Abstract
Aims:
Vaccine response is poor among children living with HIV. The gut microbiota has been identified as a potential target to improve vaccine immunogenicity, but data are scarce in the context of HIV infection.
Methods:
Pilot, double-blind, randomized placebo-controlled trial in which 24 HIV-infected children were randomized to receive a mixture of symbiotics, omega-3/6 fatty acids, and amino acids or placebo for 4 weeks, each in combination with ART, and were then immunized against influenza. Vaccine response and safety of the nutritional supplementation were the primary outcomes.
Results:
Eighteen HIV-infected children completed the follow-up period (mean age 11.5 ± 4.14 years, 61% female). The nutritional supplement was safe but did not enhance the response to the influenza vaccine. A 4-fold rise in antibody titers was obtained in only 37.5% of participants in the intervention arm vs. 40% in the placebo. No immunological or inflammatory predictors of vaccine response were identified.
Conclusions:
In this exploratory study, a 4-week course of symbiotics did not increase influenza vaccine immunogenicity in HIV-infected children. Larger studies are warranted to address the potential of modulating the microbiome in children living with HIV.
Summary
Pilot randomized clinical trial addressing the impact of a 4 weeks nutritional intervention targeting gut dysbiosis on the response to influenza immunization among HIV-infected children on ART. Despite mild changes on microbiota composition, the intervention did not affect influenza vaccine response.
Introduction
Despite achieving and maintaining viral suppression under antiretroviral treatment (ART), immunological restoration is not complete during HIV infection (1). Persistent inflammation and immunoactivation have been described both among adults and children and are a matter of concern (1–3). In HIV-infected children, innate and adaptive abnormalities (2) probably contribute to the reduction in the magnitude and durability of vaccine response (4–7). This impaired immunity leads to an increased rate of vaccine-preventable diseases in this population, greater severity and high progression-rate of vaccine-preventable infections, from measles to pneumococcal disease (8–11). Among other immunizations, influenza is amongst the vaccines with lower immunogenicity in people living with HIV (PLWHIV) (5, 12–15).
Based on the interactions that occur among bacteria and the immune system at the intestinal mucosa, the gut microbiome has been identified as a potential target for interventions aimed at increasing vaccine immunogenicity (16). Changes in the composition and function of the microbiome in PLWHIV are thought to be explained by the depletion of lymphocyte populations during acute infection at the gut mucosa, only partially restored by ART (17, 18). These abnormalities, together with microbial translocation and viral persistence, may contribute to the persistent immune dysfunction associated to chronic HIV infection (17, 19). The potential of targeting the gut microbiome in this population is therefore of interest. Studies describing the extent of gut dysbiosis among HIV-infected children have arisen controversial results (20–22). Clinical trials aiming at modulating the gut microbiome via nutritional supplementation, mostly in adults, have achieved only mild changes in the ecosystem and partial immunological effects (22–26). Because the assembling and establishment of human microbiome occurs during the first years of life (27), the potential to modulate and consequently impact the bacteria-immune system interplay might be therefore higher during childhood. However, attempts to target the microbiome in the unique population of perinatally HIV-infected children are scarce.
On the basis that the gut microbiota-immune system interactions likely influence vaccine response, we aimed to assess the potential impact of a nutritional supplementation aimed at modulating the dysbiosis of perinatally HIV-infected children on the response to influenza vaccine.
Methods
Study Design and Participants
Pilot, double blind, randomized, placebo-controlled study. Vertically HIV-infected children and adolescents aged 6–18 years, on stable ART for at least 6 months and with CD4+ T-cell counts ≥350 cells/μL, were enrolled at the HIV clinics of four Hospitals in Madrid, Spain, October 2013 to November 2014. Exclusion criteria included acute or chronic infections other that HIV, antibiotic treatment in the previous 3 months, as well as presence of other chronic conditions requiring medication. Participants were randomly assigned to receive a nutritional supplementation or placebo by a computer-generated randomized number system in blocks, daily for 4 weeks, and were then immunized against influenza infection. Peripheral blood and fecal samples were collected at baseline and after the 4-weeks intervention (±7 days). Serum samples for measurement of antibody titers against influenza were obtained immediately before and 3 months after immunization. Participants received the intramuscular quadrivalent influenza vaccine, according to recommendations by the Spanish Ministry of Health, for active immunization for the prevention of influenza A and B virus (Fluarix®, Vaxigrip tetra®). The participating clinicians, the laboratory personnel, and the study participants and family were blind to the assigned patient group.
