- 1Department of Respiratory and Critical Care Medicine, Chengdu Second People’s Hospital, Chengdu, China
- 2Department of Respiratory, The First Affiliated Hospital of Chengdu Medical College, Chengdu, China
Background: Whether all types of inhaled corticosteroids (ICSs) would increase the pneumonia risk in patients with chronic obstructive pulmonary disease (COPD) remains controversial. We aimed to assess the association between ICSs treatment and pneumonia risk in COPD patients, and the impact of medication details and baseline characteristics of patients on the association.
Methods: Four databases (PubMed, Embase, Cochrane Library, and Clinical Trials.gov) were searched to identify eligible randomized controlled trials (RCTs) comparing ICSs treatment with non-ICSs treatment on the pneumonia risk in COPD patients. Pooled results were calculated using Peto odds ratios (Peto ORs) with corresponding 95% confidence intervals (CIs).
Results: A total of 59 RCTs enrolling 103,477 patients were analyzed. All types of ICSs significantly increased the pneumonia risk (Peto OR, 1.43; 95% CI, 1.34–1.53). Subgroup analysis showed that there was a dose-response relationship between ICSs treatment and pneumonia risk (low-dose: Peto OR, 1.33; 95% CI, 1.22–1.45; medium-dose: Peto OR, 1.50; 95% CI, 1.28–1.76; and high-dose: Peto OR, 1.64; 95% CI, 1.45–1.85). Subgroup analyses based on treatment durations and baseline characteristics (severity, age, and body mass index) of patients were consistant with the above results. Subgroup analysis based on severity of pneumonia showed that fluticasone (Peto OR, 1.75; 95% CI, 1.44–2.14) increased the risk of serious pneumonia, while budesonide and beclomethasone did not.
Conclusions: ICSs treatment significantly increased the risk of pneumonia in COPD patients. There was a dose-response relationship between ICSs treatment and pneumonia risk. The pneumonia risk was related with COPD severity.
Introduction
Chronic obstructive pulmonary disease (COPD) is currently the third leading cause of death in the world, and acute exacerbations contribute substantially to this (GBD 2015 Chronic Respiratory Disease Collaborators, 2017; Viniol and Vogelmeier, 2018; López-Campos et al., 2019). Treatment and prevention of repeated exacerbations have been identified as a priority by the Global Initiative for Chronic Obstructive Lung Disease (GOLD). Currently, the management of patients with stable COPD mainly relies on inhaled agents such as inhaled corticosteroids (ICSs), long-acting muscarinic antagonist (LAMA), long-acting β-agonist (LABA), etc. Among them, ICSs have been recommended by GOLD as first-line maintenance treatment in patients with repeated exacerbations to relieve the frequency and severity of acute exacerbations of COPD, and improve their quality of life (Yang et al., 2017).
Some recent studies have raised concerns about increased pneumonia risk associated with long-term use of ICSs (Dong et al., 2014; Kew and Seniukovich, 2014; Morjaria et al., 2017; Janson et al., 2018; Yang et al., 2019; Zhang et al., 2020). However, the association between various types of ICSs and the pneumonia risk remains controversial, as the conclusions of the previous published meta-analyses are different (Sin et al., 2009; Singh et al., 2009; Kew and Seniukovich, 2014; Festic et al., 2016; Yang et al., 2019). However, the reliability and generalizability of these studies might be weakened by their small sample size, since a large number of important randomized controlled trials (RCTs) after 2017 were not included in these meta-analyses (Bhatt et al., 2017; Papi et al., 2017; Siler et al., 2017; Vestbo et al., 2017; Betsuyaku et al., 2018; Chapman et al., 2018; Ferguson et al., 2018a, Ferguson et al., 2018b; Frith et al., 2018; Lipson et al., 2018; Papi et al., 2018; Ichinose et al., 2019; Kerwin et al., 2019; Rabe et al., 2020). Moreover, none of these studies assessed the difference in the pneumonia risk in COPD patients with different demographic characteristics (including severity of airflow limitation, age, body mass index [BMI], etc.).
The aim of this meta-analysis was to objectively reappraise the pneumonia risk and serious pneumonia associated with various types of ICSs in COPD patients through all available RCTs. We also aimed to assess the impact of medication details (including dosage level and treatment duration) and demographic characteristics (severity, age, and body mass index) of patients on this association.
Methods
Protocol and Guidance
This meta-analysis was carried out according to the Preferred Reporting Items for Systematic review and Meta-Analysis (Moher et al., 2009). Ethics committee approval is not applicable for this meta-analysis. The study was registered with PROSPERO prospectively (#CRD42020213586).
Search Strategy
Two reviewers (Hong Chen and Jian Sun) independently searched the databases of PubMed, Embase, Cochrane Library, and Clinical Trials.gov from inception until February 2021, using the following terms: (“chronic obstructive pulmonary disease” OR “COPD” OR “pulmonary disease, chronic obstructive” or “chronic obstructive airway disease” OR “airflow obstruction, chronic” OR “chronic airflow obstruction” OR “chronic obstructive lung disease” OR “emphysema” OR “Bronchitis”) AND (“inhaled corticosteroids” OR “ICS” OR “budesonide” OR “fluticasone” OR “mometasone” OR “beclomethasone” OR “triamcinolone” OR “ciclesonide”). Articles in English were included. Disagreements regarding eligibility were resolved by discussion by two investigators and, if necessary, consultation with a third investigator (Hao Yan).
Eligibility Criteria
We included eligible studies based on the PICOS (Participants [P], Interventions [I], Comparators [C], Outcomes [O], and Study design [S]) criteria (Shamseer et al., 2015): 1) Participants: patients aged 40 yr or over, with stable, moderate (GOLD stage II) to very severe (GOLD stage IV) COPD. Patients with other respiratory diseases, such as asthma, bronchiectasis were excluded. 2) Interventions: various types and doses of ICSs as the intervention treatment. 3) Comparisons: non-ICSs treatment as a control treatment. 4) Outcome: Pneumonia for this meta-analysis was defined as an adverse event based on the Medical Dictionary for Regulatory Activities (MedDRA, version 10.0) pneumonia-related preferred terms, including “pneumonia,” “lobar pneumonia,” “bronchopneumonia,” “pneumonia pneumococcal,” or “pneumonia staphylococcal” (Rennard et al., 2009; Sin et al., 2009). One or more of the above MedDRA terms reported in the adverse events list or the safety profiles by the RCTs would be identified as pneumonia data, and included in our analysis. Serious pneumonia was defined as a pneumonia leading to mechanical ventilation or death, or requiring hospital admission (Aaron et al., 2007; Pascoe et al., 2015). 5) Study design: only RCTs were included. Non-RCTs, such as retrospective studies, reviews, case reports and case-control studie, were excluded.
Data Extraction and Quality Assessment
Two reviewers (Qiang Huang and Yongqi Liu) independently identified references and extracted data from eligible RCTs. Any disagreements would be resolved by discussion to reach a consensus, and consulted a third reviewer if necessary. The risk of bias of the included RCTs was assessed by two independent reviewers (Hong Chen and Mengxin Yuan) using the Cochrane risk of bias tool (Higgins et al., 2011). Any disagreements would be resolved by discussion and consultation (Hao Yan).
Subgroup Analyses
Subgroup analyses were conducted based on: 1) types of ICSs (fluticasone, budesonide, mometasone furoate and beclomethasone); 2) doses of ICSs (low-dose [defined as 100–250 μg/d of fluticasone propionate or equivalent], medium-dose [defined as >250–500 μg/d of fluticasone propionate or equivalent], and high-dose [defined as >500 μg/d of fluticasone propionate or equivalent]); 4) treatment durations (short-term ICSs treatment [defined as ≤6 mo] and long-term ICSs treatment [defined as >6 mo]); 5) severity (moderate COPD [GOLD stage II], severe COPD [GOLD stage III] and very severe COPD [GOLD stage IV]); 6) age of patients (<65 yr old and ≥65 yr old); 7) body mass index (BMI) of patients (≥25 and <25).
Statistical Analysis
The Review Manager 5.3 software was used to calculate the pooled results. Considering Peto odds ratio (Peto OR) could provide the best confidence interval (CI) when events are rare (Bradburn et al., 2007), the pooled results for the comparison of ICSs treatment vs non-ICSs treatment were calculated using Peto ORs. Sensitivity analysis was performed after excluding those studies with high risk of bias. Subgroup analyes based on the baseline demographic characteristics (severity, age, and body mass index) of the patients were conducted using the individual patient level data, which was extracted from the baseline data of the included RCTs (mean or median for lung function, age and BMI). This method of analyzing the individual patient level data was used by Sobieraj et al. (Sobieraj et al., 2018) previously. A two tailed p-value <0.05 was considered to be statistically significant. Statistical heterogeneity was further measured using the I2 test, and I2 ≥50% indicated a substantial heterogeneity (Higgins et al., 2003).
