- 1Department of Paediatric Gastroenterology and Nutrition, Royal Hospital for Children and Young People, Edinburgh, United Kingdom
- 2Child Life and Health, College of Medicine and Veterinary Medicine, University of Edinburgh, Edinburgh, United Kingdom
Evidence-based guidelines have been developed outlining the concomitant use of anti-tumor necrosis factor alpha (anti-TNF) agents and immunomodulators including azathioprine (AZA) and methotrexate (MTX) in both adult and pediatric populations. However, there exists a paucity of data guiding evidence-based strategies for their withdrawal in pediatric patients in sustained remission. This narrative review focuses on the available pediatric evidence on this question in the context of what is known from the larger body of evidence available from adult studies. The objective is to provide clarity and practical guidance around who, what, when, and how to step down pediatric patients with inflammatory bowel disease (IBD) from combination immunotherapy. Outcomes following withdrawal of either of the two most commonly used anti-TNF therapies [infliximab (IFX) or adalimumab (ADA)], or immunomodulator therapies, from a combination regimen are examined. Essentially, a judicious approach must be taken to identify a significant minority of patients who would benefit from treatment rationalization. We conclude that step-down to anti-TNF (rather than immunomodulator) monotherapy after at least 6 months of sustained clinical remission is a viable option for a select group of pediatric patients. This group includes those with good indicators of mucosal healing, low or undetectable anti-TNF trough levels, lack of predictors for severe disease, and no prior escalation of anti-TNF therapy. Transmural healing and specific human leukocyte antigen (HLA) typing are some of the emerging targets and tools that may help facilitate improved outcomes in this process. We also propose a simplified evidence-based schema that may assist in this decision-making process. Further pediatric clinical studies are required to develop the evidence base for decision-making in this area.
Introduction
Given recent advances in the management of inflammatory bowel disease (IBD), a higher proportion of patients are being exposed to both biological and immunomodulator therapies earlier in their treatment course and for longer periods of time. First-line anti-tumor necrosis factor alpha (anti-TNF) treatment with infliximab (IFX) (so-called “top-down” strategy) for children with moderate-to-severe Crohn's disease (CD), for instance, has recently been shown to convey advantages over conventional therapy in a 2020 randomized control trial (RCT) (1). While evidence-based guidelines have been developed outlining the concomitant use of anti-TNF agents and immunomodulators [including thiopurines and methotrexate (MTX)] in both adult and pediatric populations, there exists a paucity of data guiding evidence-based strategies for their subsequent withdrawal in pediatric patients who enter sustained remission (2–5) (Table 1). This pertinent question around combination therapy is a significant one for patients, clinicians, and health funding institutions alike, and is particularly crucial in the pediatric inflammatory bowel disease (PIBD) population who potentially have many years or decades of medication exposure ahead. Concerns around issues of economic cost, inconvenience, risks of opportunistic infections, and malignancies such as lymphomas and melanomas must be balanced against the risks of disease relapse, loss of therapeutic response, detrimental progression of disease, and need for surgical intervention (17–24).
Clinical observation and primarily adult-based data suggest that a significant proportion, up to 25–40%, of patients with quiescent disease on combination therapy may be stepped down to monotherapy with an immunomodulator and maintain remission for many years. Relapse rates approximate 50% between 1 and 2 years post step-down to monotherapy with either agent (17–19, 25–32). Beyond 5 years after withdrawal of either therapy, 50–80% relapse rates are reported—with higher rates and more rapid relapse typically seen following anti-TNF vs. immunomodulator withdrawal (7, 33, 34).
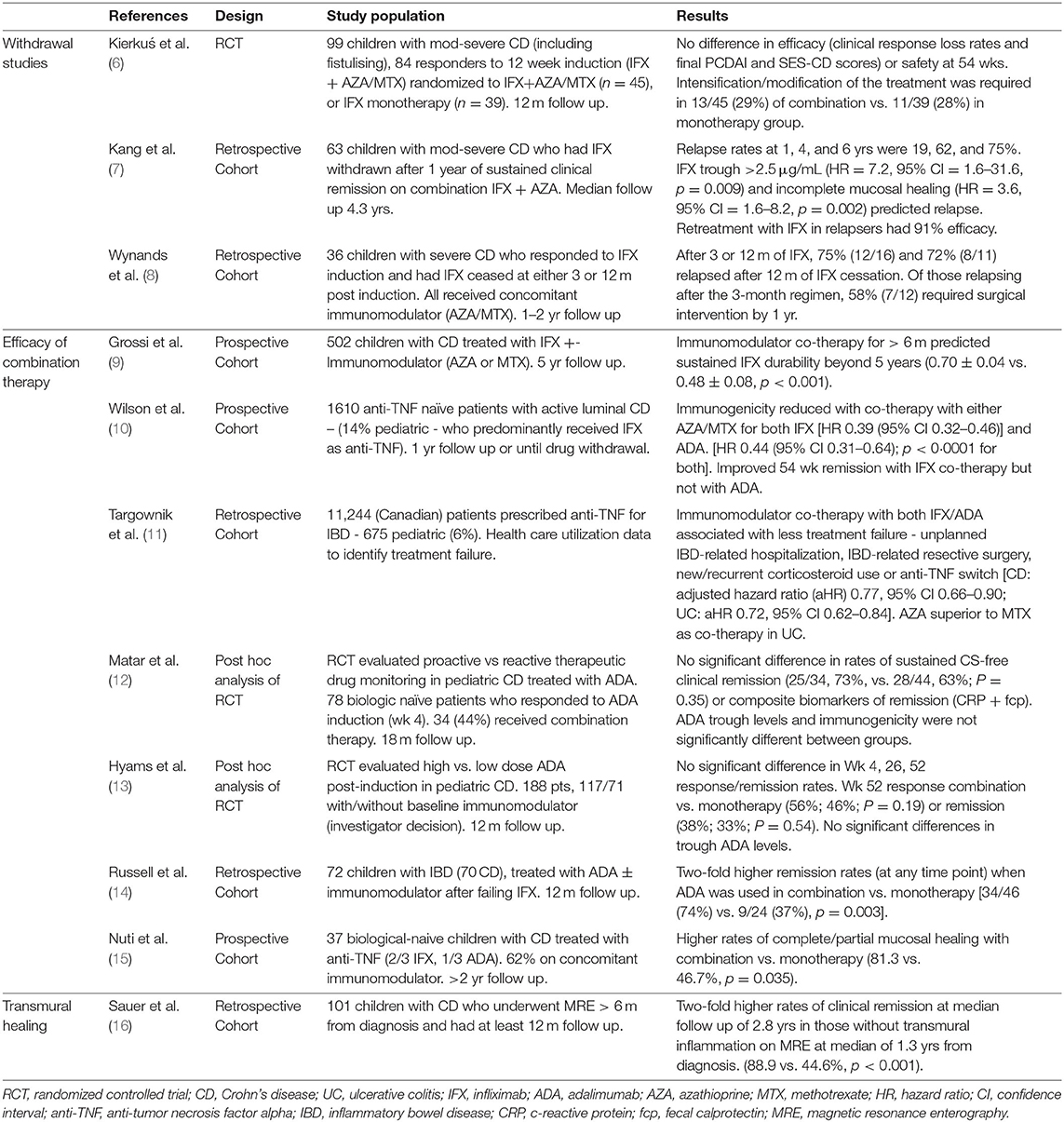
The current clinical challenge lies in the judicious selection of patients for whom drug withdrawal will prove beneficial. This involves a multifactorial assessment of where the threshold lies such that the benefits of cessation outweigh ongoing treatment. Holistically, any philosophy around “treat to target” and individualized medicine in IBD must assimilate this question around who can have therapy withdrawn, as well as the timing and manner of how this should best be done. The complex interplay between the perceptions and prejudices of both the physician and of patient (the child and their guardians) will also influence the decision (35–37).
Rationale for Combination Therapy and Which Combination to Use
Evidence supporting combination immunotherapy focuses on achieving enhanced durability of the biological agent via avoidance of anti-drug antibodies and higher sustained drug levels, with additional synergistic effects likely (38, 39). Improved response and remission rates follow, and ultimately improved disease control may then be achieved.
