Abstract
Bioactive plant derived compounds are important for a wide range of therapeutic applications, and some display promising anticancer properties. Further evidence suggests that phytochemicals modulate autophagy and apoptosis, the two crucial cellular pathways involved in the underlying pathobiology of cancer development and regulation. Pharmacological targeting of autophagy and apoptosis signaling using phytochemicals therefore offers a promising strategy that is complementary to conventional cancer chemotherapy. In this review, we sought to highlight the molecular basis of the autophagic-apoptotic pathway to understand its implication in the pathobiology of cancer, and explore this fundamental cellular process as a druggable anticancer target. We also aimed to present recent advances and address the limitations faced in the therapeutic development of phytochemical-based anticancer drugs.
Introduction
Cancer is responsible for 9.6 million deaths in 2018 and is listed as the second leading cause of death globally. Cancer thus poses a pivotal public health concern worldwide (WHO, 2018). During the 20th century, the cancer death rate was found to markedly increase, primarily because of abnormal lifestyles, such as excessive tobacco use (Siegel et al., 2020), physical and chemical carcinogens (Bhatia et al., 2020), alcohol use (Sanford et al., 2020), unhealthy diet (Khaltaev and Axelrod, 2020), and biological carcinogens (Hartwig et al., 2020). Delaying cancer treatment initiation increases patient mortality (Hanna et al., 2020). However, increased awareness about the need for lifestyle modification, early detection, and treatment may have contributed to a decline in cancer prevalence (i.e., by 1.5%, on average, per year from 2013 to 2017) (Henley et al., 2020). Cancer treatment options, such as chemotherapy, radiation therapy, hormone therapy, gene therapy, immunotherapy, photodynamic therapy, targeted therapy, surgery, palliative care, and a combination of these, are increasing in both number and efficiency across multiple types of cancer and for various patients (Markham et al., 2020). The main goal of cancer therapy is to stimulate the death of abnormal cells and preserve normal cells (Schirrmacher, 2019). Chemotherapy is the backbone of many cancer treatments. It aids in the reduction of tumor size and kills cancer cells at primary sites or metastasizing sites (Sak, 2012; Alfarouk et al., 2015). However, response to treatment varies substantially according to the type of cancer or even with the same type of cancer (Sak, 2012). Resistance to chemotherapeutic agents poses a major problem in cancer treatment, ultimately limiting the efficiency of anticancer drugs, which causes therapeutic failure and eventually death (Alfarouk et al., 2015). Chemotherapy resistance can be attributed to numerous mechanisms, including multi-drug resistance, alterations of cell death mechanisms (autophagy and apoptosis), changes in drug metabolism, epigenetic and drug targets, enhanced DNA repair and gene amplification, tumor cell heterogeneity, drug efflux and metabolism, and tumor microenvironment stress-induced genetic or epigenetic alterations as a cellular response to drug exposure (Wang et al., 2019). Among these mechanisms, alterations in autophagy (‘self-eating’) and apoptosis (‘self-killing’), which are two self-destructive processes that have propelled scientific innovation, are the vital causes of chemotherapy resistance (Thorburn et al., 2014). Autophagy, an evolutionarily conserved and regulated cellular recycling mechanism, has emerged as a key player in metabolic and therapeutic stresses. In fact, this mechanism attempts to maintain or restore metabolic homeostasis via the catabolic degradation of unnecessary proteins and injured or aged organelles (Santana-Codina et al., 2017). The role of autophagy in cancer treatment is paradoxical; it may act as a pro-survival or pro-death mechanism to counteract or mediate the cytotoxic effect of anticancer agents (Santana-Codina et al., 2017). Autophagy primarily functions as a tumor suppressor by modulating reactive oxygen species (ROS) within cells and maintaining genetic instability (Levine and Kroemer, 2008). Moreover, accumulating evidence suggests that faulty autophagy is linked to malignant transformation of cancer stem cells (Moosavi et al., 2018). Under these conditions, autophagy stimulation might be a critical approach to halt early tumor formation and development (Moosavi et al., 2018). However, autophagy can promote the growth and survival of current tumors during migration and epithelial-to-mesenchymal transition. Further, this process can help cancer stem cells escape immune surveillance and make cancer cells resistant to anoikis (Moosavi et al., 2018; Rahman et al., 2020). In this regard, inhibition of autophagy increases chemotherapy-induced cytotoxicity. Therefore, autophagy, a double-edge sword that works in a context-dependent manner, blocks the early stages of tumorigenesis while becoming a driver of tumor invasion and metastasis at later stages (Moosavi et al., 2018). The molecular mechanisms regulating the switch between these different modes of action are poorly understood (Kardideh et al., 2019). Nonetheless, the interplay between apoptosis and autophagy can be leveraged to improve cancer therapy (Tompkins and Thorburn, 2019). Cancer cells become chemotherapy-resistant by escaping some of the potential apoptotic mechanisms, such as downregulated pro-apoptotic signals, upregulated anti-apoptotic signals, and faulty apoptosis initiation and implementation. However, the functional relationship between apoptosis and autophagy is complex and has recently been deciphered at the molecular level. Therefore, modulating the key factors in the autophagic and apoptotic pathways may be a novel therapeutic strategy for enhancing chemotherapy efficiency.
The potential roles of phytochemicals in the modulation of autophagy and apoptosis have recently been reviewed (Deng et al., 2019). However, autophagy and apoptosis induction and/or inhibition are extremely complex processes that require thorough exploration. Nevertheless, a better understanding of the crosstalk between autophagy and apoptosis will enable further developments of novel anticancer therapeutic strategies. In this review, we summarize the molecular mechanisms of autophagy and apoptosis in cancer. Given the pivotal role of phytochemicals in cancer therapy, we sought to discuss various phytochemicals that could regulate autophagy and apoptosis-related signaling pathways to enhance cancer chemotherapy outcomes.
Methods
A literature-based search was accomplished to collect published databases and relevant methodological contributions of the molecular mechanism of phytochemicals in autophagy-apoptosis modulation and cancer prevention has been conducted using PubMed, Scopus, Google Scholar, Web of Science, and Google that includes all original research articles written in English on multifunctional role of phytochemicals. Searching was conducted using various keywords including autophagy, apoptosis, natural compounds, cancer, phytochemical, neurodegenerative diseases, solid tumors and lymphomas, heart/cardiovascular diseases, perspectives role autophagy in cancer therapy and so on. All figures were generated using Adobe Illustrator software.
Molecular Mechanism of Autophagy in Cancer
Autophagy is a cellular process that breaks down or degrades unwanted or aggregated dysfunctional cellular components through fusion with lysosomes; this cellular process is known to play an essential role in maintaining cellular function as well as homeostasis (Krishnan et al., 2020). Autophagy preserves an active interlink in cell defense as well as a cytostatic link in cancer cell progression (Rahman and Rhim, 2017). Generally, the process of autophagy might be introduced by the generation of pre-autophagosomal structures known as phagophore assembly sites (PAS) (Hurley and Young, 2017; Rahman and Rhim, 2017). Phosphatidylinositol 3-phosphate (PI3K), which is associated with the endoplasmic reticulum (ER), plays an essential role in the initiation of PAS formation (Kotani et al., 2018). AMP-activated protein kinase (AMPK), mammalian target of rapamycin (mTOR), and unc-51 like autophagy activating kinase-1 (ULK1) have been demonstrated to facilitate phagophore formation during autophagy induction (Alers et al., 2012), with Vps34, Vps15/p150, and Beclin-1 as recruiters for phagophore formation (Velazquez and Jackson, 2018). After phagophores are formed, phagocytosis occurs. This process is subsequently followed by expansion and sealing to elongate the membrane for autophagosome formation (Rubinsztein et al., 2012). Mature autophagosomes bind to lysosomes, resulting in autolysosome formation (Kardideh et al., 2019). Eventually, autolysosomes containing inner cargos are degraded by acid hydrolases and produce nutrients; other recycling metabolites subsequently preserve cellular homeostasis (Figure 1). The fate of cancer cells is thus dependent on autophagy (Wei and Huang, 2019). Autophagy decides whether the cancer is suppressed or promoted under certain conditions. mTOR plays an important role in protecting or activating oncogenic cells through the induction of autophagy. However, chemotherapy drugs have been found to suppress tumor cells by modulating autophagic pathways. Furthermore, inhibition of this pathway regulates cancer progression, and the influence of autophagy becomes either a cellular survival or death function (Jung et al., 2020). The metabolism of malignant cells is intensely altered to retain their proliferation and survival under adverse microenvironmental conditions. Autophagy plays an essential role in maintaining metabolic adaptations in cancer cells (Goldsmith et al., 2014). Although autophagy is recognized to sustain neoplastic cell metabolism under stress, the mutual association between cancer cell metabolism and autophagy remains unknown. mTOR and AMPK have been identified as the main signaling components that modulate autophagy via the regulation of amino acid and glucose levels (Alers et al., 2012). However, specific metabolites, ROS, growth factors, palmitate, oxygen concentration, ATP to ADP ratio, specific amino acid levels, and oncogenes regulate autophagy initiation and autophagosome formation. Further, they regulate this fine balance by assimilating these autophagy-related signals in cancer (Singh and Cuervo, 2011; Panda et al., 2015). Prominently, autophagy has been frequently identified to play a “dual role” as it can either hinder or stimulate cancer initiation and progression (Patra et al., 2020; Rahman et al., 2020a). In the present review, we outline the dual role of autophagy in tumorigenesis and emphasize our recent understanding of autophagy regulation of cancer cell activation and metabolism to control tumor growth and progression.
FIGURE 1
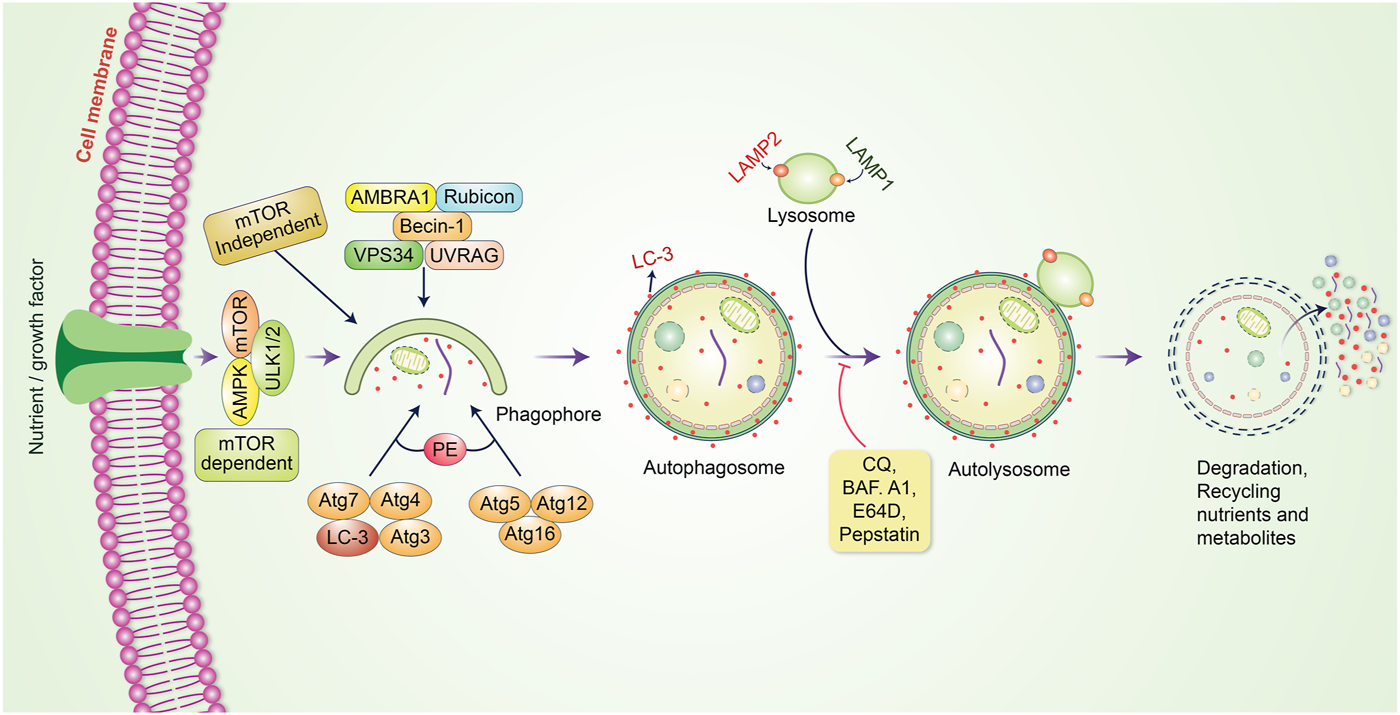
Molecular mechanism of the autophagic pathway. Autophagy is initiated by the formation of a pre-autophagosomal structure. PI3K-AMPK and mammalian target of rapamycin (mTOR) contribute to the formation of the pre-autophagosomal structure. ULK1, Vps34 and the Beclin-1 complex help to activate phagophore formation. After phagophore nucleation is elongated, subsequent binding to autophagosome occurs. Binding between mature autophagosome and lysosome results in autolysosome formation. Finally, autolysosomes are eliminated through acid hydrolases, which produce nutrients and recycling metabolites.
Molecular Mechanism of Apoptosis in Cancer
Apoptosis or programmed cell death is one of the predominant strategies for blocking or avoiding cancer or cancer formation (Lopez and Tait, 2015). Focusing on apoptosis is most effective for different cancer types because escaping apoptosis is a trademark of cancer and is indifferent to the type of cancer. Apoptosis is generally a central pathway that is associated with intrinsic and extrinsic pathways (Elmore, 2007). However, these extrinsic and intrinsic pathways could be involved in the same station, which is known as the execution pathway (Goldar et al., 2015) (Figure 2). To initiate apoptosis in apoptotic cells, the extrinsic pathway uses extracellular signals to induce apoptosis via stimulation of Fas ligand, tumor necrosis factor (TNF), and TNF-related apoptosis-inducing ligand (TRAIL), which interact with the extracellular transmembrane domain of death receptors (DR) (Guicciardi and Gores, 2009). Finally, caspases participate in the extrinsic pathway and are generally typified as starter, stimulator, or executioner caspases owing to their involvement and participation in the apoptotic signaling pathways. The intrinsic apoptotic pathway is directly involved in mitochondria-mediated proteins. Different stimuli, such as adequate Ca2+, impaired DNA molecules, oxidative stress (OS), surplus oxidants, deprivation of growth factors, and drug treatment and irradiation, have been associated with this pathway (Ghavami et al., 2004; Hassan et al., 2020). When Bax/Bak is incorporated into the mitochondrial membrane, it triggers the release of cytochrome c from the mitochondrial inner membrane into the cytosol (Kim, 2005). The intrinsic pathway of cell death is caused by Bcl-2 family proteins, which are pro-apoptotic and anti-apoptotic proteins, including Bcl-2 and Bcl-xL (Ghobrial et al., 2005). Apaf-1 and procaspase-9 combine with cytochrome c to form an apoptosome. Both mitochondria-dependent (intrinsic) and independent (extrinsic) pathways are connected at the same point, called the execution pathway (Elmore, 2007). The extrinsic and intrinsic phases are linked at the same point after caspase-8 is triggered. Activated caspase-8 in the extrinsic mechanism regulates the activation of BH3 interacting-domain (BID), a pro-apoptotic protein alternatively called BH3-only protein. BID then stimulates and oligomerizes the pro-apoptotic proteins, BAX and BAK, resulting in an intrinsic apoptotic phase (Green and Llambi, 2015).
