- Department of “A”, Children's Hospital, Zhejiang University School of Medicine, National Clinical Research Center for Child Health, Hangzhou, China
Background: Previous studies have reported that the incidence of pediatric inflammatory bowel disease (IBD) is related to vitamin D, but it is still unclear. This study intends to calculate the relationship between pediatric IBD and vitamin D.
Methods: A comprehensive literature search from inception to January 2023 was performed in the PubMed, EMBASE, Medline, Web of Science, and Google Scholar databases. Relevant data were extracted as required and used for subsequent calculations.
Results: Sixteen papers were included, and there was no significant difference between the average vitamin D level in IBD patients and healthy controls. In addition, the overall pooled results showed that C-reactive protein (CRP) was 2.65 higher before vitamin D supplementation than after supplementation [SMD = 2.65, 95% CI = (2.26, 3.04)]. Moreover, patients with IBD in remission were 0.72 higher before vitamin D supplementation than after supplementation [OR = 0.72, 95% CI = (0.52, 1.00)].
Conclusion: This study suggested that there was no obvious relationship between pediatric IBD and vitamin D, while vitamin D supplementation can improve disease activity. Therefore, follow-up still needs many prospective studies to confirm the relationship between pediatric IBD and vitamin D.
Introduction
Inflammatory bowel disease (IBD) has two main forms, Crohn's disease (CD) and ulcerative colitis (UC). IBD can be a debilitating condition to live with, yet it is largely invisible to all but the afflicted individual (1). It is considered to be a relatively new disease because it emerged only in the past 150 years. IBD occurs in children and adolescents and can adversely affect nutrition and growth in children (2). The estimated pediatric IBD incidence in Asia and the Middle East varies from 0.5 to 11.4/100,000 person years, which has seen a sharp increase in incidence over the preceding decade (3). In addition, the epidemiological trend has been consistently observed in both adult and pediatric Asian populations (3).
Recent research has indicated that the individual's genetic susceptibility, external environment, intestinal microbiome and immunodeficiencies are all involved and functionally integrated in the pathogenesis of IBD (4–6). Overall, the pathogenesis of IBD is still controversial.
In the field of IBD research, it was found that vitamin D deficiency was associated with IBD (7). Vitamin D is a lipophilic compound synthesized in the skin under the sun, and it is hydroxylated to 25-hydroxyvitamin D [25(OH)D] in the liver and further hydroxylated in the kidneys to 1,25-dihydroxyvitamin D [1,25(OH)2D], which is the active metabolite (8). Some studies have shown that vitamin D protects the gut epithelial barrier by suppressing gut epithelial cell apoptosis (9), and other studies have shown that immunological dysregulation in IBD is characterized by epithelial damage (10). Some hormones produced by the intestines, such as melatonin, can enhance the intestinal mucosal barrier and alter the composition of intestinal bacteria to regulate the immune response (11). The complexity and multiplicity of the gut microbiota play an important role in the pathophysiology of IBD by influencing the immune system, host metabolism and gastrointestinal development (12). Additionally, vitamin D has effects on both innate and adaptive immune pathways and may even play a role in promoting immune tolerance (13). In addition, vitamin D may have an important effect on gut microbiome composition and function and consequent effects on IBD clinical (14).
Vitamin D deficiency [defined as a serum 25(OH)D ≤ 20 ng/ml or 50 nmol/L] is prevalent among IBD patients (15). Vitamin D deficiency and insufficiency are common in children worldwide (16). Recent research suggested that 40.4% of 55,844 European children are vitamin D deficient, and 13.0% are severely deficient (17). Even in the United States, 50% of children ages 1%–5% and 70% of children ages 6–11 are vitamin D deficient (18). In addition, the percentage of Canadian children with vitamin D deficiency already increased to 19.4%, and the others with severe deficiency increased to 36.8% (19). Increasing evidence suggests that low levels of vitamin D are associated with important clinical parameters and outcomes in IBD patients. The possible reasons for vitamin D deficiency in IBD patients include insufficient sunlight exposure, restricted dietary intake, intestinal inflammation leading to inadequate absorption of nutrients and bile acid malabsorption (20), or as a side effect of immunosuppressive treatment with thiopurines (21). Such researchers provide supporting evidence for the relationship between vitamin D and IBD (20). Research on the mechanisms of action has provided emerging evidence that vitamin D deficiency may be implicated in disease severity (22).
In this study, we conducted a meta-analysis and systematic review to analyze the relationships between pediatric IBD and vitamin D. The results of this study may provide new insight into the cause of pediatric IBD.
Methods
Sources and methods of data retrieval
We performed a comprehensive literature search that included studies from inception to January 2023; the relevant studies included PubMed, EMBASE, Medline, Web of Science, and Google Scholar databases. The following terms were used to search to cover as many articles as possible: ((Inflammatory bowel disease) OR (Crohn's disease) OR (ulcerative colitis)) AND ((Vitamin D) OR (25(OH)D) OR (Cholecalciferol) OR (25-Hydroxyvitamin D) OR (Ergocalciferol) OR (Dihydrotachysterol) OR(hydroxycholecalciferol)) AND ((children) OR(child)OR (pediatric)). Inclusion and exclusion criteria were listed according to the requirements of this study. Articles were considered to be included if the following criteria were met: (1) published as full English research articles; (2) younger than 18 years old; (3) unified definition and diagnosis of IBD; and (4) supporting data of vitamin D. Articles that did not meet the above criteria or duplicate publications were excluded. 25(OH)D is the main form of vitamin D, and 25(OH)D is used to test and represent the vitamin D level. Moreover, a 25(OH)D unit is defined as 1 ng/ml = 2.5 nmol/L, and a 25(OH)D level lower than 50 nmol/L is defined as deficiency. (The molecular weight of vitamin D is 401, according to the formula n = m*M, 1 ng/ml = 1,000 ng/L = 1,000/401 nmol/ml ≈ 2.5 nmol/ml.).