Infliximab
Both pediatric and adult guidelines (for CD) recommend combination therapy particularly where IFX is the anti-TNF agent used (2, 3). This follows largely from the influential SONIC RCT in adults (n = 508, moderate-severe CD) that demonstrated superiority of combination with AZA over IFX monotherapy based on proposed mechanisms described above. In this group of steroid refractory patients, who were naïve to both anti-TNF and thiopurines, induction therapy with the combination showed almost two-fold higher rates of mucosal healing at 6 months [relative risk (RR) 1.82; 95% confidence interval (CI) 1.10–3.26] with lower rates of serious adverse events (39).
The UC-SUCCESS trial—the solitary RCT in ulcerative colitis (UC) comparing combination therapy (IFX/AZA) vs. monotherapy with either IFX or AZA in 239 anti-TNF naïve adults—reported superior remission rates at 16 weeks [39.7% (31/78) vs. 22.1% (17/77), p = 0.017 for IFX, 23.7% (18/76), p = 0.032 for AZA] (40). Week 16 mucosal healing rates were not statistically significantly different between the combination and IFX monotherapy arms [62.8% (49/78) vs. 54.6% (42/77), p = 0.295]; however, the AZA monotherapy group was not unexpectedly inferior in this regard vs. the combination group [36.8% (28/76), p = 0.001]. There was less anti-drug antibody formation in the combination arm [(3% (1/31) vs. 19% (7/37) in the IFX monotherapy group]. Short study duration and incompleteness of IFX-antibody analysis were important limitations of the study. However, it provides rare RCT level evidence in the adult UC context and, overall, favors combination IFX/AZA over monotherapy with these limitations in mind.
While the body of evidence is sparse, a 2014 Cochrane review reported no evidence for benefit of IFX plus MTX vs. IFX monotherapy for induction of remission in refractory CD (41). However, the COMMIT trial [the only prospective RCT comparing anti-TNF (IFX) plus MTX vs. anti-TNF monotherapy for induction of remission in CD, n = 126] and the 2007 prospective study of Vermeire et al. of 174 CD patients with on-demand IFX dosing provide evidence for MTX in reducing anti-drug antibodies and enhancing anti-TNF levels, with the latter showing equivalent efficacy for MTX and AZA in this regard (42, 43). While COMMIT showed significantly higher drug levels and five-fold lower rates of anti-drug antibodies to IFX in the combination arm (4 vs. 20%, p = 0.01) over the 12-month study period, it must be noted that there were no significant differences in clinical outcomes seen within that time frame. A caveat to the COMMIT results is that a large proportion of participants were given corticosteroids during the induction phase, followed by a prescribed taper and discontinuation by week 14. There is no published RCT comparing the two immunomodulators head to head in CD although a pediatric one is currently in progress (44).
Pediatric Data
Specific pediatric data are limited but some evidence for children can be extracted from the prospective PANTS cohort study with 12 months follow-up of 1,610 anti-TNF naïve patients with active luminal CD−14% of whom were pediatric (6–18 years) (45). Treatment failure was primarily predicted by low anti-TNF drug levels, commencing from week 14, which correlated to higher rates of immunogenicity. Immunogenicity was mitigated by using combination therapy with either type of immunomodulator (AZA/MTX) and for both IFX [HR 0.39 (95% CI 0.32–0.46)] and adalimumab (ADA) [HR 0.44 (95% CI 0.31–0.64); p < 0.0001 for both]. This is in the context, as shown from various other studies, of IFX having far higher rates of anti-drug antibody formation than ADA (63 vs. 29%). A caveat to extrapolating the outcomes with ADA treatment here into the pediatric population is that the vast majority of the 219 children included in the PANTS cohort were managed with IFX.
In a prospective cohort of 502 children with CD by Grossi et al., immunomodulator co-therapy for >6 months predicted sustained IFX durability beyond 5 years (0.70 ± 0.04 vs. 0.48 ± 0.08, p < 0.001) (9). MTX co-therapy was superior in this regard in this study, but the relative numbers of patients using MTX vs. azathioprine (AZA) was low. Neither age, gender, nor disease extent/location predicted durability.
European pediatric UC guidelines also recommend an immunomodulator where IFX is the biological agent used but, in contrast to their less prescriptive CD guidance, favor thiopurines over methothexate (2, 4). The 2018 NASPGHAN (North American Society for Pediatric Gastroenterology Hepatology and Nutrition) position paper on this topic is also non-prescriptive in terms of which combination to apply with IFX for both UC and CD, but favors MTX in males largely given concerns around lymphoma risk (10, 38).
Adalimumab
The evidence overall is less demonstrative regarding the benefits for co-therapy with ADA. Adult CD guidelines recommending against combination therapy with ADA to achieve clinical remission and response are largely based on results from the DIAMOND RCT that included 176 participants with 12 months follow-up (3, 46). Addition of AZA (25–100 mg/day) to ADA for induction of remission inpatients naïve to both with active, moderate-severe CD provided no benefit over monotherapy in achieving and maintaining clinical remission over the 12 months. Endoscopic improvement was more likely at the 6-month mark in the combination arm (84.2 vs. 63.8%, p = 0.019). While mucosal healing was more likely attained sooner in the combination arm, this benefit was not maintained at 12 months [endoscopic improvement at 12 months; 79.6% (combination) vs. 69.8% (monotherapy), p = 0.36]. Meta-analyses of the adult literature on this question have not found significant benefit to combination therapy with ADA (47–49).
Similarly, the PANTS study (ADA treatment in this study was essentially but not purposely confined to adult patients) showed no difference in clinical outcomes at 1 year follow-up when ADA was used with or without an immunomodulator. The longer-term outcomes of this patient cohort are pending publication and the effect of the significant reduction in immunogenicity when ADA is used in combination may become more apparent with time.
A 2020 retrospective study from Targownik et al. examining long-term outcomes in more than 11,000 Canadian patients (6% of whom were pediatric) treated with an anti-TNF showed significantly improved clinical efficacy when adalimumab was combined with either immunomodulator in both UC and CD (11). Combination was associated with a significant decrease in treatment ineffectiveness—unplanned IBD-related hospitalization, IBD-related resective surgery, new/recurrent corticosteroid use, or anti-TNF switch [CD: adjusted hazard ratio (aHR) 0.77, 95% CI 0.66–0.90; UC: aHR 0.72, 95% CI 0.62–0.84]. These key outcome measures were equivalent whether IFX or ADA was the anti-TNF employed. An increased likelihood of treatment failure was observed, in terms of the aforementioned outcome measures, when co-therapy was with MTX rather than AZA (CD: aHR 1.22, 95% CI 0.96–1.54; UC: aHR 1.53, 95% CI: 1.01–2.28). This extensive, real-world study of longer-term outcomes adds to the case for thiopurines over MTX.
Pediatric Data
Post-hoc analyses of pediatric CD cohorts in both the PAILOT and IMAgINE-1 studies report no significant benefits of combination therapy with ADA (12, 13) (see Table 1). An important caveat here is that this was not the primary purpose of either study; the rates of combination therapy were relatively low, so the results for this context may not have been adequately powered. Of note, neither study found evidence for enhanced ADA levels or reduced immunogenicity with co-immunosuppression, whether thiopurines or MTX was used.
A 2011 multicenter, United Kingdom retrospective study in 72 children with CD showed two-fold higher remission rates when ADA was used in combination rather than as monotherapy at 12 months follow-up (74 vs. 37%, p = 0.003) (14). A prospective study by Nuti et al. of 37 biological-naive children with CD treated with anti-TNF (2/3 IFX, 1/3 ADA) showed higher rates of complete or partial mucosal healing at 9–12 months when a co-immunosuppression strategy was used (81.3 vs. 46.7%, p = 0.035) (15). Interestingly, the rates of clinical remission based on PCDAI values were not statistically different between the two groups at follow-up (74% in combination group vs. 64% in monotherapy group, no p-value), indicative of the now well-appreciated discrepancy between clinical and endoscopic outcome measures.
Immunogenicity appears to be far less of an issue with newer biological agents such as vedolizumab and ustekinemab. Combination therapy with these agents generally has not shown improved outcomes, at least in part due to the low immunogenicity (<6% rates reported) of both drugs (50–52). However, high-quality data are again lacking, particularly in the pediatric setting.
