- 1Department of Pediatrics, Thammasat University Hospital, Thammasat University, Pathum Thani, Thailand
- 2Department of Pediatrics, Faculty of Medicine, Thammasat University, Pathum Thani, Thailand
- 3Center of Excellence in Applied Epidemiology, Thammasat University, Pathum Thani, Thailand
- 4Department of Clinical Epidemiology, Faculty of Medicine, Thammasat University, Pathum Thani, Thailand
Introduction: The number of pediatric COVID-19 infections is increasing; however, the data on long COVID conditions in children is still limited. Our study aimed to find the prevalence of long COVID in children during the Delta and Omicron waves, as well as associated factors.
Methods: A single-center prospective cohort study was conducted. We included 802 RT-PCR-confirmed COVID-19 pediatric patients in the Delta and Omicron periods. Long COVID was defined as having symptoms for ≥3 months after infection. Parents and/or patients were interviewed by phone. Multivariable logistic regression was performed to find associated factors with long COVID.
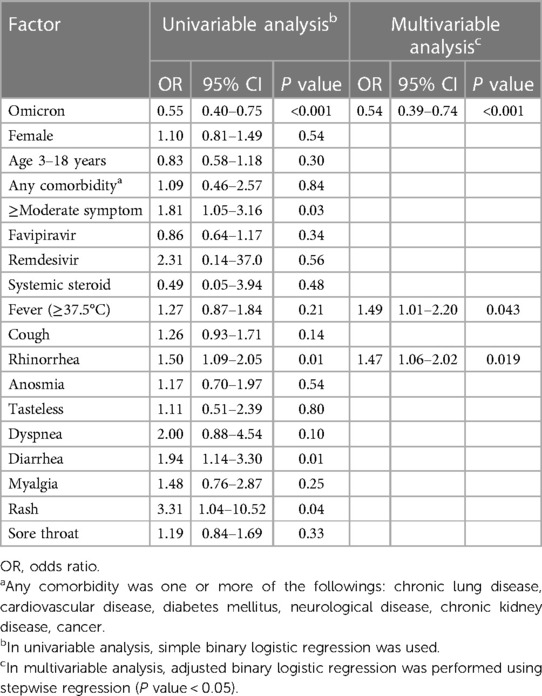
Results: The overall prevalence of long COVID was 30.2%. The Delta period had more prevalence than the Omicron (36.3% vs. 23.9%). Common symptoms for patients 0–3 years’ old were loss of appetite, rhinorrhea, and nasal congestion. Conversely, patients 3–18 years’ old had hair loss, dyspnea on exertion, rhinorrhea, and nasal congestion. However, there was no significant negative impact on daily life. Most symptoms improved after a 6-month follow-up. Factors associated with long COVID-19 conditions were infection during the Omicron period (adjusted OR: 0.54; 95% CI: 0.39–0.74, P < 0.001), fever (adjusted OR: 1.49, 95% CI: 1.01–2.20, P = 0.04) and rhinorrhea (adjusted OR: 1.47, 95% CI: 1.06–2.02, P = 0.02).
Conclusion: Infection during the Omicron wave has a lower prevalence of long COVID. The prognosis is often favorable, and most symptoms gradually become less. However, pediatricians may schedule appointments to surveil long COVID in children with fever or rhinorrhea as an initial symptom.
Introduction
Since mid-December 2019, a novel coronavirus infection, first detected in Wuhan, China, has rapidly spread throughout the world (1). The infection was first recognized as severe pneumonia with acute respiratory disease syndrome (ARDS), further delineated to be severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and now commonly known as COVID-19. The disease's manifestations vary, ranging from asymptomatic seroconversion to critical illness or death.
During the early outbreak, infection prevalence in children was low, and symptoms were usually mild or asymptomatic (2). One year after, new variants emerged and spread rapidly. The Alpha and Delta variants were associated with more pediatric cases, increasing concerns that children would be more vulnerable and sicker (3, 4). Even with the recent Omicron variant showing fewer symptoms, worries have persisted among parents regarding the possibility of acute illness as well as long-term sequelae in their children (5).
Long COVID is an umbrella term that encompasses physical and mental health consequences experienced by patients for four or more weeks following acute COVID-19 illness (6). Long COVID may have significant health and quality of life consequences, regardless of the initial disease severity. The prevalence and symptoms of long COVID in the pediatric population has varied widely among countries and age groups. For example, in Latvia, 51% of outpatients aged ≤18 years were diagnosed with long COVID (7). In the UK, 12.9% of children aged from 2 to 11 and 14.5% of those 12–16 had symptoms five weeks after the initial infection (8). In Australia, only 8% of children had post-acute COVID-19 symptoms (2).
