- 1School of Chinese Materia Medica, Beijing University of Chinese Medicine, Beijing, China
- 2Department of Pharmacy, Beijing Shijitan Hospital Affiliated to Capital University of Medical Sciences, Beijing, China
- 3Beijing Research Institute of Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China
Matrine (MT) is a naturally occurring alkaloid and an bioactive component of Chinese herbs, such as Sophora flavescens and Radix Sophorae tonkinensis. Emerging evidence suggests that MT possesses anti-cancer, anti-inflammatory, anti-oxidant, antiviral, antimicrobial, anti-fibrotic, anti-allergic, antinociceptive, hepatoprotective, cardioprotective, and neuroprotective properties. These pharmacological properties form the foundation for its application in the treatment of various diseases, such as multiple types of cancers, hepatitis, skin diseases, allergic asthma, diabetic cardiomyopathy, pain, Alzheimer’s disease (AD), Parkinson’s disease (PD), and central nervous system (CNS) inflammation. However, an increasing number of published studies indicate that MT has serious adverse effects, the most obvious being liver toxicity and neurotoxicity, which are major factors limiting its clinical use. Pharmacokinetic studies have shown that MT has low oral bioavailability and short half-life in vivo. This review summarizes the latest advances in research on the pharmacology, toxicology, and pharmacokinetics of MT, with a focus on its biological properties and mechanism of action. The review provides insight into the future of research on traditional Chinese medicine.
Introduction
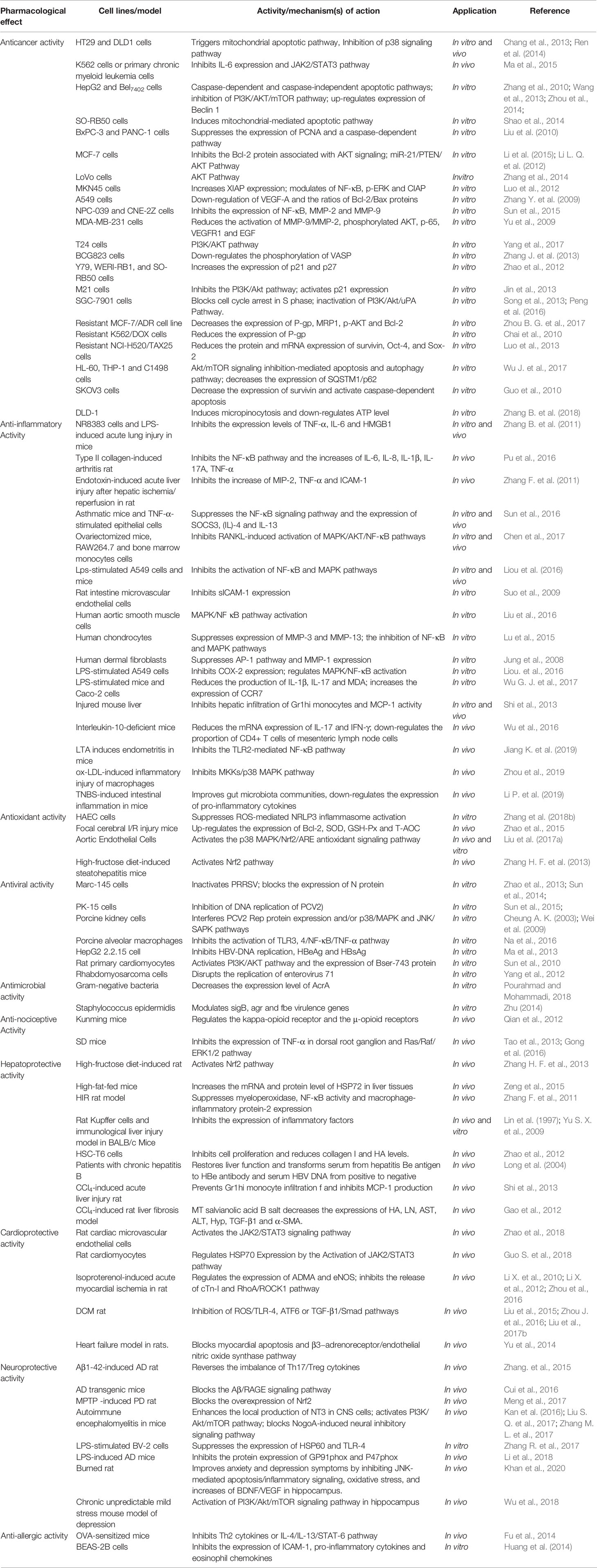
Matrine (MT) (Figure 1) is a naturally occurring alkaloid and a bioactive component of Chinese herbs, including Sophora flavescens and Radix Sophorae tonkinensis (Gu et al., 2019; You et al., 2019). These herbs have a long medicinal history in China and many Eastern Asian countries. In recent years, in vitro and in vivo studies have demonstrated that MT exhibits linear pharmacokinetics between 5 and 2000 ng/mL (Zhang X. L. et al., 2009), and possesses a wide range of pharmacological effects, including anti-cancer, anti-inflammatory, anti-bacterial, anti-parasitic, anti-virus, anti-fibrotic and, sedative properties (Table 1). These pharmacological effects have been exploited for the treatment of hepatitis (Gong et al., 2015); cardiac diseases (Liu et al., 2017b); skin diseases (Liu J. Y. et al., 2007) and many cancers, such as hepatocellular carcinoma (Wang L. et al., 2013); gastric cancer (Luo et al., 2007); breast cancer (Zhou B. G. et al., 2017), and pancreatic cancer (Huang and Xin 2017). Previous reviews reported the chemical composition and anticancer properties of MT (Huang and Xu 2016). However, to date, there are no published comprehensive and systematic reviews on MT. In this review, studies on pharmacology, toxicity, and pharmacokinetics of MT are presented so as to provide comprehensive and updated information on research on MT in the past few decades, and to investigate the therapeutic potential and safety of its components in clinical application.
Pharmacology
Anticancer Effects of MT
Induction of Apoptosis
Apoptosis is a physiological process of autonomous, programmed cell death that is used to remove malignant cells such as cancer cells without causing damage to normal cells or surrounding tissues. Apoptosis induced by MT has been reported in diverse human cancer cells. Treatment of human hepatocellular carcinoma HepG2 and Bel7402 cells with MT at doses of 0.2–3.2 mg/mL for 24, 48, and 72 h caused a dose- and time-dependent apoptosis (Wang L. et al., 2013). Moreover, MT inhibited cell growth and induced apoptosis in HT29 cells by regulating the expressions of apoptosis-related genes. Incubation with MT enhanced apoptosis in HT29 cells via the release of cytochrome c in cytoplasm and the up-regulation of apoptotic-related genes including caspase-3, caspase-9, and Bax/Bcl-2 ratio (Chang et al., 2013). In another study, MT treatment significantly inhibited cell proliferation and induced apoptosis in K562 cells (primary chronic myeloid leukemia cells) through regulation of genes related to the JAK2/STAT3 signaling pathway (Ma et al., 2015). In that study, inhibition of the expression of IL-6 expression subsequently decreased the protein expressions of downstream JAK2 and STAT3 (Ma et al., 2015).
MT effectively induced programmed cell death in HepG2 cells through caspase-dependent and caspase-independent apoptotic pathways involving the loss of mitochondrial membrane potential, reactive oxygen species (ROS) generation, Bid-mediated AIF nuclear translocation, and cytochrome c release from the mitochondria. Moreover, MT treatment up-regulated the expression of apoptosis-related proteins Fas/Fas-L and cleaved caspase-3, while down-regulating the expressions of procaspase-3, procaspase-8, and procaspase-9 (Zhou et al., 2014). It has been reported that MT inhibited the proliferation of SO-RB50 cells and initiated apoptosis by activating the caspase family of proteins, resulting in disruption of mitochondrial permeability transition pores (Shao et al., 2014). Moreover, MT treatment triggered apoptosis in human pancreatic cancer cells (BxPC-3 and PANC-1) via decreases in the expressions of PCNA and a caspase-dependent pathway (Liu et al., 2010).
The AKT signaling pathway is an important cell signaling pathway upstream of many genes that regulate cell survival, proliferation, angiogenesis, and metabolism (Manning and Cantley, 2007). Previous studies have shown that MT exerts anti-cancer effects in human breast cancer cells (MCF-7) by inhibiting Bcl-2 protein associated with the AKT signaling pathway (Li et al., 2015). A similar study indicated that MT inhibited the growth of breast cancer MCF-7 cells by modulating the miR-21/PTEN/AKT pathway (Li L. Q. et al., 2012). Interestingly, MT also inhibited human colon cancer LoVo cell proliferation through inactivation of the AKT pathway (Zhang et al., 2014). Furthermore, Guo et al., demonstrated that MT effectively inhibited the proliferation of human ovarian cancer cells (SKOV3), and its potential mechanism might be to decrease the expression of survivin and activate caspase-dependent apoptosis (Guo et al., 2010).
The X- chromosome-linked inhibitor of apoptosis protein (XIAP), an anti-apoptotic factor from the IAP family, suppresses caspase activity (Wrzesień et al., 2004). Recent studies have demonstrated that MT treatment induced apoptosis in human gastric cancer cells (MKN45) by increasing the XIAP protein expression level, modulating NF-κB, phosphorylated extracellular signal-regulated kinase (p-ERK) and CIAP (Luo et al., 2012). Interestingly, Zhang B. et al. (2018) reported that MT could induce macropinocytosis in human colon adenocarcinoma cells (DLD-1) and down-regulate ATP level in the cells, leading to non-apoptotic cell death. This provides a new strategy for the development of MT as an anticancer drug.
Anti-Metastatic Effect of MT
Tumor metastasis is a complex process that entails decreased tumor cell adhesion and degradation and remodeling of the extracellular matrix (ECM) (Hsieh et al., 2014). Studies have shown that MT significantly inhibited A549 cell growth and migration through the down-regulation of vascular endothelial growth factor A (VEGF-A) and changes in the ratio of Bcl-2/Bax proteins (Zhang Y. et al., 2009). In another study, MT at doses of 12.5, 25, 50, 100, and 200 µg/mL, inhibited the migration and invasion of nasopharyngeal carcinoma cells (NPC-039 and CNE-2Z) through suppression of the expression of NF-κB and down-regulation of downstream matrix metalloproteinase-2 and metalloproteinase-9 (MMP-2 and MMP-9) proteins (Sun and Min, 2015). It has been demonstrated that MT effectively inhibited the invasion of highly-metastatic human breast cancer MDA-MB-231 cell in vitro by reducing the activation of MMP-9/MMP-2, enhancing the phosphorylation of AKT, and decreasing the activities of p-65, VEGFR1, and epidermal growth factor (EGF) (Yu P., et al., 2009). In addition, Yang et al. reported that MT dose-dependently inhibited the growth and invasion of bladder cancer T24 cells via the PI3K/AKT pathway, and regulation of invasion-related genes (Yang Y. et al., 2017).
It has been demonstrated that MT significantly inhibited cell proliferation and invasion in colorectal cancer cells (HT29 and DLD1) via reduction in the activation of the p38 signaling pathway (Ren, 2014). Another study demonstrated that MT significantly down-regulated the expression and phosphorylation of vasodilator-stimulated phosphoprotein (VASP), which is up-regulated in gastric cancer cells (BCG823), thereby suppressing tumor cell migration and adhesion (Zhang J. et al., 2013).
Effect of MT on Cell Cycle
Impairment of cell cycle regulation is an important process in malignant transformation. Cyclins, cyclin-dependent kinases, and their inhibitors are involved in the regulation of cell cycle progression (Shi et al., 2003). Previous studies showed that MT dose-dependently inhibited the proliferation of human retinoblastoma cells (Y79, WERI-RB1, and SO-RB50), and induced cell cycle arrest at the G0/G1 phase in a time-dependent manner. The intracellular mechanisms involved are related to increased levels of the CDK inhibitors p21 and p27, and the decreased levels of the cyclin D1 protein (Zhao et al., 2012). Melanoma M21 cells also showed similar effects (Jin et al., 2013). Moreover, studies have revealed that MT caused cell cycle arrest in S phase in gastric cancer SGC-7901 cells (Song et al., 2013; Peng et al., 2016). In addition, MT has been reported to up-regulate the expressions of p53 and p21, and down-regulate the expressions of CDK2, CDK4, cyclin D1, cyclin E, and phosphorylated Rb, leading to the arrest of vascular smooth muscle cells (VSMCs) at the G0/G1 phase (Zhu et al., 2010).
Reversion of Multidrug Resistance
Chemotherapy is the major treatment for various cancers. However, excessive expression of multi-drug resistance (MDR) in tumor cells has seriously affected the success of chemotherapy. The regulation of MDR gene expression is a complex process that is primarily associated with increases in a variety of adenosine triphosphate (ATP)-binding cassette transporters, including P-glycoprotein (P-gp), and multidrug resistance-related protein (MRP) (Hien et al., 2010; Kanagasabai et al., 2011). Previous studies have demonstrated that MT (0-2.5 mg/mL) increased the intracellular accumulation of adriamycin (ADR) and triggered its apoptotic effects in the resistant MCF-7/ADR cell line through decreased expressions of P-gp, MRP1, p-AKT, and Bcl-2 (Zhou B. G. et al., 2017). Another study also found that MT up-regulated the intracellular accumulation of doxorubicin (DOX), and induced apoptosis of K562/DOX cells through a reduction in the expression of P-gp (Chai et al., 2010). Moreover, recent studies have shown that excessive expression of survivin is closely associated with MDR (Wang Q. P. et al., 2013). Decreased mRNA and protein expressions of survivin, Oct-4, and Sox-2 may be the molecular mechanism involved in the MT-mediated reversal of paclitaxel (TAX) resistance in NCI-H520/TAX25 cells (Luo et al., 2013).
Induction of Autophagy
Autophagy is an important process of cell death through which cells degrade and circulate their own components, including rapid caspase-independent self-digestion, nuclear condensation, organelle swelling, and lysosomal degradation (Yang et al., 2011; Kondratskyi et al., 2017). In addition, apoptosis and autophagy are regulated by subtle crosstalk, and their signaling pathways are interrelated in various diseases (Wu et al., 2014). Previous studies have reported that MT inhibited the growth of HepG2 cells via induction of apoptosis and autophagy. Following MT treatment, HepG2 hepatoma cells showed obvious morphological changes, including the occurrence of a large number of autophagic vacuoles (AVs) of different sizes, and the up-regulated expression of Beclin 1, which was the first identified autophagy-inducing mammalian gene (Zhang et al., 2010). Moreover, Wang et al. showed that the inhibition of autophagy promoted apoptosis of human hepatoma cells induced by MT (Wang L. et al., 2013). In acute myeloid leukemia cell lines (HL-60, THP-1 and C1498), treatment with MT at doses of 0.25-3 g/L for 12-48 h resulted in cytotoxicity via induction of Akt/mTOR signaling inhibition-mediated apoptosis and autophagy, which is involved in increased expression of LC3-II and decreased SQSTM1/p62 ratio (Wu J. et al., 2017). Therefore, MT seems to affect both autophagy and apoptosis through crosstalk.
