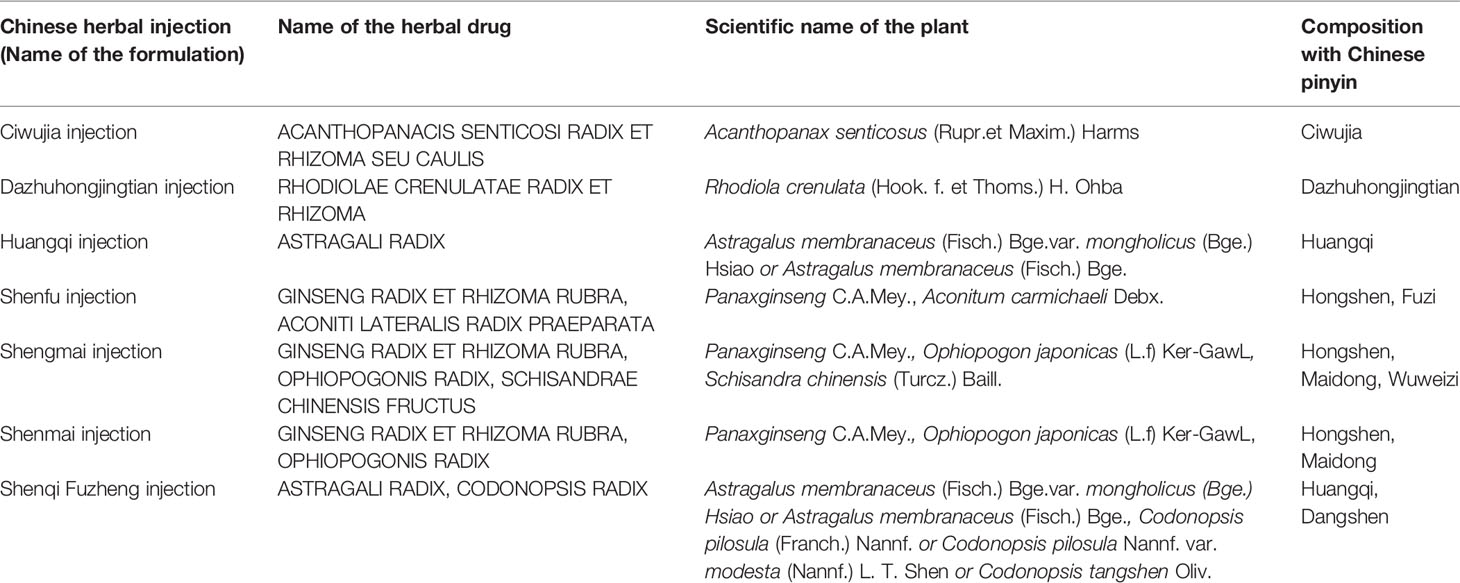
- 1Department of Clinical Chinese Pharmacy, School of Chinese Materia Medica, Beijing University of Chinese Medicine, Beijing, China
- 2Center for Evidence-Based and Translational Medicine, Zhongnan Hospital of Wuhan University, Wuhan, China
Background: Given the severity of pulmonary heart disease and the wide utilization of Chinese herbal injections, this network meta-analysis was devised to assess the comparative efficacy of seven Chinese herbal injections (Ciwujia injection, Dazhuhongjingtan injection, Huangqi injection, Shenfu injection, Shengmai injection, Shenmai injection, and Shenqi Fuzheng injection) that were combined with Western medicines in the treatment of pulmonary heart disease.
Methods: A literature search was performed in PubMed, Cochrane Library, EMBASE, Chinese Biological Medicine Database, China National Knowledge Infrastructure, Wanfang Database, and the Chinese Scientific Journal Database from their inception to July14, 2019. This network meta-analysis was conducted in accordance with a priori eligibility criteria and methodological quality recommendations. Data analysis was performed with WinBUGS 1.4.3 and Stata 13.0 software focusing on clinical effectiveness rate, arterial blood gas analysis, hemorheology and hemodynamic indexes and right ventricular dimensions. In addition to the odds ratio or mean difference in various outcomes, the ranking probability of interventions calculated by the surface under the cumulative ranking area curve was demonstrated. The surface under the cumulative ranking area was equal to the rank of the intervention and was aimed to assess the best intervention.
Results: Ultimately, 118 randomized controlled trials including 10,085 patients were included. Integrating the outcome results, all eligible Chinese herbal injections plus Western medicines were superior to Western medicines alone, especially Shenfu injection+ Western medicines, Shenmai injection+ Western medicines, and Shenqi Fuzheng injection+ Western medicines. Regarding safety, the drip rate was an essential element for clinicians to consider during treatment.
Conclusions: In conclusion, Shenfu injection+ Western medicines, Shenmai injection+ Western medicines and Shenqi Fuzheng injection+ Western medicines may be potential optimal treatments for pulmonary heart disease. A larger sample size and high-quality randomized controlled trials are needed to confirm and support this network meta-analysis.
Introduction
Pulmonary heart disease (PHD), a pathologic condition that increases pulmonary vascular resistance and pulmonary artery pressure, is caused by lesions in bronchial and lung tissue and the pulmonary vascular system and leads to the irreversible development of pulmonary hypertension and ultimate overload of the right heart or even right heart failure (Forfia et al., 2013; Wang et al., 2016).With an estimated average prevalence of 0.46% worldwide for PHD, the heavy burden of these patients and their families has also been placed on society (Yun et al., 2016). Moreover, the estimated mortality rate among hospitalized patients is between 12.5% and 14.5% globally (Ye and Lu, 2004; He and Liu, 2014). At present, the predominant treatment for PHD is Western medicine (WM), including oxygen therapy, antibiotics, diuretics, vasodilators, antiarrhythmic agents, and others. However, the efficacy of these treatments is unsatisfactory (Chen et al., 2013; Shi et al., 2015).
The use of traditional Chinese medicine combined with WM has been extensively promoted in routine practice in China. In light of traditional Chinese medicine theories, PHD is an aspect of “lung distension” and “dyspnea” and is caused by the lungs and heart. The clinical principle mainly emphasizes strengthening bodily resistance to eliminate pathogenic factors (Yu, 2009; Ruan et al., 2018). Chinese herbal injections are an indispensable part of traditional Chinese medicine and play a vital role in treating PHD. For the past twenty years, their effectiveness has been confirmed in clinical trials (Liao et al., 2004; China association of traditional Chinese medicine of lung diseases, 2014; Yun et al., 2016).
However, no clinical trials have focused on the comparative efficacy of administering various Chinese herbal injections simultaneously, which may cause difficulties for clinicians when choosing an optimal regimen. Network meta-analysis (NMA) can help fill this void, as NMA is an extension of conventional pairwise meta-analysis and can synthesize the available evidence to enable a simultaneous comparison and assessment of the best intervention amongst those that lack head-to-head evaluations (Lu and Ades, 2004; Singh et al., 2016; Cipriani et al., 2018; Cai et al., 2018). In this context, this study incorporated seven Chinese herbal injections, namely, Ciwujia, Dazhuhongjingtan, Huangqi, Shenfu, Shengmai, Shenmai, and Shenqi Fuzheng injections, to comprehensively evaluate the efficacy of these injections combined with WM using NMA. The goal of this NMA was to inform clinical practice and provide additional insights for the selection of PHD treatments.
Methods
This NMA was performed in accordance with The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions (Hutton et al., 2015). A completed PRISMA checklist is included as an additional file (Presentation File).