The study protocol was approved by the Independent Ethics Committee at all participating Institutions (approval number 173/13) and all parents and participants above 12 years of age provided written informed consent/assent. Full details on the study protocol have been previously published (22).
Nutritional Intervention
A 20 mg powder combination of a specifically design nutritional supplementation containing pre/probiotics, oligosaccharides, glutamine, AM3, and vitamin D (PMT25341) (23) or placebo (skimmed milk powder) was administered daily. Both products were prepared by Nutricion Médica, S.L. in identically appearing sealed envelopes.
Laboratory Assessments
Fasting blood samples were drawn at baseline and at post-intervention for real-time measurements of plasma HIV-1 viral load and immunological studies, by standard flow cytometric methods, and included T-cell activation (HLA-DR+/CD38+), senescence (CD28−CD57+) and exhaustion (PD-1) markers. Stained cells were run on a Gallios flow cytometer (Beckman Coulter, Inc., Münster, Germany), and data analyzed using Kaluza software (Beckman Coulter, Inc, Münster, Germany). A panel of inflammatory biomarkers and cytokines including IL-17A, IL-10, IL-6, and IP-10 was determined using a multiplex immunoassays by Invitrogen™ ProcartaPlex™ (ThermoFisher) and ELISAs for IL-7 (R&D IL-7 Quantikine HS ELISA Kit), sCD14 (Quantikine ELISA Kit DC140, RD), and zonuline (Abyntek Biopharma S.L. ELISA Kit). The plasma kynurenine to tryptophan ratio (KT ratio) was determined by mass spectrometry, using a liquid chromatography system consisting of a degasser, binary pump and autosampler (1290 Infinity, Agilent Technologies, Santa Clara, CA, USA) coupled to a triple quadrupole mass spectrometer (6460, AgilentTechnologies).
A serum sample of every participant was sent to the Pediatric HIV BioBank-HGUGM processed and stored at −80° using standard procedures for subsequent determinations. Antibody titers against the three viral haemagglutinin (HA) components of influenza vaccine used in seasons 2013–2014 and 2014–2015, (A/H1N1, A/California/07/09, A/H3N2, A/Victoria/361/2011 and A/Texas/50/2012, B/Massachusetts/2/12), and were determined by Haemagglutination Inhibition Assay, using both guinea-pig and turkey erythrocytes. All samples were studied simultaneously in order to prevent titer differences between assays. A 4-fold rise in titer was interpreted as significant.
Fecal samples were collected in sterile tubes with RNAlater (Life Technologies) and stored at −80°C until use. Total DNA was extracted and the V3-V4 region of the 16S rRNA gene were amplified from total DNA. The amplicon libraries were constructed following Illumina instructions, quantified with Qubit Fluorometer (ThermoFisher, Waltham, MA, USA) and sequenced using the Kit v3 (2 × 230 cycles) in a MiSeq platform (Illumina) as previously published (22).
Statistical Analysis
We used the Mann-Whitney U test for the between-group comparisons of continuous variables and Wilcoxon signed-rank matched-pairs test to evaluate differences in numerical outcomes between time-points. Fold changes between baseline and week four measurements were calculated to analyze correlations between inflammatory and T-cell biomarkers and vaccine response. Statistical analysis was performed using Stata v17.0 (StataCorp LP, College Station, TX) and Prism v.7.0, GraphPad, Inc., La Jolla, CA).
Results
Twenty-four vertically HIV-infected children and adolescents on ART and virologically suppressed were recruited and randomized, but only 18 completed the follow-up period. Two patients withdrew during follow-up period and four were not immunized within the per protocol ±7 days period after supplementation. Eight patients in the intervention arm and 10 in the placebo group were immunized against influenza and had available serum samples. Median age was 11.5 ± 4.14 years, 61% were female. Patients on the intervention group were younger and had a higher CD4 nadir, but no other significant differences at baseline in terms of immunological or inflammatory biomarkers, including CD4 counts (Table 1).
