- 1Department of Pediatrics, Showa Medical University, Tokyo, Japan
- 2Division of Neonatology, Center for Maternal-Fetal, Neonatal, and Reproductive Medicine, National Center for Child Health and Development, Tokyo, Japan
- 3Department of Pediatrics and Neonatology, Kameda Medical Center, Chiba, Japan
- 4Department of Pediatrics, Nara Medical University, Nara, Japan
- 5Department of Pediatrics, Fujita Health University School of Medicine, Aichi, Japan
- 6Department of Neonatology, Tokyo Metropolitan Children’s Medical Center, Tokyo, Japan
- 7Department of Pediatrics, Yokohama City University, Yokohama, Japan
- 8Department of Hygiene, Public Health, and Preventative Medicine, Showa Medical University, Tokyo, Japan
Background: Necrotizing enterocolitis (NEC) remains a major cause of morbidity and mortality in extremely low birth weight infants (ELBWIs), despite widespread donor human milk (DHM) use. This study examined NEC cases among DHM recipients to explore potential contributing factors.
Methods: We retrospectively analyzed 1,425 infants registered in Japan's human milk bank database (2018–2023). NEC cases at Bell stage ≥ II were confirmed by attending physicians. Infants who received DHM only after NEC onset were excluded. Cases were categorized by onset timing and associated clinical factors.
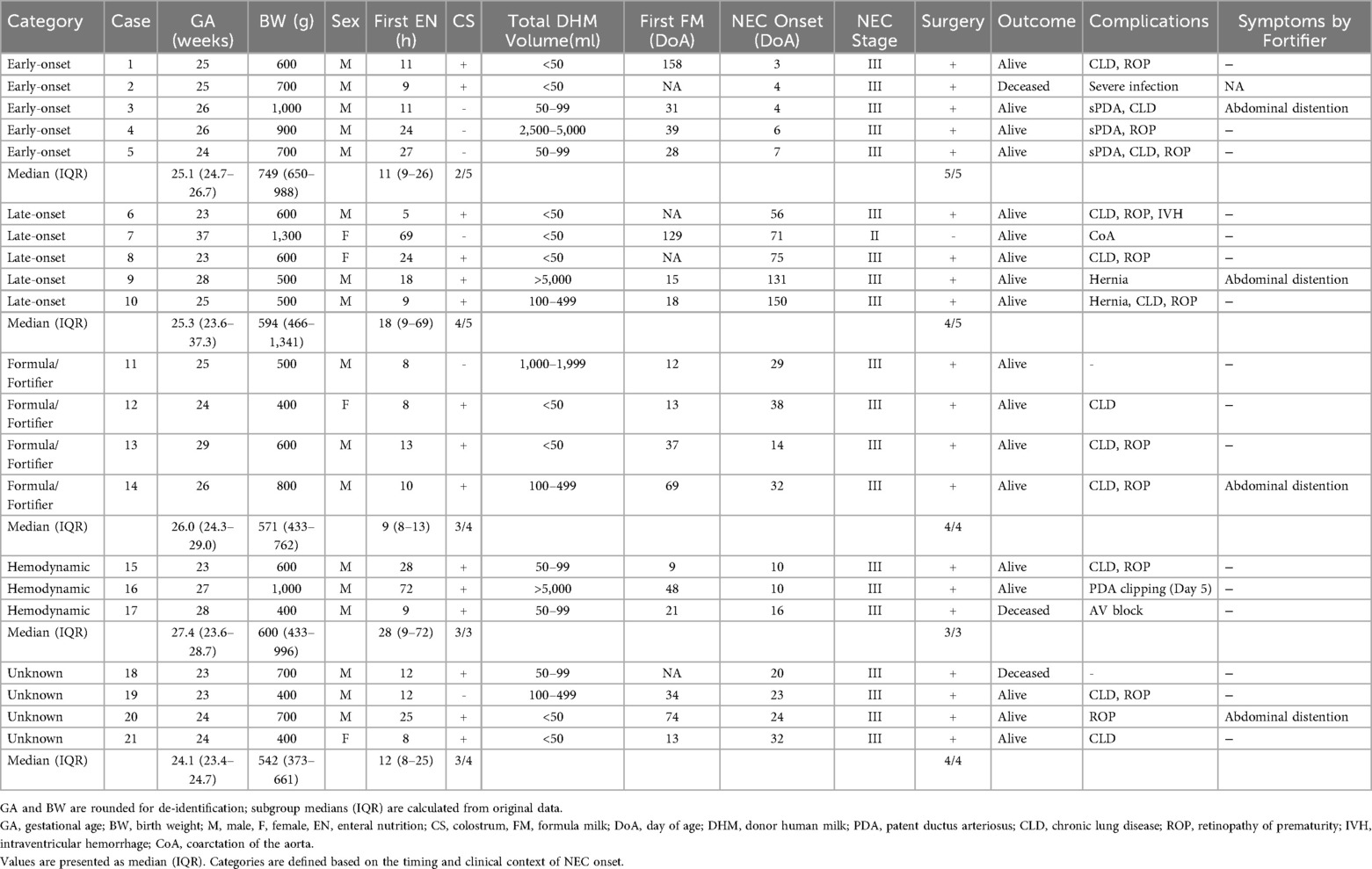
Results: Among 1,324 very low birth weight infants, 21 (1.58%) developed NEC, with 20 requiring surgical intervention. Median gestational age and birth weight were 25.1 weeks and 637 g, respectively. NEC onset was classified as follows: within 7 days of birth (n = 5), after 2 months (n = 5), after formula or fortifier use (n = 6), associated with hemodynamic changes (n = 7), or of unknown etiology (n = 4). Common factors included symptomatic PDA, congenital heart disease, infection, formula exposure, and ophthalmologic procedures.
