- 1The First Clinical College, Anhui Medical University, Hefei, China
- 2Department of Nephrology, Second Affiliated Hospital of Anhui Medical University, Hefei, China
- 3Department of Epidemiology and Biostatistics, School of Public Health, Anhui Medical University, Hefei, China
- 4Inflammation and Immune Mediated Diseases Laboratory of Anhui Province, Hefei, China
Background: Although multiple randomized controlled trials (RCTs) and systematic review and meta-analysis were performed to investigate the efficiency and safety of nonsteroidal anti-inflammatory drugs (NSAIDs) and opioids in the treatment of acute renal colic, the therapeutic regimen of renal colic is still controversial. Therefore, the aim of this study was to derive a more concise comparison of the effectiveness and safety between NSAIDs and opioids in the treatment for patients with acute renal colic by a systematic review and meta-analysis.
Design: We searched PubMed, Embase, and Cochrane Central Register of controlled trials for seeking eligible studies. The pooled mean difference (MD) or risk ratio (RR) with 95% confidence interval (CI) was calculated using the random effects model. The primary outcome was assessed according to the Grading of Recommendations Assessment, Development and Evaluation.
Results: A total of 18 studies involving 3,121 participants were included in the systematic review and meta-analysis. No significant difference between the NSAID and opioid groups was observed, with changes in the visual analog scale (VAS) at 0–30 min (MD = 0.79, 95% CI: −0.51, 2.10). NSAIDs in the form of intravenous administration (IV) had no better effect on the changes in the VAS at 0–30 min, when compared to opioids (MD = 1.25, 95% Cl: −4.81, 7.3). The NSAIDs group in the form of IV had no better outcome compared to the opioids group, as well as the VAS at 30 min (MD = −1.18, 95% Cl: −3.82, 1.45; MD = −2.3, 95% Cl: −5.02, 0.42, respectively). Moreover, similar results of this outcome were also seen with the VAS at 45 min (MD = −1.36, 95% Cl: −5.24, 2.52). Besides, there was a statistical difference in the incidence of later rescue (RR = 0.76, 95% CI: 0.66, 0.89), drug-related adverse events (RR = 0.44, 95% CI: 0.27, 0.71), and vomiting (RR = 0.68, 95% CI: 0.49, 0.96).
Conclusion: There is no significant difference between the NSAIDs and opioids in the treatment of renal colic in many outcomes (e.g., the VAS over different periods using different injection methods at 30 and 60 min), which has been focused on in this study. However, the patients who were treated using NSAIDs by clinicians can benefit from fewer side effects.
Introduction
Renal colic, a sort of pain that is hard to bear for patients when it attacks, along with a high incidence of 0.5% each year in Europe and North America, is characterized by pain in the waist or upper abdomen with paroxysmal attack (Patti and Leslie, 2021). Timely pain management is usually performed by using nonsteroidal anti-inflammatory drugs (NSAIDs) and with opioids as a drug of choice for renal colic patients, for reducing acute and unbearable pain in patients who have been severely affected by renal colic (Schmidt and Kroeger, 2016; Zamanian et al., 2016). Nevertheless, the antinociceptive effects of both these drugs are different, as is the pharmacological mechanism. For instance, NSAIDs have been associated with inhibiting the release of prostaglandins, further reducing the pressure of kidneys in order to increase the diuretic effect (Larkin et al., 1999).
However, the application of NSAIDs or opioids depends on the clinical workers' preference to these drugs (Sackner et al., 1974). A study of Passik (2009) indicated that this reluctance to their application may be caused by worries that are relevant to the possible adverse events and other potential risks, including fatal opioid overdose, the development of tolerance and dependence syndrome, and the harmful use of opioid and diversion. Some previous studies have revealed that renal colic patients could get more benefits from applying NSAIDs compared to opioids (Masoumi et al., 2014; Rezaei et al., 2020). Meanwhile, randomized controlled trials (RCTs) have shown a completely opposite conclusion, which hold the view that NSAIDs has a similar analgesic effect to opioids for pain relief in the management of renal colic (Majidi and Derakhshani, 2020).
Compared with a previous meta-analysis on a similar topic, this study has the advantage of including more new RCTs, more abundant outcome indicators, and a detailed collection of analgesic effects of two different drugs at different times in important outcomes, which is believed to play a guiding role in clinical practice.
Moreover, there are a variety of problems about the agents used clinically in the treatment of acute renal colic, that is, the routes of administration [intramuscular administration (IM) and intravenous administration (IV)], dosage of agents, and the safety and effectiveness of agents appropriate on patients with acute renal colic (Marthak et al., 1991). Relevant studies have shown that different routes of administration for pain management could have an influence on the adverse events and others (Fanelli et al., 2014; Daoust et al., 2015). Therefore, based on the uncertainty of pain management of NSAIDs and opioids for renal colic patients, this systematic review and meta-analysis was performed to investigate the efficacy and safety of NSAIDs and opioids in the treatment of renal colic and obtain an optimal management for patients, with less adverse events under the premise of relieving pain.
Methods
Search Strategy
Three electronic databases, including PUBMED, Embase, and Cochrane Central Register of controlled trials, were screened up to February 1, 2021, for seeking eligible studies on comparison of efficacy and safety of NSAIDs and opioids in patients undergoing treatment for renal colic. The medical terms and keywords we used are as follows: “renal colic,” “Nonsteroidal drugs,” “non-steroidal anti-inflammatory drugs,” and “opioids.” The detailed search strategies are shown in Supplementary Method S1.
Inclusion and Exclusion Criteria
We screened relevant RCT studies according to the following criteria: 1) all the patients with acute renal colic involved in the study were older than 16 years; 2) patients were administered only NSAIDs and opioids in the treatment of renal colic without a request of dose or route of drugs; 3) sufficient data were available about outcomes such as changes in the visual analog scale (VAS) or numerical rating scale (NRS) at 0–30 min, the VAS or NRS over different time periods (at 15, 30, 45, 60 min), need for rescue analgesia, and adverse events involving vomit and dizziness.
Patients' pain was assessed by using the VAS or NRS, which rates the amount of pain from 0 to 10, where 0 means no pain and 10 means the most pain. The adverse drug reactions include the overall incidence of adverse drug events and the incidence of dizziness and vomiting.
The exclusion criteria were as follows: 1) participants presenting to the hospital because of acute renal colic with more than 12 h after disease onset; 2) duplicate studies; 3) data were not available.
Data Extraction
Titles and abstracts of the articles were screened according to the preset inclusion and exclusion criteria. Subsequently, the eligible studies were registered through reading of the full text of the articles. Two authors (Xie-Yuan Leng and Chang-Ning Liu) individually collected the data and information from included studies involving research design, the average age, the proportion of gender, intervention measures, the comparison of analgesic effect, the route of administration, and adverse events. We contacted the original author for inquiry of the missing data. Additionally, two outcomes that changed in the VAS at 0–30 min and the VAS over different time periods (at 15, 30, 45, and 60 min) were defined as the primary outcomes, and the other outcomes were considered as secondary outcomes in this systematic review and meta-analysis. Besides, the scores of 100 subscales were converted into 10 subscales, so as to achieve the unity of data units. Any discrepancies were resolved in discussions with the third reviewer (Hao-Dong Peng).
Quality Assessment
Two reviewers (Xie-Yuan Leng and Chang-Ning Liu) independently completed quality assessment of included RCTs by adopting the Cochrane Collaboration's tool, which involves seven items that evaluated quality via ranking “low risk of bias,” “unclear risk of bias,” or “high risk of bias.”
