- 1Molecular and Cell Biology Research Center, Hemoglobinopathy Institute, Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran
- 2Gastrointestinal Cancer Research Center, Non-Communicable Diseases Institute, Mazandaran University of Medical Sciences, Sari, Iran
Background: Vitamin D acts as a pro-hormone with a wide range of beneficial effects. It is reported that vitamin D deficiency is a risk factor for COVID-19 severity in children. In the present study, we decided to assess 25 hydroxy (OH) vitamin D status in children with mild, moderate, or severe confirmed COVID-19 and also compare them with those of a healthy control group using existing data.
Methods: Relevant studies were extracted using online international databases including Scopus, Science Direct, PubMed, Web of Science, ProQuest, and Google Scholar search engine between Jan 2019 and 2024. The quality of all papers is determined by the NOS checklist. Heterogeneity between the results of primary studies was evaluated with the I-square index. Egger's test, funnel plot, and sensitivity analysis were applied. The statistical analysis was done using Stata version 17.
Results: In 12 documents, the status of vitamin D was examined between case and control groups. By combining the results of these studies using random effect model, the standardized mean difference (SMD) vitamin D level in the COVID-19 children compared to the control group was estimated to be −0.88 (98% CI: −1.24, −0.51), which was statistically significant. In the present study, the odd ratio of vitamin D deficiency and vitamin D disorder (insufficiency and deficiency) in children with moderate COVID-19 compared to asymptomatic children with COVID-19 were estimated to be 3.58 (1.10, 11.63) and 2.52 (0.99, 6.41) respectively which was higher than in asymptomatic children with COVID-19. In addition, vitamin D deficiency and vitamin D disorder in children with moderate COVID-19 compared to the children with mild COVID-19 were estimated to be 2.12 (0.90, 4.98) and 1.82 (0.78, 4.22) respectively, which was higher than in children with mild COVID-19. Also, vitamin D deficiency and vitamin D disorder in children with mild COVID-19 compared to asymptomatic children with COVID-19 were estimated to be 2.02 (0.60, 6.78) and 1.64 (0.53, 5.07) respectively, which was higher than in asymptomatic children.
Conclusions: Combining the results of these studies, the effect size of the relationship between vitamin D and COVID-19 in children is significant. During the COVID-19 pandemic (except for the Omicron peak), children were less affected by the severity of COVID-19. The standardized mean difference (SMD) vitamin D level in children with COVID-19 was significantly 0.88 units lower than the control group. Also, the odds ratio of moderate COVID-19 in children with vitamin D deficiency was significantly 3.58 times higher than in asymptomatic children with COVID-19.
1 Introduction
In December 2019, a new coronavirus (CoV) infection started with pneumonia epidemics in Wuhan, Hubei, and China, and spread throughout the world, resulting in the COVID-19 pandemic (1, 2). COVID-19 infection has been reported in different age groups and results in high mortality in patients (3). The first confirmed cases among children were reported from January 20 to February 6, 2020, in children (≤18 years) (4). In children, COVID-19 usually occurs with milder symptoms; however, cases with more severe disease have been reported (5). The most common symptoms of acute respiratory infection in the pediatric age group are fever, watery diarrhea, frequent runny noses, fever, cough, nausea and vomiting (6).
It has been reported that hyper-inflammation increases the mortality risk in severe COVID-19 patients (7) and vitamin D has a protective effect against acute respiratory interstitial pneumonia, and influenza A infections (8). Vitamin D acts as a pro-hormone (prototypical secosteroid) with a wide range of beneficial effects, including immune-modulator, anti-inflammatory, anti-fibrotic, epithelial integrity protective properties and antioxidant effects. Vitamin D upregulates IL-4 in Th2 cells and inhibits inflammatory cytokine expression and Th1 cell proliferation. It is suggested that vitamin D deficiency is associated with overexpression of Th1 cytokines (9, 10). The finding showed that vitamin D produces cathelicidin (an endogenous antimicrobial peptide), reduces the synthesis of dipeptidyl peptidase-4 receptor (DPP-4/CD26), and plays a protective role with antibacterial, antiviral, and antioxidant properties (11).
Angiotensin-converting enzyme II (ACE2) is the cell receptor for SARS-CoV-2 and is not as robust in children as it is in adults. 1,25(OH)2D3, The active form of vitamin D, can induce ACE2 expression which plays a role in protecting against severe infection and its level is associated with asymptomatic or mild-to-moderate symptoms of COVID-19 (3).
It is reported that vitamin D deficiency, an important public health problem, is a risk factor for COVID-19 severity in children. It has been reported that vitamin D supplementation is associated with reduced mortality risk, length of hospital stay, and ICU admission rates in COVID-19 patients (12). Different cutoffs of vitamin D exist. Following recent consensus, deficiency was defined as 25(OH)D concentration of ≤50 nmol/L (20 ng/ml) and vitamin D insufficiency or deficiency are common findings in children (13).
In the present study, we decided to assess 25 hydroxy (OH) vitamin D status in children with mild, moderate, or severe confirmed COVID-19 and also compare them with those of a healthy control group using existing data.
2 Material and methods
This study was designed and conducted based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, but its protocol was not registered.
The primary outcome was the assessment of 25-Hydroxy vitamin D status in case (COVID-19 patients) and control (healthy) groups. The secondary outcome was the evaluation of 25-Hydroxy vitamin D in patients with asymptomatic, mild, moderate, and severe COVID-19.
2.1 Search strategy
In the present study, two researchers (M.M and T.M) conducted the literature search independently. The published articles were collected via a systematic search of the literature databases such as Scopus, Science Direct, Pubmed, Web of Science, Proquest, and Google Scholar search engine between January 2019 and 2024.
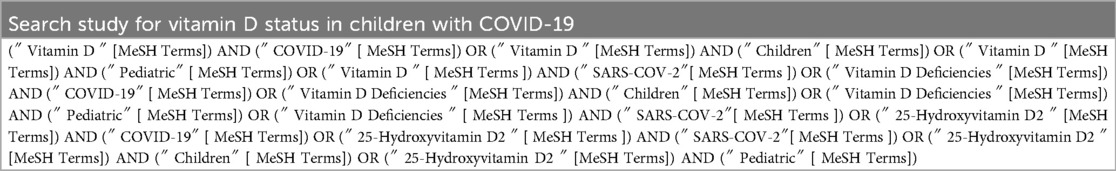
Our search terms included “ vitamin D”, “vitamin D Deficiencies”, “25-Hydroxyvitamin D2”, “COVID-19”, “SARS-COV-2”, “Children”, and “Pediatric” by the combination of “OR”, and “AND” Boolean Operators in the Title/Abstract/Keywords field (Table 1). In addition, the article references were screened to find additional related studies and increase search sensitivity. Finally, all collected references are entered into reference management software (EndNote). A reference list of all related studies was also reviewed for any other related publications. One of the team's researchers randomly evaluated the search results and reported that no relevant study was ignored. The search was restricted to original Articles/Abstracts published in the English language that reported the association between vitamin D status and COVID-19 infection in children.
All these steps were done by two authors (M.M and TM) and any disagreements with article selection were resolved through discussion, and a third author (A.N) was available to resolve the disagreement. In addition, the article references were screened to find additional related studies and increase search sensitivity. Finally, all collected references are entered into reference management software (EndNote).
2.2 Inclusion criteria
“P” signifies children patients (1 month-18 years) with COVID-19; “I (E)” means 25-Hydroxy vitamin D. “C” Healthy groups (control). “O” means the assessment of 25-Hydroxy vitamin D in case (COVID-19 patients) and control (healthy) groups and the assessment of 25-Hydroxy vitamin D in asymptomatic, moderate, mild, and severe groups.
