Clinic: Dartmouth-Hitchcock Medical Center, Lebanon, NH, United States
- 8Department of Pediatrics, University of New Mexico Health Sciences Center, Albuquerque, NM, United States
- 9Department of Pediatrics, Division of Hospitalist Medicine, John A. Burns School of Medicine, University of Hawai'i at Manoa, Honolulu, HI, United States
- 10ECHO IDeA States Pediatric Clinical Trials Network Data Coordinating and Operations Center, University of Arkansas for Medical Sciences, Little Rock, AR, United States
- 11Department of Pediatrics, Warren Alpert Medical School, Brown University, Providence, RI, United States
- 12College of Medicine, University of Arkansas for Medical Sciences, Fayetteville, AR, United States
- 13Institute for Research on Equity and Community Health, Thomas Jefferson University, Newark, DE, United States
- 14Children's Mercy Hospital - Kansas City Department of Infectious Diseases, Kansas University Medical Center, University of Missouri Kansas City, Kansas City, MO, United States
- 15Department of Pediatrics, West Virginia University, Morgantown, WV, United States
- 16Division of Organizational Development and Innovation, Southcentral Foundation, Anchorage, AK, United States
- 17Department of Pediatrics, University of Nebraska Medical Center, Omaha, NE, United States
- 18Department of Pediatric Infectious Disease, ECHO IDeA States Pediatric Clinical Trials Network Data Coordinating and Operations Center, University of Arkansas for Medical Sciences, Little Rock, AR, United States
- 19Department of Pediatrics, Medical University of South Carolina, Charleston, SC, United States
Introduction: Research capacity building is a critical component of professional development for pediatrician scientists, yet this process has been elusive in the literature. The ECHO IDeA States Pediatric Clinical Trials Network (ISPCTN) seeks to implement pediatric trials across medically underserved and rural populations. A key component of achieving this objective is building pediatric research capacity, including enhancement of infrastructure and faculty development. This article presents findings from a site assessment inventory completed during the initial year of the ISPCTN.
Methods: An assessment inventory was developed for surveying ISPCTN sites. The inventory captured site-level activities designed to increase clinical trial research capacity for pediatrician scientists and team members. The inventory findings were utilized by the ISPCTN Data Coordinating and Operations Center to construct training modules covering 3 broad domains: Faculty/coordinator development; Infrastructure; Trials/Research concept development.
Results: Key lessons learned reveal substantial participation in the training modules, the importance of an inventory to guide the development of trainings, and recognizing local barriers to clinical trials research.
Conclusions: Research networks that seek to implement successfully completed trials need to build capacity across and within the sites engaged. Our findings indicate that building research capacity is a multi-faceted endeavor, but likely necessary for sustainability of a unique network addressing high impact pediatric health problems. The ISPCTN emphasis on building and enhancing site capacity, including pediatrician scientists and team members, is critical to successful trial implementation/completion and the production of findings that enhance the lives of children and families.
Introduction
Clinical trial funding has historically been confined to large academic centers with largely urban populations and limited age groups of children (1). Likewise, populations under-represented in pediatric trials often are rural, medically underserved, and economically disadvantaged (2). Involvement of medically underserved and rural populations is critical to addressing health conditions affecting the most vulnerable populations of children in the country. These groups often have high rates of infant mortality (3), asthma (4), and childhood obesity (5).
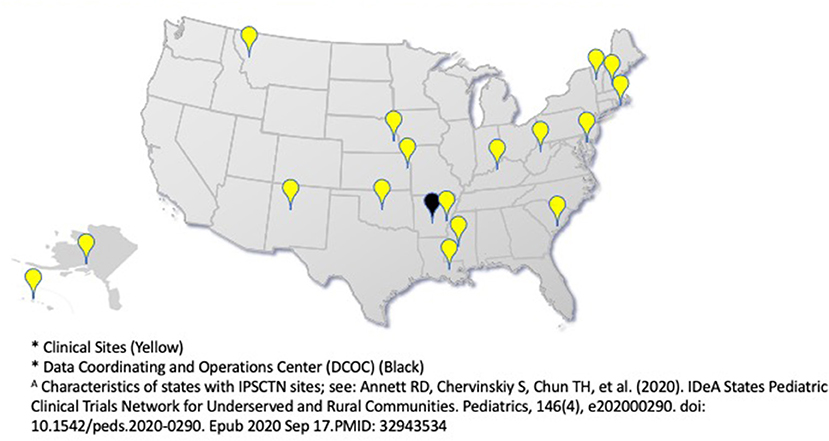
The ECHO IDeA States Pediatric Clinical Trials Network (ISPCTN), funded and established by the National Institutes of Health (NIH) in 2016 as a component of the NIH Environmental Influences on Child Health Outcomes (ECHO) program, is unique in its geographic composition and diverse in its ethnic and racial makeup (Figure 1). Characterization of these differences have recently been published (6). Clinical trial networks, such as the ISPCTN, represent an effective, efficient, and cost effective method for the creation of high quality, generalizable research (7). Networks typically consist of formal arrangements between individuals, institutions, and key stakeholders designed to facilitate the development, implementation, operation and completion of clinical trials (8, 9). As a new network charged to produce impactful pediatric research, building research capacity among sites was an initial ISPCTN priority to ensure that the nascent network could meet the challenges of conducting state-of-the-art research for underserved pediatric populations.
