- 1Department of Urology, Yantai Yuhuangding Hospital, Qingdao University, Yantai, China
- 2The Second Clinical Medical College, Binzhou Medical University, Yantai, China
- 3Department of Pharmacy, Yantai Yuhuangding Hospital, Qingdao University, Yantai, China
- 4Department of Urology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
Objective: Pediatric urolithiasis is a common condition, and medical expulsive therapy has grown to be accepted by many parents. We carried out a meta-analysis to identify the efficacy and safety of α-adrenergic blockers for the treatment of pediatric urolithiasis.
Methods: We identified related articles from the PubMed, Embase, and Cochrane Library databases. All published randomized controlled trials (RCTs) describing the use of α-adrenergic blockers and placebo treatment for pediatric distal urolithiasis were involved. The outcomes included stone expulsion rate, stone expulsion time, pain episodes, need for analgesia, adverse events, and related subgroup analyses.
Results: A total of nine RCTs were involved in our study, including 586 patients. We found that α-adrenergic blockers could significantly increase the rate of stone expulsion [odds ratio (OR), 3.49; 95% confidence interval (CI), 2.38–5.12; p < 0.00001], reduce the stone expulsion time [mean difference (MD), −5.15; 95% CI, −8.51 to −1.80; p = 0.003], and decrease pain episodes (MD, −1.02; 95% CI, −1.33 to −0.72; p < 0.00001) and analgesia demand (MD, −0.92; 95% CI, −1.32 to −0.53; p < 0.00001) but had a higher incidence of side effects (MD, 2.83; 95% CI, 1.55 to 5.15; p = 0.0007). During subgroup analyses, different medications (tamsulosin, doxazosin, and silodosin) also exhibited better efficiencies than placebo, except for doxazosin, which showed no difference in expulsion time (MD, −1.23; 95% CI, −2.98 to 0.51; p = 0.17). The three kinds of α-adrenergic blockers also appeared to be better tolerated, except for tamsulosin with its greater number of adverse events (MD, 2.85; 95% CI, 1.34 to 6.03; p = 0.006). Silodosin led to a better expulsion rate than tamsulosin (OR, 0.42; 95% CI, 0.20 to 0.92; p = 0.03). In addition, α-adrenergic blockers increased the stone expulsion rate regardless of stone size and decreased the expulsion time of stones measuring <5 mm (MD, −1.71; 95% CI, −2.91 to −0.52; p = 0.005), which was not the case for stones measuring >5 mm in expulsion time (MD, −3.61; 95% CI, −10.17 to 2.96; p = 0.28).
Conclusion: Our review suggests that α-adrenergic blockers are well-tolerated and efficient for treating pediatric distal urolithiasis. We also conclude that silodosin is the best choice of drug, offering a better expulsion rate, but it remains to be evaluated further by future studies.
Introduction
The occurrence of urolithiasis in children has become more and more frequent, with an incidence of about 0.1–5% (1–3). The reasons for urinary stone formation in children are various and include metabolic, environmental, and nutritional factors (4, 5). The typical presentations of urolithiasis are hematuria, dysuria, and pain in older children, whereas younger children present with non-specific symptoms like irritability (6).
The appropriate treatment for urolithiasis is generally selected according to stone size, location, and composition and the urinary tract anatomy (7). Over time, the main surgical technique has also evolved from open stone surgery to minimally invasive procedures with the development of endoscopic equipment (8). Medical expulsive therapy (MET) has also been accepted as initial management for small distal ureteric stones to avoid the risk of anesthesia and associated costs (9, 10), and α-adrenergic blockers are the preferred medication choice in this context (11).
In recent years, α-adrenergic blockers have been recommended for the treatment of distal ureter stones (12). Many studies have shown that α-adrenergic blockers achieve great success in both the spontaneous stone expulsion rate and time in adults (13–18). In addition, α-adrenergic blockers have also been applied to treat urolithiasis in children as MET (19), but few strong evidence-based studies on the feasibility of using α-adrenergic blockers in the treatment of urolithiasis in children exist to date.
In this systematic review and meta-analysis, we assessed the efficacy of α-adrenergic blockers as a medical treatment for distal ureteral stones in pediatric patients using data from published randomized controlled trials (RCTs).
Methods
Search Strategy
This analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses guideline (20). RCTs covering α-adrenergic blockers in the treatment of pediatric distal urolithiasis were collected by three authors from the PubMed (1997 to November 2021), Embase (1997 to November 2021), and Cochrane Library (1997 to November 2021) literature databases. Table 1 summarizes the search strategy using the Populations, Interventions, Comparators, Outcomes, and Study designs. The search terms used were as follows: “alpha-adrenergic blockers,” “distal ureter,” “ureteral stone,” “calculi,” “urolithiasis,” “children,” “pediatric,” and “randomized controlled trials.” We reviewed all search results to confirm the availability of studies and to extract further information. The references of related articles were also searched for eligible studies.
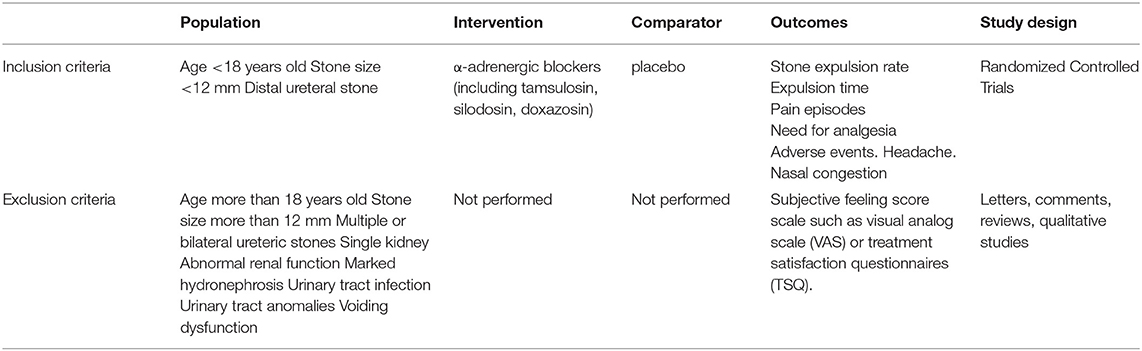
Table 1. Search strategy according to populations, interventions, comparators, outcomes, and study designs (PICOS).
Inclusion Criteria
Studies were included in this analysis if they met the following criteria: 1) the study analyzed the efficiency of α-adrenergic blockers and placebo treatment in pediatric distal urolithiasis; 2) a full-text version of the article could be obtained; 3) the study was an RCT and provided complete and accurate data, including its sample size and the valuable results of each indicator; 4) patients were <18 years of age. The related details of inclusion criteria are shown in Table 1. If a study was published in multiple journals or at different times, the most comprehensive version of the study was included in this investigation. If the same group of participants was studied by one group of researchers in several experiments, all studies were included. Compared with retrospective studies, RCTs have stricter inclusion and exclusion criteria.
Quality Assessment
The quality of all included RCTs was evaluated using the Cochrane Handbook (21). The quality of individual studies was determined according to its assessment methods, including patient allocation, concealment of allocation, and blinding method. Each included study was assessed using the guidelines published in the Cochrane Handbook for Systematic Reviews of Interventions, version 5.4.0 (22). All studies were classified based on the following quality assessment criteria: (+) the study met all quality criteria and had a low risk of bias; (?) the study met most quality criteria and had a moderate risk of bias; or (–) the study met few quality criteria and had a high risk of bias. Different opinions about the classifications of studies were solved by discussion among all authors.
Data Extraction
We collected valuable information from all included articles, including the name of the first author, the publication time, the study type, the capacity of the study sample, the treatment of patients, and data of study outcomes (i.e., stone expulsion rate, expulsion time, adverse events, pain episodes, analgesia requirement, and stone size). The main outcome was the stone expulsion rate, and the secondary outcome was the stone expulsion time; however, other outcomes like pain episodes and analgesia demand were also analyzed, and the safety of different medications was identified by adverse events. The stone expulsion rate was defined by the ratio of pediatric patients with complete expulsion and incomplete expulsion, which was tested using plain kidney ureter bladder, non-contrast CT, and ultrasonography. The stone expulsion time was defined as the time to stone passage, which was confirmed by visual observation of some or all of the stone.
Statistical Analysis and Meta-Analysis
The data of this study were statistically analyzed using the Review Manager software (version 5.4.0; Cochrane Collaboration, London, UK) (22). Changes in the stone expulsion time, stone expulsion rate, pain episodes, and adverse events were analyzed to declare the effect of α-adrenergic blockers in the treatment of pediatric urolithiasis. Continuous data were analyzed by the mean difference (MD), and dichotomous data were evaluated by odds ratios (ORs) with 95% confidence intervals (95% CIs) (23). A fixed-effects model was applied if the study was deemed to be homogenous; if the p > 0.05, then a random-effects model was used. Inconsistent results were analyzed with the I2 statistic. If the p < 0.05, the results were considered to indicate statistical significance.
