- Department of Obstetrics and Gynecology, Shengjing Hospital of China Medical University, Shenyang, China
Aim: Anti-angiogenesis agents have been added as maintenance therapy in ovarian cancer over the past decade. The aim of this meta-analysis was to analyze the efficacy of anti-angiogenesis therapy in newly diagnosed and relapsed ovarian cancer.
Methods: PubMed, Embase, and Cochrane databases were searched for all phase III randomized controlled trials (RCTs) that assessed the efficacy and toxicity of anti-angiogenesis agents in ovarian cancer. Overall survival (OS) and progression-free survival (PFS) were used to evaluate the effectiveness of anti-angiogenesis therapy in ovarian cancer.
Results: A total of 6097 patients with newly diagnosed ovarian cancer from 5 phase III RCTs and 2943 patients with relapsed ovarian cancer from 6 phase III RCTs were included in this meta-analysis. The pooled results showed that anti-angiogenesis maintenance therapy significantly improved PFS (hazard ratio [HR], 0.84; 95% confidence interval [CI], 0.76–0.93; p = 0.001), but not OS (HR, 0.98; 95% CI, 0.91–1.05; p = 0.49) compared with placebo in patients with newly diagnosed ovarian cancer. In patients with relapsed ovarian cancer, the pooled results showed a significant improvement on OS (HR, 0.89; 95% CI, 0.82–0.98; p = 0.02) and PFS (HR, 0.61; 95% CI, 0.52–0.72; p < 0.001). The pooled results also showed that the anti-angiogenesis agents were associated with an increase in the occurrence of severe hypertension, neutropenia, diarrhea, thrombocytopenia, headache, and bleeding in ovarian cancer. However, infrequent fatal adverse events occurred in the anti-angiogenesis groups.
Conclusions: Study results suggest that anti-angiogenesis agents were an effective therapy for newly diagnosed and relapsed ovarian cancer, especially for relapsed ovarian cancer. Anti-angiogenesis agents may be associated with some severe but not fatal adverse events.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42021283647
Introduction
Ovarian cancer is the fifth lethal cancer of all malignancies, and it is the first leading cause of death from gynecologic cancer (Siegel et al., 2021). Despite standard treatment with surgery combined with platinum-based chemotherapy, the majority of patients relapse within 5 years (Bartoletti et al., 2020).
Angiogenesis plays a key role in the growth and progression of malignant tumors through a complex process (Coleman et al., 2013; Monk et al., 2016a), which is distinctly related to vascular endothelial growth factor (VEGF) and the angiopoietin-Tie2 receptor complex (Gavalas et al., 2013). The VEGF pathway has been widely studied in carcinogenesis, with the agents bevacizumab, cediranib, pazopanib, and nintedanib having been proved to treat solid tumors through inhibition of the VEGF pathway (Ferrara and Adamis, 2016; Jayson et al., 2016). Angiopoietin 1 (Ang1) and angiopoietin 2 (Ang2) interact with the Tie2 receptor, increasing blood vessel density (Falcón et al., 2009). Trebananib has been proved to target this pathway by neutralizing Ang1 and Ang2 and then preventing their interaction with the Tie2 receptor (Coxon et al., 2010).
Several anti-angiogenic agents have been researched in ovarian cancer. Bevacizumab was proved to be effective in improving the progression-free survival (PFS) of patients with ovarian cancer in the first phase III randomized controlled trial (RCT) (Perren et al., 2011). Then, more RCTs were designed to evaluate the effect of anti-angiogenesis therapy in ovarian cancer. However, the prognoses of patients with newly diagnosed or relapsed ovarian cancer reported by these RCTs are not consistent.
From the results of phase III RCTs of anti-angiogenesis agents that have reported the final PFS, overall survival (OS), and safety analysis, we performed a meta-analysis aiming to assess the efficacy of anti-angiogenesis maintenance therapy for patients with newly diagnosed or relapsed ovarian cancer.
Methods
Literature Search
In this meta-analysis performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, a search was carried out of the Embase, PubMed, Web of Science, and Cochrane databases. The meta-analysis included all publications that reported the results of phase III RCTs before January 30, 2021. The comprehensive search strings were “ovarian neoplasm,” “ovarian cancer,” “ovarian malignancy,” “ovarian carcinoma”, and associated terms; as well as “anti-angiogenesis,” “bevacizumab,” “nintedanib,” “pazopanib,” “nintedanib,” “cediranib,” “trebananib.” Searches were performed without any restriction on language or publication year. Manual searches for other potential studies were conducted using the reference lists of the selected studies.
Eligibility Criteria
Phase III RCTs were included if they met the following inclusion criteria in accordance with PICOS (population, intervention, comparison, outcomes and study design) guidelines: 1) patients with newly diagnosed or relapsed ovarian cancer; 2) patients administered anti-angiogenesis agents as a maintenance treatment; 3) the comparison made was anti-angiogenesis agent vs placebo; 4) OS and PFS were compared between the group receiving anti-angiogenesis treatment and the placebo group; and 5) the study design included phase III RCTs.
Studies were excluded if they were non-RCTs, phase I or II RCTs, review articles, case reports, editorials, letters, or conference abstracts. We also excluded any studies that lacked OS or PFS and those with patient populations that were duplicated in another study.
Selection and Extraction
Two of the authors (Wang and Zhang) independently identified and selected the studies according with the inclusion and exclusion criteria. The following data elements were extracted from each trial: first author, publication year, clinical trial acronym, medication, disease setting, study period, follow-up time, total patients, PFS, and OS. The Cochrane Collaboration tool was used to assess the risk of bias in the trial (Higgins et al., 2011). Disagreements between the 2 reviewers were identified and resolved by referral to a third author (Song) and by consensus.
Statistical Analysis
We extracted hazard ratios (HRs) and 95% confidence intervals (CIs) of the OS and PFS from all the included trials. We also calculate the odds ratio (OR) to determine the patients’ severe toxicity profile (G3-G4 toxicity) of the anti-angiogenesis agents administered. The meta-analysis was performed using Stata software, version 12.0 (StataCorp) and Review Manager 5.3. Pooled HRs were obtained using random-effects models to reduce the heterogeneity between studies (DerSimonian and Laird, 1986). Heterogeneity between studies was evaluated using the χ2 test and I2 statistic, and I2 values of less than 25%, 25–75%, and greater than 75% were considered low, moderate, and high heterogeneity, respectively (Higgins et al., 2003). The robustness of the main findings were assessed using sensitivity analyses (Copas and Shi, 2000). We also performed subgroup analyses to confirm if the heterogeneity results were moderate or higher. Funnel plots with Begg’s and Egger’s regressions were used to visually examine the effect of publication bias (Begg and Mazumdar, 1994; Egger et al., 1997). A p value less than 0.05 was considered significant, and all p values were 2-sided.
