- 1Department of Neurosurgery, Xijing Hospital, Airforce Military Medical University (Fourth Military Medical University), Xi’an, China
- 2Nursing Department of Zhengzhou People's Hospital, Zhengzhou, China
- 3Department of Neurosurgery, Xi’an Children’s Hospital, Xi’an, China
Background: The study introduced the Pediatric Quality of Life Inventory™ (PedsQL™) brain tumor module for the first time in China. Further, the Chinese version of the PedsQL™ brain tumor module was developed and its feasibility, reliability, and validity were investigated.
Methods: A total 129 cases completed the assessment. Feasibility was evaluated according to the percentage of missing items and the time required to complete the questionnaire. Internal consistency, retest reliability, and split-half reliability were tested to confirm reliability. We evaluated validity by testing content validity, construct validity, and criterion-related validity. The consistency between the child-self and parent-proxy reports was analyzed by calculating the correlation coefficient (r value) between them.
Results: The Cronbach's alpha values for all subscales were above 0.7 and many subscales scored more than 0.9. The intra-class correlation coefficients of retest reliability were higher than 0.9. The split-half reliability scores for all subscales were higher than 0.6. The factor-item correlations ranged between 0.575–0.922 in the child report and 0.492–0.949 in the parent report. Exploratory factor analyses produced five factors corresponding to each subscale in the child report and six factors in the parent report.
Conclusion: The feasibility, reliability, and validity of the Chinese PedsQL™ brain tumor module were ascertained through this study. This module can be used to effectively monitor children with brain tumors and conduct descriptive or exploratory studies to determine the risk factors affecting their quality of life. This would help develop a new basis for formulating measures to improve patient prognosis and quality of life.
1. Introduction
Brain tumors and other central nervous system (CNS) tumors have surpassed leukemia to become the most common cancers in children (1). With the development of neurosurgical technology, the five-year survival rate of pediatric brain tumor patients (PBTs) has increased to nearly 80% (2). The improvement in survival rate suggests that the quality of life of PBTs deserves more attention. Children receiving treatment usually experience symptoms such as pain, nausea, and fatigue. Many children may still experience issues with the nervous and endocrine systems even after treatment (3). In addition to physical problems, many PBTs may also have psychological problems, such as cognitive impairment and social disorder, resulting in a serious decline in their quality of life (4–6).
Health-related quality of life (HRQOL) has become an international research hotspot in the medical field. HRQOL is a multidimensional concept that includes physical, psychological, and social health (7). With the extensive development of HRQOL research, the research on quality of life gradually began to be applied to PBTs. To effectively evaluate the quality of life of patients with brain tumors, an efficient evaluation scale is necessary. Therefore, experts have developed some scales specifically used to evaluate HRQOL of patients with brain tumors, such as the head and neck cancer-specific scale of the Functional Assessment of Cancer Therapy (FACT) (8), the head and neck questionnaire (UWQOL) of the University of Washington (9), and the head and neck cancer-specific module of the European Cancer Research and Treatment Organization (EORTC QLQ-BN20) (10); however, these scales are not applicable to children with brain tumors.
The Pediatric Quality of Life Inventory ™ (PedsQL™) is a special quality of life measurement tool for children developed by Varni et al. (7). The inventory has good reliability and validity and has been used in many previous studies (11–14). Since its development in 1978, PedsQL™ has formed a complete modular evaluation system for children's quality of life. The scale consists of general core scales for measuring the common part of children's quality of life and disease-specific modules for measuring children's quality of life with different diseases. The PedsQL™ brain tumor module is one of the few effective tools to accurately evaluate HRQOL in children with brain tumors (15). Each set of scales was divided into four exclusive scales based on age: 2–4 years old, 5–7 years old, 8–12 years old, and 13–18 years old. While the 2–4 years old scale only includes parents' report, all other scales include both children's self-assessment report and parents' report (16). In 2006, Palmer et al. (15) first confirmed that the PedsQL™ brain tumor module has good reliability and validity in evaluating the quality of life of PBTs, and can effectively reflect the cognitive, neurological, endocrine system, and social and emotional problems of children (15). More than 10 countries including Japan and France have introduced the PedsQL™ brain tumor module and formed a variety of versions suitable for different cultures and language environments, with good applicability (17, 18). However, this module has not yet been introduced in China. The present study is the first to introduce the PedsQL™ brain tumor module in China, and has developed the Chinese version of the PedsQL™ brain tumor module and investigated its feasibility, reliability, and validity. Through this, we intended to provide a new method for the evaluation of the quality of life of children with brain tumors in China and lend a new basis for formulating measures to improve the prognosis and quality of life of children.
2. Method
2.1. Patients’ recruitment
Parents and their children with brain tumors, treated in the Department of Neurosurgery of Xijing Hospital from January 2010 to September 2020, were recruited for this study. This study was approved by the ethics committee of Xijing Hospital and the number is KY20202071-F-1.The inclusion criteria were as follows: (a) Children diagnosed with brain tumors; (b) Age range of 2–18 years; (c) Course of disease should be more than 1 month; (d) At least a parent of the children with brain tumor with basic Chinese listening and speaking ability, and with an ability of communicating with the researcher without obstacles; and (e) Children and their parents should have provided informed consent to participate in the study and should participate voluntarily. The exclusion criteria were as follows: (a) Children with other major diseases affecting their quality of life and (b) Children and/or parents who cannot correctly understand the content of the scale or fill in the scale correctly.