Results
Characteristics of Included Studies
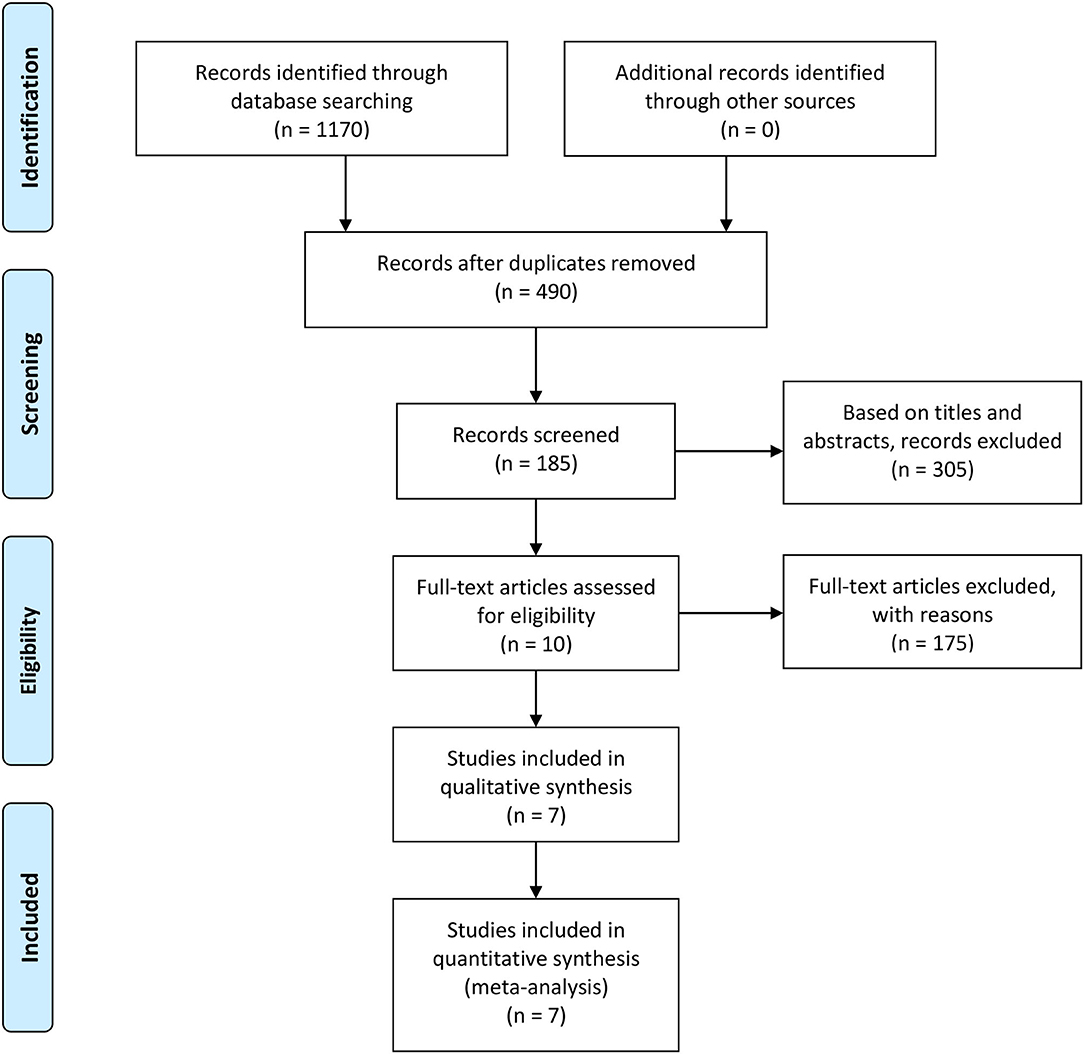
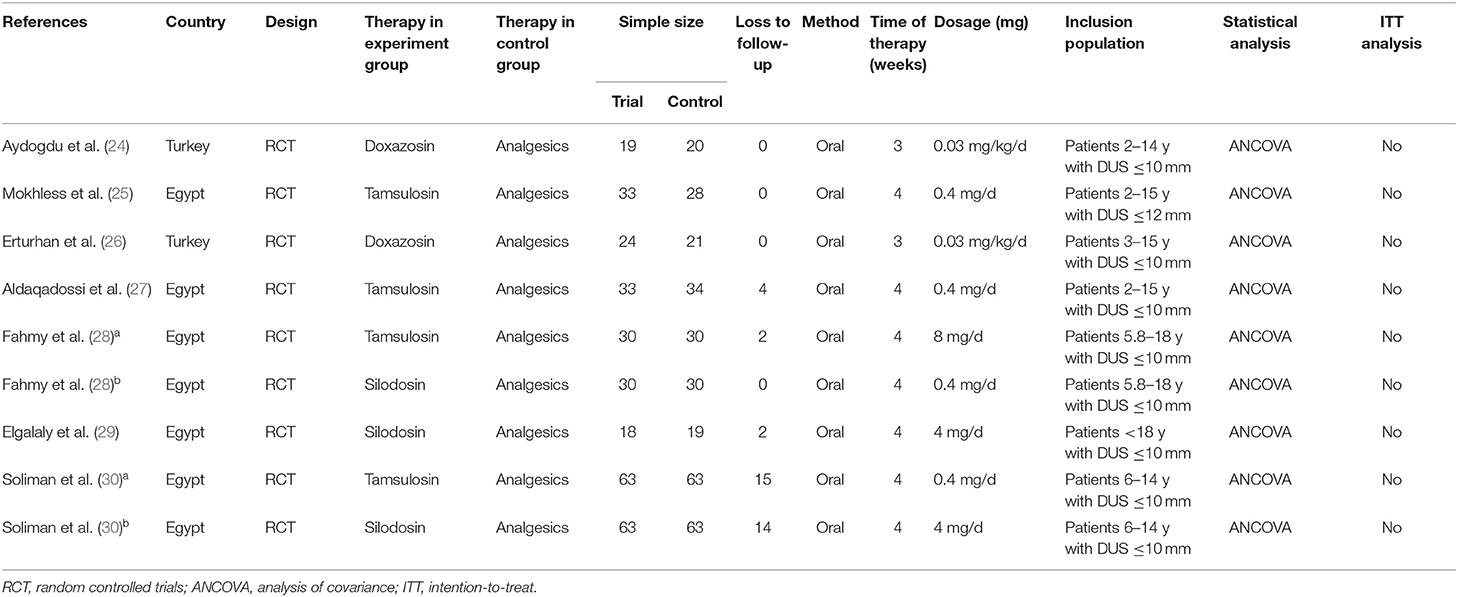
A total of 185 articles found to meet the inclusion criteria after their titles and abstracts were read were included. After reviewing the tables and figures in each article, however, 175 articles were excluded due to a lack of useful data. Then, when we reviewed the remaining 10 articles, 3 articles were further excluded because of their article designs. Finally, 7 high-quality articles were included in our review (24–30). Because 2 intervention groups were set in 2 articles, we regarded these 2 articles as 4 individual RCTs (28, 30). The 2 different RCTs in each study were marked as a and b. The details of the study selection process are shown in Figure 1, and the characteristics and features of 9 included RCTs are presented in Table 2.