Results
Study Selection
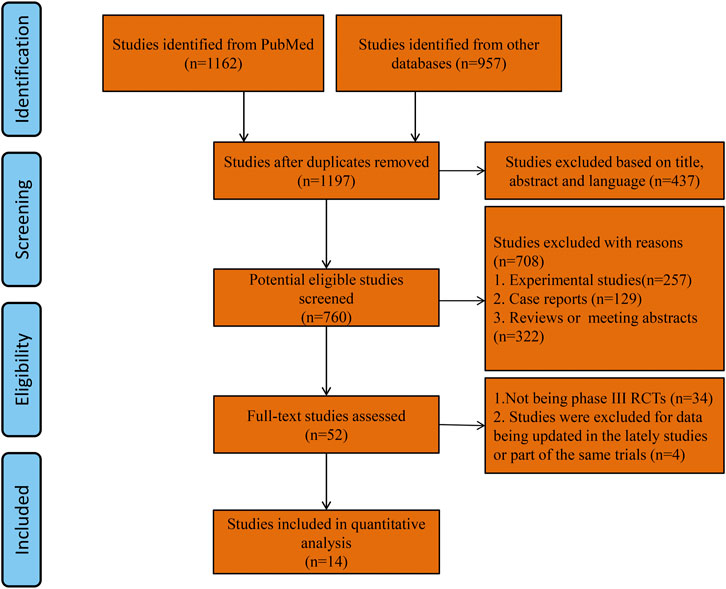
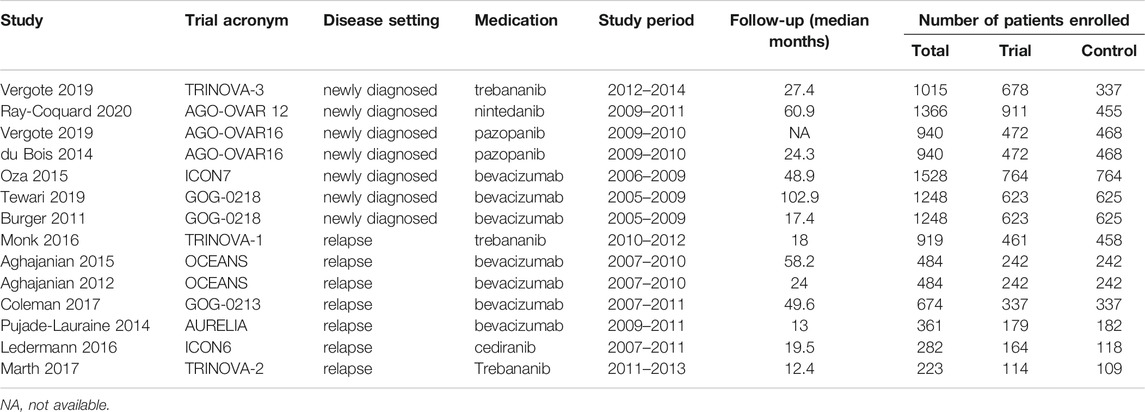
After the initial comprehensive search, a total of 1197 studies were retrieved. Using the abstracts or titles of the articles during the preliminary screening, 52 full-text papers were further scrutinized. Thirty-four publications were excluded because they were not phase III RCTs. Three studies (Perren et al., 2011; du Bois et al., 2016; Monk et al., 2014) were excluded because the survival outcome data were updated in the lately three studies with the same trials. One study was excluded because the anti-angiogenesis treatment trial was conducted in the subgroup of a rare gynecologic ovarian cancer (Gore et al., 2019). Eventually, 11 phase III RCTs were selected as they fulfilled all the study inclusion criteria. A total of 6097 patients with newly diagnosed ovarian cancer from 5 phase III RCTs and 2943 patients with relapsed ovarian cancer from 6 phase III RCTs were included in this meta-analysis. As the OS and PFS data of 3 trials were reported separately in different publications, we included 14 publications in our study (Figure 1). Five RCTs reported the results of patients with newly diagnosed ovarian cancer (anti-angiogenesis group = 3,448; placebo group = 2649; total = 6097 patients): ICON7, GOG-0218, AGO-OVAR16, AGO-OVAR 12, TRINOVA-3 (Ray-Coquard et al., 2020; Vergote et al., 2019a; Vergote et al., 2019b; du Bois et al., 2014; Tewari et al., 2019; Burger et al., 2011; Oza et al., 2015). The other 6 RCTs reported the results of patients with recurrent ovarian cancer (anti-angiogenesis group = 1497; placebo group = 1446; total = 2943 patients): OCEANS, ICON6, TRINOVA-1, TRINOVA-2, GOG-0213, AURELIA (Aghajanian et al., 2012; Pujade-Lauraine et al., 2014; Aghajanian et al., 2015; Monk et al., 2016b; Ledermann et al., 2016; Coleman et al., 2017; Marth et al., 2017). The risk of bias was globally low that was illustrated using “Risk of bias graph” (Supplementary Figure S1). Characteristics of the individual studies and enrolled population are summarized in Table 1.
Anti-Angiogenesis Maintenance Treatment in Newly Diagnosed Ovarian Cancer
Five trials (Ray-Coquard et al., 2020; Vergote et al., 2019a; Vergote et al., 2019b; du Bois et al., 2014; Tewari et al., 2019; Burger et al., 2011; Oza et al., 2015) reported the effects of anti-angiogenesis maintenance therapy in patients with newly diagnosed ovarian cancer. A total of 3,448 patients received chemotherapy followed by anti-angiogenesis maintenance therapy, whereas 2649 patients received maintenance therapy with placebo. There was no difference in OS between the two groups (HR, 0.98; 95% CI, 0.91–1.05; p = 0.49). However, the pooled results revealed a significant improvement in PFS for patients receiving anti-angiogenesis maintenance therapy compared with placebo (HR, 0.84; 95% CI, 0.76–0.93; p = 0.001) (Figure 2).
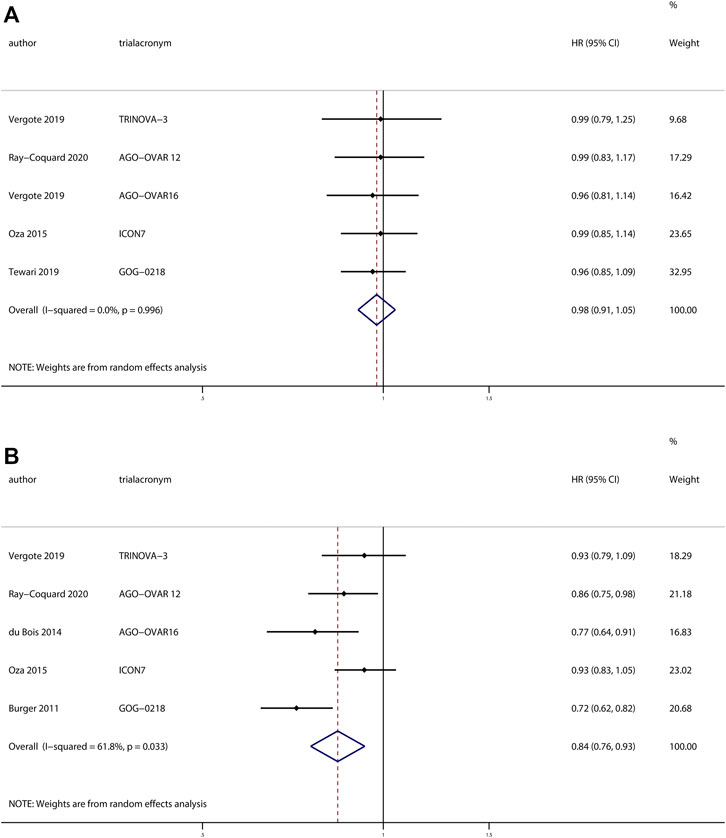
FIGURE 2. Anti-angiogenesis maintenance treatment vs placebo in newly diagnosed ovarian cancer. (A) Overall survival (OS). (B) Progression-free survival (PFS).
No heterogeneity was observed in OS (χ2 = 0.18, p > 0.99, I2 = 0%), but a moderate degree of heterogeneity existed in terms of PFS (χ2 = 10.48, p = 0.03, I2 = 61.8%), sensitivity analyses revealed the same results when we excluded individual studies one by one (Supplementary Figure S2).
Funnel plots showed little evidence of asymmetry in those assessing OS (Begg’s test, p > 0.99; Egger’s test, p = 0.42) and PFS (Begg’s test, p = 0.46; Egger’s test, p = 0.57), which revealed the absence of publication bias (Supplementary Figure S3).