FIGURE 2
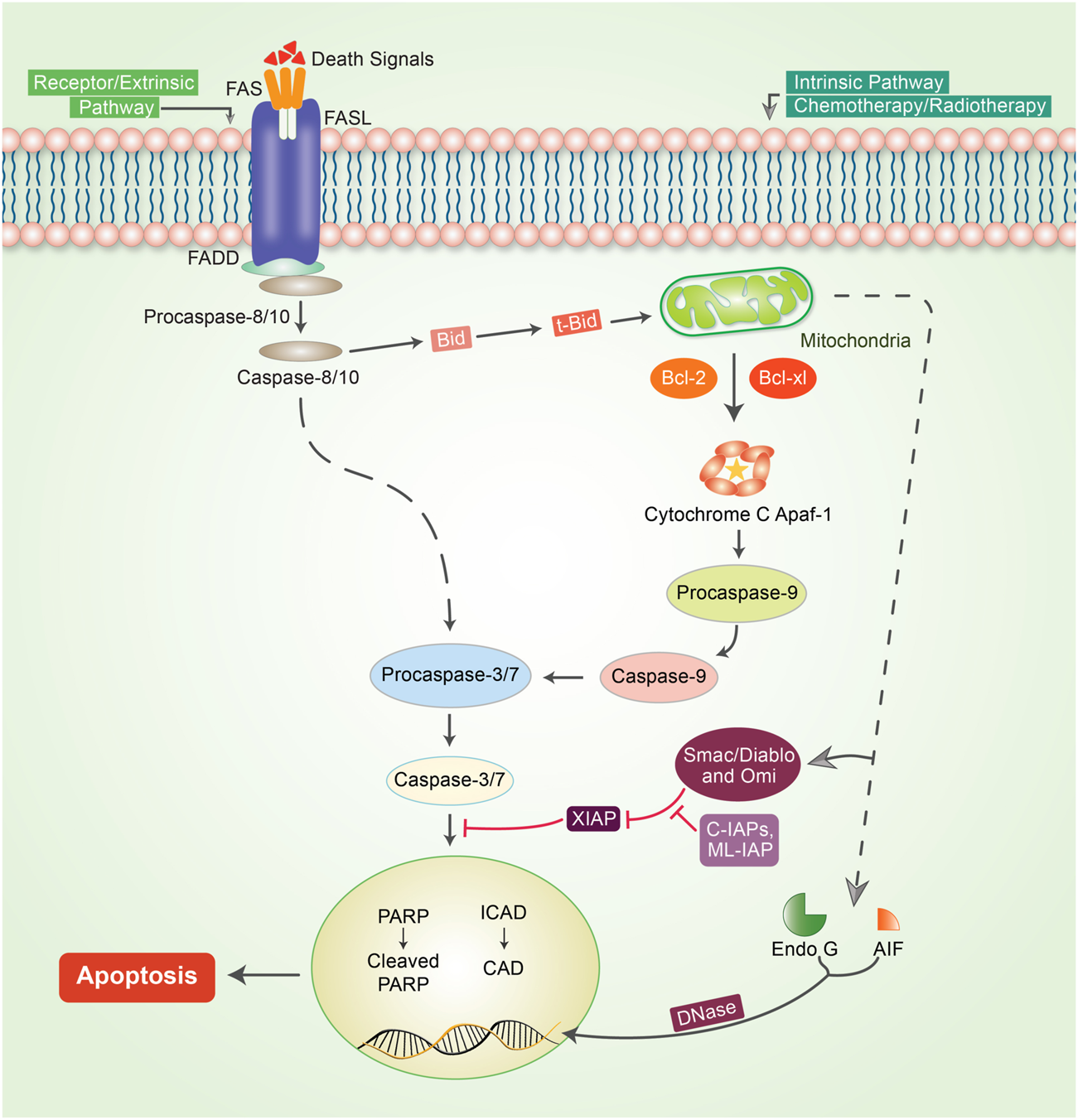
Mechanism of the apoptotic pathway in cancer. To initiate apoptosis, two central pathways are involved in this mechanism: the intrinsic pathway and extrinsic pathway. The extrinsic pathway of apoptosis is well defined by the TNF-α/TNFR1 and FasL/FasR models. Herein, the death receptor is induced by an adaptor protein; adaptor proteins are comprised of FADD (Fas-associated death domain) and TRADD (TNF receptor-associated death domain). The signaling that occurs through the extrinsic pathway causes the attachment of DRs to specific death ligands (DLs), thereby forming a death-inducing signaling cascade (DISC). The complex pathway of caspase-8 activation follows a predefined system that actively enables caspase-8 to detach from the DISC, whether or not the pro-domain of caspase-8 is retained as part of the DISC to initiate the signaling phases of apoptosis. However, in most apoptotic cells, proteins are customarily engaged in intrinsic phases that involve caspase-9, SMAC/DIABLO, Bcl-2, Bcl-w, Aven, Nox, and MYC. Mitochondrial dysfunction is followed by the loss of inner membrane mitochondrial potential, adequate formation of superoxide ions, impaired mitochondrial biogenesis formation, release of intra-membrane proteins, and matrix calcium glutathione burst, which enumerate the important potential for cancer therapeutic strategies by triggering the intrinsic phases of apoptosis in tumor cells. The execution phase of apoptosis initiator caspases, such as caspase-8/-9 or caspase-activated dnase (CAD), Poly (ADP-ribose polymerase (PARP), and other caspases such as caspase-3, -6, -7, and caspase-10, are typified as upregulator or executioner caspases. Caspase-3 is the most essential and effective of all effector caspases because it can be activated by all initiator caspases.
Phytochemicals Modulate Autophagy-Apoptosis Signaling in Several Cancers
Autophagy plays an essential role in cancer treatment, especially in chemotherapy, by removing dysfunctional organelles and intracellular components and inducing lysosomal degradation. This self-digestion mechanism strengthens cellular defense to protect cells from various intracellular and extracellular stresses and regulate redox balance to provide genomic and cytoplasmic stability. Emerging evidence supports the dual role of autophagy in cancer (i.e., as a promoter and an inhibitor of tumor development). However, the induction of autophagy in cancer is still a potential strategy; this is because it induces type II programmed cell death. During cancer initiation, autophagy regulators, such as mTOR and AMPK, are negatively modulated by tumor-suppressing factors, which cause autophagy induction (Comel et al., 2014). However, these autophagy regulators are activated by several oncogenes that suppress autophagy and promote cancer formation (Choi et al., 2013). Autophagy also suppresses carcinogenesis by regulating ROS, and excessive ROS production promotes tumor generation (Ávalos et al., 2014; Filomeni et al., 2015). Owing to their multifaceted therapeutic activities, phytochemicals have proven to be promising for treating many cancers (Mitra and Dash, 2018). In some cases, metabolites and synthetic products from natural compounds have demonstrated better chemopreventive effects than their original compounds (Aung et al., 2017). Our model and emerging evidence indicate that phytochemicals targeting the autophagic-apoptotic pathways are promising agents for cancer treatment for both pathways, or are dependent- and -independent of target-specific molecular mechanisms in cancer cells (Figure 3). Several phytochemicals and their autophagic-apoptotic effects are summarized in Table 1.
FIGURE 3
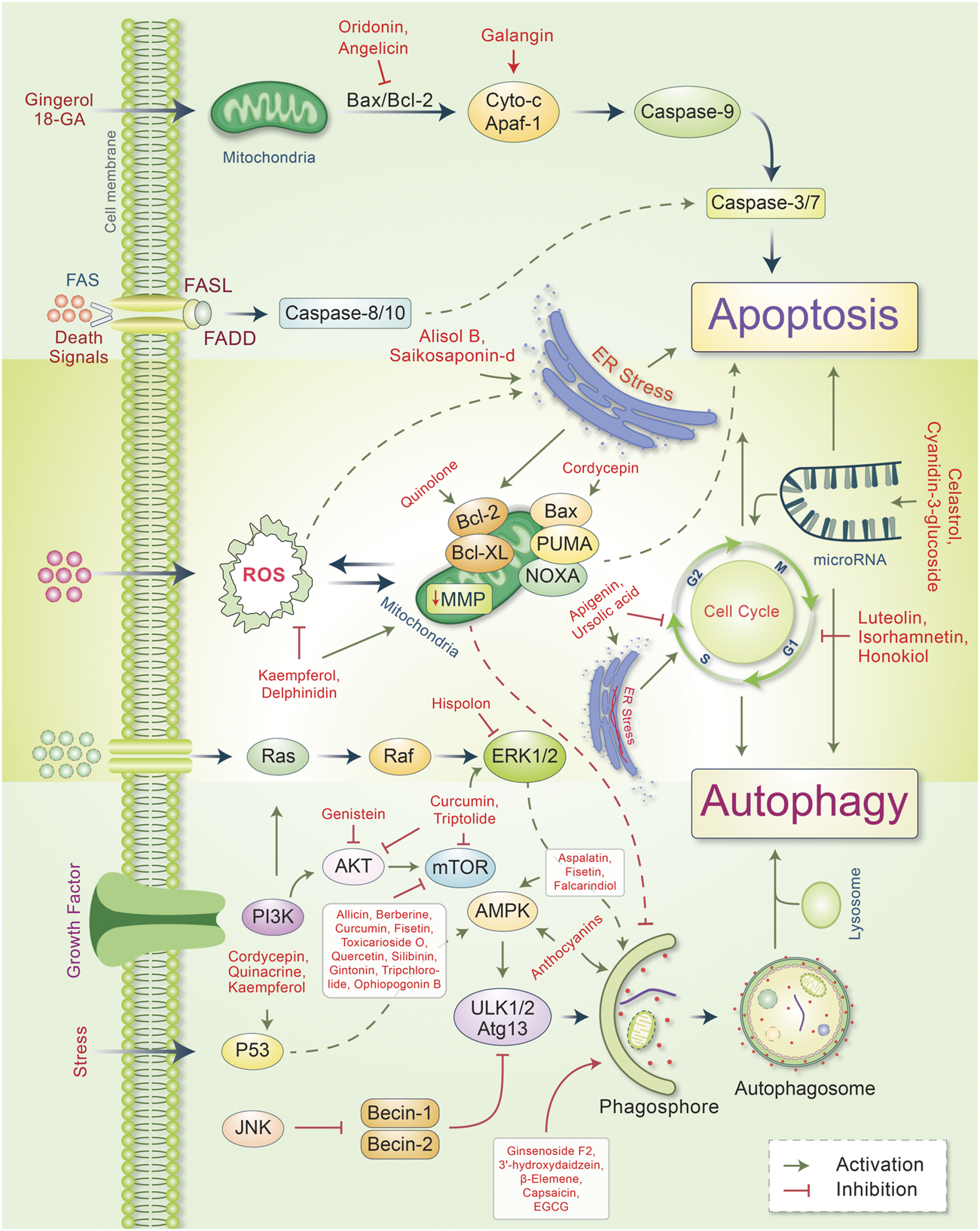
Major phytochemicals induce the signal transduction pathways that regulate autophagic and apoptotic cell death in cancer. Phytochemicals have been found to activate both the intrinsic and extrinsic apoptotic pathways by inducing a dysfunction in mitichrondria-caspase-9 and FAS-ligand-caspase-8 mediated apoptotic cell death, respectively. Phytochemicals induce ER stress and apoptotic cell death. However, some phytochemicals modulate mitichrondrial biogenesis and ensure apoptosis-autophagic cell death. Phytochemicals regulate the cell cycle and microRNA as well as cause apoptosis-autophagic cell death in cancer cells. Some phytochemicals activate autophagic signaling and inhibits cell growth and autophagy. For a detailed explanation, see the text.
TABLE 1
| Phytochemicals | Doses/Conc | Cancer model | Molecular effects | References |
|---|---|---|---|---|
| Resveratrol | 10–100 μM | Human colon carcinoma cell lines SW480, SW620, B103, and HCT116 | Activate procaspase-3, 8/FADD | Delmas et al. (2003); Rahman et al. (2012a) |
| Eriocalyxin B (EriB) | 1.4 μM | Human pancreatic cancer cellPANC-1, SW1990 CAPAN-2, and CAPAN-1 | Caspase 8,9 activation and downstream regulation of caspases 3, 7, PARP | Li et al. (2012) |
| β-Elemene | 10 μM | Human breast cancer cell lines Bcap37, MBA-MD-231 | Conservation of LC3-I to LC3-II | Guan et al. (2014) |
| Oblongifolin C | 15 μM | Human breast carcinoma cell lines HeLa or MEF | Activation of CASP3 and cleaved PARP | Lao et al. (2014) |
| Apigenin | 10 μM | Colorectal cancer cells HCT-116, SW480, HT-29 and LoVo | Activate NAG-1, p53, p21 | Zhong et al. (2010) |
| Allicin | 1 μg/ml | Human gastric cancer cell line MGC-803, BGC-823 and SGC-7901 | Increase expression of p38 and cleaved Of caspase 3 | Zhang et al. (2015) |
| Anthocyanins | 50 µM | Breast cancer cell lines MCF-MDA-MB-231 and MDA-MB-453 | Inhibit the expression of VEGF, suppressed the MMP-9,MMP-2 and uPA expression | Hui et al. (2010) |
| Aspalathin | 0.2 µM | Ovarian cancer cell Caov-3 | Inhibit Dox, decrease expression of p53 and induce AMPK and Foxo1 | Lin et al. (2017) |
| Baicalein | 200 µM | Human HCC cell lines SMMC-772 and Bel-7402 | Downregulate Bcl 2, increase ER stress | Wang et al. (2014) |
| Berberine | 100 nM | Human glioma cell lines U251 and U87 GBM | Inhibition of AMPK/mTOR/ULK1 | Peng et al. (2008); Wang et al. (2010); Yu et al. (2014); Guamán Ortiz et al. (2015); Wang et al. (2016a) |
| Capsaicin | 150 µM | Human nasopharyngeal carcinoma cell line NPC-TW01 | Downstream of PI3K/Akt/mTOR, increase caspase-3 activity | Lin et al. (2017b) |
| Celastrol | 1.5 μM | Human prostate cancer cell lines LNCaP, 22Rv1, DU145 and PC-3 | Upstream of miR-101 | Guo et al. (2015) |
| Cordycepin | 200 µM | Human brain cancer cellSH-SY5Y and U-251 | Upregulates ROS, p53, and LC3II | Chaicharoenaudomrung et al. (2018) |
| Curcumin | 25 µM | Malignant mesotheloma cancer cell line MM-B1, H-Meso-1, and MM-F1 | Increase Bax/bcl-2 ratio, p53 expression, activation of caspase 9, cleavage of PARP-1 | Masuelli et al. (2017) |
| Epigallocatechin gallate (EGCG) | 100 nM | Vascular endothelial cell line U-937 | Reduce TNF-α, inhibit VCAM1, LC3A, LC3B | Yamagata et al. (2015) |
| Evodiamine | 10 µM | Gastric cancer cell line SGC-7901 | Activates beclin-2, Bax, downregulates Bcl-2 | Rasul et al. (2012) |
| Fisetin | 40–120 µM | Prostate cancer cell lines PC3 and DU145 | Supressed Mtor and inhibit Akt, activate AMPK | Suh et al. (2010) |
| Genistein | 50–100 µM | Ovarian cancer cell line A2780 | Reduces Akt/mTOR phosphorylation | Gossner et al. (2007) |
| Gingerol | 300 µM | Human colon cancer cell lines SW-480 and HCT116 | Inhibition of JNK, ERK1-2, and P38 MAPK | Shukla and Singh, (2007); Baliga et al. (2011); Radhakrishnan et al. (2014) |
| Ginsenoside F2 | 100 µM | Breast cancer cell lines MCF-7 | Elevated Atg-7 | Mai et al. (2012) |
| Cleaved PARP | ||||
| Hispolon | 25–100 µM | Cervical cancer cell lines Hela and SiHa | Downregulated lysosomal protease Cathepsin S(CTSS) | Chen et al. (2012) |
| 3′-hydroxydaidzein (3′-ODI) | 100 µM | Mouse melanoma cell line B16F1 | Reduce the α-MSH | Kim et al. (2013) |
| Toxicarioside O | 50 nM | Human colorectal cancer cell lines HCT116 and SW480 | Inhibition of the Akt/mTOR | Huang et al. (2017) |
| Upstream SIRT1↑ | ||||
| Falcarindiol | 6 µM | Human breast cancer cell lines MDA-MB-231,MDA-MB-468 and Her2 | FAD induce expression of GRP78 | Minto and Blacklock, (2008); Jin et al. (2012); Lu et al. (2017) |
| Oleanolic acid | 100 μg/ml | Human pancreatic cancer cell line Panc‐28 | Modulate JNK and mTOR pathway | Pollier and Goossens, (2012); Liu et al. (2014) |
| Honokiol | 40 μM | Human glioblastoma cell lines LN229, GBM8401 and U373 | Reduction of p-PI3K, p-Akt and Ki67 | Cheng et al. (2016) |
| Magnolol | 40 μM | Human glioblastoma cell lines LN229, GBM8401 and U373 | Reduction of p-PI3K, p-Akt and Ki67 | Cheng et al. (2016) |
| Alisol B | 30 μM | Breast cancer cell lines MCF-7, SK-BR-3, and HeLa | Activation of Ca2+/AMPK/Mtor | Law et al. (2010) |
| Luteolin | 100 µM | Human liver cancer SMMC-7721 | Increase expression of caspase-8, decrease bcl-2 | Cao et al. (2017) |
| α-Mangostin | 5–10 µM | Human brain cancer cell lines, GBM8401 and DBTRG05MG | Activation of AMPK | Chao et al. (2011) |
| Oridonin | 8–32 μmol/L | Human hepatocellular carcinoma cell line BEL-7402 | Activation of caspase-3 | Zhang et al. (2006) |
| Down-regulation of Bcl-2 and Up-regulation of Bax | ||||
| Quercetin | 15 µM | Lymphoma cell lines BC3, BCBL1 and BC1 | Inhibits PI3K/Akt/mTOR and Wnt/β-catenin | Granato et al. (2017) |
| Rottlerin | 1–2 µM | Breast cancer cell lines CD44/CD24 | Enhance expression of LC3 | Kumar et al. (2013) |
| 6-Shogaol | 55.4 μM | Lung cancer cell line A549 | Inhibition af Akt and mTOR downstream | Hung et al. (2009) |
| Silibinin (silybin) | 50 µM | RCC cell lines ACHN and 786-O | Inhibit mTOR and activate AMPK | Li et al. (2015) |
| Sulforaphane | 40 µM | Human pancreatic cancer cell lines MIA PaCa-2,Panc-1 | Increase ROS level | Naumann et al. (2011) |
| γ-tocotrienol | 10 μmol/L | Breast cancer cell lines MCF-7 and MDA-MB-231 | Activate AMPK, down regulate Ang-1/Tie-2 | Ling et al. (2012); Tang et al. (2019) |
| Thymoquinone | 40–60 µM | Oral cancer cell lines SASVO3,SCC-4, OCT,SAS | Increase expression of LC3-II, Bax expression | Chu et al. (2014) |
| Tripchlorolide | 200 nM | Lung cancer cell line A549/DDP | Inhibition of PI3K/Akt/mTOR | Chen et al. (2017a) |
| Tetrandrine | 0–4 μM | Hepatocellular carcinoma cell lines Huh7, HCCLM9 and Hep3B | Inhibits Wnt/β-catenin | Zhang et al. (2018) |
| Decreases MTA1 | ||||
| N-desmethyldauricine | 150 μM | Lung cancer cell line H1299 | Inhibition of Ulk-1/PERK/AMPK/mTOR | Law et al. (2017) |
| Quinacrine | 15 μM | Colon cancer cell lines HCT-116/HCT-116/HCT-116 | Activation of p53, p21, and inhibition of topoisomerase | Mohapatra et al. (2012) |
| Chloroquine | 50 μM | Pancreatic cancer cell line MiaPaCa2 and S2VP10 | Decrease the level of O2 | Frieboes et al. (2014) |
| Tangeritin | 10 μM | Breast cancer cell lines MCF7, MDA–MB–468 and MCF10A | Induce CYP1 and CYP1A1/CYP1B1 protein expression | Surichan et al. (2018) |
| Myricetin | 100 μM/L | Prostate cancer cell lines PC3, DU145 | Knockdown the interaction between P1M1/CXCR4 | Ye et al. (2018) |
| Galangin | 15 μM | Human kidney cancer cell line A498 | Inhibition of PI3K/Akt/mTOR signaling | Zhu et al. (2018) |
| Isorhamnetin | 100 μM | Colon cancer cell lines HCT116 and SW480 | Increase ROS | Wu et al. (2018) |
| Hesperetin | 350 μM | Lung cancer cell line H522 | Knockdown caspase-3/9,p53,Bax | Elango et al. (2018) |
| Upregulate Fas, FADD and caspase-8 | ||||
| Delphinidin | 80 μM | Breast cancer cell lines MDA-MB-453 and BT474 | Suppression of mTOR | Chen et al. (2018) |
| Activation of the AMPK | ||||
| Epigallocatechingallate (EGCG) | 500 μM | Human glioblastoma cell lines T98G and U87MG | Increase ROS | Grube et al. (2018) |
| Epicatechin-3-O-gallate (ECG) | 36 µM | Prostate cancer cell lines LNCaP and PC-3 | Diminished the progression of carcinofenic cell | Siddiqui et al. (2011); Stadlbauer et al. (2018) |
| Cyanidin‐3‐glucoside (C3G) | 20 μM | Human breast cancer MDA‐MB‐231 and Hs‐578T | Inhibiting STAT3/VEGF and miR124 mediated downregulation STAT3 | Ma and Ning, (2019) |
| Benzyl isothiocyanate (BITC) | 6.5 μM | Pancreatic cell lines BxPC-3 and PanC-1 | Decrease the phosphorylation of PI3K/Akt/FOXO1/PDK1/mTOR/FOXO3a | Boreddy et al. (2011) |
| Phenethyl isothiocyanates (PEITC) | 10 μM | Breast cancer cell lines MDA-MB-231 and MCF-7 | Reduction of HER2, EGFR and STAT3 expression | Gupta and Srivastava, (2012) |
| Piperlongumine (PL) | 6 µM | Lung cancer cell lines A549 and A549/DTX | Regulate PI3K/Akt/mTOR | Bezerra et al. (2008); Raj et al. (2011); Wang et al. (2015) |
| Saikosaponin-d | 10 µM | Breast cancer cell lines HeLa and MCF-7 | Calcium mobilization, induce CaMKKβ-AMPK-mTOR | Hsu et al. (2004); Tundis et al. (2009); Wong et al. (2013) |
| Guttiferone K | 20 µM | Human HCCs HuH7 and HepG2 | Reduce phosphorylation of Akt/mTOR, increase ROS | Xu et al. (2008) |
| Wu et al. (2015) | ||||
| Licochalcone A | 20 or 50 µM | Breast cancer cell line MCF-7 | Suppression of PI3K/Akt/mTOR pathway | Xue et al. (2018) |
| Ophiopogonin B | 10 μM | Lung cancer (NSCLC) cell lines NCI-H157 and NCI-H460 | Inhibition of PI3K, Akt, mTOR | Chen et al. (2013a) |
