- 1Department of Pediatrics Hematology and Oncology Nursing, West China Second University Hospital, Sichuan University, Chengdu, China
- 2Department of Pediatrics, West China Second University Hospital, Sichuan University, Chengdu, China
- 3Key Laboratory of Obstetric & Gynecologic and Pediatric Diseases and Birth Defects of Ministry of Education, Sichuan University, Chengdu, China
Background: We aimed to determine the association between red blood cell transfusions (RBCT) and bronchopulmonary dysplasia (BPD) in neonates.
Methods: A systematic review and meta-analysis were conducted using data obtained from literature search of PubMed, Embase, and Web of Science from their inception till May 1, 2022. Two reviewers independently selected potentially relevant studies, and after data extraction, they assessed the methodological quality of the included studies using the Newcastle–Ottawa scale. Data were pooled using random-effects models in Review Manager 5.3. Subgroup-analysis was performed based on the number of transfusions and adjusted results.
Results: Of the 1,011 identified records, 21 total case-control, cross-sectional, and cohort studies were selected, which included a total of 6,567 healthy controls and 1,476 patients with BPD. The pooled unadjusted odds ratio ([OR], 4.01; 95% confidence interval [CI] 2.31–6.97) and adjusted OR (5.11; 95% CI 3.11–8.4) showed significant association between RBCT and BPD. A substantial heterogeneity was noted, which could be due to different variables controlled for in each study. The subgroup analysis showed that heterogeneity may be partially explained by the extent of transfusion.
Conclusion: The association between BPD and RBCT remains unclear based on the current data due to the substantial heterogeneity among the results. Well-designed studies are still needed in the future.
Introduction
Bronchopulmonary dysplasia (BPD) is a common complication in preterm infants. This complication can lead to asthma, wheezing, limitation of activity tolerance, and recurrent low respiratory tract infections (1, 2). Infants with lower birth weight (BW) and gestational age (GA) are at risk for developing BPD. In addition, maternal smoking, chorioamnionitis, male sex, oxygen therapy, duration of mechanical ventilation, and co-morbidities, including sepsis, necrotizing enterocolitis, and pulmonary hemorrhage have been found to be associated with BPD (3–5). Epidemiological studies have reported that approximately 80% of preterm infants born between 22 and 24 weeks of gestation will develop BPD to a certain degree (6). As a result of an increased survival rate of extremely preterm infants, the incidence of BPD has increased (7, 8); however, no specific or preventive treatment has developed.
Red blood cell transfusions (RBCT) are commonly used in neonatal intensive care units. A previous survey reported that the vast majority of extremely low BW infants and 58% of preterm infants (<32 weeks of GA) received RBCT in the neonatal period (9–11). However, the effects of RBCT on short and long-term outcomes remain unclear. Recent studies have reported an association between RBCT and intra-ventricular hemorrhage, retinopathy of prematurity, and necrotizing enterocolitis (12–14). Few observational studies have found that patients with BPD receive higher RBCT volume and the number of RBCT is associated with BPD development (15–17). Vecchio et al. instituted new guidelines for administering RBCT in their neonatal intensive care unit, and the transfusion rate was concordant with a lower incidence of BPD (18). However, other studies found no relationship between BPD and RBCT (19, 20). Therefore, we aimed to conduct a systematic review and meta-analysis of the available literature to investigate the association between RBCT and BPD.
Methods
Literature search
We performed this systematic review and meta-analysis in accordance with the Meta-analysis of Observational Studies in Epidemiology (MOOSE) and the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (21, 22). We searched the electronic databases PubMed, Embase, and Web of Science from their inception till May 1, 2022. The keywords “red blood cell,” OR “blood transfusion,” AND “BPD,” OR “bronchopulmonary dysplasia”, were employed as search term. Only articles published in English language were included. We also searched the reference lists of review articles for additional eligible studies. Detailed strategy for online database search is described in the supplemental files.
