- 1Department of Pediatrics, Longquanyi District of Chengdu Maternity & Child Health Care Hospital, Chengdu, Sichuan, China
- 2Division of Vascular Surgery, Department of General Surgery, West China Hospital, Sichuan University, Chengdu, China
- 3Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, Department of Pediatrics, West China Second University Hospital, Chengdu, China
- 4Department of Pediatrics, West China Second University Hospital, West China Second University Hospital-Tianfu·Sichuan Provincial Children’s Hospital, Sichuan, China
Background: Cyanosis is a common clinical finding in infants and children. Particularly, central cyanosis can be associated with significant and potentially life-threatening diseases. Acquired methemoglobinemia is a rare but severe or even fatal cause of cyanosis in infants. Due to its rarity, timely diagnosis and appropriate management, particularly in infants, can be challenging in a clinical setting.
Case report: We report the case of a previously healthy 49-day-old female infant who presented with central cyanosis. Four hours prior to presentation, her guardian had inappropriately prepared her milk formula using spinach juice. This critical clue hinted to us that this infant might suffer from acquired methemoglobinemia. Upon blood sampling, her blood appeared chocolate brown in color. Furthermore, arterial blood gas analysis revealed abnormal findings, with a significantly elevated percentage of methemoglobinemia at 44.7%. Regarding the history of inappropriate formula preparation using vegetable juice and the abnormal finding of methemoglobinemia, a diagnosis of acquired methemoglobinemia was proposed. Other causes of methemoglobinemia were further excluded. Treatment with methylene blue and vitamin C was immediately initiated. Encouragingly, the cyanosis of this infant resolved 1 h later, with normal results of the repeated blood gas analysis. This infant was discharged home 2 days later and had no abnormal findings during the follow-up.
Conclusion: In this study, we reported a rare case of acquired methemoglobinemia in a 49-day-old infant. Inappropriate preparation of the infant milk formula with spinach juice was the potential cause of methemoglobinemia in this case, which presented with central cyanosis. Our findings also suggested that pediatricians should be aware of acquired methemoglobinemia as a potential cause of cyanosis in infants.
Introduction
Cyanosis is a bluish-purple discoloration of the tissues caused by an increased concentration of deoxygenated hemoglobin in the capillary bed, resulting from various conditions (1). This condition is a common clinical finding in infants and children. Particularly, central cyanosis can be associated with significant and potentially life-threatening diseases. Therefore, it is crucial to identify the underlying causes of cyanosis in the clinical setting, which is essential for appropriate management. The causes of cyanosis in infants include respiratory, pulmonary, cardiac, metabolic, neurologic, infectious, and hematologic disorders (2). Respiratory causes most often account for previously healthy infants with the new onset of central cyanosis (1). Cardiac and pulmonary vascular etiologies might also be responsible for cyanosis, particularly in young infants. Moreover, other severe conditions produce central cyanosis through disordered breathing caused by neurologic disease or decreased ability for hemoglobin to carry oxygen, such as methemoglobinemia.
Methemoglobinemia is a rare disorder associated with the oxidation of Fe2+ of hemoglobin (Hb) to Fe3+ of methemoglobin. The presence of the Fe3+ state results in allosteric changes that irreversibly permit oxygen binding. As a result, the corresponding ferroglobins in the tetramer shift the oxygen dissociation curve of Hb to the left. This shift leads to increased affinity of the ferrous iron for oxygen, thus impairing oxygen release to the tissue, resulting in hypoxia with the so-called functional anemia without a decrease in Hb. Regarding the causes of methemoglobinemia, it can be classified into congenital or acquired forms. Congenital methemoglobinemia is caused by autosomal recessive variants in the CYB5R3 gene and autosomal dominant variants in the globin genes known as HbM disease (3–5). Patients with congenital methemoglobinemia are asymptomatic except for cyanosis, but some patients may have severe morbidity which can be fatal in neonates. As the most common form of methemoglobinemia, patients with acquired methemoglobinemia mainly suffer from various exogenous substances that increase methemoglobin formation, including dapsone, antimalarial agents, topical anesthetics, inhaled nitric oxide, rasburicase, nitrates, and nitrites (5–14). Notably, acquired methemoglobinemia can be severe or even fatal, depending on the proportion of methemoglobin. Given the rarity of this disorder, the diagnosis of methemoglobinemia often remains delayed in clinical settings; therefore, the appropriate management, particularly in infants, may be challenging (5, 15).