2.2. Translation process
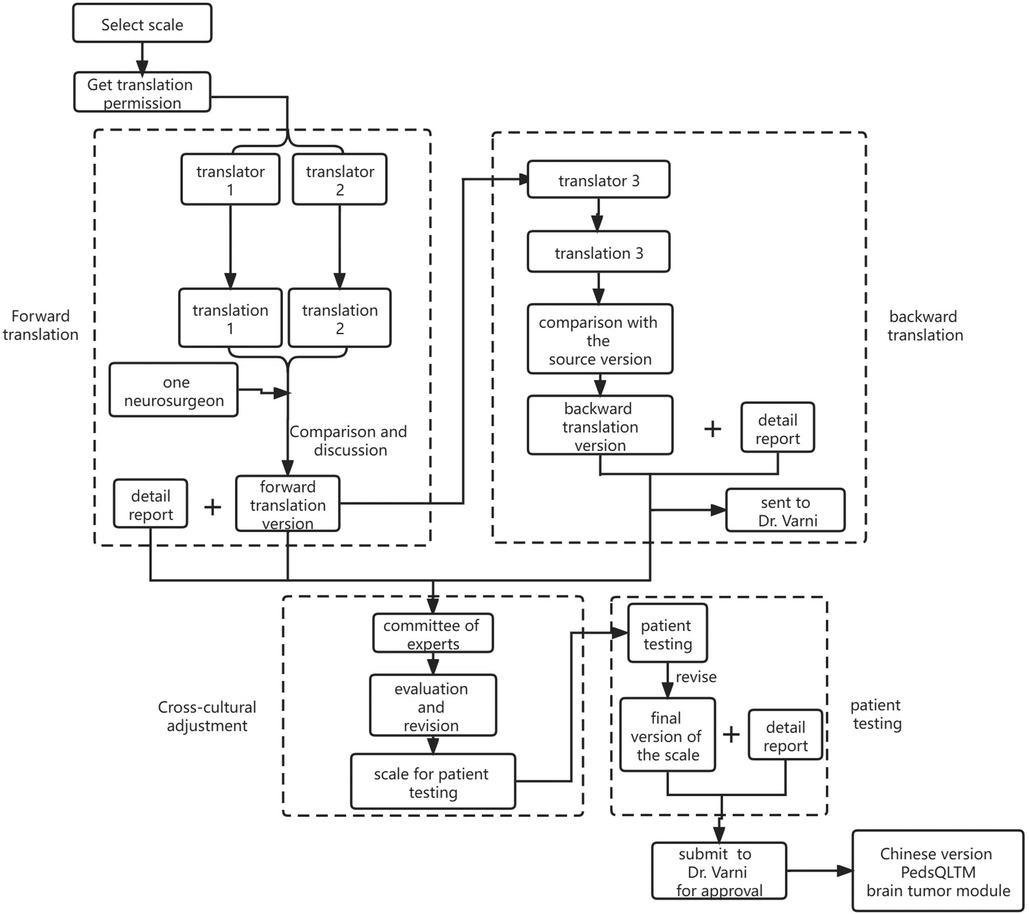
After obtaining consent from the Mapi Research Trust and Dr. Varni, we began to translate the PedsQL™ brain tumor module (English version) into Chinese, following the Mapi Research Trust linguistic validation guidelines (19). A specific flowchart is shown in Figure 1.
The forward translation of this study was completed by two native Chinese translators, of whom one is a bilingualist with clinical professional knowledge and full understanding of the relevant contents of the scale and the other, a translator majoring in English. The entire process was completed by the two translators independently. After that, the two translators and a neurosurgeon jointly compared the two translations, discussed and refined the parts with obvious differences. Based on the principle of equivalent translation of the concept of the source scale and simplicity of the language after translation, they raised questions to Dr. Varni regarding disagreements, forming a single reconciled version.
The backward translation was completed by a professional translator whose mother tongue was English and the skilled language was Chinese. He translated the version obtained from forward translation back to English and without consulting the PedsQL™ brain tumor module in the whole process. After comparison with the source version and modification, the back-translated version was sent to Dr. Varni for obtaining his review before patient testing.
After performing cross-cultural adaptation of the scale, we started patient testing. Six children with brain tumors participated in patient testing, along with their parents: one each for 2–4 years old and 5–7 years old ranges and two each for 8–12 years old and 13–18 years old age ranges. During the cognitive interview, we found that children and their families generally had a good understanding of the content and expression of the questionnaire. The time for children to complete the questionnaire was 4–9 min and their parents needed 3–6 min. The final version of the Chinese adaptation of the PedsQL™ brain tumor module was formed after obtaining the results. All the documents above were sent back to Dr. Varni for a final crosscheck and consent.
2.3. PedsQL™ brain tumor module
The PedsQL™ brain tumor module consists of six modules and 24 items: cognitive problems (7 items), pain and hurt (3 items), movement and balance (3 items), procedural anxiety (3 items), nausea (5 items), and worry (3 items). The parent proxy-report for the 2–4 years old group did not include the cognitive problem module. All child and parent reports containing responses for the questions used in the 8–18 years old group were based on a 5-point Likert-scale (0 = never a problem; 1 = almost never a problem; 2 = sometimes a problem; 3 = often a problem; 4 = almost always a problem). To facilitate the understanding and cooperation of the 5–7 years old group, the scale was simplified to a 3-point Likert scale (0 = not at all a problem; 2 = sometimes a problem; 4 = a lot of a problem). Items were reverse-scored and linearly transformed to a 0–100 scale (0 = 100, 1 = 75, 2 = 50, 3 = 25, 4 = 0) as higher scores indicate better HRQOL. Scale scores were computed as the sum of the items divided by the number of items answered, and the summary score was calculated as the sum of all the 24 items divided by the number of items answered.
2.4. PedsQL 4.0 generic core scales
The PedsQL 4.0 Generic Core Scales contains four modules and 23 items: physical functioning (8 items), emotional functioning (5 items), social functioning (5 items), and school functioning (5 items). The format, response scale, and scoring method were identical to those of the PedsQL™ brain tumor module. The scale was translated into Chinese and has good reliability and validity (20).