Results
Eligible Trials and Study Descriptions
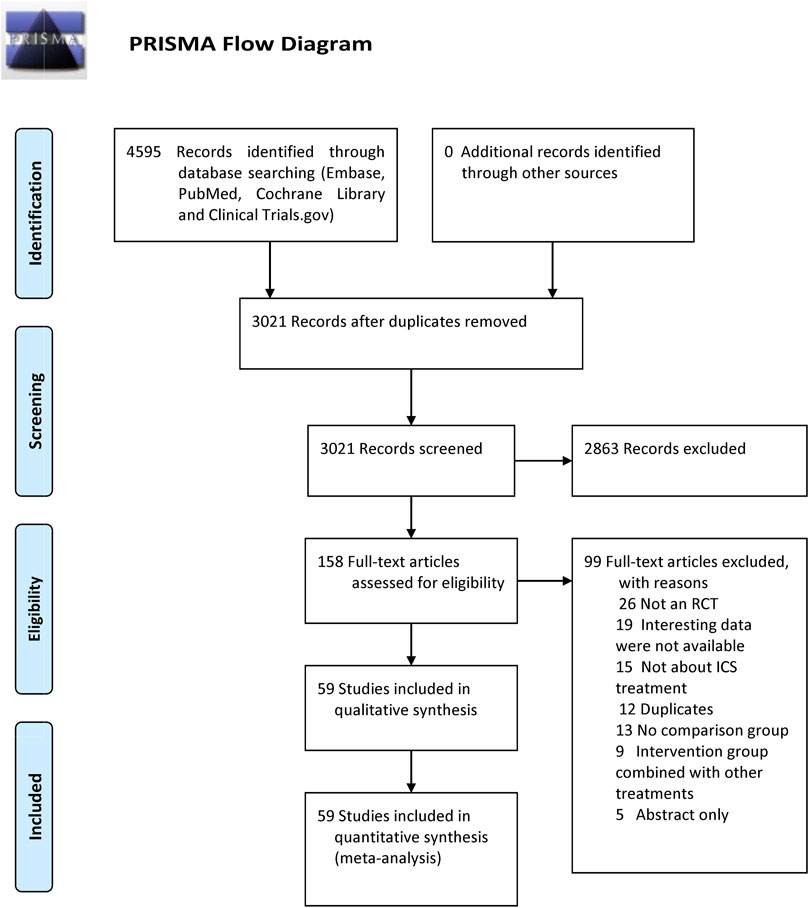
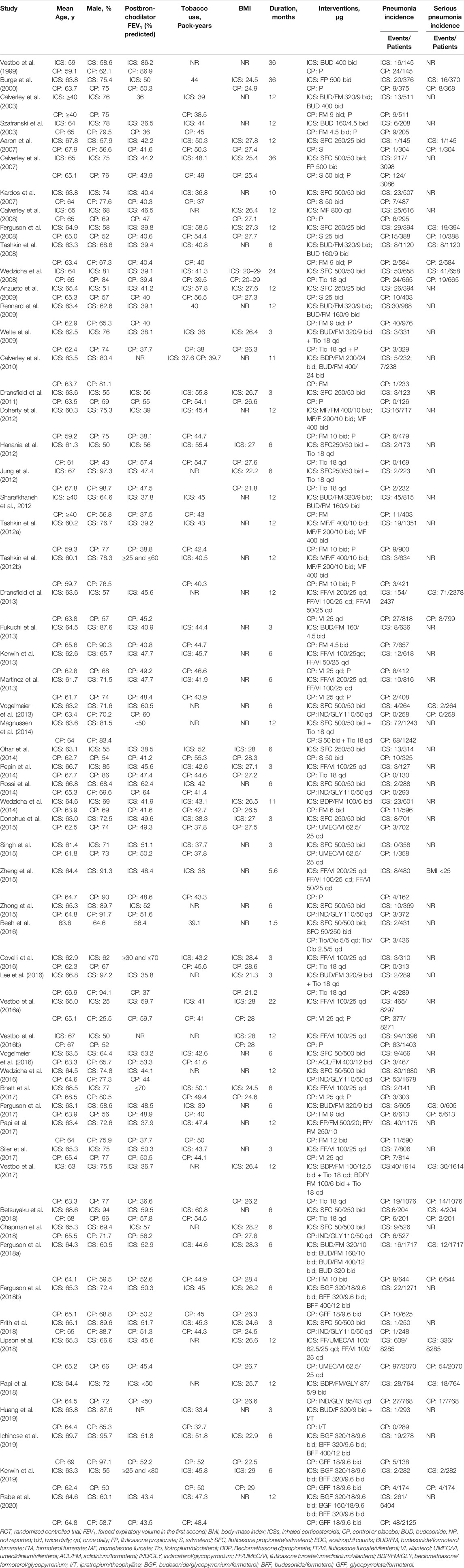
Our search identified 4,595 citations. After evaluating these citations, we included 59 RCTs (Vestbo et al., 1999; Burge et al., 2000; Calverley et al., 2003; Szafranski et al., 2003; Aaron et al., 2007; Calverley et al., 2007; Kardos et al., 2007; Calverley et al., 2008; Ferguson et al., 2008; Tashkin et al., 2008; Wedzicha et al., 2008; Anzueto et al., 2009; Rennard et al., 2009; Welte et al., 2009; Calverley et al., 2010; Dransfield et al., 2011; Doherty et al., 2012; Hanania et al., 2012; Jung et al., 2012; Sharafkhaneh et al., 2012; Tashkin et al., 2012a, Tashkin et al., 2012b; Dransfield et al., 2013; Fukuchi et al., 2013; Kerwin et al., 2013; Martinez et al., 2013; Vogelmeier et al., 2013; Magnussen et al., 2014; Ohar et al., 2014; Pepin et al., 2014; Rossi et al., 2014; Wedzicha et al., 2014; Donohue et al., 2015; Singh et al., 2015; Zheng et al., 2015; Zhong et al., 2015; Beeh et al., 2016; Covelli et al., 2016; Lee et al., 2016; Vestbo et al., 2016a; Vestbo et al., 2016b; Vogelmeier et al., 2016; Wedzicha et al., 2016; Bhatt et al., 2017; Ferguson et al., 2017; Papi et al., 2017; Siler et al., 2017; Vestbo et al., 2017; Betsuyaku et al., 2018; Chapman et al., 2018; Ferguson et al., 2018a; Ferguson et al., 2018b; Frith et al., 2018; Lipson et al., 2018; Papi et al., 2018; Huang et al., 2019; Ichinose et al., 2019; Kerwin et al., 2019; Rabe et al., 2020). These trials enrolled 103,477 patients, of whom 60,733 received ICSs treatment and 42,744 received non-ICSs treatment. The flowchart is shown in Figure 1. The studies included were published between 1999 and 2020, with sample size raging from 249 to 16,568 patients. All studies provided data on pneumonia, 14 of which provided data on serious pneumonia. Among the studies, 35 RCTs (67,109 patients) compared fluticasone and control, 17 RCTs (25,071 patients) compared budesonide and control, four RCTs (5,413 patients) compared mometasone and control, and four RCTs (5,884 patients) compared beclomethasone and control, respectively. No RCTs investigated triamcinolone or ciclesonide and the pneumonia risk in COPD patients. The detailed characteristics of the included RCTs are summarized in Table 1.
Assessment of Risk of Bias
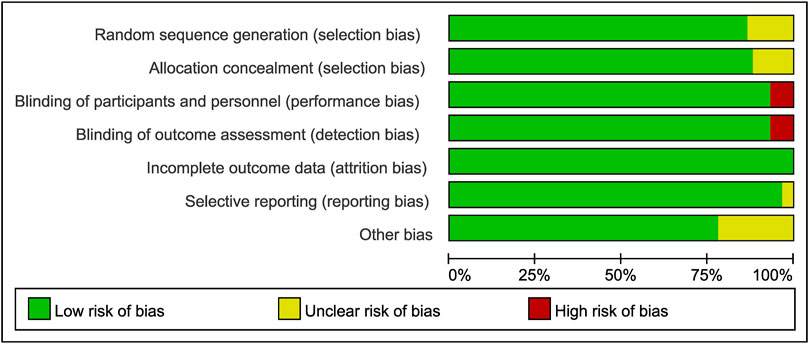
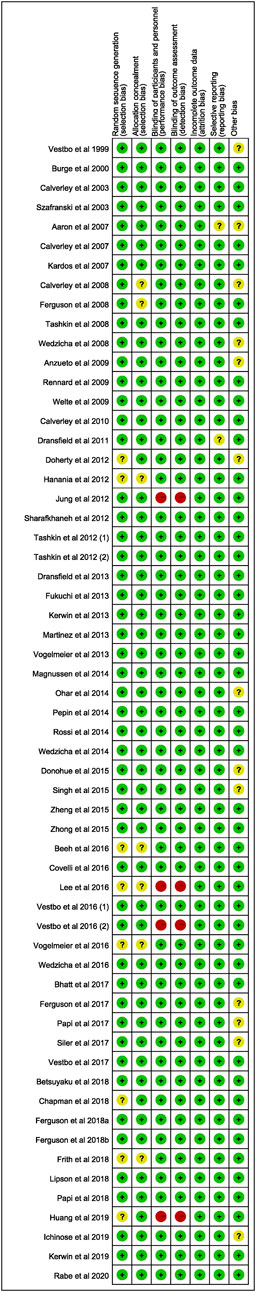
All included studies were assessed using the Cochrane Collaboration risk of bias assessment tool. The results are presented in Figures 2, 3. Thirty-five RCTs were assessed as being at low risk of bias for all aspects. Four had a high risk of bias for performance bias (blinding of participants and personnel) and detection bias (Blinding of outcome assessment). Twenty-two had an unclear risk for random sequence generation, selective reporting, allocation concealment, or other bias (Figures 2, 3).
Various Types of ICSs and Pneumonia Risk
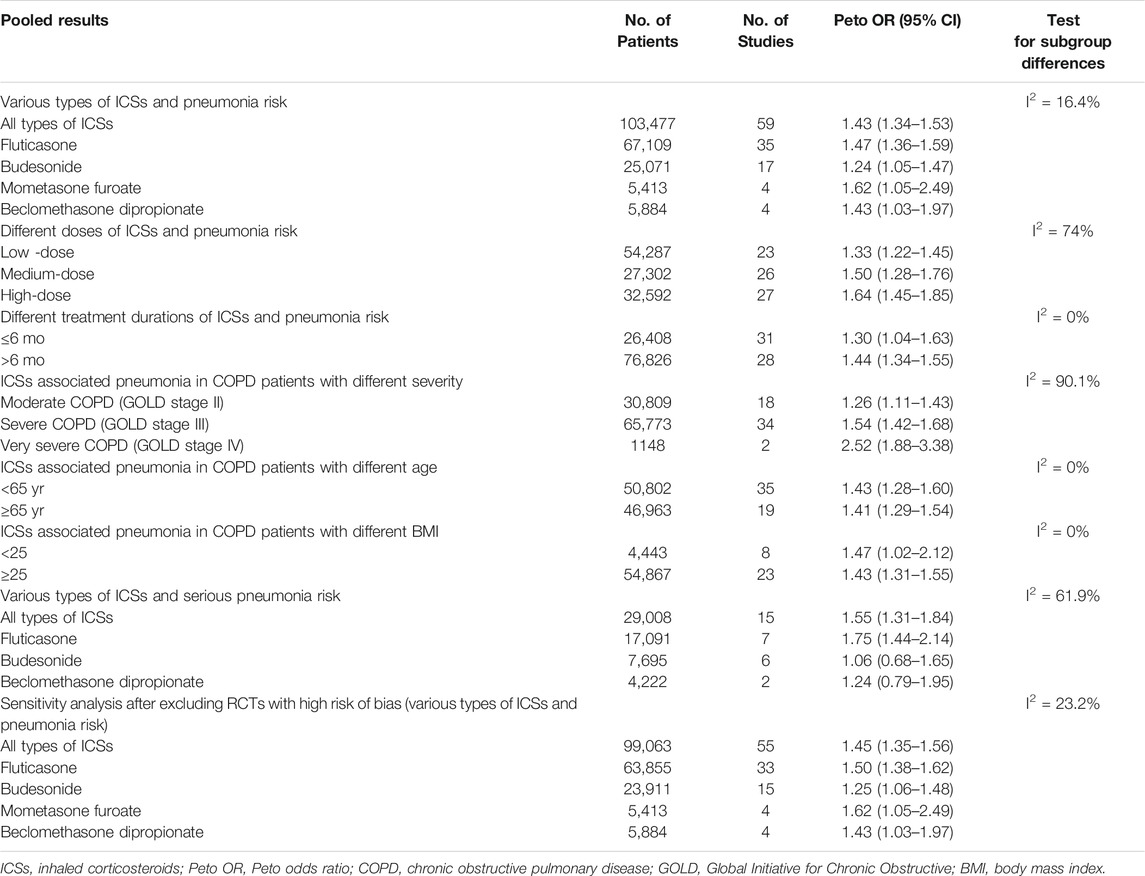
Compared with non-ICSs treatment, ICSs treatment significantly increased the pneumonia risk (Peto OR, 1.43; 95% CI, 1.34–1.53). Subgroup analysis based on types of ICSs showed that all types of ICSs increased the pneumonia risk ([fluticasone: Peto OR, 1.47; 95% CI, 1.36–1.59]; [budesonide: Peto OR, 1.24; 95% CI, 1.05–1.47]; [mometasone: Peto OR, 1.62; 95% CI, 1.05–2.49]; [beclomethasone: Peto OR, 1.43; 95% CI, 1.03–1.97]). Test for subgroup differences (I2 = 16.4%) indicated that there was no significant difference in the pneumonia risk associated with different types of ICSs (Table 2 and Figure 4).