Table 1
| Placebo N = 10 | Nutritional intervention N = 8 | P | |
|---|---|---|---|
| Female (N, %) | 6 (60) | 5 (62.5) | 1.000 |
| Age (years), mean (SD) | 13.6 (3.5) | 9 (3.5) | 0.033 |
| Black (N, %) | 4 (40) | 4 (50) | 0.772 |
| CD4 count (cells/mm3) | 628 [486–736] | 692 [517–861] | 0.447 |
| CD4/CD8 ratio | 1.45 [0.75–1.93] | 1.81 [1.36–1.90] | 0.447 |
| CD4 Nadir (cells/mm3) | 333 [206–376] | 519 [410–1,086] | 0.007 |
| PI based ART (N, %) | 7 (70) | 7 (87.5) | 0.588 |
| Lopinavir/ritonavir | 2 (20) | 6 (75) | |
| Atazanavir/ritonavir | 2 (20) | 0 (0) | |
| Darunavir/ritonavir | 1 (10) | 1 (12.5) | |
| NNRTI based ART (N, %) | 3 (30) | 1 (12.5) | 0.543 |
| Efavirenz | 3 (30) | 1 (12.5) | |
| INI based ART (N, %) | 2 (20) | 0 (0) | 0.892 |
| Raltegravir | 2 (20) | 0 (0) | |
| Time on ART (years) | 12.8 (8.6–15.5) | 8.6 (8.2–13.4) | 0.067 |
| HLADR+CD38+CD8 T cells | 1.9 [0.96–3.77] | 3.28 [1.66–9.17] | 0.248 |
| CD57+CD28- CD8 T cells | 17 [11–25] | 28 [22–32] | 0.248 |
| Interleukin 6 | 0.6 [0.29–1.23] | 0.33 [0.10–0.67] | 0.306 |
| Interleukin 7 | 114.6 [96–128] | 84.9 [43.5–127.5] | 0.204 |
| IP-10 | 47.2 [31.7–67.9] | 43.5 [36.9–62.4] | 1.000 |
| sCD14 | 2.13 [2.0–2.2] | 2.33 [1.7–2.5] | 0.328 |
| zonulin | 4.3 [2.7–5.2] | 3.9 [1.4–8.8] | 0.922 |
| KT ratio | 320 [263–423] | 296 [253–395] | 0.614 |
| 4-fold increase antibody titers against Influenza A H1N1, N (%) | 4 (40) | 3 (37.5) | 1.000 |
| 4-fold increase antibody titers against Influenza A H3N2, N (%) | 1 (10) | 0 (0) | 0.923 |
| 4-fold increase antibody titers against Influenza B, N (%) | 2 (20) | 1 (12.5) | 0.547 |
Clinical characteristics and laboratory parameters of study participants.
ART, antiretroviral treatment; IP-10, Interferon gamma-induced protein; NNRTI, non-nucleoside reverse transcriptase analogs; INI, integrase inhibitors; PI, protease inhibitors; sCD14, soluble CD14; KT ratio, kynurenine to tryptophan ratio.
Statistical test used: Mann-Whitney U/Wilcoxon signed rank test/Fisher's exact test.
Bold indicates statistically significant (p < 0.05).
Reported adherence was good over the study period, with no tolerance issues, and no adverse events were registered.
Response to all components of the trivalent vaccine were poor. For the H1N1 component, a 4-fold rise in antibody titers after immunization was obtained in 3/8 (37.5%) of participants in the intervention arm vs. 4/10 (40%) in the placebo group. For the H3N2 component and for Influenza B responses were even lower (Table 1). Individual trajectories of participants serologies according to intervention arm are shown in Figure 1.