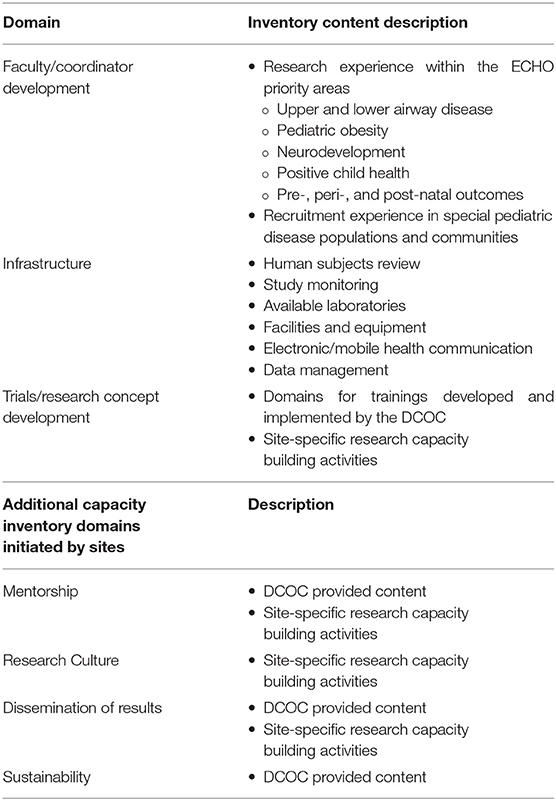
Capacity building has been defined as “a process of individual and institutional development which leads to higher levels of skills and greater ability to perform useful research” (10). Within the ISPCTN, building capacity was broadly operationalized to include faculty/coordinator development, enhancement and expansion of infrastructure, and enrichment of trials/research concept development (Table 1). These broad domains align with existing literature (11, 12). Additional elements crucial for long term success include building a research culture, providing mentorship, developing mechanisms for results dissemination, and supporting ongoing sustainability (8, 13–16). Extant literature largely focuses upon capacity building for allied health professionals or capacity building in global health settings (14, 17–20). Unfortunately, limited information exists regarding building research capacity for pediatric clinical trial operations (9, 21–24).
What can be determined from the existing literature, however, is that several barriers to building research capacity include a lack of funding, insufficient physical resources, limited research experience and expertise, competing priorities, administrative barriers, and lack of time for faculty and coordinators (8). To overcome barriers and achieve the goal of building pediatric trials capacity, an “all teach-all learn” model (25) integrated capacity building activities and locally developed training modules across all sites within this single award. The all teach-all learn model arose from quality improvement work and supports bidirectional learning, particularly focused on community health improvement (26).
Here we aim to describe findings from an ISPCTN pediatric research capacity inventory and to highlight the parallel development of a professional development curriculum, as well as qualitative reports of site-specific learning activities aimed at enhancing pediatric research capacity. Three primary capacity domains are presented: faculty/coordinator development, enhancement and expansion of organization-institutional infrastructure, and clinical trials/research concept development.
Materials and Methods
Assessment Inventory
In the 1st year of ISPCTN, each awardee site principal investigator and affiliated sites were sent a REDCap site assessment inventory, developed by the Data Coordinating and Operations Center, in December 2016 (~2 months after sites received initial funding). The inventory was completed by each site and affiliated site(s) prior to Network trial initiation. The domains of the inventory sought to identify and describe existing infrastructure and was used as a tool to inform the overall capacity building needs of the Network prior to trial initiation, thus did not meet the 45 CRF 46 definition of research. The inventory consisted of 57 multiple-choice and open-ended questions.
Inventory domains and description of content questions are outlined in Table 1. Special pediatric populations were ascertained using the total reported number of active pediatric patients seen at each site annually. Sites then reported subgroups from ECHO priority areas (airway, obesity, neurodevelopment, prenatal/perinatal/postnatal, positive child health) and patient demographic characteristics. Recruitment capacities at sites were characterized by languages spoken at associated clinics, need and ability to provide multi-language recruitment materials, hours of operation, recruitment methods and requirements needed for recruitment activities.
Other site capacities were inventoried. These included a human subjects review domain that ascertained information regarding regularity of Institutional Review Board (IRB) meetings and possible obstacles to timely reviews. The study monitoring domain collected site information including location of source documents stored in medical records and the ability to accommodate monitor visits, including work space and access to medical record (paper/electronic). The laboratory domain assessed site access to a local laboratory for specimen processing and dedicated equipment (e.g., centrifuge, refrigerator, and freezer). Sites were also assessed for imaging capabilities, including the availability of pediatric facilities for X-ray and MRI.