Herein, we report a rare case of acquired methemoglobinemia in a 49-day-old infant who presented with central cyanosis, attributed to inappropriate preparation of milk formula with spinach juice. Pediatricians need to recognize acquired methemoglobinemia as a potential cause of cyanosis in infants.
Case presentation
A previously healthy 49-day-old female (Chinese, Han ethnicity) was admitted to the pediatric emergency department of our hospital with her parents reporting she presented with cyanosis persisting for 4 h. She was immediately transferred to our inpatient department under nasal cannula oxygen inhalation. On arrival, the physical examination revealed a body temperature of 36.0°C, heart rate of 158 beats per minute, blood pressure of 125/62 mmHg, respiratory rate of 54 per minute, and initial SaO2 of 77%. Central cyanosis was noted, which was evident in the lips, nail beds, earlobes, and mucous membranes (Figure 1A). Capillary refill time and skin temperature were normal. The skin exhibited normal elasticity, without cutaneous mottling, petechiae, and ecchymoses. Pupils were sensitive to light reflex, the anterior fontanelle was flat, and the sign of three depressions was negative. On auscultation, heart sounds were normal, with no murmurs. Neurological examination revealed no signs of meningeal irritation or positive Babinski reflexes, and both patellar and ankle reflexes were normal. No other abnormalities were found in the physical examinations. Moreover, no apparent abnormalities were found during her fetal period. After her birth, the infant had good formula feeding and normal growth and development. The infant had not been exposed to any suspected drugs or toxins, did not have diarrhea, or was undernourished. Her neonatal screening was normal, including G6PD deficiency. Her mother denied a history of respiratory, cardiovascular, and neurological disease, and no family members had a history of related diseases. At the same time, the people living in the same household have shown no relevant clinical manifestations. Notably, 4 h ago, this infant's guardian inappropriately prepared the infant's milk formula with spinach juice. The spinach juice was prepared in advance and kept in the refrigerator for 12–24 h. The mean level of nitrate concentration in Spinacia in our area was 1–2 mg/kg. This critical clue hinted that this infant might suffer from acquired methemoglobinemia, associated with nitrates and nitrites from vegetable juice, which increases methemoglobin formation. Moreover, her blood presented with a chocolate-brown color (Figure 1C) when drawn for laboratory tests, compared with its color when clinical conditions recovered (Figure 1D) and consistent with the color change of blood among patients with methemoglobinemia (Figure 1E) (16).
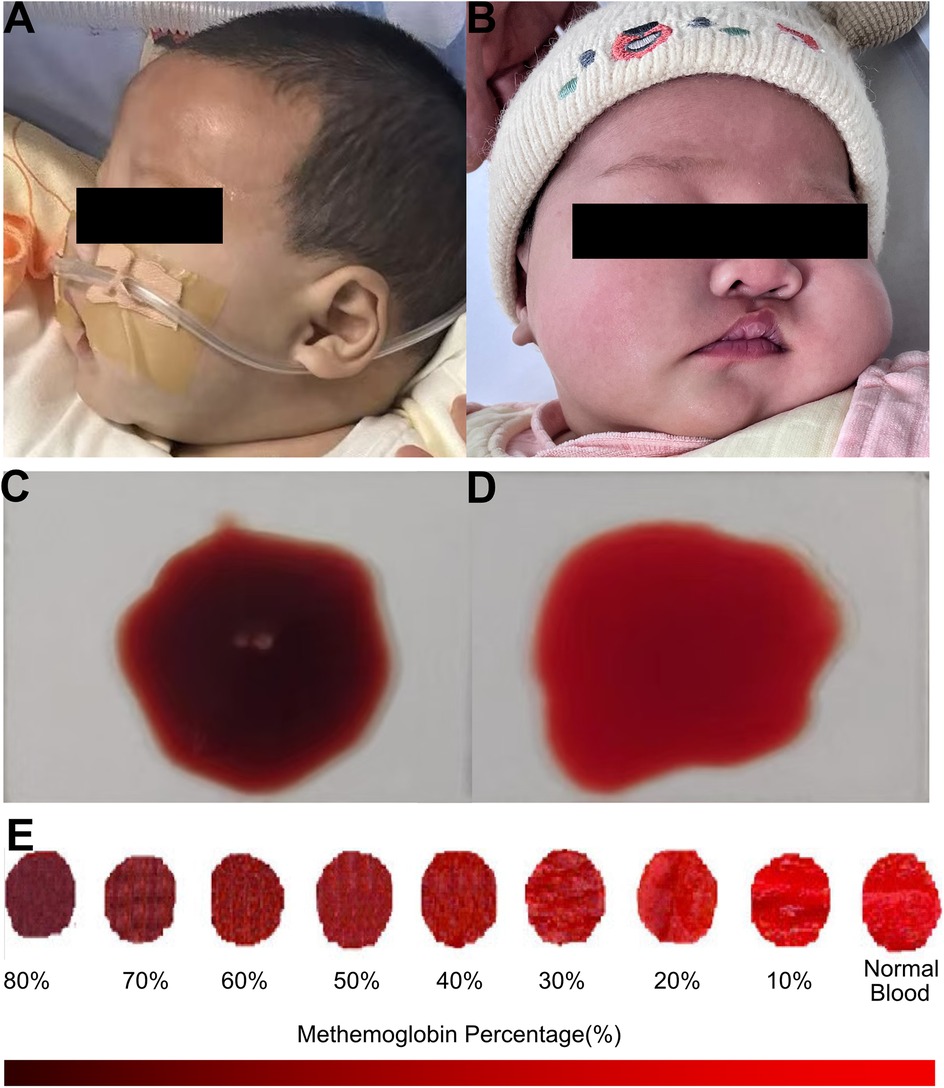