Study selection
Studies were selected by screening titles, abstracts and full texts. Studies were included if they had a control group, and reported mean and standard deviation [(SD) or the data can be converted to mean and SD] of the number and total volume of RBCT between BPD patients and controls, as well as the odds ratio (OR) of RBCT for BPD. Case reports, letters to the editors, articles with incomplete data, and studies with less than 10 samples were excluded from analysis.
Data extraction
One reviewer (J.Z.) extracted data from the selected studies into a predetermined form. The first author, published year, study design, country, group size, sample size, BPD diagnosis and transfusion data were recorded. Another reviewer (L.T.) checked the data to ensure the accuracy. Any disagreements were settled with a third reviewer (T.T.Z.).
Quality assessment
Two authors (J.Z. and L.T.) independently evaluated the quality of each included study using the Newcastle–Ottawa scale (NOS) for case-control or cohort studies (23). This scale covers three parts of the study design: patient selection (4 points), comparability of the study groups (2 points), and exposure/outcome (3 points). A modified NOS (24) with a total of 10 points was used for cross-sectional studies. Studies with a score below six points were considered to be of low quality.
Statistical analysis
We pooled the weighted mean difference and 95% confidence interval (CI) of total number of administered RBCT and total RBCT volume in patients with BPD and those without BPD, and the OR of RBCT for BPD, using a random effect model. A two-tailed p < 0.05 was considered statistically significant. Heterogeneity across studies was assessed using the chi-square test and I2. An I2 value > 75% indicated high heterogeneity. Publication bias was assessed visually based on the symmetry of funnel plots. Sensitivity analyses were performed by excluding one study at a time, to test the stability of the results. We calculated the mean and SD of the number or volume of RBCT scans using sample size, median and interquartile range (IQR) according to Hozo et al. (25), when needed. Data analyses were performed using Review Manager Software version 5.3.
Results
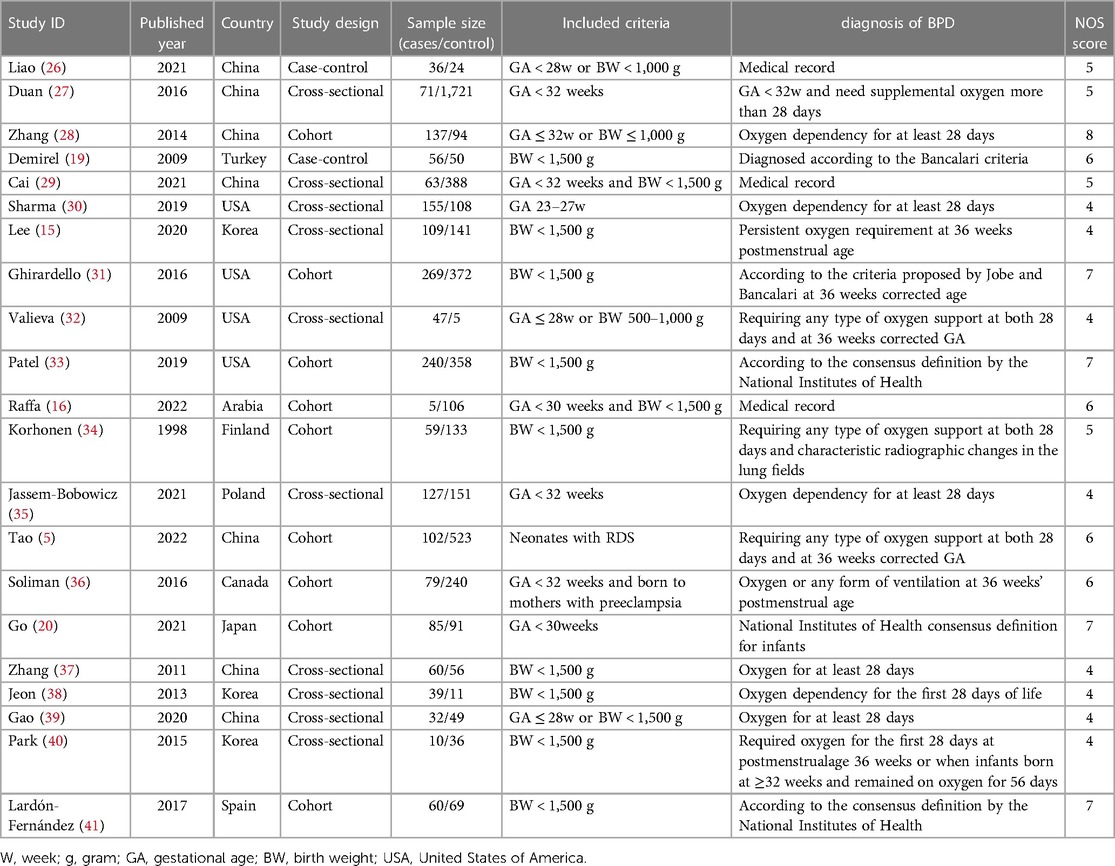
A total of 1,011 articles were retrieved from the databases using systematic and manual searches. After removing the duplicates, and performing screening of abstracts and full-text articles, 21 studies (5, 15, 16, 19, 20, 26–41) were included. These studies included a total of 6,567 control infants and 1,476 patients with BPD, and were eligible for the meta-analysis. (Supplementary Material Figure S1). The characteristics of eligible studies are presented in Table 1.