Search Strategy
In this NMA, a comprehensive data search was conducted using the following electronic databases from their inception to July14, 2019: PubMed, Cochrane Library, EMBASE, Chinese Biological Medicine Database, China National Knowledge Infrastructure, Wanfang Database, and the Chinese Scientific Journal Database. The method of combining MeSH terms with free text search terms was applied to the search. Using PubMed as an example, two reviewers developed the search strategy as follows:(randomized controlled trial[Publication Type] OR controlled clinical trial[Publication Type] OR random*[All fields]) AND (ciwujia[Title/Abstract]OR acanthopanax[Title/Abstract] OR dazhuhongjintian[Title/Abstract] OR rhodiola[Title/Abstract]OR huangqi[Title/Abstract] OR astragalus[Title/Abstract] OR shenfu[Title/Abstract] OR shengmai[Title/Abstract] OR shenmai[Title/Abstract] OR shenqi fuzheng[Title/Abstract] OR yiqifumai[Title/Abstract]) AND (pulmonary heart disease[MeSH terms] OR pulmonary heart disease*[Title/Abstract] OR corpulmonale[Title/Abstract]) (See the Presentation File for more details about the search strategy).
Inclusion Criteria
Types of Studies
Randomized controlled trials (RCTs) that reported the efficacy of the seven Chinese herbal injections combined with WM for treating PHD were eligible. No limitation on language, publication year, or publication status was applied. If a study was published more than once, we included only the first publication.
Types of Participants
Patients who suffered from PHD and were diagnosed according to the specific diagnostic criteria were included. Gender, ration and nationality were unrestricted. Patients would be excluded if they had severe complications.
Types of Interventions
Eligible RCTs were not limited to two-arm RCTs. All RCTs included WM, including treatments to control respiratory tract infection and improve respiratory and heart failure, as well as anti-arrhythmic drugs. The experimental group was administered one of the eligible Chinese herbal injections and WM, while the control group was administered the same WM alone or in combination with another Chinese herbal injection. If patients had complications during the therapeutic process, the appropriate therapy needed to be adopted. No restriction was placed on dosage or duration, but for a study to be eligible, it needed to include the specific dosage of the Chinese herbal injection.
Types of Outcomes
(1) Clinical effectiveness rate. The clinical effectiveness rate was calculated with the following formula: (number of remarkable recovery patients + number of basic recovery patients)/total number of patients * 100%. To be considered a remarkable recovery, patients needed to show complete amelioration of clinical symptoms and improvement of cardiac function by two levels. For a basic recovery, patients needed to show relief from clinical symptoms and an improvement in cardiac function by one level. Unaltered or worsened clinical symptoms and cardiac function were regarded as deterioration. Cardiac function classification conformed to the standard issued by the New York Heart Association in the United States. (2) Arterial blood gas analysis (partial pressure of arterial oxygen, partial pressure of arterial carbon dioxide). (3) Hemorheology (the level of whole blood viscosity and the level of fibrinogen). (4) Hemodynamics (mean pulmonary arterial pressure). (5) Right ventricular dimension. RCTs were eligible if they reported one of the aforementioned outcomes. The safety of the intervention (adverse drug reactions/adverse drug events (ADRs/ADEs)) was also summarized.
Data Extraction and Quality Assessment
The initial literature screening process was conducted by two reviewers through reading titles and abstracts. Then, the full-text versions of potential articles were obtained for further assessment. Any discrepancies between the two reviewers were resolved by discussion or consultation with a third reviewer. Next, data were extracted in accordance with the predesigned form, including the first author name, publication year, patient characteristics (sample size, gender, age, patients' baseline, and disease duration), intervention details, duration, outcomes, study design and the domains of risk of bias.
The Cochrane Collaboration risk of bias tool was used to evaluate the quality of the eligible RCTs. The following items were accessed: sequence generation (selection bias), allocation concealment (selection bias), blinding of patients and personnel (performance bias), blinding of outcomes assessors (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other biases. Each bias had three levels: “low risk”, “unclear risk” and “high risk”. Quality assessment was performed by two reviewers, and any conflicts during this process were solved by discussion or consultation.
Data Analysis
For each outcome, we carried out a Bayesian NMA to compare efficacy between eligible Chinese herbal injections. The calculation was performed by WinBUGS 1.4.3 software, and correlative graphical representation was depicted using Stata 13.0 software. The OR ratios (OR) and mean differences (MD) with their 95% confidence intervals (95% CI) were produced in a random-effects model for binary and continuous outcomes, respectively. When the OR included 1.00 or the MD included 0.00, the meta-analysis result was deemed not statistically significant. In the analysis process, the number of iterations was set to 200,000, and the first 10,000 were used for the annealing algorithm to eliminate the impact of the initial value.
Furthermore, a surface under the cumulative ranking area (SUCRA) curve was used to estimate the ranking probabilities for each intervention, which ranged from 0 to 100%.Interventions with larger SUCRA values were considered better interventions (Chang and Guo, 2017; Cai et al., 2018). To determine the most efficacious injection for the PHD treatment, a cluster analysis for different outcomes was carried out (Tang et al., 2016). A funnel plot was also depicted to estimate the publication bias of outcomes included in more than10 RCTs. The network graph is displayed as well.
This NMA was based on previous publications, and thus, ethical approval was unnecessary.
Results
Search Results
A total of 2,421 records were identified from the seven databases. Then, 1,159 records were removed for duplication, and 258 records were excluded though scanning titles and abstracts because they were reviews, animal experiments or irrelevant studies. The full texts of the remaining records were screened, and 886 records were excluded for the following reasons: (1) It was not an RCT, or it was an RCT with inappropriate randomization (n = 28). (2) It was a retrospective study (n = 3). (3) The intervention did not meet the inclusion criteria (n = 620). (4) The RCT did not mention a standard diagnostic criterion (n = 128). (5) The outcomes did not meet the inclusion criteria (n = 46). (6) The report contained duplicate data (n = 48). (7) It was an RCT without an available full-text report (n = 13). Ultimately, 118 RCTs were included in this NMA (Presentation File). They were all conducted in China and published from 1996 to 2017.
Inclusion Studies and Characteristics
One hundred and eighteen two-arm RCTs containing 10,085 patients (5,241 patients in the experimental group, 4,844 patients in the control group) were eligible for this NMA. Among the patients, the majority were middle-aged and elderly people, and 6,408 (63.5%) of the 10,085 patients were men. A total of eight interventions were evaluated: Ciwujia injection+WM, Dazhuhongjingtian injection+WM, Huangqi injection+WM, Shenfu injection+WM, Shenmai injection+WM, Shenmai injection+WM, Shenqi Fuzheng injection+WM and WM (Detailed information of included Chinese herbal injection were showed in the Table 1). WM involving therapy for controlling respiratory tract infections, improving respiratory and heart failure, and regulating electrolyte and acid-base balance were adopted in the control groups of all eligible RCTs. The interventions of the experimental group were as follows: on the basis of their corresponding control group, three RCTs administered Ciwujia injections, one RCT administered Dazhuhongjingtian injections, 31 RCTs administered Huangqi injections, 11 RCTs administered Shenfu injections, 15 RCTs administered Shengmai injections, 54 RCTs administered Shenmai injections, and three RCTs administered Shenqi Fuzheng injections. Among the eligible RCTs, 23 RCTs exceed the specification dosage when administering a Chinese herbal injection (Huangqi injection, 20 RCTs; Shenfu injection, two RCTs; Shengmai injection, one RCT). In addition, 26 eligible RCTs did not follow the specification for utilizing menstruum (Dazhuhongjingtian injection, one RCT; Shenfu injection, four RCTs; Shengmai injection, four RCTs; Shenmai injection, 17 RCTs). Fifteen RCTs were prescribed a Chinese herbal injection based on syndrome differentiation. The eligible Chinese herbal injections were given via intravenous drip once a day, except for two RCTs, which reported an administration twice a day, and one RCT that did not mention it. The RCT duration ranged from 7 to 42 days. In terms of outcomes, 83.1% of the RCTs reported a clinical effectiveness rate, 25.4% of the RCTs mentioned arterial blood gas analysis, 15.3% of the RCTs evaluated hemorheology results, 5.9% of the RCTs tested the hemodynamic dimension and 4.2% of the RCTs measured the right ventricular dimension. Table 2 summarizes the characteristics of the eligible RCTs, and Figure 1 illustrates the network graphs of the various eligible outcome comparisons.
