- 1The Dyna and Fala Weinstock Department of Pediatric Hematology Oncology, Hadassah Hebrew University Medical Center, Jerusalem, Israel
- 2Department of Pathology, Hadassah Hebrew University Medical Center, Jerusalem, Israel
- 3Hadassah Hebrew University Medical Center, Nuclear Medicine Institute, Jerusalem, Israel
- 4Center for Melanoma and Cancer Immunotherapy, Hadassah Hebrew University Medical Center, Sharett Institute of Oncology, Jerusalem, Israel
Clear Cell Sarcoma (CCS), also referred to as malignant melanoma of soft parts, is a rare and aggressive malignant tumor. It comprises 1% of all soft tissue sarcomas and is known to be radio- and chemotherapy resistant. CCS shares morphological and immunohistochemical features with malignant melanoma, including melanin biosynthesis and melanocytic markers. However, it is distinct for the presence of EWSR1-ATF1 translocation which activates MITF transcription factor. We report here of an aggressive case of CCS in a 9-year-old patient, which demonstrates the critical role of molecular analysis in the diagnosis and treatment of uncommon cancer variants in the era of personalized medicine. The EWSR1-ATF1 translocation induces pathological c-Met activation, and so, following unsuccessful CTLA4 and PD-1 blockade immunotherapy, the child received cabozantinib, a small molecule tyrosine kinase inhibitor, with the intent to block c-Met oncogenic effect. In parallel, active immunization, using hapten di-nitrophenyl modified autologous tumor cells was administered with monotherapy PD-1 inhibitor nivolumab. Under this “triplet” therapy, the patient attained an initial partial response and was progression-free for 2 years, in good performance status and resumed schooling. Based on our observation, cabozantinib can be used as an effective and potentially life-prolonging treatment in CCS. We suggest that priming the child’s immune system using her autologous tumor and combating T cell exhaustion with PD-1 blockade may have synergized with the targeted therapy. Combining targeted and immunotherapy is a rapidly growing practice in solid tumors and provides a glimpse of hope in situations that previously lacked any treatment option.
Introduction
Clear Cell Sarcoma (CCS), is a rare and aggressive malignant tumor. It was first defined by Enzinger (1) in a series of 21 cases, collected over 25 years. He then identified a distinct type of sarcoma that arises mostly from tendons and aponeuroses of extremities. Due to the presence of melanin pigment, immunohistochemical staining for S-100 and other melanocytic markers, CCS resembles melanoma and was granted the name of “melanoma of soft parts” (2). The disease, comprising only 1% of all soft tissue sarcomas, appears mostly in extremities (3), while head and neck CCS are rarer, and comprise only 1–2% of reported cases (4). Complete surgical excision and a small tumor size are the main prognostic factors, since there is poor response to radio- and chemotherapy (5–8). The prognosis of head and neck cases, especially in a site that does not allow complete resection, is grim (5, 9). Reviewing the literature, only few studies and reports of head and neck CCS cases are found (3, 10–13). Metastases are common at presentation, or appear as late relapses- 30 and 63%, respectively (14, 15). Lymphatic spread is common, as well as lung metastases (16). The 5-years survival rate of metastatic CCS is only 20% [5–7]. CCS is characterized in most cases by the t(12;22)(q13;q12) translocation, that involves the Ewing sarcoma gene (EWS) on chromosome 22, with the cAMP regulated transcription factor ATF1 on chromosome 12, a member of the CREB family (17–20). This translocation has a direct impact on the MITF pathway resulting in c-Met aberrant expression (21). This abnormal expression plays, most probably, an important role in the malignant transformation of CCS progenitor cell. Hence, c-Met can be the molecular target of novel directed therapy for CCS. Herein, we present an effective treatment for metastatic clear cell sarcoma by targeting c-Met with cabozantinib, a semi-selective signal blocker.