All included participants had a GA of less than 32 weeks at birth or BW of less than 1,500 g. Studies included populations from different countries, with being China and the USA being the most common. Although all studies reported the association between BPD and RBCT, their objectives varied. In half of the included studies the primary objective was to investigate risk factors of BPD including RBCT. Two studies collected data based on medical records (16, 26), and others defined BPD as neonates requiring oxygen support at 28 days of life or at 36 weeks of corrected GA. Study design included cross-sectional (n = 10), cohort (n = 9), and case–control (n = 2).
The NOS scores of the included studies ranged from 4 to 8, and detailed information is provided in the supplemental files. In all included studies, exposed and non-exposed controls were drawn from the same community. However, common weaknesses were lack of adequate confounder adjustments such as neonatal sepsis, maternal diseases during pregnancy, and outcome assessment based on medical records without a description of blind.
The pooled ORs obtained using a random-effect model showed a significant association between RBCT and BPD in both crude analysis (OR 4.01, 95% CI: 2.31–6.97) and adjusted analysis (OR 5.11, 95% CI: 3.11–8.40). There was a substantial heterogeneity between studies (I2 = 56%) (Figure 1). In addition, we conducted subgroup-analyses of the number of transfusions, and the association remained significant (Figure 2).
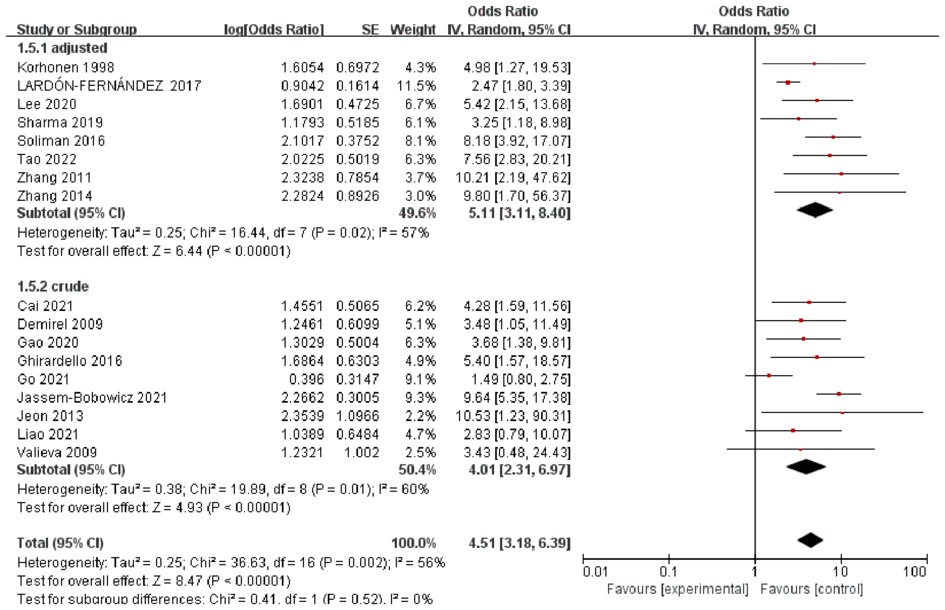
Figure 1. Forest plot for the association between RBCT and the development of BPD, stratified on crude or adjusted results.