Conclusion: NEC can develop despite DHM use, often in association with early infections, PDA, or fortification. Strategies to further reduce NEC incidence should include management of hemodynamic instability, delayed formula introduction, and use of exclusive human milk-based diets. Further research should explore potential roles of ophthalmologic interventions and human milk fortifiers in NEC development.
Introduction
Even in the 2015 national survey, the in-hospital mortality rate of extremely low birth weight infants (ELBWIs) in NICUs remained below 10%, demonstrating that Japan's neonatal care is among the best in the world. However, the proportion of necrotizing enterocolitis (NEC) and focal intestinal perforation as causes of death has shown an increasing trend—from 7.2% in 2005 to 14.1% in 2010, and 16.2% in 2015. The mortality rate for infants diagnosed with NEC was reported to be 39.6% (1). Additionally, approximately 40% of NEC survivors develop neurodevelopmental impairment (NDI), with significantly higher incidence in the NEC group compared to those without NEC (2). Therefore, preventive strategies against NEC are essential to further improve the prognosis of ELBWIs.
Various strategies have been introduced to reduce NEC, with some reports even documenting complete elimination of NEC cases (3). These strategies include establishing human milk banks, standardizing enteral nutrition, using probiotics, and oropharyngeal administration of colostrum (4). In Japan, NICUs have long encouraged the use of mothers' own milk, and both probiotics and oropharyngeal colostrum application are already widely practiced (5, 6), which may contribute to the country's relatively low NEC incidence.
Breast milk contains immunologically active substances such as secretory IgA, macrophages, lactoferrin, human milk oligosaccharides (HMOs), and exosomes, which offer superior NEC prevention compared to formula milk. Although donor human milk (DHM) undergoes pasteurization—reducing levels of secretory IgA and lactoferrin—studies indicate that HMOs and exosomes retain both their quantity and activity (7, 8). In California, the increased use of DHM in hospitals (from 27 hospitals in 2007 to 55 in 2013) was associated with a decrease in NEC incidence from 6.6% to 4.3% (9).