Case presentation
A 9-year-old girl was evaluated for a bleeding mass in her left external auditory canal. Initial pathology report revealed a malignant tumor composed of hyperchromatic spindle and ovoid cells, focally demonstrating pigment, with areas of necrosis. Vascular invasion was demonstrated. The tumor cells stained for pan-melanoma markers including MART-1, HMB-45, and SOX-10 (Figure 1). The findings were compatible with malignant melanoma, pigmented Schwannoma or CCS. Imaging studies revealed a 3 × 2 cm mass extending and invading the mastoid bone. The child underwent left mastoidectomy and lymph node sampling. The tumor mass was only partially resected and on CT scan 4 weeks later a recurring mass was revealed with new enlarged lymph nodes at the posterior cervical and retro-auricular chains. The rapid, aggressive course of the disease raised the concern that another surgical procedure will only delay systemic therapy. A combination of CTLA-4 and PD-1 blocking antibodies (ipilimumab and nivolumab) was selected based on case reports of benefit in CCS and immune responsiveness of MITFhigh melanomas (22, 23). After four courses of therapy, imaging showed new 7-mm lung metastases and enlargement of the cervical lymph nodes. Fresh tissue was retrieved to establish a primary autologous tumor cell culture and chemotherapy with weekly carboplatin was administered to prevent fast, uncontrolled growth of the disease that proved to be immune checkpoint non-responsive. Thereafter, the patient received eight doses (every 3 months) of intra-dermal injections of 25 × 106 irradiated autologous tumor cells admixed with the hapten di-nitrophenyl to prime an initial tumor-specific immune response. This treatment was given based on histologic resemblance to malignant melanoma, and previous encouraging results (24), especially with combination of check-point inhibitor (25).
