- 1Department of Pharmacy, Guizhou Provincial People’s Hospital, Guiyang, China
- 2Department of Pharmacy, Hospital of Chengdu Office of People’s Government of Tibetan Autonomous Region, Chengdu, China
- 3Global Health Nursing, Graduate School of Nursing Science, St. Luke’s International University, Tokyo, Japan
- 4Evidence Based Social Science Research Centre, School of Public Health, Lanzhou University, Lanzhou, China
- 5Department of Laboratory Medicine, Experimental Cancer Medicine, Karolinska Institute, Stockholm, Sweden
- 6Department of Respiratory, Guizhou Provincial People’s Hospital, Guiyang, China
Background Revefenacin (REV) is a novel once-daily long-acting muscarinic antagonist (LAMA) in the treatment of moderate to very severe chronic obstructive pulmonary disease (COPD). This systematic review incorporating a dose-response meta-analysis aimed to assess the efficacy and safety of REV.
Methods PubMed, Embase, Cochrane Library, China National Knowledge Infrastructure, VIP database, and Wanfang database were searched from their inception to April 2020. We included randomized controlled trials (RCTs) which evaluated the efficacy and safety of REV in COPD patients. Two reviewers independently performed study screening, data extraction, and risk of bias assessment. Outcomes consisted of the mean change in trough Forced Expiratory Volume in 1 second (FEV1) from baseline, adverse events (AEs), and serious adverse events (SAEs). A dose-response meta-analysis using the robust error meta-regression method was conducted. We used Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach to assess the quality of evidence.
Results Nine RCTs (3,121 participants) were included in this systematic review. The meta-analyses indicated that 175 μg/day REV could significantly improve the trough FEV1 (MD=143.67, 95%CI: 129.67 to 157.68; I2=96%; 809 participants; studies=4; low quality) without increasing the risk of AEs (OR=0.98, 95%CI: 0.81 to 1.18; I2=34%; 2,286 participants; studies=7; low quality) or SAEs (OR=0.89, 95%CI: 0.55 to 1.46; I2=0%; 2,318 participants; studies=7; very low quality) compared to placebo. Furthermore, the effect of REV in increasing trough FEV1 was dose-dependent with an effective threshold of 88 μg/day (R2 = 0.7017). Nevertheless, only very low-quality to low-quality evidence showed that REV at a dose of 175 μg/day was inferior to tiotropium regarding the long-term efficacy, and its safety profile was not superior to tiotropium or ipratropium.
Conclusion Current evidence shows that REV is a promising option for the treatment of moderate to very severe COPD. Due to most evidence graded as low quality, further studies are required to compare the efficacy, long-term safety and cost-effectiveness between REV and other LAMAs in different populations.
Clinical Trial Registration: [PROSPERO], identifier [CRD42020182793]
Introduction
Chronic obstructive pulmonary disease (COPD) is a common, preventable and treatable disease that is characterized by persistent respiratory symptoms and airflow limitation due to airway and/or alveolar abnormalities (GOLD., 2021). Significant exposure to noxious particles or gases and host factors including abnormal lung development usually contribute to the pathogenesis (GOLD., 2021). Based on Burden of Obstructive Lung Disease (BOLD) and other large scale epidemiological studies, a meta-analysis estimated that the number of COPD cases was 384 million in 2010, with a global prevalence of 11.7% (95% confidence interval (CI): 8.4–15.0%) (Adeloye et al., 2015). Around 3.2 million people died from COPD each year, making it the third leading cause of death worldwide (World Health Organization, 2007; Burney et al., 2015; Global Burden of Disease Study Collaborators, 2015; Halpin et al., 2019). In the latest Global Burden of Disease (GBD) analysis, COPD entered the top 10 causes of years of life lost (YLL), increasing from the 11th position in 2007 to seventh in 2017 (GBD 2017 Causes of Death Collaborators, 2018). Another GBD study also predicted that deaths from COPD would rise to 4.4 million per year in 2040 and by then, COPD would be the fourth most important cause of YLL (Foreman et al., 2018). With the increasing exposure to risk factors (e.g., smoking) and aging of the world’s population, the prevalence of COPD is expected to rise over the next 40 years and by 2060 there may be more than 5.4 million deaths from COPD and its related conditions annually (Lopez et al., 2006; GBD 2017 Causes of Death Collaborators, 2018; World Health Organization, 2020), which will induce a substantial and elevated economic burden (Lozano et al., 2012; Vos et al., 2012). In the European Union, COPD accounted for 56% (38.6 billion Euros) of the cost on respiratory disease which took up about 6% of the total annual healthcare budget (European Respiratory Society on behalf of the Forum of International Respiratory Societies (FIRS), 2017). In the United States, the estimated direct and indirect costs of COPD were $32 billion and $20.4 billion, respectively (Guarascio et al., 2013).
In absence of conclusive evidence supporting any existing medications which can modify the long-term decline in lung function for COPD (Anthonisen et al., 1994; Burge et al., 2000; Pauwels et al., 1999; Tashkin et al., 2008; Vestbo et al., 1999), the purpose of pharmacological therapy for COPD is to ameliorate symptoms, reduce the frequency and severity of exacerbations, and improve exercise tolerance and health status. As the first-line therapy to address COPD symptoms and prevent exacerbations (GOLD., 2021), long-acting muscarinic antagonists (LAMAs) can improve the effectiveness of pulmonary rehabilitation (Casaburi et al., 2005; Kesten et al., 2008) and reduce exacerbation and related hospitalization (Karner et al., 2014; Melani A.S., 2015) by durably blocking the bronchoconstrictor effects of acetylcholine on M3 muscarinic receptors expressed in airway smooth muscle (Melani A.S., 2015). Revefenacin (REV), a novel once-daily LAMA for nebulization, was approved for the treatment of COPD by the United States Food and Drug Administration (FDA) in November 2018 (Highlights Of Prescribing Information, 2021). Several randomized trials (Donohue et al., 2019a; Donohue et al., 2019b; Donohue et al., 2019c; Ferguson et al., 2019; Krishna et al., 2017; Mahler et al., 2019; Quinn et al., 2018; Sethi et al., 2020; Siler et al., 2020; Theravance Biopharma, 2021a; Theravance Biopharma, 2021b) investigating the use of REV concluded that it was effective and safe in the treatment of COPD. Nevertheless, evidence has not been systematically assessed. To better understand and interpret available evidence, we conducted a systematic review incorporating a dose-response meta-analysis to evaluate the efficacy and safety of REV in patients with COPD.
Materials and Methods
We reported our study following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Supplementary Table S1). The study was prospectively registered on International Prospective Register of Systematic Review (PROSPERO, CRD42020182793).
Search Strategy
PubMed, Embase, the Cochrane Central Register of Controlled Trials (CENTRAL) were searched using the search strategies detailed in Supplementary Table S2, from their inception to April 2020. ClinicalTrials.gov was also searched using the term of “Revefenacin”. The China National Knowledge Infrastructure (CNKI), VIP database, and Wanfang database were also searched with Chinese terms. We reviewed the references from relevant review articles and included studies to find additional studies.
Eligibility Criteria
We included studies meeting the following criteria: 1) Randomized controlled trials (RCTs) published in English or Chinese; 2) participants with confirmed moderate to very severe COPD (Stage 2, three or four according to the GOLD Guidelines); 3) the intervention was REV irrespective of dosage and schedule; 4) the comparisons included placebo, tiotropium (TIO), and ipratropium (IPR); 5) studies reporting at least one of the following outcomes: the mean change from baseline in trough forced expiratory volume in 1 s (FEV1) as the efficacy outcome; adverse events which were subdivided into total adverse events (AEs) and serious adverse events (SAEs) by ICH GCP standards as the safety endpoints. We excluded duplicated studies or conference abstract without available raw data.
