- 1College of Pharmaceutical Sciences and Chinese Medicine, Southwest University, Chongqing, China
- 2College of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 3Institute of Tibetan Medicine, Tibetan Traditional Medical College, Lhasa, China
- 4Key Laboratory on Health Cultivation of the Ministry of Education, Beijing University of Chinese Medicine, Beijing, China
Background: Lamiophlomis rotata (LR) showed favorable clinical effect and safety on rheumatoid arthritis (RA), but its active ingredients and mechanisms against RA remain unknown. The aim of this work was to explore the active ingredients and mechanisms of LR against RA by network pharmacology.
Methods: Compounds from LR were identified using literature retrieval and screened by absorption, distribution, metabolism, excretion, and toxicity (ADMET) evaluation. Genes related to the selected compounds or RA were identified using public databases, and the overlapping genes between compounds and RA target genes were identified using Venn diagram. Then, the interactions network between compounds and overlapping genes was constructed, visualized, and analyzed by Cytoscape software. Finally, pathway enrichment analysis of overlapping genes was carried out on Database for Annotation, Visualization, and Integrated Discovery (DAVID) platform.
Results: A total of 148 compounds in LR were identified, and ADMET screen results indicated that 67 compounds exhibited good potential as active ingredients. A total of 90 compounds-related genes and 1,871 RA-related genes were identified using public databases, and 48 overlapping genes between them were identified. Cytoscape results suggested that the active ingredients and target genes of LR against RA consisted of 23 compounds and 48 genes, and luteolin and AKT1 were the uppermost active ingredient and hub gene, respectively. DAVID results exhibited that the mechanisms of LR against RA were related to 34 signaling pathways, and the key mechanism of LR against RA might be to induce apoptosis of synovial cells by inactivating PI3K-Akt signaling pathway.
Conclusion: The active ingredients and mechanisms of LR against RA were firstly investigated using network pharmacology. This work provides scientific evidence to support the clinical effect of LR on RA, and a research basis for further expounding the active ingredients and mechanisms of LR against RA.
Introduction
Rheumatoid arthritis (RA), a chronic autoimmune disease, can cause cartilage and bone damage as well as disability. RA is characterized by joint inflammation, but is more like a syndrome that consists of extra-articular manifestations, such as rheumatoid nodules, pulmonary involvement or vasculitis, and systemic comorbidities (Smolen et al., 2016). RA can present at any age, affects about 1% of the population, and carries a huge emotional and financial burden for both the individual and society (McInnes and Schett, 2017). Because inflammation is the main driving factor to cause clinical symptoms, joint damage, disability, and comorbidity in RA patients, anti-inflammation is a key therapeutic strategy (Smolen et al., 2007). At present, the anti-RA drugs include disease-modifying antirheumatic drugs and non-steroidal anti-inflammatory drugs in western country (Smolen et al., 2016). However, traditional Chinese medicine (TCM) plays a vital complementary role in treating RA in China (Zhang et al., 2010). For the past few years, TCM has been increasingly important strategy for treatment of RA in China due to its good therapeutic effect and low toxic side effects.
Chinese Pharmacopoeia shows that Lamiophlomis rotata (Benth.) Kudo (LR) can be used to treat RA, and LR patent medicines (Duyiwei capsule or tablet) are legally allowed to trade in China. It was reported that LR showed favorable clinical effect and safety on RA (Ye et al., 2007), and a meta-analysis indicated that LR was effective and safe in treating bleeding, pain, and inflammation (Wang et al., 2008). In addition, animals experiment indicated that LR could significantly inhibit the formation of primary and secondary arthritis in rats (Wang et al., 2013). At present, the active ingredients and mechanisms of LR against RA has not been reported. Therefore, the studies on active ingredients and mechanisms of LR against RA should be strengthened to provide scientific evidence to support its clinical application in treating RA.
Network pharmacology, a systematic analytical method, can analyze the interaction network of multiple factors such as drugs, protein target, diseases, and genes (Hopkins, 2007). Network pharmacology can decipher the mechanism of drugs action with a holistic perspective, which emphasizes the paradigm shift from “one target, one drug” to “network target, multicomponent therapeutics” (Hopkins, 2008). The characteristic is also shared by TCM, and the holistic theory has long been central to TCM treatments of various diseases (Li et al., 2014). Therefore, network pharmacology is a very advantageous technology to explore TCM-related issues. At present, network pharmacology has been widely used to investigate the active ingredients and mechanisms of TCM against various diseases (Tang et al., 2015; Chen et al., 2018).
