- 1Department of Medical Oncology and Radiation Sickness, Peking University Third Hospital, Beijing, China
- 2Department of Gastrointestinal Oncology, Peking University Cancer Hospital and Institute, Beijing, China
- 3Research Center of Clinical Epidemiology, Peking University Third Hospital, Beijing, China
Objective: The occurrence, development, and prognosis of serious adverse events (SAEs) associated with anticancer drugs in clinical trials have important guiding significance for real-world clinical applications. However, to date, there have been no studies investigating SAEs reporting in randomized clinical trials of colorectal cancer treatments. This article systematically reviewed the SAEs reporting of phase III randomized clinical trials of colorectal cancer treatments and analyzed the influencing factors.
Methods: We reviewed all articles about phase III randomized clinical trials of colorectal cancer treatments published in the PubMed, Embase, Medline, and New England Journal of Medicine databases from January 1, 1993, to December 31, 2018, and searched the registration information of clinical trials via the internet sites such as “clinicaltrials.gov”. We analyzed the correlation between the reported proportion (RP) of SAEs in the literature and nine elements, including the clinical trial sponsor and the publication time. Chi-square tests and binary logistic regression were used to identify the factors associated with improved SAEs reports. This study was registered on PROSPERO.
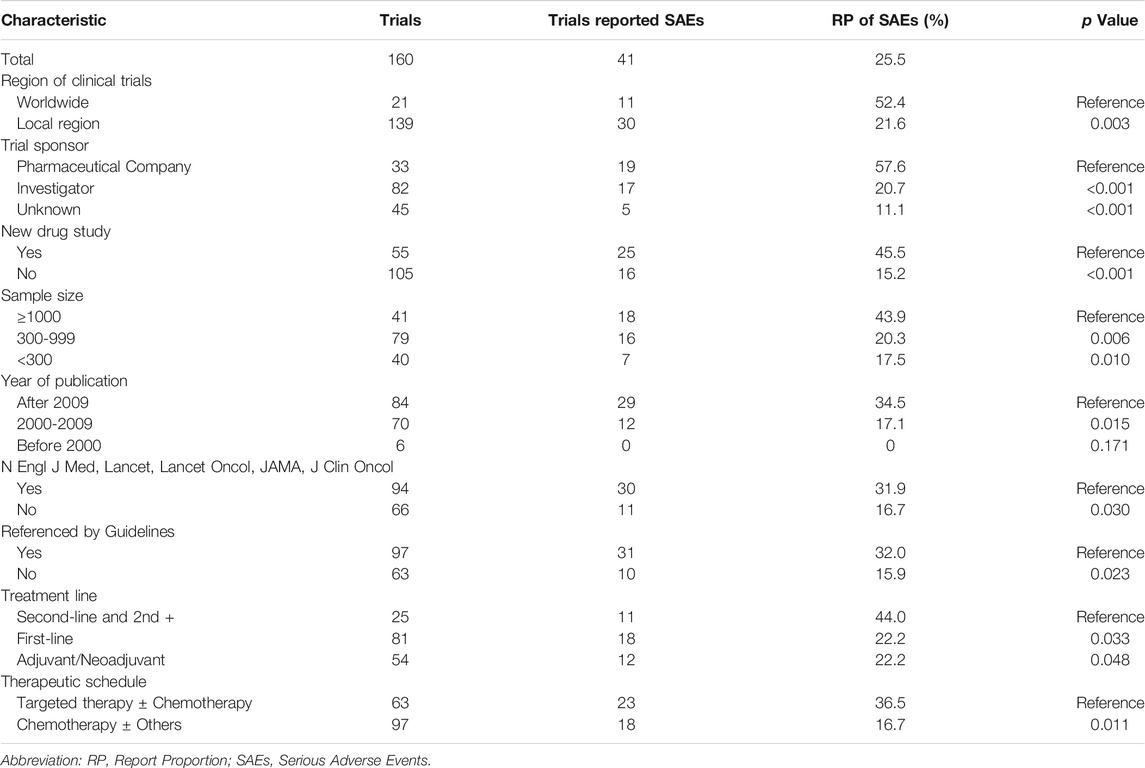
Results: Of 1560 articles identified, 160 were eligible, with an RP of SAEs of 25.5% (41/160). In forty-one publications reporting SAEs, only 14.6% (6/41) described the pattern of SAEs in detail. In clinical trials sponsored by pharmaceutical companies, the RP of SAEs was significantly higher than that in those sponsored by investigators (57.6 versus 20.7%, p < 0.001). From 1993 to 2018, the RP of SAEs gradually increased (none (0/6) before 2000, 17.1% (12/70) from 2000 to 2009, and 34.5% (29/84) after 2009). The average RP of SAEs published in the New England Journal of Medicine (N Engl J Med), the Lancet, the Journal of the American Medical Association (JAMA), the Lancet Oncology (Lancet Oncol), and the Journal of Clinical Oncology (J Clin Oncol) was significantly higher than that published in other journals (31.9 versus 16.7%, p = 0.030). In the clinical trials referenced by clinical guidelines, the RP of SAEs was higher than that in non-referenced clinical trials (32.0 versus 15.9%, p = 0.023). Binary logistic regression analysis showed that pharmaceutical company sponsorship, new drug research, and sample size greater than 1000 were positive influencing factors for SAEs reporting.