All cohort or case-control studies were entered into this meta-analysis for the assessment of 25-Hydroxy vitamin D in the case and control groups.
2.3 Exclusion criteria
1. Studies that scored less than 5 based on the quality assessment checklist were excluded from the meta-analysis.
2. Duplicate publications, reviews, animal research, case reports, in vitro, and in-silico studies were removed from the meta-analysis.
3. Research of adult groups (14).
4. Research of specific groups (15).
5. Studies that did not report the disease severity (mild/moderate or severe) (16–20).
6. Studies were not case-control (16, 21).
2.4 Study selection
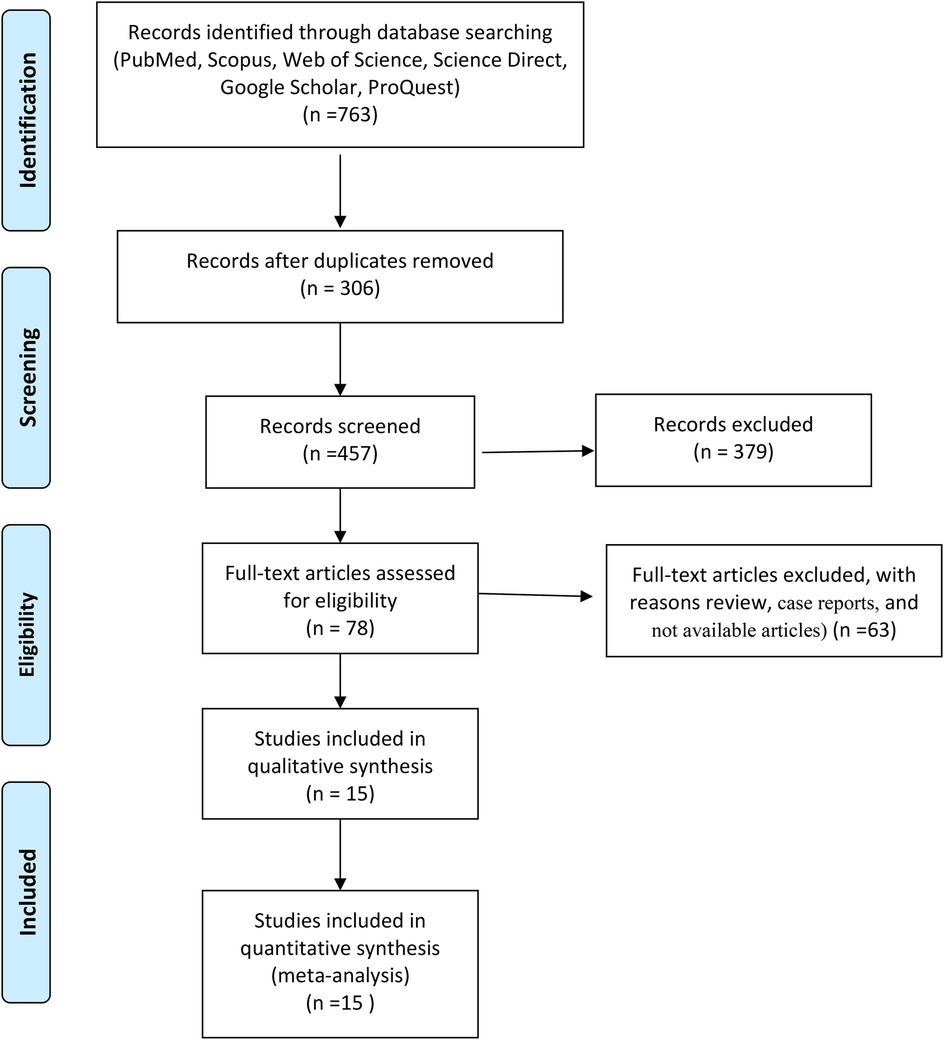
First, the full text or summary of articles, documents, and reports is extracted from different databases. After removing duplicates, the irrelevant articles were excluded from this study. Then, full-text articles were assessed for eligibility and next, review studies were removed from this meta-analysis. Finally, data was extracted from full-text articles based on inclusion and exclusion criteria (Figure 1).
2.5 Data extraction
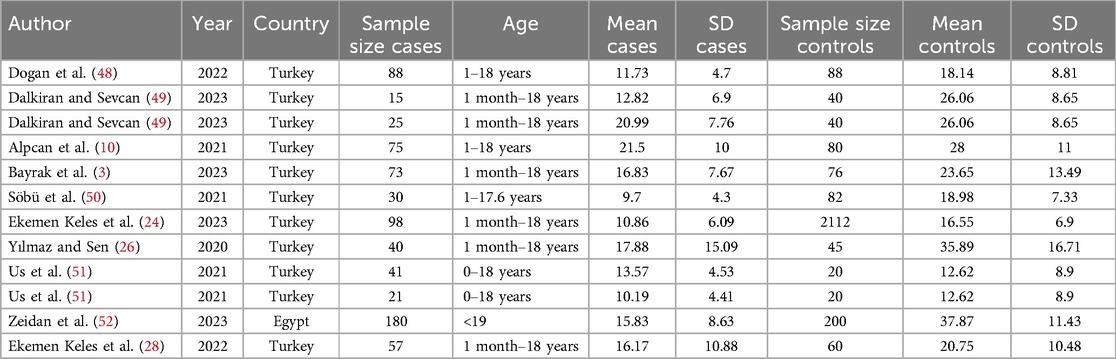
For data extraction, two researchers independently screened the studies based on article title, first author's name, year of study, place of study, type of study, the total number of cases and controls, mean (SD) of vitamin D in the case group, mean (SD) of vitamin D in the control group, the median of vitamin D in the case group, the median of vitamin D in the control group, number of asymptomatic, mild, moderate and severe patients with normal vitamin D, number of asymptomatic, mild, moderate and severe patients with vitamin D insufficiency, and number of asymptomatic, mild, moderate and severe patients with vitamin D deficiency.
2.6 Quality evaluation
Considering all the prospective cohort studies entered in our meta-analysis, the Newcastle–Ottawa scale (NOS) checklist was applied to assess the quality of studies. Based on this checklist, nine questions assigning a score and covering the type of study, sample size, study objectives, study population, sampling method, data analysis method, presentation of findings in an appropriate way, and presentation of results based on objectives were designed. This checklist has three parts: selection, comparability, and exposure (range 0–9). Only the studies that scored at least 5 points were included in the study. Maximum criterion scores (2); maximum comparability scores (2); and maximum exposure has 3 scores (22, 23).
2.7 Analysis
In the present study, Stata version 17 (Stata Corp, College Station, TX, USA) was applied for data analysis. In this study, to estimate the odd ratio, a two-by-two table was designed for each of the primary studies. Data were combined based on the random effect model and DerSimonian–Laird method. The odds ratio with a 95% confidence interval is presented in a Forest plot.
The standardized mean difference (SMD) vitamin D level, the number of samples, the mean and the standard deviation of vitamin D were extracted from primary studies. Using the random effect model and DerSimonian–Laird method, the SMD of vitamin D between the case and control groups was estimated with a 95% confidence interval. The criterion for judging the significant differences between SMD of vitamin D does not include the zero between the upper and lower confidence interval of the SMD.
For assessing the heterogeneity index between studies, the Cochran (Q) and I2 tests were used. Egger's test and funnel plot were applied to assess publication bias and sensitivity analysis was performed to determine the influence of individual studies on the overall estimate.