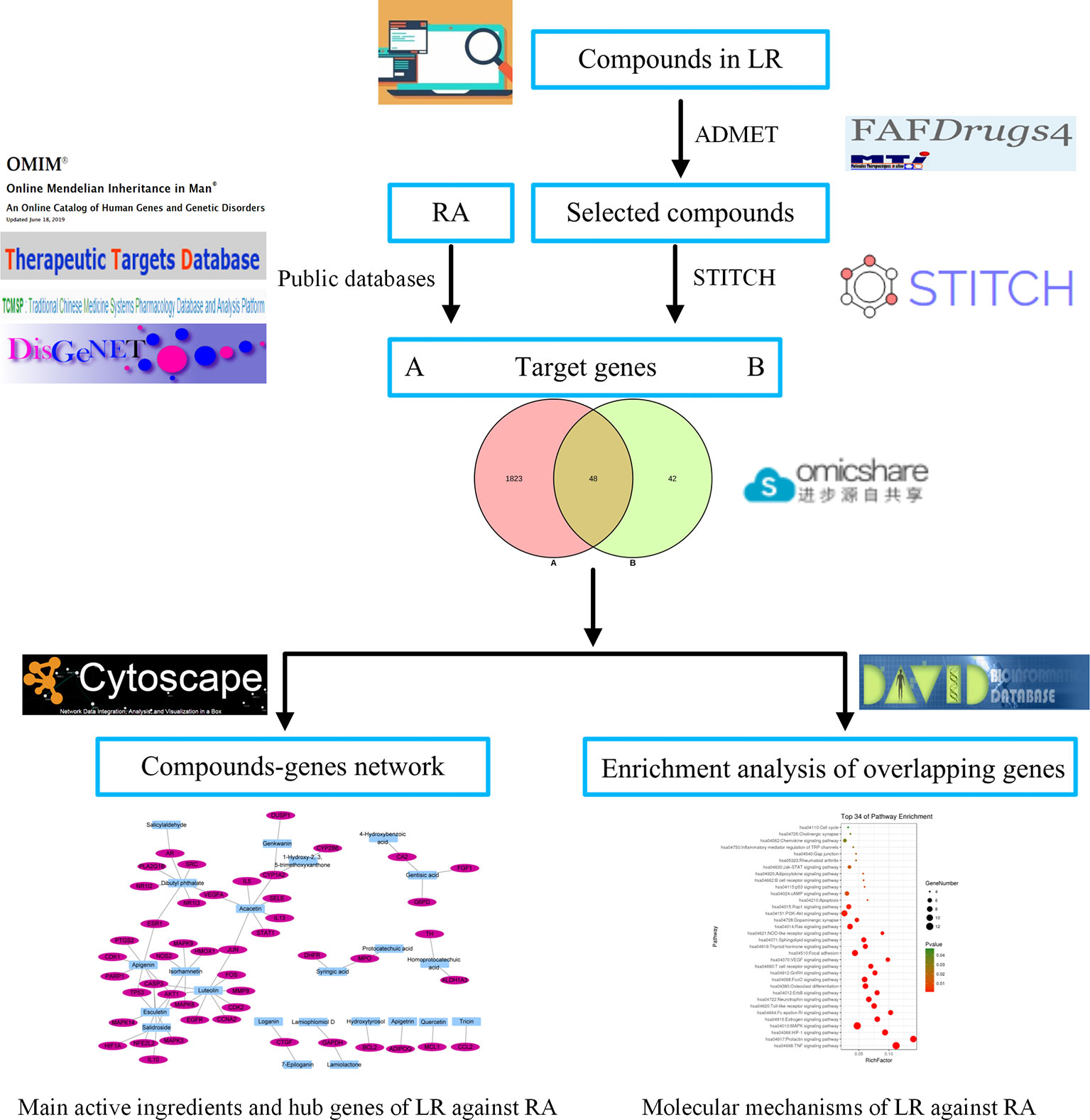
In this work, network pharmacology was used to investigate the active ingredients and mechanisms of LR against RA. First, compounds from LR were identified using literature retrieval, and were screened by absorption, distribution, metabolism, excretion, and toxicity (ADMET) evaluation. Then, genes related to selected compounds or RA were identified using public databases, and the overlapping genes between compounds and RA target genes were identified. Third, the key active ingredients and hub genes of LR against RA were identified by analyzing the interactions between compounds and overlapping genes. Finally, pathway enrichment analysis of overlapping genes was carried out to explore the molecular mechanisms of LR against RA. The workflow is shown in Figure 1.
Materials and Methods
Compounds Database Construction and ADMET Evaluation
The information of compounds from LR were collected by retrieving literatures in CNKI (http://www.cnki.net/), WANFANG DATA (http://www.wanfangdata.com.cn/), Baidu Xueshu (http://xueshu.baidu.com/), Web of Science and Google Scholar, and the SMILES and molecular formulas of compounds were identified using SciFinder (https://scifinder.cas.org/), PubChem (https://pubchem.ncbi.nlm.nih.gov/), or ChemSpider (http://www.chemspider.com/) with the aid of compounds names or structure. Then, compounds were screened by applying ADMET criteria of FAFDrugs4 (http://fafdrugs4.mti.univ-paris-diderot.fr/) (Miteva et al., 2006) with the aid of SMILES, and the “PhysChem Filters” of FAFDrugs4 was set as “Drug-Like Soft.” Compounds were selected out as potential active ingredients when the result of ADMET evaluation was “Accepted.”
Target Genes Linked to Selected Compounds or RA
Based on SMILES, target genes of the identified compounds were predicted using STITCH (http://stitch.embl.de/) (Szklarczyk et al., 2016) with the “Homo sapiens” setting. To get more credible target genes of each compound, compound with the highest “Tanimoto score,” usually 1.000 (match via InChIKey), was used to predict the genes of target compound, and the target genes were screened by setting “minimum required interaction score” as “high confidence (0.700)” during performing STITCH prediction (Lee et al., 2018).
RA-related target genes were identified by retrieving public databases including Online Mendelian Inheritance in Man (OMIM, https://omim.org/), Therapeutic Target Database (TTD, http://bidd.nus.edu.sg/group/cjttd/) (Li et al., 2018), Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP, http://lsp.nwu.edu.cn/tcmsp.php) (Ru et al., 2014), and DisGeNET (http://www.disgenet.org/). The overlapping genes between compounds and RA target genes were identified and visualized by Venn diagram, plotted using the OmicShare tools, a free online platform for data analysis (www.omicshare.com/tools).
Network Construction of Interactions Between Compounds and Overlapping Genes
The interactions between compounds and overlapping genes were obtained based on the results of STITCH prediction, and the network of the interactions was constructed, visualized, and analyzed by Cytoscape ver. 3.7.1 (https://cytoscape.org/). Nodes in network indicate compounds and genes, and edges suggest interactions between compounds and genes (Lee et al., 2018). The key active ingredients and hub genes of LR against RA were selected out by setting “Degree value” of compounds or genes, identified by analyzing topological structure of network. Degree value of compounds or genes represents the edges numbers of compounds or genes in network. The bigger degree value of compounds or genes are, the more important compounds or genes are for the therapeutic effect of LR on RA.
Pathway Enrichment Analysis of Overlapping Genes
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of overlapping genes was carried out on Database for Annotation, Visualization, and Integrated Discovery ver. 6.8 (https://david.ncifcrf.gov/) with the “Homo sapiens” setting. The results of KEGG pathway enrichment were used to decipher the potential molecular mechanisms of LR against RA. Bubble chart of interested KEGG pathways was plotted by the OmicShare tools.
Results
Potential Active Ingredients From LR
A total of 148 compounds in LR were identified by literatures retrieval, and the names, molecular formulas of these compounds are listed in Supplementary Table S1. The ADMET screen results of 148 compounds showed that the results of 67 compounds were “Accepted,” indicating that the 67 compounds exhibited good potential as active ingredients. These compounds are listed in Table 1.
Target Genes Linked to the 67 Compounds or RA
As shown in Supplementary Table S2, a total of 90 genes related to 25 compounds from abovementioned 67 compounds were identified using STITCH prediction, and no genes linked to another 42 compounds were identified based on STITCH prediction. As listed in Supplementary Table S3, a total of 1,871 RA-related genes were identified by retrieving OMIM, TTD, TCMSP, and DisGeNET databases. The results of Venn diagram (Figure 2) suggested 48 overlapping genes were identified by matching 90 compounds-related genes with 1,871 RA-related genes.
