- 1School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2The Affiliated Hospital of Southwest Medical University, Luzhou, China
- 3School of Nursing, Southwest Medical University, Luzhou, China
Background: Over the past five years the Chinese herbal formula (CHF) medicine, Xiaoer-Feike granules (XFG), has become a widely used adjuvant therapy for acute lower respiratory infections (ALRI). Considering the rapid popularization and application of XFG, and the lack of systematic evidence evaluating its effectiveness and safety in treating ALRI, it is necessary to conduct a meta-analysis to determine its benefits for patients.
Methods: This study systematically identified randomized controlled trials (RCTs) of XFG treatments for ALRI through July 2019 using four English-databases (PubMed, Cochrane Library, Ovid, and Web of Science) and four Chinese-databases (Sino-med database, China National Knowledge Infrastructure (CNKI), VIP database, and the WANFANG database). We then performed a quality assessment and data analysis with Review Manager 5.3.5 and Stata 15.1.
Results: Twenty-one RCTs involving 3425 patients were randomly divided into an XFG group and a conventional medicine (CM) group. The results showed that the clinical efficacy rate (CER) of the XFG group was significantly higher than that of the CM group (RR=1.17, 95% CI =1.13-1.22, P< 0.00001). In comparison with the CM group, the XFG group had strikingly shortened: resolution time of cough (RTC) (MD = -1.92; 95% CI =-2.33, -1.51, P<0.00001); resolution time of rale (RTR) (MD = -1.68; 95% CI =-2.27, -1.10, P<0.00001); resolution time of fever (RTF) (MD = -1.46; 95% CI =-1.92, -1.00, P<0.00001); resolution time of inflammatory lesions (RTIL) (MD = -2.43, 95% CI =-2.94, -1.93, P< 0.00001); and hospital stays (HS) (MD = -2.26, 95% CI =-3.03, -1.49, P< 0.00001). At the cellular and molecular level, the CD4, CD8, CD4/CD8, IL-6, TNF-α, and CRP levels were significantly improved when CM was complemented with XFG. In addition, no significant difference was observed between the XFG and CM groups in terms of the adverse events (AE) (RR =0.97, 95% CI= 0.61-1.54, P= 0.89).
Conclusions: The findings of this meta-analysis support the use of XFG in the treatment of ALRI. However, these results should be treated with caution due to the significant heterogeneity and publication bias of existing data. Further well-designed and high-quality RCTs are needed to interrogate the efficacy and safety of XFG.
Introduction
Acute lower respiratory infections (ALRI), primarily including pneumonia and bronchiolitis with typical clinical symptoms such as fever, cough, asthma, rale, and so forth, are among the most severe infectious diseases (Emam et al., 2019). ALRI leads to nearly one million deaths in children under five years of age every year, a much higher rate than all other childhood illnesses (UNICEF/WHO. UNICEF, 2016). Among ALRIs, pneumonia is the number one killer of children. In particular, ALRI primarily affects patients in developing countries due to limited health care and poorer control of infection risk factors. In some parts of the world, one child dies of pneumonia every 35 seconds, and the combined number in developing countries accounts for more than 99% of childhood pneumonia deaths (UNICEF/WHO. UNICEF, 2006). Despite a significant decline in child deaths from ALRI between 2000 and 2015, 19% of all childhood deaths were still attributable to ALRI (Bryce et al., 2005; Rudan et al., 2008). Children with ALRI not only endure a lower quality of life, these conditions can also cause significant medical and financial burdens on families and society (Jiang et al., 2016).
The pathogenic microorganisms of ALRI include bacteria (Streptococcus pneumoniae, Staphylococcus aureus, Escherichia coli, Klebsiella Pseudomonas aeruginosa, etc.), fungi (Candida albicans and aspergilloma), viruses (RSV, IFV, IFB, and CMV), mycoplasma, Chlamydia trachomatis, Rickettsia, and protozoal pneumonia. Bacterial and viral infections predominate ALRI. Currently, conventional medicines (CM) consist of defervescence, antitussive, expectorant (ambroxol and clenbuterol), aerosol, and anti-infection agents along with a variety of antibiotics, such as ceftriaxone sodium, ceftizoxime sodium, levofloxacin, and azithromycin, which have shown results in reducing ALRI mortality (He and Yang, 2018). However, because patients are often desperate for a speedy recovery, they are often irrationally prescribed antibiotics. This overuse of antibiotics worldwide has gradually enhanced the drug resistance of ALRI pathogens, which has significantly increased ALRI infection rates (Zhang L. et al., 2018). In the long run, the benefits from CM as led by antibiotics are likely to be challenged. Thus, research on novel therapies that can substantially enhance the effect of CM and reduce antibiotic use is of great social and clinical importance.
In recent years increasing numbers of parents in East Asia have sought complementary and alternative medicine for their children. In China, Chinese herbal formulas (CHFs), based on the basic theories of traditional Chinese medicine (TCM), are widely accepted and prescribed for children with ALRI. For example, XFG was first recorded in the 2015 edition of the Chinese pharmacopoeia and is one of the most effective CHFs used for ALRI. XFG consists of 22 ingredients: Ginseng radix et rhizoma, Poria, Atractylodis macrocephalae rhizoma, Citri reticulatae Pericarpium, Rhei radix et rhizome, Lycii cortex, Glehniae radix, Glycyrrhizae radix et rhizome, Artemisiae annuae herba, Ophiopogonis radix, Cinnamomic ramulus, Zingiberis rhizoma, Aconiti radix, Trichosanthis Fructus, Farfarae flos, Asteris radix et rhizome, Mori cortex, Astragali radix, Lycii fructu, Arisaema cum Bile, Galli gigerii endothelium corneum, and Trionycis carapax. XFG exerts its therapeutic effects mostly by inhibiting pathogenic microorganisms, enhancing immune function, reducing inflammation, relieving bronchospasm, and improving digestion and appetite (Chu, 2018; Hu et al., 2018; Wei and Li, 2018). Increasing numbers of clinical studies have indicated that XFG provides more benefits to preschool children with ALRI than CM alone (Yan, 2017). There is abundant evidence demonstrating that XFG can significantly shorten the duration of cough, asthma, fever, rale, hospital stays (HS), and antibiotic use and promote recovery from ALRI (Zhao and Chen, 2017; Liao and Lou, 2018). However, considering the rapid popularization and application of XFG, and the lack of systemic evidence to support the effectiveness and safety of XFG in treating ALRL, it is necessary to conduct a meta-analysis and rigorously assess the efficacy and safety of XFG to provide more objective and reliable evidence for the use of XFG in clinical settings.
Methods
Search Strategy
The databases we searched in this review included PubMed, WANFANG, Ovid, Web of Science, Cochrane Library, China National Knowledge Infrastructure (CNKI), VIP database, Sino-med database through July 2019. Considering that XFG was primarily used in China, we searched the above Chinese-language electronic databases to obtain as many clinical trials as possible. The search was restricted to trials published in Chinese and English. For the English-language databases, we used the following search strategies: Subject terms= (“Feike” or “pediatric lung cough” or “pediatric pulmonary cough”). For the Chinese databases, we used Subject terms= (“Xiaoer-Feike”). We also manually searched for articles that met our inclusion criteria from other sources that were not included in the above databases. Eligible studies were screened out by two reviewers independently. When a discrepancy occurred between the two investigators, it was resolved by discussion.