Standardization of enteral nutrition has also been shown in systematic reviews to significantly reduce NEC risk (risk ratio 0.22; 95% confidence interval 0.13–0.36) (10). However, this is not feasible when relying solely on mothers' own milk, highlighting the need for a human milk banking system. By incorporating human milk banks and standardized enteral nutrition into existing practices such as oropharyngeal colostrum administration and probiotic use, further reductions in NEC incidence in Japan are anticipated. In Japan, DHM is processed using Holder pasteurization. Milk from three donor mothers is pooled per batch. DHM is distributed within 6 months from the date of expression. This study analyzed NEC cases using a database of infants who received DHM to explore clinical patterns and contributing factors that may inform future prevention strategies.
Materials and methods
In Japan, all infants who received DHM are registered in the human milk bank database. Parental consent has been obtained for inclusion in the human milk bank registration system, and this consent includes permission for the data to be analyzed and published in scientific research. The human milk bank registry captures infants who received donor human milk (DHM); infants who did not receive DHM (including those exclusively fed mother's own milk) are not included in this database. We analyzed data from 2018 to 2023. Infants who began receiving DHM after developing NEC were excluded from this analysis, even though they were included in the database. Due to the limited number of NEC cases (n = 21), only descriptive statistics were used. Continuous variables are presented as medians and interquartile ranges, and categorical variables as counts and percentages. No inferential statistical tests were performed, as the small sample size precluded meaningful quantitative analysis.
The following categories were used in this study:
• Birth weight and gestational age
• Postnatal age (hours/days) at initiation of enteral feeding with DHM, mother's own milk (MOM), and formula
• NEC diagnosis at Bell stage II or higher (Yes/No)
• For NEC cases, the postnatal day of diagnosis and associated clinical course
NEC cases were categorized by onset circumstances:
1. Onset within 7 days of birth
2. Onset after approximately 2 months
3. Onset following formula feeding or fortification with cow's milk-based fortifiers
4. Cases associated with hemodynamic changes
5. Cases without a clear associated factor
These categories were selected to reflect the timing and context of NEC onset, aiming to explore recurring clinical patterns rather than infer causality.
Results
A total of 1,425 infants (690 females, 735 males) were registered in the human milk bank database as of December 31, 2023. This included 1,324 very low birth weight infants, of whom 774 were classified as ELBWIs. Among these, 25 cases (1.75%) were recorded as NEC. A flowchart of case selection and exclusions is shown in Figure 1. Baseline clinical characteristics of the NEC cases are summarized by onset category in Table 1. To comply with de-identification requirements, gestational age (GA) and birth weight (BW) in Table 1 are presented in rounded values (completed weeks for GA and the nearest 100 g for BW). However, the median and interquartile range (IQR) values for each subgroup were calculated from the original data and are therefore shown with exact values.Consistent with the severity distribution (Bell stage IIb–III), nearly all infants required surgical intervention. NEC onset details were verified via email communication with attending physicians. Two cases were later determined not to have developed NEC, and two received DHM after NEC onset. Thus, 21 cases (1.58%; 19/1,324) were confirmed as Bell stage II or higher NEC, with 20 requiring surgical intervention. The total volume of DHM indicates that while the majority of infants received relatively small volumes of DHM (under 100 ml), a subset required over 1,000 ml, and in three cases, more than 5,000 ml was administered.
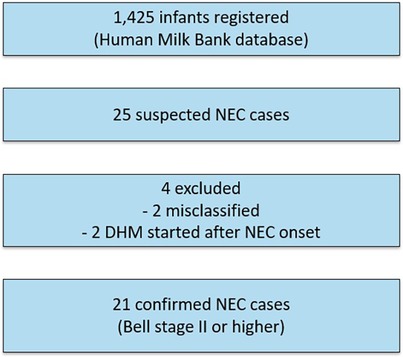
Figure 1. Flowchart of case selection. A total of 1,425 infants were registered in the Human Milk Bank database. Among these, 25 were reported as suspected NEC cases. Four cases were excluded (two were misclassified and two had donor human milk introduced only after NEC onset). The final study cohort therefore consisted of 21 confirmed cases of NEC at Bell stage II or higher.