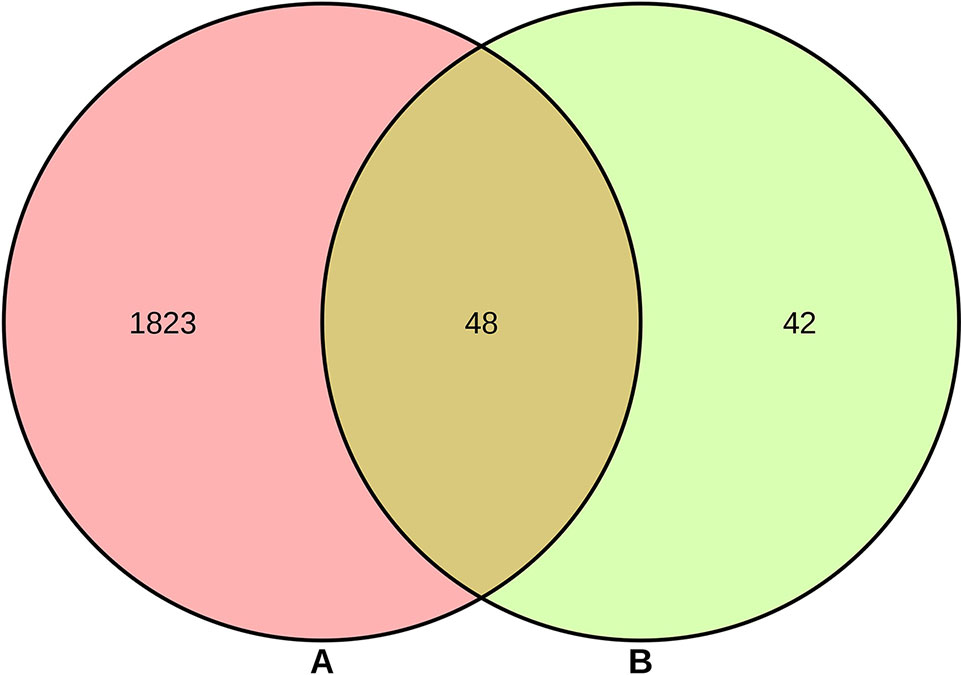
Figure 2 Overlapping genes between 1,871 rheumatoid arthritis (RA)-related genes (A) and 90 compounds-related genes (B).
Key Active Ingredients and Hub Genes of LR Against RA
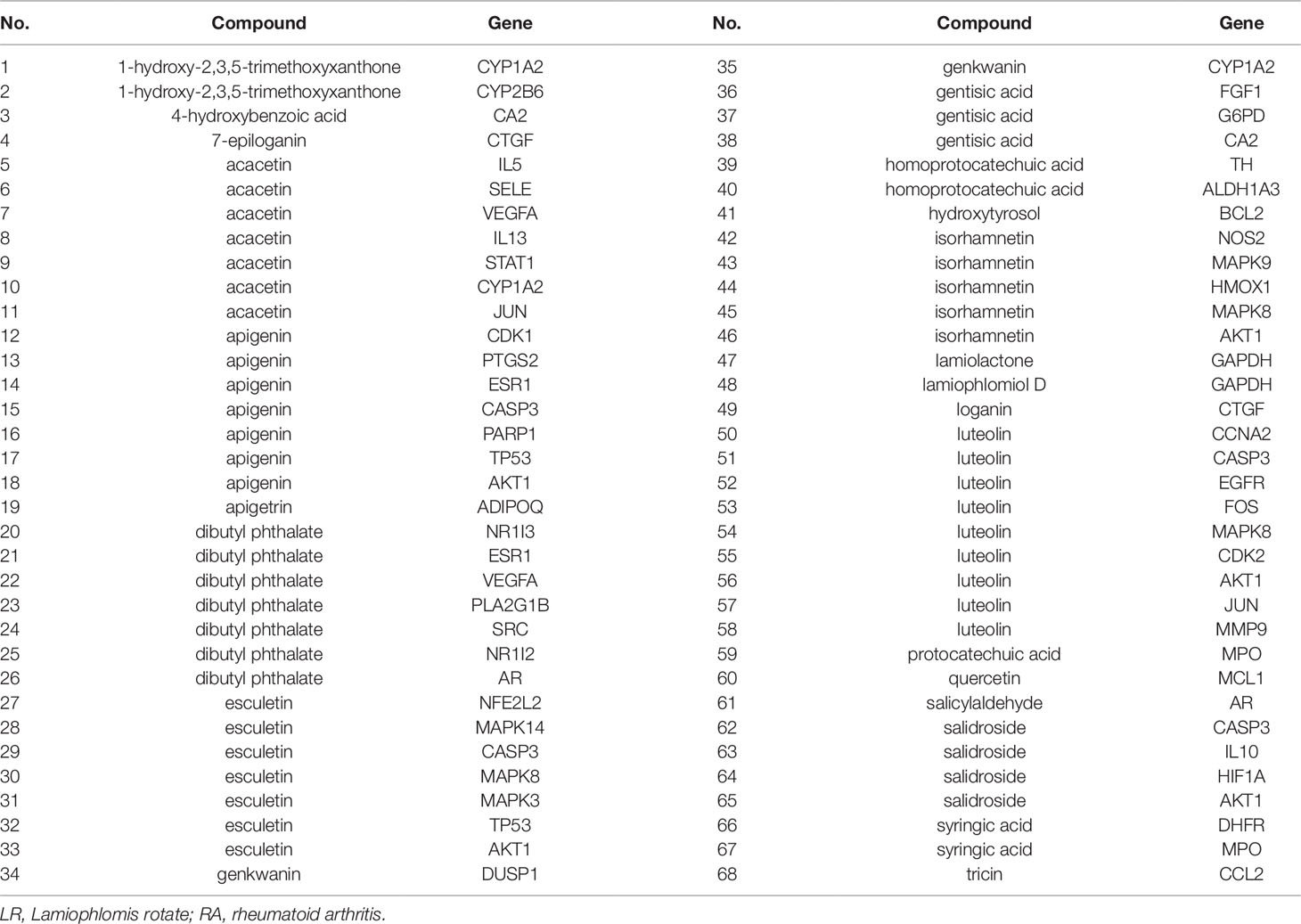
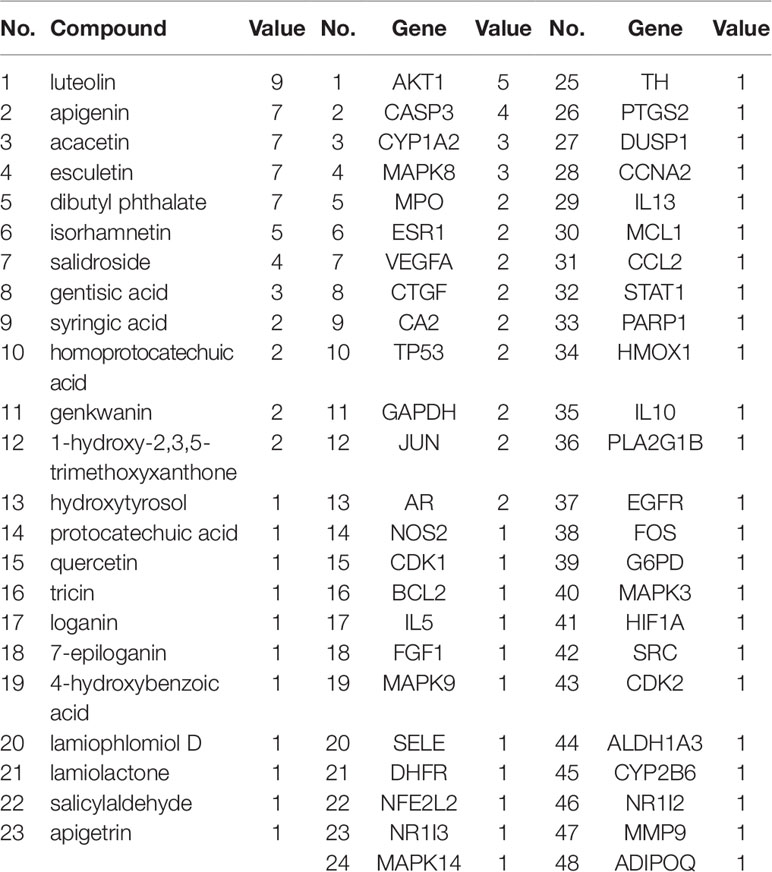
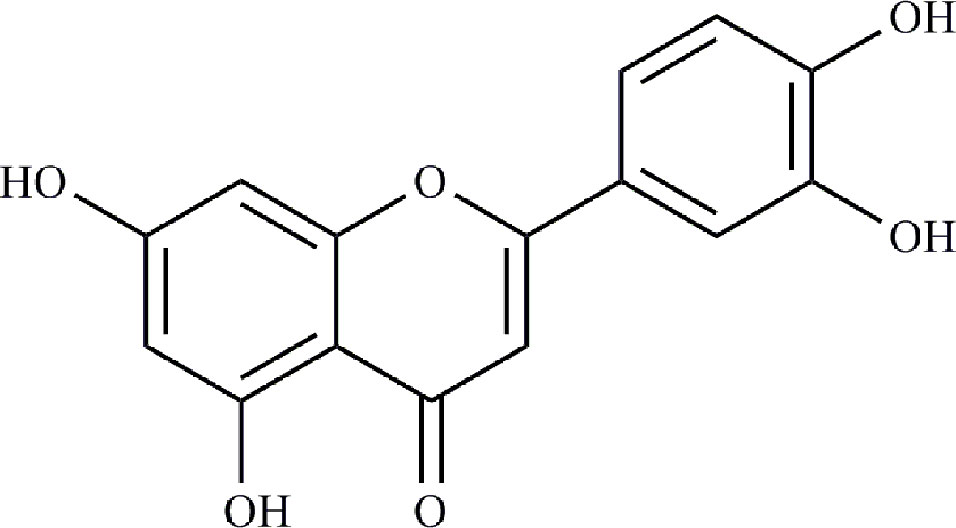
The interactions between 48 overlapping genes and compounds were identified based on the results of STITCH prediction, and 23 compounds were finally identified. The interactions between 48 overlapping genes and 23 compounds are listed in Table 2, and were visualized by network, which includes with 71 nodes and 68 edges (Figure 3). The results suggested that the therapeutic effect of LR on RA was directly related to the 23 compounds and 48 genes. The 23 compounds were categorized as nine flavonoids (luteolin, apigenin, acacetin, isorhamnetin, genkwanin, 1-hydroxy-2,3,5-trimethoxyxanthone, quercetin, tricin, and apigetrin), five phenolic acids (gentisic acid, syringic acid, homoprotocatechuic acid, protocatechuic acid, and 4-hydroxybenzoic acid), four iridoids (loganin, 7-epiloganin, lamiophlomiol D, and lamiolactone), two volatile oil (dibutyl phthalate and salicylaldehyde), one coumarin (esculetin), one phenylethanoid glycoside (salidroside), and one polyphenol (hydroxytyrosol). Based on the degree value of each compound or gene (Table 3), it was very easy to distinguish the contribution difference of 23 compounds and 48 genes to LR against RA. Luteolin (Figure 4), connected to nine genes, was considered as the uppermost active ingredient of LR against RA. AKT1, connected to five compounds, was considered as the hub gene of LR against RA.