In this study, articles were independently screened by two authors, and both of them subsequently screened full articles. If disagreement appeared, another author evaluated the disagreement again and formed a final result after the trade.
Article assessment
We assessed the possibility of publication bias by constructing a funnel plot of each trial's effect size against the standard error using Egger's test to assess funnel plot asymmetry; if p < 0.05, there was publication bias.
Risk of bias and quality assessment were assessed through the STROBE checklist for the included studies (23). In addition, the study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (24).
Data extraction
Data were extracted to numbers (Apple Distribution International, USA) for effective statistical analysis. The following data were obtained from the included studies: basic characteristics, including author, published year, country, detection method of 25(OH)D and diagnostic criteria of IBD; 25(OH)D levels [mean ± standard deviation (SD)] of IBD and controls; number of individuals with IBD and controls; IBD patients in remission and C-reactive protein (CRP) changes. The criteria for remission of IBD patients were serum levels of 25-hydroxyvitamin D increased, all relevant symptoms improved, inflammatory markers were not changed, and there were no adverse events such as overdose. Finally, all data were double-checked by two authors.
Statistical analysis
In this study, we used the standardized mean difference (SMD) to combine the mean and SD values for 25(OH)D levels. The odds ratio [OR, 95% confidence intervals (CIs)] was used to calculate the ratio of IBD patients in remission. In addition, statistical heterogeneity was assessed