2.5. Data collection and quality control
Two nurses with at least five years of pediatric neurosurgery clinical nursing experience and two research students performed this investigation. All interviewers were trained before the investigation in interviewing and administering questionnaires. This study was approved by the ethics committee of Xijing Hospital. The researchers explained the purpose, significance, and research process of this investigation to the children and their parents, and informed them that they had the right to fill in the questionnaire voluntarily and withdraw from the study freely. After obtaining informed consent, the researchers explained the filling method of the questionnaire and distributed the PedsQL™ brain tumor module and PedsQL™ 4.0 generic core scales. The children and their parents completed the questionnaire independently. Among children aged 5–7 or those unable to read or write, the questionnaires were administered by their parents or researcher. After that, two students reviewed all the questionnaires and supplemented the missing items through repeated interviews or telephone interviews.
2.6. Statistical analysis
The feasibility was evaluated according to the percentage of missing items and the time required to complete the questionnaire. Three methods were used to test the reliability: (1) Internal consistency: Cronbach's alpha was used to evaluate the internal consistency; alpha value of 0.7–0.8 indicates that the scale was reliable and alpha value of 0.8–0.9 indicates that the reliability of the scale was very good. (2) Retest reliability: Assuming that there is no change in the status of a group of patients in a short time, each object was measured twice with the same scale, and the two results were tested using the intra-class correlation coefficient (ICC). The ICC is a value between 0 and 1. The higher the consistency of the two measurement results, the closer the ICC is to 1, the higher the test-retest reliability, and the better the stability of the scale and therefore, the more reliable the results. We selected 18 children who were hospitalized for more than 1 week and their parents to measure the test-retest reliability. (3) Split-half reliability: The items in the scale were randomly divided into two halves to obtain two total scores. The correlation coefficient between the two total scores was recorded as R, and 2R/(1 + R) was the split half reliability of the scale. When this coefficient is greater than 0.6, the split half reliability is good (20). The supplementary test-retest reliability cannot measure the defects of objects that change over time.
We also used three methods to test the validity of the Chinese version of the PedsQL™ brain tumor module: (1) Content validity: The content validity of the scale was analyzed by calculating the Pearson correlation coefficient (r value) between the items and subscales of the scale. The r value is between 0 and 1, and the higher the r value, the better the content validity. (2) Construct validity: In this study, exploratory factor analysis was used to test the structural validity of the scale. First, the Kaiser-Meyer-Olkin (KMO) value was calculated to judge whether the scale is suitable for factor analysis. Then, we determined the number of factors, extracted the common factors by principal component analysis, carried out rotation transformation, and sought the best analysis effect. The cumulative variance contribution rate of the extracted common factors should be greater than 40% and each item has a high factor compliance (>0.4) on its common factors (15). (3) Criterion-related validity: The Chinese version of the PedsQL 4.0, generic core scales (2–18 years old) was used as the calibration standard to test the calibration correlation validity. The higher the correlation coefficient (r value) between them, the better the correlation degree of the effective standard and the r value is between 0 and 1 (21).
The consistency between the child-self and parent-proxy reports was analyzed by calculating the correlation coefficient (r value) between them. All analyses were performed using SPSS 23.0, and statistical significance was set at p < 0.05. Power analysis using the findings from the original English version demonstrated that the minimum requisite sample size was 85 subjects (15). Besides, the sample size should be more than 100 to meet the minimum sample size for exploratory factor analysis.
3. Results
3.1. Sample characteristics
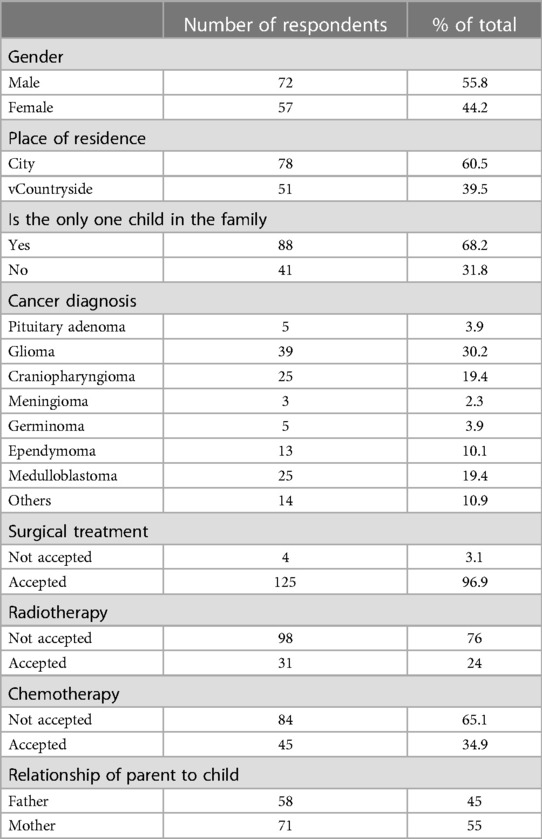
A total of 129 patients and their parents were included in the study and completed the assessment. The children were 2–18 years old, with a median age of eight years. The course of the disease ranged from 1 month to 125 months, with a median time of 35 months. A total of 125 patients received surgery; 31, radiotherapy; 45, chemotherapy; and 3, no treatment. See Table 1 for further details.
3.2. Descriptive statistics and feasibility
The maximum value for all scales was 100, whereas the minimum value ranged from 0 to 25. The parent reports' scores were higher than the child reports' scores in all subscales (Table 2). The time for children to complete the questionnaire was 4–9 min and their parents needed 3–6 min.
