- 1State Key Laboratory of Agriculture Microbiology, College of Veterinary Medicine, Huazhong Agriculture University, Wuhan, China
- 2Department of Veterinary Pharmacology, College of Veterinary Medicine, Huazhong Agricultural University, Wuhan, China
- 3National Reference Laboratory of Veterinary Drug Residues and MAO Key Laboratory for Detection of Veterinary Drug Residues, Huazhong Agriculture University, Wuhan, China
A Corrigendum on
Evaluation of Marbofloxacin in Beagle Dogs After Oral Dosing: Preclinical Safety Evaluation and Comparative Pharmacokinetics of Two Different Tablets
by Lei, Z., Liu, Q., Yang, B., Khaliq, H., Ahmed, S., Fan, B., et al. (2018). Front. Pharmacol. 9:306. doi: 10.3389/fphar.2018.00306
In the original article, there was a mistake in Figure 4 as published. Information in Figure 4A was lost, due to a decimal error in the figure axis. The corrected Figure 4 appears below. The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
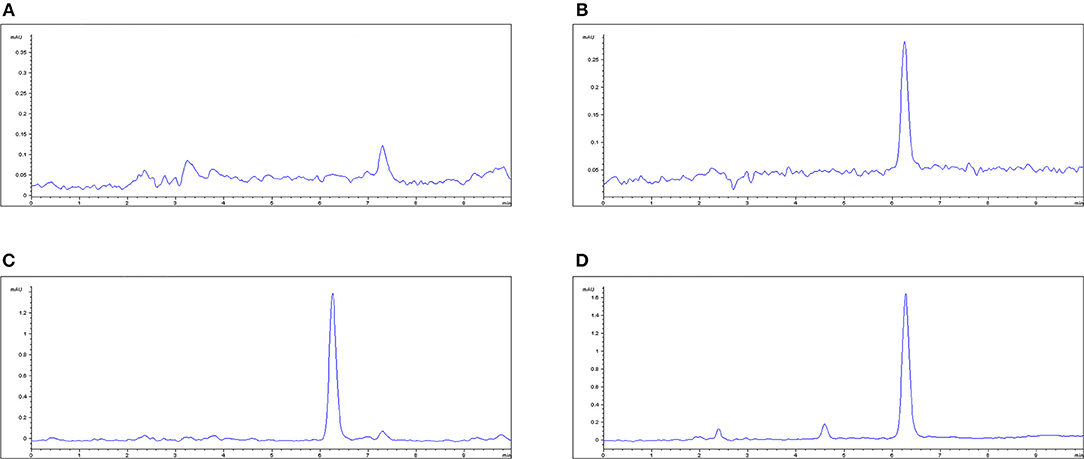
Figure 1. The HPLC method for MBF quantification in plasma. (A) Blank plasma sample, (B) plasma sample at the LLOQ of 0.05 μg/ml, (C) plasma sample after oral administration of Petsen at the point of 16 h, (D) plasma sample after i.v administration of MBF at the point of 16 h. MBF at the peak time of 6.3 min.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Keywords: fluoroquinolones, marbofloxacin, pharmacokinetics, beagle dogs, bioavailability, toxicity
Citation: Lei Z, Liu Q, Yang B, Khaliq H, Ahmed S, Fan B, Cao J and He Q (2018) Corrigendum: Evaluation of Marbofloxacin in Beagle Dogs After Oral Dosing: Preclinical Safety Evaluation and Comparative Pharmacokinetics of Two Different Tablets. Front. Pharmacol. 9:1385. doi: 10.3389/fphar.2018.01385
Received: 25 October 2018; Accepted: 12 November 2018;
Published: 28 November 2018.
Edited and reviewed by: Yurong Lai, Gilead (United States), United States
Copyright © 2018 Lei, Liu, Yang, Khaliq, Ahmed, Fan, Cao and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiyue Cao, Y2Fvaml5dWUyQDE2My5jb20=
Qigai He, aGU2MjhAbWFpbC5oemF1LmVkdS5jbg==
 Zhixin Lei
Zhixin Lei Qianying Liu
Qianying Liu Bing Yang1,2
Bing Yang1,2 Saeed Ahmed
Saeed Ahmed Jiyue Cao
Jiyue Cao