3 Results
3.1 Comparison of the vitamin D between case and control groups
The average of vitamin D level in the case and control group was examined in 12 evidence (Table 2). In 11 studies, the SMD of vitamin D in the case group was lower than the control group, while in 10 studies, the observed differences were statistically significant. According to the results of heterogeneity indices (I-square: 91.84%, Q: 134.86, P-value < 0.001), the heterogeneity between primary studies was high. Using DerSimonian–Laird method and a random effect model, the results of 12 primary studies were combined, and the standardized mean difference (SMD) vitamin D level in the COVID-19 children compared to the control group was estimated to be −0.88 (98% CI: −1.24, −0.51), which was statistically significant (Figure 2).

Figure 2. The pooled estimates of SMD of vitamin D between the case and control groups according to primary studies and overall estimate with a 95% confidence interval.
Based on the funnel plot diagram (Figure 3) and the results of Egger's test (β = 1.74, P-value: 0.551), it can be stated that there is no publication bias to estimate the SMD of vitamin D between the case and the control group. Also, the results of the sensitivity analysis show that there are no differences between the effect of each primary study on the overall estimate of SMD of vitamin D between the case and control groups (Figure 4).
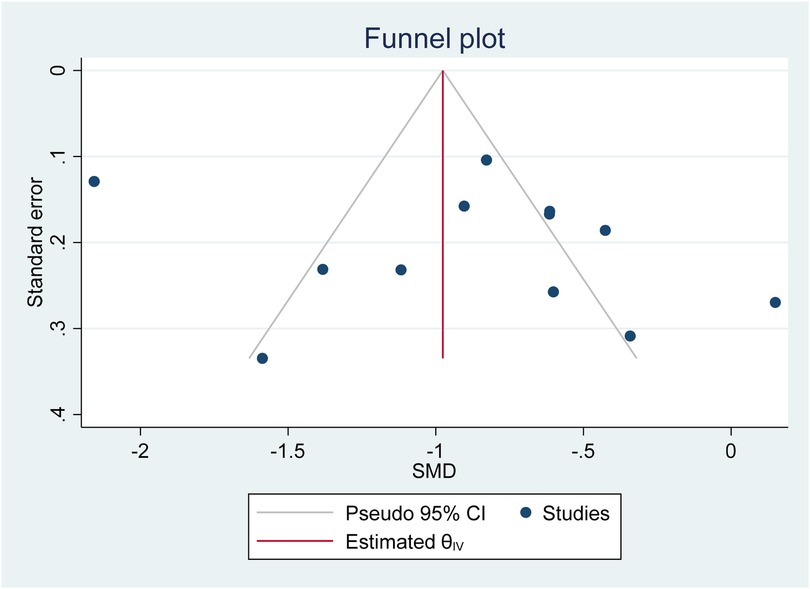
Figure 3. Investigate the publication bias of primary studies in estimating the SMD of vitamin D between the case and control group.
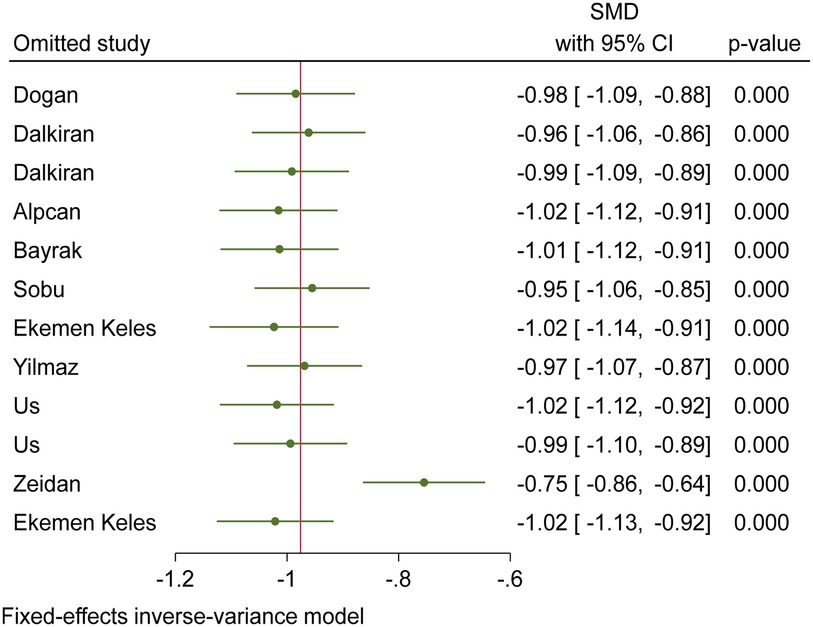
Figure 4. Sensitivity analysis chart to investigate the effect of each of the primary studies on the overall estimation of SMD of vitamin D between the case and control group.
3.2 Vitamin D and COVID-19 severity
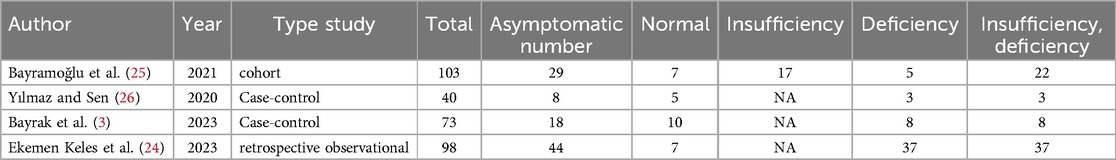
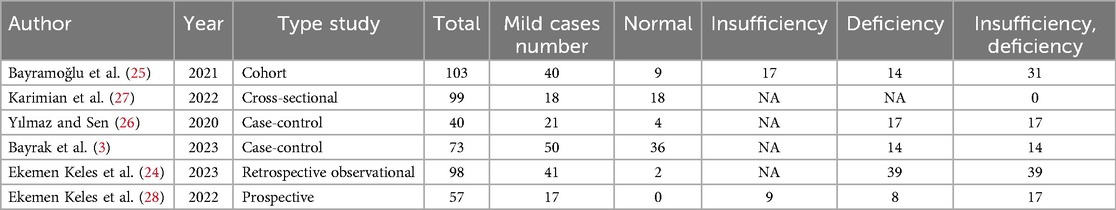
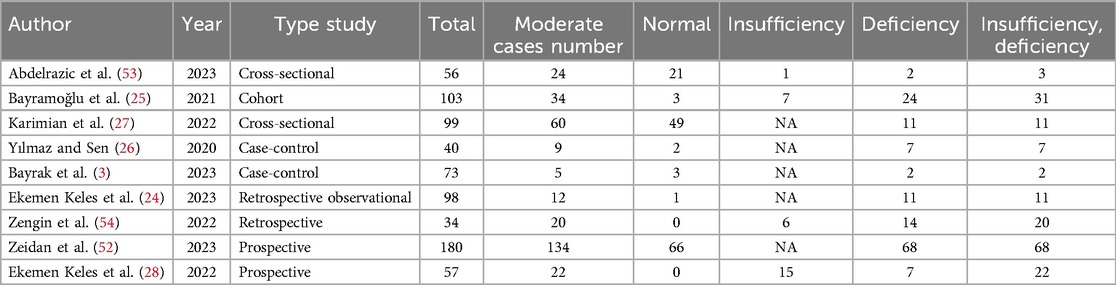
Vitamin D (deficiency, insufficiency, and normal) was evaluated in COVID-19 children (Tables 3–6). The comparison of vitamin D deficiency between children with moderate COVID-19 compared to asymptomatic children with COVID-19 was investigated in four primary studies. In three of these studies, the odds ratio for moderate COVID-19 in children with vitamin D deficiency [ranged from 0.83 in Ekemen Keles (24) to 11.2 in Bayramoglu's study (25)] was higher than in asymptomatic children with COVID-19. Differences were statistically significant only in the study of Bayramoglu et al. (25). By combining the results of these studies, the odds ratio for contracting vitamin D deficiency among children with moderate COVID-19 (OR: 3.58, 95% CI: 1.10, 11.63) was 3.58 times that of asymptomatic children; which is statistically significant. It should be noted that the heterogeneity between the studies was not significant (I-square: 29.35%, Q: 4.25, P-value: 0.24) (Figure 5).