Study Selection and Data Extraction
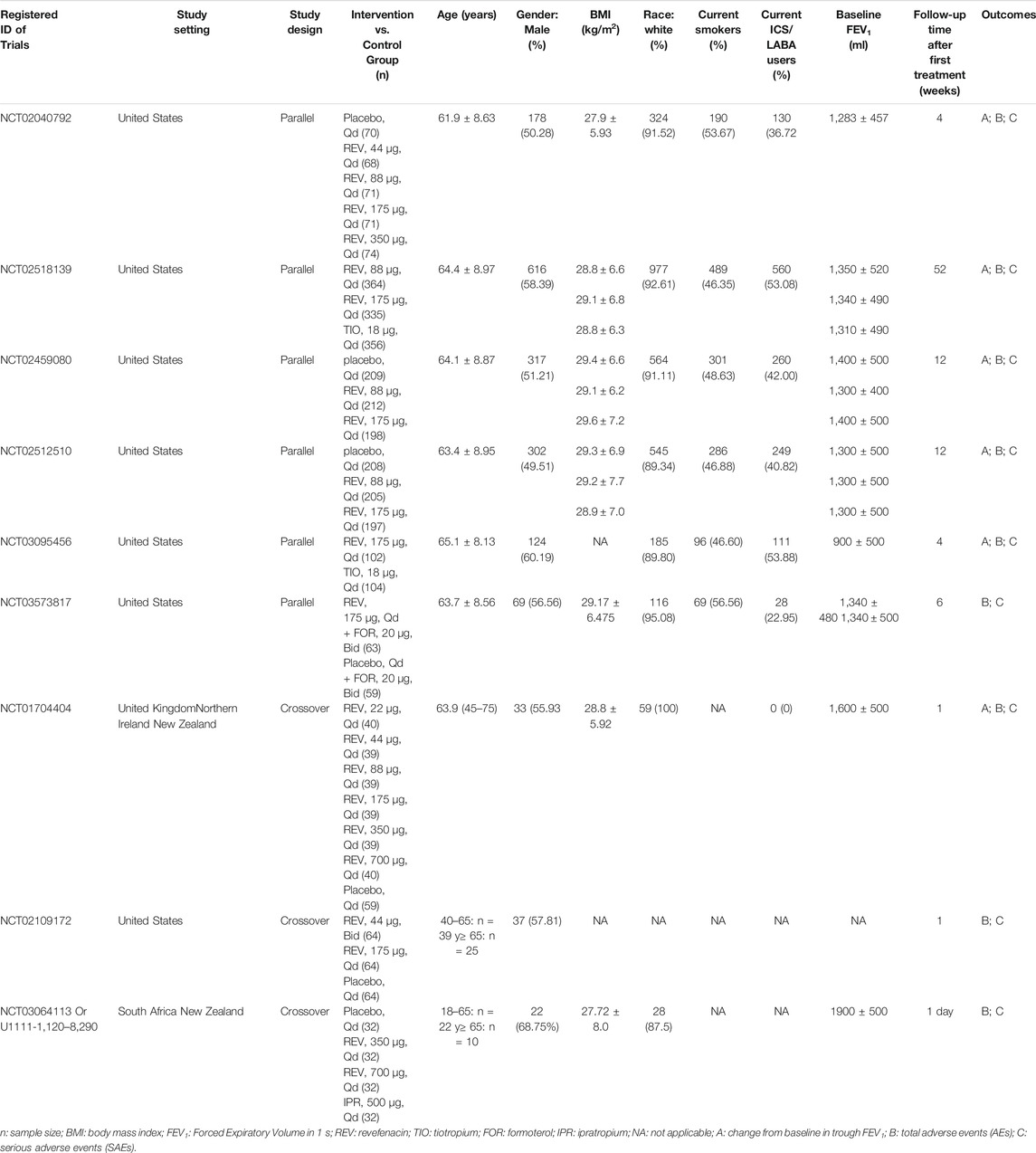
Two authors independently screened the titles and abstracts of all studies searched using predetermined inclusion criteria. The full texts of any potentially relevant articles were retrieved for detailed review. We resolved any disagreements by discussion. We used a pre-designed data collection form to extract data from each eligible study. The following data were extracted: 1) authors; 2) year of publication; 3) country or region where the study conducted; 4) study design and use of control; 5) number of participants in each group; 6) population characteristics (e.g., gender, age, body mass index (BMI), race, etc.); 7) outcomes and their definitions, categorical or numerical data for assessment of included outcomes; 8) Sources of funding.
Risk of Bias Assessment
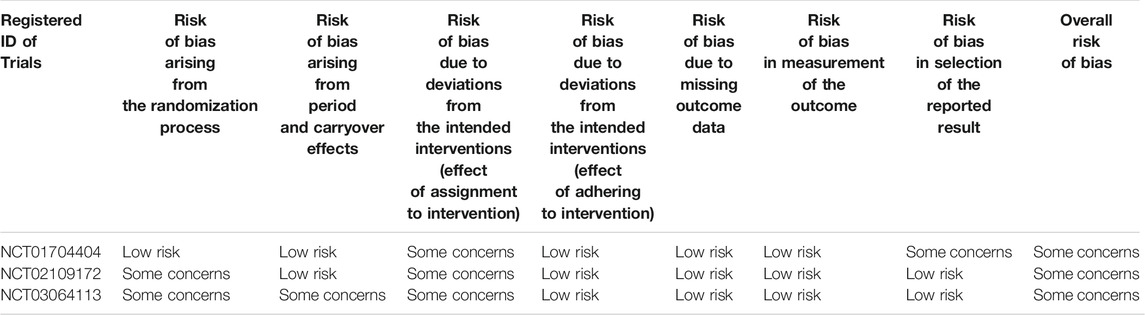
Two authors independently assessed the risk of bias of each included RCT using the checklist developed by Cochrane Collaboration (Higgins et al., 2011; Higgins et al., 2020), including random sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other bias. We categorized the judgement to be low, high or unclear risk of bias and created a “risk of bias summary” using the Review Manager Software (RevMan 5.3). As for crossover studies, a revised tool to assess the risk of bias in crossover trials (RoB 2) was used to assess the risk of bias (Higgins et al., 2021). Any disagreements about the risk of bias were resolved by discussion.
Statistical Synthesis
If more than one study reported the same outcome, a pairwise meta-analysis was conducted. To compare the differences between REV and control groups, odds ratios (ORs) were used for the incidence of AEs or SAEs and mean differences (MDs) were calculated for FEV1, with corresponding 95% confidence intervals (CIs). We choose to use OR since a recent study have pointed out that it is better than risk ratio (RR) in clinical trials, where RR are not a portable estimator (Doi et al., 2020). As to the change from baseline in trough FEV1, per-protocol analyses were performed according to the data of patients who completed the trial. As to the AEs and SAEs, we conducted analyses based on the safety population which included all subjects who were randomized into the study and received at least one dose of study drug. For studies with zero-events in either of the arms, the continuity correction (add 0.5) was employed to estimate the OR and variance; for studies with zero-events in both arms, we impute OR = 1 for them while use continuity correction to estimate the variance (Xu et al., 2021). In addition, considering the unstable nature of rare events, as suggested by the guideline, we employed a sensitivity analysis by using Mantel-Haenszel risk difference (RD) estimator for the meta-analyses (Xu et al., 2021). We pooled ORs with the Mantel-Haenszel method, and MDs with the inverse variance method using RevMan 5.3, respectively. Statistical heterogeneity among studies was examined by the Chi-square test and quantified by the I2 statistic (Higgins et al., 2011). A fixed-effects model was applied to synthesize data when heterogeneity was not significant (I2<30%), while a random-effects model was used when heterogeneity was significant (I2>30%) and could not be explained by subgroup analyses or in terms of clinical or methodological features of the trials. We explored sources of heterogeneity based on the subgroup analyses including type of control groups and different dose of REV. The sensitivity analyses were performed by omitting the crossover studies.
The robust error meta-regression method (Xu et al., 2018) was used to summarize relationship between the dosage and response (efficacy and safety) of REV. This was achieved by treating the dosage as dependent variable (dose) while the efficacy and safety as the independent variables of study level. Under this meta-regression method, each study was regarded as a cluster within a whole population, as a solution to pool the dose-response relationship and to address the potential correlations among within-study effects. The potential dose-response relationship was fitted through a restricted cubic spline function with three random knots automatically generated. The Wald test by assuming the coefficients of non-linear terms to zero was employed to investigate whether a non-linear relationship exists (Xu et al., 2019).
The Quality of Evidence Assessment
We used the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach to rate the quality of evidence, which rated evidence from systematic review and meta-analysis as high, moderate, low, or very low quality, by considering risk of bias, indirectness, inconsistency, imprecision, and publication bias (Guyatt et al., 2011).