Conclusion: Although the RP of SAEs increased over time, SAEs reporting in clinical trials needs to be further improved. The performance, outcomes and prognosis of SAEs should be reported in detail to guide clinical practice in the real world.
Introduction
Colorectal cancer is the third most commonly diagnosed malignancy worldwide (Fitzmaurice et al., 2017). Chemotherapy and targeted therapy play an important role in standard treatments for colorectal cancer. Fluorouracil-based adjuvant chemotherapy significantly improved the disease-free survival (DFS) and overall survival (OS) of stage II/III colorectal cancer (Group et al., 2007; André et al., 2009; Iveson et al., 2018). Combination chemotherapy with bevacizumab or cetuximab as the initial treatment significantly improved the median progression free survival (mPFS) and median overall survival (mOS) of metastatic colorectal cancer (Saltz et al., 2008; Van Cutsem et al., 2009; Qin et al., 2018). Fruquintinib and regorafenib in the 3 + line significantly prolonged the mOS and mPFS of advanced colorectal cancer (Grothey et al., 2013; Li et al., 2015; Li et al., 2018). Based on the results of clinical trials that have confirmed the efficacy of many chemotherapeutic and targeted drugs, experts have formed guidelines and consensuses to guide the diagnosis and treatment of colorectal cancer in the real world. Reporting the occurrence, development, and prognosis of adverse events (AEs), especially serious AEs (SAEs), is particularly crucial for reducing or avoiding the toxicity of regimens in real-world clinical practice, improving patients’ quality of life, and decreasing the psychological and economic burden of patients. During the past 20 years, SAEs have attracted increasing attention as the number of SAEs reported to the U.S. Food and Drug Administration (FDA) increased by 2.6 times from 1998 to 2005 (Moore et al., 2007) and by 2 times from 2006 to 2014 (Sonawane et al., 2018). Guidelines indicate that clinical trials should report AEs and SAEs in a consistent manner (Wallace et al., 2016).
AEs reporting is relatively higher in cancer clinical trial publications, but the reporting quality is low. A review showed that 96% of cancer clinical studies reported AEs, but oncology-specific reporting standards were lacking (Sivendran et al., 2014). Another article reviewed 325 randomized clinical trials, all of which reported the occurrence of AEs. Nevertheless, the AEs collection and analysis methods were highly heterogeneous, and the quality of AEs reporting did not improve significantly over time (Péron et al., 2013). In addition, there was a considerable discrepancy between the final published AEs data and the sponsors’ database (Scharf and Colevas, 2006). Although there have been some reviews of AEs reports, analysis of SAEs reports on colorectal cancer clinical trials is scarce, and the report proportion of SAEs in publications is unknown.