| Norcantharidin | 40 μM | Human MHCC-97H (97H) and HepG2 HCC cells | Inhibition of c-Met, mTOR | Sun et al. (2017a) |
| Juglanin | 10 μM | Breast cancer cell lines MCF-7 and SKBR3 | Regulation of ROS, JNK | Sun et al. (2017b) |
| Isoliquiritigenin | 25 μM | Human ovarian cancer cell lines, OVCAR5 and ES-2 | Cleaved caspase-3, increased LC3B-II, and Beclin-1 level | Chen et al. (2017b) |
| Cucurbitacin B | 200 μM | Breast cancer cell line MCF-7 | Increase γH2AX, phosphorylation of ATM/ATR, ROS | Chen et al. (2005); Ren et al. (2015) |
| Carnosol | 25 µM | Human breast cancer cell line MDA-MB-231 | Increase p21/WAF1 and downregulate p27 | Al Dhaheri et al. (2014) |
| Kaempferol | 50 or 100 μM | Colorectal cancer cell lines HCT116, HCT15, and SW480 | Generated ROS and p53 signal | Choi et al. (2018) |
| Ursolic acid | 10–40 µM | Prostate cancer cell lines PC3 | Increases Beclin-1/Atg5 and inhibits Akt/mTOR | Shin et al. (2012) |
| Triptolide | 200 nM | Human pancreatic cancer cell line S2-013, S2-VP10, and Hs766T | Inhibits of Akt-mTOR-P70S6K | Mujumdar et al. (2010) |
Phytochemicals that activate autophagy and apoptosis in various in vitro and in vivo cancer models.
Phytochemicals in Autophagy Signaling
Apigenin is a flavonoid derivative that modulates several kinase pathways and inhibits the cell cycle at the G2/M phase. Studies have shown that apigenin can inhibit cell growth and induce autophagy in time-and dose-dependent manners in HepG2 cells (Zhong et al., 2010). Autophagy was also found to be mediated via the inhibition of the PI3K/Akt/mTOR pathway in HepG2 cells (Yang et al., 2018). An organic sulfur compound, allicin, acts as an antitumor agent that activates autophagic cell death by inhibiting the PI3K/mTOR signaling pathway (Sak, 2012). Allicin also inhibits the expression of p53 and Bcl-2, and upregulates the Beclin-1 signaling and AMPK/TSC2 signaling pathways (Chu et al., 2012). Anthocyanins (ACNs) present in black soybeans induce autophagy; however, their underlying mechanism have yet to be determined (Choe et al., 2012). Aspalathin is a polyphenolic dihydrochalcone C-glucoside that plays a critical role in inhibiting Dox-induced cardiotoxicity and decreasing P53 expression. Aspalatin triggered autophagy-related genes and decreased p62 by inducing the AMPK and Fox pathways (Johnson et al., 2017). Berberine is an isoquinoline alkaloid that exerts anticancer activity for autophagy induction by inhibiting the AMPK/mTOR/ULK1 pathway (Wang et al., 2016a). Celastrol is another triterpenoid that is effective against human prostate cancer. Celastrol blocks the AR signaling pathway, which induces autophagy and downregulates the expression of miR-101 (Guo et al., 2015). Cordycepin generates ROS in cancer cells and enhances p53 and LC3I/II expression, thereby modulating autophagy (Chaicharoenaudomrung et al., 2018). Cordycepin inhibits renal carcinoma in the migration of the Caki-1 cell line by reducing microRNA-21 expression and Akt phosphorylation, and increasing PTEN phosphatase levels (Yang et al., 2017). In addition, cordycepin induces autophagy via Bax activation in ovarian cancer cell lines, including SKOV-3 and OVCAR-3 (Jang et al., 2019). Curcumin has been shown to increase ROS and DNA damage in cancer cells. Further, curcumin increased the phosphorylation of ERK1/2 and p38 MAPK, inhibited Akt and P54 JNK (Masuelli et al., 2017), and eventually induced autophagy in NSLCA549 cells (Liu et al., 2018). Evodiamine, a quinolone alkaloid, mediates autophagy activation by upregulating Beclin-1 and Bax expression and downregulating Bcl-2 (Rasul et al., 2012). Fisetin is a naturally occurring flavonoid that is reported to suppress the mTOR signaling pathway via the inhibition of Akt and activation of AMPK, and autophagic programmed cell death in prostate cancer cells (Suh et al., 2010). Similarly, genistein displayed chemopreventive and chemotherapeutic effects in cancer cells. Treating ovarian cancer cells with genistein led to a reduction in Akt phosphorylation and induced autophagy, thereby contributing to glucose uptake reduction in cancer cells (Gossner et al., 2007). Ginsenoside F2 showed anti-proliferative activity and initiated the autophagic process in breast cancer stem cells. Concurrently, ginsenoside F2 elevated Atg-7 levels, induced the formation of acidic vascular organelles, and recruited GFP-tagged LC3-II to autophagosomes (Mai et al., 2012). Hispolon, a phenolic compound isolated from Phellinus igniarius (L.) Quél., exhibited apoptotic and anti-tumor effects in cervical cancer cell lines and notably induced autophagy. Treatment with hispolon inhibited metastasis by downregulating lysosomal protease cathepsin S (CTSS) (Chen et al., 2012). Further, hispolon was found to mechanistically block the ERK pathway and enhance LC3 conversion and acidic vesicular organelle formation (Hsin et al., 2017). 3′-hydroxydaidzein (3′-ODI) is another phytochemical derivative that induces autophagy. In fact, it was found to significantly reduce α-MSH-mediated melanogenesis in melanoma cells (Kim et al., 2013). Toxicarioside O, a natural product derived from the Antira toxicaria Lesch., showed anticancer potency through autophagy induction via the subsequent reduction of the Akt/mTOR pathway (Huang et al., 2017). Falcarindiol (FAD), a natural polyene (Minto and Blacklock, 2008) promotes autophagy in response to ER stress (Jin et al., 2012) while α-mangostin mediates autophagic cell death via AMPK activation in human glioblastoma cells (Chao et al., 2011). The bioflavonoid, quercetin, possesses anticancer and anti-inflammatory properties. In hyperactive primary effusion lymphoma (PEL), quercetin reduced the release of cytokines and inhibited PI3K/Akt/mTOR and STAT3 pathway-induced autophagy, ultimately resulting in PEL cell death (Granato et al., 2017). In breast cancer steam cells, rottlerin (Rott) enhanced the expression of LC3, Beclin-1, and Atg12 aggregation during autophagy. Silibinin (silybin) is a chemoprotective flavonoid that might exhibit anti-metastatic effects on renal cell carcinoma (RCC). Silibinin increased the expression of LC3-II, which not only suppressed mTOR regulation but also activated the AMPK pathway (Li et al., 2015). Sulforaphane (SFN) is a group of phytochemicals that are referred to as isothiocyanates (Uddin et al., 2020). Multiple studies have shown that autophagy in SFN-induced cell death eliminates highly resistant pancreatic carcinoma cells by releasing ROS, without exhibiting cytotoxic effects (Naumann et al., 2011; Uddin et al., 2020). Gintonin has been found to stimulate autophagic flux via the Akt/mTOR/p70S6K-mediated pathway in primary cortical astrocytes (Rahman et al., 2020b). Ursolic acid (UA), a pentacyclic triterpenoid, showed anti-proliferative effects via G1 phase arrest and induced autophagy regulation through the beclin-1 and Akt/mTOR pathways (Shin et al., 2012). Tripchlorolide is present in tripterygium. Treatment with tripchlorolide was found to attenuate the expression of the PI3K/Akt/mTOR signaling pathway (Chen et al., 2017a). Tetrandrine is a bisbenzylisoquinoline alkaloid isolated from the Chinese medicinal herb, Stephania tetrandra S. Moore. Tetrandrine plays an important role in the suppression of human hepatocellular carcinoma, inhibits the Wnt/β-catenin pathway, and reduces MTA1 expression, which eventually causes autophagy (Zhang et al., 2018). N-desmethyldauricine is a novel inducer of autophagy that is mediated by the inhibition of Ulk-1/PERK/AMPK mTOR and causes calcium accumulation, leading to autophagic cell death (Law et al., 2017). Quinacrine displayed anticancer properties in breast cancer cells by enhancing p53 and p21 regulation and inhibiting topoisomerase activity (Mohapatra et al., 2012). The anti-proliferative activity of tangeritin initiates anticancer activity by modulating autophagy and inducing the CYP1 enzyme and CYP1A1/CYP1B1 proteins in MDA-MB-468 and MCF-7 cells (Surichan et al., 2018). Multiple studies have indicated that licochalcone A treatment activates the LC3-II signaling pathway and suppresses the PI3K/Akt/mTOR pathway to promote autophagy in MCF-7 cells (Xue et al., 2018). In addition, ophiopogonin B was found to induce autophagy by inhibiting the PI3K/Akt/mTOR signaling pathway (Chen et al., 2013a). Anticancer activity was also exhibited by juglanin, which is generally extracted from green husks. Juglanin-mediated treatment attenuated G2/M phase arrest and induced autophagy by regulating the ROS/JNK signaling pathway in human breast cancer (Sun et al., 2017a). Cucurbitacin B (Cuc B) is another natural tetracyclic triterpene compound that is generally used as an anti-inflammatory drug (Chen et al., 2005). Treatment with Cuc B increases γH2AX protein expression, promotes DNA damage through phosphorylation of ATM/ATR, and concurrently increases the level of ROS that induces autophagy in MCF-7 cells (Ren et al., 2015).
Phytochemicals in Apoptosis Signaling
Angelica polymorpha Maxim, which contains angelicin, increases cellular cytotoxicity and induces apoptosis by decreasing the expression of anti-apoptotic proteins, including Bcl-xL, Bcl-2, and Mcl-1 in SH-SY5Y human neuroblastoma cells (Rahman et al., 2012b; Rahman et al., 2012b). As FAD-induced cell death is known to be caused by caspase-dependent modulation, FAD is suggested to have a synergistic effect on several approved cancer drugs designed to kill cancer cells (Lu et al., 2017). Alisol B induces autophagy by modulating the CaMKK-AMPK-mTOR signaling pathway, calcium mobilization, and enhanced ER stress, leading to apoptotic cell death (Law et al., 2010). Luteolin is a flavonoid found in various plants and is known to play a leading role in hepatocellular carcinoma cell lines through G0/G1 phase cell cycle arrest. Studies have shown that treatment with luteolin induces apoptosis by increasing caspase-8 expression, reducing Bcl-2 at the mRNA level, improving the conversion of LC3B-I to LC3B-II, and decreasing the viability of SMMC-7721 cells (Cao et al., 2017). In the human carcinoma BEL-7402 cell line, oridonin-mediated apoptosis was found to be driven by the activation of caspase-3 as well as reduced Bcl-2 expression and Bax upregulation, which can inhibit cell growth (Zhang et al., 2006). Prolonged treatment with Rott in breast CSCs suppressed the phosphorylation of Akt and mTOR, and upregulated the phosphorylation of AMPK, eventually upregulating apoptosis (Kumar et al., 2013). Several natural plant extracts derived from Dioscorea nipponica Makino, Melandrium firmum (Sieb. & Zucc.) Rohrb., and Saussurea lappa (Decne.) Sch. Bip. have been found to induce anti-proliferative effects and apoptotic cell death in human neuroblastoma cells (Rahman et al., 2013; Rahman et al., 2014; Rahman et al., 2015). γ-Tocotrienol, a vitamin E isomer (Ling et al., 2012), is known to target Ang-1/Tie-2 and exert anti-cancer effects through the activation of AMPK signaling, leading to apoptotic cell death in human prostate cancer cell lines (Tang et al., 2019). Triptolide induced apoptosis in pancreatic cancer cells, causing the inactivation of Akt/mTOR/p70S6K and upregulation of the ERK1/2 pathway (Mujumdar et al., 2010). Kaempferol is a flavonoid compound that generates ROS and p53 signals and regulates p38 phosphorylation as well as caspase activation, thereby inducing apoptosis of colorectal cancer cells (Choi et al., 2018). Myricetin is a natural flavonoid found in various fruits and vegetables. A previous report suggested that myricetin attenuated tumor cell growth by promoting apoptotic cell death (Cao et al., 2018). Myricetin exerts pro-apoptotic and cytotoxic effects on prostate cancer cells by inhibiting P1M1 and downregulating the interaction between P1M1 and CXCR4 (Ye et al., 2018). Galangin induced apoptosis in kidney cancer cells by increasing the expression of Bax and Cyt-c and decreasing Bcl-2 expression (Zhu et al., 2018). In a human breast cancer cell line, isorhamnetin inhibited tumor growth by inducing cell cycle arrest in the S-phase and displayed strong cytotoxic effects via the ROS-dependent apoptotic pathway (Wu et al., 2018). In H522 cells, Hesperet induced apoptotic cell death by downregulating caspase-3/9, p53, and Bax expression and upregulating Fas, FADD, and caspase-8 expression (Elango et al., 2018). Cyanidin-3-glucoside (C3G) is an ACN found in fruits. C3G exerts anti-inflammatory properties and induces miR-124 expression. Concurrently, miR-124 regulation downregulates STAT3 and inhibits angiogenesis induced by C3G in human breast cancer (Ma and Ning, 2019). Benzyl isothiocyanate (BITC) is present in cruciferous vegetables. Administering BITC to mice caused decreased phosphorylation of PI3K/Akt/FOXO1/PDK1/mTOR/FOXO3a, which suppressed pancreatic cancer cell growth and induced apoptosis (Boreddy et al., 2011). Several studies have reported that glucosinolate-derived phenethyl isothiocyanates (PEITC) are promising anti-tumorigenic agents. In fact, PEITC-treated mice were found to exhibit reduced expression of HER2, EGFR, and STAT3, and enhanced apoptosis through the cleavage of caspase 3 and PARP (Gupta and Srivastava, 2012). NCTD inhibits c-Met and mTOR and exhibits anticancer properties (Sun et al., 2017b).