Results
Search Results
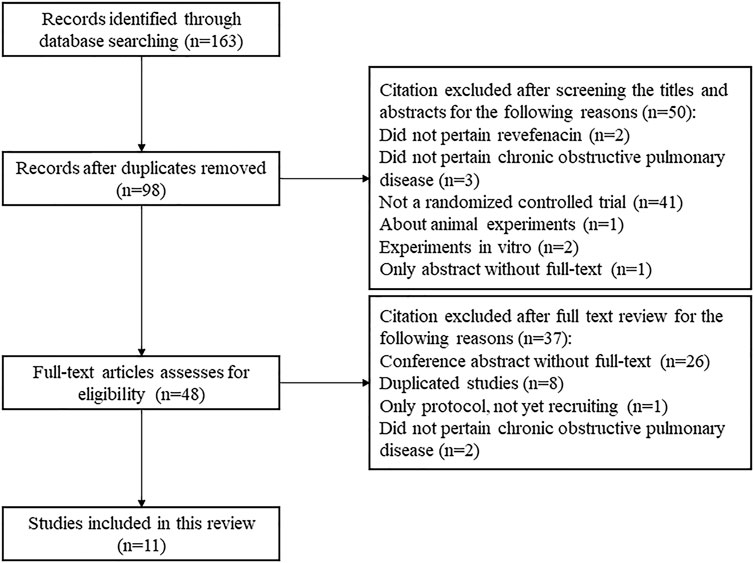
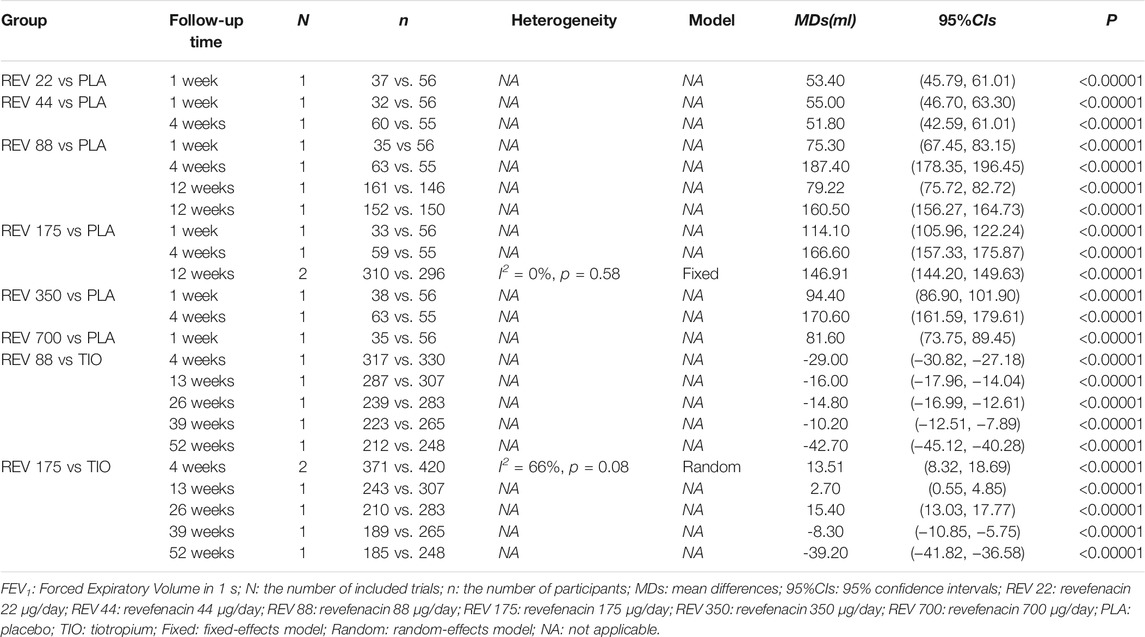
A total of 163 publications were obtained from literature search and the selection process is shown in Figure 1. Eleven articles (Donohue et al., 2019a; Donohue et al., 2019b; Donohue et al., 2019c; Ferguson et al., 2019; Krishna et al., 2017; Mahler et al., 2019; Quinn et al., 2018; Sethi et al., 2020; Siler et al., 2020; Theravance Biopharma, 2021a; Theravance Biopharma, 2021b) reporting nine RCTs with 3,121 participants were included in this systematic review. As shown in Table 1, two RCTs were multicenter studies, and the other seven were single-center studies. Both parallel (n = 6) and crossover study design (n = 3) were used. The dosage of REV in intervention group ranged from 22 to 700 μg/day, and it was compared with placebo (7 RCTs, 701 participants), IPR (1 RCT, 32 participants), and TIO (2 RCTs, 460 participants). The follow-up time ranged from 1 day to 52 weeks after the first treatment. Two RCTs identified from ClinicalTrials.gov are yet to be published in full and thus the baseline characteristics of their enrolled participants were unclear. For the other seven RCTs, the mean age and mean BMI of participants were 61.4–65.1 years and 27.9–29.6 kg/m2, respectively, and the proportion of ICS/LABA users varied from 0 to 53.88%.
Quality of Included Studies
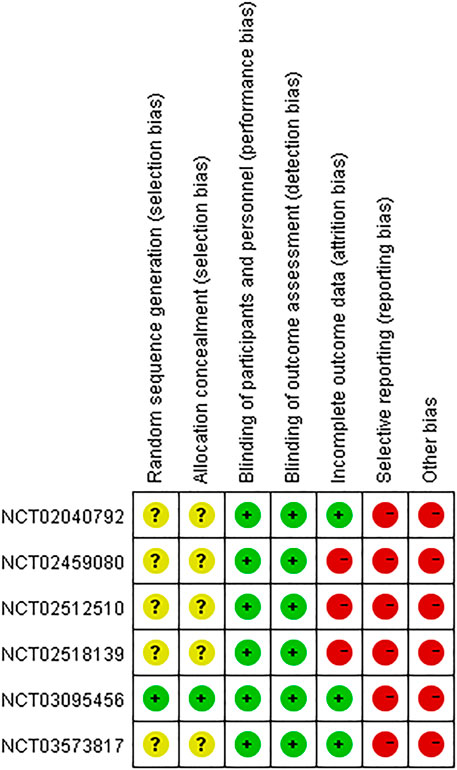
As shown in Figure 2, one study (NCT03095456) had low risk of selection bias for clearly describing the methods (centralized randomization) of randomization and allocation concealment, while the others were unclear because the information about selection participants was not reported. Triple (participant, care provider, and investigator) and quadruple (participant, care provider, investigator, and outcome assessor) blinding methods were applied in three RCTs (NCT02040792, NCT02459080, NCT02512510) and three RCTs (NCT02040792, NCT03095456, NCT03573817), respectively, therefore all the included studies had low risk of performance bias and detection bias. Four studies (NCT02040792, NCT03095456, NCT03573817, NCT02109172) had low risk of attribution bias, as there was no loss of follow-up or missing data was appropriately addressed (e.g., applying ITT analysis which could underestimate the efficacy of the intervention). Nevertheless, other three studies (NCT02518139, NCT02459080, NCT02512510) had high risk of attribution bias due to high loss of follow-up (>15%). Although all the studies mentioned registration information and had an available protocol, data from some outcomes of interest (i.e., AEs, SAEs, FEV1) in six studies (NCT02040792, NCT02518139, NCT02459080, NCT02512510, NCT03095456, NCT03573817) were inconsistent with the information on ClinicalTrial.gov. Therefore, the reporting bias risk of these studies was high. Since Theravance Biopharma, Inc. supported all the studies and their employees participated in the executing and writing process of six studies (NCT02040792, NCT02518139, NCT02459080, NCT02512510, NCT03095456, NCT03573817), the risk of bias caused by conflict of interest was high. Due to the limited number of the included studies for the same outcome, publication bias investigation was not performed. As to the three crossover studies (NCT01704404, NCT02109172, and NCT03064113), the overall risk of bias was assessed as “some concerns” (Table 2).
Results From the Meta-analysis
The Change From Baseline in Trough FEV1
Six trials involving 2,093 participants reported the change from baseline in trough FEV1. Among them, four trials (NCT02040792, NCT02459080, NCT02512510, and NCT01704404) compared REV with placebo at different doses, one trial (NCT02518139) compared REV with TIO at different follow-up time (4-weeks, 13-weeks, 26-weeks, 39-weeks, and 52-weeks), whereas the rest one (NCT03095456) made plain comparison between REV and TIO. In subgroup analyses, we found that both dose and therapeutic course of REV contribute to the heterogeneity, so the results were presented according to the control group, the dose and the therapeutic course (Table 3). In contrast to placebo, all different doses of REV could significantly improve the trough FEV1. Yet this effect would be weakened with the longer course of treatments. Despite that trials NCT02459080 and NCT02512510 reported the change from baseline in trough FEV1 for 88 μg/day REV vs. placebo at 12-weeks, the heterogeneity between the two trials was significantly high (I2 = 100%). Therefore, we described their respective results rather than the pooling results. In the dose-response meta-analysis, there was a potential non-linear association (R2 = 0.7017) of the REV dose with the change from baseline in trough FEV1 (Figure 3). The predicted dose-specific mean changes from baseline in trough FEV1 were 27.43 (95%CI: 13.55–68.41) ml at a dose of 22 μg/day, 54.41 (95%CI: 22.50–86.31) ml at a dose of 44 μg/day, 97.96 (95%CI: 77.72–118.21) ml at a dose of 88 μg/day, 119.47 (95%CI: 104.21–134.74) ml at a dose of 175 μg/day, 121.86 (95%CI: 112.79–130.92) ml at a dose of 350 μg/day, and 126.63 (95%CI: 112.13–141.12) ml at a dose of 700 μg/day. Interestingly, 88 μg/day seemed to be a threshold dose above which the change from baseline in trough FEV1 began to slow down (Figure 3). Patients who received 175 μg/day REV experienced improvement of trough FEV1 on average of 143.67 ml higher than those who received placebo (MD = 143.67, 95%CI: 129.67 to 157.68; I2 = 96%; 809 participants; studies = 4; low quality; Table 4). Patients treated with 175 μg/day REV gained increment of trough FEV1 on average of 13.51 ml higher than TIO at 4 weeks (MD = 13.51, 95%CI: 8.32 to 18.69; I2 = 66%; 791 participants; studies = 2; very low quality; Table 4), but this effect was reversed at 52 weeks (MD = -39.2, 95%CI: 41.82 to 36.58; 433 participants; study = 1; low quality; Table 4). The sensitivity analyses showed that the results including crossover studies were consistent with those omitting crossover studies (Supplementary Table S3).