We systematically reviewed SAEs reporting from publications of colorectal cancer clinical trials, to further draw researchers’ attention to SAEs reporting. The SAEs reporting was influenced by many social factors, such as regional policy, preciseness and awareness of investigators, purpose of sponsor, so this article analyzed the possible influencing factors of SAEs reporting. Because the results of phase III randomized clinical trials were the most instructive in the real world, herein we just reviewed phase III randomized clinical trials.
Materials and Methods
This study included randomized phase III colorectal cancer clinical trials whose intervention measures contained anticancer pharmaceuticals and whose results were published in PubMed, Embase, Medline, and the New England Journal of Medicine from January 1, 1993, to December 31, 2018. We analyzed several possible factors that may affect SAEs reporting in the literature. These factors included the region where the clinical trial was conducted, the sponsor of the clinical trial, whether the trial researched new drugs, the publication date which may reflect the change of policy and awareness of investigators, factors related with the rigorous of the clinical trials such as sample size, the type of journal and whether the clinical guidelines referenced the results of the study, and factors owned by clinical trials themselves, such as treatment line, therapeutic schedule.
Literature Search Strategy
A review of citations from PubMed, Embase, Medline, and New England Journal of Medicine for studies published between January 1, 1993 and December 31, 2018, was performed to identify eligible colorectal cancer clinical trial publications for the analysis. The search terms were as follows: “colorectal cancer” [All fields] or “colon cancer” [All fields] or “rectal cancer” [All fields], and “phase 3” [All fields] or “phase III” [All fields]. We used the filters as follows: “subjects = cancer,” “article type = clinical trial,” “language = English,” “species = humans,” and “publications dates = 1/1/1993-12/31/2018.” Endnote X4 (Clarivate, Philadelphia, PA, United States) was used to manage the publications. We searched the registration information of clinical trials via the following internet sites: http://www.clinicaltrials.gov, http://www.isrctn.com/search, http://www.anzctr.org.au, https://www.umin.ac.jp/, http://apps.who.int/en/. The inclusion criteria were as follows: 1) phase III randomized colorectal cancer clinical trials, 2) intervention measure contained chemotherapy and/or target therapy, 3) the articles showed the efficacy and/or safety of the clinical trial, 4) published in English. The exclusion criteria were as follows: 1) the same research published repeatedly, 2) reviews, meta-analysis, molecular analysis and cost analysis, 3) subgroup analysis of the research already included, 4) intervention measure contained immune therapy (because the AEs spectrum of chemotherapy and immunotherapy is different), 5) clinical trials aimed to observe the efficacy or AEs of accompanying regimens along with anticancer therapy. The primary objective was the reported proportion (RP) of SAEs. The secondary objectives were the performance, outcomes and prognosis of SAEs.
The Criteria of AEs, SAEs and SAE Reporting
According to the National Cancer Institute Common Toxicity Criteria (NCI-CTC) version 5.0 (Common terminology criteria for adverse events (CTCAE) v5.0, 2017), an AE is any unfavorable and unintended sign (including an abnormal laboratory finding), symptom, or disease temporally associated with the use of a medical treatment or procedure that may or may not be considered related to the medical treatment or procedure. Grade 3 AEs are defined as: 1) severe or medically significant but not immediately life-threatening, 2) hospitalization or prolongation of hospitalization indicated, 3) disabling, 4) limiting self-care activities of daily living (ADL). Grade 4 AEs are defined as: 1) life-threatening consequences, 2) urgent intervention indicated. Grade 5 AEs are death related to AEs.