Phytochemicals in Autophagic-Apoptotic Signaling
β-Elemene is a natural chemical compound collected from different medicinal plants, such as Curcuma WenYuJin (Edris, 2009). β-Elemene exerts cytoprotective activity by converting LC3-I into LC3-II to form autolysosomes that activate autophagy and significantly reduce the in vitro growth of human breast cancer cells via apoptosis (Guan et al., 2014). Capsaicin is another naturally occurring phytochemical that exerts antitumor potency by downregulating the PI3K/Akt/mTOR pathway. Capsaicin instigates the autophagy process by increasing the expression of the autophagy markers, LC3-II and Atg5, and enhances the degradation of p62 and Fap-1, while increasing caspase-3 activity (Lin et al., 2017a). The Morus alba L. root extract containing oxyresveratrol was previously found to accumulate ROS and induce autophagic and apoptotic cell death via the FOXO-Caspase-3 pathway in human neuroblastoma cells (Kwon et al., 2015; Rahman et al., 2017). Gingerol possesses antioxidant, anti-inflammatory, and anti-tumor properties (Shukla and Singh, 2007; Baliga et al., 2011), and inhibits colon cancer cell proliferation by activating the caspase-dependent pathway and concurrently cleaving PARP, which induces autophagy (Radhakrishnan et al., 2014). Concurrent treatment with honokiol (Hono) and magnolol (Mag) decreased the expression of cyclin A, D1, and cyclin-dependent kinase, which arrests cell cycle progression and reduces p-PI3K, p-Akt, and Ki67 expression in U87MG and LN229 human glioma cells. Both Hono-and Mag-mediated treatments exert synergistic anti-tumor effects by inhibiting cell proliferation. Accordingly, they induce autophagy and apoptosis in human GMB cells (Cheng et al., 2016). 6-Shogaol disrupts the Akt/mTOR mediated signaling pathway; blocking of Akt is beneficial to apoptotic cell death. 6-Shogaol induces autophagy through the inhibition of Akt overexpression and exhibits anticancer activity against non-small cell lung cancer (Hung et al., 2009). Thymoquinone (TQ), a major component of black cumin, exhibits potent cytotoxic effects in several cancer cell lines. In SASVO3 cells, TQ was found to mediate cell death caused by the enhancement of Bax expression and increase autophagic vacuoles and LC3-II protein expression following apoptosis and autophagy (Chu et al., 2014). In our previously published study, we revealed that the gap-junction inhibitor, 18α-Glycyrrhetinic acid (18-GA), induces apoptosis and autophagy. 18-GA-induced autophagy has been shown to induce Atg5, Atg7, and LC3II accumulation through p62 degradation (Rahman et al., 2016b). 18-GA was also found to destabilize the Bcl-2/Beclin-1 interaction and the cleavage of Beclin-1, ultimately highlighting the occurrence of mitochondrial-mediated apoptosis in SH-SY5Y cells (Figure 4). 18-GA is also known to activate several MAPKs and arrest the cell cycle, which leads to the activation of apoptosis. 18-GA may thus be used as a therapeutic target for the apoptosis-autophagy pathway in neuroblastoma.
FIGURE 4
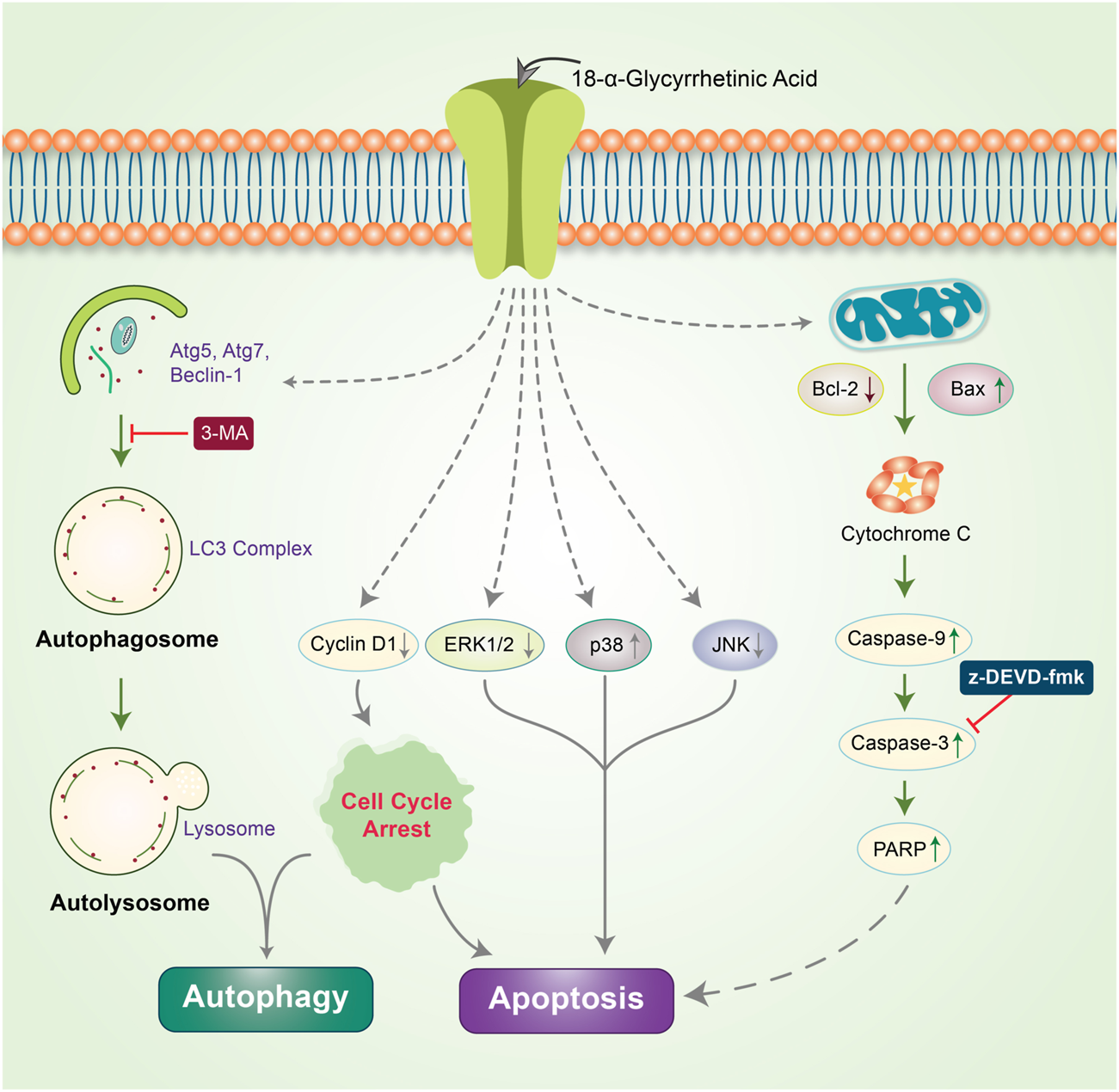
Anticancer effects of 18-GA in autophagy-apoptosis modulation in neuroblastoma cells. 18-GA encouraged caspase-induced apoptosis by depolarizing the mitochondria membrane potential (MMP). 18-GA also induced early autophagy through Atg5 and Atg7 activation and converted LC3I to LC3II. The autophagy inhibitor, 3-MA, inhibited 18-GA-mediated autophagy. Nonetheless, 18-GA caused the downregulation of ERK1/2, JNK, and cyclinD1 protein and the upregulation of p38 MAPK, which activated apoptosis in neuroblastoma cancer.
Delphinidin is an anthocyanidin monomer with strong anti-oxidative characteristics. In HER-2 positive breast cancer cells, delphinidin enhances apoptosis and autophagy by suppressing mTOR and activating the AMPK signaling pathway (Chen et al., 2018). Emerging evidence has shown that epicatechin-3-O-gallate (EGCG) promotes autophagy and apoptosis in different cancer lines (Siddiqui et al., 2011; Grube et al., 2018; Stadlbauer et al., 2018). Previously, OxyR was found to simultaneously activate apoptosis and autophagy in NB. OxyR also reduces PI3K/Akt/mTOR signaling and enhances cytotoxicity by increasing autophagy levels (Figure 5) (Rahman et al., 2017). OxyR-induced cell death was found to occur independent of apoptosis induction due to alterations in the levels of PI3K/Akt/mTOR and p38 MAPK activity in SH-SY5Y cells.
FIGURE 5
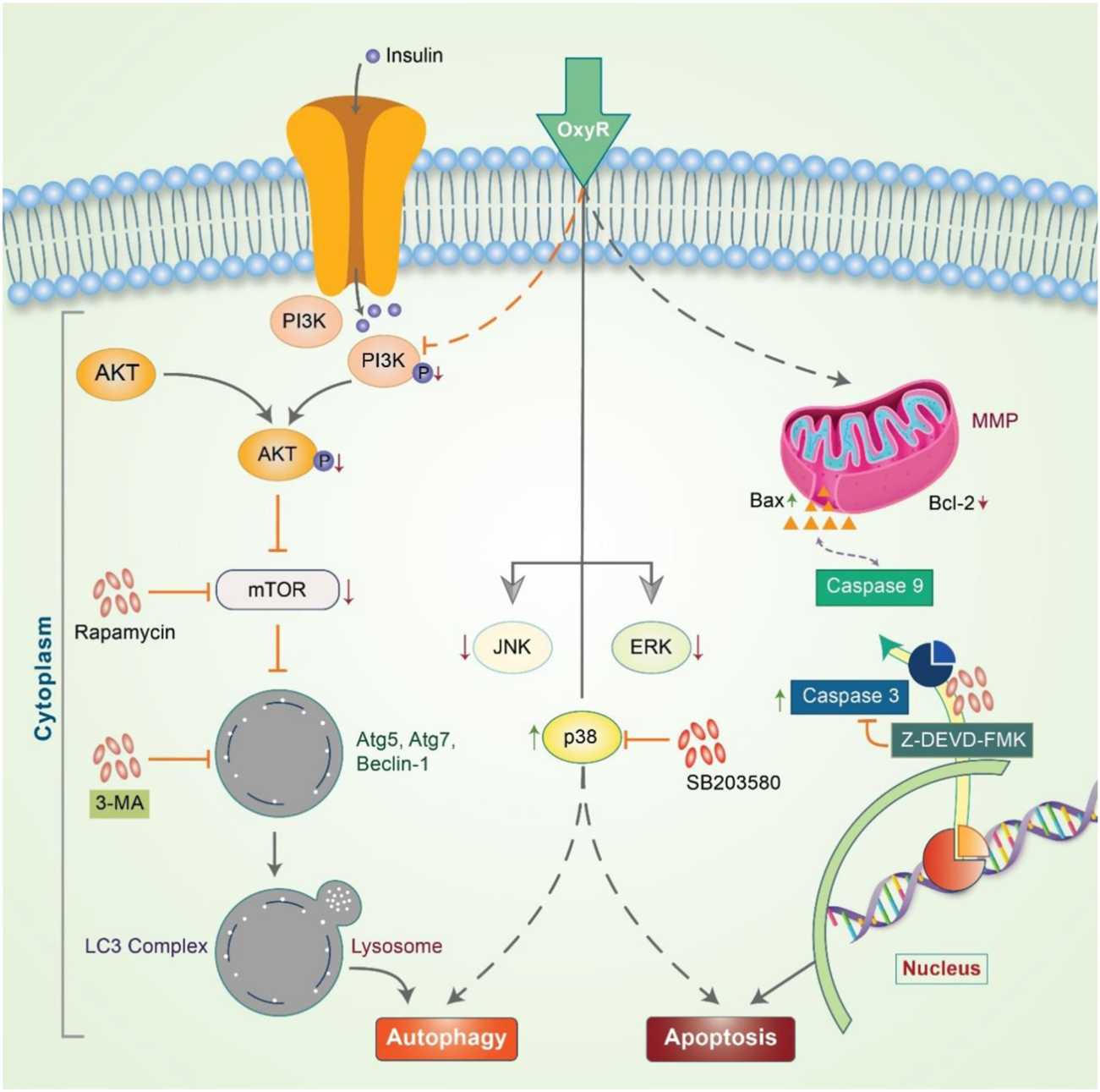
Oxyresveratrol controls the autophagy-apoptosis signal to modulate neuroblastoma cells. OxyR activates PI3K/Akt/mTOR and the inhibition of mTOR by rapamycin blocks autophagy, indicating an mTOR-dependent autophagic pathway. OxyR led to arrest at the G2/M phase of the cell cycle and activated mitochondria-mediated caspase-3 dependent apoptosis. OxyR was also revealed to increase Bax/Bcl-2 ratio without generating ROS or activating p53. When the p38 inhibitor, SB203580, was applied, OxyR was found to activate autophagy-apoptosis signaling in neuroblastoma cells.
Saikosaponin-d is reported to induce intracellular calcium accumulation and autophagy by activating the CaMKKβ-AMPK-mTOR pathway. Nonetheless, ER stress and UPR activation by saikosaponin-d have been demonstrated to trigger apoptosis and autophagic cell death (Wong et al., 2013). Isoliquiritigenin (ISL) hinders the viability of ovarian cancer cell lines (OVCAR5) and the ES-2 model. ISL also induced autophagy in OVCAR5 via cell cycle arrest at the G2/M phase, cleaved caspase-3, and increased LC3B-II and Beclin-1 expression (Chen et al., 2017b). Guttiferone K (GUTK) isolated from garcinia yunnanensis Hu (Xu et al., 2008) was found to reduce Akt phosphorylation and inhibit the mTOR pathway. GUTK also enhanced ROS and triggered the phosphorylation of JNK in EBSS, which induced autophagy and apoptosis under nutrient-deficient conditions (Wu et al., 2015).
Phytochemicals Modulate Autophagy-Apoptosis Through ROS Signaling
ROS, such as O2•−, H2O2, and •OH, are generated as metabolic by-products by biological systems; such generation may trigger detrimental as well as useful health outcomes (Covarrubias et al., 2008; Sena and Chandel, 2012). An optimum level of ROS is required for different biological processes, such as cell signaling, activation of proteins, immune function and transcriptional factors, and the regulation of apoptosis and differentiation (Rajendran et al., 2014). However, overproduction of ROS may have damaging effects on various proteins, lipids, and nucleic acids (Wu et al., 2013). Thus, an imbalance in ROS levels may be the cause of several diseases, such as cancer. Cellular ROS levels are also critical for cancer progression (Aggarwal et al., 2019). ROS-mediated DNA damage may play a critical role in the initiation and progression of carcinogenesis. Reversible DNA damage may allow an internal repair system to normalize the adverse effects of ROS. However, irreversible damage may not permit the proper functioning of the repair system. As a result, the cells undergo apoptosis, which has a considerable effect in cancer therapy (Aggarwal et al., 2019).