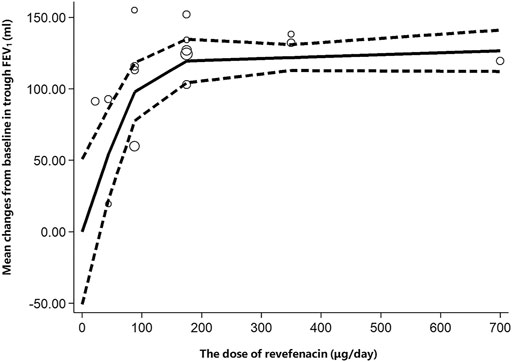
FIGURE 3. Increase in dose (μg/day) of revefenacin and change from baseline in trough FEV1 (ml). The solid line is the nonlinear prediction of the mean change from baseline in trough FEV1 and the dotted lines indicate the 95% confidence interval. The threshold is 88 μg/day. FEV1: Forced Expiratory Volume in 1 s.
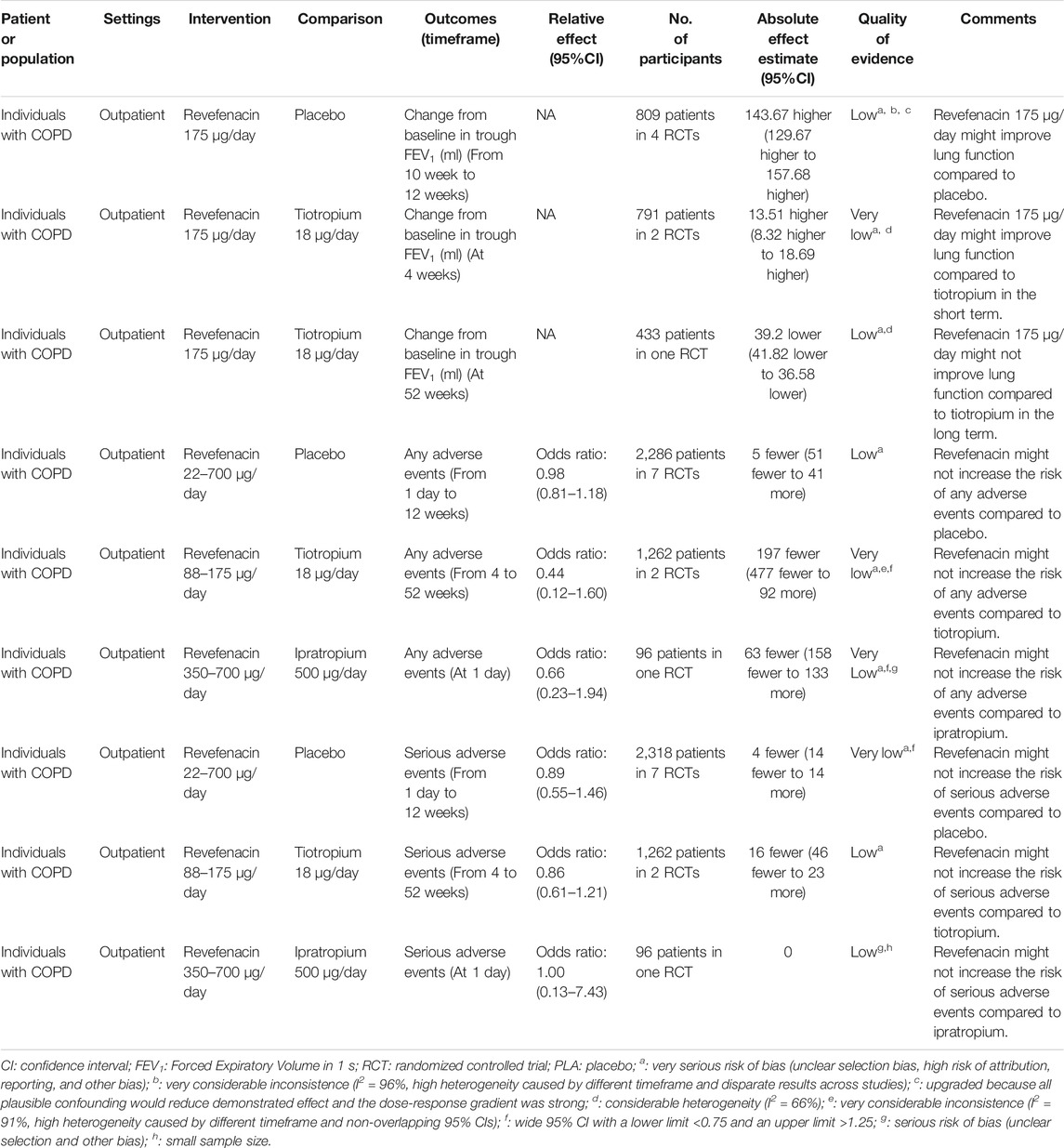
TABLE 4. GRADE summary of findings for intervention versus controls in patients with chronic obstructive pulmonary disease (COPD).
The Incidence of Any Adverse Events
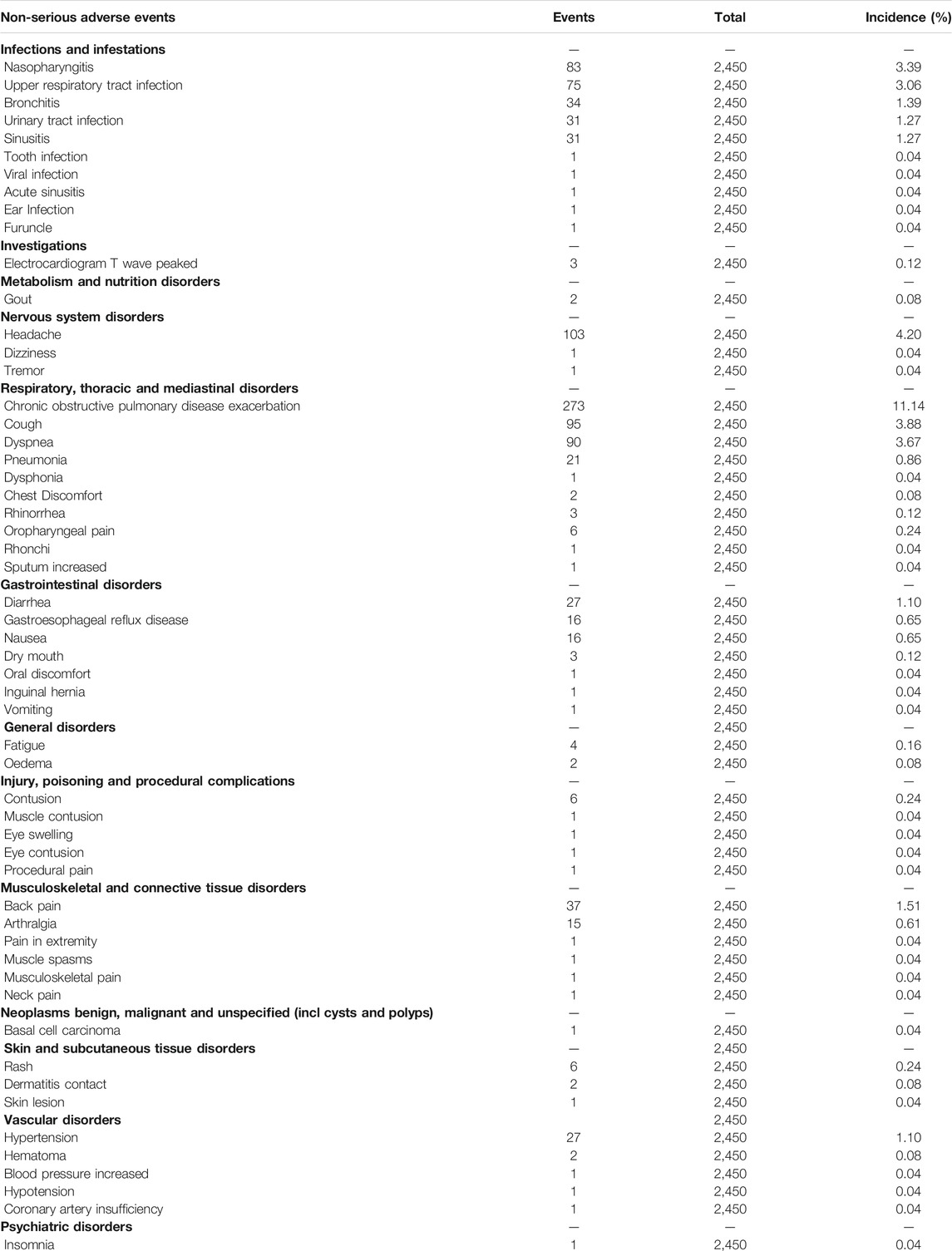
The AEs were reported in all trials including 3,121 participants. As presented in Table 5, most AEs were mild, transient, and reversible. A limited association (R2 = 0.1787) of the REV dose with the total AEs incidence was present (Supplementary Figure S1). The predicted dose-specific RRs of the REV dose were 1.03 (95%CI: 1.00–1.07) at a dose of 22 μg/day, 1.02 (95%CI: 0.99–1.06) ml at a dose of 44 μg/day, 1.00 (95%CI: 0.97–1.04) ml at a dose of 88 μg/day, 0.96 (95%CI: 0.92–1.01) ml at a dose of 175 μg/day, 0.89 (95%CI: 0.81–0.97) ml at a dose of 350 μg/day, and 0.76 (95%CI: 0.64–0.90) ml at a dose of 700 μg/day. On average, the decrease in total AEs was 0.05% (RR = 0.9995, 95%CI: 0.9992–0.9998; p = 0.009) between 0 and the maximum dose. Furthermore, tests of interaction showed no evidence of different therapeutic course subgroup effect for total AEs in comparison of REV vs. PLA (Supplementary Figure S2). Notably, the incidence of total AEs in REV group was significantly lower than that in TIO group at 4 weeks (OR = 0.22, 95%CI: 0.11–0.45, p < 0.0001), while the difference became not significant at 52 weeks (OR = 0.82, 95%CI: 0.61–1.10, p = 0.19). Patients who received REV were the equivalent likely to undergo total AEs as placebo patients (OR = 0.98, 95%CI: 0.81 to 1.18; I2 = 34%; 2,286 participants; studies = 7; low quality; Figure 4, Table 3), TIO patients (OR = 0.44, 95%CI: 0.12 to 1.60; I2 = 91%; 1,262 participants; studies = 2; very low quality; Supplementary Figure S3, Table 4), or IPR patients (OR = 0.66, 95%CI: 0.23 to 1.94; 96 participants; study = 1; very low quality; Figure 4, Table 4). The sensitivity analyses showed that the results including crossover studies were consistent with those omitting crossover studies (Supplementary Figure S4).
