- 1Oxford Chinese Medicine Research Centre, University of Oxford, Oxford, United Kingdom
- 2Department of Physiology, Anatomy and Genetics, University of Oxford, Oxford, United Kingdom
- 3Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences, Beijing, China
Xin Su Ning (XSN) is a China patented and certified traditional Chinese herbal medicine used to treat premature ventricular contractions (PVCs) since 2005. XSN is formulated with 11 herbs, designed to treat arrhythmia with phlegm-heat heart-disturbed syndrome (PHHD) according to Chinese medicine theory. The rational compatibility of the 11 herbs decides the therapeutic outcome of XSN. Due to the multicomponent nature of traditional Chinese medicine, it is difficult to use conventional pharmacology to interpret the therapeutic mechanism of XSN in terms of clear-cut drug molecule and target interactions. Network pharmacology/systematic pharmacology usually consider all the components in a formula with the same weight; therefore, the proportion of the weight of the components has been ignored. In the present study, we introduced a novel coefficient to mimic the relative amount of all the components in relation with the weight of the corresponding herb in the formula. The coefficient is also used to weigh the pharmacological effect of XSN on all relative biological pathways. We also used the cellular electrophysiological data generated in our lab, such as the effect of liensinine and isoliquiritigenin on NaV1.5 channels; we therefore set sodium channel as one of the targets of these two components, which would support the clinical efficacy of XSN in treating tachyarrhythmia. Combining the collected data and our discovery, a panoramagram of the pharmacological mechanism of XSN was established. Pathway enrichment and analysis showed that XSN treated PHHD arrhythmia through multiple ion channels regulation, protecting the heart from I/R injury, inhibiting the apoptosis of cardiomyocyte, and improving glucose and lipid metabolism.
Introduction
Arrhythmia is a disease featuring the abnormalities of frequency or rhythm of heart excitement caused by abnormal cardiac electrophysiological activities generated by the electrical conduction system of the heart. Symptoms of arrhythmia often include dizziness, breathlessness, and palpitations (Barsky, 2001). The presence of cardiac arrhythmias may at times suggest a specific underlying heart disease or even noncardiac pathological changes. The most common and important cause of cardiac arrhythmias is coronary artery disease (Atarashi and Hayakawa, 1996). In recent decades, even though plenty of non-chemical therapies have been developed, anti-arrhythmic drug is still the most common treatment. However, the proarrhythmic effect of the anti-arrhythmic drugs has been causing concerns on the safety of the arrhythmic patients.
As a complementary and alternative medicine, Chinese medicine plays an increasing role in the treatment of arrhythmias (Dong et al., 2017). Many proprietary Chinese medicines, such as Xin Su Ning capsule (Yuan and Zhou, 2000; Wang and Lu, 2008; Lin et al., 2011; Li and Zhang, 2015; Zhai et al., 2017), Wenxin Keli (Kalifa and Avula, 2012; Li and Guihua, 2018), and Shensong Yangxin capsule (Wang et al., 2011; Liu et al., 2014), have showed clear clinical antiarrhythmic efficacy comparable with the single chemical compound anti-arrhythmic drugs. However, the Chinese-patented antiarrhythmic medicines have rare adverse reactions being recorded.
Based on the theories of traditional Chinese medicine (TCM), a disease can be categorized from four fundamental dimensions, which is consisted of four pairs of relative concepts including yin–yang, exterior–interior, excess–deficiency, and cold–heat (Lu et al., 2004; Jiang et al., 2012). In terms of excess–deficiency categorization, arrhythmia can be divided into two main types of syndromes: excess syndrome and deficient syndrome (Chen and Ba, 2010). Deficiency syndrome is a TCM condition with weakness and lack of energy. Up to present, most of the marketed anti-arrhythmic Chinese medicines have been used to treat deficient syndrome, such as Wenxin Keli (Wang et al., 2016) and Shensong Yangxin capsule (Gu et al., 2005) for the deficiency of Qi and Yin, Xinbao Wan for the deficiency of Yang (Chen and Wu, 1996; Dong et al., 2005), and Tianwang Buxin Wan for the deficiency of Yin (Ni et al., 2019). Excess syndrome is a typical TCM syndrome caused by the accumulation of pathological phlegm and dampness in the body. Cardiac arrhythmia with typical clinical manifestation of phlegm-heat heart-disturbance (PHHD) syndrome takes a large proportion of the clinical treatment, and to the best of our knowledge, XSN is the only medicine for treating PHHD arrhythmia (Yuan and Zhou, 2000).
Xin Su Ning (XSN) is a multi-herbal medicine patented and launched in China since 2005 for treating cardiac ventricular arrhythmia, especially arrhythmias induced by cardiac ischemia and viral myocarditis (Zhai et al., 2017). XSN is comprised of 11 herbs: Coptidis Rhizoma (Huanglian, Coptis chinensis Franch.), Pinelliae Rhizoma (Banxia, Pinellia ternata [Thunb.] Makino), Poria (Fuling, Poria cocos [Schw.] Wolf), Aurantii Fructus Immaturus (Zhishi, Citrus aurantium L.), Dichroae Radix (Changshan, Dichroa febrifuga Lour.), Nelumbinis Plumula (Lianzixin, Nelumbo nucifera Gaertn.), Sophorae flavescentis Radix (Kushen, Sophora flavescens Ait.), Artemisiae annuae Herba (Qinghao, Artemisia annua L.), Ginseng Radix et Rhizoma (Renshen, Panax ginseng C. A. Mey.), Ophiopogonis Radix (Maidong, Ophiopogon japonicus (L. f) Ker Gawl.), and Nardostachyos Radix et Rhizoma (Gancao, Glycyrrhiza uralensis Fisch.).
However, the complexity of the chemical composition of multi-herbal TCM formula brings great difficulties to the pharmacological research. Network pharmacology has been one of the approaches to reveal the complex pharmacological mechanisms behind the multitargeting properties by the multicomponent medicines (Li et al., 2011). TCM formulas are well known by the characteristics of multi-herbal/component, which would exert its clinical efficacy through multiple targeting, hence multi-pharmacological actions (Leung et al., 2014). However, most of the studies were centered on attributing the edge between component and target and considering the weight values of all the components as the same (Shao and Zhang, 2013). A good TCM formula would be formed with the right herbs in an accurate proportion that would produce the best possible clinical efficacy with none or minimal toxicity. Therefore, each herb in a formula would be indispensable in evaluating the clinical efficacy of the formula as well as in a network pharmacological mechanism mapping. Furthermore, in terms of compound formula which consists of multiple herbs, the target spectrum (target distribution of all monomer components) determines the therapeutic range of the formula, and high-weight components may determine the therapeutic direction.
In this study, we tried to introduce a parameter, weight coefficient, to mimic the proportion of all the encompassed components and resort their effects on different targets and pathways. We used the data from multiple bioassay databases, combining with the results of pharmacological assays of high-weight coefficient components; a closer-to-the-fact pharmacological panorama was constructed.
Materials and Methods
XSN Chemical Component Library Building
All of the chemical monomer components in the 11 herbs of XSN were retrieved from the book Chemical Components of Source Plants in Traditional Chinese Medicine (Zhou et al., 2009) and the database: TCM Systems Pharmacology Database and Analysis Platform (TcmSP™, http://lsp.nwu.edu.cn/tcmsp.php) (Ru et al., 2014). In addition, four important pharmacology-related properties of the collected components were also obtained from admetSAR (Cheng et al., 2012; Yang et al., 2018), including MW, ALogP, Hbond donor count, and Hbond acceptor count, Rotation bond count, and human oral bioavailability (HOB). The proportion of each herb in XSN formula was retrieved from the patent of XSN (Wang and Mao, 2018), and the contents of the main active components in all the 11 herbs were retrieved from published papers, and all the other components without quantitative data were set to be equal to the lowest quantity value of known components (details see Supplement Table 1).
Calculation of Weight Coefficients
Component weight coefficient:
wi,j is the weight coefficient of component j in herb i, and mi is the weight of herb i in XSN formula, n is the total count of herbs in XSN, Ci,j is the content of component j in herb i, and Mj is the molecular weight of component j, and pj(OB) is the predicted probability of positive human OB of component j.
The weight coefficient is the product of three elements: is the proportion of each herb in the XSN formula, represented the molar concentration of each component in 1 gram of raw herb in XSN formula, and pj(OB) is the probability of the bioavailability of each component reaching 30% of the total oral administration. Therefore, the coefficient wi,j is a relative quantity among all the components of the 11 herbs in XSN formula to reflect the proportional relationships of the components existed in the body.
Since the activity of the multiple herbal medicine involved in multiple targeting, and each target was usually bound by multiple components. Weight coefficient of one single target in this paper was represented by the sum of all the weight coefficients of the components interacting with this target. Furthermore, weight coefficient of each pathway was also represented by the sum of all the weight coefficients of all targets in this pathway.
Establishment of the Relationships Between Component-Target and Zheng Target
All the relative targets of each component in the 11 herbs of XSN were retrieved from BindingDB database (https://www.bindingdb.org/) as the component-target relationship library of XSN (Gilson et al., 2016). Since Zheng (TCM syndrome) is represented by the series of characteristics with clinical manifestations and symptoms (hereinafter referred to as TCM symptom), all the 10 TCM symptoms of PHHD arrhythmia referred in the indications of XSN capsule and the standard names of their corresponding modern medicine symptoms (MM symptom) were collected from SymMap database (https://www.symmap.org/) (Wu et al., 2018). All the target genes relative to each MM symptom above were obtained from the Human Phenotype Ontology Database (https://hpo.jax.org/app/) as the target-Zheng relationship library of PHHD arrhythmia.