SAEs were diagnosed according to NCI-CTC version 5.0 (Common terminology criteria for adverse events (CTCAE) v5.0, 2017) as follows:
An SAE is any untoward medical occurrence that, at any dose: 1) results in death, 2) is life-threatening, 3) an event is considered life-threatening if it is suspected that the individual is at substantial risk of dying at the time of the AEs, 4) requires inpatient hospitalization or prolongation of existing hospitalization (an admission and/or overnight stay or an event that prolongs hospitalization), 5) results in persistent or significant disability/incapacity (includes an AEs that resulted in a substantial disruption of a person’s ability to conduct normal life functions, i.e., significant, persistent or permanent change in, impairment of, damage to or disruption in the individual’s body function/structure, physical activities, and/or quality of life), 6) is a congenital anomaly/congenital disability, 7) is medically significant (other important medical events may be considered serious when, based on appropriate medical judgment, they might jeopardize the individual and/or may require medical or surgical intervention to prevent the event from meeting a criterion for an SAE).
Herein we mainly discussed the SAEs reporting. If the publication pointed out the occurrence of SAEs, even the incidence was zero, it was judged to have reported SAEs. SAEs consisted of many events not only death, so if the publication just only reported death and didn’t mention “SAEs,” it wasn’t judged to have reported SAEs in this review. And reporting Grade 3/4 AEs were not identified as having reported SAEs.
Data Extraction
The data were collected independently by two investigators (Yanhong Yao and Zhentao Liu) who screened eligible publications and searched the registry of clinical trials. The collected data included performance, outcomes and prognosis of SAEs, the region where the clinical trial was conducted, the sponsor of the clinical trial, whether the trial researched new drugs, the sample size, the publication date, the type of journal, whether the clinical guidelines [including the National Comprehensive Cancer Network (NCCN), American Society of Clinical Oncology (ASCO), European Society for Medical Oncology (ESMO) and Chinese Society of Clinical Oncology (CSCO)] referenced the study results, the treatment lines, and treatment schedules. Professor Baoshan Cao checked the data if inconsistencies existed between the results collected by the two investigators.
Statistical Analysis
We used SPSS version 19.0 (IBM, New York, United States) to analyze the data, and differences were considered statistically significant when the two-sided p values were less than 0.05. Frequencies and percentages were calculated for counting data. The