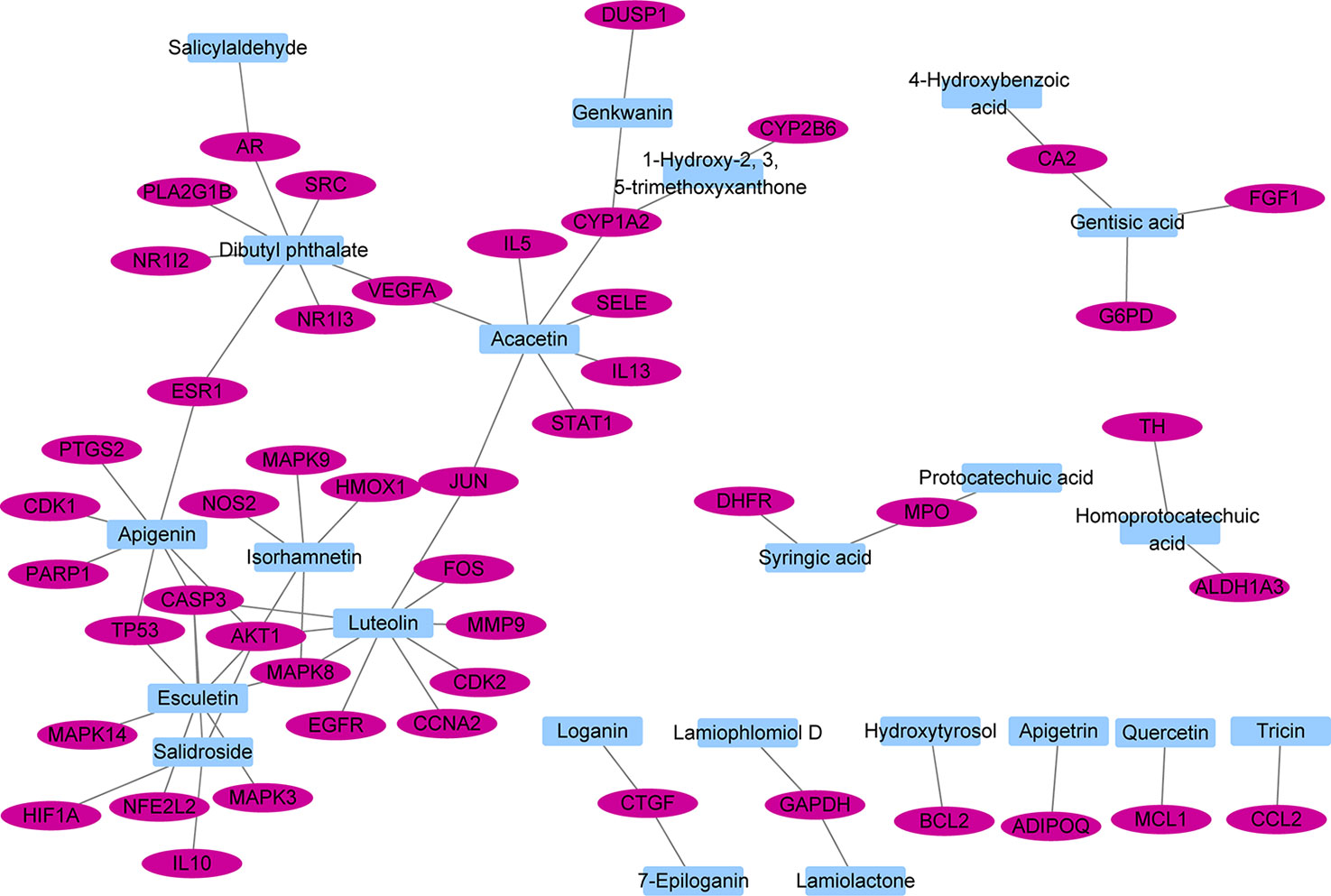
Figure 3 Network with 71 nodes and 68 edges linking 23 compounds in Lamiophlomis rotata and 48 target genes related to rheumatoid arthritis.
Potential Molecular Pathways of LR Against RA
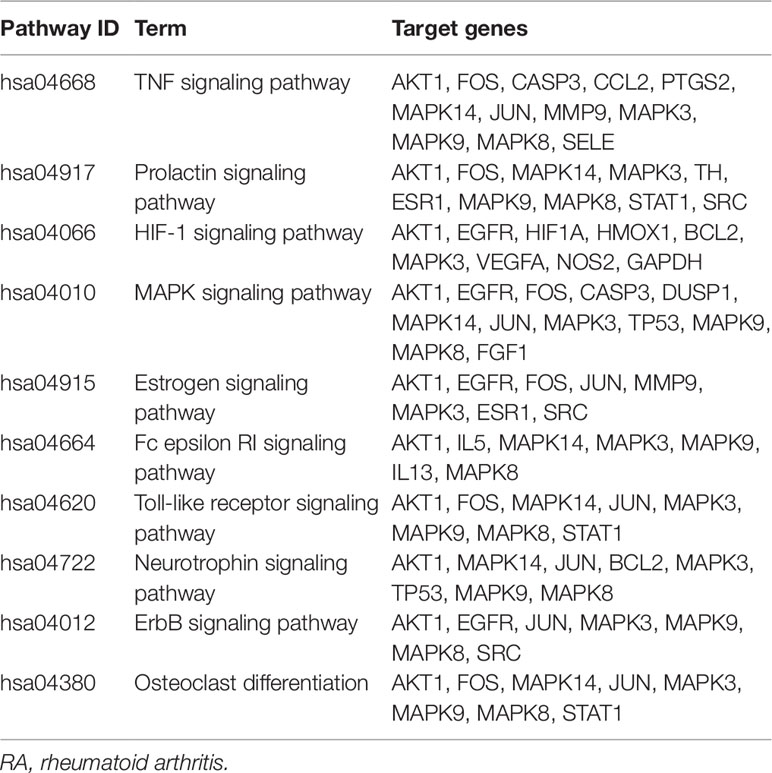
The results of KEGG pathway enrichment analysis indicated that 48 overlapping genes were significantly enriched in 74 signaling pathways (p < 0.05). Based on the extensive literature retrieval, the 34 signaling pathways (Figure 5) were directly related to occurrence and development of RA, indicating that these signaling pathways might be the mechanisms of LR against RA. The detailed information of top 10 pathways is shown in Table 4. In addition, the hub gene AKT1 of LR against RA was directly enriched in 27 signaling pathways of the 34 signaling pathways. Coincidently, AKT1 plays a role in almost all of the 27 signaling pathways by PI3K-Akt signaling pathway, suggesting that PI3K-Akt signaling pathway might be the hub signaling pathway of LR against RA.
