Abstract
Puerariatuberosa (Roxb. ex Willd.) DC. (Fabaceae), also known as Indian Kudzu (vidari kand), is a perennial herb distributed throughout India and other Asian countries. Traditionally, tuber and leaves of this plant have extensively been reported for nutritional and medicinal properties in Ayurveda as well as in Chinese traditional practices. The objective of the present review is to compile and update the published data on traditional uses, pharmacological potential, and phytochemistry of compounds isolated from the plant Pueraria tuberosa. P. tuberosa extracts and its purified compounds possess multiple activities such as anticancer, anticonvulsant, antidiabetic, antifertility, anti-inflammatory, antioxidant, anti-stress, antiulcerogenic, cardioprotective, hypolipidemic, hepatoprotective, immunomodulatory, nephroprotective, nootropic, neuroprotective, and wound healing. Tuber and leaf extracts of P. tuberosa contain several bioactive constituents such as puerarin, daidzein, genistein, quercetin, irisolidone, biochanin A, biochanin B, isoorientin, and mangiferin, which possess an extensive range of pharmacological activities. The extensive range of pharmacological properties of P. tuberosa provides opportunities for further investigation and presents a new approach for the treatment of ailments. Many phytochemicals have been identified and characterized from P. tuberosa; however, some of them are still unexplored, and there is no supporting data for their activities and exact mechanisms of action. Therefore, further investigations are warranted to unravel the mechanisms of action of individual constituents of this plant.
Introduction
As per the World Health Organization (WHO) estimation, about 65–80% of people all over the world seek herbal therapies to cure primary health conditions (Robinson and Zhang, 2011). Surprisingly, only 15% of the global flora has been assessed for pharmacological potential (De Luca et al., 2012). WHO has published four volumes of the monographs on selected medicinal plants to support the research in the field of herbal medicine (WHO, 2009). In India, Ayurveda, Unani, Siddha, Homeopathy, and Folk medicine are commonly used as traditional alternative medicine practices for treating different ailments. Among the modern civilizations, India has long been known for its rich treasure of medicinal plants, and about more than 7,000 plant remedies have been categorized and documented by the AYUSH system of medicine (National Medicinal Plants Board, Government of India, 2020). One of the medicinally important plants discussed in this review is Pueraria tuberosa (Roxb. ex Willd.) DC. (Fabaceae), also known as Indian Kudzu (vidari kand). It is a rapidly growing large perennial climber with big tuberous roots (Figures 1–4) (Indian Medicinal Plant Database) and is distributed throughout India, Pakistan, and Nepal (Keung, 2002). Lianas of P. tuberosa has also been found to grow at 4,000 feet in the Himalayan mountain series (Pueraria tuberosa—Vikaspedia, 2020). In Ayurveda, it is known as vidari (vidari kand). The tuber of this plant is sweet (Ayurvedic pharmacopoeia of India, 2001) and is widely used in the treatment of fever, menorrhagia, skin diseases, wounds, bronchial asthma, and jaundice. Apart from the traditional uses of this plant as mentioned in ancient literature like Sushruta Samhita (Sanskrit: सुश्रुत संहिता), several studies have been reported on different pharmacological activities of P. tuberosa extracts and its purified compounds, viz., anticancer (Adedapo et al., 2017), anticonvulsant (Basavaraj et al., 2011), antidiabetic (Oza and Kulkarni, 2018a), antifertility (Gupta et al., 2005), anti-inflammatory (Tripathi et al., 2013), antioxidant (Shukla et al., 2018a), anti-stress (Verma et al., 2012), antiulcerogenic (Gindi et al., 2010), cardioprotective (Patel et al., 2018), hypolipidemic (Tanwar et al., 2008), hepatoprotective (Xia et al., 2013), immunomodulatory (Patel et al., 2016), nephroprotective (Shukla et al., 2018b), nootropic (Rao et al., 2008), neuroprotective (Xing et al., 2011), and wound healing activities (Kambhoja and Murthy, 2007). Previously, Maji et al. (2014) broadly highlighted the phytochemical and therapeutic potential of P. tuberosa in various pharmacological activities. However, the information about the doses of plant extracts used and the models implied for the studies (in vitro or in vivo) in different pharmacological activities was missing. In addition, chemical structures of only few phytoconstituents isolated from P. tuberosa have been given. Therefore, this review is aimed to provide an up-to-date summary of the literature on traditional uses, doses, and types of studies used to confirm pharmacological activities and phytochemical constituents isolated from P. tuberosa plant with their chemical structures and IUPAC names.
FIGURE 1
FIGURE 2
FIGURE 3
FIGURE 4
Methodology
Relevant literature for this review on P. tuberosa has been sourced from PubMed, ScienceDirect, Web of Science, PubChem, Google Scholar, SciFinder, and Scopus database. The articles published in English before September 2020 on traditional uses, pharmacology of extracts, and various phytoconstituents isolated from different parts of P. tuberosa were included in this review. The keywords used for retrieving relevant studies were Pueraria tuberosa plant, Indian Kudzu, vidari kand, tuber extract, traditional uses, phytochemical constituents, pharmacological activity, and in silico, in vitro, and in vivo studies.
Data inclusion criteria included (a) published/peer-reviewed scientific manuscripts; (b) ethnopharmacological studies; (c) tuber extracts with different solvents; (d) studies on the mechanism of actions of plant extracts and their phytoconstituents; (e) in silico, in vitro, and in vivo studies. Exclusion criteria included (a) repetitive studies and information not meeting the inclusion criteria; (b) studies performed with extracts of other Pueraria species; (c) opinion to the editors, case studies, abstracts of the conferences, any unpublished data, and reports.
Synonyms (Ayurvedic pharmacopoeia of India, 2001)
Assamese: Bhedeleton, Bhuikumra
Bengali: Bhuinkumra, Bhumikusmanda, Vidari
English: Indian kudzu
Gujrati: Bhoikolu, Bhonykoru, Eagio, Sakharvel, Vidarikanta,
Hindi: विदारीकंद (Vidarikanda), बनकुमड़ा (Bankumara)
Kannada: Gumadi belli, Gumadigida, Nelagumbala Gudde, Nelagumbala, Nelagumbula
Malayalam: Mudakku
Marathi: Bhuikohala, Ghodvel
Oriya: Bhuiankakharu
Punjabi: Siali, Surala
Sanskrit: भूमिकुष्माण्ड (Bhumikusmanda), गजवाजिप्रिया (Gajavajipriya), कन्दपलाश (Kandapalash), स्वादुकन्दा (Svadukanda), विदारी (Vidari), इक्षुगन्धा (Iksu-Gandha).
Tamil: Nilapoosani
Telugu: Darigummadi, Nelagummuda
Scientific Classification (Rawtal et al., 2019)
Kingdom: Plantae
Subkingdom: Trachebionta
Superdivision: Spermatophyta
Division: Magnoliophyta
Subclass: Rosidae
Order: Fabales
Family: Fabaceae
Genus: Pueraria DC.
Species: Pueraria tuberosa
Traditional Uses
In Ayurveda, vidari kand (Pueraria tuberosa) has been described as a plant having good nutritional value. Besides, the plant also possesses aphrodisiac, diuretic, galactagogue (Kirtikar and Basu, 1935), energizing (Maji et al., 2014), and spermatogenic (Chauhan et al., 2013) properties. It has been prescribed for treatment for all three doshas (i.e., for the complications of three different energies, viz., Vata, Kapha, and Pitta) of human body (Ayurvedic pharmacopoeia of India, 1999; Dalal et al., 2013). The powdered form of tuber is primarily used in combination with cow’s milk as a galactagogue agent to abrogate lack of milk production after childbirth and also as an anabolic agent along with Piper longum L. (Piperaceae) powder to cure malnutrition in children. For relieving excessive menstruation, the powder is used with honey. A mixture of powdered P. tuberosa and wheat or barley fried in ghee (clarified butter) with milk has been advised for sexual enervation and strength. For spermatorrhoea, fresh tuber juice of this plant with cumin seeds and sugar has been used therapeutically (Puri, 2003).
Traditionally, P. tuberosa has been used along with other medicinal plants in different combinations to prepare therapeutic Ayurvedic formulation. Some of the important Ayurvedic formulations utilizing P. tuberosa are “Ashwagandharishta”, a traditional remedy for epilepsy (Tanna et al., 2012), “Maha visagarbha taila”, a traditional remedy for sciatica and joint disorders (Kumawat et al., 2017), and “Nityananda rasa”, “Sarasvatarista”, “Satavaryadi ghrta” (Ayurvedic pharmacopoeia of India, 2001), “Marma gutika” (Kumar, 2016), and “Vidaryadi ghrita” (Sharma et al., 2018).
Traditional uses of Pueraria species, namely, Pueraria montana var. thomsonii (Benth.) (Fabaceae) and Pueraria montana var. lobata (Willd.) (Fabaceae), have been reported for their medicinal properties such as antiemetic, antitoxic, cold, countering the effect of alcohol abuse, anti-stress agent, neck stiffness, hypohidrosis, migraines, hypoglycemia, and certain cardiovascular diseases in the Chinese Medicinal Herbs, a book written by Li Shih Chen (Li, 2003; Croom, 2004).
Pharmacology
In phytopharmacological/ethnopharmacological research, scientific community should follow best practices in designing and conducting studies and reporting the results of analyzing pharmacological properties of the plant extracts and compounds of natural origin (Heinrich et al., 2020). Therefore, while reporting biological activities of any plant/herbal product, detailed information about the characterization of the plant extracts, their phytoconstituents, doses, duration of treatment, type of models used in the studies, toxicological data, and so forth should be clearly presented for the benefit of research community (Heinrich et al., 2020). Various pharmacological activities of the tuber extracts of P. tuberosa have been explored, and a graphical summary of these activities is shown in Figure 5 and Table 1.
FIGURE 5
TABLE 1
| Extract | Dose tested | Pharmacological activity | Model used for study (in vivo or in vitro) | Reference |
|---|---|---|---|---|
| Aqueous | 50 mg/100 g b/w | Antidiabetic | In vivo | Srivastava et al. (2015); Srivastava et al. (2017); Srivastava et al. (2018); Srivastava et al. (2019) |
| 50 mg/100 g b/w for 35 days | ||||
| 50 mg/100 g b/w for 10 days | ||||
| Ethanol | 100–400 mg/kg b/w for 5 days | Immunomodulatory | In vivo | Patel et al. (2016) |
| Tuber powder | 250 mg/kg b/w | Immunomodulatory | In vivo | Shilpashree et al. (2015) |
| Aqueous | 250 mg/ml given orally to rats for 14 days | Hepatoprotective | In vivo | Pandey et al. (2019) |
| Ethanol and methanol | 125, 250, 500, and 1,000 μg/ml | Antioxidant | In vitro | Likhitkar and Pande (2017) |
| Aqueous | 200, 400, and 700 μg/ml for 24, 48, and 72 h | Anticancer | In vitro | Adedapo et al. (2017) |
| Hydroalcoholic | 64 and 128 µg/ml for 24 h | Anticancer | In vitro | Aruna et al. (2018) |
| Ethyl acetate | 31.5–500 μg/ml for 72 h | Anticancer | In vitro | Satpathy et al. (2020) |
| Aqueous | 50–100 mg/100 g b/w for 20 days | Antidiabetic nephropathy | In vivo | Shukla et al. (2017); (2018a); (2018b) |
| Hydroalcoholic | 20–40 mg/100 g b/w for 14 days | Antidiabetic nephropathy | In vivo | Tripathi et al. (2017) |
| Methanolic | 20 mg/kg b/w for 14 days and 40 mg/kg b/w for 7 days | Antidiabetic nephropathy | In vivo | Yadav et al. (2019) |
| Methanolic | 20 and 40 mg/100 g b/w for 2 days | Nephroprotective | In vivo | Yadav et al. (2016a) |
| Butanol and ethyl acetate | 50 mg/100 g b/w for 5 days | Nephroprotective | In vivo | Yadav et al. (2016b) |
| Methanolic | 200 mg/ml | Antibacterial | In vitro | Pandya et al. (2019) |
| Hydroalcoholic | 50, 100, and 200 mg/kg b/w for 30 days | Neuroprotective | In vivo | Umarani et al. (2016) |
Pharmacological activities of tuber extract of Pueraria tuberosa.
b/w: body weight.
Nephroprotective Activity
Several studies have shown that P. tuberosa plant possesses nephroprotective activities. Oral administration of methanolic tuber extract to cisplatin- (8 mg/kg body weight) induced kidney damaged rats showed a dose-dependent protective effect (Nagwani and Tripathi, 2010). Tuber extract significantly reduced blood urea nitrogen, serum creatinine, glutathione, and superoxide dismutase (SOD) levels. The extract could control deoxyribonucleic acid (DNA) damage and catalase activities, cellular necrosis, and tubular swelling and prevent coagulation of proteins, in contrast to the control group. The nephroprotection of tuber extract of the plant has been attributed to its free radical scavenging activity (Nagwani and Tripathi, 2010). Feeding of biscuits made up of powder of P. tuberosa tuber for 10 days showed significant recovery in cisplatin-induced nephrotoxicity in Swiss mice. However, at higher dose, aspartate aminotransferase and alanine aminotransferase levels were temporarily elevated, so monitoring of liver functions, periodically, is imperative when continuing this regimen for longer periods such as a food supplement for cancer patients undertaking cisplatin chemotherapy (Tripathi et al., 2012). The methanolic extract of P. tuberosa ameliorated glycerol-induced acute kidney injury in rats by affecting the lipid peroxidation, SOD, and catalase activity with a lesser accumulation of hyaline casts and a lesser degree of tubular necrosis on histology of the kidney (Yadav et al., 2016a). Water decoction of P. tuberosa has also been reported to significantly reverse cisplatin-induced nephrotoxicity in rats (Yadav et al., 2016b). Hydroalcoholic tuber extracts of P. tuberosa showed nephroprotective activity in sodium arsenate- (1 mg/kg body weight) induced oxidative kidney tissue damage in rats (Rani et al., 2017). The nephroprotective effect through free radical scavenging activity was supported in a study, where streptozotocin- (STZ-) induced diabetic nephropathic rats, treated with aqueous tuber extract of P. tuberosa, exhibited an upsurge in activity of antioxidant enzymes, lowered oxidative stress, apoptosis, and urinary albumin excretion in a concentration-dependent manner (Shukla et al., 2018a). Methanolic tuber extract of the plant showed substantial protection in diabetic nephropathy induced by the administration of alloxan in rats (120 mg/kg body weight) by decreasing urea and creatinine and improving physiology of the kidney (Yadav et al., 2019). The supplementation of tuber extract of the P. tuberosa showed protection of kidney from oxidative stress and cellular injury. It also improved kidney physiology and parameters of kidney function test by reducing cellular apoptosis. These studies indicate that P. tuberosa extracts have nephron-protective potential and might lead to promising therapeutic agents for treating kidney diseases.
Antioxidant Activity
Methanolic and hexane tuber extract of P. tuberosa exhibited a strong free radical scavenging activity in a concentration-dependent fashion. These results showed that the methanolic extract of this plant exhibited better activity than the hexane extract in trapping hydroxyl radicals and inhibited lipid peroxidation, which indicated potent antioxidant property (Pandey et al., 2007). Hot water tuber extract of the plant P. tuberosa, supplemented with milk in Swiss mice, showed potent antioxidant activities in liver and red blood cells. Besides, a remarkable difference in glutathione levels was also observed in the control (172 μg/ml) and supplemented groups (P. tuberosa: 1,212 μg/ml and P. tuberosa + milk: 1,308.2 μg/ml). P. tuberosa along with milk has antioxidant property as evidenced by higher phagocytic activity, increased immunoglobulin levels, and reduced glutathione and lipid peroxidation (Sawale et al., 2013). P. tuberosa extracted with chloroform, acetone, methanol, and hot water was used to determine its antioxidant potential by using ferric reducing antioxidant power (FRAP) assay, metal chelating, phosphomolybdenum, and free radical scavenging using DPPH (2,2′-diphenyl-1-picrylhydrazyl radical) and ABTS (3-ethylbenzothiazoline-6-sulfonic acid) assay. The results showed that acetone extract of P. tuberosa has potent antioxidant activity (Viji and Paulsamy, 2015).