To date, information about long COVID in children remains scarce and inconsistent (7, 9). To describe long-term outcomes in this population comprehensively, including assessing physical and mental health outcomes, telehealth has become essential in screening and follow-up. To our knowledge, no study has compared symptoms of long COVID in pediatric populations between different viral variants; very few studies have mentioned the factors associated with these symptoms. Thus, we created this study to determine the prevalence, clinical characteristics, and associated factors of long COVID by using phone interviews, comparing the Delta and Omicron variant waves. Our data may aid in early detection with appropriate management and provide essential information to overcome parental concerns.
Materials and methods
Study design
We conducted a single-center study, which aimed to evaluate the long COVID in children. All COVID-19 patients were <18 years of age and treated at the Thammasat University Hospital. We included first-infection patients with initial infections from September 2021 to March 2022. Diagnosis was performed using nasopharyngeal RT-PCR tests. Based on data from the Thai Government and Ministry of Public Health (MOPH), cases between July 2021 to December 2021 were classified as the Delta dominant period, and from January 2022, it has been considered as the Omicron dominant period (10). The report of SARS-CoV-2 variant surveillance from the Department of Medical Science, MOPH showed the Delta variants of 88%–100% from September to the mid-half of December 2021. Then, the proportion of Omicron variants rapidly increased, accounting for 94%–100% after the mid-half of January 2022 (11), which was consistent with our variant testing among 363 adult and pediatric cases in the study period (Omicron 97.8%: sublineages BA.1 64.8% and BA.2 35.2%). The exclusion criteria were (1) patient mortality or loss of follow-up within three months after infection, (2) unable to communicate in the Thai language, (3) disinclined to consent by phone, and (4) unable to be contacted by phone. This protocol was approved by the Human Research Ethics Committee of Thammasat University No 1 (Faculty of Medicine): MTU-EC-PE-1-326/64.
There were two data sources in our study. First, the baseline characteristics data of included pediatric patients such as age, gender, body weight, comorbidities, infection source, presenting symptoms, as well as severity and treatment data during patient acute illness, were retrieved from the cohort of all patients under investigation (PUI) criteria for COVID-19 at Thammasat University Hospital. Disease severity classification was based on the National Institute of Health (NIH): asymptomatic, mild, moderate, severe, and critically ill (12). All patients were treated with standard protocols according to recommendations from the Department of Medical Service, MOPH (13). Anti-viral therapy was not used for asymptomatic patients. For mild symptoms with comorbidity, favipiravir was prescribed for five days. For moderate symptoms to critical illness, remdesivir and/or systemic steroids were indicated. We also verified individual vaccine status before getting COVID-19 infection using the vaccine database of the MOPH immunization center. Fully vaccinated was defined as at least two-dose regimen according to the Thai Government recommendation.
Second, post-COVID-19 follow-up symptoms of pediatric patients were collected prospectively at three and six months after infection. Informed consent was requested from parents as well as children older than 7 years. Data were collected through a phone survey instead of in-person outpatient visits to ensure the participants’ comfort and reduce hospital visits during the outbreak of COVID-19. Due to diverse symptoms among ages, we designed two specific patient questionnaires: one for those 0–3 years of age and another for 3–18 years of age. To obtain accurate information, questions were asked by trained research assistants. For infants or young children who could not provide information, parents provided data, but for adolescents, they were encouraged to answer by themselves. For the purposes of our study, we defined long COVID as having symptoms for more than three months after infection onset. At the time of phone survey, an appointment for an outpatient visit was also offered to all participants to evaluate an alternative diagnosis. All data were collected in the online standard platform, Research Electronic Data Capture (REDCap) (14, 15).
Outcomes
The primary outcome was the prevalence of long COVID, comparing the Delta and Omicron waves. We also followed patient symptoms at six months. Unfortunately, there were very few responses in the Omicron group; we suspect that this occurred because symptoms may have disappeared or not been as severe. It is possible that the increased lack of phone follow-up during this time was because there was a proliferation of scam phone calls in Thailand, and many people would not answer calls from unknown numbers. Another important objective was to explore factors associated with long COVID to help inform the risk of long COVID in children.
Statistical analyses
Sample size estimation was based on the formula of a single proportion. We expected the prevalence of long COVID in our cohort to be 50%. With the assumption of 5% precision and 95% confidence level, the study needed to include at least 485 pediatric patients.