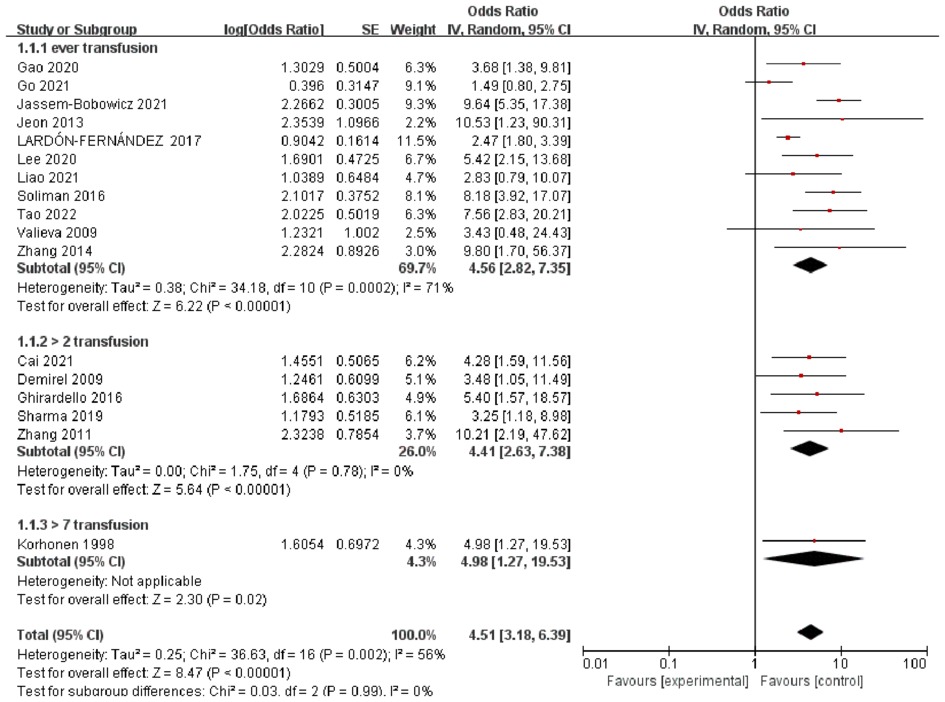
Figure 2. Forest plot for the association between RBCT and the development of BPD, stratified on number of transfusion.
A pooled analysis of RBCT including 227 cases and 465 controls (Supplementary Material Figure S2) found that patients with BPD were more likely to have more RBCT than those without BPD (standard mean difference 2.62, 95% CI 0.99–4.24, I2 = 89%, p < 0.01).
Three studies including 255 cases and 500 controls were pooled for the total transfusion volumes. The standard mean difference of total RBCT volumes was 55.48 (95% CI: 3.82–107.4, I2 = 95%, p < 0.01) between patients with BPD and controls. (Supplementary Material Figure S2).
Except for the analysis of the total transfusion volume, there were no apparent changes in the pooled results when any single study was removed. Visual examination of the funnel plots of the studies included in this meta-analysis showed no apparent publication bias.
Discussion
The aim of this systematic review was to evaluate the association between RBCT and BPD based on the available evidence to provide suggestions for future investigations. The meta-analysis of the unadjusted data showed a significant association between RBCT and BPD. The odds of BPD among infants having RBCT were 4.01 times higher than the odds of those without transfusion with statistically significant adjusted estimate (OR 5.11, 95% CI, 3.11–8.40). These estimate association between RBCT and BPD for different numbers of transfusions did not differ between groups. Our study provides data on the total volume of transfusions, and the number of transfusions between BPD and non-BPD groups.