As antioxidant phytochemicals can inhibit the growth of different cancer cells, they could serve as good candidates for anticancer therapy (Barrajón-Catalán et al., 2010; Sak, 2014). Depending on the concentration, exposure time, and ability of oxidative stress-inducing compounds, ROS signaling may act as an autophagic activator or apoptotic initiator in target cancer cells (Chirumbolo et al., 2018). EGCG is the most abundant polyphenol in green tea. EGCG has been found to induce apoptosis and autophagy in human mesothelioma cell death through prompting ROS (Satoh et al., 2013). Ha et al. represented that ROS generation is important in quercetin-meiated apoptotic cell death in Jurkat T cells has been targeted via BCL-XL antiapoptotic action protein (Ha et al., 2019). Epicatechins as shown to modulate autophagy and endoplasmic reticulum (ER) stress-induced apoptostic cell death of human various diseases (Zhang et al., 2020a). Gallic acid, 3,4,5-trihydroxy-benzoic acid found in red wine and grapes, acts as an auto-oxidation in addition to produce H2O2 and O2− lead to intrinsic mitochondria-mediated apoptosis in prostate cancer cells (Russell Jr et al., 2012). Gallic acid prevents lung cancer cell growth via elevating ROS level as well as GSH depleting (Wang et al., 2016b). Gallic acid additionally encourages apoptosis through ROS-mediated activation of JNK pathways (Chen et al., 2013b). Oxidation of catechin-derived quinone has also been observed to result in anti-tumor activities in several human cancer cells through apoptotic as well as autophagic cell death via modulating ROS (Saibu et al., 2014; Lee et al., 2017). Thus, activation of oxidative stress signaling may not always be associated with unexpected side effects. A high dose of EGCG exerts pro-oxidant effects, which ultimately leads to autophagy activation and increased antitumor activity (Yang et al., 1998; Tsai et al., 2018; Bimonte et al., 2019). EGCG induces apoptosis in cancer cells through different mechanisms, including the suppression of PI3K/Akt signaling (Liu et al., 2016), reduction in mitochondrial membrane potential (Li et al., 2009) and expression of anti-apoptotic proteins, including Bcl-2, xIAP, and Bcl-xl (Wu et al., 2009). Previously, quercetin was found to promote ROS-stimulated apoptosis and autophagy in different cancers (Choi et al., 2008; Bi et al., 2016) by activating caspase-3 and inhibiting anti-apoptotic proteins, such as Bcl-2 and Bcl-xl. Additionally, quercetin reduces apoptosis in addition to decrease intervertebral disc degeneration through SIRT-mediated autophagy induction (Wang et al., 2020). In cancer cells, curcumin enhances TRAIL-induced apoptosis via ROS-mediated DR5 upregulation (Jung et al., 2005) and activates autophagy through the ROS-ERK1/2-p38 MAPK signaling pathway (Lee et al., 2011). Resveratrol has also been demonstrated to possess beneficial effects (Moni et al., 2018) it promotes apoptosis via ROS-dependent caspase activation (Shankar et al., 2007) and Bax/caspase-3 (Whitlock and Baek, 2012) and induces apoptosis associated with mitochondrial dysfunction in cancer cells (Lin et al., 2012). As depicted in Figure 6, phytochemicals are important modulators of cancer cell control owing to the autophagy-apoptosis pathways.
FIGURE 6
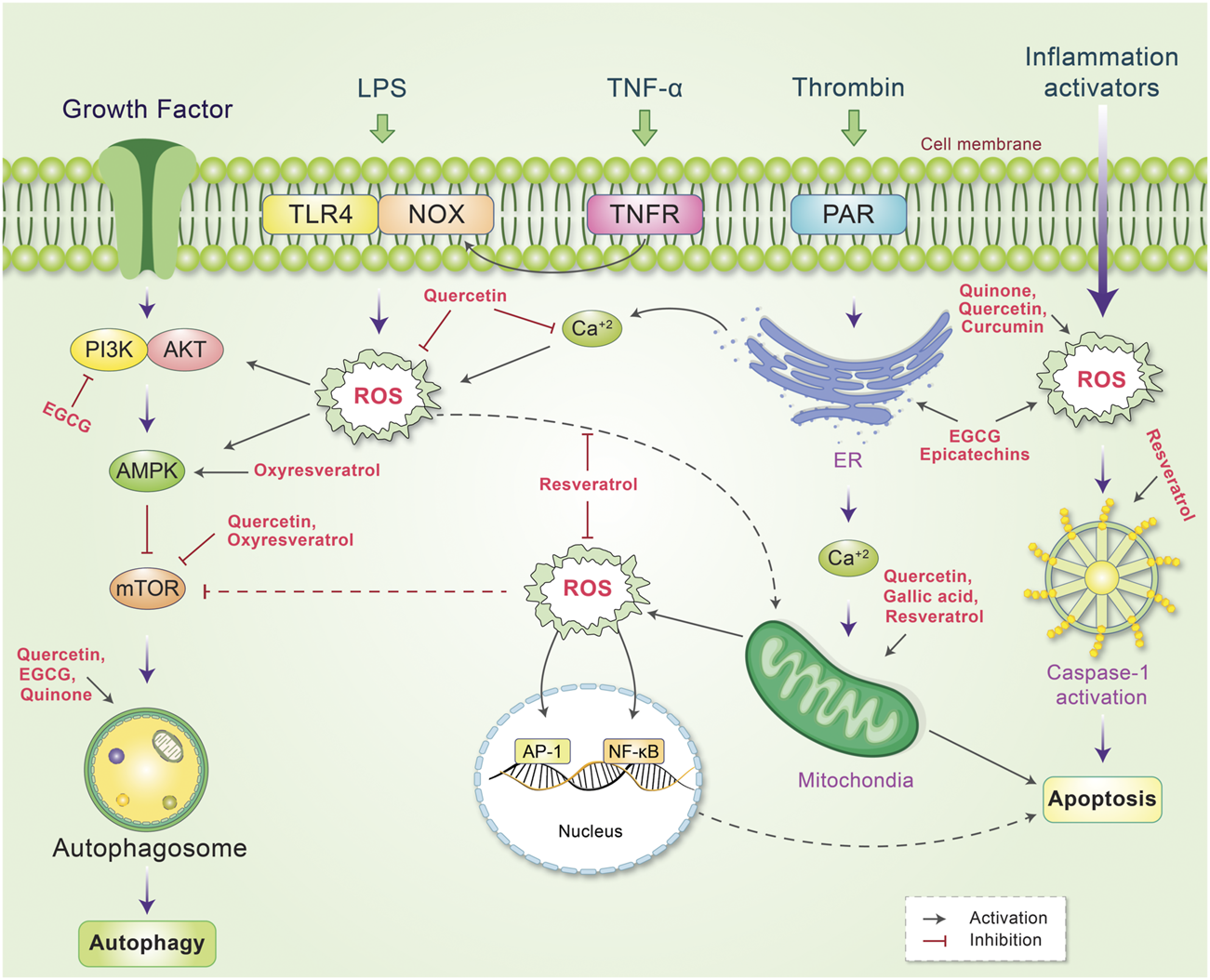
Schematic representation of the mechanism of action of phytochemicals and reactive oxygen species (ROS), which lead to the control of several signaling pathways. ROS is produced by several internal and external stimuli. Extranally, ROS is activated through growth factors, LPS, TNF-α, thrombin, and inflammation. Different phytochemicals have been found to scavenge or decrease cellular ROS level by inhibiting or stimulating their action. Internally, phytochemicals inhibit PI3K or mTOR, which activates autophagy and reduces ROS production. Some phytochemicals have also been found to activate mitochrondrial ROS production while other phytochemicals scavenge ROS and protect against DNA damage. ROS production mediated by ER and inflammation activators is also reduced by phytochemicals, which modulate the autophagy-apoptosis pathways.
Therapeutic Targets of Phytochemicals in Autophagy-Apoptosis Modulation for Cancer Prevention
Phytochemicals and naturally occurring compounds are well-known to ameliorate several human diseases owing to their pharmacological activities (Hannan et al., 2020; Rahman et al., 2020b). The most well-known anticancer agents, including taxol, resveratrol, vincristine, quercetin, vinblastine, tetrandrine, and arteannuin, modulate the autophagy-apoptosis pathway (Sun et al., 2019). Polyphenolic compounds and alkaloids are particularly dominant among all other cancer therapeutics (Newman and Cragg, 2016). Polyphenols play a greater role in apoptotic, autophagic, and cytostatic activities owing to their antioxidant properties, thereby serving as preventative cancer therapies (Focaccetti et al., 2019). Polyphenols can easily bind to cell membranes and trigger numerous signaling pathways, including caspases, epidermal growth factor (EGF), Bcl-2 family proteins, mitogen-activated protein kinase (MAPK), microRNAs (miRNAs), nuclear factor (NF)-κB, phosphatidylinositol-3-kinase PI3K/Akt/mTOR, and epidermal growth factor receptor (EGFR) (Sun et al., 2019). MicroRNAs (miRNAs) have also been demonstrated to regulate gene expression and are targeted as novel therapeutic approaches to control cancer; phytochemicals, such as resveratrol, silibinin, curcumin, genistein, and EGCG can be employed as apoptotic inducers, autophagy modulators, and cell cycle inhibitors (Lancon et al., 2012; Estrela et al., 2017; Jahanafrooz et al., 2018). miRNAs have been predicted to be critical for modulating cancer cell differentiation, invasion, proliferation, autophagy, and apoptosis via the regulation of oncogenic gene expression (Karius et al., 2012). Further, the MAPK and PI3K/Akt/mTOR signaling pathways have been shown to activate NF-κB in numerous cancer cell lines by modulating several phytochemicals in the autophagy-apoptosis pathway (Chao et al., 2017). Matrix metalloproteinase (MMP)-2 and MMP-9 modulate the autophagy-apoptosis pathway and control cancer through the action of different polyphenols (Balli et al., 2016).
Based on scientific evidence, phytochemicals present substantial anticancer potential for bench to bedside drug development. In fact, preclinical screening models can be used to assess their preliminary toxicity, safety, pharmacokinetics, and efficacy, which may serve as useful information for clinical trials (Zhang et al., 2020b). The preclinical efficacy of several phytochemicals, including ursolic acid, baicalein, genistein, 6-Shogaol, apigenin, thymoquinone, allicin, dicumarol, epigallocatechin, alpinumisoflavone, sulforaphane, curcumin, emodin, withaferin A, resveratrol, gingerol, physapubescin B, nimbolide, licochalcone A, glycyrrhizin, and hispidulin, has been demonstrated using numerous animal models (Choudhari et al., 2020). Despite several assessments of phytochemicals against cancer in the clinical trial setting, most trials continue to be in the early stage as numerous anti-cancer chemicals are currently being investigated. The most important phytochemicals under clinical trial investigation for various cancers include sulforaphane, resveratrol, lycopene, epigallocatechin, curcumin, and berberine; these phytochemicals aim to target the autophagy-apoptosis pathway (Choudhari et al., 2020).
Limitations of Targeting the Autophagy-Apoptosis Crosstalk Using Phytochemicals in Anticancer Drug Development
Increasing evidence suggests that phytochemicals could exhibit anticancer effects by modulating various signaling pathways, such as autophagy and apoptosis (Figure 3). These two significant cellular pathways are largely responsible for determining the fate of cancerous cells (Su et al., 2013). However, such finding is mainly based on in vitro and preclinical in vivo investigations that may not necessarily guarantee clinical outcomes. Moreover, many phytochemicals target multiple signaling pathways that may be shared among multiple cellular systems. These multitargeted effects of phytochemicals may generate positive outcomes, but can also lead to unanticipated effects, thereby challenging the development of phytochemical-based anticancer drugs. Although many phytochemicals are not specific in their action and exert multitarget effects, it is uncertain whether their anticancer effects are autophagy-dependent or merely a response to mitigate the adverse conditions that support the survival of cells in the tumor microenvironment (Patra et al., 2020). Although autophagy and apoptosis are two critical cellular pathways in cancer biology, their specific roles remain unclear. However, because autophagy plays a critical role in cellular protein homeostasis and other quality control systems, modulating this crucial pathway may hamper cellular physiology. As autophagy is considered to be a double-edge sword, targeting this pathway may result in unprecedented outcomes.
Conclusion and Future Perspectives
As the incidence of cancer increases on a daily basis, new strategies are being discovered to ensure this fatal disease is managed therapeutically. The major challenge in developing target-specific anticancer drugs is inextricably linked to the complexity of cancer pathobiology. Autophagy and apoptosis are two vital cellular pathways involved in cancer development and regulation. In addition, crosstalk is known to occur across signaling pathways, including those associated with autophagy and apoptosis. Many cancer types are becoming resistant to chemotherapy due to defects in signaling pathways, particularly apoptosis. As an alternative cell fate mechanism, autophagy could be explored for the development of target-specific anticancer drugs. Further investigations, both in vitro and in vivo, are however necessary to better understand cancer pathobiology, which will enable the full potential of autophagy-apoptosis-targeted drug design to be exploited.
Scientists have always been interested in the use of plant products and their derivatives as successful sources of anticancer therapeutics. In fact, there is increasing evidence suggesting the emerging anticancer potential of phytochemicals that modulate several signaling pathways, including autophagy and apoptosis. The anticancer effects of phytochemicals have been observed to be selective and specific to cancer cells, and involve the modulation of autophagy and apoptosis. As a result, many phytochemicals are promising sources of anticancer drugs. The most notable phytochemicals that have exerted their anticancer potential in vitro and in vivo through modulating the autophagy-apoptosis pathway (i.e., sulforaphane, resveratrol, lycopene, epigallocatechin, curcumin, and berberine) are currently being investigated in clinical trials for different cancer types.
Because autophagy plays a context-dependent role in cancer patients, targeting this crucial cellular pathway may not always be beneficial. Furthermore, several phytochemicals target multiple signaling pathways that may be shared among multiple cellular systems, thereby posing a challenge to the development of phytochemical-based anticancer drugs. This issue could however be resolved through in vitro and in vivo studies on phytochemical-mediated autophagy-apoptosis modulation. In addition, an integrated system pharmacology and computational approach could be employed to better understand the anticancer effects of phytochemicals. As the clinical application of phytochemicals is limited by their poor bioavailability, improvements can be achieved by employing nanotechnology-based drug delivery. Based on the highlights in this review, the potential as well as the challenges of phytochemical-mediated targeting of autophagy and apoptosis could unravel new approaches and strategies for the development of novel anticancer therapeutics to treat several cancer types.
Eventually, upcoming challenges as well as possible perspectives have been demonstrated in the hope of improving anticancer effectiveness in addition to accelerate the translational improvement of precise nanomedicine or nanotechnology for targeted cancer therapy based on autophagy-apoptosis pathway. Nanoparticle-based drug delivery systems (NDDSs) have been comprehensively used in the diagnosis, therapy, as well as cancer imaging because of their features of extraordinary cancer-targeting efficiency and low toxic properties. Nevertheless, because of the problems of poor patient prognosis, high variability, as well as multidrug resistance (MDR), NDDSs have currently been challenged remarkable experiments. Indeed, combined targets of nanoscience along with naturally occurring bioactive compounds are very attractive as well as developing rapidly in recent times in combination with conventional drugs for improving clinical outcomes. Therefore, it would be urgently required to necessary with designing novel treatment approaches to investigate in-depth the early diagnosis and pathogenesis of cancer thereby targeting phytochemicals through autophagy-apoptosis pathway.