These findings indicate that MT inhibits the growth of various cancer cells and regulates the expressions of genes and proteins associated with apoptosis, autophagy, cell invasion, metastasis, and cell cycle arrest.
Anti-Inflammatory Effect of MT
The anti-inflammatory effect of MT been well confirmed. This effect is exerted via regulation of the expressions of inflammatory cytokines and chemokines such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1, IL-2, IL-4, IL-5, and IL-10; pro-inflammatory transcription factors, i.e., nuclear factor kappa-B (NF-κB), and inflammatory mediators, i.e., nitric oxide and matrix metalloproteinases (NO and MMPs). MT has been used for the treatment of rheumatoid arthritis, hepatitis, atopic dermatitis, endometritis, and enteritis (Zhang B. et al., 2011; Zhang L. et al., 2015; Jiang K. et al., 2019).
The potential mechanisms of the anti-inflammatory effects of MT may involve the following:
1. Down-regulation of the expressions of inflammatory cytokines by inhibiting NF-κB.MT exerts its anti-cancer activity, partly at least, through the inhibition of NF-κB. It also plays an extensive role in regulating the inflammatory response through the NF-κB signaling pathway. In a type II collagen-induced arthritis rat model, MT exerted its anti-arthritis effects via down-regulation of pro-inflammatory cytokines and proteins (IL-6, IL-8, IL-1β, IL-17A, and TNF-α) and down-regulation of NF-κB (Pu et al., 2016). In addition, MT inhibited lipopolysaccharide (LPS)-induced increases in macrophage inflammatory protein-2 (MIP-2), TNF-α, soluble intercellular adhesion molecule-1 (ICAM-1), and NO through inhibition of the activity of NF-κB (Zhang F. et al., 2011). It has been reported that MT inhibited ovalbumin (OVA)-induced airway hyperresponsiveness (AHR) in mice by decreasing the production of IL-4 and IL-13, and increasing the expression of interferon (IFN)-γ. Furthermore, the study showed that MT suppressed the expression levels of SOCS3 in asthmatic mice and TNF-α-stimulated epithelial cells (BEAS-2B and MLE-12) by inhibiting the NF-κB signaling pathway (Sun et al., 2016). Estrogen withdrawal results in up-regulation of pro-inflammatory cytokines, thereby inducing overactivation of osteoclasts inflammation. Recent studies have shown that MT significantly prevented ovariectomy-induced osteoporosis and osteoclastogenesis in vivo, while reducing serum levels of IL-6, TNF-α, and tartrate-resistant acid phosphatase 5B (TRACP5B). Moreover, MT significantly inhibited osteoclast differentiation of RAW264.7 and bone marrow monocytes cells though the inhibition of NF-κB ligand (RANKL)-induced activation of MAPK/AKT/NF-κB pathways (Chen et al., 2017). Another study showed that MT has a protective effect on Staphylococcus aureus lipoic acid (LTA) induced endometritis by inhibiting the TLR2-mediated NF-κB pathway (Jiang K. et al., 2019).
2. Decreases in the expressions of endothelial cell adhesion molecules (ECAMs). Decreased expressions of ECAMs have been shown to reduce the vascular complications induced by inflammation (Hu et al., 2013). The transcription and expression of intercellular adhesion molecule-1 (ICAM-1) could be decreased via treatment with MT in LPS-stimulated human lung epithelial A549 cells via inhibition of the activation of NF-κB and MAPK pathways (Liou et al., 2016). Moreover, MT decreased LPS-induced increases in IL-6, IL-8, and soluble intercellular adhesion molecule-1 (sICAM-1) in rat intestine microvascular endothelial cells (RIMECs) (Suo et al., 2009). In addition, it has been found that MT treatment inhibited TNF-α-induced up-regulation of vascular cell adhesion molecule-1 (VCAM−1) and ICAM−1 in human aortic smooth muscle cells through the activation of MAPK/NF-κB pathway (Liu et al., 2016).
3. Reduction of the expressions of MMPs. Matrix metalloproteinases (MMPs) are involved in tumorigenesis, tumor invasion and inflammation-related diseases, e.g., osteoarthritis. It is known that MMP-3 and MMP-13 are responsible for the degradation of extracellular matrix and are associated with cartilage degradation. Studies demonstrated that MT effectively suppressed IL-1β-induced expressions of MMP-3 and MMP-13 in human articular cartilage chondrocytes via inhibition of the activation of the NF-κB and MAPK signaling pathways (Lu et al., 2015). Moreover, MT significantly inhibited the mRNA and protein expressions of MMP-1 induced by phorbol myristate acetate (PMA) through suppression of the activation of the AP-1 signaling pathway (Jung et al., 2008).
4. Suppression of the production of cyclooxygenase-2 (COX-2). COX-2 induces inflammation and enhances capillary permeability through multiple stimuli, including injury, tumorigenesis, migration, and control of prostaglandin production (Flower, 2003). Studies have shown that MT possesses significant COX-1 and COX-2 inhibitory activity (Ao et al., 2009). Moreover, MT treatment alleviated the LPS-induced inflammatory response and inhibited the gene expression of COX-2 in LPS-stimulated A549 cells through regulation of the activation of MAPK/NF-κB (Liou et al., 2016).
5. Other mechanisms. Besides the above-mentioned mechanisms, MT also moderates inflammation in other ways. In mice and Caco-2 cell models, MT alleviated LPS-induced inflammation and oxidative stress through reduction in the production of IL-1β, IL-17, and malondialdehyde (MDA), while increasing the expressions of chemokine receptor 7 (CCR7) (Wu G. J. et al., 2017). Furthermore, MT exhibited anti-fibrotic effects, as demonstrated via suppression of hepatic infiltration of Gr1hi monocytes and inhibition of the activity of monocyte chemoattractant protein-1 (MCP-1) (Shi et al., 2013). Moreover, MT effectively reduced the mRNA expressions and cytokines (IL-17 and IFN-γ) in interleukin-10-deficient mice, and down-regulated the proportion of CD4+ T cells of mesenteric lymph node cells (Wu et al., 2016). It was also found that MT inhibited the oxidized low-density lipoprotein (ox-LDL)-induced inflammatory injury of macrophages by inhibiting the MAP kinase kinases (MKKs)/p38 MAPK signaling pathway (Zhou et al., 2019). It is worth noting that MT effectively alleviated colonic injury and intestinal inflammation by improving gut microbiota communities (i.e., Bacilli and Mollicutes), down-regulating the expression of pro-inflammatory cytokines (IL-1 and TNF-α), and increasing serum immunoglobulin G (IgG) (Li P. et al., 2019). This provides a new direction and reference for the development of MT targeting intestinal flora.
Antioxidant Property of MT
Excessive generation of ROS is involved in various pathophysiological processes such as cancer, aging, chronic inflammation, neurodegenerative diseases, and degenerative rheumatic diseases. It has been reported that MT suppressed advanced glycation end products (AGEs)-induced ROS production in human aortic endothelial cells (HAEC) (Zhang et al., 2018b). In the middle cerebral artery occlusion (MCAO)-induced focal cerebral ischemia-reperfusion (I/R) injury model, MT pretreatment for 7 consecutive days effectively decreased Bax and, caspase-3 expressions, and reduced MDA levels, while it up-regulated Bcl-2, superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), catalase (CAT), and total antioxidant capacity (T-AOC), thereby attenuating MCAO-induced cerebral I/R injury (Zhao et al., 2015). In addition, MT markedly increased the expression levels of antioxidant enzymes such as NADPH quinone oxidoreductase-1(NQO-1) and heme oxygenase-1(HO-1), and decreased AGEs-induced ROS production through activation of the p38 mitogen-activated protein kinase/nuclear factor E2-related factor-2/antioxidant response elements (MAPK/Nrf2/ARE) antioxidant signaling pathway (Liu et al., 2017a). In a D-galactose- (D-gal-) induced aging mouse model, Sun et al. found that MT inhibited oxidative stress damage in the liver, plasma, and brain of mice by increasing T-AOC, T-SOD, CAT, and decreasing MDA levels. Meanwhile, MT suppressed the activation of p19/p21 and p16 pathways in the liver and hippocampus of D-gal-induced mice. These results suggest that MT plays an anti-aging role by inhibiting oxidative stress and cell senescence (Sun et al., 2018).
Antiviral Property of MT
Porcine reproductive and respiratory syndrome (PRRS) is a devastating swine disease caused by a genetically diverse RNA virus that directly affects the economics of the swine industry. Previous studies have shown that MT suppressed porcine reproductive and respiratory syndrome virus (PRRSV) infection in Marc-145 cells through direct inactivation of PRRSV, and interference with its cellular replication (Zhao et al., 2013). Subsequent studies found that MT significantly blocked the expression of N protein in Marc-145 cells and inhibited PRRSV-induced apoptosis through the suppression of caspase-3 activation (Sun et al., 2014). Moreover, Sun et al. reported that the exposure of porcine kidney cell line (PK-15) to MT resulted in a dose-dependent inhibition of DNA replication of porcine circoviruses 2 (PCV2) (Sun et al., 2015). The anti-PCV2 mechanism of MT may be related to interference with PCV2 Rep protein expression and/or interference with host cell p38/MAPK and JNK/SAPK signaling pathways (Cheung, 2003; Wei et al., 2009). Another study showed that MT exerted anti-PRRSV/PCV2 co-infection activity in vitro via the inhibition of the activation of TLR3 and 4/NF-κB/TNF-α pathways (Na et al., 2016). These results indicate that MT may be considered a major potential therapeutic drug for PRRS.
Previous studies found that MT inhibited wild-type and entecavir-resistant hepatitis B virus (HBV) in vitro, and effectively inhibited HBV replication in vivo (Liu et al., 2018). Moreover, MT interfered with infection of coxsackievirus group B 3 (CVB3) (Liu et al., 2003). Similarly, it has been found that MT inhibited CVB3 virus-induced neonatal rat cardiomyopathy, reduced the rate of apoptosis, and up-regulated the expression of phosphorylated protein kinase Bser-743 protein. The results showed that MT repaired CVB3 virus-induced cell damage through up-regulation of the phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) signaling pathway (Sun et al., 2010). The in vitro inhibitory effect of MT in a model of human laryngeal carcinoma epithelial cells (Hep-2 cells) infected with respiratory syncytial virus, has been reported (Ma et al., 2002). Furthermore, MT exerted antiviral effects by inhibiting the viral RNA copy number on rhabdomyosarcoma cells, and disrupting the replication of enterovirus 71 in the mouse model (Yang et al., 2012). These results indicate that MT has a broad-spectrum antiviral potential that merits further investigation.
Antimicrobial Effect of MT
MT showed a remarkable bacteriostatic effect on Candida albicans SC5314. The bioactive minimum inhibitory concentration (MIC80) and effective concentration (EC50) values of MT were 1 and 2 mg/mL, respectively. In addition, MT reversed fluconazole -resistance of Candida albicans 215, either in the biofilm phenotype or in the free-floating form. The MIC80 and EC50 of MT were 3 and 6 mg/mL, respectively. These results indicated that MT suppressed Candida-related infections by controlling yeast-to-hypha conversion (Shao et al., 2015). Similarly, it has been reported that MT exerted strong inhibitory effects on E. coli, Bacillus subtilis, and S. aureus with MICs of 12.5, 12.5, and 25 μg/mL, respectively (Liu et al., 2011). Zhao et al. investigated the antibacterial effects of MT on drug-resistant E. coli and methicillin-resistant Staphylococcus aureus (MRSA) isolated from the uterus of dairy cows. The results showed that MT significantly inhibited the growth of drug-resistant E. coli (MIC = 12.5 mg/mL) and MRSA strains (MIC = 25 mg/mL) (Zhao and Yu, 2017). AcrAB-TolC is a three-component pump that includes cytoplasmic membrane proteins (AcrB), periplasmic proteins (AcrA), and outer membrane protein channels (TolC); over-activation of this pump resulted in multidrug-resistant (MDR) strains.
MT modulated sigB, agr, and fbe virulence genes of Staphylococcus epidermidis isolated from milk samples of clinical mastitis, down-regulated sigB and fbe genes, up-regulated the agr gene, and promoted the atlE expression of gene and synthesis of phenol-soluble peptide (PSMs), resulting in rapid shedding of bacteria from the biofilm (Zhu, 2014). In recent years, it has been reported that the Panton-Valentine leukocidin (PVL) toxin of Staphylococcus aureus may be related to the pathogenic mechanism of bovine mastitis. Jia et al. found that MT inhibited PVL-induced apoptosis of bovine mammary epithelial cells (BMEC) by down-regulating the expression of cleaved caspase-3, cleaved caspase-8, and cleaved caspase-9 proteins (Jia et al., 2020). Furthermore, a subinhibitory concentration of MT inhibited the Staphylococcus aureus secretion of α-hemolysin in a none dose-dependent manner, thus reducing the inflammatory damage of α-hemolysin-induced BMEC cells (Feng et al., 2018). These results suggest that MT may be developed into a novel drug for the prevention and treatment of bovine mastitis. Generally speaking, MT may be considered a potential antibacterial drug for further research and development.
Anti-Nociceptive Activity
Studies have demonstrated that subcutaneous injections of MT at doses of 1-10 mg/kg reduced the number of acetic acid-induced writhing responses induced, with ED50 of 4.7 mg/kg (Kamei et al., 1997). Other studies have reported that subcutaneous injections of MT at dose of 10-100 mg/kg dose-dependently inhibited hot water induced tail-flick response in mice, with pick analgesic effect after 30 min (Xiao et al., 1999). These results imply that MT produced anti-nociceptive effects mainly through the kappa-opioid receptor, and partially through the μ-opioid receptors. However, after an intravenous injection of MT at doses of 10, 20, and 40 mg/kg, the percentage inhibition values of acetic acid-induced writhing responses in mice were 15, 25, and 55, respectively, indicating that the anti-nociceptive effect was not significant, but it had obvious anti-inflammatory effects (Qian et al., 2012). Moreover, another study showed that MT reduced neuropathic pain in a dose-dependent manner by inhibiting the expression of tumor necrosis factor-α in dorsal root ganglion (Tao et al., 2013). Studies have shown that MT (15-60 mg/kg, i.p.) had an anti-nociceptive effect on mechanical stimulation and cold stimulation of vincristine induced neuropathic pain in model mice (Dun L. L. et al., 2014). In addition, it has been shown that MT reduced vincristine-induced neuropathic pain in mice through mechanisms associated with down-regulation of expression of spinal cord Ras, phosphorylated c-Raf, p-ERK 1/2, TNF-α, and IL – 6, and up-regulation of the expression of IL-10 (Gong et al., 2016). Overall, MT has potent analgesic effects which should be exploited in drug research and development for the benefit patients suffering from pain.