Network Construction and Analysis
Two networks were constructed: the networks of PHHD target and XSN component targets. All the networks were visualized by Cytoscape 3.7.0 (http://www.cytoscape.org), which is an open-source project for complex network construction and visualizing and analyzing (Shannon et al., 2003). Pathway enrichment was carried out using Reactome pathway database (https://www.reactome.org) (Fabregat et al., 2017; Fabregat et al., 2018); the pathways with FDR < 0.05 were kept in the result.
Chemicals and Solutions
All the chemical agents were purchased from Sigma–Aldrich, and all high-weight coefficient components were obtained from Chengdu Herbpurify Co., Ltd. The intracellular buffer contained (in mM): KCl 120, MgCl2 2, CaCl2 1, Na2ATP 3, EGTA 11, HEPES 10, and pH 7.2 corrected with 5M NaOH. The extracellular buffer contained (in mM): NaCl 112, NaH2PO4•H2O 1, KCl 5.4, HEPES 5, NaHCO3 24, glucose 10, MgCl2 1.2, CaCl2 1.8, and pH 7.4 corrected with 5M NaOH. Components experimented in this paper were dissolved in the external buffer, and for the dose–response research, the concentrations of the compounds studied were ranging from 1 to 100 μM in the external buffer solution.
Electrophysiological Research
CHL cells stably expressing the α-subunit of human NaV1.5 (SCN5A) were used for electrophysiological assays. Patch clamp assays were carried out at room temperature (∼ 22 to 24°C), and the cells were superfused with the extracellular buffer at a rate of 2 ml/min. Patch pipettes were pulled from borosilicate glass (Harvard Apparatus, UK) using a DMZ-Universal Puller (Zeitz-Instruments, Germany); the average pipette resistance was 3–5 MΩ. The control currents were recorded 5 minutes after the whole-cell configuration was achieved using an Axopatch™ 200B Amplifier (Molecular Devices, USA).
Data Analysis
Data was analyzed and illustrated using pCLAMP 10.3 software (Axon Instruments, Inc.) and Origin 9.1. For each protocol and condition, at least five cells were tested. Data values are presented as mean ± standard error of the mean (SEM). The difference between the control and the effect of a compound was statistically tested using Student’s unpaired t-test. P < 0.05 was deemed to be statistically significant. Where results are not statistically significant, the actual p-value is provided.
Results
XSN Component Library
Nine hundred sixty-three monomer components from 11 herbs of XSN were collected from Chemical Components of Source Plants in Traditional Chinese Medicine and TCMSP database (Supplement Table 1). Chemical properties including AlogP, Hbond donor and acceptor count, molecular weight (MW), human OB, and rotatable bond count were calculated by AdmetSar 2.0 as described above, analyzed and shown as Figures 1A–F (Supplement Table 1). The component accounts of each herb were analyzed and shown in Figure 1G. The content of monomer components was obtained from published chemical constituent research indexed by PubMed (https://www.ncbi.nlm.nih.gov/pubmed) and CNKI (http://www.cnki.net/) (Supplement Table 1). The contents of 47 of 963 components above were quantified in the reports, and the content values were abstracted from the references. The contents of the remaining 916 components were set as the lowest value of the content reported components. The most abundant component berberine was 2.82×106 times that of neohesperidin which was with the lowest content in the 47 reported components. The weight coefficients for all the components were calculated, and top 40 with references were listed and shown in Figure 1H with higher weight components.
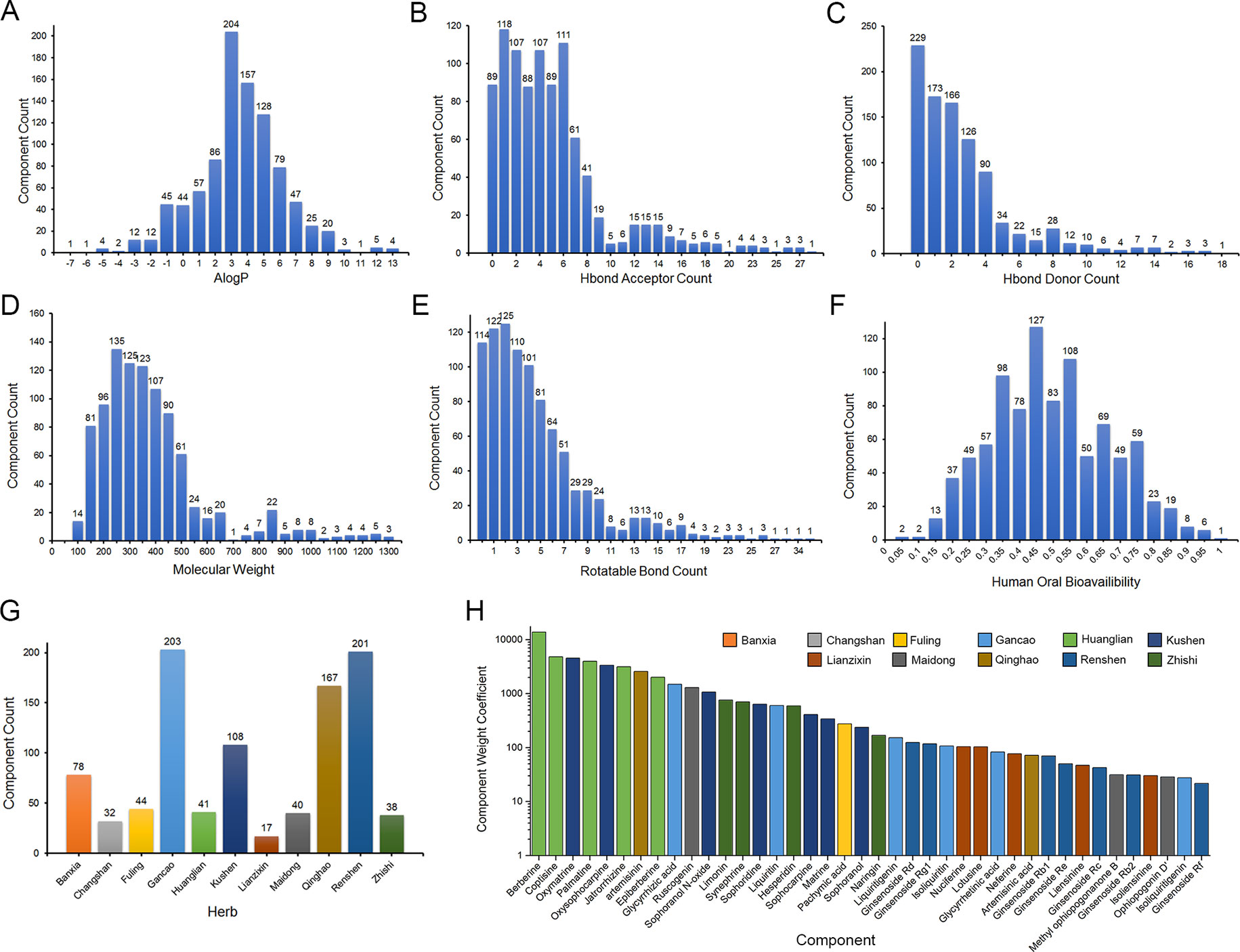
Figure 1 Chemical properties statistics of components in the 11 herbs of XSN. (A) AlogP, (B) Hbond donor count, (C) Hbond acceptor count, (D) Molecular weight (MW), (E) human oral bioavailability, (F) rotatable bond count, (G) component count of each herb collected in XSN component library, and (H) weight coefficient of top 40 weight components in 11 herbs. The different colors means the source herb of components, which is same as (G).
XSN Target Spectrum
Two thousand eight hundred thirty-five component-target relationship data were obtained, covering 487 monomer components and 618 targets (Supplement Table 2). The classification of targets was analyzed using the ChEMBL hierarchical target classification system (Bento et al., 2014), and the classification of enzymes were updated according to the ENZYME database (Bairoch et al., 2004). Major and secondary classifications of target spectrum were shown as Figure 2 (Supplement Table 3). The proportion of targets were grouped by the means of target count in Figure 2, which shows the proportion of different target categories and also shows the differences between statistics by target count and weight coefficient, especially for cytochrome P450, hydrolase, nuclear receptor, enzymes, oxidoreductase, protease, protein kinase, and VGIC (proportion > 5% and fold change >3).