Different Doses of ICSs and Pneumonia Risk
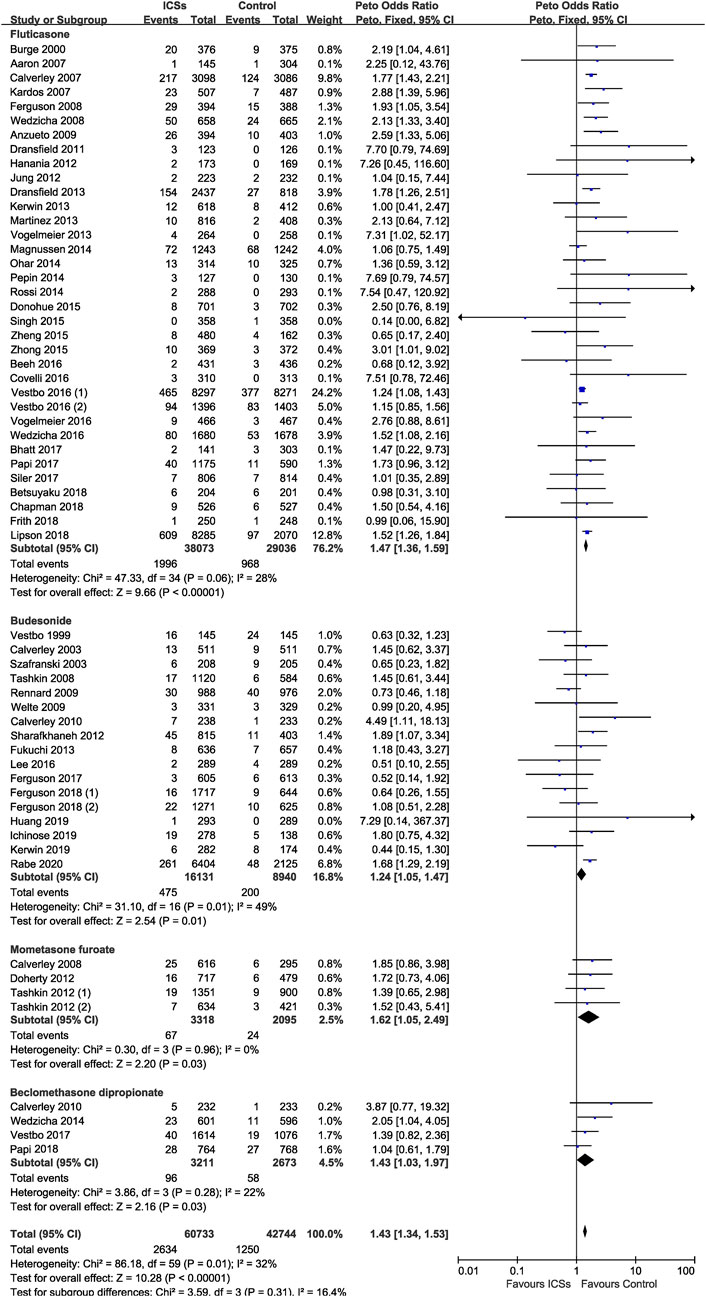
Of the included trials, 23 RCTs (54,287 patients), 26 RCTs (27,302 patients), and 27 RCTs (32,592 patients) assessed high-dose, medium-dose, and low-dose ICSs and pneumonia risk, respectively. Subgroup analysis showed that there was a dose-response relationship between ICSs treatment and pneumonia risk. Low-dose (Peto OR, 1.33; 95% CI, 1.22–1.45), medium-dose (Peto OR, 1.50; 95% CI, 1.28–1.76), and high-dose (Peto OR, 1.64; 95% CI, 1.45–1.85) ICSs all significantly increased the pneumonia risk. Test for subgroup differences (I2 = 74%) indicated that there was a significant difference in the pneumonia risk associated with different doses of ICSs (Table 2 and Figure 5).
Different Treatment Durations of ICSs and Pneumonia Risk
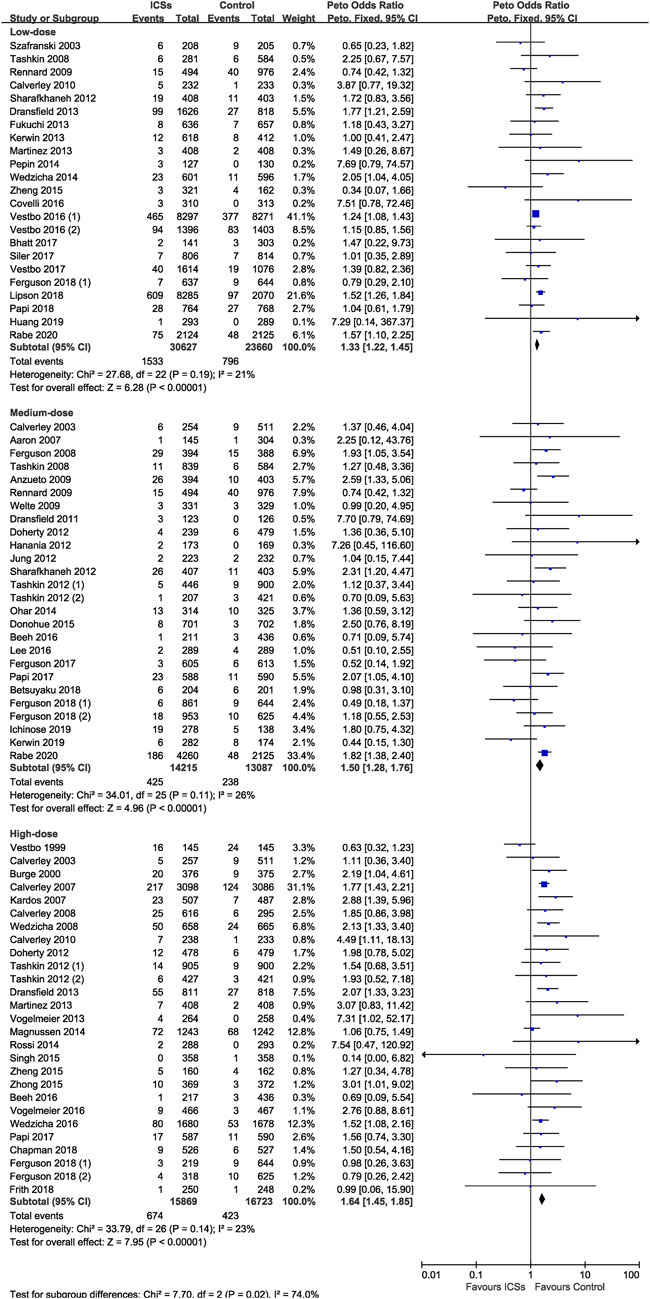
Of the included trials, 31 RCTs (26,408 patients), and 28 RCTs (76,826 patients) assessed short-term ICSs treatment and long-term ICSs treatment and pneumonia risk. Subgroup analysis showed that both short-term ICSs treatment (Peto OR, 1.30; 95% CI, 1.04–1.63) and long-term ICSs treatment (Peto OR, 1.44; 95% CI, 1.34–1.55) significantly increased the pneumonia risk. Test for subgroup differences (I2 = 0%) indicated that there was no significant difference in the pneumonia risk associated with different treatment durations of ICSs (Table 2 and Figure 6).