The Quality of Eligible Studies
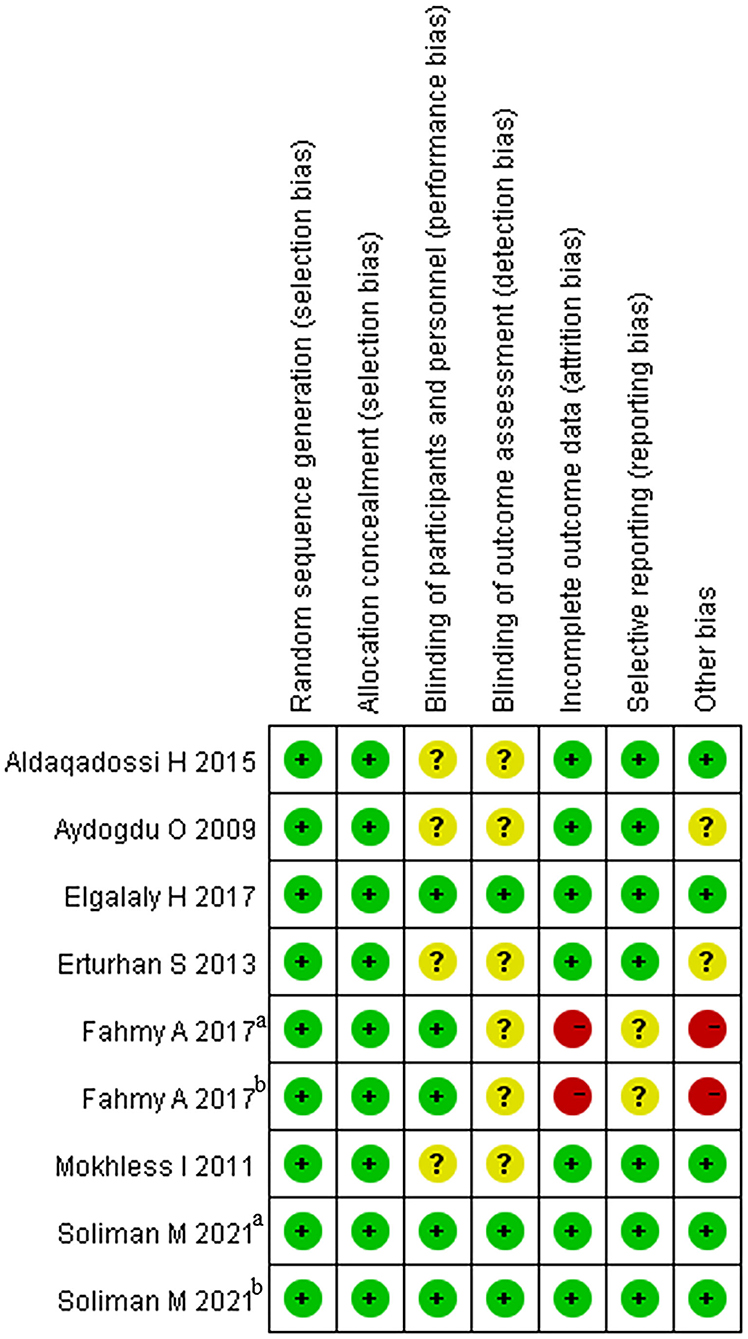
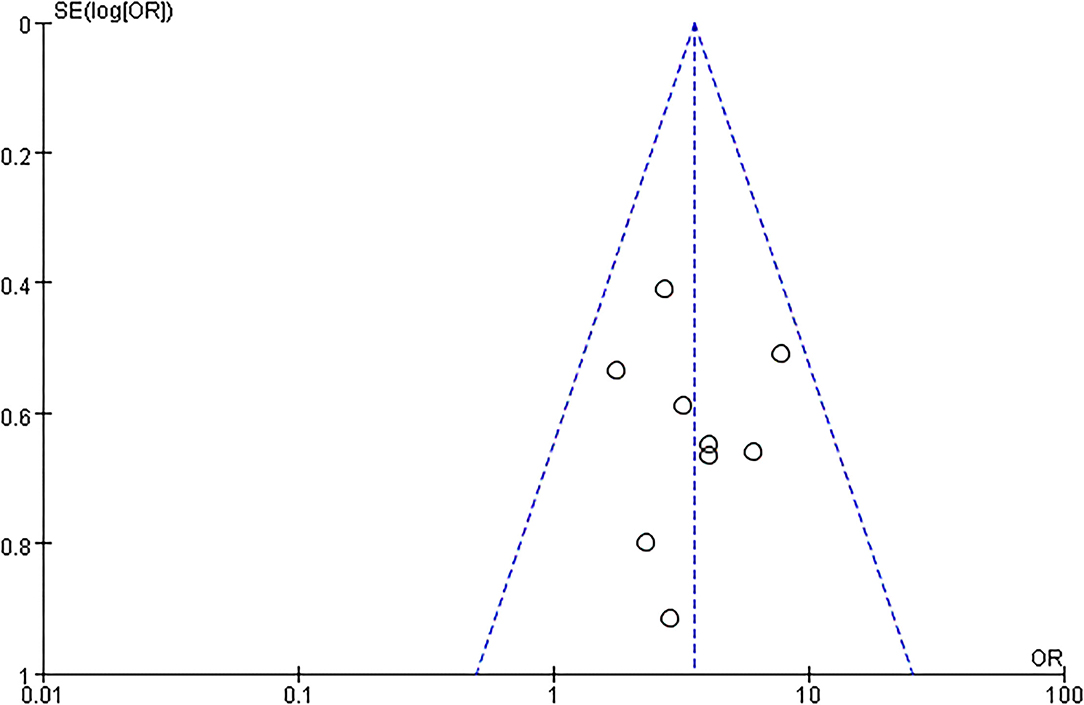
The articles included in our study were all RCTs, including 5 that were blinded RCTs (28–30). As a blinding method was not mentioned in 4 articles (24–27), the grade of the blinding method was “?” Two RCTs offered less useful data, so its quality grade was “–” (28). Details are shown in Figure 2. The capacity of each RCT was calculated. The funnel plot showed that the publication bias of all studies was not identifiable in this study (Figure 3).
Efficacy
Stone Expulsion Rate
All included articles (9 RCTs) contained data on the stone expulsion rate from 586 patients, including 294 patients in the experimental group and 292 participants in the control (placebo) group. We deemed the trials to be homogenous given that p > 0.05, so a fixed-effects model was selected for analysis and showed that the OR was 3.49 and the 95% CI was 2.38–5.12 (p < 0.00001) (Figure 4A). The result suggested that α-adrenergic blockers could improve the stone expulsion rate more effectively than placebo.
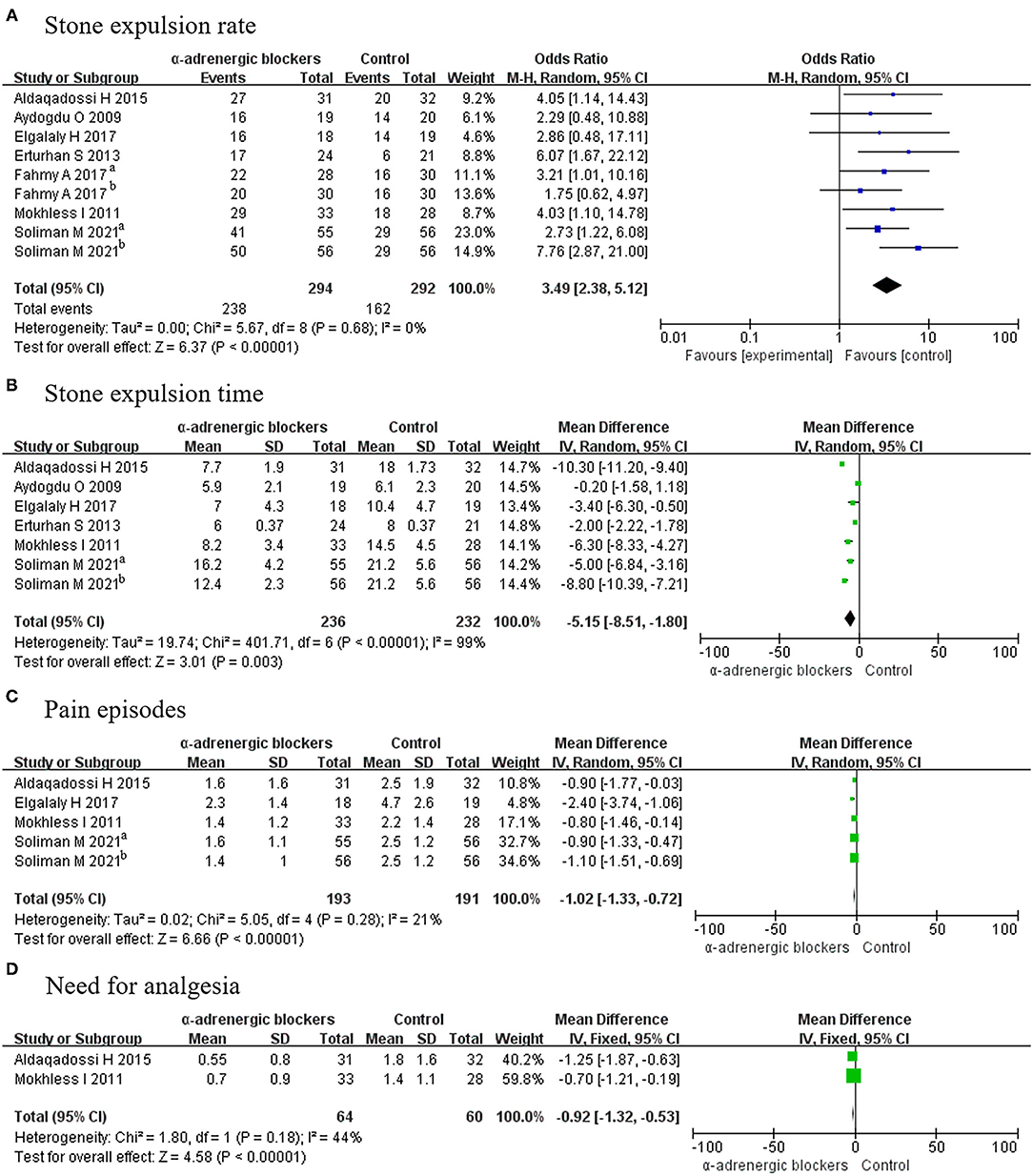
Figure 4. Forest plots showing the result of (A) stone expulsion rate, (B) stone expulsion time, (C) pain episodes, and (D) need for analgesia. M–H, Mantel–Haenszel; CI, confidence interval; df, degrees of freedom.