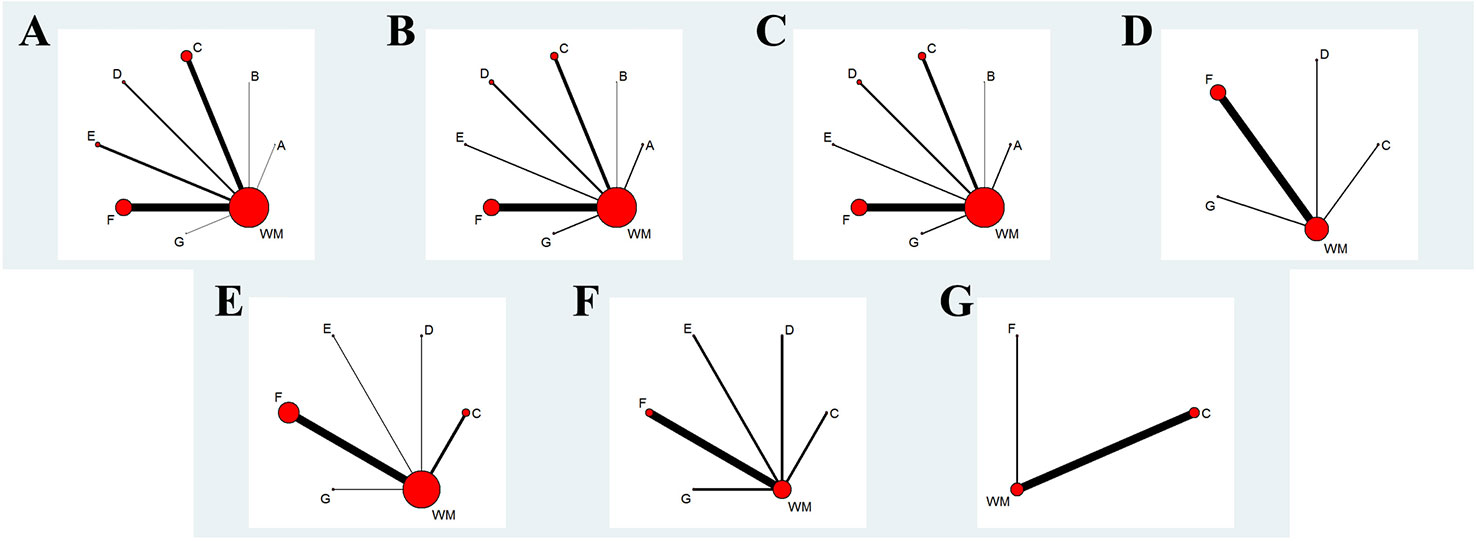
Figure 1 Network graph of different outcomes. (A) The Clinical Effectiveness Rate; (B) Partial Pressure of Arterial Oxygen; (C) Partial Pressure of Arterial Oxygen Carbon Dioxide; (D) The Level of Whole Blood Viscosity; (E) The Level of Fibrinogen; (F) Mean Pulmonary Arterial Pressure; (G) Right Ventricular Dimension. A: Ciwujia injection+WM; B: Dazhuhongjingtian injection+WM; C: Huangqi injection+WM; D: Shenfu injection+WM; E: Shengmai injection+WM; F: Shenmai injection+WM; G: Shenqi Fuzheng injection+WM.
Methodological Quality
The eligible RCTs were performed using randomization; however, only seven RCTs stated their specific random method, and these studies were evaluated as “low risk”. One of the eligible RCTs claimed a double-blind design and was classified as “low risk” regarding performance bias. In addition, two RCTs were conducted in a single-blind manner and were assessed as “high risk” regarding performance bias because the color and usage of the Chinese herbal injections increased the potential for blinding to be broken. The remaining RCTs were evaluated as “unclear risk” for their selection, performance and detection bias due to insufficient information. Concerning attribution bias, all of the eligible RCTs provided complete data, indicating that they were “low risk”. In addition, one RCT did not report all outcomes in accord with its design and was assessed as “high risk”. The others were deemed “low risk”. In addition, other biases were also found, namely, whether a significant difference existed between the experimental and control groups. Fifteen of the eligible RCTs did not report the baseline, which may have had an impact on the results; thus, these 15 RCTs were evaluated as “high risk”. The others were considered “low risk” (Presentation file).
Network Meta-Analysis
The Clinical Effectiveness Rate
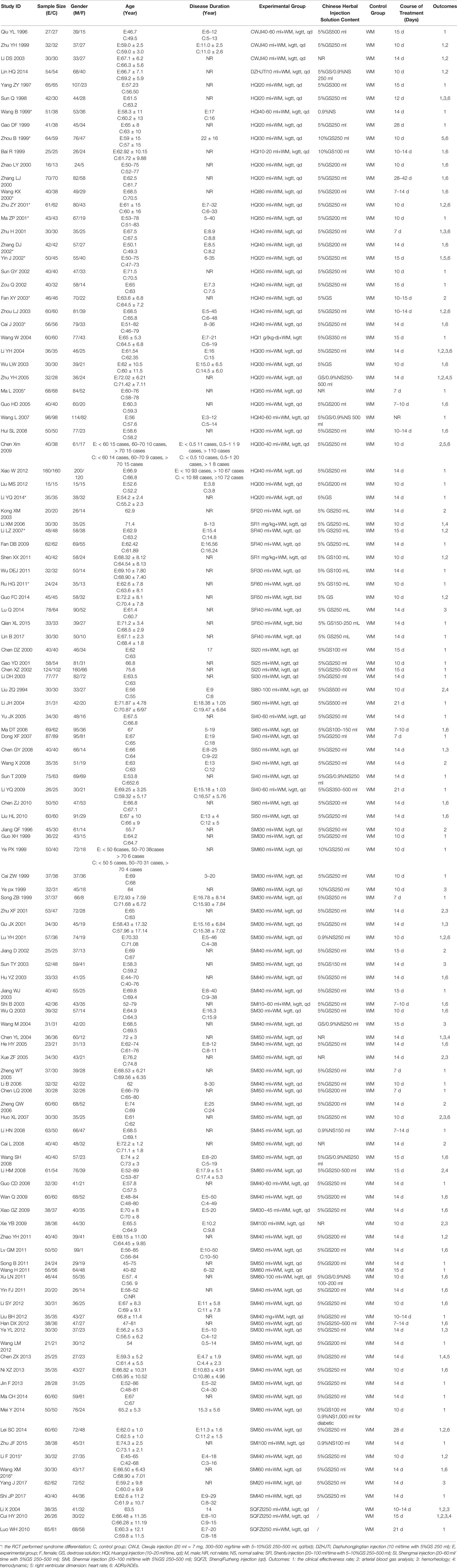
A total of 98 RCTs reported the clinical effectiveness rate (Ciwujia injection, three RCTs; Dazhuhongjingtian, one RCT; Huangqi injection, 28 RCTs; Shenfu injection, nine RCTs; Shengmai injection, 13 RCTs; Shenmai injection, 41 RCTs; Shenqi Fuzheng injection, three RCTs). Table 3 demonstrates the OR of this NMA, indicating that the combination of an eligible Chinese herbal injection and WM was superior to WM alone. The ORs of the comparisons were significantly different as follows: Ciwujia injection+WM vs. WM (OR = 0.27, 95% CI: 0.13–0.55), Dazhuhongjingtian injection+WM vs. WM (OR = 0.35, 95% CI: 0.11–0.91), Huangqi injection+WM vs. WM (OR = 0.23, 95% CI: 0.18–0.29), Shenfu injection+WM vs. WM (OR = 0.21, 95% CI: 0.12–0.35), Shengmai injection+WM vs. WM (OR = 0.29, 95% CI: 0.22–0.39), Shenmai injection+WM vs. WM (OR = 0.24, 95% CI: 0.20–0.30), and Shenqi Fuzheng injection+WM vs. WM (OR = 0.25, 95% CI: 0.11–0.54).