Investigators of the ICON7 study defined the high-risk tumors (International Federation of Gynecology and Obstetrics [FIGO] stage IV, or FIGO stage III if residual tumor after debulking surgery was >1.0 cm), and investigators of three trials (Ray-Coquard et al., 2020; du Bois et al., 2014; Oza et al., 2015) reported the effects of anti-angiogenesis therapy in high-risk and non-high-risk patients. We evaluated these data into subgroup analyses. The results revealed a significant improvement in PFS for patients receiving anti-angiogenesis maintenance treatment (HR, 0.80; 95% CI, 0.65–0.97; p = 0.02), but no significant difference in OS (HR, 0.94; 95% CI, 0.65–1.36; p = 0.74) compared with placebo in high-risk patients. However, in the complementary subgroup of non-high-risk patients, there were no significant differences in OS (HR, 1.02; 95% CI, 0.80–1.29; p = 0.90) or PFS (HR, 0.87; 95% CI, 0.72–1.06; p = 0.17).
Among these trials, the AGO-OVAR16 trial evaluated 472 patients who received anti-angiogenesis maintenance treatment after completion of standard first-line chemotherapy, whereas the other four trials contained a total of 2976 patients who received anti-angiogenesis agents in combination with chemotherapy followed by anti-angiogenesis maintenance treatment. We pooled the results of the four trials and found a significant improvement in PFS for patients receiving anti-angiogenesis maintenance therapy (HR, 0.85; 95% CI, 0.76–0.96; p = 0.01), but no significant difference in OS (HR, 0.98; 95% CI, 0.90–1.06; p = 0.58) compared with placebo. The results of the subgroup analyses are depicted in Figure 3.
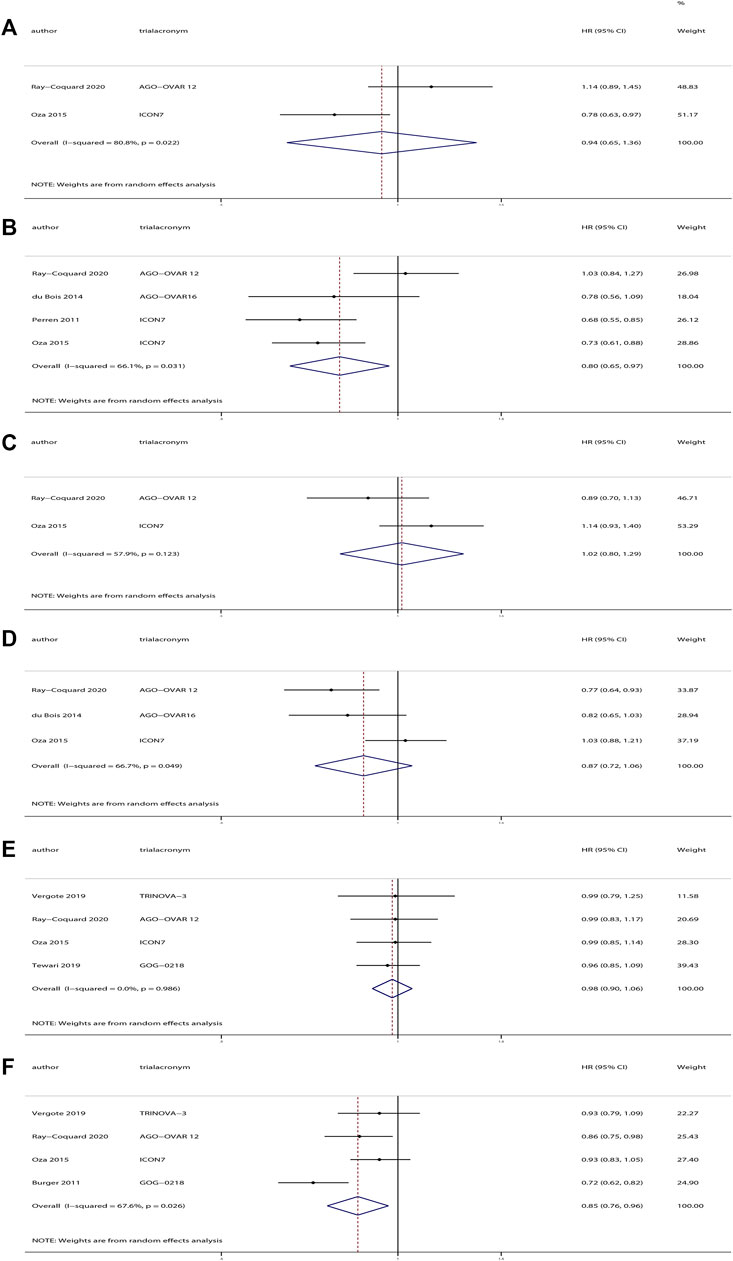
FIGURE 3. Subgroup analyses of anti-angiogenesis treatment vs placebo in newly diagnosed ovarian cancer. (A) Overall survival (OS) of patients with high-risk tumors. (B) Progression-free survival (PFS) of patients with high-risk tumors. (C) OS of patients with non-high-risk tumors. (D) PFS of patients with non-high-risk tumors. (E) OS of patients who received anti-angiogenesis treatment in combination with chemotherapy followed by anti-angiogenesis maintenance treatment vs. placebo. (F) PFS of patients who received anti-angiogenesis treatment in combination with chemotherapy followed by anti-angiogenesis maintenance treatment vs. placebo.
Anti-Angiogenesis Maintenance Treatment in Relapsed Ovarian Cancer
The investigators of six trials with 2943 patients reported the effects of anti-angiogenesis maintenance therapy for relapsed ovarian cancer. A total of 1497 patients with recurrent ovarian cancer received chemotherapy followed by anti-angiogenesis maintenance therapy, whereas 1446 women received placebo. The pooled results revealed a significant improvement in OS (HR, 0.89; 95% CI, 0.82–0.98; p = 0.02) and PFS (HR, 0.61; 95% CI, 0.52–0.72; p < 0.001) (Figure 4) between the two groups.
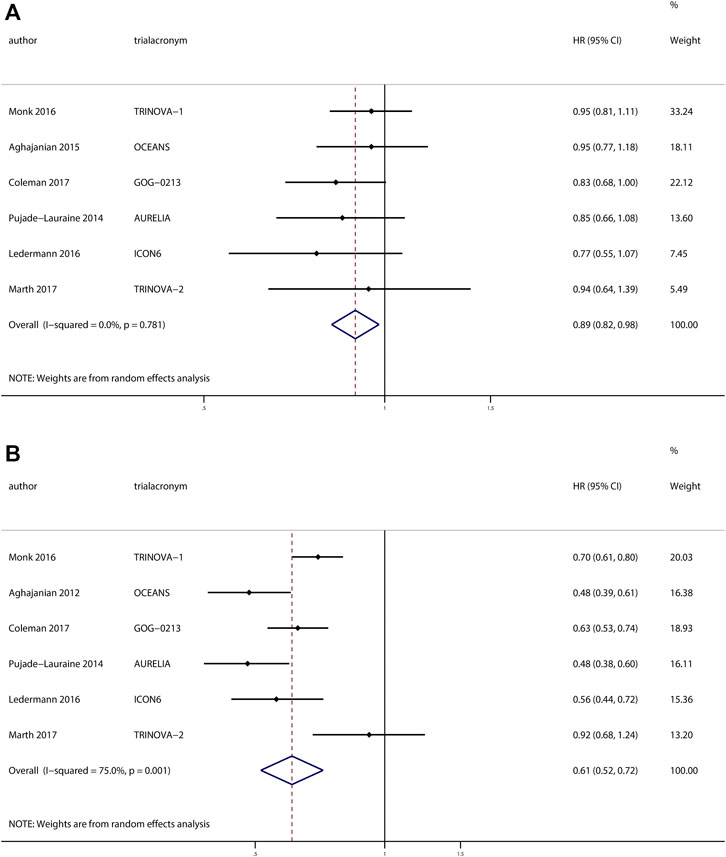
FIGURE 4. Anti-angiogenesis maintenance treatment vs placebo in relapsed ovarian cancer. (A) Overall survival (OS). (B) Progression-free survival (PFS).