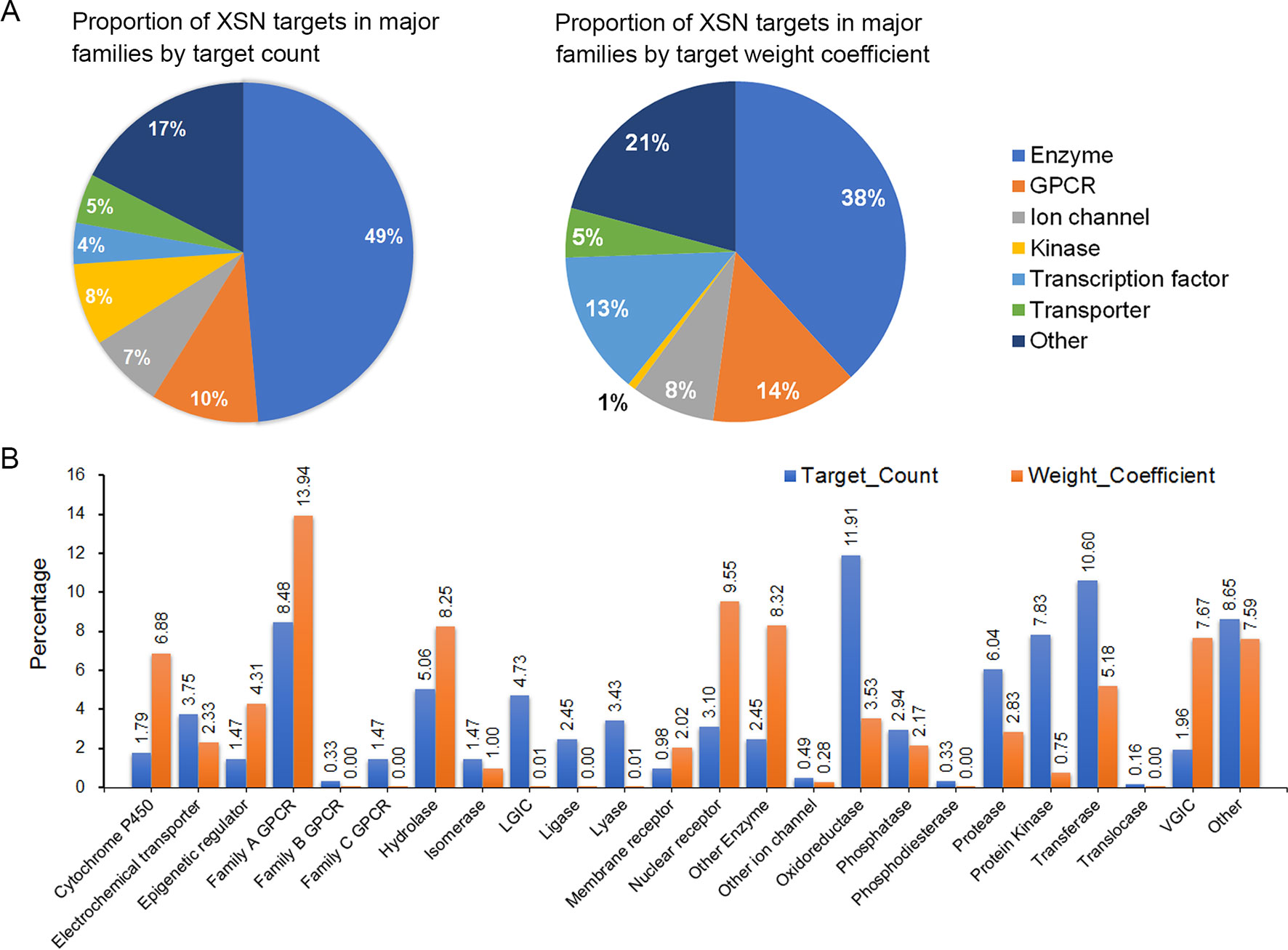
Figure 2 The targeting spectrum of XSN. (A) Statistic and major classifications of XSN target spectrum by target count and (B) statistic and secondary classifications of XSN target spectrum. The X axis of (B) is labeled with targets including family A GPCR, rhodopsin-like GPCRs; family B GPCR, secretin-like GPCRs; family C GPCR, metabotropic glutamate receptor family; LGIC, ligand-gated ion channel; VGIC, voltage-gated ion channel.
PHHD-Arrhythmia Target Spectrum
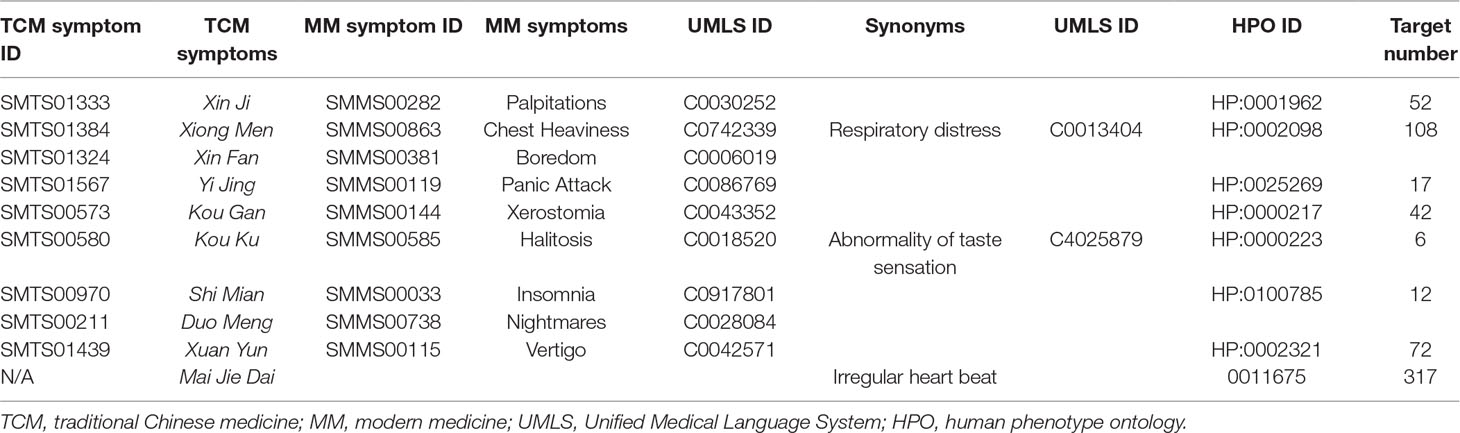
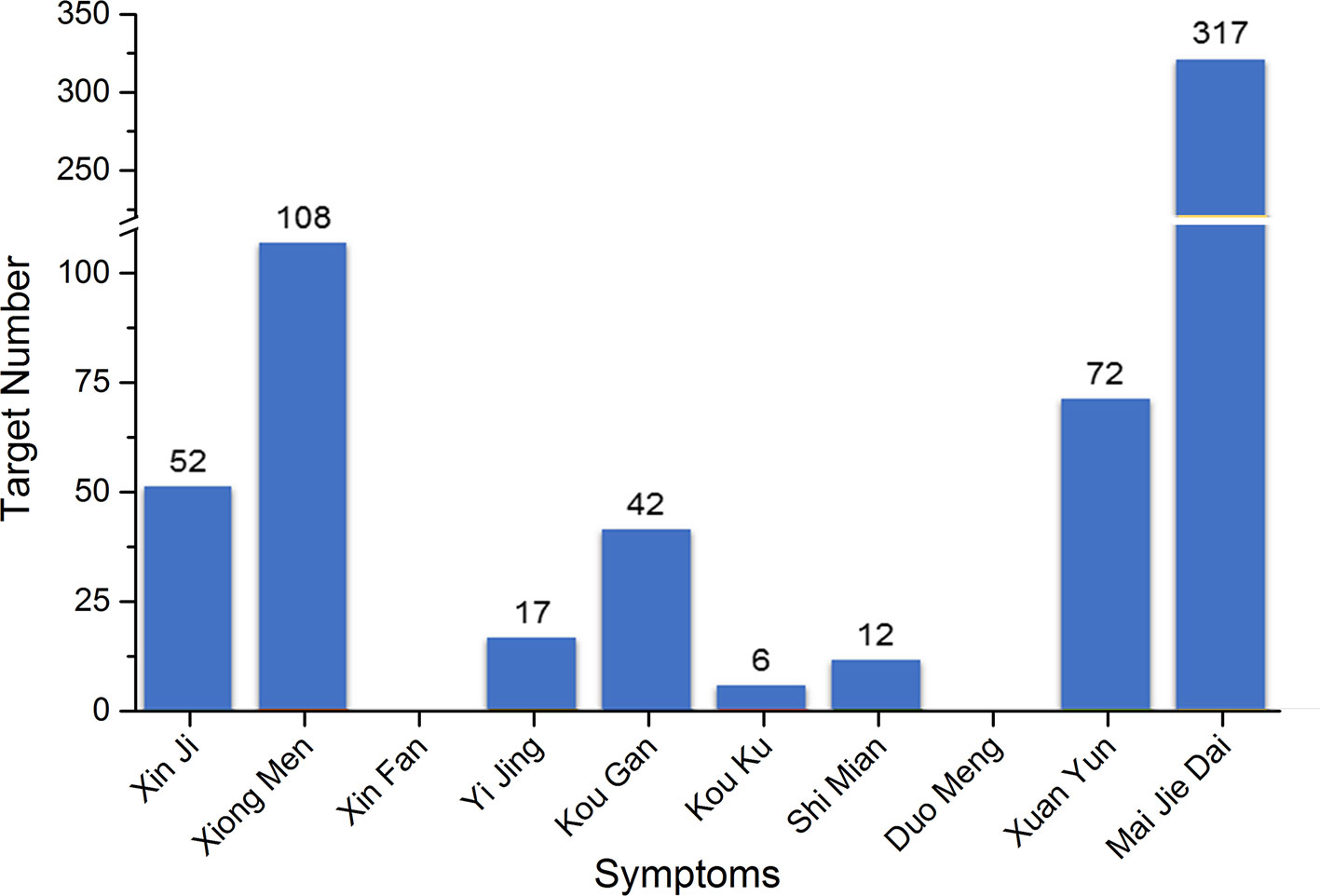
Since Zheng (TCM syndrome) is represented by the series of characteristics with clinical manifestations and symptoms (hereinafter referred to as TCM symptom); all the 10 TCM symptoms of PHHD arrhythmia and their relative modern medicine symptoms (MM symptom) were collected: Xin Ji (palpitations), Xiong Men (respiratory distress), Xin Fan (boredom), Yi Jing (panic attack), Kou Gan (xerostomia), Kou Ku (bitter taste in the mouth), Shi Mian (insomnia), Duo Meng (dreaminess), Xuan Yun (vertigo), and Mai Jie Dai (knotted or regularly intermittent pulse) (Table 1, Supplement Table 4).
TCM symptom Kou Ku means bitter taste in the mouth, but not bad breath in the mouth; therefore, the MM symptom halitosis retrieved from SymMap was replaced by another keyword: abnormality of taste sensation. Mai Jie Dai included two types of pulses: Jie Mai is knotted or bound pulse, which is slow, relaxed and stops at irregular intervals. Jie Mai represents an irregular beat or palpitation stemming from the heart. Dai Mai means the pulse is with regularly intermittent abnormality, which also suggests the patients with this pulse have advanced heart disease according to modern medicine. Therefore, an alternative keyword irregular heartbeat was used to represent the TCM symptom Mai Jie Dai.