The overall body of evidence favors commencement of combination therapy as the default regimen for achieving sustained disease remission, particularly for younger patients and in those with more severe disease. Enhanced anti-TNF efficacy, earlier achievement of mucosal healing, and long-term durability are especially important in these patients. While the data are certainly more compelling for combination with IFX, immunogenicity data and real-world clinical outcomes mean combination therapy with adalimumab may emerge as more standard care, rather than the exception in recalcitrant cases only. Patient selection for, and timing of, withdrawal follows as the next key decision for this ever-growing patient population on anti-TNF therapy managed with co-immunosuppression.
Deciding Who and When to Withdraw—Assessing Relapse Risk
Pariente et al. synthesized potential key risk factors for relapse post step-down from a combination regimen from various adult studies (53). Deep remission at withdrawal and sustained duration (>2 years) of disease control on combination anti-TNF and immunomodulator treatment were the key factors predicting successful step-down. Complicated, extensive disease with various markers of incomplete disease control (including clinical, biochemical, and endoscopic indicators, and prior need for anti-TNF regime escalation) predicts relapse. Surrogate markers of mucosal healing, such as fecal calprotectin, and therapeutic drug monitoring (TDM) levels may also help stratify patients into those most likely to step down successfully. The STORI study, a multicenter, prospective cohort of 115 adult patients with CD who had IFX withdrawn from combination therapy with thiopurines following at least 6 months of corticosteroid-free remission reported a fecal calprotectin level > 300 mcg/g at step-down as a strong predictor of earlier relapse (HR 2.5, 95% CI 1.5–2.8) (54). Observational adult cohort data such as that from Brooks et al. and Bots et al. report avoidance of relapse at 2 and 4 years follow-up, respectively, using more stringent cutoff levels of 50 and 25 mcg/g for calprotectin preceding anti-TNF withdrawal (55, 56).
While pediatric data on specific calprotectin cutoff levels and relapse risk post step-down are sparse, a level below the 100 mcg/g associated with “deep healing” in this population would be in keeping with the evidence around improved outcomes post withdrawal in the context of presumed mucosal healing (57). Pragmatically, pediatric patients with more severe disease—especially those diagnosed at a younger age that have extensive disease, growth failure, fistulizing or perianal phenotypes, steroid refractoriness, and previous surgical resection in CD—will benefit most from early combination therapy and subsequent delayed withdrawal (1, 7). Predictors of severity of outcomes in PIBD have recently been more clearly delineated (58, 59). These risk factors should be carefully considered when determining the weighted risks of relapse vs. continuation of combination therapy at an individual patient level.
Transmural Healing
Transmural healing, distinct from mucosal healing, has also been identified as a sensitive prognostic tool and potential treatment target in IBD, particularly in Crohn's (a transmural disease by definition) with small bowel involvement (60–62). Up to a quarter of pediatric CD patients may have mucosal healing but with ongoing (“deeper”) transmural inflammation (60–63). Although seemingly self-evident, early work by Sauer et al. showed almost two-fold higher rates of clinical remission at a median follow-up of 2.8 years in a pediatric CD cohort who had no transmural inflammation on magnetic resonance enterography (MRE) at a median of 1.3 years from diagnosis (88.9 vs. 44.6%, p < 0.001) (16). A recent meta-analysis including adult and pediatric studies showed transmural healing as a strong prognostic indicator of improved longer-term outcomes in key domains such as sustained remission, need for escalation of therapy, avoidance of CD-related hospitalization, and surgery (64).
None of the studies analyzing predictive factors for relapse post step-down from combination to monotherapy evaluated transmural healing specifically, but this finding (albeit likely in a small percentage of patients) may reasonably be assumed to help predict those patients who will tolerate therapeutic rationalization longer term.
HLA Typing
There is emerging evidence around the potential utility of specific human leukocyte antigen (HLA) typing and other specific genetic markers in predicting risk of immunogenicity to anti-TNF therapies. These markers may factor in assessing risk of relapse, with or without drug withdrawal, at an individual level. Subsequent analysis from the PANTS cohort (adult and pediatric) and work from the adult European consortium ABIRISK (Anti-Biopharmaceutical Immunization: prediction and analysis of clinical relevance to minimize the RISK) identified HLA-DQA1*05 and a variant in the gene C-X-C motif chemokine 12 (CXCL12) as two key markers predicting immunogenicity (65, 66). Carriage of the HLA-DQA1*05 allele in the PANTS cohort predicted a two-fold higher rate of immunogenicity to anti-TNF drugs (HR 1.90; 95% CI 1.60–2.25; p = 5.88 × 10−13). Stratifying further, 90% of patients treated with IFX monotherapy carrying this “risk” allele developed drug antibodies by week 52. Conversely, those without this allele who were treated with ADA in combination with an immunomodulator had a 10% rate of anti-drug antibody development over the same period.
The ABIRISK cohort of 560 patients with autoimmunity (multiple sclerosis n = 147, rheumatoid arthritis n = 229, IBD n = 184) on anti-TNF and other biologic therapeutics showed a 1.5- and 4-fold risk for immunogenicity in heterozygotes and homozygotes for HLA-DQA1*05, respectively. They found that patients homozygous for a minor allelic variant (rs10508884) in the CXCL12 gene also had four-fold higher rates of anti-drug antibody development and that CXCL12 protein levels above the median correlated with significantly higher rates of immunogenicity (aHR = 2.329, 95% CI 1.106–4.90, p = 0.026). This genetic profiling of immunogenicity risk could be used in concert with TDM to enhance the durability of anti-TNF therapies, which is crucial in pediatric IBD. It would prove an excellent example of the widespread and practical use of pharmacogenomics in the clinic. It would add further information to the decision matrix in determining who will benefit most from withdrawal and which therapy should be first withdrawn.
TDM and Timing of Withdrawal
Logically, studies in adult and pediatric IBD alike have found that patients in clinical remission with low or undetectable anti-TNF trough levels have significantly reduced relapse risk post withdrawal (7, 35, 67, 68). Although the time required to achieve mucosal healing may be the ideal starting point at which to consider step-down from combination to monotherapy, a minimum of 6 months duration of combination therapy and corticosteroid-free clinical remission is supported by the STORI study, as well as the RCTs by Roblin and Van Assche in adult cohorts, as an appropriate time frame to start withdrawal planning (18, 19, 54). This apparent “sweet spot” (if not minimum duration) of 6 months combination anti-TNF and immunomodulator is re-enforced by the few pediatric studies on this topic and has been established as a commonly utilized threshold in the pediatric guidelines (2, 7, 9).
The ECCO/ESPGHAN consensus statement recommends consideration for step-down from combination to monotherapy with an anti-TNF agent after 6 months given the child or adolescent is in complete remission with mucosal healing. They suggest, “if proven effective,” continuation of one of the two treatment modalities “at least for several years” (2). Furthermore, the ongoing requirement for biological therapy should be interrogated on an annual basis as a minimum.
Deciding What to Withdraw
Immunomodulator Withdrawal From Combination Therapy
A recent Cochrane meta-analysis failed to identify any eligible adult or pediatric studies investigating the outcomes of withdrawal of anti-TNF therapies from a combination regimen in patients with Crohn's disease in remission, and there are few high-quality studies assessing relapse with immunomodulator withdrawal (17). The two RCTs (125 participants) in adults from Roblin and Van Assche included in the meta-analysis compared discontinuation of azathioprine from a combination regimen to continuation of combination therapy and followed patients for 1 and 2 years, respectively, post intervention, after at least 6 months of remission (18, 19). They showed equivalent relapse rates in those that continued combination therapy (27/56, 48%) and in those who continued IFX alone [27/55 (49%), RR 1.02, 95% CI 0.68–1.52]. Dohos et al. included the prospective RCT DIAMOND-2 study that examined outcomes following thiopurine withdrawal from maintenance ADA after 6 months of remission in adult CD in their meta-analysis on this question (69, 70). The pooled data again showed no statistically significant difference in relapse rates between those stepped down to monotherapy and those continuing the combination regime (RR 1.30, 95% CI 0.81-−2.08, p = 0.269; I2 = 0.0%, p = 0.641). Meta-analysis by Chalhoub et al. specifically addressed response and relapse with ADA monotherapy vs. combination in adult CD (47). They found no significant differences in relapse rates between groups and similarly reported no differences in serious adverse events and opportunistic infections.
