- 1Department of Cognitive Sciences, Psychology, Education and Cultural Studies, University of Messina, Messina, Italy
- 2Child and Adolescent Neurology and Psychiatric Section, Department of Clinical and Experimental Medicine, Catania University, Catania, Italy
Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS) are clinical conditions characterized by the sudden onset of obsessive–compulsive disorder and/or tics, often accompanied by other behavioral symptoms in a group of children with streptococcal infection. PANDAS-related disorders, including pediatric acute-onset neuropsychiatric syndrome (PANS), childhood acute neuropsychiatric symptoms (CANS), and pediatric infection triggered autoimmune neuropsychiatric disorders (PITANDs), have also been described. Since first defined in 1998, PANDAS has been considered a controversial diagnosis. A comprehensive review of the literature was performed on PubMed and Scopus databases, searching for diagnostic criteria and diagnostic procedures of PANDAS and related disorders. We propose a test panel to support clinicians in the workout of PANDAS/PANS patients establishing an appropriate treatment. However, further studies are needed to improve our knowledge on these acute-onset neuropsychiatric conditions.
Introduction
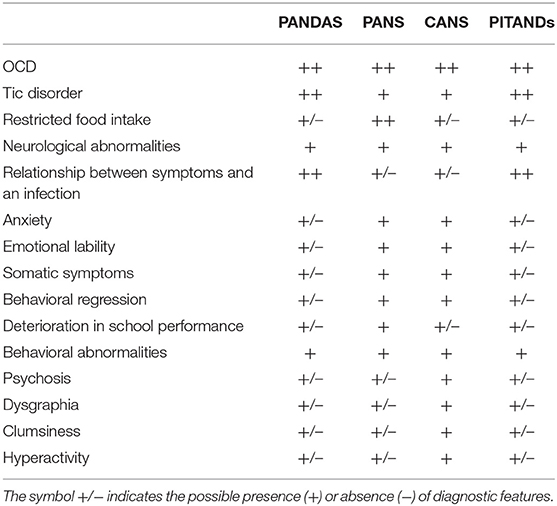
Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection (PANDAS) was first described by Swedo et al. in a group of 50 patients with an acute, sudden onset of obsessive–compulsive disorder (OCD) and/or tics, often accompanied by attention deficit/hyperactivity, separation anxiety, oppositional behaviors, and emotional lability, in the context of a previous streptococcal infection (1). Five working criteria for the diagnosis of PANDAS (Table 1) were proposed:
1. Presence of diagnostic criteria for OCD and/or tic disorder;
2. Pediatric onset, between 3 years and the beginning of puberty;
3. Episodic course of symptom severity, characterized by acute, severe onset and dramatic symptom exacerbations;
4. Temporal relationship between symptom onset and/or exacerbation and group A b-hemolytic streptococcal infections (GABHS); and
5. Association with neurologic abnormalities, such as motor hyperactivity, tics or choreiform movements.
Personality changes, cognitive disturbances, motor abnormalities, sensory sensitivity, behavioral regression, and, occasionally, psychosis are frequently observed (2, 3). The pathogenesis of PANDAS syndrome is hypothesized to be related to GABHS infections via molecular mimicry, where antibodies produced against streptococcal proteins also may cross-react with brain proteins, particularly in the basal ganglia (4). PANDAS exhibits immunological similarities to Sydenham's chorea (SC), a classic infection-triggered autoimmune disorder that also presents with a high degree of OCD comorbidity (5). Unlike SC, PANDAS has been considered a controversial diagnosis, because of contradictory results of various immunologic and epidemiologic studies, and the absence of clinical characteristics and biomarkers that differentiate PANDAS from childhood-onset OCD or tic disorders (6–8).
Swedo et al. (9) proposed a modification of the PANDAS diagnostic criteria termed “pediatric acute-onset neuropsychiatric syndrome (PANS).”
PANS is characterized by an abrupt onset of OCD and/or severe eating restrictions along with at least other concomitant cognitive, motor, behavioral, or affective symptoms (9). PANS criteria (Table 1) describe a clinically distinct presentation, defined as follows:
1. Abrupt, dramatic onset of OCD or severely restricted food intake (<48 h).
2. Concurrent presence of additional neuropsychiatric symptoms, with similarly severe and acute onset, from at least two of the following categories:
A. Anxiety;
B. Emotional lability and/or depression;
C. Irritability, aggression, and/or severely oppositional behaviors;
D. Behavioral (developmental) regression;
E. Deterioration in school performance;
F. Sensory or motor abnormalities including heightened sensitivity to sensory stimuli, hallucinations, dysgraphia, complex motor, and/or vocal tics; and
G. Somatic signs or symptoms, including sleep disturbances, enuresis, or urinary frequency.
3. Symptoms are not better explained by a known neurologic or medical disorder, such as Sydenham chorea, systemic lupus erythematosus, Tourette syndrome, or others.
PANS symptoms overlap with a variety of psychiatric disorders, such as OCD, Tourette's syndrome, ADHD, depression, and bipolar disorder. Many clinical features are also shared between PANDAS and PANS. However, certain aspects of these two syndromes remain elusive, and there is a lack of a global agreement on their symptomatology and course. It is important to specify that PANDAS and PANS cannot occur in comorbidities. Furthermore, a streptococcal infection is required to make a diagnosis of PANDAS, but not for PANS.
Another PANDAS-related disorder but with a non-streptococcal infectious trigger was termed Pediatric Infection Triggered Autoimmune Neuropsychiatric Disorders (PITANDs). This acronym was coined to describe a subset of children with OCD who had a sudden onset of their psychiatric symptoms, typically following infection with a variety of agents (Varicella, Mycoplasma pneumoniae, etc.) (10) (Table 1). At this time, however, PITANDS diagnosis is no longer used.
Another group of authors, based on conflicting scientific support for the PANDAS syndrome, proposed the broader term “childhood acute neuropsychiatric syndrome” (CANS) for patients with acute, fulminant childhood onset of PANDAS symptoms but with no specificity regarding infections or autoimmunity (11) (Table 1). Ultimately, CANS diagnosis may be used in patients who meet the diagnostic criteria of PANDAS or PANS.
In sum, all these clinical conditions are characterized by a possible autoimmune etiology; however, the diagnosis is essentially based on the typical clinical presentation.
The aim of this study was (1) to collect and review the available data about the diagnostic evaluation of PANDAS and related disorders and (2) to focus on diagnostic accuracy of available investigations for diagnosis of these pediatric clinical conditions.
Materials and Methods
Search Strategy
A review of the PubMed and Scopus electronic databases was conducted on February 2021 for the following key terms: “Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections,” “Pediatric acute-onset neuropsychiatric syndrome,” “PANDAS,” “PANS” combined with “diagnosis,” “laboratory tests,” “diagnostic criteria,” to capture a broad range of potential articles. We did not restrain the period of search and reviewed all results. We included clinical studies with full-text display in English, which investigated the available diagnostic tools of these conditions, relevant for the clinician to these searches. Reference lists of all included papers were also checked for other relevant papers to the topic under review. Exclusion criteria were represented by studies written in other languages than English, letters to the editor, editorials, comments, animal studies, adult-focused studies, and contents not related to the topic of our review. At first, reviews, systematic reviews, textbooks, case reports, and case series were examined for any further publications but later excluded to this search.