Article Inclusion and Data Extraction
This systematic review was conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analyses Statement (PRISMA). We selected eligible studies based on the following inclusion criteria: (1) the experimental group was treated with the XFG included in the Chinese Pharmacopoeia (edition 2015) regardless of the pharmaceutical company, (2) the control group was treated with conventional medicines alone, and (3) the clinical efficacy rate (CER), resolution time of cough (RTC), resolution time of rale (RTR), resolution time of fever (RTF) and HS were used as the primary outcomes by referring to guidelines or consensus views or the evaluation criteria. (4) In addition to the CER, at least one other outcome was employed. The exclusion criteria were: (1) non-RCTs or only one outcome; (2) the XFG group received the combination treatment of XFG and acupuncture therapy or other TCM; (3) studies that did not have control groups, or studies in which control subjects received TCM treatment including herbal medicine, acupuncture, or acupoint injection therapy; (4) the XFG that was not recorded in the Chinese Pharmacopoeia; and (5) systematic review, important data reports, and case reports were addressed. Two reviewers independently checked for relevant literature, including the first author’s last name, year of publication, sample size, gender distribution, age, disease duration, intervention, duration of treatment, dose, outcomes and adverse events, and clinical diagnosis.
Quality Assessment
Two reviewers independently evaluated the methodological qualities of the trials according to the Cochrane manual. We identified a list of seven categories for risk of bias: selection bias, performance bias, detection bias, attrition bias, reporting bias, and other bias. Each item was classified into low bias risk, high bias risk, and unclear bias risk. Disagreements between the reviewers were settled through discussion.
Data Synthesis and Analysis
In this review, statistical analyses were conducted by a reviewer manager (version 5.3.5), and we used OR with 95% CI for the analyses of dichotomous data, whereas the continuous data were presented as MD or sMD with 95% CI. The data were merged according to the Mantel-Haenszel (fixed-effects) model and the DerSimonian and Laird (random-effects) model. The heterogeneity between studies was determined by the chi-square test. With the I2 statistic, an I2< 25% indicating that heterogeneity may not be important, values between 25% and 50% represent moderate inconsistency, and I2 > 50% suggest severe heterogeneity. We defined P≥ 0.1 and I2< 50 as indications that the results are consistent and that the fixed-effects model would be used, while I2> 50% was an indicator of significant heterogeneity among trials. Then, a random-effects model was used to pool the results to minimize the influence of potential clinical heterogeneity. We evaluated the robustness of publication bias and sensitivity analyses to evaluate the robustness of the merged results with Stata 15.1. All the statistical tests were two tailed, and the differences were statistically significant at P< 0.05.
Results
Search Results and Study Characteristics
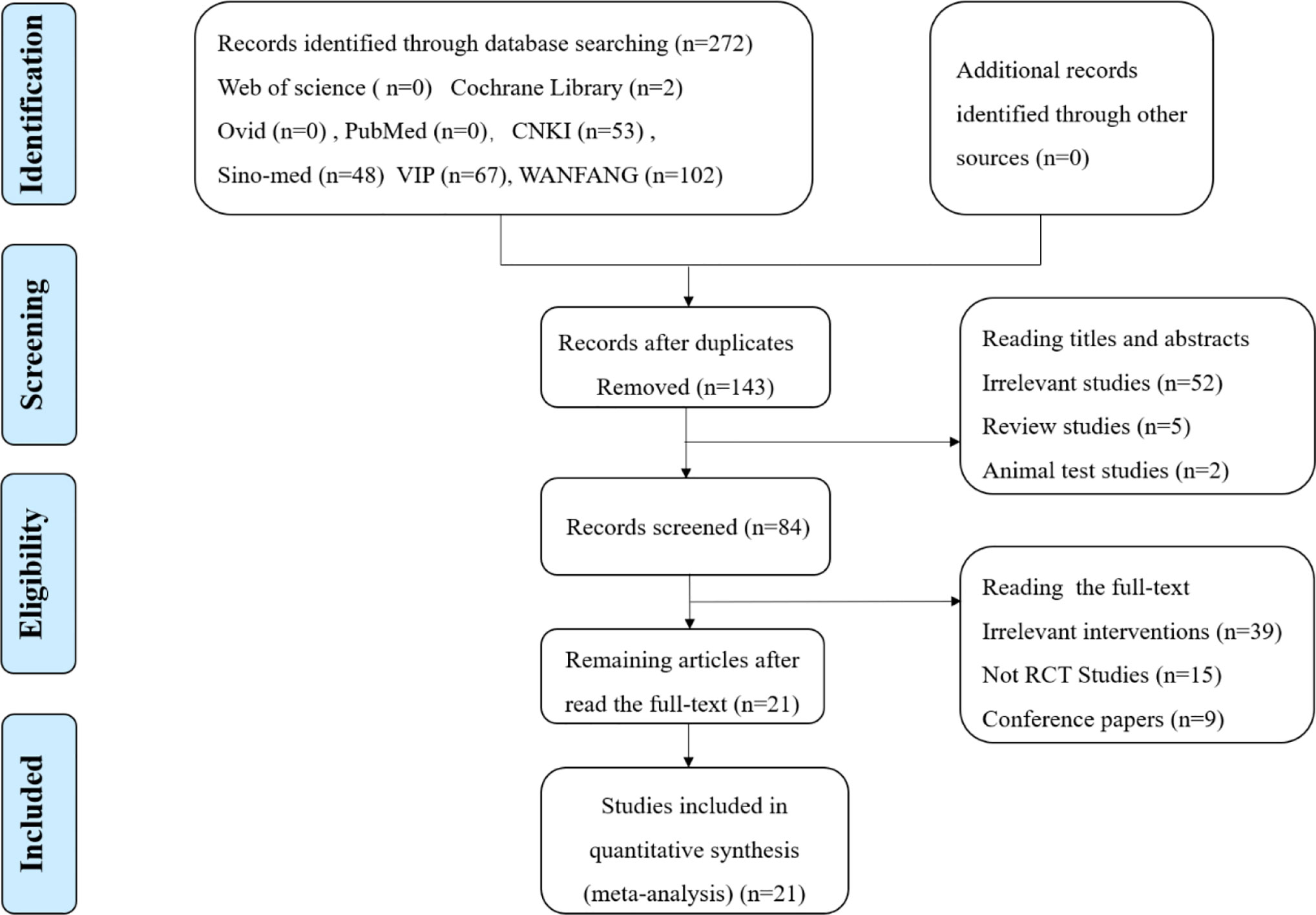
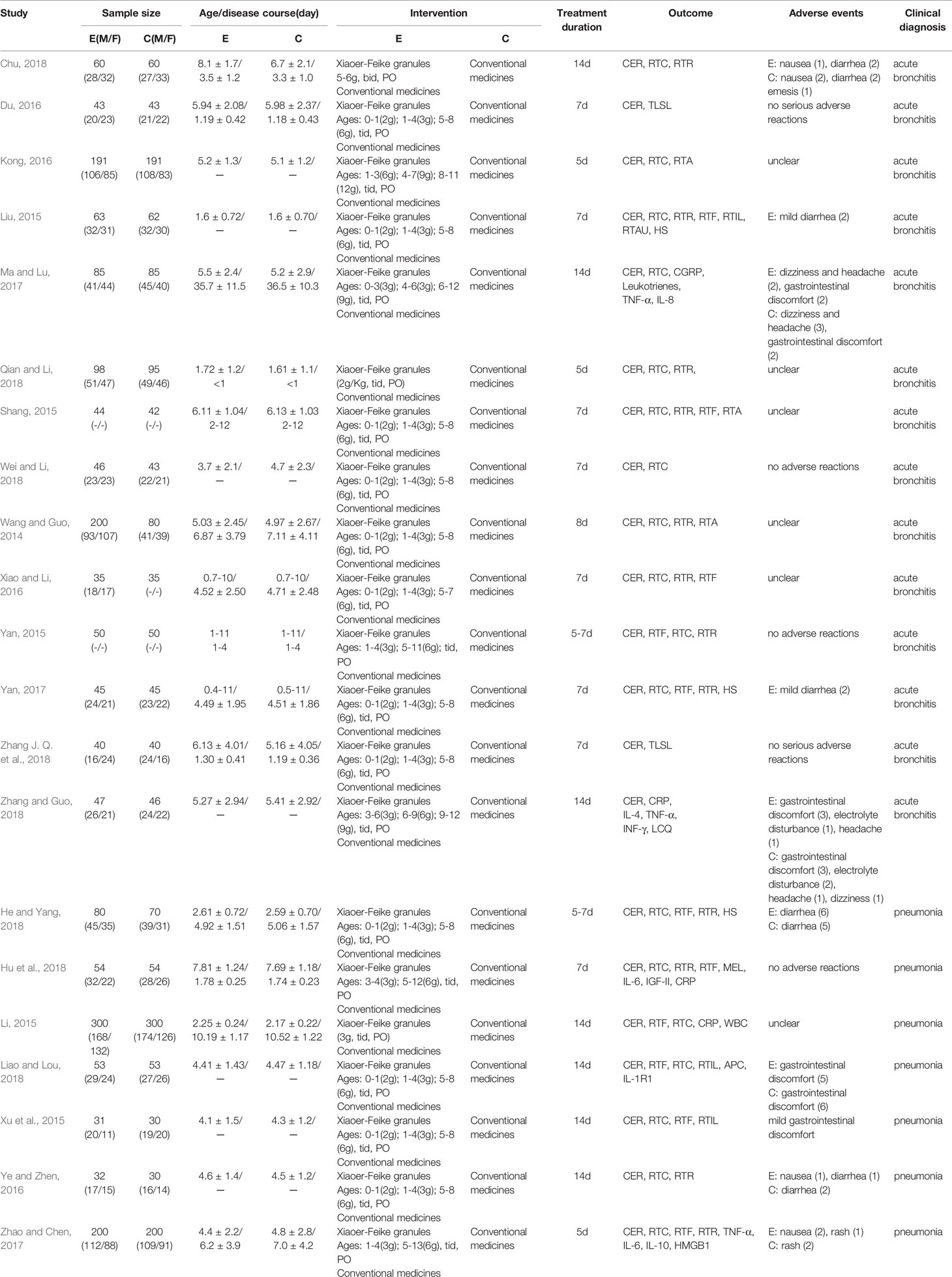
A total of 272 papers were obtained from a database search of the following sources: Web of Science (n=0), Cochrane Library (n=2), Ovid (n=0), PubMed (n=0), CNKI (n=53), Sino-med (n=48), VIP (n=67), WANFANG (n=102), from which 129 duplicated publications were removed. Fifty-nine citations of irrelevant topics were excluded after reading the titles and abstracts (irrelevant studies (n=52), review studies (n=5), and animal test studies (n=2)), and 63 studies were ruled out following a screening of the full text (irrelevant interventions (n=39), not RCT studies (n=15), and conference papers (n=9). Finally, 21 RCTs published between 2014 and 2018 involving 3425 patients with ALRI were eligible according to the inclusion criteria. Two reviewers extracted data from the literature, including information on general trial characteristics (first author’s last name, publication date); baseline patient and disease data (number of patients in each group, age and disease course); interventions (XFG, conventional medicine, treatment duration, and dose), outcome definitions, detailed adverse reactions, and clinical diagnosis. The sample size was 60 to 600 with significant age differences. The treatment duration ranged from 5 to 14 days. The XFG was provided by two Chinese pharmaceutical companies, Tian-sheng Pharmaceutical Group co., LTD, and Chang-chun People Pharmaceutical g=Group co., LTD. Twelve trials reported a specific number of adverse events. Of the 21 trials, 14 included patients diagnosed with acute bronchitis, and the other 7 studies selected pneumonia patients. Generally, the basic characteristics of the two groups were consistent, and no significant difference was found before the intervention. The procedure of the literature search is shown in Figure 1, and the general characteristics of the selected studies are listed in Table 1.
Methodological Quality Assessment
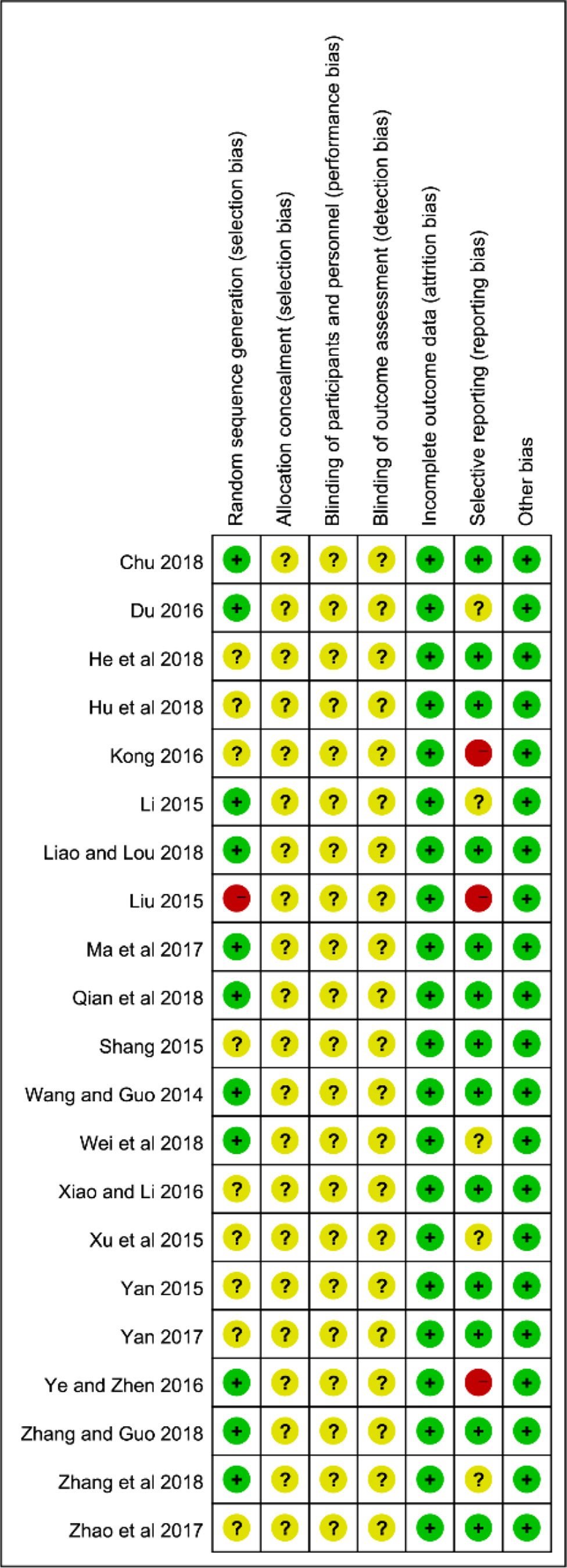
The specific randomized methods were detailed in 11 studies by random number tables in the assessments of selection bias, and we considered them as low-risk (Wang and Guo, 2014; Li, 2015; Du, 2016; Ye and Zhen, 2016; Ma and Lu, 2017; Chu, 2018; Liao and Lou, 2018; Qian and Li, 2018; Wei and Li, 2018; Zhang and Guo, 2018; Zhang J. Q. et al., 2018). One study (Liu, 2015) was high-risk due to its use of the registration order to generate a random sequence. The remaining 9 articles did not offer any specific information regarding the generation of random sequences. Almost all the studies failed to give the specific allocation concealment, performance bias, and detection bias. The reporting bias was at high risk in 3 documents (Liu, 2015; Kong, 2016; Ye and Zhen, 2016) because they failed to report the pre-listed outcomes. On the whole, the 21 studies fell in the middle because of the high proportion of the unclear risk of biases in the majority of studies. The specific results of the bias assessment are summarized in Figure 2.
Primary Outcomes
CER
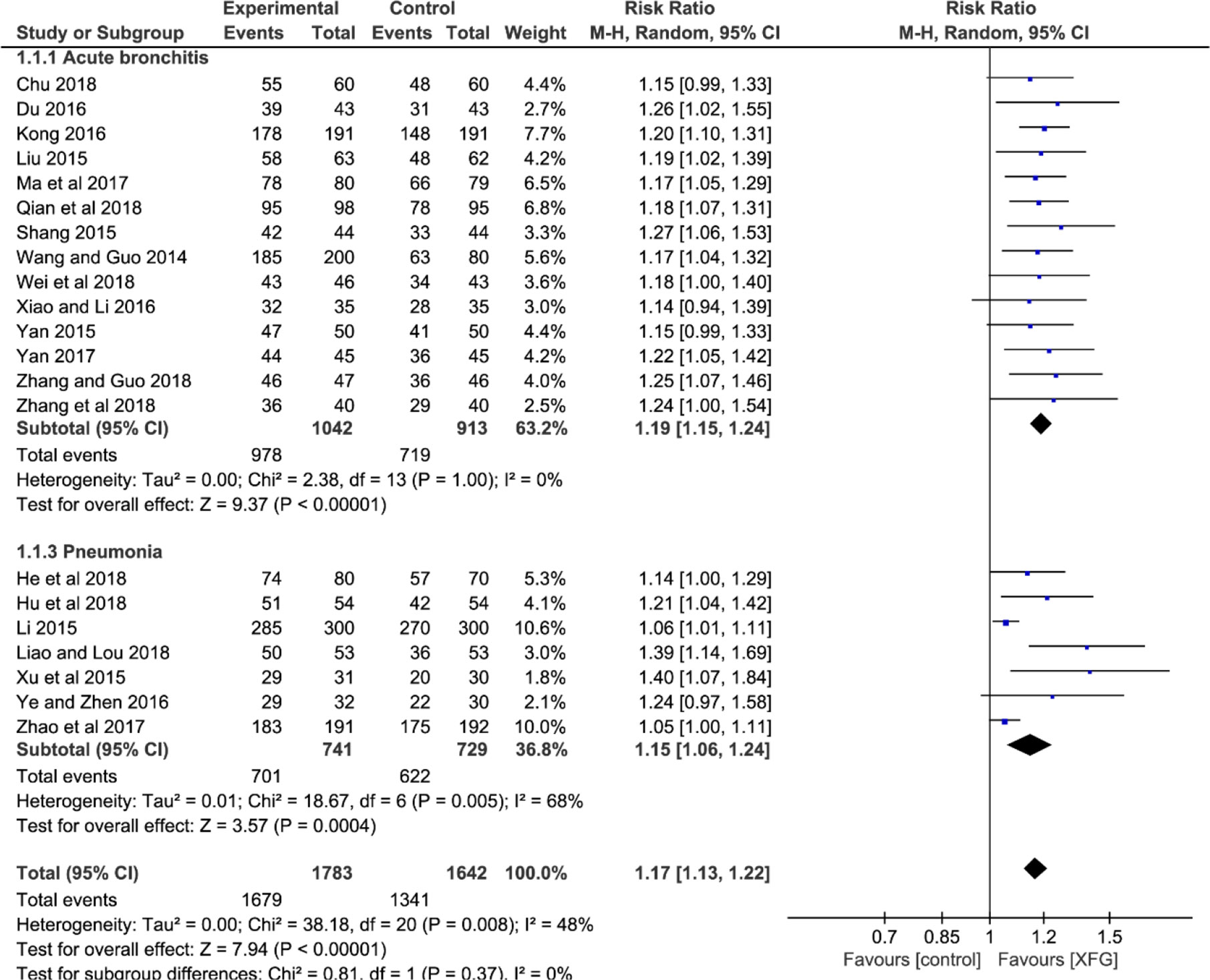
All the studies reported CER involving 1783 patients in the experimental groups who were treated with a combination of XFG and CM, and 1642 patients in the control groups were treated with CM alone. The heterogeneity test suggested that the fixed-effects model was more suitable (P=0.008, I2 = 48%). The result showed a statistically significant difference between the two groups, which indicated that adding XFG provided more benefits to ALRI patients. (RR=1.17, 95% CI =1.13-1.22, and P< 0.00001), and no significant difference was found between acute bronchitis and pneumonia groups (P= 0.37, I2 = 0%), as shown in Figure 3.