chi-square test was used to assess the association between RPs of SAEs and collected items, and Fisher’s exact test was used if the theoretical number was less than 5 or the sample size was less than 40. A binary logistic regression model was used to identify items associated with SAEs reporting. The dependent variable was whether reported SAEs, and the independent variables were the positive influencing factors for SAEs reporting based chi-square test. The method of the independent variables entering the regression equation was “Backwald”.
Results
Characteristics of Selected Publications
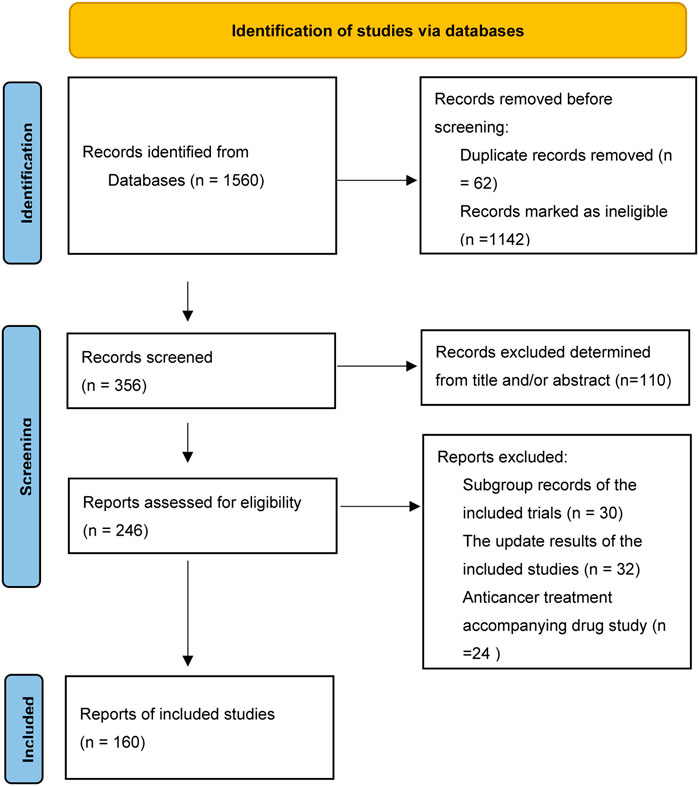
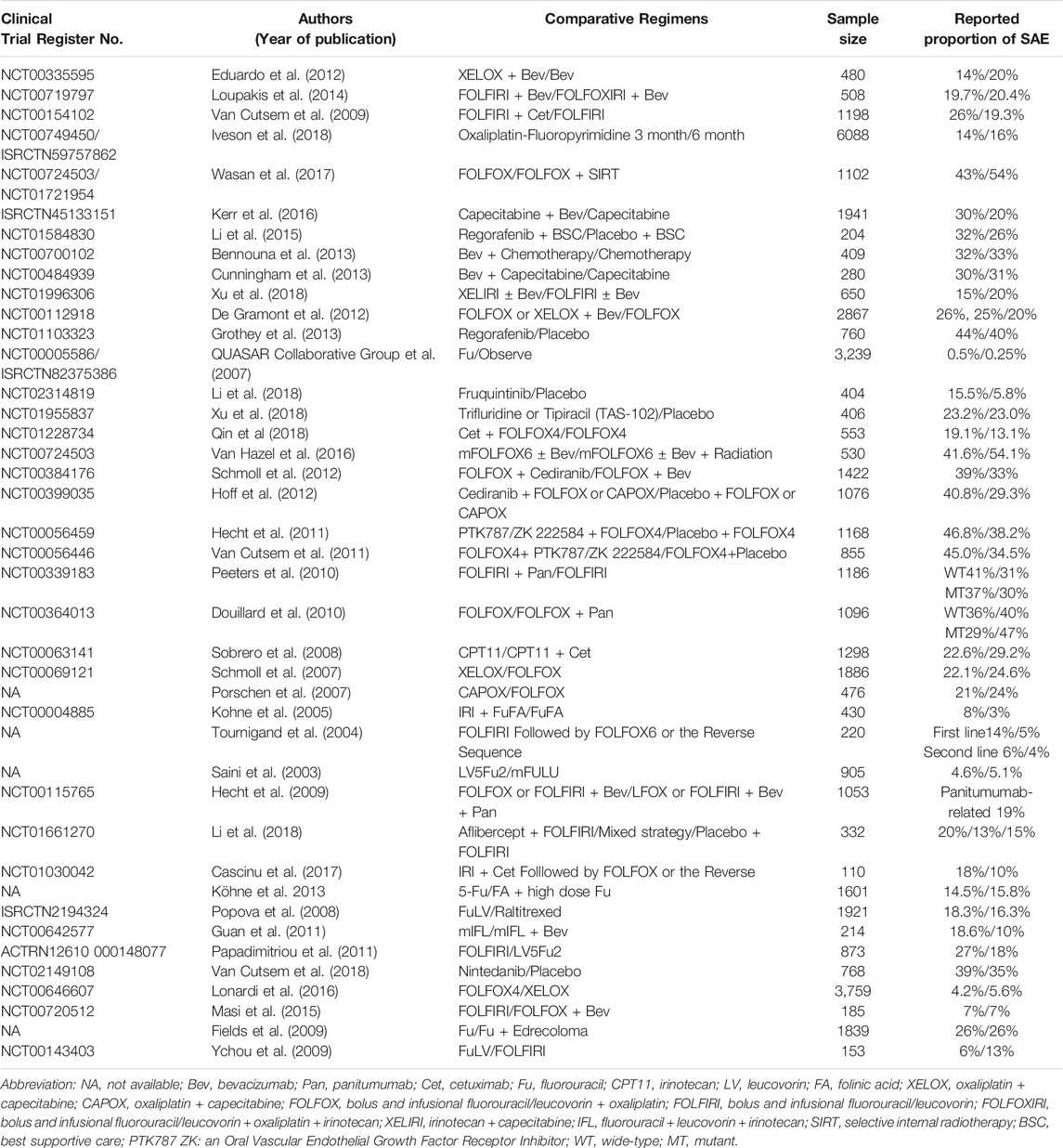
From the 1560 publications initially collected by the two investigators, a total of 160 publications (Supplementary Material) were included in this analysis according to the eligible criteria (Figure 1). The characteristics of the 160 included publications were listed in Table 1. There were more trials conducted in local region (139, 86.9%) than worldwide (21, 13.1%). Ninety-four (58.8%) articles were published in journals such as N Engl J Med, Lancet, JAMA, Lancet Oncol and J Clin Oncol, and one hundred fifty-four (96.3%) articles were published after 2000. The sample size of one hundred and twenty (75.0%) articles was greater than 300. One hundred and six (66.2%) clinical trials researched treatment of metastatic colorectal cancer.