Data Extraction and Synthesis
A total of 783 articles were found through the databases. Data were searched and extracted independently by one author with assistance of the others. Of the 783 papers originally founded, 727 articles were ruled out based on exclusion criteria, and 56 were identified as potentially pertinent and included in the review.
Results
Laboratory Tests
There is currently no biomarker for the establishment of the diagnosis of these pediatric conditions. However, all patients meeting diagnostic criteria for PANDAS or PANS should have a series of laboratory tests: complete blood cell count with manual differential, indicator for liver and kidney disease, inflammatory markers such us erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), metabolic panel, and urinalysis (12, 13). The diagnosis of PANDAS is based on evidence of recent or current streptococcal infection, which is confirmed with the positive swab from the throat and/or increased titers of antistreptolysin-O (ASO) or anti-DNase B. Several studies showed simultaneously increased levels of ASO and anti-DNAse B in patients with PANDAS compared to controls (3, 14–20) (Table 2). However, the positive results do not differentiate between the carrier state and acute infection, they only indicate exposure to the streptococcal infection (13). Furthermore, false positive or false negative of either ASO or anti-DNase B results is possible. In case of suspected diagnosis of PANS, other infectious triggers should be considered. Mycoplasma pneumoniae, viruses including influenza and Epstein–Barr virus, and Borrelia burgdorferi (Lyme disease) have also been reported as a potential infectious trigger and should be included in the infectious agent's evaluation for PANS (12, 24).
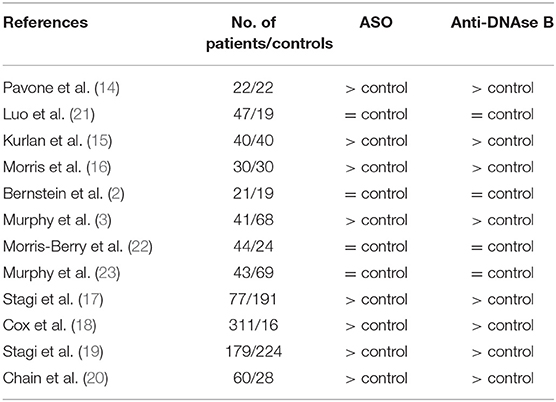
Table 2. Studies that investigated titers of antistreptolysin-O (ASO) and anti-DNase B in patients with PANDAS.
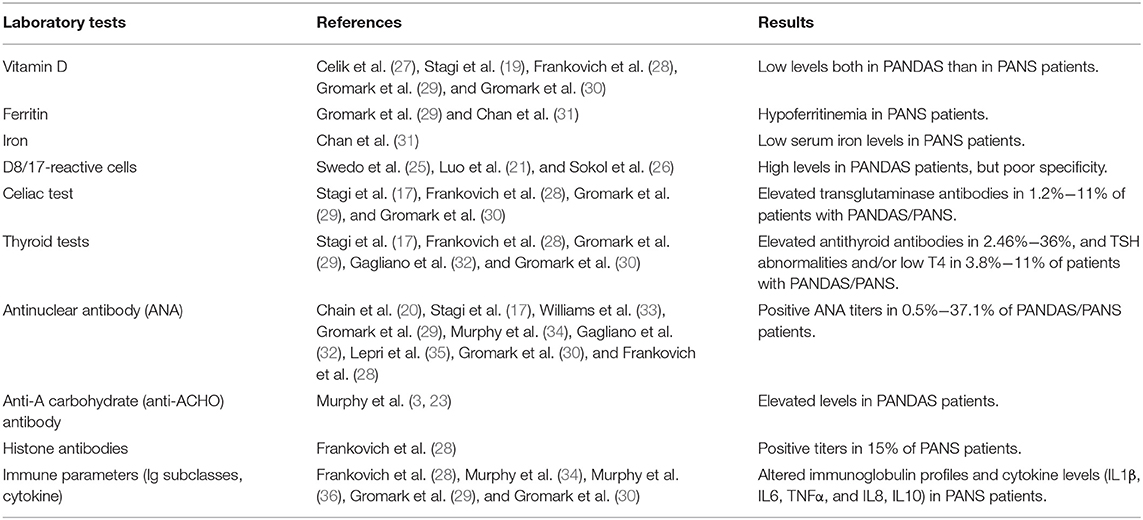
Additional laboratory investigations were performed in clinical studies regarding PANDAS disease. D8/D17, a monoclonal antibody directed against a B lymphocyte surface antigen, has been proposed as a potential marker of susceptibility to PANDAS (21, 25, 26) (Table 3). However, D8/D17 is no longer used owing to poor specificity for PANDAS diagnosis (37–39). Given its role in the modulation of innate immunity and autoimmunity, vitamin D levels have been investigated in several studies, with detection of low levels in PANDAS (19, 27) as well as in PANS patients (28–30) (Table 3). Evidence from a recent study also suggested that lower vitamin D levels may be associated with the presence and severity of comorbid ADHD in children and adolescents with chronic tic disorders (40). Low vitamin D levels may occur in various neurodevelopmental disorders such as autism spectrum disorder (41), making unlikely the usefulness of Vitamin D dosage as potential biomarker of PANDAS condition. Recent studies have also evaluated ferritin and iron levels in patients with PANS. Hypoferritinemia was documented in two different pediatric PANS cohorts (29, 31), associated in one case with low serum iron levels (31) (Table 3). Abnormalities of thyroid function and thyroid autoimmune diseases, as well as the association with celiac disease, have been explored in a few studies (17, 28–30, 32). Elevated transglutaminase and antithyroid antibodies have been documented in, respectively, 1.2–11% and 2.46–36% of patients affected with PANDAS or PANS, whereas TSH abnormalities and/or low T4 have been found in 3.8–11% (17, 28–30, 32) (Table 3). Moreover, evaluation for autoimmune disease through measurements of serum antinuclear antibody (ANA) or others (b2-glycoprotein antibodies, anticardiolipin antibodies, antiphospholipid antibody, and lupus anticoagulant) has yielded conflicting results. Positive ANA titers have been found in 0.5–37.1% of PANDAS/PANS patients (17, 20, 28–30, 32–35), while no other autoantibodies were detected (17, 35) (Table 3). Furthermore, Murphy et al. (3, 23) have documented elevated anti-A carbohydrate (anti-ACHO) antibody levels in PANDAS patients compared to healthy controls, while Frankovich et al. (28) have reported positive histone antibodies in 15% of PANS patients (Table 3). Finally, other studies concerning immune parameters (Ig subclasses and cytokine) have been shown altered immunoglobulin profiles and cytokine levels in PANS patients (IL1β, IL6, TNFα, IL8, and IL10) (28–30, 34, 36) (Table 3). Regarding these additional diagnostic tests, it should be specified that none of these biomarkers are specific for the diagnosis, but nevertheless should be examined for the differential diagnosis.