RTC
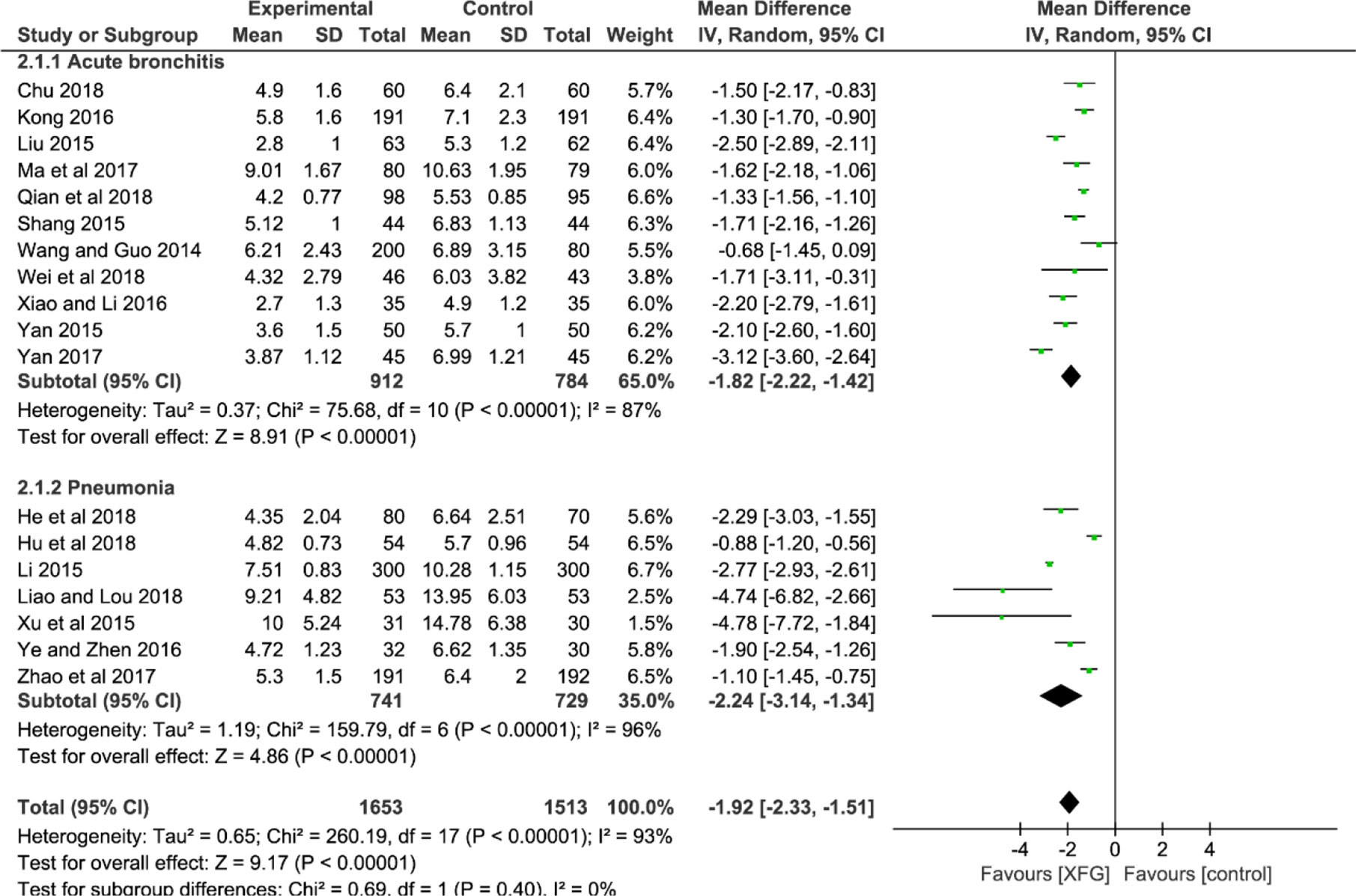
Eighteen studies used the RTC as an outcome after treatment (Wang and Guo, 2014; Li, 2015; Liu, 2015; Shang, 2015; Xu et al., 2015; Yan, 2015; Kong, 2016; Xiao and Li, 2016; Ye and Zhen, 2016; Ma and Lu, 2017; Yan, 2017; Zhao and Chen, 2017; Chu, 2018; He and Yang, 2018; Hu et al., 2018; Liao and Lou, 2018; Qian and Li, 2018; Wei and Li, 2018). An MD with a random-effects model was used to synthesize the data due to its striking heterogeneity (P<0.00001, I2 = 93%). The result suggested that the RTC of the XFG group was reduced more effectively than that of the CM group (MD = -1.92; 95% CI =-2.33, -1.51; and P<0.00001). Additionally, there was no significant difference between the two subgroups (p=0.40, I2 = 0%), as shown in Figure 4.
RTR
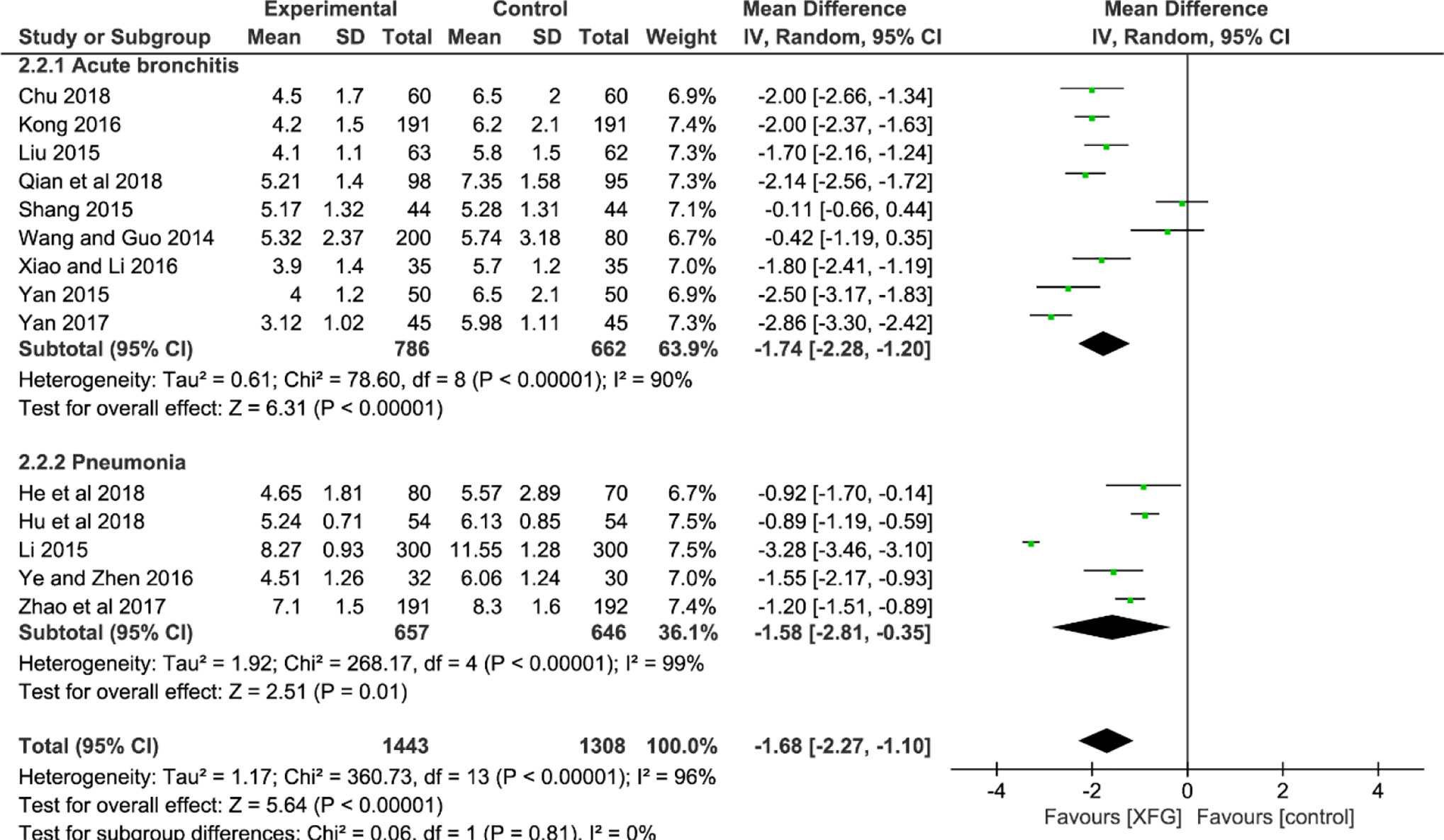
The RTR was reported in 14 studies (Wang and Guo, 2014; Li, 2015; Liu, 2015; Shang, 2015; Yan, 2015; Kong, 2016; Xiao and Li, 2016; Ye and Zhen, 2016; Yan, 2017; Zhao and Chen, 2017; Chu, 2018; He and Yang, 2018; Hu et al., 2018; Qian and Li, 2018). The result indicated a significant difference between the two groups with significant heterogeneity, suggesting that the RTR of the XFG group was shorter than that of the CM group (MD = -1.68, 95% CI =-2.27, -1.10, and P<0.00001). No significant difference was observed between the two subgroups (P=0.81, I2 = 0%), as shown in Figure 5.
RTF
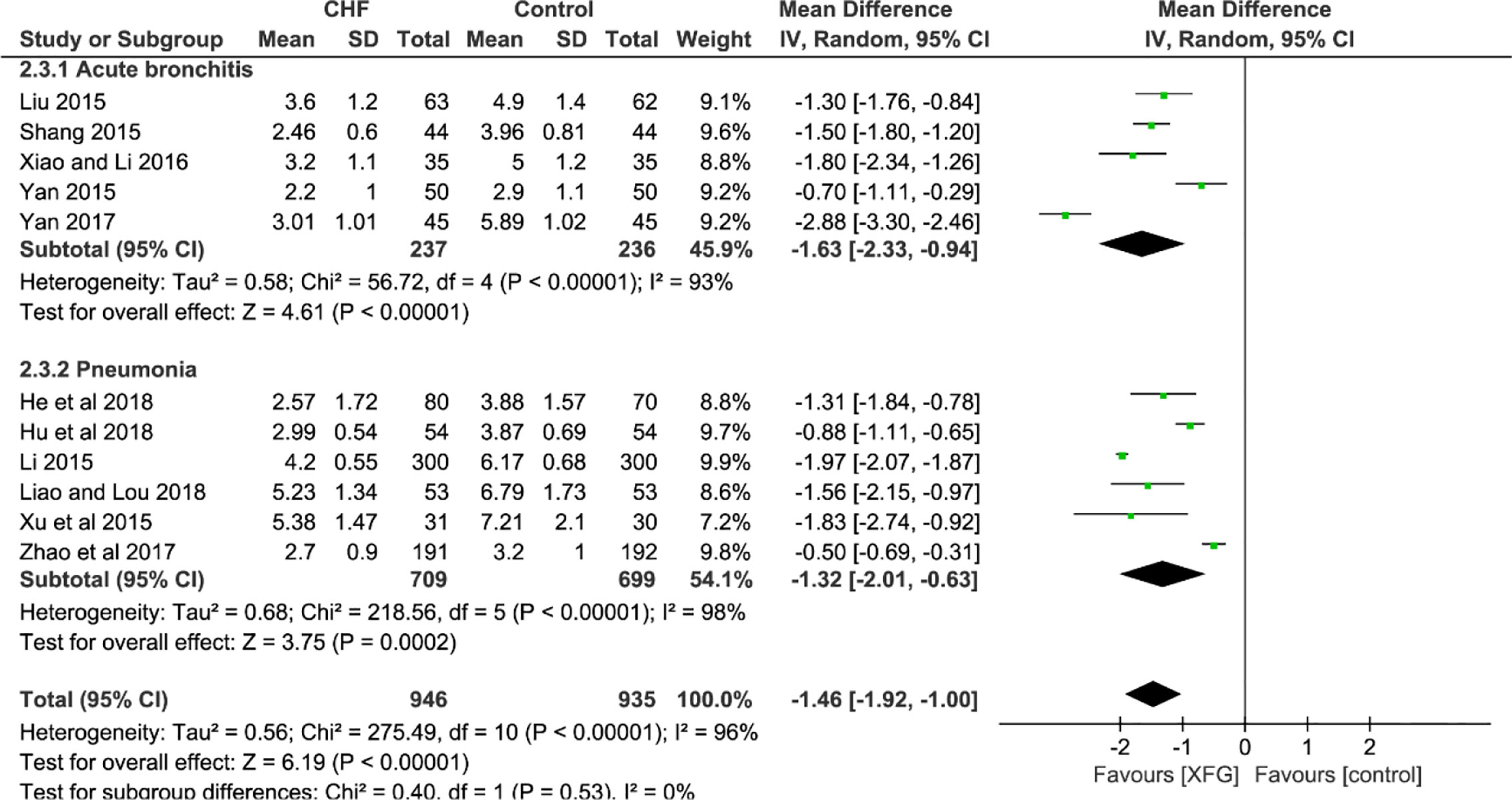
Eleven studies employed the outcome of the RTF (Liu, 2015; Shang, 2015; Yan, 2015; Li, 2015; Xu et al., 2015; Xiao and Li, 2016; Yan, 2017; Zhao and Chen, 2017; He and Yang, 2018; Hu et al., 2018; Liao and Lou, 2018). In a pooled analysis of the 11 trials, the addition of XFG led to a greater decrease in the RTF when compared with the CM therapy alone (MD=-1.46, 95% CI =-1.92, -1.00, and P< 0.00001), with high heterogeneity (P< 0.00001, I2 = 96%), and there was no significant difference between the two subgroups (P=0.53, I2 = 0%), as shown in Figure 6.