Antidiabetic Activity
Oral gavage of ethyl acetate tuber extract of P. tuberosa (250 mg/kg body weight) to alloxan-induced diabetic rats for seven days showed a pronounced decrease in blood glucose levels (Raghuwanshi and Jain, 2011). Studies suggested that chloroform, petroleum ether, ethanol, and aqueous tuber extracts of P. tuberosa confer significant antidiabetic activity in STZ- (50 mg/kg body weight) induced diabetic rats by a single intraperitoneal injection (Tripathi and Kohli, 2013). Water extract of root of P. tuberosa showed significant inhibition of dipeptidyl peptidase-4 (DPP-IV) that causes an enhanced half-life of active glucagon-like peptide-1 hormone. This hormone regulates glucose-dependent insulin release from β-cells of the pancreas in rats (Srivastava et al., 2015). In Srivastava et al.’s next study, they found that P. tuberosa water extract increased the glucose homeostatic potential through DPP-IV inhibitory pathway and the bioactive components robinin and puerarone, and this inhibitory activity was also confirmed by in silico molecular docking (Srivastava et al., 2017). Aqueous extract of tuber of P. tuberosa has further been reported to act as incretin receptor agonist and downregulated β-cells apoptosis and protected STZ-induced diabetes in rats (Srivastava et al., 2018). Aqueous tuber extract of the plant showed an elevated expression of nephrin and SOD and a declined expression of cysteinyl aspartate specific proteinase 3 (caspase-3), interleukin 6 (IL-6), nuclear factor kappa B (NF-κB), protein kinase C epsilon type (PKCε), tumor necrosis factor alpha (TNF-α), vascular endothelial growth factor (VEGF), matrix metalloproteinase-9 (MMP-9), and hypoxia-inducible factor 1-alpha in STZ-induced diabetic rats (Srivastava et al., 2019). In another experiment, it has been shown that administration of P. tuberosa water extract in alloxan-induced rat diabetic model resulted in decrease in SGOT (serum glutamic oxaloacetic transaminase), SGPT (serum glutamic pyruvic transaminase), and alkaline phosphates level and improved deformed hepatocytes and significant decrease in blood glucose levels as well as apoptosis (Pandey et al., 2019). The tuber extract contains different bioactive compounds that may act as agonists on glucagon-like peptide-1 hormone released from intestine and can also protect β-cells of the pancreas. It also resulted in decreased expression of different inflammatory and apoptotic markers during hypoxic injury to β-cells as evidenced by decreased apoptosis of β-cells. The extract also inhibited DPP-IV enzyme as an incretins receptor agonist, and hence it is emanating from the above studies that P. tuberosa has antidiabetic potential.
Anti-Stress Activity
Adult male Wistar rats subjected to cold immobilization stress, pretreated with 70% hydroethanolic tuber extract of P. tuberosa (200 and 400 mg/kg body weight) for 5 days, showed significant protection from gastric mucosal damage, reduced corticosterone level in the blood, and no enlargement of spleen and adrenals as compared to Withania somnifera (L.) Dunal (Solanaceae) rhizome extract (100 mg/kg body weight). These studies established the anti-stress effect of P. tuberosa (Pramanik et al., 2011). In a human trial, hypertensive patients were divided into two groups: group 1 was given capsules with 0.75 g tuber powder, whereas group 2 was given placebo capsules with lactose powder administered for 12 weeks. Group 1, treated with 1.5 g (twice a day) tuber powder of P. tuberosa for 12 weeks, showed a gradual decrease in systolic, diastolic, and mean blood pressure as well as a tolerant decrease in fibrinogen and increased plasma fibrinolytic activity (Verma et al., 2012). In stress-mediated disorders, the hypothalamic-pituitary-adrenal (HPA) axis is dysregulated which changes the levels of corticosteroids in plasma and monoamine in the brain. The extract of this plant might act on mucosal layer of the gastrointestinal, cardiovascular, and nervous (HPA) system, suggestive of anti-stress activity by a reduction in stress hormones.
Antidiabetic Nephropathic Activity
STZ-induced diabetic rats with nephropathy were given tuber extract of P. tuberosa (30 mg/100 g, body weight) for 20 days and exhibited a significant reduced severity of diabetic nephropathy by enhanced expression and activity of MMP-9 and degrading the accumulation of extracellular matrix in kidney tissue (Tripathi et al., 2017). Levels of nephrin, a biomarker of early glomerular injury, in the kidney of diabetic nephropathic rats were restored after treatment with tuber extract of P. tuberosa (Shukla et al., 2017). The diabetic nephropathic inflammatory response is mediated by NF-κB and its activated phosphorylated derivative (pNF-κB). Improved levels of these transcription factors and inflammatory cytokines (IL-6 and TNF-α) in the kidney of STZ-induced (55 mg/kg body weight) diabetic nephropathic rats were observed, and treatment with extracts from the tuber of P. tuberosa significantly negated these changes in a dose-dependent manner (Shukla et al., 2018b). Amelioration of renal damage was evaluated by renal functional tests, histopathology, and oxidative stress in alloxan-induced diabetic nephropathy. P. tuberosa methanolic extract showed renal protection by decreasing urea and creatinine and improved kidney physiology and histopathology changes through antioxidant mechanisms (Yadav et al., 2019). These studies are indicative of nephro-protection offered by P. tuberosa in diabetic nephropathy; however, this protective effect needs to be further explored, including studies on the protection of renal and glomerular cells mediated by different signaling pathway in the antidiabetic nephropathy.
Anti-Inflammatory Activity
The ethyl acetate and methanolic tuber extracts of P. tuberosa showed considerable anti-inflammatory potential compared to the control and standard drugs, ibuprofen, and nitrofurazone ointment in the rat paw edema method (Kambhoja and Murthy, 2007). The methanolic tuber extract of the plant significantly prevented the carrageenan-induced inflammation by lowering the glutathione content, catalase, SOD activity, and enhancing lipid peroxidase and C-reactive proteins in rats in a sequential manner (Tripathi et al., 2013). Isoorientin, isolated from the tuber of P. tuberosa plant, showed significant anti-inflammatory activity in LPS-treated mouse macrophage (RAW 264.7) cell line. It was also effective against carrageenan-induced inflammation on paw edema and air pouch mouse models. These studies revealed the downregulation in the expression of proinflammatory genes such as inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), TNF-α, and inactivation of NF-κB. Moreover, there was activation of antioxidant enzymes, catalase and glutathione-S-transferase (Anilkumar et al., 2017). The anti-inflammatory property of extracts of P. tuberosa in these studies appears to be mediated by lipid peroxidation, inactivation of the NF-κB pathway, and downregulation of proinflammatory cytokines.
Immunomodulatory Activity
Immunomodulatory activities of plant extract (0.4%) with milk as a carrier given to Swiss mice for 28 days were evaluated. The result showed a significantly higher phagocytic activity and immunoglobulin concentration, reduced glutathione content, and thiobarbituric acid reactive substances level compared to the control (Sawale et al., 2013). Reversed phase high-performance liquid chromatography (RP-HPLC) analysis of ethanolic tuber extract of the plant revealed that bioactive compounds involved in the immunomodulatory activities are genistein (1.37%), daidzein (1.70%), and puerarin (8.31%). Oral administration of these extracts builds up innate and humoral immune responses against sheep red blood cells challenged rats (Maji et al., 2014). The immunomodulatory activity of petroleum ether extract of P. tuberosa was evaluated by carbon clearance assay (Granulopectic index). The extract and Withania somnifera (L.) Dunal (Solanaceae) at 250 mg/kg body weight (Medicinal Plant Names Services, e) exhibited enhanced phagocytic activity of peritoneal macrophages to clear the carbon particles (Shilpashree et al., 2015). The ethanolic extract of tuber increased the phagocytic activity of macrophages in the mice model. The extract also inhibited both the cell mediated and humoral immunity, which supports its potent immunomodulatory activity (Patel et al., 2016).
Anticancer Activity
There is no significant toxicity of mangiferin isolated from tuber of P. tuberosa on normal cell lines (mouse fibroblast NIH-3T3, RAW 264.7, HEK293, and mouse lymphocytes) in cell viability assay in vitro; however, it is cytotoxic to various cancer cell lines like K562, MCF7, HEPG2, Jurkat cells, and A549 (Bulugonda et al., 2017). Furthermore, the anticancer and apoptotic potential of the hydroalcoholic tuber extract of P. tuberosa was investigated by cell viability assay. The extract showed a 50% inhibition of cell viability against human colon carcinoma (HT-29) cells at a concentration of 63.91 µg/ml. Cells also exhibited DNA fragmentation that is the hallmark of apoptosis, apoptotic cell death, and increased expression of certain proapoptotic genes (Aruna et al., 2018). The silver nanoparticles biosynthesized with aqueous extract of the P. tuberosa showed in vitro anticancer potential on different cancer cell lines (breast MCF-7 and MDA-MB-231; ovarian SKOV-3; brain U-87 cancer). However, the mechanism behind this activity needs exploration for therapeutic use (Satpathy et al., 2018). Antioxidant-enriched fraction also exhibited in vitro cytotoxicity in the breast (MCF-7 and MDA-MB-231) and ovarian (SKOV-3) cancer cells (Satpathy et al., 2020).
Other Pharmacological Properties
P. tuberosa has been attributed as one of the most sought plants that proved to be effective against multiple diseases and ailments. Alcoholic and aqueous extracts of P. tuberosa tuber were studied for nootropic effect in mice and rat models of amnesia induced by scopolamine and diazepam. The inflexion ratio observed was considerably high and comparable with piracetam, the standard drug in an elevated plus-maze experiment. Flavonoids present in the P. tuberosa tuber extracts have been reported for nootropic effect by interacting with cholinergic, adrenergic, serotonergic, and GABAnergic system (Rao et al., 2008). The neuroprotective properties of this plant were also studied in chronic foot-shock stressed rat model showing unpredictable and inescapable nature of physiological malfunctions, increase in anxiety level, decrease in male sexual indices, and behavioral changes. All these symptoms were abolished by this plant’s tuber extract (Pramanik et al., 2010). Neurotoxicity induced by sodium arsenate was ameliorated by hydroalcoholic extract which strengthens its memory and restores muscle strength and locomotor activity. Biochemical and histopathological changes are suggestive of the protective property of the extract in maintaining normal functional status of the brain in arsenate neurotoxicity (Umarani et al., 2016).
Alcoholic tuber extract of P. tuberosa was studied for anticonvulsant activity in pentalene tetrazole, strychnine, and maximal electroshock-induced convulsions in animals. Different doses of the extract (50, 100, and 200 mg/kg body weight) were compared with the standard drug, diazepam (5 mg/kg body weight). The medium and high doses exhibited potent anticonvulsant activity as compared to the control group (Basavaraj et al., 2011). The ethanolic and methanolic extract of leaf, stem, and tuber of P. tuberosa showed a wide range of antimicrobial activity against bacteria, Escherichia coli, Bacillus cereus, Salmonella paratyphi, and Staphylococcus aureus, as well as fungus, Candida albicans, Aspergillus fumigates, and Alternaria solani, on agar diffusion assay (Sadguna et al., 2015). The tuber extracts of P. tuberosa with different solvents exhibited a wide range of antimicrobial activity on selected bacterial and fungal pathogens (Aruna et al., 2016). The chloroform and water extracts of tuber of P. tuberosa showed significant antibacterial activity against Klebsiella pneumoniae and Staphylococcus aureus and methanolic extract on Staphylococcus aureus and Streptococcus agalactiae (Pandya et al., 2019). The metabolites in P. tuberosa extracts may be behind the mechanism involved in the antimicrobial action, which may interact with the microbial cell membrane resulting in microbial cell death. The antiulcerogenic activity of aqueous leaf extract of P. tuberosa on cold restraint stress, pyloric ligation, and ethanol-induced gastric ulcer rat models was observed. There was significant inhibition in gastric lesions by 76.6% in cold restraint stress, 80.1% in pyloric ligation, and 70.6% in ethanol-induced rat models (Gindi et al., 2010).
In metabolic disorders also, P. tuberosa extracts exhibited a hypolipidemic effect. Oral administration of butanol tuber extract of P. tuberosa at a dose of 150 mg/kg body weight showed a pronounced protective effect against CCl4-induced hepatotoxicity in adult male rats (Shukla et al., 1996). Rats maintained on high cholesterol diet upon the treatment demonstrated a substantial reduction in serum cholesterol, triglycerides (TG), low-density lipoproteins (LDL), and very-low-density lipoproteins (VLDL) levels (Tanwar et al., 2008). These results were corroborated in another study where nonalcoholic fatty liver disease (NAFLD), induced in rats by feeding a high fat diet, was treated with water extract of this plant. Antioxidant activity with reduced lipid peroxidation and enhanced activities of SOD and catalase enzymes were observed. A similar finding was observed by Tripathi et al. in the NAFLD rats model which also showed a reduction in serum TG and cholesterol values (Tripathi and Aditi, 2020). The ethanolic extract of P. tuberosa showed a dose-dependent immunosuppressant activity as evident by a decrease in antibody titer and also a reduction in hematological parameters in the drug-induced myelosuppression model (Babu et al., 2016). Crude powder (3 g daily) of P. tuberosa tuber was given to a human patient with ischemic heart disease for twelve months. The case study demonstrated an overall significant cardioprotective effect; resting mean blood pressure was reduced from 96.66 to 90.00 mm Hg without affecting the resting heart rate, and the heart rate at peak exercise was also reduced, indicating better exercise tolerance (Verma et al., 2009).
P. tuberosa root extract, given to male Wistar rats (100 mg/rat per day) for 60 days, affected the fertility of rats as shown by a reduction in weight of testes, epididymis, prostate, and the seminal vesicle. Studies also showed a considerable decrease in the quantity of mature Leydig cells, cauda epididymis, and sperm motility (Gupta et al., 2005). The antioxidant-enriched fraction from the tuber extract of P. tuberosa against menopausal osteoporosis in ovariectomy-induced osteoporosis in rats was studied and found that it improved biochemical parameters, controlled the increased body weight, and decreased uterus weight following ovariectomy as well as restoration of typical bone structure and trabecular width of the femur (Satpathy et al., 2020). Incision and excision wounds were treated with methanolic and ethyl acetate tuber extract of P. tuberosa. The extracts showed potent wound healing property in comparison to the control and the group of rats treated with standard drugs, ibuprofen, and nitrofurazone ointment (Kambhoja and Murthy, 2007).
Phytochemistry
The crude tuber extracts of P. tuberosa are known to contain alkaloids, anthracene, anthocyanidins, anthraquinone, glycosides, carbohydrates, catecholic compounds, coumarins, flavonoids, glycosides, hexose sugars, saponins, steroids, terpenoids, and volatile oils (Ratnam and Venkata Raju, 2009; Rawtal et al., 2019). Therefore, many studies have been undertaken to individually analyze and characterize the activities of different phytoconstituents of the plant. Vaishnav et al. could grow a callus culture of P. tuberosa and identified four isoflavanoids, viz., puerarin [1], daidzein [2], genistin [3], and genistein [4] (Vaishnav et al., 2006; Satpathy et al., 2017). Lupinoside PA4 [5] was isolated from methanolic extract of P. tuberosa using HPLC, and its structure was determined by 1D, 2D NMR, and Q-TOF-MS (Dey et al., 2007). Pandey and Tripathi extracted tuberosin [6], 3-O-methylanhydrotuberosin [7], and puerarostan [8] from ethanolic tuber extract; the same was confirmed by UV, IR, and NMR spectral data (Pandey and Tripathi, 2010). β-Sitosterol [9] was quantified in the methanolic root extract of P. tuberosa by high-performance thin layer chromatography (HPTLC) method (Mhaske et al., 2009). Liquid chromatography–mass spectrometry (LC–MS) analysis of ethanolic extract was found to contain puerarin, daidzein, biochanin A [10], and biochanin B [11] (formononetin) (Chauhan et al., 2013). Daidzin [12], irisolidone [13], 4-methoxypuerarin [14], puerarone [15], quercetin [16], and tectoridin [17] are the flavonoid compounds and p-coumaric acid [18], which have been reported to be isolated from tuber of P. tuberosa (Maji et al., 2014) and aqueous tuber decoction shown to contain daidzein, genistin, hydroxytubersone [19], puerarin, puetuberosanol [20], robinin [21], tuberosin, and tuberostan [22] (Shukla et al., 2017). Mass spectrometry and 2D-NMR techniques were used to isolate isoorientin [23] and mangiferin [24] from methanolic extract from P. tuberosa (Sumalatha et al., 2015). Phytochemical analysis of P. tuberosa extract using HPTLC revealed the presence of carbohydrates, proteins, alkaloids, flavonoids, saponins, phenols, and tannins (Viji and Paulsamy, 2018). Satpathy et al. showed the presence of 23 bioactive molecules including stigmasterol [25], β-sitosterol, and stigmasta-3,5-dien-7-one by gas chromatography–mass spectrometry analysis of antioxidant-enriched fraction prepared from P. tuberosa (Satpathy et al., 2020). We have listed various phytoconstituents isolated from P. tuberosa and provided detailed information about their chemical structures, IUPAC names, and pharmacological activities, as well as associated references, in Table 2. The chemical structures of phytochemical compounds from P. tuberosa were drawn using “ChemDraw JS 19.0”; https://chemdrawdirect.perkinelmer.cloud/js. IUPAC (International Union of Pure and Applied Chemistry) names have been taken from PubChem database.