Figure 1. Clinical findings of the infant with acquired methemoglobinemia. (A) This infant presented with central cyanosis (B) and had no abnormal findings during the follow-up. (C) Blood presented with a chocolate-brown color. (D) Blood color was normal when clinical conditions recovered. (E) An obvious change of blood in color from red to dark brown among patients with methemoglobinemia, reproduced with the free permission of Copyright © 2009 American College of Emergency Physicians (16).
Furthermore, laboratory tests showed abnormal findings of arterial blood gas analysis, including a significantly elevated percentage of methemoglobinemia at 44.7% (normal range, 0%–6%) and decreased level of Hb was 91 g/L (normal range, 120–145 g/L), with a normal level of pH value, partial pressure of carbon dioxide (PaCO2), partial pressure of oxygen (PaO2), bicarbonate (HCO3−), and base excess or deficit. The diagnosis of acquired methemoglobinemia was proposed, based on the history of inappropriately preparing the infant formula using vegetable juice and the abnormal finding of methemoglobinemia. Although clinicians must identify a hereditary cause for methemoglobinemia, the treatment of the acute patient must be initiated as soon as possible which does not require the distinction between acquired and inherited causes at the same time (17). Therefore, the emergency treatment of methylene blue was immediately initiated with a dose of 1.5 mg/kg, in combination with vitamin C (1,000 mg twice a day) and nasal cannula oxygen inhalation (0.5 L/min). Encouragingly, the cyanosis of this infant resolved 1 h later. At the same time, other causes of acquired methemoglobinemia were further excluded by the unremarkable findings of blood routine test, C reaction protein (CRP), erythrocyte sedimentation rate (ESR), procalcitonin (PCT), liver function, renal function, blood creatase levels, blood glucose, blood ammonia, and blood fat. In addition, other causes of cyanosis in infants were further excluded, such as respiratory causes (such as upper airway obstruction, bronchiolitis, pneumonia, pneumothorax), circulatory causes (congenital heart disease, pulmonary hypertension, pulmonary hemorrhage), neurologic causes, by negative findings of chest x-ray, echocardiography, electrocardiogram, and physical examination of the respiratory, cardiovascular, and neurological system. Moreover, efforts were made to classify her methemoglobinemia as the acquired form as her family members did not present with a long-life history of cyanosis, dusky skin, or blue sclera, which might suggest the congenital form. Regrettably, her parents refused whole-exome sequencing.
Encouragingly, the cyanosis of this infant resolved 1 h later after the therapy of methylene blue and Vitamin C was carried out. The repeated blood gas analysis also recovered to normal. In turn, the outcome of this infant also confirmed our primary diagnosis of acquired methemoglobinemia. During the follow-up, this infant was discharged home 2 days later and had no abnormal findings of skin color, arterial blood gas, or blood routine test (Figure 1B).