by Cochran's Q test and the I2 statistic. For heterogeneous results, publication bias was estimated by funnel plot and Egger's test (P = 0.164). The fixed-model (Mantel and Haenszel) method (if I2 ≤ 50%, P > 0.1) or random-model (M-H heterology) method (if I2 > 50%, P ≤ 0.1) was performed to obtain pooled estimates. To enhance the credibility of the results, meta-regression was used to look for potential sources of heterogeneity (Monte Carlo permutation test). All analyses were carried out using Review Manager (Version 5.3) and Stata (version 15.1).
Results
Basic characteristics
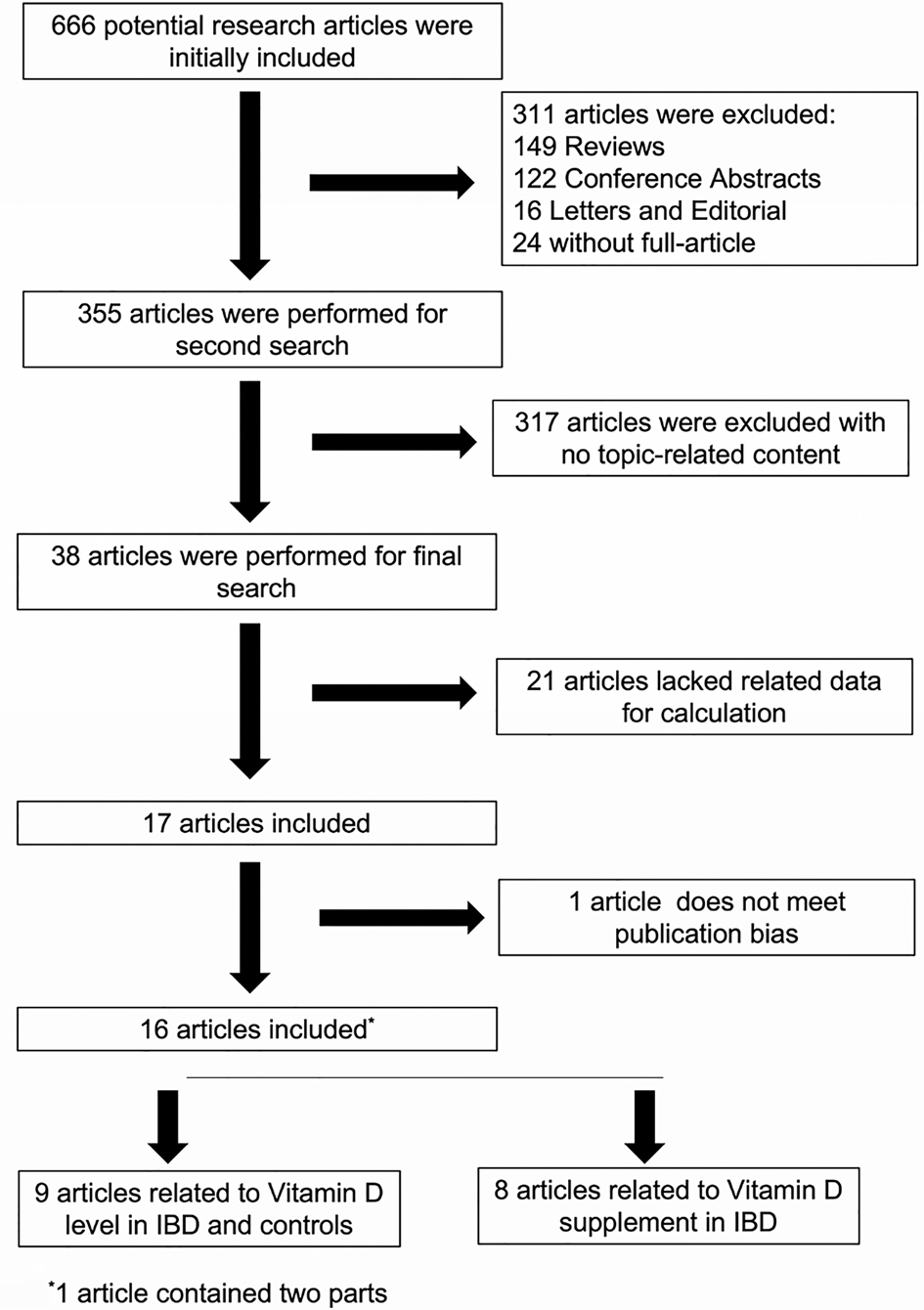
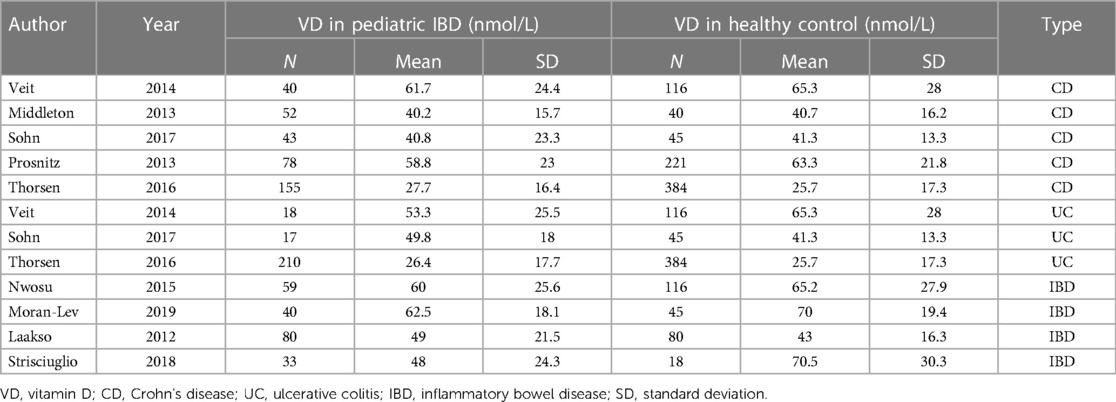
In total, we searched 666 potential related articles, of which 16 papers met the inclusion criteria (19, 25–40). One of the articles from El-Matary was included in our study at the beginning but was eliminated in the later publication bias assessment (Figure 1). The flowchart that describes the process of study selection is shown in Figure 2. Nine articles reported on vitamin D levels in IBD patients and healthy controls, and eight articles reported on vitamin D supplementation in IBD patients. Of these, one article contained two parts. Detailed information on all included studies, including the type of study used, source of recruitment population, gender ratio, and age distribution, are listed in Tables 1.1, 1.2. In this study, there was no publication bias or abnormal sensitivity analysis. In addition, meta-regression showed that heterogeneity did not come from location, patient or healthy volunteers.
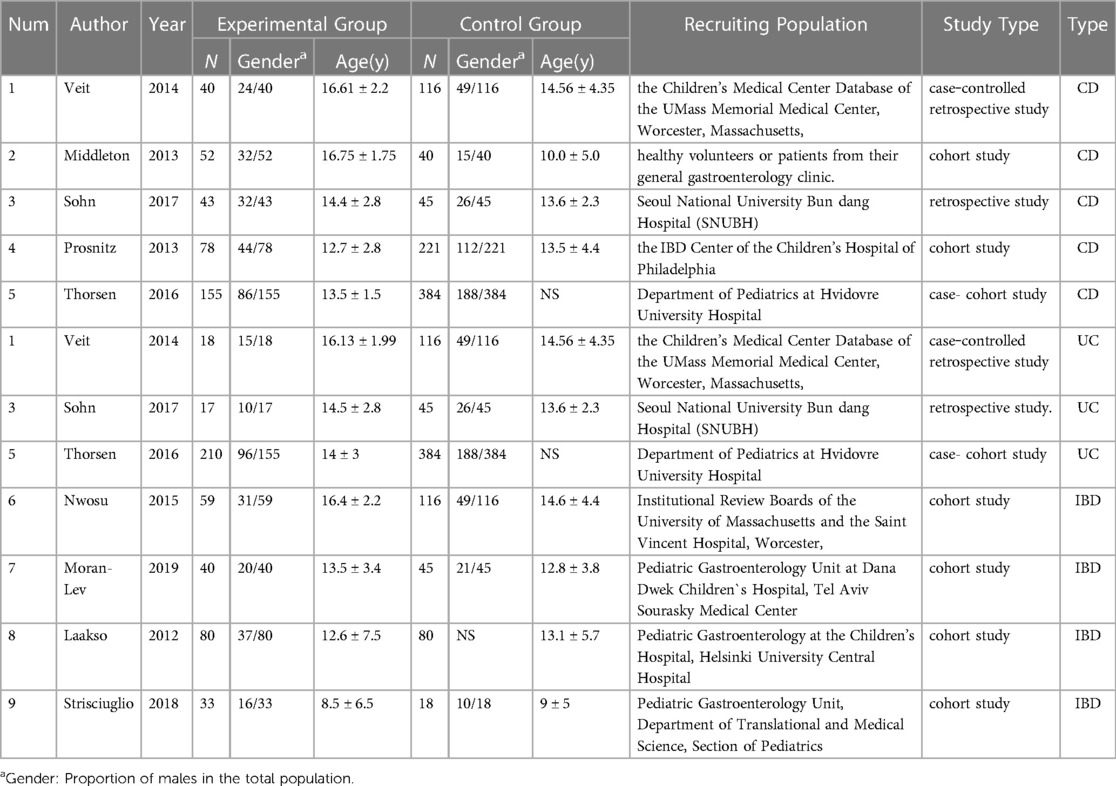
Table 1.1. Specific information on the 9 studies on vitamin D levels in IBD patients and healthy controls.