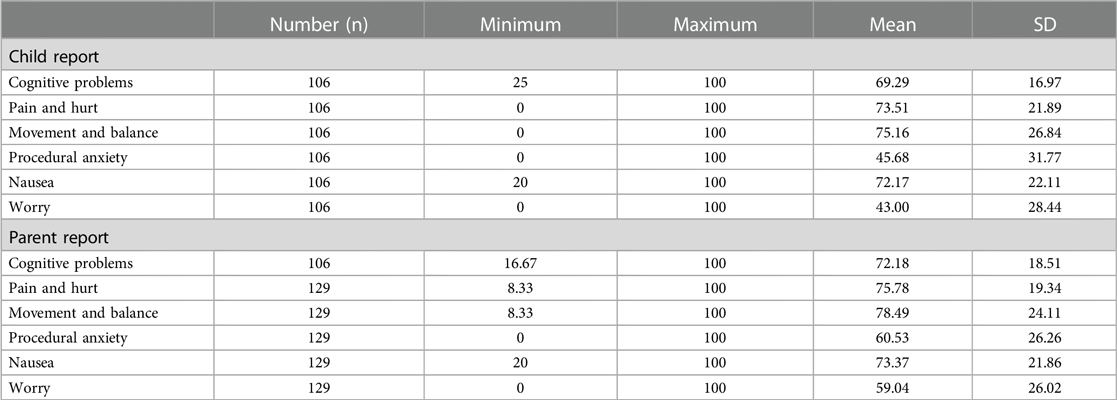
Table 2. Scale descriptives for Chinese version of PedsQL™ brain tumor module child and parent reports.
3.3. Reliability
Internal consistency and retest reliability are shown in Table 3. The Cronbach's coefficient alpha values for all subscales were above 0.7 and many subscales scored more than 0.9, proving that the Chinese version of the PedsQL™ brain tumor module has good internal consistency. We chose 18 children, who were hospitalized for more than one week, and their parents to measure the retest reliability. As shown in Table 3, all the ICCs were higher than 0.9, indicating that all the subscales had high agreement in this test. For split-half reliability, all subscale scores were higher than 0.6 (Table 4).
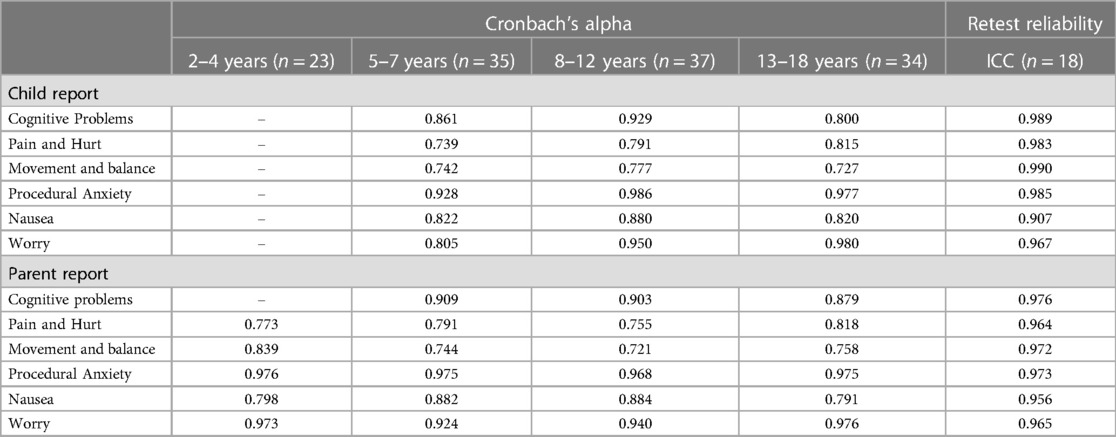
Table 3. Internal consistency and retest reliability of Chinese version of PedsQL™ brain tumor module child and parent reports.
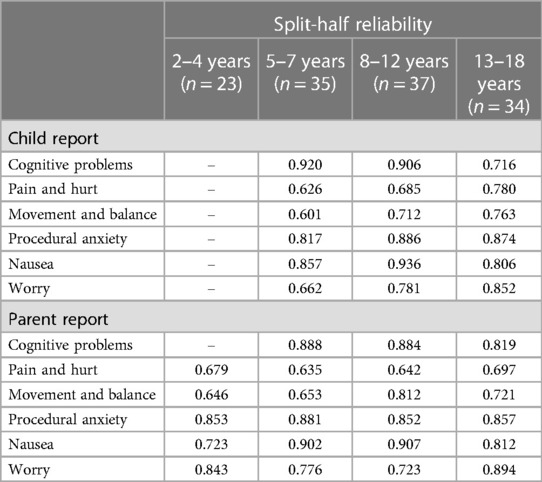
Table 4. Split-half reliability of Chinese version of pedsQL™ brain tumor module child and parent reports.