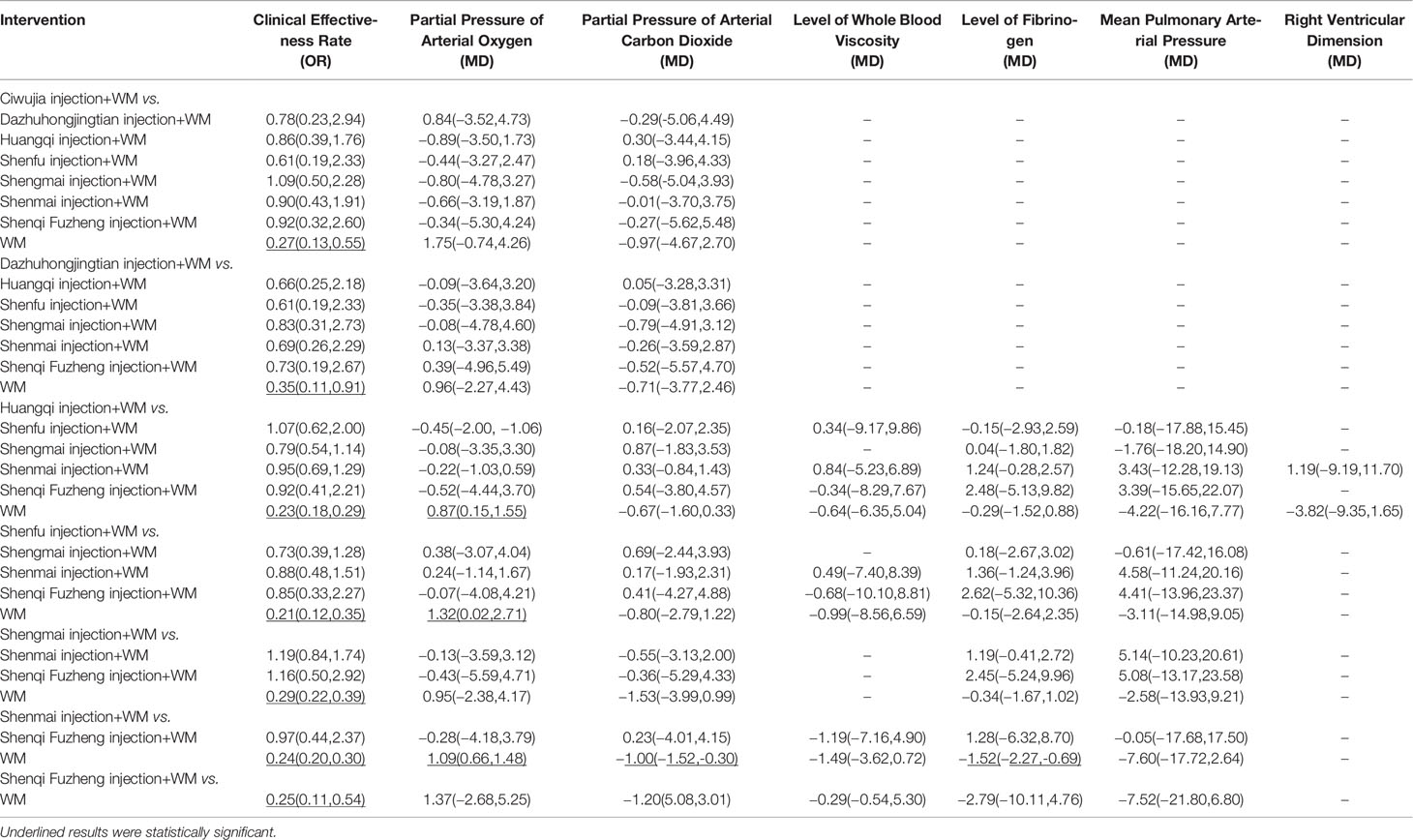
Rankings of the analysis results are illustrated in Table 4 and Figure 2, suggesting that Shenfu injection+WM had the highest clinical effectiveness rate, with a probability of 74.4%. Huangqi injection+WM (70.4%) was the second highest, and Shenmai injection+WM (63.0%) was the third highest.
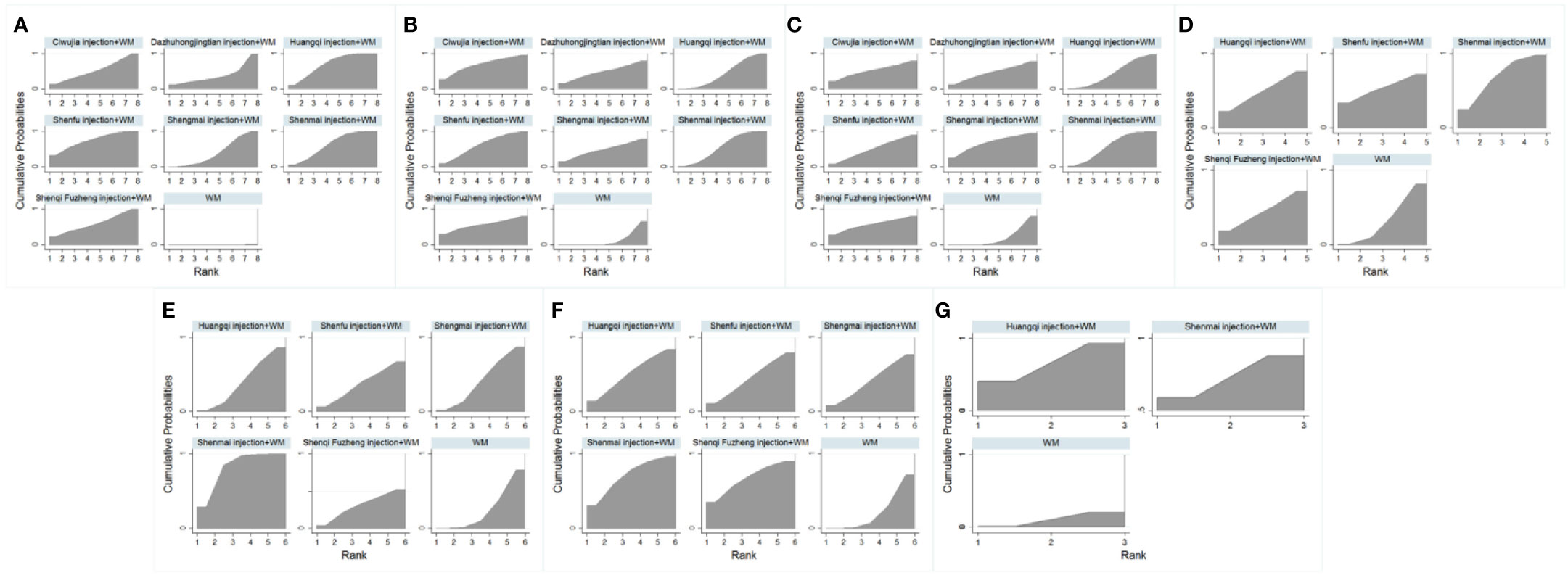
Figure 2 Surface under the cumulative ranking curve plots for all the different outcome interventions. (A) The Clinical Effectiveness Rate; (B) Partial Pressure of Arterial Oxygen; (C) Partial Pressure of Arterial Oxygen Carbon Dioxide; (D) The Level of Whole Blood Viscosity; (E) The Level of Fibrinogen; (F) Mean Pulmonary Arterial Pressure; (G) Right Ventricular Dimension.