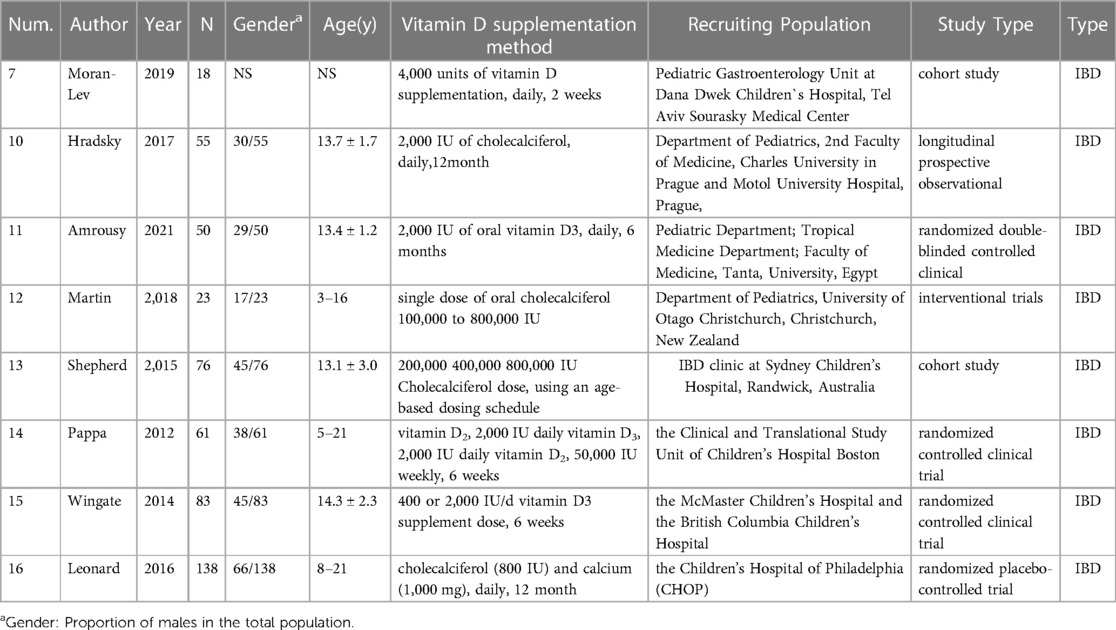
Table 1.2. Specific information on the 7 studies about the CRP levels in IBD patients and changes in remission IBD patients before and after VD supplementation.
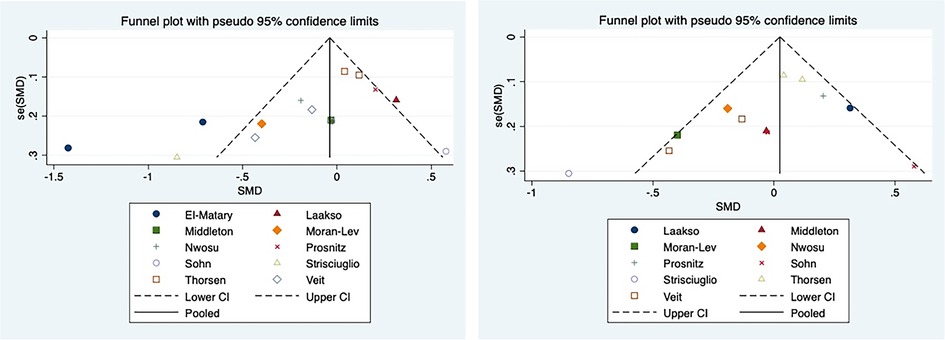
Figure 1. Funnel plot including (left) and excluding (right) El Matary 2011. In meta-analysis, publication bias assessment mainly depends on the Egger test. It is generally believed that there is publication bias if p < 0.05. In the funnel chart, it was found that one study, El Matary 2011, was far outside the funnel chart and affected the final Egger’s test results (Figure 1 left), and the Egger’s test results we used, P = 0.037, indicated that there was significant publication bias. After removing El Matary 2011's study, the new Egger's test was conducted on the basis of the remaining 12 studies, P = 0.164, indicating no significant publication bias (Figure 1 right).
Vitamin D levels in IBD patients and healthy controls
The corresponding data are listed in Table 2. Finally, 9 articles were included, containing 825 IBD patients (368 CD, 245 UC, 212 unclassified) and 1,610 healthy controls. In addition, the mean value of 25(OH)D was 48.18 nmol/L in IBD patients and 51.44 nmol/L in controls. The average 25(OH)D level was slightly higher in the control group. According to the characteristics of the enrolled patients, we conducted an overall comparison and subgroup comparison. The overall pooled results showed that there was no significant difference between the average 25(OH)D level in IBD patients and healthy controls [SMD = 0.03, 95% CI = (−1.60, 1.65)] (Figure 3). In the subgroup analysis, the results showed that the average 25(OH)D levels of CD, UC and unclassified were not significantly different compared with the control group. [SMD = 0.07, 95% CI = (−2.26, 2.41)] [SMD = 0.79, 95% CI = (−1.97, 3.54)] [SMD = −1.71, 95% CI = (−5.70, 2.28)] (Figure 3). In the forest plot, there is an intersection between the rhombus and the line, indicating that the combined results are not statistically significant.

Figure 3. The results showed that there was no significant difference between the average 25(OH)D level in IBD patients and healthy controls [SMD = 0.03, 95% CI = (−1.60, 1.65)]. In the forest plot, there is an intersection between the rhombus and the line, indicating that the combined results are not statistically significant.
The effect of vitamin D supplementation on IBD
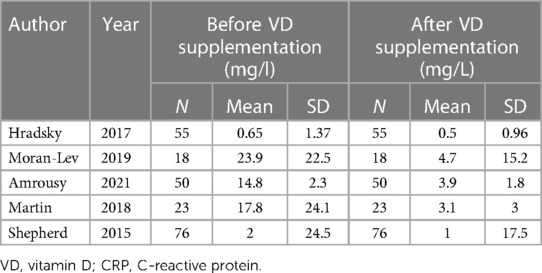
A total of 5 articles described the changes in CRP after vitamin D supplementation in IBD patients, and 5 articles described changes in IBD patients who were in remission. The corresponding data are listed in Tables 3, 4. Five articles contained 222 IBD patients. The mean value of CRP was 11.83 mg/L before vitamin D supplementation and 2.64 mg/L after vitamin D supplementation. The overall pooled results showed that CRP was 2.65 higher before vitamin D supplementation than after supplementation [SMD = 2.65, 95% CI = (2.26, 3.04)] (Figure 4). In the forest plot, there is no intersection between the rhombus and the linfie, indicating that the combined results are statistically significant.