Facilities questions elicited information on infrastructure available for research, including neonatal intensive care unit (NICU) presence at the site, dedicated pediatric research space, investigational pharmacy, storage for lab supplies, practice management system, and medical records. Information was obtained on electronic/mobile health communication and if sites were tracking mobile device usage in their patient community. The data management domain included the availability of EHR resources.
Curriculum Development
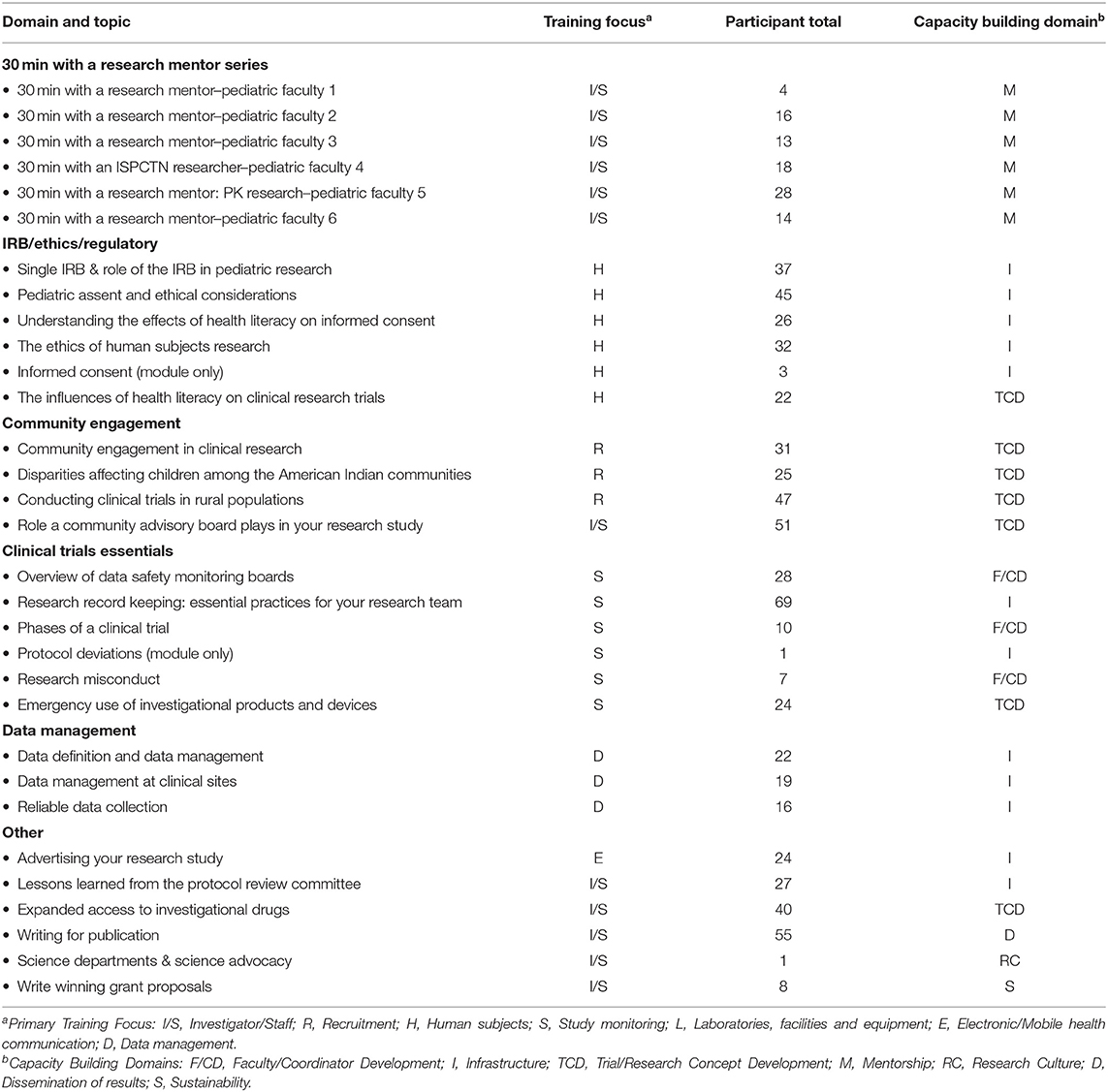
Professional development curriculum and site-developed training modules were created by the DCOC. The professional development curriculum was comprised of learning themes (Table 2). These were developed from DCOC expert input, based upon research trainings offered through the Arkansas Translational Research Institute and guided by the ISPCTN mission that includes engaging rural and underserved communities. Thus, core learning themes included: clinical trials essential elements, Institutional Review Board/ethics/regulatory teachings, data management, and community engagement. To further foster the development of pediatric scientists, a learning theme providing opportunities for interaction with a pediatric researcher was implemented. Finally, several specific professional development offerings were created from participant requests.
Results
Inventory Findings
Overall, 17 ISPCTN sites and 7 affiliates are predominantly at academic medical centers (83%; 20 of 24 total responses), with sites also including Tribal health organizations and primary care centers.