No heterogeneity was observed in OS (χ2 = 2.47, p = 0.78, I2 = 0%), but a moderate degree of heterogeneity existed in terms of PFS (χ2 = 19.99, p = 0.001, I2 = 75.0%). We performed a sensitivity analysis based on the PFS and obtained the same results when we excluded studies one by one (Supplementary Figure S4).
Visual inspection of the funnel plots shows little evidence of asymmetry in plots assessing OS (Begg’s test, p = 0.45; Egger’s test, p = 0.43) and PFS (Begg’s test, p > 0.99; Egger’s test, p = 0.67), which revealed the absence of publication bias (Supplementary Figure S5).
Among these trials, patients from three trials (Aghajanian et al., 2012; Ledermann et al., 2016; Coleman et al., 2017) received platinum-based therapy followed by anti-angiogenesis for maintenance, whereas patients from the other three trials (Pujade-Lauraine et al., 2014; Monk et al., 2016b; Marth et al., 2017) received non–platinum-based therapy followed by anti-angiogenesis therapy for maintenance. Hence, we performed subgroup analyses based on platinum-based therapy or non–platinum-based therapy. With respect to OS, we observed a significant improvement in OS for patients receiving platinum-based therapy followed by anti-angiogenesis treatment for maintenance (HR, 0.86; 95% CI, 0.76–0.98; p = 0.03) compared with placebo, but no significant difference in OS for patients receiving non–platinum-based therapy followed by anti-angiogenesis treatment for maintenance (HR, 0.92; 95% CI, 0.81–1.05; p = 0.20) compared with placebo. At the same time, we found both an improved PFS (HR, 0.56; 95% CI, 0.48–0.66; p < 0.001) for patients receiving platinum-based therapy followed by anti-angiogenesis for maintenance compared with placebo and an improved PFS (HR, 0.67; 95% CI, 0.49–0.92; p = 0.01) for patients receiving non–platinum-based therapy followed by anti-angiogenesis for maintenance therapy compared with placebo.
Subgroups based on specific medications were also analyzed. Three trials (Aghajanian et al., 2012; Pujade-Lauraine et al., 2014; Coleman et al., 2017) researched bevacizumab, and the pooled results revealed a significant improvement in OS (HR, 0.87; 95% CI, 0.77–0.99; p = 0.03) and PFS (HR, 0.53; 95% CI, 0.44–0.65; p < 0.001) compared with placebo. However, trebananib and cediranib did not demonstrate superiority compared with placebo. The subgroup analysis results are depicted in Figure 5.
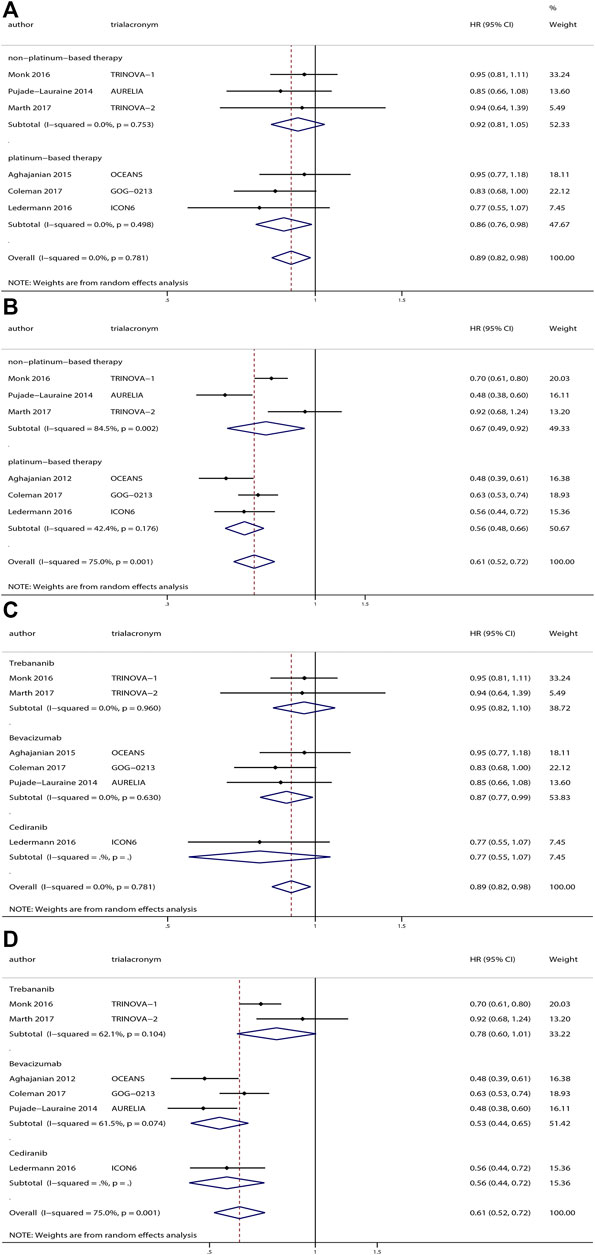
FIGURE 5. Subgroup analyses of anti-angiogenesis treatment vs placebo in relapsed ovarian cancer. Subgroup analyses based on platinum-based therapy or non–platinum-based therapy: (A) overall survival (OS) and (B) progression-free survival (PFS). Subgroup based on drugs: (C) OS and (D) PFS.
Safety of Anti-Angiogenesis Maintenance Therapy
All the included trials investigated the adverse events of anti-angiogenesis therapy in patients. The most common adverse events were hypertension, diarrhea, headache, and proteinuria. In addition, we pooled and analyzed the results from individual trials regarding the severe adverse events (Grade ≥3).
Of the five trials that evaluated patients with newly diagnosed ovarian cancer, we analyzed the results of severe adverse events. The pooled results revealed that there were significant differences in terms of severe hypertension, neutropenia, diarrhea, thrombocytopenia, headache, proteinuria, hypokalemia, and bleeding in patients with newly diagnosed ovarian cancer who received anti-angiogenesis therapy compared with placebo (Supplementary Figure S6).
The investigators of six trials reported adverse events of anti-angiogenesis maintenance therapy in patients with relapsed ovarian cancer, and we analyzed the results of severe adverse events. We observed significant differences in severe hypertension, headache, proteinuria, bleeding, localized edema, and ascites in patients with relapsed ovarian cancer patients who received anti-angiogenesis therapy compared with placebo (Supplementary Figure S7).
Regarding to the occurrence of severe nausea, vomiting, fatigue, abdominal pain, and asthenia, we observed no significant differences between the anti-angiogenesis and placebo groups regardless of whether or not the ovarian cancer was newly diagnosed or relapsed based on the pooled analyses.
Discussion
Ovarian cancer is usually detected during the advanced stages due to the atypical symptoms seen during earlier stages; it has the worst prognosis of all the gynecologic malignancies (Dinkelspiel et al., 2015). Almost all patients with advanced ovarian cancer will undergo relapse, and then chemoresistance commonly occurs (Roane et al., 2019). In previous years, targeted agents have been used to treat advanced ovarian cancer and have proved to be effective. These include the poly (ADP-ribose) polymerase (PARP) inhibitors (Tomao et al., 2019; Ruscito et al., 2020). At the same time, anti-angiogenesis maintenance therapy has been researched in some phase III trials, however, the reported results were not consistent.