Stone Expulsion Time
Five articles covering 6 RCTs, in which a total of 236 experimental patients and 232 control participants were considered, reported on the stone expulsion time. A random-effects model was chosen to analyze these data because p < 0.05. The MD was −5.15, with a 95% CI of −8.51 to −1.80 (p = 0.003) (Figure 4B). It was concluded that α-adrenergic blockers had a shorter stone expulsion time than placebo.
Pain Episodes
Four articles covering 5 RCTs offering a total of 384 patients, including 193 patients in the experimental group and 191 participants in the control group, reported on pain outcomes. A fixed-effects model was chosen for analysis and revealed that the MD was −1.02 and the 95% CI was −1.33 to −0.72 (p < 0.00001) (Figure 4C). This result meant that α-adrenergic blockers experienced less pain than placebo.
Need for Analgesia
Only 2 RCTs offering 124 patients—including 64 experimental patients and 60 control participants—reported on the need for analgesia, and a fixed-effects model indicated that the experimental group required less placebo (MD, −0.92; 95% CI, −1.32 to −0.53; p < 0.00001) (Figure 4D).
Safety
Adverse Events
Six articles covering 7 RCTs, in which 236 patients received α-adrenergic blockers and 232 patients only received placebo, described the related details of adverse events. Because p > 0.05, a fixed-effects model was selected, and it was found that the OR was 2.83 and the 95% CI was 1.55–5.15 (p = 0.0007) (Figure 5A), which meant that the α-adrenergic blockers led to more adverse events than did placebo.
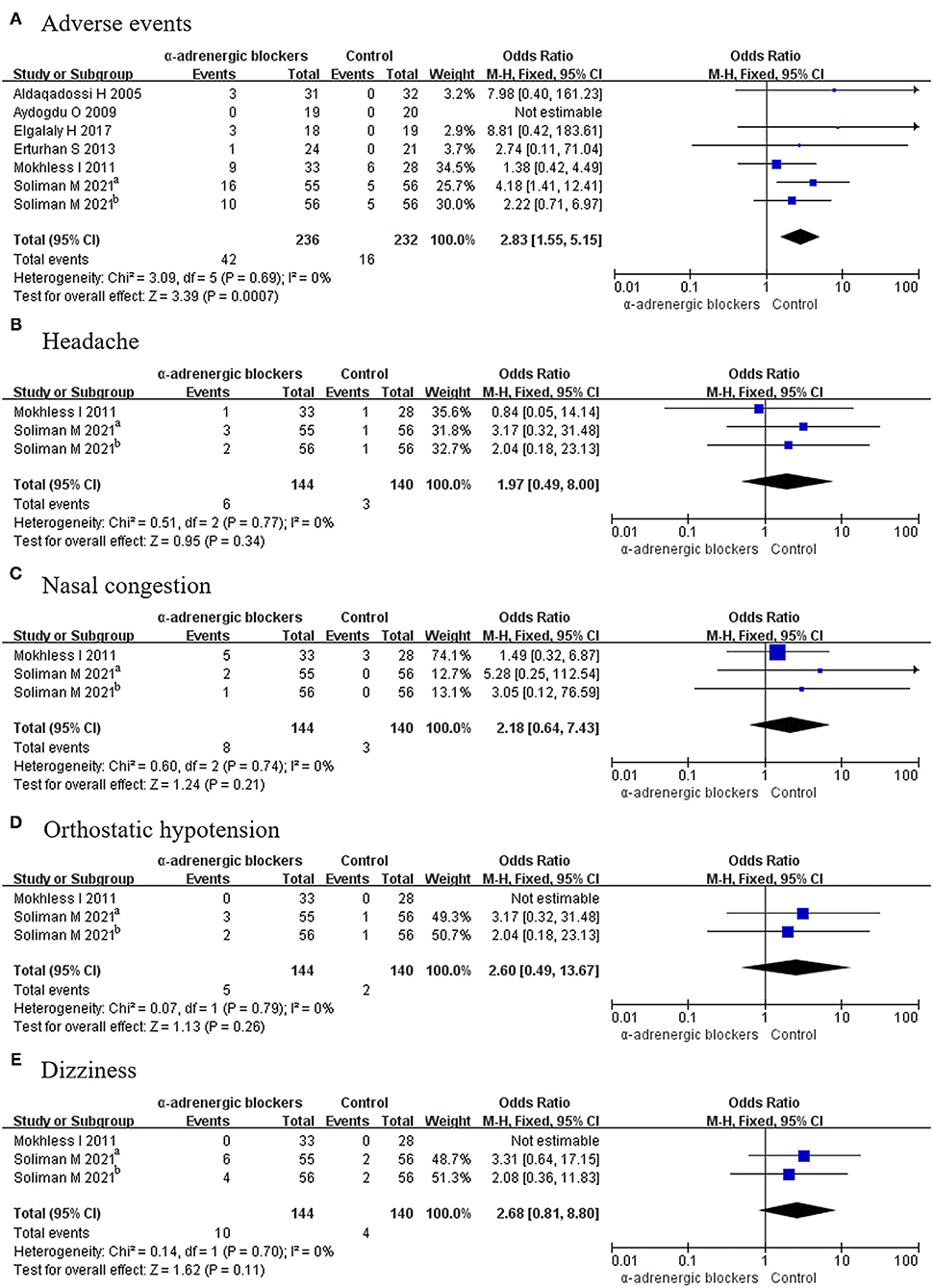
Figure 5. Forest plots showing the result of (A) adverse events, (B) headache, (C) nasal congestion, (D) orthostatic hypotension, and (E) dizziness. M–H, Mantel–Haenszel; CI, confidence interval; df, degrees of freedom.
Headache
Two articles covering 3 RCTs offered data on headache from 284 patients, including 144 patients in the experimental group and 140 participants in the control group. Given that p > 0.05, a fixed-effects model was used to compare the rate of headache between the 2 groups, and the OR was 1.97 and the 95% CI was 0.49–8.00, with a p > 0.05 (Figure 5B). This result signified that there was no difference in the rate of headache between the groups.
Nasal Congestion
Because p > 0.05, a fixed-effects model was used to analyze the rate of nasal congestion. A total of 3 RCTs were considered, among which 144 patients accepted α-adrenergic blockers and 140 patients accepted placebo. The results showed that the OR was 2.18 and the 95% CI was 0.64–7.43 (p > 0.05) (Figure 5C). This indicated that there was no difference in the occurrence of nasal congestion between the 2 groups.
Orthostatic Hypotension
Three RCTs discussed orthostatic hypotension, which occurred in 5 patients in the experimental group and 2 participants in the control group. Given that p > 0.05, a fixed-effects model was selected, and there was no difference between the groups, as the OR was 2.60 and the 95% CI was 0.49–13.67 (p > 0.05) (Figure 5D).
Dizziness
Dizziness was described in 3 RCTs involving 284 patients. A fixed-effects model was chosen given that p > 0.05, and the OR was 2.68 and the 95% CI was 0.81–8.80 (p > 0.05). No difference was found between α-adrenergic blockers and placebo (Figure 5E).
Subgroup Analysis
Different Medications
In this study, 3 kinds of α-adrenergic blockers, including tamsulosin, silodosin, and doxazosin, were used to treat pediatric urolithiasis. Subgroup analysis was performed in view of these different drugs.
Efficiency
Stone Expulsion Rate
In this assessment, 4 RCTs using tamsulosin, 3 RCTs using silodosin, and 2 RCTs using doxazosin were absorbed. The expulsion rate associated with tamsulosin was greater than that of placebo (OR, 2.80; 95% CI, 1.67–4.69; p < 0.0001) (Figure 6A). There was a significant difference in this parameter between silodosin and placebo (OR, 4.92; 95% CI, 2.48–9.77; p < 0.00001) (Figure 6B), and the same result was also found for doxazosin (OR, 4.04; 95% CI, 1.50–10.86; p = 0.006) (Figure 6C).