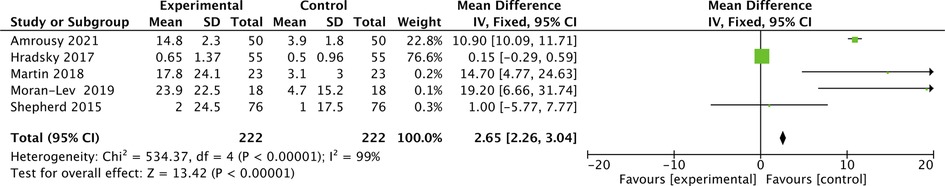
Figure 4. The results showed that CRP was 2.65 higher before vitamin D supplementation than after supplementation [SMD = 2.65, 95% CI = (2.26, 3.04)]. In the forest plot, there is no intersection between the rhombus and the line, indicating that the combined results are statistically significant.
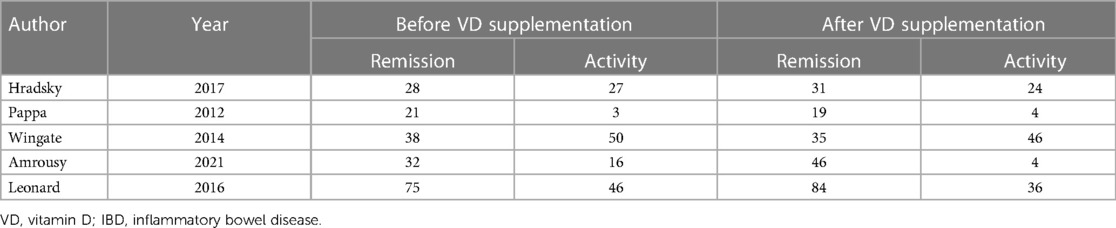
In addition, in 5 articles describing IBD in remission, the proportion of patients with IBD in remission was 57.7% before vitamin D supplementation and 65.3% after vitamin D supplementation. The overall pooled results showed that patients with IBD in remission were 0.72 higher before vitamin D supplementation than after supplementation [OR = 0.72, 95% CI = (0.52, 1.00)] (Figure 5). In the forest plot, there is no intersection between the rhombus and the line, indicating that the combined results are statistically significant.
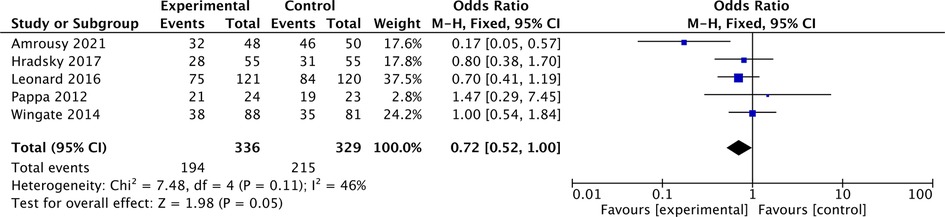
Figure 5. The results showed that patients with IBD in remission were 0.72 higher before vitamin D supplementation than after supplementation [OR = 0.72, 95% CI = (0.52, 1.00)]. In the forest plot, there is no intersection between the rhombus and the line, indicating that the combined results are statistically significant.
Discussion
In previous clinical studies, comparison results of vitamin D between pediatric IBD patients and controls were inconsistent. In this study, the results suggested that vitamin D levels were not significantly different between IBD patients and controls. However, in the other results, vitamin D supplementation decreased CRP levels and increased the proportion of patients who achieved remission. Combining these results, we hypothesized that vitamin D might be related to pediatric IBD. However, due to the existence of heterogeneity, our study did not directly reach the link between pediatric IBD and vitamin D.
Vitamin D exerts biological activity through vitamin D receptors, and VDR plays an important role in mediating the pathogenesis of pediatric IBD (42). The gene encoding VDR is recognized as a promising candidate for indicating the development of IBD (43). Robert et al. reported that vitamin D signaling contributes to innate immune responses through VDR (44). As a direct target, VDR can interfere with the inflammasome by binding to interleukin 1β (IL-1β) and ultimately mediate IBD (45). In addition, vitamin D induces NOD2 gene expression to regulate autotrophic function, which is also crucial for the pathogenesis of IBD (46). Therefore, there is basic research evidence for vitamin D-mediated IBD.
Vitamin D possesses strong antibacterial activity and inhibits lipopolysaccharide (LPS) to induce inflammation by downregulating inflammatory cytokines (47). In particular, vitamin D stimulates the production of pattern recognition receptors, cytokines and antimicrobial peptides, including β-defensins and cathepsin (48, 49). Moreover, LL-37 is the only human catalytin that has potential antimicrobial activity against both gram-positive and gram-negative bacteria, as well as some viruses (50). Indeed, cell experiments have shown that active vitamin D can inhibit the production of pro-inflammatory cytokines, including TNF-α, IL-1β, IL-6, IL-8 and IL-12, and promote the production of IL-10, an anti-inflammatory cytokine (51, 52). Low vitamin D status is often associated with systemic low-grade inflammation, as reflected by elevated C-reactive protein (CRP) levels. Linear and nonlinear Mendelian randomization (MR) analyses have been used to explore the bidirectional association between serum 25(OH)D and CRP concentrations. The observed association between 25(OH)D and CRP is likely to be caused by vitamin D deficiency, and correction of low vitamin D status may reduce chronic inflammation (53).