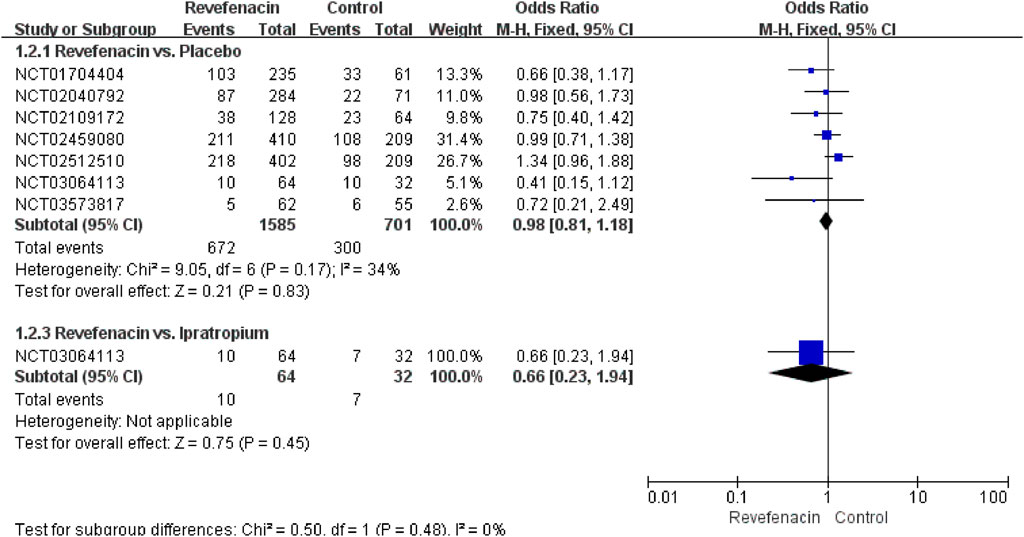
FIGURE 4. Total adverse events for revefenacin in patients with chronic obstructive pulmonary disease.
The Incidence of SAEs
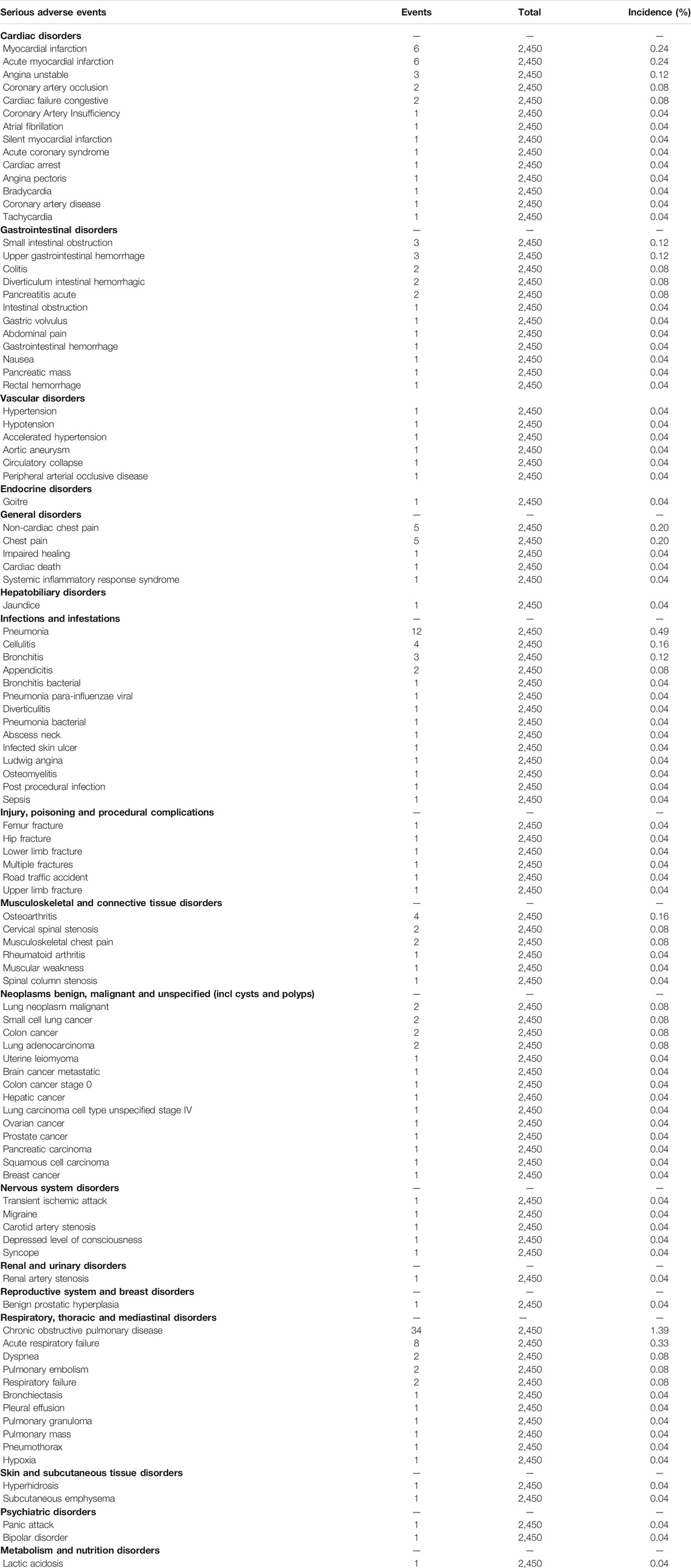
All the nine trials reported 200 SAEs, and the most common SAEs was COPD worsening or exacerbation (1.39%, Table 6). A weak association (R2 = 0.1325) of the REV dose with the SAEs incidence existed (Supplementary Figure S5). The predicted dose-specific RRs of the REV dose were 0.99 (95%CI: 0.95–1.04) at a dose of 22 μg/day, 0.97 (95%CI: 0.93–1.02) ml at a dose of 44 μg/day, 0.94 (95%CI: 0.88–0.99) ml at a dose of 88 μg/day, 0.86 (95%CI: 0.78–0.96) ml at a dose of 175 μg/day, 0.74 (95%CI: 0.60–0.90) ml at a dose of 350 μg/day, and 0.54 (95%CI: 0.36–0.81) ml at a dose of 700 μg/day. The average decrement in risk of SAEs between 0 and the maximum dose was 0.1% (RR = 0.9990, 95%CI: 0.9984–0.9998; p = 0.020). Yet we found no evidence of different therapeutic course effect for this outcome in comparison of REV vs. PLA (Supplementary Figure S6). Patients treated with REV were the similar likely to experience SAEs as placebo patients (OR = 0.89, 95%CI: 0.55 to 1.46; I2 = 0%; 2,318 participants; studies = 7; very low quality; Figure 5, Table 4), TIO patients (OR = 0.86, 95%CI: 0.61 to 1.21; I2 = 0%; 1,262 participants; studies = 2; low quality; Figure 5, Table 4), or IPR patients (OR = 1.00, 95%CI: 0.13 to 7.43; 96 participants; study = 1; low quality; Figure 5, Table 4). These results were consistent with the sensitivity analyses by using Mantel-Haenszel RD (Supplementary Figure S7). The sensitivity analyses showed that the results including crossover studies were consistent with those omitting crossover studies (Supplementary Figure S8).