TABLE 2
| Purified compound studied | Model used for study (in silico/in vitro/in vivo) | Dose tested | Pharmacological activity | Conclusion | References |
|---|---|---|---|---|---|
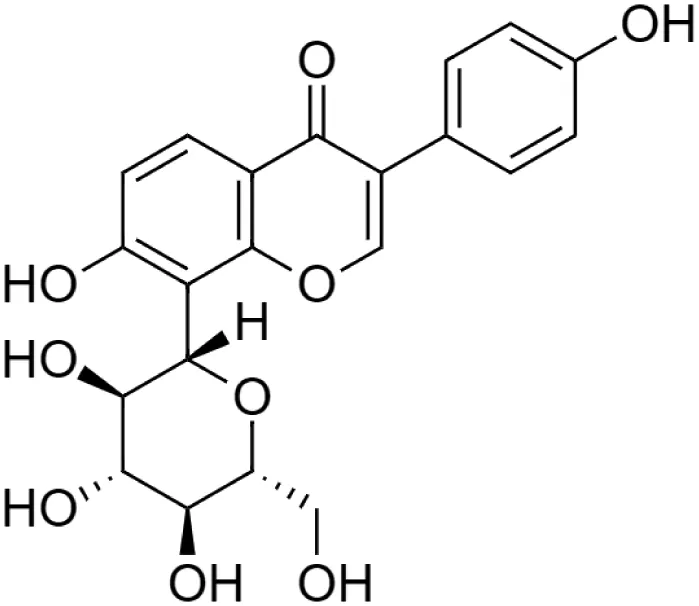 Puerarin [1] (C21H20O9) Puerarin [1] (C21H20O9)IUPAC name–[7-hydroxy-3-(4-hydroxyphenyl)-8-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]chromen-4-one] | In vivo | 100 mg/kg b/w for 7 days | Nephroprotective | Suppression of oxidative stress production and S-nitrosylation of proteins in the diabetic kidneys and MMP-9 | Zhong et al. (2014) |
| In vivo | 20, 40, and 80 mg/kg b/w/day for 8 weeks | Antidiabetic | Hypoglycemic effect which supports its antidiabetic property and renal protective effects via the mechanism of attenuating SIRT1/FOXO1 pathway | Xu et al. (2016) | |
| In vivo | 2.5 mg/kg b/w/day for 2 weeks | Antioxidant | Suppressed macrophage activation by inhibiting IκB, ERK, and p38 activity and reactive oxygen species production | Tanaka et al. (2016) | |
| In vitro | 10 and 50 µM | Anticancer | Suppressed MCF-7 and MDA-MB-231 cell LPS-stimulated migration, invasion and adhesion by inhibition of the NF-kB pathway and phosphorylation of ERK | Liu et al. (2017) | |
| In vivo | 500 mg/kg b/w/day for 6 weeks | Antidiabetic | Improved insulin resistance and reduced diabetic foot ulcers | Yu et al. (2017) | |
| In vitro | 0.01, 0.1, 1, 10, and 100 μmol/L | Anticancer | Puerarin-induced apoptosis in human bladder cancer cells was mediated by activation of the mTOR/p70S6K signaling pathway | Jiang et al. (2018) | |
| In vivo | 25, 50, and 100 mg/kg b/w/day for 12 weeks | Antidiabetic | Hypoglycemic effects, prevented cataract development and progression in diabetic rats by reducing oxidative stress through the NRF2/HO-1 signaling pathway | Zhang and Li (2019) | |
| In vivo | 50 mg/kg b/w/day for 14 weeks | Anti-inflammatory | Reduced inflammatory regulators (TNF-α, IL-1β, COX2, and MMP-14) and inhibited HDAC1/HDAC3 signaling | Guo et al. (2019) | |
| In vivo | 5, 10, 20, and 40 mg/kg b/w for 12 weeks | Nephroprotective | Protects podocytes from diabetes-induced injury through HMOX1 and SIRT1-mediated upregulation of autophagy | Li et al. (2020) | |
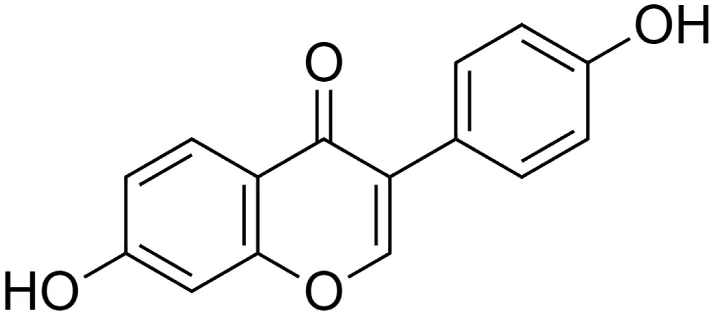 Daidzein [2] (C15H10O4) Daidzein [2] (C15H10O4)IUPAC name–[7-hydroxy-3-(4-hydroxyphenyl)chromen-4-one] | In vitro/in vivo | In vitro: 12.50–50 μM; in vivo: 1 g/kg b/w for 12 weeks | Anti-inflammatory | Reduced adipose tissue inflammation through the upregulation of PPARγ, which might result in alleviating insulin resistance in obesity | Sakamoto et al. (2014) |
| In vitro/in vivo | In vitro: 0.5–100 µM; in vivo: 10 mg/kg and 20 mg/kg b/w for 27 days | Anticancer | Reduced viability of bladder carcinoma RT112 cells by inducing G1/S phase arrest and apoptosis and inhibited tumor growth | He et al. (2016) | |
| In vitro/in vivo | In vitro: 12.50–400 μM; in vivo: 10 mg/kg and 20 mg/kg b/w for 27 days | Anticancer | Reduced the cell viability and colony formation in concentration-dependent manner and inhibited tumor growth | Zheng et al. (2017) | |
| In vitro/in vivo | In vitro: 0.78–200 μM; in vivo: 10–40 µg/kg b/w | Anticancer | Induced G2/M cell cycle arrest and suppressed the ovarian tumor growth | Hua et al. (2018) | |
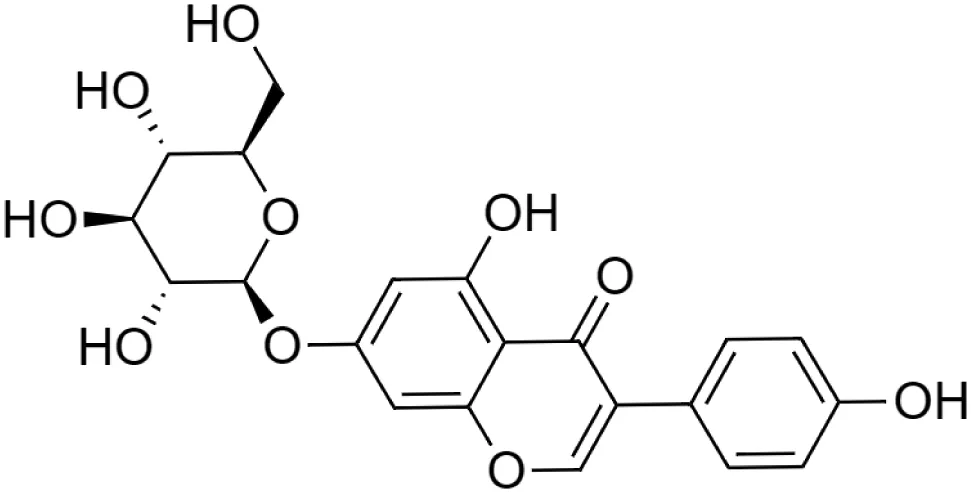 Genistin [3] (C21H20O10) Genistin [3] (C21H20O10)IUPAC name–[5-hydroxy-3-(4-hydroxyphenyl)-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxychromen-4-one] | In vivo | 20, 40, and 60 mg/kg b/w | Cardioprotective | Significantly attenuated the release of LDH, CK in a dose-dependent manner | Gu et al. (2016) |
| In vitro | 50 and 100 µM for 48 h | Antiadipogenic and antilipogenic | Activated AMP-activated protein kinase a (AMPKα), and inhibited sterol regulatory element-binding transcription factor-1c (SREBP-1c) | Choi et al. (2020) | |
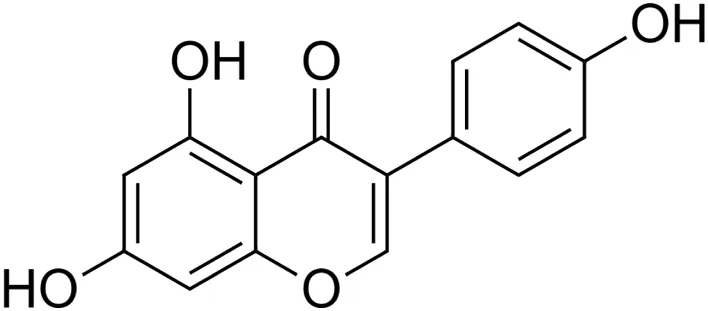 Genistein [4] (C15H10O5) Genistein [4] (C15H10O5)IUPAC name–[5,7-dihydroxy-3-(4-hydroxyphenyl)chromen-4-one] | In vivo | 10 and 20 mg/kg b/w 3 times a week for 10 weeks | Nephroprotective | Reduced renal inflammation, oxidative stress, and apoptosis in diabetic mice | Elmarakby et al. (2011) |
| In vitro | 10 nmol/L to 5 μmol/L | Antidiabetic | Acted on pancreatic β-cells, activation of the cAMP/PKA signaling cascade | Liu et al. (2006) | |
| In vivo | 0.2, 1, and 5 mg/kg b/w once daily | Wound healing | Suppression of FoxO1, iNOS activity, and oxidative stress | Tie et al. (2013) | |
| In vitro | 5, 10, and 25 µM for 24 h | Antioxidant | Activated AMPK and increased PTEN expression | Park et al. (2010) | |
| In vivo | 2.5–10 mg/kg b/w for 14 days | Neuroprotective | Reduced the infarct volume, improved the neurological deficit, and prevented cell apoptosis after ischemia | Qian et al. (2012) | |
| In vivo | 10 mg/kg b/w 1 h before surgery | Nootropic | Ameliorated aβ-induced impairment of short-term spatial memory in rats through an estrogenic pathway and reduced oxidative stress | Bagheri et al. (2011) | |
| In vivo | 1 mg/kg b/w from day 16 until day 60 | Anti-stress | Lowered blood pressure, restored ACE, PKC-bII, and eNOS expression, and preserved renal ultrastructural integrity | Palanisamy and Venkataraman (2013) | |
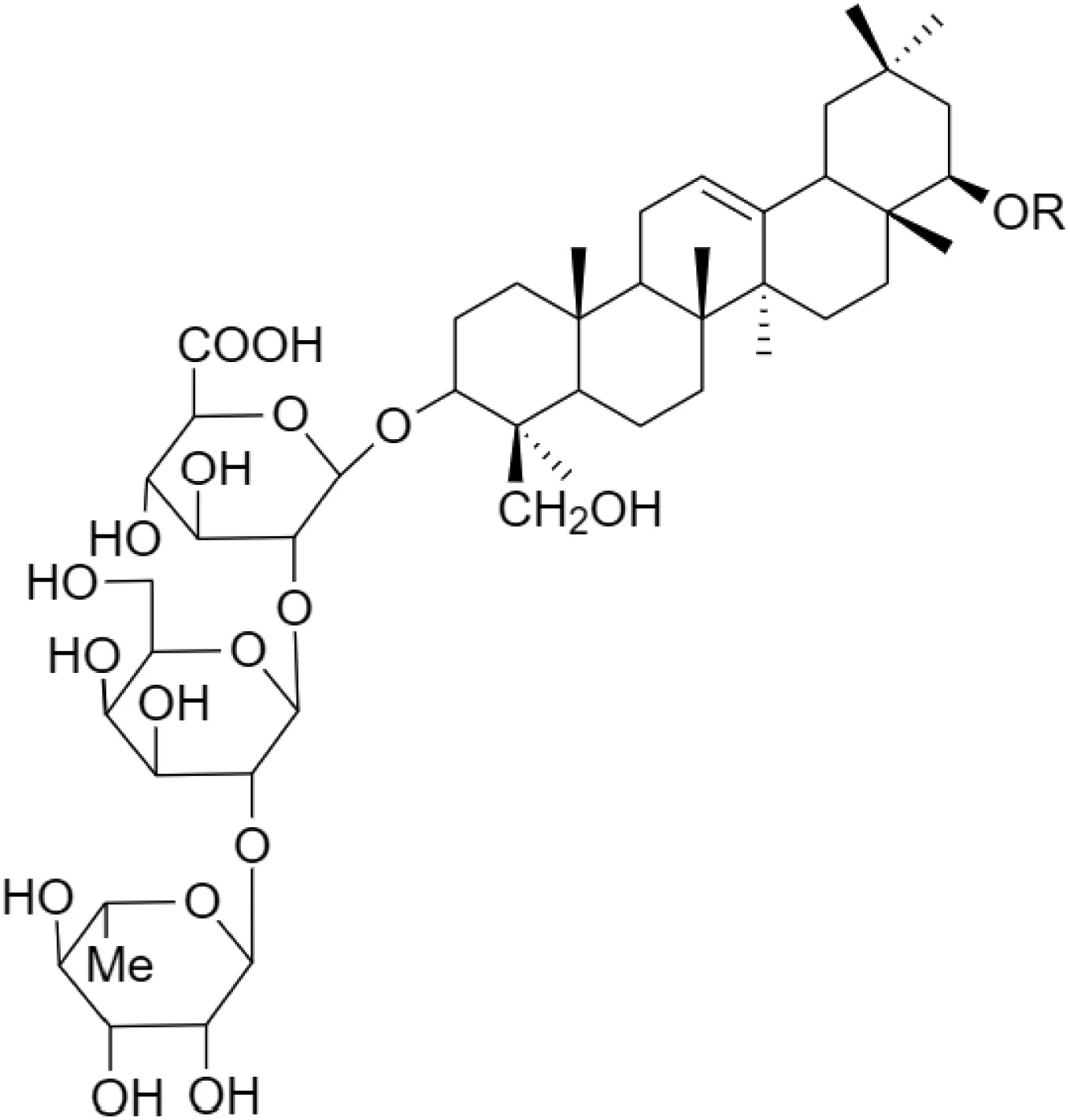 Lupinoside PA4 [5]Dey et al. (2007) Lupinoside PA4 [5]Dey et al. (2007) | In vitro/in vivo | In vitro: 20 ng/ml; in vivo: 1.5 mg/200 g b/w for 12 days | Antidiabetic | Stimulated IR-β and akt phosphorylation | Dey et al. (2007) |
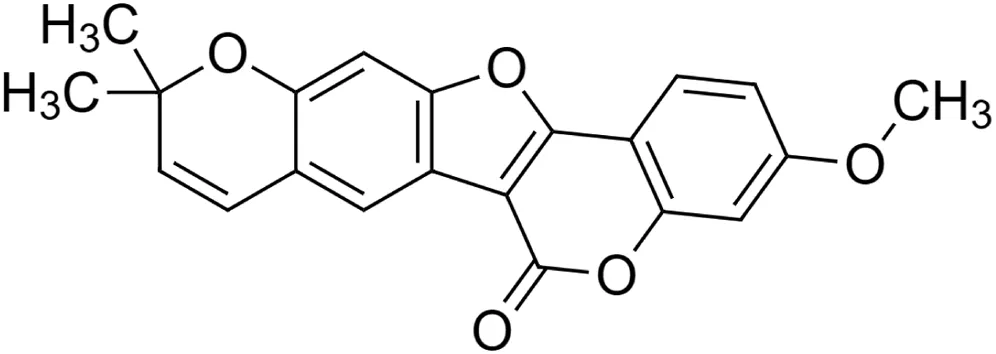
 Tuberosin [6] (C20H18O5) Tuberosin [6] (C20H18O5)IUPAC name–[7,7-dimethyl-8,12,20-trioxapentacyclo [11.8.0.02,11.04,9.014,19]henicosa-2 (11),3,5,9,14 (19),15,17-heptaene-1,17-diol] | In vitro | 50, 100, 300, and 600 ng/ml | Antioxidant | Inhibited LPS-induced NO production in a concentration-dependent manner, expression of iNOS proteins | Pandey and Tripathi (2010) |
 3-O-methylanhydrotuberosin [7] (C21H18O4) 3-O-methylanhydrotuberosin [7] (C21H18O4)IUPAC name–[17-methoxy-7,7-dimethyl-8,12,20-trioxapentacyclo [11.8.0.02,11.04,9.014,19]henicosa-1(13),2(11),3,5,9,14 (19),15,17-octaene] | Pharmacological activity not reported | ||||
 Puerarostan [8] (C21H18O6) Puerarostan [8] (C21H18O6)IUPAC name–[3,9-dihydroxy-4-methoxy-8-(3-methylbut-2-enyl)-[1]benzofuro [3,2-c]chromen-6-one] | Pharmacological activity not reported | ||||
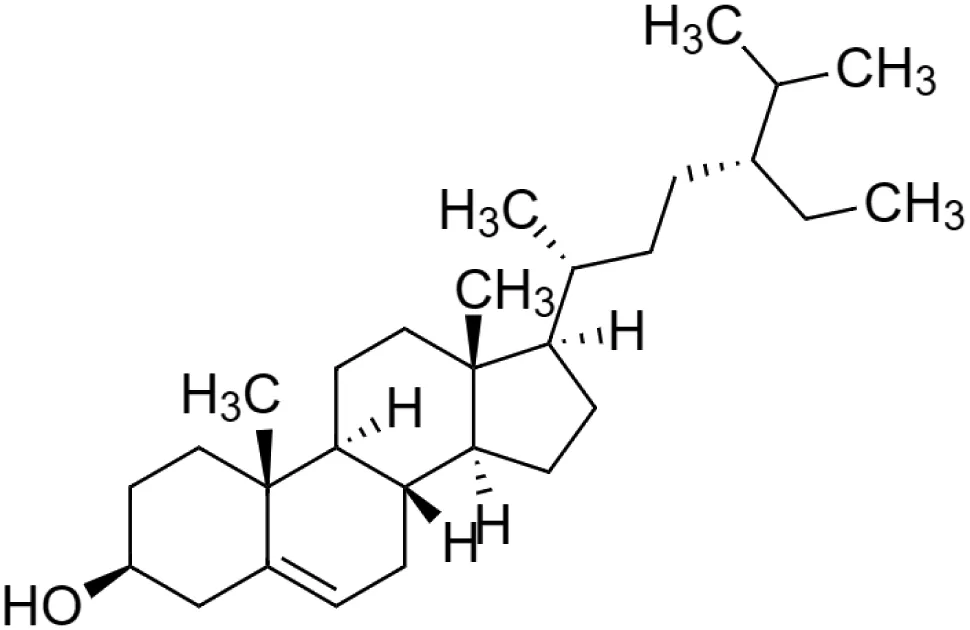 β-sitosterol [9] (C29H50O) β-sitosterol [9] (C29H50O)IUPAC name–[(3S,8S,9S,10R,13R,14S,17R)-17-[(2R,5R)-5-ethyl-6-methylheptan-2-yl]-10,13-dimethyl-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta [a]phenanthren-3-ol] | In vivo | 20 mg/kg b/w | Anti-colitis | Ameliorated HFD-induced colitis by inhibiting the binding of LPS to toll-like receptor 4 in the NF-κB pathway | Kim et al. (2014) |
| In vitro | 0.25 µg/ml and 2.5 µg/ml | Antiproliferative for mast cell | Decreased thymic stromal lymphopoietin (TSLP) induced mast cell proliferation | Han et al. (2015) | |
| In vivo | 20 mg/kg b/w | Nephroprotective | β-sitosterol showed significant positive changes to nephrotoxicity-induced rats; altered biochemical parameters were restored to near normal | Sharmila et al. (2016) | |
 Biochanin a [10] (C16H12O5) Biochanin a [10] (C16H12O5)IUPAC name–[5,7-dihydroxy-3-(4-methoxyphenyl)chromen-4-one] | In vivo | 10, 20, and 40 mg/kg b/w for 28 days | Antidiabetic | Increased NAD-dependent deacetylase sirtuin-1 (SIRT1) expression in pancreatic tissue | Oza and Kulkarni (2018b) |