Secondary Outcomes
HS and RTIL
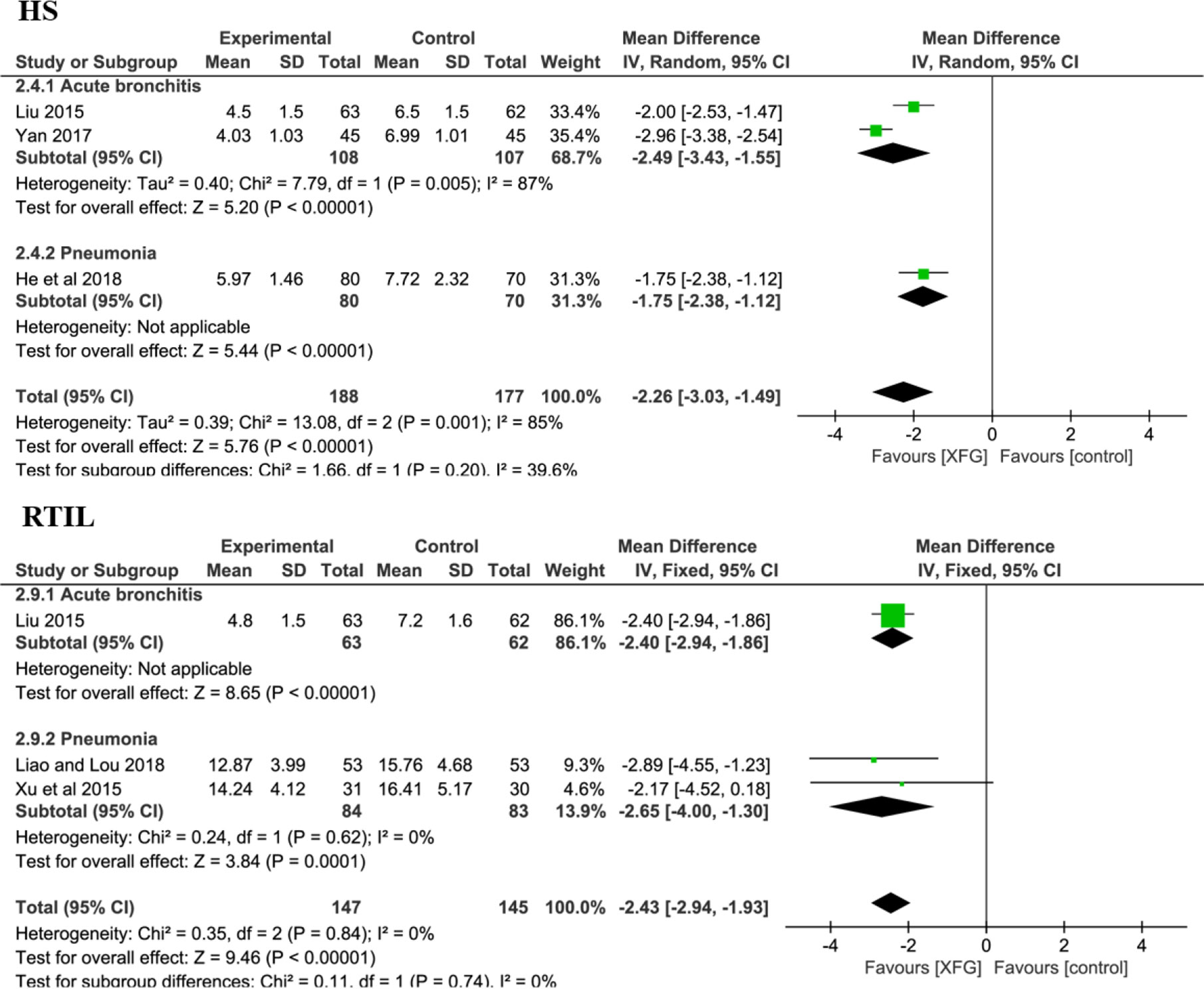
There were 3 RCTs (Liu, 2015; Yan, 2017; He and Yang, 2018) with a total of 365 patients on the HS (XFG group: 188 and CM group: 177). Compared with CM therapy alone, adding XFG to ALRI treatment significantly reduced the duration of the HS (MD = -2.26, 95% CI =-3.03, -1.49, P< 0.00001). Additionally, the RTIL was determined by X-ray in three studies involving 221 patients (Liu, 2015; Liao and Lou, 2018; Xu et al., 2015). The fixed-effects model was employed after a heterogeneity test (P=0.74; I2 = 0%). Significantly, the meta-analysis results favored the XFG group, indicating that the XFG group was better than the CM group at accelerating the disappearance of inflammatory lesions (MD = -2.43, 95% CI =-2.94, -1.93, and P< 0.00001), as shown in Figure 7.
Immune Cells and Cytokines
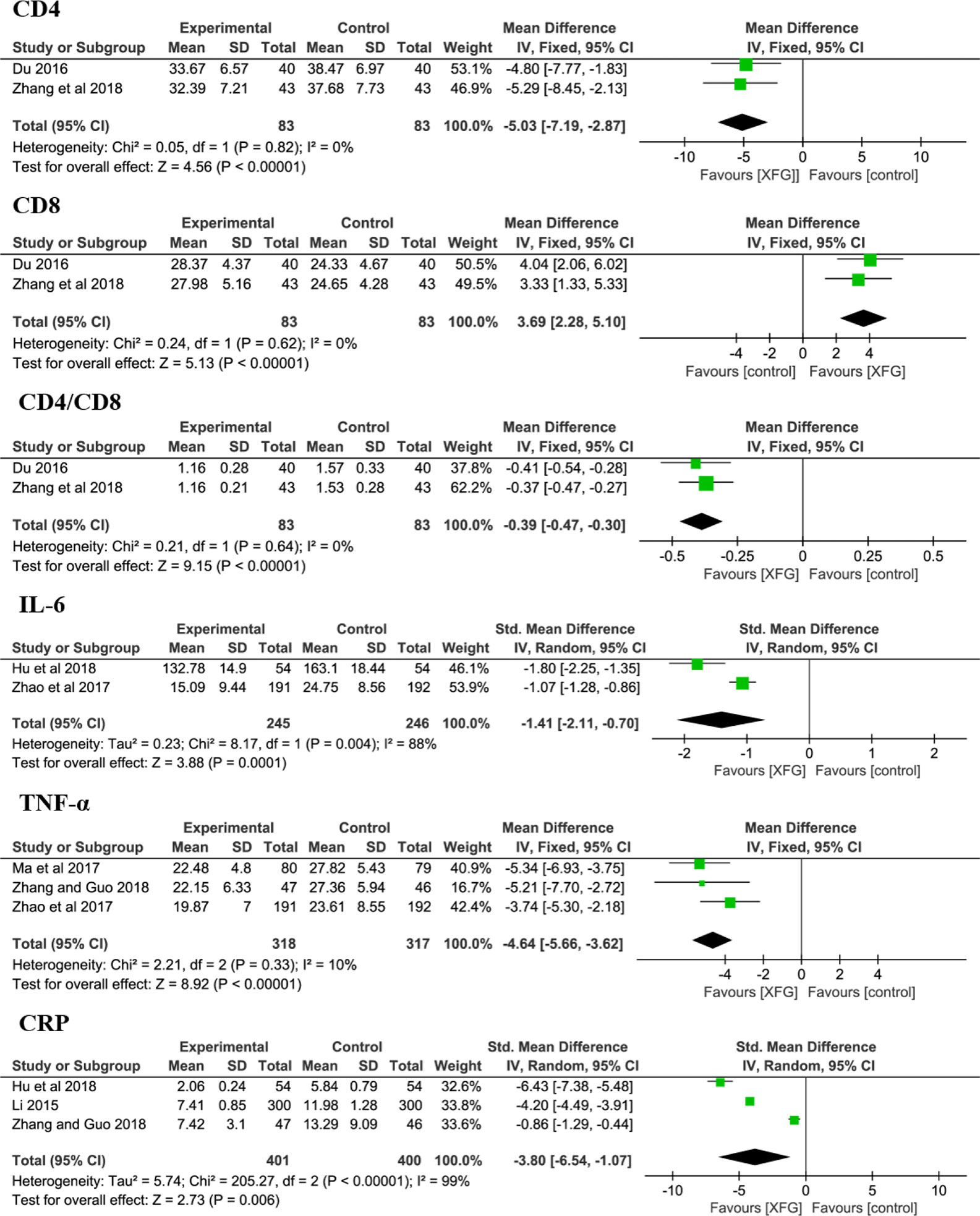
At the cellular and molecular levels, the immune cells (CD4 and CD8) and cytokines (IL-6, TNF-α and CRP levels) were determined in some trials (Li, 2015; Du, 2016; Zhao and Chen, 2017; Ma and Lu, 2017; Hu et al., 2018; Zhang and Guo, 2018; Zhang J. Q. et al., 2018). The results suggested that the levels of CD4 (MD = -5.03, 95% CI =-7.19, -2.83, P< 0.00001), CD8 (MD = 3.69, 95% CI =2.28, 5.10, P< 0.00001), CD4/CD8 (MD = -0.39, 95% CI =-0.47, -0.30, P< 0.00001), IL-6 (sMD = -1.41, 95% CI =-2.11, -0.70, P=0.0001), TNF-α (MD = -4.64, 95% CI =-5.66, -3.62, P< 0.00001) and CRP (sMD = -3.80, 95% CI =-6.54, -1.07, P=0.006) were significantly improved when complemented with XFG, indicating that adding XFG greatly reduced inflammation and enhanced the immune functions of the patient, as shown in Figure 8.
Adverse Events
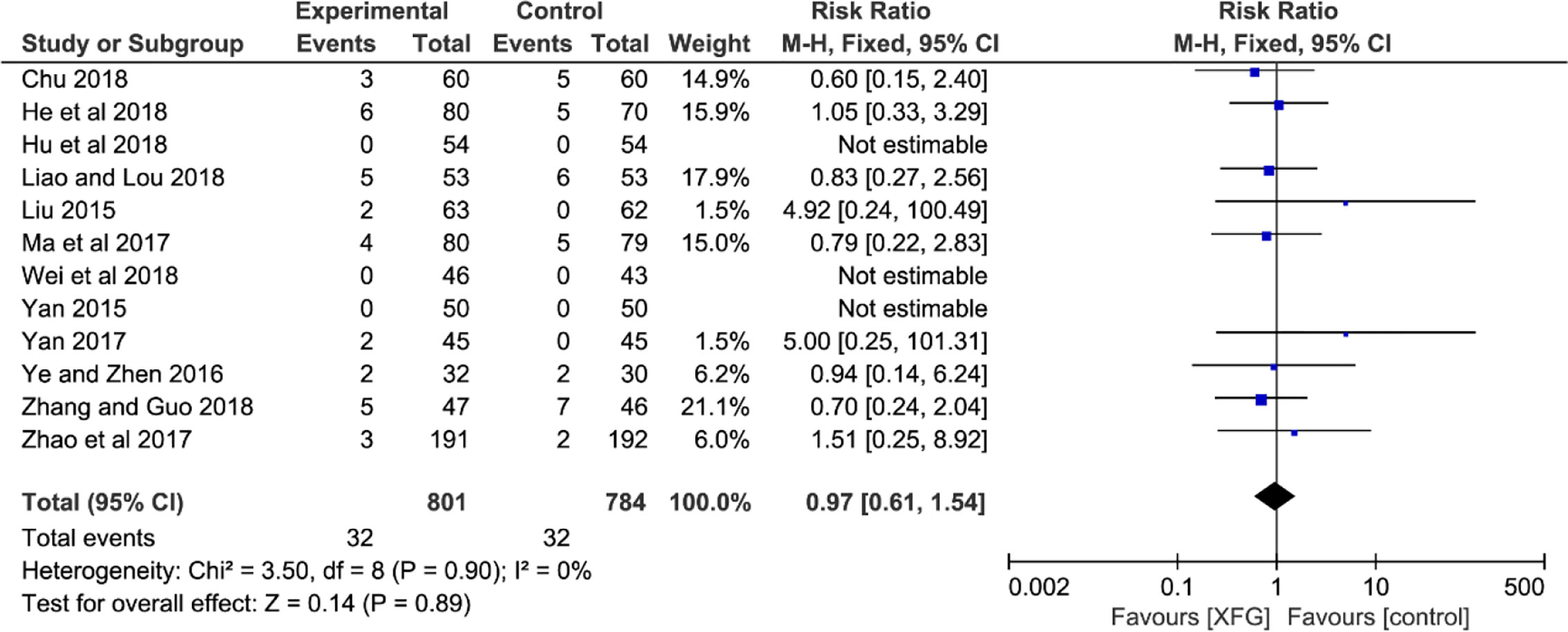
Adverse events were mentioned in fifteen trials (Liu, 2015; Xu et al., 2015; Yan, 2015; Du, 2016; Ye and Zhen, 2016; Ma and Lu, 2017; Yan, 2017; Zhao and Chen, 2017; Chu, 2018; He and Yang, 2018; Hu et al., 2018; Liao and Lou, 2018; Wei and Li, 2018; Zhang J. Q. et al., 2018; Zhang and Guo, 2018). However, the remaining six trials did not record any adverse reaction information. Only three of the fifteen trials reported that there were no serious adverse reactions (Xu et al., 2015; Du, 2016; Zhang J. Q. et al., 2018). One of the trials reported mild gastrointestinal discomfort without statistics. Therefore, twelve studies detailed adequate information on adverse events (XFG: 32/801, 4.0%; CM: 32/784, 4.1%), and three reported no adverse events during trials (Yan, 2015; Hu et al., 2018; Wei and Li, 2018). The most frequent adverse reactions mentioned in these studies were gastrointestinal discomfort symptoms (nausea, diarrhea, and emesis) and dizziness, headache, and rash. In addition, one trial (Zhang and Guo, 2018) reported 3 cases with electrolyte disturbance (XFG: 1/47, 2.1%; CM: 2/46, 4.3%). In general, all the adverse reactions were mild, and no serious adverse reactions were reported. The pooled result indicated no statistical difference between the two groups, suggesting that adding XFG did not increase the adverse events (RR =0.97, 95% CI= 0.61-1.54, and P= 0.89), as shown in Figure 9.
Subgroup Analysis
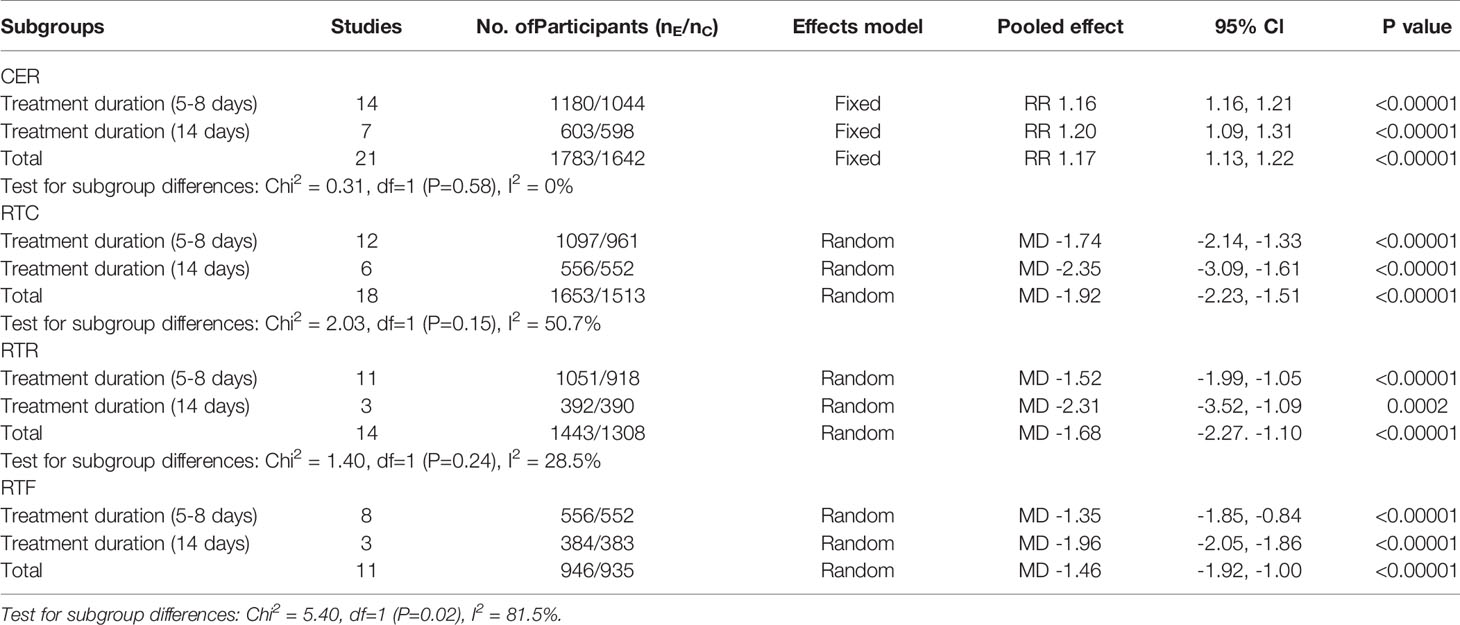
Subgroup analyses of the treatment durations were introduced to determine the subgroup differences according to the primary outcomes, including the CER, RTC, RTR, and RTF. For the CER, RTC, and RTR, the results across the various subgroups were highly consistent, and the benefits of XFG were significant. However, a significant difference was observed between the “5-8 days” and “14 days” groups in terms of the RTF. Generally, the “14 day” group showed a lower effect size value and a narrower confidence interval than that of the “5-8 days” group, which indicated that “14 days” of treatment was more likely to bring the maximum therapeutic effect to the ALRI patients in terms of improving the RTF. Despite the significant difference favoring the 14-day group, it is unreasonable to conclude that 14 days of XFG was more effective than 5-8 days because the number of studies between subgroups varied widely, and there are some differences between bronchitis and pneumonia. In terms of the dose, most studies followed the packaging instructions for the drug provided by the two pharmaceutical companies (ages: 0-1 (2g); 1-4 (3g); 5-8 (6g), tid, PO). Therefore, considering the large age difference between the studies and the ALRI severity difference, it is reasonable to adjust the medication according to different conditions, as shown in Table 2.