Statistical Analysis
The measurement tools for pain used in the included studies included the VAS and NRS of 100 mm and 10 cm, with 100–0 and 10–0, respectively, to indicate the most severe pain to no pain. In this study, the scores of 100 subscales were converted into 10 subscales, so as to achieve the unity of data units. Dichotomous outcomes were expressed as relative risks (RR) with 95% confidence interval (CI), and continuous outcomes were expressed as mean difference (MD) with 95% CI (Deeks, 2002; Cumpston et al., 2019). We applied Knapp–Hartung adjustments to reduce the uncertainty of inter-study heterogeneity estimation. The combined data were processed first and the uncertainty heterogeneity within the study was reduced. The subgroup analysis was conducted to compare the NSAIDs to opioids, so as to analyze the source of heterogeneity according to the different routes of administration, NSAID type, and opioids type on patients. The primary outcome was assessed according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework based on five items including the risk of bias, inconsistency, indirectness, imprecision, and publication bias (Guyatt et al., 2008). The funnel plots were used to evaluate the possible publication bias of the efficacy and safety of the drug. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for the reporting of systematic reviews and meta-analysis was employed (Liberati et al., 2009).
Results
Literature Search and Characteristics of Included Studies
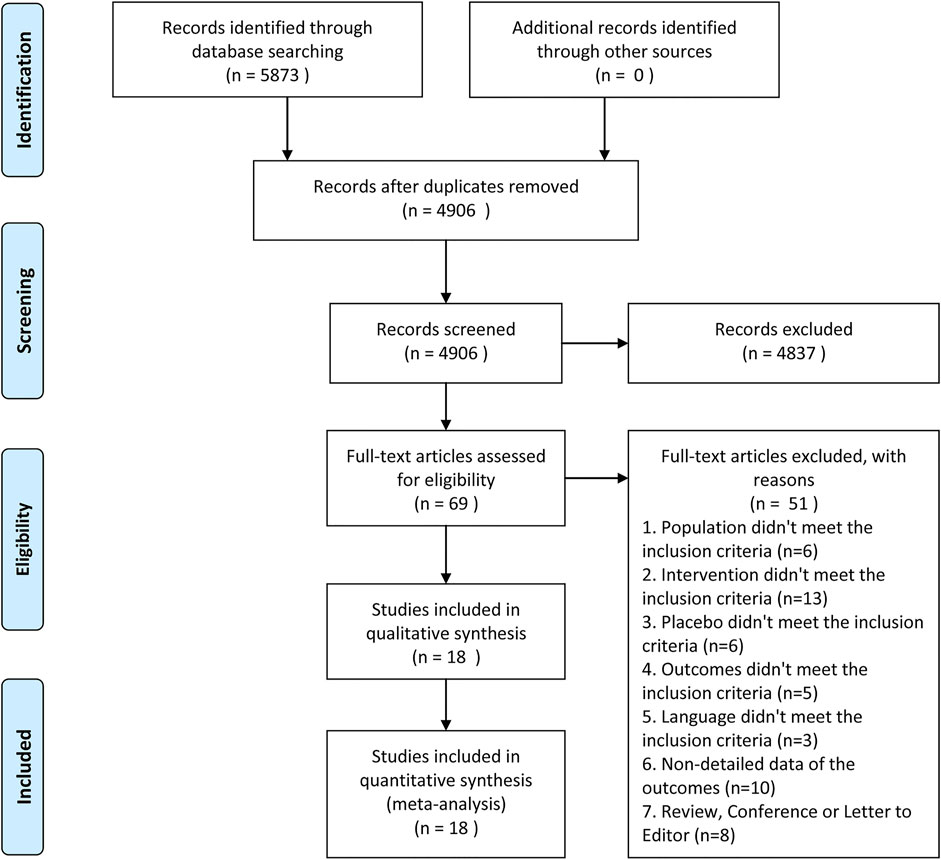
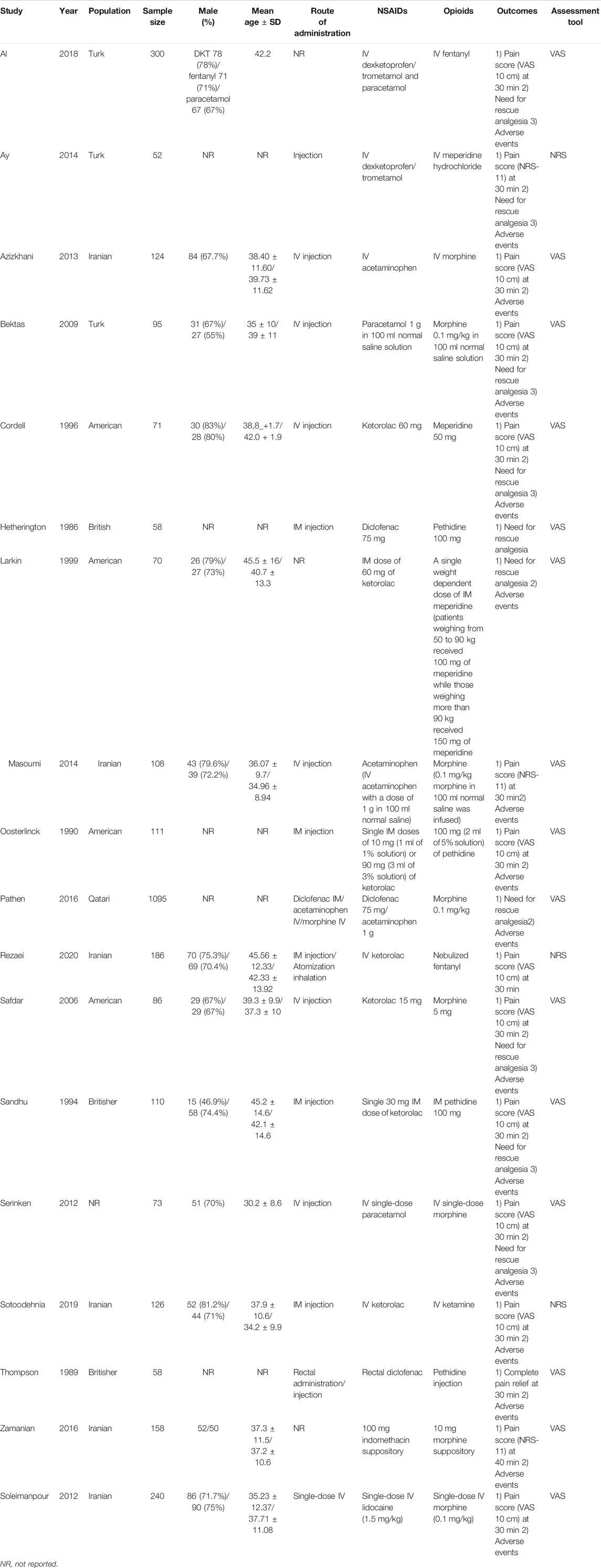
There were 5,873 articles obtained from the initially screened databases, and finally, a total of 18 studies (Hetherington and Philp, 1986; Thompson et al., 1989; Oosterlinck et al., 1990; Sandhu et al., 1994; Cordell et al., 1996; Larkin et al., 1999; Safdar et al., 2006; Bektas et al., 2009; Serinken et al., 2012; Soleimanpour et al., 2012; Azizkhani et al., 2013; Ay et al., 2014; Masoumi et al., 2014; Pathan et al., 2016a; Zamanian et al., 2016; Al et al., 2018; Sotoodehnia et al., 2019; Rezaei et al., 2020) were identified, including 3,121 participants, who met eligible criteria in this systematic review and meta-analysis through the process of deleting duplicate articles, and removing ineligible studies with reasons. All the details of screening the articles are illustrated in Figure 1. In addition, Table 1 demonstrates basic information of the included studies, mainly consisting of a summary of patient characteristics, administration of drugs (NSAIDs and opioids), and outcomes.
Quality Assessment of Included Studies
A total of 18 included studies in this systematic review and meta-analysis were rated according to the Cochrane Collaboration's tool in Supplementary Figure S1.