Pediatric Data
Work by Kierkuś et al. provides one of the few pediatric randomized investigations into this question, albeit limited significantly by short follow-up intervals (6). Step-down to anti-TNF monotherapy (IFX) for 6 months after 6 months of combination therapy in 84 of 99 children (mean age 14.5 ± 2.5 years) with moderate to severe Crohn's disease (including fistulizing disease) who responded to the initial 12-week induction with combination therapy showed no significant difference in outcomes at 12 months. Relapse rates equated to 30% in both groups at this early follow-up time frame. There were no statistically significant differences in adverse events in either group, although the number of serious events was low in both combination (n = 4, 9%) and withdrawal groups (n = 5, 13%). Overall safety data were equivalent between groups, and this study did not identify factors predicting either the need for intensification or successful withdrawal.
Anti-TNF Withdrawal From Combination Therapy
Arguments in support for earlier withdrawal of anti-TNF, including significant cost savings, reduced risk of opportunistic infections, and other serious side effects, are in part negated by the high relapse rates from clinical studies quoted here. Introduction of biosimilars into the therapeutic armamentarium has substantially reduced the cumulative financial costs. A 2016 United Kingdom IBD audit reported savings of 10–30%, exceeding £5,000/patient/year by switching to biosimilars, with larger savings likely to follow longer term (71, 72). Further systematic lowering of biosimilar anti-TNF prices to 2021 means that biosimilar prices in 2021 are only 20–25% of the bio-originator anti-TNFs in 2015–2016.
The consequences of clinical relapse at the patient level must also factor into the equation. Adult data from two Hungarian studies, where regulations mandated cessation of biological treatment after 12 months maintenance in those responding to induction treatment, warn of the risks of premature cessation of anti-TNF therapies. A study of outcomes 12 months post IFX cessation in UC reported that re-initiation of IFX was necessary in 35% (18/51) at a median of 4 months, with 6% (1/18) of relapsers requiring colectomy.(28). A similar prospective observational study in CD patients (n = 121; 87 withdrawing IFX, 34 withdrawing ADA) showed re-induction required in 45% (54/121) at a median of 6 months post withdrawal and 9% (5/54) of relapsers requiring surgery by 1 year follow-up (29). However, where anti-TNF therapy is more readily available, this is not a common practice, and successful re-induction rates with the same biologic approximates 90% in these studies in agreement with those discussed below.
Pediatric Data
Pediatric data on anti-TNF withdrawal are limited to small observational studies. The retrospective study of Wynands et al. in children (10.7 ± 2 years) was an early warning against premature de-escalation to sole immunomodulator treatment (AZA) in those with more severe CD (8). Of 36 patients who achieved remission after either a pre-determined 3 or 12 months of IFX, 75% (12/16) and 72% (8/11), respectively, relapsed after 12 months of drug cessation. Of those relapsing after the 3-month regimen, 58% (7/12) required surgical intervention by 1 year of follow-up.
Kang et al. found relapse rates at 1, 4, and 6 years of 19, 62, and 75%, respectively, in children with moderate-severe CD who had IFX withdrawn after 1 year of sustained clinical remission on combination IFX and AZA (7). IFX trough >2.5 μg/ml (HR 7.2, 95% CI 1.6–31.6, p = 0.009) and incomplete mucosal healing (HR 3.6, 95% CI 1.6–8.2, p = 0.002) predicted relapse. Retreatment with IFX in relapsers showed efficacy rates of 91% in keeping with larger studies in adults.
Safety Concerns and Immunosuppressive Withdrawal
Impetus for expediting rationalization of combination immunosuppressive therapy also stems from concerns around the potential multiplier effect of treatments for increasing the risk of opportunistic infections. The case–control study of Toruner et al. across all ages—that included 100 consecutive IBD patients with opportunistic infections each matched with 2 IBD patients without an opportunistic infection over a 15 year study period (1998–2003)—reported a five-fold increased risk of infection [odds ratio (OR) 14.5 (95% CI 4.9–43) vs. 2.9 (95% CI 1.5–5.3)] for two or more immunosuppressives (corticosteroids, thiopurines, and/or IFX) vs. monotherapy with either of the three (73). Of note, age was identified as an important relative risk factor with those >50 years old more susceptible (OR, 3.0; 95% CI 1.2–7.2, relative to those <25 years old). While a case of EBV-associated lymphoma and a severe systemic fungal infection were included, most were mild cutaneous or gastrointestinal infections. All cases responded to treatment. Severity of the opportunistic infection was not correlated with type or number of immunosuppressives in this study. Subsequent meta-analyses (that included adult studies only) have reported no increased risk of serious opportunistic infections with combination anti-TNF and immunomodulator therapy above monotherapy (74, 75). In the context of the current COVID-19 pandemic, evidence from the international SECURE-IBD registry of adult and pediatric patients indicates that combination anti-TNF and thiopurine therapy confers a four-fold higher risk of severe COVID-19 above anti-TNF monotherapy, implicating thiopurines as the primary factor in this heightened risk (76). The low rates of severe COVID-19 infection (<1%) in children reported make this somewhat less pertinent for pediatric gastroenterologists. Overall, while an opportunistic infection in an individual patient may be a devastating outcome, for PIBD patients, the vast majority of infections are very mild (77).
Concerns around lymphoma risk associated with AZA use for >2 years in young males and the role of primary EBV in contributing to this risk of lymphoproliferative disease (particularly hemophagocytic lymphohistiocytosis) that have arisen from extensive population studies such as CESAME and DEVELOP have prompted both North American pediatric and European adult guidance for gastroenterologists to “consider” EBV status and gender in the combination therapy decision (38, 78–80). The common preference in clinical practice is for longer-term anti-TNF monotherapy or alternative co-therapy (MTX) in the EBV naïve and in young males. At present, a robust evidence base for doing so is lacking.
With conflicting results on the efficacy of combining methotrexate with biologicals mentioned previously, step-down to anti-TNF monotherapy represents a reasonable step for selected children in sustained remission as long-term thiopurines carry a small but additional, long-term risk. This is reflected in current, real-world PIBD practice as indicated in a 2021 survey from 62 pediatric gastroenterology centers where withdrawal of immunomodulators as the initial step-down from combination therapy was the preferred option for 88% (59/67) of physicians (36).
Re-Treatment Outcomes in Relapsers
In the realization that relapse is a possible, if not probable, outcome for many patients who have undergone treatment rationalization (even in those stratified as the lowest risk) beyond a 5-year follow-up period, it is worth considering the re-treatment outcome data. Most of the data here focus on re-treatment outcomes following anti-TNF withdrawal given concerns around immunogenicity, loss of response, and potential adverse events on re-introduction. In terms of success of retreatment with the withdrawn anti-TNF, the body of evidence from the aforementioned studies in adults and pediatrics alike consistently indicates efficacy rates exceeding 85%, with negligible rates of serious adverse events with re-induction (27, 54–56, 81). Concerns around increased risk of serious infusion reactions after a period of cessation of anti-TNF therapy (primarily IFX) was not borne out in any of these studies, with total infusion reactions typically <5% and significantly lower rates of serious reactions. Concomitant immunomodulator therapy and lack of anti-drug antibodies are potential factors mitigating such risks (82, 83). Planned, regular follow-up post step-down is clearly crucial to avoid delayed recognition and response to disease relapse. Serial fecal calprotectin monitoring (at 3–6 monthly scheduling) allows for earlier relapse detection (84, 85).
Looking beyond biochemical and histopathologic parameters, the developmental phase and patient factors around the acceptability of relapse at a specific time must also be carefully considered before embarking on a planned withdrawal. Optimization of growth and pubertal development, avoidance of interruption of educational/vocational requirements, and consideration of the psychosocial implications of potential relapse should factor in the decision-making conversations with the patient and their family.