Hepatoprotective Effect of MT
It has been demonstrated that MT at doses of 40, 80, and 160 mg/kg/day for 28 days mitigated high-fructose diet (HFD)-induced hepatic steatosis, and reduced the expression of aspartate aminotransferase (AST) and alanine aminotransferase (ALT), while reducing malondialdehyde and increasing reduced glutathione. Moreover, MT significantly promoted Nrf2 translocation to the nucleus, and subsequently increased the protein expressions of the antioxidative enzymes, CAT, SOD, and GSH-Px (Zhang H. F. et al., 2013). Furthermore, Zhang et al. have shown that MT exerted antioxidant capacity not only by reducing MDA levels and increasing liver GSH production, but also via stimulating Nrf2 translocation and the expressions of its downstream antioxidant enzymes GSH-Px, SOD, CAT, HO-1, and NQO-1, suggesting that the hepatoprotective effects of MT on the liver may be related to its restoration of liver redox balance (Zhang H. F. et al., 2013).
In a high-fat-fed mouse model, MT at a dose of 100 mg/kg/day for 28 days, reduced glucose intolerance and plasma insulin levels, hepatic triglyceride levels, and obesity, without affecting caloric intake. Moreover, MT increased the mRNA and protein levels of heat shock protein-72 (HSP72) in liver tissues (Zeng et al., 2015). Furthermore, it has been reported that MT (100 mg/kg/day for 4 weeks) effectively reduced glucose intolerance and plasma insulin levels in high-fructose-fed mice by inhibiting endoplasmic reticulum (ER) stress-associated de novo lipogenesis (DNL) and increasing HSP72 protein expression in the liver (Mahzari et al., 2018). These results suggest that the hepatoprotective drug MT may be a promising novel anti-type 2 diabetes drug, and the liver is its important target organ.
In another experiment, MT at a dose of 100 mg/kg/day for 7 days, was shown to attenuate endotoxin-induced acute liver injury after hepatic ischemia/reperfusion (HIR) in rats by suppressing myeloperoxidase, nuclear factor κB activity, and macrophage-inflammatory protein-2 expression in a dose-dependent manner (Zhang F. et al., 2011). This indicates that MT might be used for the treatment of HIR-induced liver injury. It has been demonstrated that various doses of MT (50 or 100 mg/kg/day for 5 days; 20, 40, or 80 mg/kg/day for 10 days attenuated LPS-induced liver injury through modulation inflammation (Lin et al., 1997; Yu S. X. et al., 2009). Furthermore, MT at a dose of 10 mg/kg (i.p.) exhibited hepatoprotective effect against α-naphthyl isothiocyanate (ANIT)-induced liver damage via the inhibition of the ANIT-induced increase in serum ALT, alkaline phosphatase (ALP), total bilirubin (Tbil), and γ-GT (Zhai et al., 2007).
Fibrosis is one of the common causes of chronic organ failure. It is characterized by the accumulation of extracellular matrix and the destruction of normal tissue structures (Wynn, 2007). Recent evidence indicates that MT has been used as an anti-fibrotic agent to treat hepatic disorders. The anti-fibrotic effects of MT (10 or 30 mg/kg/day, given 5 times twice in a week for 3 weeks) has been attributed in part to the inhibition of Gr1hi monocytes to injured liver, and the inhibition of production/activity of monocyte chemo attractant protein-1 (MCP-1) (Shi et al., 2013). Moreover, administration of MT salvianolic acid B salt at a dose of 25, 50, or 100 mg/kg/day for 56 days led to significant amelioration of fibrotic changes and decreased expressions of HA, LN, AST, ALT, hydroxyproline (Hyp), transforming growth factor beta 1 (TGF-β1), and alpha-smooth muscle actin (α-SMA). The depletion of reduced glutathione (GSH), and SOD accumulation in liver tissues were inhibited by MT salvianolic acid B salt. These results indicate that treatment with MT salvianolic acid B salt mitigated carbon tetrachloride-induced fibrosis, implying that MT is a potential anti-fibrotic agent (Gao et al., 2012). In addition, MT (100 mg/day for 90 days) was found to be effective in improving the clinical symptoms and signs of patients with chronic hepatitis B, restoring liver function and transforming serum from the hepatitis Be antigen to the HBe antibody and serum HBV DNA from positive to negative. The total effective rate of the MT group was 86.7%, and no serious side effects were observed except for a few patients with mild pain at the intramuscular injection site of MT (Long et al., 2004).
Cardioprotective Effect of MT
Previous studies showed that the JAK2/STAT3 signaling pathway is involved in the prevention of myocardial ischemia/reperfusion (I/R) injury (Boengler et al., 2008). Indeed, MT activates the JAK2/STAT3 pathway. Studies have shown that MT inhibited hypoxia/reoxygenation (H/R)-induced apoptosis of cardiac microvascular endothelial cells (CMECs) in rats, through increases in the phosphorylation of the JAK2/STAT3 signaling pathway-related proteins (Zhao et al., 2018). A similar study suggested that MT effectively up-regulated the expression of heat shock protein 70 (HSP70) and reduced lactate dehydrogenase (LDH) release, decreased activity of creatine kinase-myocardial band (CK-MB), and mitigated cardiomyocytes apoptosis via activation of the JAK2/STAT3 signaling pathway (Guo Q. P. et al., 2018). These data indicate that MT can be used as a potential JAK2/STAT3 signaling pathway activator in studies of its cardioprotective effects. Furthermore, another study confirmed that MT possesses a protective effect on rat heart failure via blocking myocardial apoptosis and β3−adrenoreceptor/endothelial nitric oxide synthase pathways (Yu et al., 2014). Moreover, results have shown that MT plays an important role in cardiovascular protection in isoproterenol-induced acute myocardial injury via its antioxidant properties. The protective effect of MT on isoproterenol-induced acute myocardial ischemia in rats was associated with the regulation of asymmetric dimethylarginine (ADMA) and endothelial nitric oxide synthase (eNOS) (Li X. et al., 2010; Li X. et al., 2012). Moreover, MT up-regulated the expressions of transforming growth factor-β1 (TGF-β1) and insulin-like growth factor-1 (IGF-1), while inhibiting the release of cardiac troponin (cTn-I) and suppressing activation of inflammatory mediator RhoA/ROCK1, thereby exerting a protective effect on isoproterenol-induced acute myocardial ischemia (Zhou et al., 2016).
Diabetic cardiomyopathy (DCM) is one of the causes of disability or death in diabetic patients, and it is often accompanied by persistent hyperglycemia, metabolic disorders, and cardiac fibrosis (Cai and Kang, 2003; Cai, 2007). Administration of MT (200 mg/kg/day for 10 days, po) improved cardiac dysfunction in DCM rats, most likely via suppression of the activation of the ROS/TLR-4 signaling pathway and reduction of the expression of myocyte apoptosis-related proteins such as caspase-8 and caspase-3 (Liu et al., 2015). It was reported that MT significantly improved cardiac function and compliance by inhibiting the ATF6 signaling pathway in DCM rats (Liu et al., 2017b). In addition, MT inactivated the TGF-β1/Smad signaling pathway by inhibiting the expression of TGF-β1 and phosphorylation of Smad2/3 in a dose-dependent manner, thereby exerting anti-fibrotic effects in DCM rats (Zhang et al., 2018a).
These results indicate that MT ameliorates the symptoms of diabetic cardiomyopathy through inhibition of the ROS/TLR-4, ATF6, or TGF-β1/Smad signaling pathways. Therefore, MT may be considered a potential therapeutic agent for diabetic cardiomyopathy and its complications.
Neuroprotective Effect of MT
Deposition of the amyloid-β protein (Aβ) in the tight structure between neurons is closely linked to the pathogenesis of AD, and its accumulation in the hippocampus and neocortex may lead to neuronal death and eventually irreversible cognitive impairment and behavioral changes (Dong et al., 2018). Studies have shown that MT, at doses of 100 and 200 mg/kg/day reduced cognitive dysfunction in AD rats through a dose-dependent reversal of imbalance in Th17/Treg cytokines ratio induced by a Aβ1-42 injection, while up-regulating Foxp3 mRNA expression and decreasing RORγt expression (Zhang Y. et al., 2015). In a similar study, it was also shown that MT repaired cognitive deficits in AD transgenic mice by blocking the Aβ/RAGE signaling pathway (Cui et al., 2016). Ni et al. also reported that MT injection reduced ibotenic acid (IBO)-induced increases in IL-1β content in AD rats, thereby protecting mitochondrial structure, improving energy metabolism, delaying neuronal apoptosis, and suppressing AD (Ni et al., 2006). Li et al. (2018) reported that the administration of MT effectively alleviated the learning and memory impairment and neuroinflammation of the LPS-induced AD mice model, with a potential mechanism involved in the inhibition of NADPH oxidase subunits GP91phox and P47phox protein expression. Yang et al. reported that MT could maintain and even strengthen the cellular nutrition of Aβ42 monomers by inhibiting the aggregation of Aβ42 monomers and synergizing with Aβ42 monomers. In addition, MT promotes the dissociation of immature Aβ42 oligomers to protect the morphological integrity of human neuroblastoma cell lines (SH-SY5Y). The underlying mechanism may be that the presence of MT-like metabolites in the human brain negatively regulates the formation of toxic Aβ42 oligomers (Yang et al., 2020). These results confirm that MT can be considered an effective multi-target compound for the prevention and treatment of AD. Furthermore, MT exerted a protective effect against PD induced in rats with 1−methyl−4−phenyl−1,2,3,6−tetrahydropyridine (MPTP), through blockage of the MPTP-induced overexpression of Nrf2 (Meng et al., 2017).
In an animal model of multiple sclerosis (MS), MT treatment (200 mg/kg/day) effectively improved the clinical symptoms of experimental autoimmune encephalomyelitis (EAE). Zhang M. L. et al. (2017) reported that MT enhanced local production of neurotrophin 3 (NT3) in microglia, astrocytes, and oligodendrocyte precursor cells, thereby protecting neural cells from tissue damage caused by CNS inflammation. It was shown for the first time that MT treatment promoted the differentiation and myelination oligodendrocytes during EAE via the activation of the PI3K/Akt/mTOR signaling pathway (Liu S. Q. et al., 2017). By directly activating the cAMP/PKA signaling pathway in astrocytes, MT up-regulated the production of the brain-derived neurotrophic factor (BDNF) and effectively protected nerve axons from CNS inflammation-induced damage. Furthermore, it was found that MT reduced the expression of NogoA, its receptor complex NgR/p75NTR/LINGO-1, and its downstream RhoA/ROCK signaling pathway, thereby promoting neuro-regeneration in damaged CNS during EAE (Kan et al., 2015; Kan et al., 2016). Studies have demonstrated that MT increased LPS-stimulated BV-2 cell viability and prevented microglial activation by suppressing the expression of heat shock protein 60 (HSP60) and toll-like receptor 4 (TLR-4), indicating that MT can be used to treat neurodegenerative diseases involving microglial activation (Zhang R. et al., 2017). It was demonstrated that MT alleviated anxiety and depression symptoms in a mouse model of burn injury by inhibiting JNK-mediated apoptosis/inflammatory signaling, oxidative stress, and reversing the burn-induced down-regulation of BDNF/VEGF in the hippocampus (Khan et al., 2020). Furthermore, Wu et al. (2018) demonstrated that MT possessed antidepressant-like effect on mice by activating the PI3K/Akt/mTOR signaling pathway in the hippocampus.
Based on these studies, MT may be used as an effective neuroprotective drug for the treatment of AD, PD, and CNS inflammation, neurobehavioral disorders, and it prevents LPS-induced neuronal injury.
Anti-Allergic Effect of MT
Asthma is an allergic lung inflammatory disease whose occurrence is related to the expression levels of IL, IgE, and eosinophil. The Th2 type cytokines, including interleukin IL-4, IL-5, and IL-13, are essential factors for the initiation and transmission of inflammatory and allergic reactions (Agrawal and Shao, 2010; Bosnjak et al., 2011). To assess the anti-allergic activity of matrine, the potential mechanism of its action was studied by using an in vivo OVA-sensitized mice model and in vitro human bronchial epithelial cells (BEAS-2B). The results demonstrated that matrine alleviated eosinophil infiltration, airway hyperresponsiveness (AHR), and airway inflammation by inhibiting Th2 cytokines or the IL-4/IL-13/STAT-6 pathway in asthmatic mice. In addition, MT pretreatment significantly reduced the production of pro-inflammatory cytokines and eosinophil chemokines in activated beas-2b cells, and inhibited the expression of ICAM-1, thereby suppressing the adhesion of eosinophil to inflammatory BEAS-2B cells (Fu et al., 2014; Huang et al., 2014). These findings suggest that MT could be developed into a drug for the treatment of allergic asthma.
Toxicology
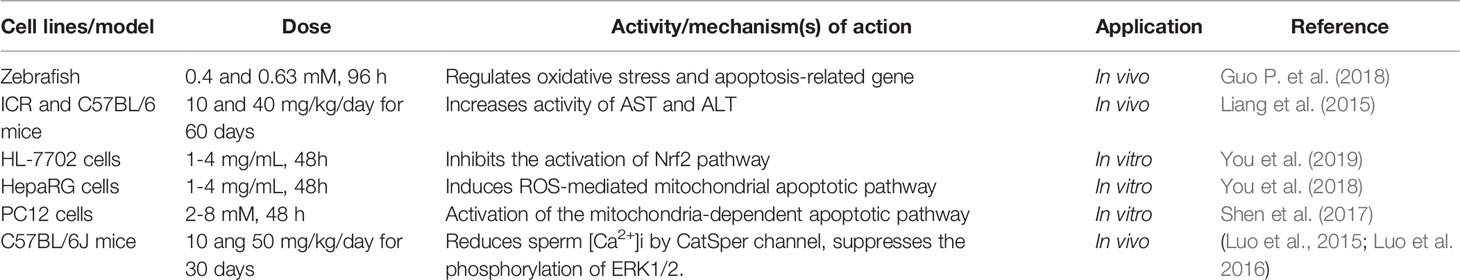
Through in vivo experiments, the toxicity and target organs of Radix Sophorae tonkinensis extracts in ICR mice were studied, and the toxicities of its major bioactive components were compared. In these studies, administration of MT at doses of 118 and 154 mg/kg/day for 21 days produced major toxicity in the liver (Wang L. et al., 2017). In human normal liver HL-7702 cells, MT at doses of 2.5-5 mg/mL for 24 h, increased the contents of ALT, AST, ALP, LDH, and MDA, promoted the induction of cell apoptosis, and decreased the level of GSH (Zhang Q. et al., 2011). Furthermore, another study showed that MT at doses of 0.4 and 0.63 mM for 96 h induced a significant increase in zebrafish hepatocyte apoptosis, and down-regulated the oxidative stress-related gene zgc: 136383 and anti-apoptotic gene EIF4BP3 (Guo S. et al., 2018). Previous studies confirmed that MT at doses of 1-4 mg/mL for 48h inhibited cell viability and induced cell cycle arrest and apoptosis in HepaRG and HL-7702 cells, most probably through a mechanism involving inhibition of the Nrf2 pathway and activation of the ROS-mediated mitochondrial apoptotic pathway (You et al., 2018; You et al., 2019).