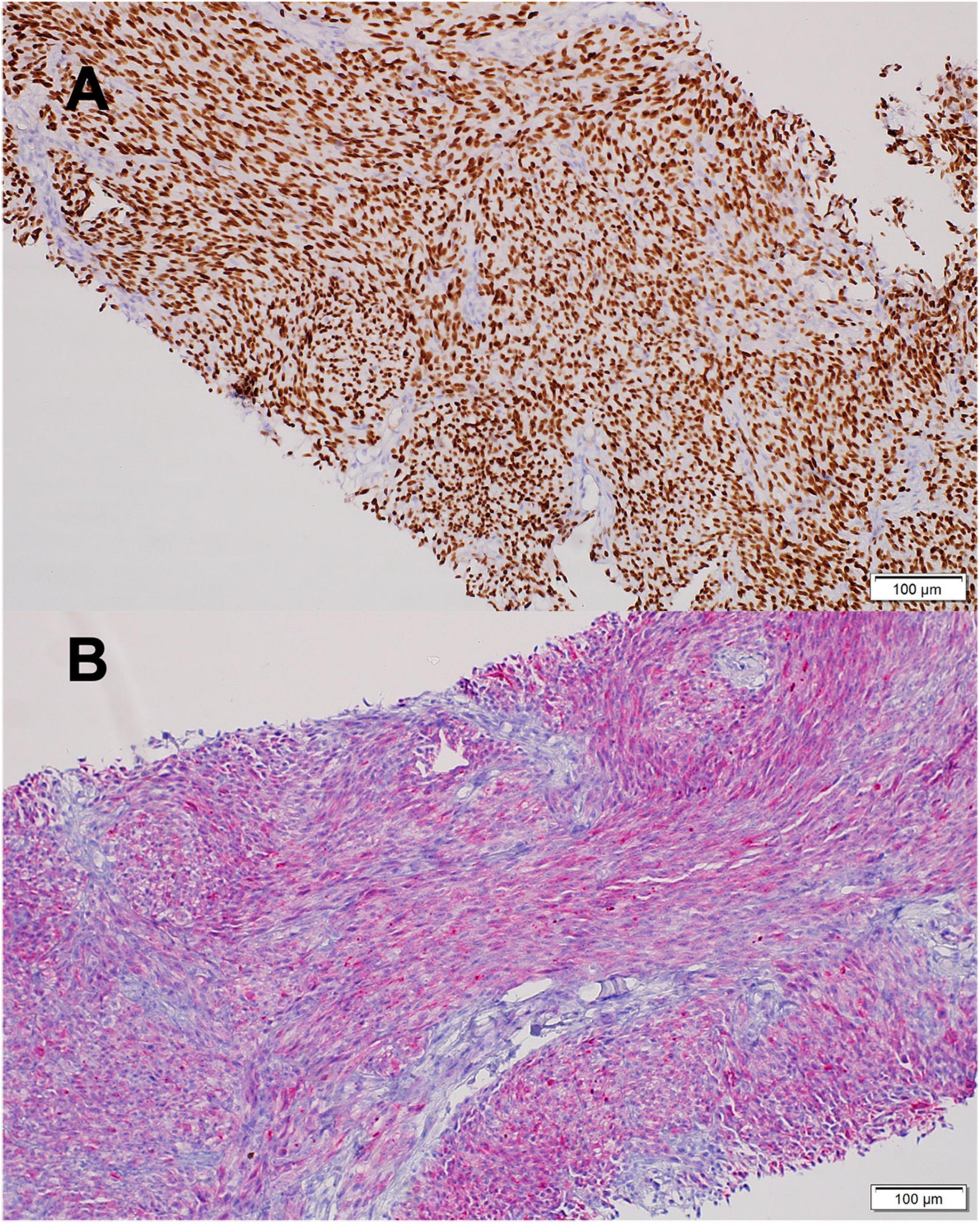
Figure 1. Clear Cell Sarcoma histology. (A) SOX10 positive staining. (B) MART1 positive staining. Scale bars 100 mm. Images were acquired by brightfield Olympus BX53 microscope with X20 air objective. (Olympus Life Science, Waltham, MA, United States), and Llumins 5MP Bright Field Camera (Llumins, Johnannesburg, South Africa). Software- Llumins ToupView (Llumins).
Genome analysis (FoundationOne®CDx, Foundation Medicine, Cambridge, MA) revealed EWSR1-ATF1 translocation, which finalized the diagnosis of CCS.
At this point cabozantinib 40 mg/day was started. Dose reductions were made to reach a level that allowed the child to resume daily activity. The treatment was given continuously for 24 months, with a daily dose of 40–20 mg/day. The patient presented with several adverse effects (classified by the CTCAE criteria): intermittent abdominal pain (grade 1) accompanied by moderate anorexia (grade 2) and weight loss, mild hair whitening, and hypothyroidism (grade 2), which are known adverse effects of cabozantinib treatment. Imaging studies showed a mild decrease in size of her lung and cervical lymph nodes, and no disease progression for 24 months-consisted with stable disease by RESICT criteria (Figure 2). The patient has a Lansky performance score of 100, and resumed schooling. Unfortunately, after 2 years, disease progressed again. Once the disease escaped therapy, it progressed rapidly, until the patient’s death.
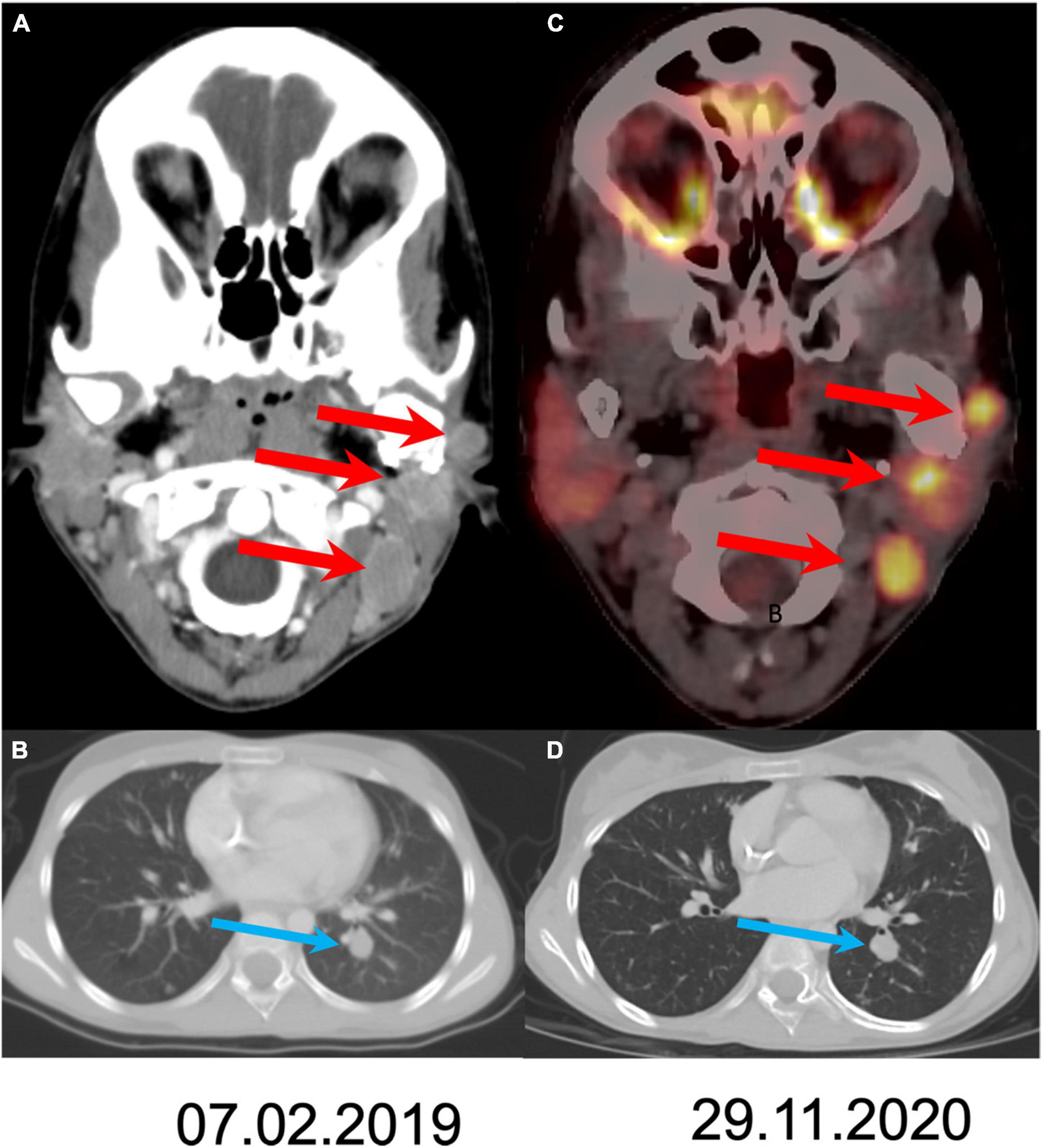