| In vitro | 5–20 μM for 1 h | Antitoxic against 2,3,7,8-tetrachlorodibenzo-p-dioxin | Inhibited the TCDD-induced loss of triglycerides in 3T3-L1 adipocytes, showing increased differentiation of 3T3-L1 preadipocytes to adipocytes when compared with the cells exposed to TCDD alone | Choi et al. (2019) | |
| In vitro | 2–4 µM | Vasodilatory | Interfered with the cGMP pathway in isolated coronary arteries and vasodilatory effect | Migkos et al. (2020) | |
| In vitro | 2.5–100 μM | Anti-inflammatory | Suppressing iNOS, COX-2, MyD88, and TLR-4 protein expressions and akt and ERK1/2 pathway activation | Berköz et al. (2020) | |
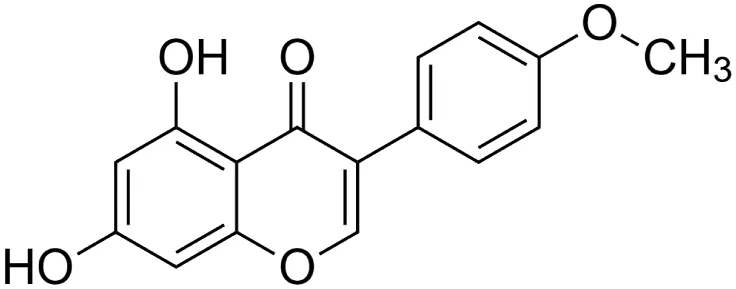
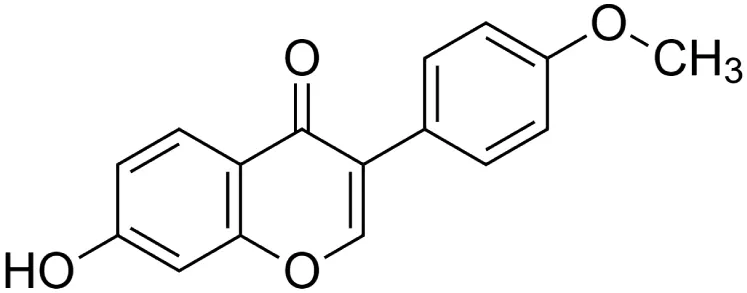 Biochanin B [11] (formononetin) (C16H12O4) Biochanin B [11] (formononetin) (C16H12O4)IUPAC name–[7-hydroxy-3-(4-methoxyphenyl)chromen-4-one] | In vitro | 3.45 μmol/L in Vero cells and 3.98 μmol/L in SK-N-SH cells | Anti-enterovirus 71 | Inhibited EV71-induced COX-2 expression and PGE2 production via MAPKs pathway including ERK, p38, and JNK | Wang et al. (2015) |
| In vivo | 1 and 100 µM for 2 weeks | Hair growth activity | Topical formononetin treatment induced hair regrowth in the depilated telogenic C57BL/6 mice and restored the length of hair shafts and size of hair follicles | Kim et al. (2016) | |
| In vivo | 5, 10, and 20 mg/kg b/w | Antidiabetic | Inhibited apoptosis of β-cell of the pancreas and promoted islet β-cell regeneration | Qiu et al. (2017) | |
| In vivo | 15, 50, and 75 mg/kg b/w for 5 days | Nephroprotective | Promoted proliferation of surviving renal tubular cells and inhibited apoptosis after cisplatin-induced acute kidney injury | Huang et al. (2017) | |
| In vitro | 0–280 μM for 24 and 48 h | Anticancer | Significant increase in Bax/Bcl-2 ratio accompanied with elevated level of cleaved-caspase-3 and cleaved-caspase-9 after formononetin treatment | Zhang et al. (2018) | |
| In vitro | 2.5, 5, and 10 µM for 24 h | Neuroprotective | Inhibited neuroinflammation in BV2 microglia cells stimulated with LPS and also suppressed production of the proinflammatory cytokines TNF-α, IL-6, and IL-1β from the cells | El-Bakoush and Olajide (2018) | |
| In vivo | 10, 20, and 40 mg/kg b/w for 28 days | Antidiabetic | Reduced insulin resistance and attenuated hyperglycemia in type II diabetes, which could be due to increased expression of SIRT1 in pancreatic tissues | Oza and Kulkarni (2018a) | |
| In vitro | 30 μM for 24 h | Anti-inflammatory | Inhibited HMGB1 release by decreased HMGB1 acetylation via upregulating SIRT1 in a PPARδ-dependent manner | Hwang et al. (2018) | |
| In vitro | 5 mM | Cardioprotective | Pretreatment with formononetin reduced myocardial tissue injury, improved cardiac function, and decreased apoptosis in heart tissue | Huang et al. (2018) | |
| In vitro | 25–100 µM for 24 h | Nephroprotective | Formononetin-treated cells were morphologically normal compared to the cells undergoing cisplatin-induced death and showed potent protective effect against cisplatin-mediated LLC-PK1 cell (renal tubular epithelial cell) death | Lee et al. (2018) | |
| In vivo | 25, 50, and 100 mg/kg b/w for 8 days | Anti-colitis | After formononetin administration, there was less infiltration of neutrophils and macrophages in the injured colonic tissue and also a significant decrease in the level of inflammatory cytokines TNF-α and IL-1β in the colon of mice with acute colitis | Wu et al. (2018) | |
| In vitro/in vivo | In vitro: 150 μmol/L for 12, 24, and 48 h; in vivo: 50 mg/kg b/w for 4 weeks | Anticancer | Inhibited MDA-MB-468 cell survival in a dose- and time-dependent manner, and tumor volume shrank from 472.7 to 253.6 mm3 on day 30 in xenograft model | Zhou et al. (2019) | |
| In vivo | 10 mg/kg b/w | Anticancer | The tumor inhibition rate was 50.17% in the mice treated with formononetin | Zhang et al. (2019) | |
| In vivo | 100 mg/kg b/w for 14 weeks | Hepatoprotective | Promoted lysosome biogenesis and autophagosome-lysosome fusion, relieving the blockade in autophagic flux and further induced lipophagy | Wang et al. (2019) | |
| In vivo | 10–50 mg/kg b/w for 10 days | Hepatoprotective | Ameliorated hepatic cholestasis by upregulating expression of SIRT1 and activating PPARa | Yang et al. (2019) | |
| In vivo | 20 and 40 mg/kg b/w for 10 weeks | Neuroprotective | Reduced the levels of inflammation cytokines IL-1β and TNF-α and tau hyperphosphorylation in mice hippocampus | Fu et al. (2019) | |
| In vivo | 31.25 μg/ml | Anti-inflammatory | LPS-induced inflammation in zebrafish was attenuated by formononetin mainly by restraining the MyD88 or TRIF MAPK/ERK and MAPK/JNK pathways | Luo et al. (2019) | |
| In vivo | 25 mg/kg b/w for 10 days | Anti-stress | Reduced the neural excitability and the protective upregulation of GABAA receptors | Wang et al. (2019) | |
| In vivo | 10, 20, and 40 mg/kg b/w for 16 weeks | Nephroprotective | Enhanced creatinine clearance and reduced oxidative stress burden along with increased SIRT1 expression in kidney tissues | Oza and Kulkarni (2019) | |
| In vivo | 10 mg/kg b/w | Anticancer | Inhibited EGFR-Akt axis and promoted FBW7-mediated Mcl-1 ubiquitination | Yu et al. (2020) | |
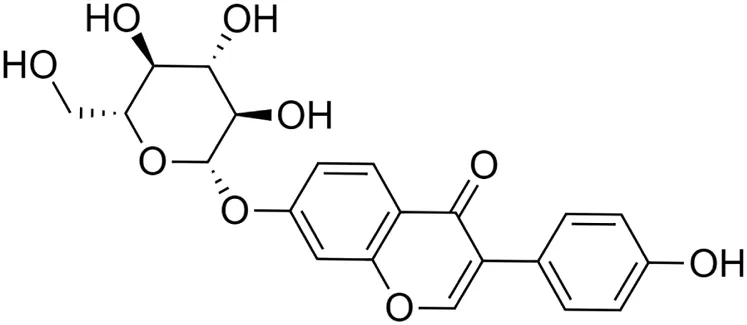 Daidzin [12] (C21H20O9) Daidzin, daidzein and their metabolites, O-desmethylangolensin (O-DMA) and equol Daidzin [12] (C21H20O9) Daidzin, daidzein and their metabolites, O-desmethylangolensin (O-DMA) and equolIUPAC name (Daidzin)–[3-(4-hydroxyphenyl)-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxychromen-4-one] | In vitro | 5–100 µM | Antioxidant | Stimulated catalase and total superoxide dismutase (CuZn- and Mn-SOD) activity, and mRNA and protein expression | Choi and Kim (2014) |
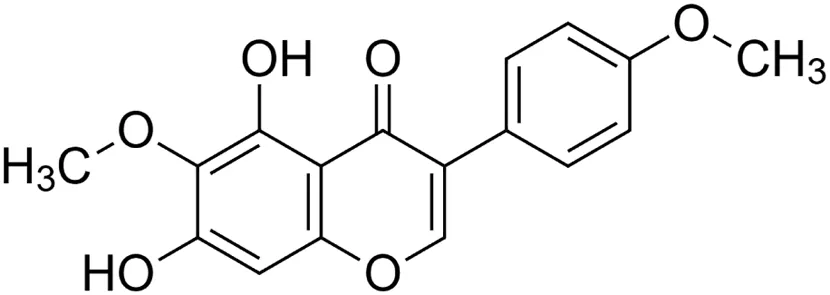 Irisolidone [13] (C17H14O6) Irisolidone [13] (C17H14O6)IUPAC name–[5,7-dihydroxy-6-methoxy-3-(4-methoxyphenyl)chromen-4-one] | In vitro/in vivo | In vitro: 1, 5, and 10 μM for 12 h; in vivo: 50, 100, and 200 mg/kg b/w for 30 days | Anti-ischemia | In vitro, increased cell viability and attenuated apoptosis; in vivo, inhibited mitochondrial membrane potential (MMP) and increased total ATPase activity | Yin et al. (2016) |
| In vitro/in vivo | In vitro: 5 or 10 μM for 90 min; in vivo: 20–50 mg/kg for 4 days | Anti-gastritic | Pretreatment with irisolidone decreased the area of hemorrhagic ulcerative lesions caused by ethanol and suppressed stomach myeloperoxidase activity, CXCL4 secretion, and NF-κB activation | Kang et al. (2017) | |
| In vivo | 20 mg/kg b/w | Anti-colitic | Alleviated colon shortening and myeloperoxidase activity in mice with TNBS-induced colitis | Jang et al. (2019) | |
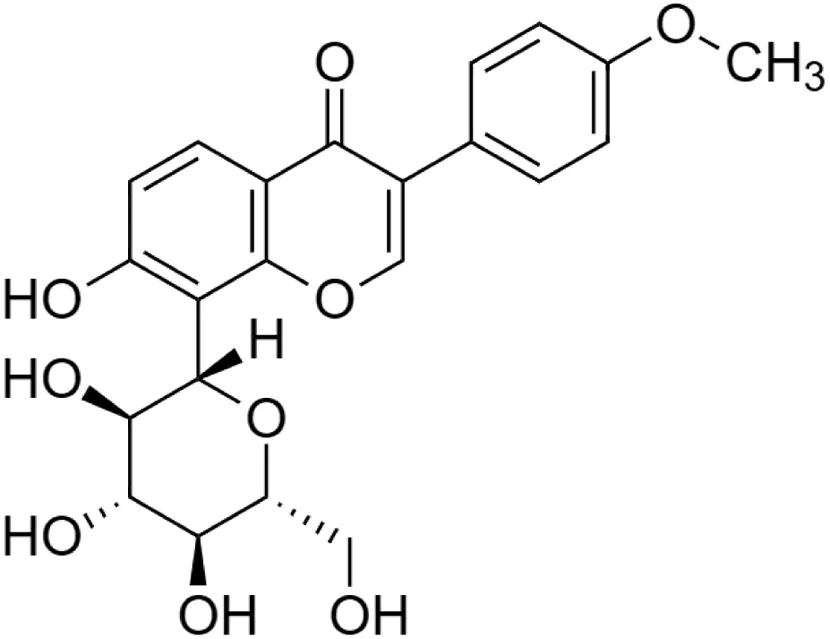 4-Methoxypuerarin [14] (C22H22O9) 4-Methoxypuerarin [14] (C22H22O9)IUPAC name–[7-hydroxy-3-(4-methoxyphenyl)-8-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]chromen-4-one] | In silico | — | Weak DNA binding affinity | Glycosylation of 4′-methoxypuerarin, caused steric hindrance to weaken the DNA binding affinity and had no significant inhibition on DNA amplification | Chen et al. (2020) |
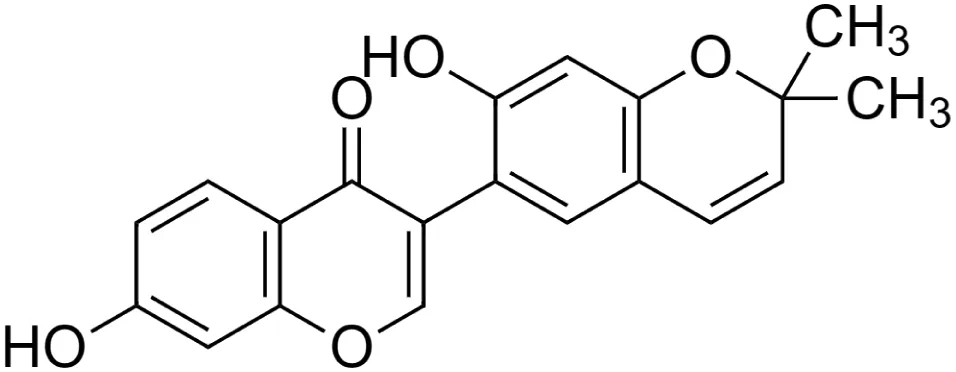 Puerarone [15] (C20H16O5) Puerarone [15] (C20H16O5)IUPAC name–[7-hydroxy-3-(7-hydroxy-2,2-dimethylchromen-6-yl)chromen-4-one] | In silico | — | Antidiabetic | Strong affinity to VEGFR-1 and VEGFR-2 along with 93.881% human intestinal absorption | Srivastava et al. (2017) |