Sensitivity Analysis
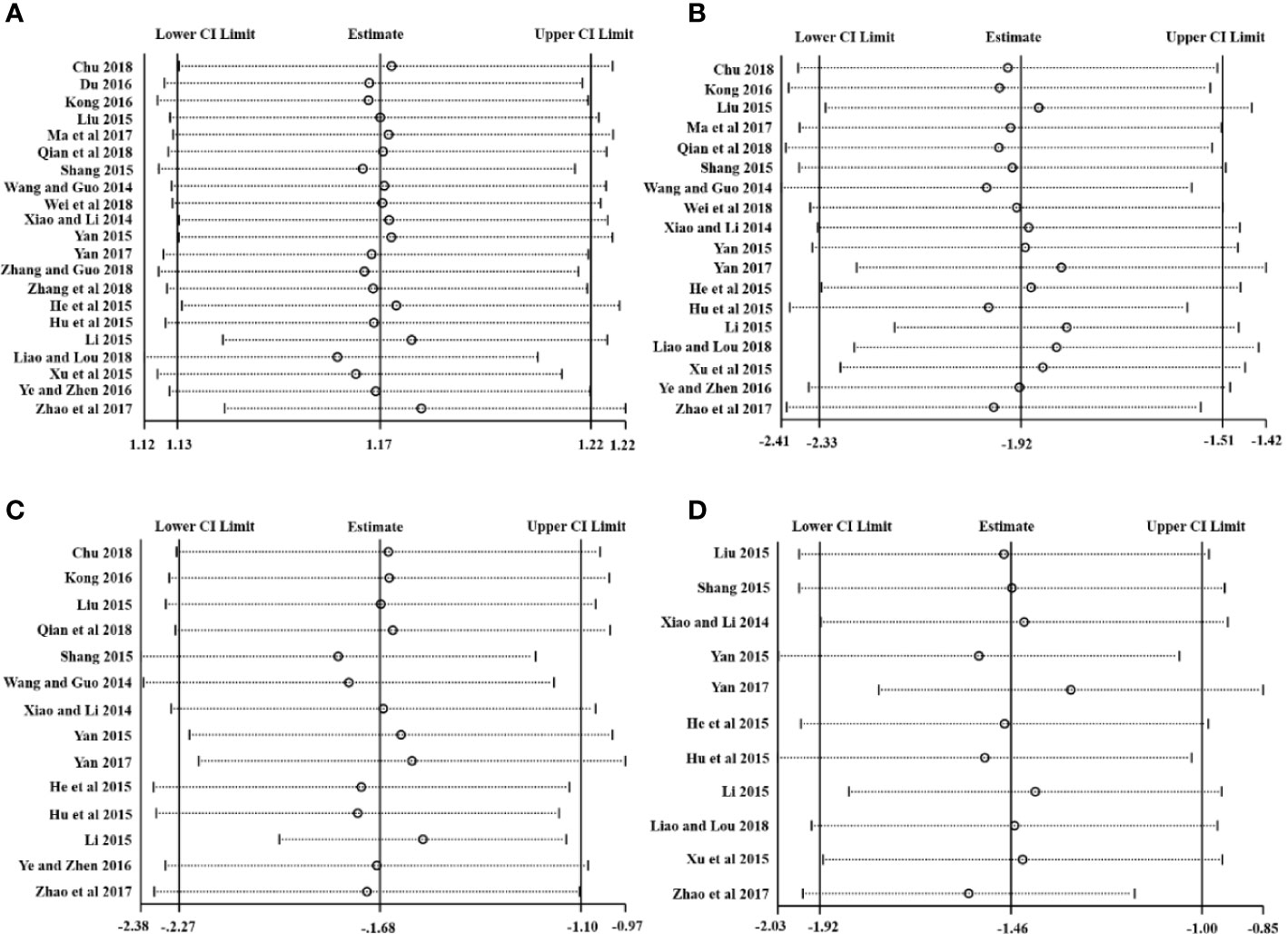
Stata 15.1 was employed for sensitivity analysis of the primary outcomes including the CEF, RTC, RTR, and RTF. The centralized small circles in the sensitivity analysis plots suggested that the results were not reversed by removing any study for each outcome, indicating that the results were reliable, as shown in Figure 10.
Publication Bias
We used Stata15.1 software to detect possible publication bias according to primary outcomes, and the trim and filling method was used to cope with the striking publication bias if the P<0.05. The result of Egger’s test suggested that there was no publication bias in terms of the RTC (P >| t |=0.542, 95% CI= −2.6 to 4.79) and RTF (P >| t |=0.48, 95% CI= -4.17 to 8.21). However, significant publication bias was observed in the CER (P >| t |=0.0001, 95% CI= 1.61-2.84) and the RTR (P >| t |=0.04, 95% CI= 0.35 to 12.56). The trim and filling method was then used to evaluate the reliability of results affected by significant publication bias. After two iterations, no potential missing study was filled. The result suggested that the MD and 95% CI of the RTR (MD = -1.68; 95% CI=-2.26 to -1.0; P<0.0001) vs. (MD = -1.68; 95% CI=-2.26 to -1.0; P<0.0001) before and after applying the trim and filling method was consistent, indicating that the result was stable without a flip. For the CER, after four iterations, nine studies marked with squares were filled in. The results showed that the RR and 95% CI (RR=1.17, 95% CI =1.13-1.22, P< 0.00001) versus (RR = 1.13; 95% CI=1.09-1.17; P<0.0001) before and after applying the trim and filling method was consistent. Generally, the publication bias induced by CER and RTR had no significant effect on the pooled results, as shown in Figure 11.
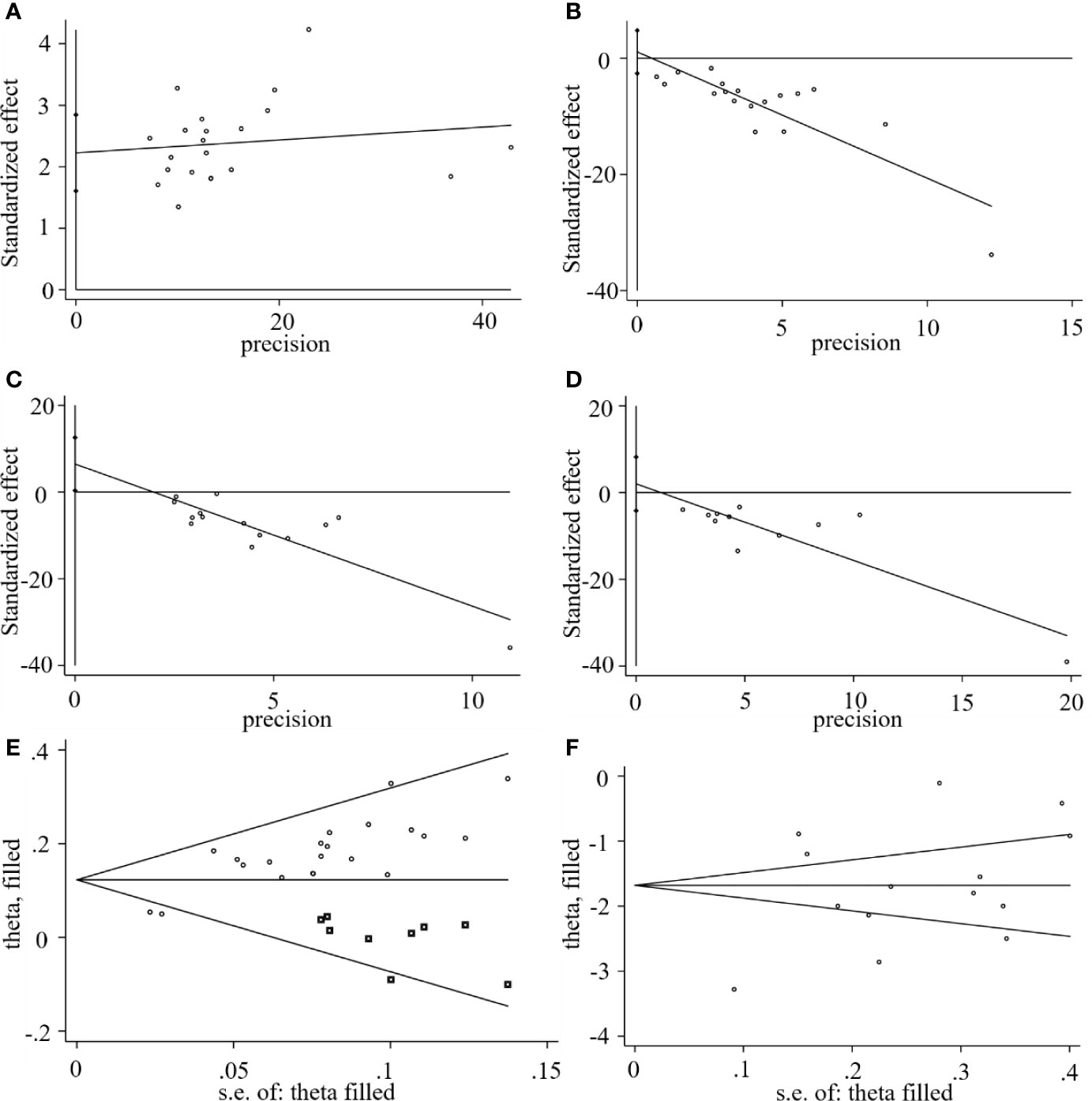
Figure 11 Egger’s publication bias plots of the (A) CER, (B) RCT, (C) RTR, (D) RTF, and filled funnel plots of the (E) CER, and (F) RTR.
Discussion
This meta-analysis assessed the evidence from 21 RCTs. A total of 3425 ALRI patients were randomly assigned to receive either an XFG addition with CM or CM alone. The findings of this study suggested that XFG supplementation of the ALRI treatment was effective and safe, and it was associated with significant improvements in the RTC, RTR, RTF, RTIL, CD4, CD8, IL-6, TNF-α, and CRP. Both the symptoms and immunity-related outcomes of the patients were significantly improved when CM was complemented with XFG, which indirectly reduced the use of antibiotics. However, substantial heterogeneity was present in the three primary outcomes (RTC, RTR, and RTF). According to the sensitivity analysis, two studies (Li, 2015; Yan, 2017) significantly increased the overall heterogeneity. A possible explanation for this finding is that Li, 2015 had a larger sample size compared with the other 20 included studies, while Yan, 2017 had a small sample size. In addition, differences in age and the severity of the disease appear to be another important source of heterogeneity.
XFG is a patented preparation. Two Chinese pharmaceutical companies, Tian-sheng Pharmaceutical Group Co., LTD, and Chang-chun People Pharmaceutical Group Co., LTD, produce XFG according to the guidelines outlined in the latest edition of the Chinese pharmacopoeia, which consists of Ginseng radix et rhizoma, Poria, Atractylodis macrocephalae rhizoma, Citri reticulatae pericarpium, Rhei radix et rhizome, Lycii cortex, Glehniae radix, Glycyrrhizae radix et rhizome, Artemisiae annuae herba, Ophiopogonis radix, Cinnamomic ramulus, Zingiberis rhizoma, Aconiti radix, Trichosanthis fructus, Farfarae flos, Asteris radix et rhizome, Mori cortex, Astragali radix, Lycii fructu, Arisaema cum Bile, Galli gigerii endothelium corneum, Trionycis carapax, and saccharose. The procedure for making this preparation is as follows. First, ten herbs are mixed, including Astragali radix, Lycii cortex, Glehniae radix, Glehniae radix, Glycyrrhizae radix et rhizome, Artemisiae annuae herba, Cinnamomic ramulus, Trichosanthis fructus, Asteris radix et rhizome, and Mori cortex. They are decocted with water twice, for 2 hours per decoction, and the decoctions are combined, filtered, and concentrated to form a thin extract with a relative density of 1.26-1.30 (80°C). Second, the other twelve ingredients are pulverized into a powder. Third, in addition to the above thin extract, the powder and saccharose are mixed, dried, and made to reach 1000g. This preparation method for XFG is stable and repeatable (Figure 12).