Figure 2. Comparison of disease extent at the initiation of treatment and after 21 months of follow-up. Axial CT slides at staging (A,B) show three metastases (arrows) in the left neck and lung spread. Follow up after 21 months shows stable disease both in the neck (C; fused PET/CT axial slide) and in the lung (D).
Discussion
This case is the first known report of successful stabilization of an aggressive metastatic clear cell sarcoma, under the administration of targeted multi kinase inhibitor and immuno-therapy for 24 months.
This case emphasizes the importance of genomic molecular analysis in the diagnosis and treatment of rare tumors. The EWSR1-ATF1 translocation established a clear distinction between CCS and malignant cutaneous melanoma, and navigated our team toward finding a novel treatment for this patient. EWSR1-ATF1 translocation replaces the kinase-dependent regulatory region of ATF1 with the N-terminal of EWSR1. This chimera results in an oncoprotein that mimics Melanocytic Stimulating Hormone/CREB signaling pathway and aberrantly activates MITF transcription factor (21). The MITF-regulated genes play a role in oncogenesis by activating the c-Met gene (26), which encodes a receptor tyrosine kinase that is normally expressed on stromal and mesenchymal cells and mediates signaling from hepatocyte growth factor. Few studies showed that c-Met is aberrantly expressed in several malignant tumors, such as small cell lung cancer and sarcomas (27, 28), and affects cell survival, growth, invasion and metastasis (29, 30). Previous studies showed that in CCS, EWS-ATF1 product is required for c-Met expression, and that malignant features such as survival, proliferation, chemotaxis and invasion are dependent on c-Met signaling in cellular models (31). Hence, we may assume that c-Met signaling has probably a connection to the viability of the malignancy.