The microbiome and vitamin D deeply influence each other and the immune system in many different ways. The rapid corresponding immune response is activated in the innate and adaptive immune systems during hypovitaminosis D and microbiome dysbiosis (54).
This study also concluded that vitamin D supplementation was helpful in improving IBD activity, which may be closely related to the improvement of intestinal local immune function and tight junctions by vitamin D (55). However, due to the lack of sufficient prospective studies, there are also doubts about the causal relationship between the two. In the included articles, IBD treatment included vitamin D supplementation and basic treatment recommended by the guidelines. It is possible that in the course of disease treatment, as basic treatment takes effect, the damaged intestinal mucosa will be repaired, and the absorption efficiency of vitamin D will be improved, which will lead to an increase in the level of vitamin D.
This study failed to draw the results of the correlation between vitamin D and pediatric IBD. Our statistical results are the same as those of Veit but different from those of Middleton and Prosnitz. The main reasons may be related to heterogeneity and research objects, including race, age distribution, gender distribution, and so on. The main reason may be related to heterogeneity. There was obvious heterogeneity in the overall results and the subgroup analysis results. The author's analysis may have the following aspects. First, although the patients included in the study were all children, they had a large age span. Some patients are already adolescents, so the basic vitamin D level itself will be different. However, as there were not enough studies included and the included articles were not stratified for age, this study could not perform subgroup analysis based on age. Second, the regions of the patients included in the study were different. It can be seen that different dietary cultures and light intensities will also lead to different levels of basic vitamin D levels, which will also bring heterogeneity to the analysis. In addition, there were some differences in the dose and range of vitamin D supplementation, and the difference in the basic treatment plan had a greater impact on the prognosis of the disease and changes in vitamin D levels. Hormones, immunosuppressants, and biological agents have different remission rates for IBD, and the role of vitamin D supplementation in the treatment process needs to be confirmed by more prospective studies and basic studies. Finally, different vitamin D detection methods will more or less interfere with the analysis.
Although there were some shortcomings in this study, there were also some points worthy of recognition. This study systematically summarized the relationship between vitamin D and pediatric IBD for the first time, providing part of the basis for clarifying the relationship between the two.
In conclusion, this study suggested that there was no obvious relationship between pediatric IBD and vitamin D, while vitamin D supplementation can improve disease activity. We thought that the existence of heterogeneity may affect the results of this study to some extent. Therefore, follow-up still needs many prospective studies to confirm the relationship between pediatric IBD and vitamin D.
Author contributions
YS and DT prepared and wrote the manuscript and made the tables and figures. YS, JZ and QY prepared and revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Conrad MA, Rosh JR. Pediatric inflammatory bowel disease. Pediatr Clin North Am. (2017) 64(3):577–91. doi: 10.1016/j.pcl.2017.01.005
3. Huang JG, Aw MM. Pediatric inflammatory bowel disease in Asia: epidemiology and natural history. Pediatr Neonatol. (2020) 61(3):263–71. doi: 10.1016/j.pedneo.2019.12.008
4. Kelsen JR, Sullivan KE. Inflammatory bowel disease in primary immunodeficiencies. Curr Allergy Asthma Rep. (2017) 17(8):57–69. doi: 10.1007/s11882-017-0724-z
5. Franzin M, Stefančič K, Lucafò M, Decorti G, Stocco G. Microbiota and drug response in inflammatory bowel disease. Pathogens. (2021) 10(2):211. doi: 10.3390/pathogens10020211
6. Lin Y, Luo L, Lin H, Li X, Huang R. Potential therapeutic targets and molecular details of anthocyan-treated inflammatory bowel disease: a systematic bioinformatics analysis of network pharmacology. RSC Adv. (2021) 11(14):8239–49. doi: 10.1039/D0RA09117K
7. Fatahi S, Alyahyawi N, Albadawi N, Mardali F, Dara N, Sohouli MH, et al. The association between vitamin D status and inflammatory bowel disease among children and adolescents: a systematic review and meta-analysis. Front Nutr. (2023) 9:1007725. doi: 10.3389/fnut.2022.1007725
8. Wong RS, Tung KTS, Mak RTW, Leung WC, Yam JC, Chua GT, et al. Vitamin D concentrations during pregnancy and in cord blood: a systematic review and meta-analysis. Nutr Rev. (2022) 80(12):2225–36. doi: 10.1093/nutrit/nuac023
9. He L, Liu T, Shi Y, Tian F, Hu H, Deb DK, et al. Gut epithelial vitamin D receptor regulates microbiota-dependent mucosal inflammation by suppressing intestinal epithelial cell apoptosis. Endocrinology. (2018) 159(2):967–79. doi: 10.1210/en.2017-00748