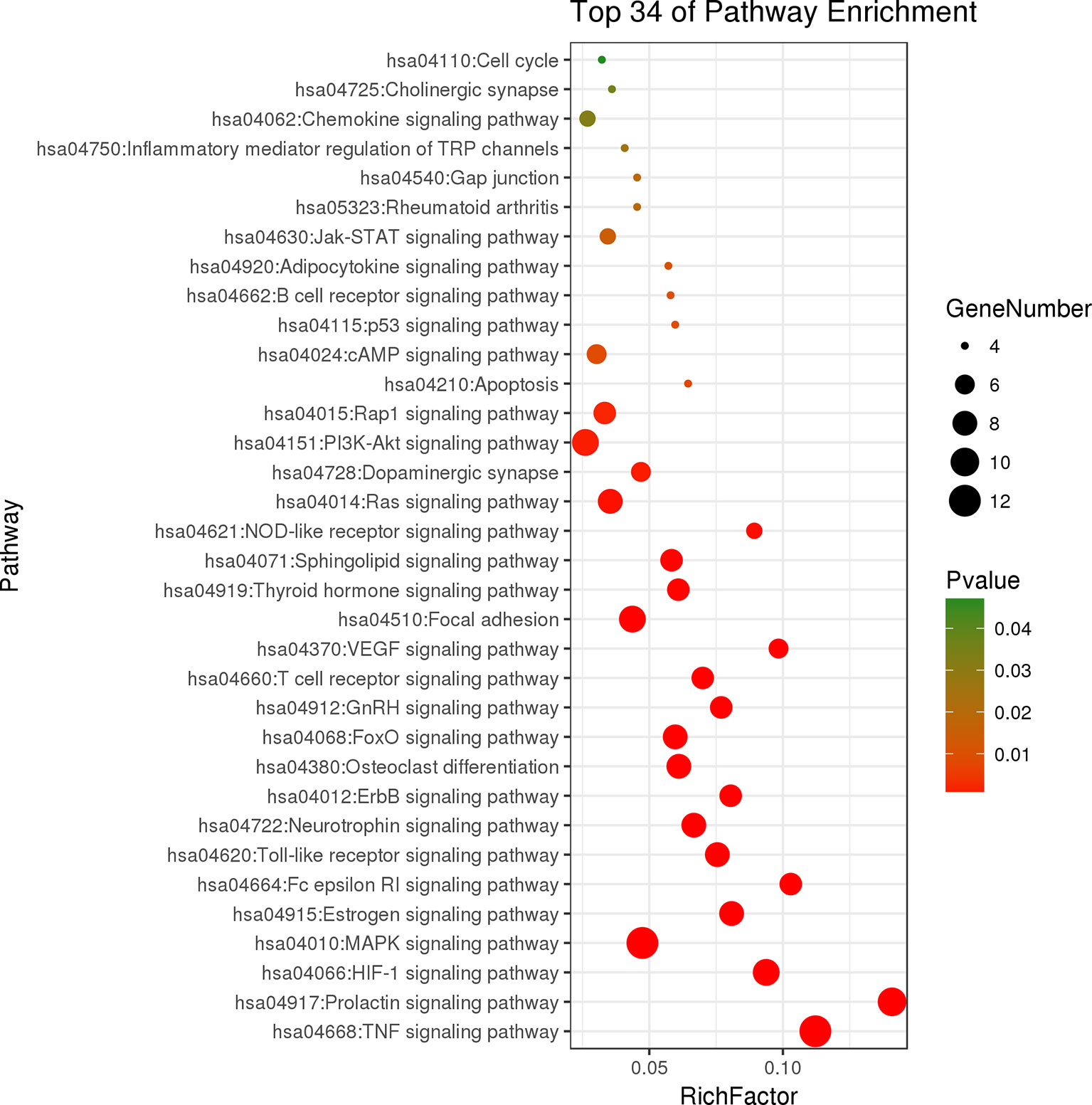
Figure 5 Bubble chart of 34 signaling pathways related to occurrence and development of rheumatoid arthritis.
Discussion
Compounds-genes network suggested that the therapeutic effect of LR on RA was directly related to 23 compounds, including nine flavonoids, five phenolic acids, four iridoids, two volatile oil, one coumarin, one phenylethanoid glycoside, and one polyphenol. The ratio of flavonoids to 23 compounds was close to 40%, suggesting that flavonoids were more important than other kinds of compounds for the therapeutic effect of LR on RA. Based on the degree value of each compound in compounds-genes network, luteolin was considered as the uppermost active ingredient of LR against RA. It was reported that flavonoids were the key active ingredients group of LR (Lin et al., 2003), and flavonoids are used to control the quality of LR patent medicines (Duyiwei capsule or tablet) in Chinese Pharmacopoeia. Studies suggested that luteolin inhibited the proliferation and partially blocked the pathogenic function of synovial fibroblasts in RA (Hou et al., 2009; Lou et al., 2015). Meanwhile, TCMSP suggests that luteolin is related to occurrence and development of RA (Ru et al., 2014). Additionally, it was reported that the quantity of luteolin in LR was about 0.9% (Yi and Sun, 2016), and the clinical dosage of LR patent medicines is 9 g/day based on Chinese Pharmacopoeia, suggesting that the daily intake of luteolin 81 mg in clinic. Study indicated that luteolin showed obvious anti-RA effect on mice with collagen type II-induced RA at a dose of 1 mg/kg/day (Impellizzeri et al., 2013), which is far lower than the equivalent dose of luteolin in mice, suggesting that the quantity of luteolin in LR is high enough to be of pharmacological relevance.
Compounds-genes network showed that the therapeutic effect of LR on RA was directly related to 48 genes. The results of KEGG pathway enrichment analysis of 48 genes suggested that 34 signaling pathways were directly linked to occurrence and development of RA, indicating that these signaling pathways might be the mechanisms of LR against RA. The relationships of the top 10 pathways with RA were briefly discussed as follows. TNF signaling pathway: The occurrence and development of RA can be suppressed by inhibiting the overexpression of TNF-α, and antibody therapy against TNF-α can effectively reduce the arthritis and synovitis symptoms of RA patients (Matsuno et al., 2002). Prolactin signaling pathway and estrogen signaling pathway: Sex hormones such as estrogen and prolactin have long been thought to be directly related to occurrence and development of RA, and recent evidence indicated that estrogen and prolactin showed both anti- and pro-inflammatory effects in RA (Tang et al., 2017). HIF-1 signaling pathway: Clinical research exhibited that HIF-1alpha level was strongest in the sub-lining layer of RA synovium and was linked to synovium inflammation and angiogenesis in RA patients (Brouwer et al., 2009). MAPK signaling pathway: It was reported that andrographolide showed protective effects on RA through inhibiting MAPK pathways, suggesting that MAPK signaling pathway was related to occurrence and development of RA (Li et al., 2017). Fc epsilon RI signaling pathway: Report indicated that IgE, the initiation factor in Fc epsilon RI signaling pathway, may be involved in some extra-articular manifestations of RA (Meretey et al., 1982). Toll-like receptor signaling pathway: Previous reports indicated that Toll-like receptor and the signaling pathway were intensively linked to RA pathogenesis (Takagi, 2011). Neurotrophin signaling pathway: Report suggested that the level of mesencephalic astrocyte-derived neurotrophic factor was closely related to occurrence and development of RA (Ma et al., 2018). ErbB signaling pathway: It was reported that ErbB-2 was involved in occurrence and development of RA (Jiang et al., 2012). Osteoclast differentiation: Reports exhibited that activated RA synovial fibroblasts played a vital role in rheumatoid bone destruction by expressing osteoclast differentiation factor (Shigeyama et al., 2000).