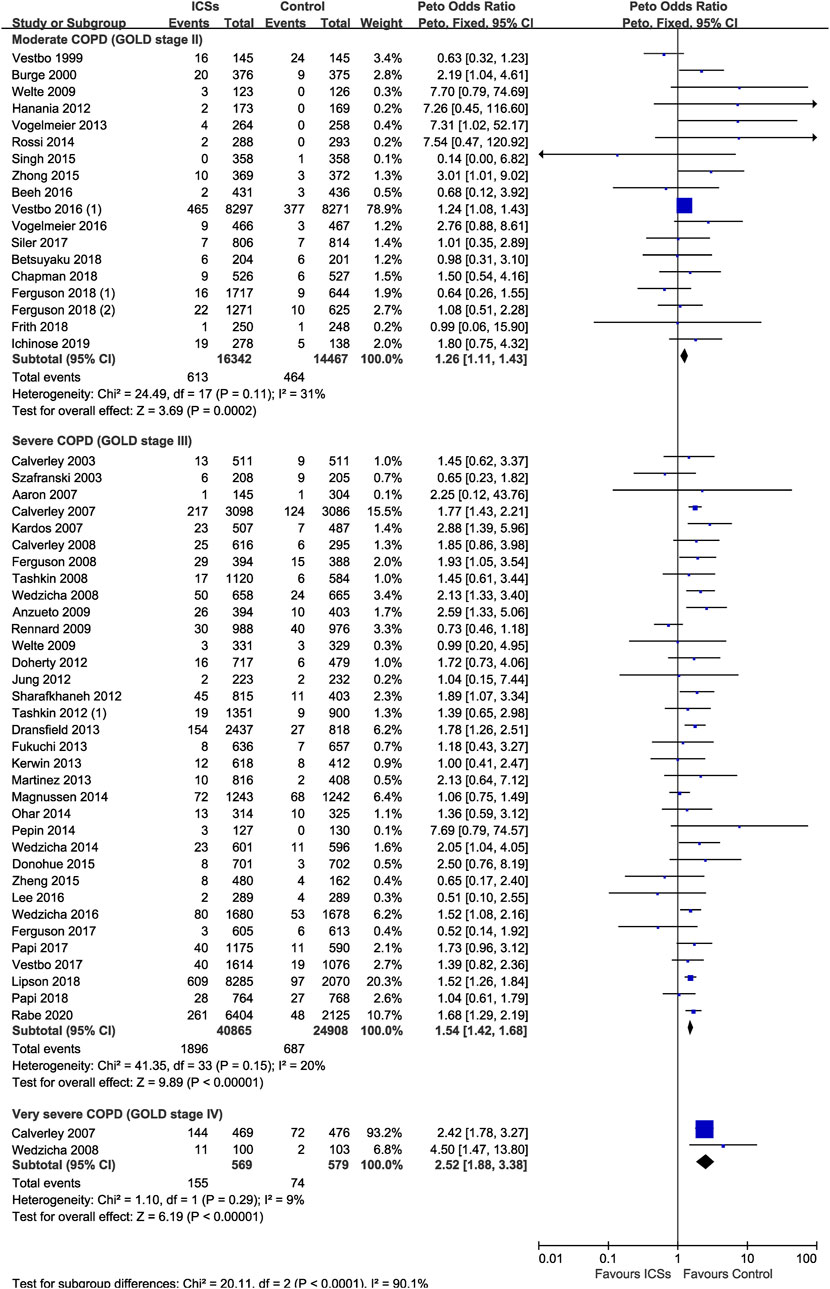
ICSs Associated Pneumonia in COPD Patients With Different Severity
Eighteen RCTs (30,809 patients), 34 RCTs (65,773 patients) and two RCTs (1,148 patients) assessed ICSs associated pneumonia in moderate, severe, and very severe COPD patients, respectively. Subgroup analysis showed that the pneumonia risk was related with COPD severity. ICSs treatment significantly increased the pneumonia risk in all severity subgroups of COPD patients: ([Moderate COPD: Peto OR, 1.26; 95% CI, 1.11–1.43]; [Severe COPD: Peto OR, 1.54; 95% CI, 1.42–1.68]; [Very severe COPD: Peto OR, 2.52; 95% CI, 1.88–3.38]) (Table 2 and Figure 7). Test for subgroup differences (I2 = 90.1%) indicated that there was a significant difference in the pneumonia risk in patients with different severity.
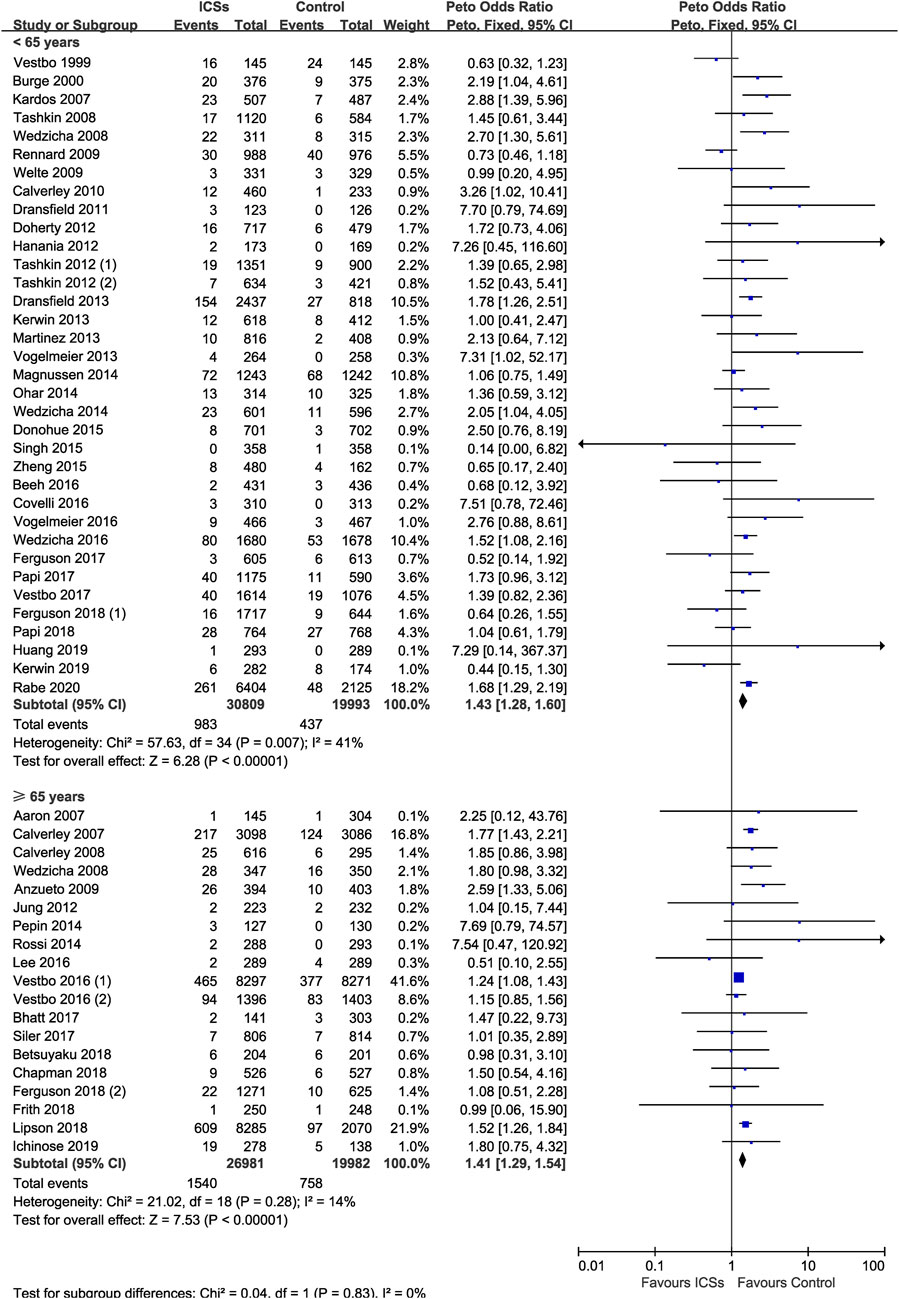
ICSs Associated Pneumonia in COPD Patients With Different Age
Thirty-five RCTs (50,802 patients) and 19 RCTs (46,963 patients) assessed ICSs associated pneumonia in patients with different age. Subgroup analysis showed that ICSs treatment significantly increased the pneumonia risk in patients both age subgroups: ([<65 yr old: Peto OR, 1.43; 95% CI, 1.28–1.60]; [≥65 yr old: Peto OR, 1.41; 95% CI, 1.29–1.54]). Test for subgroup differences (I2 = 0%) indicated that there was no significant difference in the pneumonia risk in patients with different age (Table 2 and Figure 8).
ICSs Associated Pneumonia in COPD Patients With Different BMI
Eight RCTs (4,443 patients) and 23 RCTs (54,867 patients) assessed ICSs associated pneumonia in patients with different BMI. Subgroup analysis showed that ICSs treatment significantly increased the pneumonia risk in patients both BMI subgroups: ([<25: Peto OR, 1.47; 95% CI, 1.02–2.12]; [≥25: Peto OR, 1.43; 95% CI, 1.31–1.55]). Test for subgroup differences (I2 = 0%) indicated that there was no significant difference in the pneumonia risk in patients with different BMI (Table 2 and Figure 9).
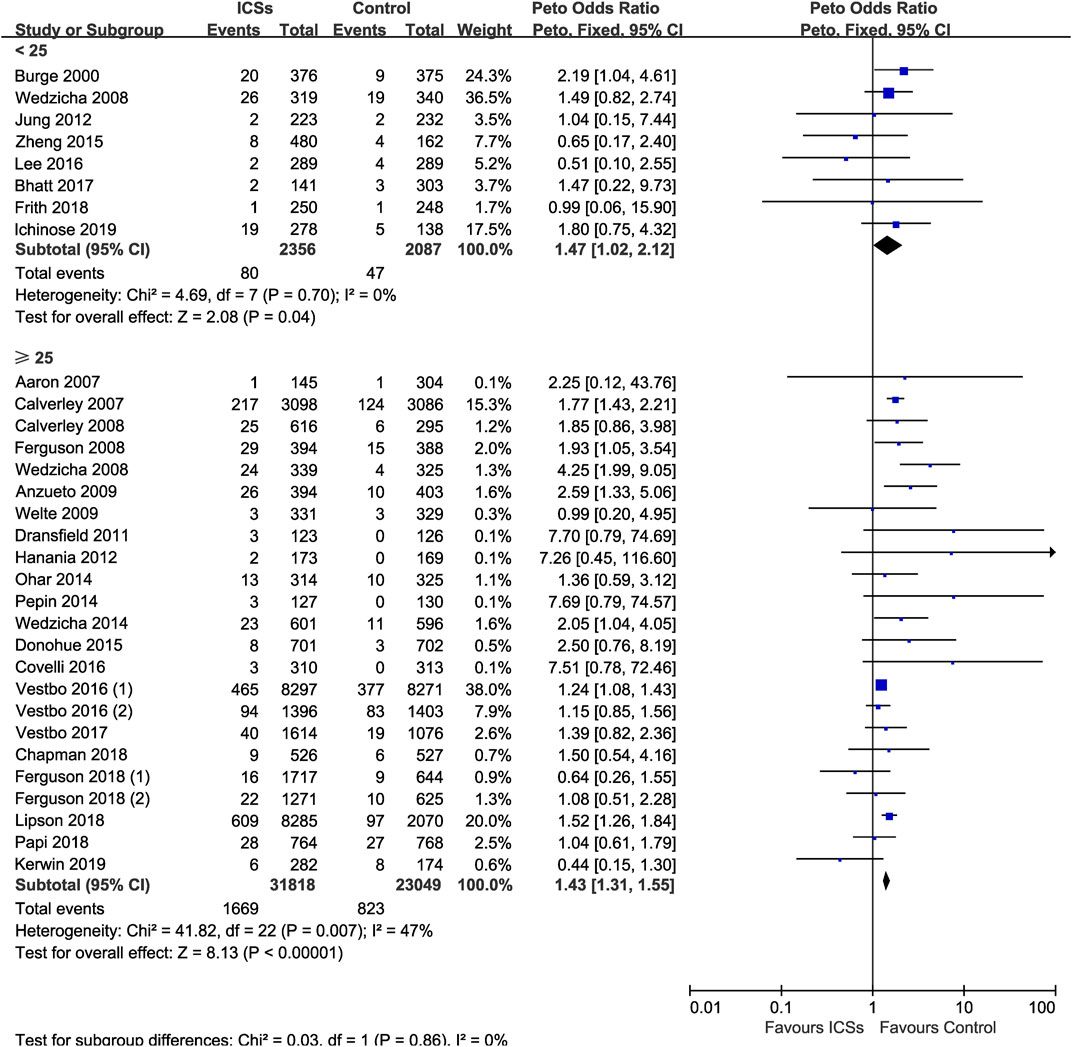
FIGURE 9. Inhaled corticosteroids associated pneumonia in COPD patients with different body mass index.