Neural Autoimmunity
Based on the evidence for autoantibodies in SC, it is hypothesized that the underlying pathology in PANDAS involves an immune-mediated mechanism with molecular mimicry. Therefore, several studies have investigated the role of cross-reactive antibodies in patients with PANDAS and related disorders. Accumulating evidence with controversial results showed that autoantibodies target antigens in the basal ganglia (14, 16, 22, 38, 42–50). Autoimmune targets identified to overlap between SC and PANDAS were used to develop the Cunningham Panel, a commercially available set of blood tests utilized for measuring immune dysfunction related to neuropsychiatric conditions associated with an infection trigger (18, 51, 52). This panel aims to titer autoantibodies to dopamine receptors D1 and D2, tubulin and lysoganglioside-GM1, and calcium/calmodulin-dependent protein kinase II (CaMKII). Despite the fact that multiple studies have analyzed biomarker levels in patient populations and comparison samples (18, 22, 33, 45–49, 53), only a few studies have measured all five assays of this panel of antibodies simultaneously (18, 20, 50, 52, 54). Cox et al. (18) suggested a significant correlation of streptococcal-associated tics and OCD with elevated anti-D1R and antilysoganglioside antineuronal antibodies in serum concomitant with higher activation of CaMKII in human neuronal cells. In another study performed in children with PANDAS-chronic tics and OCD (50), CaMKII activation at the GABHS exacerbation was identified in some subjects. While the clinical use of the Cunningham Panel in diagnosing PANS or PANDAS is not supported by Hesselmark and Bejerot (54), results reported by other recent studies suggested the clinical utility of the Cunningham Panel (20, 52). Despite multiple studies, the clinical reliability of these autoantibodies remains unclear, given the various and often poorly reproducible techniques used for their analysis. Finally, based on the results of a small pilot study (55), Xu et al. (56) investigated the cellular effects of antibodies on cholinergic interneurons (CINs) from 27 children with PANDAS and 23 control subjects. PANDAS serum IgG showed selectively elevated binding to CINs of the striatum, offering novel evidence for striatal CINs as a critical cellular target for antibodies in these patients (56). However, future research is needed to improve our knowledge about these molecular targets of pathogenic antibodies in PANDAS patients.
Lumbar Puncture
Lumbar puncture (LP) with a complete cerebrospinal fluid (CSF) evaluation and assays for antineuronal antibodies should be considered if there are MRI or electroencephalographic (EEG) abnormalities, or encephalopathic symptoms (12, 13). Diagnostic criteria for possible autoimmune encephalitis include a subacute onset of working memory deficits, altered mental status or psychiatric symptoms associated at least to one of the following: (1) new focal CNS findings, (2) seizures not explained by a previously known seizure disorder, (3) CSF pleocytosis, and (4) MRI features suggestive of encephalitis. Clearly, other possible alternative causes must be ruled out (57). There is a heterogeneous presentation of neuropsychiatric features of autoimmune encephalitis in the pediatric population. Symptoms of autoimmune encephalitis can include cognitive regression/impairment, memory changes, seizures, sleep disturbance, autonomic instability, speech changes or mutism, and involuntary movement (58). LP may be crucial for a correct differential diagnosis.
Electroencephalography and Others Electrophysiological Investigations
Although sleep disturbances are frequently reported in PANDAS or PANS patients, little data are currently available in literature regarding electrophysiological investigations in these patients. EEGs may be a useful tool in demonstrating possible signs of abnormal brain activity. Intermittent or persistent focal or generalized EEG alterations were reported in 22 patients (56%) affected by PANS (32). Focal epileptiform activity was reported by Gamucci et al. (59) in a pediatric PANDAS and PANS sample, but EEG monitoring was not available for all participants. Similarly, polysomnography (PSG) evaluations showed several sleep disruptions in children with PANS, such as rapid eye movement (REM) sleep motor disinhibition (60) or periodic limb movement during REM sleep (61). However, there are few data regarding the application of electrophysiological investigations in this population. Further studies into the sleep disturbances of patients with PANS are needed.
Neuroimaging
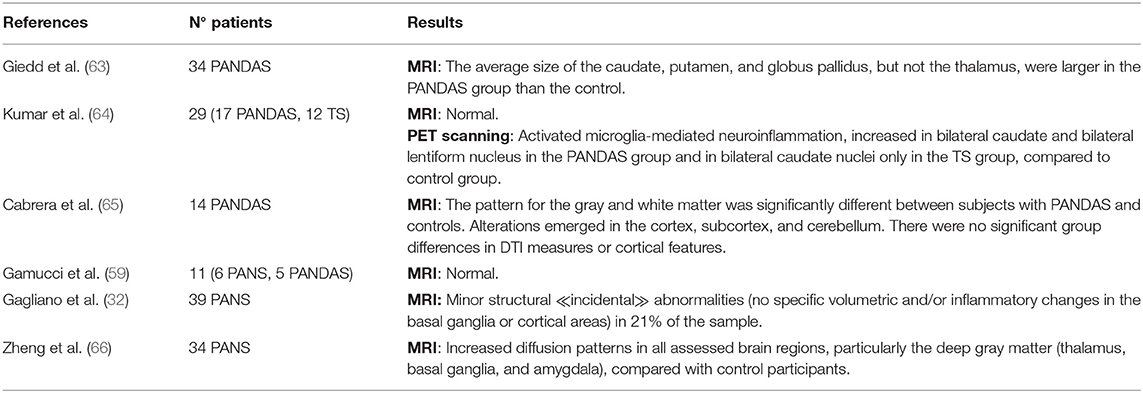
Neuroimaging studies on pediatric patients affected by PANDAS or PANS are limited. After the demonstration of a reduction of magnetic resonance images (MRI) and basal ganglia volumes after plasmapheresis in a single case study of an adolescent with PANDAS (62), the cerebral MRI of 34 children with PANDAS were compared to those from 82 healthy controls. The average size of the caudate, putamen, and globus pallidus, but not of the thalamus or total cerebrum, were significantly greater in the PANDAS group than the control (63) (Table 4). Furthermore, volumetric increases in basal ganglia structures found among the children with PANDAS were like those reported for Sydenham's chorea (SC) (67). The neuroimaging studies are consistent with the hypothesis of a selective cross-reactive antibody-mediated inflammation of the basal ganglia underlying the development of post-streptococcal OCD or tics (63). Using voxel-based morphometry, diffusion tensor imaging (DTI), and surface analysis, Cabrera et al. (65) documented neuroanatomical differences for the gray and white matter pattern between subjects with PANDAS and healthy controls, but no significant group differences in DTI measures or cortical features (65) (Table 4). Other neuroimaging studies on PANDAS or PANS patients showed no brain MRI anomalies (59, 64) or described the detection of minor structural “incidental” abnormalities not located in the basal ganglia or cortical areas (32) (Table 4). A recent case–control study of 34 patients with PANS demonstrated increased diffusion patterns in all assessed brain regions, particularly the deep gray matter (thalamus, basal ganglia, and amygdala). These cerebral microstructural differences are consistent with the cardinal clinical symptoms of these patients, suggesting a unifying inflammatory process involving the central nervous system, particularly the basal ganglia (66) (Table 4). Finally, by using positron emission tomography (PET) imaging with (11)C-[R]-PK11195 (PK), a ligand that binds to the transporter protein expressed by activated microglia, Kumar et al. (64) presented increased PK binding potential values, suggesting underlying neuroinflammation, in bilateral caudate for both PANDAS and Tourette syndrome (TS) patients, and in bilateral lentiform nuclei in patients with PANDAS only (Table 4).