Continuous data were described by the mean and standard deviation or median and interquartile range, as appropriate. Percentages were given as categorical data. Student t-test, Mann-Whitney U-test, χ2 test, or Fisher exact test were used to compare baseline characteristics, acute symptoms, prevalence of long COVID, and long COVID symptoms between Delta and Omicron dominant periods.
We used binary logistic regression to determine factors associated with long COVID symptoms in univariable and multivariable models. Stepwise backward procedure for P value <0.05 was used. Additionally, we did sensitivity analyses by excluding vague symptoms, such as rhinitis and congestion, from the long COVID definition and then explored the factor associated with long COVID. To evaluate the vaccine effect on long COVID, we excluded children <5 years old in the analysis, which not included in the national vaccine program at the study period. Statistical significance was determined if P value <0.05 (two-sided). All analyses were performed using Stata v.17.0 (StataCorp LLC., Texas, USA).
Results
In our data, 844 RT-PCR-confirmed COVID-19 children were eligible. We excluded 41 cases due to no telephone response. One patient with neurological comorbidities (porencephaly, spastic cerebral palsy, and epilepsy) passed away from COVID-19 pneumonia and was also excluded. There were 802 patients left who completed the interview for long COVID.
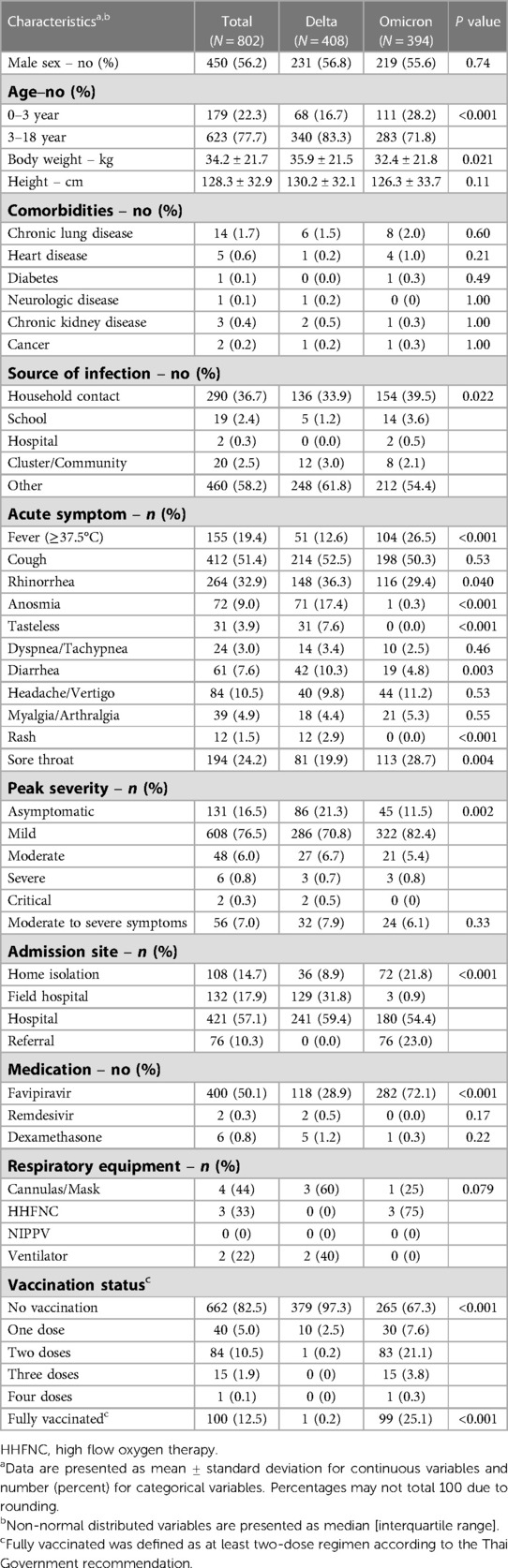
Table 1 shows the characteristics of patients at the time of COVID-19 infection, comparing Delta and Omicron periods. Of 802 patients, 179 (22.3%) were aged 0–3 years and 623 (77.7%) in the 3–18 age group. Chronic lung disease was the predominant comorbidity (1.7%). Fever was found less often in the Delta as compared to the Omicron period (12.6% vs. 26.5%, P < 0.001), but rhinorrhea (36.3% vs. 29.4%, P = 0.040), anosmia (17.4% vs. 0.3%, P < 0.001), tastelessness (7.6% vs. 0%, P < 0.001), diarrhea (10.3% vs. 4.8%, P = 0.003), and rash (2.9% vs. 0%, P < 0.001) were more common. Children were generally found to be asymptomatic or had mild symptoms in both periods. Notably, we only saw critical patients during the Delta period but not Omicron. Admission sites differed between the two waves due to a change in Thai government policy: during the Omicron dominant period, patients were permitted to home or community isolate versus previous mandatory hospital admission during the Delta era. Favipiravir was prescribed more often during the Omicron wave; this is likely because favipiravir was given based on the symptom of fever, which presented more often during this time period. The proportion of fully vaccinated cases significantly increased in the Omicron periods (0.2% vs. 25.1%, P < 0.001). The common initial regimens were two-doses of BNT162b2 (59 patients), CoronaVac or BBIBP-CorV (22 patients), and ChAdOx1 (10 patients).