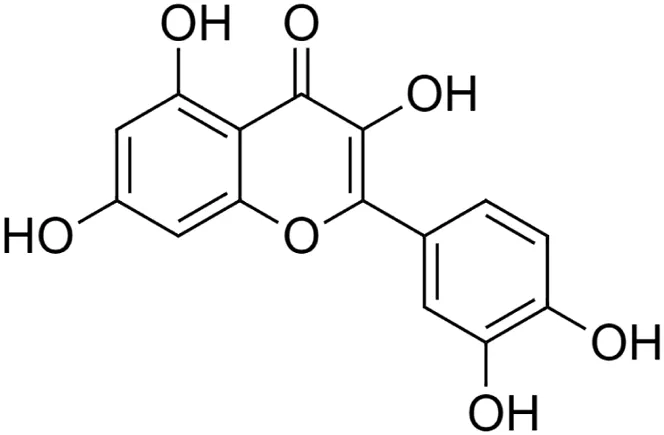 Quercetin [16] (C15H10O7) Quercetin [16] (C15H10O7)IUPAC name–[2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxychromen-4-one] | In vivo | 15 mg/kg b/w for 7 days | Hepatoprotective | Accelerated the regeneration after partial hepatectomy | Kanter et al. (2016) |
| In vitro/in vivo | In vitro: 5–100 μM; in vivo: 100 mg/kg b/w for 30 days | Neuroprotective | Protected neuronal cells from amyloid beta induced oxidative stress | Li et al. (2017) | |
| In vivo | 100 mg/kg b/w for 6 days | Intestinal damage repair | Increased intestinal and mucosal weight and prevented methotrexate-induced intestinal damage | Sukhotnik et al. (2018) | |
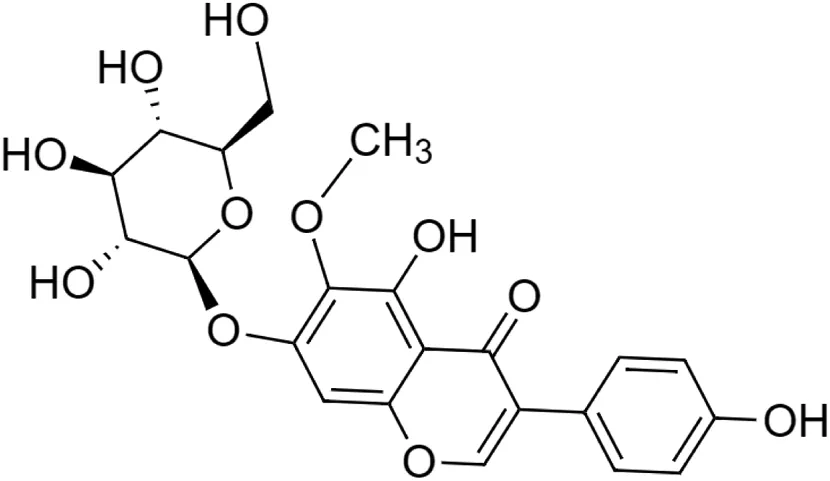 Tectoridin [17] (C22H22O11) Tectoridin [17] (C22H22O11)IUPAC name–[5-hydroxy-3-(4-hydroxyphenyl)-6-methoxy-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxychromen-4-one] | In vivo | 25–400 mg/kg b/w | Anti-alcoholism | Strongest clearance rate of ethanol | Zhang et al. (2019) |
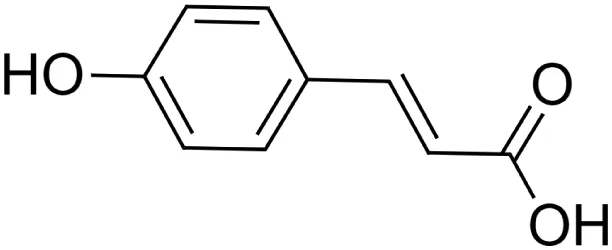 p-coumaric acid [18] (C9H8O3) p-coumaric acid [18] (C9H8O3)IUPAC name–[(E)-3-(4-hydroxyphenyl)prop-2-enoic acid] | In vivo | 100 mg/kg b/w for 7 days | Immunomodulatory | Decreased the expression of inflammatory mediator TNF-α and circulating immune complexes | Pragasam et al. (2013) |
| In vivo | 8 mg/kg b/w for 7 days | Cardioprotective | Prevented cardiac hypertrophy, by virtue of its antihypertrophic, antilipidemic, and free radical scavenging | Roy and Prince (2013) | |
| In vivo | 100 mg/kg b/w for 3 weeks | Nephroprotective | Cadmium metal chelating activity | Navaneethan and Rasool (2014) | |
| In vivo | 100 mg/kg b/w | Antidiabetic | Modulated glucose and lipid metabolism via GLUT2 activation in the pancreas | Amalan et al. (2016) | |
| In vivo | 30 mg/kg b/w | Neuroprotective | Increased the total activity of fEPSP dose-dependently after high frequency stimulation and attenuated scopolamine-induced blockade of fEPSP in the hippocampal CA1 long-term potentiation area | Kim et al. (2017) | |
| In vivo | 100 mg/kg b/w for 26 days | Anti-arthritic | Suppressed the paw edema, body weight loss and inflammatory cytokine and chemokine levels (TNF-α, IL-1β, IL-6, and MCP-1) in serum and ankle joint of arthritic rats | Neog et al. (2017) | |
| In vivo | 50 mg/kg b/w | Hepatoprotective | Suppressed hepatic apoptosis via ROS-mediated DNA damage and inflammation by modulating the mitogen-activated protein kinase (MAPK) signaling axis in an ROS-dependent manner | Cha et al. (2018) | |
| In vivo | 100 mg/kg b/w for 2 weeks | Neuroprotective | Pretreatment with p-coumaric acid significantly reduced malondialdehyde (MDA) levels, whole-brain infarction volume, and hippocampal neuronal death together and increased catalase and superoxide dismutase activities | Sakamula and Thong-asa (2018) | |
| In vitro/in vivo | In vitro: 0–4,000 µmol/L for 24 and 72 h; in vivo: 100 mg/kg b/w for 30 weeks | Anticancer | Downregulated Grp78 and activated UPR mediated apoptosis both in vitro and in vivo models of colon cancer | Sharma et al. (2018) | |
| In vivo | 50 and 100 µmol/L | Antioxidant | Significantly increased the survival rate of Caenorhabditis elegans under the oxidative stress condition and also increased lifespan by 20% for both 50 and 100 µmol/L compared to the control | Yue et al. (2019) | |
| In vivo | 50 mg/kg b/w for 6 weeks | Antidiabetic | Enhanced anti-inflammatory, anti-osteoclastogenic, and antioxidant defense systems in streptozotocin-treated mice | Bhattarai et al. (2019) | |
| In vivo | In vitro: 10–100 μM; in vivo: 50, 100, and 200 mg/kg b/w | Hepatoprotective | No effect on cell viability up to 60–80 μM concentrations on HepG2 cells in vitro, p-coumaric acid at 200 mg/kg exhibited higher protection on ethanol-induced hepatic injury in rats | Sabitha et al. (2020) | |
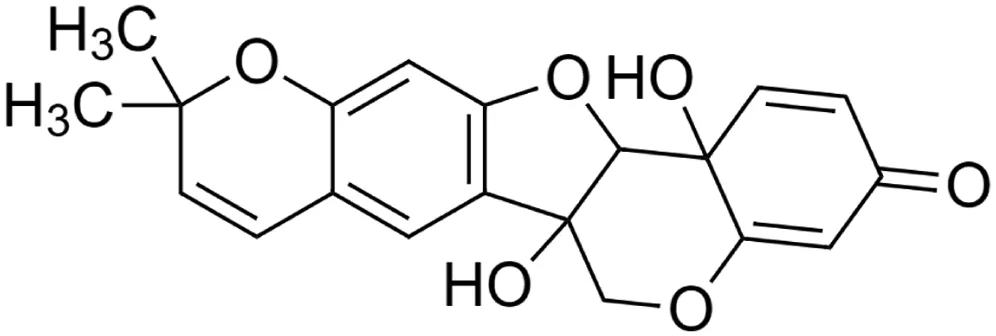 Hydroxytuberosone [19] (C20H18O6) Hydroxytuberosone [19] (C20H18O6)IUPAC name–[1,14-dihydroxy-7,7-dimethyl-8,12,20-trioxapentacyclo [11.8.0.02,11.04,9.014,19]henicosa-2 (11),3,5,9,15,18-hexaen-17-one] | In vivo | Topical application | Wound healing | Excision and incision wound model | Kambhoja and Murthy (2007) |
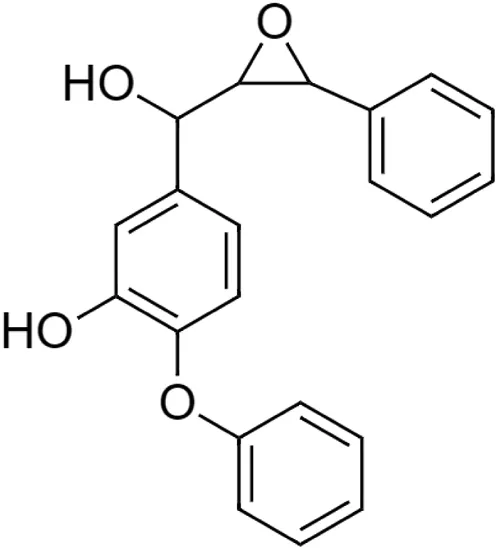 Puetuberosanol [20] (C21H18O4) Puetuberosanol [20] (C21H18O4)IUPAC name–[5-[hydroxy-(3-phenyloxiran-2-yl)methyl]-2-phenoxyphenol] | Pharmacological activity not reported | ||||
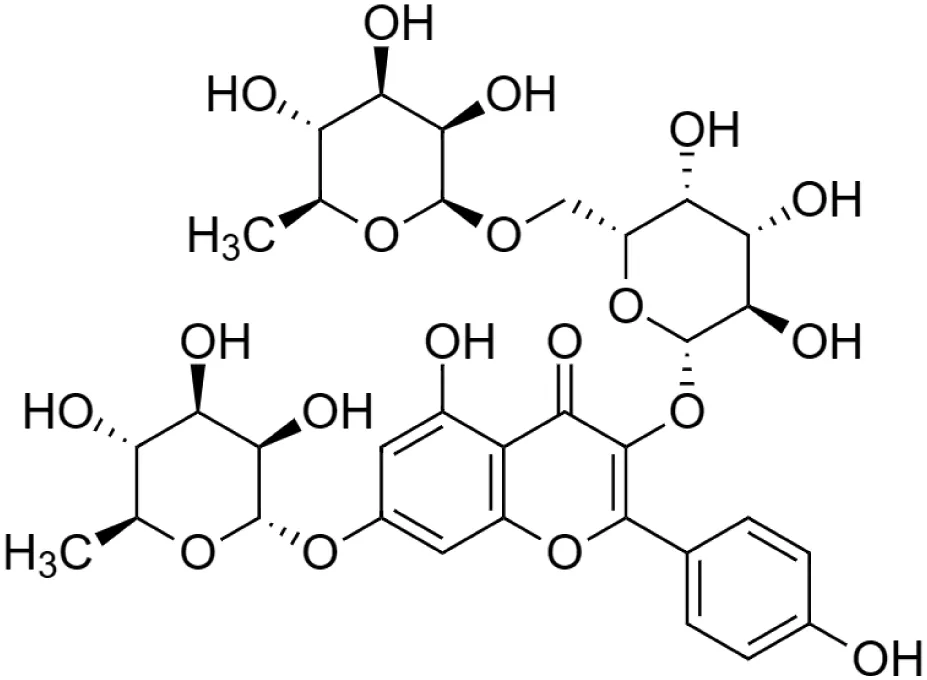 Robinin [21] (C33H40O19) Robinin [21] (C33H40O19)IUPAC name–[5-hydroxy-2-(4-hydroxyphenyl)-7-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy-3-[(2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-[[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxymethyl]oxan-2-yl]oxychromen-4-one] | In vivo | 50 mg/kg b/w for 10 days | Cardioprotective | Modulation of TGF-β1 signaling pathway in doxorubicin-induced cardiac toxicity in Sprague Dawley rats | Janeesh and Abraham (2014) |
| In vitro | 6 μg/ml | Immunomodulatory | Inhibited TLR4-NF-κB signaling pathway | Janeesh et al. (2014) | |
| In vitro | 0.125–0.50 mg/ml | Antioxidant | The total antioxidant capacity (TAC) in robinin was significantly higher and best maintained the follicular morphology | Dos Santos Morais et al. (2019) | |
 Tuberostan [22] (C21H16O5) Tuberostan [22] (C21H16O5)IUPAC name–[17-methoxy-7,7-dimethyl-8,12,20-trioxapentacyclo [11.8.0.02,11.04,9.014,19]henicosa-1(13),2(11),3,5,9,14 (19),15,17-octaen-21-one] | In silico | — | Antidiabetic | In molecular docking study, tuberostan showed best interaction for GLP-1R with binding energy at 8.15 kcal/mol and dissociation constant at 1061624.125 pM | Srivastava et al. (2018) |
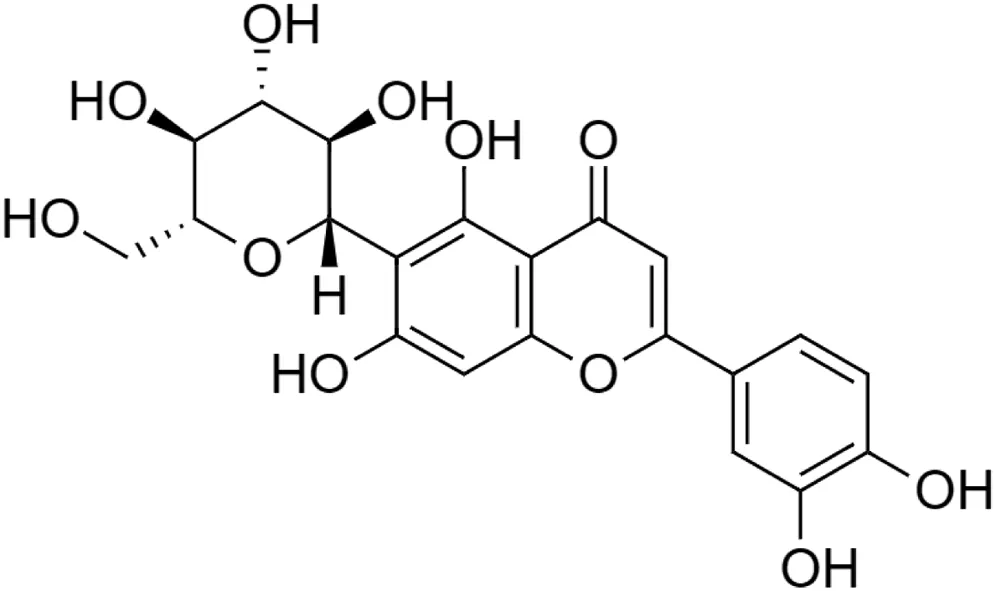 Isoorientin [23] (C21H20O11) Isoorientin [23] (C21H20O11)IUPAC name–[2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-6-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]chromen-4-one] | In vitro | 0.1–100 µM | Anti-inflammatory | Inhibited COX-2 activity by 64% | Sumalatha et al. (2015) |
| In vitro/in vivo | In vitro: 25 nM and 100 μM for 16 hours; in vivo: 10 mg/kg and 20 mg/kg b/w for 24 h | Anti-inflammatory | Inhibited the expression of COX-2 in vitro and decreased the expression of COX-2, TNF-α, IL-1β, iNOS, and 5-LOX in dose-dependent manner in carrageenan-induced inflammation in mice | Anilkumar et al. (2017) | |
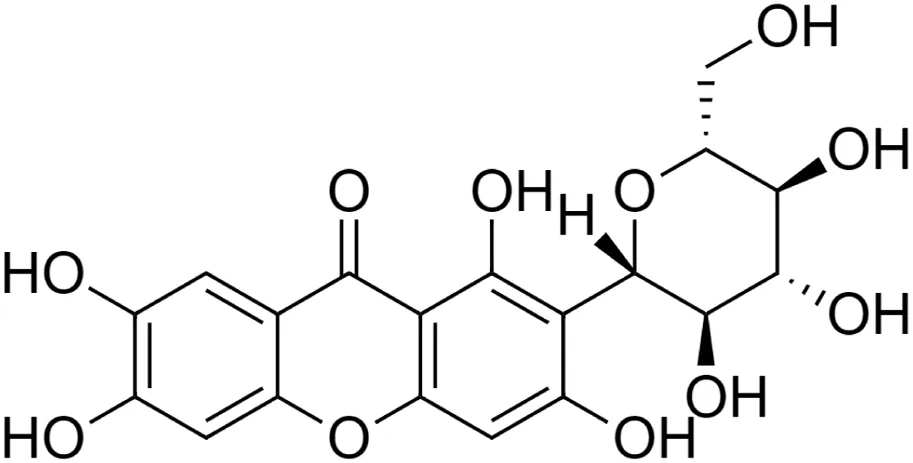 Mangiferin [24] (C19H18O11) Mangiferin [24] (C19H18O11)IUPAC name–[1,3,6,7-tetrahydroxy-2-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]xanthen-9-one] | In vitro | 100 µM | Anti-inflammatory | Inhibited COX-1 and COX-2 activity by 79.4% and 45.9%, respectively | Sumalatha et al. (2015) |
| In vitro/in vivo | In vitro: 20 and 40 μM for 16 h; in vivo: 10 mg/kg and 20 mg/kg b/w for 24 h | Anti-inflammatory | Reduced expression of inflammatory mediator (COX-2, iNOS, and TNF-α) and increased anti-inflammatory cytokine (IL-10) and increased size of blood vessels were significantly reduced, and cell infiltration was less compared to mice treated with carrageenan alone | Bulugonda et al. (2017) | |
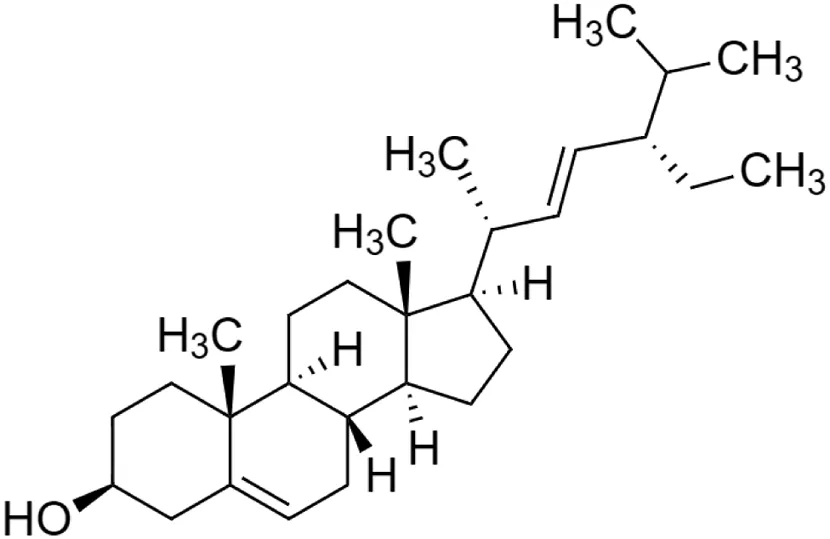 Stigmasterol [25] (C29H48O) Stigmasterol [25] (C29H48O)IUPAC name–[(3S,8S,9S,10R,13R,14S,17R)-17-[(E,2R,5S)-5-ethyl-6-methylhept-3-en-2-yl]-10,13-dimethyl-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta [a]phenanthren-3-ol] | In vivo | 200 mg/kg and 400 mg/kg b/w | Chemo-preventive | Induced a significant decrease in 7,12-dimethylbenz[a]anthracene (DMBA)-induced skin tumor | Ali et al. (2015) |
Pharmacological activities of phytoconstituents of Pueraria tuberosa.