Other Diagnostic Evaluations
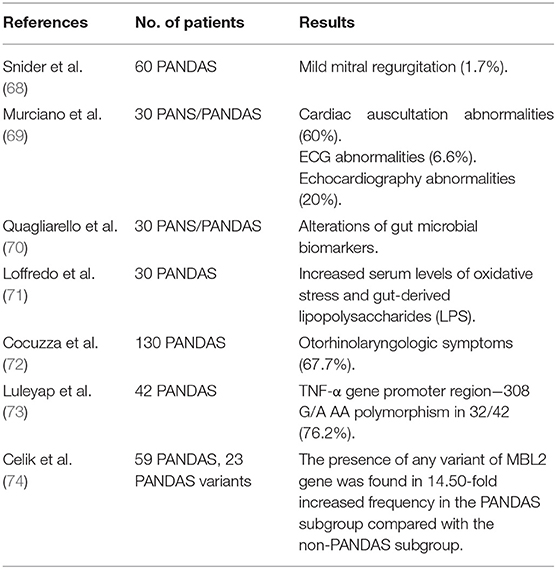
PANDAS syndrome is characterized by several clinical features that may involve multiple districts, but there are only a few data in the literature about these aspects. Considering the detection of significant echocardiographic findings in patients with rheumatic fever and SC, the cardiologic involvement in the PANDAS subgroup was first investigated by Snider et al. (68). Children with PANDAS were assessed by echocardiography, and only one patient (1.7%) was found to have mild mitral regurgitation (68) (Table 5). In contrast, full cardiological assessment was performed through clinical examination, electrocardiography (ECG), and echocardiography in a selected pediatric population diagnosed with PANS/PANDAS (69). A significant number of children presented systolic murmurs (60%), ECG abnormalities (6.6%), and mild mitral valve involvement (20%), suggesting that a cardiologic screening should be performed in these patients (69) (Table 5).
Recently, there has been a growing interest in alterations of gut microbiota (GM), particularly regarding its possible correlations with neuroinflammation and the development of psychiatric disorders. Quagliarello et al. (70) investigated the gut microbiota profiling in PANDAS patients, suggesting that alterations of gut microbial biomarkers can lead to a proinflammatory state (Table 5). A recent study performed by Loffredo et al. (71) also shows that children affected by PANDAS have high circulating levels of soluble NOX2-dp (sNOX-2-dp), isoprostanes, and lipopolysaccharide (LPS), as markers of oxidative stress that could be involved in the process of neuroinflammation (Table 5). Overall, these observations may lead to the conclusion that alterations in the gut microbiota may represent one of the factors enhancing PANDAS development (75).
Otorhinolaryngology symptoms (ENT) in a pediatric population affected by PANDAS were found in about 67.7% of patients, suggesting that PANDAS patients may undergo a specific otolaryngologic consultation (72) (Table 5). Finally, genetic investigations in patients with PANDAS showed a positive relationship with the TNF-α gene promoter region−308 G/A AA polymorphism (73) and exon variants of the MBL2 gene (Mannose-binding lectin) (74) (Table 5).
Neuropsychological Assessment
Neurocognitive functioning of patients affected by PANDAS and related disorders has not been extensively studied in literature. Lewin et al. (76) evaluated neurocognitive profiles in 26 youth with PANDAS, and observed severe impairment in visuospatial recall memory, inhibitory control, fine motor speed, and graphomotor function. Reduced sustained attention and response suppression have also been reported in patients with PANDAS (77). Bernstein et al. (2) compared clinical characteristics of children with PANDAS and children with non-PANDAS OCD. There was no significative difference between the two-diagnostic group on OCD severity score, while the tic severity score was significantly higher in the PANDAS group compared with the non-PANDAS group (2). A recent study has also demonstrated relative difficulties with aspects of executive functions and motor skills in patients with PANDAS (78). Instead, the neuropsychological profile of PANS subjects was analyzed in a few studies. Murphy et al. (34) demonstrated visuospatial deficit in a cohort of 43 youth with PANS. A study of cognitive functioning in patients with PANDAS and related disorders has found a more compromised neuropsychological profile in patients with PANS with respect to patients with PANDAS, particularly for visual-motor abilities, short- and long-term memory, and processing speed (59). In some studies (34, 79–81), PANS patients have been assessed using PANS scales, in association to other self-report and clinician administered measures for a comprehensive clinical evaluation. Furthermore, the use of specific PANS-related scales do not seem to have added value to clarify the diagnostics of Tourette syndrome [European Clinical guidelines for Tourette syndrome and other tic disorders Part 1 Assessment-2,0 (82)].
Discussion
Although there is a body of evidence that supports the existence of PANDAS and related conditions, it remains a controversial diagnosis (83). However, some authors who have investigated the PANDAS hypothesis do support the existence of these conditions (16, 22, 42, 44, 46–50, 53, 54, 84, 85). More studies failed to identify significant differences in specific serum autoantibodies between PANDAS patients and healthy controls (16, 42, 46, 47). Until now, none of the studied diagnostic approaches is sufficient to confirm the diagnosis. The responsibility of evaluating these patients falls to primary care clinicians and child psychiatrists (12). As shown, the diagnosis for the PANDAS syndrome and related conditions is clinical and it requires a complete medical and psychiatric history, a thorough physical examination, a comprehensive neuropsychological assessment, and a series of lab tests. More specific blood tests should also be performed in case of suspected alternative medical explanations for the neuropsychiatric symptoms. If PANS is suspected, it is recommended to consider other possible infectious agents such us Mycoplasma pneumoniae, EBV, and Borrelia burgdorferi. Two unrelated cases of PANS onset in patients with a COVID-19 infection have lately been reported (86). Furthermore, a recent study explored the effects of the COVID-19 pandemic during the lockdown on children affected by PANS and showed an increase in symptoms during the block in 71% of the sample (87). Therefore, COVID-19 infection should also be considered in the differential diagnosis. It is important to underline that various research groups have reported different results about lab tests, due to the use of different analytical techniques.
Based on present data, the diagnostic evaluation of children who meet PANDAS or PANS diagnostic criteria would benefit from including a cardiologic screening with ECG and EEG examination. Given its poor specificity, the clinical use of the Cunningham Panel in diagnosing PANS or PANDAS is not supported for patients with mild forms of the disease. Instead, antineuronal antibodies in serum and CSF, and MRI are indicated for the differential diagnosis with autoimmune encephalitis (7, 12). Based on the results obtained from the previously performed diagnostic investigations, it may be necessary to carry out further paraclinical investigations such as echocardiography or PSG.
By definition, PANDAS and related disorders are always a diagnosis of exclusion. Differential diagnosis is performed with other similar clinical conditions and requires a multidisciplinary approach. Given these diagnostic difficulties, there is a clear need to a better definition of PANDAS/PANS syndrome, to establish more precise diagnostic guidelines and indications. With respect to the previously published PANS diagnostic guidelines (12) and other proposed diagnostic protocols (59, 88), we propose a test panel to support clinicians in the management of PANDAS/PANS patients to promptly initiate a treatment when appropriate (Supplementary Figure 1).