Five hundred two targets in total for PHHD arrhythmia were collected as PHHD-arrhythmia target spectrum; statistics of target count for each TCM symptom were shown in Figure 3 (Supplement Table 4).
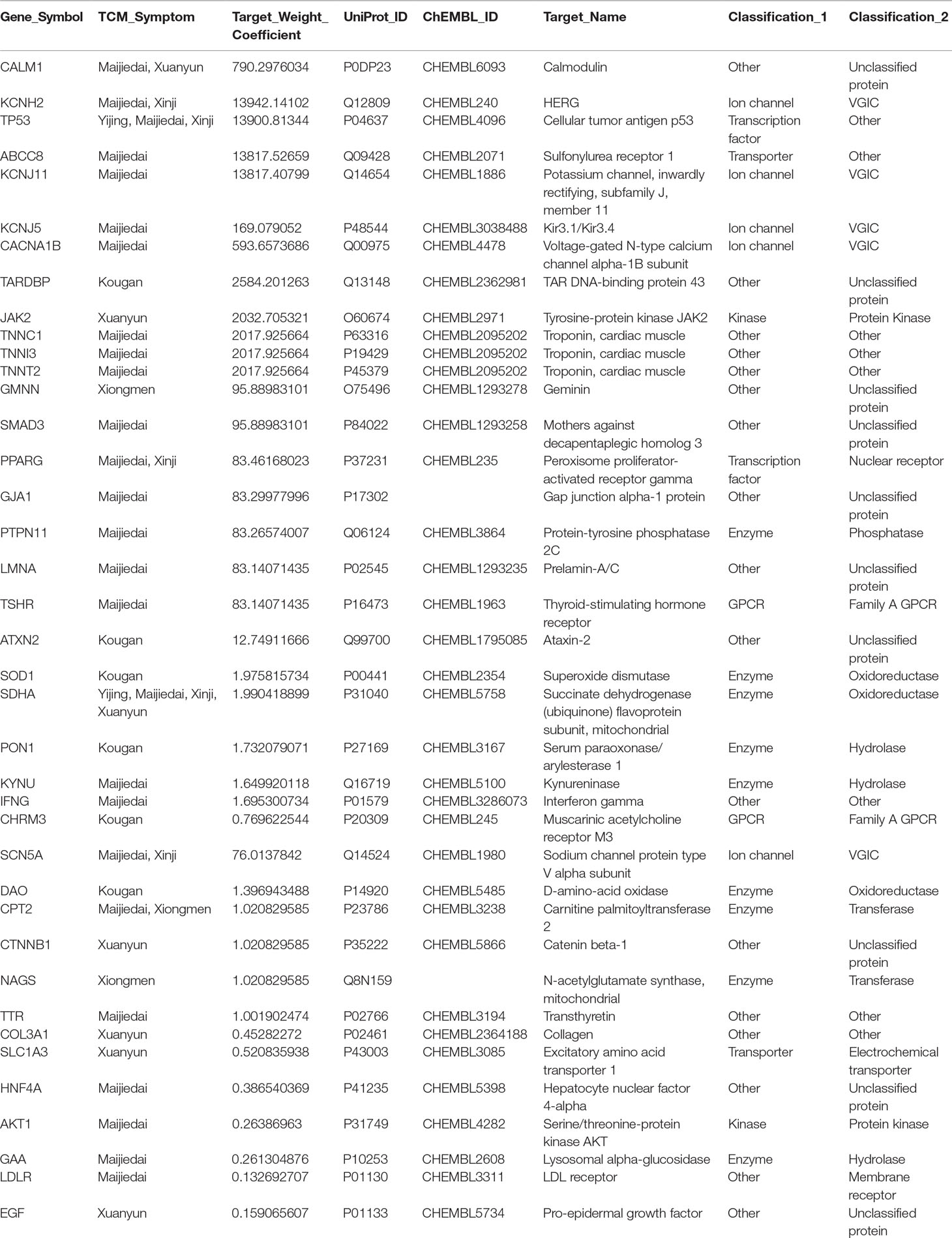
To obtain the pharmacological network of XSN on treating PHHD arrhythmia, 40 targets were obtained in the common set of XSN target spectrum and PHHD-arrhythmia target spectrum (Table 2). All the components interact with the 40 targets above consist of the panoramagram of the pharmacological mechanism of XSN for treating PHHD arrhythmia.
Pharmacodynamic Research of High-Weight Components in XSN
Based on our previous study, XSN is a class III anti-arrhythmic drug supported by the prolongation of the action potential of cardiac myocytes through blocking hERG channel (Ma et al., 2016; Wang et al., 2017), and it also showed class I antiarrhythmic property of blocking sodium channels (Wang et al., 2019b). However, the available pharmacological research data of all the components in the 11 herbs of XSN reported and recorded in all the databases are not sufficient to correlate with the clinical antiarrhythmic efficacy and our discovery that inhibit human cardiac sodium current, NaV1.5 channel (encoded by gene SCN5A) with total target weight coefficient of 1.32 (Wang et al., 2019b). Comparing with the effect of hERG channel (encoded by KCNH2), total target weight coefficient of hERG is 14838.89; both of them are with similar IC50 values generated by XSN, which can be considered as a combination of all the components. Thus, we screen the set of high-weight components with reported content in corresponding herbs on inhibiting NaV1.5.
Two high-weight active components: liensinine (LSN, PubChem CID: 160644) and isoliquiritigenin (ISL, PubChem CID: 638278) were detected using electrophysiological approaches. LSN is the No. 35 high-weight component, which blocked human NaV1.5 channel dose-dependently with an IC50 of 3.58 ± 0.36 μM (Figure 4). ISL reduced peak INa concentration dependently, with a median inhibitory concentration (IC50) of 10.11 μM ± 1.12 μM. The positive control amiodarone (AMD) blocks NaV1.5 channel with IC50 = 5.05 ± 0.55 μM.
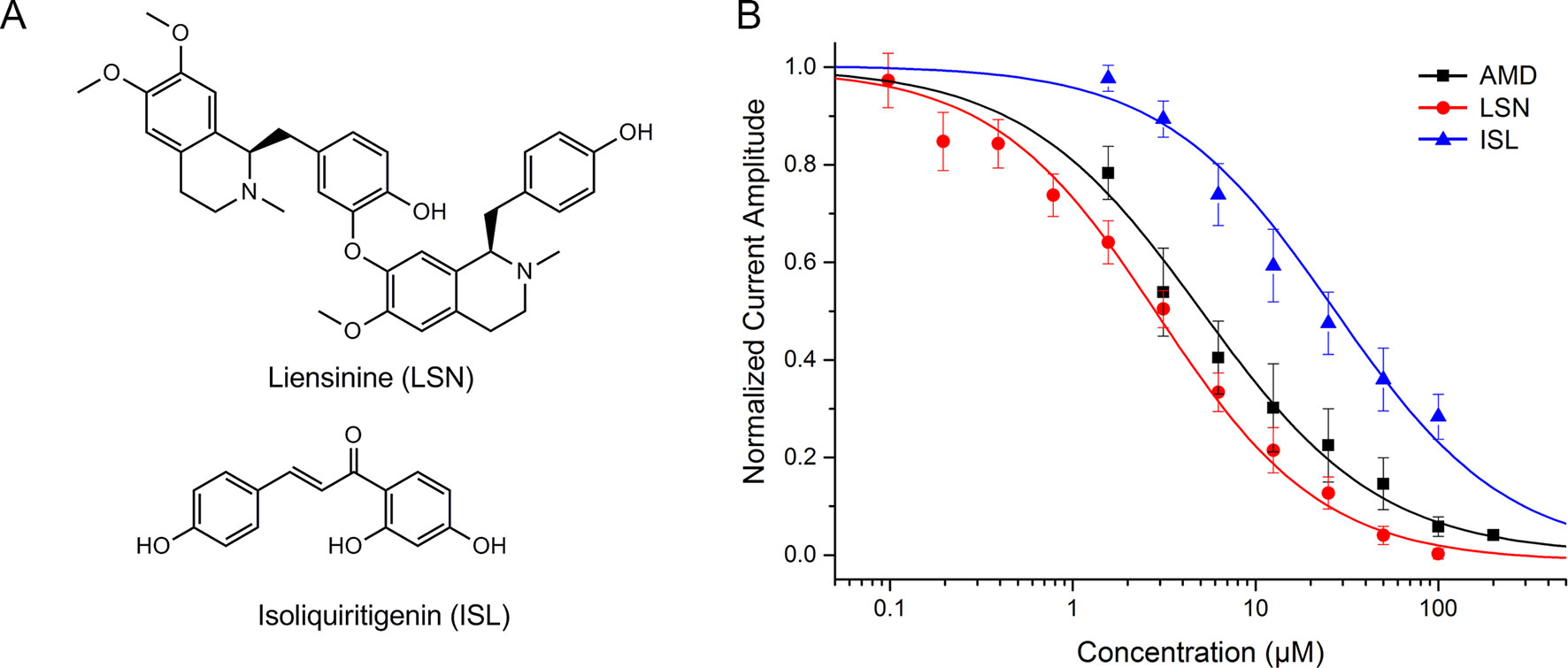
Figure 4 (A) Chemical structure of liensinine (LSN) and isoliquiritigenin (ISL). (B) Dose–response curve of the inhibition on human NaV1.5 channel by amiodarone (AMD, n=5), LSN (n=6), and ISL (n=8).