3.4. Validity
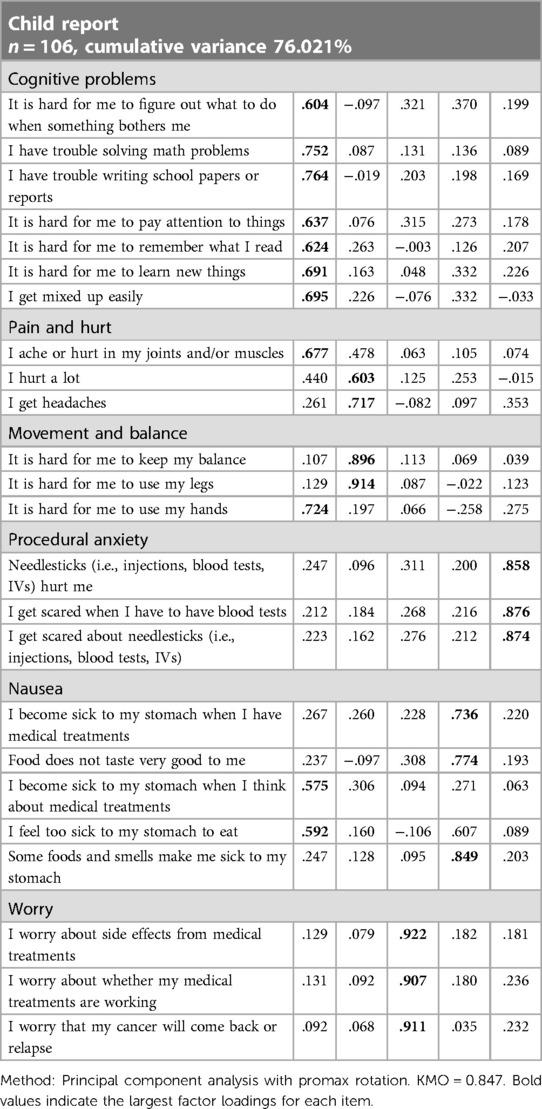
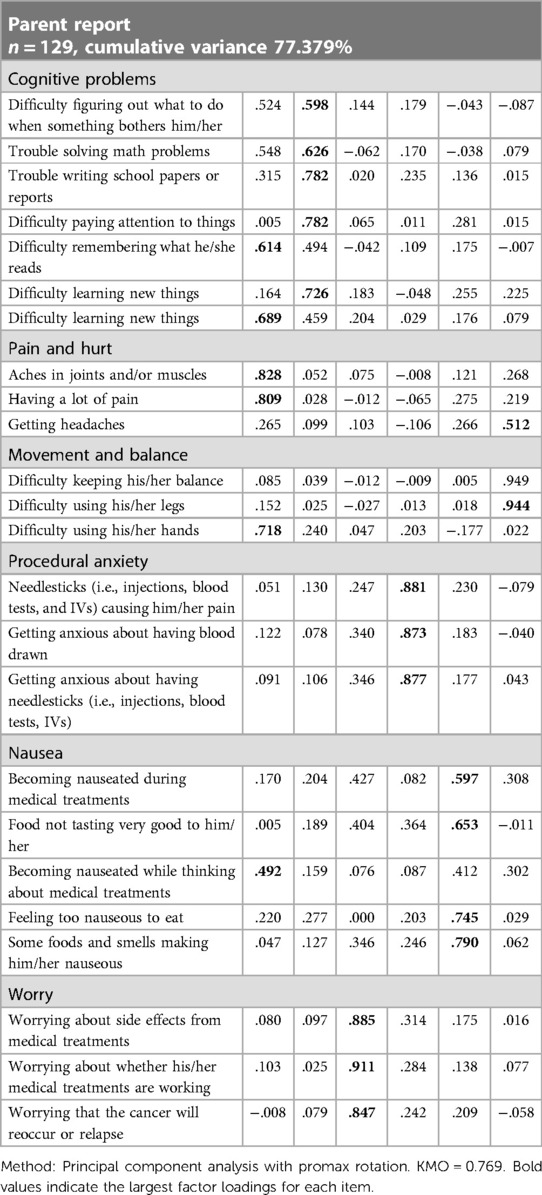
As shown in Table 5, there is a certain correlation between each item and each subscale. Except for items 8 and 13 in the children's report and item 13 in the parents' report, the other correlation coefficients are all greater than 0.6, indicating that the Chinese version of the PedsQL™ brain tumor module has good content validity. The results of the factor analysis for the Chinese version of the PedsQL™ brain tumor module to test the construct validity are presented in Tables 6, 7. Exploratory factor analyses produced five factors corresponding to each subscale in the child report and six factors in the parent report. The factor-item correlations were between 0.575 and 0.922 in the child report and between 0.492 and 0.949 in the parent report. We used the Chinese version of the PedsQL™ 4.0 generic core scale as a calibration standard to test the calibration correlation validity of the Chinese version of the PedsQL™ brain tumor module. There was a high degree of consistency between the total scores of the two scales. There was good consistency between all the subscales and the total score of the calibration scale (Table 8).