b/w: body weight.
Toxicology of Pueraria tuberosa
The acute (single dose of 2,000 and 5,000 mg/kg body weight) and repeated dose (250, 500, 1,000, and 2,000 mg/kg body weight for 28 days) toxicity studies with water extract of the tuber of P. tuberosa were conducted in rats as per OECD (Organization for Economic Co-Operation and Development) guidelines. The survival rate and biochemical and histological changes were studied. No adverse effect was reported in single-dose acute toxicity, but in repeated dose toxicity studies, 100% mortality was observed on day 21 at 2,000 mg/kg body weight, and histological examination of the visceral organs showed that this mortality could be due to hepatotoxicity (Pandey et al., 2018). However, histological evaluation of different organs using hematoxylin and eosin staining did not observe any morphological alterations in the spleen, adrenal glands, and heart. The size and shapes in crypts and villi of the intestine and semeniferous tubules were intact with normal spermatozoa count in testis (Pandey et al., 2019). In another experiment on acute toxicity study of poly-herbal formulation (containing P. tubrosa), “Dhatryadi Ghrita” methanolic extract did not show any untoward effects in mice (Pal and Mishra, 2019).
Conclusion and Future Directions
The scientific community worldwide has shown an interest in discovering the disease combating potential of natural flora and bioactive compounds therein. A wide pool of literature suggests that these phytochemicals hold the immense potential of eliminating diseases, and many such plant-based drugs have long been used in many parts of the world. Markedly, the tuber and leaf of P. tuberosa plant have been used from ancient times in the traditional practices. Previous literature has shown that leaf and tuber extracts of the plant contain several bioactive constituents that possess an extensive range of pharmacological activities. Some of the isolated compounds, namely, puerarin, irisolidone, genistein, daidzein, biochanin A, biochanin B, isoorientin, and mangiferin, have been studied for various medicinal purposes and demonstrated several pharmacological activities like anticancerous, antidiabetic, anti-inflammatory, antioxidant, antiviral, cardioprotective, fibrinolytic, hepatoprotective, hypolipidemic, immunomodulatory, neuroprotective, nephroprotective, nootropic, vasodilatory, and wound healing. The bioactive constituents of P. tuberosa can individually or synergistically exert their therapeutic effects. Apart from puerarin, daidzein, genistein, irisolidone, and biochanin, many more compounds have been identified from P. tuberosa; however, underlying mechanisms of action of compounds isolated from this plant are not completely known. Thus, exploration of pharmacological mechanisms of individual bioactive constituents and their toxicity/clinical studies shall be the focus of future investigations. The extensive range of pharmacological properties of P. tuberosa could provide us a new interesting path for future research and may present new perspectives for the disease management.
Statements
Author contributions
RB was responsible for the methodology, writing the original draft, and data curation. BC and SR were responsible for data curation and reviewing and editing the manuscript. NK was responsible for conceptualization, data curation, writing, reviewing, and editing the manuscript.
Funding
RB was supported by CSIR-JRF fellowship, and NK lab was supported by grants from CSIR-IMTECH.
Acknowledgments
The authors gratefully acknowledge the Foundation for Revitalization of Local Health Traditions and the University of TransDisciplinary Health Sciences and Technology (FRLHT-TDU) for providing the permission to use images of P. tuberosa plant from Indian Medicinal Plants database.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1
AdedapoA. A.FagbohunO. A.DawurungC.OyagbemiA. A.OmobowaleT. O.YakubuM. A. (2017). The aqueous tuber extract of Pueraria tuberosa (Willd.) D.C. caused cytotoxic effect on HT 29 cell lines with down regulation of nuclear factor-kappa B (NF-κB). J. Compl. Integr. Med.16 (4), 1–8. 10.1515/jcim-2016-0119
2
AliH.DixitS.AliD.AlqahtaniS. M.AlkahtaniS.AlarifiS. (2015). Isolation and evaluation of anticancer efficacy of stigmasterol in a mouse model of DMBA-induced skin carcinoma. Drug Des. Dev. Ther.9, 2793–2800. 10.2147/DDDT.S83514
3
AmalanV.VijayakumarN.IndumathiD.RamakrishnanA. (2016). Antidiabetic and antihyperlipidemic activity of p-coumaric acid in diabetic rats, role of pancreatic GLUT 2: in vivo approach. Biomed. Pharmacother.84, 230–236. 10.1016/j.biopha.2016.09.039
4
AnilkumarK.ReddyG. V.AzadR.Sastry YarlaN.DharmapuriG.SrivastavaA.et al (2017). Evaluation of anti-inflammatory properties of isoorientin isolated from tubers of pueraria tuberosa. Oxid. Med. Cell. Longev.2017, 1–7. 10.1155/2017/5498054
5
ArunaM. R.KumarD. J. M.SenbagamD.SenthilkumarB. (2016). Investigation on phytochemical and antimicrobial properties of tuber extracts of pueraria tuberosa linn. J. Pure Appl. Microb.10 (2), 1573–1578.
6
ArunaM. R.Mukesh KumarD. J.PalaniP.SenbagamD.SenthilkumarB. (2018). Effects of pueraria tuberosa linn hydroalcoholic tuber extract on expression of apoptosis associated proteins in HT-29 human colon carcinoma cell line. Int. J. Curr. Microbiol. Appl. Sci.7 (6), 3863–3873. 10.20546/ijcmas.2018.706.455
7
Ayurvedic pharmacopoeia of India (1999). Ayurvedic pharmacopoeia of India.Part-1, 2. New Delhi, India: Ministry of Health and Family Planning, Department of Health, Government of India, 1–190.
8
Ayurvedic pharmacopoeia of India (2001). Ayurvedic pharmacopoeia of India Part-1., 2. New Delhi, India: Ministry of Health and Family Planning, Department of Health, Government of India, 183–184.
9
BabuP. V.BandiS.RajuM.TiwariV. K. (2016). Antioxidant and immunosuppressant activity of pueraria tuberosa. IJPPR.8 (1), 23–34.
10
BagheriM.JoghataeiM. T.MohseniS.RoghaniM. (2011). Genistein ameliorates learning and memory deficits in amyloid β(1-40) rat model of Alzheimer's disease. Neurobiol. Learn. Mem.95 (3), 270–276. 10.1016/j.nlm.2010.12.001
11
BasavarajP.ShivakumarB.ShivakumarH. (2011). Evaluation of anticonvulsant activity of alcoholic extract of tubers of Pueraria tuberosa (Roxb). Adv. in Pharm. and Toxicol.12 (I), 1–9.
12
BerközM.KrośniakM.Özkan-YılmazF.Özlüer-HuntA. (2020). Prophylactic effect of Biochanin A in lipopolysaccharide-stimulated BV2 microglial cells. Immunopharmacol. Immunotoxicol.1. 1010.1080/08923973.2020.1769128
13
BhattaraiG.MinC. K.JeonY. M.BashyalR.PoudelS. B.KookS. H.et al (2019). Oral supplementation with p-coumaric acid protects mice against diabetes-associated spontaneous destruction of periodontal tissue. J. Periodontal. Res.54 (6), 690–701. 10.1111/jre.12678
14
BulugondaR. K.KumarK. A.GangappaD.BeedaH.PhilipG. H.Muralidhara RaoD.et al (2017). Mangiferin from Pueraria tuberosa reduces inflammation via inactivation of NLRP3 inflammasome. Sci. Rep.7, 42683–42714. 10.1038/srep42683
15
ChaH.LeeS.LeeJ. H.ParkJ. W. (2018). Protective effects of p-coumaric acid against acetaminophen-induced hepatotoxicity in mice. Food Chem. Toxicol.121, 131–139. 10.1016/j.fct.2018.08.060
16
ChauhanN. S.SharmaV.ThakurM.Christine Helena Frankland SawayaA.DixitV. K. (2013). Pueraria tuberosa DC extract improves androgenesis and sexual behavior via FSH LH cascade. Sci. World. J.2013, 1–10. 10.1155/2013/780659
17
ChenX.HeZ.WuX.MaoD.FengC.ZhangJ.et al (2020). Comprehensive study of the interaction between Puerariae Radix flavonoids and DNA: from theoretical simulation to structural analysis to functional analysis. Spectrochim. Acta Mol. Biomol. Spectrosc.231. 10.1016/j.saa.2020.118109
18
ChoiE. J.KimG. H. (2014). The antioxidant activity of daidzein metabolites, O‑desmethylangolensin and equol, in HepG2 cells. Mol. Med. Rep.9 (1), 328–332. 10.3892/mmr.2013.1752
19
ChoiE. M.SuhK. S.ParkS. Y.ChinS. O.RheeS. Y.ChonS. (2019). Biochanin A prevents 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced adipocyte dysfunction in cultured 3T3-L1 cells. J. Environ. Sci. Heal.54 (9), 865–873. 10.1080/10934529.2019.1603746
20
ChoiY. R.ShimJ.KimM. J. (2020). Genistin: a novel potent anti-adipogenic and anti-lipogenic agent. Molecules25 (9). 10.3390/molecules25092042
21
CroomE. M. (2004). Pueraria. The Genus pueraria. Econ. Bot.58 (3), 490. 10.1663/0013-0001(2004)058[0490:dfabre]2.0.co;2
22
DalalP. K.TripathiA.GuptaS. K. (2013). Vajikarana: treatment of sexual dysfunctions based on Indian concepts. Indian J. Psych.55, S273. 10.4103/0019-5545.105550
23
De LucaV.SalimV.AtsumiS. M.YuF. (2012). Mining the biodiversity of plants: a revolution in the making. Science336 (6089), 1658–1661. 10.1126/science.1217410
24
DeyD.PalB. C.BiswasT.RoyS. S.BandyopadhyayA.MandalS. K.et al (2007). A lupinoside prevented fatty acid induced inhibition of insulin sensitivity in 3T3 L1 adipocytes. Mol. Cell. Biochem.300 (1–2), 149–157. 10.1007/s11010-006-9378-1
25
Dos Santos MoraisM. L. G.de BritoD. C. C.PintoY.Mascena SilvaL.Montano VizcarraD.SilvaR. F.et al (2019). Natural antioxidants in the vitrification solution improve the ovine ovarian tissue preservation. Reprod. Biol.19 (3), 270–278. 10.1016/j.repbio.2019.07.008
26
El-BakoushA.OlajideO. A. (2018). Formononetin inhibits neuroinflammation and increases estrogen receptor beta (ERβ) protein expression in BV2 microglia. Int. Immunopharm.61, 325–337. 10.1016/j.intimp.2018.06.016
27
ElmarakbyA. A.IbrahimA. S.FaulknerJ.MozaffariM. S.LiouG. I.AbdelsayedR. (2011). Tyrosine kinase inhibitor, genistein, reduces renal inflammation and injury in streptozotocin-induced diabetic mice. Vasc. Pharmacol.55 (5–6), 149–156. 10.1016/j.vph.2011.07.007
28
FuX.QinT.YuJ.JiaoJ.MaZ.FuQ.et al (2019). Formononetin ameliorates cognitive disorder via PGC-1α pathway in neuroinflammation conditions in high-fat diet-induced mice. CNS Neurol. Disord. - Drug Targets18 (7), 566–577. 10.2174/1871527318666190807160137
29
GindiS.AwenB. Z.BaburaoC.KhaggaM. (2010). Anti ulcerogenic & ulcer healing studies of aqueous extract of Pueraria tuberosa leaves on rats. Int. J. Pharma Bio Sci.1 (4), 651–661.
30
GuM.ZhengA. B.JinJ.CuiY.ZhangN.CheZ. P.et al (2016). Cardioprotective effects of genistin in rat myocardial ischemia-reperfusion injury studies by regulation of P2X7/NF-κB pathway. Evid. Based Complement Alternat. Med.2016, 5381290. 10.1155/2016/5381290
31
GuoC. J.XieJ. J.HongR. H.PanH. S.ZhangF. G.LiangY. M. (2019). Puerarin alleviates streptozotocin (STZ)-induced osteoporosis in rats through suppressing inflammation and apoptosis via HDAC1/HDAC3 signaling. Biomed. Pharmacother.115, 108570. 10.1016/j.biopha.2019.01.031
32
GuptaR. S.SharmaR.SharmaA.ChoudharyR.BhatnagerA. K.JoshiY. C.et al (2005). Antifertility effects of Puerariaï¿¿tuberosa. Root extract in male rats. Pharmaceut. Biol.42 (8), 603–609. 10.1080/13880200490902491
33
HanN. R.KimH. M.JeongH. J. (2015). The potential anti-proliferative effect of β-sitosterol on human mast cell line-1 cells. Can. J. Physiol. Pharmacol.93 (11), 979–983. 10.1139/cjpp-2015-0166
34
HeY.WuX.CaoY.HouY.ChenH.WuL.et al (2016). Daidzein exerts anti-tumor activity against bladder cancer cells via inhibition of FGFR3 pathway. Neoplasma.63 (4), 523–531. 10.4149/neo_2016_405
35
HeinrichM.AppendinoG.EfferthT.FürstR.IzzoA. A.KayserO.et al (2020). Best practice in research - overcoming common challenges in phytopharmacological research. J. Ethnopharmacol.246, 112230. 10.1016/j.jep.2019.112230
36
HuaF.LiC. H.ChenX. G.LiuX. P. (2018). Daidzein exerts anticancer activity towards SKOV3 human ovarian cancer cells by inducing apoptosis and cell cycle arrest, and inhibiting the Raf/MEK/ERK cascade. Int. J. Mol. Med.41 (6), 3485–3492. 10.3892/ijmm.2018.3531
37
HuangD.WangC.DuanY.MengQ.LiuZ.HuoX.et al (2017). Targeting Oct2 and P53: formononetin prevents cisplatin-induced acute kidney injury. Toxicol. Appl. Pharmacol. N.326, 15–24. 10.1016/j.taap.2017.04.013
38
HuangZ.LiuY.HuangX. (2018). Formononetin may protect aged hearts from ischemia/reperfusion damage by enhancing autophagic degradation. Mol. Med. Rep.18 (6), 4821–4830. 10.3892/mmr.2018.9544
39
HwangJ. S.KangE. S.HanS. G.LimD. S.PaekK. S.LeeC. H.et al (2018). Formononetin inhibits lipopolysaccharideinduced release of high mobility group box 1 by upregulating SIRT1 in a PPARδ-dependent manner. Peer J.6 (1, e4208). 10.7717/peerj.4208
40
Indian Medicinal Plant DatabaseFoundation for Revitalisation of Local Health Traditions (FRLHT).Available at: http://www.medicinalplants.in/searchpage/showdetails/xplant_id/5e61a3ccb898ce34f62ec050b012b2f2/keywords/vidari/languages/HI.