The present scheme may also be helpful in the differential diagnosis with other childhood-onset neuropsychiatric disorders (i.e., autoimmune acute encephalitis) to define their diagnosis as soon as possible. In order to understand which of the available diagnostic tests are most discriminating for the PANDAS/PANS diagnosis, more systematic data should be collected, which can later be used to set the most appropriate diagnostic protocol(s). In conclusion, larger studies are desirable, to establish more precise diagnostic guidelines and indications.
Author Contributions
AP and MS drafted the manuscript. MG, RB, CV, and RR performed critical editing. AP, RR, and CV participated in constructive outline, discussions, and editing. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2021.746639/full#supplementary-material
Supplementary Figure 1. Proposed test panel for patients with suspected PANDAS and related disorders.
References
1. Swedo SE, Leonard HL, Garvey M, Mittleman B, Allen AJ, Perlmutter S, et al. Paediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: clinical description of the first 50 cases. Am J Psychiatry. (1998) 155:264–71.
2. Bernstein GA, Victor AM, Pipal AJ, Williams KA. Comparison of clinical characteristics of paediatric autoimmune neuropsychiatric disorders associated with streptococcal infections and childhood obsessive-compulsive disorder. J Child Adolesc Psychopharmacol. (2010) 20:333–40. doi: 10.1089/cap.2010.0034
3. Murphy TK, Storch EA, Lewin AB, Edge PJ, Goodman WK. Clinical factors associated with paediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. J Pediatr. (2012) 160:314–9. doi: 10.1016/j.jpeds.2011.07.012
4. Cunningham MW. Streptococcus and rheumatic fever. Curr Opin Rheumatol. (2012) 24:408–16. doi: 10.1097/BOR.0b013e32835461d3
5. Swedo SE, Rapoport JL, Cheslow DL, Leonard HL, Ayoub EM, Hosier DM, et al. High prevalence of obsessive-compulsive symptoms in patients with Sydenham's chorea. Am J Psychiatry. (1989) 146:246–9. doi: 10.1176/ajp.146.2.246
6. Perrin EM, Murphy ML, Casey JR, Pichichero ME, Runyan DK, Miller WC, et al. Does group A beta-haemolytic streptococcal infection increase risk for behavioural and neuropsychiatric symptoms in children? Arch Pediatr Adoles Med. (2004) 158:848–56. doi: 10.1001/archpedi.158.9.848
7. Chiarello F, Spitoni S, Hollander E, Matucci Cerinic M, Pallanti S. An expert opinion on PANDAS/PANS: highlights and controversies. Int J Psychiatry Clin Pract. (2017) 21:91–8. doi: 10.1080/13651501.2017.1285941
8. Wilbur C, Bitnun A, Kronenberg S, Laxer RM, Levy DM, Logan WJ, et al. PANDAS/PANS in childhood: controversies and evidence. Paediatr Child Health. (2018) 2018:1–7. doi: 10.1093/pch/pxy145
9. Swedo SE, Leckman JF, Rose NR. From research subgroup to clinical syndrome: modifying the PANDAS criteria to describe PANS (paediatric acute-onset neuropsychiatric syndrome). Pediatr Therapeut. (2012) 2:2. doi: 10.4172/2161-0665.1000113
10. Allen AJ, Leonard HL, Swedo SE. Case study: a new infection-triggered, autoimmune subtype of paediatric OCD and Tourette's syndrome. J Am Acad Child Adoles Psychiatry. (1995) 34:307–11. doi: 10.1097/00004583-199503000-00015
11. Singer HS, Gilbert DL, Wolf DS, Mink JW, Kurlan R. Moving from PANDAS to CANS. J. Paediatr. (2012) 160:725–31. doi: 10.1016/j.jpeds.2011.11.040
12. Chang K, Frankovich J, Cooperstock M, Cunningham MW, Latimer ME, Murphy TK, et al. Clinical evaluation of youth with paediatric acute-onset neuropsychiatric syndrome (PANS): recommendations from the 2013 PANS Consensus Conference. J Child Adolesc Psychopharmacol. (2015) 25:3–13. doi: 10.1089/cap.2014.0084
13. Dop D, Marcu IR, Padureanu R, Niculescu CE, Padureanu V. Paediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (review). Exp Ther Med. (2021) 21:94. doi: 10.3892/etm.2020.9526
14. Pavone P, Bianchini R, Parano E, Incorpora G, Rizzo R, Mazzone L, et al. Anti-brain antibodies in PANDAS versus uncomplicated streptococcal infection. Paediatr Neurol. (2004) 30:107–10. doi: 10.1016/S0887-8994(03)00413-2
15. Kurlan R, Johnson D, Kaplan EL, Tourette Syndrome Study Group. Streptococcal infection and exacerbations of childhood tics and obsessive-compulsive symptoms: a prospective blinded cohort study. Pediatrics. (2008) 121:1188–97. doi: 10.1542/peds.2007-2657
16. Morris CM, Pardo-Villamizar C, Gause CD, Singer HS. Serum autoantibodies measured by immunofluorescence confirm a failure to differentiate PANDAS and Tourette syndrome from controls. J Neurol Sci. (2009) 276:45–8. doi: 10.1016/j.jns.2008.08.032
17. Stagi S, Rigante D, Lepri G, Bertini F, Matucci-Cerinic M, Falcini F. Evaluation of autoimmune phenomena in patients with paediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS). Autoimmun Rev. (2014) 13:1236–40. doi: 10.1016/j.autrev.2014.08.009
18. Cox CJ, Zuccolo AJ, Edwards EV, Mascaro-Blanco A, Alvarez K, Stoner J, et al. Antineuronal antibodies in a heterogeneous group of youth and young adults with tics and obsessive-compulsive disorder. J Child Adoles Psychopharmacol. (2015) 25:76–85. doi: 10.1089/cap.2014.0048
19. Stagi S, Lepri G, Rigante D, Matucci Cerinic M, Falcini F. Cross-sectional evaluation of plasma vitamin D levels in a large cohort of italian patients with paediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. J Child Adolesc Psychopharmacol. (2018) 28:124–9. doi: 10.1089/cap.2016.0159