MT has been reported to exert neurotoxic effects. Wang et al. reported that the half-lethal dose (LD50) of MT administered by intraperitoneal injection in Kunming mice was 157.13 mg/kg. In addition, histopathological observation showed that small softening foci were formed in the brain tissue of mice, and part of the nerve nuclei were necrotic or even broken, indicating that one of the main toxic target organs of MT is the nervous system (Wang et al., 2010). A study showed that when given at doses of 10 and 40 mg/kg/day for 60 days, MT inhibited the central nervous system of ICR mice, and impaired their balance and coordination (Liang et al., 2015). Investigation of the relationship between MT-induced neurotoxicity and oxidative stress in PC12 cells indicated that MT induced high level of oxidative stress after treatment with graded concentrations of MT (2-8 mM) for 48 h, resulting in a reduction of SOD and an increase in ROS and MDA, as well as activation of the mitochondria-dependent apoptotic pathway (Shen et al., 2017). In terms of reproductive toxicity, Luo et al. (2015) was the first to show that MT (100-200 µM) suppressed mouse sperm function by reducing sperm [Ca2+]i and inhibiting the phosphorylation of ERK1/2. Moreover, further studies indicated that MT (10 ang50 mg/kg/day for 30 days) inhibited mouse sperm function through the [Ca2+]i-related mechanism via the CatSper channel, which is the main channel for controlling extracellular Ca2+ influx in mouse sperm (Luo et al., 2015; Luo et al., 2016). Moreover, it was found that the vinegar-processing method reduces the oral toxicity of Sophora alopecuroides L. containing MT and other MT-type alkaloids mainly due to a sharp decrease of cytisine (Zhang C. X. et al., 2018). These results indicated that the toxicity of Sophora alopecuroides L. might be related to dose and/or drug-drug interactions. In summary, the toxic effects of MT in clinical use remains unclear. These findings highlight the need to assess the risk of human exposure to MT.
Pharmacokinetics
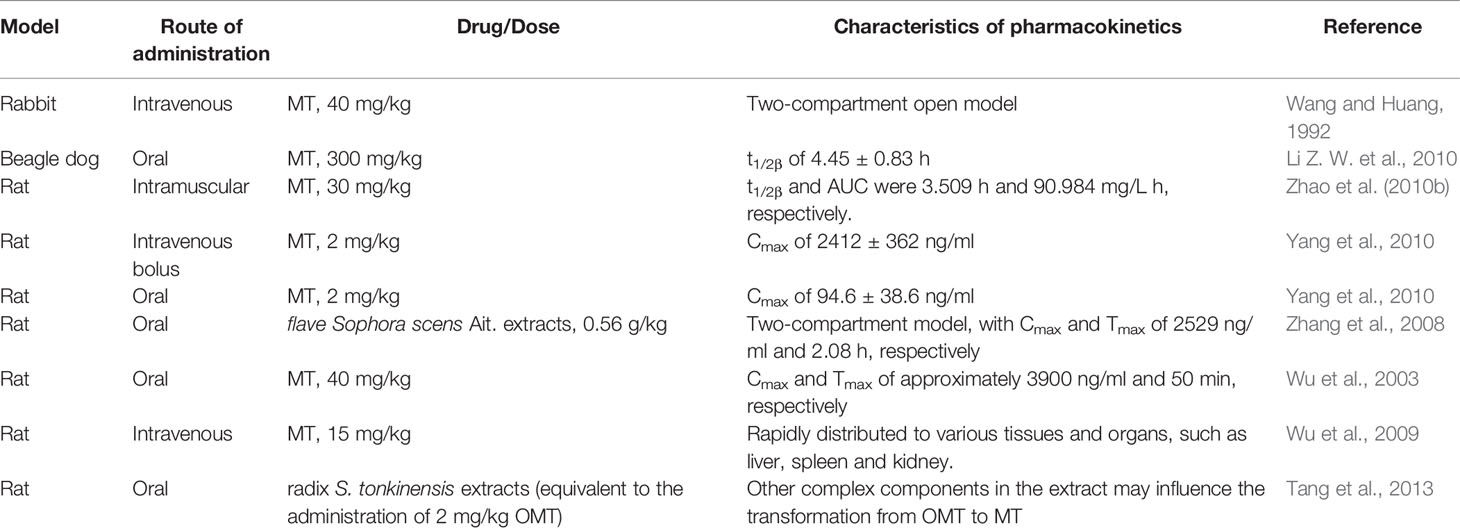
The absorption, distribution, metabolism, and excretion (ADME) processes of drugs in vivo are regulated by a variety of factors (dose, administration, and drug interaction). Wang and Huang (1992) was the first to report that intravenous administration of MT to rabbits at a dose of 40 mg/kg resulted in plasma concentration-time profiles consistent with the two-compartment open model, and the relationship between its effect and the effect of compartment concentration was consistent with the sigmoid Emax model (Wang and Huang, 1992). After oral administration of MT at doses of 300 mg/kg in beagle dogs, the drug was rapidly distributed and was eliminated from plasma with a terminal half-life (t1/2β) of 4.45 ± 0.83 h (Li Z. W. et al., 2010). Moreover, Zhao et al. (2010a) studied the pharmacokinetics of intramuscularly administered MT (30 mg/kg) in rats. The results showed that intramuscular administration of MT resulted in better absorption than oral administration, and the distribution from the central compartment to the peripheral compartment was faster. Moreover, its absolute bioavailability was higher than that of oral administration, and its t1/2β and areas under concentration time curve (AUC) were 3.509 h and 90.984 mg/L h, respectively. It has been speculated that the pharmacological action was stronger with intramuscular administration than oral administration, and the maintenance time was also longer (Zhao et al., 2010a). It has also been shown that after an intravenous bolus injection of MT (2 mg/kg) to rats, the blood concentration of MT reached a maximum of 2412 ± 362 ng/ml, and subsequently quickly decreased. When the same dose of MT was orally administered to rats, it was easily absorbed and reached Cmax of 94.6 ± 38.6 ng/ml after approximately 105 min (Yang et al., 2010). These results show that the Cmax and Tmax values of MT after oral administration were not significantly different from those reported by Wu et al., 2003; Zhang et al., 2008. Zhang et al. showed that the pharmacokinetics of MT in rats after oral administration of flave Sophora scens Ait. extracts (0.56 g/kg) was consistent with the two-compartment model, with Cmax and Tmax of 2529 ng/ml and 2.08 h, respectively (Zhang et al., 2010). In addition, Wu et al. performed pharmacokinetic studies on rats after oral administration of MT at a dose of 40 mg/kg, and reported Cmax and Tmax of approximately 3900 ng/ml and 50 min, respectively (Wu et al., 2003). In the Caco-2 cell model, the absorption permeability of MT was regulated by pH, while in the rat intestinal perfusion model, the absorption of MT was significantly different in the four intestinal segments (ileum, colon, duodenum, and jejunum) of the rat. In addition, Zhao et al. used in vitro rat liver microsomal model studies to reveal that MT could not be metabolized by CYP450 and UGT enzymes, indicating that MT may not undergo extensive first-pass metabolism after oral administration (Zhao et al., 2010a).
It has been suggested that the tissue distribution characteristics of MT solution (MS), MT liposome (ML), and MT stealth liposome (LML) were significantly different after intravenous administration of MT in rats at a single dose of 15 mg/kg. After administration of MT, it can be rapidly distributed to various tissues and organs, such as liver, spleen, and kidney. However, compared with the MS group, the LML group significantly reduced the uptake of MT by the liver and spleen, and improved the bioavailability of MT in rats (Wu et al., 2009). Tang et al. used an ultra-performance liquid chromatography–tandem mass spectrometry method to systematically study the pharmacokinetic behaviors of radix Sophorae tonkinensis extracts. The results indicated that oral administration of radix S. tonkinensis extracts exhibited different pharmacokinetic behavioral changes, when compared with pure oxymatrine (OMT). It was speculated that the potential mechanism was that other complex components in the extracts affected the transformation of OMT to MT (Tang et al., 2013). Moreover, after oral administration of kushen-gancao decoction, the concentrations of MT in the serum of rats was much lower than that of glycyrrhetinic acid (GA), suggesting that the binding of MT to organs was higher, while the absorption and blood distribution were lower (Wang et al., 2014).
Drug-Drug Interactions of MT
Recently, enhancement of efficacy and/or toxicity due to drug-drug interactions has been a key consideration in the development of new drugs in pre-clinical and clinical investigations (Pai et al., 2018). Pu et al. showed that MT combined with cisplatin, 5-fluorouracil, and paclitaxel, respectively, effectively inhibited the proliferation of A549 cells and showed dose-dependent anticancer effects (Pu et al., 2018). It has been reported that a combination of MT and cisplatin synergistically inhibited proliferation, and induced apoptosis of rhabdomyosarcoma (RMS) RD cells, through a mechanism involving inhibition of mRNA expression of XIAP mRNA (Li et al., 2016). Another study found that MT and cisplatin synergistically suppressed the growth of urothelial bladder cancer cells (EJ, T24, BIU and 5637 cells) by inhibiting the VEGF/PI3K/Akt signaling pathway (Liao et al., 2017). Moreover, the protein expression levels of topoisomerase (TOPO) I, Bax, and caspase-3 were up-regulated in HT29 cells treated with a combination of MT and irinotecan (Duan et al., 2017). Rong et al. reported that MT combined with platinum-based doublet chemotherapy (PBDC) had a lower adverse reaction rate, higher response rate (RR), disease control rate (DCR) and mean survival time (MST), and better quality of life (QOL) than PBDC alone (Rong et al., 2015). It has been reported that the co-administration of MT and oxymatrine synergistically inhibits ATP production and cell proliferation of human umbilical vein endothelial cells (ECV304), thereby achieving indirect antitumor effects against tumor angiogenesis (Wang S. J. et al., 2017). All of these studies showed that MT could strengthen the efficacy of many anticancer drugs through drug-drug interactions.
In a rat hepatic stellate cell line (HSC-T6), it was reported that a combination of MT and glycyrrhizin (100 µM MT+glycyrrhizin) inhibited proliferation of activated HSCs and reduced levels of collagen I and hexadecenoic acid (HA). It was also found that combination of MT and glycyrrhizin, 1 mg/mL MT and 1 mg/mL glycyrrhizin, given at a dose of 0.1 mL/100g body weight significantly reduced serum laminin (LN), HA and procollagen type-III (PC-III) levels in a rat model of CCl4-induced liver fibrosis, when compared with MT or glycyrrhizin alone (Zhao et al., 2012). Administration of a mixture of 1 mg/mL glycyrrhizin and 1 mg/mL MT to mice at a dose of 0.5 mL/20g body weight decreased mortality of acetaminophen-overdosed mice, reduced acetaminophen-induced hepatotoxicity, and decreased the area and number of glutamyl transpeptidase (-GT)+positive foci, thereby restoring liver function and preventing liver cancer (Wan et al., 2009). Furthermore, in vitro experiments showed that a combination of MT with lamivudine dose-dependently inhibited HBV-DNA replication and secretions of hepatitis Be antigen (HBeAg) and hepatitis B surface antigen (HBsAg) in HepG2 2.2.15 cell (Ma et al., 2013). Another study showed that combination of sub-MIC MT and erythromycin had a synergistic inhibitory effect on plankton and adhesion of Staphylococcus epidermidis (Guan et al., 2013). It has been reported that a combination of MT and ciprofloxacin significantly decreased the expression level of AcrA, resulting in the inhibition of multidrug-resistant phenotypes in Gram-negative bacteria (Pourahmad and Mohammadi, 2018). In a non-tumorigenic human skin keratinocytes (HaCaT) model, MT combined with acitretin negatively regulates the phosphorylation of PI3K/Akt/mTOR signaling pathway, and plays a synergistic role in inducing autophagy and cell cycle G0/G1 phase arrest (Jiang W. W. et al., 2019). Studies have shown that the combination of MT and lycopene possessed a synergistic protective effect on a LPS-induced acute lung injury (ALI) mouse model through the inhibition of the NF-κB pathway (Li W.W. et al., 2019). Rong et al. reported that MT combined with other drugs (such as Cisplatin, interleukin-11, and bleomycin) has a synergistic effect, which can effectively improve the control of malignant pleural effusion and reduce the incidence of adverse reactions (Rong et al., 2015). Taken together, these results indicated that MT combined therapy showed excellent efficacy.
Qi et al. (2012) studied the effect of glycyrrhizin on MT pharmacokinetics in rats. Compared with the MT alone administration group, the Cmax of MT in the MT and glycyrrhizin combination group decreased from 6.861 ± 0.635 mg/L to 4.122 ± 0.965 mg/L, and the AUC of MT from 47.105 ± 7.062 mg/L h decreased to 35.508 ± 5.024 mg/L h (Qi et al., 2012). Another similar study also suggested that the Cmax and AUC of MT reduced by 32.8% and 34.9% after the combination of Sophorae flavescentis radix (Kushen) and Glycyrrhizae radix et rhizoma (Gancao) (Shi et al., 2015). Furthermore, Zhao et al. (2010a) reported that the AUC and Cmax of MT were significantly decreased when MT was administered in combination with ceftiofur hydrochloride (Zhao et al., 2010b). These results indicated that the combination of MT and other drugs (such as glycyrrhizin and ceftiofur hydrochloride) results in a serious decrease in the plasma concentration and bioavailability of MT, which may adversely affect the pharmacological effects of MT. Therefore, in the clinical combination, we should fully evaluate the pharmacological effects, toxicity, and pharmacokinetic characteristics of MT to obtain better efficacy and drug safety.