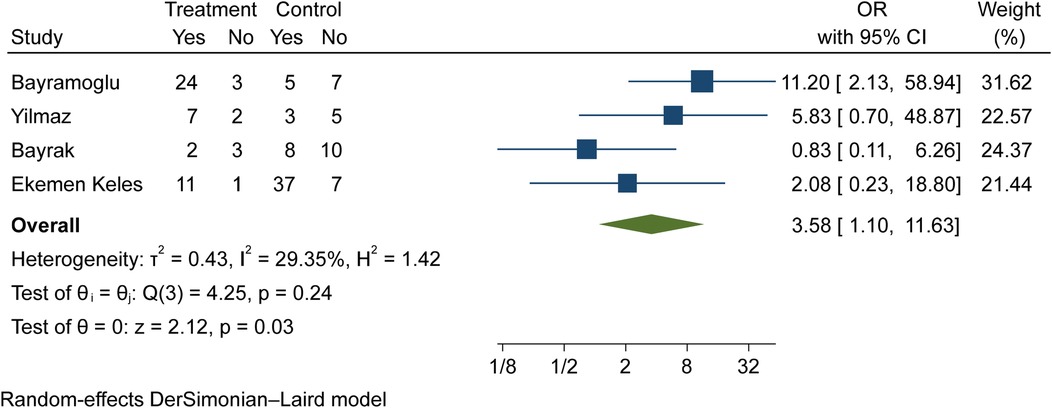
Figure 5. Comparison of vitamin D deficiency among children with moderate COVID-19 compared to the asymptomatic children with COVID-19.
The comparison of vitamin D disorder in children with moderate COVID-19 compared to asymptomatic children with COVID-19 was investigated in four primary studies. In three of these studies, the odds ratio for moderate COVID-19 among children with vitamin D disorder was higher (from 0.83 in the Ekemen Keles study (24) to 5.83 in Yilmaz's study (26)) than in asymptomatic children with COVID-19. Despite the high effect size, statistically significant differences were not observed in the primary studies. By combining the results of these studies, the odds ratio of contracting moderate COVID-19 among children with vitamin D disorder (OR: 2.52, 95% CI: 0.99, 6.41) was 2.52 times that of asymptomatic children with COVID-19, which is not statistically significant. It should be noted that the heterogeneity between the studies was not significant (I-square: 0%, Q: 1.91, P-value: 0.59) (Figure 6).
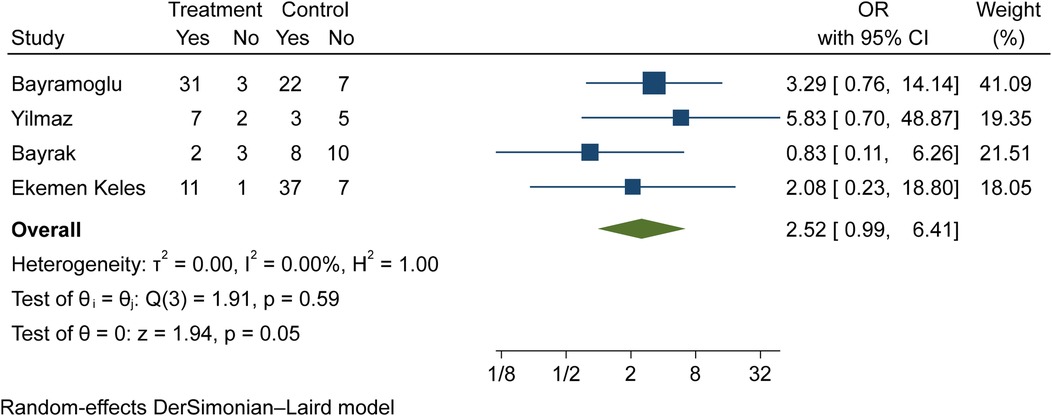
Figure 6. Comparison of vitamin D disorder (insufficiency and deficiency) among children with moderate COVID-19 compared to the asymptomatic children with COVID-19.
The comparison of vitamin D deficiency in children with moderate COVID-19 compared to children with mild COVID-19 was investigated in six primary studies. In three of these studies, the odds ratio of contracting moderate COVID-19 in children with vitamin D deficiency [ranged from 1.71 in Bayrak's study (3) to 8.59 in Karimian's study (27)] was higher than in mild COVID-19 group. Although the observed differences were significant only in the study of Bayramoglu et al. (25). Also, the study of Ekemen Keles (28) has been excluded from the analysis. By combining the results of the preliminary study, the odds ratio of contracting moderate COVID-19 in children with vitamin D deficiency (OR: 2.12, 95% CI: 0.90, 4.98) was 2.12 times that of children with mild COVID-19, which is not statistically significant. It should be noted that the heterogeneity between the studies was not significant (I-square: 0%, Q: 4.57, P-value: 0.47) (Figure 7).
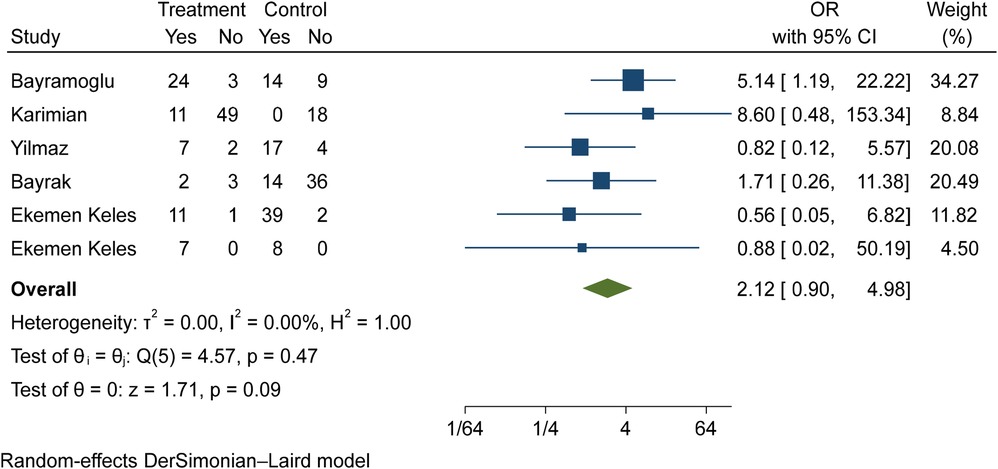
Figure 7. Comparison of vitamin D deficiency among children with moderate COVID-19 compared to the children with mild COVID-19.
The comparison of vitamin D disorder in children with moderate COVID-19 compared to children with mild COVID-19 was investigated in six primary studies. In three of these studies, the odds ratio of moderate COVID-19 in children with vitamin D disorder was higher [ranging from 1.71 in Bayrak's study (3) to 8.59 in Karimian's study (27)] than in children with mild COVID-19. There were no statistically significant differences between the studies. Also, the study of Ekemen Keles (28) has been excluded from the analysis. By combining the results of the preliminary study, the odds ratio of contracting moderate COVID-19 in children with vitamin D disorder (OR: 1.82, 95% CI: 0.78, 4.22) was 1.82 times that of children with mild COVID-19, which is not statistically significant. It should be noted that the heterogeneity between the studies was not significant (I-square: 0%, Q: 3.15, P-value: 0.68) (Figure 8).