20. Chain JL, Alvarez K, Mascaro-Blanco A, Reim S, Bentley R, Hommer R, et al. Autoantibody biomarkers for basal ganglia encephalitis in sydenham chorea and paediatric autoimmune neuropsychiatric disorder associated with streptococcal infections. Front Psychiatry. (2020) 11:564. doi: 10.3389/fpsyt.2020.00564
21. Luo F, Leckman JF, Katsovich L, Findley D, Grantz H, Tucker DM, et al. Prospective longitudinal study of children with tic disorders and/or obsessive-compulsive disorder: relationship of symptom exacerbations to newly acquired streptococcal infections. Paediatrics. (2004) 113:e578–85. doi: 10.1542/peds.113.6.e578
22. Morris-Berry CM, Pollard M, Gao S, Thompson C, Singer HS. Anti-streptococcal, tubulin, and dopamine receptor 2 antibodies in children with PANDAS and Tourette syndrome: single-point and longitudinal assessments. J Neuroimmunol. (2013) 264:106–13. doi: 10.1016/j.jneuroim.2013.09.010
23. Murphy TK, Lewin AB, Parker-Athill EC, Storch EA, Mutch PJ. Tonsillectomies and adenoidectomies do not prevent the onset of paediatric autoimmune neuropsychiatric disorder associated with group A streptococcus. Paediatr Infect Dis J. (2013) 32:834–8. doi: 10.1097/INF.0b013e31829062e2
24. Murphy TK, Gerardi DM, Leckman JF. Paediatric acute-onset neuropsychiatric syndrome. Psychiatr Clin North Am. (2014) 37:353–74. doi: 10.1016/j.psc.2014.06.001
25. Swedo SE, Leonard HL, Mittleman BB, Allen AJ, Rapoport JL, Dow SP, et al. Identification of children with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections by a marker associated with rheumatic fever. Am J Psychiatry. (1997) 154:110–2. doi: 10.1176/ajp.154.1.110
26. Sokol MS, Ward PE, Tamiya H, Kondo DG, Houston D, Zabriskie JB. D8/17 expression on B lymphocytes in anorexia nervosa. Am J Psychiatry. (2002) 159:1430–2. doi: 10.1176/appi.ajp.159.8.1430
27. Çelik G, Taş D, Tahiroglu A, Avci A, Yüksel B, Çam P. Vitamin D deficiency in obsessive-compulsive disorder patients with paediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: a case control study. Noro psikiyatriarsivi. (2016) 53:33–37. doi: 10.5152/npa.2015.8763
28. Frankovich J, Thienemann M, Pearlstein J, Crable A, Brown K, Chang K. Multidisciplinary clinic dedicated to treating youth with paediatric acute-onset neuropsychiatric syndrome: presenting characteristics of the first 47 consecutive patients. J Child Adolesc Psychopharmacol. (2015) 25:38–47. doi: 10.1089/cap.2014.0081
29. Gromark C, Harris RA, Wickström R, Horne A, Silverberg-Mörse M, Serlachius E, et al. Establishing a paediatric acute-onset neuropsychiatric syndrome clinic: baseline clinical features of the paediatric acute-onset neuropsychiatric syndrome cohort at Karolinska Institutet. J Child Adolesc Psychopharmacol. (2019) 29:625–33. doi: 10.1089/cap.2018.0127
30. Gromark C, Hesselmark E, Djupedal IG, Silverberg M, Horne A, Harris RA, et al. A two-to-five year follow-up of a paediatric acute-onset neuropsychiatric syndrome cohort. Child Psychiatry Hum Dev. (2021) 1–11. doi: 10.1007/s10578-021-01135-4
31. Chan A, Karpel H, Spartz E, Willett T, Farhadian B, Jeng M, et al. Hypoferritinemia and iron deficiency in youth with paediatric acute-onset neuropsychiatric syndrome. Paediatr Res. (2021) 89:1477–84. doi: 10.1038/s41390-020-1103-3
32. Gagliano A, Galati C, Ingrassia M, Ciuffo M, Alquino MA, Tanca MG, et al. Paediatric acute-onset neuropsychiatric syndrome: a data mining approach to a very specific constellation of clinical variables. J Child Adolesc Psychopharmacol. (2020) 30:495–511. doi: 10.1089/cap.2019.0165
33. Williams KA, Swedo SE, Farmer CA, Grantz H, Grant PJ, D'Souza P, et al. Randomized, controlled trial of intravenous immunoglobulin for paediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. J Am Acad Child Adoles Psychiatry. (2016) 55:860–7.e2. doi: 10.1016/j.jaac.2016.06.017
34. Murphy TK, Patel PD, McGuire JF, Kennel A, Mutch PJ, Parker-Athill EC, et al. Characterization of the paediatric acute-onset neuropsychiatric syndrome phenotype. J Child Adolesc Psychopharmacol. (2015) 25:14–25. doi: 10.1089/cap.2014.0062
35. Lepri G, Rigante D, Bellando Randone S, Meini A, Ferrari A, Tarantino G, et al. Clinical-serological characterization and treatment outcome of a large cohort of Italian children with paediatric autoimmune neuropsychiatric disorder associated with streptococcal infection and paediatric acute neuropsychiatric syndrome. J Child Adolesc Psychopharmacol. (2019) 29:608–14. doi: 10.1089/cap.2018.0151
36. Murphy TK, Brennan EM, Johnco C, Parker-Athill EC, Miladinovic B, Storch EA, et al. A double-blind randomized placebo-controlled pilot study of azithromycin in youth with acute-onset obsessive-compulsive disorder. J Child Adolesc Psychopharmacol. (2017) 27:640–51. doi: 10.1089/cap.2016.0190
37. Leonard HL, Swedo SE. Paediatric autoimmune neuropsychiatric disorders associated with streptococcal infection (PANDAS). Int J Neuropsychopharmacol. (2001) 4:191–8. doi: 10.1017/S1461145701002371
38. van Toorn R, Weyers HH, Schoeman JF. Distinguishing PANDAS from Sydenham's chorea: case report and review of the literature. Eur J Paediatr Neurol. (2004) 8:211–6. doi: 10.1016/j.ejpn.2004.03.005
39. da Rocha FF, Correa H, Teixeira AL. Obsessive-compulsive disorder and immunology: a review. Prog Neuropsychopharmacol Biol Psychiatry. (2008) 32:1139–46. doi: 10.1016/j.pnpbp.2007.12.026
40. Bond M, Moll N, Rosello A, Bond R, Schnell J, Burger B, et al. Vitamin D levels in children and adolescents with chronic tic disorders: a multicentre study. Eur Child Adoles Psychiatry. (2021). doi: 10.1007/s00787-021-01757-y. [Epub ahead of print].