Primary Outcomes
Changes in Visual Analog Scale at 0–30 min
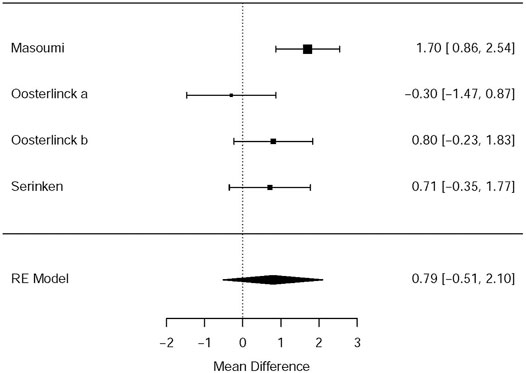
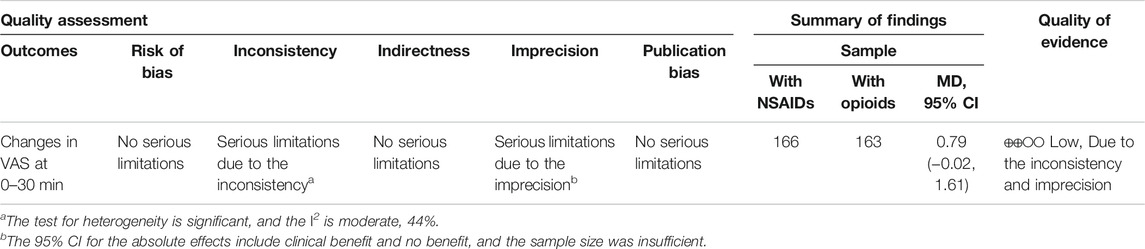
Three RCTs (Oosterlinck et al., 1990; Serinken et al., 2012; Masoumi et al., 2014) presented the outcome that the changes of the VAS at the 30th minute after applying NSAIDs and opioids as the treatment of acute renal colic. Based on these included studies, no marked significance between the NSAIDs group and opioids group was observed in terms of the analgesic effect for patients with renal colic (MD = 0.79, 95% CI: −0.51, 2.1, I2 = 61%, NSAIDs vs. opioids). More data about the changes in the VAS at the 30th min are presented in Figure 2. The overall evidence of changes of the VAS at the 30th min was assessed to be of low quality using the GRADE assessment framework (Table 2).
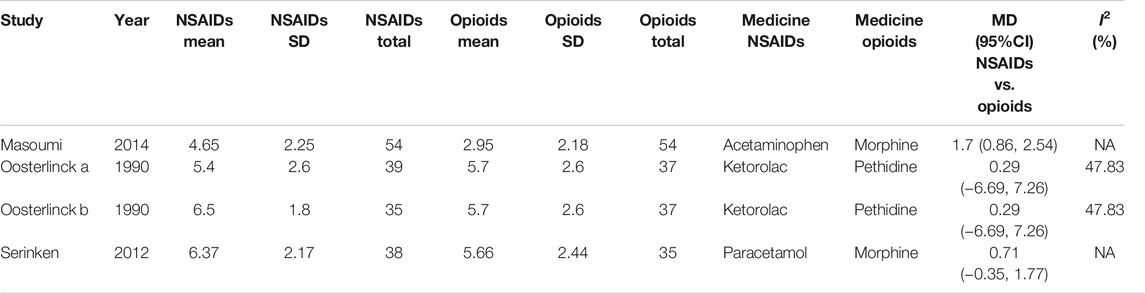
Besides, the information of the subgroup analysis by the different routes of administration and NSAID type are presented in Figure 3. The results presented (Serinken et al., 2012; Masoumi et al., 2014) that opioids in the form of IV administration didn't have significant difference on changes in the VAS at 0–30 min in the renal colic patients, when compared with the NSAIDs through the same route (MD = 1.25, 95% Cl: −4.81, 7.30, I2 = 48%, NSAIDs vs. opioids) (Figure 3). Conversely, no statistical significance was evident with the primary outcome (Oosterlinck et al., 1990) in comparison between the NSAID and opioid groups when administered IM (MD = 0.29, 95% Cl: −6.69, 7.26, I2 = 48%, NSAIDs vs. opioids) (Figure 3).
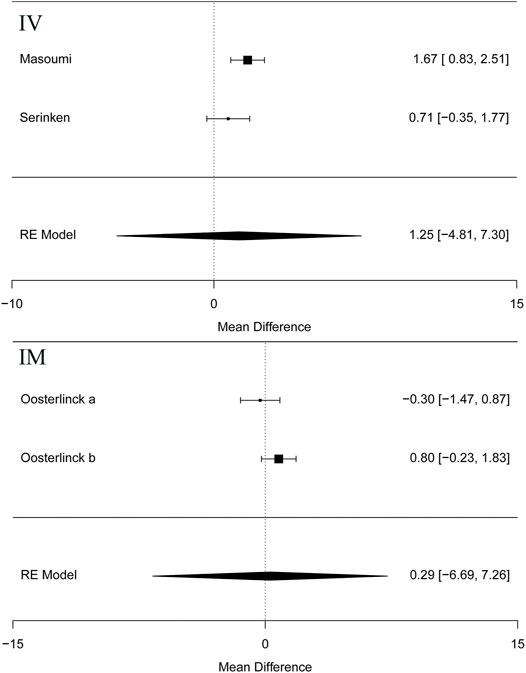
FIGURE 3. The results of meta-analysis for changes in pain scores (VAS) at 0–30 min based on different routes.
Based on the subgroup analysis of different NSAIDs and opioids in Table 3, it is shown that the changes in the VAS at 0–30 min had no statistical difference (MD = 0.29, 95% CI: −6.69, 7.26, I2 = 48%, NSAIDs vs. opioids) between ketorolac and pethidine, provided by the data of two RCTs of Oosterlinck et al. (1990). However, when compared with morphine from only one study (Masoumi et al., 2014), acetaminophen (MD = 1.70, 95% CI: 0.86, 2.54, I2 = NA, NSAIDs vs. opioids) can significantly lower the changes in the VAS at 0–30 min, but paracetamol (MD = 0.71, 95% Cl: −0.35, 1.77, I2 = NA, NSAIDs vs. opioids) demonstrated no significant difference (Table 3).