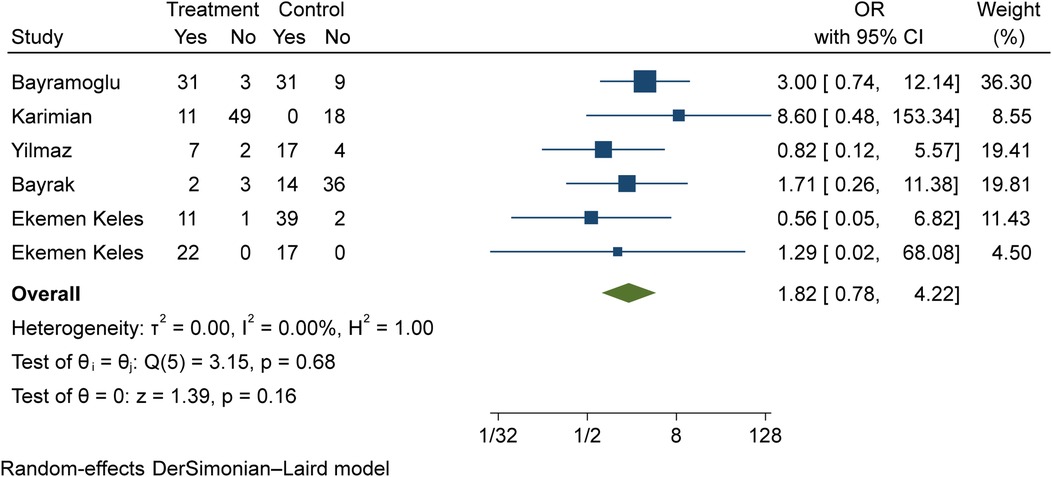
Figure 8. Comparison of vitamin D disorder among children with moderate COVID-19 compared to the children with mild COVID-19.
The comparison of vitamin D deficiency in children with mild COVID-19 compared to asymptomatic children with COVID-19 was investigated in four primary studies. In three studies, the odds ratio of mild COVID-19 in children with vitamin D deficiency [ranged from 2.18 in Bayramoglu's study (25) to 7.08 in Yilmaz's study (26)] was higher than in asymptomatic children with COVID-19. Although the observed differences were significant only in the study of Yilmaz et al. (26). By combining the results of these studies, the odds ratio of contracting mild COVID-19 in children with vitamin D deficiency (OR: 2.02, 95% CI: 0.60, 6.78) was 2.02 times that of asymptomatic children with COVID-19, which is not statistically significant. It should be noted that the heterogeneity between studies has been significant (I-square: 63.36%, Q: 8.19, P-value: 0.04) (Figure 9).
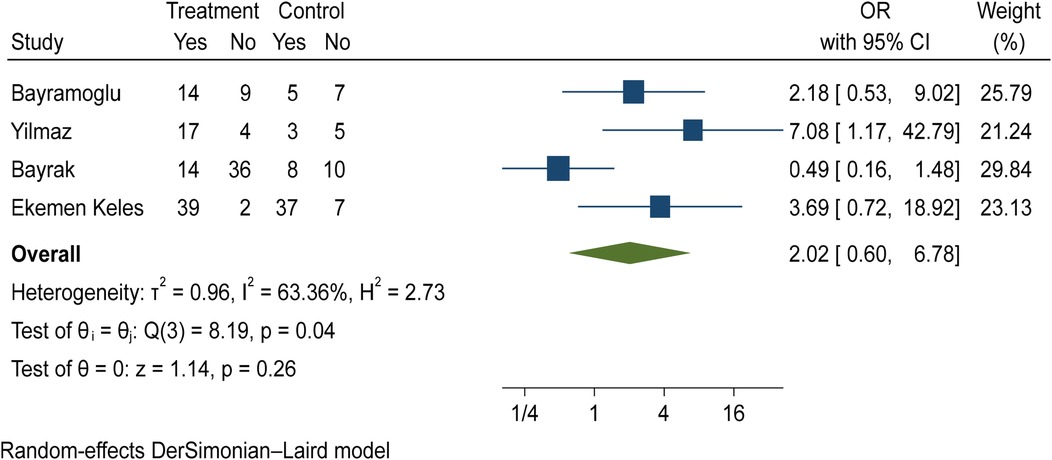
Figure 9. Comparison of vitamin D deficiency among children with mild COVID-19 compared to asymptomatic children with COVID-19.
The comparison of vitamin D disorder between children with mild COVID-19 compared to asymptomatic children with COVID-19 was investigated in four primary studies. In three of these studies, the odds ratio of mild COVID-19 among children with vitamin D disorder was higher [ranging from 1.09 in Bayramoglu's study (25) to 7.08 in Yilmaz's study (26)] than in asymptomatic children with COVID-19. Although the observed differences were significant only in the study of Yilmaz et al. (26). By combining the results of these four primary studies, the odds ratio of mild COVID-19 in children with vitamin D disorder (OR: 1.64, 95% CI: 0.53, 5.07) was 1.64 times that of asymptomatic children with COVID-19, which is not statistically significant. It should be noted that the heterogeneity between studies has been significant (I-square: 62.73%, Q: 8.05, P-value: 0.04) (Figure 10).
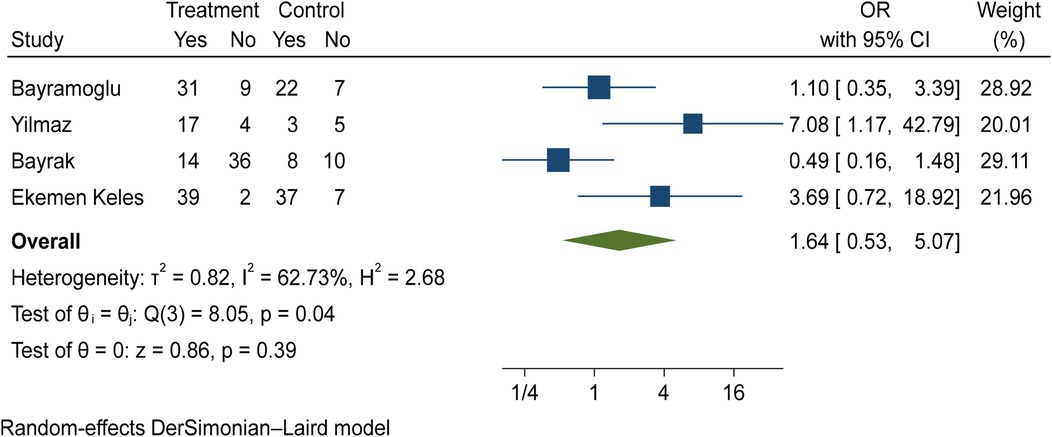
Figure 10. Comparison of vitamin D disorder among children with mild COVID-19 compared to asymptomatic children with COVID-19.