Figure 1
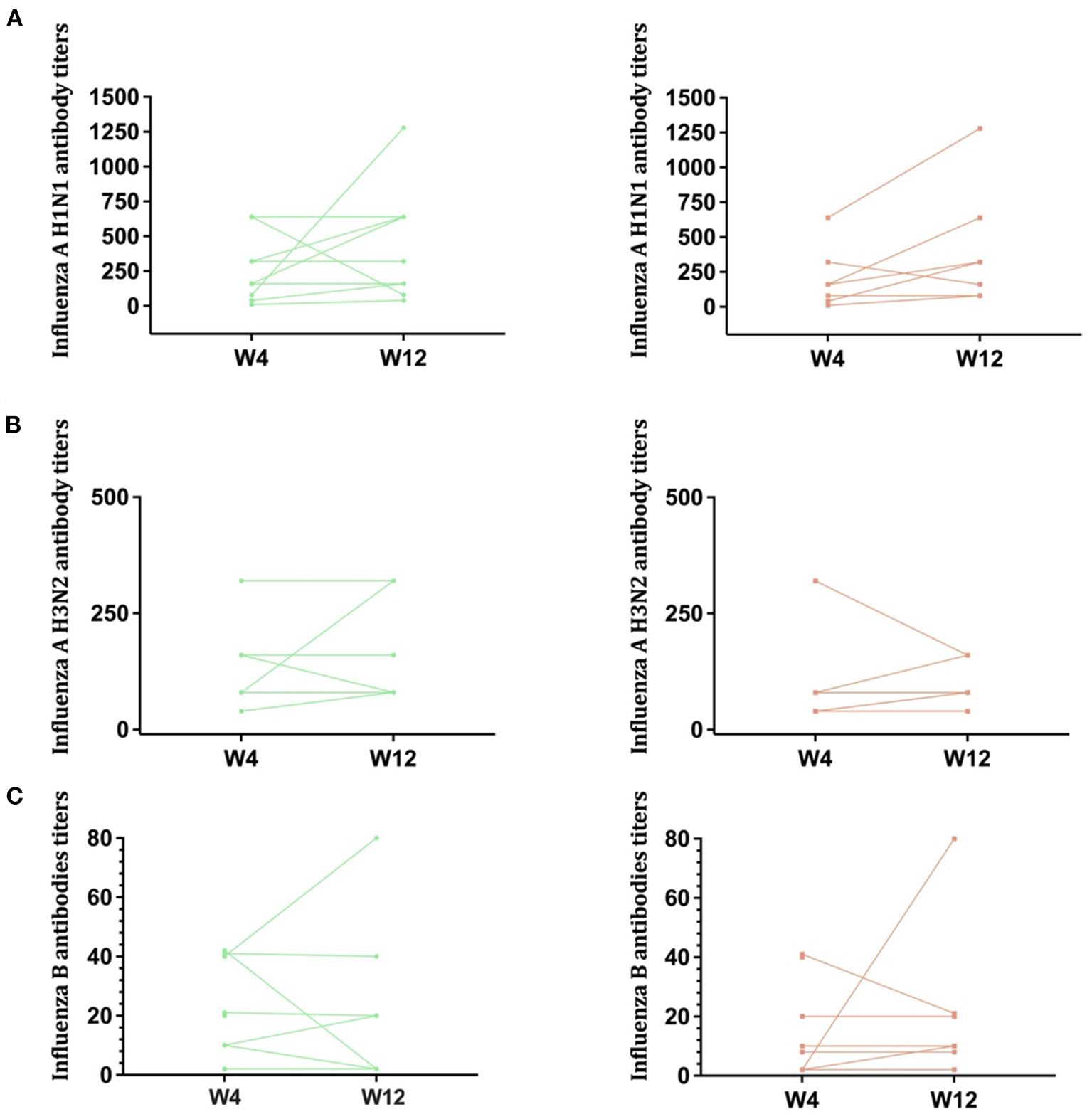
Antibody titers against influenza. Antibody titers against Influenza A H1N1 (A), Influenza A H3N2 (B), and Influenza B (C) are shown in this figure, according to randomization group (Left: placebo, Right: intervention arm). Lines represent individual trajectories.
Results regarding fecal microbiota structure in comparison with uninfected controls have been previously published (22). In summary, the intervention did not lead to clear differences on alpha diversity. The differences in beta diversity present at baseline between groups disappeared after the intervention, suggesting that PMT25341 attenuated the compositional changes in the microbiota associated with HIV infection. However, changes over time within the groups were non-significant, and the nutritional supplementation did not achieve a significant increase in CD4 counts or any immunological marker. IL-6, IL-7, IL-10, Zonulin, Intestinal Fatty Acid Binding Protein, soluble CD14 (sCD14), or the KT ratio remain stable over time, with no differences between study arms (26).
No associations were found between the influenza antibody titers and the CD4/CD8 ratio, which has been suggested as a marker of immune reconstitution and vaccine response among people living with HIV (28, 29). None of the studied immune activation/senescence markers or the inflammatory and microbial translocation markers was identified as a predictor of vaccine response, including the KT ratio that has been shown to predict vaccine immunogenicity among adults living with HIV (29) (data not shown).