Our meta-analysis included 11 trials enrolling a total of 9,040 patients with ovarian cancer. Five of these trials reported anti-angiogenesis maintenance therapy in newly diagnosed ovarian, and the other six trials researched anti-angiogenesis in relapsed ovarian cancer.
Each of the five trials we included reported PFS and the final OS results. We found that all of the trials reported no significant differences based on OS in patients with newly diagnosed ovarian cancer, although GOG-0218, AGO-OVAR 12, and AGO-OVAR16 observed that bevacizumab, nintedanib, or pazopanib, respectively, could improve the PFS. However, ICON7 and TRINOVA-3 did not observe an improved PFS. The ICON7 study performed a subgroup analysis of patients with stage IIIB to stage IV disease and found an improvement in PFS irrespective of residual cancer or stage status and an improved OS in high-risk patients (Oza et al., 2015). Trebananib was the targeted drug in newly diagnosed ovarian cancer in the TRINOVA-3 study, the results of which showed that it did not improve OS or PFS compared with placebo. Our pooled results indicated an improved PFS in patients with newly diagnosed ovarian cancer who received anti-angiogenesis maintenance therapy compared with placebo. However, there was no significant difference in OS in patients with newly diagnosed ovarian cancer between the anti-angiogenesis and placebo groups. In addition, we conducted subgroup analyses based on high-risk or non-high-risk tumours. We obtained similar results that anti-angiogenesis maintenance therapy could improve PFS but not OS in high-risk patients. However, in the subgroup analysis of non-high-risk patients, there were no significant differences in OS or PFS between the anti-angiogenesis and placebo groups. We also pooled the results of trials that combined anti-angiogenesis with first-line chemotherapy followed by anti-angiogenesis maintenance therapy, which revealed a significant improvement in PFS but not OS for patients receiving anti-angiogenesis maintenance compared with placebo.
From the previously mentioned results, we found that, although anti-angiogenesis treatment could improve PFS, patients with newly diagnosed ovarian cancer did not benefit from anti-angiogenesis treatment in terms of OS. The most likely explanation for this was that OS might be affected by many factors. Yang et al. found that patients with long-term survival likely have higher mutation frequency, such as BRCA mutation (Yang et al., 2018). Iwase et al. found that OS from the time of the first recurrence might be not associated with the initial FIGO stage because the OS could be prolonged in the patients with relapsed ovarian cancer who were sensitive to chemotherapy, regardless of the original stage (Iwase et al., 2015). Some authors have demonstrated that primary complete or optimal cytoreductive surgery (Chan et al., 2003; Kaern et al., 2005), preoperative disease burden (Horowitz et al., 2015; Hamilton et al., 2018; Javellana et al., 2019), multiple lines of prior therapy (Pignata et al., 2017), and disease-free interval are independent prognostic factors for OS (Kajiyama et al., 2012; Hoppenot et al., 2018). Unexpected crossover, inconsistent treatment, and survival time after progression may not reflect the real effect of new drugs on OS (Tewari et al., 2019). However, we couldn’t balance factors such as the patients’ genetic variant status, therapy after recurrence, or response to the chemotherapy when we performed the meta-analysis.
Although the pooled results didn’t support the role of anti-angiogenesis therapy in newly diagnosed ovarian cancer, OS has improved in the past several decades. The 5-year relative survival rate for ovarian cancer was 36% between 1975 and 1997. This rate has increased year by year, and it improved significantly to 49% between 2010 and 2016 (Siegel et al., 20152015). This improvement reflects the advances in treatment of ovarian cancer, including improved surgical interventions and multiple lines of therapy for recurrence. The US Food and Drug Administration (FDA) has reported that novel agents are approved depending on the PFS combined with comprehensive factors of quality of life and toxicities (Herzog et al., 2017). As an improved PFS was observed in our meta-analysis, this may be a possible reason for application of anti-angiogenesis treatment in these patients.
When we performed analyses of patients with relapsed ovarian cancer, we found anti-angiogenesis maintenance therapy could improve both OS and PFS compared with placebo. All the trials reported a significantly improved PFS in patients who received anti-angiogenesis maintenance therapy except for the TRINOVA-2 trial. Although the individual trials didn’t observe an improved OS, we found a significant improvement in OS when we pooled all the individual results. At the same time, there was no heterogeneity or publication bias observed in OS. The most likely explanation is that when increasing the sample size, the efficiency was improved which reduced the possibility of a false negative.
In the subgroup analyses, OCEANS, GOG-0213, and ICON6 administered anti-angiogenesis treatment to patients who received platinum-based therapy, whereas patients in the TRINOVA-1, AURELIA, and TRINOVA-2 trials received non–platinum-based therapy. The pooled results revealed that anti-angiogenesis maintenance therapy could improve PFS both in patients who received platinum-based and non–platinum-based therapy. However, an improved OS was observed in patients who received platinum-based therapy plus anti-angiogenesis maintenance therapy but not in patients who received non–platinum-based therapy. The TRINOVA-1 and TRINOVA-2 trials evaluated trebananib plus paclitaxel or pegylated liposomal doxorubicin, respectively. An improved PFS was observed in TRINOVA-1 but not in TRINOVA-2. The ICON6 trial found an improved PFS in patients who received platinum-based therapy followed by treatment with cediranib. The previous review included two RCTs of bevacizumab use in relapsed ovarian cancer, and found a statistically significant improvement in PFS but not in OS (Rossi et al., 2017). In our meta-analysis, three trials (Aghajanian et al., 2012; Pujade-Lauraine et al., 2014; Coleman et al., 2017)researched bevacizumab as maintenance therapy, and we pooled the results which revealed a significant improvement in both PFS and OS in patients with relapsed ovarian cancer.
Stark et al. reported no decreased health-related quality of life in patients with relapsed ovarian cancer who received anti-angiogenesis therapy (Stark et al., 2017). Higher frequency of serious hypertension occurred in older patients who received bevacizumab therapy (Sorio et al., 2017). In a meta-analysis of bevacizumab treatment for advanced or metastatic breast cancer, the quality of life was similar between the bevacizumab and control groups (Miyashita et al., 2020).
All the trials we included compared the adverse events between anti-angiogenesis treatment and placebo. In the ICON7, OCEANS, GOG-0213, and AURELIA trials, a higher frequency of severe adverse events occurred in patients who received bevacizumab. But the occurrence rate of fatal adverse events was not significant difference between bevacizumab and placebo groups. And in the GOG-0218 trial, there were no significant differences in the occurrence rate of fatal adverse events between the bevacizumab group (2.3%) and the control group (1.0%). In the TRINOVA-1, TRINOVA-2, and TRINOVA-3 trials, the occurrence rate of severe adverse events was similar in the trebananib and placebo group. In the AGO-OVAR 12 trial, more severe adverse events were observed in patients who received nintedanib (81%) than in those who received placebo (67%). However, the occurrence of fatal adverse events was similar in the two groups (3 vs. 4%, respectively). In the AGO-OVAR16 trial, some severe adverse events were more frequently observed in the pazopanib group, such as hypertension, neutropenia, diarrhea, fatigue, and thrombocytopenia. However, the occurrence rate of fatal adverse events was not significant. In the ICON6 trial, the incidence of drug discontinuation was higher in the cediranib group. Most trials reported a grade of greater than or equal to 3 adverse events including hypertension, neutropenia, nausea, fatigue, diarrhea, thrombocytopenia, headache, proteinuria, and dyspnea. We pooled the results and found that severe hypertension, neutropenia, diarrhea, thrombocytopenia, headache, proteinuria, hypokalemia, and bleeding were more frequently observed in patients with newly diagnosed ovarian cancer and that severe hypertension, headache, proteinuria, bleeding, localized edema, and ascites were more likely to occur in patients with relapsed ovarian cancer who received anti-angiogenesis treatment compared with placebo. However, all the trials reported infrequent fatal adverse events in both groups.