The overall median gestational age and birth weight of these 21 NEC cases were 25.1 weeks (IQR 23.9–27.0 weeks) and 637 g (IQR 484.5–755.5 g), respectively.
The NEC cases were categorized (with some overlaps) as follows (Table 1):
1. Onset within 7 days of birth (5 cases)
2. Onset after approximately 2 months (5 cases)
3. Onset after initiating formula or fortifiers (6 cases; 2 overlapping with category 2)
4. Associated with hemodynamic changes (7 cases; 3 overlapping with category 1, 1 overlapping with category 2)
5. No clear associated factor (4 cases)
Median values are also shown in Table 1 to provide descriptive context; however, given the small group sizes (n = 3–5 per category), these values should be interpreted with caution and no statistical comparisons were performed.
1. NEC Onset Within 7 Days of Birth (5 Cases)
Case No.1 was born in poor condition due to non-reassuring fetal status, developed intraventricular hemorrhage on day 1, and NEC on day 3. Case No.2 involved severe neonatal infection and intraventricular hemorrhage on day 3, followed by NEC on day 4. Three cases (case No. 3,4,5) developed NEC on days 4, 6, and 7, respectively, with symptomatic patent ductus arteriosus (PDA) suspected. Symptomatic PDA is defined as presence of clinical signs such as hyperdynamic precordium, widened pulse pressure, tachycardia, or tachypnea, along with echocardiographic evidence of ductal significance—defined as at least one of the following: ductal diameter ≥1.5 mm, left atrium-to-aortic root ratio ≥1.5, diastolic flow velocity in the left pulmonary artery ≥0.2 m/sec, or absence of diastolic flow in the anterior cerebral artery—and requiring at least one course of indomethacin treatment. Several early-onset cases were associated with severe congenital infection, non-reassuring fetal status, or symptomatic PDA. Notably, three early NEC cases occurred at the same institution, suggesting possible differences in PDA management protocols.
2. NEC onset after approximately 2 months (5 cases)
One term infant (37 weeks, 1,300 g; case No.7) with congenital heart disease developed NEC due to hemodynamic abnormalities. Two cases (case No.6 and 8) were born at 23 weeks of gestation and were suspected to develop NEC following ophthalmologic exams. Systemic absorption of mydriatic agents may have contributed to circulatory instability. Two others (case No.9 and 10) developed NEC at 131 and 150 days of age after inguinal hernia incarceration. Both had transitioned from DHM to formula on days 15 (No.9) and 18 (No.10).
3. NEC after formula or fortifier introduction (6 cases)
Two cases (case No.9 and 10) overlapped with category 2 and were likely unrelated to formula. Case No. 11 and 12 began formula on days 12 and 13 and developed NEC on days 29 and 38, respectively, without other known causes. Case No.13 and 14 started cow's milk-based fortifier and developed NEC on days 14 and 32, respectively, with no other identified risk factors. In Japan, only cow's milk-based fortifier is available.
4. Associated with hemodynamic changes (7 cases, 4 overlapping)
Includes five cases with symptomatic PDA (NEC onset on days 4, 6, 7, 10, and 10), one case with hypoplastic left heart syndrome (case No.7; overlapping with category 2), and one with complete AV block (case No.17). In cases of symptomatic PDA and congenital heart disease, increased pulmonary blood flow and reduced systemic circulation were presumed to have led to decreased intestinal perfusion, which was considered a contributing factor to NEC onset.
5. No clear associated factor (4 cases)
These cases (case No. 18–21) had no identifiable risk factors. Although they were exclusively fed human milk, they developed to NEC between 20 and 32 days of age. All of them were extremely immature (23 and 24 weeks of gestation).
Taken together, these findings reaffirm the central role of developmental immaturity in NEC susceptibility.