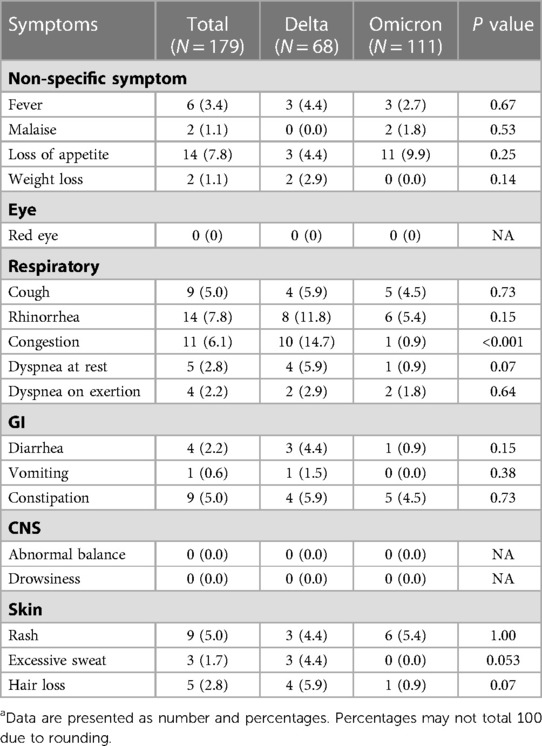
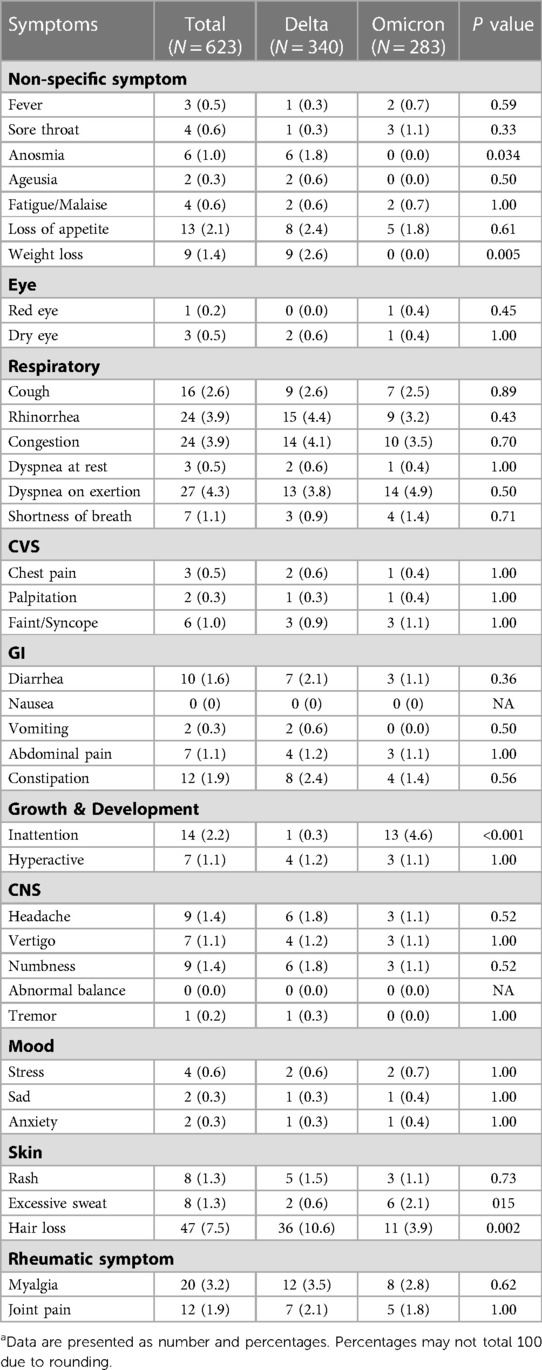
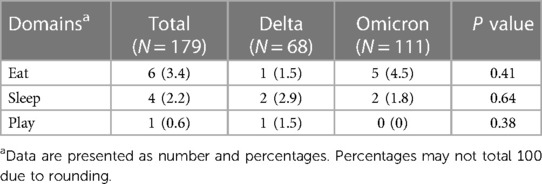
The overall prevalence of long COVID was 30.2% (95% CI: 27.0–33.5) (Figure 1). The Delta period had more prevalence than the Omicron (36.3% vs. 23.9%). Prevalence in all age, 0–3 year, and 3–18 year groups were significantly predominant during the Delta period. Patients 0–3 years’ old had more symptoms than those aged 3–18 years (33.3% vs. 29.3%). Tables 2, 3 show long COVID symptoms in the two different age cohorts. In both waves, three months after COVID-19 infection, common symptoms for the younger group were loss of appetite, rhinorrhea, and nasal congestion. Nasal congestion was found more often during Delta (14.7% vs. 0.9%, P < 0.001). On the contrary, children aged 3–18 years during Delta had more anosmia (1.8% vs. 0%, P = 0.03), weight loss (2.6% vs. 1.4, P = 0.005), and hair loss (10.6% vs. 3.9%, P = 0.002) when compared to the Omicron period. Moreover, inattention was more common during Omicron (4.6% vs. 0.3%, P = 0.001). However, there was no difference impact on daily life of long COVID symptoms between infection periods in both age groups (Tables 4 and 5). Follow-up by telehealth six months after initial infection showed that symptoms improved over time (Supplementary Tables S1 and S2).
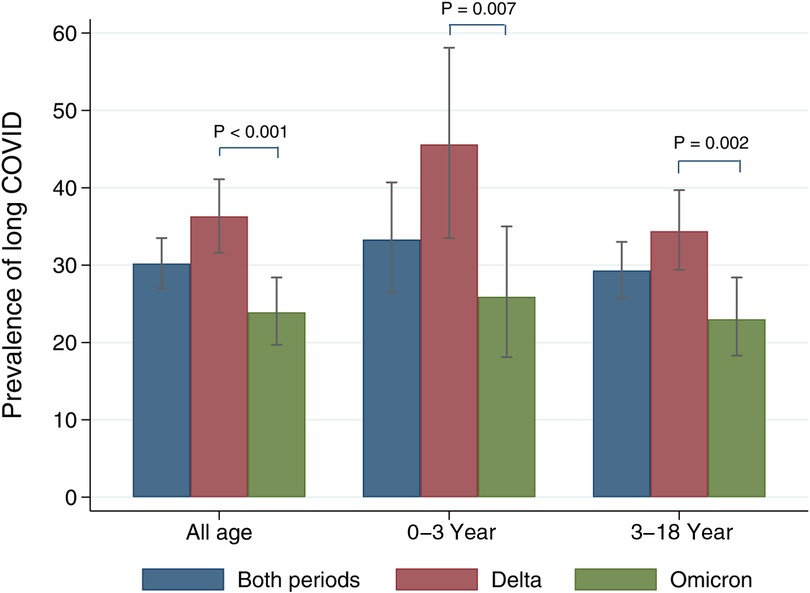
Figure 1. Prevalence of long COVID stratified by age groups and COVID-19 variant periods. The grey bar indicates 95% confidence intervals.