Current systemic therapies for clear cell sarcoma are of limited benefit (32–36). Tyrosine kinase inhibitors were given in the past to treat patients with CCS in different clinical trials including crizotinib (32, 33), sorafenib (34) tivantinib (35) and sunitinib (33, 36). Generally, responses were poor, partial and very short-lasting with only limited disease stabilization. Early analyses of novel clinical trials investigating sunitinib or chemotherapy in combination with nivolumab are encouraging (37) and clear cell sarcoma will be considered as subgroup in Immunosarc II with further analyses to follow- although stability of disease lasted less 6 months in most cases.
Cabozantinib is an oral small-molecule-inhibitor of multiple tyrosine kinases. Several studies published showed distinct inhibition of MET and VEGFR2, and suppression of metastases, angiogenesis and tumor growth (38–40). The FDA has already approved cabozantinib for renal cell carcinoma (41) and for progressive metastatic medullary thyroid cancer (42). The Children Oncology Group published a phase I study of cabozantinib for resistant solid tumor malignancies (e.g., medullary thyroid carcinoma, wilms tumor, synovial sarcoma), with good tolerability (43). Recently, data emerging in clinical trials evaluating cabozantinib as soft tissue sarcoma treatment, show promising results, with high tolerability of treatment and effective stabilization of the disease (44, 45) and when cabozantinib is combined with immune checkpoint inhibitors (46).
The initial success we had with cabozantinib may have been predictable. The durability of the response is the surprising aspect and the therapeutic achievement of this case. Cabozantinib might prove to be a “game changer” in the treatment approach and the natural history of CCS. The question arises is there contribution of the immunotherapy to the lasting effect of cabozantinib? Early reports imply there is (46). In kidney cancer this combination is now the standard of care. Our experience demonstrates the role of Next Generation Sequencing and molecular diagnosis in enabling targeted therapy in rare forms of solid cancers. Furthermore, it shows that rational prescription of a tyrosine kinase inhibitor based on molecular tumor vulnerability may be significantly augmented by immunotherapy and should be sought in any cancer which lacks good therapeutic options.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
RSM and GG initiated the case report and substantially contributed to its conception and design, acquisition, analysis and interpretation of the data, and wrote the manuscript. NP, JG, and ML contributed to the acquisition of data and analyses. All authors critically reviewed the manuscript and approved the submitted version.
Acknowledgments
We wish to thank the patient’s family for their kind cooperation and support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Enzinger F. Clear-cell sarcoma of tendons and aponeuroses. An analysis of 21 cases. Cancer. (1965) 18:1163–74. doi: 10.1002/1097-0142(196509)18:9<1163::AID-CNCR2820180916>3.0.CO;2-0
2. Chung EB, Enzinger FM. Malignant melanoma of soft parts. A reassessment of clear cell sarcoma. Am J Surg Pathol. (1983) 7:405–13. doi: 10.1097/00000478-198307000-00003
3. Chen G, Sun S, Du Z, Sun Y, Pan Z, Che X, et al. Intra-extracranial primary clear cell sarcoma: The first report and review of the literature. World Neurosurg. (2019) 126:e1140–6. doi: 10.1016/j.wneu.2019.02.216
4. Bisceglia M, Carosi I, Lelli G, Pasquinelli G, Martinelli GN. Clear cell sarcoma of soft tissues. Clinico-pathological and ultrastructural analysis of a case in the head-neck region and a literature review. Pathologica. (1998) 90: 391–6.