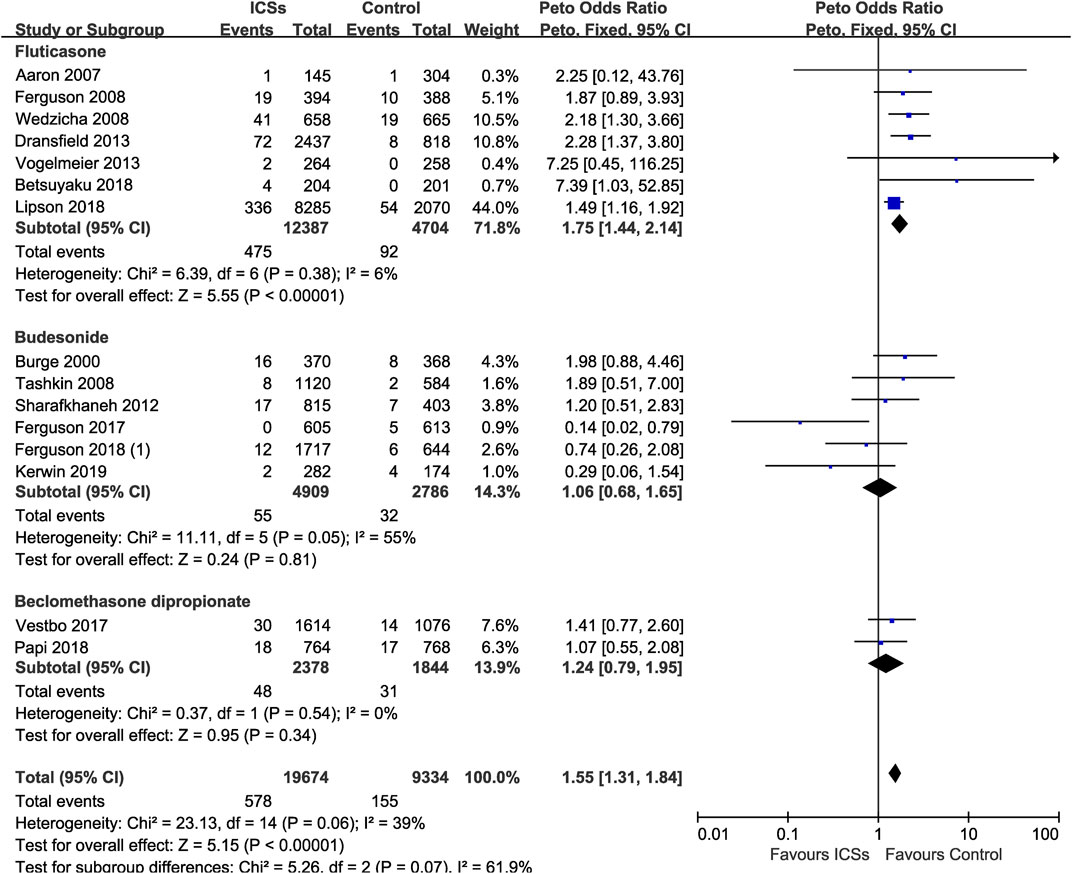
Various Types of ICSs and Serious Pneumonia Risk
Of the included trials, 15 RCTs (29,008 patients) offered data on serious pneumonia associated with ICSs treatment. Compared with non-ICSs treatment, ICSs treatment significantly increased the serious pneumonia risk (Peto OR, 1.55; 95% CI, 1.31–1.84). Of the included RCTs, seven RCTs (17,091 patients) assessed fluticasone and serious pneumonia risk, six RCTs (7,695 patients) assessed budesonide and two RCTs (4,222 patients) assessed beclomethasone, respectively. Subgroup analysis showed that only fluticasone significantly increased the serious pneumonia risk (Peto OR, 1.75; 95% CI, 1.44–2.14) while budesonide (Peto OR, 1.06; 95% CI, 0.68–1.65) and beclomethasone (Peto OR, 1.24; 95% CI, 0.79–1.95) did not. Test for subgroup differences (I2 = 61.9%) indicated that there was a significant difference in the serious pneumonia risk associated with different types of ICSs (Table 2 and Figure 10).
Sensitivity Analysis
After excluding four RCTs (4,414 patients) with high risk of bias, the pooled results were similar in magnitude and direction to those (pooled results of association between various types of ICSs and pneumonia risk) obtained from all included RCTs (Table 2).
Discussion
In this meta-analysis of 59 RCTs (including 103,477 patients), all types of ICSs, not only fluticasone, increased the pneumonia risk in COPD patients in a dose-dependent manner, and the risk was particularly evident in more severe COPD patients. Moreover, fluticasone increased the risk of serious pneumonia, while budesonide and beclomethasone did not. To our knowledge, this study was the first meta-analysis which revealed the pneumonia risk associated ICSs treatment was related with COPD severity. In addition, there was a dose-response relationship between the pneumonia risk and ICSs treatment.
At present, ICSs are widely used in the maintenance treatment of COPD patients. Since numerous COPD patients use ICSs every day, both its efficacy and safety should be considered. Although some studies have reported that fluticasone increases the pneumonia risk in COPD patients, whether other types of ICSs would increase the pneumonia risk in COPD patients remains controversial (Sin et al., 2009; Singh et al., 2009; Kew and Seniukovich, 2014; Festic et al., 2016; Yang et al., 2019; Zhang et al., 2020). In addition, it is still unclear whether different medication details and baseline characteristics (severity, age, and body mass index) of patients would affect the incidence of pneumonia after ICSs treatment.
Our results first revealed that all types of ICSs significantly increased the pneumonia risk in COPD patients regardless of treatment duration. The dose-response relationship further confirmed the causality of ICSs treatment and increased pneumonia risk in COPD patients. Moreover, our results revealed that the pneumonia risk was related with COPD severity. However, age and BMI may not be the determinants of ICSs associated pneumonia. In addition, we found that COPD patients receiving different types of ICSs may have different risk of serious pneumonia. Only fluticasone increased the risk of serious pneumonia, while other types of ICSs did not. We speculated that this may be due to the different pharmacodynamics and pharmacokinetic characteristics of different types of ICSs. Previous studies reported that fluticasone could exhibit a longer retention in the airway mucosa and thus have a more prolonged suppression of local immunity of patients (Brattsand and Miller-Larsson, 2003; Dalby et al., 2009).
Compared With Other Studies
Several previous meta-analyses (Sin et al., 2009; Singh et al., 2009; Kew and Seniukovich, 2014; Festic et al., 2016; Yang et al., 2019; Zhang et al., 2020) also assessed the pneumonia risk associated with ICSs treatment. However, there were major differences between our meta-analysis and the previous ones in terms of selected studies, statistical analyses, and outcomes. First, we included data of some recent large-scale RCTs (Bhatt et al., 2017; Papi et al., 2017; Siler et al., 2017; Vestbo et al., 2017; Betsuyaku et al., 2018; Chapman et al., 2018; Ferguson et al., 2018a; Ferguson et al., 2018b; Frith et al., 2018; Lipson et al., 2018; Papi et al., 2018; Ichinose et al., 2019; Kerwin et al., 2019; Rabe et al., 2020) which were published after some of the previous meta-analyses. In addition, varied search strategy may be an important reason for the difference in the number of RCTs included in different meta-analyses. We systematically searched four large databases for relevant RCTs, including PubMed, Embase, Cochrane Library, and Clinical Trials.gov. In particular, we systematically searched the online supplementary documents of relevant RCTs. Indeed, pneumonia risk was not the primary outcome in most RCTs, some researchers provided data on pneumonia risk in the online supplementary documents rather than in the text. Second, compared with the previous meta-analyses, we conducted more subgroup analyses based on the baseline demographic characteristics of the patients (severity, age and BMI) to clarify possible varied pneumonia risk in different patients receiving ICSs treatment. Third, our results indicated that all types of ICSs, not only fluticasone, increase the pneumonia risk in COPD patients in a dose-dependent manner, and the risk is particularly evident in more severe patients.
In 2009, Singh et al. (Singh et al., 2009) performed a meta-analysis (18 RCTs, 16,996 patients) and concluded that ICSs (fluticasone and budesonide) treatment significantly increased the pneumonia risk in COPD patients. However, their study failed to provide some important information on ICSs associated pneumonia due to a lack of subgroup analyses based on medication details of ICSs (including dose, type and treatment duration), and subgroup analyses based on the baseline demographic characteristics of patients. In 2009, Sin et al. conducted a meta-analysis of budesonide and pneumonia risk (seven RCTs, 7,042 patients) and found that budesonide treatment for 12 mo did not increase the pneumonia risk in COPD patients. In 2014, a meta-analysis performed by Kew et al. (Kew and Seniukovich, 2014) (43 RCTs, 31,397 patients) suggested that both fluticasone and budesonide increased the serious pneumonia risk in COPD patients. However, that study did not further examine the association between other types of ICSs (momethasone and beclomethasone) and the pneumonia risk, nor conduct subgroup analyses based on the baseline demographic characteristics of patients. In 2016, another meta-analysis (29 RCTs, 33,472 patients) performed by Festic et al. (Festic et al., 2016) also revealed that ICSs increased the pneumonia risk in COPD patients. However, that study also limited by a smaller sample size and absent subgroup analyses. In addition, Yang et al. (Yang et al., 2019) conducted a meta-analysis (25 RCTs, 49,982 patients) and found ICSs significantly increased the pneumonia risk and serious pneumonia risk in COPD patients. However, their study also did not analyse the impact of baseline demographic characteristics of patients on the pneumonia risk. Moreover, in 2020, a meta-analysis (18 RCTs, 49,828 patients) performed by Zhang et al. (Zhang et al., 2020) also investigated the association between different types of ICSs and the pneumonia risk, and suggested that fluticasone increased the pneumonia risk while budesonide or beclomethasone did not. However, their results might be limited by the smaller sample size, since much fewer RCTs (especially RCTs on budesonide and beclomethasone) were included in their meta-analysis. In contrast, we searched more databases, used more search terms, and put less restrictions on literature search, which made more relevant RCTs were identified.