Discussion
Results from this pilot study confirm that influenza vaccine response is extremely poor among HIV-infected children, with H1N1 being the component that elicits the most intense response. Despite being safe, a 4-week nutritional intervention failed to improve vaccine immunogenicity. These results suggest that targeting the gut microbiota might require more powerful or longer interventions, if aiming at achieving systemic effects.
HIV-infected children are at heightened risk for severe influenza illness (6, 7, 14), and immunization is recommended in most guidelines, even when immunogenicity seems particularly low in this population according to most series (5, 14). In the design of an exploratory trial, we understood this fact may have maximized our possibilities to achieve an effect, as the margin of improvement is greater for influenza vaccine compared to more immunogenic vaccines. However, the fact that no changes in CD4, CD8 or activation markers were observed (26) also supports the hypothesis of the shortness of the intervention, as T cell subpopulations typically evolve slowly. In the study by Cahn et al. that showed lower CD4 decline and a significant decrease of the immune activation markers in ART-naïve adults, probiotics were administered over a 52 weeks period (25). The fact that, for security reasons, a basal CD4 T cell count above 350 cell/mm was required for inclusion, may have excluded from participation those patients with potentially a greater margin of benefit. However, our previous experience using this nutritional supplement, designed to include components suggested to enhance gut epithelial barrier integrity, as well as stimulate immune recovery, precisely among ART naïve adults with CD4 < 350 counts/uL, and for 48 weeks, showed no clear immunological benefits in addition to ART (23). Modulating an established ecosystem might require additional interventions to the solely nutritional supplementation (30, 31), and the optimal supplements are still to be defined. Vaccine response was in fact extremely poor in our study compared to other series addressing also influenza immunization in children living with HIV (14, 15). This fact is even more shocking taking into account the good immunological status of the children included in this pilot trial, as previous studies have suggested that immunological status determines vaccine response (7, 32). The identification of predictors of vaccine response has been identified as an urgent need for this population that grows with the virus (32). Markers available to routine clinical practice would allow us to personalize vaccination schedules. In PLWHIV, both the CD4/CD8 ratio and the KT ratio, an indirect marker of the activity of the Indoleamine 2,3-dioxygenase-1 (IDO) that catabolizes tryptophan (T) to kynurenine (K), have been suggested to predict vaccine immunogenicity (29). None of these markers correlated here with vaccine response, and none improved due to the intervention, in line with the fact that vaccine response was not modified either.
Sample size is the main limitation of this exploratory trial, as the study might be underpowered to detect any differences. There were some unexpected differences between both groups, with younger patients with higher CD4 nadir in the intervention group. Despite the good immunological situation of all participants, the fact that IgG levels were unmeasured at baseline is also a limitation. The inclusion of patients with CD4 > 350 cell/mm impairs us to extrapolate results to immunological non-responders, which may be the population that hypothetically benefits most from an intervention addressing immune dysfunction. No adverse events occurred during the study period. Despite reported adherence was good, compliance with an additional treatment could have been suboptimal. Adherence issues are common among children and adolescents, and should be balanced when attempting to design strategies to improve quality of life in PLWHIV, especially during childhood and adolescence. Although adherence was reinforced during the trial, to what extent it may have affected the lack of differences found remains unknown.
To our knowledge, this exploratory trial is the first exploring the effect of a dietary intervention to improve vaccine response among vertically HIV-infected children. Response to influenza immunization, poor among participants, was not enhanced by means of pre/probiotic supplementation. Further studies and innovative approaches are required to address the potential transferability to clinical settings of microbiota modulation as a useful tool to improve the health of children living with HIV.
Funding
This work was funded by the Instituto de Salud Carlos III-Fondos FEDER (grant number CB21/17/00025), Acción Estratégica en Salud (PI13/0422, PI17/01283, PI18/00154, and PI18CIII/00009). TS and SS-V have been funded by the Instituto de Salud Carlos III-Fondos FEDER (BA21/00022 and BA21/00017). The funding bodies did not have a role in the design or conduct of the study, the analysis and interpretation of the results, and the writing of the report or the decision to publish.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: European Bioinformatics Institute (http://www.ebi.ac.uk/) database (accession number: PRJEB35283, secondary accession: ERP119574).