41
JaneeshP. A.AbrahamA. (2014). Robinin modulates doxorubicin-induced cardiac apoptosis by TGF-β1 signaling pathway in Sprague Dawley rats. Biomed. Pharmacother.68 (8), 989–998. 10.1016/j.biopha.2014.09.010
42
JaneeshP. A.SasikalaV.DhanyaC. R.AbrahamA. (2014). Robinin modulates TLR/NF-κB signaling pathway in oxidized LDL induced human peripheral blood mononuclear cells. Int. Immunopharm.18 (1), 191–197. 10.1016/j.intimp.2013.11.023
43
JangH. M.ParkK. T.NohH. D.LeeS. H.KimD. H. (2019). Kakkalide and irisolidone alleviate 2,4,6-trinitrobenzenesulfonic acid-induced colitis in mice by inhibiting lipopolysaccharide binding to toll-like receptor-4 and proteobacteria population. Int. Immunopharm.73, 246–253. 10.1016/j.intimp.2019.05.008
44
JiangK.ChenH.TangK.GuanW.ZhouH.GuoX.et al (2018). Puerarin inhibits bladder cancer cell proliferation through the mTOR/p70S6K signaling pathway. Oncol Lett.15 (1), 167–174. 10.3892/ol.2017.7298
45
KambhojaS.MurthyK. R. K. (2007). Wound healing and anti-inflammatory activity of Pueraria tuberosa (Roxb Ex wild) DC. Biomed.2 (2), 229–232.
46
KangG. D.LeeS. Y.JangS. E.HanM. J.KimD. H. (2017). Irisolidone attenuates ethanol-induced gastric injury in mice by inhibiting the infiltration of neutrophils. Mol. Nutr. Food Res.61 (2). 10.1002/mnfr.201600517
47
KanterM.TuncerI.ErbogaM.AtanassovaP.TakirM.KostekO. (2016). The effects of quercetin on liver regeneration after liver resection in rats. Folia Morphol.75 (2), 179–187. 10.5603/FM.a2015.0086
48
KeungW. M. (2002). Pueraria. The Genus pueraria.Boca Raton, FL: CRC Press. 10.1201/9780203300978
49
KimH. B.LeeS.HwangE. S.MaengS.ParkJ. H. (2017). p-Coumaric acid enhances long-term potentiation and recovers scopolamine-induced learning and memory impairments. Biochem. Biophys. Res. Commun.492 (3), 493–499. 10.1016/j.bbrc.2017.08.068
50
KimK. A.LeeI. A.GuW.HyamS. R.KimD. H. (2014). β-Sitosterol attenuates high-fat diet-induced intestinal inflammation in mice by inhibiting the binding of lipopolysaccharide to toll-like receptor 4 in the NF-κB pathway. Mol. Nutr. Food Res.58 (5), 963–972. 10.1002/mnfr.201300433
51
KimM.ChoiY.LeeJ.KimK.YangW. M. (2016). Topical treatment of hair loss with formononetin by modulating apoptosis. Planta Med.82 (1–2), 65–69. 10.1055/s-0035-1557897
52
KirtikarK. R.BasuB. D. (1935). Indian medicinal plants.Allahabad, India: Allahabad Lalit Mohan Basu.
53
KumarP. G. (2016). Wild edible plants of Hassan District, Karnataka: a role in ayurvedic formulation. Int. J. Herb. Med.4 (1), 16–24.
54
KumawatR. B.SharmaR. A.MaliP. C.ChandrawatP. (2017). Ethnophormacological screening of some selected medicinal plants. Res. J. Recent Sci.6 (5), 32–41.
55
LeeH.LeeD.KangK. S.SongJ. H.ChoiY. K. (2018). Inhibition of intracellular ROS accumulation by formononetin attenuates cisplatin-mediated apoptosis in LLC-PK1 cells. Int. J. Mol. Sci.19 (3). 10.3390/ijms19030813
56
legumesAvailable at: http://www.legumes-online.net/ildis/aweb/td076/td_16078.htm (Accessed July 7, 2020).
57
LiS.-C. (2003). Chinese medical herbs: a modern edition of a classic sixteenth-century manual.Mineola, NY: Dover Publications Inc.
58
LiX.ZhuQ.ZhengR.YanJ.WeiM.FanY.et al (2020). Puerarin attenuates diabetic nephropathy by promoting autophagy in podocytes. Front. Physiol.11. 10.3389/fphys.2020.00073
59
LiY. L.GuoH.ZhaoY. Q.LiA. F.RenY. Q.ZhangJ. W. (2017). Quercetin protects neuronal cells from oxidative stress and cognitive degradation induced by amyloid β-peptide treatment. Mol. Med. Rep.16 (2), 1573–1577. 10.3892/mmr.2017.6704
60
LikhitkarM.PandeM. (2017). Antioxidant activity of methanolic and ethanolic extracts of Pueraria tuberosa plant. Int J Ind Herbs Drugs2 (1), 1–5.
61
LiuD.ZhenW.YangZ.CarterJ. D.SiH.ReynoldsK. A. (2006). Genistein acutely stimulates insulin secretion in pancreatic beta-cells through a cAMP-dependent protein kinase pathway. Diabetes55 (4), 1043–1050. 10.2337/diabetes.55.04.06.db05-1089
62
LiuX.ZhaoW.WangW.LinS.YangL. (2017). Puerarin suppresses LPS-induced breast cancer cell migration, invasion and adhesion by blockage NF-κB and Erk pathway. Biomed. Pharmacother.92, 429–436. 10.1016/j.biopha.2017.05.102
63
LuoL.ZhouJ.ZhaoH.FanM.GaoW. (2019). The anti-inflammatory effects of formononetin and ononin on lipopolysaccharide-induced zebrafish models based on lipidomics and targeted transcriptomics. Metabolomics15 (12, 153). 10.1007/s11306-019-1614-2
64
MajiA. K.PanditS.BanerjiP.BanerjeeD. (2014). Natural product research., 28. Abingdon, UK: Taylor & Francis, 2111–2127. 10.1080/14786419.2014.928291
65
MajiA. K.MahapatraS.BanerjeeD. (2014). In-vivo immunomodulatory potential of standardized pueraria tuberosa extract and its isoflavonoids. Int. J. Pharm. Pharmaceut. Sci.6 (1), 861–867.
66
MhaskeH. P.VaidyaV. V.KekareM. B.ChampanerkarP. A.ParekhS. A. (2009). Quantification of {β-sitosterol and puerarin from Pueraria tuberosa DC. by using high performance thin layer chromatography. Asian J. Chem.21 (5), 3449–3454.
67
MigkosT.PourováJ.VopršalováM.AugerC.Schini-KerthV.MladěnkaP. (2020). Biochanin A, the most potent of 16 isoflavones, induces relaxation of the coronary artery through the calcium channel and cGMP-dependent pathway. Planta Med.86 (10), 708–716. 10.1055/a-1158-9422
68
NagwaniS.TripathiY. B. (2010). Amelioration of cisplatin induced nephrotoxicity by PTY: a herbal preparation. Food Chem. Toxicol.48 (8–9), 2253–2258. 10.1016/j.fct.2010.05.057
69
National Medicinal Plants Board, Government of IndiaRetrieved from https://www.nmpb.nic.in/about-us July 7, 2020).
70
NavaneethanD.RasoolM. (2014). p-Coumaric acid, a common dietary polyphenol, protects cadmium chloride-induced nephrotoxicity in rats. Ren. Fail.36 (2), 244–251. 10.3109/0886022X.2013.835268
71
NeogM. K.Joshua PragasamS.KrishnanM.RasoolM. (2017). p-Coumaric acid, a dietary polyphenol ameliorates inflammation and curtails cartilage and bone erosion in the rheumatoid arthritis rat model. Biofactors43 (5), 698–717. 10.1002/biof.1377
72
OzaM. J.KulkarniY. A. (2018b). Biochanin A improves insulin sensitivity and controls hyperglycemia in type 2 diabetes. Biomed. Pharmacother.107, 1119–1127. 10.1016/j.biopha.2018.08.073
73
OzaM. J.KulkarniY. A. (2019). Formononetin attenuates kidney damage in type 2 diabetic rats. Life Sci.219, 109–121. 10.1016/j.lfs.2019.01.013
74
OzaM. J.KulkarniY. A. (2018a). Formononetin treatment in type 2 diabetic rats reduces insulin resistance and hyperglycemia. Front. Pharmacol.9, 73. 10.3389/fphar.2018.00739
75
PalR. S.MishraA. (2019). Evaluation of acute toxicity of the methanolic extract of dhatryadi ghrita in wistar rats. Open Pharmacol. J.9 (1), 1–4. 10.2174/1874143601909010001
76
PalanisamyN.VenkataramanA. C. (2013). Beneficial effect of genistein on lowering blood pressure and kidney toxicity in fructose-fed hypertensive rats. Br. J. Nutr.109 (10), 1806–1812. 10.1017/S0007114512003819
77
PandeyH.SrivastavaS.SinghS.TripathiY. B. (2019). Histopathological study of different organs of charles foster strain rat under the exposure of Pueraria tuberosa. BioRxiv.671529. 10.1101/671529
78
PandeyH.SrivastavaS.TripathiY. B. (2019). Herbal tablet of Pueraria tuberosa water extract suppresses the alloxan induced liver damage and hyperglycemia in rats. BioRxiv.671594. 10.1101/671594
79
PandeyH.SrivastavaS.TripathiY. B.KumarR. (2018). Preclinical acute and repeated dose toxicity of Pueraria tuberosa (PTWE) on charles foster rats. Int. J. Pharmaceut. Sci. Res.9 (11), 4572. 10.13040/IJPSR.0975-8232.9(11).4572-81
80
PandeyN.TripathiY. B. (2010). Antioxidant activity of tuberosin isolated from Pueraria tuberose Linn. J. Inflamm.7, 47. 10.1186/1476-9255-7-47
81
PandeyN.ChaurasiaJ. K.TiwariO. P.TripathiY. B. (2007). Antioxidant properties of different fractions of tubers from Pueraria tuberosa Linn. Food Chem.105 (1), 219–222. 10.1016/j.foodchem.2007.03.072
82
PandyaK. B.PatelH. B.BhattP. R.PatelU. D.ModiC. M. (2019). In vitro antibacterial activity of sixteen medicinal plants collected from nearby region of Junagadh, Gujarat (India). J. Pharm. Innov.8 (3), 662–667.
83
ParkC. E.YunH.LeeE. B.MinB. I.BaeH.ChoeW.et al (2010). The antioxidant effects of genistein are associated with AMP-activated protein kinase activation and PTEN induction in prostate cancer cells. J. Med. Food.13 (4), 815–820. 10.1089/jmf.2009.1359
84
PatelJ.DoshiN.BhaleraoA.BonagiriR. (2016). Immunomodulatory activity of ethanolic extract of Pueraria Tuberosa Immunomodulatory activity of ethanolic extract of Pueraria Tuberosa D.C. Int. J. Sci. Eng. Res.7 (11), 708–713.
85
PatelR. V.MistryB. M.ShindeS. K.SyedR.SinghV.ShinH. S. (2018). Therapeutic potential of quercetin as a cardiovascular agent. Eur. J. Med. Chem.155, 889–904. 10.1016/j.ejmech.2018.06.053
86
PragasamS. J.VenkatesanV.RasoolM. (2013). Immunomodulatory and anti-inflammatory effect of p-coumaric acid, a common dietary polyphenol on experimental inflammation in rats. Inflammation36 (1), 169–176. 10.1007/s10753-012-9532-8
87
PramanikS. S.SurT. K.DebnathP. K.BhattacharyyaD. (2010). Effect of Pueraria tuberosa tuber extract on chronic foot shock stress in Wistar rats, Nepal Med. Coll. J.12 (4), 234–238.
88
PramanikS. S.SurT. K.DebnathP. K.PramanikT.BhattacharyyaD. (2011). Effect of Pueraria tuberosa on cold immobilization stress induced changes in plasma corticosterone and brain monoamines in rats. J. Nat. Remedies.11 (1), 69–75. 10.18311/jnr/2011/52
89
Pueraria tuberosa-VikaspediaAvailable at: https://vikaspedia.in/agriculture/crop-production/package-of-practices/medicinal-and-aromatic-plants/pueraria-tuberosa.(Retrieved from September 21 2020).
90
PuriH. S. (2003). Rasayana: Ayurvedic Herbs for Longevity and Rejuvenation.3 (2), (Hoboken, NJ: CRC Press). 303–307.
91
QianY.GuanT.HuangM.CaoL.LiY.ChengH.et al (2012). Neuroprotection by the soy isoflavone, genistein, via inhibition of mitochondria-dependent apoptosis pathways and reactive oxygen induced-NF-κB activation in a cerebral ischemia mouse model. Neurochem. Int.60 (8), 759–767. 10.1016/j.neuint.2012.03.011
92
QiuG.TianW.HuanM.ChenJ.FuH. (2017). Formononetin exhibits anti-hyperglycemic activity in alloxan-induced type 1 diabetic mice. Exp. Biol. Med.242 (2), 223–230. 10.1177/1535370216657445
93
RaghuwanshiR.JainB. (2011). Hypoglycemic effect of Pueraria tuberosa tubers in healthy and alloxan diabetic Rats. J. Chem. Biol. Phys. Sci.2 (1), 270–272.
94
RaniV. U.SudhakarM.RameshA. (2017). Protective effect of Pueraria tuberosa Linn. in arsenic induced nephrotoxicity in rats. Asian J. Pharmaceut. Res.7 (1), 15. 10.5958/2231-5691.2017.00003.x
95
RaoN. V.PujarB.NimbalS. K.ShantakumarS. M.SatyanarayanaS. (2008). Nootropic activity of tuber extract of Pueraria tuberosa (roxb). Indian J. Exp. Biol.46 (8), 591–598.
96
RatnamV.Venkata RajuR. R. (2009). Preliminary phytochemical and antimicrobial properties of pueraria tuberosa (Willd.) DC: a potential medicinal plant. Ethnobotanical Leaflets.13, 1051–1059.
97
RawtalB.SahatpureN.SakharwadeS. (2019). Pueraria tuberosa (vidarikanda): an emerging cosmeceutical herb. Int. J. Sci.4 (7), 130–137.
98
RobinsonM. M.ZhangX. (2011). The world medicines situation 2011, traditional Medicines : global situation , issues and challenges.3rd Edn. (Geneva, Switzerland: World Health Organization), 1–14.
99
RoyA. J.Stanely Mainzen PrinceP. (2013). Preventive effects of p-coumaric acid on cardiac hypertrophy and alterations in electrocardiogram, lipids, and lipoproteins in experimentally induced myocardial infarcted rats. Food Chem. Toxicol.60, 348–354. 10.1016/j.fct.2013.04.052
100
SabithaR.NishiK.GunasekaranV. P.AgilanB.DavidE.AnnamalaiG.et al (2020). p-Coumaric acid attenuates alcohol exposed hepatic injury through MAPKs, apoptosis and Nrf2 signaling in experimental models. Chem. Biol. Interact.321, 109044. 10.1016/j.cbi.2020.109044
101
SadgunaV.SarikhaK.KomuraiahT. R.MustafaM. (2015). Anti-microbial activity of pueraria tuberosa DC, an economically and medicinally important plant. Int. J. Curr. Microbiol. App. Sci.4 (5), 152–159Available at: https://www.ijcmas.com/vol-4-5.