Limitations and Strengths
The major strength of our study was that we conducted a comprehensive literature search including all currently available RCTs, thus ensured the generalizability of the conclusions. Moreover, the multiple subgroup analyses based on the medication details (dose and treatment duration) and baseline of patients (severity, age and BMI of patients) enhanced the reliability of the conclusions, and also provided implications for the clinical practice. As far as we know, our study is the first meta-analysis which systematically assesses the association between various types of ICSs and the pneumonia risk based on baseline characteristics of patients.
This meta-analysis had several limitations. First, none of the included RCTs were specifically designed to monitor pneumonia event, therefore, there may be underreporting of pneumonia incidence. However, the underestimate of the pneumonia risk could not substantially impact the pooled results of this meta-analysis, since underreporting of pneumonia incidence might occur equally in ICSs treatment groups and non-ICSs treatment groups. Moreover, in the sensitivity analysis, after removing four non double-blind RCTs, the results were consistent with the previous pooled results. Second, the pooled results of momethasone (four RCTs, 5,413 patients) and beclomethasone (four RCTs, 5,884 patients) may weakened by the relatively small sample size. Third, some studies were excluded because of incomplete data or non-English literature, which may lead to inevitable selection bias.
Conclusions
ICSs treatment significantly increased the risk of pneumonia in COPD patients. There was a dose-response relationship between ICSs treatment and pneumonia risk. The pneumonia risk was related with COPD severity.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Author Contributions
HC and HY conceived and designed this study. HC and JS searched and selected studies. QH and YL extracted essential information. HC and MY assessed the risk of bias. HC and CM conducted the statistical analysis. HC wrote the original draft. All authors approved the final version to be published.
Funding
This study was supported by the Chengdu Science and Technology Project (No. 2015-HM0100621-SF).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to express our appreciation to all authors listed in these all primary studies which were included in the current meta-analysis.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.691621/full#supplementary-material
References
Aaron, S. D., Vandemheen, K. L., Fergusson, D., Maltais, F., Bourbeau, J., Goldstein, R., et al. (2007). Tiotropium in Combination with Placebo, Salmeterol, or Fluticasone-Salmeterol for Treatment of Chronic Obstructive Pulmonary Disease. Ann. Intern. Med. 146 (8), 545–555. doi:10.7326/0003-4819-146-8-200704170-00152
Anzueto, A., Ferguson, G. T., Feldman, G., Chinsky, K., Seibert, A., Emmett, A., et al. (2009). Effect of Fluticasone Propionate/salmeterol (250/50) on COPD Exacerbations and Impact on Patient Outcomes. COPD: J. Chronic Obstructive Pulm. Dis. 6 (5), 320–329. doi:10.1080/15412550903140881
Beeh, K. M., Derom, E., Echave-Sustaeta, J., Grönke, L., Hamilton, A., Zhai, D., et al. (2016). The Lung Function Profile of Once-Daily Tiotropium and Olodaterol Via Respimat® Is superior to that of Twice-Daily Salmeterol and Luticasone Propionate Via Accuhaler® (ENERGITO® Study). Copd 11, 193–205. doi:10.2147/copd.s95055
Betsuyaku, T., Kato, M., Fujimoto, K., Kobayashi, A., Hayamizu, T., Hitosugi, H., et al. (2018). A Randomized Trial of Symptom-Based Management in Japanese Patients with COPD. Copd 13, 2409–2423. doi:10.2147/copd.s152723
Bhatt, S., Dransfield, M., Cockcroft, J., Wang-Jairaj, J., Midwinter, D., Rubin, D., et al. (2017). A Randomized Trial of Once-Daily Fluticasone Furoate/vilanterol or Vilanterol Versus Placebo to Determine Effects on Arterial Stiffness in COPD. Copd 12, 351–365. doi:10.2147/copd.s117373
Bradburn, M. J., Deeks, J. J., Berlin, J. A., and Russell Localio, A. (2007). Much Ado about Nothing: a Comparison of the Performance of Meta-Analytical Methods with Rare Events. Statist. Med. 26, 53–77. doi:10.1002/sim.2528
Brattsand, R., and Miller-Larsson, A. (2003). The Role of Intracellular Esterification in Budesonide Once-Daily Dosing and Airway Selectivity. Clin. Ther. 25 (Suppl. C), C28–C41. doi:10.1016/s0149-2918(03)80304-1
Burge, P. S., Calverley, P. M., Jones, P. W., Spencer, S., Anderson, J. A., and Maslen, T. K. (2000). Randomised, Double Blind, Placebo Controlled Study of Fluticasone Propionate in Patients with Moderate to Severe Chronic Obstructive Pulmonary Disease: The ISOLDE Trial. BMJ 320 (7245), 1297–1303. doi:10.1136/bmj.320.7245.1297
Calverley, P. M. A., Anderson, J. A., Celli, B., Ferguson, G. T., Jenkins, C., Jones, P. W., et al. (2007). Salmeterol and Fluticasone Propionate and Survival in Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 356 (8), 775–789. doi:10.1056/nejmoa063070
Calverley, P. M. A., Kuna, P., Monsó, E., Costantini, M., Petruzzelli, S., Sergio, F., et al. (2010). Beclomethasone/formoterol in the Management of COPD: A Randomised Controlled Trial. Respir. Med. 104, 1858–1868. doi:10.1016/j.rmed.2010.09.008
Calverley, P. M., Boonsawat, W., Cseke, Z., Zhong, N., Peterson, S., and Olsson, H. (2003). Maintenance Therapy with Budesonide and Formoterol in Chronicobstructive Pulmonary Disease. Eur. Respir. J. 22 (6), 912–919. doi:10.1183/09031936.03.00027003
Calverley, P. M., Rennard, S., Nelson, H. S., Karpel, J. P., Abbate, E. H., Stryszak, P., et al. (2008). One-year Treatment with Mometasone Furoate in Chronic Obstructive Pulmonary Disease. Respir. Res. 9, 73. doi:10.1186/1465-9921-9-73
Chapman, K. R., Hurst, J. R., Frent, S.-M., Larbig, M., Fogel, R., Guerin, T., et al. (2018). Long-Term Triple Therapy De-escalation to Indacaterol/Glycopyrronium in Patients with Chronic Obstructive Pulmonary Disease (SUNSET): A Randomized, Double-Blind, Triple-Dummy Clinical Trial. Am. J. Respir. Crit. Care Med. 198 (3), 329–339. doi:10.1164/rccm.201803-0405oc
Covelli, H., Pek, B., Schenkenberger, I., Scott-Wilson, C., Emmett, A., and Crim, C. (2016). Efficacy and Safety of Fluticasone Furoate/vilanterol or Tiotropium in Subjects with COPD at Cardiovascular Risk. Int. J. Chron. Obstruct Pulmon Dis. 11, 1–12. doi:10.2147/COPD.S91407
Dalby, C., Polanowski, T., Larsson, T., Borgström, L., Edsbäcker, S., and Harrison, T. W. (2009). The Bioavailability and Airway Clearance of the Steroid Component of Budesonide/formoterol and Salmeterol/fluticasone after Inhaled Administration in Patients with COPD and Healthy Subjects: A Randomized Controlled Trial. Respir. Res. 10, 104. doi:10.1186/1465-9921-10-104
Doherty, D., Tashkin, D., Kerwin, E., Knorr, B. A., Shekar, T., Banerjee, S., et al. (2012). Effects of Mometasone Furoate/formoterol Fumarate Fixed-Dose Combination Formulation on Chronic Obstructive Pulmonary Disease (COPD): Results From a 52-week Phase III Trial in Subjects with Moderate-To-Very Severe COPD. Copd 7, 57–71. doi:10.2147/copd.s27320
Dong, Y.-H., Chang, C.-H., Wu, F.-L. L., Shen, L.-J., Calverley, P. M. A., Löfdahl, C.-G., et al. (2014). Use of Inhaled Corticosteroids in Patients with COPD and the Risk of TB and Influenza. Chest 145 (6), 1286–1297. doi:10.1378/chest.13-2137
Donohue, J. F., Worsley, S., Zhu, C.-Q., Hardaker, L., and Church, A. (2015). Improvements in Lung Function with Umeclidinium/vilanterol Versus Fluticasone Propionate/salmeterol in Patients with Moderate-To-Severe COPD and Infrequent Exacerbations. Respir. Med. 109 (7), 870–881. doi:10.1016/j.rmed.2015.04.018
Dransfield, M. T., Bourbeau, J., Jones, P. W., Hanania, N. A., Mahler, D. A., Vestbo, J., et al. (2013). Once-daily Inhaled Fluticasone Furoate and Vilanterol Versus Vilanterol Only for Prevention of Exacerbations of COPD: Two Replicate Double-Blind, Parallel-Group, Randomised Controlled Trials. Lancet Respir. Med. 1 (3), 210–223. doi:10.1016/s2213-2600(13)70040-7
Dransfield, M. T., Cockcroft, J. R., Townsend, R. R., Coxson, H. O., Sharma, S. S., Rubin, D. B., et al. (2011). Effect of Fluticasone Propionate/salmeterol on Arterial Stiffness in Patients with COPD. Respir. Med. 105 (9), 1322–1330. doi:10.1016/j.rmed.2011.05.016
Ferguson, G. T., Anzueto, A., Fei, R., Emmett, A., Knobil, K., and Kalberg, C. (2008). Effect of Fluticasone Propionate/salmeterol (250/50μg) or Salmeterol (50μg) on COPD Exacerbations. Respir. Med. 102 (8), 1099–1108. doi:10.1016/j.rmed.2008.04.019