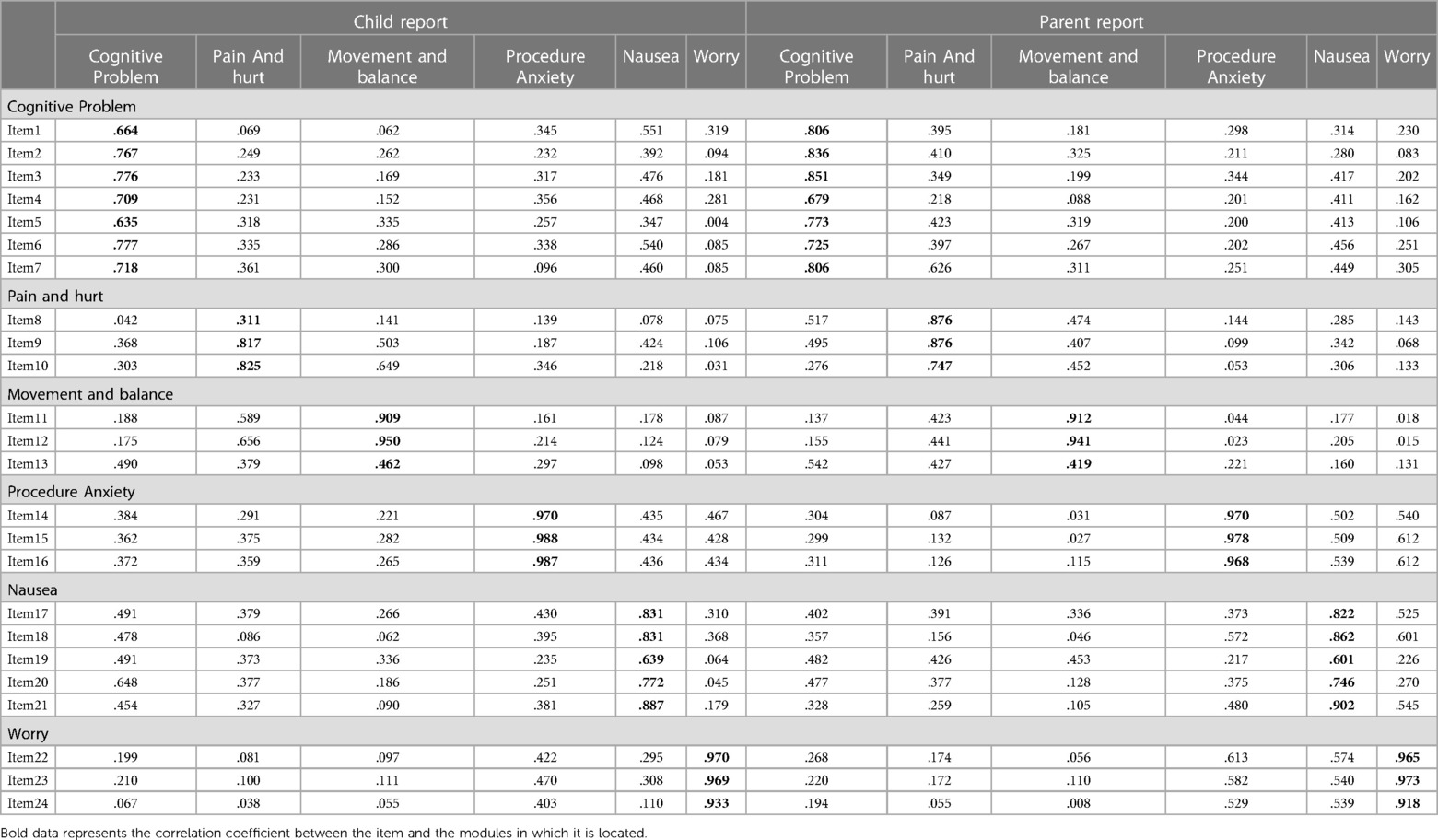
Table 5. Content validity of Chinese version of PedsQL™ brain tumor module child and parent reports.
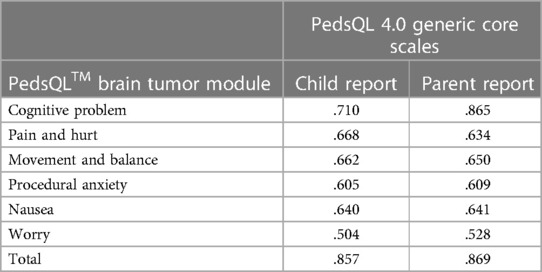
Table 8. Criterion-related validity of Chinese version of PedsQL™ brain tumor module child and parent reports.
3.5. Consistency between child-self report and parent-proxy report
As shown in Table 9, ICCs between the same subscales of child-self reports and parent-proxy reports were all higher than 0.6. The ICCs of the total scores were as high as 0.89.
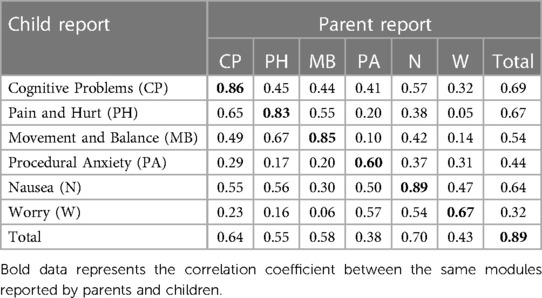
Table 9. The consistency between Chinese version of PedsQL™ brain tumor module child and parent reports.
4. Discussion
Currently, increasing attention is being paid to research on HRQOL of patients worldwide, and an increasing number of scales have been introduced in China to specifically study the HRQOL of children with certain diseases (21–24). However, there are no measurement tools that are be specifically suitable for PBTs in China. Our study is the first to develop the Chinese version of the PedsQL™ brain tumor module and demonstrate its feasibility, reliability, and validity in PBTs. Through the present study, we proved that this questionnaire can effectively reflect the HRQOL of PBTs.
We completed the translation according to the MAPI Research Trust linguistic validation guidelines. Adhering to the principles of conceptual equivalence, semantic equivalence, idiom equivalence, and experience equivalence, we added cultural adaptation following backward translation. After the patient testing, to ensure that the PBTs and their parents could easily and correctly understand the questionnaire, the Chinese version of the PedsQL™ brain tumor module was officially developed. The children who could not complete the questionnaire independently were helped by the investigator. Due to our strict inclusion criteria and the fact that the researchers helped the patients, no item was missed in both the child and parent reports. The minimal missing item responses indicated that PBTs and their parents could provide good quality data on the patients' HRQOL through the questionnaire. During patient testing, only a short time was required for the patients and their parents to complete the questionnaire. These results suggest that the Chinese version of the PedsQL™ brain tumor module has good feasibility and is convenient for use in the fast-paced work of outpatient clinics.
For all the scales for both child and parent reports, the Cronbach's alpha approached or exceeded the standard of 0.70, thus indicating its reliability. Most of the subscales exceeded an alpha of 0.90, indicating that this scale could be used for individual analysis and comparative analysis of HRQOL between the groups in clinical trials (25). Moreover, all the subscales in both the child and parent reports showed sufficient retest reliability and split-half reliability. These results show that the Chinese version of the PedsQL™ brain tumor module has good reliability.