Statements
Author contributions
Idea and conceptualization by MAR. Figures are drawing by MHR. Writing and original draft preparation by MAH, RD, RI, MJU, AAMS, and MHR. Visualization and supervision by HR. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by NRF Research Program (2016M3C7A1913845) and the Korea Research Fellowship (KRF) Program (M.A.R. 2016H1D3A1908615; M.A.H. 2018H1D3A1A01074712 to.; R.I. 2020H1D3A1A04104782), and additionally supported by (M.J.U. 2020R1I1A1A01072879 and 2020H1D3A2A02110924) funded by the Ministry of Science and ICT, Republic of Korea, and the KIST Institutional Programs (2E30962).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1
AggarwalV.TuliH. S.VarolA.ThakralF.YererM. B.SakK.et al (2019). Role of reactive oxygen species in cancer progression: molecular mechanisms and recent advancements. Biomolecules. 9, 735. 10.3390/biom9110735
2
Al DhaheriY.AttoubS.RamadanG.ArafatK.BajboujK.KaruvantevidaN.et al (2014). Carnosol induces ROS-mediated beclin1-independent autophagy and apoptosis in triple negative breast cancer. PLoS One. 9, e109630. 10.1371/journal.pone.0109630
3
AlersS.LofflerA. S.WesselborgS.StorkB. (2012). Role of AMPK-mTOR-ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol. Cell Biol.32, 2–11. 10.1128/Mcb.06159-11
4
AlfaroukK. O.StockC.-M.TaylorS.WalshM.MuddathirA. K.VerduzcoD.et al (2015). Resistance to cancer chemotherapy: failure in drug response from ADME to P-gp. Cancer Cel Int.15, 71. 10.1186/s12935-015-0221-1
5
AungT. N.QuZ. P.KortschakR. D.AdelsonD. L. (2017). Understanding the effectiveness of natural compound mixtures in cancer through their molecular mode of action. Int. J. Mol. Sci.18, 656. 10.3390/ijms18030656
6
ÁvalosY.CanalesJ.Bravo-SaguaR.CriolloA.LavanderoS.QuestA. F. G. (2014). Tumor suppression and promotion by autophagy. Biomed. Res. Int.2014, 1. 10.1155/2014/603980
7
BaligaM. S.HaniadkaR.PereiraM. M.D'SouzaJ. J.PallatyP. L.BhatH. P.et al (2011). Update on the chemopreventive effects of ginger and its phytochemicals. Crit. Rev. Food Sci. Nutr.51, 499–523. 10.1080/10408391003698669
8
BalliU.CetinkayaB. O.KelesG. C.KelesZ. P.GulerS.SogutM. U.et al (2016). Assessment of MMP-1, MMP-8 and TIMP-2 in experimental periodontitis treated with kaempferol. J. Periodontal Implan. 46, 84–95. 10.5051/jpis.2016.46.2.84
9
Barrajón-CatalánE.Fernández-ArroyoS.SauraD.GuillénE.Fernández-GutiérrezA.Segura-CarreteroA.et al (2010). Cistaceae aqueous extracts containing ellagitannins show antioxidant and antimicrobial capacity, and cytotoxic activity against human cancer cells. Food Chem. Toxicol.48, 2273–2282. 10.1016/j.fct.2010.05.060
10
BezerraD. P.PessoaC.MoraesM. O.AlencarN. M.MesquitaR. O.LimaM. W.et al (2008). In vivo growth inhibition of sarcoma 180 by piperlonguminine, an alkaloid amide from the Piper species. J Appl Toxicol.28, 599–607. 10.1002/jat.1311
11
BhatiaK.Bhumika, DasA. (2020). Combinatorial drug therapy in cancer - new insights. Life Sci.258, 118134. 10.1016/j.lfs.2020.118134
12
BiY.ShenC.LiC.LiuY.GaoD.ShiC.et al (2016). Inhibition of autophagy induced by quercetin at a late stage enhances cytotoxic effects on glioma cells. Tumour Biol.37, 3549–3560. 10.1007/s13277-015-4125-4
13
BimonteS.AlbinoV.PiccirilloM.NastoA.MolinoC.PalaiaR.et al (2019). Epigallocatechin-3-gallate in the prevention and treatment of hepatocellular carcinoma: experimental findings and translational perspectives. Drug Des. Dev. Ther.13, 611–621. 10.2147/DDDT.S180079
14
BoreddyS. R.PramanikK. C.SrivastavaS. K. (2011). Pancreatic tumor suppression by benzyl isothiocyanate is associated with inhibition of PI3K/AKT/FOXO pathway. Clin Cancer Res.17, 1784–1795. 10.1158/1078-0432.ccr-10-1891
15
CaoZ.ZhangH.CaiX.FangW.ChaiD.WenY.et al (2017). Luteolin promotes cell apoptosis by inducing autophagy in hepatocellular carcinoma, cellular physiology and biochemistry. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol.43, 1803–1812. 10.1159/000484066
16
CaoJ.ChenH.LuW.WuY.WuX.XiaD.et al (2018). Myricetin induces protective autophagy by inhibiting the phosphorylation of mTOR in HepG2 cells, anatomical record (hoboken, N. Anat Rec (Hoboken).301, 786–795. 10.1002/ar.23754
17
ChaicharoenaudomrungN.JaroonwitchawanT.NoisaP. (2018). Cordycepin induces apoptotic cell death of human brain cancer through the modulation of autophagy. Toxicol. Vitro. 46, 113–121. 10.1016/j.tiv.2017.10.002
18
ChaoA. C.HsuY. L.LiuC. K.KuoP. L. (2011). α-Mangostin, a dietary xanthone, induces autophagic cell death by activating the AMP-activated protein kinase pathway in glioblastoma cells. J. Agric. Food Chem.59, 2086–2096. 10.1021/jf1042757
19
ChaoW.DengJ. S.LiP. Y.LiangY. C.HuangG. J. (2017). 3,4-Dihydroxybenzalactone suppresses human non-small cell lung carcinoma cells metastasis via suppression of epithelial to mesenchymal transition, ROS-mediated PI3K/AKT/MAPK/MMP and NF kappa B signaling pathways. Molecules.22, 537. 10.3390/molecules22040537
20
ChenJ. C.ChiuM. H.NieR. L.CordellG. A.QiuS. X. (2005). Cucurbitacins and cucurbitane glycosides: structures and biological activities. Nat. Prod. Rep.22, 386–399. 10.1039/b418841c
21
ChenK.-L.ChangW.-S. W.CheungC. H. A.LinC.-C.HuangC.-C.YangY.-N.et al (2012). Targeting cathepsin S induces tumor cell autophagy via the EGFR-ERK signaling pathway. Cancer Lett.317, 89–98. 10.1016/j.canlet.2011.11.015
22
ChenC. Y.ChenK. C.YangT. Y.LiuH. C.HsuS. L. (2013a). Gallic acid induces a reactive oxygen species-provoked c-jun NH2-terminal kinase-dependent apoptosis in lung fibroblasts. Evid. Based Complement. Alternat Med.2013, 613950. 10.1155/2013/613950
23
ChenM.DuY.QuiM.WangM.ChenK.HuangZ.et al (2013b). Ophiopogonin B-induced autophagy in non-small cell lung cancer cells via inhibition of the PI3K/Akt signaling pathway. Oncol. Rep.29, 430–436. 10.3892/or.2012.2131
24
ChenH. Y.HuangT. C.ShiehT. M.WuC. H.LinL. C.HsiaS. M. (2017a). Isoliquiritigenin induces autophagy and inhibits ovarian cancer cell growth. Int. J. Mol. Sci.18, 2025. 10.3390/ijms18102025
25
ChenL. M.SongT. J.XiaoJ. H.HuangZ. H.LiY.LinT. Y. (2017b). Tripchlorolide induces autophagy in lung cancer cells by inhibiting the PI3K/AKT/mTOR pathway and improves cisplatin sensitivity in A549/DDP cells. Oncotarget. 8, 63911–63922. 10.18632/oncotarget.19201
26
ChenJ.ZhuY.ZhangW.PengX.ZhouJ.LiF.et al (2018). Delphinidin induced protective autophagy via mTOR pathway suppression and AMPK pathway activation in HER-2 positive breast cancer cells. BMC Cancer. 18, 342. 10.1186/s12885-018-4231-y
27
ChengY. C.HuengD. Y.HuangH. Y.ChenJ. Y.ChenY. (2016). Magnolol and honokiol exert a synergistic anti-tumor effect through autophagy and apoptosis in human glioblastomas. Oncotarget. 7, 29116–29130. 10.18632/oncotarget.8674
28
ChirumboloS.BjørklundG.LysiukR.VellaA.LenchykL.UpyrT. (2018). Targeting cancer with phytochemicals via their fine tuning of the cell survival signaling pathways. Int. J. Mol. Sci.19. 10.3390/ijms19113568
29
ChoeY.-J.HaT. J.KoK.-W.LeeS.-Y.ShinS. J.KimH.-S. (2012). Anthocyanins in the black soybean (Glycine max L.) protect U2OS cells from apoptosis by inducing autophagy via the activation of adenosyl monophosphate-dependent protein kinase. Oncol. Rep.28, 2049–2056. 10.3892/or.2012.2034
30
ChoiE. J.BaeS. M.AhnW. S. (2008). Antiproliferative effects of quercetin through cell cycle arrest and apoptosis in human breast cancer MDA-MB-453 cells. Arch. Pharm. Res.31, 1281–1285. 10.1007/s12272-001-2107-0
31
ChoiA. M. K.RyterS. W.LevineB. (2013). Autophagy in human health and disease. N. Engl. J. Med.368, 651–662. 10.1056/NEJMra1205406
32
ChoiJ. B.KimJ. H.LeeH.PakJ. N.ShimB. S.KimS. H. (2018). Reactive oxygen species and p53 mediated activation of p38 and caspases is critically involved in kaempferol induced apoptosis in colorectal cancer cells. J. Agric. Food Chem.66, 9960–9967. 10.1021/acs.jafc.8b02656
33
ChoudhariA. S.MandaveP. C.DeshpandeM.RanjekarP.PrakashO. (2020). Phytochemicals in cancer treatment: from preclinical studies to clinical practice. Front. Pharmacol.10, 1614. 10.3389/fphar.2019.01614
34
ChuY.-L.HoC.-T.ChungJ.-G.RajasekaranR.SheenL.-Y. (2012). Allicin induces p53-mediated autophagy in hep G2 human liver cancer cells. J. Agric. Food Chem.60, 8363–8371. 10.1021/jf301298y
35
ChuS. C.HsiehY. S.YuC. C.LaiY. Y.ChenP. N. (2014). Thymoquinone induces cell death in human squamous carcinoma cells via caspase activation-dependent apoptosis and LC3-II activation-dependent autophagy. PLoS One. 9, e101579. 10.1371/journal.pone.0101579
36
ComelA.SorrentinoG.CapaciV.Del SalG. (2014). The cytoplasmic side of p53's oncosuppressive activities. FEBS Lett.588, 2600–2609. 10.1016/j.febslet.2014.04.015
37
CovarrubiasL.Hernández-GarcíaD.SchnabelD.Salas-VidalE.Castro-ObregónS. (2008). Function of reactive oxygen species during animal development: passive or active?Developmental Biol.320, 1–11. 10.1016/j.ydbio.2008.04.041
38
DelmasD.RébéC.LacourS.FilomenkoR.AthiasA.GambertP.et al (2003). Resveratrol-induced apoptosis is associated with Fas redistribution in the rafts and the formation of a death-inducing signaling complex in colon cancer cells. J. Biol. Chem.278, 41482–41490. 10.1074/jbc.M304896200
39
DengS.ShanmugamM. K.KumarA. P.YapC. T.SethiG.BishayeeA. (2019). Targeting autophagy using natural compounds for cancer prevention and therapy. Cancer. 125, 1228–1246. 10.1002/cncr.31978
40
EdrisA. E. (2009). Anti-cancer properties of Nigella spp. essential oils and their major constituents, thymoquinone and beta-elemene. Curr. Clin. Pharmacol.4, 43–46. 10.2174/157488409787236137
41
ElangoR.AthinarayananJ.SubbarayanV. P.LeiD. K. Y.AlshatwiA. A. (2018). Hesperetin induces an apoptosis-triggered extrinsic pathway and a p53- independent pathway in human lung cancer H522 cells. J. Asian Nat. Prod. Res.20, 559–569. 10.1080/10286020.2017.1327949
42
ElmoreS. (2007). Apoptosis: a review of programmed cell death. Toxicol. Pathol.35, 495–516. 10.1080/01926230701320337
43
EstrelaJ. M.MenaS.ObradorE.BenllochM.CastellanoG.SalvadorR.et al (2017). Polyphenolic phytochemicals in cancer prevention and therapy: bioavailability versus bioefficacy. J. Med. Chem.60, 9413–9436. 10.1021/acs.jmedchem.6b01026
44
FilomeniG.De ZioD.CecconiF. (2015). Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ. 22, 377–388. 10.1038/cdd.2014.150
45
FocaccettiC.IzziV.BenvenutoM.FaziS.CiuffaS.GigantiM. G.et al (2019). Polyphenols as immunomodulatory compounds in the tumor microenvironment: friends or foes?Int. J. Mol. Sci.20, 1714. 10.3390/ijms20071714
46
FrieboesH. B.HuangJ. S.YinW. C.McNallyL. R. (2014). Chloroquine-mediated cell death in metastatic pancreatic adenocarcinoma through inhibition of autophagy. JOP. 15, 189–197. 10.6092/1590-8577/1900
47
GhavamiS.KerkhoffC.LosM.HashemiM.SorgC.Karami-TehraniF. (2004). Mechanism of apoptosis induced by S100A8/A9 in colon cancer cell lines: the role of ROS and the effect of metal ions. J. Leukoc. Biol.76, 169–175. 10.1189/jlb.0903435
48
GhobrialI. M.WitzigT. E.AdjeiA. A. (2005). Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin.55, 178–194. 10.3322/canjclin.55.3.178
49
GoldarS.KhanianiM. S.DerakhshanS. M.BaradaranB. (2015). Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pac. J. Cancer Prev.16, 2129–2144. 10.7314/apjcp.2015.16.6.2129
50
GoldsmithJ.LevineB.DebnathJ. (2014). Autophagy and cancer metabolism. Method Enzymol.542, 25–57. 10.1016/B978-0-12-416618-9.00002-9
51
GossnerG.ChoiM.TanL.FogorosS.GriffithK.KuenkerM.et al (2007). Genistein-induced apoptosis and autophagocytosis in ovarian cancer cells. Gynecol. Oncol.105, 23–30. 10.1016/j.ygyno.2006.11.009
52
GranatoM.RizzelloC.Gilardini MontaniM. S.CuomoL.VitilloM.SantarelliR.et al (2017). Quercetin induces apoptosis and autophagy in primary effusion lymphoma cells by inhibiting PI3K/AKT/mTOR and STAT3 signaling pathways. J. Nutr. Biochem.41, 124–136. 10.1016/j.jnutbio.2016.12.011
53
GreenD. R.LlambiF. (2015). Cell death signaling. Cold Spring Harbor Perspect. Biol.7, a00608. 10.1101/cshperspect.a006080
54
GrubeS.EwaldC.KöglerC.Lawson McLeanA.KalffR.WalterJ. (2018). Achievable central nervous system concentrations of the green tea catechin EGCG induce stress in glioblastoma cells in Vitro. Nutr. Cancer. 70, 1145–1158. 10.1080/01635581.2018.1495239
55
Guamán OrtizL. M.CroceA. L.ArediaF.SapienzaS.FiorilloG.SyedaT. M.et al (2015). Effect of new berberine derivatives on colon cancer cells. Acta Biochim. Biophys. Sinica. 47, 824–833. 10.1093/abbs/gmv077
56
GuanC.LiuW.YueY.JinH.WangX.WangX.-J. (2014). Inhibitory effect of β-elemene on human breast cancer cells. Int. J. Clin. Exp. Pathol.7, 3948
57
GuicciardiM. E.GoresG. J. (2009). Life and death by death receptors. FASEB j.23, 1625–1637. 10.1096/fj.08-111005
58
GuoJ.HuangX.WangH.YangH. (2015). Celastrol induces autophagy by targeting AR/miR-101 in prostate cancer cells. PLoS One. 10, e0140745. 10.1371/journal.pone.0140745
59
GuptaP.SrivastavaS. K. (2012). Antitumor activity of phenethyl isothiocyanate in HER2-positive breast cancer models. BMC Med.10, 80. 10.1186/1741-7015-10-80
60
HaE. J.KimK. Y.KimC. E.JunD. Y.KimY. H. (2019). Enhancement of quercetin-induced apoptosis by cotreatment with autophagy inhibitor is associated with augmentation of BAK-dependent mitochondrial pathway in Jurkat T cells. Oxid Med. Cel Longev. 2019, 7989276. 10.1155/2019/7989276
61
HannaT. P.KingW. D.ThibodeauS.JalinkM.PaulinG. A.Harvey-JonesE.et al (2020). Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ. 371, m4087. 10.1136/bmj.m4087
62
HannanM. A.DashR.HaqueM. N.MohibbullahM.SohagA. A.RahmanM. A.et al (2020). Neuroprotective potentials of marine algae and their bioactive metabolites: pharmacological insights and therapeutic advances. Mar. Drugs. 18, 347. 10.3390/md18070347
63
HartwigA.ArandM.EpeB.GuthS.JahnkeG.LampenA.et al (2020). Mode of action-based risk assessment of genotoxic carcinogens. Arch. Toxicol.94, 1787–1877. 10.1007/s00204-020-02733-2
64
HassanM.WatariH.AbuAlmaatyA.OhbaY.SakuragiN. (2020). Apoptosis and molecular targeting therapy in cancer, BioMed Research International.2020, 2451249. 10.1155/2020/2451249
65
HenleyS. J.WardE. M.ScottS.MaJ.AndersonR. N.FirthA. U.et al (2020). Annual report to the nation on the status of cancer, part I: National cancer statistics. Cancer. 126, 2225–2249. 10.1002/cncr.32802
66
HsinM. C.HsiehY. H.WangP. H.KoJ. L.HsinI. L.YangS. F. (2017). Hispolon suppresses metastasis via autophagic degradation of cathepsin S in cervical cancer cells. Cell Death Dis. 8. 3089. 10.1038/cddis.2017.459
67
HsuY. L.KuoP. L.ChiangL. C.LinC. C. (2004). Involvement of p53, nuclear factor kappaB and Fas/Fas ligand in induction of apoptosis and cell cycle arrest by saikosaponin d in human hepatoma cell lines. Cancer Lett.213, 213–221. 10.1016/j.canlet.2004.03.044
68
HuangY.-H.SunY.HuangF.-Y.LiY.-N.WangC.-C.MeiW.-L.et al (2017). Toxicarioside O induces protective autophagy in a sirtuin-1-dependent manner in colorectal cancer cells. Oncotarget. 8, 52783. 10.18632/oncotarget.17189
69
HuiC.BinY.XiaopingY.LongY.ChunyeC.MantianM.et al (2010). Anticancer activities of an anthocyanin-rich extract from black rice against breast cancer cells in vitro and in vivo. Nutr. Cancer. 62, 1128–1136. 10.1080/01635581.2010.494821
70
HungJ. Y.HsuY. L.LiC. T.KoY. C.NiW. C.HuangM. S.et al (2009). 6-Shogaol, an active constituent of dietary ginger, induces autophagy by inhibiting the AKT/mTOR pathway in human non-small cell lung cancer A549 cells. J. Agric. Food Chem.57, 9809–9816. 10.1021/jf902315e
71
HurleyJ. H.YoungL. N. (2017). Mechanisms of autophagy initiation. Annu. Rev. Biochem.86, 225–244. 10.1146/annurev-biochem-061516-044820
72
JahanafroozZ.MotamedN.RinnerB.MokhtarzadehA.BaradaranB. (2018). Silibinin to improve cancer therapeutic, as an apoptotic inducer, autophagy modulator, cell cycle inhibitor, and microRNAs regulator. Life Sci.213, 236–247. 10.1016/j.lfs.2018.10.009
73
JangH. J.YangK. E.HwangI. H.HuhY. H.KimD. J.YooH. S.et al (2019). Cordycepin inhibits human ovarian cancer by inducing autophagy and apoptosis through Dickkopf-related protein 1/β-catenin signaling. Am. J. Transl Res.11, 6890–6906.