The relationship between RBCT and BPD may be explained by several mechanisms. When immature lungs are exposed to a hyperoxic environment, this could lead to a disruption of the alveolar-capillary barrier, overexpression of inflammatory mediators, vascular leakage, and cell death (42). RBCT increases adult hemoglobin in neonates, and shifts the oxygen disassociation curve to the right, increasing lung susceptibility to hyperoxia. Additionally, iron levels and other inflammatory mediators in stored blood products can promote free radical generation, infection, and fibrosis (43). Patel et al. (33) reported that very low BW infants undergoing multiple RBCT had excessive iron stores compared to non-transfused infants, and those who received more transfusions were more prone to developing BPD. This finding supports the hypothesis that free radicals and iron overload play key roles in the pathogenesis of BPD. Evidence also suggests that RBCT can potentially impact the lungs via an acute inflammatory response (28). Some neonates that received RBCT required mechanical ventilation, and the ventilator parameters could not be reduced in these patients (28). The hypothesis of transfusion related acute lung injury may be explained by this fact. The production of inflammatory cytokines and immunoactivation of the endothelium observed after the transfusion of RBCT in preterm infants may be a direct causal link between transfusion and major neonatal morbidity (44).
Investigations exploring the association between RBCT and BPD should consider several other factors based on our study. First, the number or the volume of transfusions showed substantial heterogeneity across studies, ranging from one to more than seven. We grouped the studies according to the number of transfusions performed. Although the ORs of BPD did not differ within the groups, heterogeneity was significantly reduced in each subgroup. As hypothesized, a higher number of transfusions may increase the risk of BPD. This might be explained by the small number of studies and variables that were adjusted differently between studies. The second factor that could have influenced the results on the association between RBCT and BPD may be the timing of transfusion. Duan et al. (27) investigated the risk factors for BPD and showed that early but not late anemia increased the risk of BPD. Early exposure to free radicals and iron load during this critical period would influence lung development. However, none of the included studies that described RBCT provided information about the timing of transfusion.
RBCT in neonates could be a lifesaving intervention in certain emergencies, but it is also known to cause adverse events, such as dissemination of infectious disease and inappropriate immune responses (45). Our findings raise the awareness of the risks associated with RBCT. This is the first study to systematically review pooled evidence for an association between BPD and RBCT. We used rigorous methods to search for potential studies, select studies, extract data, and pool the results to identify potential confounders. However, our study has a few limitations. The GA and BW from the included studies varied, and there was limited data to perform subgroup analysis with these parameters. Additionally, the definition of BPD differed among included studies, and misclassification of BPD could lead to possible bias. Primary outcomes in the majority of included studies did not investigate the association between BPD and RBCT, therefore, they may not have adequately controlled for potential confounders such as neonatal co-morbidities. Because more ill neonates often require closer monitoring and have more complications of prematurity and longer hospital stays, more RBCT and higher rates of BPD will be observed in these patients. Furthermore, although we performed a subgroup analysis of crude and adjusted analyses, the adjusted variables were not consistent between studies. There were insufficient data to perform meta-regression to identify potential effectors. The number of studies was lower than needed to interpret the results of subgroup analysis.
Conclusion
This is the most comprehensive review and analysis of the evidence on the relationship between RBCT and BPD. However, substantial heterogeneity across the body of studies was clearly identified. Many aspects, such as lack of or inadequate confounder adjustments, severity of BPD, and the number of transfusions significantly varied. The association between RBCT and BPD needs more studies to confirmit in the future. This review provides empirical evidence that demonstrating the importance of accounting for potential confounders when interpreting data in this area.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
Conception of the work: JZ, TZ Literature search: JZ, LT. Data analysis: JZ., TZ. Drafting the article: JZ, LT, Critical revision of the article: JL. Final approval of the version to be published: All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1095889/full#supplementary-material.