5. Kawai A, Hosono A, Nakayama R, Matsumine A, Matsumoto S, Ueda T, et al. Clear cell sarcoma of tendons and aponeuroses: A study of 75 patients. Cancer. (2007) 109:109–16. doi: 10.1002/cncr.22380
6. Deenik W, Mooi WJ, Rutgers EJ, Peterse JL, Hart AA, Kroon BB. Clear cell sarcoma (malignant melanoma) of soft parts: A clinicopathologic study of 30 cases. Cancer. (1999) 86:969–75. doi: 10.1002/(SICI)1097-0142(19990915)86:6<969::AID-CNCR11>3.0.CO;2-Z
7. Li AB, Jiang BJ, Wang HH, Yang YS, Zhang XB, Lan GH, et al. Prognostic factors for survival in patients with clear cell sarcoma: An analysis of the surveillance, epidemiology, and end results (SEER) database. Med Sci Monit. (2019) 25:6950–6. doi: 10.12659/MSM.916705
8. Gantzer J, Eberst L, Csasier P, Brahmi M. Tailored approaches to rare sarcomas: current challenges and future prospects. Expert Rev Precis Med Drug Dev. (2018) 3:95–105. doi: 10.1080/23808993.2018.1454260
9. Tsuchiya H, Tomita K, Yamamoto N, Mori Y, Asada N. Caffeine-potentiated chemotherapy and conservative surgery for high-grade soft-tissue sarcoma. Anticancer Res. (1998) 18:3651–6.
10. Feasel PC, Cheah AL, Fritchie K, Winn B, Piliang M, Billings SD. Primary clear cell sarcoma of the head and neck: a case series with review of the literature. J Cutan Pathol. (2016) 43:838–46. doi: 10.1111/cup.12755
11. Silverman DA, Smith SW, Essig GF Jr., Kang SY, Schoenfield L, Kim LR. Clear cell sarcoma of the nasal soft tissue envelope. Otolaryngol Case Rep. (2019) 13:100127. doi: 10.1016/j.xocr.2019.100127
12. Inoue S, Chepeha DB, Lucas DR, Faisal S, Stewart RC, Mushtaq R, et al. Clear cell sarcoma of the jaw: a case report and review of the literature. J Pediatr Hematol Oncol. (2013) 35:402–5. doi: 10.1097/MPH.0b013e3182580d1f
13. Hicks MJ, Saldivar VA, Chintagumpala MM, Horowitz ME, Cooley LD, Barrish JP, et al. Malignant melanoma of soft parts involving the head and neck region: review of literature and case report. Ultrastruct Pathol. (1995) 19:395–400. doi: 10.3109/01913129509021912
14. Obiorah IE, Ozdemirli M. Clear cell sarcoma in unusual sites mimicking metastatic melanoma. World J Clin Oncol. (2019) 10:213–21. doi: 10.5306/wjco.v10.i5.213
15. Ferrari A, Casanova M, Bisogno G, Mattke A, Meazza C, Gandola L, et al. Clear cell sarcoma of tendons and aponeuroses in pediatric patients: A report from the italian and german soft tissue sarcoma cooperative group. Cancer. (2002) 94:3269–76. doi: 10.1002/cncr.10597
16. Yang L, Chen Y, Cui T, Knösel T, Zhang Q, Geier C, et al. Identification of biomarkers to distinguish clear cell sarcoma from malignant melanoma. Hum Pathol. (2012) 43:1463–70. doi: 10.1016/j.humpath.2011.10.022
17. Bridge JA, Sreekantaiah C, Neff JR, Sandberg AA. Cytogenetic findings in clear cell sarcoma of tendons and aponeuroses. Malignant melanoma of soft parts. Cancer Genet Cytogenet. (1991) 52:101–6. doi: 10.1016/0165-4608(91)90059-4
18. Reeves BR, Fletcher CD, Gusterson BA. Translocation t(12;22)(q13;q13) is a nonrandom rearrangement in clear cell sarcoma. Cancer Genet Cytogenet. (1992) 4:122–7. doi: 10.1016/0165-4608(92)90336-7
19. Langezaal SM, Graadt can Roggen JF, Cleton-Jansen AM, Baelde JJ, Hogendoorn PC. Malignant melanoma is genetically distinct from clear cell sarcoma of tendons and aponeurosis (malignant melanoma of soft parts). Br J Cancer. (2001) 84:535–8. doi: 10.1054/bjoc.2000.1628