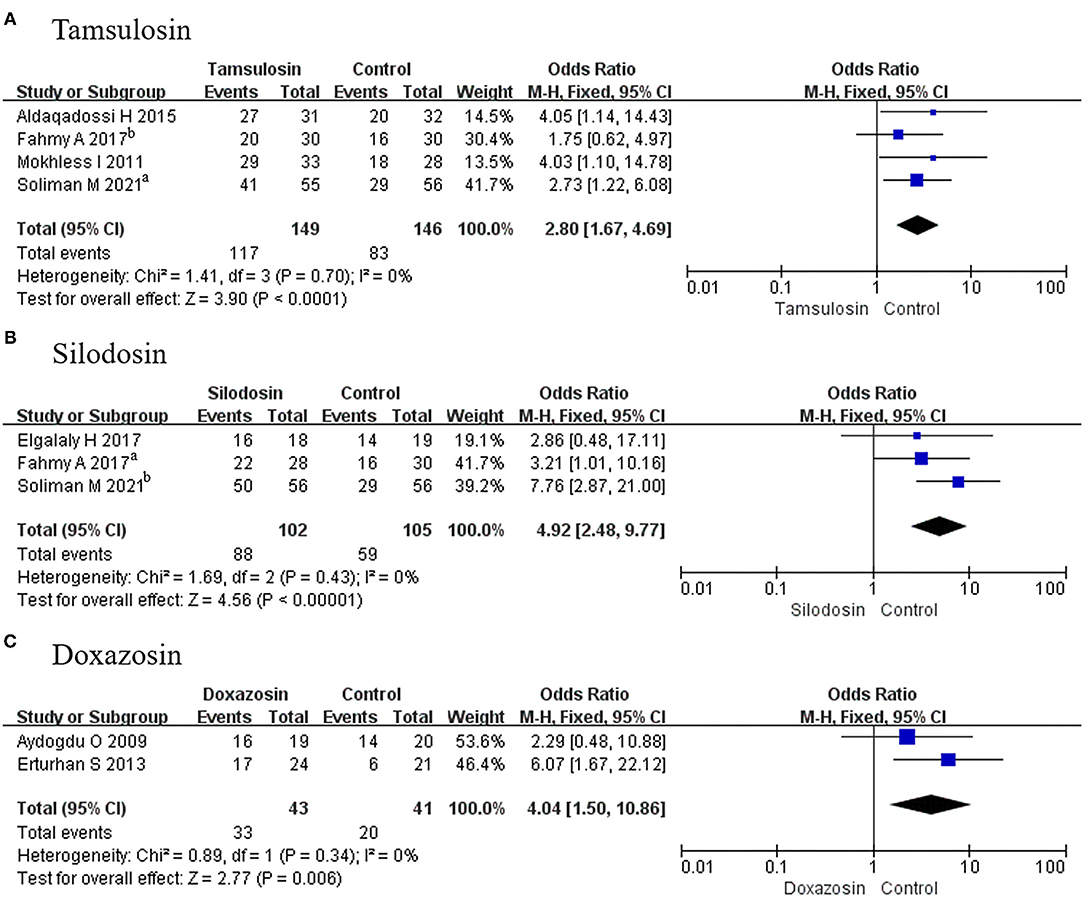
Figure 6. Forest plots showing the result of (A) subgroup analyses of tamsulosin in stone expulsion rate, (B) subgroup analyses of silodosin in stone expulsion rate, and (C) subgroup analyses of doxazosin in stone expulsion rate. M–H, Mantel–Haenszel; CI, confidence interval; df, degrees of freedom.
Stone Expulsion Time
In this analysis, 2 RCTs using tamsulosin, 2 RCTs using silodosin, and 2 RCTs using doxazosin were considered. It was found that the stone expulsion time was less for tamsulosin (MD, −7.27; 95% CI, −10.92 to −3.63; p < 0.0001) (Figure 7A) and silodosin (MD, −6.24; 95% CI, −11.53 to −0.96; p = 0.02), compared with placebo (Figure 7B). However, it was not different for doxazosin (MD, −1.23; 95% CI, −2.98 to 0.51; p = 0.17) (Figure 7C).
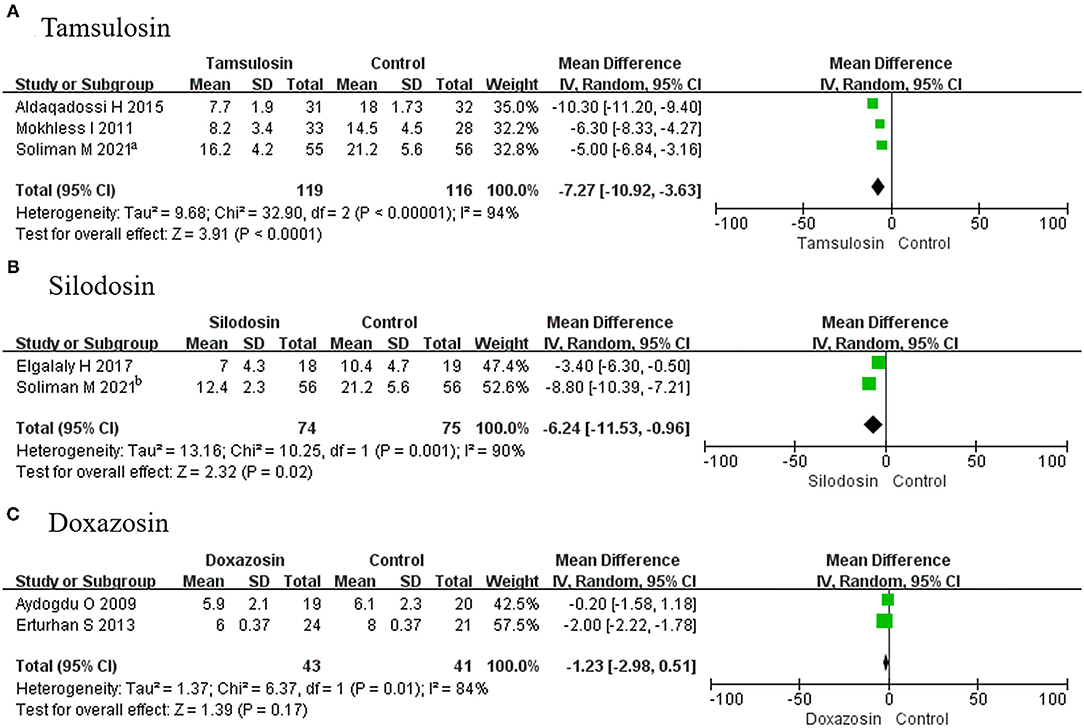
Figure 7. Forest plots showing the result of (A) subgroup analyses of tamsulosin in stone expulsion time, (B) subgroup analyses of silodosin in stone expulsion time, and (C) subgroup analyses of doxazosin stone in expulsion time. M–H, Mantel–Haenszel; CI, confidence interval; df, degrees of freedom.
Pain Episodes
A total of 3 RCTs using tamsulosin and 2 RCTs using silodosin were included in this group. We found that pain episodes were less frequent among patients receiving tamsulosin (MD, −0.87; 95% CI, −1.21 to −0.54; p < 0.00001) (Figure 8A) and silodosin (MD, −1.59; 95% CI, −2.82 to −0.35; p = 0.01) (Figure 8B), compared with placebo.
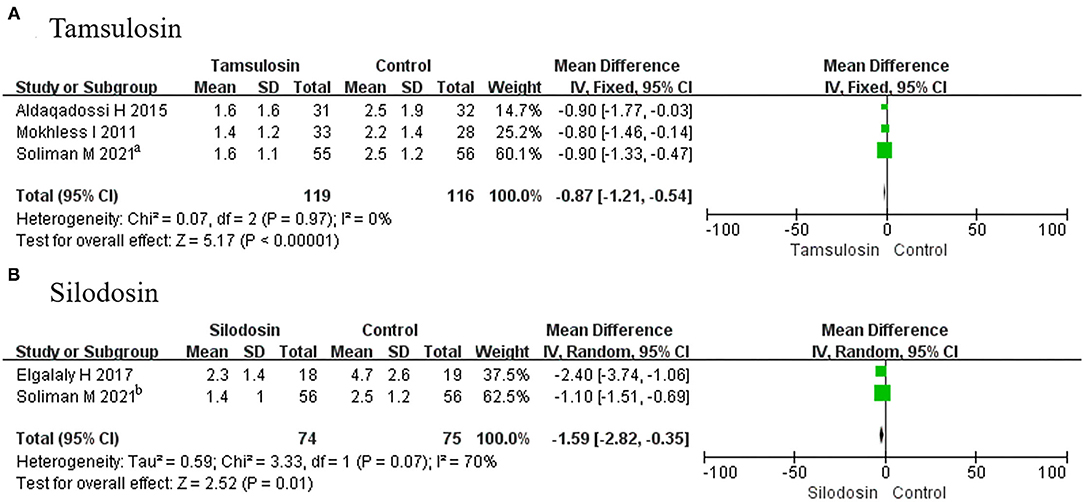
Figure 8. Forest plots showing the result of (A) subgroup analyses of tamsulosin in pain episodes and (B) subgroup analyses of silodosin in pain episodes. M–H, Mantel–Haenszel; CI, confidence interval; df, degrees of freedom.
Adverse Events
A total of 3 RCTs using tamsulosin and 2 RCTs using silodosin reported adverse events. Given that p > 0.05, fixed-effects models were adopted in the analysis of tamsulosin and silodosin, and we found a significant difference for tamsulosin (OR, 2.85; 95% CI, 1.34–6.03; p = 0.006) (Figure 9A), compared with placebo. In contrast, there was no difference recorded for silodosin (OR, 2.80; 95% CI, 0.98–7.99; p = 0.05) (Figure 9B) or doxazosin (MD, 2.74; 95% CI, 0.11–71.04; p = 0.54) (Figure 9C).