References
1. Stoll BJ, Hansen NI, Bell EF, Shankaran LA, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD neonatal research network. Pediatrics. (2010) 126(3):443–56. doi: 10.1542/peds.2009-2959.20732945
2. Keller RL, Feng R, DeMauro SB, Ferkol T, Hardie W, Rogers EE, et al. Bronchopulmonary dysplasia and perinatal characteristics predict 1-year respiratory outcomes in newborns born at extremely low gestational age: a prospective cohort study. J Pediatr. (2017) 187:89–97.e3. doi: 10.1016/j.jpeds.2017.04.026.28528221
3. Hartling L, Liang YY, Lacaze-Masmonteil T. Chorioamnionitis as a risk factor for bronchopulmonary dysplasia: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. (2012) 97(1):F8–F17. doi: 10.1136/adc.2010.210187.21697236
4. Bose C, Van Marter LJ, Laughon M, O'Shea TM, Allred EN, Karna P, et al. Fetal growth restriction and chronic lung disease among infants born before the 28th week of gestation. Pediatrics. (2009) 124(3):e450–8. doi: 10.1542/peds.2008-3249
5. Tao Y, Han X, Guo WL. Predictors of bronchopulmonary dysplasia in 625 neonates with respiratory distress syndrome. J Trop Pediatr. (2022) 68(3):fmac037. doi: 10.1093/tropej/fmac037.35595255
6. Younge N, Goldstein RF, Bann CM, Hintz SR, Patel RM, Smith PB, et al. Survival and neurodevelopmental outcomes among periviable infants. N Engl J Med. (2017) 376(7):617–28. doi: 10.1056/NEJMoa1605566.28199816
7. Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA. (2015) 314(10):1039–51. doi: 10.1001/jama.2015.10244.26348753
8. Doyle LW, Carse E, Adams AM, Ranganathan S, Opie G, Cheong JLY, et al. Ventilation in extremely preterm infants and respiratory function at 8 years. N Engl J Med. (2017) 377(4):329–37. doi: 10.1056/NEJMoa1700827.28745986
9. Keir AK, Yang J, Harrison A, Pelausa E, Shah PS; Canadian Neonatal Network. Temporal changes in blood product usage in preterm neonates born at less than 30 weeks’ gestation in Canada. Transfusion. (2015) 55(6):1340–6. doi: 10.1111/trf.12998
10. Banerjee J, Asamoah FK, Singhvi D, Kwan AWG, Morris JK, Aladangady N. Haemoglobin level at birth is associated with short term outcomes and mortality in preterm infants. BMC Med. (2015) 13:16. doi: 10.1186/s12916-014-0247-6.25622597
11. Howarth C, Banerjee J, Aladangady N. Red blood cell transfusion in preterm infants: current evidence and controversies. Neonatology. (2018) 114(1):7–16. doi: 10.1159/000486584.29550819
12. Baer VL, Lambert DK, Henry E, Snow GL, Christensen RD. Red blood cell transfusion of preterm neonates with a grade 1 intraventricular hemorrhage is associated with extension to a grade 3 or 4 hemorrhage. Transfusion. (2011) 51(9):1933–9. doi: 10.1111/j.1537-2995.2011.03081.x.21382049
13. Hengartner T, Adams M, Pfister RE, Snyers D, McDougall J, Waldvogel S, et al. Associations between red blood cell and platelet transfusions and retinopathy of prematurity. Neonatology. (2020) 117(5):1–7. doi: 10.1159/000512020.33291117
14. Marin T, Moore J, Kosmetatos N, Roback JD, Weiss P, Higgins M, et al. Red blood cell transfusion-related necrotizing enterocolitis in very-low-birthweight infants: a near-infrared spectroscopy investigation. Transfusion. (2013) 53(11):2650–8. doi: 10.1111/trf.12158.23480548
15. Lee EY, Kim SS, Park GY, Lee SH. Effect of red blood cell transfusion on short-term outcomes in very low birth weight infants. Clin Exp Pediatr. (2020) 63(2):56–62. doi: 10.3345/kjp.2019.00990.32024329