41. Barone R, Alaimo S, Messina M, Pulvirenti A, Bastin J, Ferro A, et al. A subset of patients with autism spectrum disorders show a distinctive metabolic profile by dried blood spot analyses. Front Psychiatry. (2018) 9:636. doi: 10.3389/fpsyt.2018.00636
42. Singer HS, Loiselle CR, Lee O, Minzer K, Swedo S, Grus FH. Anti-basal ganglia antibodies in PANDAS. Mov Disord. (2004) 19:406–15. doi: 10.1002/mds.20052
43. Dale RC, Heyman I, Giovannoni G, Church AW. Incidence of anti-brain antibodies in children with obsessive-compulsive disorder. Br J Psychiatry J Ment Sci. (2005) 187:314–9. doi: 10.1192/bjp.187.4.314
44. Singer HS, Hong JJ, Yoon DY, Williams PN. Serum autoantibodies do not differentiate PANDAS and Tourette syndrome from controls. Neurology. (2005) 65:1701–7. doi: 10.1212/01.wnl.0000183223.69946.f1
45. Kirvan CA, Swedo SE, Snider LA, Cunningham MW. Antibody-mediated neuronal cell signaling in behaviour and movement disorders. J Neuroimmunol. (2006) 179:173–9. doi: 10.1016/j.jneuroim.2006.06.017
46. Morer A, Lázaro L, Sabater L, Massana J, Castro J, Graus F. Antineuronal antibodies in a group of children with obsessive-compulsive disorder and Tourette syndrome. J Psychiatr Res. (2008) 42:64–8. doi: 10.1016/j.jpsychires.2006.09.010
47. Gause C, Morris C, Vernekar S, Pardo-Villamizar C, Grados MA, Singer HS. Antineuronal antibodies in OCD: comparisons in children with OCD-only, OCD + chronic tics and OCD+PANDAS. J Neuroimmunol. (2009) 214:118–24. doi: 10.1016/j.jneuroim.2009.06.015
48. Brilot F, Merheb V, Ding A, Murphy T, Dale RC. Antibody binding to neuronal surface in Sydenham chorea, but not in PANDAS or Tourette syndrome. Neurology. (2011) 76:1508–13. doi: 10.1212/WNL.0b013e3182181090
49. Dale RC, Merheb V, Pillai S, Wang D, Cantrill L, Murphy TK, et al. Antibodies to surface dopamine-2 receptor in autoimmune movement and psychiatric disorders. Brain. (2012) 135:3453–68. doi: 10.1093/brain/aws256
50. Singer HS, Mascaro-Blanco A, Alvarez K, Morris-Berry C, Kawikova I, Ben-Pazi H, et al. Neuronal antibody biomarkers for Sydenham's chorea identify a new group of children with chronic recurrent episodic acute exacerbations of tic and obsessive-compulsive symptoms following a streptococcal infection. PLoS ONE. (2015) 10:e0120499. doi: 10.1371/journal.pone.0120499
51. Cunningham MW. (2014). Rheumatic fever, autoimmunity, and molecular mimicry: the streptococcal connection. Int Rev Immunol. 33:314–29. doi: 10.3109/08830185.2014.917411
52. Shimasaki C, Frye RE, Trifiletti R, Cooperstock M, Kaplan G, Melamed I, et al. Evaluation of the Cunningham Panel™ in paediatric autoimmune neuropsychiatric disorder associated with streptococcal infection (PANDAS) and paediatric acute-onset neuropsychiatric syndrome (PANS): Changes in antineuronal antibody titers parallel changes in patient symptoms. J Neuroimmunol. (2020) 339:577138. doi: 10.1016/j.jneuroim.2019.577138
53. Singer HS, Gause C, Morris C, Lopez P. Serial immune markers do not correlate with clinical exacerbations in paediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. Paediatrics. (2008) 121:1198–205. doi: 10.1542/peds.2007-2658
54. Hesselmark E, Bejerot S. Biomarkers for diagnosis of paediatric acute neuropsychiatric syndrome (PANS) - sensitivity and specificity of the Cunningham panel. J Neuroimmunol. (2017) 312:31–7. doi: 10.1016/j.jneuroim.2017.09.002
55. Frick LR, Rapanelli M, Jindachomthong K, Grant P, Leckman JF, Swedo S, et al. Differential binding of antibodies in PANDAS patients to cholinergic interneurons in the striatum. Brain Behav Immun. (2018) 69:304–11. doi: 10.1016/j.bbi.2017.12.004
56. Xu J, Liu RJ, Fahey S, Frick L, Leckman J, Vaccarino F, et al. Antibodies from children with PANDAS bind specifically to striatal cholinergic interneurons and alter their activity. Am J Psychiatry. (2021) 178:48–64. doi: 10.1176/appi.ajp.2020.19070698
57. Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. (2016) 15:391–404. doi: 10.1016/S1474-4422(15)00401-9
58. Shekunov J, Blacker CJ, Vande Voort JL, Tillema JM, Croarkin PE, Romanowicz M. Immune mediated paediatric encephalitis - need for comprehensive evaluation and consensus guidelines. BMC Neurol. (2020) 20:44. doi: 10.1186/s12883-020-1605-y
59. Gamucci A, Uccella S, Sciarretta L, D'Apruzzo M, Calevo MG, Mancardi MM, et al. PANDAS and PANS: clinical, neuropsychological, and biological characterization of a monocentric series of patients and proposal for a diagnostic protocol. J Child Adoles Psychopharmacol. (2019) 29:305–12. doi: 10.1089/cap.2018.0087
60. Gaughan T, Buckley A, Hommer R, Grant P, Williams K, Leckman JF, et al. Rapid eye movement sleep abnormalities in children with paediatric acute-onset neuropsychiatric syndrome (PANS). J Clin Sleep Med. (2016) 12:1027–32. doi: 10.5664/jcsm.5942
61. Santoro JD, Frankovich J, Bhargava S. Continued presence of period limb movements during REM sleep in patients with chronic static pediatric acute-onset neuropsychiatric syndrome (PANS). J Clin Sleep Med. (2018) 14:1187–92. doi: 10.5664/jcsm.7222
62. Giedd JN, Rapoport JL, Leonard HL, Richter D, Swedo SE. Case study: acute basal ganglia enlargement and obsessive-compulsive symptoms in an adolescent boy. J Am Acad Child Adolesc Psychiatry. (1996) 35:913–5. doi: 10.1097/00004583-199607000-00017
63. Giedd JN, Rapoport JL, Garvey MA, Perlmutter S, Swedo SE. MRI assessment of children with obsessive-compulsive disorder or tics associated with streptococcal infection. Am J Psychiatry. (2000) 157:281–3. doi: 10.1176/appi.ajp.157.2.281
64. Kumar A, Williams MT, Chugani HT. Evaluation of basal ganglia and thalamic inflammation in children with paediatric autoimmune neuropsychiatric disorders associated with streptococcal infection and Tourette syndrome: a positron emission tomographic (PET) study using 11C-[R]-PK11195. J Child Neurol. (2015) 30:749–56. doi: 10.1177/0883073814543303
65. Cabrera B, Romero-Rebollar C, Jiménez-Ángeles L, Genis-Mendoza AD, Flores J, Lanzagorta N, et al. Neuroanatomical features and its usefulness in classification of patients with PANDAS. CNS Spectr. (2019) 24:533–43. doi: 10.1017/S1092852918001268
66. Zheng J, Frankovich J, McKenna ES, Rowe NC, MacEachern SJ, Ng NN, et al. Association of paediatric acute-onset neuropsychiatric syndrome with microstructural differences in brain regions detected via diffusion-weighted magnetic resonance imaging. JAMA Network Open. (2020) 3:e204063. doi: 10.1001/jamanetworkopen.2020.4063