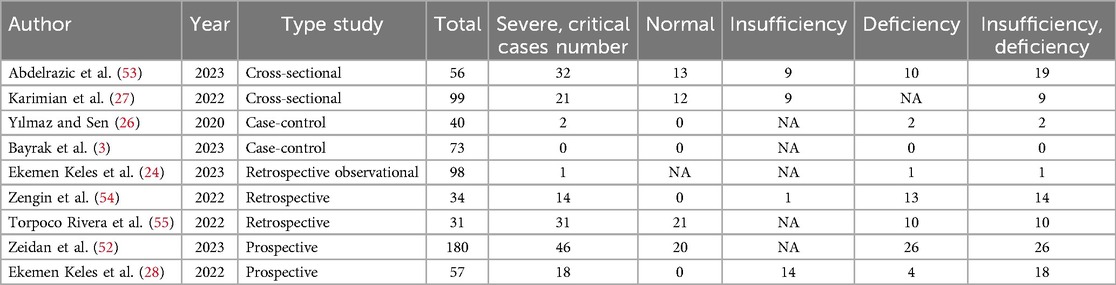
The comparison of vitamin D disorder between children with severe COVID-19 and those with mild COVID-19 has been extracted from three primary studies. Ekemen Keles' study was not investigated because all children with severe COVID-19 (18 out of 18 people) and mild COVID-19 (17 out of 17 people) were in the vitamin D disorder group. In two studies [Karimian et al.'s study (27) and Yilmaz et al.'s study (26)], the odds ratio of severe COVID-19 among children with vitamin D deficiency was higher than among children with mild COVID-19. The odds ratio in Karimian et al. (27) and Yilmaz's studies (26) were 28.12 and 1.29 respectively and the differences observed in Karimian et al.'s (27) study were statistically significant. It should be noted that because the target group of this study was children, the incidence of severe COVID-19 was low. Therefore, the evidence of vitamin D disorder was not high.
Discussion
Our study evaluated the status of vitamin D between children with COVID-19 and healthy groups. The SMD of vitamin D in the COVID-19 children compared to the control group was estimated to be −0.88 (98% CI: −1.24, −0.51), which was statistically significant. Also, the odds ratio of moderate COVID-19 among children with vitamin D disorder was higher than in asymptomatic children with COVID-19.
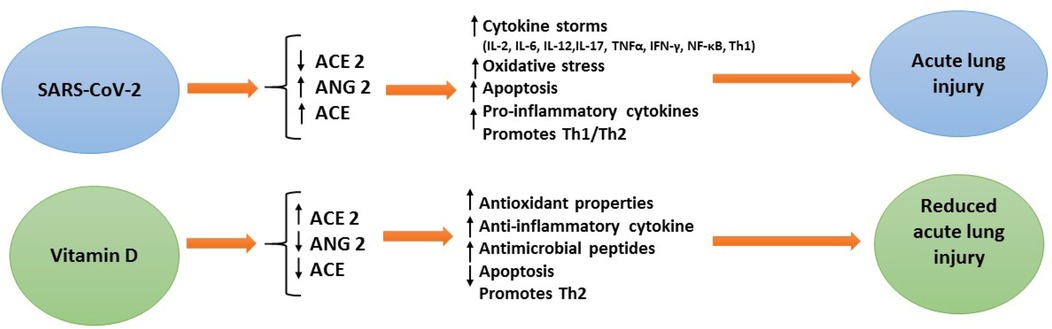
Vitamin D deficiency is emerging as a global public health problem worldwide. In some countries, vitamin D supplementation has been proposed as a preventive measure for respiratory tract infections (RTIs) in children (26, 29). It has been shown that 1,25-OH2-vitamin D inhibits cellular infection in human nasal epithelial cells and can fight SARS-CoV-2 infections (30). Vitamin D has numerous effects in modulating the immune response. The expression of the vitamin D receptor (VDR) in several immune cells such as dendritic cells, antigen-presenting cells, neutrophils, B cells and T cells, regulates both innate and adaptive immunity (31). It has been reported that patients with COVID-19 reduce plasma cells, pro-inflammatory cytokines [including IL-6, IL-10, granulocyte-colony stimulating factor, macrophage inflammatory protein, tumor necrosis factor-α (TNF-α)] (32), and vitamin D has been shown to increase macrophages' response, anti-inflammatory cytokine production, and activating Toll-like receptors and thus suppress the cytokine storm and pathogenic inflammation during COVID-19 infection (33, 34) (Figure 11).
In addition, it has been reported that the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein enters host cells after it binds to angiotensin-converting enzyme 2 (ACE2) receptors (35). Vitamin D can balance the inflammatory response by increasing the expression and concentration of ACE2, which has anti-inflammatory properties and is capable of trapping and inactivating virus particles. Genetic variations in the vitamin D receptor (VDR) gene or ACE2 may also affect COVID-19 disease outcomes (36). A study (37) suggested that sufficient vitamin D boosts the innate immune system and improves the adaptive immune response so reduces the risk of acute respiratory tract infection (such as respiratory syncytial virus, tuberculosis, and influenza) (12, 38). Yilmaz and Şen (26) investigated the correlation between vitamin D and COVID-19 in a pediatric age group. They also showed that vitamin D was significantly lower in COVID-19 patients than in the controls. Martineau et al. (39) reported that 25(OH)-D vitamin is generally protective against acute respiratory tract infection. In the study of Li et al. (40) with 1,582 children, the group with community-acquired pneumonia was compared with the control group, and the low 25(OH)-D vitamin was found to be lower in the group with community-acquired pneumonia. In addition, in a case-control study conducted by Velarde López et al. (41) 25(OH)-D vitamin deficiency was found to be significantly low in children with lower respiratory tract infections. Previous studies in critically ill children have shown an association between severe disease and vitamin D deficiency (26, 27). Moreover, a recent study reported that a single dose of vitamin D given to pediatric patients with vitamin D deficiency could reduce the incidence of septic shock in specific patient populations (42) Akoğlu et al. (43) also found that the 25-hydroxyvitamin D3 (vitamin D3) was significantly lower in the moderately severe disease group than the mild disease group (P = 0.044).
A study evaluating Indian children found that acute lower respiratory infection was significantly higher among children with vitamin D deficiency in a six-month follow-up time (44). Banajeh investigated 79 cases between 2 and 59 months and found that vitamin D deficiency was associated with reduced circulating neutrophils and hypoxemia (45). Akoğlu et al. (43) reported that patients with moderate COVID-19 severity had lower 25(OH) D as compared to the mild disease group. An epidemiological study reported that 25(OH) D < 20 ng/ml almost doubled the risk for SARS-CoV-2 infection and hospitalization (46). Merzon et al. defined in their study that <30 ng/ml was a risk factor for infection and hospitalization, independent of demographic characteristics and previous medical conditions (46). In the study conducted by Carpagnano et al. (47), mortality was shown to be 50% higher in patients hospitalized with COVID-19 infection with acute respiratory failure in patients with 25(OH)-D < 10 ng/ml. During the COVID-19 pandemic (except for the Omicron peak), children were less affected by the severity of COVID-19. Combining the results of these studies, the effect size of the relationship between vitamin D and COVID-19 severity in children is significant. The standardized mean difference (SMD) vitamin D level in children with COVID-19 was significantly 0.88 units lower than the control group. Also, the odds ratio of moderate COVID-19 in children with vitamin D deficiency was significantly 3.58 times higher than in asymptomatic children with COVID-19.
Limitation
This study has some limitations:
At first, the relationship of zinc and vitamin D to disease incidence, contribution to treatment and clinical severity was not examined, which can be the subject of a new approach. Second, weight and some clinical symptoms such as abdominal pain, pharyngeal pain, loss of taste, and myalgia cannot be evaluated in all groups. Third, most studies were from Turkey and Egypt. Therefore, this limitation should be explained in the interpretation of the results.