Arterial Blood Gas Analysis
The arterial blood gas analysis of the partial arterial oxygen and partial pressure of arterial carbon dioxide were subjected to meta-analysis. Both were measured in 30 RCTs (Ciwujia injection, two RCTs; Dazhuhongjingtian, one RCT; Huangqi injection, six RCTs; Shenfu injection, four RCTs; Shengmai injection, two RCTs; Shenmai injection,13 RCTs; and Shenqi Fuzheng injection, two RCTs). Huangqi injection+ WM (MD = 0.87, 95% CI: 0.15–1.55), Shenfu injection+ WM (MD = 3.88, 95% CI: 1.10–8.05) and Shenmai injection+ WM (MD = 1.09, 95% CI: 0.66–1.48) were significantly different from WM alone in boosting the partial pressure of arterial oxygen (Table 3).
Ranking analysis revealed that Ciwujia injection+WM was the optimal combination with a probability of 69.1%, and the other beneficial interventions were Shenfu injection+WM (62.3%) and Shenqi Fuzheng injection+WM (56.6%) (Table 4 and Figure 2).
Shenmai injection+WM (MD = −1.00, 95% CI: −1.52- −0.30) was significantly different from WM alone in reducing the partial pressure of arterial carbon dioxide (Table 3).
Ranking analysis showed that Shengmai injection+WM was the optimal combination with a probability of 67.9%. Other beneficial interventions were Shenfu injection+WM (59.3%) and Shenqi Fuzheng injection+WM (56.9%) (Table 4 and Figure 2).
Hemorheology
The hemorheology index, which includes whole blood viscosity and fibrinogen levels, were evaluated in this NMA. Nine RCTs tested whole blood viscosity levels (Huangqi injection, one RCT; Shenfu injection, one RCT; Shenmai injection, six RCTs; and Shenqi Fuzheng injection, one RCT). No significant differences were observed amongst the various interventions (Table 3).
Ranking analysis demonstrated that Shenmai injection+WM performed well in decreasing the whole blood viscosity level, with a probability of 69.1% (Table 4 and Figure 2).
Fourteen RCTs tested fibrinogen levels (Huangqi injection, three RCT; Shenfu injection, one RCT; Shengmai injection, one RCT; Shenmai injection, eight RCTs; and Shenqi Fuzheng injection, one RCT). Shenmai injection+WM (MD = −1.52, 95% CI: −2.27 −0.69) was significantly different from WM alone with respect to lowering fibrinogen levels (Table 3).
The ranking analysis demonstrated that Shenmai injection+WM was more efficacious than the other treatments, with a probability of 82.2% (Table 4 and Figure 2).
Hemodynamics
The hemodynamic index of this NMA focused on the mean pulmonary arterial pressures measured in seven RCTs (Huangqi injection, one RCT; Shenfu injection, one RCT; Shengmai injection, one RCT; Shenmai injection, three RCTs; and Shenqi Fuzheng injection, one RCT). No significant difference was observed between the various interventions (Table 3).
In the ranking analysis, Shenmai injection+WM was more effective in decreasing mean pulmonary arterial pressures with a probability of 71.2% (Table 4 and Figure 2).
Right Ventricular Dimension
Five RCTs reported right ventricular dimensions (Huangqi injection, four RCTs and Shenmai injection, one RCT). None of the treatments produced significant decreases in right ventricular dimensions (Table 3).
Based on SUCRA, the ranking analysis revealed that Shenmai injection+WM could achieve a better impact in this outcome with a probability of 73.3% (Table 4 and Figure 2).
Cluster Analysis
The cluster analysis based on SUCRA is illustrated in Figure 3. First, the cluster analysis was conducted on arterial blood gas analysis. Among the eligible treatments, Shenmai injection+WM and Shenqi Fuzheng injection+WM achieved superior effects over the others in improving arterial blood gas levels, and WM alone ranked towards the bottom. Next, cluster analyses were performed on the clinical effectiveness rate and other outcomes. The results revealed that Shenfu injection+WM, Shenmai injection+WM, and Shenqi Fuzheng injection+WM were the highest ranked amongst the eligible interventions.
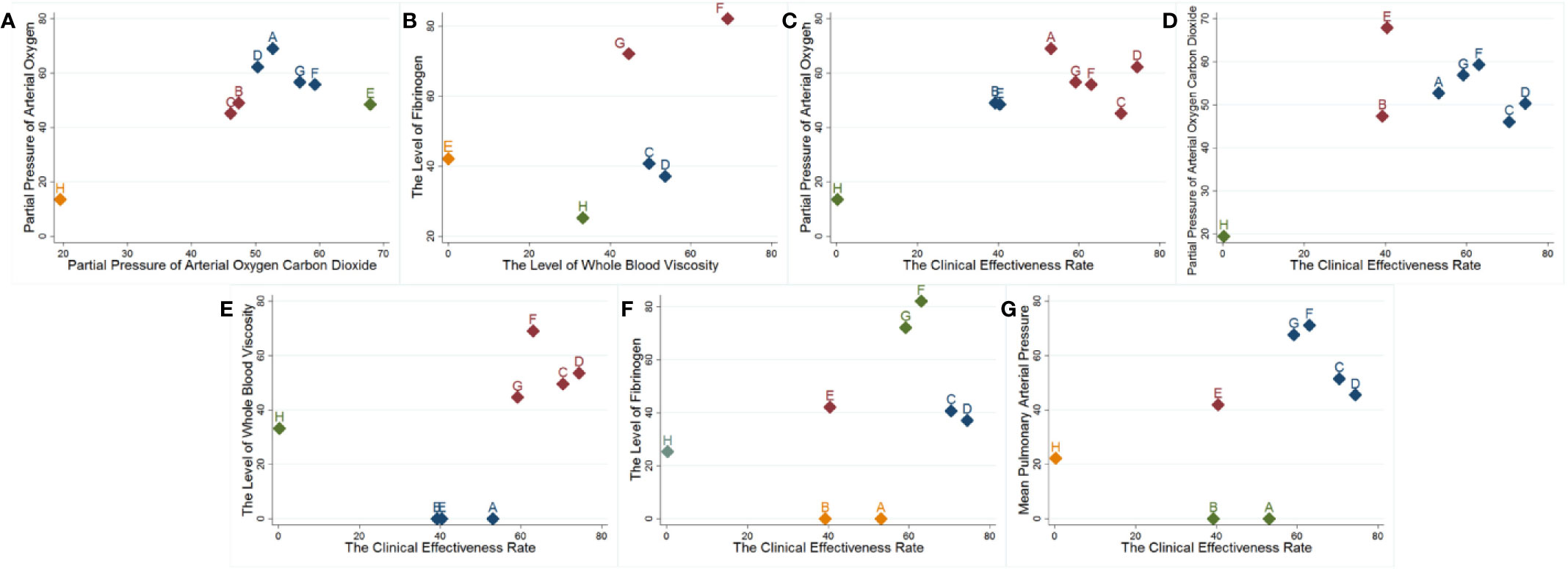
Figure 3 Cluster analysis plots. (A, B) Arterial blood gas analysis; (C–G) Cluster analysis on the clinical effectiveness rate and other outcomes. A: Ciwujia injection+WM; B: Dazhuhongjingtian injection+WM; C: Huangqi injection+WM; D: Shenfu injection+WM; E: Shengmai injection+WM; F: Shenmai injection+WM; G: Shenqi Fuzheng injection+WM.