The Performance, Outcomes and Prognosis of SAEs
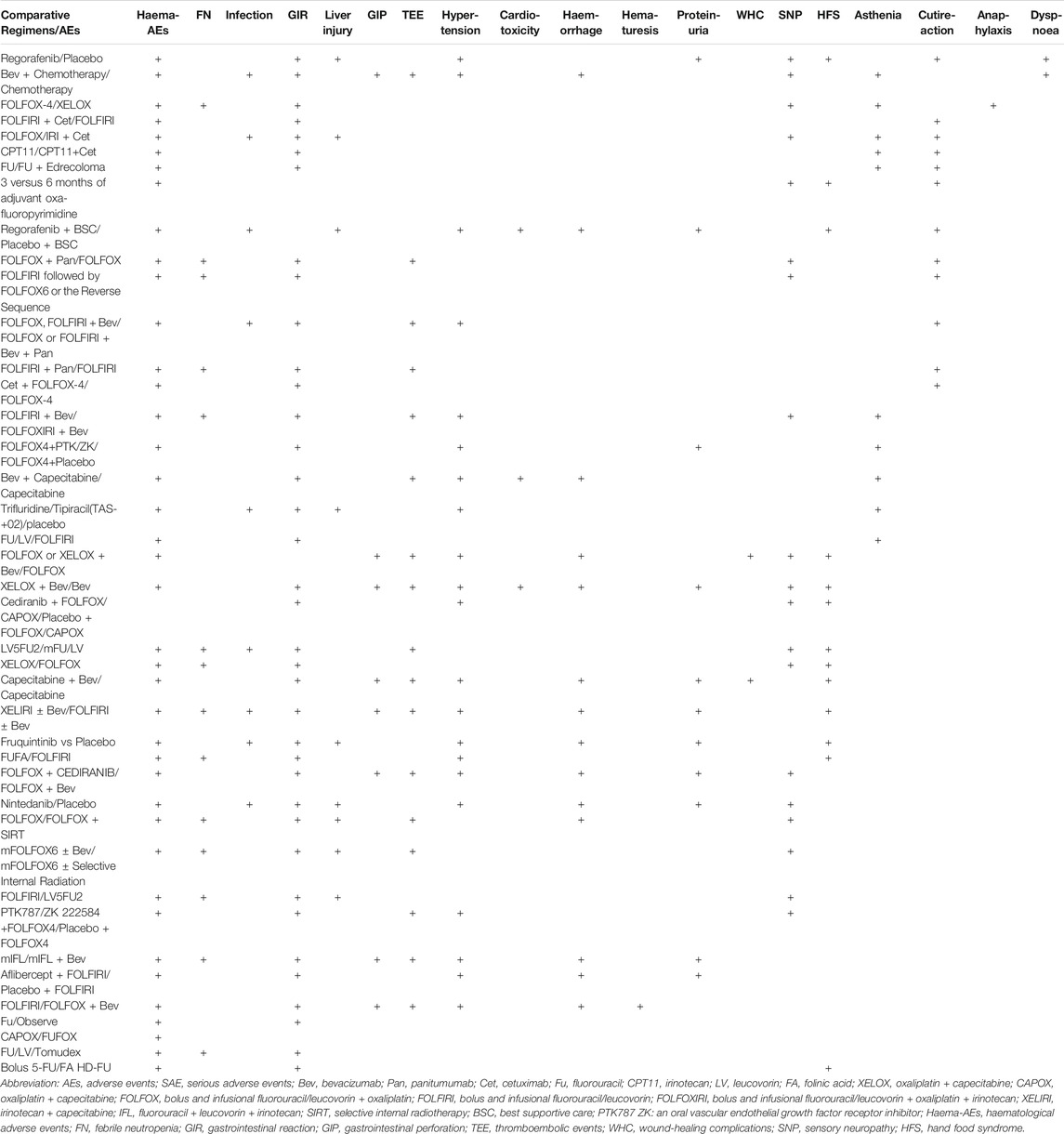
Forty-one (25.5%) of the 160 included publications reported SAEs (Table 2). Only six publications described the performance of SAEs in detail. None described the detailed treatment process for the SAEs. All of the publications that reported SAEs listed grade 3/4 AEs (Table 3). Grade 3/4 hematological toxicity (40/41) and gastrointestinal reactions (37/41) were the most common. Hypertension, proteinuria, and gastrointestinal perforation were more common for anti-vascular drugs. Hand-foot syndrome (HFS) was more common for capecitabine and regorafenib. Skin reactions were more common for cetuximab and panitumumab.
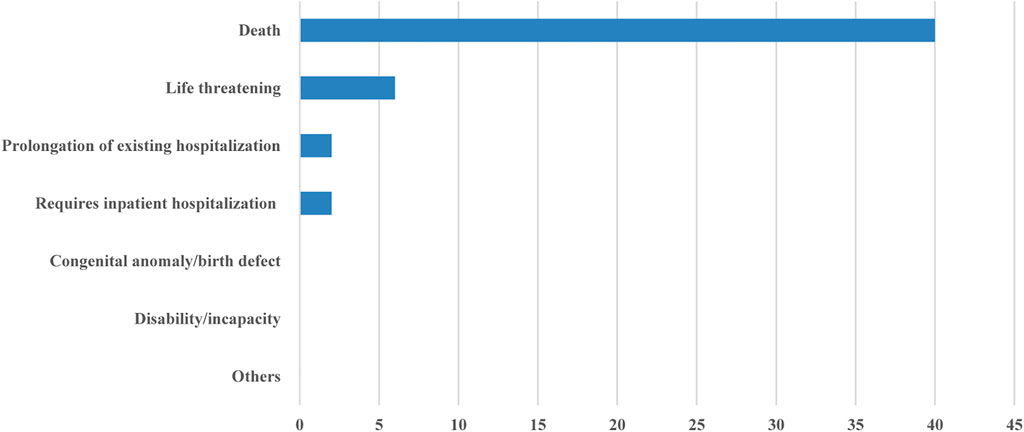
Of the forty-one publications that reported SAEs, forty publications reported whether the SAEs resulted in death, and thirty-seven publications reported the relationship between death and the treatment, and only fifteen reported the relationship between the non-death SAEs and the anticancer treatment. The proportion of deaths caused by SAEs was as follows: less than 1% in nineteen clinical trials, 1–5% in sixteen clinical trials, and 5–10% in five clinical trials. Six publications reported whether the SAEs were life-threatening, and only two publications reported the prognosis of SAEs in detail (Figure 2).