Faculty and Coordinator Development
All ISPCTN site principal investigators reported having clinical trials expertise [100% (n = 24) reporting previous experience with clinical trials] in ECHO disease priority domains; with 17 of 24 principal investigators reporting participation in a clinical trials network. However, variability was observed. ECHO domains with the greatest site investigator trial expertise were perinatal outcomes (n = 17), obesity (n = 17), and airway diseases (n = 18) (range 71–75% of investigators reporting trial experience in these domains). Less trial experience was observed in positive child health and neurodevelopment [67% (n = 16) and 50% (n = 14) of investigators reporting trial experience, respectively]. Among study coordinators, less trial experience in ECHO domains was reported [38–42% (n = 9–10) with previous experience].
Experience With Recruitment Approaches
A broad range of recruitment approaches were identified. Most popular methods were flyer, mailings and attending health fairs, with 71–88% (n = 17–21) of sites favoring these approaches. Recruitment methods utilized by <10% of sites included: provider recruitment (n = 2), referral from hospital/clinic staff (n = 2), websites (n = 2), 3rd party recruitment companies (n = 1), patient registries (n = 1), e-newsletters (n = 1), word of mouth (n = 1) and Instagram© (n = 1). E-media use included Facebook© (54%; n = 13) and Twitter© (25%; n = 6).
Affiliate Needs
Knowledge gaps in regional affiliates that some sites had partnered with to promote clinical trials recruitment were identified. Local study conduct procedures and follow-up education were identified to increase the number of trained clinical investigators and research coordinators conducting clinical trials for children in rural and underserved communities.
Infrastructure (Facilities and Equipment)
Most ISPCTN sites had facilities critical to implementation of pediatric trials. NICUs were identified at 79% (n = 19) of sites, while other on-site facilities were frequently present [on-site pharmacy was reported at 93% (n = 20) of sites; neuroimaging facilities on site ranged from 83 to 92%; n = 20–22]. However, research pharmacy capacity for investigational agents was reported at fewer sites (79%; n = 19). Infrastructure for biosample storage and shipping, research refrigerator and freezer availability, and refrigerated centrifuges were frequently reported [92–96% (n = 22–23) of sites].
Access to Electronic Health Records
A majority of our sites use electronic medical records (20 of 24 total responses reporting use of electronic medical records), with EPIC and Cerner being the most common (22 of 30 sites and subsites) of those using electronic medical records.
Electronic/Mobile Health Communication
Patient communication through email occurs at many sites (75%; n = 18 of 24), though text messaging is less often used (38%; n = 9). However, across all sites, the estimated percent of patients with an email address was 60% (median). A high level of enthusiasm was evident for using e-communication for collection of research data [96% (n = 23) of sites expressing interest in this modality], though relatively few actively collect information on patient mobile capabilities (25%; n = 6).
Trials/Research Concept Development
The DCOC provided a curriculum in an effort to increase the capacity for investigators and coordinators to develop research concepts. Training domain, content area, training focus and number of attendees for DCOC-built modules are presented in Table 2. There is no information available on participants who viewed the archived recordings of these trainings. The range of participants for each live module varied, as these were voluntary trainings. Due to the diverse location of sites, modules included a combination of operational as well as conceptual topics. The DCOC facilitated communication and collaboration across the network sites, resulting in shared content, practices, and resources through an all teach-all learn model, which provided opportunities for bi-directional learning, as well as access national expertise for specific gaps and resource needs. These modules covered a wide range of basic and applied skills and were implemented beginning the 1st year of Network operations and into the 2nd year.
Site-Specific Learning Activities
These learning and capacity building activities were developed utilizing a combination of ISPCTN and local site resources. Each network site focused on areas of local need, as determined by site leadership, and shared between sites and the Network. Table 3 highlights the qualitative findings of learning activities developed and conducted by sites, and grouped by the capacity building inventory domains. Network funding played a role in sites initiating interaction with local infrastructure resources to support pediatric trials. Table 3 provides more nuanced information on site generated topics including mentoring, constructing an institutional research culture and activities for promotion of sustainability.
Discussion
Developing research capacity in a new multisite network involves many intersecting priorities, including prioritization of activities at the site and Network levels. Clinical trials capacity building for the ISPCTN benefited from guidelines in the extant literature (13, 27). The ISPCTN site assessment inventory during the initial year of funding revealed considerable pediatric-specific research capacity. This Network, including academic and non-academic sites, was led by principal investigators with clinical trials experience. Most of the Network sites had existing capacity for pediatric imaging and biosample storage facilities, as well as clinical services that could be engaged in research (e.g., NICUs). With this enhanced understanding of within and between site capacity variability, the DCOC developed and implemented training modules to complement and enhance knowledge and skills across ISPCTN faculty and coordinators in many of the identified domains needed for research skill development. As evident in Table 2, considerable efforts from the DCOC were directed to building and presentation of modules. Attendance by site faculty/coordinators suggests a high level of utilization. The skill-focused approach to capacity building, leveraging pre-existing site expertise, has allowed the Network members to build expertise more uniformity than if each site were providing local education. Having training content available across all sites, regardless of size or local expertise, formed a foundation of shared research mission and helped create shared experiences that fostered further lines of communication between site teams. Thus, key principles for capacity building, such as site-to-site collaboration, were emphasized through the inventory and subsequent training modules (27).