Publication Bias
Publication bias was detected by funnel plots for outcomes included in more than 10 RCTs. Visual inspections showed that the eligible RCTs showed symmetry in the funnel plot of the clinical effectiveness rate, whereas the funnel plots for arterial blood gas analysis and fibrinogen levels were distributed asymmetrically and were out of line. Hence, a potential publication bias did exist (Figure 4).
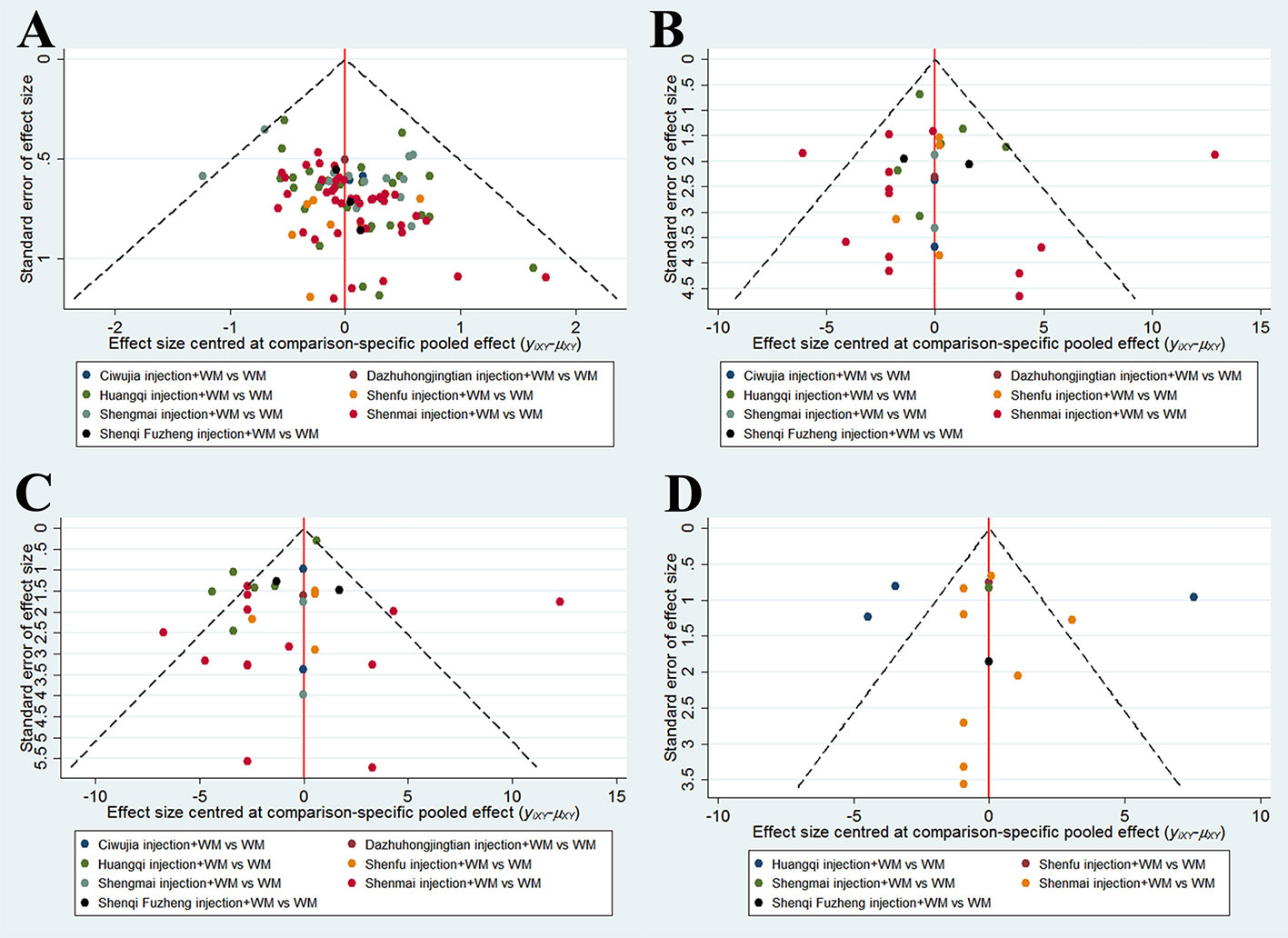
Figure 4 Funnel plots. (A) The Clinical Effectiveness Rate; (B) Partial Pressure of Arterial Oxygen; (C) Partial Pressure of Arterial Oxygen Carbon Dioxide; (D) The Level of Fibrinogen.
Adverse Drug Reactions (ADRs)/Adverse Drug Events (ADEs)
Amongst the eligible RCTs, 27 RCTs did not observe any ADRs/ADEs during the treatment, and 11 RCTs recorded ADRs/ADEs. Moreover, the remaining RCTs did not report ADRs/ADEs in their studies. Amongst the 11 RCTs, the intervention of the control group was WM, and three administered Huangqi injection+WM in the treatment group. In Zhu's research, three fever cases occurred in the treatment group. In Yin's research, nausea occurred in the treatment group. In Chen's research, one low fever case occurred in the treatment group. All of the above mentioned symptoms spontaneously resolved. One RCT reported two cases of xerostomia in the Shenmai injection+WM treatment group, which resolved with a slower drip rate. Seven RCTs reported ADRs/ADEs in the Shenmai injection+WM treatment group. In Wan's and Yin's research studies, the treatment group experienced two cases of xerostomia; in Xiao's research, three cases experienced pain at the injection site, and two cases experienced dizziness and palpitation in the treatment group; in Lv's research, one case of xerostomia and one case of tachycardia occurred in the treatment group; in Xu's research, one patient's heart rate decreased and two cases of headache, palpitation, and nausea in the corresponding control group were noted; in Lei's research, one case of rush and two cases of dizziness occurred in the treatment group, and three cases of rush, two cases of headache, and two cases of palpitations occurred in the corresponding control group; and in Wang's research, the treatment group and the control group reported one case of palpitations respectively. All of the aforementioned symptoms were relieved with a lower drip rate, which did not influence the RCTs.
Discussion
The severity of PHD has been widely recognized due to its high mortality rates and heavy economic burden (Yun et al., 2016). Currently, a combination of Chinese herbal injections with WM have been reported to achieve better curative effects in PHD patients amongst various treatments, and its efficacy was verified by clinical trials and pairwise meta-analyses (Li et al., 2008; Shi et al., 2015; Wu et al., 2018). As the aim of this study was to discern the comparative effectiveness of Chinese herbal injections simultaneously, this NMA incorporated 118 RCTs, which included 10,085 patients, comparing the efficacy of seven Chinese herbal injections combined with WM versus WM alone, namely, Ciwujia injection+WM, Dazhuhongjingtian injection+WM, Huangqi injection+WM, Shenfu injection+WM, Shenmai injection+WM, Shenmai injection+WM, Shenqi Fuzheng injection+WM verse WM.