Ferguson, G. T., Papi, A., Anzueto, A., Kerwin, E. M., Cappelletti, C., Duncan, E. A., et al. (2018a). Budesonide/formoterol MDI with Co-suspension Delivery Technology in COPD: The TELOS Study. Eur. Respir. J. 52 (3), 1801334. doi:10.1183/13993003.01334-2018
Ferguson, G. T., Rabe, K. F., Martinez, F. J., Fabbri, L. M., Wang, C., Ichinose, M., et al. (2018b). Triple Therapy with Budesonide/glycopyrrolate/formoterol Fumarate with Co-suspension Delivery Technology Versus Dual Therapies in Chronic Obstructive Pulmonary Disease (KRONOS): A Double-Blind, Parallel-Group, Multicentre, Phase 3 Randomised Controlled Trial. Lancet Respir. Med. 6 (10), 747–758. doi:10.1016/s2213-2600(18)30327-8
Ferguson, G. T., Tashkin, D. P., Skärby, T., Jorup, C., Sandin, K., Greenwood, M., et al. (2017). Effect of Budesonide/formoterol Pressurized Metered-Dose Inhaler on Exacerbations Versus Formoterol in Chronic Obstructive Pulmonary Disease: The 6-month, Randomized RISE (Revealing the Impact of Symbicort in Reducing Exacerbations in COPD) Study. Respir. Med. 132, 31–41. doi:10.1016/j.rmed.2017.09.002
Festic, E., Bansal, V., Gupta, E., and Scanlon, P. D. (2016). Association of Inhaled Corticosteroids with Incident Pneumonia and Mortality in COPD Patients; Systematic Review and Meta-Analysis. COPD: J. Chronic Obstructive Pulm. Dis. 13 (3), 312–326. doi:10.3109/15412555.2015.1081162
Frith, P. A., Ashmawi, S., Krishnamurthy, S., Gurgun, A., Hristoskova, S., Pilipovic, V., et al. (2018). Efficacy and Safety of the Direct Switch to Indacaterol/glycopyrronium From Salmeterol/fluticasone in Non‐frequently Exacerbating COPD Patients: The FLASH Randomized Controlled Trial. Respirology 23 (12), 1152–1159. doi:10.1111/resp.13374
Fukuchi, Y., Samoro, R., Fassakhov, R., Taniguchi, H., Ekelund, J., Carlsson, L.-G., et al. (2013). Budesonide/formoterol Via Turbuhaler Versus Formoterol Via Turbuhaler in Patients with Moderate to Severe Chronic Obstructive Pulmonary Disease: Phase III Multinational Study Results. Respirology 18 (5), 866–873. doi:10.1111/resp.12090
GBD 2015 Chronic Respiratory Disease Collaborators (2017). Global, Regional, and National Deaths, Prevalence, Disability-Adjusted Life Years, and Years Lived With Disability for Chronic Obstructive Pulmonary Disease and Asthma, 1990-2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet Respir. Med. 5 (9), 691–706. doi:10.1016/S2213-2600(17)30293-X
Hanania, N. A., Crater, G. D., Morris, A. N., Emmett, A. H., O’Dell, D. M., and Niewoehner, D. E. (2012). Benefits of Adding Fluticasone Propionate/salmeterol to Tiotropium in Moderate to Severe COPD. Respir. Med. 106 (1), 91–101. doi:10.1016/j.rmed.2011.09.002
Higgins, J. P. T., Altman, D. G., Gotzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Higgins, J. P. T., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring Inconsistency in Meta-Analyses. BMJ 327, 557–560. doi:10.1136/bmj.327.7414.557
Huang, K., Guo, Y., Kang, J., An, L., Zheng, Z., Ma, L., et al. (2019). The Efficacy of Adding Budesonide/formoterol to Ipratropium Plus Theophylline in Managing Severe Chronic Obstructive Pulmonary Disease: An Open-Label, Randomized Study in China. Ther. Adv. Respir. Dis. 13, 1753466619853500. doi:10.1177/1753466619853500
Ichinose, M., Fukushima, Y., Inoue, Y., Hataji, O., Ferguson, G. T., Rabe, K. F., et al. (2019). Long-Term Safety and Efficacy of Budesonide/Glycopyrrolate/Formoterol Fumarate Metered Dose Inhaler Formulated Using Co-suspension Delivery Technology in Japanese Patients with COPD. Copd 14, 2993–3002. doi:10.2147/copd.s220861
Janson, C., Johansson, G., Ställberg, B., Lisspers, K., Olsson, P., Keininger, D. L., et al. (2018). Identifying the Associated Risks of Pneumonia in COPD Patients: ARCTIC An Observational Study. Respir. Res. 19 (1), 172. doi:10.1186/s12931-018-0868-y
Jung, K. S., Park, H. Y., Park, S. Y., Kim, S. K., Kim, Y.-K., Shim, J.-J., et al. (2012). Comparison of Tiotropium Plus Fluticasone Propionate/salmeterol with Tiotropium in COPD: A Randomized Controlled Study. Respir. Med. 106 (3), 382–389. doi:10.1016/j.rmed.2011.09.004
Kardos, P., Wencker, M., Glaab, T., and Vogelmeier, C. (2007). Impact of Salmeterol/fluticasone Propionate Versus Salmeterol on Exacerbations in Severe Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 175 (2), 144–149. doi:10.1164/rccm.200602-244oc
Kerwin, E. M., Ferguson, G. T., Mo, M., DeAngelis, K., and Dorinsky, P. (2019). Bone and Ocular Safety of Budesonide/glycopyrrolate/formoterol Fumarate Metered Dose Inhaler in COPD: A 52-week Randomized Study. Respir. Res. 20 (1), 167. doi:10.1186/s12931-019-1126-7
Kerwin, E. M., Scott-Wilson, C., Sanford, L., Rennard, S., Agusti, A., Barnes, N., et al. (2013). A Randomised Trial of Fluticasone Furoate/vilanterol (50/25 μg; 100/25 μg) on Lung Function in COPD. Respir. Med. 107 (4), 560–569. doi:10.1016/j.rmed.2012.12.014
Kew, K. M., and Seniukovich, A. (2014). Inhaled Steroids and Risk of Pneumonia for Chronic Obstructive Pulmonary Disease. Cochrane Database Syst. Rev. 3, CD010115. doi:10.1002/14651858.CD010115.pub2
Lee, S.-D., Xie, C.-m., Yunus, F., Itoh, Y., Ling, X., Yu, W.-c., et al. (2016). Efficacy and Tolerability of Budesonide/formoterol Added to Tiotropium Compared with Tiotropium Alone in Patients with Severe or Very Severe COPD: A Randomized, Multicentre Study in East Asia. Respirology 21 (1), 119–127. doi:10.1111/resp.12646
Lipson, D. A., Barnhart, F., Brealey, N., Brooks, J., Criner, G. J., Day, N. C., et al. (2018). Once-Daily Single-Inhaler Triple Versus Dual Therapy in Patients with COPD. N. Engl. J. Med. 378 (18), 1671–1680. doi:10.1056/nejmoa1713901
López-Campos, J. L., Soler-Cataluña, J. J., and Miravitlles, M. (2020). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2019 Report: Future Challenges. Archivos de Bronconeumología 56, 65–67. doi:10.1016/j.arbres.2019.06.001
Magnussen, H., Disse, B., Rodriguez-Roisin, R., Kirsten, A., Watz, H., Tetzlaff, K., et al. (2014). Withdrawal of Inhaled Glucocorticoids and Exacerbations of COPD. N. Engl. J. Med. 371 (14), 1285–1294. doi:10.1056/nejmoa1407154
Martinez, F. J., Boscia, J., Feldman, G., Scott-Wilson, C., Kilbride, S., Fabbri, L., et al. (2013). Fluticasone Furoate/vilanterol (100/25; 200/25 μg) Improves Lung Function in COPD: A Randomised Trial. Respir. Med. 107 (4), 550–559. doi:10.1016/j.rmed.2012.12.016
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). PRISMA Group, Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Plos Med. 6 (7), e1000097. doi:10.1371/journal.pmed.1000097
Morjaria, J. B., Rigby, A., and Morice, A. H. (2017). Inhaled Corticosteroid Use and the Risk of Pneumonia and COPD Exacerbations in the UPLIFT Study. Lung 195 (3), 281–288. doi:10.1007/s00408-017-9990-8
Ohar, J. A., Crater, G. D., Emmett, A., Ferro, T. J., Morris, A. N., Raphiou, I., et al. (2014). Fluticasone Propionate/salmeterol 250/50 μg Versus Salmeterol 50 μg After Chronic Obstructive Pulmonary Disease Exacerbation. Respir. Res. 15, 105. doi:10.1186/s12931-014-0105-2
Papi, A., Dokic, D., Tzimas, W., Mészáros, I., Olech-Cudzik, A., Koroknai, Z., et al. (2017). Fluticasone Propionate/formoterol for COPD Management: A Randomized Controlled Trial. Copd 12, 1961–1971. doi:10.2147/copd.s136527
Papi, A., Vestbo, J., Fabbri, L., Corradi, M., Prunier, H., Cohuet, G., et al. (2018). Extrafine Inhaled Triple Therapy Versus Dual Bronchodilator Therapy in Chronic Obstructive Pulmonary Disease (TRIBUTE): A Double-Blind, Parallel Group, Randomised Controlled Trial. The Lancet 391 (10125), 1076–1084. doi:10.1016/s0140-6736(18)30206-x
Pascoe, S., Locantore, N., Dransfield, M. T., Barnes, N. C., and Pavord, I. D. (2015). Blood Eosinophil Counts, Exacerbations, and Response to the Addition of Inhaled Fluticasone Furoate to Vilanterol in Patients with Chronic Obstructive Pulmonary Disease: A Secondary Analysis of Data From Two Parallel Randomised Controlled Trials. Lancet Respir. Med. 3 (6), 435–442. doi:10.1016/s2213-2600(15)00106-x
Pepin, J.-L., Cockcroft, J. R., Midwinter, D., Sharma, S., Rubin, D. B., and Andreas, S. (2014). Long-Acting Bronchodilators and Arterial Stiffness in Patients with COPD. Chest 146 (6), 1521–1530. doi:10.1378/chest.13-2859
Rabe, K. F., Martinez, F. J., Ferguson, G. T., Wang, C., Singh, D., Wedzicha, J. A., et al. (2020). Triple Inhaled Therapy at Two Glucocorticoid Doses in Moderate-To-Very-Severe COPD. N. Engl. J. Med. 383 (1), 35–48. doi:10.1056/nejmoa1916046
Rennard, S. I., Tashkin, D. P., McElhattan, J., Goldman, M., Ramachandran, S., Martin, U. J., et al. (2009). Efficacy and Tolerability of Budesonide/Formoterol in One Hydrofluoroalkane Pressurized Metered-Dose Inhaler in Patients with Chronic Obstructive Pulmonary Disease. Drugs 69 (5), 549–565. doi:10.2165/00003495-200969050-00004