Panoramagram of the Pharmacological Mechanism of XSN
Including the pharmacological effects of LSN and ISL, a panoramagram of the integrative pharmacological mechanism of XSN was interpreted (shown as Figure 5). The pharmacological network consisted of 963 components, 618 targets, and 10 symptoms. This panoramagram illustrated the network pharmacological relationships among the XSN formula, herbs, components, targets, symptoms, and PHHD arrhythmia.
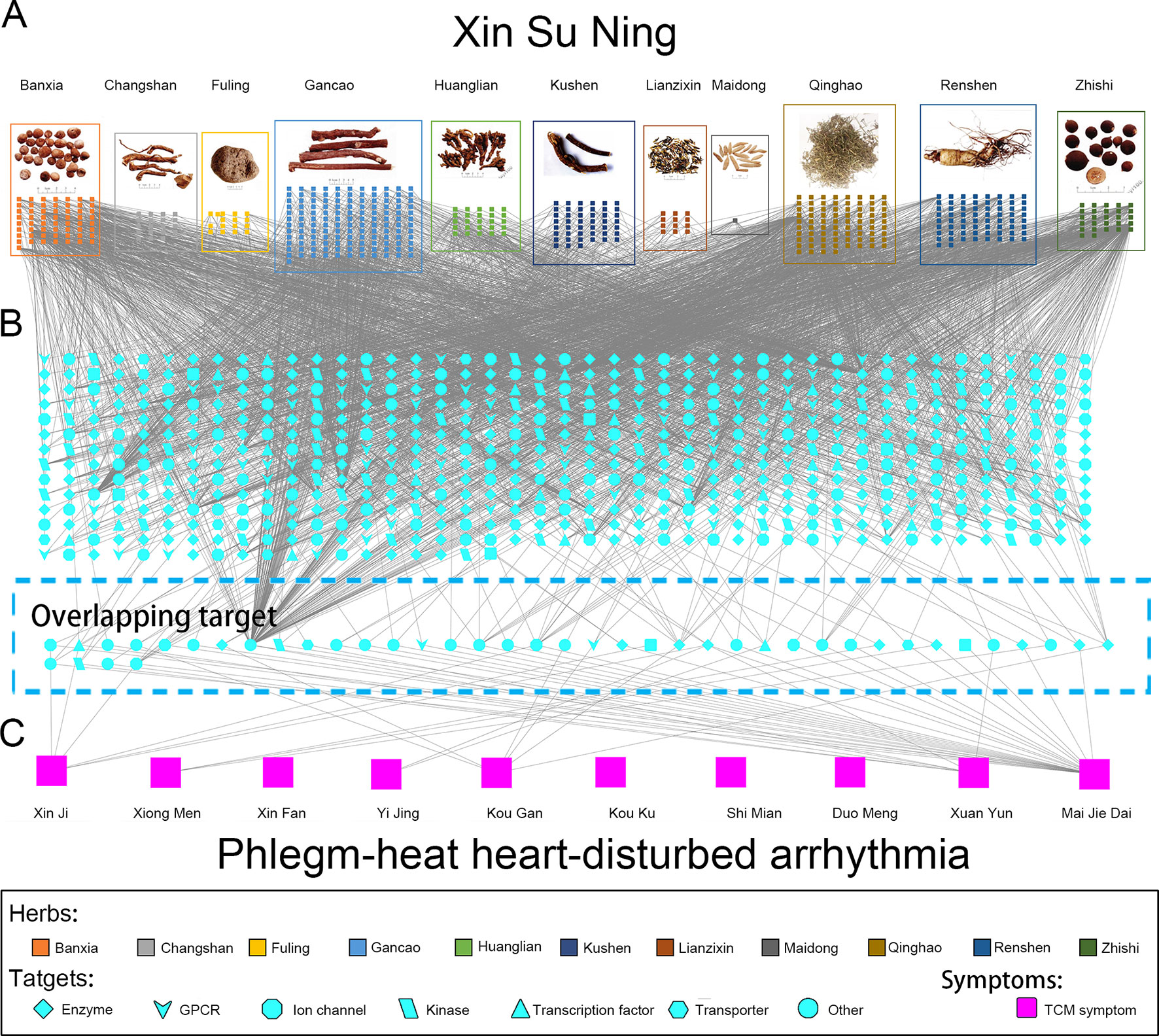
Figure 5 Panoramagram of the pharmacological mechanism of XSN for treating PHHD arrhythmia. This panoramagram illustrated the connections among the XSN formula, herbs, components, targets, symptoms, and PHHD arrhythmia. (A) XSN formula and components. All the 11 herbs were shown as figures in the box with different colors, and the components of each herb were shown as small square with the same color as their source herb. (B) XSN target spectrum. All the targets were corresponding to the component in panel A; the 41 targets showed in the dash line box in the bottom is the overlapping target set between XSN target spectrum and PHHD-arrhythmia target spectrum. Targets were shown in different shapes according to the classifications as indicated in the keys. (C) Arrhythmia with PHHD Zheng and relative TCM symptoms. Ten TCM symptoms of PHHD arrhythmia were represented by large pink squares in the bottom panel.
Pathways Involved in the Pharmacological Mechanism of XSN on Treating PHHD Arrhythmia
All the 40 common targets between XSN target spectrum and PHHD-arrhythmia target spectrum were selected to carry out pathway enrichment with Reactome application in Cytoscape. 117 pathways with FDR less than 0.05 were obtained and resort by total weight coefficients (Figure 6, Supplement Table 5).
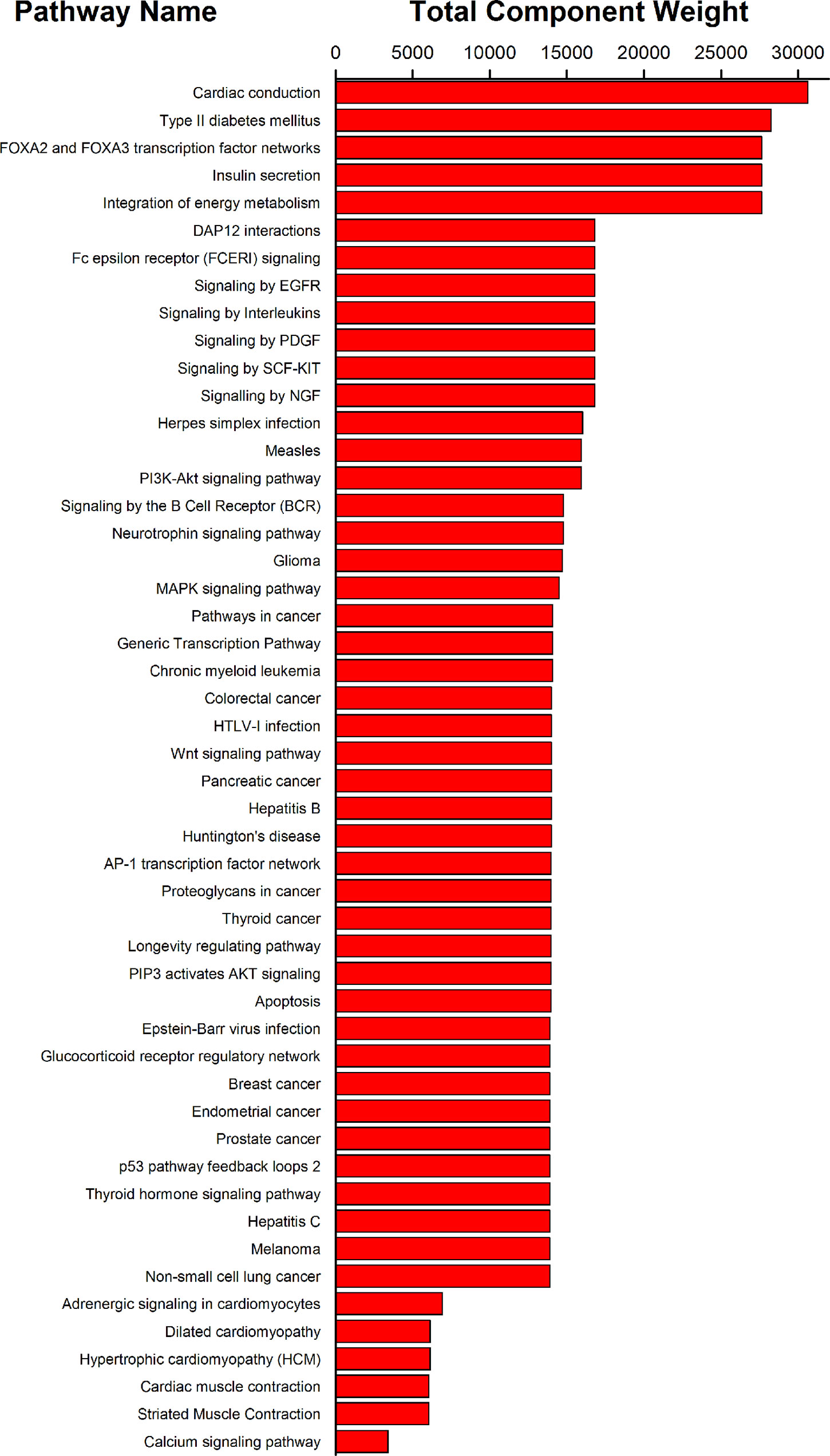
Figure 6 Pathway enrichment results of 40 targets of XSN on treating PHHD arrhythmia. Top 50 pathways were shown here; and all were sorted by pathway weight coefficient.
To better understand the antiarrhythmic therapeutic efficacy of XSN, cardiac conduction pathway is calculated to hold the highest total weight coefficient in the list of resorting pathway total weight coefficient. It was shown in Figure 7 and all the components in XSN involved in regulating this pathway were marked in the figure.