Table 5. Negative impact on daily life of long COVID symptoms in 3–18-year-old patients at 3 months.
Factors associated with long COVID-19 symptoms are displayed in Table 6. In univariable analyses, infections during the Omicron era were significantly associated with a reduction in the risk of developing long COVID-19 (crude odd ratio (OR) 0.55; 95% CI: 0.40–0.75, P < 0.001). During both waves, other significant factors associated with developing long COVID were having moderate to severe symptoms (crude OR: 1.81; 95% CI: 1.05–3.16, P = 0.03), rhinorrhea (crude OR: 1.5; 95% CI: 1.09–2.05, P = 0.01), diarrhea (crude OR: 1.94; 95% CI: 1.14–3.30, P = 0.01), and rash (crude OR: 3.31; 95% CI: 1.04–10.52, P = 0.04). After performing multivariable analyses with stepwise backward elimination for both eras, significant factors associated with long COVID-19 conditions were COVID-19 infection during the Omicron period (adjusted OR: 0.54; 95% CI: 0.39–0.74, P < 0.001), fever (adjusted OR: 1.49, 95% CI: 1.01–2.20, P = 0.04) and rhinorrhea (adjusted OR: 1.47, 95% CI: 1.06–2.02, P = 0.02). The sensitivity analyses excluding vague symptoms showed that the Omicron period was the only robust predictor associated with less occurrence of long COVID compared to the Delta period (Supplementary Table S3). Additionally, vaccination was not associated with occurrence of long COVID-19 in ≥5-year-old patients (N = 559) (crude OR: 0.94; 95% CI: 0.58–1.53, P = 0.81).