This NMA extensively evaluated these treatments and revealed that all eligible Chinese herbal injections plus WM had a positive effect in PHD patients. Three principal findings were observed as new evidence for the efficacy of Chinese herbal injections in treating PHD: (1) According to the OR/MD and cluster analysis results, all eligible Chinese herbal injections plus WM were superior to WM alone, particularly in promoting the clinical effectiveness rate, improving respiratory failure and reducing pulmonary arterial hypertension. (2) In contrast, Shenfu injection+WM, Shenmai injection+WM, and Shenqi Fuzheng injection+WM exhibited outstanding efficacies compared with the others. (3) It is essential for clinicians to pay more attention to drip rates during treatment. In addition, this NMA could not draw a specific conclusion regarding the safety of the Chinese herbal injections due to insufficient information.
In addition to pulmonary arterial hypertension, which is a precondition of PHD, limited respiratory and cardiac function and heart overload are predisposing factors (Tao and Zhang, 2004; Chen et al., 2013). Shenfu is a Chinese herbal medicine that is extracted from Hongshen (Ginseng radix et rhizoma rubra) and Fuzi (Aconm lateralis radix praeparaia) and functions by building up vital energy and relieving depletion. Although no experiments have shown that Shenfu injection could decrease pulmonary arterial hypertension, pharmacological experiments have already revealed that Shenfu injection has a specific influence on hemorheology. For instance, Shenfu injection is capable of lowering plasma viscosity, speeding blood flow velocity, alleviating platelet aggregation and relieving pulmonary artery thrombosis (Tao and Zhang, 2004). It also excels at alleviating bronchial smooth muscle spasms, protecting impaired lung tissue, and improving oxyhemoglobin saturation to enhance respiratory function (Sui, 2015). Moreover, Shenfu injection can modify the weak immune functions in PHD patients as well (Tian and Han, 2011; Sui, 2015). In addition, several pairwise meta-analyses showed that Shenfu injection plus WM improved the clinical effectiveness rate and respiratory and cardiac functions and lowered fibrinogen levels (Li et al., 2008). Shenmai contains Ginseng radix et rhizoma and Ophiopogonis radix and has an outstanding capacity to nourish and benefit (Cao et al., 2010). Pharmacological experiments confirmed that Shenmai injection had the ability to reduce pulmonary vascular resistance, improve the partial pressure of arterial oxygen, and enhance upper airway contractility through resisting upper airway fatigue, thus improving respiratory function (He et al., 2005; Li et al., 2006; Huang and Hu, 2017). It can also improve cardiac function by reducing the load on the heart and improving the oxygen supply for the myocardium (Li et al., 2006). With respect to immune function, Shenmai injection can boost CD3+, CD4+, and CD8+ T-lymphocyte levels (Sun, 2014; Huang and Hu, 2017). Shenqi Fuzheng contains Codonopsis radix and Astragali radix and is beneficial to strength and helpful in restoring vitality. No pharmacological experiments have verified its capacity in the lung, but its functions regarding positive inotropic effects, vasodilation and restraining heart failure have been confirmed (Wang and Zhang, 2016). Moreover, Shenqi Fuzheng injection enhanced the immune system via inhibition of T-lymphocytes (Liao and Xing, 2016).
In addition to the efficacy of Chinese herbal injections, their safety should also be considered. Though the occurrences of ADRs/ADEs in this NMA were low, approximately two-thirds of eligible RCTs did not report ADRs/ADEs, which meant their occurrence has not attracted clinical attention. While describing ADRs/ADEs, this NMA observed that an appropriate drip rate is essential in treatment. In addition, dosage, appropriate solution and syndrome differentiation should also be emphasized (Tan et al., 2014; Liu et al., 2016; Yang et al., 2018). This NMA has summarized this information (Presentation file).
This NMA was the first to apply a Bayesian model in the evaluation of Chinese herbal injection efficacy in the treatment of PHD to help in choosing a proper regimen. Bayesian NMA is considered the most applicable approach for a multiple-intervention NMA, as it enhances the relationship between the eligible RCTs and improves data utilization. In this NMA, a comprehensive literature search was performed to ensure the sample size of the NMA. Additionally, this NMA formulated strict eligibility criteria that control for the consistency between eligible RCTs on disease situations and interventions to reduce clinical heterogeneity. While the heterogeneity cannot be eliminated entirely, this NMA reduced it in this way. Notably, however, the pre-retrieval found that most relevant RCTs did not report the WM dosage; therefore, this NMA restricted the WM types and did not limit specific dosages. If the dosage description is included, then the quality of the NMA will be improved as well. Moreover, the kinds of outcomes varied because PHD involves lung and heart failure; therefore, on the basis of reading the clinical trials before the NMA was performed, seven representative outcomes that were measured in the highest number of studies were selected. This NMA used the clinical effectiveness rate to reflect the recovery condition of the PHD patients, arterial blood gas analysis to determine their functions on respiration, and hemorheology and hemodynamic index data to discern the pulmonary vasculature characteristics. Moreover, analyzing the right ventricular dimension reflected the right heart features.
Although the results of this NMA are promising, its limitations are worth mentioning. First, the eligible RCTs were conducted in China, and non-Chinese publications were excluded, resulting in a potential publication bias, which is illustrated in the funnel plot. Second, the credibility of this NMA was reduced because most eligible RCTs were carried out without adequate randomization, allocation and blinding. Third, the sample sizes of the outcomes need improvement. A small sample size may not detect a significant difference in comparisons. If the sample size is increased and the number of RCTs focused on different kinds of Chinese herbal injection was balanced, then the statistical strength of the data and the credibility of the NMA would be enhanced. In this context, further high quality and large scale RCTs are required to support this NMA.
Conclusion
In general, this NMA performed a comprehensive evaluation and summary of Chinese herbal injections for treating PHD for the first time and proposed several findings. The results manifested that eligible Chinese herbal injections plus WM were superior to WM alone, especially Shenfu injection+WM, Shenmai injection+WM and Shenqi Fuzheng injection+WM. It is imperative for clinicians to incorporate the patients' symptoms and the Chinese herbal injections' efficacies when diagnoses are made. Larger sample sizes and high quality RCTs are needed to confirm and support this NMA.
Data Availability Statement
All datasets presented in this study are included in the article/Supplementary Material.
Author Contributions
JW and KW did conception and design of the network meta-analysis. KW, YW, XZe, MN, SL, ZM, and XZh performed the network meta-analysis. DZ, XD, and JW assessed the quality of the network meta-analysis. KW, XD, JW, YW, HW, and XZh analyzed study data. KW, XD, and HW wrote the paper. All authors read and approved the final version of the manuscript.
Funding
This study received funding from the National Natural Science Foundation of China (No. 81473547 and No. 81673829) and the Young Scientists Training Program of Beijing University of Chinese Medicine.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer XL declared a shared affiliation, with no collaboration, with the authors to the handling editor at the time of review.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.00634/full#supplementary-material.
Data Sheet 1 | This file contains seven parts, which includes items regarding the PRISMA checklist for network meta-analysis and corresponding pages of this study, the search strategy of traditional Chinese medicine injections and English databases, information about the included randomized controlled trials and Chinese herbal injections, and a reference list of the eligible randomized controlled trials. This file also contains two figures, a flow chart of the included randomized controlled trial searches and a risk of bias graph.
Abbreviations
95% CI, 95% credible interval; ADRs/ADEs, adverse drug reactions/adverse drug events; CHIs, Chinese herbal injections; PHD, Pulmonary heart disease; MD, mean difference; NMA, network meta-analysis; OR, odds ratio; RCTs, randomized controlled trials; WM, western medicine; SUCRA, surface under the cumulative ranking area curve.