102
SakamotoY.NakaA.OharaN.KondoK.IidaK. (2014). Daidzein regulates proinflammatory adipokines thereby improving obesity-related inflammation through PPARγ. Mol. Nutr. Food Res.58 (4), 718–726. 10.1002/mnfr.201300482
103
SakamulaR.Thong-asaW. (2018). Neuroprotective effect of p-coumaric acid in mice with cerebral ischemia reperfusion injuries. Metab. Brain Dis.33 (3), 765–773. 10.1007/s11011-018-0185-7
104
SatpathyS.PatraA.AhirwarB.HussainM. D. (2018). Antioxidant and anticancer activities of green synthesized silver nanoparticles using aqueous extract of tubers of Pueraria tuberosa. Artificial Cells. Nanomed. and Biotechnol.46 (Suppl. 3), S71–S85. 10.1080/21691401.2018.1489265
105
SatpathyS.PatraA.HussainM. D.AhirwarB. (2017). Simultaneous estimation of genistein and daidzein in Pueraria tuberosa (Willd.) DC by validated high-performance thin-layer chromatography (HPTLC) densitometry method. J. Liq. Chromatogr. Relat. Technol.40 (10), 499–505. 10.1080/10826076.2017.1329743
106
SatpathyS.PatraA.HussainM. D.KaziM. (2020). Antioxidant enriched fraction from Pueraria tuberosa alleviates ovariectomized-induced osteoporosis in rats, and inhibits growth of breast and ovarian cancer cell lines in vitro. BioRxiv.10.1101/2020.09.21.305953
107
SawaleP. D.SinghR. R. B.KapilaS.AroraS.RastogiS.RawatA. K. S. (2013). Immunomodulatory and antioxidative potential of herb (Pueraria tuberosa) in mice using milk as the carrier. Int. J. Dairy Technol.66 (2), 202–206. 10.1111/1471-0307.12011
108
SharmaS.AgrawalM.LalM. (2018). Cultivation of “vidarikand” (Pueraria tuberosa dc): a drug of potential importance. Int. J. Inf. Retr. Res. (IJIRR).5 (5), 5460–5462.
109
SharmaS. H.RajamanickamV.NagarajanS. (2018). Antiproliferative effect of p-Coumaric acid targets UPR activation by downregulating Grp78 in colon cancer. Chem. Biol. Interact.291, 16–28. 10.1016/j.cbi.2018.06.001
110
SharmilaR.SindhuG.ArockianathanP. M. (2016). Nephroprotective effect of β-sitosterol on N-diethylnitrosamine initiated and ferric nitrilotriacetate promoted acute nephrotoxicity in Wistar rats. J. Basic Clin. Physiol. Pharmacol.27 (5), 473–482. 10.1515/jbcpp-2015-0085
111
ShilpashreeV. K.DangR.DasK. (2015). Evaluation of phytochemical investigation and immunomodulatory activity of four different plant species of vidari by carbon clearance test on wister rats. Ann. Phytomed.4 (1), 94–98.
112
ShuklaR.BanerjeeS.TripathiY. B. (2018a). Antioxidant and Antiapoptotic effect of aqueous extract of Pueraria tuberosa (Roxb. Ex Willd.) DC. On streptozotocin-induced diabetic nephropathy in rats. BMC Compl. Alternative Med.18 (1), 156. 10.1186/s12906-018-2221-x
113
ShuklaR.BanerjeeS.TripathiY. B. (2018b). Pueraria tuberosa extract inhibits iNOS and IL-6 through suppression of PKC-α and NF-kB pathway in diabetes-induced nephropathy. J. Pharm. Pharmacol.70 (8), 1102–1112. 10.1111/jphp.12931
114
ShuklaR.PandeyN.BanerjeeS.TripathiY. B.TripathiY. B. (2017). Effect of extract of Pueraria tuberosa on expression of hypoxia inducible factor-1α and vascular endothelial growth factor in kidney of diabetic rats. Biomed. Pharmacother.93, 276. 10.1016/j.biopha.2017.06.045
115
ShuklaS.JonathanS.SharmaA. (1996). Protective action of butanolic extract of Pueraria tuberosa DC. against carbon tetrachloride induced hepatotoxicity in adult rats. Phytother Res.10 (7), 608–609. 10.1002/(SICI)1099-1573(199611)10:7<608::AID-PTR842>3.0.CO;2-1
116
SrivastavaS.PandeyH.SinghS. K.TripathiY. B. (2019). Anti-oxidant, anti-apoptotic, anti-hypoxic and anti-inflammatory conditions induced by PTY-2 against STZ-induced stress in islets. Biosci Trends13 (5), 382–393. 10.5582/bst.2019.01181
117
SrivastavaS.ShreeP.PandeyH.TripathiY. B. (2018). Incretin hormones receptor signaling plays the key role in antidiabetic potential of PTY-2 against STZ-induced pancreatitis. Biomed. Pharmacother.97, 330–338. 10.1016/j.biopha.2017.10.071
118
SrivastavaS.ShreeP.TripathiY. B. (2017). Active phytochemicals of Pueraria tuberosa for DPP-IV inhibition: in silico and experimental approach. J. Diabetes Metab. Disord.16 (1), 46. 10.1186/s40200-017-0328-0
119
SrivastavaS.KoleyT. K.SinghS. K.TripathiY. B. (2015). The tuber extract of pueraria tuberosa Linn. competitively inhibits DPP-IV activity in normoglycemic rats. Int. J. Pharm. Pharmaceut. Sci.7 (9), 227–231.
120
SukhotnikI.MoatiD.ShaoulR.LobermanB.PollakY.SchwartzB. (2018). Quercetin prevents small intestinal damage and enhances intestinal recovery during methotrexate-induced intestinal mucositis of rats. Food Nutr. Res.62. 10.29219/fnr.v62.1327
121
SumalathaM.MunikishoreR.RammohanA.GunasekarD.KumarK. A.ReddyK. K.et al (2015). Isoorientin, a selective inhibitor of cyclooxygenase-2 (COX-2) from the tubers of pueraria tuberosa. Nat Prod Commun.10 (10), 1703–1704. 10.1177/1934578x1501001017
122
TanakaT.MoriyamaT.KawamuraY.YamanouchiD. (2016). Puerarin suppresses macrophage activation via antioxidant mechanisms in a CaPO. J. Nutr. Sci. Vitaminol.62, 425–431. 10.3177/jnsv.62.425
123
TannaI.AgheraH.AshokB.ChandolaH. (2012). Protective role of Ashwagandharishta and flax seed oil against maximal electroshock induced seizures in albino rats. Ayu.33 (1), 114–118. 10.4103/0974-8520.100327
124
TanwarY.GoyalS.RamawatK. (2008). Hypolipidemic effects of tubers of Indian kudzu (Pueraria tuberosa). J Herb Med Toxicol.2, 21–25.
125
TieL.AnY.HanJ.XiaoY.XiaokaitiY.FanS.et al (2013). Genistein accelerates refractory wound healing by suppressing superoxide and FoxO1/iNOS pathway in type 1 diabetes. JNB (J. Nutr. Biochem.).24 (1), 88–96. 10.1016/j.jnutbio.2012.02.011
126
TripathiA. K.KohliS. (2013). Anti-diabetic activity and phytochemical screening of crude extracts of PuerariaTuberosa DC. (FABACEAE) grown in India on STZ -induced diabetic rats. Asian J. Med. Pharmaceut. Res.3 (3), 66–73.
127
TripathiY. B.AditiP. (2020). Antioxidative and hypolipidemic effect of pueraria tuberosa water extract (ptwe) in rats with high fat diet induced non-alcoholic fatty liver disease (nafld). Int. J. Pharmaceut. Sci. Res.11 (1), 378–386. 10.1017/CBO9781107415324.004
128
TripathiY. B.NagwaniS.MishraP.JhaA.RaiS. P. (2012). Protective effect of Pueraria tuberosa DC. Embedded biscuit on cisplatin-induced nephrotoxicity in mice. J. Nat. Med.66 (1), 109–118. 10.1007/s11418-011-0559-1
129
TripathiY. B.ShuklaR.PandeyN.PandeyV.KumarM. (2017). An extract of Pueraria tuberosa tubers attenuates diabetic nephropathy by upregulating matrix metalloproteinase-9 expression in the kidney of diabetic rats. J. Diabetes.9 (2), 123–132. 10.1111/1753-0407.12393
130
TripathiY.PandeyN.YadavD.PandeyV. (2013). Anti-inflammatory effect of Pueraria tuberosa extracts through improvement in activity of red blood cell anti-oxidant enzymes. AYU.34 (3), 297. 10.4103/0974-8520.123131
131
UmaraniV.SudhakarM.RameshA.LakshmiB. V. S.SandhyaraniD. (2016). Protective effect of hydroalcoholic extract of Pueraria tuberosa against arsenic induced neurotoxicity in rats. Int. J. Res. Pharm. Chem.6 (2), 350–362.
132
VaishnavK.GoyalS.RamawatK. G. (2006). Isoflavonoids production in callus culture of Pueraria tuberosa, the Indian kudzu. Indian J. Exp. Biol.44 (12), 1012–1017.
133
VermaS. K.JainV.SinghD. P. (2012). Effect of Pueraria tuberosa DC. (Indian Kudzu) on blood pressure, fibrinolysis and oxidative stress in patients with stage 1 hypertension. Pakistan J. Biol. Sci.15 (15), 742–747. 10.3923/pjbs.2012.742.747
134
VermaS. K.JainV.VyasA.SinghD. P. (2009). Protection against stress induced myocardial ischemia by Indian kudzu (Pueraria tuberosa)-a case study. Int. J. Herb. Med.3 (1), 59–63.
135
VijiZ.PaulsamyS. (2018). Preliminary phytochemical screening and HPTLC finger printing analysis of traditional medicinal plant Pueraria tuberosa (Roxb. ex Willd.) DC. Kong. Res. J.5 (1), 56–59. 10.26524/krj254
136
VijiZ.PaulsamyS. (2015). In-Vitro antioxidant properties and total phenolic, flavonoid and tannin contents of Pueraria tuberosa (Roxb. Ex Willd.) DC. RJPBCS.7 (2428), 2428–2438.
137
WangH.ZhangD.GeM.LiZ.JiangJ.LiY. (2015). Formononetin inhibits enterovirus 71 replication by regulating COX- 2/PGE2 expression. Virol. J.12 (1). 10.1186/s12985-015-0264-x
138
WangX. S.GuanS. Y.LiuA.YueJ.HuL. N.ZhangK.et al (2019). Anxiolytic effects of Formononetin in an inflammatory pain mouse model. Mol. Brain12 (1). 10.1186/s13041-019-0453-4
139
WangY.ZhaoH.LiX.WangQ.YanM.ZhangH.et al (2019). Formononetin alleviates hepatic steatosis by facilitating TFEB-mediated lysosome biogenesis and lipophagy. J. Nutr. Biochem.73. 10.1016/j.jnutbio.2019.07.005
140
WHO (2009). WHO monographs on selected medicinal plants., Vol. 4. (Geneva, Switzerland: WHO)
141
WuD.WuK.ZhuQ.XiaoW.ShanQ.YanZ.et al (2018). Formononetin administration ameliorates dextran sulfate sodium-induced acute colitis by inhibiting NLRP3 inflammasome signaling pathway. Mediat. Inflamm.2018, 9878120. 10.1155/2018/9878120
142
XiaD. Z.ZhangP. H.FuY.YuW. F.JuM. T. (2013). Hepatoprotective activity of puerarin against carbon tetrachloride-induced injuries in rats: a randomized controlled trial. Food Chem. Toxicol.59, 90–95. 10.1016/j.fct.2013.05.055
143
XingG.DongM.LiX.ZouY.FanL.WangX.et al (2011). Neuroprotective effects of puerarin against beta-amyloid-induced neurotoxicity in PC12 cells via a PI3K-dependent signaling pathway. Brain Res. Bull.85 (3–4), 212–218. 10.1016/j.brainresbull.2011.03.024
144
XuX.ZhengN.ChenZ.HuangW.LiangT.KuangH. (2016). Puerarin, isolated from Pueraria lobata (Willd.), protects against diabetic nephropathy by attenuating oxidative stress. Gene591 (2), 411–416. 10.1016/j.gene.2016.06.032
145
YadavD.SharmaA.SrivastavaS.TripathiY. B. (2016b). Nephroprotective potential of standardized herbals described in Ayurveda: a comparative study. J. Chem. Pharmaceut. Res.8 (8), 419–427.
146
YadavD.KumarM.TripathiY. B. (2016a). Methanolic extract of tubers of Pueraria tuberosa Linn. ameliorates glycerol induced acute kidney injury in rats. J. Chem. Pharmaceut. Res.8 (2), 133–139.
147
YadavD.PandeyV.SrivastavaS.TripathiY. B.KumarM. (2019). Methanolic extract of Pueraria tuberosa Linn ameliorates renal injury and oxidative stress in rats with alloxan-induced diabetes. J. emerg. technol.6, 557–574.
148
YangS.WeiL.XiaR.LiuL.ChenY.ZhangW.et al (2019). Formononetin ameliorates cholestasis by regulating hepatic SIRT1 and PPARα. Biochem. Biophys. Res. Commun.512 (4), 770–778. 10.1016/j.bbrc.2019.03.131
149
YinM. S.XuS. H.WangY.JieL.ZhangQ.ZhengW. M.et al (2016). Methylamine irisolidone, a novel compound, increases total ATPase activity and inhibits apoptosis in vivo and in vitro. J. Asian Nat. Prod. Res.18 (6), 562–575. 10.1080/10286020.2015.1133610
150
YuQ.HanW.ZhuY.ZhaiH. (2017). Effect of puerarin on type II diabetes mellitus with orthopaedic footwear. Pak. J. Pharm. Sci.30 (5), 1899–1903.
151
YuX.GaoF.LiW.ZhouL.LiuW.LiM. (2020). Formononetin inhibits tumor growth by suppression of EGFR-Akt-Mcl-1 axis in non-small cell lung cancer. J. Exp. Clin. Canc. Res.39 (1). 10.1186/s13046-020-01566-2
152
YueY.ShenP.XuY.ParkY. (2019). p-Coumaric acid improves oxidative and osmosis stress responses in Caenorhabditis elegans. J. Sci. Food Agric.99 (3), 1190–1197. 10.1002/jsfa.9288
153
ZhangD.LiM. (2019). Puerarin prevents cataract development and progression in diabetic rats through Nrf2/HO-1 signaling. Mol. Med. Rep.20 (2), 1017–1024. 10.3892/mmr.2019.10320
154
ZhangJ.LiuL.WangJ.RenB.ZhangL.LiW. (2018). Formononetin, an isoflavone from Astragalus membranaceus inhibits proliferation and metastasis of ovarian cancer cells. J. Ethnopharmacol.221, 91–99. 10.1016/j.jep.2018.04.014
155
ZhangQ.-Y.ZhengW.Gul KhaskheliS.HuangW. (2019). In Vivo evaluation of tectoridin from puerariae flos on anti-alcoholism property in rats. Journal of Food and Nutrition Research7 (6), 458–464. 10.12691/jfnr-7-6-8
156
ZhangY.ChenC.ZhangJ. (2019). Effects and significance of formononetin on expression levels of HIF-1α and VEGF in mouse cervical cancer tissue. Oncol. Lett.18 (3), 2248–2253. 10.3892/ol.2019.10567
157
ZhengW.SunR.YangL.ZengX.XueY.AnR. (2017). Daidzein inhibits choriocarcinoma proliferation by arresting cell cycle at G1 phase through suppressing ERK pathway in vitro and in vivo. Oncol. Rep.38 (4), 2518–2524. 10.3892/or.2017.5928
158
ZhongY.ZhangX.CaiX.WangK.ChenY.DengY. (2014). Puerarin attenuated early diabetic kidney injury through down-regulation of matrix metalloproteinase 9 in streptozotocin-induced diabetic rats. PLoS One9 (1). 10.1371/journal.pone.0085690
159
ZhouQ.ZhangW.LiT.TangR.LiC.YuanS.et al (2019). Formononetin enhances the tumoricidal effect of everolimus in breast cancer MDA-MB-468 cells by suppressing the mTOR pathway. Evid. base Compl. Alternative Med.2019, 1–8. 10.1155/2019/9610629
Summary
Keywords
in vivo studies, pharmacological properties, phytochemical constituents, traditional uses, Pueraria tuberosa
Citation
Bharti R, Chopra BS, Raut S and Khatri N (2021) Pueraria tuberosa: A Review on Traditional Uses, Pharmacology, and Phytochemistry. Front. Pharmacol. 11:582506. doi: 10.3389/fphar.2020.582506
Received
12 July 2020
Accepted
16 November 2020
Published
27 January 2021
Volume
11 - 2020
Edited by
Lyndy Joy McGaw, University of Pretoria, South Africa
Reviewed by
Francis-Alfred Unuagbe Attah, University of Ilorin, Nigeria
Ravishankar Ramesh Patil, Amity Institute of Biotechnology, Amity University, India
Updates
Copyright
© 2021 Bharti, Chopra, Raut and Khatri.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Neeraj Khatri, neeraj@imtech.res.in
This article was submitted to Ethnopharmacology, a section of the journal Frontiers in Pharmacology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.