To the best of our knowledge, this meta-analysis was the first to assess the effects of anti-angiogenesis maintenance therapy in both patients with newly diagnosed and relapsed ovarian cancer. All the studies included in the meta-analysis were well-designed, high-quality, phase III RCTs.
Inevitably, there are some limitations in our meta-analysis. First, potential heterogeneity existed among the individual studies for the inconsistent tumor characteristics of the enrolled population, such as preoperative disease burden, tumor stage, residual tumor after primary surgery, multiple lines of prior therapy, or disease-free interval; these characteristics were not consistent among the different trials. Because the number of studies was limited, we could not perform stratified pooled analyses for each characteristic. Second, the patients in different trials were administered different anti-angiogenesis drugs. We couldn’t pool the results of studies involving the same drugs due to the limited numbers of trials, except for bevacizumab in relapsed ovarian cancer. Third, the majority of included trials reported OS, which was defined as death regardless of cause. The disease-specific OS is more persuasive, however, we could not obtain this from the included trials. Finally, the number of the trials performed on anti-angiogenesis maintenance therapy in ovarian cancer was limited, and more high-quality phase III RCTs are needed to confirm or update our conclusion. Due to the conflicting results of PFS and OS, future RCTs would be better designed to unify the tumor characteristics of ovarian cancer patients, such as histopathologic type and residual tumors, in an attempt to find the best indication for anti-angiogenesis use.
Conclusion
The results of our study suggest that anti-angiogenesis maintenance therapy may be associated with improved PFS and OS in patients with relapsed ovarian cancer. However, in patients with newly diagnosed ovarian cancer, anti-angiogenesis therapy may be associated with improved PFS, but not OS. Infrequent fatal adverse events occurred in the anti-angiogenesis and placebo groups.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
YW and YL conceived the manuscript idea and designed the study methodology. YW, SZ, and ZS performed the literature search and data collection. LO and YL screened full texts and analyzed data. YW wrote the manuscript. All authors read and approved the final manuscript.
Funding
YW has received research funding by 345 Talent Project from Shengjing Hospital of China Medical University.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.726278/full#supplementary-material
Supplementary Figure 1 | Risk of bias graph. (A) Newly diagnosed ovarian cancer. (B) Relapsed ovarian cancer.
Supplementary Figure 2 | Sensitivity analyses of the pooled analysis of anti-angiogenesis treatment vs placebo in newly diagnosed ovarian cancer in terms of progression-free survival (PFS).
Supplementary Figure 3 | Publication bias for overall survival (OS) analysis of anti-angiogenesis treatment vs placebo in newly diagnosed ovarian cancer. (A) Begg’s test. (B) Egger’s test. Publication bias for progression-free survival (PFS) analysis. (C) Begg’s test. (D) Egger’s test.
Supplementary Figure 4 | Sensitivity analyses of the pooled analysis of anti-angiogenesis treatment vs. placebo in relapsed ovarian cancer in terms of progression-free survival (PFS).
Supplementary Figure 5 | Publication bias for overall survival (OS) analysis of anti-angiogenesis treatment vs placebo in relapsed ovarian cancer. (A) Begg’s test. (B) Egger’s test. Publication bias for progression-free survival (PFS) analysis. (C) Begg’s test. (D) Egger’s test.
Supplementary Figure 6 | Severe adverse events of anti-angiogenesis maintenance treatment vs. placebo in newly diagnosed ovarian cancer.
Supplementary Figure 7 | Severe adverse events of anti-angiogenesis maintenance treatment vs. placebo in relapsed ovarian cancer.
References
Aghajanian, C., Blank, S. V., Goff, B. A., Judson, P. L., Teneriello, M. G., Husain, A., et al. (2012). OCEANS: a Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Chemotherapy with or without Bevacizumab in Patients with Platinum-Sensitive Recurrent Epithelial Ovarian, Primary Peritoneal, or Fallopian Tube Cancer. J. Clin. Oncol. 30 (17), 2039–2045. doi:10.1200/JCO.2012.42.0505
Aghajanian, C., Goff, B., Nycum, L. R., Wang, Y. V., Husain, A., and Blank, S. V. (2015). Final Overall Survival and Safety Analysis of OCEANS, a Phase 3 Trial of Chemotherapy with or without Bevacizumab in Patients with Platinum-Sensitive Recurrent Ovarian Cancer. Gynecol. Oncol. 139 (1), 10–16. doi:10.1016/j.ygyno.2015.08.004
Bartoletti, M., Pelizzari, G., Gerratana, L., Bortot, L., Lombardi, D., Nicoloso, M., et al. (2020). Bevacizumab or PARP-Inhibitors Maintenance Therapy for Platinum-Sensitive Recurrent Ovarian Cancer: A Network Meta-Analysis. Int. J. Mol. Sci. 21 (11). doi:10.3390/ijms21113805
Begg, C. B., and Mazumdar, M. (1994). Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics 50 (4), 1088–1101. doi:10.2307/2533446
Burger, R. A., Brady, M. F., Bookman, M. A., Fleming, G. F., Monk, B. J., Huang, H., et al. (2011). Incorporation of Bevacizumab in the Primary Treatment of Ovarian Cancer. N. Engl. J. Med. 365 (26), 2473–2483. doi:10.1056/NEJMoa1104390
Chan, J. K., Loizzi, V., Lin, Y. G., Osann, K., Brewster, W. R., and DiSaia, P. J. (2003). Stages III and IV Invasive Epithelial Ovarian Carcinoma in Younger versus Older Women: what Prognostic Factors Are Important? Obstet. Gynecol. 102 (1), 156–161. doi:10.1016/s0029-7844(03)00399-5
Coleman, R. L., Brady, M. F., Herzog, T. J., Sabbatini, P., Armstrong, D. K., Walker, J. L., et al. (2017). Bevacizumab and Paclitaxel-Carboplatin Chemotherapy and Secondary Cytoreduction in Recurrent, Platinum-Sensitive Ovarian Cancer (NRG Oncology/Gynecologic Oncology Group Study GOG-0213): a Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 18 (6), 779–791. doi:10.1016/S1470-2045(17)30279-6
Coleman, R. L., Monk, B. J., Sood, A. K., and Herzog, T. J. (2013). Latest Research and Treatment of Advanced-Stage Epithelial Ovarian Cancer. Nat. Rev. Clin. Oncol. 10 (4), 211–224. doi:10.1038/nrclinonc.2013.5