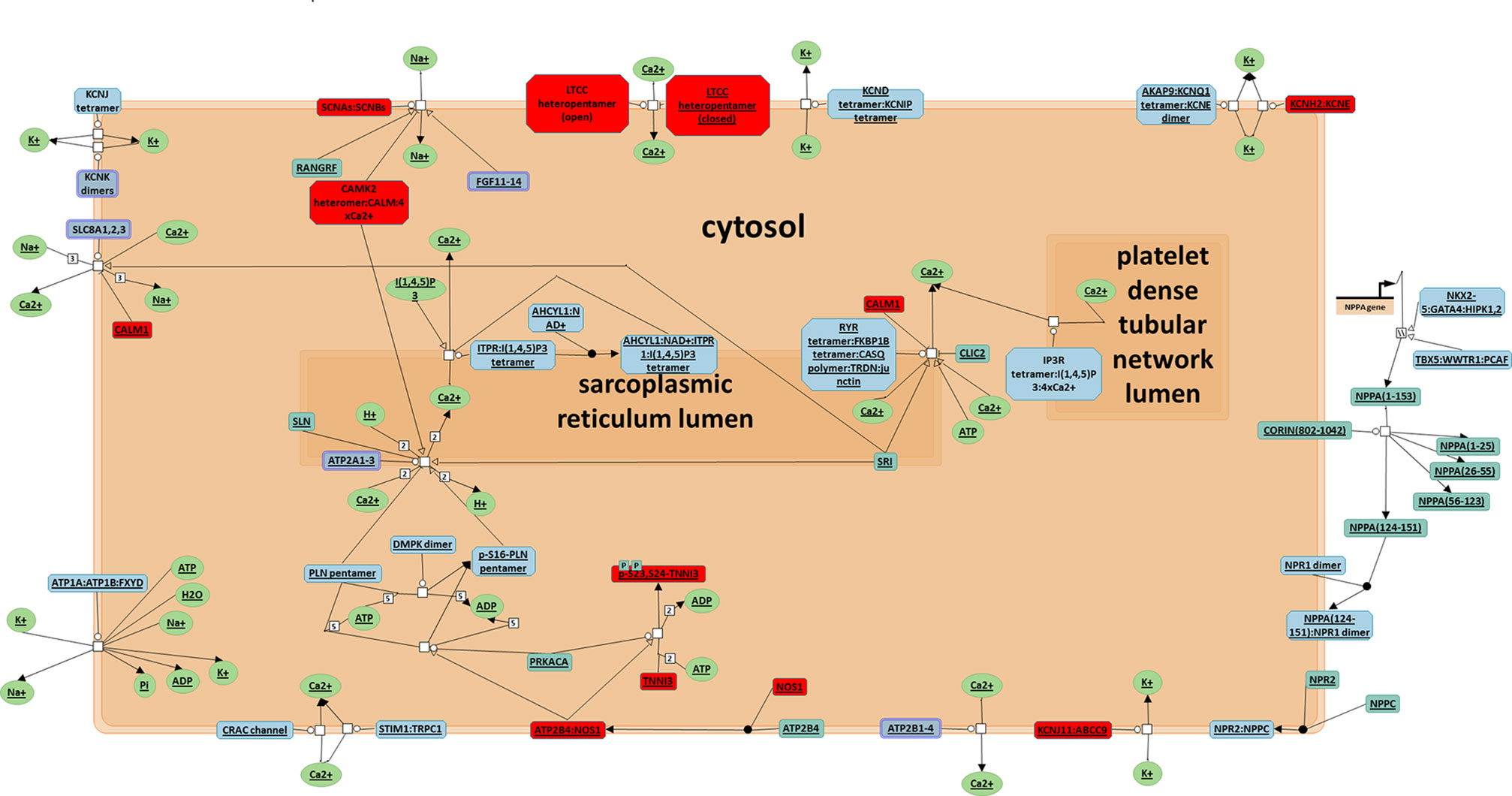
Figure 7 Cardiac conduction pathway. Top 1 pathway in the rank of total target weight coefficient, all the target regulated in this pathway were marked in red (Jassal, 2014).
Discussion
Tan-Re-Rao-Xin Zheng is named as PHHD syndrome in English. Based on the traditional Chinese Medicine theories, PHHD is defined as that heat having existed in the body for a period of time without distributing leads to the body's fluids being concentrated as Phlegm. The concept Heart in the Chinese medicine mainly refers to the circulatory system and nervous system, and they are easily to be blocked or obstructed to cause malfunction (Jiao et al., 2015). This is why PHHD is classified as an “excess” syndrome; the heat and the phlegm must be cleared to treat the symptoms in circulatory system and neuronal system successfully.
Physical symptoms of PHHD are usually consist of the followings (Yongdun et al., 2005; Ren et al., 2012; Xing et al., 2015): (1) insomnia, difficulty in falling asleep and frequent dream-disturbed waking. (2) Palpitations, the heart rhythm becomes out of control, e.g., PVCs or tachycardia. However, it depends on the severity of how much the heart had been disturbed by the phlegm fire; the clinical manifestation could range from incidental PVC to frequent PVC or from normal heart rate to tachycardia. In the region of the heart, there is often a sensation of heat or discomfort and oppressed chest, feeling difficult to breath. (3) Tongue, tongue body is red, especially at the tip, which is often swollen and painful. The tongue coating is yellow and thick. There is very often a mid-line crack extending to the tip, with yellow moss on it. Hypertension—high blood pressure—is often a feature of heart phlegm fire. (4) Pulse, slippery, and rapid and overflowing which means “excess”; it often appears after the first stage of infectious disease and shows excess heat. Complexion is red; eyes are bloodshot. Some mental symptoms could also happen, like boredom or even hypomania which also depends on the severity of phlegm-heat disturbing the neural system (which is also part of the Heart in TCM theory). Therefore, arrhythmia with obvious clinical signs of PHHD is a particular condition that the patient suffering from phlegm-heat, and the circulation system would be mostly influenced.
XSN capsule originated from the famous compound formula Huanglian Wendan (HLWD) Decoction recorded in 150 years ago, which has the effect of removing phlegm, blood stasis, and fire toxin. Huanglian, Banxia, Fuling, Gancao, and Zhishi are from the original formula HLWD decoction; Changshan, Lianzixin, Qinghao, and Kushen were added to make XSN formula more inclined to the circulation system. Therefore, XSN was not only a combination of anti-arrhythmic monomer components, but also a complex therapeutic system to regulate biological/pathological process relative to PHHD syndrome.
The compatibility principle of the herbs in XSN also reflects another key property of Chinese herbal medicine: amount/proportion of herbs in compound formula. For a better understanding of the pharmacological mechanism of XSN, we introduced a novel parameter, weight coefficient, to mimic the compatible combination of all the chemical components in the 11 herbs in XSN. As mentioned in methods section, weight coefficient is the product of proportion of herbs in the compound formula, content of each component in relative herb and the predicted probability of human OB of each component. Weight coefficient is not the absolute concentration of each chemical component but a relative content among them, which means the ranked order of weight coefficient of each component can be considered as the extent how much a component involved in the pharmacological mechanism of XSN. The weight coefficient of each component reflects its molar concentration in the blood; therefore, the higher the weight coefficient, the more molecules will bind to the targets. However, the binding affinity of the component to the target remains to be tested pharmacologically.
In this study, we used network pharmacological approach to visualize the complex pharmacological mechanism of XSN on treating PHHD arrhythmia. All the data were collected from databases and books to assure the quality and reliability of all the relationships in the pharmacological network. Based on our previous study, XSN can be classified as class III antiarrhythmic drug, and with some pharmacological property of class I drug (Sweeney et al., 2019; Wang et al., 2019a; Wang et al., 2019b). The application of XSN resulted in significant morphological changes of the ECG trace of isolated rat heart featured by the suppression of the R wave amplitude. The QRS complex represents ventricular depolarization as a result of activation of NaV1.5. Our previous study attributed the decrease in R wave amplitude to XSN dependent inhibition of INa; when whole-cell sodium currents were elicited using a depolarizing pulse protocol, it was evident that XSN inhibited INa concentration dependently and reversibly. However, the data from reported research recorded in the databases were not sufficient to support all the antiarrhythmic effect of XSN; we, therefore, carried out a small scale component screening on the target of class I antiarrhythmic drug, human NaV1.5 channel. Given previous collected in this paper did not provide enough weight coefficient on NaV1.5 channel, some unidentified high-content components should target NaV1.5. Thus, we screened a series of high-content components listed in Figure 1H, to replenish the component-target information. LSN and ISL were identified as active components on NaV1.5 channel in the present study. Briefly speaking, the pharmacologically defined inhibition of NaV1.5 by the active components of the 11 herbs in XSN suggests that Lien and ISL bind to the channel in its inactivated state which reduces the INa amplitude. The total weight coefficient of NaV1.5 is 76.013, which is still much lower than hERG 13942.141. The reason is understandable that, based on the content of components in the mixture of raw herbs of XSN, the weight of Huanglian (334g/2,377g mixture of raw herbs) is much more than Lianzixin (42g/2,377g mixture of raw herbs) and Gancao (167g/2,377g mixture of raw herbs); meanwhile, the concentration of berberine is much higher than LSN and ISL in relative herbs (details see Supplement Table 1). Secondly, the experimental inhibitory effect of NaV1.5 by XSN was carried on directly on isolated heart or cell lines without influenced by absorption, distribution, metabolism, and excretion; the predicted oral bioavailability also lower down the weight coefficients of LSN and ISL. Technically, more active components inhibiting NaV1.5 were still undiscovered, and further research on XSN is still needed.
Including the pharmacological effects of LSN and ISL, we drew a panoramagram of the integrative pharmacological mechanism of XSN with 475 components targeting 617 targets. Among the 617 targets, 40 targets were relative to PHHD arrhythmia. To explain the mechanism, we carried out pathway enrichment instead of subnetwork analysis, 116 pathways were obtained, and top 50 were shown as Figure 6.