Conclusions and Future Perspectives
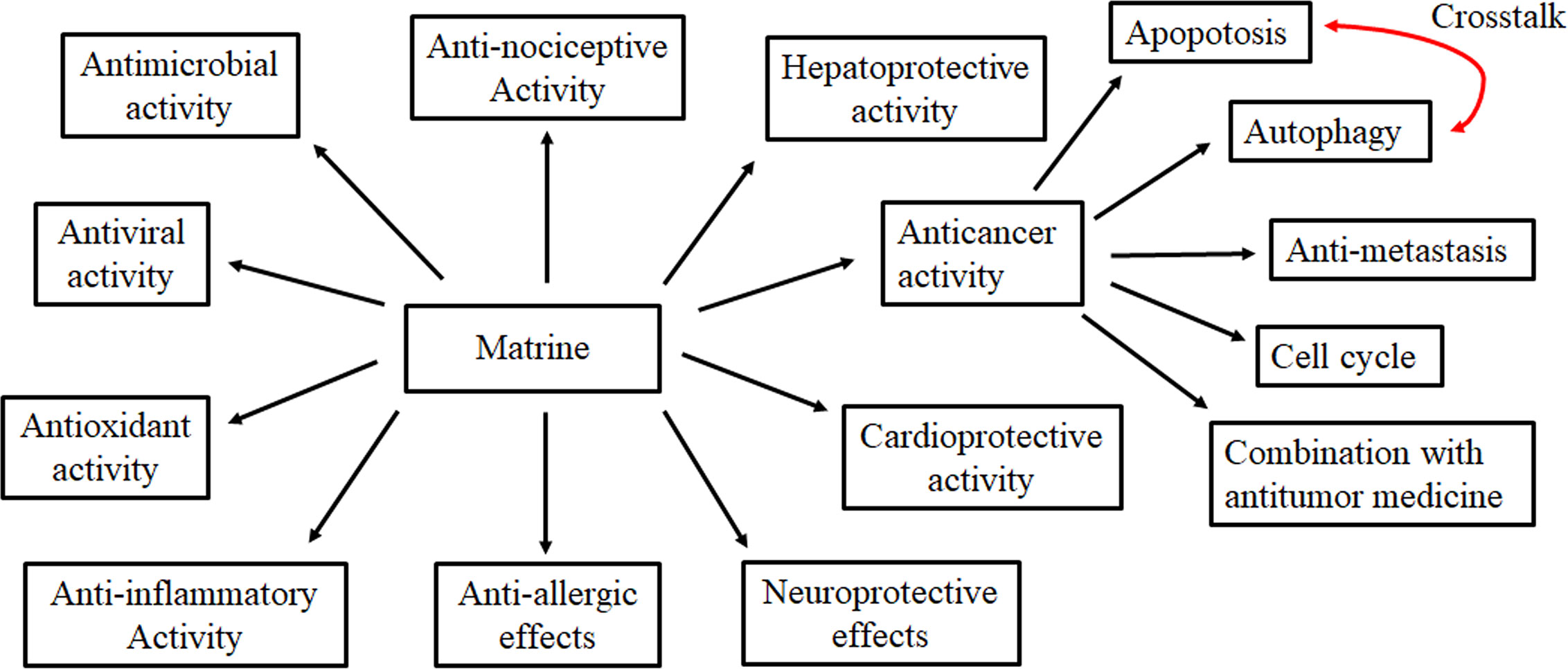
MT is a quinolizidine alkaloid with potential pharmacological benefits, including anti-cancer, anti-inflammatory, anti-oxidant, antiviral, antimicrobial, anti-fibrosis, anti-allergic, antinociceptive, hepatoprotective, cardioprotective, and neuroprotective effects (Figure 2). Previous studies have shown that MT may be one of the valuable options for the prevention and therapy of cancers, hepatitis, skin diseases, allergic asthma, diabetic cardiomyopathy, analgesic, AD, PD, and CNS inflammation. In addition, MT exerts significant anti-cancer effects, through a mechanism involving the regulation of gene and protein expressions of pathways involved in apoptosis, autophagy, cell invasion, and metastasis, as well as cell cycle arrest. Furthermore, studies have suggested that MT should be used in combination with a variety of chemotherapy drugs and alternative therapies to treat cancer more effectively. Therefore, in the clinical combination of MT, it is necessary to assess the effect of MT on the metabolism of other drugs.
Detailed studies of MT with respect to the underlying mechanisms of its effects have shown that its anti-tumor effects are associated with the inhibition of various proteins and genes that are overexpressed in cancer such as IL-6, JAK2, STAT3, PCNA, VEGF-A, NF-κB, MMP-9/MMP-2, phosphorylated AKT, p-65, VEGFR1, epidermal growth factor (EGF), VASP, and SQSTM1/p62. However, the expression levels of Bax, Fas, XIAP, mitochondrial membrane potential, p-ERK, CIAP, p53, p21, p27, P-gp, MRP, caspase-3, caspase-9, and LC3-II are up-regulated. The effect is primarily mediated by signaling pathways involving JAK2/STAT3, AKT, miR-21/PTEN/AKT, Akt/mTOR, and NF-κB. Consistently, these data have strongly established that MT has the capacity to influence various events involved in cell apoptosis, autophagy, cell metastasis, invasion, and cell cycle arrest. These data clearly support its traditional use in cancer therapy. In this paper, a wide range of pharmacological effects and molecular mechanisms of MT have been systematically summarized to further support the viewpoint that MT possesses a broad application prospect (Table 1).
Unfortunately, MT has been reported to have serious side effects, including hepatotoxicity, neurotoxicity, and reproductive and developmental toxicity, thereby limiting its clinical use (Table 2). The potential toxic mechanisms involve the activation of receptor-mediated and mitochondrial-dependent apoptotic pathways, as well as the inhibition of the Nrf2 and ERK pathways (Figure 3). It is worth noting that MT salvianolic acid B salt treatment at doses of 25, 50, or 100 mg/kg/day for 56 days ameliorated fibrosis induced by carbon tetrachloride injection, but without significant hepatotoxicity (Gao et al., 2012). The mechanism involved might be that MT and salvianolic acid B combined to form a salt which enhanced the efficacy of the treatment and reduced the risk of toxicity. Further studies are needed on the mechanism involved. In addition, as summarized in Tables 1 and 2, the toxic doses of MT significantly affected the mitochondrial apoptotic pathway, Nrf2 pathway and ERK1/2 pathway. These findings indicate that MT-induced toxicity has an underlying mechanism similar to that of the pharmacological effects of MT. Therefore, further studies are needed for a closer investigation of the relationship between the efficacy and toxicology of MT. Moreover, the appropriate clinical dose range and exposure mechanism for different dosing times and patient disease conditions need to be determined. Taken together, while pursuing greater efficacy, attention should be paid to reducing the toxicity of MT. Future studies should focus on the combination with other drugs, determination of a single dose range, structural optimization, formulation, and establishment of a toxicity warning system.
In this study, we summarize and discuss the factors affecting MT ADME based on the latest understanding of MT pharmacokinetics (Table 3). At present, oral and injection routes are widely used in clinical applications of MT. The oral administration is relatively simple and convenient, and can meet the treatment of some specific diseases. After intravenous administration of MT, it is quickly absorbed, and it reaches the site of action through blood circulation, with high bioavailability, no first-pass effect, and accurate dose. However, in future drug development and application, attention should be paid to the emergence of other toxicity problems caused by increase in the content of MT.
This paper reviewed the progress of research on MT in recent years, highlighting its preponderant pharmacological activity and inevitable side effects. MT possesses a variety of pharmacological effects, including antioxidant, anti-inflammatory, antiviral, antinociceptive, neuroprotective, and cardioprotective properties. Although the pharmacological mechanism of MT has been widely investigated, the mechanism of its anti-tumor action and its synergistic treatment with other drugs needs to be further studied. In general, future drug development of MT needs to focus on ameliorating its oral bioavailability, reducing toxicity, and improving efficacy.
Author Contributions
LY and CY wrote the manuscript. YD, WW, MS, JL, and BM analyzed the data, while LP, YZ, and ZZ made the pictures and tables. XD, XY, and JN designed the research and revised the primary manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was financially supported by the National Natural Science Foundation of China (81703715).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Agrawal, D. K., Shao, Z. (2010). Pathogenesis of allergic airway inflammation. Curr. Allergy Asthma Rep. 2010 (10), 39–48. doi: 10.1007/s11882-009-0081-7
Ao, C., Araki, N., Tawata, S. (2009). Cyclooxygenase Inhibitory Compounds with Antioxidant Activities from Sophora subprostrata. Asian. J. Chem. 21, 745–754.
Boengler, K., Hilfiker-Kleiner, D., Drexler, H., Heusch, G., Schulz, R. (2008). The myocardial JAK/STAT pathway: from protection to failure. Pharmacol. Ther. 120, 172–185. doi: 10.1016/j.pharmthera.2008.08.002
Bosnjak, B., Stelzmueller, B., Erb, K. J., Epstein, M. M. (2011). Treatment of allergic asthma: modulation of Th2 cells and their responses. Respir. Res. 2011, 12, 114. doi: 10.1186/1465-9921-12-114
Cai, L., Kang, Y. J. (2003). Cell death and diabetic cardiomyopathy. Cardiovasc. Toxicol. 3, 219. doi: 10.1385/CT:3:3:219
Cai, L. (2007). Diabetic cardiomyopathy and its prevention by metallothionein: experimental evidence, possible mechanisms and clinical implications. Curr. Med. Chem. 14, 2193–2203. doi: 10.2174/092986707781389646
Chai, S., To, K. K., Ge, L. (2010). Circumvention of multi-drug resistance of cancer cells by Chinese herbal medicines. Chin. Med. UK. 5, 26. doi: 10.1002/jcb.26502
Chang, C., Liu, S. P., Fang, C. H., He, R. S., Wang, Z., Zhu, Y. Q., et al. (2013). Effects of MT on the proliferation of HT29 human colon cancer cells and its antitumor mechanism. Oncol. Lett. 6, 699–704. doi: 10.3892/ol.2013.1449
Chen, X., Zhi, X., Pan, P., Cui, J., Cao, L. H., Weng, W. Z., et al. (2017). Matrine prevents bone loss in ovariectomized mice by inhibiting RANKL-induced osteoclastogenesis. J. Off. Publ. Fed. Am. Soc. Exp. Biol. 31, 4855–4865. doi: 10.1096/fj.201700316R
Cheung, A. K. (2003). Transcriptional analysis of porcine circovirus type 2. J. Virol. 305, 168–180. doi: 10.1006/viro.2002.1733
Cui, L., Cai, Y., Cheng, W., Liu, G., Zhao, J. H., Cao, H., et al. (2016). A Novel, Multi-Target Natural Drug Candidate, Matrine, Improves Cognitive Deficits in Alzheimer’s Disease Transgenic Mice by Inhibiting Aβ Aggregation and Blocking the RAGE/Aβ Axis. Mol. Neurobiol. 54, 1939–1952. doi: 10.1007/s12035-016-9783-8
Dong, K. K., Park, J., Han, D., Yang, G., Kim, A., Woo, J., et al. (2018). Molecular and functional signatures in a novel Alzheimer’s disease mouse model assessed by quantitative proteomics. Mol. Neurodegener. 13, 2. doi: 10.1186/s13024-017-0234-4
Duan, L., Deng, L. J., Wang, D. B., Ma, S. C., Li, C. M., Zhao, D., et al. (2017). Treatment mechanism of matrine in combination with irinotecan for colon cancer. Oncol. Lett. 14, 2300–2304. doi: 10.3892/ol.2017.6407
Dun, L. L., Li, Y. X., Xu, Y. Q., Zhou, R., Yu, J. Q. (2014). Antinociceptive effect of matrine on vincristine-induced neuropathic pain model in mice. Neurol. Sci. 35. doi: 10.1007/s10072-013-1603-6
Feng, F., Ma, W. W., Luo, H. X., Guan, C. P., Zhou, X. Z. (2018). Effect of matrine on reducing damage to bovine mammary epithelial cells induced by Staphylococcus aureus alpha-hemolysin. Pol. J. Vet. Sci. 21, 409–413. doi: 10.24425/122610
Flower, R. J. (2003). The development of COX2 inhibitors. Nat. Rev. Drug Dscov. 2, 179. doi: 10.1038/nrd1034
Fu, Q., Wang, J., Ma, Z. Q., Ma, S. P. (2014). Anti-asthmatic effects of matrine in a mouse model of allergic asthma. Fitoterapia 94, 183–189. doi: 10.1016/j.fitote.2013.12.014
Gao, H. Y., Li, G. Y., Lou, M. M., Li, X. U., Wei, X. Y., Wang, J. H. (2012). Hepatoprotective effect of Matrine salvianolic acid B salt on Carbon Tetrachloride-Induced Hepatic Fibrosis. J. Inflamm. (Lond) 9, 16. doi: 10.1186/1476-9255-9-16
Gong, X. B., Yuan, G., Guo, Q., Vondran, F. W. R., Schwartlander, R., Efimova, E., et al. (2015). Effect of MT on primary human hepatocytes in vitro. Cytotechnology 67, 255–265. doi: 10.1007/s10616-013-9680-1
Gong, S. S., Li, Y. X., Zhang, M. T., Du, J., Ma, P. S., Yao, W. X., et al. (2016). Neuroprotective effect of matrine in mouse model of vincristine-induced neuropathic pain. Neurochem. Res. 41, 3147–3159. doi: 10.1007/s11064-016-2040-8
Gu, Y. M., Lu, J. Y., Sun, W., Jin, R. M., Ohira, T., Zhang, Z., et al. (2019). Oxymatrine and its metabolite matrine contribute to the hepatotoxicity induced by radix Sophorae tonkinensis in mice. Exp. Ther. Med. 17, 2519–2528. doi: 10.3892/etm.2019.7237
Guan, Y., Zhou, H. N., Shi, J. J., Liu, L., Wang, C. Z. (2013). Preliminary study on inhibitory effects of sub-MIC matrine alone or in combination with erythromycin On Staphylococcus epidermidis biofilm. Chin. J. Microbiol. Immunol. 33, 86–90. doi: 10.3760/cma.j.issn.0254-5101.2013.02.003
Guo, Q. S., Huang, X., Li, S. L., et al. (2010). Effects of matrine on apoptosis of human ovarian cancer cell line SKOV 3 and its mechanism. Chin. Pharmacol. Bull. 26, 1104–1107. doi: 10.1007/s00343-010-9055-9
Guo, Q. P., Chen, G. Y., Zhou, Q., Jin, R. M. (2018). Comparison of hepatotoxicity and toxic mechanisms of matrine and oxymatrine using in vivo and in vitro models. Chin. J. Comp. Med. 28, 44–50. doi: 10.3969/j.issn.1671-7856.2018.01.008
Guo, S., Gao, C., Xiao, W., Zhang, J., Qu, Y. S., Li, J., et al. (2018). Matrine Protects Cardiomyocytes from Ischemia/Reperfusion Injury by Regulating HSP70 Expression Via Activation of the JAK2/STAT3 Pathway. Shock 50, 664–670. doi: 10.1097/SHK.0000000000001108
Hien, T. T., Kim, H. G., Han, E. H., Kang, K. W., Jeong, H. G. (2010). Molecular mechanism of suppression of MDR1 by puerarin from Pueraria lobata via NF-kappaB pathway and cAMP-responsive element transcriptional activity-dependent up-regulation of AMP-activated protein kinase in breast cancer MCF-7/adr cells. Mol. Nutr. Food. Res. 54, 918. doi: 10.1002/mnfr.200900146
Hsieh, S. C., Tsai, J. P., Yang, S. F., Tang, M. G., Hsieh, Y. H. (2014). Metformin inhibits the invasion of human hepatocellular carcinoma cells and enhances the chemosensitivity to sorafenib through a down-regulation of the ERK/JNK-mediated NF-κB-dependent pathway that reduces uPA and MMP-9 expression. Amino. Acids 46, 2809–2822. doi: 10.1007/s00726-014-1838-4
Hu, G., Liu, J., Zhen, Y. Z., Wei, J., Tu, P (2013). Rhein inhibits the expression of vascular cell adhesion molecule 1 in human umbilical vein endothelial cells with or without lipopolysaccharide stimulation. Am. J. Chin. Med. 41, 473–485. doi: 10.1142/S0192415X13500341
Huang, M., Xin, W. (2017). Matrine inhibiting pancreatic cells epithelial-mesenchymal transition and invasion through ROS/NF-κB/MMPs pathway. Life Sci. 192, 55–61. doi: 10.1016/j.lfs.2017.11.024