Based on the degree value of each gene in compounds-genes network, AKT1 was considered as the hub gene of LR against RA. AKT1 was directly enriched in 27 signaling pathways of the abovementioned 34 signaling pathways. Coincidently, AKT1 plays a role in almost all of the 27 signaling pathways by PI3K-Akt signaling pathway, suggesting that PI3K-Akt signaling pathway might be the hub signaling pathway of LR against RA. Joint synovium is the main diseased region in RA patients, and its out-of-control proliferation to cartilage and bone causes release of inflammatory cytokines, resulting in occurrence of RA. Therefore, inducing apoptosis of synovial cells is also a feasible strategy for treating RA by preventing development of inflammation (Park et al., 2010). It was reported that PI3K-Akt signaling pathway was abnormally activated in RA synovium, resulting in the overexpression of anti-apoptotic genes such as FLIP, Bcl-2, and Mcl-1 (Harris et al., 2009). The overexpression of these anti-apoptotic genes lead to out-of-balance apoptosis of synovial cells, which induced occurrence and development of RA (Smith et al., 2010). Reports indicated that luteolin, the uppermost active ingredient of LR against RA, inhibited the proliferation of synovial fibroblasts in RA by blocking PI3K-Akt signaling pathway (Hou et al., 2009). Therefore, the key mechanism of LR against RA might be to induce apoptosis of synovial cells by inactivating PI3K-Akt signaling pathway.
Conclusion
The active ingredients and mechanisms of LR against RA were firstly investigated using network pharmacology. The findings of this work suggested that the active ingredients and target genes of LR against RA consisted of 23 compounds and 48 genes, and luteolin and AKT1 were the uppermost active ingredient and hub gene of LR against RA, respectively. The mechanisms of LR against RA were related to 34 signaling pathways, and the key mechanism of LR against RA might be to induce apoptosis of synovial cells by inactivating PI3K-Akt signaling pathway. This work provides scientific evidence to support the clinical effect of LR on RA, and a research basis for further expounding the active ingredients and mechanisms of LR against RA.
Data Availability Statement
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Author Contributions
YJ, YZ, and TL conceived and designed this work, and wrote and revised the whole manuscript. MZ collected the data. YJ, MZ, FL, and RY analyzed the data.
Funding
This work was supported by the Regional Collaborative Innovation Center Project of Tibetan Medicine (No. 2018XTCX045).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2019.01435/full#supplementary-material
References
Brouwer, E., Gouw, A. S., Posthumus, M. D., van Leeuwen, M. A., Boerboom, A. L., Bijzet, J., et al. (2009). Hypoxia inducible factor-1-alpha (HIF-1alpha) is related to both angiogenesis and inflammation in rheumatoid arthritis. Clin. Exp. Rheumatol. 27, 945–951.
Chen, L., Cao, Y., Zhang, H., Lv, D., Zhao, Y., Liu, Y., et al. (2018). Network pharmacology-based strategy for predicting active ingredients and potential targets of Yangxinshi tablet for treating heart failure. J. Ethnopharmacol. 219, 359–368. doi: 10.1016/j.jep.2017.12.011
Harris, S. J., Foster, J. G., Ward, S. G. (2009). PI3K isoforms as drug targets in inflammatory diseases: lessons from pharmacological and genetic strategies. Curr. Opin. Investig. Drugs 10, 1151–1162.
Hopkins, A. L. (2007). Network pharmacology. Nat. Biotechnol. 25, 1110–1111. doi: 10.1038/nbt1007-1110
Hopkins, A. L. (2008). Network pharmacology: the next paradigm in drug discovery. Nat. Chem. Biol. 4, 682–690. doi: 10.1038/nchembio.118
Hou, Y., Wu, J., Huang, Q., Guo, L. (2009). Luteolin inhibits proliferation and affects the function of stimulated rat synovial fibroblasts. Cell Biol. Int. 33, 135–147. doi: 10.1016/j.cellbi.2008.10.005
Impellizzeri, D., Esposito, E., Di Paola, R., Ahmad, A., Campolo, M., Peli, A., et al. (2013). Palmitoylethanolamide and luteolin ameliorate development of arthritis caused by injection of collagen type II in mice. Arthritis Res. Ther. 15, R192. doi: 10.1186/ar4382
Jiang, Y., Tu, S. H., Xia, Y. K., Chen, Z., Chang, D., Yang, H. W., et al. (2012). Regulatory effect of tripterygium wilfordii polyglycoside on expression of epidermal growth factor receptor family in collagen induced arthritis rats. Chin. J. Rheumatol. 16, 187–190. (in Chinese). doi: 10.3760/cma.j.issn.1007-7480.2012.03.010
Lee, A. Y., Park, W., Kang, T. W., Cha, M. H., Chun, J. M. (2018). Network pharmacology-based prediction of active compounds and molecular targets in Yijin-Tang acting on hyperlipidaemia and atherosclerosis. J. Ethnopharmacol. 221, 151–159. doi: 10.1016/j.jep.2018.04.027
Li, S., Fan, T. P., Jia, W., Lu, A., Zhang, W. (2014). Network pharmacology in traditional chinese medicine. Evid. Based Complement. Alternat. Med. 2014, 138460. doi: 10.1155/2014/138460
Li, Z. Z., Tan, J. P., Wang, L. L., Li, Q. H. (2017). Andrographolide benefits rheumatoid arthritis via inhibiting MAPK pathways. Inflammation 40, 1599–1605. doi: 10.1007/s10753-017-0600-y
Li, Y. H., Yu, C. Y., Li, X. X., Zhang, P., Tang, J., Yang, Q., et al. (2018). Therapeutic target database update 2018: enriched resource for facilitating bench-to-clinic research of targeted therapeutics. Nucleic Acids Res. 46, D1121–D1127. doi: 10.1093/nar/gkx1076
Lin, T. M., Gu, Y., Fang, K. Q., Zhang, S. Q., Jiang, Y. P. (2003). Analgesic effects of different extraction components from Lamiophlomis rotata (Benth.) kudo, a medicinal plant in XiZang (Tibet) in mice. J. Fourth Mil. Med. Univ. 24, 444–446. (in Chinese). doi: 10.3321/j.issn:1000-2790.2003.05.021