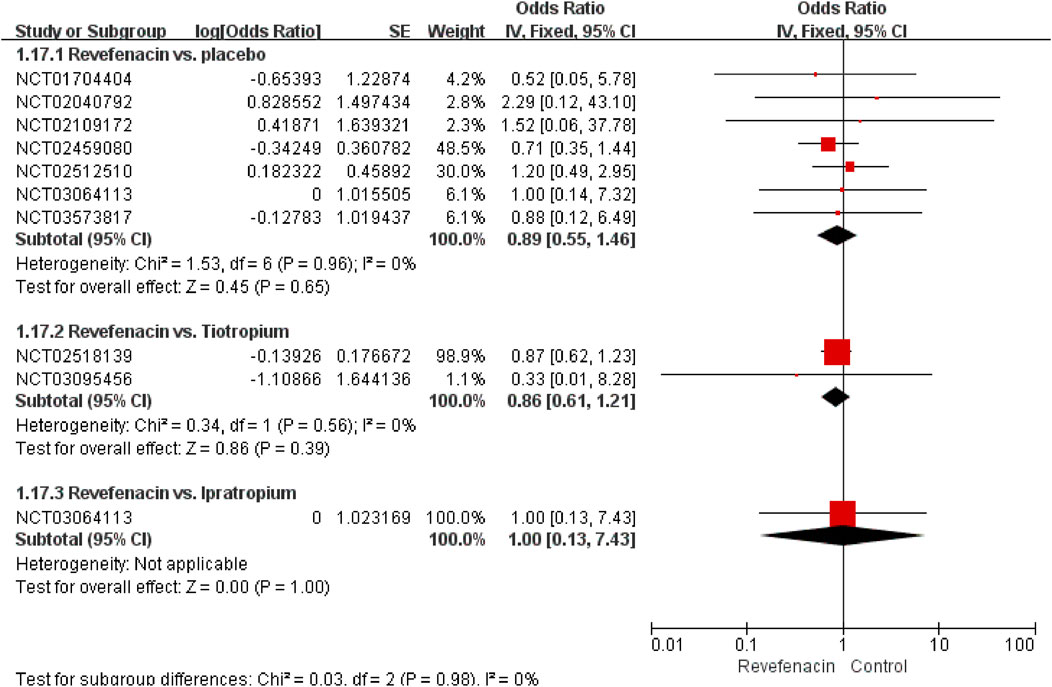
FIGURE 5. Serious adverse events for revefenacin in patients with chronic obstructive pulmonary disease.
Discussion
This systematic review summarized the evidence of efficacy and safety of REV in patients with moderate to very severe COPD and used a novel meta-analysis method to account for the dose-response relationship of the trough FEV1, AEs, and SAEs with REV dose. Low-quality evidence suggests that, compared to placebo, 175 μg/day REV might improve the lung function (increment of trough FEV1 on average of 143.67 ml higher than placebo) without elevating the risk of AEs or SAEs. However, only very low-quality to low-quality evidence demonstrates that the safety profile of REV at a dose of 175 μg/day is similar to TIO and IPR but its long-term efficacy is inferior to TIO (decrease of trough FEV1 on average of 39.2 ml lower than TIO). The effect of REV in increasing trough FEV1 was correlated to the dose with a threshold value of 88 μg/day. Notably, the efficacy of REV would be weakened with the extension of therapeutical course.
Despite the serious risk of bias and inconsistence, the confidence rating of evidence regarding the efficacy of REV vs. placebo might be enhanced by the dose-response gradient which was consistent with the results in vitro (Pulido-Rios et al., 2013). The novel robust error meta-regression method had some merits of reducing the probability of type I error caused by repeated analyses, so it was utilized to investigate the dose-response relationship. This relationship was non-linear and included three phases based on REV dose: 0–88 μg/day, 88–175 μg/day, and 175–700 μg/day. The change from baseline in trough FEV1 dramatically escalated with the increasing dose of REV from 0 to 88 μg/day. Thereafter, the growth rate started to slow down and achieved a plateau phase when the dose exceeded 175 μg/day due to a ceiling effect. Our finding is coincided with current suggestion where 88 and 175 ug/day REV are considered as appropriate doses for investigating longer-term safety and efficacy of REV (Krishna et al., 2017). To explore the heterogeneity of trough FEV1 regarding 88 μg/day of REV vs. placebo at 12-weeks, we compared the baseline of participants in trial NCT02512510 with that in trial NCT02459080. Unfortunately, there was no significant difference in baseline characteristics. Hence, one possible reason for explaining the heterogeneity is that a dose of 88 μg/day was the threshold of the dose-response curve and some patients in the study might not receive the full benefits of the treatment, suggesting that a higher dose would be more optimal for all participants. Different from efficacy, there was no significant dose-response relationship between dose and the incidence of AEs or SAEs and the safety profile of REV was comparable to placebo. In addition, a previous study also reported that concurrent long-acting β-agonists (LABA) would slightly raise the incidence of AEs for patients receiving REV at a dose of 88 μg/day rather than those receiving REV at a dose of 175 μg/day (Donohue et al., 2019c). Thereby 175 μg/day has been approved as a standard dose by the United States FDA (Highlights Of Prescribing Information, 2021).
The effect of REV at a dose of 175 μg/day in improving the trough FEV1 was superior to TIO within 26 weeks but then got inferior after 39 weeks. On one hand, the disproportionate number of poor performers who discontinued TIO during the final 3 months of treatment (Donohue et al., 2019b) could partially account for this phenomenon. On the other hand, the distinct mechanism of drug action should also be considered, as REV exhibits pharmacological effects through selective inhibition of M3 receptor at the smooth muscle leading to bronchodilation, while TIO blocks both M3 and M1 receptors to take more prolonged effects (Li and Yang, 2019). Given that REV with novel biphenyl carbamate tertiary amine structure is different from TIO with quaternary ammonium feature (Donohue et al., 2019d; Montuschi and Ciabattoni, 2015), REV was supposed to have higher metabolic lability and more rapid systemic clearance than TIO (Babu and Morjaria, 2017; GlaxoSmithKline. Incruse, 2021) in terms of minimizing systemically mediated AEs. Nonetheless, this systematic review did not show any significant advantage of REV in reducing the risk of AEs or SAEs compared to TIO or IPR, which might be ascribed to underpowered sample size. Although present evidence showed that REV was not preferable to TIO both in efficacy and safety, certain COPD patients with chronic muscle weakness, or cognitive or visual impairment or diminished manual dexterity may still particularly benefit from the use of this once-daily nebulized delivery LAMA (Bonini and Usmani, 2015; Tashkin D. P., 2016). As the evidence about the efficacy and safety of REV vs. TIO was mainly from two trials (NCT02518139 and NCT03095456) with high risk of attribution, reporting, and other bias, its confidence rating was graded as very low to low quality.
This systematic review also found the therapeutical course would influence the efficacy in improving trough FEV1, which could be explained by the progression of COPD with longer follow-up time. Considering the limited data from trials, we did not evaluate the association of reduced efficacy with treatment course. Furthermore, the proportion of ICS/LABA users varied a lot among all the included trials, which probably brought heterogeneity to the results of meta-analyses. Nevertheless, a subgroup analysis (Sethi et al., 2020) found that REV produced similar improvements from baseline in trough FEV1 in the non-LABA and LABA groups despite more AEs reported in the LABA.
There are several limitations in this study. As we only included RCTs, the results may not have good generalizability for strict inclusion criteria and small sample size. Particularly, the representativeness of participants was compromised because all the trials were conducted in the United States, the United Kingdom, Northern Ireland, New Zealand, and South Africa where most of the participants were white. In addition, these trials were not sensitive to assess treatment-related rare AEs (incidence ≤0.01%) due to relatively lower power of test and shorter follow-up term. Furthermore, the quality of evidence was subpar for the high risk of attribution and reporting bias in primary studies. Moreover, the language restriction for English and Chinese could also reduce the generalizability of our results. Therefore, prospective, multicenter, RCTs with larger samples, different populations, and better methodological design are urgently needed in this field. Although the course of treatment would influence the efficacy of REV, we performed the dose-response meta-analysis without adjusting this confounder due to limited data from the trials, suggesting that the non-linearity relationship between dosage and improvements in the through FEV1 of REV should be interpreted with caution. Finally, even though study design and concomitant medication such as formoterol in NCT03573817 would also be the possible source of heterogeneity, we did not assess the effect of these factors on the results due to small quantity of trials with the same outcomes.