10. Gubatan J, Moss AC. Vitamin D in inflammatory bowel disease: more than just a supplement. Curr Opin Gastroenterol. (2018) 34(4):217–25. doi: 10.1097/MOG.0000000000000449
11. Vaghari-Tabari M, Moein S, Alipourian A, Qujeq D, Malakoti F, Alemi F, et al. Melatonin and inflammatory bowel disease: from basic mechanisms to clinical application. Biochimie. (2022) 209:20–36. doi: 10.1016/j.biochi.2022.12.007
12. Luthold RV, Fernandes GR, Franco-de-Moraes AC, Folchetti LG, Ferreira SR. Gut microbiota interactions with the immunomodulatory role of vitamin D in normal individuals. Metab Clin Exp. (2017) 69:76–86. doi: 10.1016/j.metabol.2017.01.007
13. Guan Q. A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J Immunol Res. (2019) 2019:7247238. doi: 10.1155/2019/7247238
14. Singeap AM, Girleanu I, Diculescu M, Gheorghe L, Ciocîrlan M, Gheorghe C, et al. Risk factors for extraintestinal manifestations in inflammatory bowel diseases—data from the Romanian national registry. J Gastrointestin Liver Dis. (2021) 30(3):346–57. doi: 10.15403/jgld-3818
15. Cediel G, Pacheco-Acosta J, CastiUo-Durdn C. Vitamin D deficiency in pediatric clinical practice. Deficiencia de vitamina D en la práctica clínica pediátrica. Arch Argent Pediatr. (2018) 116(1):e75–81. doi: 10.5546/aap.2018.eng.e75
16. Cashman KD, Dowling KG, Škrabáková Z, Gonzalez-Gross M, Valtueña J, De Henauw S, et al. Vitamin D deficiency in Europe: pandemic? Am J Clin Nutr. (2016) 103(4):1033–44. doi: 10.3945/ajcn.115.120873
17. Holick MF. The vitamin D deficiency pandemic: approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. (2017) 18(2):153–65. doi: 10.1007/s11154-017-9424-1
18. Sarafin K, Durazo-Arvizu R, Tian L, Phinney KW, Tai S, Camara JE, et al. Standardizing 25-hydroxyvitamin D values from the Canadian health measures survey. Am J Clin Nutr. (2015) 102(5):1044–50. doi: 10.3945/ajcn.114.103689
19. El Amrousy D, El Ashry H, Hodeib H, Hassan S. Vitamin D in children with inflammatory bowel disease: a randomized controlled clinical trial. J Clin Gastroenterol. (2021) 55(9):815–20. doi: 10.1097/MCG.0000000000001443
20. Vernia P, Burrelli Scotti G, Dei Giudici A, Chiappini A, Cannizzaro S, Afferri MT, et al. Inadequate sunlight exposure in patients with inflammatory bowel disease. J Dig Dis. (2018) 19(1):8–14. doi: 10.1111/1751-2980.12567
21. Fletcher J. Vitamin D deficiency in patients with inflammatory bowel disease. Br J Nurs. (2016) 25(15):846–51. doi: 10.12968/bjon.2016.25.15.846
22. Fletcher J, Cooper SC, Ghosh S, Hewison M. The role of vitamin D in inflammatory bowel disease: mechanism to management. Nutrients. (2019) 11(5):1019. Published 2019 May 7. doi: 10.3390/nu11051019
23. Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLoS Med. (2007) 4(10):e297. doi: 10.1371/journal.pmed.0040297
24. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
25. El-Matary W, Sikora S, Spady D. Bone mineral density, vitamin D, and disease activity in children newly diagnosed with inflammatory bowel disease. Dig Dis Sci. (2011) 56(3):825–9. doi: 10.1007/s10620-010-1380-5
26. Veit LE, Maranda L, Fong J, Nwosu BU. The vitamin D status in inflammatory bowel disease. Plos One. (2014) 9(7):e101583. doi: 10.1371/journal.pone.0101583
27. Middleton JP, Bhagavathula AP, Gaye B, Alvarez JA, Huang CS, Sauer CG, et al. Vitamin D status and bone mineral density in African American children with crohn's disease. J Pediatr Gastroenterol Nutr. (2013) 57(5):587–93 doi: 10.1097/MPG.0b013e31829e0b89
28. Nwosu BU, Maranda L. Vitamin D status and adiposity in pediatric malabsorption syndromes. Digestion. (2015) 92(1):1–7. doi: 10.1159/000381895
29. Moran-Lev H, Galai T, Yerushalmy-Feler A, Weisman Y, Anafy A, Deutsch V, et al. Vitamin D decreases hepcidin and inflammatory markers in newly diagnosed inflammatory bowel disease paediatric patients: a prospective study. J Crohns Colitis. (2019) 13(10):1287–91. doi: 10.1093/ecco-jcc/jjz056
30. Sohn J, Chang EJ, Yang HR. Vitamin D Status and bone mineral density in children with inflammatory bowel disease compared to those with functional abdominal pain. J Korean Med Sci. (2017) 32(6):961–7. doi: 10.3346/jkms.2017.32.6.961
31. Laakso S, Valta H, Verkasalo M, Toiviainen-Salo S, Viljakainen H, Mäkitie O. Impaired bone health in inflammatory bowel disease: a case-control study in 80 pediatric patients. Calcif Tissue Int. (2012) 91(2):121–30. doi: 10.1007/s00223-012-9617-2
32. Prosnitz AR, Leonard MB, Shults J, Zemel BS, Hollis BW, Denson LA, et al. Changes in vitamin D and parathyroid hormone metabolism in incident pediatric crohn's disease. Inflamm Bowel Dis. (2013) 19(1):45–53. doi: 10.1002/ibd.22969
33. Thorsen SU, Jakobsen C, Cohen A, Lundqvist M, Thygesen LC, Pipper C, et al. Perinatal vitamin D levels are not associated with later risk of developing pediatric-onset inflammatory bowel disease: a danish case-cohort study. Scand J Gastroenterol. (2016) 51(8):927–33. doi: 10.3109/00365521.2016.1144218
34. Strisciuglio C, Cenni S, Giugliano FP, Miele E, Cirillo G, Martinelli M, et al. The role of inflammation on vitamin D levels in a cohort of pediatric patients with inflammatory bowel disease. Digestive & Liver Disease. (2017) 49(4):e260. doi: 10.1016/j.dld.2017.09.050