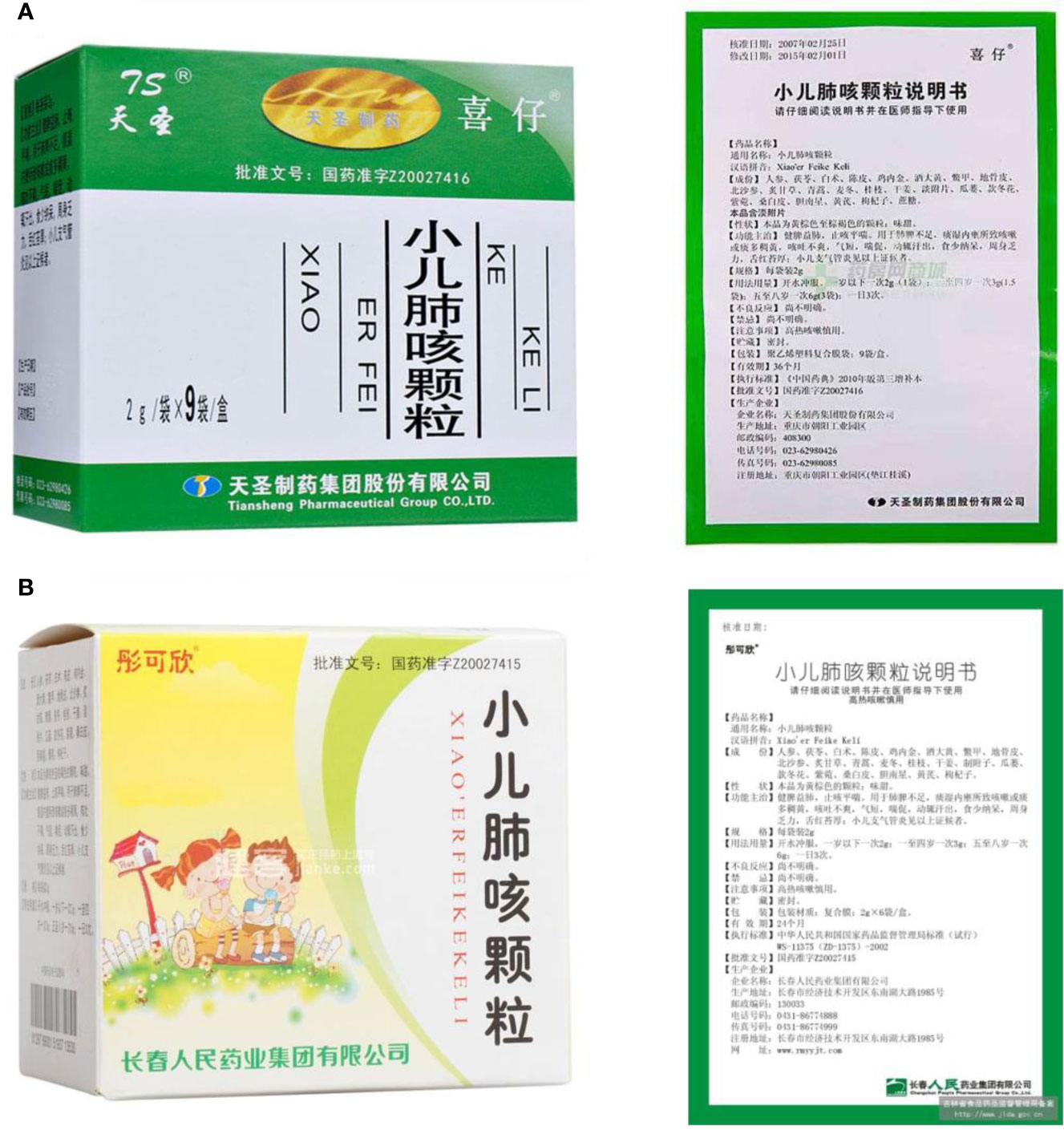
Figure 12 XFG and its instructions from (A) Tian-sheng Pharmaceutical Group Co., LTD, Chong-qing, China, and (B) Chang-chun People Pharmaceutical Group Co., LTD, Chang-chun, China.
The primary pharmacological effects of the 22 ingredients include lung injury protection, enhancing immune function, anti-microbial, anti-inflammatory, and anti-asthmatic activities, neuroprotection, and antitussive and expectorant effects. The majority of the 22 ingredients have strong anti-microbial and anti-inflammatory properties, which significantly enhanced resistance to pathogenic microorganisms in ALRI patients. Seven herbs, namely, Citri reticulatae pericarpium, Glycyrrhizae radix et rhizome, Glycyrrhizae radix et rhizome, Trichosanthis fructus, Farfarae flos, Trichosanthis fructus, and Mori cortex, are widely used for antitussive, expectorant, and anti-asthmatic effects by TCM physicians for thousands of years. Modern pharmacology has also confirmed these effects on respiratory disease (Ruan and Yue, 2004; Shi et al., 2009; Sun et al., 2012; Wang et al., 2014; Kim et al., 2017; Singh et al., 2018; Fu et al., 2019; Li et al., 2019). Seven ingredients (Ginseng radix et rhizoma, Poria, Atractylodis macrocephalae rhizoma, Ophiopogonis radix, Astragali radix, Astragali radix, and Trionycis carapax) showed biological activity in immune enhancement, especially Ginseng radix et rhizome, which significantly increased the viability and proliferation of spleen cells by promoting proliferation from CD19(+) B cells and increased the surface expression of CD25, CD69, and interleukin-2, which significantly enhanced the immune system of ALRI patients. Two herbs (Lycii cortex and Cinnamomic ramulus) showed striking neuroprotection effects, with kukoamine A (Zhang et al., 2016) from Cortex lycii reducing the malondialdehyde level and increasing the glutathione level, superoxide dismutase, catalase (CAT) activities, and the expression of BDNF. Three herbs significantly improved acute lung injury in mice (Asteris radix et rhizome, Astragali radix, and Lycii fructus) due to mechanisms that enhanced the activation of Nrf2, inducing human cathelicidin antimicrobial peptide LL-37 in respiratory epithelial cells, promoting the repair of vascular endothelial cells, and inhibiting NF-κB signaling pathway. On the whole, the TCM efficacy of XFG was consistent with its pharmacological effects, and XFG plays a therapeutic role through coordinated cooperation among the 22 ingredients. Therefore, based on TCM theory, through a multiple-target pathway, multi-level and holistic therapy, XFG was found to be a reliably effective and safe complementary therapy in ALRI treatment, as shown in Tables 3, 4.
Primary Chemical Compositions
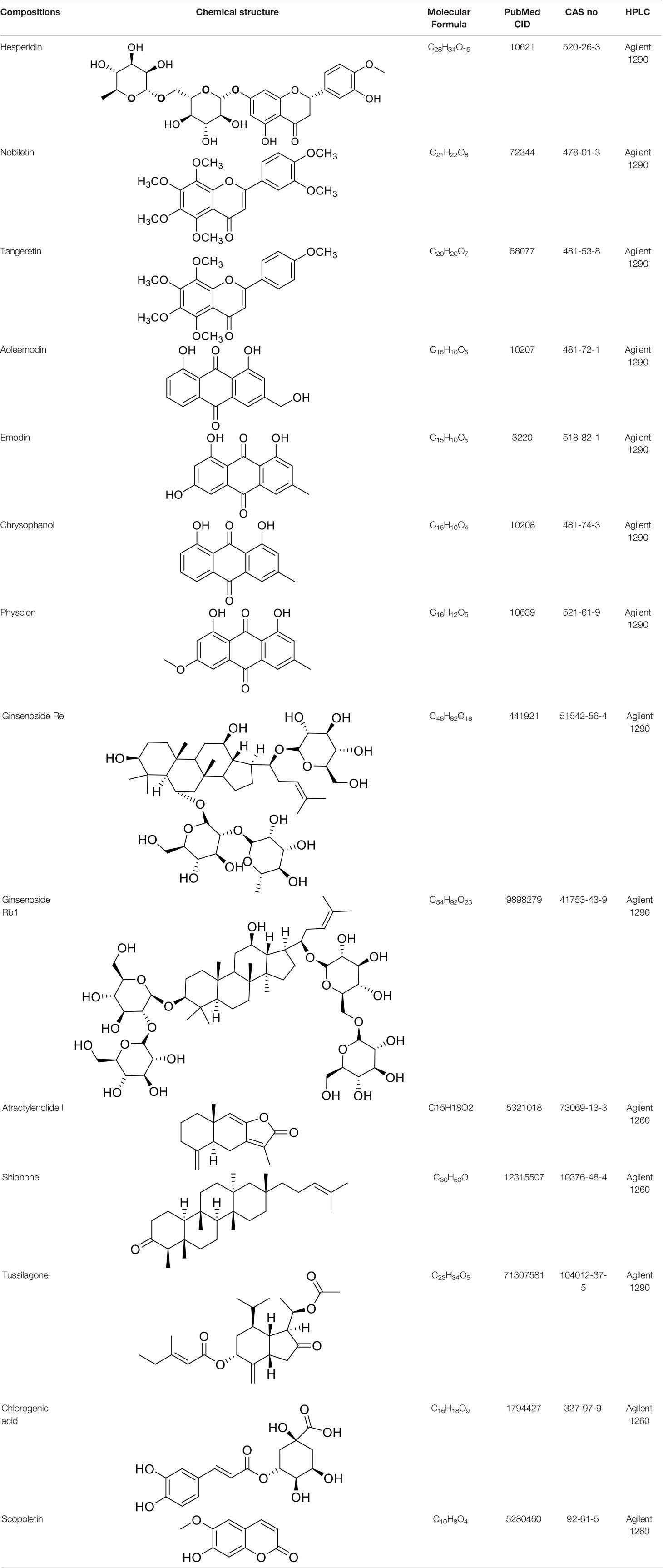
HPLC analysis was performed to determine the primary chemical compositions of XFG. We determined a total of 17 chemical compounds (hesperidin, nobiletin, tangeretin, aoleemodin, emodin, rhein, chrysophanol, physcion, ginsenoside Rg1, ginsenoside Re, ginsenoside Rb1, atractylenolide I, atractylenolide III, shionone, chlorogenic acid, scopoletin, and tussilagone). After a comparison with the respective reference samples, fourteen potential active compounds were detected, as shown in Table 5.
Implications for Further Study
This review has evaluated the curative effects of XFG, but most of the included studies used CER, RTC, RTR, and RTF as the primary outcomes. A few studies reported on the immune cells (CD4, CD8, and WBC) and cytokine levels (TNF-α, INF-γ, IL-4, IL-6, IL-8, IL-10, and CRP, etc.), (Li, 2015; Du, 2016; Ma and Lu, 2017; Zhao and Chen, 2017; Hu et al., 2018; Zhang and Guo, 2018; Zhang J. Q. et al., 2018). This indicates that the therapeutic mechanisms of XFG are associated with immune enhancement and inflammation reduction. However, this explanation is far from conclusive, because these cellular and molecular parameters were only reported in three studies. The therapeutic effects of XFG are multiple-target, multi-level, and holistic, and therefore, at the cellular and molecular levels, the mechanisms of action of XFG warrant further investigation. Although the evidence analyzed in this study indicated that XFG is an effective and safe adjuvant therapy in treating ALRI, we found significant heterogeneity and publication bias. Therefore, high-quality, multicenter, double-blind RCTs are needed to provide reliable evidence that will guide the rational use of XFG in clinical settings.
Limitations
Several limitations should be highlighted in our meta-analysis. First, we searched only the primary English and Chinese databases. Therefore, some studies that meet our inclusion criteria in other languages or databases may have been excluded. All the included trials declared randomizations, but only eleven studies described a specific randomization method. Blinded assessments were not detailed in all the included documents, which may have exerted a potential impact on the objectivity of the ALRI outcomes. Second, the inclusion criteria of these studies had small sample sizes with low-quality designs, which may have led them to overvalue the benefits of XFG. Additionally, there may be a degree of selective reporting bias because the majority of studies were not officially registered. Third, only a few eligible studies reported the HS, RTIL, cellular, and cytokine outcomes. Fourth, six papers did not mention any information on adverse reactions. Thus, insights as to the safety of using XFG to treat ALRI are limited. Fifth, although the RTC and RTR were widely used to evaluate the curative effect of XFG for ALRI in hospitalized patients, this was still somewhat subjective. Although these limitations may undermine the quality of the evidence, the trials are nevertheless comparable, and the documents were selected using strict inclusion criteria. Since the patients of the selected studies were primarily from China, the conclusion of this meta-analysis might not apply to other ethnic groups. Therefore, large sample trials of a higher-quality, with well-designed considerations of different ethnic groups should be conducted in the future to provide more reliable evidence regarding the efficacy and safety of using XFG to treat ALRI.