Ethics statement
The studies involving human participants were reviewed and approved by Hospital Gregorio Marañón Ethics Committee and all participating Hospitals, Madrid, Spain. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author contributions
TS: study conception and design, funding acquisition, recruitment, coordination, data analysis, and draft and final manuscript writing. IC: methodology, laboratory determinations, analysis and interpretation, funding acquisition, and writing. MG-E: laboratory determinations, analysis, and interpretation. LE-G and MM: recruitment and data collection at Hospital La Paz. MM-F: study design, coordination, funding acquisition, and writing. LP: recruitment and data collection at Hospital and H Getafe. MG: study design, laboratory analysis, statistical analysis and interpretation, and writing. NJ-H: sequencing and laboratory analysis. JR: recruitment and data collection at Hospital, and H. Clínico. MN: study coordination, recruitment and data collection at Hospital, and H Gregorio Marañón. SS-V: study conception and design, statistical analysis, interpretation, and writing. CC: conceptualization, study coordination, funding acquisition, and writing. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors would like to particularly acknowledge all the children and adolescents as well as their families for their participation in this study. They acknowledge the Spanish Pediatric HIV infection Group CORISPE and the Pediatric HIV BioBank integrated in the Spanish AIDS Research Network and collaborating Centers [supported by the Instituto de Salud Carlos III, Spanish Health Ministry (Grant n° RD06/0006/0035)] for its collaboration and cession of clinical information and samples used in this work. Nutricion Médica S.L., manufactured and packaged the nutritional product under investigation. Final results of this work have been presented at the following meetings: 36rd Annual Meeting of the European Society for Pediatric Infectious Diseases (ESPID 2018), Malmö, Sweden, 28th May-June 2nd, 2018. (Ref. ESP18-0517).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Deeks SG Phillips AN . HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ. (2009) 338:288–92. 10.1136/bmj.a3172
2.
Carrasco I Tarancon-Diez L Vázquez-Alejo E Jiménez De Ory S Sainz T Apilanez M et al . Innate and adaptive abnormalities in youth with vertically acquired HIV through a multicentre cohort in Spain. J Int AIDS Soc. (2021) 24:e25804. 10.1002/jia2.25804
3.
Sainz T Serrano-Villar S Díaz L Tome MIG Gurbindo MD de José MI et al . The CD4/CD8 ratio as a marker T-cell activation, senescence and activation/exhaustion in treated HIV-infected children and young adults. AIDS. (2013) 27:1513–6. 10.1097/QAD.0b013e32835faa72
4.
Helfand RF Witte D Fowlkes A Garcia P Yang C Fudzulani R et al . Evaluation of the immune response to a 2-dose measles vaccination schedule administered at 6 and 9 months of age to HIV-infected and HIV-uninfected children in Malawi. J Infect Dis. (2008) 198:1457–65. 10.1086/592756
5.
Madhi SA Dittmer S Kuwanda L Venter M Cassim H Lazarus E et al . Efficacy and immunogenicity of influenza vaccine in HIV-infected children: a randomized, double-blind, placebo controlled trial. AIDS. (2013) 27:369–79. 10.1097/QAD.0b013e32835ab5b2
6.
Cagigi A Cotugno N Giaquinto C Nicolosi L Bernardi S Rossi P et al . Immune reconstitution and vaccination outcome in HIV-1 infected children present knowledge and future directions. Hum Vaccin Immunother. (2012) 8:1784–94. 10.4161/hv.21827
7.
Kosalaraksa P Srirompotong U Newman RW Lumbiganon P Wood JM . Serological response to trivalent inactive influenza vaccine in HIV-infected children with different immunologic status. Vaccine. (2011) 29:3055–60. 10.1016/j.vaccine.2011.01.091
8.
Adetokunboh OO Ndwandwe D Awotiwon A Uthman OA Wiysonge CS . Vaccination among HIV-infected, HIV-exposed uninfected and HIV-uninfected children: a systematic review and meta-analysis of evidence related to vaccine efficacy and effectiveness. Hum Vaccin Immunother. (2019) 15:2578–89. 10.1080/21645515.2019.1599677
9.