From our inventory we learned that capacity for pediatric trials rests upon several factors, including the type of trial (e.g., randomized controlled, pragmatic public health directed), other necessary physical attributes (e.g., presence an investigational pharmacy), and site training needs. Imperative in the inventory findings, we find three necessary elements that are of greatest importance: (a) having site leadership with trial experience; (b) providing skills-focused training for investigators and coordinators; and (c) supporting site infrastructure, such as protected time for development and conduct of trials. Our inventory provided the Network with simple metrics about sites and identified several areas where variability was evident (e.g., 17% of sites not using an electronic medical record). An inventory approach to determining site and Network capacity for clinical trials has not been evident in the extant literature on capacity building, thus the current presentation fills a gap in providing domains and context that may be a useful startup activity for new research teams and new networks. For the ISPCTN, the inventory findings were disseminated to sites in two ways: a document was developed and disseminated, and findings shared/discussed at a steering committee meeting. This approach was intended to motivate site teams to action that would facilitate the successful conduct of trials through active support and engagement in Network trials.
In the initial year of funding sites were invited to share and build upon tools and activities established, as well as draw upon and contribute to the centralized activities provided by the DCOC. The ability for sites to augment Network knowledge as a whole, and for the Network to augment the capacity of sites, was a crucial element of the capacity building approach adopted. Best practices were identified and shared, exemplifying the bi-directional commitment to accelerating pediatric trial capacity development. The ISPCTN was thus built upon existing site capacities for pediatric trials research, which could be more rapidly advanced together, while developing research capacity and Network culture, essential for successful implementation of multi-site pediatric trials.
From the assessment inventory and curriculum development, several important lessons stand out.
Lesson 1: Research Education Matters
Educational opportunities provided by a central coordinating center, such as online training modules, provides an efficient, effective mechanism for engaging multiple sites, establishing shared operating procedures, and providing uniform knowledge for pediatric trials. Sites within the Network brought a range of individual and institutional expertise, from those sites where individuals had limited trials research knowledge and limited staff expertise, to those that had participated in networked trial groups (e.g., the Pediatric Trials Network, Pediatric Emergency Care Applied Research Network). Institutional resources for research have been supported by the ISPCTN, serving as a stimulus to support the development of pediatrician scientists. Centralized training has facilitated the development of common trials knowledge for pediatric faculty and staff. Module utilization suggests several training topics received generally greater participation (e.g., research record keeping), indicating training gaps that were not necessarily anticipated. The modules have provided a foundation that is being further built upon with the implementation of network trials and associated trial-specific trainings. We anticipate that both individual and institutional trials expertise will continue to develop as a range of pediatric trials are opened across the Network. Pediatric academic research training is a continuous and career long endeavor that needs to constantly be updated through professional development activities, which the ISPCTN recognizes and is addressing.
Lesson 2: Assessing Local Site Resources/Experience Before Trial Launch
Characterizing infrastructure prior to the initiation of pediatric clinical trials provides necessary information, yet alone is insufficient for operationalizing trials for children in rural and medically underserved communities. This is particularly true across a network that have diverse pediatric health issues that are the focus for trials. The inventory findings suggest the need for teams with less trials research background or for those who have not received training in clinical trials need to be provided with learning opportunities that increase research skills necessary for successful trial completion. Missing in our inventory, however, was a clearer characterization of site leaders' experiences with different types of trials (e.g., industry trials, investigator-initiated trials, adaptive and pragmatic trials). While multi-site trials experience is clearly essential and important for building capacity, it is also heterogeneous, and opportunities for scholarly productivity from some trials may be limited to more seasoned investigators.
Lesson 3: Identify Site Facilitators and Barriers to Trial Implementation
Capacity building for pediatrician scientists and coordinators must include a determination of local resources and barriers to research. Barriers may include (a) protected research time in order to establish and develop pediatrician scientists and (b) implementation of pediatric trials within rural and medically underserved communities. While this presentation addresses network capacity and development from that perspective, the ability to establish a research culture within a site and within rural communities is an important element of sustainability. Local institutions and departments developing new programs may identify administrative challenges, such as developing grant budgets and contracts. Departmental priorities for clinical productivity and teaching, as well as balancing professional commitments, can have an impact upon scholarly productivity. Similarly, building sustained relationships with underserved communities requires identification of community champions and the development of trusting relationships between investigators and community members. These and other factors (e.g., sufficient mentoring, protected time) will be important to assess in future capacity assessment inventories, as they play an indirect, but instrumental role in the success of the network.