16. Raffa LH, Aljohani W. Evaluation of the effect of blood transfusion on retinopathy of prematurity at a tertiary care center in western Saudi Arabia. Cureus. (2022) 14(4):e24495. doi: 10.7759/cureus.24495.35651468
17. Duan J, Kong XY, Li QP, Hua S, Zhang S, Feng Z, et al. Association between hemoglobin levels in the first 3 days of life and bronchopulmonary dysplasia in preterm infants. Am J Perinatol. (2016) 33(10):998–1002. doi: 10.1055/s-0036-1583189.27120476
18. Vecchio AD, Henry E, D'Amato G, Cannuscio A, Corriero L, Motta M, et al. Annamaria cannuscio instituting a program to reduce the erythrocyte transfusion rate was accompanied by reductions in the incidence of bronchopulmonary dysplasia, retinopathy of prematurity and necrotizing enterocolitis. J Matern Fetal Neonatal Med. (2013) 26(Suppl 2):77–9. doi: 10.3109/14767058.2013.830836.24059559
19. Demirel N, Bas AY, Zenciroglu A. Bronchopulmonary dysplasia in very low birth weight infants. Indian J Pediatr. (2009) 76(7):695–8. doi: 10.1007/s12098-009-0110-5.19381510
20. Go H, Ohto H, Nollet KE, Sato K, Ichikawa H, Kume Y, et al. Red cell distribution width as a predictor for bronchopulmonary dysplasia in premature infants. Sci Rep. (2021) 11(1):7221. doi: 10.1038/s41598-021-86752-.33790386
21. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. Jama. (2000) 283(15):2008–12. doi: 10.1001/jama.283.15.2008.10789670
22. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097.19621072
23. Wells GA, Shea B, O’Connell D, et al. Ottawa Hospital Research Institute. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed 2 May, 2022).
24. Modesti PA, Reboldi G, Cappuccio FP, Agyemang C, Remuzzi G, Rapi S, et al. Panethnic differences in blood pressure in Europe: a systematic review and meta-analysis. PLoS One. (2016) 11(1) :e0147601. doi: 10.1371/journal.pone.0147601
25. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. (2005) 5:13. doi: 10.1186/1471-2288-5-13.15840177
26. Liao ZX, Zhao X, Rao HP, Kang Y. Analysis of correlative risk factors for blood transfusion therapy for extremely low birth weight infants and extreme preterm infants. Am J Transl Res. (2021) 13(7):8179–85. eCollection 2021. doi: 10.1038/srep22717
27. Duan J, Kong XY, Li QP, Hua S, Zhang S, Zhang X. Association between anemia and bronchopulmonary dysplasia in preterm infants. Sci Rep. (2016) 6:22717. doi: 10.1038/srep22717.26936610
28. Zhang ZQ, Huang XM, Lu H. Association between red blood cell transfusion and bronchopulmonary dysplasia in preterm infants. Sci Rep. (2014) 4:4340. doi: 10.1038/srep04340.24614152
29. Cai HW, Jiang L, Liu YS, Shen T, Yang Z, Wang S, et al. Development and verification of a risk prediction model for bronchopulmonary dysplasia in very low birth weight infants. Transl Pediatr. (2021) 10(10):2533–43. doi: 10.21037/tp-21-445.34765477
30. Sharma A, Xin YM, Chen XG, Sood BG. Early prediction of moderate to severe bronchopulmonary dysplasia in extremely premature infants. Pediatr Neonatol. (2020) 61(3):290–9. doi: 10.1016/j.pedneo.2019.12.001.32217025
31. Ghirardello S, Dusi E, Cortinovis I, Villa S, Fumagalli M, Agosti M, et al. Effects of red blood cell transfusions on the risk of developing complications or death: an observational study of a cohort of very low birth weight infants. Am J Perinatol. (2017) 34(1):88–95. doi: 10.1055/s-0036-158430.27249797
32. Valieva OA, Strandjord TP, Mayock DE, Juul SE. Effects of transfusions in extremely low birth weight infants: a retrospective study. J Pediatr. (2009) 155(3):331–37.e1. doi: 10.1016/j.jpeds.2009.02.026.19732577