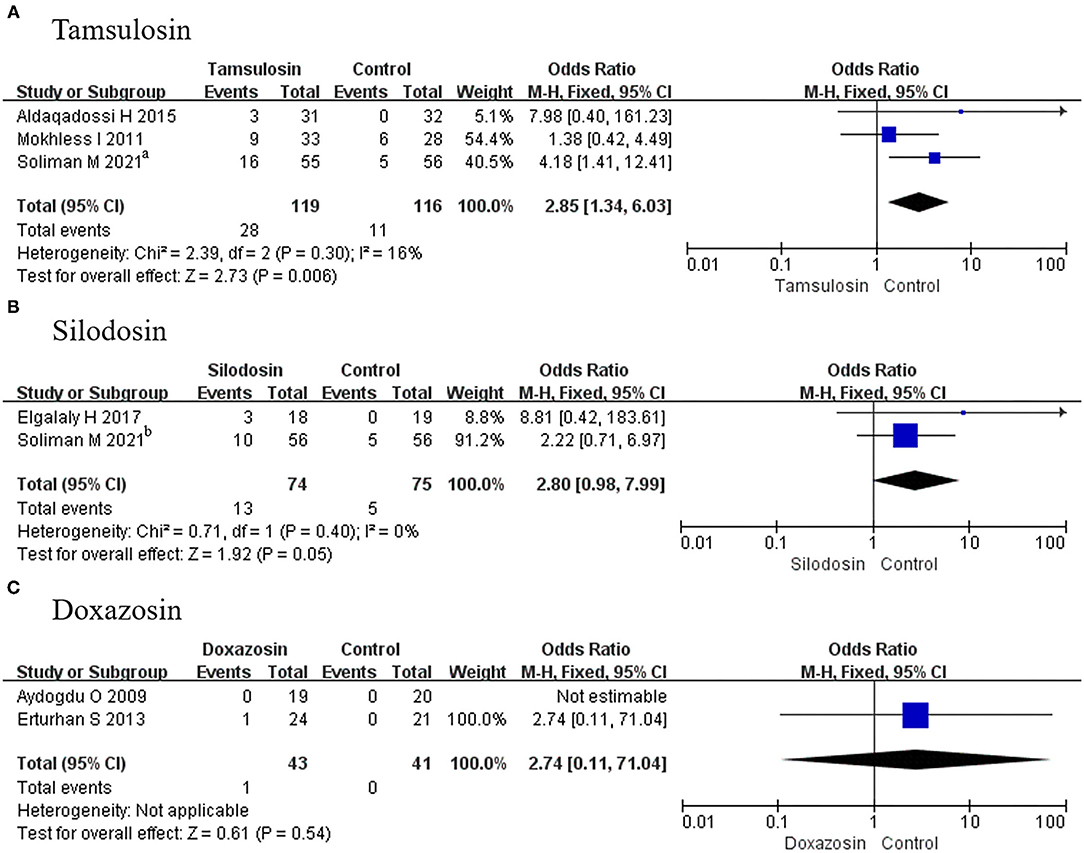
Figure 9. Forest plots showing the result of (A) subgroup analyses of tamsulosin in adverse events, (B) subgroup analyses of silodosin in adverse events, and (C) subgroup analyses of doxazosin in adverse events. M–H, Mantel–Haenszel; CI, confidence interval; df, degrees of freedom.
Tamsulosin and Silodosin
Two articles not only described the efficiency between α-adrenergic blockers and placebo but also offered a comparison of data for tamsulosin and silodosin. A total of 169 patients were involved in this subgroup analysis, and a fixed-effects model was used for analysis given that p > 0.05. Compared with tamsulosin, silodosin appeared to ensure a better expulsion rate, as the OR was 0.42 and the 95% CI was 0.20–0.92 (p = 0.03) (Figure 10).
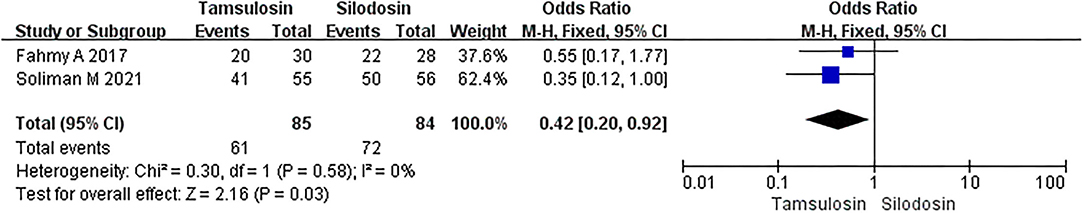
Figure 10. Forest plots showing the result of stone expulsion rate between tamsulosin and silodosin. M–H, Mantel–Haenszel; CI, confidence interval; df, degrees of freedom.
Different Stone Sizes
As different stone sizes were analyzed in 3 RCTs, stone size was divided into 2 groups of <5 and >5 mm. When the stone size was <5 mm, α-adrenergic blockers could increase the stone expulsion rate (OR, 6.28; 95% CI, 1.50–26.29; p = 0.01) (Figure 11A) and decrease the stone expulsion time (MD, −1.71; 95% CI, −2.91 to −0.52; p = 0.005) (Figure 11B). However, if the stone size was >5 mm, then α-adrenergic blockers could only increase the stone expulsion rate (OR, 3.88; 95% CI, 1.29–11.68; p = 0.02) (Figure 11C) and were useless for improving the stone expulsion time (MD, −3.61; 95% CI, −10.17 to 2.96; p = 0.28) (Figure 11D).
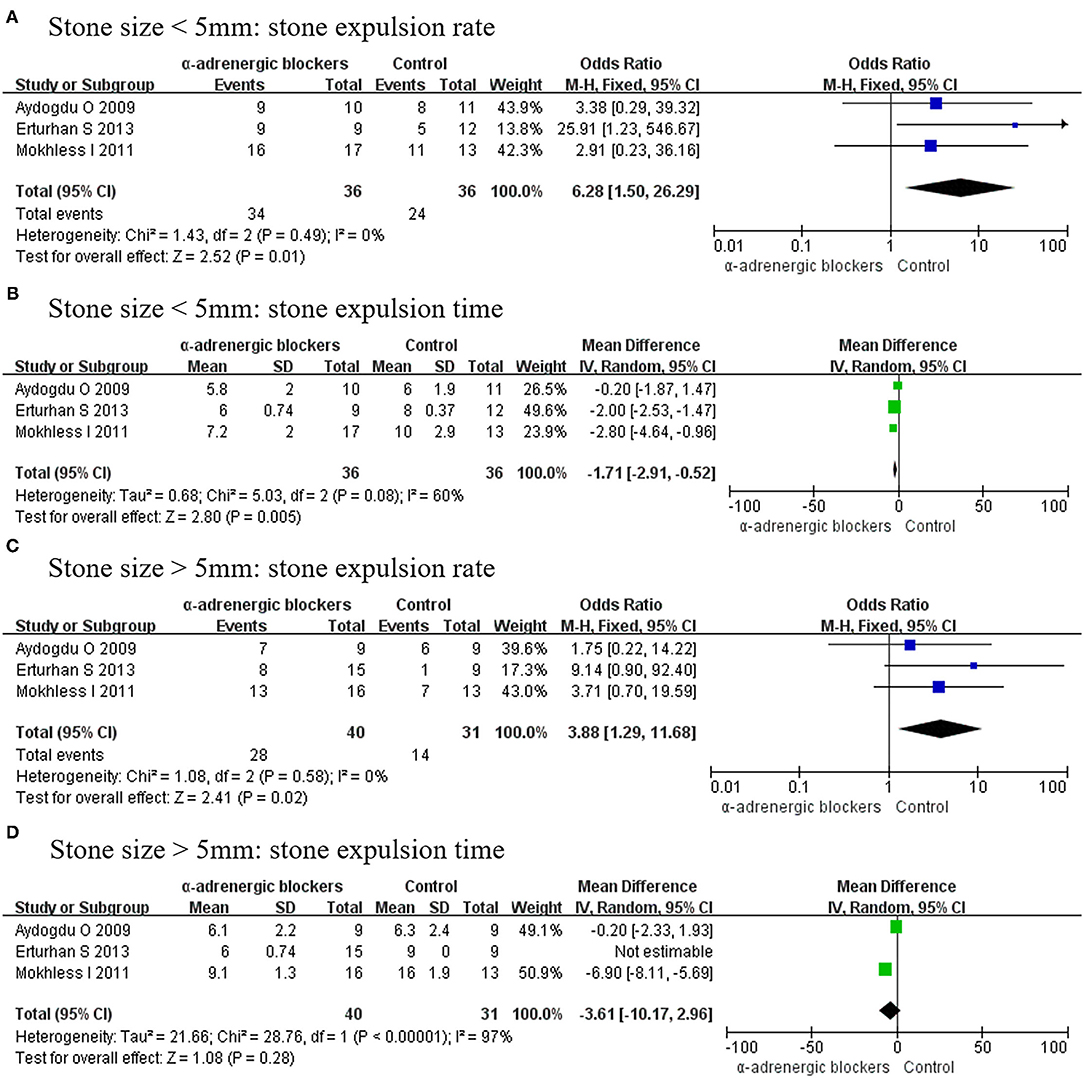
Figure 11. Forest plots showing the result of (A) stone expulsion rate in patients with stone size <5 mm, (B) stone expulsion time in patients with stone size <5 mm, (C) stone expulsion rate in patients with stone size >5 mm, and (D) stone expulsion time in patients with stone size >5 mm. M–H, Mantel–Haenszel; CI, confidence interval; df, degrees of freedom.