Seth A Deepa S Dutta R Chandra J . Evaluation of immune response to measles component of MMR vaccine in children with HIV infection receiving antiretroviral therapy. Pediatr Infect Dis J. (2016) 35:e8–11. 10.1097/INF.0000000000000934
10.
Al-Attar I Reisman J Muehlmann M McIntosh K . Decline of measles antibody titers after immunization in human immunodeficiency virus-infected children. Pediatr Infect Dis J. (1995) 14:149–51. 10.1097/00006454-199502000-00013
11.
Zizza A Banchelli F Guido M Marotta C di Gennaro F Mazzucco W et al . Efficacy and safety of human papillomavirus vaccination in HIV-infected patients: a systematic review and meta-analysis. Sci Rep. (2021) 11:4954. 10.1038/s41598-021-83727-7
12.
Richardson K Weinberg A . Reduced immunogenicity of influenza vaccines in HIV-infected compared with uninfected pregnant women is associated with regulatory T cells. AIDS. (2011) 25:595–602. 10.1097/QAD.0b013e32834411a8
13.
Parmigiani A Alcaide ML Freguja R Pallikkuth S Frasca D Fischl MA et al . Impaired antibody response to influenza vaccine in HIV-infected and uninfected aging women is associated with immune activation and inflammation. PLoS ONE. (2013) 8:e79816. 10.1371/journal.pone.0079816
14.
MacHado AA MacHado CM Boas LSV Lopes MC de Fátima Barbosa Gouvêa A de Menezes Succi RC et al . Short communication: immunogenicity of an inactivated influenza vaccine and postvaccination influenza surveillance in HIV-infected and noninfected children and adolescents. AIDS Res Hum Retroviruses. (2011) 27:999–1003. 10.1089/aid.2010.0306
15.
Esposito S Tagliaferri L Daleno C Valzano A Picciolli I Tel F et al . Pandemic influenza A/H1N1 vaccine administered sequentially or simultaneously with seasonal influenza vaccine to HIV-infected children and adolescents. Vaccine. (2011) 29:1677–82. 10.1016/j.vaccine.2010.12.047
16.
Lynn DJ Benson SC Lynn MA Pulendran B . Modulation of immune responses to vaccination by the microbiota: implications and potential mechanisms. Nat Rev Immunol. (2022) 22:33–46. 10.1038/s41577-021-00554-7
17.
Vujkovic-Cvijin I Dunham RM Iwai S Maher MC Albright RG Broadhurst MJ et al . Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci Transl Med. (2013) 5:193ra91. 10.1126/scitranslmed.3006438
18.
Vázquez-Castellanos JF Serrano-Villar S Latorre A Artacho A Ferrús ML Madrid N et al . Altered metabolism of gut microbiota contributes to chronic immune activation in HIV-infected individuals. Mucosal Immunol. (2015) 8:760–72. 10.1038/mi.2014.107
19.
Serrano-Villar S Rojo D Martínez-Martínez M Deusch S Vázquez-Castellanos JF Bargiela R et al . Gut bacteria metabolism impacts immune recovery in HIV-infected individuals. EBioMedicine. (2016) 8:203–16. 10.1016/j.ebiom.2016.04.033
20.
Sessa L Reddel S Manno E Quagliariello A Cotugno N Del Chierico F et al . Distinct gut microbiota profile in ART-treated perinatally HIV-infected patients associated with cardiac and inflammatory biomarkers. AIDS. (2019) 33:1001–11. 10.1097/QAD.0000000000002131
21.
Kaur US Shet A Rajnala N Gopalan BP Moar P Himanshu D et al . High Abundance of genus Prevotella in the gut of perinatally HIV-infected children is associated with IP-10 levels despite therapy. Sci Rep. (2018) 8:17679. 10.1038/s41598-018-35877-4
22.
Sainz T Gosalbes MJ Talavera A Jimenez-Hernandez N Prieto L Escosa L et al . Effect of a nutritional intervention on the intestinal microbiota of vertically HIV-infected children: the pediabiota study. Nutrients. (2020) 12:2112. 10.3390/nu12072112
23.
Serrano-Villar S de Lagarde M Vázquez-Castellanos J Vallejo A Bernadino JI Madrid N et al . Effects of immunonutrition in advanced human immunodeficiency virus disease: a randomized placebo-controlled clinical trial (Promaltia study). Clin Infect Dis. (2019) 68:120–30. 10.1093/cid/ciy414
24.