Discussion
NEC remains a major challenge in neonatal care due to its high mortality. DHM has been reported to reduce NEC incidence by approximately 50% compared to formula (risk ratio 0.53, 95% CI 0.37–0.76) (11), making it an essential preventive measure. Our observed NEC incidence of 1.58% (21/1,324 VLBW infants) was essentially identical to the rate of 1.6% recently reported in a nationwide cohort of Japanese VLBW infants (12). This similarity suggests that the NEC incidence in our DHM-based registry is consistent with national and international observations, further supporting the descriptive nature of our findings rather than indicating a differential risk attributable to DHM use.
This study aimed to identify factors contributing to NEC onset despite DHM use. Known NEC risk factors include poor birth conditions, mechanical ventilation, asphyxia, hypotension, hypothermia, fetal growth restriction, PDA, congenital heart disease, and formula feeding. This study identified cases where congenital infection, PDA, heart disease, and formula use on the first few weeks were likely contributors. Ophthalmologic exams and human milk fortifier use were observed among the cases and may warrant further investigation as possible contributing factors.
While median NEC onset is around 10 days (range: 7–23.5 days) (13), early- and late-onset cases suggest distinct etiologies. Early-onset NEC was linked to severe infection, non-reassuring fetal status and symptomatic PDA—especially in one facility, indicating a need to review PDA management there. Late-onset NEC followed hernia incarceration or ophthalmologic procedures. Systemic effects of mydriatic agents used during exams warrant further investigation.
Although not statistically significant, these cases raise a hypothesis that the timing of formula introduction may influence NEC risk; however, further studies are needed as the broader literature reports mixed findings. Exclusive human milk-based diets (EHMD), including human milk-derived fortifiers, have shown lower NEC rates than cow's milk-based alternatives (14, 15).
In the hemodynamic group, the initiation of enteral feeding tended to be delayed, possibly reflecting clinical decisions to postpone feeding until circulatory stability was achieved. In the unknown group, the main common feature was extreme prematurity and very low birth weight, despite early enteral feeding and colostrum administration. Thus, developmental immaturity may represent the primary underlying risk factor in these cases.
Although DHM and MOM are widely considered protective against NEC, they do not guarantee absolute protection; anecdotal and cohort data show that NEC can still occur despite exclusive human milk feeding. One possible explanation is interindividual variation in milk bioactive components. In particular, DSLNT—a specific human milk oligosaccharide—has been shown to reduce NEC-like injury in neonatal rat models (16), and its concentration was significantly lower in MOM samples fed to preterm infants who later developed NEC (17). These findings underscore the role of milk compositional variability in NEC risk and further support the notion that protective associations do not imply direct causation.
Because the registry scope is limited to DHM recipients, direct comparisons with non-DHM or exclusively MOM-fed infants were not feasible. To avoid implying causation, we interpret our findings descriptively and contextualize the observed NEC incidence against national cohorts and prior studies. Future work will prioritize linkage with external datasets (e.g., national neonatal registries) to enable comparative analyses across DHM, MOM, and non-DHM groups, including subgroup analyses among severe cases (Bell IIb–III).
Limitations
This study has several limitations. First, due to the retrospective design and small sample size (n = 21), we did not perform statistical comparisons or multivariable modeling. For patient confidentiality, GA and BW were rounded in the individual case listings, whereas subgroup medians and IQRs were derived from the original data. This approach ensures anonymity while preserving accuracy in the descriptive statistics. Although limited modeling may be possible even with small datasets, we opted for a purely descriptive approach to avoid overinterpretation. Accordingly, our findings should be interpreted as exploratory observations rather than definitive associations.
Second, NEC case classification was based on temporal context and clinical features, which often overlapped. These categorizations were intended to identify patterns, not to imply causation. Terms such as “associated factors” has been used throughout the manuscript. Notably, developmental risk factors such as extreme prematurity and low birth weight were present in all cases and remain the most consistent predictors of NEC.
Third, several important variables were not captured in the database, including detailed DHM dosage and duration, volume of mother's own milk, specific timing of feeding advancement, and use of oropharyngeal colostrum or probiotics. However, most Japanese NICUs routinely implement probiotics and oropharyngeal colostrum administration, and feeding advancement speed has not been shown to significantly affect NEC or mortality risk in previous studies (18).