Discussion
This study investigated the prevalence of long COVID in pediatric patients and its associated factors: overall prevalence was 30.2%. Long COVID was significantly higher during the Delta era in all age groups, and it occurred more often in patients aged 0–3- years versus those 3–18 years. Infection during the Omicron period showed a lower prevalence of long COVID. Furthermore, fever and rhinorrhea at the time of acute illness were associated with long COVID.
Previous studies reported a variety of prevalence for long COVID and with different symptoms. A systematic review and meta-analysis in children and adolescents showed a prevalence of 25% (95% CI: 18–33%) (16). The most dominant clinical symptoms were mood symptoms (16.5%), fatigue (9.7%), sleep disorder (8.4%), headache (7.8%), and respiratory symptoms (7.6%). The authors mentioned high heterogeneity and high probabilities of bias due to a lack of standardized definitions, recall, nonresponse, and a difference in follow-up and age groups. Our study found a prevalence of 30.2% for long COVID, a similar range to previous reports. However, our main symptoms were respiratory, not mood or fatigue which are quite subjective to monitor. Most of the studies (16 out of 21) in the systematic review and meta-analysis included a large number of adolescent cases. Older children can typically describe their symptoms well, unlike young ones who cannot completely verbalize their symptoms. Thus, our common symptoms differed. We also confirmed that the Omicron variant had a lower prevalence than Delta, consistent with findings in adult studies (17).
Associated factors in this study were COVID-19 infection in the Omicron period, clinical symptoms of fever, and rhinorrhea. Infection during the Omicron period was a robust predictor associated with less frequency of long COVID in our sensitivity analyses. Similarly, a recent study from a pediatric post-COVID clinic in Italy showed that the Omicron variant had a lower risk of long COVID than the previous variants (Wild, Alfa, and Delta variants) (18). Another study revealed that the risk factors for persistent symptoms were when the children were older and had a history of allergic disease (19). The symptoms of rhinorrhea may be a part of the allergic disease that was unchecked before the COVID-19 infection. One-fourth of them were diagnosed with allergic rhinitis at our hospital. Others were checked at their own health care provider. Patients with allergic disease might have an increased risk of long COVID, resulting from T helper 2 immunological response and may be linked with mast cell activation (20, 21).
While there are fewer cases of long COVID at this time, pediatricians should schedule appointments to evaluate the risk of long COVID, particularly if patients present with fever or rhinorrhea as these patients may actually have an allergic disease. Nonetheless, the symptom of long COVID in children is not severe and has good prognosis, so parents should not be as concerned. Similar to the data from the nationwide cohort studies, long COVID in children is not highly prevalent and mainly short of duration (22, 23).
COVID-19 vaccination before infection seems to reduce long COVID in adult patients. Preliminary evidence suggested that two doses can reduce symptoms better than one dose (24). A recent systematic review (25), which searched studies from January 2020 to August 2022, included 16 observational studies in 614,392 patients. Ten studies showed a significant reduction in long COVID incidence; however, the authors did not conduct meta-analyses due to high heterogeneity. The result should be interpreted for early variants because most studies included patients in the pre-Omicron era. Unlike the adult data, our study could not demonstrate the association between receiving vaccine before infection and long COVID occurrence. One explanation may be a mild degree of long COVID symptoms in children, especially in the Omicron period. Other might be the low-vaccine coverage among the participants during the study period. Thus, we cannot find the positive effect of vaccination.
The strength of this study is its large sample size, which has helped further identify the prevalence, signs, symptoms, and risk factors of long COVID in children, during the Delta and Omicron waves, specifically in Asia. In addition, all COVID-19 cases were confirmed by RT-PCR, and we defined long COVID as having signs and symptoms persisting more than three months after initial infection.
However, our major limitation was the general lack of consensus for any standardized definitions and criteria for the definitive diagnosis of long COVID as this is new phenomenon, leading to difficulty in representing and comparing the true prevalence of the condition. We used phone interviews; thus, performing a physical examination or laboratory investigations to confirm the definitive diagnosis of particular complaints was impossible. Regardless of our efforts to offer an appointment for an in-person visit for all participants, most patients (73%) failed to show up for further appointments because their symptoms improved, as well as fear of contracting more infections from the hospital, which was the real-life situation. Therefore, we were unable to identify the definitive pathogen responsible for the symptoms. Our nonresponsive population may have altered the true prevalence of long COVID in children. Finally, there was no control group in children with similar demographics without COVID-19 infection to help elucidate whether these constellations of symptoms were or were not possibly related to isolation and lockdowns.