Conclusion
The findings of this meta-analysis support the use of XFG in the treatment of ALRI. However, the results should be treated with caution due to the significant heterogeneity and publication bias of the studies examined. Therefore, further well-designed and high-quality RCTs are needed to interrogate the efficacy and safety of XFG.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Author Contributions
QY and LL conceived this review and completed the manuscript. DL performed the literature searches electronically and manually. DY and LC performed the study selection and data extraction and assessed the risk of bias. Y-pL and H-pC critically revised the paper. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the National Natural Science Foundation of China (No.: 81973436) and Research Premotion Project of Chengdu University of TCM (No: CXTD2018011).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.496348/full#supplementary-material
References
Ahn, C. B., Je, J. Y. (2012). Anti-inflammatory activity of the oriental herb medicine, Arisaema cum Bile, in LPS-induced PMA-differentiated THP-1 cells. Immunopharmacol. Immunotoxicol. 34, 379–384. doi: 10.3109/08923973.2011.608683
Albano, M., Crulhas, B. P., Alves, F. C. B., Pereira, A. F. M., Andrade, B. F. M. T., Barbosa, L. N., et al. (2019). Antibacterial and anti-biofilm activities of cinnamaldehyde against S. epidermidis. Microb. Pathog. 126, 231–238. doi: 10.1016/j.micpath.2018.11.009
Arokiyaraj, S., Vincent, S., Saravanan, M., Lee, Y., Oh, Y. K., Kim, K. H. (2017). Green synthesis of silver nanoparticles using Rheum palmatum root extract and their antibacterial activity against Staphylococcus aureus and Pseudomonas aeruginosa. Artif. Cells Nanomed. Biotechnol. 45, 372–379. doi: 10.3109/21691401.2016.1160403
Bose, S., Kim, H. (2013). Evaluation of In Vitro Anti-Inflammatory Activities and Protective Effect of Fermented Preparations of Rhizoma Atractylodis Macrocephalae on Intestinal Barrier Function against Lipopolysaccharide Insult. Evid.-Based Comple. Alt. 2013, 1–16. doi: 10.1155/2013/363076
Bryce, J., Boschi-Pinto, C., Shibuya, K., Black, R. E. (2005). WHO estimates of the causes of death in children. Lancet 365, 1147–1152. doi: 10.1016/S0140-6736(05)71877-8
Casquete, R., Castro, S., Martín, A., Ruiz-Moyano Seco de Herrera, J., Saraiva, R., Córdoba, M., et al. (2015). Evaluation of the effect of high pressure on total phenolic content, antioxidant and antimicrobial activity of citrus peels. Innovative Food Sci. Emerg. Technol. 31, 37–44. doi: 10.1016/j.ifset.2015.07.005
Charlie-Silva, I., Fraceto, L. F. (2018). Progress in nano-drug delivery of artemisinin and its derivatives: towards to use in immunomodulatory approaches. Artif. Cells Nanomed. Biotechnol. 16, 1–10. doi: 10.1080/21691401.2018.1505739
Chen, J. N., Shan, G. S., Zhao, Q. M., Jia, T. Z., Gao, H. (2017). Comparison on bile acids and clearing heat effects among Arisaema cum Bile processed with different biles. Drugs Clin. 32, 567–571. doi: 10.7501/j.issn.1674-5515.2017.04.004
Chen, L., Li, W., Qi, D., Wang, D. (2018). Lycium barbarum polysaccharide protects against LPS-induced ARDS by inhibiting apoptosis, oxidative stress, and inflammation in pulmonary endothelial cells. Free Radic. Res. 52, 480–490. doi: 10.1080/10715762.2018.1447105
Chen, Y., Dong, J., Liu, J., Xu, W., Wei, Z., Li, Y., et al. (2019). Network Pharmacology-Based Investigation of Protective Mechanism of Aster tataricus on Lipopolysaccharide-Induced Acute Lung Injury. Int. J. Mol. Sci. 20, 543–558. doi: 10.3390/ijms20030543
Cheng, Y., Mai, J. Y., Hou, T. L., Ping, J., Chen, J. J. (2016). Antiviral activities of atractylon from Atractylodis Rhizoma. Mol. Med. Rep. 14, 3704–3710. doi: 10.3892/mmr.2016.5713
Cheng, X., Liao, M., Diao, X., Sun, Y., Zhang, L. (2018). Screening and identification of metabolites of two kinds of main active ingredients and hepatotoxic pyrrolizidine alkaloids in rat after lavage Farfarae Flos extract by UHPLC-Q-TOF-MS mass spectrometry. Biomed. Chromatogr. 32. doi: 10.1002/bmc.4047
Chu, L. F. (2018). Clinical effect of Xiaoer-Feike granules in convalescence of coughing due to wind-heat. Shenzhen J. Integrated Tradit. Chin. Western Med. 28, 25–26. doi: 10.16458/j.cnki.1007-0893.2018.08.011
Du, J. (2016). Chinese medicine pediatric pulmonary cough particles in adjuvant therapy for acute bronchitis in children. Chin. Arch. Tradit. Chin. Med. 34, 2076–2078. doi: 10.13193/j.issn.1673-7717.2016.11.042
Efferth, T. (2018). Beyond malaria: The inhibition of viruses by artemisinin-type compounds. Biotechnol. Adv. 36, 1730–1737. doi: 10.1016/j.biotechadv.2018.01.001
Emam, A. A., Shehab, M. M. M., Allah, M., Elkoumi, M. A., Abdelaal, N. M., Mosabah, A. A. A., et al. (2019). Interleukin-4 -590C/T gene polymorphism in Egyptian children with acute lower respiratory infection: A multicenter study. Pediatr. Pulmonol. 54, 297–302. doi: 10.1002/ppul.24235
Friedman, M. (2017). Chemistry, Antimicrobial Mechanisms, and Antibiotic Activities of Cinnamaldehyde against Pathogenic Bacteria in Animal Feeds and Human Foods. J. Agric. Food. Chem. 65 (48), 10406–10423. doi: 10.1021/acs.jafc.7b04344
Fu, M., Zou, B., An, K., Yu, Y., Tang, D., Wu, J., et al. (2019). Anti-asthmatic activity of alkaloid compounds from Pericarpium Citri Reticulatae (Citrus reticulata ‘Chachi’). Food. Funct. 10, 903–911. doi: 10.1039/c8fo01753k
Giner, E. M., Máñez, S., Recio, M. C., Giner, R. M., Cerdá-Nicolás, M., Ríos, J. L. (2000). In vivo studies on the anti-inflammatory activity of pachymic and dehydrotumulosic acids. Planta. Med. 66, 221–227. doi: 10.1055/s-2000-8563
Guo, L., Sun, Y. L., Wang, A. H., Xu, C. E., Zhang, M. Y. (2012). Effect of polysaccharides extract of rhizoma atractylodis macrocephalae on thymus, spleen and cardiac indexes, caspase-3 activity ratio, Smac/DIABLO and HtrA2/Omi protein and mRNA expression levels in aged rats. Mol. Biol. Rep. 39, 9285–9290. doi: 10.1007/s11033-012-1677-x
Guo, Y., Fu, R., Qian, Y., Zhou, Z., Liu, H., Qi, J., et al. (2019). Comprehensive screening and identification of natural inducible nitric oxide synthase inhibitors from Radix Ophiopogonis by off-line multi-hyphenated analyses. J. Chromatogr. A. 1592, 55–63. doi: 10.1016/j.chroma.2019.01.029
Ha, S. K., Park, H. Y., Eom, H., Kim, Y., Choi, I. (2012). Narirutin fraction from citrus peels attenuates LPS-stimulated inflammatory response through inhibition of NF-kappa B and MAPKs activation. Food Chem. Toxicol. 50, 3498–3504. doi: 10.1016/j.fct.2012.07.007
Hadjipavlou-Litina, D., Garnelis, T., Athanassopoulos, C. M., Papaioannou, D. (2009). Kukoamine A analogs with lipoxygenase inhibitory activity. J. Enzyme. Inhib. Med. Chem. 24, 1188–1193. doi: 10.1080/14756360902779193
Hagenlocher, Y., Feilhauer, K., Schäffer, M., Bischoff, S. C., Lorentz, A. (2016). Citrus peel polymethoxyflavones nobiletin and tangeretin suppress LPS- and IgE-mediated activation of human intestinal mast cells. Eur. J. Nutr. 56, 1609–1620. doi: 10.1007/s00394-016-1207-z
Han, S. H., Lee, H. H., Lee, I. S., Moon, Y. H., Woo, E. R. (2002). A new phenolic amide from Lycium chinense Miller. Arch. Pharm. Res. 25, 433–437. doi: 10.1007/BF02976596
He, R., Yang, Z. Y. (2018). Observation on the efficacy of Xiaoer-Feike particles as adjuvant treatment for bronchopneumonia in children. J. Qiqihar Med. Univ. 39, 1126–1127. doi: 10.3969/j.issn.1002-1256.2018.10.004
Hu, X. L., Niu, Y. X., Zhang, Q., Tian, X., Gao, L. Y., Guo, L. P., et al. (2015). Neuroprotective effects of Kukoamine B against hydrogen peroxide-induced apoptosis and potential mechanisms in SH-SY5Y cells. Environ. Toxicol. Pharmacol. 40, 230–240. doi: 10.1016/j.etap.2015.06.017
Hu, B., Guo, Q., Wang, X. W. (2018). Clinical study on Xiaoer-Feike granules combined with terbutaline in treatment of acute bronchial pneumonia in children. Drugs Clin. 33, 2576–2580. doi: 10.7501/j.issn.1674-5515.2018.10.023
Huang, X. Z., Yao, D. J. (1984). Trichosanthis injection is mainly used to treat asthmatic bronchitis and pulmonary heart disease asthma in 40 cases. Beijing J. Tradit. Chin. Med. 25, 22–22.
Huang, G. J., Deng, J. S., Liao, J. C., Hou, W.-C., Wang, S.-Y., Sung, P.-J., et al. (2012). Inducible nitric oxide synthase and cyclooxygenase-2 participate in anti-inflammatory activity of imperatorin from Glehnia littoralis. J. Agric. Food. Chem. 60, 1673–1681. doi: 10.1021/jf204297e
Jiang, W. J., Yin, F., Zhou, W. F. (2016). Clinical significance of different virus load of human bocavirus in patients with lower respiratory tract infection. Sci. Rep. 6. doi: 10.1038/srep20246
Jung, J. S., Shin, J. A., Park, E. M., Lee, J. E., Kang, Y. S., Min, S. W., et al. (2010). Anti-inflammatory mechanism of ginsenoside Rh1 in lipopolysaccharide-stimulated microglia: critical role of the protein kinase A pathway and hemeoxygenase-1 expression. J. Neurochem. 115, 1668–1680. doi: 10.1111/j.1471-4159.2010.07075.x
Keser, S., Keser, F., Karatepe, M., Kaygili, O., Tekin, S., Turkoglu, I., et al. (2019). Bioactive contents, In vitro antiradical, antimicrobial and cytotoxic properties of rhubarb (Rheum ribes L.) extracts. Nat. Prod. Res. 19, 1–5. doi: 10.1080/14786419.2018.1560294
Khiveh, A., Hashempur, M. H., Shakiba, M., Lotfi, M. H., Shakeri, A., Kazemeini, S., et al. (2017). Effects of rhubarb (Rheum ribes L.) syrup on dysenteric diarrhea in children: a randomized, double-blind, placebo-controlled trial. J. Integr. Med. 15, 365–372. doi: 10.1016/S2095-4964(17)60344-3
Kim, Y. R., Yang, C. S. (2018). Protective roles of ginseng against bacterial infection. Microb. Cell. 5, 472–481. doi: 10.15698/mic2018.11.654
Kim, H. J., Lee, H. J., Jeong, S. J., Lee, H. J., Kim, S. H., Park, E. J. (2011). Cortex Mori radicis extract exerts antiasthmatic effects via enhancement of CD4(+) CD25(+) Foxp3(+) regulatory T cells and inhibition of Th2 cytokines in a mouse asthma model. J. Ethnopharmacol. 138, 40–46. doi: 10.1016/j.jep.2011.08.021
Kim, S. H., Hong, J. H., Lee, J. E., Lee, Y. C. (2017). 18β-Glycyrrhetinic acid, the major bioactive component of glycyrrhizae radix, attenuates airway inflammation by modulating Th2 cytokines, GATA-3, STAT6, and Foxp3 transcription factors in an asthmatic mouse model. Environ. Toxicol. Pharmacol. 52, 99–113. doi: 10.1016/j.etap.2017.03.011
Ko, E. J., Joo, H. G. (2010). Stimulatory Eeffets of Ginsan on the proliferation and Viability of Mouse Spleen Cells. Korean J. Physiol. Pharmacol. 14, 133–137. doi: 10.4196/kjpp.2010.14.3.133
Kong, J. (2016). The curative effect of Xiaoer-Feike granules on acute children bronchitis. J. Today Health 15, 45–45.
Lee, D. G., Park, Y., Kim, M. R., Jung, H. J., Seu, Y. B., Hahm, K.-S., et al. (2004). Anti-fungal effects of phenolic amides isolated from the root bark of Lycium chinens. Biotechnol. Lett. 26, 1125–1130. doi: 10.1023/B:BILE.0000035483.85790.f7
Lee, J. H., Kim, Y. G., Choi, P., Ham, J., Park, J. G., Lee, J. (2018). Antibiofilm and antivirulence activities of 6-gingerol and 6-shogaol against candida albicans due to hyphal inhibition. Front. Cell. Infect. Microbiol. 8, 299. doi: 10.3389/fcimb.2018.00299
Li, W. X., Li, F. Y., Li, D. (2008). Comparative study on the influence of aqueous extracts from different processed endothelium corneum gigeriae gallis ongastric juice and pepsinum. Hunan J. Tradit. Chin. Med. 24, 100–101. doi: 10.16808/j.cnki.issn1003-7705.2008.02.065
Li, R., Sakwiwatkul, K., Yutao, L., Hu, S. (2009). Enhancement of the immune responses to vaccination against foot-and-mouth disease in mice by oral administration of an extract made from Rhizoma Atractylodis Macrocephalae (RAM). Vaccine 27, 2094–2098. doi: 10.1016/j.vaccine.2009.02.002
Li, Y., Meng, T., Hao, N., Tao, H., Zou, S., Li, M., et al. (2017). Immune regulation mechanism of Astragaloside IV on RAW264.7 cells through activating the NF-κB/MAPK signaling pathway. Int. Immunopharmacol. 49, 38–49. doi: 10.1016/j.intimp.2017.05.017
Li, J., Gao, L., Gao, Y. (2018a). Exploration in targets action of antitussive and expectorant bioactive components from Farfarae Flos based on network pharmacology. Chin. Tradit. Herbal Drugs 49, 179–187. doi: 10.7501/j.issn.0253-2670.2018.01.025
Li, J., Zhang, Z. Z., Lei, Z. H., Qin, X. M., Li, Z. Y. (2018b). NMR based metabolomic comparison of the antitussive and expectorant effect of Farfarae Flos collected at different stages. J. Pharm. Biomed. Anal. 150, 377–385. doi: 10.1016/j.jpba.2017.12.028
Li, Z., Liu, Z., Uddandrao, V. V. S., Ponnusamy, P., Balakrishnan, S., Brahmanaidu, P., et al. (2019). Asthma-alleviating potential of 6-gingerol: effect on cytokines, related mRNA and c-Myc, and NFAT1 expression in ovalbumin-sensitized asthma in rats. J. Environ. Pathol. Toxicol. Oncol. 38, 41–50. doi: 10.1615/JEnvironPatholToxicolOncol.2018027172
Li, J. (2015). Clinical study on Xiaoer-Feike granules combined with western medicine in the treatment of pneumonia with lung spleen qi deficiency syndrome in 300 cases. J. New Chin. Med. 47, 156–158. doi: 10.13457/j.cnki.jncm.2015.12.069
Liao, Z., Lou, L. Y. (2018). Study on the effect of Xiaoer-Feike granules as adjuvant treatment for children with mycoplasma pneumonia and the influence on the APC and IL-1R1. Maternal Child Health Care China. 33, 369–37. doi: 10.7620/zgfybj.j.issn.1001-4411.2018.02.44
Liu, Y. M., Liu, B., Wang, J. F. (2005). Extraction of crude polysaccharide from Radix Glehniae and its effect on Yin deficiency mice. Chin. J. Biol. Med. 26, 224–225. doi: 10.3969/j.issn.1005-1678.2005.04.012
Liu, K. Y., Zhou, B., Liu, H. J. (2011). Study on preparation technique and quality control of Ziwan-Zhike dropping pills. Chin. J. Exp. Tradit. Med. Formulae. 17, 19–22. doi: 10.3969/j.issn.1005-9903.2011.13.006
Liu, D., Su, J., Lin, J., Qian, G., Chen, X., Song, S., et al. (2018). Activation of AMPK-dependent SIRT-1 by astragalus polysaccharide protects against ochratoxin A-induced immune stress in vitro and in vivo. Int. J. Biol. Macromol. 120, 683–692. doi: 10.1016/j.ijbiomac.2018.08.156
Liu, W. G. (2015). Observation of efficacy of pediatric lung cough granules in treatment of children with acute bronchitis. Eval. Anal. drug-use hospitals China. 15, 1173–1175. doi: 10.14009/j.issn.1672-2124.2015.09.016
Lu, X., Tong, W., Wang, S., Li, J., Zheng, J., Fan, X., et al. (2017). Comparison of the chemical consituents and immunomodulatory activity of ophiopogonis radix from two different producing areas. J. Pharm. Biomed. Anal. Feb 5 134, 60–70. doi: 10.1016/j.jpba.2016.11.025
Ma, W. G., Lu, Y. F. (2017). Mechanism and effect of Xiaoer-Feike granules on post-infection cough. Chin. J. Exp. Tradit. Med. Formulae. 23, 204–209. doi: 10.13422/j.cnki.syfjx.2017140204
Park, E. K., Choo, M. K., Han, M. J., Kim, D. H. (2004). Ginsenoside Rh1 possesses antiallergic and anti-inflammatory activities. Int. Arch. Allergy Immunol. 133, 113–120. doi: 10.1159/000076383
Prieto, J. M., Recio, M. C., Giner, R. M., Máñez, S., Giner-Larza, E. M., Ríos, J. L. (2003). Influence of traditional Chinese anti-inflammatory medicinal plants on leukocyte and platelet functions. J. Pharm. Pharmacol. 55, 1275–1282. doi: 10.1211/0022357021620
Qian, X. M., Li, T. (2018). Xiaoer-Feike granules in the treatment of infantile acute bronchitis for 98 Cases. Chin. Med. Modern Distance Educ. China. 16, 44–46. doi: 10.3969/j.issn.1672-2779.2018.02.019
Qin, L., Tan, H. L., Wang, Y. G., Xu, C. Y., Feng, J., Li, M., et al. (2018). Astragalus membranaceus and Salvia miltiorrhiza ameliorate lipopolysaccharide-induced acute lung injury in rats by regulating the Toll-like receptor 4/Nuclear factor-kappa B signaling pathway. Evid.-Based Comple. Alt. 2018:3017571. doi: 10.1155/2018/3017571
Ruan, Y., Yue, X. (2004). Study on the effect of cough and expectoration of decoction of Trichosanthis Fructus. Forum. Tradit. Chin. Med. 19, 48.