To conclude, based on the findings of our systematic review and dose-response meta-analysis of RCTs, REV appears to be a promising option for the treatment of moderate to very severe COPD. Considering the low confidence rating of evidence, further studies are warranted to compare the efficacy, long-term safety and cost-effectiveness between REV and other LAMAs (TIO) in different populations. Although most studies used the FEV1 to evaluate the efficacy of REV in treatment of COPD, but FEV1 should just be set as a surrogate outcome. Therefore, the clinical benefit of REV in patients with COPD should be further evaluated. And researchers should increase focus on those important endpoints (e.g., death, exacerbations requiring antibiotics or oral steroids, hospitalizations due to exacerbation of COPD, exacerbations requiring a short course of an oral steroid or antibiotic, etc.) and patient-reported outcomes in the further research due to few trials reporting such related endpoints.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
JZ, YX and XL did the literature search, collected the data, and drafted the manuscript. JS-K, RH, WZ, LG, YH, RZ, HZ, and JH revised the final manuscript. All authors conceived and designed the study, analyzed and interpreted the data, did the quality assessment, and revised and approved the manuscript for submission.
Funding
This work was supported by National Natural Science Foundation of China (No. 72064004) and Doctoral Foundation of Guizhou Provincial People’s Hospital (GZSYBS (2019) No.09).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Chang Xu at Qatar University for helping with the statistical analysis.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.667027/full#supplementary-material
References
Adeloye, D., Chua, S., Lee, C., Basquill, C., Papana, A., Theodoratou, E., et al. (2015). Global and Regional Estimates of COPD Prevalence: Systematic Review and Meta-Analysis. J. Glob. Health 5, 020415. doi:10.7189/jogh.05-020415
Anthonisen, N. R., Connett, J. E., Kiley, J. P., Altose, M. D., Bailey, W. C., Buist, A. S., et al. (1994). Effects of Smoking Intervention and the Use of an Inhaled Anticholinergic Bronchodilator on the Rate of Decline of FEV1. The Lung Health Study. JAMA 272, 1497–1505. doi:10.1001/jama.1994.03520190043033
Babu, K. S., and Morjaria, J. B. (2017). Umeclidinium in Chronic Obstructive Pulmonary Disease: Latest Evidence and Place in Therapy. Ther. Adv. Chronic Dis. 8, 81–91. doi:10.1177/2040622317700822
Bonini, M., and Usmani, O. S. (2015). The Importance of Inhaler Devices in the Treatment of COPD. COPD Res. Pract. 1, 1–9. doi:10.1186/s40749-015-0011-0
Burge, P. S., Calverley, P. M., Jones, P. W., Spencer, S., Anderson, J. A., and Maslen, T. K. (2000). Randomised, Double Blind, Placebo Controlled Study of Fluticasone Propionate in Patients with Moderate to Severe Chronic Obstructive Pulmonary Disease: the ISOLDE Trial. BMJ 320, 1297–1303. doi:10.1136/bmj.320.7245.1297
Burney, P. G., Patel, J., Newson, R., Minelli, C., and Naghavi, M. (2015). Global and Regional Trends in COPD Mortality, 1990-2010. Eur. Respir. J. 45, 1239–1247. doi:10.1183/09031936.00142414
Casaburi, R., Kukafka, D., Cooper, C. B., Witek, T. J., and Kesten, S. (2005). Improvement in Exercise Tolerance with the Combination of Tiotropium and Pulmonary Rehabilitation in Patients with COPD. Chest 127, 809–817. doi:10.1378/chest.127.3.809
Doi, S. A., Furuya-Kanamori, L., Xu, C., Lin, L., Chivese, T., and Thalib, L. (2020). Questionable Utility of the Relative Risk in Clinical Research: a Call for Change to Practice. J. Clin. Epidemiol. S0895-4356 (20), 31171–31179. doi:10.1016/j.jclinepi.2020.08.019
Donohue, J. D., Feldman, G., Sethi, S., Barnes, C. N., Pendyala, S., Bourdet, D., et al. (2019a). Cardiovascular Safety of Revefenacin, a Once-Daily, Lung-Selective, Long Acting Muscarinic Antagonist for Nebulized Therapy of Chronic Obstructive Pulmonary Disease: Evaluation in Phase 3 Clinical Trials. Pulm. Pharmacol. Ther. 57, 101808. doi:10.1016/j.pupt.2019.101808
Donohue, J. D., Kerwin, E., Sethi, S., Haumann, B., Pendyala, S., Dean, L., et al. (2019b). Maintained Therapeutic Effect of Revefenacin over 52 Weeks in Moderate to Very Severe Chronic Obstructive Pulmonary Disease (COPD). Respir. Res. 20, 241. doi:10.1186/s12931-019-1187-7
Donohue, J. D., Kerwin, E., Sethi, S., Haumann, B., Pendyala, S., Dean, L., et al. (2019c). Revefenacin, a Once-Daily, Lung-Selective, Long-Acting Muscarinic Antagonist for Nebulized Therapy: Safety and Tolerability Results of a 52-week Phase3 Trial in Moderate to Very Severe Chronic Obstructive Pulmonary Disease. Respir. Med. 153, 38–43. doi:10.1016/j.rmed.2019.05.010
Donohue, J. F., Mahler, D. A., and Sethi, S. (2019d). Revefenacin: A Once-Daily, Long-Acting Bronchodilator for Nebulized Treatment of COPD. Int. J. Chron. Obstruct Pulmon Dis. 14, 2947–2958. doi:10.2147/COPD.S157654
European Respiratory Society on behalf of the Forum of International Respiratory Societies (FIRS) (2017). The Global Impact of Respiratory Disease. 2nd Edition. Sheffield, England, European Respiratory Society, Available at: https://www.who.int/gard/publications/The_Global_Impact_of_Respiratory_Disease.pdf (accessed January 5, 2021).
Ferguson, G. T., Feldman, G., Pudi, K. K., Barnes, C. N., Moran, E. J., Haumann, B., et al. (2019). Improvements in Lung Function with Nebulized Revefenacin in the Treatment of Patients with Moderate to Very Severe COPD: Results from Two Replicate Phase III Clinical Trials. Chronic Obstr Pulm. Dis. 6, 154–165. doi:10.15326/jcopdf.6.2.2018.0152
Foreman, K., Marquez, N., Dolgert, A., Fukutaki, K., Fullman, N., McGaughey, M., et al. (2018). Forecasting Life Expectancy, Years of Life Lost, and All-Cause and Cause-specific Mortality for 250 Causes of Death: Reference and Alternative Scenarios for 2016-40 for 195 Countries and Territories. Lancet 392, 2052–2090. doi:10.1016/S0140-6736(18)31694-5
GBD 2017 Causes of Death Collaborators (2018). Global, Regional, and National Age-sex-specific Mortality for 282 Causes of Death in 195 Countries and Territories, 1980-2017: a Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 392, 1736–1788. doi:10.1016/S0140-6736(18)32203-7
Global Burden of Disease Study Collaborators (2015). Global, Regional, and National Age-Sex Specific All-Cause and Cause-specific Mortality for 240 Causes of Death, 1990-2013: a Systematic Analysis for the Global Burden of Disease Study 2013. Lancet 385, 117–171. doi:10.1016/S0140-6736(14)61682-2
GOLD (2021). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2021 Report). Available at: https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf (accessed January 5, 2021).
Guarascio, A. J., Ray, S. M., Finch, C. K., and Self, T. H. (2013). The Clinical and Economic burden of Chronic Obstructive Pulmonary Disease in the USA. Clinicoecon Outcomes Res. 5, 235–245. doi:10.2147/CEOR.S34321
Guyatt, G., Oxman, A. D., Akl, E. A., Kunz, R., Vist, G., Brozek, J., et al. (2011). GRADE Guidelines: 1. Introduction - GRADE Evidence Profiles and Summary of Findings Tables. J. Clin. Epidemiol. 64, 383–394. doi:10.1016/j.jclinepi.2010.04.026
Halpin, D. M. G., Celli, B. R., Criner, G. J., Firth, P., Lopez Varela, M. V., Salvi, S., et al. (2019). The GOLD Summit on Chronic Obstructive Pulmonary Disease in Low- and Middle-Income Countries. Int. J. Tuberc. Lung Dis. 23, 1131–1141. doi:10.5588/ijtld.19.0397
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Higgins, J. P. T., Li, T., and Sterne, J. (2021). Revised Cochrane Risk of Bias Tool for Randomized Trials (RoB 2) Additional Considerations for Crossover Trials. (updated March 2021). Available at: https://www.riskofbias.info/welcome/rob-2-0-tool (accessed August 4, 2021).
Highlights Of Prescribing Information, (2021). YUPELRI® (Revefenacin) Inhalation Solution, for Oral Inhalation. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210598s000lbl.pdf (accessed January 5, 2021).
Incruse, Glaxo. Smith. Kline. (2021). Ellipta® (Umeclidinium Inhalation Powder) [prescribing Information]. Research Triangle Park. Available at: http://bit.ly/2szZfSf (accessed January 5, 2021).
J. P. T. Higgins, J. Thomas, J. Chandler, M. Cumpston, T. Li, M. J. Pageet al. (Editors) (2020). Cochrane Handbook for Systematic Reviews of Interventions, version 6.1(updated September 2020). Available at: www.training.cochrane.org/handbook (accessed January 5, 2021).