Huang, J., Xu, H. (2016). Matrine: Bioactivities and Structural Modifications. Curr. Top. Med. Chem. 16, 3365–3378. doi: 10.2174/1568026616666160506131012
Huang, W. C., Chan, C. C., Wu, S. J., Chen, L. C., Shen, J. J., Kuo, M. L., et al. (2014). Matrine attenuates allergic airway inflammation and eosinophil infiltration by suppressing eotaxin and Th2 cytokine production in asthmatic mice. J. Ethnopharmacol. 151, 470–477. doi: 10.1016/j.jep.2013.10.065
Jia, F., Ma, W. W., Zhang, X. J., Wang, D., Zhou, X (2020). Matrine and baicalin inhibit apoptosis induced by Panton-Valentine leukocidin of Staphylococcus aureus in bovine mammary epithelial cells. J. Dairy Sci. 103. doi: 10.3168/jds.2019-17619
Jiang, K., Guo, S., Yang, J., Liu, J. F., Shaukat, A., Zhao, G., et al. (2019). Matrine alleviates Staphylococcus aureus lipoteichoic acid-induced endometritis via suppression of TLR2-mediated NF-κB activation. Int. Immunopharmacol. 70, 201–207. doi: 10.1016/j.intimp.2019.02.033
Jiang, W. W., Wang, Y. M., Zhu, S. M., Zhang, C. L. (2019). Role and mechanism of matrine alone and combined with acitretin for HaCaT cells and psoriasis-like murine models. Chin. Med. J. 132, 2079–2088. doi: 10.1097/CM9.0000000000000412
Jin, H., Sun, Y., Wang, S., Cheng, X. (2013). MT Activates PTEN to Induce Growth Inhibition and Apoptosis inV600EBRAF Harboring Melanoma Cells. Int. J. Mol. Sci. 14, 16040. doi: 10.3390/ijms140816040
Jung, E., Lee, J., Huh, S., Lee, J., Hwang, H., Kim, Y., et al. (2008). Matrine inhibits PMA-induced MMP-1 expression in human dermal fibroblasts. Biofactors 33, 121–128. doi: 10.1002/biof.5520330204
Kamei, J., Xiao, P., Ohsawa, M., Kubo, H., Ohmiya, S. (1997). Antinociceptive effects of (+)-matrine in mice. Eur. J. Pharmacol. 337, 223–226. doi: 10.1016/S0014-2999(97)01273-9
Kan, Q. C., Lv, P., Zhang, X. J., Xu, Y. M., Zhang, G. X., Zhu, L. (2015). Matrine protects neuro-axon from CNS inflammation-induced injury. Exp. Mol. Pathol. 98, 124–130. doi: 10.1016/j.yexmp.2015.01.001
Kan, Q. C., Zhang, H. J., Zhang, Y., Li, X., Xu, Y. M., Thome, R., et al. (2016). Matrine Treatment Blocks NogoA-Induced Neural Inhibitory Signaling Pathway in Ongoing Experimental Autoimmune Encephalomyelitis. Mol. Neurobiol. 54, 1–15. doi: 10.1007/s12035-016-0333-1
Kanagasabai, R., Krishnamurthy, K., Druhan, L. J., Ilangovan, G. (2011). Forced Expression of Heat Shock Protein 27 (Hsp27) Reverses P-Glycoprotein (ABCB1)-mediated Drug Efflux and MDR1 Gene Expression in Adriamycin-resistant Human Breast Cancer Cells. J. Biol. Chem. 286, 289–300. doi: 10.1074/jbc.m111.249102
Khan, A., Shal, B., Naveed, M., Nasir, B., Irshad, B., Ali, H., et al. (2020). Matrine alleviates neurobehavioral alterations via modulation of JNK-mediated caspase-3 and BDNF/VEGF signaling in a mouse model of burn injury. Psychopharmacology 237, 2327–2343. doi: 10.1007/s00213-020-05537-5
Kondratskyi, A., Kondratska, K., Skryma, R., Klionsky, D. J., Prevarskaya, N. (2017). Ion channels in the regulation of autophagy. Autophagy 14, 2–21. doi: 10.1080/15548627.2017.1384887
Li, X., Zhou, R., Zheng, P., Yan, L., Wu, Y., Xiao, X., et al. (2010). Cardioprotective effect of matrine on isoproterenol-induced cardiotoxicity in rats. J. Pharm. Pharmacol. 62, 514–520. doi: 10.1211/jpp.62.04.0015
Li, Z. W., Li, G. F., Zhang, J. H. (2010). Pharmacokinetics and Bioavailability of Sustained-release Tablets of Matrine in Dogs. J. Chin. Med. Mater. 33, 99–102. doi: 10.13863/j.issn1001-4454.2010.08.038
Li, L. Q., Li, X. L., Wang, L., Du, W. J., Guo, R., Liang, H. H., et al. (2012). MT inhibits breast cancer growth via miR-21/PTEN/Akt pathway in MCF-7 cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 30, 631–641. doi: 10.1159/000341444
Li, X., Wang, X., Guo, Y., Deng, N., Zheng, P., Xu, Q. B., et al. (2012). Regulation of endothelial nitric oxide synthase and asymmetric dimethylarginine by matrine attenuates isoproterenol-induced acute myocardial injury in rats. J. Pharm. Pharmacol. 64, 1107–1118. doi: 10.1111/j.2042-7158.2012.01502.x
Li, H., Li, X., Bai, M., Suo, Y., Caoet, X. Y. (2015). MT inhibited proliferation and increased apoptosis in human breast cancer MCF-7 cells via up-regulation of Bax and down-regulation of Bcl-2. Int. J. Clin. Exp. Pathol. 8, 14793.
Li, L., Xue, T., Xu, W., Zhou, B. (2016). Effect of matrine combined with cisplatin on the expression of XIAP in human rhabdomyosarcoma RD cells. Oncol. Lett. 12, 3793–3798. doi: 10.3892/ol.2016.5150
Li, J., Yao, Y., Han, H. Q., Li, W. Q., Li, N., Miao, Z. H., et al. (2018). Effect of Matrine on Learning and Memory Function and Neuroinflammation in LPS-induced Alzheimer’s Disease Mice Mode. Chin. J. Exp. Tradit. Med. Formul. 24, 134–139. doi: 10.13422/j.cnki.syfjx.20182430
Li, P., Lei, J., Hu, G., Chen, X. M., Liu, Z. F., Yang, J. (2019). Matrine Mediates Inflammatory Response via Gut Microbiota in TNBS-Induced Murine Colitis. Front. Physiol. 10, 28. doi: 10.3389/fphys.2019.00028
Li, W. W., Wang, T. Y., Cao, B., Bin, L., Liu, X. Y (2019). Synergistic protection of matrine and lycopene against lipopolysaccharide−induced acute lung injury in mice. Mol. Med. Rep. 20, 1–8. doi: 10.3892/mmr.2019.10278
Liang, P., Yuan, T. J., Gu, L. L., Lu, H (2015). Study of Hepatotoxicity and Neural Behavioral Changes of Sophora Flavescens and Matrine in Mice. Chin. J. Modern Appl. Pharmacy. 32, 1444–1448. doi: 10.13748/j.cnki.issn1007-7693.2015.12.008
Liao, X. Z., Tao, L. T., Liu, J. H., Gu, Y. Y., Xie, J., Chen, Y. L., et al. (2017). Matrine combined with cisplatin synergistically inhibited urothelial bladder cancer cells via down-regulating VEGF/PI3K/Akt signaling pathway. Cancer Cell Int. 17, 124. doi: 10.1186/s12935-017-0495-6
Lin, W., Zhang, J. P., Hu, Z. L., Qian, D. H. (1997). Inhibitory effect of matrine on lipopolysacchride-induced tumor necrosis factor and interleukin-6 production from rat Kupffer cells. Acta Pharm. Sinica. 32, 93.
Liou, C. J., Lai, Y. R., Chen, Y. L., Chang, Y. H., Li, Z. Y., Huang, W. C., et al. (2016). Matrine Attenuates COX-2 and ICAM-1 Expressions in Human Lung Epithelial Cells and Prevents Acute Lung Injury in LPS-Induced Mice. Mediat. Inflamm. 2016, 3630485. doi: 10.1155/2016/3630485
Liu, M., Liu, X. Y., Cheng, J. F. (2003). Advance in the pharmacological research on matrine. China J. Chin. Mater. Med. 28, 801–804.
Liu, J. Y., Hu, J. H., Zhu, Q. G., Li, F. Q., Wang, J., Sun, H. J. (2007). Effect of MT on the expression of substance P receptor and inflammatory cytokines production in human skin keratinocytes and fibroblasts. Int. Immunopharmacol. 7, 816. doi: 10.1016/j.intimp.2007.02.003
Liu, T., Song, Y., Chen, H., Pan, S. H., Sun, X. Y. (2010). MT inhibits proliferation and induces apoptosis of pancreatic cancer cells in vitro and in vivo. Biol. Pharm. Bull. 33, 1740. doi: 10.1248/bpb.33.1740
Liu, F., Ding, Z., O-Yang, Y., Wang, F., Deng, L. (2011). Determination of Antibacterial Activity of Sophora alopecuroides. J. Beijing Univ. Chem. Technol. (Natural Sci. Ed) 38, 84–88. doi: 10.13543/j.cnki.bhxbzr.2011.02.003
Liu, Z. W., Wang, J. K., Qiu, C., Guan, G. C., Deng, Z. R. (2015). Matrine pretreatment improves cardiac function in rats with diabetic cardiomyopathy via suppressing ROS/TLR-4 signaling pathway. Acta Pharmacol. Sinica. 36, 323–333. doi: 10.1038/aps.2014.127
Liu, J., Zhang, L., Ren, Y., Gao, Y. L., Kang, L., Lu, S. P. (2016). Matrine inhibits the expression of adhesion molecules in activated vascular smooth muscle cells. Mol. Med. Rep. 13, 2313. doi: 10.3892/mmr.2016.4767
Liu, S. Q., Zhang, M. L., Zhang, H. J., Liu, F. Z., Chu, R. G., Guang, X., et al. (2017). Matrine promotes oligodendrocyte development in CNS autoimmunity through the PI3K/Akt signaling pathway. Life Sci. 180, 36–41. doi: 10.1016/j.lfs.2017.05.010
Liu, Z., Lv, Y., Zhang, Y., Liu, F. Q., Zhu, L., Pan, S., et al. (2017a). Matrine-Type Alkaloids Inhibit Advanced Glycation End Products Induced Reactive Oxygen Species-Mediated Apoptosis of Aortic Endothelial Cells In Vivo and In Vitro by Targeting MKK3 and p38MAPK Signaling. J. Am. Heart Assoc. 6, e007441. doi: 10.1161/JAHA.117.007441
Liu, Z., Zhang, Y., Tang, Z., Xu, J., Ma, M. J., Pan, S., et al. (2017b). Matrine attenuates cardiac fibrosis by affecting ATF6 signaling pathway in diabetic cardiomyopathy. Eur. J. Pharmacol. 804, 21–30. doi: 10.1016/j.ejphar.2017.03.061
Liu, Y., Yao, W., Si, L., Hou, J., Wang, J. B., Xu, Z. H., et al. (2018). Chinese herbal extract Su-duxing had potent inhibitory effects on both wild-type and entecavir-resistant hepatitis B virus (HBV) in vitro and effectively suppressed HBV replication in mouse model. Antiviral Res. 155, 39–47. doi: 10.1016/j.antiviral.2018.04.017
Long, Y., Lin, X. T., Zeng, K. L. (2004). Efficacy of matrine in the treatment of chronic hepatitis B. Chin. J. Nat. Med. 3, 148–150. doi: 10.3969/j.issn.1006-3110.2005.04.041
Lu, S., Xiao, X., Cheng, M. (2015). Matrine inhibits IL-1β-induced expression of matrix metalloproteinases by suppressing the activation of MAPK and NF-κB in human chondrocytes in vitro. Int. J. Clin. Exp. Pathol. 8, 4764–4772. doi:10.1016/S1359-6349(09)71986-X
Luo, C., Zhong, H. J., Zhu, L. M., Wu, X. G., Ying, J. E., Wang, X. H., et al. (2012). Inhibition of MT against gastric cancer cell line MNK45 growth and its anti-tumor mechanism. Mol. Biol. Rep. 39, 5459–5464. doi: 10.1007/s11033-011-1346-5
Luo, C., Zhu, Y. T., Jiang, T. J., Lu, X. Y., Zhang, W., Jing, Q. F., et al. (2007). MT induced gastric cancer MKN45 cells apoptosis via increasing pro-apoptotic molecules of Bcl-2 family. Toxicology 229, 245. doi: 10.1016/j.tox.2006.10.020
Luo, S. X., Deng, W. Y., Wang, X. F., Han, H. F., Chen, L. L., Chen, B. B., et al. (2013). Molecular mechanism of indirubin-3’-monoxime and MT in the reversal of paclitaxel resistance in NCI-H520/TAX25 cell line. Chin. Med. J. Peking. 126, 925. doi: 10.3760/cma.j.issn.0366-6999.20123583
Luo, T., Zou, Q. X., He, Y. Q., Wang, H. F., Zeng, X. H (2015). Matrine Inhibits Mouse Sperm Function by Reducing Sperm [Ca2+]i and Phospho-ERK1/2. Cell. Physiol. Biochem. 35, 374–385. doi: 10.1159/000369703
Luo, T., Zou, Q. X., He, Y. Q., Wang, H. F., Wang, T., Liu, M., et al. (2016). Matrine compromises mouse sperm functions by a [Ca2+]i-related mechanism. Reprod. Toxicol. 60, 69–75. doi: 10.1016/j.reprotox.2016.02.003
Ma, S. C., Du, J., But, P. P., Sun, X., Lu, X. Z., Zhou, M., et al. (2002). Antiviral Chinese medicinal herbs against respiratory syncytial virus. J. Ethnopharmacol. 79, 205–211. doi: 10.1016/s0378-8741(01)00389-0
Ma, Z. J., Li, Q., Wang, J. B., Zhao, Y. L., Xiao, X. H. (2013). Combining Oxymatrine or Matrine with Lamivudine Increased Its Antireplication Effect against the Hepatitis B Virus In Vitro. Evidence-Based Complement. Altern. Med. 186573. doi: 10.1155/2013/186573
Ma, L., Zhu, Z., Jiang, L., et al. (2015). MT suppresses cell growth of human chronic myeloid leukemia cells via its inhibition of the interleukin-6/Janus activated kinase/signal transducer and activator of transcription 3 signaling cohort. Leukemia Lymphoma. 56, 2923. doi: 10.3109/10428194.2015.1007507
Mahzari, A., Zeng, X. Y., Zhou, X., Sun, X., Lu, X. Z., Zhou, M., et al. (2018). Repurposing matrine for the treatment of hepatosteatosis and associated disorders in glucose homeostasis in mice. Acta Pharmacol. Sin. 39, 1–7. doi: 10.1038/s41401-018-0016-8
Manning, B. D., Cantley, L. C. (2007). AKT/PKB signaling: navigating downstream. Cell 129, 1261. doi: 10.1016/j.cell.2007.06.009
Meng, F., Wang, J., Ding, F., Xie, Y. L., Zhang, Y. J., Zhu, J., et al. (2017). Neuroprotective effect of matrine on MPTP-induced Parkinson’s disease and on Nrf2 expression. Oncol. Lett. 13, 296–300. doi: 10.3892/ol.2016.5383
Na, S., Sun, P., Lv, H., Sun, Y. G., Guo, J. H., Wang, Z. R., et al. (2016). Matrine displayed antiviral activity in porcine alveolar macrophages co-infected by porcine reproductive and respiratory syndrome virus and porcine circovirus type 2. Sci. Rep. 6, 24401. doi: 10.1038/srep24401
Ni, J. J., Wu, Z. M., Ling, S. C., Zhu, X., Lu, P. B. (2006). Effect of matrine injection IL-1β level and ultrastructural changes of hippocampal neuron in Alzheimer’s disease rat. Chin. J. Anat. 29, 608–611.