Rudan, I., Boschi-Pinto, C., Biloglav, Z., Mulholand, K., Campbell, H. (2008). Epidemiology and etiology of childhood pneumonia. Bull. WHO. 86, 408–416. doi: 10.1097/INF.0b013e3181950942
Shang, Y. L. (2015). The curative effect of Xiaoer-Feike granules on acute children bronchitis. J. Med. Aesthetics Beauty 6, 739–739.
Shi, Q., Liu, Z., Yang, Y., Geng, P., Zhu, Y. Y., Zhang, Q., et al. (2009). Identification of anti-asthmatic compounds in Pericarpium citri reticulatae and evaluation of their synergistic effects. Acta Pharmacol. Sin. 30, 567–575. doi: 10.1038/aps.2009.36
Singh, R. P., Gangadharappa, H. V., Mruthunjaya, K. (2018). Phytosome complexed with chitosan for gingerol delivery in the treatment of respiratory infection: In vitro and in vivo evaluation. Eur. J. Pharm. Sci. 122, 214–229. doi: 10.1016/j.ejps.2018.06.028
Smolarz, H. D., Swatko-Ossor, M., Ginalska, G., Medyńska, E. (2013). Antimycobacterial effect of extract and its components from Rheum rhaponticum. J. AOAC Int. 96, 155–160. doi: 10.5740/jaoacint.12-010
Su, G., Chen, X., Liu, Z., Yang, L. (2015). Oral Astragalus (Huang qi) for preventing frequent episodes of acute respiratory tract infection in children. Cochrane Database Syst. Rev. 12, CD011958. doi: 10.1002/14651858.CD011958
Sui, Z. Y., Wang, A. J., Li, Q. (2015). Effect of crude and honeyed Mori Cortex on serum NO, LP0, IL-4 and IFN- in asthmatic rats. Chin. J. Exp. Tradit. Med. Formulae 21, 95–98. doi: 10.13422/j.cnki.syfjx.2015070095
Sun, J., Meng, B., Zhao, Q. (2012). A general overview of the pharmacological properties of Trichosanthis Kirilowii Maxim. Shandong J. Tradit. Chin. Med. 31, 461–463.
UNICEF/WHO. UNICEF (2006). Pneumonia: the forgotten killer of children. World Health Organization Press. Available at: http://whqlibdoc.who.int/publications/2006/9280640489_eng.pdf.
UNICEF/WHO. UNICEF (2016). Ending child deaths from pneumonia and diarrhoea. World Health Organization Press. Available at: https://weshare.unicef.org/Package/2AMZIFSBQHY#/SearchResult&ALID=2AMZIFSBQHY&VBID=2AMZVNKVZM0Z.
Wang, D., Guan, H. Q. (2010). Research of influence of rat immunologic function by Carapax Trionycis decoction. J. Liaoning Univ. TCM 12, 103–104. doi: 10.13194/j.jlunivtcm.2010.07.105.wangd.068
Wang, Q. Q., Guo, Q. (2014). Clinical effects of pediatric lung cough particles on acute bronchitis in children. Chin. Foreign Med. Res. 12, 28–30. doi: 10.14033/j.cnki.cfmr.2014.33.017
Wang, X. H., Huang, W. M. (2014). Astragalus polysaccharides exert protective effects in newborn rats with bronchopulmonary dysplasia by upregulating the expression of EGFL7 in lung tissue. Int. J. Mol. Med. 34, 1529–1536. doi: 10.3892/ijmm.2014.1951
Wang, Z. Z., Meng, J. J., Xi, Y. J. (2013). Maturation of murine bone marrow dendritic cells induced by acidic Ginseng Polysaccharides. Int. J. Biol. Macromol. 53, 93–100. doi: 10.1016/j.ijbiomac.2012.11.009
Wang, X. L., Hao, J. L., Zhang, G. S. (2014). Study on antitussive, expectorant and antiasthmatic effect of aqueous extracts and chemical split fractions of Mori Cortex. World Sci. Technol./Modernization Tradit. Chin. Med. Mater. Medica. 16, 1951–1956.
Wei, W., Li, T. (2018). Clinical curative effect of Xiaoer-Feike granules combined with ambrocol oral solution on infantile bronchitis. Chin. Pediatr. Integrated Tradit. Western Med. 10, 53–55. doi: 10.3969/j.issn.1674-3865.2018.01.015
Xiao, X. P., Li, X. Y. (2016). Clinical application and analysis of Xiaoer-Feike granules combined with cefotaxime granules for children with acute bronchitis. Nei Mongol J. Tradit. Chin. Med. 17, 89–89. doi: 10.16040/j.cnki.cn15-1101.2016.17.085
Xu, H. M., Hu, A. H., Li, Z., Hu, G. H. (2015). Observation of integrated traditional and western medicine treating 31 cases of child prolonged mycoplasmal pneumonia. J. Pediatr. TCM 11, 25–27.
Xu, W., Zhang, M., Liu, H., Wei, K., He, M., Li, X., et al. (2017). Antiviral activity of aconite alkaloids from Aconitum carmichaelii Debx. Nat. Prod. Res. 22, 1–5. doi: 10.1080/14786419.2017.1416385
Xu, X. P., Wang, W., Li, R. G. (2017). Overview of TCM for the treatment of gastric mucosal injury. J. Pract. Tradit. Chin. Internal Med. 31, 79–82. doi: 10.13729/j.issn.1671-7813.2017.02.31
Yan, H. G. (2015). Effect of pediatric lung cough particles on acute bronchitis in children. Chin. J. Pract. Med. 42, 17–18. doi: 10.3760/cma.j.issn.1674-4756.2015.07.006
Yan, Q. (2017). Curative effect analysis of pediatric pulmonary cough particles on children with acute bronchitis. Clin. Res. Pract. 8, 107–108. doi: 10.19347/j.cnki.2096-1413.201708056
Yang, B., Xiao, Y. Q., Liang, R. X. (2008). Studie son expectorant compounds in volatile oil from root and rhizome of Aster tataricus. China J. Chin. Mater. Medica. 33, 281–283. doi: 10.3321/j.issn:1001-5302.2008.03.015
Ye, Z. Q., Zhen, J. L. (2016). Efficacy of Xiaoer-Feike granules in the treatment of bacterial pneumonia in children during recovery period. China Pharma. 19, 140–142. doi: 10.3969/j.issn.1008-049X.2016.01.043
Yu, S. J., Tseng, J. (1996). Fu-Ling, a Chinese herbal drug, modulates cytokine secretion by human peripheral blood monocytes. Int. J. Immunopharmacol. 18, 37–44. doi: 10.1016/0192-0561(95)00103-4
Yu, P., Cheng, S., Xiang, J., Yu, B., Zhang, M., Zhang, C., et al. (2015). Expectorant, antitussive, anti-inflammatory activities and compositional analysis of Aster tataricus. J. Ethnopharmacol. 164, 328–333. doi: 10.1016/j.jep.2015.02.036
Zhang, J. Z., Guo, C. Q. (2018). Clinical observation of Xiaoer-Feike granules combined with montelukast sodium chewable tablets for treating children with post-Infectious cough in 47 cases. China Pharma. 27, 67–70. doi: 10.3969/j.issn.1006-4931.2018.04.023
Zhang, D. X., Zhang, Y. J., Gan, Z. W., Ma, X. (2004). Study on anti-fatigue and immunomodulatory effects of extracts from Carapax Trionycis. Chin. J. Public Health Jul. 20, 834–834. doi: 10.11847/zgggws2004-20-07-41
Zhang, J. L., Huang, W. M., Zeng, Q. Y. (2015). Atractylenolide I protect mice from lipopolysaccharide-induced acute lung injury. Eur. J. Pharmacol. 15765, 94–99. doi: 10.1016/j.ejphar.2015.08.022
Zhang, Y., Cheng, Z., Wang, C., Ma, H., Meng, W., Zhao, Q. (2016). Neuroprotective effects of kukoamine A against radiation-induced rat brain injury through inhibition of oxidative stress and neuronal apoptosis. Neurochem. Res. 41, 2549–2558. doi: 10.1007/s11064-016-1967-0
Zhang, H., Zheng, L., Yuan, Z. (2019). Lycium barbarum polysaccharides promoted proliferation and differentiation in osteoblasts. J. Cell. Biochem. 120, 5018–5023. doi: 10.1002/jcb.27777
Zhang, J., Qin, W., Lyu, W., Shen, W., Wang, X., Sun, B. (2014). Inhibitory effect of kukoamine B on lung inflammatory responses in mice with sepsis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 26, 493–497. doi: 10.3760/cma.j.issn.2095-4352.2014.07.010
Zhang, J. Q., He, W. H., Wen, X. (2018). Curative effect comparison of Xiaoer-Feike granules combined with azithromycin with azithromycin. Jilin Med. J. 39, 2095–2097. doi: 10.3969/j.issn.1004-0412.2018.11.040
Zhang, L., Liu, B. H., Wang, C. B. (2018). Pharmaceutical analysis of different antibiotic regimens in the treatment of lower respiratory tract infection. Exp. Ther. Med. 16, 2369–2374. doi: 10.3892/etm.2018.6437
Zhang, X., Li, Y., Cheng, J., Liu, G., Qi, C., Zhou, W., et al. (2014). Immune activities comparison of polysaccharide and polysaccharide-protein complex from Lycium barbarum L. Int. J. Biol. Macromol. 65, 441–445. doi: 10.1016/j.ijbiomac.2014.01.020
Zhao, Y. F., Chen, Y. H. (2017). Clinical observation of Xiaoer-Feike granules combined with ambroxol hydrochloride oral solution for infantile pneumonia. J. New Chin. Med. 49, 98–100. doi: 10.13457/j.cnki.jncm.2017.08.031
Zhao, L., Tan, S., Zhang, H., Liu, P., Tan, Y. Z., Li, J. C., et al. (2018). Astragalus polysaccharides exerts anti-infective activity by inducing human cathelicidin antimicrobial peptide LL-37 in respiratory epithelial cells. Phytother. Res. 32, 1521–1529. doi: 10.1002/ptr.6080
Zheng, F. H., Wei, P., Huo, H. L., Xing, X. F., Chen, F. L., Tan, X. M., et al. (2015). Neuroprotective effect of gui zhi (ramulus cinnamomi) on ma huang- (herb ephedra-) induced toxicity in rats treated with a ma huang-gui zhi herb pair. Evid.-Based Comple. Alt. 2015, 1–9. doi: 10.1155/2015/913461
Keywords: Chinese herbal formula, Xiaoer-Feike granules, acute lower respiratory infection, meta-analysis, randomized controlled trials
Citation: You Q, Li L, Li D, Yang D, Chen L, Chen H-p and Liu Y-p (2020) Meta-Analysis on the Chinese Herbal Formula Xiaoer-Feike Granules as a Complementary Therapy for Children With Acute Lower Respiratory Infections. Front. Pharmacol. 11:496348. doi: 10.3389/fphar.2020.496348
Received: 06 September 2019; Accepted: 24 August 2020;
Published: 22 October 2020.
Edited by:
Pulok Kumar Mukherjee, Institute of Bio-Resources and Sustainable Development (IBSD), IndiaReviewed by:
Ahmed Negida, University of Portsmouth, United KingdomEsraa Ghanem, Al-Azhar University, Egypt
Copyright © 2020 You, Li, Li, Yang, Chen, Chen and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong-ping Chen, Y2hlbi1ob25ncGluZ0AxMjYuY29t; You-ping Liu, eW91cGluZ2xpdUBjZHV0Y20uZWR1LmNu
†These authors have contributed equally to this work
 Qiang You
Qiang You Lan Li
Lan Li Dan Li
Dan Li Dan Yang1
Dan Yang1 Hong-ping Chen
Hong-ping Chen