VAS Over Different Time Periods
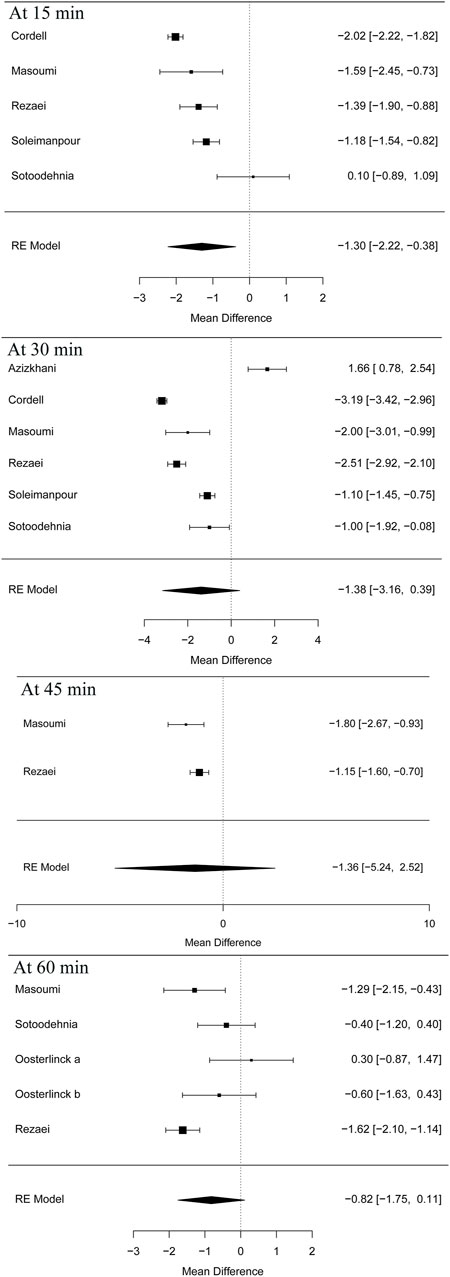
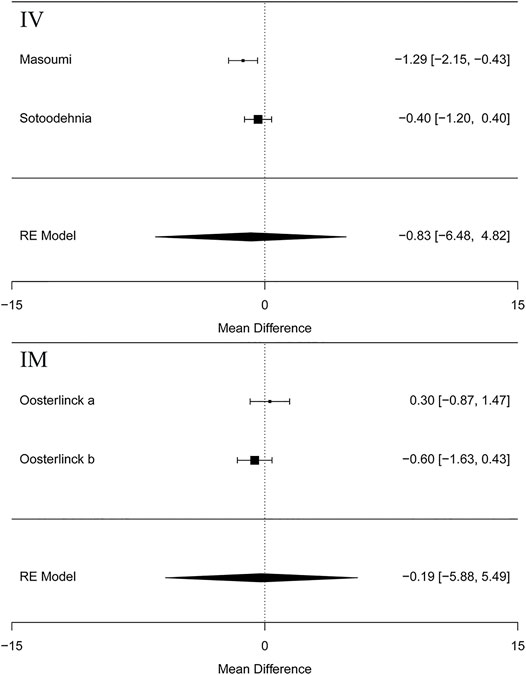
Five articles (Cordell et al., 1996; Masoumi et al., 2014; Sotoodehnia et al., 2019; Rezaei et al., 2020) in this meta-analysis presented the result of the VAS at 15 min (MD = −1.30 95% CI: −2.22, −0.38, I2 = 88.8%, NSAIDs vs. opioids) and demonstrated that the NSAIDs group greatly improved former outcomes compared to the opioids group, as is shown in Figure 4. However, the VAS at 30 min (Cordell et al., 1996; Soleimanpour et al., 2012; Azizkhani et al., 2013; Masoumi et al., 2014; Sotoodehnia et al., 2019; Rezaei et al., 2020) (MD = −1.38, 95% CI: −3.16, 0.39, I2 = 98%, NSAIDs vs. opioids) indicated that there was no significant difference between the opioid and NSAID groups. Moreover, similar results were also seen in the VAS at 45 min (Masoumi et al., 2014; Rezaei et al., 2020) (MD = −1.36, 95% CI: −5.24, 2.52, I2 = 40.7%, NSAIDs vs. opioids) (Figure 4). Besides, the outcome of the VAS at 60 min involved data (Oosterlinck et al., 1990; Masoumi et al., 2014; Sotoodehnia et al., 2019; Rezaei et al., 2020) from both forms of IV and IM administration, and the total result of this outcome shows that using NSAIDs had a similar benefit to using opioids in the treatment of renal colic (MD = −0.82, 95% CI: −1.75, 0.11, I2 = 72%, NSAIDs vs. opioids) (Figure 4). Figure 5 shows the details of the VAS over different time periods. Regarding the subgroup analysis of the VAS at 60 min (Figure 5), there was no significant difference between the NSAID and opioid groups in the treatment of renal colic (MD = −0.83, 95% CI: −6.48, 4.82, I2 = 47.8%, NSAIDs vs. opioids) (Masoumi et al., 2014; Sotoodehnia et al., 2019). This is similar to the primary outcome in the form of IM (Oosterlinck et al., 1990) (MD = −0.19, 95% CI: −5.88, 5.49, I2 = 48.4%, NSAIDs vs. opioids) (Figure 5).
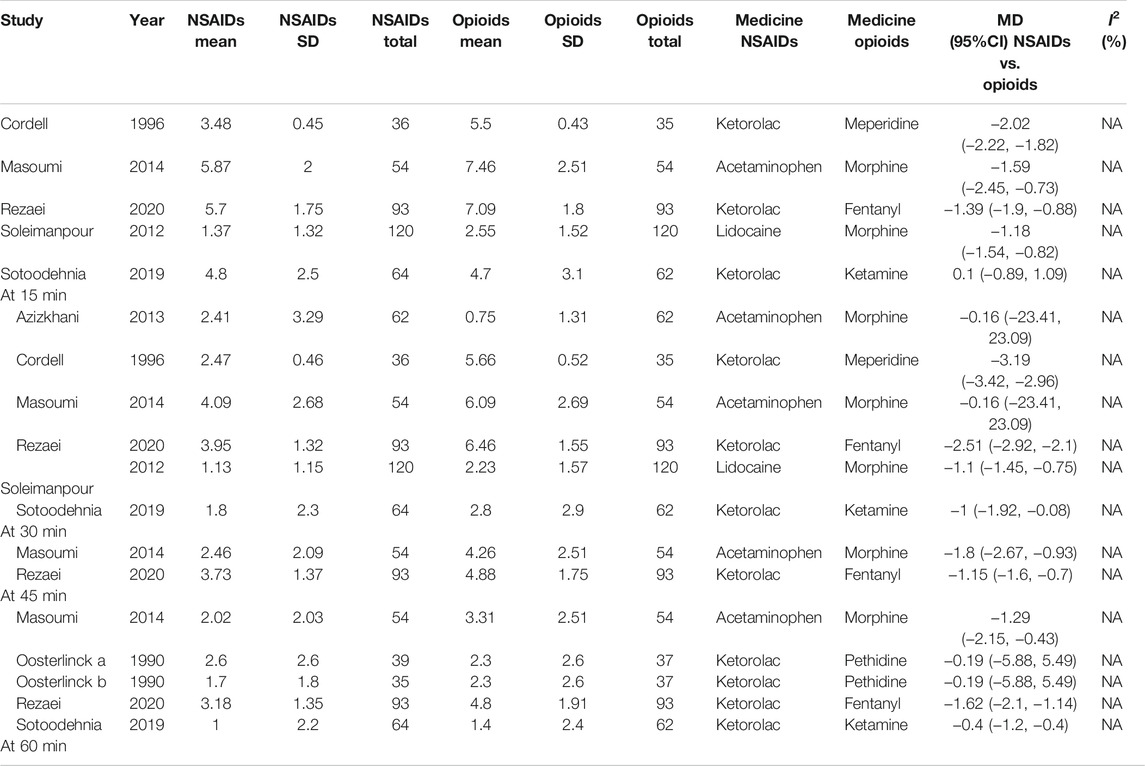
At 15 min, according to the subgroup analysis of different NSAIDs and opioids shown in Table 4, it is not difficult to find that using ketorolac is better than meperidine as per one study (Cordell et al., 1996) (MD = −2.02, 95% CI: −2.22, −1.82, I2 = NA, NSAIDs vs. opioids). Three other studies (Soleimanpour et al., 2012; Masoumi et al., 2014; Rezaei et al., 2020) also proved that acetaminophen performed better than morphine (MD = −1.59, 95% CI: −2.45, −0.73, I2 = NA, NSAIDs vs. opioids), ketorolac better than fentanyl (MD = −1.39, 95% CI: −1.90, −0.88, I2 = NA, NSAIDs vs. opioids), and lidocaine better than morphine (MD = −1.18, 95% CI: −1.54, −0.82, I2 = NA, NSAIDs vs. opioids). However, one study showed that there was no significant difference between ketorolac and ketamine at this time (Sotoodehnia et al., 2019).
Similarly, four articles (Cordell et al., 1996; Soleimanpour et al., 2012; Sotoodehnia et al., 2019; Rezaei et al., 2020) as shown in Table 4 reported the indicated significant differences on the VAS at 30 min for subjects with acute renal colic. They used ketorolac–meperidine (MD = −3.19, 95% CI: −3.42, −2.96, I2 = NA, NSAIDs vs. opioids), ketorolac–fentanyl (MD = −2.51, 95% CI: −2.92, −2.1, I2 = NA, NSAIDs vs. opioids), lidocaine–morphine (MD = −1.1, 95% CI: −1.45, −0.75, I2 = NA), and ketorolac–ketamine (MD = −1, 95% CI: −1.92, −0.08, I2 = NA, NSAIDs vs. opioids), respectively, and showed that NSAIDs had more advantages in the treatment of acute renal colic at 30 min. However, two RCTs (Azizkhani et al., 2013; Masoumi et al., 2014) showed that there was no significant difference between acetaminophen and morphine at this time (MD = −0.16, 95% CI: −23.41, 23.09, I2 = NA, NSAIDs vs. opioids).
It happens that there is a similar case, at 45 min; two studies (Masoumi et al., 2014; Rezaei et al., 2020) showed the superiority of acetaminophen over morphine (MD = −1.8, 95% CI: −2.67, −0.93, I2 = NA, NSAIDs vs. opioids) and ketorolac over fentanyl (MD = −1.15, 95% CI: −1.6, −0.7, I2 = NA, NSAIDs vs. opioids).