Rossi, A., van der Molen, T., Olmo, R. d., Papi, A., Wehbe, L., Quinn, M., et al. (2014). INSTEAD: A Randomised Switch Trial of Indacaterol Versus Salmeterol/fluticasone in Moderate COPD. Eur. Respir. J. 44 (6), 1548–1556. doi:10.1183/09031936.00126814
Shamseer, L., Moher, D., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., et al. (2015). Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015: Elaboration and Explanation. BMJ 349, g7647. doi:10.1136/bmj.g7647
Sharafkhaneh, A., Southard, J. G., Goldman, M., Uryniak, T., and Martin, U. J. (2012). Effect of Budesonide/formoterol pMDI on COPD Exacerbations: A Double-Blind, Randomized Study. Respir. Med. 106, 257–268. doi:10.1016/j.rmed.2011.07.020
Siler, T. M., Nagai, A., Scott-Wilson, C. A., Midwinter, D. A., and Crim, C. (2017). A Randomised, Phase III Trial of Once-Daily Fluticasone Furoate/vilanterol 100/25 μg Versus Once-Daily Vilanterol 25 μg to Evaluate the Contribution on Lung Function of Fluticasone Furoate in the Combination in Patients with COPD. Respir. Med. 123, 8–17. doi:10.1016/j.rmed.2016.12.001
Sin, D. D., Tashkin, D., Zhang, X., Radner, F., Sjöbring, U., Thorén, A., et al. (2009). Budesonide and the Risk of Pneumonia: A Meta-Analysis of Individual Patient Data. The Lancet 374 (9691), 712–719. doi:10.1016/S0140-6736(09)61250-2
Singh, D., Worsley, S., Zhu, C. Q., Hardaker, L., and Church, A. (2015). Umeclidinium/vilanterol Versus Fluticasone Propionate/salmeterol in COPD: A Randomised Trial. BMC Pulm. Med. 15, 91. doi:10.1186/s12890-015-0092-1
Singh, S., Amin, A. V., and Loke, Y. K. (2009). Long-term Use of Inhaled Corticosteroids and the Risk of Pneumonia in Chronic Obstructive Pulmonary Disease. Arch. Intern. Med. 169 (3), 219–229. doi:10.1001/archinternmed.2008.550
Sobieraj, D. M., Weeda, E. R., Nguyen, E., Coleman, C. I., White, C. M., Lazarus, S. C., et al. (2018). Association of Inhaled Corticosteroids and Long-Acting β-Agonists as Controller and Quick Relief Therapy with Exacerbations and Symptom Control in Persistent Asthma. JAMA 319 (14), 1485–1496. doi:10.1001/jama.2018.2769
Szafranski, W., Cukier, A., Ramirez, A., Menga, G., Sansores, R., Nahabedian, S., et al. (2003). Efficacy and Safety of Budesonide/formoterol in the Management of Chronic Obstructive Pulmonary Disease. Eur. Respir. J. 21 (1), 74–81. doi:10.1183/09031936.03.00031402
Tashkin, D. P., Doherty, D. E., Kerwin, E., Matiz-Bueno, C. E., Knorr, B., Shekar, T., et al. (2012a). Efficacy and Safety Characteristics of Mometasone Furoate/formoterol Fumarate Fixed-Dose Combination in Subjects with Moderate to Very Severe COPD: Findings From Pooled Analysis of Two Randomized, 52-week Placebo-Controlled Trials. Int. J. Chron. Obstruct Pulmon Dis. 7, 73–86. doi:10.2147/COPD.S29444
Tashkin, D. P., Doherty, D. E., Kerwin, E., Matiz-Bueno, C. E., Knorr, B., Shekar, T., et al. (2012b). Efficacy and Safety of a Fixed-Dose Combination of Mometasone Furoate and Formoterol Fumarate in Subjects with Moderate to Very Severe COPD: Results From a 52-week Phase III Trial. Int. J. Chron. Obstruct Pulmon Dis. 7, 43–55. doi:10.2147/COPD.S27319
Tashkin, D. P., Rennard, S. I., Martin, P., Ramachandran, S., Martin, U. J., Silkoff, P. E., et al. (2008). Efficacy and Safety of Budesonide and Formoterol in One Pressurized Metered-Dose Inhaler in Patients with Moderate to Very Severe Chronic Obstructive Pulmonary Disease. Drugs 68 (14), 1975–2000. doi:10.2165/00003495-200868140-00004
Vestbo, J., Anderson, J. A., Brook, R. D., Calverley, P. M. A., Celli, B. R., Crim, C., et al. (2016a). Fluticasone Furoate and Vilanterol and Survival in Chronic Obstructive Pulmonary Disease with Heightened Cardiovascular Risk (SUMMIT): A Double-Blind Randomised Controlled Trial. The Lancet 387 (10030), 1817–1826. doi:10.1016/s0140-6736(16)30069-1
Vestbo, J., Leather, D., Diar Bakerly, N., New, J., Gibson, J. M., McCorkindale, S., et al. (2016b). Effectiveness of Fluticasone Furoate-Vilanterol for COPD in Clinical Practice. N. Engl. J. Med. 375 (13), 1253–1260. doi:10.1056/nejmoa1608033
Vestbo, J., Papi, A., Corradi, M., Blazhko, V., Montagna, I., Francisco, C., et al. (2017). Single Inhaler Extrafine Triple Therapy versus Long-Acting Muscarinic Antagonist Therapy for Chronic Obstructive Pulmonary Disease (TRINITY): a Double-Blind, Parallel Group, Randomised Controlled Trial. The Lancet 389 (10082), 1919–1929. doi:10.1016/s0140-6736(17)30188-5
Vestbo, J., Søorensen, T., Lange, P., Brix, A., Torre, P., and Viskum, K. (1999). Long-term Effect of Inhaled Budesonide in Mild and Moderate Chronic Obstructive Pulmonary Disease: A Randomised Controlled Trial. The Lancet 353 (9167), 1819–1823. doi:10.1016/s0140-6736(98)10019-3
Viniol, C., and Vogelmeier, C. F. (2018). Exacerbations of COPD. Eur. Respir. Rev. 27, 170103. doi:10.1183/16000617.0103-2017
Vogelmeier, C. F., Bateman, E. D., Pallante, J., Alagappan, V. K. T., D'Andrea, P., Chen, H., et al. (2013). Efficacy and Safety of Once-Daily QVA149 Compared with Twice-Daily Salmeterol-Fluticasone in Patients with Chronic Obstructive Pulmonary Disease (ILLUMINATE): A Randomised, Double-Blind, Parallel Group Study. Lancet Respir. Med. 1 (1), 51–60. doi:10.1016/s2213-2600(12)70052-8
Vogelmeier, C., Paggiaro, P. L., Dorca, J., Sliwinski, P., Mallet, M., Kirsten, A.-M., et al. (2016). Efficacy and Safety of Aclidinium/formoterol Versus Salmeterol/fluticasone: A Phase 3 COPD Study. Eur. Respir. J. 48 (4), 1030–1039. doi:10.1183/13993003.00216-2016
Wedzicha, J. A., Banerji, D., Chapman, K. R., Vestbo, J., Roche, N., Ayers, R. T., et al. (2016). Indacaterol-Glycopyrronium Versus Salmeterol-Fluticasone for COPD. N. Engl. J. Med. 374 (23), 2222–2234. doi:10.1056/nejmoa1516385
Wedzicha, J. A., Calverley, P. M. A., Seemungal, T. A., Hagan, G., Ansari, Z., Stockley, R. A., et al. (2008). The Prevention of Chronic Obstructive Pulmonary Disease Exacerbations by Salmeterol/fluticasone Propionate or Tiotropium Bromide. Am. J. Respir. Crit. Care Med. 177 (1), 19–26. doi:10.1164/rccm.200707-973oc
Wedzicha, J. A., Singh, D., Vestbo, J., Paggiaro, P. L., Jones, P. W., Bonnet-Gonod, F., et al. (2014). Extrafine Beclomethasone/formoterol in Severe COPD Patients with History of Exacerbations. Respir. Med. 108 (8), 1153–1162. doi:10.1016/j.rmed.2014.05.013
Welte, T., Miravitlles, M., Hernandez, P., Eriksson, G., Peterson, S., Polanowski, T., et al. (2009). Efficacy and Tolerability of Budesonide/formoterol Added to Tiotropium in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 180 (8), 741–750. doi:10.1164/rccm.200904-0492oc
Yang, M., Chen, H., Zhang, Y., Du, Y., Xu, Y., Jiang, P., et al. (2017). Long-term Use of Inhaled Corticosteroids and Risk of Upper Respiratory Tract Infection in Chronic Obstructive Pulmonary Disease: A Meta-Analysis. Inhalation Toxicol. 29 (5), 219–226. doi:10.1080/08958378.2017.1346006
Yang, M., Du, Y., Chen, H., Jiang, D., and Xu, Z. (2019). Inhaled Corticosteroids and Risk of Pneumonia in Patients with Chronic Obstructive Pulmonary Disease: A Meta-Analysis of Randomized Controlled Trials. Int. Immunopharmacology 77, 105950. doi:10.1016/j.intimp.2019.105950
Zhang, Q., Li, S., Zhou, W., Yang, X., Li, J., and Cao, J. (2020). Risk of Pneumonia with Different Inhaled Corticosteroids in COPD Patients: A Meta-Analysis. COPD: J. Chronic Obstructive Pulm. Dis. 17, 462–469. doi:10.1080/15412555.2020.1787369
Zheng, J., de Guia, T., Wang-Jairaj, J., Newlands, A. H., Wang, C., Crim, C., et al. (2015). Efficacy and Safety of Fluticasone Furoate/vilanterol (50/25 Mcg; 100/25 Mcg; 200/25 Mcg) in Asian Patients with Chronic Obstructive Pulmonary Disease: A Randomized Placebo-Controlled Trial. Curr. Med. Res. Opin. 31 (6), 1191–1200. doi:10.1185/03007995.2015.1036016
Keywords: inhaled corticosteroids, chronic obstructive pulmonary disease, adverse event, pneumonia, meta-analysis
Citation: Chen H, Sun J, Huang Q, Liu Y, Yuan M, Ma C and Yan H (2021) Inhaled Corticosteroids and the Pneumonia Risk in Patients With Chronic Obstructive Pulmonary Disease: A Meta-analysis of Randomized Controlled Trials. Front. Pharmacol. 12:691621. doi: 10.3389/fphar.2021.691621
Received: 06 April 2021; Accepted: 14 June 2021;
Published: 29 June 2021.
Edited by:
Djuro Kosanovic, I.M. Sechenov First Moscow State Medical University, RussiaReviewed by:
Srikanth Karnati, Julius Maximilian University of Würzburg, GermanyKay Tetzlaff, University Hospital of Tübingen, Germany
Copyright © 2021 Chen, Sun, Huang, Liu, Yuan, Ma and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Yan, ZXl5cmVzcGlyYXRvcnltZWRpQDE2My5jb20=
 Hong Chen
Hong Chen Jian Sun2
Jian Sun2 Hao Yan
Hao Yan