35. Hradsky O, Soucek O, Maratova K, Matyskova J, Copova I, Zarubova K, et al. Supplementation with 2000 IU of cholecalciferol is associated with improvement of trabecular bone mineral density and muscle power in pediatric patients with IBD. Inflamm Bowel Dis. (2017) 23(4):514–23. doi: 10.1097/MIB.0000000000001047
36. Pappa HM, Mitchell PD, Jiang H, Kassiff S, Filip-Dhima R, DiFabio D, et al. Treatment of vitamin D insufficiency in children and adolescents with inflammatory bowel disease: a randomized clinical trial comparing three regimens. J Clin Endocrinol Metab. (2012) 97(6):2134–42. doi: 10.1210/jc.2011-3182
37. Wingate KE, Jacobson K, Issenman R, Carroll M, Barker C, Israel D, et al. 25-Hydroxyvitamin D concentrations in children with Crohn's disease supplemented with either 2000 or 400 IU daily for 6 months: a randomized controlled study. J Pediatr. (2014) 164(4):860–5. doi: 10.1016/j.jpeds.2013.11.071
38. Martin NG, Rigterink T, Adamji M, Wall CL, Day AS. Single high-dose oral vitamin D3 treatment in New Zealand children with inflammatory bowel disease. Transl Pediatr. (2019) 8(1):35–41. doi: 10.21037/tp.2018.11.01
39. Shepherd D, Day AS, Leach ST, Lopez R, Messenger R, Woodhead HJ, et al. Single high-dose oral vitamin D3 therapy (stoss): a solution to vitamin D deficiency in children with inflammatory bowel disease? J Pediatr Gastroenterol Nutr. (2015) 61(4):411–4. doi: 10.1097/MPG.0000000000000823
40. Leonard MB, Shults J, Long J, Baldassano RN, Brown JK, Hommel K, et al. Effect of low-magnitude mechanical stimuli on bone density and structure in pediatric crohn's disease: a randomized placebo-controlled trial. J Bone Miner Res. (2016) 31(6):1177–88. doi: 10.1002/jbmr.2799
41. Rigterink T, Appleton L, Day AS. Vitamin D therapy in children with inflammatory bowel disease: a systematic review. World J Clin Pediatr. (2019) 8(1):1–14. doi: 10.5409/wjcp.v8.i1.1
42. Yang L, He X, Li L, Lu C. Effect of vitamin D on helicobacter pylori infection and eradication: a meta-analysis. Helicobacter. (2019) 24(5):e12655. doi: 10.1111/hel.12655
43. Sasson AN, Ingram RJM, Zhang Z, Taylor LM, Ananthakrishnan AN, Kaplan GG, et al. The role of precision nutrition in the modulation of microbial composition and function in people with inflammatory bowel disease. Lancet Gastroenterol Hepatol. (2021) 6(9):754–69. doi: 10.1016/S2468-1253(21)00097-2
44. White JH. Vitamin D deficiency and the pathogenesis of crohn's disease. J Steroid Biochem Mol Biol. (2018) 175:23–8. doi: 10.1016/j.jsbmb.2016.12.015
45. Deng Z, Yang Z, Peng J. Role of bioactive peptides derived from food proteins in programmed cell death to treat inflammatory diseases and cancer. Crit Rev Food Sci Nutr. (2021):1–19. doi: 10.1080/10408398.2021.1992606
46. Watanabe D, Kamada N. Contribution of the gut Microbiota to intestinal fibrosis in crohn's disease. Front Med (Lausanne). (2022) 9:826240. doi: 10.3389/fmed.2022.826240
47. Gatera VA, Lesmana R, Musfiroh I, Judistiani RTD, Setiabudiawan B, Abdulah R. Vitamin D inhibits lipopolysaccharide (LPS)-induced inflammation in A549 cells by downregulating inflammatory cytokines. Med Sci Monit Basic Res. (2021) 27:e931481. doi: 10.12659/MSMBR.931481
48. Dimitrov V, White JH. Vitamin D signaling in intestinal innate immunity and homeostasis. Mol Cell Endocrinol. (2017) 453:68–78. doi: 10.1016/j.mce.2017.04.010
49. Lu EM. The role of vitamin D in periodontal health and disease. J Periodontal Res. (2023) 58(2):213–24. doi: 10.1111/jre.13083
50. Dürr UH, Sudheendra US, Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim Biophys Acta. (2006) 1758(9):1408–25. doi: 10.1016/j.bbamem.2006.03.030
51. Charoenngam N, Holick MF. Immunologic effects of vitamin D on human health and disease. Nutrients. (2020) 12(7):2097. doi: 10.3390/nu12072097
52. Calton EK, Keane KN, Newsholme P, Soares MJ. The impact of vitamin D levels on inflammatory status: a systematic review of immune cell studies. PLoS One. (2015) 10(11):e0141770. doi: 10.1371/journal.pone.0141770
53. Zhou A, Hyppönen E. Vitamin D deficiency and C-reactive protein: a bidirectional mendelian randomization study. Int J Epidemiol. (2023) 52(1):260–71. doi: 10.1093/ije/dyac087
54. Murdaca G, Gerosa A, Paladin F, Petrocchi L, Banchero S, Gangemi S. Vitamin D and microbiota: is there a link with allergies? Int J Mol Sci. (2021) 22(8):4288. doi: 10.3390/ijms22084288
Keywords: 25-hydroxyvitamin D, meta-analysis, pediatric inflammatory bowel disease, vitamin D, c-Reactive protein
Citation: Sun Y-h, Tian D-d, Zhou J-m and Ye Q (2023) Association between vitamin D level and pediatric inflammatory bowel disease: A systematic review and meta-analysis. Front. Pediatr. 11:1155004. doi: 10.3389/fped.2023.1155004
Received: 31 January 2023; Accepted: 6 April 2023;
Published: 24 April 2023.
Edited by:
Andrew S Day, University of Otago, New ZealandReviewed by:
Mostafa Vaghari-Tabari, Tabriz University of Medical Sciences, IranGiuseppe Murdaca, University of Genoa, Italy
© 2023 Sun, Tian, Zhou and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qing Ye cWluZ3llQHpqdS5lZHUuY24=
Specialty Section: This article was submitted to Pediatric Gastroenterology, Hepatology and Nutrition, a section of the journal Frontiers in Pediatrics
 Yan-hong Sun
Yan-hong Sun Dan-dan Tian
Dan-dan Tian Qing Ye
Qing Ye