Copas, J., and Shi, J. Q. (2000). Meta-analysis, Funnel Plots and Sensitivity Analysis. Biostatistics 1 (3), 247–262. doi:10.1093/biostatistics/1.3.247
Coxon, A., Bready, J., Min, H., Kaufman, S., Leal, J., Yu, D., et al. (2010). Context-dependent Role of Angiopoietin-1 Inhibition in the Suppression of Angiogenesis and Tumor Growth: Implications for AMG 386, an Angiopoietin-1/2-Neutralizing Peptibody. Mol. Cancer Ther. 9 (10), 2641–2651. doi:10.1158/1535-7163.MCT-10-0213
DerSimonian, R., and Laird, N. (1986). Meta-analysis in Clinical Trials. Control. Clin. Trials 7 (3), 177–188. doi:10.1016/0197-2456(86)90046-2
Dinkelspiel, H. E., Champer, M., Hou, J., Tergas, A., Burke, W. M., Huang, Y., et al. (2015). Long-term Mortality Among Women with Epithelial Ovarian Cancer. Gynecol. Oncol. 138 (2), 421–428. doi:10.1016/j.ygyno.2015.06.005
du Bois, A., Floquet, A., Kim, J. W., Rau, J., del Campo, J. M., Friedlander, M., et al. (2014). Incorporation of Pazopanib in Maintenance Therapy of Ovarian Cancer. J. Clin. Oncol. 32 (30), 3374–3382. doi:10.1200/JCO.2014.55.7348
du Bois, A., Kristensen, G., Ray-Coquard, I., Reuss, A., Pignata, S., Colombo, N., et al. (2016). Standard First-Line Chemotherapy with or without Nintedanib for Advanced Ovarian Cancer (AGO-OVAR 12): a Randomised, Double-Blind, Placebo-Controlled Phase 3 Trial. Lancet Oncol. 17 (1), 78–89. doi:10.1016/S1470-2045(15)00366-6
Egger, M., Davey Smith, G., Schneider, M., and Minder, C. (1997). Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ 315 (7109), 629–634. doi:10.1136/bmj.315.7109.629
Falcón, B. L., Hashizume, H., Koumoutsakos, P., Chou, J., Bready, J. V., Coxon, A., et al. (2009). Contrasting Actions of Selective Inhibitors of Angiopoietin-1 and Angiopoietin-2 on the Normalization of Tumor Blood Vessels. Am. J. Pathol. 175 (5), 2159–2170. doi:10.2353/ajpath.2009.090391
Ferrara, N., and Adamis, A. P. (2016). Ten Years of Anti-vascular Endothelial Growth Factor Therapy. Nat. Rev. Drug Discov. 15 (6), 385–403. doi:10.1038/nrd.2015.17
Gavalas, N. G., Liontos, M., Trachana, S. P., Bagratuni, T., Arapinis, C., Liacos, C., et al. (2013). Angiogenesis-related Pathways in the Pathogenesis of Ovarian Cancer. Int. J. Mol. Sci. 14 (8), 15885–15909. doi:10.3390/ijms140815885
Gore, M., Hackshaw, A., Brady, W. E., Penson, R. T., Zaino, R., McCluggage, W. G., et al. (2019). An International, Phase III Randomized Trial in Patients with Mucinous Epithelial Ovarian Cancer (mEOC/GOG 0241) with Long-Term Follow-Up: and Experience of Conducting a Clinical Trial in a Rare Gynecological Tumor. Gynecol. Oncol. 153 (3), 541–548. doi:10.1016/j.ygyno.2019.03.256
Hamilton, C. A., Miller, A., Casablanca, Y., Horowitz, N. S., Rungruang, B., Krivak, T. C., et al. (2018). Clinicopathologic Characteristics Associated with Long-Term Survival in Advanced Epithelial Ovarian Cancer: an NRG Oncology/Gynecologic Oncology Group Ancillary Data Study. Gynecol. Oncol. 148 (2), 275–280. doi:10.1016/j.ygyno.2017.11.018
Herzog, T. J., Ison, G., Alvarez, R. D., Balasubramaniam, S., Armstrong, D. K., Beaver, J. A., et al. (2017). FDA Ovarian Cancer Clinical Trial Endpoints Workshop: A Society of Gynecologic Oncology White Paper. Gynecol. Oncol. 147 (1), 3–10. doi:10.1016/j.ygyno.2017.08.012
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's Tool for Assessing Risk of Bias in Randomised Trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring Inconsistency in Meta-Analyses. BMJ 327 (7414), 557–560. doi:10.1136/bmj.327.7414.557
Hoppenot, C., Eckert, M. A., Tienda, S. M., and Lengyel, E. (2018). Who Are the Long-Term Survivors of High Grade Serous Ovarian Cancer? Gynecol. Oncol. 148 (1), 204–212. doi:10.1016/j.ygyno.2017.10.032
Horowitz, N. S., Miller, A., Rungruang, B., Richard, S. D., Rodriguez, N., Bookman, M. A., et al. (2015). Does Aggressive Surgery Improve Outcomes? Interaction between Preoperative Disease burden and Complex Surgery in Patients with Advanced-Stage Ovarian Cancer: an Analysis of GOG 182. J. Clin. Oncol. 33 (8), 937–943. doi:10.1200/JCO.2014.56.3106
Iwase, H., Takada, T., Iitsuka, C., Nomura, H., Abe, A., Taniguchi, T., et al. (2015). Clinical Features of Long-Term Survivors of Recurrent Epithelial Ovarian Cancer. Int. J. Clin. Oncol. 20 (1), 143–149. doi:10.1007/s10147-014-0687-1
Javellana, M., Hoppenot, C., and Lengyel, E. (2019). The Road to Long-Term Survival: Surgical Approach and Longitudinal Treatments of Long-Term Survivors of Advanced-Stage Serous Ovarian Cancer. Gynecol. Oncol. 152 (2), 228–234. doi:10.1016/j.ygyno.2018.11.007
Jayson, G. C., Kerbel, R., Ellis, L. M., and Harris, A. L. (2016). Antiangiogenic Therapy in Oncology: Current Status and Future Directions. Lancet 388 (10043), 518–529. doi:10.1016/S0140-6736(15)01088-0
Kaern, J., Aghmesheh, M., Nesland, J. M., Danielsen, H. E., Sandstad, B., Friedlander, M., et al. (2005). Prognostic Factors in Ovarian Carcinoma Stage III Patients. Can Biomarkers Improve the Prediction of Short- and Long-Term Survivors? Int. J. Gynecol. Cancer 15 (6), 1014–1022. doi:10.1111/j.1525-1438.2005.00185.x
Kajiyama, H., Shibata, K., Mizuno, M., Umezu, T., Suzuki, S., Yamamoto, E., et al. (2012). Long-term Clinical Outcome of Patients with Recurrent Epithelial Ovarian Carcinoma: Is it the Same for Each Histological Type? Int. J. Gynecol. Cancer 22 (3), 394–399. doi:10.1097/IGC.0b013e31823eed2c
Ledermann, J. A., Embleton, A. C., Raja, F., Perren, T. J., Jayson, G. C., Rustin, G. J. S., et al. (2016). Cediranib in Patients with Relapsed Platinum-Sensitive Ovarian Cancer (ICON6): a Randomised, Double-Blind, Placebo-Controlled Phase 3 Trial. Lancet 387 (10023), 1066–1074. doi:10.1016/S0140-6736(15)01167-8
Marth, C., Vergote, I., Scambia, G., Oberaigner, W., Clamp, A., Berger, R., et al. (2017). ENGOT-ov-6/TRINOVA-2: Randomised, Double-Blind, Phase 3 Study of Pegylated Liposomal Doxorubicin Plus Trebananib or Placebo in Women with Recurrent Partially Platinum-Sensitive or Resistant Ovarian Cancer. Eur. J. Cancer 70, 111–121. doi:10.1016/j.ejca.2016.09.004
Miyashita, M., Hattori, M., Takano, T., Toyama, T., and Iwata, H. (2020). Risks and Benefits of Bevacizumab Combined with Chemotherapy for Advanced or Metastatic Breast Cancer: a Meta-Analysis of Randomized Controlled Trials. Breast Cancer 27 (3), 347–354. doi:10.1007/s12282-020-01052-9