Additionally, with regard to the subgroup analysis by NSAIDs and opioids, at 60 min, a study (Oosterlinck et al., 1990) showed that there was no significant difference between ketorolac and pethidine (MD = −0.19, 95% CI: −5.88, 5.49, I2 = NA, NSAIDs vs. opioids). However, three other studies (Masoumi et al., 2014; Sotoodehnia et al., 2019; Rezaei et al., 2020) that used acetaminophen–morphine (MD = −1.29, 95% CI: −2.15, −0.43, I2 = NA, NSAIDs vs. opioids), ketorolac–fentanyl (MD = −1.62, 95% CI: −2.10, −1.14, I2 = NA, NSAIDs vs. opioids), and ketorolac–ketamine (MD = −0.40, 95% CI: −1.20, −0.40, I2 = NA, NSAIDs vs. opioids), respectively, showed that NSAIDs were more effective than opioids (Table 4).
Secondary Outcomes
Need for Rescue Analgesia
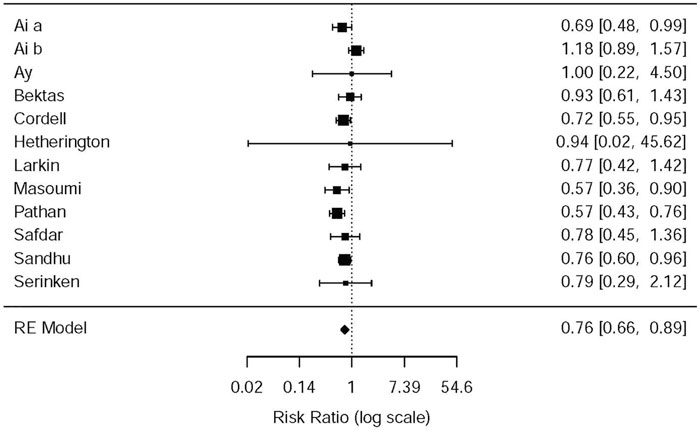
In the light of the 11 included studies (Hetherington and Philp, 1986; Sandhu et al., 1994; Cordell et al., 1996; Larkin et al., 1999; Safdar et al., 2006; Bektas et al., 2009; Serinken et al., 2012; Ay et al., 2014; Masoumi et al., 2014; Pathan et al., 2016a; Al et al., 2018), there were a total of 2,262 patients who needed rescue analgesia during the therapy of acute renal colic. The difference among nine RCTs, to be exact, involved the standards of rescue, administration of NSAIDs or opioids (e.g., dose of drugs, time of using analgesic) and the criteria that rescue succeed. The difference was observed with data from the above studies that opioids were more likely to receive later rescue when they were used for analgesia (RR = 0.76, 95% CI: 0.66, 0.89, I2 = 42.3%, NSAIDs vs. opioids). The relevant data about the need for rescue analgesia is presented in Figure 6 (please refer for details).
Some studies were performed by the subgroup analysis on the NSAID and Opioid types. Three studies (Sandhu et al., 1994; Masoumi et al., 2014; Pathan et al., 2016b) used morphine–acetaminophen (RR = 0.57, 95% CI: 0.36, 0.9, I2 = NA, NSAIDs vs. opioids); morphine–diclofenac (RR = 0.57, 95% CI: 0.43,0.76, I2 = NA, NSAIDs vs. opioids), and pethidine–ketorolac (RR = 0.76, 95% CI: 0.6, 0.96, I2 = NA, NSAIDs vs. opioids), respectively; all of them showed that morphine is easier to use for rescue analgesia than NSAIDs. Several other studies, as given in Table 5, showed no significant statistical difference between the NSAIDs and opioids.
Drug-Related Adverse Events
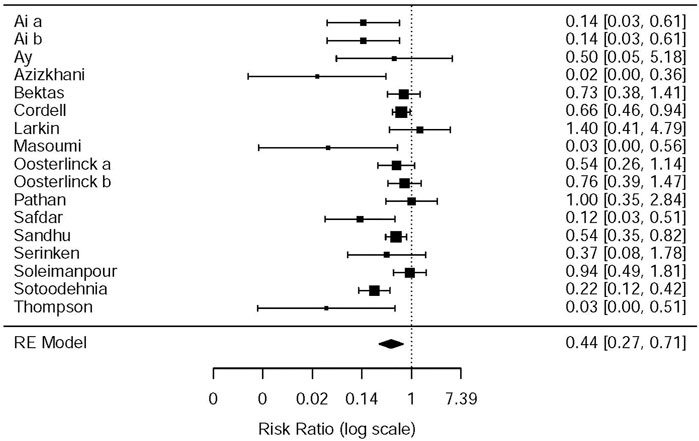
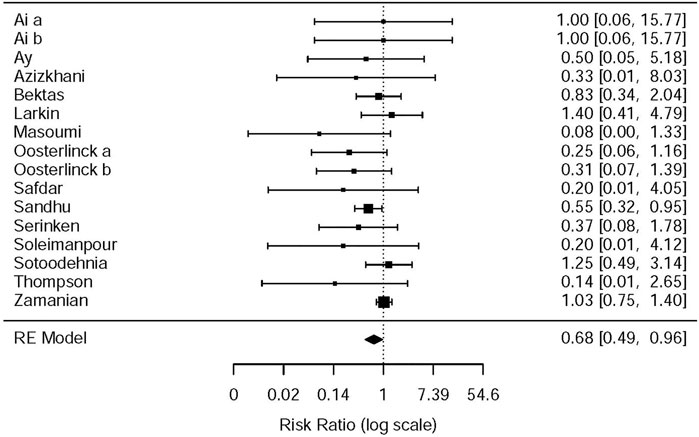
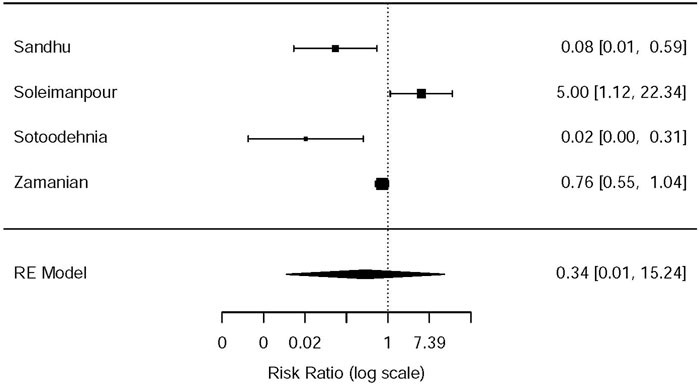
This systematic review and meta-analysis collected all adverse events described in 15 original articles (Thompson et al., 1989; Oosterlinck et al., 1990; Sandhu et al., 1994; Cordell et al., 1996; Larkin et al., 1999; Safdar et al., 2006; Bektas et al., 2009; Serinken et al., 2012; Soleimanpour et al., 2012; Azizkhani et al., 2013; Masoumi et al., 2014; Ay et al., 2014; Pathan et al., 2016a; Al et al., 2018; Sotoodehnia et al., 2019), which differ in types and standards of adverse events caused by drugs. We synthesized all data about drug-related adverse events and found that it was more possible for opioids to lead to adverse events in the treatment of patients with acute renal colic than the NSAIDs group (RR = 0.44, 95% CI: 0.27, 0.71, I2 = 65.7%, NSAIDs vs. opioids) (Figure 7). According to drug-related adverse events, the subgroup analysis was conducted to investigate the difference of both therapeutic regimens in renal colic patients. A total of 15 studies (Thompson et al., 1989; Oosterlinck et al., 1990; Sandhu et al., 1994; Larkin et al., 1999; Safdar et al., 2006; Bektas et al., 2009; Soleimanpour et al., 2012; Serinken et al., 2012; Azizkhani et al., 2013; Masoumi et al., 2014; Ay et al., 2014; Zamanian et al., 2016; Al et al., 2018; Sotoodehnia et al., 2019) referred to vomiting caused by the medications, and there were remarkable differences among the patients of the NSAIDs group (RR = 0.68, 95% CI: 0.49, 0.96, I2 = 22.6%, NSAIDs vs. opioids) (Figure 8). However, the participants in the opioids group and NSAIDs group showed no significance in dizziness comparing with those in the NSAIDs group (RR = 0.34, 95% CI: 0.01, 15.24, I2 = 90.3%, NSAIDs vs. opioids) on the basis of four RCTs (Sandhu et al., 1994; Soleimanpour et al., 2012; Zamanian et al., 2016; Sotoodehnia et al., 2019) (Figure 9).