We have completed enough tests to prove the scale's validity, such as content, construct, and criterion-related validity. The present study is the first to ascertain the scale's content validity by calculating the Pearson correlation coefficient (r value) between the items and subscales, which had not been confirmed in the original or other versions of the PedsQL™ brain tumor module. Except for three items, the correlation coefficients between all the other items and their subscales were greater than 0.6, indicating that the scale has good content validity in both child and parent reports. As expected, the Chinese version of the PedsQL™ brain tumor module performs well in terms of construct validity and calibration correlation validity, which is completely consistent with previous studies (15, 17, 18).
In our study, we observed that the scores of procedural anxiety and worry subscales were relatively lower than those of other subscales, which was consistent with the findings of Caru et al. (17) and Palmer et al. (15). This may be due to the severity of symptoms and treatment of PBTs (26), or trying to avoid hospital visits (27). Current studies have different opinions on the consistency of parents' and children's reports (28–30). The subjects' responses to the scale also depended on the scale and the subject population. In our study, the parent reports' scores were higher than child reports' scores in all subscales. The reason is perhaps that a portion of children in China are a little bit more ashamed to express their true thoughts to their parents. Good concordance between child reports and parent reports was observed for all subscales in our study. Children's age and cognitive development may pose some limitations in the completion of the questionnaire, so parents were required to complete the questionnaire, which will serve as references and supplements.
Indeed, there were some limitations in our present research. Although we included PBTs of all age groups and many kinds of cancers, we only conducted the study at one hospital, which cannot fully reflect the response of all Chinese children to this scale. Further multicenter collaborative studies are needed to expand the sample size in more representative hospitals.
5. Conclusion
To conclude, we developed the Chinese version of the PedsQL™ brain tumor module and verified its feasibility, reliability, and validity. This version was a first instrument used specifically to measure the HRQOL of PBTs in China. This module can be used to effectively monitor PBTs and conduct descriptive or exploratory studies to determine the risk factors affecting their HRQOL. We believe that wide application of Chinese version of the PedsQL™ brain tumor module provides an important method for formulating measures to improve patient prognosis and HRQOL. And this is also a new start for future multicenter researches in order to improve the HRQOL of PBTs in China.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of Xijing Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
JW: Conceptualization, Writing – original draft. JL: Investigation, Software, Writing – original draft. XJ: Conceptualization, Formal Analysis, Writing – review & editing. PS: Data curation, Formal Analysis, Methodology, Writing – original draft. XL: Resources, Validation, Visualization, Writing – review & editing. GW: Conceptualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
The present work was supported by the Natural Science Foundation of China (NSFC) grant to Xiaosheng He (Nos. 81971156 and 2020XC015).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ostrom QT, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2014–2018. Neuro-Oncology. (2021) 23(12, Suppl 2):iii1–iii105. doi: 10.1093/neuonc/noab200
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. (2021) 71(1):7–33. doi: 10.3322/caac.21654
3. Hidalgo ET, Orillac C, Kvint S, McQuinn MW, Dastagirzada Y, Phillips S, et al. Quality of life, hypothalamic obesity, and sexual function in adulthood two decades after primary gross-total resection for childhood craniopharyngioma. Child’s Nervous System. (2020) 36(2):281–9. doi: 10.1007/s00381-019-04161-9
4. Bhat SR, Goodwin TL, Burwinkle TM, Lansdale MF, Dahl GV, Huhn SL, et al. Profile of daily life in children with brain tumors: an assessment of health-related quality of life. J Clin Oncol. (2005) 23(24):5493–500. doi: 10.1200/JCO.2005.10.190
5. Bitterlich C, Vordermark D. Analysis of health-related quality of life in patients with brain tumors prior and subsequent to radiotherapy. Oncol Lett. (2017) 14(2):1841–6. doi: 10.3892/ol.2017.6310
6. Schulte F, Russell KB, Cullen P, Embry L, Fay-McClymont T, Johnston D, et al. Systematic review and meta-analysis of health-related quality of life in pediatric CNS tumor survivors. Pediatr Blood Cancer. (2017) 64:e26442. doi: 10.1002/pbc.26442
7. Varni JW, Limbers CA. The pediatric quality of life inventory: measuring pediatric health-related quality of life from the perspective of children and their parents. Pediatr Clin N Am. (2009) 56(4):843–63. doi: 10.1016/j.pcl.2009.05.016
8. Weitzner MA, Meyers CA, Gelke CK, Byrne KS, Cella DF, Levin VA. The Functional Assessment of Cancer Therapy (FACT) scale. Development of a brain subscale and revalidation of the general version (FACT-G) in patients with primary brain tumors. Cancer. (1995) 75(5):1151–61. doi: 10.1002/1097-0142(19950301)75:5%3C1151::aid-cncr2820750515%3E3.0.co;2-q