Another limitation of the present study is that there is a possibility that vitamin D in the patients with COVID-19 was low before contracting the disease, and it is not possible to prove this in this meta-analysis due to the lack of access to vitamin D data.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
TM: Methodology, Writing – original draft. MM: Formal analysis, Methodology, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mousavi T, Moosazadeh M. Association of hospitalization rate, mortality, and CD4T cell count with comorbidity of COVID-19 and HIV: a systematic review and meta-analysis. AIDS Res Hum Retrovir. (2023) 39(7):332–9. doi: 10.1089/aid.2022.0076
2. Valadan R, Golchin S, Alizadeh-Navaei R, Haghshenas M, Zargari M, Mousavi T, et al. Differential gene expression analysis of common target genes for the detection of SARS-CoV-2 using real time-PCR. AMB Express. (2022) 12(1):112. doi: 10.1186/s13568-022-01454-2
3. Bayrak H, Öztürk D, Bolat A, Ünay B. Association between vitamin D levels and COVID-19 infection in children: a case-control study. Turk Arch Pediatr. (2023) 58(3):250. doi: 10.5152/TurkArchPediatr.2023.22217
4. Choi S-H, Kim HW, Kang J-M, Kim DH, Cho EY. Epidemiology and clinical features of coronavirus disease 2019 in children. Clin Exp Pediatr. (2020) 63(4):125. doi: 10.3345/cep.2020.00535
5. Cui X, Zhao Z, Zhang T, Guo W, Guo W, Zheng J, et al. A systematic review and meta-analysis of children with coronavirus disease 2019 (COVID-19). J Med Virol. (2021) 93(2):1057–69. doi: 10.1002/jmv.26398
6. Mehraeen E, Oliaei S, SeyedAlinaghi S, Karimi A, Mirzapour P, Afsahi AM, et al. COVID-19 in pediatrics: a systematic review of current knowledge and practice. Infect Disord Drug Targets. (2022) 22(5):47–57. doi: 10.2174/1871526521666210929121705
7. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395(10229):1054–62. doi: 10.1016/S0140-6736(20)30566-3
8. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. (2020) 323(13):1239–42. doi: 10.1001/jama.2020.2648
9. Hughes D, Norton R. Vitamin D and respiratory health. Clin. Exp. Immunol. (2009) 158(1):20–5. doi: 10.1111/j.1365-2249.2009.04001.x
10. Alpcan A, Tursun S, Kandur Y. Vitamin D levels in children with COVID-19: a report from Turkey. Epidemiol Infect. (2021) 149:e180. doi: 10.1017/S0950268821001825
11. Jain SK, Parsanathan R. Can vitamin D and L-cysteine co-supplementation reduce 25 (OH)-vitamin D deficiency and the mortality associated with COVID-19 in African Americans? J Am Coll Nutr. (2020) 39(8):694–9. doi: 10.1080/07315724.2020.1789518
12. Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. (2020) 12(4):988. doi: 10.3390/nu12040988
13. Fahrni O, Wilhelm-Bals A, Posfay-Barbe KM, Wagner N. Hypovitaminosis D in migrant children in Switzerland: a retrospective study. Eur J Pediatr. (2021) 180(8):2637–44. doi: 10.1007/s00431-021-04143-7
14. Meltzer DO, Best TJ, Zhang H, Vokes T, Arora V, Solway J. Association of vitamin D status and other clinical characteristics with COVID-19 test results. JAMA Netw Open. (2020) 3(9):e2019722. doi: 10.1001/jamanetworkopen.2020.19722
15. Beyazgül G, Bağ Ö, Yurtseven İ, Coşkunol F, Başer S, Çiçek D, et al. How vitamin D levels of children changed during COVID-19 pandemic: a comparison of pre-pandemic and pandemic periods. J Clin Res Pediatr Endocrinol. (2022) 14(2):188. doi: 10.4274/jcrpe.galenos.2022.2021-10-6
16. Yu L, Ke H-J, Che D, Luo S-L, Guo Y, Wu J-L. Effect of pandemic-related confinement on vitamin D status among children aged 0–6 years in Guangzhou, China: a cross-sectional study. Risk Manag Healthc Policy. (2020) 13:2669–75. doi: 10.2147/RMHP.S282495
17. Cavarzere P, Pausilli R, Nicolussi Prinicpe L, Gaudino R, Guzzo A, Cantalupo G, et al. Decreased vitamin D levels in the pediatric population after COVID-19 lockdown. Ital J Pediatr. (2023) 49(1):113. doi: 10.1186/s13052-023-01515-7
18. Yoldaş MA, Atasoy Hİ. The importance of vitamins in pediatric COVID-19 patients. Northwestern Med J. (2022) 2(2):123–8. doi: 10.54307/NWMJ.2022.58077
19. Rustecka A, Maret J, Drab A, Leszczyńska M, Tomaszewska A, Lipińska-Opałka A, et al. The impact of COVID-19 pandemic during 2020–2021 on the vitamin D serum levels in the paediatric population in Warsaw, Poland. Nutrients. (2021) 13(6):1990. doi: 10.3390/nu13061990
20. Katz J, Yue S, Xue W. Increased risk for COVID-19 in patients with vitamin D deficiency. Nutrition. (2021) 84:111106. doi: 10.1016/j.nut.2020.111106
21. Hussein AM, Galal I, Amin M, Moshnib A, Makhlouf N, Makhlouf H, et al. Prevalence of vitamin D deficiency among patients attending post COVID-19 follow-up clinic: a cross-sectional study. Eur Rev Med Pharmacol Sci. (2022) 26(8):3038–45. doi: 10.26355/eurrev_202204_28635
22. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
23. Moosazadeh M, Mousavi T. Combination therapy of tocilizumab and steroid for COVID-19 patients: a meta-analysis. J Med Virol. (2022) 94(4):1350–6. doi: 10.1002/jmv.27489
24. Keles YE, Ciftdoğan DY, Colak A, Aksay AK, Sahin A, Ustundag G, et al. Vitamin D concentrations in pediatric patients with COVID-19. Top Clin Nutr. (2023) 38(4):285–93. doi: 10.1097/TIN.0000000000000351
25. Bayramoğlu E, Akkoç G, Ağbaş A, Akgün Ö, Yurdakul K, Selçuk Duru HN, et al. The association between vitamin D levels and the clinical severity and inflammation markers in pediatric COVID-19 patients: single-center experience from a pandemic hospital. Eur J Pediatr. (2021) 180:2699–705. doi: 10.1007/s00431-021-04030-1
26. Yılmaz K, Şen V. Is vitamin D deficiency a risk factor for COVID-19 in children? Pediatr Pulmonol. (2020) 55(12):3595–601. doi: 10.1002/ppul.25106
27. Karimian P, Tahami MS, Sayyahfar S, Delavar MA. Association of vitamin D and severity of COVID-19 in children. Eur J Transl Myol. (2022) 32(2):10453. doi: 10.4081/ejtm.2022.10453
28. Keleş YE, Yılmaz D, Taşar S, Üstündağ G, Şahin A, Tuz AE, et al. Can serum 25-hydroxy vitamin D levels predict the severity of multisystem inflammatory syndrome in children and COVID-19? J Clin Res Pediatr Endocrinol. (2023) 15(2):190. doi: 10.4274/jcrpe.galenos.2023.2022-10-1
29. Lips P, Cashman KD, Lamberg-Allardt C, Bischoff-Ferrari HA, Obermayer-Pietsch B, Bianchi ML, et al. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: a position statement of the European calcified tissue society. Eur J Endocrinol. (2019) 180(4):P23–54. doi: 10.1530/EJE-18-0736