Monk, B. J., Minion, L. E., and Coleman, R. L. (2016). Anti-angiogenic Agents in Ovarian Cancer: Past, Present, and Future. Ann. Oncol. 27, i33–i39. doi:10.1093/annonc/mdw093
Monk, B. J., Poveda, A., Vergote, I., Raspagliesi, F., Fujiwara, K., Bae, D. S., et al. (2014). Anti-angiopoietin Therapy with Trebananib for Recurrent Ovarian Cancer (TRINOVA-1): a Randomised, Multicentre, Double-Blind, Placebo-Controlled Phase 3 Trial. Lancet Oncol. 15 (8), 799–808. doi:10.1016/S1470-2045(14)70244-X
Monk, B. J., Poveda, A., Vergote, I., Raspagliesi, F., Fujiwara, K., Bae, D. S., et al. (2016). Final Results of a Phase 3 Study of Trebananib Plus Weekly Paclitaxel in Recurrent Ovarian Cancer (TRINOVA-1): Long-Term Survival, Impact of Ascites, and Progression-free Survival-2. Gynecol. Oncol. 143 (1), 27–34. doi:10.1016/j.ygyno.2016.07.112
Oza, A. M., Cook, A. D., Pfisterer, J., Embleton, A., Ledermann, J. A., Pujade-Lauraine, E., et al. (2015). Standard Chemotherapy with or without Bevacizumab for Women with Newly Diagnosed Ovarian Cancer (ICON7): Overall Survival Results of a Phase 3 Randomised Trial. Lancet Oncol. 16 (8), 928–936. doi:10.1016/S1470-2045(15)00086-8
Perren, T. J., Swart, A. M., Pfisterer, J., Ledermann, J. A., Pujade-Lauraine, E., Kristensen, G., et al. (2011). A Phase 3 Trial of Bevacizumab in Ovarian Cancer. N. Engl. J. Med. 365 (26), 2484–2496. doi:10.1056/NEJMoa1103799
Pignata, S., Scambia, G., Bologna, A., Signoriello, S., Vergote, I. B., Wagner, U., et al. (2017). Randomized Controlled Trial Testing the Efficacy of Platinum-free Interval Prolongation in Advanced Ovarian Cancer: The MITO-8, MaNGO, BGOG-Ov1, AGO-Ovar2.16, ENGOT-Ov1, GCIG Study. J. Clin. Oncol. 35 (29), 3347–3353. doi:10.1200/JCO.2017.73.4293
Pujade-Lauraine, E., Hilpert, F., Weber, B., Reuss, A., Poveda, A., Kristensen, G., et al. (2014). Bevacizumab Combined with Chemotherapy for Platinum-Resistant Recurrent Ovarian Cancer: The AURELIA Open-Label Randomized Phase III Trial. J. Clin. Oncol. 32 (13), 1302–1308. doi:10.1200/JCO.2013.51.4489
Ray-Coquard, I., Cibula, D., Mirza, M. R., Reuss, A., Ricci, C., Colombo, N., et al. (2020). Final Results from GCIG/ENGOT/AGO-OVAR 12, a Randomised Placebo-Controlled Phase III Trial of Nintedanib Combined with Chemotherapy for Newly Diagnosed Advanced Ovarian Cancer. Int. J. Cancer 146 (2), 439–448. doi:10.1002/ijc.32606
Roane, B. M., Arend, R. C., and Birrer, M. J. (2019). Review: Targeting the Transforming Growth Factor-Beta Pathway in Ovarian Cancer. Cancers (Basel) 11 (5). doi:10.3390/cancers11050668
Rossi, L., Verrico, M., Zaccarelli, E., Papa, A., Colonna, M., Strudel, M., et al. (2017). Bevacizumab in Ovarian Cancer: A Critical Review of Phase III Studies. Oncotarget 8 (7), 12389–12405. doi:10.18632/oncotarget.13310
Ruscito, I., Bellati, F., Ray-Coquard, I., Mirza, M. R., du Bois, A., Gasparri, M. L., et al. (2020). Incorporating Parp-Inhibitors in Primary and Recurrent Ovarian Cancer: A Meta-Analysis of 12 Phase II/III Randomized Controlled Trials. Cancer Treat. Rev. 87, 102040. doi:10.1016/j.ctrv.2020.102040
Siegel, R. L., Miller, K. D., and Jemal, A. (20152015). Cancer Statistics, 2015. CA Cancer J. Clin. 65 (1), 5–29. doi:10.3322/caac.21254
Siegel, R. L., Miller, K. D., Fuchs, H. E., and Jemal, A.Cancer Statistics (2021). Cancer Statistics, 2021. CA A. Cancer J. Clin. 71 (1), 7–33. doi:10.3322/caac.21654
Sorio, R., Roemer-Becuwe, C., Hilpert, F., Gibbs, E., García, Y., Kaern, J., et al. (2017). Safety and Efficacy of Single-Agent Bevacizumab-Containing Therapy in Elderly Patients with Platinum-Resistant Recurrent Ovarian Cancer: Subgroup Analysis of the Randomised Phase III AURELIA Trial. Gynecol. Oncol. 144 (1), 65–71. doi:10.1016/j.ygyno.2016.11.006
Stark, D. P., Cook, A., Brown, J. M., Brundage, M. D., Embleton, A. C., Kaplan, R. S., et al. (2017). Quality of Life with Cediranib in Relapsed Ovarian Cancer: The ICON6 Phase 3 Randomized Clinical Trial. Cancer 123 (14), 2752–2761. doi:10.1002/cncr.30657
Tewari, K. S., Burger, R. A., Enserro, D., Norquist, B. M., Swisher, E. M., Brady, M. F., et al. (2019). Final Overall Survival of a Randomized Trial of Bevacizumab for Primary Treatment of Ovarian Cancer. J. Clin. Oncol. 37 (26), 2317–2328. doi:10.1200/JCO.19.01009
Tomao, F., Bardhi, E., Di Pinto, A., Sassu, C. M., Biagioli, E., Petrella, M. C., et al. (2019). Parp Inhibitors as Maintenance Treatment in Platinum Sensitive Recurrent Ovarian Cancer: An Updated Meta-Analysis of Randomized Clinical Trials According to BRCA Mutational Status. Cancer Treat. Rev. 80, 101909. doi:10.1016/j.ctrv.2019.101909
Vergote, I., du Bois, A., Floquet, A., Rau, J., Kim, J. W., Del Campo, J. M., et al. (2019). Overall Survival Results of AGO-OVAR16: A Phase 3 Study of Maintenance Pazopanib versus Placebo in Women Who Have Not Progressed after First-Line Chemotherapy for Advanced Ovarian Cancer. Gynecol. Oncol. 155 (2), 186–191. doi:10.1016/j.ygyno.2019.08.024
Vergote, I., Scambia, G., O'Malley, D. M., Van Calster, B., Park, S. Y., Del Campo, J. M., et al. (2019). Trebananib or Placebo Plus Carboplatin and Paclitaxel as First-Line Treatment for Advanced Ovarian Cancer (TRINOVA-3/engot-ov2/GOG-3001): a Randomised, Double-Blind, Phase 3 Trial. Lancet Oncol. 20 (6), 862–876. doi:10.1016/S1470-2045(19)30178-0
Keywords: anti-angiogenesis, meta-analysis, newly diagnosed ovarian cancer, relapsed ovarian cancer, adverse events
Citation: Wang Y, Zhang S, Song Z, Ouyang L and Li Y (2021) Anti-Angiogenesis Maintenance Therapy in Newly Diagnosed and Relapsed Ovarian Cancer: A Meta-analysis of Phase III Randomized Controlled Trials. Front. Pharmacol. 12:726278. doi: 10.3389/fphar.2021.726278
Received: 16 June 2021; Accepted: 29 October 2021;
Published: 17 November 2021.
Edited by:
Robert Clarke, University of Minnesota Twin Cities, United StatesReviewed by:
Federica Tomao, European Institute of Oncology (IEO), ItalyJanina Markowska, Poznan University of Medical Sciences, Poland
Copyright © 2021 Wang, Zhang, Song, Ouyang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Li, bGVleWFuODg4OEB5ZWFoLm5ldA==
 Yizi Wang
Yizi Wang Shitai Zhang
Shitai Zhang Zixuan Song
Zixuan Song Ling Ouyang
Ling Ouyang Yan Li
Yan Li