Fourth, the assessment of NEC-associated clinical factors relied on documentation and attending physicians' judgment, which may introduce some subjectivity. We aim to incorporate surgical and pathological data in future analyses to improve diagnostic accuracy. Additionally, two cases involving inguinal hernia incarceration were excluded from the final analysis, as their pathophysiology differed from classical NEC.
Fifth, our registry does not include non-DHM infants; thus, between-group comparisons could not be performed.
Despite these limitations, the study offers valuable insights into the clinical context and diversity of NEC onset among DHM recipients, and may help generate hypotheses for future prospective research.
Conclusion
Extremely low gestational age and birth weight were common to all NEC cases in this study, underscoring their role as the most consistent and well-established risk factors for NEC. These developmental vulnerabilities likely contributed to the intestinal immaturity and impaired perfusion that predispose preterm infants to NEC, regardless of feeding practices.
While this study was descriptive in nature and limited by a small sample size, several recurring clinical features suggest that early-onset severe infections, hemodynamic instability related to PDA, and the timing of formula or fortifier introduction may play a role in NEC development, even among infants receiving DHM.
Further prospective studies are needed to determine whether delaying formula introduction, using exclusive human milk-based diets when fortification is required, and optimizing circulatory management could contribute to reducing NEC incidence in this vulnerable population.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Showa Medical University Hospital, 21-084-A. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
KM: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Writing – original draft, Writing – review & editing. YW: Data curation, Writing – review & editing. MS: Conceptualization, Data curation, Formal analysis, Writing – review & editing. YT: Conceptualization, Investigation, Methodology, Writing – review & editing. MM: Conceptualization, Project administration, Supervision, Writing – review & editing. JS: Conceptualization, Supervision, Visualization, Writing – review & editing. SN: Supervision, Writing – review & editing. HD: Conceptualization, Data curation, Formal analysis, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Children and Families Agency Program (Grant Number 23DA0901).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Miyazawa T, Arahori H, Ota S, Tanaka K, Fujii Y, Kawano S. Mortality and morbidity of extremely low birth weight infants in Japan, 2015. Pediatr Int. (2023) 65:e15493. doi: 10.1111/ped.15493
2. Matei A, Montalva L, Goodbaum A, Sharma R, Kim S, Patel V. Neurodevelopmental impairment in necrotising enterocolitis survivors: systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. (2020) 105:F432–9. doi: 10.1136/archdischild-2019-317830
3. Chandran S, Anand AJ, Rajadurai VS, Lim SB, Tan WC, Ng YP. Evidence-based practices reduce necrotizing enterocolitis and improve nutrition outcomes in very low-birth-weight infants. JPEN J Parenter Enteral Nutr. (2021) 45:1408–16. doi: 10.1002/jpen.2058
4. Barr PA, Mally PV, Caprio MC. Standardized nutrition protocol for very low-birth-weight infants resulted in less use of parenteral nutrition and associated complications, better growth, and lower rates of necrotizing enterocolitis. JPEN J Parenter Enteral Nutr. (2019) 43:540. doi: 10.1002/jpen.1453
5. Isayama T. The clinical management and outcomes of extremely preterm infants in Japan: past, present, and future. Transl Pediatr. (2019) 8(3):199–211. doi: 10.21037/tp.2019.07.10