33. Patel RM, Knezevic A, Yang J, Shenvi N, Hinkes M, Roback JD, et al. Enteral iron supplementation, red blood cell transfusion, and risk of bronchopulmonary dysplasia in very-low-birth-weight infants. Transfusion. (2019) 59(5):1675–82. doi: 10.1111/trf.15216.30801736
34. Korhonen P, Tammela O, Koivisto AM, Laippala P, Ikonen S. Frequency and risk factors in bronchopulmonary dysplasia in a cohort of very low birth weight infants. Early Hum Dev. (1999) 54(3):245–58. doi: 10.1016/s0378-3782(98)00101-7.10321791
35. Jassem-Bobowicz JM, Klasa-Mazurkiewicz D, Żawrocki A, Stefańska K, Domżalska-Popadiuk I, Kwiatkowski S, et al. Prediction model for bronchopulmonary dysplasia in preterm newborns. Children (Basel). (2021) 8(10):886. doi: 10.3390/children8100886.34682151
36. Soliman N, Chaput K, Alshaikh B, Yusuf K. Preeclampsia and the risk of bronchopulmonary dysplasia in preterm infants less than 32 Weeks’ gestation. Am J Perinatol. (2017) 34(6):585–92. doi: 10.1055/s-0036-1594017.27919118
37. Zhang HS, Fang JP, Su HB, et al. Risk factors for bronchopulmonary dysplasia in neonates born at ≤ 1500 g (1999-2009). Pediatr Int. (2011) 53(6):915–20.21605281
38. Jeon GW, Sin JB. Risk factors of transfusion in anemia of very low birth weight infants. Yonsei Med J. (2011) 53(6):915–20. doi: 10.1111/j.1442-200X.2011.03399.x.
39. Gao SQ, Xiao TT, Ju R, Ma R, Zhang X, Dong W. The application value of lung ultrasound findings in preterm infants with bronchopulmonary dysplasia. Transl Pediatr. (2020) 9(2):93–100. doi: 10.21037/tp.2020.03.14.32477908
40. Park SH, Kim HM. The iron Status of very low birth weight infants receiving multiple erythrocyte transfusions during hospitalization in the neonatal intensive care unit. Pediatr Gastroenterol Hepatol Nutr. (2015) 18(2):100–7. doi: 10.5223/pghn.2015.18.2.100.26157695
41. Lardón-Fernández M, Uberos J, Molina-Oya M, Narbona-López E. Epidemiological factors involved in the development of bronchopulmonary dysplasia in very low birth-weight preterm infants. Minerva Pediatr. (2017) 69(1):42–9. doi: 10.23736/S0026-4946.16.04215-8.
42. Harijith AK, Bhandari V. Hyperoxia in the pathogenesis of bronchopulmonary dysplasia. In: Bhandari V, editors. Bronchopulmonary dysplasia. Respiratory medicine. Cham: Humana Press (2016). p. 3–26. doi: 10.1007/978-3-319-28486-6_1
43. Collard KJ. Is there a causal relationship between the receipt of blood transfusions and the development of chronic lung disease of prematurity? Med Hypotheses. (2006) 66(2):355–64. doi: 10.1016/j.mehy.2005.04.046.16236459
44. Keir AK, McPhee AJ, Andersen CC, Stark MJ. Plasma cytokines and markers of endothelial activation increase after packed red blood cell transfusion in the preterm infant. Pediatr Res. (2013) 73(1):75–9. doi: 10.1038/pr.2012.14.23095979
Keywords: bronchopulmonary dysplasia, transfusion, red blood cell, systematic review, meta-analysis
Citation: Tang L, Zhu TT and Zhao J (2023) Association between red blood cell transfusion and bronchopulmonary dysplasia: a systematic review and meta-analysis. Front. Pediatr. 11:1095889. doi: 10.3389/fped.2023.1095889
Received: 11 November 2022; Accepted: 21 April 2023;
Published: 31 May 2023.
Edited by:
John McGuire, University of Washington, United StatesReviewed by:
Joanna Maria Jassem-Bobowicz, Medical University of Gdansk, PolandMaryAnn Volpe, Tufts University, United States
© 2023 Tang, Zhu and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Zhao emhhb2ppbmcxOTg1MTExMUAxNjMuY29t
 Li Tang1,2,3
Li Tang1,2,3 Ting Ting Zhu
Ting Ting Zhu Jing Zhao
Jing Zhao