9. Heimans JJ, Taphoorn MJ. Impact of brain tumour treatment on quality of life. J Neurol. (2002) 249(8):955–60. doi: 10.1007/s00415-002-0839-5
10. Osoba D, Aaronson NK, Muller M, Sneeuw K, Hsu MA, Yung WK, et al. The development and psychometric validation of a brain cancer quality-of-life questionnaire for use in combination with general cancer-specific questionnaires. Qual Life Res. (1996) 5(1):139–50. doi: 10.1007/BF00435979
11. Cremeens J, Eiser C, Blades M. Factors influencing agreement between child self-report and parent proxy-reports on the Pediatric Quality of Life Inventory 4.0 (PedsQL) generic core scales. Health Qual Life Outcomes. (2006) 4:58. doi: 10.1186/1477-7525-4-58
12. Upton P, Eiser C, Cheung I, Hutchings HA, Jenney M, Maddocks A, et al. Measurement properties of the UK-English version of the Pediatric Quality of Life Inventory 4.0 (PedsQL) generic core scales. Health Qual Life Outcomes. (2005) 3:22. doi: 10.1186/1477-7525-3-22
13. Williams J, Wake M, Hesketh K, Maher E, Waters E. Health-related quality of life of overweight and obese children. J Am Med Assoc. (2005) 293(1):70–6. doi: 10.1001/jama.293.1.70
14. Musiol K, Bulska W, Brożek P, Oślizło B, Ryzak S, Dubiel J, et al. Quality of life in survivors of childhood brain tumour and the association of children’s diseases on quality of their parents life. Psychooncology. (2019) 28(5):1088–95. doi: 10.1002/pon.5061
15. Palmer SN, Meeske KA, Katz ER, Burwinkle TM, Varni JW. The PedsQL™ brain tumor module: initial reliability and validity. Pediatr Blood Cancer. (2007) 49(3):287–93. doi: 10.1002/pbc.21026
16. Varni JW, Seid M, Knight TS, Uzark K, Szer IS. The PedsQL 4.0 generic core scales: sensitivity, responsiveness, and impact on clinical decision-making. J Behav Med. (2002) 25(2):175–93. doi: 10.1023/a:1014836921812
17. Caru M, Perreault S, Levesque A, Sultan S, Desjardins L, Rondeau É, et al. Validity and reliability of the French version of the Pediatric Quality of Life Inventory™ brain tumor module. Qual Life Res. (2021) 30(8):2387–404. doi: 10.1007/s11136-021-02815-3
18. Sato I, Higuchi A, Yanagisawa T, Mukasa A, Ida K, Sawamura Y, et al. Development of the Japanese version of the pediatric quality of life inventory brain tumor module. Health Qual Life Outcomes. (2010) 8:38. doi: 10.1186/1477-7525-8-38
19. Institute MR. Linguistic validation of the PedsQL™—a quality of life questionnaire. Lyon: Mapi Research Institute (2002).
20. Ji Y, Chen S, Li K, Xiao N, Yang X, Zheng S, et al. Measuring health-related quality of life in children with cancer living in mainland China: feasibility, reliability and validity of the Chinese mandarin version of PedsQL 4.0 generic core scales and 3.0 cancer module. Health Qual Life Outcomes. (2011) 9:103. doi: 10.1186/1477-7525-9-103
21. Chang Y, Luo Y, Zhou Y, Wang R, Song N, Zhu G, et al. Reliability and validity of the Chinese mandarin version of PedsQL™ 3.0 transplant module. Health Qual Life Outcomes. (2016) 14(1):142. doi: 10.1186/s12955-016-0545-0
22. Feng L, Zhang Y, Chen R, Hao Y. The Chinese version of the Pediatric Quality of Life Inventory™ (PedsQL™) 3.0 asthma module: reliability and validity. Health Qual Life Outcomes. (2011) 9:64. doi: 10.1186/1477-7525-9-64
23. Hu J, Jiang L, Hong S, Cheng L, Kong M, Ye Y. Reliability and validity of the Chinese version of the Pediatric Quality Of Life InventoryTM (PedsQL™) 3.0 neuromuscular module in children with Duchenne muscular dystrophy. Health Qual Life Outcomes. (2013) 11:47. doi: 10.1186/1477-7525-11-47
24. Lau JT, Yu XN, Chu Y, Shing MM, Wong EM, Leung TF, et al. Validation of the Chinese version of the Pediatric Quality of Life Inventory (PedsQL) cancer module. J Pediatr Psychol. (2010) 35(1):99–109. doi: 10.1093/jpepsy/jsp035
25. Novick MR, Lewis C. Coefficient alpha and the reliability of composite measurements. Psychometrika. (1967) 32(1):1–13. doi: 10.1007/BF02289400
26. Sato I, Higuchi A, Yanagisawa T, Mukasa A, Ida K, Sawamura Y, et al. Cancer-specific health-related quality of life in children with brain tumors. Qual Life Res. (2014) 23(4):1059–68. doi: 10.1007/s11136-013-0555-x
27. Yu WY, Yang GF, Ren ZM. Survey of psychological health of parents of children with malignant tumor. Academic J First Med Coll PLA. (2004) 24(10):1197–8. 15485799.
28. Yeh CH, Chang CW, Chang PC. Evaluating quality of life in children with cancer using children’s self-reports and parent-proxy reports. Nurs Res. (2005) 54(5):354–62. doi: 10.1097/00006199-200509000-00010
29. Eiser C, Vance YH, Horne B, Glaser A, Galvin H. The value of the PedsQL™ in assessing quality of life in survivors of childhood cancer. Child Care Health Dev. (2003) 29(2):95–102. doi: 10.1046/j.1365-2214.2003.00318.x
Keywords: pediatric brain tumors, health-related quality of life, PedsQL, children, translation
Citation: Wang J, Li J, Jiang X, Sun P, Li X and Wang G (2023) Validation of the Chinese version of PedsQL™ brain tumor module. Front. Pediatr. 11:1277223. doi: 10.3389/fped.2023.1277223
Received: 14 August 2023; Accepted: 13 October 2023;
Published: 30 October 2023.
Edited by:
Luca Giacomelli, Polistudium srl, Italy© 2023 Wang, Li, Jiang, Sun, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xia Li c2p3a2xpeGlhQHNpbmEuY29t Guanyi Wang MzY0NTc4MTk1QHFxLmNvbQ==
†These authors have contributed equally to this work
 Juan Wang1,†
Juan Wang1,† Xia Li
Xia Li Guanyi Wang
Guanyi Wang