20. Zucman J, Delattre O, Desmaze C, Epstein AL, Stenman G, Speleman F, et al. EWS and ATF-1 gene fusion induced by t (12;22) translocation in malignant melanoma of soft parts. Nature Genet. (1993) 4:341–5. doi: 10.1038/ng0893-341
21. Davis IJ, Kim JJ, Ozsolak F, Widlund HR, Rozenblatt-Rosen O, Granter SR, et al. Oncogenic MITF dysregulation in clear cell sarcoma: defining the MiT family of human cancers. Cancer Cell. (2006) 9:473–84. doi: 10.1016/j.ccr.2006.04.021
22. Marcrom S, De Los Santos JF, Conry RM. Complete response to mediastinal clear cell sarcoma to pembrolizumab with radiotherapy. Clin Sarcoma Res. (2017) 7:14. doi: 10.1186/s13569-017-0079-1
23. Rabbie R, Ferguson P, Molina-Aguilar C, Adams DJ, Robles-Espinoza CD. Melanoma subtypes: genomic profiles, prognostic molecular markers and therapeutic possibilities. J Pathol. (2019) 247:539–51. doi: 10.1002/path.5213
24. Berd D, Sato T, Cohn H, Maguire HC Jr., Mastrangelo MJ. Treatment of metastatic melanoma with autologous, hapten-modified melanoma vaccine: Regression of pulmonary metastases. Int J Cancer. (2001) 94:531–9. doi: 10.1002/ijc.1506.abs
25. Cavalcante L, Chowdhary A, Sosman JA, Chandra S. Combining tumor vaccination and oncolytic viral approaches with checkpoint inhibitors: rationale, pre-clinical experience, and current clinical trials in malignant melanoma. Am J Clin Dermatol. (2018) 19:657–70. doi: 10.1007/s40257-018-0359-4
26. McGill GG, Haq R, Nishimura EK, Fisher DEC-. Met expression is regulated by Mitf in the melanocyte lineage. J Bio Chem. (2006) 281:10365–73. doi: 10.1074/jbc.M513094200
27. Knudsen BS, Vande Woude G. Showering c-MET-dependent cancers with drugs. Curr Opin Genet Dev. (2008) 18:87–96. doi: 10.1016/j.gde.2008.02.001
28. Milgliore C, Giordano S. Molecular cancer therapy: can our expectation be MET? Eur J Cancer. (2008) 44:641–51. doi: 10.1016/j.ejca.2008.01.022
29. Rong S, Segal S, Anver M, Resau JH, Vande Woude GF. Invasiveness and metastasis of NIH 3T3 cells induced by Met-hepatocyte growth factor/scatter factor autocrine stimulation. Proc Natl Acad Sci U S A. (1994) 91:4731–5. doi: 10.1073/pnas.91.11.4731
30. Boccaccio C, Sabatino G, Medico E, Girolami F, Follenzi A, Reato G, et al. The MET oncogene drives a genetic programme linking cancer to haemostasis. Nature. (2005) 434:396–400. doi: 10.1038/nature03357
31. Davis IJ, McFadden AW, Zhang Y, Coxon A, Burgess TL, Wagner AJ, et al. Identification of the receptor tyrosine kinase c-Met and its ligand, hepatocyte growth factor, as therapeutic targets in clear cell sarcoma. Cancer Res. (2010) 70:639–45. doi: 10.1158/0008-5472.CAN-09-1121
32. Schöffski P, Wozniak A, Stacchiotti S, Rutkowski P, Blay JY, Lindner LH, et al. Activity and safety of crizotinib in patients with advanced clear-cell sarcoma with MET alterations: European organization for research and treatment of cancer phase II trial 90101 ‘CREATE’. Ann Oncol. (2017) 28:3000–8. doi: 10.1093/annonc/mdx527
33. Smrke A, Frezza AM, Giani C, Somaiah N, Brahmi M, Czarnecka AM, et al. Systemic treatment of advanced clear cell sarcoma: results from a retrospective international series from the world sarcoma network. ESMO open. (2022) 7:100522. doi: 10.1016/j.esmoop.2022.100522
34. Mir O, Boudou-Rouquette P, Larousserie F, Babinet A, Dumaine V, Anract P, et al. Objective response to sorafenib in advanced clear-cell sarcoma. Ann Oncol. (2012) 23:807–9. doi: 10.1093/annonc/mds005