Discussion
Pediatric urolithiasis happens more frequently in developing countries compared with developed countries (31). Generally, pediatric patients undergo treatment more than once given the high recurrence rate of urolithiasis. Given the cost and procedural risks, multiple medicines are used widely instead of surgery (32), including placebo, α-adrenergic blockers (e.g., tamsulosin, silodosin, doxazosin), calcium channel blockers (e.g., nifedipine), and other adjuvant medications (e.g., steroids or tolterodine). Analgesics could relieve pain to allow the body to expulse the stone autonomously, while α-adrenergic blockers can inhibit uncoordinated frequency and maintain propulsive contractions to accelerate stone expulsion and reduce pain levels (33). However, the efficacy of α-adrenergic blockers for pediatric urolithiasis in the distal ureter was not clear.
In our meta-analysis, we compared α-adrenergic blockers (tamsulosin, doxazosin, and silodosin) and placebo in the treatment of pediatric urolithiasis with a stone size of <12 mm. Among 9 RCTs including 294 patients in the experimental group and 292 participants in the control group, we recorded a significant efficiency of α-adrenergic blockers in treating pediatric urolithiasis. Notably, α-adrenergic blockers could improve the stone expulsion rate and decrease the expulsion time obviously, and the pain episodes of children receiving α-adrenergic blockers were fewer in number than those of children receiving placebo only, so the need for analgesics in the experimental group was less. In addition, we also carried out some subgroup analyses according to different medicines and stone sizes. Tamsulosin, silodosin, and doxazosin all showed immense efficiency in improving the expulsion rate, while both tamsulosin and silodosin showed a significant beneficial effect on expulsion time, but doxazosin showed no difference compared with placebo. Both tamsulosin and silodosin could reduce the frequency of pain episodes remarkably. Although it has been confirmed previously that silodosin leads to a better expulsion rate, shorter expulsion time, and fewer pain episodes in adult patients (34), in this study, we have compared tamsulosin and silodosin in pediatric patients for the first time, confirming again that silodosin had a better expulsion rate than tamsulosin. The explanation for this result was that the two main different subtypes of α-adrenergic receptors distributed in the human ureter, including α-1D and α-1A receptors, contribute to stone expulsion (35–37). Silodosin had an equal affinity to that of tamsulosin for the α-1D receptor subtype, but the affinity of silodosin for the α-1A receptor subtype is ~17-fold greater than that of tamsulosin (38). Therefore, it is no surprise that silodosin showed a better efficiency than tamsulosin and doxazosin in pediatric urolithiasis. In addition, during the subgroup analyses according to stone size, the expulsion rate and expulsion time were improved obviously in cases of stones measuring <5 mm, but the expulsion time was not different between the two groups for those measuring >5 mm. In summary, α-adrenergic blockers can enhance the stone expulsion efficiency, especially for smaller stones, which is a finding that contrasts with the current guidelines. The main reason for this discordant result may be the small sizes of the experimental and control groups.
In view of safety, a total of seven RCTs referred to the tolerance of α-adrenergic blockers. According to the collected data, adverse events were frequently observed in the experimental group. According to the subgroup analyses of different medicines, silodosin and doxazosin showed no differences compared with placebo, but tamsulosin appeared to trigger more side effects. When focusing only on common side effects of α-adrenergic blockers, including orthostatic hypotension, headache, dizziness, and nasal congestion, there were also no differences between the two groups.
A previous article including 3 RCTs and 2 retrospective cohort studies reported that MET could accelerate stone expulsion (39), but the inclusion criteria of this study were not limited to only treatment with α-adrenergic blockers (tamsulosin, doxazosin), and other medications, including calcium channel blockers or adjuvant medications, were also included. Furthermore, because of the limited available data, the conclusion in said article was not convincing, with a high degree of publication bias and an uncertain risk of significant bias. Overall, this previous study did not effectively conclude the effect of α-adrenergic blockers in pediatric urolithiasis. Two other articles have also reviewed the efficiency of α-adrenergic blockers (tamsulosin and doxazosin) in pediatric urolithiasis (40, 41). In the first study (40), only 3 RCTs were involved, including a total of 145 patients, and the results were limited by the scale of included trials with a high degree of publication bias, and the expulsion rate and pain episodes were analyzed without side effects analysis, which led to an incomplete result. A total of 406 patients from 4 RCTs and 1 cohort study were analyzed in the other review (41) but, owing to the still-larger number of patients included in our study, some contrasting results could be pointed out. Considering expulsion time, our study indicated that α-adrenergic blockers showed better efficiency than placebo, whereas this difference was not found in the aforementioned review. Moreover, tamsulosin showed more side effects compared with placebo in our study. Some other valuable information, like pain episodes, analgesia demand, and different stone sizes, was also analyzed in our study yet ignored in the previous one. The present study may therefore change some minds about drug selection in pediatric urolithiasis by modifying some inaccurate results in the literature and adding comparisons. We have also described the advantages of silodosin in pediatric urolithiasis compared with tamsulosin for the first time.
Importantly, there are some limitations that need to be considered in our study. First, our included articles were all from Turkey and Egypt, which could result in publication bias. We will focus in the future on publications from different regions of the world to broaden our conclusion. Second, the qualities of included studies were heterogeneous. Different randomization processes and blinding methods were accepted, and the measurement methods of outcomes in different studies were not all the same. In all, our results should be verified by further studies.
Conclusion
Our study suggested that α-adrenergic blockers could promote stone expulsion efficiency in pediatric urolithiasis, especially for small stones measuring <5 mm, with a favorable safety profile. We also found that silodosin was the best medication choice, leading to a better expulsion rate, but it remains to be evaluated by further studies.
Author Contributions
JW and ZZ designed the research, interpreted the data, and revised the paper. FS, XB, DC, HY, KS, and DW performed the data extraction and carried out the meta-analysis. FS drafted the paper. All authors approved the submitted and final versions.