Pai, M. P., Kiser, J. J., Gubbins, P. O., Rodvold, K. A. (2018). Introduction to Drug-Drug Interactions. Drug Interact. Infect. Dis.: Mech. Models Drug Interact. 1–13. doi: 10.1007/978-3-319-72422-5_1
Peng, X., Zhou, D., Wang, X., Hu, Z. F., Yan, Y., Huang, J. R., et al. (2016). Matrine Suppresses Proliferation and Invasion of SGC7901 Cells through Inactivation of PI3K/Akt/uPA Pathway. Ann. Clin. Lab. Sci. 46, 457–462.
Pourahmad, R. J., Mohammadi, P. (2018). The effect of total alkaloid extract of local Sophora alopecuroides on MIC and intracellular accumulation of ciprofloxacin, and acrA expression in ciprofloxacin high resistance Escherichia coli clones. J. Glob. Antimicrob. Resist. 12, 55–60. doi: 10.1016/j.jgar.2017.09.005
Pu, J., Fang, F. F., Li, X. Q., Shu, Z. H., Jiang, Y. P (2016). Matrine Exerts a Strong Anti-Arthritic Effect on Type II Collagen-Induced Arthritis in Rats by Inhibiting Inflammatory Responses. Int. J. Mol. Sci. 17, 1410. doi: 10.3390/ijms17091410
Pu, J., Tang, X., Zhuang, X., Hu, Z., He, K. M., Wu, Y. F., et al. (2018). Matrine induces apoptosis via targeting CCR7 and enhances the effect of anticancer drugs in non-small cell lung cancer in vitro. Innate Immun. 24, 395–399. doi: 10.1177/1753425918800555
Qi, L. Y., Huang, L., Yang, B. C., Pan, D. Y., Lu, W. F., Chen, Z. L., et al. (2012). Effects of glycyrrhizin on the pharmacokinetics of matrine in rats. China Pharmacy. 23, 985–986. doi: 10.6039/j.issn.1001-0408.2012.11.10
Qian, L. W., Dai, W. H., Zhou, G. Q., Wang, L. L., Wang, H. S. (2012). Analgesic and Anti-inflammatory Effects of Sophora flavescens and Sophora radiata. Chin. Tradit. Patent Med. 34, 1593–1596.
Ren, H. T., Zhang, S. Q., Ma, H. B., Wang, Y. L., Liu, D., Wang, X. J., et al. (2014). Matrine reduces the proliferation and invasion of colorectal cancer cells via reducing the activity of p38 signaling pathway. Acta Biochim. Biophys. Sin. 46, 1049–1055. doi: 10.1093/abbs/gmu101
Rong, B., Zhao, C., Gao, W., Yang, S. (2015). Matrine promotes the efficacy and safety of platinum-based doublet chemotherapy for advanced non-small cell lung cancer. Int. J. Clin. Exp. Med. 8, 14701–14717.
Shao, Q., Zhao, X., Yao, L. (2014). MT inhibits the growth of retinoblastoma cells (SO-Rb50) by decreasing proliferation and inducing apoptosis in a mitochondrial pathway. Mol. Biol. Rep. 41, 3475–3480. doi: 10.1007/s11033-014-3209-3
Shao, J., Wang, T., Yan, Y., Shi, G., Cheng, H., Wu, D., et al. (2015). Matrine reduces yeast-to-hypha transition and resistance of a fluconazole-resistant strain of Candida albicans. J. Appl. Microbiol. 117, 618–626. doi: 10.1111/jam.12555
Shen, F., Liang, P., Lu, H. (2017). Cytotoxicity and mitochondrial injury mechanism of matrine on PC12 cells. Chin. J. Pharmacol. Toxicol. 31, 873–879.
Shi, C., Yu, L., Yang, F., Yan, J., Zeng, H. H. (2003). A novel organoselenium compound induces cell cycle arrest and apoptosis in prostate cancer cell lines. Biochem. Bioph. Res. Co. 309, 578–583. doi: 10.1016/j.bbrc.2003.08.032
Shi, D., Zhang, J., Qiu, L., Li, J. Z., Zhang, J. P. (2013). Matrine Inhibits Infiltration of the Inflammatory Gr1(hi) Monocyte Subset in Injured Mouse Liver through Inhibition of Monocyte Chemoattractant Protein-1. Evid. Based. Complement. Alternat. Med. 2013, 580673–580673. doi: 10.1155/2013/580673
Shi, L., Tang, X., Dang, X., Wang, Q. H., Wang, X. R., He, P., et al. (2015). Investigating herb–herb interactions: The potential attenuated toxicity mechanism of the combined use of Glycyrrhizae radix et rhizoma (Gancao) and Sophorae flavescentis radix (Kushen). J. Ethnopharmacol. 165, 243–250. doi: 10.1016/j.jep.2015.02.022
Song, S., Zhu, S., Zhang, Z., Mo, Z. Y., Luo, Z. G. (2013). A Study on the Inhibitory Effect of MT on Gastric Cancer SGC-7901 Cells. Afr. J. Tradit. Complem. 10, 435–438. doi: 10.4314/ajtcam.v10i6.5
Sun, B., Min, X. U. (2015). MT inhibits the migratory and invasive properties of nasopharyngeal carcinoma cells. Mol. Med. Rep. 11, 4158. doi: 10.3892/mmr.2015.3276
Sun, Y. M., Chu, G. L., Chen, X., et al. (2010). Protective Effect of Matrine on Cardiomyocytes Infected with Coxsackie Virus B3 and Its Relationship with Phosphatidylinositol 3-Kinase/Protein Kinase B Singnal Pathway. J. Appl. Clin. Pediatr. 25, 1428–1431.
Sun, N., Wang, Z. W., Wu, C. H., Li, E., He, J. P., Wang, S. Y., et al. (2014). Antiviral activity and underlying molecular mechanisms of Matrine against porcine reproductive and respiratory syndrome virus in vitro. Res. Vet. Sci. 96, 323–327. doi: 10.1016/j.rvsc.2013.12.009
Sun, N., Yu, T., Zhao, J. X., Sun, Y. G., Jiang, J. B., Duan, Z. B., et al. (2015). Antiviral activities of natural compounds derived from traditional chinese medicines against porcine circovirus type 2 (PCV2). Biotechnol. Bioprocess Engineer. 20, 180–187. doi: 10.1007/s12257-014-0520-8
Sun, D., Jing, W., Yang, N., Ma, H. X. (2016). Matrine suppresses airway inflammation by down-regulating SOCS3 expression via inhibition of NF-κB signaling in airway epithelial cells and asthmatic mice. Biochem. Biophys. Res. Commun. 477, 83–90. doi: 10.1016/j.bbrc.2016.06.024
Sun, K., Yang, P., Zhao, R., Bai, Y. T., Guo, Z. J. (2018). Matrine Attenuates D-Galactose-Induced Aging-Related Behavior in Mice via Inhibition of Cellular Senescence and Oxidative Stress. Oxid. Med. Cell Longev., 7108604. doi: 10.1155/2018/7108604
Suo, Z., Liu, Y., Ferreri, M., Zhang, T., Liu, Z. J., Mu, X., et al. (2009). Impact of matrine on inflammation related factors in rat intestinal microvascular endothelial cells. J. Ethenopharmacol. 125, 404. doi: 10.1016/j.jep.2009.07.023
Tang, L., Dong, L. N., Peng, X. J., Li, Y., Shi, J., Zhou, F. Y., et al. (2013). Pharmacokinetic characterization of oxymatrine and matrine in rats after oral administration of radix Sophorae tonkinensis extract and oxymatrine by sensitive and robust UPLC–MS/MS method. J. Pharmaceut. Biomed. 83, 179–185. doi: 10.1016/j.jpba.2013.05.003
Tao, R., Wang, J., Xia, L. J., Wei, X. H., Li, H. Q. (2013). Matrine Inhibits the Expression Of TNF-α in the Dorsal Root Ganglion in Rats. Chin. J. Pain Med. 19, 414–419.
Wan, X. Y., Luo, M., Li, X. D., He, P. (2009). Hepatoprotective and anti-hepatocarcinogenic effects of glycyrrhizin and matrine. Chem. Biol. Interact. 181, 15–19. doi: 10.1016/j.cbi.2009.04.013
Wang, X. H., Huang, S. K. (1992). Pharmacokinetics and pharmacodynamics of matrine and oxymatrine. Acta Pharmaceut. Sinica. 27, 572–576. doi: 10.16438/j.0513-4870.1992.08.003
Wang, X. Y., Liang, L., Chang, J. L., Yang, M. H., Li, Z. G. (2010). Toxicity of matrine in Kunming mice. J. South Med. Univ. 30, 2154–2155.
Wang, L., Gao, C., Yao, S., Xie, B. S. (2013). Blocking Autophagic Flux Enhances Matrine-Induced Apoptosis in Human Hepatoma Cells. Int. J. Mol. Sci. 14, 23212–23230. doi: 10.3390/ijms141223212
Wang, Q. P., Wang, Y., Wang, X. D., Mo, X. M., Gu, J., Lu, Z. Y., et al. (2013). Survivin up-regulates the expression of breast cancer resistance protein (BCRP) through attenuating the suppression of p53 on NF-κB expression in MCF-7/5-FU cells. Int. J. Biochem. Cell. B. 45, 2036. doi: 10.1016/j.biocel.2013.06.026
Wang, Q., Shi, L., Tang, X., Dang, X. L., Zhang, Y. (2014). Pharmacokinetic study of multiple active constituents from Kushen–Gancao Decoction after oral administration in rat by HPLC–MS/M. J. Chromatogr. B. 965, 19–26. doi: 10.1016/j.jchromb.2014.05.038
Wang, L., Lu, J., Sun, W., Gu, Y. M., Zhang, C. C., Jin, R. M., et al. (2017). Hepatotoxicity induced by radix, Sophorae tonkinensis, in mice and increased serum cholinesterase as a potential supplemental biomarker for liver injury. Exp. Toxicol. Pathol. 69, 193–202. doi: 10.1016/j.etp.2017.01.003
Wang, S. J., Sun, W. W., Zhang, J. N., Zhao, J. K., Ren, S., Zhang, W. J., et al. (2017). Effect of Matrine and Oxymatrine on Proliferation and Energy Metabolism of ECV304. Nat. Prod. Res. Dev. 29, 741–746. doi: 10.16333/j.1001-6880.2017.5.004
Wei, L., Zhu, Z., Wang, J., Liu, J. (2009). JNK and p38 mitogen-activated protein kinase pathways contribute to porcine circovirus type 2 infection. J. Virol. 83, 6039–6047. doi: 10.1128/JVI.00135-09
Wrzesień, K. A., Smolewski, P., Sobczak-Pluta, A., Wierzbowska, A., Robak, T. (2004). The inhibitor of apoptosis protein family and its antagonists in acute leukemias. Apoptosis 9, 705–715. doi: 10.1023/b:appt.0000045788.61012.b2
Wu, X., Yamashita, F., Hashida, M., Chen, X. G., Hu, Z. D. (2003). Determination of matrine in rat plasma by high-performance liquid chromatography and its application to pharmacokinetic studie. Talanta 59, 0–971. doi: 10.1016/s0039-9140(03)00009-2
Wu, W. Y., Huang, J., Liu, S., Li, X. H., Cai, Y. (2009). Study on pharmacokinetics and tissue distribution of stealth matrine liposomes in rats. China J. Chin. Mater. Med. 34, 751–755.
Wu, H., Che, X., Zheng, Q., Wu, A., Pan, K., Shao, A. W., et al. (2014). Caspases: A Molecular Switch Node in the Crosstalk between Autophagy and Apoptosis. Int. J. Biol. Sci. 10, 1072–1083. doi: 10.7150/ijbs.9719
Wu, C., Xu, Z., Gai, R., Huang, K. H. (2016). Matrine ameliorates spontaneously developed colitis in interleukin-10-deficient mice. Int. Immunopharmacol. 36, 256–262. doi: 10.1016/j.intimp.2016.04.038
Wu, J., Hu, G., Dong, Y., Ma, R. Y., Yu, Z. J., Jiang, S. F., et al. (2017). MT induces Akt/mTOR signalling inhibition-mediated autophagy and apoptosis in acute myeloid leukaemia cells. J. Cell. Mol. Med. 21, 1171. doi: 10.1111/jcmm.13049
Wu, G. J., Zhou, W., Zhao, J., Pan, X., Sun, Y. J., Xu, H., et al. (2017). Matrine alleviates lipopolysaccharide-induced intestinal inflammation and oxidative stress via CCR7 signal. Oncotarget. 8, 11621. doi: 10.18632/oncotarget.14598
Wu, Z. H., You, Z. C., Chen, P., Chen, C., Chen, F., Shen, J. H., et al. (2018). Matrine exerts antidepressant-like effects on mice: Role of the Hippocampal PI3K/Akt/mTOR Signaling. Int. J. Neuropsychopharmacol. 21, 764–776. doi: 10.1093/ijnp/pyy028
Wynn, T. A. (2007). Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J. Clin. Invest. 117, 524–529. doi: 10.1172/JCI31487
Xiao, P., Kubo, H., Ohsawa, M., Higashiyama, K., Nagase, H., Yan, Y. N., et al. (1999). kappa-Opioid receptor-mediated antinociceptive effects of stereoisomers and derivatives of (+)-matrine in mice. Planta Med. 65, 230–233. doi: 10.1055/s-1999-14080
Yang, Z., Gao, S., Yin, T., Kulkarni, K. H., Teng, Y., You, M., et al. (2010). Biopharmaceutical and pharmacokinetic characterization of matrine as determined by a sensitive and robust UPLC–MS/MS method. J. Pharm. Biomed. Anal. 51, 1120–1127. doi: 10.1016/j.jpba.2009.11.020
Yang, Z. J., Cheng, E. C., Huang, S., Sinicrope, F. A. (2011). The Role of Autophagy in Cancer: Therapeutic Implications. Mol. Cancer Ther. 10, 1533. doi: 10.1158/1535-7163.MCT-11-0047
Yang, Y., Xiu, J., Zhang, X., Zhang, L., Yan, K., Qin, C., et al. (2012). Antiviral effect of matrine against human enterovirus 71. Molecules 17, 10370–10376. doi: 10.3390/molecules170910370
Yang, Y., Guo, J. X., Shao, Z. Q., Gao, J. P. (2017). MT inhibits bladder cancer cell growth and invasion in vitro through PI3K/AKT signaling pathway: An experimental study. Asian. Pac. J. Trop. Med. 10, 489–492. doi: 10.1016/j.apjtm.2017.05.009
Yang, B., Li, H. L., Zhang, T. Y., Wang, Z. X., Li, H., Zhang, Y. J., et al. (2020). Nonlinear and mixed inhibitory effect of matrine on the cytotoxicity of oligomeric amyloid-β protein. Neurochem. Int. 137, 104746. doi: 10.1016/j.neuint.2020.104746
You, L. T., Yang, C. J., Leng, X., Dong, X. X., Liu, Y., Sai, N., et al. (2018). Study on the toxicity of matrine on HepaRG cell line. Global Tradit. Chin. Med. 11, 1867–1874.