Research Needs in PIBD Regarding Combination Immunotherapy Use and Withdrawal
In order to optimize outcomes for pediatric patients with IBD and improve the evidence base for decision-making on this topic, some of the unmet research questions include, but are not limited to
• short-, medium-, and longer-term outcomes after immunomodulator withdrawal from a combination regime;
• risk stratification and approach to clinical relapse in this context;
• head-to-head studies of co-immunosuppression strategies (thiopurines and methotrexate) with anti-TNF therapies in both pediatric CD and UC;
• efficacy of co-immunosuppression with newer biologics and small molecules;
• outcomes, risk stratification, and optimization of timing and approach to withdrawal of anti-TNF therapies for patients in long-term remission;
• clinical utility of genetic profiling including HLA typing in predicting individual response, durability, immunogenicity to anti-TNF treatment, and how to tailor co-immunosuppression based on these data; and
• clarification of treatment targets including “deep” healing and transmural healing and how these may be incorporated into routine clinical practice around the approach to treatment monitoring and withdrawal.
Conclusions
Based on the available adult and pediatric data, it is reasonable to suggest step-down to anti-TNF monotherapy after at least 6 months of sustained clinical remission in a select group of patients (Figure 1). Unacceptably high relapse rates after anti-TNF withdrawal, rare but concerning long-term complications of thiopurines, and the advent of more affordable anti-TNF biosimilar therapies are some of the key factors that make initial immunomodulator withdrawal the more logical approach. While clear pediatric evidence is lacking, there are various factors to consider in selecting those who will benefit from step-down without sacrificing overall treatment efficacy and anti-TNF durability.
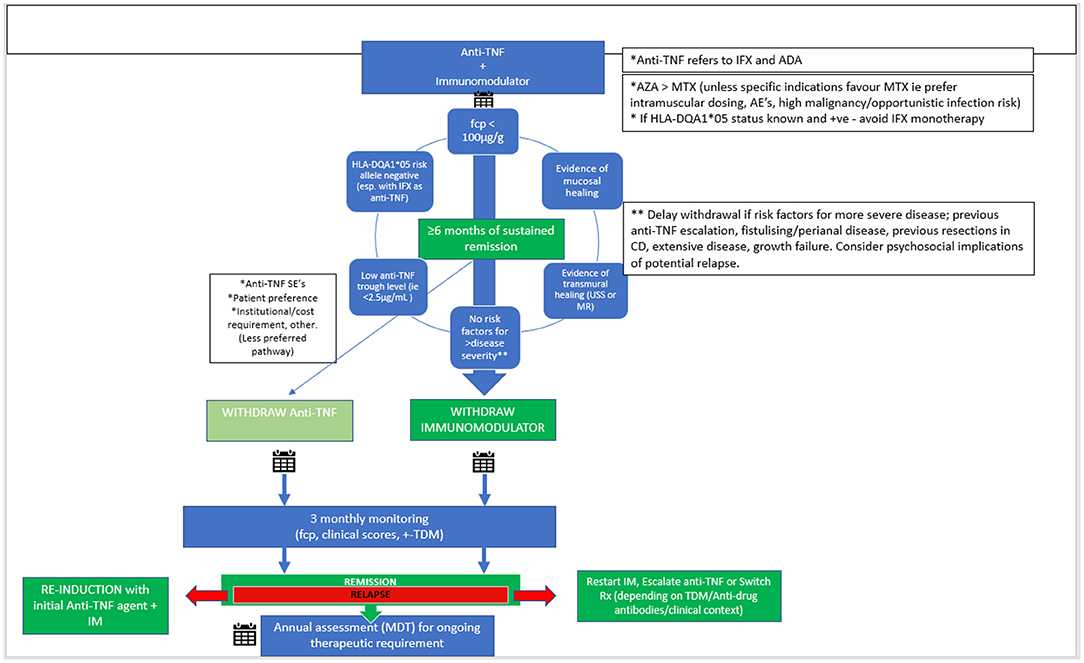
Figure 1. Proposed scheme for withdrawal from combination therapy in PIBD. Anti-TNF, anti-tumor necrosis factor; IFX, Infliximab; ADA, Adalimumab; AZA, Azathioprine; MTX, Methotrexate; CD, Crohn's disease; AE's, adverse events; SE's, side effects; fcp, fecal calprotectin; TDM, therapeutic drug monitoring; IM, Immunomodulator.
Summarizing the available published evidence, the best predictors of a successful step-down to monotherapy include:
• low or undetectable anti-TNF trough levels in the context of quiescent disease;
• FCP < 100 mcg/g, or other combined indicators of mucosal and/or transmural healing;
• no significant predictors for severe disease (i.e., fistulizing, perianal, previous resections in CD, extensive disease);
• no previous escalation of the anti-TNF regimen required; and
• minimum of 6 months of combination therapy prior to step-down.
Further long-term, high-quality evidence is required to help guide the decision-making process around this important question for patients and physicians alike. HLA and genetic risk profiling for immunogenicity to biologics may help fine-tune the patient selection process. We are currently undertaking a large, multi-center clinical study, as part of the Paediatric IBD Porto Group, into immunosuppressive withdrawal and hope to provide further information including long-term follow-up outcomes in this area of interest, and at times controversy, in PIBD.
Author Contributions
JM and RR conceptualized, designed and produced the initial manuscript and revisions for the final submission. PH and DW provided critical revisions to the manuscript up to the final submission. All authors contributed to the article and approved the submitted version.
Funding
RR and PH were supported by an NHS Research Scotland Career Researcher Clinician award. JM was supported by a joint Edinburgh Children's Hospital Charity/Catherine McEwan Foundation research fellowship (Grant No. 2019-66).
Conflict of Interest
DW has received speaker's fees, travel support, and participated in medical board meetings with AbbVie, Roche, and Nestle Heath Sciences. RR has received speaker's fees, travel support, and participated in medical board meetings with Abbvie, Janssen, Takeda, Celltrion, Pharmacosmos, and Nestle.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jongsma MME, Aardoom MA, Cozijnsen MA, Van Pieterson M, De Meij T, Groeneweg M, et al. First-line treatment with infliximab versus conventional treatment in children with newly diagnosed moderate-to-severe Crohn's disease: an open-label multicentre randomised controlled trial. Gut. (2020). doi: 10.1136/gutjnl-2020-322339
2. Van Rheenen PF, Aloi M, Assa A, Bronsky J, Escher JC, Fagerberg UL, et al. The medical management of paediatric Crohn's disease: an ECCO-ESPGHAN guideline update. J Crohns Colitis. (2020) jjaa161. doi: 10.1093/ecco-jcc/jjaa161
3. Torres J, Bonovas S, Doherty G, Kucharzik T, Gisbert JP, Raine T, et al. ECCO guidelines on therapeutics in Crohn's disease: medical treatment. J Crohns Colitis. (2019) 14:4–22. doi: 10.1093/ecco-jcc/jjz180
4. Turner D, Ruemmele FM, Orlanski-Meyer E, Griffiths AM, de Carpi JM, Bronsky J, et al. Management of paediatric ulcerative colitis, part 1: ambulatory care—an evidence-based guideline from European Crohn's and Colitis Organization and European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. (2018) 67:257–91. doi: 10.1097/MPG.0000000000002035
5. Harbord M, Eliakim R, Bettenworth D, Karmiris K, Katsanos K, Kopylov U, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. (2017) 11:769–84. doi: 10.1093/ecco-jcc/jjx009
6. Kierkuś J, Iwańczak B, Wegner A, Dadalski M, Grzybowska-Chlebowczyk U, Łazowska I, et al. Monotherapy with infliximab versus combination therapy in the maintenance of clinical remission in children with moderate to severe Crohn disease. J Pediatr Gastroenterol Nutr. (2015) 60:580–5. doi: 10.1097/MPG.0000000000000684
7. Kang B, Choi SY, Choi YO, Kim MJ, Kim K, Lee JH, et al. Subtherapeutic infliximab trough levels and complete mucosal healing are associated with sustained clinical remission after infliximab cessation in paediatric-onset Crohn's disease patients treated with combined immunosuppressive therapy. J Crohns Colitis. (2018) 12:644–52. doi: 10.1093/ecco-jcc/jjy021
8. Wynands J, Belbouab R, Candon S, Talbotec C, Mougenot JF, Chatenoud L, et al. 12-month follow-up after successful infliximab therapy in pediatric crohn disease. J Pediatr Gastroenterol Nutr. (2008) 46:293–8. doi: 10.1097/MPG.0b013e31815604cd
9. Grossi V, Lerer T, Griffiths A, LeLeiko N, Cabrera J, Otley A, et al. Concomitant use of immunomodulators affects the durability of infliximab therapy in children with Crohn's Disease. Clin Gastroenterol Hepatol. (2015) 13:1748–56. doi: 10.1016/j.cgh.2015.04.010