Network capacity rests upon the sites that can successfully operationalize pediatric trials while embracing principals that support research accomplishments (27). Findings presented here provide a high-level overview of site capabilities. In order to address the mission of increasing pediatric scientists, sites developed a variety of strategies for building research skills in experienced faculty, early career pediatric faculty, coordinators, pediatric residents/fellows and medical students. A more complete understanding of the scope of the training modules, the rationale that drove local development, and the support for these local activities would be valuable in developing an understanding of the diversity of local research cultures and thus the potential for sustainability. Discussion of these activities through Network presentations (via steering committee calls and meetings) has fostered collaborative research activities. Moreover, these collaborations have resulted in scientific presentations, such as those through IDeA regional conferences and academic pediatric national/international meetings.
Limitations
Research capacity building for multi-site pediatric clinical trials has been inadequately described in the literature. While our presentation serves to increase available information, there are several shortcomings with our approach. First, our approach identified research infrastructure capacities across sites, yet did not specifically focus on pediatric research needs assessments of individual sites and individual investigator research needs. Rather than an inventory, a needs assessment encompassing early career, senior faculty, and coordinators could provide greater depth of appreciation of gaps within and across sites. Additionally, sites did not provide their full educational and training materials, but may have provided topics that were unique or demonstrated a particular area of interest, and were not meant to be comprehensive of their full curricula.
Conclusions
Identifying features of ISPCTN site has been a remarkable adjunct to the competence areas that are needed in a multi-site network. Professional development has only recently been identified as a competency area in pediatrics (28). Pediatrician scientists face similar challenges in increasing their knowledge of research-specific skills, including the conduct of pediatric trials. A major emphasis in the original development of ISPCTN was not simply to develop a trials network, but to create and sustain pediatric researchers with a firm commitment to clinical trials addressing high frequency child health conditions among communities that are historically underserved and underrepresented in trials research.
Descriptions of the implementation of trials networks have typically focused upon developing network priorities and research agendas (29–32), yet have seldom addressed the capacity of the research teams to implement trials (33). Building a research network and teams have been reported to carry unique challenges and burdens (34, 35) and our experiences demonstrate the variety of needs that must be assessed and monitored over time in order to identify learning gaps that may develop at the site and/or team level. As our network matures, devoting time to continuing to develop capacity through the domains of research culture, dissemination of results, and sustainability, will be important areas of focus. Together with the ever-important need for enhancing scientific rigor, the next phase for professional development for pediatrician scientists should include measurement of qualitative indices along with trial implementation/completion and the associated scholarly work products. With the successes to date, we are confident that the ISPCTN can succeed and prosper.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
RA, AK, CT, and AA were responsible of the conceptualization and design of the manuscript contents, as well as the interpretation of data. RA drafted the initial version of the manuscript. SB, JC, KC, SC, MF, JJ, AK, JK, KK, AL, PM, MM, LPa, BP, LPy, JSh, KS, JSn, CT, and AA revised and edited the manuscript. All authors approved the final version of the manuscript.
Funding
The IDeA States Pediatric Clinical Trials Network was funded by the National Institutes of Health's Office of the Director and the National Institute of General Medical Sciences, as part of the Environmental influences on Child Health Outcomes program, under the following project award numbers: UG1OD024944, UG1OD024946, UG1OD024959, UG1OD024958, UG1OD024951, UG1OD024948, UG1OD024943, UG1OD024954, UG1OD024942, UG1OD024952, UG1OD024953, UG1OD024947, UG1OD024950, UG1OD024956, UG1OD024955, UG1OD024949, and U24OD024957.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful for the cores that facilitate capacity building through the IDeA Program Infrastructure for Clinical and Translational Research centers (Locations: American Indian/Alaska Native CTR, Delaware CTR, Great Plains CTR Network, Louisiana LS Ca-TS Center, Mississippi Center CTR, Mountain West CTR-IN, Northern New England CTR, Oklahoma CTR, Rhode Island Advance CTR, and West Virginia WVCTSI) and the Clinical and Translational Science Awards Programs (Locations: Arkansas, New Mexico, South Carolina).
Abbreviations
NIH, National Institute of Health; ECHO, Environmental Influences on Child Health Outcomes; ISPCTN, ECHO IDeA States Pediatric Clinical Trials Network; DCOC, Data Coordinating and Operations Center; IRB, Internal Review Board; MRI, Magnetic Resonance Imaging; NICU, Neonatal Intensive Care Unit; HER, Electronic Health Record.
References
1. Snowden J, Darden P, Palumbo P, Saul P, Lee J. The institutional development award states pediatric clinical trials network: building research capacity among the rural and medically underserved. Curr Opin Pediatr. (2018) 30:297–302. doi: 10.1097/MOP.0000000000000597
2. Mayer ML. Disparities in geographic access to pediatric subspecialty care. Matern Child Health J. (2008) 12:624–32. doi: 10.1007/s10995-007-0275-3
3. Ely DM, Driscoll AK, Matthews TJ. Infant mortality rates in rural and urban areas in the United States, 2014. NCHS Data Brief. (2017) 285:1–8.