Discussion
Methemoglobinemia is a rare cause of cyanosis in children, and clinicians have widely recognized its clinical manifestations. However, this disorder is rarely found in infants, especially those younger than 6 months old. Notably, the clinical presentations of younger infants with acquired methemoglobinemia might be more severe. They had low stomach acid production, large numbers of nitrite-reducing bacteria, the relatively easy oxidation of fetal hemoglobin, and immaturity of the methemoglobin reductase system (18–20). In this study, we described a rare case of a 49-day-old infant who developed acquired methemoglobinemia, presenting with central cyanosis, due to the inappropriate preparation of her milk formula with spinach juice. Encouragingly, her clinical conditions resolved 1 h after receiving the therapy of methylene blue and Vitamin C. Two days later, she was discharged home and had no abnormal findings during the follow-up.
Regarding the pathogenic basis of methemoglobinemia, the detailed information of clinical and family history, environmental and drug exposure, and evaluation of consanguinity is critical for making the diagnosis, and differential diagnosis between hereditary and acquired forms. The most common form of methemoglobinemia is acquired, associated with various conditions, including diarrhea and acidosis (21, 22) and consumption of high-nitrate water (23, 24) or high-nitrate food (spinach, carrots, beets) (20). Additionally, food-borne nitrates and nitrites exposure to certain drugs can contribute to developing this order. The relevant drugs encompass topical anesthetic agents, silver nitrate, chloroquine, sulfonamides, dapsone, phenacetin, sodium valproate, phenazopyridine, inhaled nitrous oxide, and amyl nitrite (25, 26). Infants with acquired methemoglobinemia are more likely to be exposed to nitrate, including milk formula prepared with healthy water, vegetables containing high nitrate concentration, oxidant drugs, and diarrhea. Upon the ingestion of nitrates, fecal organisms transformed nitrates into nitrites. Subsequently, nitrites are rapidly absorbed from the intestine through passive diffusion and enter the systemic circulation without undergoing the first-pass metabolism in the liver. Notably, nitrites are one of the most potent oxidant agents of ferrohemoglobin (Figure 2). Particularly, nitrate-induced methemoglobinemia is more likely to attack infants younger than 6 months old. It was speculated that these infants produced low levels of stomach acid and had amounts of nitrite-reducing bacteria. In addition, infants have higher levels of fetal hemoglobin, presenting with relatively easy oxidation than adult hemoglobin, and their methemoglobin reductase system remains immature with lower levels of erythrocyte CYB5R activity (18–20). Considering our case, she was previously healthy without infectious disease, diarrhea, or drug administration and did not have G6PD deficiency. Moreover, no nitrite poisoning was found in her family members and people living nearby, which excluded the possibility of consumption of high-nitrate water. It was confirmed that the guardian inappropriately prepared the infant’s milk formula with spinach juice, which is considered a high-nitrate food. Therefore, we speculated that the cause of acquired methemoglobinemia in this case was most likely attributed to the spinach juice-mixed milk formula. The possibility of congenital methemoglobinemia for our case was partly excluded due to the sudden onset of cyanosis and the absence of a long-life history of cyanosis, dusky skin, or blue sclera in the family members. Regrettably, her parents refused whole-exome sequencing. In addition, clinicians needed to identify the causes of cyanosis in neonates and infants, including respiratory, circulatory, neurologic, infectious, and hematologic disorders (2). Respiratory causes most often account for previously healthy infants with the new onset of central cyanosis (1), such as upper airway obstruction, bronchiolitis, pneumonia, and pneumothorax. Circulatory etiologies might also be responsible for cyanosis, particularly in young infants, such as congenital heart disease, pulmonary hypertension, and pulmonary hemorrhage. Moreover, other severe conditions produce central cyanosis through disordered breathing caused by neurologic disease or decreased ability for hemoglobin to carry oxygen. These disorders should be evaluated by detailed clinical symptoms; physical examination of the respiratory, neurologic, and cardiovascular systems; past medical history; a related family history of the disease; and targeted auxiliary examination such as pulmonary imaging, echocardiography, and electrocardiogram.