Conclusion
Long COVID in children is common, and the clinical manifestations vary among individuals and involve multiple organ systems. Nonetheless, the prognosis of long COVID is generally good, and the majority of symptoms go better with time. The Omicron variant has a lower chance of developing long COVID. Patients, who present with fever and rhinorrhea in acute COVID-19 infection, should be monitored for long COVID. Further studies are paramount to understanding the prevention, providing appropriate care, and follow-up of long COVID in pediatric patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by The Human Research Ethics Committee of Thammasat University No 1 (Faculty of Medicine). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
All authors made substantial contributions to the study and manuscript and met the criteria for authorship defined in the author instruction: TL designed the study, collected data, interpreted the results, drafted and revised the manuscript, and approved the final version of the manuscript. AS designed the study, collected data, interpreted the results, revised the manuscript, and approved the final version of the manuscript. AT designed the study, interpreted the results, revised the manuscript, and approved the final version of the manuscript. PB designed the study, interpreted the results, revised the manuscript, and approved the final version of the manuscript. PSi designed the study, collected data, interpreted the results, revised the manuscript, and approved the final version of the manuscript. CC collected data, designed the study, interpreted the results, revised the manuscript, and approved the final version of the manuscript. PSr designed the study, collected data, interpreted the results, revised the manuscript, and approved the final version of the manuscript. PT designed the study, analyzed data, interpreted the results, drafted and revised the manuscript and approved the final version of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The authors acknowledge the Thammasat Research Grant and the Research Group in Pediatrics from the Faculty of Medicine, Thammasat University for supporting this research.
Acknowledgments
The authors thank the Pediatric Department staff and healthcare workers responsible for the patient care at Thammasat University Hospital. We also thank Debra Kim Liwiski for her English language editing of this paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1127582/full#supplementary-material
References
1. She J, Liu L, Liu W. COVID-19 epidemic: disease characteristics in children. J Med Virol. (2020) 92(7):747–54. doi: 10.1002/jmv.25807
2. Say D, Crawford N, McNab S, Wurzel D, Steer A, Tosif S. Post-acute COVID-19 outcomes in children with mild and asymptomatic disease. Lancet Child Adolesc Health. (2021) 5(6):e22–e3. doi: 10.1016/S2352-4642(21)00124-3
3. Satdhabudha A, Chaiyakulsil C, Sritipsukho P, Sinlapamongkolkul P, Chaumrattanakul U, Tangsathapornpong A, et al. Epidemiological and clinical characteristics of pediatric COVID-19 in the tertiary care system in Thailand: comparative Delta and pre-Delta era. Mediterr J Hematol Infect Dis. (2022) 14(1):e2022044. doi: 10.4084/MJHID.2022.044
4. Khemiri H, Ayouni K, Triki H, Haddad-Boubaker S. SARS-CoV-2 infection in pediatric population before and during the Delta (B.1.617.2) and omicron (B.1.1.529) variants era. Virol J. (2022) 19(1):144. doi: 10.1186/s12985-022-01873-4
5. Torjesen I. COVID-19: omicron variant is linked to steep rise in hospital admissions of very young children. Br Med J. (2022) 376:o110. doi: 10.1136/bmj.o110
6. Clements W, Joseph T, Koukounaras JJC. UK NICE guidelines for EVAR: cost implications for post-COVID Australian public health. J Vasc Interv Radiol. (2021) 44:1286–8. doi: 10.1007/s00270-021-02832-2
7. Smane L, Roge I, Pucuka Z, Pavare J. Clinical features of pediatric post-acute COVID-19: a descriptive retrospective follow-up study. Ital J Pediatr. (2021) 47(1):177. doi: 10.1186/s13052-021-01127-z
8. Thomson H. Children with long COVID. New Sci. (2021) 249(3323):10–1. doi: 10.1016/S0262-4079(21)00303-1