74
JinH. R.ZhaoJ.ZhangZ.LiaoY.WangC. Z.HuangW. H.et al (2012). The antitumor natural compound falcarindiol promotes cancer cell death by inducing endoplasmic reticulum stress. Cel Death Dis.3, e376. 10.1038/cddis.2012.122
75
JohnsonR.ShabalalaS.LouwJ.KappoA. P.MullerC. J. F. (2017). Aspalathin reverts doxorubicin-induced cardiotoxicity through increased autophagy and decreased expression of p53/mTOR/p62 signaling. Molecules. 22. 1589. 10.3390/molecules22101589
76
JungE. M.LimJ. H.LeeT. J.ParkJ. W.ChoiK. S.KwonT. K. (2005). Curcumin sensitizes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis through reactive oxygen species-mediated upregulation of death receptor 5 (DR5). Carcinogenesis. 26, 1905–1913. 10.1093/carcin/bgi167
77
JungS.JeongH.YuS.-W. (2020). Autophagy as a decisive process for cell death. Exp. Mol. Med.52, 921–930. 10.1038/s12276-020-0455-4
78
KardidehB.SamimiZ.NorooznezhadF.KianiS.MansouriK. (2019). Autophagy, cancer and angiogenesis: where is the link?Cell Biosci. 9, 65. 10.1186/s13578-019-0327-6
79
KariusT.SchnekenburgerM.DicatoM.DiederichM. (2012). MicroRNAs in cancer management and their modulation by dietary agents. Biochem. Pharmacol.83, 1591–1601. 10.1016/j.bcp.2012.02.004
80
KhaltaevN.AxelrodS. (2020). Global lung cancer mortality trends and lifestyle modifications: preliminary analysis. Chin. Med J-Peking. 133, 1526–1532. 10.1097/Cm9.0000000000000918
81
KimE. S.ShinJ. H.SeokS. H.KimJ. B.ChangH.ParkS. J.et al (2013). Autophagy mediates anti-melanogenic activity of 3′-ODI in B16F1 melanoma cells. Biochem. Biophysical Res. Commun.442, 165–170. 10.1016/j.bbrc.2013.11.048
82
KimR. (2005). Recent advances in understanding the cell death pathways activated by anticancer therapy. Cancer. 103, 1551–1560. 10.1002/cncr.20947
83
KotaniT.KirisakoH.KoizumiM.OhsumiY.NakatogawaH. (2018). The Atg2-Atg18 complex tethers pre-autophagosomal membranes to the endoplasmic reticulum for autophagosome formation. Proc. Natl. Acad. Sci. USA.115, 10363–10368. 10.1073/pnas.1806727115
84
KrishnanS.ShresthaY.JayatungaD. P. W.ReaS.MartinsR.BharadwajP. (2020). Activate or inhibit? Implications of autophagy modulation as a therapeutic strategy for alzheimer's disease. Int. J. Mol. Sci.21, 673910.3390/ijms21186739
85
KumarD.ShankarS.SrivastavaR. K. (2013). Rottlerin-induced autophagy leads to the apoptosis in breast cancer stem cells: molecular mechanisms. Mol. Cancer. 12, 171. 10.1186/1476-4598-12-171
86
KwonY. H.BishayeeK.RahmanM. A.HongJ. S.LimS. S.HuhS. O. (2015). Morus alba accumulates reactive oxygen species to initiate apoptosis via FOXO-caspase 3-dependent pathway in neuroblastoma cells. Mol. Cell. 38, 630–637. 10.14348/molcells.2015.0030
87
LanconA.KaminskiJ.TiliE.MichailleJ. J.LatruffeN. (2012). Control of MicroRNA expression as a new way for resveratrol to deliver its beneficial effects. J. Agr Food Chem.60, 8783–8789. 10.1021/jf301479v
88
LaoY.WanG.LiuZ.WangX.RuanP.XuW.et al (2014). The natural compound oblongifolin C inhibits autophagic flux and enhances antitumor efficacy of nutrient deprivation. Autophagy. 10, 736–749. 10.4161/auto.28034
89
LawB. Y.WangM.MaD. L.Al-MousaF.MichelangeliF.ChengS. H.et al (2010). Alisol B, a novel inhibitor of the sarcoplasmic/endoplasmic reticulum Ca(2+) ATPase pump, induces autophagy, endoplasmic reticulum stress, and apoptosis. Mol. Cancer Ther.9, 718–730. 10.1158/1535-7163.mct-09-0700
90
LawB. Y. K.MokS. W. F.ChenJ.MichelangeliF.JiangZ. H.HanY.et al (2017). N-desmethyldauricine induces autophagic cell death in apoptosis-defective cells via Ca(2+) mobilization. Front. Pharmacol.8, 388. 10.3389/fphar.2017.00388
91
LeeY. J.KimN.-Y.SuhY.-A.LeeC. (2011). Involvement of ROS in curcumin-induced autophagic cell death. Korean J. Physiol. Pharmacol.15, 1–7. 10.4196/kjpp.2011.15.1.1
92
LeeE. B.CheonM. G.CuiJ.LeeY. J.SeoE. K.JangH. H. (2017). The quinone-based derivative, HMNQ induces apoptotic and autophagic cell death by modulating reactive oxygen species in cancer cells. Oncotarget. 8, 99637–99648. 10.18632/oncotarget.21005
93
LevineB.KroemerG. (2008). Autophagy in the pathogenesis of disease. Cell. 132, 27–42. 10.1016/j.cell.2007.12.018
94
LiW.NieS.YuQ.XieM. (2009). (-)-Epigallocatechin-3-gallate induces apoptosis of human hepatoma cells by mitochondrial pathways related to reactive oxygen species. J. Agric. Food Chem.57, 6685–6691. 10.1021/jf901396f
95
LiL.YueG. G.LauC. B.SunH.FungK. P.LeungP. C.et al (2012). Eriocalyxin B induces apoptosis and cell cycle arrest in pancreatic adenocarcinoma cells through caspase-and p53-dependent pathways. Toxicol. Appl. Pharmacol.262, 80–90.
96
LiF.MaZ.GuanZ.ChenY.WuK.GuoP.et al (2015). Autophagy induction by silibinin positively contributes to its anti-metastatic capacity via AMPK/mTOR pathway in renal cell carcinoma. Int. J. Mol. Sci.16, 8415–8429. 10.3390/ijms16048415
97
LinX.WuG.HuoW.-Q.ZhangY.JinF.-S. (2012). Resveratrol induces apoptosis associated with mitochondrial dysfunction in bladder carcinoma cells. Int. J. Urol.19, 757–764. 10.1111/j.1442-2042.2012.03024.x
98
LinC.-M.ChenH.-H.LinC.-A.WuH.-C.SheuJ. J.-C.ChenH.-J. (2017a). Apigenin-induced lysosomal degradation of β-catenin in Wnt/β-catenin signaling. Scientific Rep.7, 1–17. 10.1038/s41598-017-00409-z
99
LinY.-T.WangH.-C.HsuY.-C.ChoC.-L.YangM.-Y.ChienC.-Y. (2017b). Capsaicin induces autophagy and apoptosis in human nasopharyngeal carcinoma cells by downregulating the PI3K/AKT/mTOR pathway. Int. J. Mol. Sci.18, 1343. 10.3390/ijms18071343
100
LingM. T.LukS. U.Al-EjehF.KhannaK. K. (2012). Tocotrienol as a potential anticancer agent. Carcinogenesis. 33, 233–239. 10.1093/carcin/bgr261
101
LiuJ.ZhengL.ZhongJ.WuN.LiuG.LinX. (2014). Oleanolic acid induces protective autophagy in cancer cells through the JNK and mTOR pathways. Oncol. Rep.32, 567–572. 10.3892/or.2014.3239
102
LiuS.XuZ. L.SunL.LiuY.LiC. C.LiH. M.et al (2016). (-)-Epigallocatechin-3-gallate induces apoptosis in human pancreatic cancer cells via PTEN. Mol. Med. Rep.14, 599–605. 10.3892/mmr.2016.5277
103
LiuF.GaoS.YangY.ZhaoX.FanY.MaW.et al (2018). Antitumor activity of curcumin by modulation of apoptosis and autophagy in human lung cancer A549 cells through inhibiting PI3K/Akt/mTOR pathway. Oncol. Rep.39, 1523–1531. 10.3892/or.2018.6188
104
LopezJ.TaitS. W. G. (2015). Mitochondrial apoptosis: killing cancer using the enemy within. Br. J. Cancer. 112, 957–962. 10.1038/bjc.2015.85
105
LuT.GuM.ZhaoY.ZhengX.XingC. (2017). Autophagy contributes to falcarindiol-induced cell death in breast cancer cells with enhanced endoplasmic reticulum stress. PLoS One. 12, e0176348. 10.1371/journal.pone.0176348
106
MaX.NingS. (2019). Cyanidin-3-glucoside attenuates the angiogenesis of breast cancer via inhibiting STAT3/VEGF pathway. Phytotherapy Res.33, 81–89. 10.1002/ptr.6201
107
MaiT. T.MoonJ.SongY.VietP. Q.PhucP. V.LeeJ. M.et al (2012). Ginsenoside F2 induces apoptosis accompanied by protective autophagy in breast cancer stem cells. Cancer Lett.321, 144–153. 10.1016/j.canlet.2012.01.045
108
MarkhamM. J.WachterK.AgarwalN.BertagnolliM. M.ChangS. M.DaleW.et al (2020). Clinical cancer advances 2020: annual report on progress against cancer from the American society of clinical oncology. Jco. 38, 1081. 10.1200/jco.19.03141
109
MasuelliL.BenvenutoM.StefanoE. D.MatteraR.FantiniM.FeudisG. D.et al (2017). Curcumin blocks autophagy and activates apoptosis of malignant mesothelioma cell lines and increases the survival of mice intraperitoneally transplanted with a malignant mesothelioma cell line. Oncotarget. 8, 34405. 10.18632/oncotarget.14907
110
MintoR. E.BlacklockB. J. (2008). Biosynthesis and function of polyacetylenes and allied natural products. Prog. lipid Res.47, 233–306. 10.1016/j.plipres.2008.02.002
111
MitraS.DashR. (2018). Natural products for the management and prevention of breast cancer, evidence-based complementary and alternative medicine, Evid Based Complement Alternat Med.2018, 8324696. 10.1155/2018/8324696
112
MohapatraP.PreetR.DasD.SatapathyS. R.ChoudhuriT.WyattM. D.et al (2012). Quinacrine-mediated autophagy and apoptosis in colon cancer cells is through a p53- and p21-dependent mechanism. Oncol. Res.20, 81–91. 10.3727/096504012x13473664562628
113
MoniA.IqbalA.UddinM. J. (2018). Resveratrol attenuates inflammation through tristetraprolin expression in human hepatocytes. J. Adv. Biotechnol. Exp. Ther.1, 78–82. 10.5455/jabet.2018.d14
114
MoosaviM. A.HaghiA.RahmatiM.TaniguchiH.MocanA.EcheverríaJ.et al (2018). Phytochemicals as potent modulators of autophagy for cancer therapy. Cancer Lett.424, 46–69. 10.1016/j.canlet.2018.02.030
115
MujumdarN.MackenzieT. N.DudejaV.ChughR.AntonoffM. B.Borja-CachoD.et al (2010). Triptolide induces cell death in pancreatic cancer cells by apoptotic and autophagic pathways. Gastroenterology. 139, 598–608. 10.1053/j.gastro.2010.04.046
116
NaumannP.FortunatoF.ZentgrafH.BüchlerM. W.HerrI.WernerJ. (2011). Autophagy and cell death signaling following dietary sulforaphane act independently of each other and require oxidative stress in pancreatic cancer. Int. J. Oncol.39, 101–109. 10.3892/ijo.2011.1025
117
NewmanD. J.CraggG. M. (2016). Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod.79, 629–661. 10.1021/acs.jnatprod.5b01055
118
PandaP. K.MukhopadhyayS.DasD. N.SinhaN.NaikP. P.BhutiaS. K. (2015). Mechanism of autophagic regulation in carcinogenesis and cancer therapeutics. Semin. Cel Developmental Biol.39, 43–55. 10.1016/j.semcdb.2015.02.013
119
PatraS.MishraS. R.BeheraB. P.MahapatraK. K.PanigrahiD. P.BholC. S.et al (2020). Autophagy-modulating phytochemicals in cancer therapeutics: current evidences and future perspectives. Semin. Cancer Biol.10.1016/j.semcancer.2020.05.008
120
PengP. L.KuoW. H.TsengH. C.ChouF. P. (2008). Synergistic tumor-killing effect of radiation and berberine combined treatment in lung cancer: the contribution of autophagic cell death. Int. J. Radiat. Oncol. Biol. Phys.70, 529–542. 10.1016/j.ijrobp.2007.08.034
121
PollierJ.GoossensA. (2012). Oleanolic acid, Phytochemistry. 77, 10–15. 10.1016/j.phytochem.2011.12.022
122
RadhakrishnanE.BavaS. V.NarayananS. S.NathL. R.ThulasidasanA. K. T.SoniyaE. V.et al (2014). [6]-Gingerol induces caspase-dependent apoptosis and prevents PMA-induced proliferation in colon cancer cells by inhibiting MAPK/AP-1 signaling. PloS one. 9, e104401. 10.1371/journal.pone.0104401
123
RahmanM. A.RhimH. (2017). Therapeutic implication of autophagy in neurodegenerative diseases. BMB Rep.50, 345–354. 10.5483/BMBRep.2017.50.7.069
124
RahmanM. A.KimN. H.KimS. H.OhS. M.HuhS. O. (2012a). Antiproliferative and cytotoxic effects of resveratrol in mitochondria-mediated apoptosis in rat B103 neuroblastoma cells. Korean J. Physiol. Pha. 16, 321–326. 10.4196/kjpp.2012.16.5.321
125
RahmanM. A.KimN. H.YangH.HuhS. O. (2012b). Angelicin induces apoptosis through intrinsic caspase-dependent pathway in human SH-SY5Y neuroblastoma cells. Mol. Cell Biochem.369, 95–104. 10.1007/s11010-012-1372-1
126
RahmanM. A.KimN. H.HuhS. O. (2013). Cytotoxic effect of gambogic acid on SH-SY5Y neuroblastoma cells is mediated by intrinsic caspase-dependent signaling pathway. Mol. Cel Biochem. 377, 187–196. 10.1007/s11010-013-1584-z
127
RahmanM. A.YangH.KimN. H.HuhS. O. (2014). Induction of apoptosis by Dioscorea nipponica Makino extracts in human SH-SY5Y neuroblastoma cells via mitochondria-mediated pathway. Anim. Cell Syst. 18, 41–51. 10.1080/19768354.2014.880372
128
RahmanM. A.HongJ. S.HuhS. O. (2015). Antiproliferative properties of Saussurea lappa Clarke root extract in SH-SY5Y neuroblastoma cells via intrinsic apoptotic pathway. Anim. Cell Syst19, 119–126. 10.1080/19768354.2015.1008041
129
RahmanM. A.BishayeeK.HabibK.SadraA.HuhS. O. (2016a). 18 alpha-Glycyrrhetinic acid lethality for neuroblastoma cells via de-regulating the Beclin-1/Bcl-2 complex and inducing apoptosis. Biochem. Pharmacol.117, 97–112. 10.1016/j.bcp.2016.08.006
130
RahmanM. A.BishayeeK.HuhS. O. (2016b). Angelica polymorpha Maxim induces apoptosis of human SH-SY5Y neuroblastoma cells by regulating an intrinsic caspase pathway. Mol. Cell. 39, 119–128. 10.14348/molcells.2016.2232
131
RahmanM. A.BishayeeK.SadraA.HuhS. O. (2017). Oxyresveratrol activates parallel apoptotic and autophagic cell death pathways in neuroblastoma cells. Biochim. Biophys. Acta Gen. Subj. 1861, 23–36. 10.1016/j.bbagen.2016.10.025
132
RahmanM. A.HwangH.NahS. Y.RhimH. (2020). Gintonin stimulates autophagic flux in primary cortical astrocytes. J. Ginseng Res.44, 67–78. 10.1016/j.jgr.2018.08.004
133
RahmanM. A.RahmanM. H.HossainM. S.BiswasP.IslamR.UddinM. J.et al (2020a). Molecular insights into the multifunctional role of natural compounds: autophagy modulation and cancer prevention. Biomedicines. 8. 517. 10.3390/biomedicines8110517
134
RahmanM. A.RahmanM. R.ZamanT.UddinM. S.IslamR.Abdel-DaimM. M.et al (2020b). Emerging potential of naturally occurring autophagy modulators against neurodegeneration. Curr. Pharm. Des.26, 772–779. 10.2174/1381612826666200107142541
135
RajL.IdeT.GurkarA. U.FoleyM.SchenoneM.LiX.et al (2011). Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature. 475, 231–234. 10.1038/nature10167
136
RajendranP.NandakumarN.RengarajanT.PalaniswamiR.GnanadhasE. N.LakshminarasaiahU.et al (2014). Antioxidants and human diseases. Clin. Chim. Acta436, 332–347. 10.1016/j.cca.2014.06.004
137
RasulA.YuB.ZhongL.KhanM.YangH.MaT. (2012). Cytotoxic effect of evodiamine in SGC-7901 human gastric adenocarcinoma cells via simultaneous induction of apoptosis and autophagy. Oncol. Rep.27, 1481–1487. 10.3892/or.2012.1694
138
RenG.ShaT.GuoJ.LiW.LuJ.ChenX. (2015). Cucurbitacin B induces DNA damage and autophagy mediated by reactive oxygen species (ROS) in MCF-7 breast cancer cells. J. Nat. medicines. 69, 522–530. 10.1007/s11418-015-0918-4
139
RubinszteinD. C.ShpilkaT.ElazarZ. (2012). Mechanisms of autophagosome biogenesis. Curr. Biol.22, R29–R34. 10.1016/j.cub.2011.11.034
140
RussellL. H.Jr.MazzioE.BadisaR. B.ZhuZ. P.AgharahimiM.OriakuE. T.et al (2012). Autoxidation of gallic acid induces ROS-dependent death in human prostate cancer LNCaP cells. Anticancer Res.32, 1595–1602.