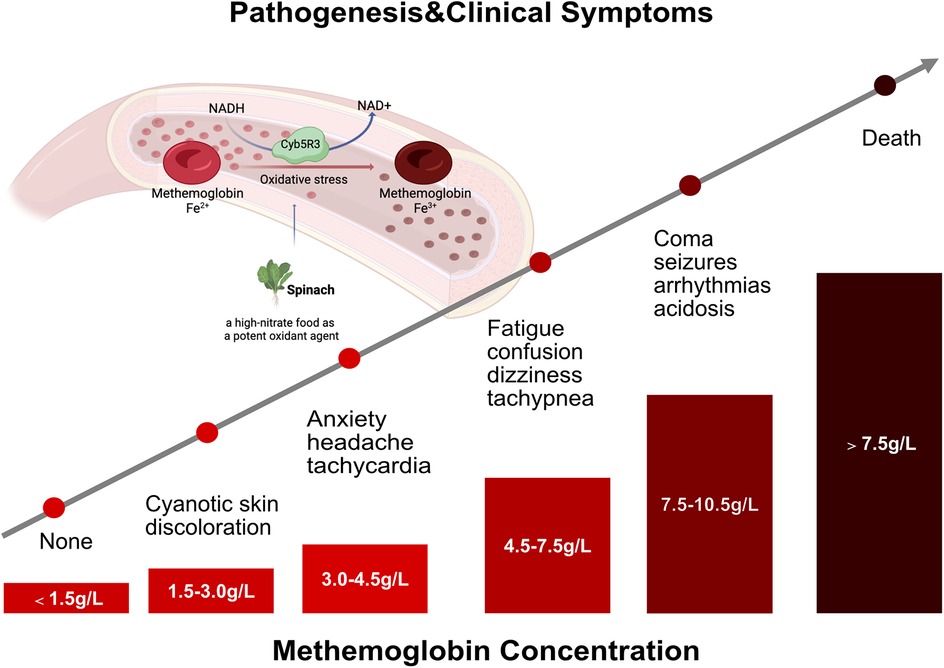
Figure 2. Clinical characteristics of patients with methemoglobinemia. The pathogenesis and clinical manifestations of patients with methemoglobinemia in line with methemoglobinemia concentration.
Recently, a multicenter study reported that the incidence of methemoglobinemia was much lower at 0.015‰; however, the overall 30-day mortality of patients with methemoglobinemia was 6.1% (6). Therefore, clinicians needed to identify the methemoglobinemia and initiate prompt management in children, especially in younger infants. Patients with methemoglobinemia might present with different clinical features in the clinical setting. However, the familiar clinical characteristics of methemoglobinemia are cyanosis, cardiac and neurologic complications, and metabolic acidosis (17, 27). Notably, the clinical symptoms of methemoglobinemia are mainly associated with methemoglobin concentration and methemoglobin level (% of total hemoglobin in patients with nonanemic). Wright et al. (28) concluded that patients with methemoglobin of 1.5 g/dl (10%–20% of total hemoglobin) might develop cyanosis. With the increased concentration and level of methemoglobin, patients gradually developed anxiety, headache, dizziness, fatigue, confusion, tachypnea, arrhythmias, acidosis, seizures, coma, and even death (Figure 2). In this study, the percentage of methemoglobinemia in our infant was significantly elevated to 44.7%, presenting with cyanosis, tachycardia, and high blood pressure. Encouragingly, these clinical symptoms resolved soon after the standard treatment of methylene blue and vitamin C was initiated with this infant.
The management of methemoglobinemia depends on clinical symptoms, methemoglobin and hemoglobin saturation percentage, and underlying conditions. First, the clinician should stop and/or remove the causative agent. In asymptomatic patients who had methemoglobin levels <20%, no treatment was usually needed as the methemoglobin could be reduced by normally metabolizing red blood cells within several hours. Otherwise, interventions should be initiated with the therapy of methylene blue and vitamin C (27). Methylene blue is the cornerstone therapy of methemoglobinemia as it accepts an electron from nicotinamide adenine dinucleotide phosphate (NADPH), reducing Fe3+ back to Fe2+ in erythrocytes. Its initial dose is 1–2 mg/kg infused intravenously over 3–5 min. It can be repeated at a dose of 1 mg/kg if the clinical conditions do not significantly resolve within 1 h. As a natural water-soluble vitamin, vitamin C reduces excessive oxidative stress, with various doses in children (0.5 g–1.0 g every 12–4 h). In addition, the additional treatment of blood transfusions, exchange transfusion, hemodialysis, and hyperbaric oxygen were also described in patients with refractory methemoglobinemia (17, 27).