9. Buonsenso D, Munblit D, De Rose C, Sinatti D, Ricchiuto A, Carfi A, et al. Preliminary evidence on long COVID in children. Acta Paediatr. (2021) 110(7):2208–11. doi: 10.1111/apa.15870
10. Corona Virus Disease (COVID-19). (2022). Available at: https://ddc.moph.go.th/viralpneumonia/eng/index.php
11. Department of Medical Science. SARS-CoV-2 variants in Thailand. (2023). Available at: https://dmsc.gdcatalog.go.th/dataset/sars-cov-2-variants
12. COVID-19 Treatment Guidelines (2022). (Available at: https://www.covid19treatmentguidelines.nih.gov/
13. COVID-19 Department of Medical Services. (2022) Available at: https://covid19.dms.go.th/
14. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. (2019) 95:103208. doi: 10.1016/j.jbi.2019.103208
15. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. (2009) 42(2):377–81. doi: 10.1016/j.jbi.2008.08.010
16. Lopez-Leon S, Wegman-Ostrosky T, Ayuzo Del Valle NC, Perelman C, Sepulveda R, Rebolledo PA, et al. Long-COVID in children and adolescents: a systematic review and meta-analyses. Sci Rep. 2022;12(1):9950. doi: 10.1038/s41598-022-13495-5
17. Wise J. COVID-19: long COVID risk is lower with omicron than delta, researchers find. Br Med J. (2022) 377:o1500. doi: 10.1136/bmj.o1500
18. Buonsenso D, Morello R, Mariani F, De Rose C, Mastrantoni L, Zampino G, et al. Risk of long COVID in children infected with omicron or pre-omicron SARS-CoV-2 variants. Acta Paediatr. (2023) 112(6):1284–86. doi: 10.1111/apa.16764
19. Osmanov IM, Spiridonova E, Bobkova P, Gamirova A, Shikhaleva A, Andreeva M, et al. Risk factors for post-COVID-19 condition in previously hospitalised children using the ISARIC global follow-up protocol: a prospective cohort study. Eur Respir J. (2022) 59(2):2101341. doi: 10.1183/13993003.01341-2021
20. Wise J. Long COVID: one in seven children may still have symptoms 15 weeks after infection, data show. Br Med J. (2021) 374:n2157. doi: 10.1136/bmj.n2157
21. Afrin LB, Weinstock LB, Molderings GJ. COVID-19 hyperinflammation and post-COVID-19 illness may be rooted in mast cell activation syndrome. Int J Infect Dis. (2020) 100:327–32. doi: 10.1016/j.ijid.2020.09.016
22. Borch L, Holm M, Knudsen M, Ellermann-Eriksen S, Hagstroem S. Long COVID symptoms and duration in SARS-CoV-2 positive children - a nationwide cohort study. Eur J Pediatr. (2022) 181(4):1597–607. doi: 10.1007/s00431-021-04345-z
23. Molteni E, Sudre CH, Canas LS, Bhopal SS, Hughes RC, Antonelli M, et al. Illness duration and symptom profile in symptomatic UK school-aged children tested for SARS-CoV-2. Lancet Child Adolesc Health. (2021) 5(10):708–18. doi: 10.1016/S2352-4642(21)00198-X
24. Notarte KI, Catahay JA, Velasco JV, Pastrana A, Ver AT, Pangilinan FC, et al. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: a systematic review. EClinicalMedicine. (2022) 53:101624. doi: 10.1016/j.eclinm.2022.101624
Keywords: long COVID, SARS-CoV-2, COVID-19, Delta variant, Omicron variant
Citation: Lokanuwatsatien T, Satdhabudha A, Tangsathapornpong A, Bunjoungmanee P, Sinlapamongkolkul P, Chaiyakulsil C, Sritipsukho P and Tantiyavarong P (2023) Prevalence and associating factors of long COVID in pediatric patients during the Delta and the Omicron variants. Front. Pediatr. 11:1127582. doi: 10.3389/fped.2023.1127582
Received: 19 December 2022; Accepted: 9 May 2023;
Published: 24 May 2023.
Edited by:
Yusra Habib Khan, Jouf University, Saudi ArabiaReviewed by:
Danilo Buonsenso, Catholic University of the Sacred Heart, ItalyLuiz Gonzaga Francisco De Assis Barros D'Elia Zanella, University of São Paulo, Brazil
© 2023 Lokanuwatsatien, Satdhabudha, Tangsathapornpong, Bunjoungmanee, Sinlapamongkolkul, Chaiyakulsil, Sritipsukho and Tantiyavarong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pichaya Tantiyavarong cGljaGF5YV90QHR1LmFjLnRo
 Tananya Lokanuwatsatien
Tananya Lokanuwatsatien Araya Satdhabudha
Araya Satdhabudha Auchara Tangsathapornpong2
Auchara Tangsathapornpong2 Chanapai Chaiyakulsil
Chanapai Chaiyakulsil Pichaya Tantiyavarong
Pichaya Tantiyavarong