10. Wilson DC, Griffiths AM. Thiopurine monotherapy in paediatric inflammatory bowel disease: 20 years after Markowitz. J Pediatr Gastroenterol Nutr. (2020) 70:758–9. doi: 10.1097/MPG.0000000000002697
11. Targownik LE, Benchimol EI, Bernstein CN, Singh H, Tennakoon A, Zubieta AA, et al. Combined biologic and immunomodulatory therapy is superior to monotherapy for decreasing the risk of inflammatory bowel disease-related complications. J Crohns Colitis. (2020) 14:1354–63. doi: 10.1093/ecco-jcc/jjaa050
12. Matar M, Shamir R, Turner D, Broide E, Weiss B, Ledder O, et al. Combination therapy of adalimumab with an immunomodulator is not more effective than adalimumab monotherapy in children with crohn's disease: a post hoc analysis of the PAILOT randomized controlled trial. Inflamm Bowel Dis. (2020) 26:1627–35. doi: 10.1093/ibd/izz294
13. Hyams JS, Dubinsky M, Rosh J, Ruemmele FM, Eichner SF, Maa JF, et al. The effects of concomitant immunomodulators on the pharmacokinetics, efficacy and safety of adalimumab in paediatric patients with Crohn's disease: a post hoc analysis. Aliment Pharmacol Ther. (2019) 49:155–64. doi: 10.1111/apt.15054
14. Russell RK, Wilson ML, Loganathan S, Bourke B, Kiparissi F, Mahdi G, et al. A British Society of Paediatric Gastroenterology, Hepatology and Nutrition survey of the effectiveness and safety of adalimumab in children with inflammatory bowel disease. Aliment Pharmacol Therap. (2011) 33:946–53. doi: 10.1111/j.1365-2036.2011.04603.x
15. Nuti F, Civitelli F, Bloise S, Oliva S, Aloi M, Latorre G, et al. Prospective evaluation of the achievement of mucosal healing with anti-TNF-α therapy in a paediatric Crohn's disease cohort. J Crohns Colitis. (2016) 10:5–12. doi: 10.1093/ecco-jcc/jjv126
16. Sauer CG, Middleton JP, McCracken C, Loewen J, Braithwaite K, Alazraki A, et al. Magnetic resonance enterography healing and magnetic resonance enterography remission predicts improved outcome in pediatric Crohn disease. J Pediatr Gastroenterol Nutr. (2016) 62:378–83. doi: 10.1097/MPG.0000000000000976
17. Boyapati RK, Torres J, Palmela C, Parker CE, Silverberg OM, Upadhyaya SD, et al. Withdrawal of immunosuppressant or biologic therapy for patients with quiescent Crohn's disease. Cochrane Database Syst Rev. (2018) 5:CD012540. doi: 10.1002/14651858.CD012540.pub2
18. Roblin X, Boschetti G, Williet N, Nancey S, Marotte H, Berger A, et al. Azathioprine dose reduction in inflammatory bowel disease patients on combination therapy: an open-label, prospective and randomised clinical trial. Aliment Pharmacol Therap. (2017) 46:142–9. doi: 10.1111/apt.14106
19. Van Assche G, Magdelaine–Beuzelin C, d'Haens G, Baert F, Noman M, Vermeire S, et al. Withdrawal of immunosuppression in Crohn's disease treated with scheduled infliximab maintenance: a randomized trial. Gastroenterology. (2008) 134:1861–8. doi: 10.1053/j.gastro.2008.03.004
20. Squires S, Naismith G, Boal A. Combining NICE guidelines with exclusion criteria to identify patients with Crohn's disease for treatment withdrawal: 12-month prospective cohort study. Gastrointest Nurs. (2016) 14:20–28. doi: 10.12968/gasn.2016.14.2.20
21. Park KT, Crandall WV, Fridge J, Leibowitz IH, Tsou M, Dykes DM, et al. Implementable strategies and exploratory considerations to reduce costs associated with anti-TNF therapy in inflammatory bowel disease. Inflamm Bowel Dis. (2014) 20:946–51. doi: 10.1097/01.MIB.0000441349.40193.aa
22. Billioud V, Ford AC, Tedesco ED, Colombel JF, Roblin X, Peyrin-Biroulet L. Preoperative use of anti-TNF therapy and postoperative complications in inflammatory bowel diseases: a meta-analysis. J Crohns Colitis. (2013) 7:853–67. doi: 10.1016/j.crohns.2013.01.014
23. Andersen N, Pasternak B, Basit S, Andersson M, Svanström H, Caspersen S, et al. Association between tumor necrosis factor-α antagonists and risk of cancer in patients with inflammatory bowel disease. JAMA. (2014) 311:2406–13. doi: 10.1001/jama.2014.5613
24. Subramaniam K, Yeung D, Grimpenet F, Joseph J, Fay K, Buckland M, et al. Hepatosplenic T-cell lymphoma, immunosuppressive agents and biologicals: what are the risks? Intern Med J. (2014) 44:287–90. doi: 10.1111/imj.12363
25. Waugh AW, Garg S, Matic K, Gramlich L, Wong C, Sadowski DC, et al. Maintenance of clinical benefit in Crohn's disease patients after discontinuation of infliximab: long-term follow-up of a single centre cohort. Aliment Pharmacol Ther. (2010) 32:1129–34. doi: 10.1111/j.1365-2036.2010.04446.x
26. Steenholdt C, Molazahi A, Ainsworth MA, Brynskov J, Thomsen OØ, Seidelin JB. Outcome after discontinuation of infliximab in patients with inflammatory bowel disease in clinical remission: an observational Danish single center study. Scand J Gastroenterol. (2012) 47:518–27. doi: 10.3109/00365521.2012.660541
27. Casanova MJ, Chaparro M, García-Sánchez V, Nantes O, Leo E, Rojas-Feria M, et al. Evolution after anti-TNF discontinuation in patients with inflammatory bowel disease: a multicenter long-term follow-up study. Am J Gastroenterol. (2017) 112:120–31. doi: 10.1038/ajg.2016.569
28. Farkas K, Lakatos PL, Nagy F, Szepes Z, Miheller P, Papp M, et al. Predictors of relapse in patients with ulcerative colitis in remission after one-year of infliximab therapy. Scand J Gastroenterol. (2013) 48:1394–8. doi: 10.3109/00365521.2013.845906
29. Molnar T, Lakatos PL, Farkas K, Nagy F, Szepes Z, Miheller P, et al. Predictors of relapse in patients with Crohn's disease in remission after 1 year of biological therapy. Aliment Pharmacol Ther. (2013) 37:225–33. doi: 10.1111/apt.12160
30. Schnitzler F, Fidder H, Ferrante M, Noman M, Arijs I, Van Assche G, et al. Long-term outcome of treatment with infliximab in 614 patients with Crohn's disease: results from a single-centre cohort. Gut. (2009) 58:492–500. doi: 10.1136/gut.2008.155812
31. D'Haens G, Baert F, van Assche G, Caenepeel P, Vergauwe P, Tuynman H, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn's disease: an open randomised trial. Lancet. (2008) 371:660–7. doi: 10.1016/S0140-6736(08)60304-9
32. Baert F, Moortgat L, Van Assche G, Caenepeel P, Vergauwe P, De Vos M, et al. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn's disease. Gastroenterology. (2010) 138:463–e11. doi: 10.1053/j.gastro.2009.09.056
33. Reenaers C, Mary JY, Nachury M, Bouhnik Y, Laharie D, Allez M, et al. Outcomes 7 years after infliximab withdrawal for patients with Crohn's disease in sustained remission. Clin Gastroenterol Hepatol. (2018) 16:234–43.e2. doi: 10.1016/j.cgh.2017.09.061
34. Treton X, Bouhnik Y, Mary JY, Colombel JF, Duclos B, Soule JC, et al. Azathioprine withdrawal in patients with Crohn's disease maintained on prolonged remission: a high risk of relapse. Clin Gastroenterol Hepatol. (2009) 7:80–85. doi: 10.1016/j.cgh.2008.08.028