References
Cai, W., Gu, Y., Cui, H., Cao, Y. Y., Wang, X. L., Yao, Y., et al. (2018). The Efficacy and Safety of Mainstream Medications for Patients With cDMARD-Naïve Rheumatoid Arthritis: A Network Meta-Analysis. Front. Pharmacol. 9, 138. doi: 10.3389/fphar.2018.00138
Cao, X. D., Ding, Z. S., Chen, J. C. (2010). Pharmacology and clinical research of Shenmai injection. Chin. J. InfTradit. Chin. Med. 17, 104–106.
Chang, L., Guo, R. (2017). Comparison of the efficacy among multiple chemotherapeutic interventions combined with radiation therapy for patients with cervix cancer after surgery: a network meta-analysis. Oncotarget 8, 49515–49533. doi: 10.18632/oncotarget.17259
Chen, H. Z., Lin, G. W., Wang, J. Y. (2013). Practical of internal medicine (Beijing: People's Medical Publishing House 2013).
China association of traditional Chinese medicine of lung diseases (2014). Guidelines for traditional Chinese medicine diagnosis and treatment of chronic pulmonary heart disease. J. Tradit. Chin. Med. 55, 526–531.
Cipriani, A., Furukawa, T. A., Salanti, G., Chaimani, A., Atkinson, L. Z., Ogawa, Y., et al. (2018). Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet 391, 1357–1366. doi: 10.1016/S0140-6736(17)32802-7
Forfia, P. R., Vaidya, A., Wiegers, S. E. (2013). Pulmonary heart disease: The heart-lung interaction and its impact on patient phenotypes. Pulm. Circul. 3, 5. doi: 10.4103/2045-8932.109910
He, Y., Liu, Y. F. (2014). TCM diagnosis and discuss on chronic pulmonary heart disease. Chin. Med. Doctor 52, 75–77.
He, T., Tan, Y. Q., Shi, J. P. (2005). The latest development of Shenmai injection experiment. Neimonggu. J. Tradit. Chin. Med. 24, 33–34.
Huang, Z. Q., Hu, T. H. (2017). Pharmacology and clinical research of Shenmai injection. J. Clin. Med. 4, 2762–2763.
Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H., Cameron, C., et al. (2015). The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann. Intern. Med. 162, 777–784. doi: 10.7326/M14-2385
Li, J. W., Zhao, Y., Liu, Z. F. (2006). Application of Shenmai injection in chronic congestive heart failure. J. Emerg. Syndromes Tradit. Chin. Med. 15, 86–87.
Li, P., Qu, W. X., Wu, J. (2008). Systematic evaluation in the treatment of chronic pulmonary heart disease by Shenfu injection. Her. Med. 27, 553.
Liao, X., Xing, R. (2016). Pharmacological action and clinical application of Shenqi Fuzheng injection. J. China Pharm. 27, 3455–3456.
Liao, M. L., Huang, L., Kong, Y. Q. (2004). New progress in treating pulmonary heart disease with traditional Chinese medicine injection. World Latest Med. Inf. 3, 1231–1232.
Liu, S., Qiao, Y., Liu, G. F. (2016). Adverse drug reactions of traditional Chinese medicine injections and irrational drug use. Cent. South. Pharm. 14, 1177–1172.
Lu, G., Ades, A. E. (2004). Combination of direct and indirect evidence in mixed treatment comparisons. Stat. Med. 23, 3105–3124. doi: 10.1002/sim.1875
Ruan, H. R., Ma, J. D., Li, J. D., Zhang, P., Li, Q. L., Sun, S. M., et al. (2018). Etiology and Pathogenesis Aualysis in Treatment of Chronic Pulmonary Heart Disease. J. Chin. Med. 33, 37–41.
Shi, L. W., Xie, Y. M., Liao, X., Chai, Y., Luo, Y. H. (2015). Shenmai injection as an adjuvant treatment for chronic cor pulmonale heart failure: a systematic review and meta-analysis of randomized controlled trials. BMC Complement Altern. Med. 15, 418. doi: 10.1186/s12906-015-0939-2
Singh, J. A., Hossain, A., Kotb, A., Wells, G. (2016). Risk of serious infections with immunosuppressive drugs and glucocorticoids for lupus nephritis: a systematic review and network meta-analysis. BMC Med. 14, 137. doi: 10.1186/s12916-016-0673-8
Sui, Y. L. (2015). The research pharmacological effect and progress of clinical applications of Shenfu injection. World Latest Med. Inf. 15, 23–24.
Tan, L. J., Wang, M., Zhu, Y. (2014). Research progress of adverse reactions of traditional Chinese medicine injections. China J. Chin. Mater. Med. 35, 3889–3898.
Tang, H., Fang, Z. W., Wang, T. S., Cui, W., Zhai, S. D., Song, Y. Q. (2016). Meta-analysis of effects of sodium-glucose cotransporter 2 (sglt2) inhibitors on cardiovascular outcomes and all-cause mortality among patients with type 2 diabetes mellitus. Am. J. Cardiol. 118, 1774–1780. doi: 10.1016/j.amjcard.2016.08.061
Tao, J., Zhang, Y. J. (2004). Experimental research progress of Shenfu injection. J. Emerg. Syndromes Tradit. Chin. Med. 13, 688–689.
Tian, Z. M., Han, Y. (2011). Research on pathogenesis of chronic pulmonary heart disease and its treatment progress. Chin. Heal. Ind. 8, 118–119.
Wang, D., Zhang, J. M. (2016). Ginseng and astragalus righting the research progress of injection treatment of chronic heart failure patients. Cardiovasc. Dis. J. Integrated Tradit. Chin. West. Med. 4, 27–29.
Wang, Y., Teng, L., Wang, H. (2016). Research in the prevention and treatment of pulmonary heart disease by traditional Chinese medicine. InfTradit. Chin. Med. 33, 125–127.
Wu, J. T., Wang, K. H., Wu, J. R., Duan, X. J., Ni, M. W., Liu, S. Y. (2018). Meta-analysis on Randomized Controlled Trials of Hangqi Injection in the Treatment of Pulmonary Heart Disease. Eval. Anal. Drug Use Hosp. China 18, 1009–1014, 1020.
Yang, F. W., Zou, J. H., Wang, Y., Sun, C. X., Ge, L., Tian, J. H., et al. (2018). Network Meta-analysis of Chinese medical injections for heart failure. China J. Chin. Mater. Med. 43, 1247–1253.
Yu, B. (2009). Progress in traditional Chinese medicine treatment of chronic pulmonary heart disease. Jiangsu J. Tradit. Chin. Med. 41, 79–81.
Keywords: network meta-analysis, Bayesian model, pulmonary heart disease, Chinese herbal injection, Shenfu injection, Shenmai injection, Shenqi Fuzheng injection
Citation: Wang K, Wu J, Wang H, Duan X, Zhang D, Wang Y, Ni M, Liu S, Meng Z, Zeng X and Zhang X (2020) Comparative Efficacy of Chinese Herbal Injections for Pulmonary Heart Disease: A Bayesian Network Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 11:634. doi: 10.3389/fphar.2020.00634
Received: 20 August 2019; Accepted: 21 April 2020;
Published: 07 May 2020.
Edited by:
Anna Rita Bilia, University of Florence, ItalyReviewed by:
Guochun Li, Nanjing University of Chinese Medicine, ChinaXinkui Liu, Beijing University of Chinese Medicine, China
Copyright © 2020 Wang, Wu, Wang, Duan, Zhang, Wang, Ni, Liu, Meng, Zeng and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiarui Wu, ZXhvZ2FteUAxNjMuY29t
 Kaihuan Wang1
Kaihuan Wang1 Jiarui Wu
Jiarui Wu Mengwei Ni
Mengwei Ni Shuyu Liu
Shuyu Liu Ziqi Meng
Ziqi Meng Xiantao Zeng
Xiantao Zeng