The NSAIDs and opioids subgroup analysis was performed on drug-related adverse events as given in Table 6. Two studies (Oosterlinck et al., 1990; Sandhu et al., 1994) showed that pethidine is more likely to produce drug-related adverse events than ketorolac (RR = 0.58, 95% CI: 0.38, 0.90, I2 = NA, NSAIDs vs. opioids). The other two studies (Azizkhani et al., 2013; Masoumi et al., 2014) showed the same results between morphine and acetaminophen (RR = 0.03, 95% CI: 0, 0.45, I2 = NA, NSAIDs vs. opioids). One study (Thompson et al., 1989) showed that pethidine is more likely to produce drug-related adverse events than diclofenac (RR = 0.03, 95% CI: 0, 0.51, I2 = NA, NSAIDs vs. opioids). Furthermore, the study by Safdar et al. (2006) showed that morphine is more likely to produce drug-related adverse events than ketorolac (RR = 0.12, 95% CI: 0.03, 0.51, I2 = NA, NSAIDs vs. opioids). The study by Sotoodehnia et al. (2019) showed that ketamine is more likely to produce drug-related adverse events than ketorolac (RR = 0.22, 95% CI: 0.12, 0.42, I2 = NA, NSAIDs vs. opioids). However, there are still seven studies (Cordell et al., 1996; Larkin et al., 1999; Bektas et al., 2009; Serinken et al., 2012; Soleimanpour et al., 2012; Ay et al., 2014; Pathan et al., 2016b) that indicated that there was no significant statistical difference in drug side effects between the opioids and NSAIDs (Table 6). All in all, most of the included studies showed that opioids were more prone to drug side effects.
Publication Bias
According to the assessment of funnel plots, no obvious publication biases were found as shown in Supplementary Figures S2–S9.
Discussion
According to a recent study, a large proportion of people suffer from acute renal colic each year, suffering unbearable pain that is described as agony of obstruction in the upper urinary tract by kidney stone(s) (Wang et al., 2020). Unfortunately, there are a myriad of patients around the world who do not adequately receive pain relief, with possible reasons of excessive regulatory restrictions (Minozzi et al., 2013). With respect to therapy of acute renal colic, NSAIDs are prescribed for patients with acute pain and have been identified as necessary agents for treating pain when clinicians made the diagnosis of renal colic, in addition to opioids (Bultitude and Rees, 2012). However, the therapeutic regimen of renal colic is still controversial, with analgesic drug as the appropriate choice for patients who need it to release their pain immediately (Zamanian et al., 2016; Zhili et al., 2020). Therefore, the aim of this study is to investigate the effect and safety of two main analgesics (NSAIDs and opioids) in the treatment of patients diagnosed with acute renal colic, by a systematic review and meta-analysis.
NSAIDs exert their analgesic effect through inhibiting the release of prostaglandins in the body, then acting on the kidneys to increase the diuretic effect, which efficiently alleviates the pain of renal colic patients (Larkin et al., 1999). The creation of renal colic was attributed to prostacyclin and prostaglandin E2, which has been presented in previous studies (Fu et al., 2019). The above demonstration accounts for using NSAIDs in patients with acute renal colic. Be divergent with NSAIDs, however, opioids work by binding to opioid receptors in the brain of the patients and then stimulating the opioid systems that result in decreased pain within a short time.
A total of 18 studies (Hetherington and Philp, 1986; Thompson et al., 1989; Oosterlinck et al., 1990; Sandhu et al., 1994; Cordell et al., 1996; Larkin et al., 1999; Safdar et al., 2006; Bektas et al., 2009; Serinken et al., 2012; Azizkhani et al., 2013; Ay et al., 2014; Masoumi et al., 2014; Pathan et al., 2016a; Zamanian et al., 2016; Al et al., 2018; Sotoodehnia et al., 2019; Rezaei et al., 2020) were included in this systematic review and meta-analysis, involving 3,121 participants who used NSAIDs or opioids for releasing pain. The primary outcome in this study—changes in the VAS at 0–30 min, showed the result of the nonsignificant difference between the NSAID and opioid groups, which is consistent with the study of Khammissa (2020). The conclusion of this outcome is consistent with previous studies (Das and Teece, 2006), but there is one article that clearly indicates significant advantage in favor of NSAIDs in the treatment of acute renal colic (Steinberg and Chang, 2016). The subgroup analysis that was performed, in light of the different routes of administration (IV & IM administration) and NSAID type, with changes in the VAS at 0–30 min and the VAS at 60 min, aims to explore the resource of heterogeneity among the included studies about certain outcomes discussed in this systematic review and meta-analysis. Additionally, this study found interesting results that all of the secondary outcomes were statistically significant for patients with renal colic, between the NSAID and opioid groups. To be specific, regarding the outcome of the VAS over different time periods. There was no significant difference in the analgesic effect of NSAIDs and opioids on renal colic in each time period except 15 min. These results indicated that opioids that are more likely to be needed later in the rescue cause drug-related side effects in patients when compared with the NSAIDs group. Furthermore, using NSAIDs has no advantages on several outcomes of the VAS at 30, 45, 60 min and for the VAS at 0–30 min, but it seems to be a better choice when used at 15 min, when compared to opioids. A single article (Masoumi et al., 2014) might account for this different result on changes in the VAS at 0–30 min.
According to previous studies and this meta-analysis (Khammissa et al., 2020), the factors of analgesic effect of these two kinds of drugs are numerous and could be divided into two types, which involve the dose and routes of administration in patients, and the differences among individuals and environments (e.g., age, gender, and pain sensitivity).
On the one hand, a plethora of publications did not elucidate the method of administration, and at present, the evidence is inadequate to dictate the risk of this factor (Daoust et al., 2015). But, on the other hand, the beneficial effects of diclofenac sodium in the form of IM has been stated by Marthak et al. (1991); meanwhile, diclofenac sodium has been identified as a remarkably favored agent by assessing the overall effectiveness of the relevant drugs in the treatment of renal colic. Moreover, the RCT conducted by Pathan et al. (2016b) also found that IM NSAID drugs could be considered as the first-line treatment, as it provides the best analgesic effect for patients with renal colic. However, compared with opioids, both IV and IM administration of NSAIDs exhibited no better outcomes regarding change in the VAS at 0–30 min and 60 min. The reason for heterogeneity is possibly attributed to the different scales in assessing patients' pain and the antalgic agents those clinicians used for patients, based on the results of this meta-analysis.
Regarding the antalgic effects and side effects of both NSAIDs and opioids, whereas some publications persist that NSAIDs is superior to opioids in terms of side effects (e.g., hypotension, respiratory depression) (Esmailian and Keshavarz, 2014; Berthelot et al., 2015), there are a few studies that indicate there are similar effects between these two kinds of agents (Fu et al., 2019). Almost all included studies in this systematic review and meta-analysis illustrated adverse effects in their participants who were suffering acute renal colic and were using analgesics, but the divergent data about adverse effects, after analyzing the subgroup analysis, showed significant differences in favor of NSAIDs when compared with opioids in the treatment of renal colic patients. Similarly, a higher risk of side effects in patients when using opioids for treatment of renal colic, such as nausea and vomiting, was reported in many systematic reviews and meta-analyses, when compared with NSAIDs (Holdgate and Pollock, 2005). Besides, a recent study raised a regime that IV lidocaine could be a substitution for mitigating pain in a condition of intolerant adverse effects of NSAIDs or opioids (Loj et al., 2018). One note of caution, we considered there are possible reasons to explain the inconsistencies of side effects included in the studies reported, that is, the analgesic effect was not satisfactory due to the underdose of the antalgics and individual variations, because severe pain of acute renal colic can also cause nausea, vomiting, and hypertension (Holdgate and Pollock, 2005).