4. Estrada RD, Ownby DR. Rural asthma: current understanding of prevalence, patterns, and interventions for children and adolescents. Curr Allergy Asthma Rep. (2017) 17:37. doi: 10.1007/s11882-017-0704-3
5. Johnson JA, Johnson AM. Urban-rural differences in childhood and adolescent obesity in the United States: a systematic review and meta-analysis. Child Obes. (2015) 11:233–41. doi: 10.1089/chi.2014.0085
6. Annett RD, Chervinskiy S, Chun TH, Cowan K, Foster K, Goodrich N, et al. IDeA states pediatric clinical trials network for underserved and rural communities. Pediatrics. (2020) 146:290. doi: 10.1542/peds.2020-0290
7. Turner MA, Attar S, de Wildt SN, Vassal G, Mangiarini L, Giaquinto C. Roles of clinical research networks in pediatric drug development. Clin Ther. (2017) 39:1939–48. doi: 10.1016/j.clinthera.2017.09.001
8. Li G, Wu Q, Jin Y, Vanniyasingam T, Thabane L. Key factors of clinical research network capacity building. J Venom Anim Toxins Incl Trop Dis 24:15. doi: 10.1186/s40409-018-0152-0
9. Slora EJ, Harris DL, Bocian AB, Wasserman RC (2010). Pediatric clinical research networks: current status, common challenges, potential solutions. Pediatrics. (2018) 126:740–5. doi: 10.1542/peds.2009-3586
10. Trostle J. Research capacity building in international health: definitions, evaluations and strategies for success. Soc Sci Med. (1992) 35:1321–4. doi: 10.1016/0277-9536(92)90035-O
11. DeCorby-Watson K, Mensah G, Bergeron K, Abdi S, Rempel B, Manson H. Effectiveness of capacity building interventions relevant to public health practice: a systematic review. BMC Public Health. (2018) 18:684. doi: 10.1186/s12889-018-5591-6
12. Lupi CS, Ownby AR, Jokela JA, Cutrer WB, Thompson-Busch AK, Catallozzi M, et al. Faculty development revisited: a systems-based view of stakeholder development to meet the demands of entrustable professional activity implementation. Acad Med. (2018) 93:1472–9. doi: 10.1097/ACM.0000000000002297
13. Cooke J. A framework to evaluate research capacity building in health care. BMC Family Practice. (2005) 6:44. doi: 10.1186/1471-2296-6-44
14. Franzen SRP, Chandler C, Siribaddana S, Atashili J, Angus B, Lang T. Strategies for developing sustainable health research capacity in low and middle-income countries: a prospective, qualitative study investigating the barriers and enablers to locally led clinical trial conduct in Ethiopia, Cameroon and Sri Lanka. BMJ Open. (2017) 7:e017246. doi: 10.1136/bmjopen-2017-017246
15. Franzen SR, Chandler C, Lang T. Health research capacity development in low and middle income countries: reality or rhetoric? A systematic meta-narrative review of the qualitative literature. BMJ Open. (2017) 7:e012332. doi: 10.1136/bmjopen-2016-012332
16. Trytten C, Wale M, Hayes M, Holmes B. Lessons learned from a health authority research capacity-building initiative. Healthc Manage Forum. (2019) 32:259–65. doi: 10.1177/0840470419849468
17. Ali R, Finlayson A, Indox Cancer Research N. Building capacity for clinical research in developing countries: the INDOX Cancer Research Network experience. Glob Health Action. (2012) 5:17288. doi: 10.3402/gha.v5i0.17288
18. Beran D, Byass P, Gbakima A, Kahn K, Sankoh O, Tollman S, et al. Research capacity building—obligations for global health partners. Lancet Global Health. (2017) 5:e567–8. doi: 10.1016/S2214-109X(17)30180-8
19. Klassen TP, Acworth J, Bialy L, Black K, Chamberlain JM, Cheng N, et al. Pediatric emergency research networks: a global initiative in pediatric emergency medicine. Eur J Emerg Med. (2010) 17:224–7. doi: 10.1097/MEJ.0b013e32833b9884
20. Rodriguez-Galindo C, Friedrich P, Alcasabas P, Antillon F, Banavali S, Castillo L, et al. Toward the cure of all children with cancer through collaborative efforts: pediatric oncology as a global challenge. J Clin Oncol. (2015) 33:3065–73. doi: 10.1200/JCO.2014.60.6376
21. Bourgeois FT, Murthy S, Pinto C, Olson KL, Ioannidis JPA, Mandl KD. Pediatric versus adult drug trials for conditions with high pediatric disease burden. Pediatrics. (2012) 130:285–92. doi: 10.1542/peds.2012-0139
22. Martinez-Castaldi C, Silverstein M, Bauchner H. Child versus adult research: the gap in high-quality study design. Pediatrics. (2008) 122:52–7. doi: 10.1542/peds.2007-2849
23. Wasserman R, Bocian A, Harris D, Slora E. Limited capacity in US pediatric drug trials: qualitative analysis of expert interviews. Paediatr Drugs. (2011) 13:119–24. doi: 10.2165/11584240-000000000-00000