141
SaibuM.SagarS.GreenI.AmeerF.MeyerM. (2014). Evaluating the cytotoxic effects of novel quinone compounds. Anticancer Res.34, 4077–4086.
142
SakK. (2012). Chemotherapy and dietary phytochemical agents, Chemotherapy research and practice. Chemother Res Pract.2012, 282570. 10.1155/2012/282570
143
SakK. (2014). Site-specific anticancer effects of dietary flavonoid quercetin. Nutr. Cancer. 66, 177–193. 10.1080/01635581.2014.864418
144
SanfordN. N.SherD. J.XuX.AhnC.D’AmicoA. V.AizerA. A.et al (2020), Alcohol use among patients with cancer and survivors in the United States, 2000-2017, J. Natl. Compr. Canc Ne. 18, 69. 10.6004/jnccn.2019.7341
145
Santana-CodinaN.ManciasJ. D.KimmelmanA. C. (2017). The role of autophagy in cancer. Annu. Rev. Cancer Biol.1, 19–39. 10.1146/annurev-cancerbio-041816-122338
146
SatohM.TakemuraY.HamadaH.SekidoY.KubotaS. (2013). EGCG induces human mesothelioma cell death by inducing reactive oxygen species and autophagy. Cancer Cel Int. 13, 19. 10.1186/1475-2867-13-19
147
SchirrmacherV. (2019). From chemotherapy to biological therapy: a review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int. J. Oncol.54, 407–419. 10.3892/ijo.2018.4661
148
SenaLaura. A.ChandelNavdeep. S. (2012). Physiological roles of mitochondrial reactive oxygen species. Mol. Cel. 48, 158–167. 10.1016/j.molcel.2012.09.025
149
ShankarS.ChenQ.SiddiquiI.SarvaK.SrivastavaR. K. (2007). Sensitization of TRAIL-resistant LNCaP cells by resveratrol (3, 4', 5 tri-hydroxystilbene): molecular mechanisms and therapeutic potential. J. Mol. Signal.2, 7. 10.1186/1750-2187-2-7
150
ShinS. W.KimS. Y.ParkJ. W. (2012). Autophagy inhibition enhances ursolic acid-induced apoptosis in PC3 cells. Biochim. Biophys. Acta. 1823, 451–457. 10.1016/j.bbamcr.2011.10.014
151
ShuklaY.SinghM. (2007). Cancer preventive properties of ginger: a brief review. Food Chem Toxicol.45, 683–690. 10.1016/j.fct.2006.11.002
152
SiddiquiI. A.AsimM.HafeezB. B.AdhamiV. M.TaraporeR. S.MukhtarH. (2011). Green tea polyphenol EGCG blunts androgen receptor function in prostate cancer. FASEB J. : official Publ. Fed. Am. Societies Exp. Biol.25, 1198–1207. 10.1096/fj.10-167924
153
SiegelR. L.MillerK. D.JemalA. (2020). Cancer statistics, 2020. CA A. Cancer J. Clin.70, 7–30. 10.3322/caac.21590
154
SinghR.CuervoA. M. (2011). Autophagy in the cellular energetic balance. Cel Metab.13, 495–504. 10.1016/j.cmet.2011.04.004
155
StadlbauerS.SteinbornC.KlemdA.HattoriF.OhmoriK.SuzukiK.et al (2018). Impact of green tea catechin ECG and its synthesized fluorinated analogue on prostate cancer cells and stimulated immunocompetent cells. Planta Med.84, 813–819. 10.1055/s-0044-102099
156
SuM.MeiY.SinhaS. (2013). Role of the crosstalk between autophagy and apoptosis in cancer. J. Oncol.2013, 102735. 10.1155/2013/102735
157
SuhY.AfaqF.KhanN.JohnsonJ. J.KhusroF. H.MukhtarH. (2010). Fisetin induces autophagic cell death through suppression of mTOR signaling pathway in prostate cancer cells. Carcinogenesis. 31, 1424–1433. 10.1093/carcin/bgq115
158
SunC. Y.ZhuY.LiX. F.TangL. P.SuZ. Q.WangX. Q.et al (2017a). Norcantharidin alone or in combination with crizotinib induces autophagic cell death in hepatocellular carcinoma by repressing c-Met-mTOR signaling. Oncotarget. 8, 114945–114955. 10.18632/oncotarget.22935
159
SunZ. L.DongJ. L.WuJ. (2017b). Juglanin induces apoptosis and autophagy in human breast cancer progression via ROS/JNK promotion. Biomed. Pharmacother.85, 303–312. 10.1016/j.biopha.2016.11.030
160
SunL. R.ZhouW.ZhangH. M.GuoQ. S.YangW.LiB. J.et al (2019). Modulation of multiple signaling pathways of the plant-derived natural products in cancer. Front. Oncol.9, 1153. 10.3389/fonc.2019.01153
161
SurichanS.ArrooR. R.TsatsakisA. M.AndroutsopoulosV. P. (2018). Tangeretin inhibits the proliferation of human breast cancer cells via CYP1A1/CYP1B1 enzyme induction and CYP1A1/CYP1B1-mediated metabolism to the product 4' hydroxy tangeretin. Toxicol In Vitro.50, 274–284. 10.1016/j.tiv.2018.04.001
162
TangK. D.LiuJ.RussellP. J.ClementsJ. A.LingM. T. (2019). Gamma-tocotrienol induces apoptosis in prostate cancer cells by targeting the ang-1/tie-2 signalling pathway. Int. J. Mol. Sci.20, 1164. 10.3390/ijms20051164
163
ThorburnA.ThammD. H.GustafsonD. L. (2014). Autophagy and cancer therapy. Mol. Pharmacol.85, 830–838. 10.1124/mol.114.091850
164
TompkinsK. D.ThorburnA. (2019). Regulation of apoptosis by autophagy to enhance cancer therapy. Yale J. Biol. Med.92, 707–718.
165
TsaiC.-Y.ChenC.-Y.ChiouY.-H.ShyuH.-W.LinK.-H.ChouM.-C.et al (2018). Epigallocatechin-3-gallate suppresses human herpesvirus 8 replication and induces ROS leading to apoptosis and autophagy in primary effusion lymphoma cells. Int. J. Mol. Sci.19, 16. 10.3390/ijms19010016
166
TundisR.BonesiM.DeguinB.LoizzoM. R.MenichiniF.ConfortiF.et al (2009). Cytotoxic activity and inhibitory effect on nitric oxide production of triterpene saponins from the roots of Physospermum verticillatum (Waldst & Kit) (Apiaceae). Bioorg. Med. Chem.17, 4542–4547. 10.1016/j.bmc.2009.05.006
167
UddinM. S.Al MamunA.JakariaM.ThangapandiyanS.AhmadJ.RahmanM. A.et al (2020). Emerging promise of sulforaphane-mediated Nrf2 signaling cascade against neurological disorders. Sci. Total Environ.707, 135624. 10.1016/j.scitotenv.2019.135624
168
VelazquezA. F. C.JacksonW. T. (2018). So many roads: the multifaceted regulation of autophagy induction. Mol. Cel Biol. 38, e00303-18. 10.1128/MCB.00303-18
169
WangN.FengY.ZhuM.TsangC. M.ManK.TongY.et al (2010). Berberine induces autophagic cell death and mitochondrial apoptosis in liver cancer cells: the cellular mechanism. J. Cell. Biochem.111, 1426–1436. 10.1002/jcb.22869
170
WangZ.JiangC.ChenW.ZhangG.LuoD.CaoY.et al (2014). Baicalein induces apoptosis and autophagy via endoplasmic reticulum stress in hepatocellular carcinoma cells. Biomed Res Int.2014, 732516. 10.1155/2014/732516
171
WangF.MaoY.YouQ.HuaD.CaiD. (2015). Piperlongumine induces apoptosis and autophagy in human lung cancer cells through inhibition of PI3K/Akt/mTOR pathway. Int. J. immunopathology Pharmacol.28, 362–373. 10.1177/0394632015598849
172
WangJ.QiQ.FengZ.ZhangX.HuangB.ChenA.et al (2016a). Berberine induces autophagy in glioblastoma by targeting the AMPK/mTOR/ULK1-pathway. Oncotarget. 7, 66944. 10.18632/oncotarget.11396
173
WangR.MaL.WengD.YaoJ.LiuX.JinF. (2016b). Gallic acid induces apoptosis and enhances the anticancer effects of cisplatin in human small cell lung cancer H446 cell line via the ROS-dependent mitochondrial apoptotic pathway. Oncol. Rep.35, 3075–3083. 10.3892/or.2016.4690
174
WangX.ZhangH.ChenX. (2019). Drug resistance and combating drug resistance in cancer. Cancer Drug Resist.2, 141–160. 10.20517/cdr.2019.10
175
WangD.HeX.WangD.PengP.XuX.GaoB.et al (2020). Quercetin suppresses apoptosis and attenuates intervertebral disc degeneration via the SIRT1-autophagy pathway. Front Cel Dev Biol. 8, 613006. 10.3389/fcell.2020.613006
176
WeiY.HuangJ. (2019). Role of estrogen and its receptors mediated-autophagy in cell fate and human diseases. J. Steroid Biochem.191, 105380. 10.1016/j.jsbmb.2019.105380
177
WhitlockN. C.BaekS. J. (2012). The anticancer effects of resveratrol: modulation of transcription factors. Nutr. Cancer. 64, 493–502. 10.1080/01635581.2012.667862
178
179
WongV. K.LiT.LawB. Y.MaE. D.YipN. C.MichelangeliF.et al (2013). Saikosaponin-d, a novel SERCA inhibitor, induces autophagic cell death in apoptosis-defective cells. Cel Death Dis.4, e720. 10.1038/cddis.2013.217
180
WuP. P.KuoS. C.HuangW. W.YangJ. S.LaiK. C.ChenH. J.et al (2009). (-)-Epigallocatechin gallate induced apoptosis in human adrenal cancer NCI-H295 cells through caspase-dependent and caspase-independent pathway. Anticancer Res.29, 1435–1442.
181
WuJ. Q.KostenT. R.ZhangX. Y. (2013). Free radicals, antioxidant defense systems, and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry.46, 200–206. 10.1016/j.pnpbp.2013.02.015
182
WuM.LaoY.XuN.WangX.TanH.FuW.et al (2015). Guttiferone K induces autophagy and sensitizes cancer cells to nutrient stress-induced cell death. Phytomedicine. 22, 902–910. 10.1016/j.phymed.2015.06.008
183
WuQ.KroonP. A.ShaoH.NeedsP. W.YangX. (2018). Differential effects of quercetin and two of its derivatives, isorhamnetin and isorhamnetin-3-glucuronide, in inhibiting the proliferation of human breast-cancer MCF-7 cells. J. Agric. Food Chem.66, 7181–7189. 10.1021/acs.jafc.8b02420
184
XuG.FengC.ZhouY.HanQ. B.QiaoC. F.HuangS. X.et al (2008). Bioassay and ultraperformance liquid chromatography/mass spectrometry guided isolation of apoptosis-inducing benzophenones and xanthone from the pericarp of Garcinia yunnanensis Hu. J. Agric. Food Chem.56, 11144–11150. 10.1021/jf802690g
185
XueL.ZhangW. J.FanQ. X.WangL. X. (2018). Licochalcone A inhibits PI3K/Akt/mTOR signaling pathway activation and promotes autophagy in breast cancer cells. Oncol. Lett.15, 1869–1873. 10.3892/ol.2017.7451
186
YamagataK.XieY.SuzukiS.TagamiM. (2015). Epigallocatechin-3-gallate inhibits VCAM-1 expression and apoptosis induction associated with LC3 expressions in TNFα-stimulated human endothelial cells. Phytomedicine. 22, 431–437. 10.1016/j.phymed.2015.01.011
187
YangG.-Y.LiaoJ.KimK.YurkowE. J.YangC. S. (1998). Inhibition of growth and induction of apoptosis in human cancer cell lines by tea polyphenols. Carcinogenesis. 19, 611–616. 10.1093/carcin/19.4.611
188
YangC.ZhaoL.YuanW.WenJ. (2017). Cordycepin induces apoptotic cell death and inhibits cell migration in renal cell carcinoma via regulation of microRNA-21 and PTEN phosphatase. Biomed. Res.38, 313–320. 10.2220/biomedres.38.313
189
YangJ.PiC.WangG. (2018). Inhibition of PI3K/Akt/mTOR pathway by apigenin induces apoptosis and autophagy in hepatocellular carcinoma cells. Biomed. Pharmacother.103, 699–707. 10.1016/j.biopha.2018.04.072
190
YeC.ZhangC.HuangH.YangB.XiaoG.KongD.et al (2018). The natural compound myricetin effectively represses the malignant progression of prostate cancer by inhibiting PIM1 and disrupting the PIM1/CXCR4 interaction, cellular physiology and biochemistry. Cell Physiol Biochem.48, 1230–1244. 10.1159/000492009
191
YuR.ZhangZ. Q.WangB.JiangH. X.ChengL.ShenL. M. (2014). Berberine-induced apoptotic and autophagic death of HepG2 cells requires AMPK activation. Cancer Cel. Int.14, 49. 10.1186/1475-2867-14-49
192
ZhangJ. F.LiuJ. J.LiuP. Q.LinD. J.LiX. D.ChenG. H. (2006). Oridonin inhibits cell growth by induction of apoptosis on human hepatocelluar carcinoma BEL-7402 cells. Hepatol. Res.35, 104–110. 10.1016/j.hepres.2006.03.007
193
ZhangX.ZhuY.DuanW.FengC.HeX. (2015). Allicin induces apoptosis of the MGC-803 human gastric carcinoma cell line through the p38 mitogen-activated protein kinase/caspase-3 signaling pathway. Mol. Med. Rep.11, 2755–2760. 10.3892/mmr.2014.3109
194
ZhangZ.LiuT.YuM.LiK.LiW. (2018). The plant alkaloid tetrandrine inhibits metastasis via autophagy-dependent Wnt/β-catenin and metastatic tumor antigen 1 signaling in human liver cancer cells. J. Exp. Clin. Cancer Res.37, 7. 10.1186/s13046-018-0678-6
195
ZhangS.CaoM.FangF. (2020a). The role of epigallocatechin-3-gallate in autophagy and endoplasmic reticulum stress (ERS)-Induced apoptosis of human diseases. Med. Sci. Monit.26, e924558. 10.12659/MSM.924558
196
ZhangZ.ZhouL.XieN.NiceE. C.ZhangT.CuiY. P.et al (2020b), Overcoming cancer therapeutic bottleneck by drug repurposing. Signal Transduct Target Ther.5, 113. 10.1038/s41392-020-00213-8
197
ZhongY.KrisanapunC.LeeS.-H.NualsanitT.SamsC.PeungvichaP.et al (2010). Molecular targets of apigenin in colorectal cancer cells: involvement of p21, NAG-1 and p53. Eur. J. Cancer46, 3365–3374. 10.1016/j.ejca.2010.07.007
198
ZhuY.RaoQ.ZhangX.ZhouX. (2018). Galangin induced antitumor effects in human kidney tumor cells mediated via mitochondrial mediated apoptosis, inhibition of cell migration and invasion and targeting PI3K/AKT/mTOR signalling pathway. J BUON.23, 795–799.
Summary
Keywords
phytochemicals, pharmacology, apoptosis, autophagy, anticancer
Citation
Rahman MA, Hannan MA, Dash R, Rahman MDH, Islam R, Uddin MJ, Sohag AAM, Rahman MH and Rhim H (2021) Phytochemicals as a Complement to Cancer Chemotherapy: Pharmacological Modulation of the Autophagy-Apoptosis Pathway. Front. Pharmacol. 12:639628. doi: 10.3389/fphar.2021.639628
Received
09 December 2020
Accepted
18 March 2021
Published
07 May 2021
Volume
12 - 2021
Edited by
Supratik Kar, Jackson State University, United States
Reviewed by
Prakash P. Praharaj, National Institute of Technology Rourkela, India
Natália Cruz-Martins, Universidade do Porto, Portugal
Sujit Kumar Bhutia, National Institute of Technology Rourkela, India
Updates
Copyright
© 2021 Rahman, Hannan, Dash, Rahman, Islam, Uddin, Sohag, Rahman and Rhim.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Md. Ataur Rahman, ataur1981rahman@hotmail.com; Hyewhon Rhim, hrhim@kist.re.kr
†These authors have contributed equally to this work
This article was submitted to Ethnopharmacology, a section of the journal Frontiers in Pharmacology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.