35. Doherty G, Katsanos KH, Burisch J, Allez M, Papamichael K, Stallmach A, et al. European Crohn's and Colitis Organisation topical review on treatment withdrawal [‘exit strategies'] in inflammatory bowel disease. J Crohns Colitis. (2017) 12:17–31. doi: 10.1093/ecco-jcc/jjx101
36. Meredith J, Henderson P, Wilson DC, Van Limbergen J, Wine E, Russell RK. Withdrawal of combination immunotherapy in paediatric inflammatory bowel disease – an international survey of practice. J Pediatr Gastroenterol Nutr. (2021) 73:54–60. doi: 10.1097/MPG.0000000000003098
37. Siegel C, Thompson KD, Walls D, Gollins J, Buisson A, Olympie A, et al. DOP032 Crohn's disease patients' perspectives towards de-escalating immunosuppressive therapy: a comparative French and American survey. ECCO Congress. (2018) 12:S053. doi: 10.1093/ecco-jcc/jjx180.069
38. Day A, Gulati A, Patel N, Boyle B, Park KT, Saeed SA. The role of combination therapy in pediatric inflammatory bowel disease: a clinical report from the North American Society for pediatric gastroenterology, hepatology and nutrition. J Pediatr Gastroenterol Nutr. (2018) 66:361–8. doi: 10.1097/MPG.0000000000001850
39. Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. (2010) 362:1383–95. doi: 10.1056/NEJMoa0904492
40. Panaccione R, Ghosh S, Middleton S, Márquez JR, Scott BB, Flint L, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology. (2014) 146:392–400. doi: 10.1053/j.gastro.2013.10.052
41. McDonald JWD, Wang Y, Tsoulis DJ, MacDonald JK, Feagan BG. Methotrexate for induction of remission in refractory Crohn's disease. Cochrane Database Syst Re. (2014) 12:CD003459. doi: 10.1002/14651858.CD003459.pub4
42. Feagan BG, McDonald JW, Panaccione R, Enns RA, Bernstein CN, Ponich TP, et al. Methotrexate in combination with infliximab is no more effective than infliximab alone in patients with Crohn's disease. Gastroenterology. (2014) 146:681–8.e1. doi: 10.1053/j.gastro.2013.11.024
43. Vermeire S, Noman M, Van Assche G, Baert F, D'Haens G, Rutgeerts P. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn's disease. Gut. (2007) 56:1226–31. doi: 10.1136/gut.2006.099978
44. Harris RE, Aloi M, de Ridder L, Croft NM, Koletzko S, Levine A, et al. Protocol for a multinational risk-stratified randomised controlled trial in paediatric Crohn's disease: methotrexate versus azathioprine or adalimumab for maintaining remission in patients at low or high risk for aggressive disease course. BMJ Open. (2020) 10:e034892. doi: 10.1136/bmjopen-2019-034892
45. Kennedy NA, Heap GA, Green HD, Hamilton B, Bewshea C, Walker GJ, et al. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn's disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol. (2019) 1253:1–13. doi: 10.1016/S2468-1253(19)30012-3
46. Matsumoto T, Motoya S, Watanabe K, Hisamatsu T, Nakase H, Yoshimura N, et al. Adalimumab monotherapy and a combination with azathioprine for Crohn's disease: a prospective, randomized trial. J Crohns Colitis. (2016) 10:1259–66. doi: 10.1093/ecco-jcc/jjw152
47. Chalhoub JM, Rimmani HH, Gumaste VV, Sharara AI. Systematic Review and meta-analysis: adalimumab monotherapy versus combination therapy with immunomodulators for induction and maintenance of remission and response in patients with Crohn's disease. Inflamm Bowel Dis. (2017) 23:1316–27. doi: 10.1097/MIB.0000000000001203
48. Kopylov U, Al-Taweel T, Yaghoobi M, Nauche B, Bitton A, Lakatos PL, et al. Adalimumab monotherapy versus combination therapy with immunomodulators in patients with Crohn's disease: a systematic review and meta-analysis. J Crohns Colitis. (2014) 8:1632–41. doi: 10.1016/j.crohns.2014.07.003
49. Jones JL, Kaplan GG, Peyrin-Biroulet L, Baidoo L, Devlin S, Melmed GY, et al. Effects of concomitant immunomodulator therapy on efficacy and safety of anti–tumor necrosis factor therapy for Crohn's disease: a meta-analysis of placebo-controlled trials. Clin Gastroenterol Hepatol. (2015) 13:2233–40. doi: 10.1016/j.cgh.2015.06.034
50. Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. (2013) 369:699–710. doi: 10.1056/NEJMoa1215734
51. Hanauer SB, Sandborn WJ, Feagan BG, Gasink C, Jacobstein D, Zou B, et al. IM-UNITI: three-year efficacy, safety, and immunogenicity of ustekinumab treatment of Crohn's disease. J Crohns Colitis. (2019) 14:23–32. doi: 10.1093/ecco-jcc/jjz110
52. Adedokun OJ, Xu Z, Marano C, O'Brien C, Szapary P, Zhang H, et al. Ustekinumab pharmacokinetics and exposure response in a phase 3 randomized trial of patients with ulcerative colitis. Clin Gastroenterol Hepatol. (2020) 18:2244–55.e9. doi: 10.1016/j.cgh.2019.11.059
53. Pariente B, Laharie D. Review article: why, when and how to de-escalate therapy in inflammatory bowel diseases. Aliment Pharmacol Ther. (2014) 40:338–53. doi: 10.1111/apt.12838
54. Louis E, Mary JY, Vernier-Massouille G, Grimaud JC, Bouhnik Y, Laharie D, et al. Maintenance of remission among patients with Crohn's disease on antimetabolite therapy after infliximab therapy is stopped. Gastroenterology. (2012) 142:63–e31. doi: 10.1053/j.gastro.2011.09.034
55. Brooks AJ, Sebastian S, Cross SS, Robinson K, Warren L, Wright A, et al. Outcome of elective withdrawal of anti-tumour necrosis factor-α therapy in patients with Crohn's disease in established remission. J Crohns Colitis. (2017) 11:1456–62. doi: 10.1016/j.crohns.2014.09.007
56. Bots SJ, Kuin S, Ponsioen CY, Gecse KB, Duijvestein M, D'Haens GR, et al. Relapse rates and predictors for relapse in a real-life cohort of IBD patients after discontinuation of anti-TNF therapy. Scand J Gastroenterol. (2019) 54:281–8. doi: 10.1080/00365521.2019.1582693
57. Weinstein-Nakar I, Focht G, Church P. Associations among mucosal and transmural healing and fecal level of calprotectin in children with Crohn's disease. Clin Gastroenterol Hepatol. (2018) 16:1089–97.e4. doi: 10.1016/j.cgh.2018.01.024
58. Ricciuto A, Aardoom M, Orlanski-Meyer E, Navon D, Carman N, Aloi M, et al. Predicting outcomes in pediatric Crohn's disease for management optimization: systematic review and consensus statements from the pediatric inflammatory bowel disease-ahead program. Gastroenterology. (2021) 160:403–36.e26. doi: 10.1053/j.gastro.2020.07.065
59. Orlanski-Meyer E, Aardoom M, Ricciuto A, Navon D, Carman N, Aloi M, et al. Predicting outcomes in pediatric ulcerative colitis for management optimization: systematic review and consensus statements from the pediatric inflammatory bowel disease-ahead program. Gastroenterology. (2021) 160:378–402.e22. doi: 10.1053/j.gastro.2020.07.066
60. Fernandes SR, Rodrigues RV, Bernardo S, Cortez-Pinto J, Rosa I, da Silva JP, et al. Transmural healing is associated with improved long-term outcomes of patients with Crohn's disease. Inflamm Bowel Dis. (2017) 23:1403–9. doi: 10.1097/MIB.0000000000001143