As an antiarrhythmic drug, XSN showed multiple therapeutic effects. From the standpoint of TCM treatment, both tip and root causes can be reflected in the pathways, which can also be considered as quick-acting and long-acting mechanisms. First, ion channels play essential role in terminating abnormal electrical activity in arrhythmic heart. Based on the results from the analysis above, the mechanism on cardiac electronic activity may involve such targets: TNNI3, CACNA1S, SCN5A, KCNH2, KCNJ11, and CALM1. NaV1.5, CaV1.2, and hERG are three key ion currents in cardiac electric conduction during depolarization and repolarization. Troponins I (encoded by TNNI3) is integral to cardiac muscle contraction, which is also used as diagnostic and prognostic indicators in the management of myocardial infarction and acute coronary syndrome. Calmodulin 1 (encoded by CALM1) mediates the regulation of plenty of enzymes, ion channels, aquaporins, and other proteins acting with calcium-binding. Secondary, as long-acting mechanism, XSN was used to treat arrhythmia caused by coronary heart disease and viral myocarditis. Protection of myocardium, such as protection of I/R injury, antithrombosis and anti-apoptotic effect should be the essential effects of XSN. Intracellular sodium accumulation is a key pathophysiological mechanism in myocardial ischaemia/reperfusion (I/R) injury. Furthermore, it is thought that, Nav1.5, alongside NHE and other transporters, contributes to this sodium overload that can be reduced with application of lidocaine, suggesting that inhibition of INa may attenuate I/R injury. Previous work has shown XSN is cardioprotective in I/R injury induced in isolated rat hearts. Given the effect of ISL in inhibiting Nav1.5, it is possible that LSN and ISL reported in the present study are responsible for some of XSN’s cardioprotective actions in this way.
Besides of calcium overload, another reason of myocardium injury was caused by apoptosis of cardiomyocyte. The p53 tumor suppressor is one of the major apoptosis signaling pathways. It regulates a wide variety of genes involved in apoptosis, growth arrest, or senescence in response to genotoxic or cellular stress. The p53 can promote apoptosis through interactions with Bcl-2 family proteins in the cytoplasm. In addition, HIF1 alpha was also downregulated by the top1 component berberine (Mao et al., 2018). Berberine inhibits doxorubicin-induced cardiomyocyte apoptosis (Lv et al., 2012).
Inflammatory response is well recognized as a critical contributor for the development and complications of atherosclerosis cardiovascular disease (ASCVD), including myocardial infarction (MI), heart failure, and stroke, which involve complex interactions between multiple biological processes.
Besides of cardiovascular pathways, several diabetic pathways were also obtained. In TCM system, disease with diabetes-related symptoms is called “Xiaoke,” which is primarily caused by deficiency of Yin with dryness and heat syndromes as the secondary cause (Tong et al., 2012). As both PHHD and Xiaoke partially shared the pathogenesis of heat, it is understandable to share some biological pathways in common. In type II diabetes mellitus, insulin secretion, integration of energy metabolism pathways, KCNJ11, and ABCC8 were the common targets, which may be regulated by XSN. The beta‐cell ATP‐sensitive potassium (KATP) channel is a key component of stimulus‐secretion coupling in the pancreatic beta‐cell. The channel coupled metabolism to membrane electrical events bringing about insulin secretion. The channel consists of four subunits of the inwardly rectifying potassium channel Kir6.2 and four subunits of the sulfonylurea receptor 1 (SUR1).
Beyond of these targets, several targets including fatty acid synthase (FASN), pyruvate kinase L/R (PKLR), acetyl-CoA carboxylase (ACC, alpha unit encoded by ACACA), transketolase (TKT), free fatty acid receptor 1(FFAR1), glucagon-like peptide 1 receptor (GLP1R), and so on were also regulated by XSN. These targets are involved in the bioprocess of glycolysis, fatty acid synthesis, and pentose phosphate pathway, which are key steps of glucose and lipid metabolism. Through these mechanisms, XSN may improve fatty acid storage and convert it into glycogen. Lipid metabolism disorder has been reported being related to the TCM heat syndrome (Lv et al., 2012), which also suggest XSN regulate glucose and lipid metabolism to act as removing the extra heat based on the TCM theories.
In summary, the mechanism of XSN on treating PHHD arrhythmia may act as follows: on quick-acting aspect, XSN balanced the ion current of the heart by regulating multiple ion channels to terminate cardiac arrhythmia; on the long-acting aspect, XSN protects the heart from I/R injury, inhibits the apoptosis of cardiomyocyte, and improves glucose and lipid metabolism. Part of the mechanism of XSN on treating PHHD syndrome by sharing pathways with insulin secretion or glucose and lipid metabolism also suggest the potential therapeutic effect of diabetes and diabetic cardiopathy.
Data Availability Statement
All datasets generated for this study are included in the manuscript/Supplementary Files.
Author Contributions
Y-LM and TW conceived and designed the study; TW and HS performed the electrophysiological experiments; XW discussed, analyzed and standardized the TCM symptoms; UP and ML analyzed and discussed the biological functions of the pathways; CC provided experimental equipment and technical support for cell culturing; TW and Y-LM wrote the paper.
Funding
This work was supported by grants from the Chinese Medicine Research Fund, University of Oxford. The grant was funded by Shaanxi Momentum Pharmaceutical Co.,Ltd.
Conflict of Interest
The authors declare that this study received funding from Shaanxi Momentum Pharmaceutical Co., Ltd. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Acknowledgments
We thank Prof. Yan Zhu (Tianjin University of Traditional Chinese Medicine) for providing the stable transfected cell lines and Dr. Hongbin Yang (East China University of Science and Technology) for technical support on AdmetSar 2.0 system.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2019.01138/full#supplementary-material
Table S1 | XSN component library.
Table S2 | XSN component target relationships.
Table S3 | XSN Target Weight Coefficient type.
Table S4 | PHHD-arrhythmia target spectrum.
Table S5 | XSN PHHD Pathway.
References
Atarashi, H., Hayakawa, H. (1996). Etiology and classification of cardiac arrhythmias. Nippon Rinsho 54, 2023–2028.
Bairoch, A., Boeckmann, B., Ferro, S., Gasteiger, E. (2004). Swiss-Prot: Juggling between evolution and stability. 5(1), 39–55. doi: 10.1093/bib/5.1.39
Barsky, A. J. (2001). Palpitations, arrhythmias, and awareness of cardiac activity. Ann. Intern. Med. 134, 832–837. doi: 10.7326/0003-4819-134-9_Part_2-200105011-00006
Bento, A.P., Gaulton, A., Hersey, A., Bellis, L.J., Chambers, J., Davies, M., et al. (2014). The ChEMBL bioactivity database: an update. Nucleic Acids Res. 42(D1), D1083–D1090. doi: 10.1093/nar/gkt1031
Chen, G., Wu, X. (1996). Treatment of refractory ventricular arrhythmia with the combined use of FDP, berberine, XinBaoWan and modified SMS. J. Shantou Univ. Med. College 2, 90–91.
Chen, W., Ba, Z. (2010). Prof. ZHANG Yi’s experience in treating severe arrhythmia. J. Tradit. Chin. Med. 30, 47–50. doi: 10.1016/S0254-6272(10)60012-X
Cheng, F., Li, W., Zhou, Y., Shen, J., Wu, Z., Liu, G., et al. (2012). admetSAR: a comprehensive source and free tool for assessment of chemical admet properties. J. Chem. Inf. Model. 52 (11), 3099–3105. doi: 10.1021/ci300367a
Dong, S., Liu, J., Han, L., Zhao, W., Luan, J. (2005). Contents of trace and constant elements and therapeutic effects of Chinese patent medicine on cardio-cerebral vascular disease: fuzzy classified principle. Chin. J. Tissue Eng. Res. 9, 254–256. doi: 10.3321/j.issn:1673–8225.2005.15.069
Dong, Y., Liao, J., Yao, K., Jiang, W., Wang, J. (2017). Application of traditional Chinese medicine in treatment of atrial fibrillation. Evid. Based Complement.Altern. Med. 2017, 1–11. doi: 10.1155/2017/1381732
Fabregat, A., Jupe, S., Matthews, L., Sidiropoulos, K., Gillespie, M., Garapati, P., et al. (2018). The Reactome pathway knowledgebase. Nucleic Acids Res. 46, D649–d655. doi: 10.1093/nar/gkx1132
Fabregat, A., Sidiropoulos, K., Viteri, G., Forner, O., Marin-Garcia, P., Arnau, V., et al. (2017). Reactome pathway analysis: a high-performance in-memory approach. BMC Bioinformatics 18, 142. doi: 10.1186/s12859-017-1559-2
Gilson, M.K., Liu, T., Baitaluk, M., Nicola, G., Hwang, L., Chong, J. (2016). BindingDB in 2015: A public database for medicinal chemistry, computational chemistry and systems pharmacology. Nucleic Acids Res. 44(D1), D1045–D1053. doi: 10.1093/nar/gkv107
Gu, C., Wu, Y., Tian, S., Gao, X., Qi, X., Jia, Z., et al. (2005). Effect of shensong yangxin capsule on ventricular premature beat and cardiovascular autonomic nervous function in patients with coronary heart disease. Zhongguo Zhong Xi Yi Jie He Za Zhi 25, 783–786. doi: 10.3321/j.issn:1003–5370.2005.09.004
Hong, Y., Huang, Y., Wu, H., Chen, Y., Li, F., Mo, H. (2005). Clinical studies on relationship between TCM syndromes of coronary heart disease and inflammatory factors. J. of Guangzhou Univ. of Tradit. Chin. Med. 22(2), 81–86
Jassal, B. (2014). Image for "Cardiac conduction". Reactome: R-HSA-5576891.2, doi: 10.3180/R-HSA-5576891.1
Jiang, M., Lu, C., Zhang, C., Yang, J., Tan, Y., Lu, A., et al. (2012). Syndrome differentiation in modern research of traditional Chinese medicine. J Ethnopharmacol 140, 634–642. doi: 10.1016/j.jep.2012.01.033
Jiao, Y., Han, Y., Li, X., Fang, Y.-G., Liu, Z.-H., Zhou, W.-N., et al. (2015). Comparison of Body, Auricular, and Abdominal Acupuncture Treatments for Insomnia Differentiated as Internal Harassment of Phlegm-Heat Syndrome: An Orthogonal Design. Evid. Based Complementary Altern. Med. 2015, 1–9. doi: 10.1155/2015/578972.