35. Wagner AJ, Goldberg JM, Dubois SG, Choy E, Rosen L, Pappo A, et al. Tivantinib (ARQ 197), a selective inhibitor of MET, in patients with microphthalmia transcription factor-associated tumors: Results of a multicenter phase 2 trial. Cancer. (2012) 118:5894–902. doi: 10.1002/cncr.27582
36. Stacchiotti S, Grosso F, Negri T, Palassini E, Morosi C, Pilotti S, et al. Tumor response to sunitinib malate observed in clear-cell sarcoma. Ann Oncol. (2010) 21:1130–1. doi: 10.1093/annonc/mdp611
37. Martin-Broto J, Hindi N, Grignani G, Martinez-Trufero J, Redondo A, Valverde C, et al. Nivolumab and sunitinib combination in advanced soft tissue sarcomas: a multicenter, single-arm, phase Ib/II trial. J Immunother Cancer. (2020) 8:e001561. doi: 10.1136/jitc-2020-001561
38. Yakes FM, Chen J, Tan J, Yamaguchi K, Shi Y, Yu P, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. (2011) 10:2298–308. doi: 10.1158/1535-7163.MCT-11-0264
39. Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. (2009) 15:220–31. doi: 10.1016/j.ccr.2009.01.027
40. Katamaya R, Kobayashi Y, Friboulet L, Lockerman EL, Koike S, Shaw AT, et al. Cabozantinib overcomes crizotinib resistance in ROS1 fusion-positive cancer. Clin Cancer Res. (2015) 21:166–74. doi: 10.1158/1078-0432.CCR-14-1385
41. Choueiri TK, Escudier B, Powles T, Tannir NM, Mainwaring PN, Donskov F, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomized, open-label, phase 3 trial. Lancet Oncol. (2016) 17:917–27. doi: 10.1016/S1470-2045(16)30107-3
42. Elisei R, Schlumberger MJ, Müller SP, Schöffski P, Brose MS, Shah MH, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. (2013) 31:3639–46. doi: 10.1200/JCO.2012.48.4659
43. Chunk MK, Widemann BC, Minard CG, Liu X, Kim A, Bernhardt MB, et al. A phase 1 study of cabozantinib in children and adolescents with recurrent or refractory solid tumors, including CNS tumors: Trial ADVL1211, a report from the Children’s Oncology Group. Pediatr Blood Cancer. (2018) 65:e27077. doi: 10.1002/pbc.27077
44. Coyne GO, Kummar S, Hu J, Ganjoo K, Chow WA, Do KT, et al. Clinical activity of single-agent cabozantinib (XL184), a multi-receptor tyrosine kinase inhibitor, in patients with refractory soft-tissue sarcomas. Clin Cancer Res. (2022) 28:279–88. doi: 10.1158/1078-0432.CCR-21-2480
45. Schöffski P, Blay JY, Ray-Coquard I. Cabozantinib as an emerging treatment for sarcoma. Curr Opin Oncol. (2020) 32:321–31. doi: 10.1097/CCO.0000000000000644
Keywords: clear cell sarcoma, immunotherapy, cabozantinib, MET inhibitor, EWSR1-ATF1 translocation
Citation: Sidlik Muskatel R, Pillar N, Godefroy J, Lotem M and Goldstein G (2022) Case report: Robust response of metastatic clear cell sarcoma treated with cabozantinib and immunotherapy. Front. Pediatr. 10:940927. doi: 10.3389/fped.2022.940927
Received: 10 May 2022; Accepted: 29 August 2022;
Published: 06 October 2022.
Edited by:
Ben Pode-Shakked, Sheba Medical Center, IsraelReviewed by:
Deepam Pushpam, All India Institute of Medical Sciences, IndiaKatrina Ingley, University College Hospital (UCH), United Kingdom
Copyright © 2022 Sidlik Muskatel, Pillar, Godefroy, Lotem and Goldstein. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rakefet Sidlik Muskatel, c2lkbGlrQGdtYWlsLmNvbQ==
 Rakefet Sidlik Muskatel
Rakefet Sidlik Muskatel Nir Pillar2
Nir Pillar2 Michal Lotem
Michal Lotem