You, L. T., Yang, C. J., Du, Y. Y., Liu, Y., Chen, G. S., Sai, N., et al. (2019). Matrine Exerts Hepatotoxic Effects via the ROS-Dependent Mitochondrial Apoptosis Pathway and Inhibition of Nrf2-Mediated Antioxidant Response, Oxid. Med. Cell. Longev. 2019, 1045345. doi: 10.1155/2019/1045345
Yu, P., Qian, L., Liu, K., Yagasaki, K., Wu, E., Zhang, G. Y. (2009). MT suppresses breast cancer cell proliferation and invasion via VEGF-Akt-NF-κB signaling. Cytotechnology 59, 219–229. doi: 10.1007/s10616-009-9225-9
Yu, S. X., Wu, J., Yang, W. D. (2009). Protective Effects of Matrine on Immunological Liver Injury Model in BALB/c Mice. J. Ningxia Med. Univ. 31, 11–15. doi: 10.16050/j.cnki.issn1674-6309.2009.01.012
Yu, J., Yang, S., Wang, X., Gan, R. T. (2014). Matrine improved the function of heart failure in rats via inhibiting apoptosis and blocking β3-adrenoreceptor/endothelial nitric oxide synthase pathway. Mol. Med. Rep. 2014. 10, 3199–3204. doi: 10.3892/mmr.2014.2642
Zeng, X. Y., Wang, H., Bai, F., Zhou, X., Li, S. P., Ren, L. P., et al. (2015). Identification of matrine as a promising novel drug for hepatic steatosis and glucose intolerance with HSP72 as an upstream target. Br. J. Pharmacol. 172, 4303. doi: 10.1111/bph.13209
Zhai, D., Zhao, Y., Chen, X., Guo, J. Q., He, H., Yu, Q. L., et al. (2007). Protective effect of glycyrrhizin, glycyrrhetic acid and matrine on acute cholestasis induced by alpha-naphthyl isothiocyanate in rats. Planta Med. 73, 128–133. doi: 10.1055/s-2006-957067
Zhang, L., Liu, W., Zhang, R., Wang, Z., Shen, Z., Chen, X., et al. (2008). Pharmacokinetic study of matrine, oxymatrine and oxysophocarpine in rat plasma after oral administration of Sophora flavescens Ait. extract by liquid chromatography tandem mass spectrometry. J. Pharm. Biomed. Anal. 47, 892–898. doi: 10.1016/j.jpba.2008.03.019
Zhang, X. L., Xu, H. R., Chen, W. L., Chu, N. N., Li, X. N., Liu, G. Y., et al. (2009). MT determination and pharmacokinetics in human plasma using LC/MS/MS. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 877, 3253–3256. doi: 10.1016/j.jchromb.2009.08.026
Zhang, Y., Zhang, H., Yu, P., Liu, Q., Liu, K., Duan, H. Y., et al. (2009). Effects of MT against the growth of human lung cancer and hepatoma cells as well as lung cancer cell migration. Cytotechnology 59, 191–200. doi: 10.1007/s10616-009-9211-2
Zhang, J. Q., Li, Y. M., Liu, T., He, W. T., Chen, Y. T., Chen, X. H., et al. (2010). Antitumor effect of MT in human hepatoma G2 cells by inducing apoptosis and autophagy. World J. Gastroentero. 16, 4281. doi: 10.3748/wjg.v16.i34.4281
Zhang, B., Liu, Z. Y., Li, Y. Y., Luo, Y., Liu, M. L., Dong, H. Y., et al. (2011). Anti-inflammatory effects of matrine in LPS-induced acute lung injury in mice. Eur. J. Pharm. Sci. 44, 573–579. doi: 10.1016/j.ejps.2011.09.020
Zhang, F., Wang, X., Tong, L., Qiao, H. Q., Li, X., You, L. G., et al. (2011). Matrine attenuates endotoxin-induced acute liver injury after hepatic ischemia/reperfusion in rats. Surg. Today 41, 1075–1084. doi: 10.1007/s00595-010-4423-9
Zhang, Q., Li, F. J., Jin, R. M., Song, Z. P. (2011). Study on the Hepatotoxicity Induced by Matrine and Oxymatrine. Chin. Arch. Tradit. Chin. Med. 29, 1222–1225.
Zhang, H. F., Shi, L. J., Song, G. Y., Cai, Z. G., Wang, C., An, R. J. (2013). Protective effects of matrine against progression of high-fructose diet-induced steatohepatitis by enhancing antioxidant and anti-inflammatory defences involving Nrf2 translocation. Food Chem. Toxicol. 55, 70–77. doi: 10.1016/j.fct.2012.12.043
Zhang, J., Su, K., Shi, W., Wang, Y., Hu, P. C., Wang, Y., et al. (2013). MT inhibits the adhesion and migration of BCG823 gastric cancer cells by affecting the structure and function of the vasodilator-stimulated phosphoprotein (VASP). Chin. Pharmacol. Bull. 34, 1084. doi: 10.1038/aps.2013.15
Zhang, S., Cheng, B., Li, H., Xu, W., Zhai, B., Pan, S., et al. (2014). MT inhibits proliferation and induces apoptosis of human colon cancer LoVo cells by inactivating Akt pathway. Mol. Biol. Rep. 41, 2101–2108. doi: 10.1007/s11033-014-3059-z
Zhang, L., Zhang, H., Zhu, Z., Jiang, L. J., Lu, X. Z., Zhou, M., et al. (2015). Matrine regulates immune functions to inhibit the proliferation of leukemic cells. Int. J. Clin. Exp. Med. 8, 5591.
Zhang, M. L., Zhang, X. J., Kang, J., Zhang, H. J., Chen, X. L., Liu, N., et al. (2017). Matrine promotes NT3 expression in CNS cells in experimental autoimmune encephalomyelitis. Neurosci. Lett. 649, 100–106. doi: 10.1016/j.neulet.2017.04.005
Zhang, R., Li, Y., Hou, X., Miao, Z. H., Wang, Y. (2017). Neuroprotective effect of heat shock protein 60 on matrine-suppressed microglial activation. Exp. Ther. Med. 14, 1832. doi: 10.3892/etm.2017.4691
Zhang, B., Wang, X., Li, Y., Wu, M., Wang, S. Y., Li, S. (2018). Matrine Is Identified as a Novel Macropinocytosis Inducer by a Network Target Approach. Front. Pharmacol. 9, 10. doi: 10.3389/fphar.2018.00010
Zhang, C. X., Chen, J., Zhang, J. H., Wei, S. J., Ji, H. Y., Wu, X. L., et al. (2018). Different processing methods change the oral toxicity induced by Sophora alopecuroides seeds and the contents of five main toxic alkaloids from the ethanol extracts determined by a validated UHPLC–MS/MS assay. Rev. Bras. Farmacogn. 28, 481–488. doi: 10.1016/j.bjp.2018.04.007
Zhang, Y., Cui, L., Guan, G., Wang, J. K., Qiu, C., Yang, T. L., et al. (2018a). Matrine suppresses cardiac fibrosis by inhibiting the TGF-β/Smad pathway in experimental diabetic cardiomyopathy. Mol. Med. Rep. 17, 1775–1781. doi: 10.3892/mmr.2017.8054
Zhang, Y., Yang, X., Qiu, C., Liu, F., Liu, P., Liu, Z. (2018b). Matrine suppresses AGE-induced HAEC injury by inhibiting ROS-mediated NRLP3 inflammasome activation. Eur. J. Pharmacol. 822, 207–211. doi: 10.1016/j.ejphar.2018.01.029
Zhang, Y., Liu, M., Sun, H., Yin, K. M. (2015). Matrine improves cognitive impairment and modulates the balance of Th17/Treg cytokines in a rat model of Aβ1-42-induced Alzheimer’s disease. Cent. Eur. J. Immunol. 40, 411–419. doi: 10.5114/ceji.2015.56961
>Zhao, C. G., Liao, D. D., He, X. Y., Li, Z. B. (2010a). Study on pharmacokinetics of matrine by intramuscular administration in rat. China J. Chin. Mater. Med. 35, 1315–1318. doi: 10.4268/cjcmm20101021
Zhao, C. G., Zuo, H., Liao, D. D., He, X. Y., Li, Z. B. (2010b). Effect of ceftiofur hydrochloride on pharmacokinetics of matrine in rats. Zhongguo Zhong Yao Za Zhi. 35, 1859–1861. doi: 10.4268/cjcmm20101420
Zhao, B., Li, B., Bai, S., Shen, L., Ren, R., Jonas, J. B., et al. (2012). Effects of MT on proliferation and apoptosis of cultured retinoblastoma cells. Graefes Arch. Clin. Exp. Ophthalmol. 250, 897–905. doi: 10.1007/s00417-011-1751-4
Zhao, J., Wan, X. Y., Luo, M., Chen, T. S., He, P. (2012). Antifibrotic effects of glycyrrhizin and matrine in vitro and in vivo. Biomed. Prevent. Nutrition. 2, 132–137. doi: 10.1016/j.bionut.2011.12.006
Zhao, X., Sun, N., Bai, X. Y., Sun, Y. G., Jiang, J. B., Li, H. Q. (2013). Inhibitory action of Matrine against porcine reproductive and respiratory syndrome virus in vitro and mechanism. Chin. J. Veterinary Sci. 33, 808–812. doi: 10.16303/j.cnki.1005-4545.2013.06.012
Zhao, P., Zhou, R. U., Zhu, X., Hao, Y. J., Li, N., Wang, J., et al. (2015). Matrine attenuates focal cerebral ischemic injury by improving antioxidant activity and inhibiting apoptosis in mice. Int. J. Mol. Med. 36, 633–644. doi: 10.3892/ijmm.2015.2260
Zhao, Q. M., Yu, Y. T. (2017). Antibacterial Activity of Matrine and Oxymatrine on Pathogenic Bacteria Causing Cow Endometritis in Vitro. Prog. Veterinary Med. 38, 65–68. doi: 10.16437/j.cnki.1007-5038.2017.01.013
Zhao, X. B., Qin, Y., Niu, Y. L., Yang, J. (2018). Matrine inhibits hypoxia/reoxygenation-induced apoptosis of cardiac microvascular endothelial cells in rats via the JAK2/STAT3 signaling pathway. Biomed. Pharmacother. 106, 117–124. doi: 10.1016/j.biopha.2018.06.003
Zhou, H., Xu, M., Gao, Y., Deng, Z. G., Zhang, W. Q., Wang, Q., et al. (2014). MT induces caspase-independent program cell death in hepatocellular carcinoma through bid-mediated nuclear translocation of apoptosis inducing factor. Mol. Cancer. 13, 59. doi: 10.1186/1476-4598-13-59
Zhou, J., Wei, S., Fu, Y., Guo, Z. L., Zhang, W. P., Dai, G. D., et al. (2016). Matrine ameliorates isoproterenol-induced acute myocardial ischemia through regulation of growth factors and RhoA/ROCK1 pathway. Int. J. Clin. Exp. Patho. 9, 21961–21969.
Zhou, B. G., Wei, C. S., Zhang, S., Zhang, Z., Gao, H. M. (2017). MT reversed multidrug resistance of breast cancer MCF-7/ADR cells through PI3K/AKT signal pathway. J. Cell. Biochem. 119, 3885–3891. doi: 10.1002/jcb.26502
Zhou, J., Ma, W., Wang, X., Liu, H. B., Miao, Y. L., Wang, J. L., et al. (2019). Matrine Suppresses Reactive Oxygen Species (ROS)-Mediated MKKs/p38-Induced Inflammation in Oxidized Low-Density Lipoprotein (ox-LDL)-Stimulated Macrophages. Med. Sci. Monit. 25, 4130–4136. doi: 10.12659/MSM.917151
Zhu, P., Chen, J. M., Chen, S. Z., Zhang, C., Zheng, S. Y., Long, G., et al. (2010). MT inhibits vascular smooth muscle cell proliferation by modulating the expression of cell cycle regulatory genes. Chin. Pharmacol. Bull. 31, 1329–1335. doi: 10.1038/aps.2010.145
Keywords: matrine, pharmacology, toxicology, pharmacokinetics, mechanisms
Citation: You L, Yang C, Du Y, Wang W, Sun M, Liu J, Ma B, Pang L, Zeng Y, Zhang Z, Dong X, Yin X and Ni J (2020) A Systematic Review of the Pharmacology, Toxicology and Pharmacokinetics of Matrine. Front. Pharmacol. 11:01067. doi: 10.3389/fphar.2020.01067
Received: 07 December 2019; Accepted: 30 June 2020;
Published: 16 September 2020.
Edited by:
Banasri Hazra, Jadavpur University, IndiaReviewed by:
Haolong Liu, Peking University Health Science Centre, ChinaSutapa Biswas Majee, NSHM Knowledge Campus, India
Copyright © 2020 You, Yang, Du, Wang, Sun, Liu, Ma, Pang, Zeng, Zhang, Dong, Yin and Ni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoxv Dong, ZHhpYW94dkAxNjMuY29t; Xingbin Yin, eXhidGNtQDE2My5jb20=; Jian Ni, bmp0Y21AMjYzLm5ldA==
†These authors have contributed equally to this work
 Longtai You
Longtai You Chunjing Yang2†
Chunjing Yang2† Xiaoxv Dong
Xiaoxv Dong Xingbin Yin
Xingbin Yin Jian Ni
Jian Ni