67. Giedd JN, Rapoport JL, Kruesi MJ, Parker C, Schapiro MB, Allen AJ, et al. Sydenham's chorea: magnetic resonance imaging of the basal ganglia. Neurology. (1995) 45:2199–202. doi: 10.1212/WNL.45.12.2199
68. Snider LA, Sachdev V, MaCkaronis JE, St Peter M, Swedo SE. Echocardiographic findings in the PANDAS subgroup. Paediatrics. (2004) 114:e748–51. doi: 10.1542/peds.2004-0308
69. Murciano M, Biancone DM, Capata G, Tristano I, Martucci V, Guido CA, et al. Focus on cardiologic findings in 30 children with PANS/PANDAS: an Italian single-center observational study. Front Paediatr. (2019) 7:395. doi: 10.3389/fped.2019.00395
70. Quagliariello A, Del Chierico F, Russo A, Reddel S, Conte G, Lopetuso LR, et al. Gut microbiota profiling and gut-brain crosstalk in children affected by paediatric acute-onset neuropsychiatric syndrome and paediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. Front Microbiol. (2018) 9:675. doi: 10.3389/fmicb.2018.00675
71. Loffredo L, Ettorre E, Zicari AM, Inghilleri M, Nocella C, Perri L, et al. Oxidative stress and gut-derived lipopolysaccharides in neurodegenerative disease: role of NOX2. Oxid Med Cell Longev. (2020) 2020:8630275. doi: 10.1155/2020/8630275
72. Cocuzza S, Marino S, Gulino A, Pustorino E, Murabito P, Maniaci A, et al. ENT involvement and orobuccal movements' disorders in Pandas patients: assessment and rehabilitations tools. Eur Rev Med Pharmacol Sci. (2019) 23:4110–7. doi: 10.26355/eurrev_201905_17912
73. Luleyap HU, Onatoglu D, Yilmaz MB, Alptekin D, Tahiroglu AY, Cetiner S, et al. Association between paediatric autoimmune neuropsychiatric disorders associated with streptococcal infections disease and tumour necrosis factor-α gene-308 g/a,−850 c/t polymorphisms in 4-12-year-old children in Adana/Turkey. Indian J Hum Genet. (2013) 19:196–201. doi: 10.4103/0971-6866.116116
74. Çelik GG, Taş DA, Tahiroglu AY, Erken E, Seydaoglu G, Ray PÇ, et al. Mannose-binding lectin 2 gene polymorphism in PANDAS patients. Noro psikiyatriarsivi. (2018) 56:99–105. doi: 10.29399/npa.22811
75. Baj J, Sitarz E, Forma A, Wróblewska K, Karakuła-Juchnowicz H. Alterations in the nervous system and gut microbiota after β-haemolytic streptococcus group a infection-characteristics and diagnostic criteria of PANDAS recognition. Int J Mol Sci. (2020) 21:1476. doi: 10.3390/ijms21041476
76. Lewin AB, Storch EA, Mutch PJ, Murphy TK. Neurocognitive functioning in youth with paediatric autoimmune neuropsychiatric disorders associated with streptococcus. J Neuropsychiatry Clin Neurosci. (2011) 23:391–8. doi: 10.1176/jnp.23.4.jnp391
77. Hirschtritt ME, Hammond CJ, Luckenbaugh D, Buhle J, Thurm AE, Casey BJ, et al. Executive and attention functioning among children in the PANDAS subgroup. Child Neuropsychol. (2009) 15:179–94. doi: 10.1080/09297040802186899
78. Colvin MK, Erwin S, Alluri PR, Laffer A, Pasquariello K, Williams KA. Cognitive, graphomotor, and psychosocial challenges in paediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS). J Neuropsychiatry Clin Neurosci. (2021) 33:90–7. doi: 10.1176/appi.neuropsych.20030065
79. Calaprice D, Tona J, Parker-Athill EC, Murphy TK. A survey of paediatric acute-onset neuropsychiatric syndrome characteristics and course. J Child Adolesc Psychopharmacol. (2017) 27:607–18. doi: 10.1089/cap.2016.0105
80. Johnson M, Fernell E, Preda I, Wallin L, Fasth A, Gillberg C, et al. Paediatric acute-onset neuropsychiatric syndrome in children and adolescents: an observational cohort study. Lancet Child Adolesc Health. (2019) 3:175–80. doi: 10.1016/S2352-4642(18)30404-8
81. Hesselmark E, Bejerot S. Clinical features of paediatric acute-onset neuropsychiatric syndrome: findings from a case- control study. BJPsych Open. (2019) 5:e25. doi: 10.1192/bjo.2019.10
82. Müller-Vahl KR, Szejko N, Verdellen C, Roessner V, Hoekstra PJ, Hartmann A, et al. European clinical guidelines for Tourette syndrome and other tic disorders: summary statement. Eur Child Adolesc Psychiatry. (2021). doi: 10.1007/s00787-021-01832-4
83. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5), Section II, Diagnostic Criteria and Codes, Obsessive-Compulsive and Related Disorders. Washington, DC: American Psychiatric Association (2013). p. 262.
84. Loiselle CR, Lee O, Moran TH, Singer HS. Striatal microinfusion of Tourette syndrome and PANDAS sera: failure to induce behavioural changes. Mov Disord. (2004) 19:390–6. doi: 10.1002/mds.10522
85. Kilbertus S, Brannan R, Sell E, Doja A. No cases of PANDAS on Follow-up of patients referred to a paediatric movement disorders clinic. Front Paediatr. (2014) 2:104. doi: 10.3389/fped.2014.00104
86. Pavone P, Ceccarelli M, Marino S, Caruso D, Falsaperla R, Berretta M, et al. SARS-CoV-2 related paediatric acute-onset neuropsychiatric syndrome. Lancet Child Adolesc Health. (2021) 5:e19–21. doi: 10.1016/S2352-4642(21)00135-8
87. Guido CA, Loffredo L, Zicari AM, Pavone P, Savasta S, Gagliano A, et al. The impact of the COVID-19 epidemic during the lockdown on children with the paediatric acute-onset neuropsychiatric syndrome (PANDAS/PANS): the importance of environmental factors on clinical conditions. Front Neurol. (2021) 12:702356. doi: 10.3389/fneur.2021.702356
Keywords: pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS), pediatric acute-onset neuropsychiatric syndrome (PANS), autoimmune, neuropsychiatric disorders, streptococcal infection, diagnostic criteria
Citation: Prato A, Gulisano M, Scerbo M, Barone R, Vicario CM and Rizzo R (2021) Diagnostic Approach to Pediatric Autoimmune Neuropsychiatric Disorders Associated With Streptococcal Infections (PANDAS): A Narrative Review of Literature Data. Front. Pediatr. 9:746639. doi: 10.3389/fped.2021.746639
Received: 24 July 2021; Accepted: 24 September 2021;
Published: 27 October 2021.
Edited by:
Kette D. Valente, Universidade de São Paulo, BrazilReviewed by:
Ruzica Kravljanac, The Institute for Health Protection of Mother and Child Serbia, SerbiaKyle Williams, Massachusetts General Hospital and Harvard Medical School, United States
Susanne Bejerot, Örebro University, Sweden
Copyright © 2021 Prato, Gulisano, Scerbo, Barone, Vicario and Rizzo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adriana Prato, YWRyaWFuYS5wcmF0b0B1bmltZS5pdA==
 Adriana Prato
Adriana Prato Mariangela Gulisano
Mariangela Gulisano Miriam Scerbo2
Miriam Scerbo2 Rita Barone
Rita Barone Carmelo M. Vicario
Carmelo M. Vicario Renata Rizzo
Renata Rizzo