Funding
This work was supported by grants from the National Nature Science Foundation of China (Nos. 81870525 and 81572835) and Taishan Scholars Program of Shandong Province (No. tsqn201909199).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kroovand RL. Pediatric urolithiasis. Urol Clin N Am. (1997) 24:173–84. doi: 10.1016/S0094-0143(05)70362-1
2. Milliner DS, Murphy ME. Urolithiasis in pediatric patients. Mayo Clin Proc. (1993) 68:241–8. doi: 10.1016/S0025-6196(12)60043-3
3. García-Nieto V, Siverio B, Monge M, Toledo C, Molini N. Urinary calcium excretion in children with vesicoureteral reflux. Nephrol Dialysis Transplant. (2003) 18:507–11. doi: 10.1093/ndt/18.3.507
4. Hesse A, Siener R. Current aspects of epidemiology and nutrition in urinary stone disease. World J Urol. (1997) 15:165–71. doi: 10.1007/BF02201853
5. Beach MA, Mauro LS. Pharmacologic expulsive treatment of ureteral calculi. Ann. Pharmacother. (2006) 40:1361–8. doi: 10.1345/aph.1G586
6. Sternberg K, Greenfield SP, Williot P, Wan J. Pediatric stone disease: an evolving experience. J Urol. (2005) 174:1711–4. doi: 10.1097/01.ju.0000179537.36472.59
7. Hübner WA, Irby P, Stoller ML. Natural history and current concepts for the treatment of small ureteral calculi. Eur Urol. (1993) 24:172–6. doi: 10.1159/000474289
8. Tan AH, Al-Omar M, Denstedt JD, Razvi H. Ureteroscopy for pediatric urolithiasis: an evolving first-line therapy. Urology. (2005) 65:153–6. doi: 10.1016/j.urology.2004.08.032
9. Pietropaolo A, Proietti S, Geraghty R, Skolarikos A, Papatsoris A, Liatsikos E, et al. Trends of 'urolithiasis: interventions, simulation, and laser technology' over the last 16 years (2000-2015) as published in the literature (PubMed): a systematic review from European section of Uro-technology (ESUT). World J Urol. (2017) 35:1651–8. doi: 10.1007/s00345-017-2055-z
10. Segura JW, Preminger GM, Assimos DG, Dretler SP, Kahn RI, Lingeman JE, et al. Ureteral Stones Clinical Guidelines Panel summary report on the management of ureteral calculi. The American Urological Association. J Urol. (1997) 158:1915–21. doi: 10.1016/S0022-5347(01)64173-9
11. Wang CJ, Huang SW, Chang CH. Efficacy of an alpha1 blocker in expulsive therapy of lower ureteral stones. J Endourol. (2008) 22:41–6. doi: 10.1089/end.2007.0133
12. Quhal F, Seitz C. Guideline of the guidelines: urolithiasis. Curr Opin Urol. (2021) 31:125–9. doi: 10.1097/MOU.0000000000000855
13. Cui Y, Chen J, Zeng F, Liu P, Hu J, Li H, et al. Tamsulosin as a medical expulsive therapy for ureteral stones: A systematic review and meta-analysis of randomized controlled trials. J Urol. (2019) 201:950–5. doi: 10.1097/JU.0000000000000029
14. Sridharan K, Sivaramakrishnan G. Efficacy and safety of alpha blockers in medical expulsive therapy for ureteral stones: a mixed treatment network meta-analysis and trial sequential analysis of randomized controlled clinical trials. Expert Rev Clin Pharmacol. (2018) 11:291–307. doi: 10.1080/17512433.2018.1424537
15. Wang RC, Smith-Bindman R, Whitaker E, Neilson J, Allen IE, Stoller ML, et al. Effect of tamsulosin on stone passage for ureteral stones: a systematic review and meta-analysis. Ann Emerg Med. (2017) 69:353–61.e3. doi: 10.1016/j.annemergmed.2016.06.044
16. Ding H, Ning Z, Dai Y, Shang P, Yang L. The role of Silodosin as a new medical expulsive therapy for ureteral stones: a meta-analysis. Renal Fail. (2016) 38:1311–9. doi: 10.1080/0886022X.2016.1215221
17. Liu C, Zeng G, Kang R, Wu W, Li J, Chen K, et al. Efficacy and safety of alfuzosin as medical expulsive therapy for ureteral stones: a systematic review and meta-analysis. PLoS ONE. (2015) 10:e0134589. doi: 10.1371/journal.pone.0134589
18. Ma L, Zou S, Yang L, Wang F. Efficacy and safety of doxazosin in medical expulsive therapy for distal ureteral stones: a systematic review and meta-analysis. Urol J. (2020) 17:449–55. doi: 10.22037/uj.v16i7.5958
19. Cohen TD, Ehreth J, King LR, Preminger GM. Pediatric urolithiasis: medical and surgical management. Urology. (1996) 47:292–303. doi: 10.1016/S0090-4295(99)80442-7
20. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
21. Vader JP. Randomised controlled trials: A User's guide. BMJ. (1998) 317:1258. doi: 10.1136/bmj.317.7167.1258
22. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. (2019) 10:Ed000142. doi: 10.1002/14651858.ED000142
23. DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. (2015) 45:139–45. doi: 10.1016/j.cct.2015.09.002
24. Aydogdu O, Burgu B, Gucuk A, Suer E, Soygur T. Effectiveness of doxazosin in treatment of distal ureteral stones in children. J Urol. (2009) 182:2880–4. doi: 10.1016/j.juro.2009.08.061
25. Mokhless I, Zahran AR, Youssif M, Fahmy A. Tamsulosin for the management of distal ureteral stones in children: a prospective randomized study. J Pediatric Urol. (2012) 8:544–8. doi: 10.1016/j.jpurol.2011.09.008
26. Erturhan S, Bayrak O, Sarica K, Seckiner I, Baturu M, Sen H. Efficacy of medical expulsive treatment with doxazosin in pediatric patients. Urology. (2013) 81:640–3. doi: 10.1016/j.urology.2012.11.031
27. Aldaqadossi HA, Shaker H, Saifelnasr M, Gaber M. Efficacy and safety of tamsulosin as a medical expulsive therapy for stones in children. Arab J Urol. (2015) 13:107–11. doi: 10.1016/j.aju.2015.02.007
28. Fahmy A, Rhasad H, Kamal A, Elsawy M. Silodosin for medical expulsive therapy in children with distal ureteral stone: A prospective randomized, placebo-controlled, single blind study study. J Urol. (2017) 197:e1217. doi: 10.1016/j.juro.2017.02.2837
29. Elgalaly H, Eliwa A, Seleem M, Salem E, Omran M, Shello H, et al. Silodosin in the treatment of distal ureteric stones in children: A prospective, randomised, placebo-controlled study. Arab J Urol. (2017) 15:194–8. doi: 10.1016/j.aju.2017.05.005
30. Soliman MG, El-Gamal O, El-Gamal S, Abdel Raheem A, Abou-Ramadan A, El-Abd A. Silodosin versus tamsulosin as medical expulsive therapy for children with lower-third ureteric stones: prospective randomized placebo-controlled study. Urol Int. (2021) 105:568–73. doi: 10.1159/000513074
31. Taylor EN, Stampfer MJ, Curhan GC. Dietary factors and the risk of incident kidney stones in men: new insights after 14 years of follow-up. J Am Soc Nephrol. (2004) 15:3225–32. doi: 10.1097/01.ASN.0000146012.44570.20
32. Lipkin M, Shah O. Medical therapy of stone disease: from prevention to promotion of passage options. Curr Urol Rep. (2009) 10:29–34. doi: 10.1007/s11934-009-0007-x
33. Sasaki S, Tomiyama Y, Kobayashi S, Kojima Y, Kubota Y, Kohri K. Characterization of α1-adrenoceptor subtypes mediating contraction in human isolated ureters. Urology. (2011) 77:762.e13-7. doi: 10.1016/j.urology.2010.09.034
34. Hsu YP, Hsu CW, Bai CH, Cheng SW, Chen KC, Chen C. Silodosin versus tamsulosin for medical expulsive treatment of ureteral stones: A systematic review and meta-analysis. PLoS ONE. (2018) 13:e0203035. doi: 10.1371/journal.pone.0203035
35. Itoh Y, Kojima Y, Yasui T, Tozawa K, Sasaki S, Kohri K. Examination of alpha 1 adrenoceptor subtypes in the human ureter. Int J Urol. (2007) 14:749–53. doi: 10.1111/j.1442-2042.2007.01812.x
36. Tomiyama Y, Kobayashi K, Tadachi M, Kobayashi S, Inada Y, Kobayashi M, et al. Expressions and mechanical functions of alpha1-adrenoceptor subtypes in hamster ureter. Eur J Pharmacol. (2007) 573:201–5. doi: 10.1016/j.ejphar.2007.06.056
37. Tsuzaka Y, Matsushima H, Kaneko T, Yamaguchi T, Homma Y. Naftopidil vs silodosin in medical expulsive therapy for ureteral stones: a randomized controlled study in Japanese male patients. Int J Urol. (2011) 18:792–5. doi: 10.1111/j.1442-2042.2011.02850.x
38. Huang W, Xue P, Zong H, Zhang Y. Efficacy and safety of silodosin in the medical expulsion therapy for distal ureteral calculi: a systematic review and meta-analysis. Br J Clin Pharmacol. (2016) 81:13–22. doi: 10.1111/bcp.12737
39. Velázquez N, Zapata D, Wang HH, Wiener JS, Lipkin ME, Routh JC. Medical expulsive therapy for pediatric urolithiasis: Systematic review and meta-analysis. J Pediatric Urol. (2015) 11:321–7. doi: 10.1016/j.jpurol.2015.04.036
40. Glina FP, Castro PM, Monteiro GG, Del Guerra GC, Glina S, Mazzurana M, et al. The use of alpha-1 adrenergic blockers in children with distal ureterolithiasis: a systematic review and meta-analysis. Int Braz J Urol. (2015) 41:1049–57. doi: 10.1590/S1677-5538.IBJU.2015.0048
Keywords: alpha-adrenergic blockers, tamsulosin, silodosin, doxazosin, pediatric urolithiasis, meta-analysis, distal ureteral stones, randomized controlled trials
Citation: Sun F, Bao X, Cheng D, Yao H, Sun K, Wang D, Zhou Z and Wu J (2022) Meta-Analysis of the Safety and Efficacy of α-Adrenergic Blockers for Pediatric Urolithiasis in the Distal Ureter. Front. Pediatr. 10:809914. doi: 10.3389/fped.2022.809914
Received: 05 November 2021; Accepted: 23 February 2022;
Published: 15 April 2022.
Edited by:
Alberto Parente, Consultant, Madrid, SpainReviewed by:
Yuval Bar-Yosef, Dana-Dwek Children's Hospital, IsraelAlfredo Berrettini, IRCCS Ca' Granda Foundation Maggiore Policlinico Hospital, Italy
Copyright © 2022 Sun, Bao, Cheng, Yao, Sun, Wang, Zhou and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jitao Wu, d2p0dXJvbG9neUAxNjMuY29t; Zhongbao Zhou, MzQ2ODk0MzgzQHFxLmNvbQ==
†These authors have contributed equally to this work and share last authorship
 Fengze Sun
Fengze Sun Xingjun Bao2†
Xingjun Bao2† Zhongbao Zhou
Zhongbao Zhou Jitao Wu
Jitao Wu