6. Kumar J, Meena J, Ranjan A, Singh R, Gupta A, Verma S. Otopharyngeal application of colostrum or mother’s own milk in preterm infants: a systematic review and meta-analysis. Nutr Rev. (2023) 81:1254–66. doi: 10.1093/nutrit/nuad002
7. Miyake H, Lee C, Chusilp S, Okada Y, Yamamoto T, Nakamura K. Human breast milk exosomes attenuate intestinal damage. Pediatr Surg Int. (2020) 36:155–61. doi: 10.1007/s00383-019-04599-7
8. Abbas S, Keir AK, Markides M, Turner C, Jones CA, Wallace H. Tailoring human milk oligosaccharides to prevent necrotizing enterocolitis among preterm infants. Front Nutr. (2021) 8:702888. doi: 10.3389/fnut.2021.702888
9. Kantorowska A, Wei JC, Cohen RS, Gould JB, Lee HC, Abrams SA. Impact of donor milk availability on breast milk use and necrotizing enterocolitis rates. Pediatrics. (2016) 137:e20153123. doi: 10.1542/peds.2015-3123
10. Jasani B, Patole S, Simmer K, Rao S, Clarke P, Deshpande G. Standardized feeding regimen for reducing necrotizing enterocolitis in preterm infants: an updated systematic review. J Perinatol. (2017) 37:827–33. doi: 10.1038/jp.2017.37
11. Quigley M, Embleton ND, Meader N, McGuire W. Donor human milk for preventing necrotizing enterocolitis in very preterm or very low-birthweight infants. Cochrane Database Syst Rev. (2024) 9(9):CD002971. doi: 10.1002/14651858.CD002971.pub6
12. Kusuda S, Bennett MV, Gould JB. Comparative analysis of necrotizing enterocolitis in preterm infants born in Japan and born to mothers of Japanese ethnicity in California. Sci Rep. (2025) 15:9943. doi: 10.1038/s41598-025-92393-y
13. Moschino L, Baraldi E, Castellani C, Zanin R, Faldella G, Perrone S. Metabolomic analysis to predict the onset and severity of necrotizing enterocolitis. BMC Gastroenterol. (2024) 24:380. doi: 10.1186/s12876-024-03453-y
14. Harris L, Lewis S, Vardaman S, Mitchell M, Barnes D, Greenberg J. Exclusive human milk diets and the reduction of necrotizing enterocolitis. Adv Neonatal Care. (2024) 24:400–7. doi: 10.1097/ANC.0000000000001183
15. Hanford J, Mannebach K, Ohler A, Brannon K, Wiley J, Mendez J. Rates of comorbidities in very low birth weight infants fed an exclusive human milk diet versus a bovine supplement diet. Breastfeed Med. (2021) 16:814–20. doi: 10.1089/bfm.2020.0345
16. Jantscher-Krenn E, Zherebtsov M, Nissan C, Goth K, Guner YS, Naidu N, et al. The human milk oligosaccharide disialyllacto-N-tetraose prevents necrotising enterocolitis in neonatal rats. Gut. (2012) 61:1417–25. doi: 10.1136/gutjnl-2011-301404
17. Masi AC, Embleton ND, Lamb CA, Young G, Granger CL, Najera J, et al. Human milk oligosaccharide DSLNT and gut microbiome in preterm infants predicts necrotising enterocolitis. Gut. (2021) 70:2273–82. doi: 10.1136/gutjnl-2020-322771
Keywords: donor human milk, necrotizing enterocolitis, human milk bank, preterm infants, fortifier, ELBWI, hemodynamic instability, PDA
Citation: Mizuno K, Wada YS, Sakurai M, Tani Y, Miyata M, Shindo J, Nishimaki S and Den H (2025) Analysis of NEC cases registered in the human milk bank database. Front. Pediatr. 13:1679676. doi: 10.3389/fped.2025.1679676
Received: 5 August 2025; Accepted: 28 August 2025;
Published: 17 September 2025.
Edited by:
Xiaocai Yan, Stanley Manne Children's Research Institute, United StatesReviewed by:
Sergio Verd, Servei Balear de Salut, SpainLorena Ostilla, Northwestern University Medical Center, United States
Copyright: © 2025 Mizuno, Wada, Sakurai, Tani, Miyata, Shindo, Nishimaki and Den. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katsumi Mizuno, a2F0c3Vvcm9iaUBtZWQuc2hvd2EtdS5hYy5qcA==
 Katsumi Mizuno
Katsumi Mizuno Yuka S. Wada2
Yuka S. Wada2 Masafumi Miyata
Masafumi Miyata