Cahn P Ruxrungtham K Gazzard B Diaz RS Gori A Kotler DP et al . The immunomodulatory nutritional intervention NR100157 reduced CD4 + T-cell decline and immune activation: a 1-year multicenter randomized controlled double-blind trial in HIV-infected persons not receiving antiretroviral therapy (the BITE study). Clin Infect Dis. (2013) 57:139–46. 10.1093/cid/cit171
25.
Gori A Rizzardini G Van'T Land B Amor KB van Schaik J Torti C et al . Specific prebiotics modulate gut microbiota and immune activation in HAART-naive HIV-infected adults: results of the “COPA” pilot randomized trial. Mucosal Immunol. (2011) 4:554–63. 10.1038/mi.2011.15
26.
Sainz T Diaz L Rojo D Clemente MI Barbas C Gosalbes MJ et al . Targeting the gut microbiota of vertically HIV-infected children to decrease inflammation and immunoactivation: a pilot clinical trial. Nutrients. (2022) 14:992. 10.3390/nu14050992
27.
Mesa MD Loureiro B Iglesia I Gonzalez SF Olivé EL Algar OG et al . The evolving microbiome from pregnancy to early infancy: a comprehensive review. Nutrients. (2020) 12:133. 10.3390/nu12010133
28.
Serrano-Villar S Sainz T Lee SA Hunt PW Sinclair E Shacklett BL et al . HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathogens. (2014) 10. 10.1371/journal.ppat.1004078
29.
Avelino-Silva VI Miyaji KT Hunt PW Huang Y Simoes M Lima SB et al . CD4/CD8 ratio and KT ratio predict yellow fever vaccine immunogenicity in HIV-infected patients. PLoS Negl Trop Dis. (2016) 10:e0005219. 10.1371/journal.pntd.0005219
30.
Serrano-Villar S Talavera-Rodríguez A Gosalbes MJ Madrid N Pérez-Molina JA Elliott RJ et al . Fecal microbiota transplantation in HIV: a pilot placebo-controlled study. Nat Commun. (2021) 12:1139. 10.1038/s41467-021-21472-1
31.
Vujkovic-Cvijin I Rutishauser RL Pao M Hunt PW Lynch SV McCune JM et al . Limited engraftment of donor microbiome via one-time fecal microbial transplantation in treated HIV-infected individuals. Gut Microbes. (2017) 8:440–50. 10.1080/19490976.2017.1334034
32.
de Armas LR George V Filali-Mouhim A Steel C Parmigiani A Cunningham CK et al . Transcriptional and immunologic correlates of response to pandemic influenza vaccine in Aviremic, HIV-infected children. Front Immunol. (2021) 12:639358. 10.3389/fimmu.2021.639358
Summary
Keywords
HIV, influenza vaccine response, children, microbiota, immunoactivation
Citation
Sainz T, Casas I, González-Esguevillas M, Escosa-Garcia L, Muñoz-Fernández MÁ, Prieto L, Gosalbes MJ, Jiménez-Hernández N, Ramos JT, Navarro ML, Mellado MJ, Serrano-Villar S and Calvo C (2022) Nutritional Supplementation to Increase Influenza Vaccine Response in Children Living With HIV: A Pilot Clinical Trial. Front. Pediatr. 10:919753. doi: 10.3389/fped.2022.919753
Received
13 April 2022
Accepted
22 June 2022
Published
19 July 2022
Volume
10 - 2022
Edited by
Maurizio Aricò, Department of Pediatrics, Italy
Reviewed by
Grazia Bossi, San Matteo Hospital Foundation (IRCCS), Italy; Carl Armon, Children's Hospital Colorado, United States
Updates
Copyright
© 2022 Sainz, Casas, González-Esguevillas, Escosa-Garcia, Muñoz-Fernández, Prieto, Gosalbes, Jiménez-Hernández, Ramos, Navarro, Mellado, Serrano-Villar and Calvo.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Talía Sainz talia.sainz@salud.madrid.org
†ORCID: María José Gosalbes orcid.org/0000-0003-0460-1105
Nuria Jiménez-Hernández orcid.org/0000-0002-9107-056X
This article was submitted to Pediatric Infectious Diseases, a section of the journal Frontiers in Pediatrics
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.