61. Laurent V, Naudé S, Vuitton L, Zallot C, Baumann C, Girard-Gavanier M, et al. Accuracy of diffusion-weighted magnetic resonance colonography in assessing mucosal healing and the treatment response in patients with ulcerative colitis. J Crohns Colitis. (2016) 11:716–23. doi: 10.1093/ecco-jcc/jjw211
62. Civitelli F, Nuti F, Oliva S, Messina L, La Torre G, Viola F, et al. Looking beyond mucosal healing: effect of biologic therapy on transmural healing in pediatric Crohn's disease. Inflamm Bowel Dis. (2016) 22:2418–24. doi: 10.1097/MIB.0000000000000897
63. Deepak P, Fletcher JG, Fidler JL, Barlow JM, Sheedy SP, Kolbe AB, et al. Radiological response is associated with better long-term outcomes and is a potential treatment target in patients with small bowel Crohn's disease. Am J Gastroenterol. (2016) 111:997–1006. doi: 10.1038/ajg.2016.177
64. Serban ED. Treat-to-target in Crohn's disease: will transmural healing become a therapeutic endpoint?. World J Clin Cases. (2018) 6:501–13. doi: 10.12998/wjcc.v6.i12.501
65. Sazonovs A, Kennedy NA, Moutsianas L, Heap GA, Rice DL, Reppell M, et al. HLA-DQA1*05 carriage associated with development of anti-drug antibodies to infliximab and adalimumab in patients with Crohn's disease. Gastroenterology. (2020) 158:189–99. doi: 10.1053/j.gastro.2019.09.041
66. Hässler S, Bachelet D, Duhaze J, Szely N, Gleizes A, Hacein-Bey Abina S, et al. Clinicogenomic factors of biotherapy immunogenicity in autoimmune disease: a prospective multicohort study of the ABIRISK consortium. PLoS Med. (2020) 17:e1003348. doi: 10.1371/journal.pmed.1003348
67. Ben-Horin S, Chowers Y, Ungar B, Kopylov U, Loebstein R, Weiss B, et al. Undetectable anti-TNF drug levels in patients with long-term remission predict successful drug withdrawal. Aliment Pharmacol Ther. (2015) 42:356–64. doi: 10.1111/apt.13268
68. Gisbert JP, Marín AC, Chaparro M. Systematic review: factors associated with relapse of inflammatory bowel disease after discontinuation of anti-TNF therapy. Aliment Pharmacol Therap. (2015) 42:391–405. doi: 10.1111/apt.13276
69. Dohos D, Hanák L, Szakács Z, Kiss S, Párniczky A, Eross B, et al. Systematic review with meta-analysis: the effects of immunomodulator or biological withdrawal from mono- or combination therapy in inflammatory bowel disease. Aliment Pharmacol Ther. (2020) 53:220–33. doi: 10.1111/apt.16182
70. Hisamatsu T, Kato S, Kunisaki R, Matsuura M, Nagahori M, Motoya S, et al. Withdrawal of thiopurines in Crohn's disease treated with scheduled adalimumab maintenance: a prospective randomised clinical trial (DIAMOND2). J Gastroenterol. (2019) 54:860–70. doi: 10.1007/s00535-019-01582-w
71. Chanchlani N, Mortier K, Williams LJ, Muhammed R, Auth MK, Cosgrove M, et al. Use of infliximab biosimilar versus originator in a pediatric united kingdom inflammatory bowel disease induction cohort. J Pediatr Gastroenterol Nutr. (2018) 67:513–9. doi: 10.1097/MPG.0000000000002011
72. Severs M, Oldenburg B, van Bodegraven AA, Siersema PD, Mangen MJ, initiative of Crohn's and Colitis. The economic impact of the introduction of biosimilars in inflammatory bowel disease. J Crohns Colitis. (2017) 11:289–96. doi: 10.1093/ecco-jcc/jjw153
73. Toruner M, Loftus EV Jr, Harmsen WS, Zinsmeister AR, Orenstein R, Sandborn WJ, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. (2008) 134:929–36. doi: 10.1053/j.gastro.2008.01.012
74. Lin Z, Bai Y, Zheng P. Meta-analysis: efficacy and safety of combination therapy of infliximab and immunosuppressives for Crohn's disease. Eur J Gastroenterol Hepatol. (2011) 23:1100–10. doi: 10.1097/MEG.0b013e32834b9544
75. Lichtenstein G, Diamond RH, Wagner C, Campana GL, Ortiz MA, Jaeger BR, et al. Clinical trial: benefits and risks of immunomodulators and maintenance infliximab for IBD-subgroup analyses across four randomized trials. Aliment Pharmacol Therap. (2009) 30:210–26. doi: 10.1111/j.1365-2036.2009.04027.x
76. Ungaro RC, Brenner EJ, Gearry RB, Kaplan GG, Kissous-Hunt M, Lewis JD, et al. Effect of IBD medications on COVID-19 outcomes: results from an international registry. Gut. (2021) 70:725–32. doi: 10.1136/gutjnl-2020-322539
77. Hyams J, Walters TD, Crandall W, Kugathasan S, Griffiths A, Blank M, et al. Safety and efficacy of maintenance infliximab therapy for moderate-to-severe Crohn's disease in children: REACH open-label extension. Curr Med Res Opin. (2011) 27:651–62. doi: 10.1185/03007995.2010.547575
78. Beaugerie L, Brousse N, Bouvier AM, Colombel JF, Lémann M, Cosnes J, et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet. (2009) 374:1617–25. doi: 10.1016/S0140-6736(09)61302-7
79. Hyams JS, Dubinsky MC, Baldassano RN, Colletti RB, Cucchiara S, Escher J, et al. Infliximab is not associated with increased risk of malignancy or hemophagocytic lymphohistiocytosis in pediatric patients with inflammatory bowel disease. Gastroenterology. (2017) 152:1901–14.e3. doi: 10.1053/j.gastro.2017.02.004
80. Rahier J, Magro F, Abreu C, Conlon C, De Munter P, D'Haens G, et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. (2014) 8:443–68. doi: 10.1016/j.crohns.2013.12.013
81. Kennedy NA, Warner B, Johnston EL, Flanders L, Hendy P, Ding NS, et al. Relapse after withdrawal from anti-TNF therapy for inflammatory bowel disease: an observational study, plus systematic review and meta-analysis. Aliment Pharmacol Therap. (2016) 43:910–23. doi: 10.1111/apt.13547
82. Lichtenstein L, Ron Y, Kivity S, Ben-Horin S, Israeli E, Fraser GM, et al. Infliximab-related infusion reactions: systematic review. J Crohns Colitis. (2015) 9:806–15. doi: 10.1093/ecco-jcc/jjv096
83. Baert F, Drobne D, Gils A, Casteele NV, Hauenstein S, Singh S, et al. Early trough levels and antibodies to infliximab predict safety and success of reinitiation of infliximab therapy. Clin Gastroenterol Hepatol. (2014) 12:1474.e91. doi: 10.1016/j.cgh.2014.01.033
84. Dai C, Jiang M, Sun M-J. Fecal calprotectin as a predictor of relapse in patients with inflammatory bowel disease. J Clin Gastroenterol. (2015) 49:715. doi: 10.1097/MCG.0000000000000337
Keywords: pediatric inflammatory bowel disease (PIBD), combination therapy, drug withdrawal, anti-TNF, immunomodulators
Citation: Meredith J, Henderson P, Wilson DC and Russell RK (2021) Combination Immunotherapy Use and Withdrawal in Pediatric Inflammatory Bowel Disease—A Review of the Evidence. Front. Pediatr. 9:708310. doi: 10.3389/fped.2021.708310
Received: 11 May 2021; Accepted: 26 July 2021;
Published: 21 September 2021.
Edited by:
Séamus Hussey, National Children's Research Centre (NCRC), IrelandReviewed by:
Jan De Laffolie, University of Giessen, GermanySalvatore Oliva, Sapienza University of Rome, Italy
Copyright © 2021 Meredith, Henderson, Wilson and Russell. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joseph Meredith, am9zZXBoam1lcmVkaXRoQGdtYWlsLmNvbQ==
 Joseph Meredith
Joseph Meredith Paul Henderson
Paul Henderson David C. Wilson1,2
David C. Wilson1,2 Richard K. Russell
Richard K. Russell