30. Greiller CL, Martineau AR. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients. (2015) 7(6):4240–70. doi: 10.3390/nu7064240
31. Panfili F, Roversi M, D’argenio P, Rossi P, Cappa M, Fintini D. Possible role of vitamin D in COVID-19 infection in pediatric population. J Endocrinol Investig. (2021) 44:27–35. doi: 10.1007/s40618-020-01327-0
32. Zhou Y, Fu B, Zheng X, Wang D, Zhao C, Qi Y, et al. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl Sci Rev. (2020) 7(6):998–1002. doi: 10.1093/nsr/nwaa041
33. Ahmed N, Araf Y, Ullah M. Prospects of vitamin D in the treatment of COVID-19 patient and improving maternal and child health during pandemic. J Adv Biotechnol Exp Ther. (2021) 4(2):133–48. doi: 10.5455/jabet.2021.d114
34. Ghelani D, Alesi S, Mousa A. Vitamin D and COVID-19: an overview of recent evidence. Int J Mol Sci. (2021) 22(19):10559. doi: 10.3390/ijms221910559
35. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. (2020) 181(2):271–80.e8. doi: 10.1016/j.cell.2020.02.052
36. Cui C, Xu P, Li G, Qiao Y, Han W, Geng C, et al. Vitamin D receptor activation regulates microglia polarization and oxidative stress in spontaneously hypertensive rats and angiotensin II-exposed microglial cells: role of renin-angiotensin system. Redox Biol. (2019) 26:101295. doi: 10.1016/j.redox.2019.101295
37. Camargo CA Jr, Ganmaa D, Frazier AL, Kirchberg FF, Stuart JJ, Kleinman K, et al. Randomized trial of vitamin D supplementation and risk of acute respiratory infection in Mongolia. Pediatrics. (2012) 130(3):e561–e7. doi: 10.1542/peds.2011-3029
38. Zisi D, Challa A, Makis A. The association between vitamin D status and infectious diseases of the respiratory system in infancy and childhood. Hormones. (2019) 18:353–63. doi: 10.1007/s42000-019-00155-z
39. Martineau AR, Jolliffe DA, Greenberg L, Aloia JF, Bergman P, Dubnov-Raz G, et al. Vitamin D supplementation to prevent acute respiratory infections: individual participant data meta-analysis. Health Technol Assess (Rockv). (2019) 23(2):1. doi: 10.3310/hta23020
40. Li P-L, Tian Y-j, Wang Y-h, Zhang C-z, Gao J, Li Y-h, et al. The prevalence of vitamin D deficiency among schoolchildren: a cohort study from Xinxiang, China. J Pediatr Endocrinol Metab. (2015) 28(5–6):629–33. doi: 10.1515/jpem-2014-0250
41. Velarde Lopez AA, Gerber JS, Leonard MB, Xie D, Schinnar R, Strom BL. Children with lower respiratory tract infections and serum 25-hydroxyvitamin D3 levels: a case–control study. Pediatr Pulmonol. (2016) 51(10):1080–7. doi: 10.1002/ppul.23439
42. Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. Br Med J. (2017):356. doi: 10.1136/bmj.i6583
43. Akoğlu HA, Bulut M, Alemdar DK, Aslan S, Çelikkalkan K, Tursun S, et al. Evaluation of childhood COVID-19 cases: a retrospective analysis. J Pediatr Infect Dis. (2021) 16(03):091–8. doi: 10.1055/s-0041-1723957
44. Chowdhury R, Taneja S, Bhandari N, Sinha B, Upadhyay RP, Bhan MK, et al. Vitamin-D deficiency predicts infections in young north Indian children: a secondary data analysis. PLoS One. (2017) 12(3):e0170509. doi: 10.1371/journal.pone.0170509
45. Banajeh SM. Nutritional rickets and vitamin D deficiency—association with the outcomes of childhood very severe pneumonia: a prospective cohort study. Pediatr Pulmonol. (2009) 44(12):1207–15. doi: 10.1002/ppul.21121
46. Merzon E, Tworowski D, Gorohovski A, Vinker S, Golan Cohen A, Green I, et al. Low plasma 25 (OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study. FEBS J. (2020) 287(17):3693–702. doi: 10.1111/febs.15495
47. Carpagnano GE, Di Lecce V, Quaranta VN, Zito A, Buonamico E, Capozza E, et al. Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19. J Endocrinol Investig. (2021) 44(4):765–71. doi: 10.1007/s40618-020-01370-x
48. Doğan A, Dumanoğlu Doğan İ, Uyanık M, Köle MT, Pişmişoğlu K. The clinical significance of vitamin D and zinc levels with respect to immune response in COVID-19 positive children. J Trop Pediatr. (2022) 68(5):fmac072. doi: 10.1093/tropej/fmac072
49. Dalkiran T, Sevcan İ. The relation between severity of COVID-19 disease and vitamin D level in children. Kırıkkale Üniv Tıp Fak Derg. (2023) 25(1):122–7. doi: 10.24938/kutfd.1261074
50. Sobu E, Karaaslan A, Cetin C, Akin Y. Vitamin D levels of COVID-19 positive sypmtomatic pediatric cases/COVID-19 pozitif semptomatik qocuklarin D vitamini duzeyleri. J Curr Pediatr. (2021) 19(1):9–15. doi: 10.4274/jcp.2021.0002
51. Us MC, Devrim Lanpir A, Özdatli Kurtuluş Ş, Yagci M, Akarsu Ö, Şahin K, et al. The role of free vitamin D and vitamin D binding protein in SARS-Cov-2 infection in children. Pediatr Int. (2023) 65(1):e15680. doi: 10.1111/ped.15680
52. Zeidan NM, Lateef HMAE, Selim DM, Razek SA, Abd-Elrehim GA, Nashat M, et al. Vitamin D deficiency and vitamin D receptor FokI polymorphism as risk factors for COVID-19. Pediatr Res. (2023) 93(5):1383–90. doi: 10.1038/s41390-022-02275-6
53. Abdelrazic MI, Rateeb AM, Eid WA, Abdelrazik EF, Abuelela IS. Impact of vitamin D deficiency on the severity of COVID 19 infection in pediatrics: a cross-sectional study. Egypt Paediatr Assoc Gaz. (2023) 71(1):37. doi: 10.1186/s43054-023-00185-8
54. Zengin N, Bal A, Goren TA, Bayturan SS, Alkan F, Akcali S. Serum vitamin D levels in relation to development of multisystem inflammatory syndrome in pediatric COVID-19. J Pediatr Infect Dis. (2022) 17(06):308–16. doi: 10.1055/s-0042-1756713
Keywords: vitamin D, children, COVID-19, SARS coV-2, pneumonia
Citation: Mousavi T and Moosazadeh M (2025) Vitamin D status in children with mild, moderate, or severe confirmed COVID-19: systematic-review and meta-analysis. Front. Pediatr. 13:1436633. doi: 10.3389/fped.2025.1436633
Received: 22 May 2024; Accepted: 22 April 2025;
Published: 13 May 2025.
Edited by:
Tim S. Nawrot, University of Hasselt, BelgiumReviewed by:
Otilia Marginean, Victor Babes University of Medicine and Pharmacy, RomaniaAngela Klain, University of Campania Luigi Vanvitelli, Italy
Paraskevi Theocharis, Guy's and St Thomas' NHS Foundation Trust, United Kingdom
Copyright: © 2025 Mousavi and Moosazadeh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mahmood Moosazadeh, bW1vb3NhemFkZWgxMzUxQGdtYWlsLmNvbQ==
 Tahoora Mousavi
Tahoora Mousavi Mahmood Moosazadeh
Mahmood Moosazadeh