Limitation
There are several potential limitations in this systematic review and meta-analysis. First, the potential influence, in terms of the dose of the agents used for acute renal colic patients, would probably trigger uncertain results on the efficacy and safety of NSAIDs and opioids in the treatment of renal colic, especially regarding the primary outcome changes in the VAS at 0–30 min. In addition, the reason for this difference is likely due to various drugs provided by the different hospitals. Further RCTs are urgently required to investigate whether different agents and dosage of drugs used for renal colic have diverse influence on patients while alleviating extreme pain.
Conclusion
According to the results of this systematic review and meta-analysis. There is no significant difference between the NSAIDs and opioids in the treatment of renal colic in many outcomes (e.g., the VAS over different time periods except 15 min, using different injection methods at 30 and 60 min). However, patients can benefit from less side effects because clinicians use NSAIDs. Based on the subgroup analysis of NSAID and opioid types, ketorolac performs better than meperidine at 15 and 30 min, with acetaminophen and morphine performing equally, and ketorolac is better than fentanyl at 30 and 60 min, and acetaminophen is also better than morphine. Ketorolac also has a better performance than opioids in terms of drug side effects and whether rescue is required.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Author Contributions
X-YL had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of data analysis. Study concept and design: X-YL, C-NL, D-GW, and H-FP. Acquisition of data: C-NL, S-CW, and H-DP. Analysis and interpretation of data: X-YL and C-NL. Drafting of the manuscript: X-YL and S-CW. Critical revision of the manuscript for important intellectual content: X-YL, D-GW, and H-FP. Statistical analysis: X-YL and C-NL. Administrative, technical, or material support: X-YL. Supervision: D-GW and H-FP.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.728908/full#supplementary-material
References
Al, B., Sunar, M. M., Zengin, S., Sabak, M., Bogan, M., Can, B., et al. (2018). Comparison of IV Dexketoprofen Trometamol, Fentanyl, and Paracetamol in the Treatment of Renal Colic in the ED: A Randomized Controlled Trial. Am. J. Emerg. Med. 36 (4), 571–576. doi:10.1016/j.ajem.2017.09.019
Ay, M. O., Sebe, A., Kozaci, N., Satar, S., Acikalin, A., Gulen, M., et al. (2014). Comparison of the Analgesic Efficacy of Dexketoprofen Trometamol and Meperidine HCl in the Relief of Renal Colic. Am. J. Ther. 21 (4), 296–303. doi:10.1097/MJT.0b013e318274db78
Azizkhani, R., Pourafzali, S. M., Baloochestani, E., and Masoumi, B. (2013). Comparing the Analgesic Effect of Intravenous Acetaminophen and Morphine on Patients with Renal Colic Pain Referring to the Emergency Department: A Randomized Controlled Trial. J. Res. Med. Sci. 18 (9), 772–776.
Bektas, F., Eken, C., Karadeniz, O., Goksu, E., Cubuk, M., and Cete, Y. (2009). Intravenous Paracetamol or Morphine for the Treatment of Renal Colic: a Randomized, Placebo-Controlled Trial. Ann. Emerg. Med. 54 (4), 568–574. doi:10.1016/j.annemergmed.2009.06.501
Berthelot, J. M., Darrieutort-Lafitte, C., Le Goff, B., and Maugars, Y. (2015). Strong Opioids for Noncancer Pain Due to Musculoskeletal Diseases: Not More Effective Than Acetaminophen or NSAIDs. Jt. Bone Spine 82 (6), 397–401. doi:10.1016/j.jbspin.2015.08.003
Bultitude, M., and Rees, J. (2012). Management of Renal Colic. BMJ 345, e5499. doi:10.1136/bmj.e5499
Cordell, W. H., Wright, S. W., Wolfson, A. B., Timerding, B. L., Maneatis, T. J., Lewis, R. H., et al. (1996). Comparison of Intravenous Ketorolac, Meperidine, and Both (Balanced Analgesia) for Renal Colic. Ann. Emerg. Med. 28 (2), 151–158. doi:10.1016/s0196-0644(96)70055-0
Cumpston, M., Li, T., Page, M. J., Chandler, J., Welch, V. A., Higgins, J. P., et al. (2019). Updated Guidance for Trusted Systematic Reviews: a New Edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 10, ED000142. doi:10.1002/14651858.ED000142
Daoust, R., Paquet, J., Lavigne, G., Piette, É., and Chauny, J. M. (2015). Impact of Age, Sex and Route of Administration on Adverse Events after Opioid Treatment in the Emergency Department: a Retrospective Study. Pain Res. Manag. 20 (1), 23–28. doi:10.1155/2015/316275
Das, D., and Teece, S. (2006). Best Evidence Topic Report. Intravenous NSAID's in the Management of Renal Colic. Emerg. Med. J. 23 (3), 225. doi:10.1136/emj.2005.034330
Deeks, J. J. (2002). Issues in the Selection of a Summary Statistic for Meta-Analysis of Clinical Trials with Binary Outcomes. Stat. Med. 21 (11), 1575–1600. doi:10.1002/sim.1188
Esmailian, M., and Keshavarz, M. (2014). Synergistic Effects of Citalopram and Morphine in the Renal Colic Pain Relief; a Randomized Clinical Trial. Emerg (Tehran) 2 (1), 26–29.
Fanelli, G., Cherubino, P., and Compagnone, C. (2014). Opioid Use for Chronic Pain Management in Italy: Results from the Orthopedic Instant Pain Survey Project. Orthop. Rev. (Pavia) 6 (2), 5309. doi:10.4081/or.2014.5309
Fu, S., Zhang, K., Gu, M., Liu, Z., Sun, W., and Xiao, M. (2019). Comparative Efficacy and Safety of Analgesics for Acute Renal Colic: A Network Meta-Analysis Protocol. Medicine (Baltimore) 98 (10), e14709. doi:10.1097/MD.0000000000014709
Guyatt, G. H., Oxman, A. D., Vist, G. E., Kunz, R., Falck-Ytter, Y., Alonso-Coello, P., et al. (2008). GRADE: an Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations. BMJ 336 (7650), 924–926. doi:10.1136/bmj.39489.470347.AD
Hetherington, J. W., and Philp, N. H. (1986). Diclofenac Sodium versus Pethidine in Acute Renal Colic. BMJ 292 (6515), 237–238. doi:10.1136/bmj.292.6515.237
Holdgate, A., and Pollock, T. (2005). Nonsteroidal Anti-inflammatory Drugs (NSAIDs) versus Opioids for Acute Renal Colic. Cochrane Database Syst. Rev. 2004 (2), CD004137. doi:10.1002/14651858.CD004137.pub3
Khammissa, R. A. G., Ballyram, R., Fourie, J., Bouckaert, M., Lemmer, J., and Feller, L. (2020). Selected Pathobiological Features and Principles of Pharmacological Pain Management. J. Int. Med. Res. 48 (5), 300060520903653. doi:10.1177/0300060520903653
Larkin, G. L., Peacock, W. F., Pearl, S. M., Blair, G. A., and D'Amico, F. (1999). Efficacy of Ketorolac Tromethamine versus Meperidine in the ED Treatment of Acute Renal Colic. Am. J. Emerg. Med. 17 (1), 6–10. doi:10.1016/s0735-6757(99)90003-7
Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gøtzsche, P. C., Ioannidis, J., et al. (2009). The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies that Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ 339, b2700. doi:10.1136/bmj.b2700
Loj, E. S., Cabrera, D., Scherber, K., Motov, S., Erwin, P. J., West, C. P., et al. (2018). Safety and Efficacy of Intravenous Lidocaine for Pain Management in the Emergency Department: A Systematic Review. Ann. Emerg. Med. 72 (2), 135–144. doi:10.1016/j.annemergmed.2017.12.014
Majidi, A., and Derakhshani, F. (2020). Intravenous Magnesium Sulfate for Pain Management in Patients with Acute Renal Colic; a Randomized Clinical Trial. Arch. Acad. Emerg. Med. 8 (1), e5. doi:10.1016/j.ajem.2020.07.035
Marthak, K. V., Gokarn, A. M., Rao, A. V., Sane, S. P., Mahanta, R. K., Sheth, R. D., et al. (1991). A Multi-centre Comparative Study of Diclofenac Sodium and a Dipyrone/spasmolytic Combination, and a Single-centre Comparative Study of Diclofenac Sodium and Pethidine in Renal Colic Patients in India. Curr. Med. Res. Opin. 12 (6), 366–373. doi:10.1185/03007999109111506
Masoumi, K., Forouzan, A., Asgari Darian, A., Feli, M., Barzegari, H., and Khavanin, A. (2014). Comparison of Clinical Efficacy of Intravenous Acetaminophen with Intravenous Morphine in Acute Renal Colic: a Randomized, Double-Blind, Controlled Trial. Emerg. Med. Int. 2014, 571326. doi:10.1155/2014/571326
Minozzi, S., Amato, L., and Davoli, M. (2013). Development of Dependence Following Treatment with Opioid Analgesics for Pain Relief: a Systematic Review. Addiction 108 (4), 688–698. doi:10.1111/j.1360-0443.2012.04005.x
Oosterlinck, W., Philp, N. H., Charig, C., Gillies, G., Hetherington, J. W., and Lloyd, J. (1990). A Double-Blind Single Dose Comparison of Intramuscular Ketorolac Tromethamine and Pethidine in the Treatment of Renal Colic. J. Clin. Pharmacol. 30 (4), 336–341. doi:10.1002/j.1552-4604.1990.tb03603.x
Passik, S. D. (2009). Issues in Long-Term Opioid Therapy: Unmet Needs, Risks, and Solutions. Mayo Clin. Proc. 84 (7), 593–601. doi:10.1016/S0025-6196(11)60748-9
Pathan, S. A., Mitra, B., and Cameron, P. A. (2016). Titrated Doses Are Optimal for Opioids in Pain Trials - Authors' Reply. Lancet 388 (10048), 961–962. doi:10.1016/S0140-6736(16)31494-5
Pathan, S. A., Mitra, B., Straney, L. D., Afzal, M. S., Anjum, S., Shukla, D., et al. (2016). Delivering Safe and Effective Analgesia for Management of Renal Colic in the Emergency Department: a Double-Blind, Multigroup, Randomised Controlled Trial. Lancet 387 (10032), 1999–2007. doi:10.1016/S0140-6736(16)00652-8
Rezaei, B., Salimi, R., Kalantari, A., and Astaraki, P. (2020). Comparison of Efficacy Nebulized Fentanyl with Intravenous Ketorolac for Renal Colic in Patients over 12 Years Old. Am. J. Emerg. Med. 44, 358–361. doi:10.1016/j.ajem.2020.04.053
Sackner, M. A., Silva, G., Banks, J. M., Watson, D. D., and Smoak, W. M. (1974). Distribution of Ventilation during Diaphragmatic Breathing in Obstructive Lung Disease. Am. Rev. Respir. Dis. 109 (3), 331–337. doi:10.1164/arrd.1974.109.3.331
Safdar, B., Degutis, L. C., Landry, K., Vedere, S. R., Moscovitz, H. C., and D'Onofrio, G. (2006). Intravenous Morphine Plus Ketorolac Is superior to Either Drug Alone for Treatment of Acute Renal Colic. Ann. Emerg. Med. 48 (2), 173–181. doi:10.1016/j.annemergmed.2006.03.013
Sandhu, D. P., Iacovou, J. W., Fletcher, M. S., Kaisary, A. V., Philip, N. H., and Arkell, D. G. (1994). A Comparison of Intramuscular Ketorolac and Pethidine in the Alleviation of Renal Colic. Br. J. Urol. 74 (6), 690–693. doi:10.1111/j.1464-410x.1994.tb07107.x
Schmidt, S., and Kroeger, N. (2016). Pain Therapy for Acute Renal Colics: Nonsteroidal Anti-inflammatory Drugs (NSAIDs) and Non-opioids. Urologe A 55 (3), 386–390. doi:10.1007/s00120-016-0027-3
Serinken, M., Eken, C., Turkcuer, I., Elicabuk, H., Uyanik, E., and Schultz, C. H. (2012). Intravenous Paracetamol versus Morphine for Renal Colic in the Emergency Department: a Randomised Double-Blind Controlled Trial. Emerg. Med. J. 29 (11), 902–905. doi:10.1136/emermed-2011-200165
Soleimanpour, H., Hassanzadeh, K., Vaezi, H., Golzari, S. E., Esfanjani, R. M., and Soleimanpour, M. (2012). Effectiveness of Intravenous Lidocaine versus Intravenous Morphine for Patients with Renal Colic in the Emergency Department. BMC Urol. 12 (13), 637–648. doi:10.1186/1471-2490-12-13
Sotoodehnia, M., Farmahini-Farahani, M., Safaie, A., Rasooli, F., and Baratloo, A. (2019). Low-dose Intravenous Ketamine versus Intravenous Ketorolac in Pain Control in Patients with Acute Renal Colic in an Emergency Setting: a Double-Blind Randomized Clinical Trial. Korean J. Pain 32 (2), 97–104. doi:10.3344/kjp.2019.32.2.97
Steinberg, P. L., and Chang, S. L. (2016). Pain Relief for Acute Urolithiasis: The Case for Non-steroidal Anti-inflammatory Drugs. Drugs 76 (10), 993–997. doi:10.1007/s40265-016-0595-y
Thompson, J. F., Pike, J. M., Chumas, P. D., and Rundle, J. S. (1989). Rectal Diclofenac Compared with Pethidine Injection in Acute Renal Colic. BMJ 299 (6708), 1140–1141. doi:10.1136/bmj.299.6708.1140
Wang, Z., Bai, Y., and Wang, J. (2020). Effects of Diuretic Administration on Outcomes of Extracorporeal Shockwave Lithotripsy: A Systematic Review and Meta-Analysis. PLoS One 15 (3), e0230059. doi:10.1371/journal.pone.0230059
Zamanian, F., Jalili, M., Moradi-Lakeh, M., Kia, M., Aghili, R., and Aghili, S. M. (2016). Morphine Suppository versus Indomethacin Suppository in the Management of Renal Colic: Randomized Clinical Trial. Pain Res. Treat. 2016, 4981585. doi:10.1155/2016/4981585
Keywords: renal colic, NSAIDs, nonsteroidal drugs, opioids, meta-analysis, systematic review
Citation: Leng X-Y, Liu C-N, Wang S-C, Peng H-D, Wang D-G and Pan H-F (2022) Comparison of the Efficacy of Nonsteroidal Anti-Inflammatory Drugs and Opioids in the Treatment of Acute Renal Colic: A Systematic Review and Meta-Analysis. Front. Pharmacol. 12:728908. doi: 10.3389/fphar.2021.728908
Received: 28 June 2021; Accepted: 20 December 2021;
Published: 27 January 2022.
Edited by:
Ahmad Reza Dehpour, Tehran University of Medical Sciences, IranReviewed by:
Hassan Soleimanpour, Tabriz University of Medical Sciences, IranMaziar Moradi-Lakeh, Iran University of Medical Sciences, Iran
Copyright © 2022 Leng, Liu, Wang, Peng, Wang and Pan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: De-Guang Wang, d2FuZ2RlZ3VhbmdAYWhtdS5lZHUuY24=; Hai-Feng Pan, cGFuaGFpZmVuZzE5ODJAc2luYS5jb20=
 Xie-Yuan Leng
Xie-Yuan Leng Chang-Ning Liu
Chang-Ning Liu Shi-Chan Wang
Shi-Chan Wang Hao-Dong Peng1
Hao-Dong Peng1 Hai-Feng Pan
Hai-Feng Pan