Lou, L., Liu, Y., Zhou, J., Wei, Y., Deng, J., Dong, B., et al. (2015). Chlorogenic acid and luteolin synergistically inhibit the proliferation of interleukin-1β-induced fibroblast-like synoviocytes through regulating the activation of NF-κB and JAK/STAT-signaling pathways. Immunopharmacol. Immunotoxicol. 37, 499–507. doi: 10.3109/08923973.2015.1095763
Ma, Y. Y., Di, Z. M., Cao, Q., Shen, Y. J., Shen, Y. X., Feng, L. J. (2018). Expression characterization of MANF during course of rat adjuvant arthritis and its relationship with inflammation. Chin. Pharmacol. Bull. 34, 537–543. (in Chinese). doi: 10.3969/j.issn.1001-1978.2018.04.020
Matsuno, H., Yudoh, K., Katayama, R., Nakazawa, F., Uzuki, M., Sawai, T., et al. (2002). The role of TNF-alpha in the pathogenesis of inflammation and joint destruction in rheumatoid arthritis (RA): a study using a human RA/SCID mouse chimera. Rheumatol. (Oxford) 41, 329–337. doi: 10.1093/rheumatology/41.3.329
McInnes, I. B., Schett, G. (2017). Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet 389, 2328–2337. doi: 10.1016/S0140-6736(17)31472-1
Meretey, K., Falus, A., Erhardt, C. C., Maini, R. N. (1982). IgE and IgE-rheumatoid factors in circulating immune complexes in rheumatoid arthritis. Ann. Rheumatol. Dis. 41, 405–408. doi: 10.1136/ard.41.4.405
Miteva, M. A., Violas, S., Montes, M., Gomez, D., Tuffery, P., Villoutreix, B. O. (2006). FAF-Drugs: free ADME/tox filtering of compound collections. Nucleic Acids Res. 34, W738–W744. doi: 10.1093/nar/gkl065
Park, S. Y., Lee, S. W., Shin, H. K., Chung, W. T., Lee, W. S., Rhim, B. Y., et al. (2010). Cilostazol enhances apoptosis of synovial cells from rheumatoid arthritis patients with inhibition of cytokine formation via Nrf2-linked heme oxygenase 1 induction. Arthritis Rheumatol. 62, 732–741. doi: 10.1002/art.27291
Ru, J., Li, P., Wang, J., Zhou, W., Li, B., Huang, C., et al. (2014). TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 6, 13. doi: 10.1186/1758-2946-6-13
Shigeyama, Y., Pap, T., Kunzler, P., Simmen, B. R., Gay, R. E., Gay, S. (2000). Expression of osteoclast differentiation factor in rheumatoid arthritis. Arthritis Rheumatol. 43, 2523–2530. doi: 10.1002/1529-0131(200011)43:11<2523::AID-ANR20>3.0.CO;2-Z
Smith, M. D., Weedon, H., Papangelis, V., Walker, J., Roberts-Thomson, P. J., Ahern, M. J. (2010). Apoptosis in the rheumatoid arthritis synovial membrane: modulation by disease-modifying anti-rheumatic drug treatment. Rheumatol. (Oxford) 49, 862–875. doi: 10.1093/rheumatology/kep467
Smolen, J. S., Aletaha, D., Koeller, M., Weisman, M., Emery, P. (2007). New therapies for the treatment of rheumatoid arthritis. Lancet 370, 1861–1874. doi: 10.1016/S0140-6736(07)60784-3
Smolen, J. S., Aletaha, D., Mclnnes, I. B. (2016). Rheumatoid arthritis. Lancet 388, 2023–2038. doi: 10.1016/S0140-6736(16)30173-8
Szklarczyk, D., Santos, A., von Mering, C., Jensen, L. J., Bork, P., Kuhn, M. (2016). STITCH 5: augmenting protein-chemical interaction networks with tissue and affinity data. Nucleic Acids Res. 44, D380–D384. doi: 10.1093/nar/gkv1277
Takagi, M. (2011). Toll-like receptor–a potent driving force behind rheumatoid arthritis. J. Clin. Exp. Hematop. 51, 77–92. doi: 10.3960/jslrt.51.77
Tang, F., Tang, Q., Tian, Y., Fan, Q., Huang, Y., Tan, X. (2015). Network pharmacology-based prediction of the active ingredients and potential targets of Mahuang Fuzi Xixin decoction for application to allergic rhinitis. J. Ethnopharmacol. 176, 402–412. doi: 10.1016/j.jep.2015.10.040
Tang, M. W., Garcia, S., Gerlag, D. M., Tak, P. P., Reedquist, K. A. (2017). Insight into the Endocrine system and the immune system: a review of the inflammatory role of prolactin in rheumatoid arthritis and psoriatic arthritis. Front. Immunol. 8, 720. doi: 10.3389/fimmu.2017.00720
Wang, L., Xu, L., Li, Y. L., Ai, C. L., Li, J. (2008). Efficacy and safety of Lamiophlomis Rotata (Benth) Kudo: a systematic review of randomized controlled trials. Chin. J. Evid.-based Med. 8, 1060–1078. (in Chinese). doi: 10.3969/j.issn.1672-2531.2008.12.011
Wang, L. J., Wang, Y., Yang, J., Chu, Y. P., Zhang, S. J. (2013). Experimental study of Lamiophlomis rotate preventing and treatment function on rat adjuvant arthritis. Chin. J. Basic Med. Trad. Chin. Med. 19, 763–766. (in Chinese).
Ye, F., Yang, H., Xu, Y. (2007). Clinical observation of 69 cases of arthritis deformans treated with Duyiwei capsule. World Chin. Med. 2, 339–340. (in Chinese). doi: 10.3969/j.issn.1673-7202.2007.06.007
Yi, Y., Sun, L. (2016). Simultaneous determination of dipsacoside B, luteolin and apigenin in zang medicine Lamiophlomis rotata by HPLC. Chin. Pharma. 27, 4733–4735. (in Chinese). doi: 10.6039/j.issn.1001-0408.2016.33.42
Keywords: Lamiophlomis rotata, rheumatoid arthritis, network pharmacology, active ingredient, mechanism, luteolin, PI3K-Akt signaling pathway
Citation: Jiang Y, Zhong M, Long F, Yang R, Zhang Y and Liu T (2019) Network Pharmacology-Based Prediction of Active Ingredients and Mechanisms of Lamiophlomis rotata (Benth.) Kudo Against Rheumatoid Arthritis. Front. Pharmacol. 10:1435. doi: 10.3389/fphar.2019.01435
Received: 21 June 2019; Accepted: 11 November 2019;
Published: 27 November 2019.
Edited by:
Yonghua Wang, Northwest A&F University, ChinaReviewed by:
Jingxiao Zhang, Luoyang Normal University, ChinaLilei Zhang, Luoyang Normal University, China
Copyright © 2019 Jiang, Zhong, Long, Yang, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunbin Jiang, eXVuYmluamlhbmdAc3d1LmVkdS5jbg==; Yanfei Zhang, MTMwMTM3OTE3MEBxcS5jb20=; Tonghua Liu, dGhsaXVAdmlwLjE2My5jb20=
†These authors have contributed equally to this work
 Yunbin Jiang
Yunbin Jiang Mei Zhong
Mei Zhong Fei Long
Fei Long Rongping Yang
Rongping Yang Yanfei Zhang
Yanfei Zhang Tonghua Liu
Tonghua Liu