Kalifa, J., Avula, U. M. R. (2012). The Chinese herb extract Wenxin Keli: atrial selectivity from the far east. Heart Rhythm 9, 132–133. doi: 10.1016/j.hrthm.2011.11.030
Leung, E. L. H., Wong, V. K. W., Jiang, Z. H., Li, T., Liu, L. (2014). Integrated network-based medicine: the role of traditional Chinese medicine in developing a new generation of medicine. Science 346, S16–S18.
Li, F., Zhang, C. (2015). Clinical study of Xinsuning capsule in the treatment of paroxysmal atrial fibrillation. Med. Front. 5, 95–97. doi: 10.3969/j.issn.2095-1752.2015.26.077
Li, M., Guihua, T. (2018). Therapeutic effects of Wenxin Keli in cardiovascular diseases: an experimental overview. Front. Pharmacol. 9, 1005. doi: 10.3389/fphar.2018.01005
Li, S., Zhang, B., Zhang, N. (2011). Network target for screening synergistic drug combinations with application to traditional Chinese medicine. BMC Syst. Biol. 5, S10. doi: 10.1186/1752-0509-5-S1-S10
Lin, L., Liu, Y., Lin, H. (2011). Observation on twenty-six cases of frequent premature ventricular contractions treated with Xinsuning capsules. People’s Mil. Surg. 54, 512.
Liu, Y., Li, N., Jia, Z., Lu, F., Pu, J. (2014). Chinese Medicine Shensongyangxin Is Effective for Patients with Bradycardia: Results of a Randomized, Double-Blind, Placebo-Controlled Multicenter Trial. Evidence-Based Complementary and Alternative Medicine 2014, 1–6. doi: 10.1155/2014/605714
Lu, A.-P., Jia, H.-W., Xiao, C., Lu, Q.-P. (2004). Theory of traditional Chinese medicine and therapeutic method of diseases. World J. Gastroenterol. 10, 1854. doi: 10.3748/wjg.v10.i13.1854
Lv, X., Yu, X., Wang, Y., Wang, F., Li, H., Wang, Y., et al. (2012). Berberine inhibits doxorubicin-triggered cardiomyocyte apoptosis via attenuating mitochondrial dysfunction and increasing Bcl-2 expression. PloS one 7, e47351. doi: 10.1371/journal.pone.0047351
Ma, Y., Wang, T., Ellory, C., Wilkins, R., Carr, C., Mao, P., et al. (2016). Investigation of the active antiarrhythmic components of the multi-herbal medicine Xin Su Ning. Proceedings of the British Pharmacological Societyhttp://www.pA2online.org/abstracts/Vol16Issue1abst093P.pdf
Mao, L., Chen, Q., Gong, K., Xu, X., Xie, Y., Zhang, W., et al. (2018). Berberine decelerates glucose metabolism via suppression of mTOR-dependent HIF-1α protein synthesis in colon cancer cells. Oncol. Rep. 39, 2436–2442. doi: 10.3892/or.2018.6318
Ni, X., Shergis, J. L., Zhang, A. L., Guo, X., Lu, C., Li, Y., et al. (2019). Traditional use of Chinese herbal medicine for insomnia and priorities setting of future clinical research. J. Altern. Complement. Med. 25, 8–15. doi: 10.1089/acm.2018.0249
Ren, Y., Zhang, M., Chen, K., You, S., Li, J., Guo, L., et al. (2012). Clinical and epidemiological investigation of TCM syndromes of patients with coronary heart disease in China. Evid. Based Complementary Altern. Med. 2012, 1–5. doi: 10.1155/2012/714517
Ru, J., Li, P., Wang, J., Zhou, W., Li, B., Huang, C., et al. (2014). TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform 6, 13. doi: 10.1186/1758-2946-6-13
Shannon, P., Markiel, A., Ozier, O., Baliga, N. S., Wang, J. T., Ramage, D., et al. (2003). Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504. doi: 10.1101/gr.1239303
Shao, L., Zhang, B. (2013). Traditional Chinese medicine network pharmacology: theory, methodology and application. J. Chin. J. Nat. Med. 11, 110–120. doi: 10.1016/S1875-5364(13)60037-0
Sweeney, O., Wang, T., Ellory, C., Wilkins, R., Ma, Y.-L. (2019). The effects of liquiritigenin on the activity of the hERG potassium channel. Br. J. Pharmacol. 176, 3067–3068.
Tong, X.-L., Dong, L., Chen, L., Zhen, Z. (2012). Treatment of diabetes using traditional Chinese medicine: past, present and future. Am. J. Chin. Med. 40, 877–886. doi: 10.1142/S0192415X12500656
Wang, A., Pu, J., Qi, X., Miao, W., Hou, Z., Cong, H., et al. (2011). Evaluation of shensongyangxin capsules in the treatment of paroxysmal atrial fibrillation: a randomized, double-blind and controlled multicenter trial. Zhonghua yi xue za zhi 91, 1677–1681. doi: 10.3760/cma.j.issn.0376-2491.2011.24.006
Wang, B., Mao, P. (2018). Use of traditional chinese medicine composition in preparation of potassium ion channel modulator medicine. US Patent App. 15/573, 583.
Wang, C., Lu, J. (2008). Observation on thirty cases of viral myocarditis treated with Xinsuning capsules. Shanxi Zhongyi 29, 1362. doi: 10.3969/j.issn.1000-7369.2008.10.068
Wang, T., Ellory, C., Wilkins, R., Ma, Y.-L. (2019a). Investigation on the mechanism and active component of sodium channel blockage effect of Xin Su Ning. Br. J. Pharmacol. 176, 3075–3076.
Wang, T., Xie, W., Ellory, C., Wilkins, R., Zhu, Y., and Ma, Y.-l. (2017). Investigation on the cardio-protective effect of Xin Su Ning on ischemia-reperfusion induced injury in isolated heart. Proceedings of the British Pharmacological Societyhttp://www.pa2online.org/abstracts/vol18issue1abst171p.pdf
Wang, T., Xie, W., Yu, J., Zhu, Y., Ma, Y.-L. (2019b). Ion channel targeted mechanisms of anti-arrhythmic Chinese herbal medicine Xin Su Ning. Front. Pharmacol. 10, 70. doi: 10.3389/fphar.2019.00070
Wang, X., Wang, Y., Feng, X., Lu, Y., Zhang, Y., Wang, W., et al. (2016). Systematic review and meta-analysis of randomized controlled trials on Wenxin keli. Drug Des. Devel. Ther. 10, 3725. doi: 10.2147/DDDT.S112333
Wu, Y., Zhang, F., Yang, K., Fang, S., Bu, D., Li, H., et al. (2018). SymMap: an integrative database of traditional Chinese medicine enhanced by symptom mapping. Nucleic Acids Res. 47, D1110–D1117. doi: 10.1093/nar/gky1021
Xing, Y., Hu, D., Zhang, T., Antzelevitch, C. (2015). Traditional Chinese medicine and vascular disease. Evid. Based Complementary Altern. Med. 2015, 1–2. doi: 10.1155/2015/430818
Yang, H., Lou, C., Sun, L., Li, J., Cai, Y., Wang, Z., et al. (2018). admetSAR 2.0: web-service for prediction and optimization of chemical ADMET properties. Bioinformatics 35(6), 1067-1069. doi: 10.1093/bioinformatics/bty707
Yuan, S., Zhou, C. (2000). Observation on sixty cases of rapid arrhythmia treated with Xinsuning capsules. J. Shandong Univ. TCM 24 (04), 290–293. doi: 10.3969/j.issn.1007-659X.2000.04.027
Zhai, J., Yin, X., Yang, X., Zhang, J. (2017). Xinsuning capsule for the treatment of premature ventricualr contraction: a multicenter randomised clinical trial. Lancet 390, S61. doi: 10.1016/S0140-6736(17)33199-9
Keywords: Xin Su Ning, network pharmacology, phlegm-heat heart-disturbance, weight coefficient, cardiac arrhythmia, electrophysiology
Citation: Wang T, Streeter H, Wang X, Purnama U, Lyu M, Carr C and Ma Y-l (2019) A Network Pharmacology Study of the Multi-Targeting Profile of an Antiarrhythmic Chinese Medicine Xin Su Ning. Front. Pharmacol. 10:1138. doi: 10.3389/fphar.2019.01138
Received: 03 June 2019; Accepted: 04 September 2019;
Published: 25 September 2019.
Edited by:
Xiu-Wei Yang,Peking University,Beijing,ChinaReviewed by:
Yu-Ping Tang,Shaanxi University of Chinese Medicine,ChinaDan Yan,Capital Medical University,China
Copyright © 2019 Wang, Streeter, Wang, Purnama, Lyu, Carr and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu-ling Ma, eXUtbGluZy5tYUBkcGFnLm94LmFjLnVr
 Taiyi Wang
Taiyi Wang Hamish Streeter
Hamish Streeter Xuan Wang1,2
Xuan Wang1,2 Ming Lyu
Ming Lyu Carolyn Carr
Carolyn Carr Yu-ling Ma
Yu-ling Ma