Conclusion
Acquired methemoglobinemia might also involve infants younger than 3 months old. In this case, inappropriately preparing the infant milk formula with spinach juice was the potential cause of methemoglobinemia, which was present with central cyanosis. Pediatricians need to recognize acquired methemoglobinemia as a potential cause of cyanosis in infants.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee on Human Subjects at Sichuan University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
LW: Writing – original draft, Writing – review & editing. YY: Writing – original draft, Writing – review & editing. DL: Writing – original draft, Writing – review & editing. PY: Writing – original draft, Writing – review & editing. KL: Writing – original draft, Writing – review & editing. HH: Funding acquisition, Writing – original draft, Writing – review & editing. YZ: Conceptualization, Investigation, Supervision, Validation, Writing – original draft, Writing – review & editing. XL: Conceptualization, Funding acquisition, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The authors declare that financial support was received for the research and/or publication of this article. This work was supported by Science-Technology Support Plan Projects in Sichuan Province (2023YFS0302, 2025ZNSFSC0704), Sichuan Provincial Department of Science and Technology Central-Guided Local Science and Technology Development Program (2023ZYD0074), and National Natural Science Foundation of China (No. 82205119).
Acknowledgments
We are grateful to the patients and families for their contributions to this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Driscoll DJ. Evaluation of the cyanotic newborn. Pediatr Clin N Am. (1990) 37(1):1–23. doi: 10.1016/s0031-3955(16)36829-8
2. Hiremath G, Kamat D. Diagnostic considerations in infants and children with cyanosis. Pediatr Ann. (2015) 44(2):76–80. doi: 10.3928/00904481-20150203-12
3. Viršilas E, Timukienė L, Liubšys A. Congenital methemoglobinemia: rare presentation of cyanosis in newborns. Clin Pract. (2019) 9(4):1188. doi: 10.4081/cp.2019.1188
4. Ward J, Motwani J, Baker N, Nash M, Ewer AK, Toldi G. Congenital methemoglobinemia identified by pulse oximetry screening. Pediatrics. (2019) 143(3):e20182814. doi: 10.1542/peds.2018-2814
5. Prchal JT. Methemoglobinemia and other dyshemoglobinemias. In: Kaushansky K, Prchal JT, Burns LJ, Lichtman MA, Levi M, Linch DC, editors. Williams Hematology, 10e. New York, NY: McGraw-Hill Education (2021). p. 859.
6. Sinha N, Lichak B, Thomas NJ, Krawiec C. A multi-center retrospective database evaluation of pediatric subjects diagnosed with methemoglobinemia. Clin Med Insights Pediatr. (2024) 18:11795565241271678. doi: 10.1177/11795565241271678
7. Park SM, Rhee MS. Prevalence and phylogenetic traits of nitrite-producing bacteria in raw ingredients and processed baby foods: potential sources of foodborne infant methemoglobinemia. Food Res Int. (2024) 178:113966. doi: 10.1016/j.foodres.2024.113966
8. Raucci U, Stanco M, Roversi M, Ponticiello E, Pisani M, Rosa M, et al. Acquired methemoglobinemia in children presenting to Italian pediatric emergency departments: a multicenter report. Clin Toxicol (Phila). (2022) 60(8):920–5. doi: 10.1080/15563650.2022.2061986
9. Gao H, Basri R, Tran MH. Acquired methemoglobinemia: a systematic review of reported cases. Transfus Apher Sci. (2022) 61(2):103299. doi: 10.1016/j.transci.2021.103299
10. Singh J, Gathwala G, Khanna A, Abrol P, Mittal K, Gehlawat VK. Acquired methemoglobinemia due to contaminated Holi colors - a rare but preventable complication. Indian J Pediatr. (2013) 80(4):351–2. doi: 10.1007/s12098-012-0823-8
11. Dahshan A, Donovan GK. Severe methemoglobinemia complicating topical benzocaine use during endoscopy in a toddler: a case report and review of the literature. Pediatrics. (2006) 117(4):e806–9. doi: 10.1542/peds.2005-1952