Karner, C., Chong, J., and Poole, P. (2014). Tiotropium versus Placebo for Chronic Obstructive Pulmonary Disease. Cochrane Database Syst. Rev. 7, CD009285. doi:10.1002/14651858.CD009285.pub3
Kesten, S., Casaburi, R., Kukafka, D., and Cooper, C. B. (2008). Improvement in Self-Reported Exercise Participation with the Combination of Tiotropium and Rehabilitative Exercise Training in COPD Patients. Int. J. Chron. Obstruct Pulmon Dis. 3, 127–136. doi:10.2147/copd.s2389
Krishna, K. P., Chris, N. B., Edmund, J. M., Brett, H., and Edward, K. (2017). A 28-day, Randomized, Double-Blind, Placebo-Controlled, Parallel Group Study of Nebulized Revefenacin in Patients with Chronic Obstructive Pulmonary Disease. Respir. Res. 18, 182.
Li, F., and Yang, J. (2019). Revefenacin for the Treatment of Chronic Obstructive Pulmonary Disease. Expert Rev. Clin. Pharmacol. 12, 293–298. doi:10.1080/17512433.2019.1587292
Lopez, A. D., Shibuya, K., Rao, C., Mathers, C. D., Hansell, A. L., Held, L. S., et al. (2006). Chronic Obstructive Pulmonary Disease: Current burden and Future Projections. Eur. Respir. J. 27, 397–412. doi:10.1183/09031936.06.00025805
Lozano, R., Naghavi, M., Foreman, K., Lim, S., Shibuya, K., Aboyans, V., et al. (2012). Global and Regional Mortality from 235 Causes of Death for 20 Age Groups in 1990 and 2010: a Systematic Analysis for the Global Burden of Disease Study 2010. Lancet 380, 2095–2128. doi:10.1016/S0140-6736(12)61728-0
Mahler, D. A., Ohar, J. A., Barnes, C. N., Moran, E. J., Pendyala, S., and Crater, G. D. (2019). Nebulized versus Dry Powder Long-Acting Muscarinic Antagonist Bronchodilators in Patients with COPD and Suboptimal Peak Inspiratory Flow Rate. Chronic Obstr Pulm. Dis. 6, 321–331. doi:10.15326/jcopdf.6.4.2019.0137
Melani, A. S. (2015). Long-acting Muscarinic Antagonists. Expert Rev. Clin. Pharmacol. 8, 479–501. doi:10.1586/17512433.2015.1058154
Montuschi, P., and Ciabattoni, G. (2015). Bronchodilating Drugs for Chronic Obstructive Pulmonary Disease: Current Status and Future Trends. J. Med. Chem. 58, 4131–4164. doi:10.1021/jm5013227
Pauwels, R. A., Lofdahl, C. G., Laitinen, L. A., Schouten, J. P., Postma, D. S., Pride, D. S., et al. (1999). Long-term Treatment with Inhaled Budesonide in Persons with Mild Chronic Obstructive Pulmonary Disease Who Continue Smoking. European Respiratory Society Study on Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 340, 1948–1953. doi:10.1056/NEJM199906243402503
Pulido-Rios, M. T., McNamara, A., Obedencio, G. P., Ji, Y., Jaw-Tsai, S., Martin, W. J., et al. (2013). In Vivo pharmacological Characterization of TD-4208, a Novel Lung-Selective Inhaled Muscarinic Antagonist with Sustained Bronchoprotective Effect in Experimental Animal Models. J. Pharmaco Exp. Ther. 346, 241–250. doi:10.1124/jpet.113.203554
Quinn, D., Barnes, C. N., Yates, W., Bourdet, D., Moran, E. J., Potgieter, P., et al. (2018). Pharmacodynamics, Pharmacokinetics and Safety of Revefenacin (TD-4208), a Long-Acting Muscarinic Antagonist, in Patients with Chronic Obstructive Pulmonary Disease (COPD): Results of Two Randomized, Double-Blind, Phase 2 Studies. Pulm. Pharmacol. Ther. 48, 71–79. doi:10.1016/j.pupt.2017.10.003
Sethi, S., Donohue, J. F., Ferguson, G. T., Barnes, C. N., and Crater, G. D. (2020). Efficacy and Safety of Revefenacin for Nebulization in Patients with Chronic Obstructive Pulmonary Disease Taking Concomitant ICS/LABA or LABA: Subgroup Analysis from Phase III Trials. Ther. Adv. Respir. Dis. 14, 1–11. doi:10.1177/1753466620905278
Siler, T. M., Moran, E. J., Barnes, C. N., and Crater, G. D. (2020). Safety and Efficacy of Revefenacin and Formoterol in Sequence and Combination via a Standard Jet Nebulizer in Patients with Chronic Obstructive Pulmonary Disease: a Phase 3b, Randomized,42-Day Study. Chronic Obstr Pulm. Dis. 7, 99–106. doi:10.15326/jcopdf.7.2.2019.0154
Tashkin, D. P. (2016). A Review of Nebulized Drug Delivery in COPD. Int. J. Chron. Obstruct Pulmon Dis. 11, 2585–2596. doi:10.2147/COPD.S114034
Tashkin, D. P., Celli, B., Senn, S., Burkhart, D., Kesten, S., Menjoge, S., et al. (2008). A 4-year Trial of Tiotropium in Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 359, 1543–1554. doi:10.1056/NEJMoa0805800
Theravance Biopharma (2021a) A 7-day Cross-Over Study of QD (Once Daily) and BID (Twice Daily) TD-4208 in Chronic Obstructive Pulmonary Disease (COPD). Available at: https://www.clinicaltrials.gov/ct2/show/NCT02109172 (accessed January 5, 2021).
Theravance Biopharma (2021b) Effects of TD-4208 on FEV1 in Subjects with Chronic Obstructive Pulmonary Disease (COPD). Available at: https://www.clinicaltrials.gov/ct2/show/NCT03064113 (accessed January 5, 2021).
Vestbo, J., Sorensen, T., Lange, P., Brix, A., Torre, P., and Viskum, K. (1999). Long-term Effect of Inhaled Budesonide in Mild and Moderate Chronic Obstructive Pulmonary Disease: a Randomised Controlled Trial. Lancet 353, 1819–1823. doi:10.1016/s0140-6736(98)10019-3
Vos, T., Flaxman, A. D., Naghavi, M., Lozano, R., Michaud, C., Ezzati, M., et al. (2012). Years Lived with Disability (YLDs) for 1160 Sequelae of 289 Diseases and Injuries 1990-2010: a Systematic Analysis for the Global Burden of Disease Study 2010. Lancet 380, 2163–2196. doi:10.1016/S0140-6736(12)61729-2
World Health Organization (2007). Global Surveillance, Prevention and Control of Chronic Respiratory diseasesA Comprehensive Approach. Geneva, Switzerland, WHO. Available from: http://www.who.int/gard/publications/GARD_Manual/en/(accessed January 5, 2021).
World Health Organization (2020). Projections of Mortality and Causes of Death, 2016 and 2060. Available at: http://www.who.int/healthinfo/global_burden_disease/projections/en/(accessed January 5, 2021).
Xu, C., and Doi, S. A. (2018). The Robust-Error Meta-Regression Method for Dose-Response Meta-Analysis. Int. J. Evi Based Healthc. 16, 138–144. doi:10.1097/XEB.0000000000000132
Xu, C., Furuya-Kanamori, L., Zorzela, L., Lin, L. F., and Vohra, S. (2021). A Proposed Framework to Guide Evidence Synthesis Practice for Meta-Analysis with Zero-Events Studies. J. Clin. Epidemiol., 135, P70-P78. doi:10.1016/j.jclinepi.2021.02.012
Keywords: chronic obstructive pulmonary disease, long-acting muscarinic antagonist, systematic review, dose-response meta-analysis, revefenacin
Citation: Zhang J, Xie Y, Kwong JS-w, Ge L, He R, Zheng W, Han J, Zhang R, Zhao H, He Y and Li X (2021) The Efficacy and Safety of Revefenacin for the Treatment of Chronic Obstructive Pulmonary Disease: A Systematic Review. Front. Pharmacol. 12:667027. doi: 10.3389/fphar.2021.667027
Received: 11 February 2021; Accepted: 06 October 2021;
Published: 20 October 2021.
Edited by:
Fabiane Raquel Motter, University of Sorocaba, BrazilReviewed by:
James Donohue, University of North Carolina at Chapel Hill, United StatesLuciane Cruz Lopes, University of Sorocaba, Brazil
Copyright © 2021 Zhang, Xie, Kwong, Ge, He, Zheng, Han, Zhang, Zhao, He and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaosi Li, bGl4aWFvc2l5eXlAMTI2LmNvbQ==
†These authors have contributed equally to the work and share first authorship
 Jiaxing Zhang
Jiaxing Zhang Yihong Xie2†
Yihong Xie2† Joey Sum-wing Kwong
Joey Sum-wing Kwong Long Ge
Long Ge