24. Bekmezian A, Teufel RJ, Wilson KM. Research needs of pediatric hospitalists. Hosp Pediatr. (2011) 1:38–44. doi: 10.1542/hpeds.2011-0006
25. Nembhard IM. All teach, all learn, all improve?: The role of interorganizational learning in quality improvement collaboratives. Health Care Manage Rev. (2012) 37:154–64. doi: 10.1097/HMR.0b013e31822af831
26. McPhail-Bell K, Matthews V, Bainbridge R, Redman-MacLaren ML, Askew D, Ramanathan S, et al. An “All Teach, All Learn” approach to research capacity strengthening in indigenous primary health care continuous quality improvement. Front Public Health. (2018) 6:107. doi: 10.3389/fpubh.2018.00107
27. Seven principles for strengthening research capacity in low- and middle-income countries: simple ideas in a complex world. ESSENCE Good practice document series. Wellcome Trust of the United Kingdom under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. Available online at: https://www.who.int/tdr/publications/Essence_report2014_OK.pdf
28. Hicks PJ, Schumacher D, Guralnick S, Carraccio C, Burke AE. Domain of competence: personal and professional development. Acad Pediatr. (2014) 14:S80–97. doi: 10.1016/j.acap.2013.11.017
29. Buchbinder R, Bourne A, Latimer J, Harris I, Whittle SL, Richards B, et al. Early development of the Australia and New Zealand Musculoskeletal Clinical Trials Network. Intern Med J. (2020) 50:17–23. doi: 10.1111/imj.14191
30. Hedt-Gauthier BL, Chilengi R, Jackson E, Michel C, Napua M, Odhiambo J, et al. Research capacity building integrated into PHIT projects: leveraging research and research funding to build national capacity. BMC Health Serv Res. (2017) 17:825. doi: 10.1186/s12913-017-2657-6
31. O'Brien MA, Grunfeld E. Building capacity in cancer knowledge translation through catalyst grants. Curr Oncol. (2019) 26:55. doi: 10.3747/co.26.4801
32. Tagoe N, Molyneux S, Pulford J, Murunga VI, Kinyanjui S. Managing health research capacity strengthening consortia: a systematised review of the published literature. BMJ Glob Health. (2019) 4:e001318. doi: 10.1136/bmjgh-2018-001318
33. Weidner A, Peterson LE, Mainous AG, Datta A, Ewigman B. The current state of research capacity in US family medicine departments. Fam Med. (2019) 51:112–9. doi: 10.22454/FamMed.2019.180310
34. Cummings JN, Kiesler S, Bosagh Zadeh R, Balakrishnan AD. Group heterogeneity increases the risks of large group size: a longitudinal study of productivity in research groups. Psychol Sci. (2013) 24:880–90. doi: 10.1177/0956797612463082
35. Hall KL, Stokols D, Moser RP, Taylor BK, Thornquist MD, Nebeling LC, et al. The collaboration readiness of transdisciplinary research teams and centers findings from the National Cancer Institute's TREC Year-One evaluation study. Am J Prev Med. (2008) 35:S161–72. doi: 10.1016/j.amepre.2008.03.035
Keywords: clinical trials, ISPCTN, pediatrics, network, research capacity building
Citation: Annett RD, Bickel S, Carlson JC, Cowan K, Cox S, Fisher MJ, Jarvis JD, Kong AS, Kosut JS, Kulbeth KR, Laptook A, McElfish PA, McNally MM, Pachter LM, Pahud BA, Pyles LA, Shaw J, Simonsen K, Snowden J, Turley CB and Atz AM (2021) Capacity Building for a New Multicenter Network Within the ECHO IDeA States Pediatric Clinical Trials Network. Front. Pediatr. 9:679516. doi: 10.3389/fped.2021.679516
Received: 11 March 2021; Accepted: 10 June 2021;
Published: 14 July 2021.
Edited by:
Steven Hirschfeld, Uniformed Services University of the Health Sciences, United StatesReviewed by:
Michel Tsimaratos, Aix Marseille Université, FranceLokesh Tiwari, All India Institute of Medical Sciences (Patna), India
Copyright © 2021 Annett, Bickel, Carlson, Cowan, Cox, Fisher, Jarvis, Kong, Kosut, Kulbeth, Laptook, McElfish, McNally, Pachter, Pahud, Pyles, Shaw, Simonsen, Snowden, Turley and Atz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Robert D. Annett, cmFubmV0dEB1bWMuZWR1
†ORCID: Robert D. Annett orcid.org/0000-0001-5782-9547
Scott Bickel orcid.org/0000-0002-0940-3063
Mark J. Fisher orcid.org/0000-0002-3331-7886
Lee M. Pachter orcid.org/0000-0002-5766-0953
Jennifer Shaw orcid.org/0000-0002-1824-6063
Kari Simonsen orcid.org/0000-0003-0233-1471
Christine B. Turley orcid.org/0000-0001-8079-9382
Andrew M. Atz orcid.org/0000-0002-4744-3832
 Robert D. Annett
Robert D. Annett Scott Bickel2†
Scott Bickel2† Barbara A. Pahud
Barbara A. Pahud Jessica Snowden
Jessica Snowden