12. Greer FR, Shannon M. Infant methemoglobinemia: the role of dietary nitrate in food and water. Pediatrics. (2005) 116(3):784–6. doi: 10.1542/peds.2005-1497
13. Sanchez-Echaniz J, Benito-Fernández J, Mintegui-Raso S. Methemoglobinemia and consumption of vegetables in infants. Pediatrics. (2001) 107(5):1024–8. doi: 10.1542/peds.107.5.1024
14. Saito T, Takeichi S, Osawa M, Yukawa N, Huang XL. A case of fatal methemoglobinemia of unknown origin but presumably due to ingestion of nitrate. Int J Legal Med. (2000) 113(3):164–7. doi: 10.1007/s004140050290
15. Iolascon A, Andolfo I, Russo R, Barcellini W, Fermo E, Toldi G, et al. Summary of joint European Hematology Association (EHA) and EuroBloodNet recommendations on diagnosis and treatment of methemoglobinemia. Hemasphere. (2021) 5(12):e660. doi: 10.1097/hs9.0000000000000660
16. Shihana F, Dissanayake DM, Buckley NA, Dawson AH. A simple quantitative bedside test to determine methemoglobin. Ann Emerg Med. (2010) 55(2):184–9. doi: 10.1016/j.annemergmed.2009.07.022
17. Iolascon A, Bianchi P, Andolfo I, Russo R, Barcellini W, Fermo E, et al. Recommendations for diagnosis and treatment of methemoglobinemia. Am J Hematol. (2021) 96(12):1666–78. doi: 10.1002/ajh.26340
18. Dusdieker LB, Getchell JP, Liarakos TM, Hausler WJ, Dungy CI. Nitrate in baby foods. Adding to the nitrate mosaic. Arch Pediatr Adolesc Med. (1994) 148(5):490–4. doi: 10.1001/archpedi.1994.02170050048009
19. Kross BC, Ayebo AD, Fuortes LJ. Methemoglobinemia: nitrate toxicity in rural America. Am Fam Physician. (1992) 46(1):183–8.1621630
20. Committee on Nutrition. Infant methemoglobinemia. The role of dietary nitrate. Pediatrics. (1970) 46(3):475–8. doi: 10.1542/peds.46.3.475
21. Hanukoglu A, Danon PN. Endogenous methemoglobinemia associated with diarrheal disease in infancy. J Pediatr Gastroenterol Nutr. (1996) 23(1):1–7. doi: 10.1097/00005176-199607000-00001
22. Jajoo V, Masavkar S, Ahmed Zaki S, Pawar P. Acquired methaemoglobinaemia in infancy associated with acute diarrhoea: a case series. Trop Doct. (2024) 54(1):39–41. doi: 10.1177/00494755231205632
23. Fewtrell L. Drinking-water nitrate, methemoglobinemia, and global burden of disease: a discussion. Environ Health Perspect. (2004) 112(14):1371–4. doi: 10.1289/ehp.7216
24. Avery AA. Infantile methemoglobinemia: reexamining the role of drinking water nitrates. Environ Health Perspect. (1999) 107(7):583–6. doi: 10.1289/ehp.99107583
25. Finan A, Keenan P, Donovan FO, Mayne P, Murphy J. Methaemoglobinaemia associated with sodium nitrite in three siblings. Br Med J. (1998) 317(7166):1138–9. doi: 10.1136/bmj.317.7166.1138
26. Coleman MD, Coleman NA. Drug-induced methaemoglobinaemia. Treatment issues. Drug Saf. (1996) 14(6):394–405. doi: 10.2165/00002018-199614060-00005
27. Skold A, Cosco DL, Klein R. Methemoglobinemia: pathogenesis, diagnosis, and management. South Med J. (2011) 104(11):757–61. doi: 10.1097/SMJ.0b013e318232139f
Keywords: cyanosis, acquired methemoglobinemia, infant, methylene blue, nitrate
Citation: Wang L, Ye Y, Li D, Li K, Yang P, Hu H, Liu X and Zhao Y (2025) Case Report: Clinical presentations of cyanosis associated with acquired methemoglobinemia in infants—a clinical challenge. Front. Pediatr. 13:1563277. doi: 10.3389/fped.2025.1563277
Received: 19 January 2025; Accepted: 28 April 2025;
Published: 13 May 2025.
Edited by:
Francesca Conti, IRCCS Azienda Ospedaliero-Universitaria di Bologna, University of Bologna, ItalyReviewed by:
Hasnaa Jalou, Riley Hospital for Children, United StatesYuejuan Xu, Shanghai Jiao Tong University, China
Copyright: © 2025 Wang, Ye, Li, Li, Yang, Hu, Liu and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongmei Zhao, emhhb2pmeXdAMTI2LmNvbQ==; Xiaoliang Liu, c2RpZ2pveUBsaXZlLmNvbQ==
 Lin Wang1
Lin Wang1 Xiaoliang Liu
Xiaoliang Liu