Analysis of the RP of SAEs
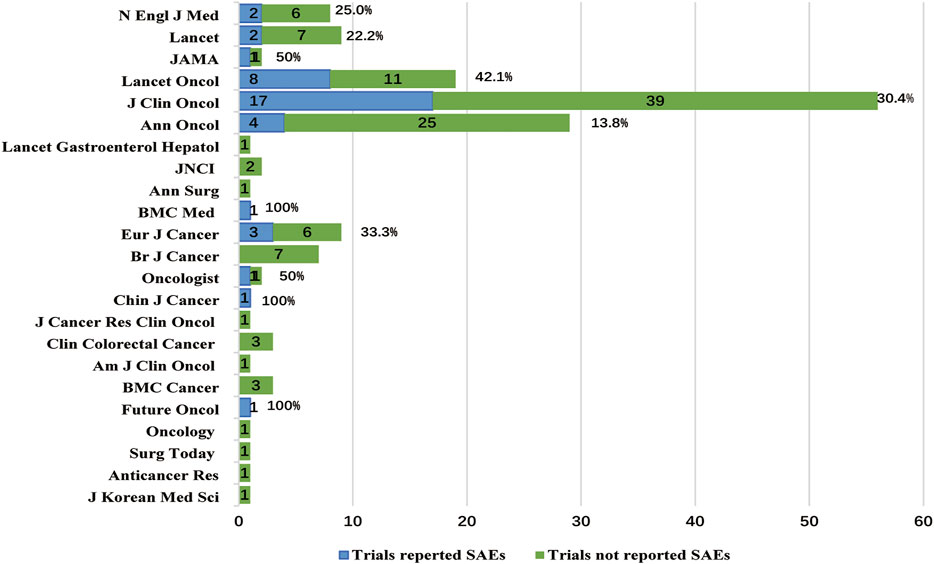
Chi-square tests (Table 4) showed that the RP of SAEs in clinical trials conducted worldwide (52.4% [11/21]) was higher than conducted in local region (21.6% [30/139], p = 0.003, Figure 3A). The RP of SAEs was more than twice in clinical trials sponsored by pharmaceutical companies (57.6% [19/33]) as much as sponsored by investigators (20.7% [17/82], p < 0.001). Clinical trials examining new drugs (45.5% [25/55]) liked to report SAEs more than those not examining new drugs (15.2% [16/105], p < 0.001). Clinical trials with larger sample sizes (≥1000, 43.9% [18/41]) seemed to have a greater RP of SAEs than those with medium sample sizes (300–999, 20.3% [16/79], p =0.006) and small sample sizes (<300, 17.5% [7/40], p = 0.010, Figure 3B). The RP of SAEs increased over time. The RP of SAEs in articles published after 2009 (34.5% [29/84]) was higher than that published from 2000 to 2009 (17.1% [12/70], p = 0.015) and published before 2000 (none [0/6], p = 0.171, Figure 3C). The RP of SAEs in clinical trials whose results were referenced by the guidelines (32.0% [31/97]) was greater than that not referenced by guidelines (15.9% [10/63]) (p = 0.023). The RPs of SAEs in studies published in famous journals were as follows: 25% [2/8] in N Engl J Med, 22.2% [2/9] in Lancet, 42.1% [8/19] in Lancet Oncol, 50.0% [1/2] in JAMA and 30.4% [17/56] in J Clin Oncol (Figure 4), with an average RP of SAEs of 31.9% (30/94), which was significantly higher than that in studies published in other journals (16.7%, [11/66], p = 0.030). The RP of SAEs was significantly higher in clinical trials about second line and above treatment than those about first line and adjuvant/neoadjuvant treatment, and higher in clinical trials researched targeted therapy ± chemotherapy than those researched other therapeutic schedules (Table 4).
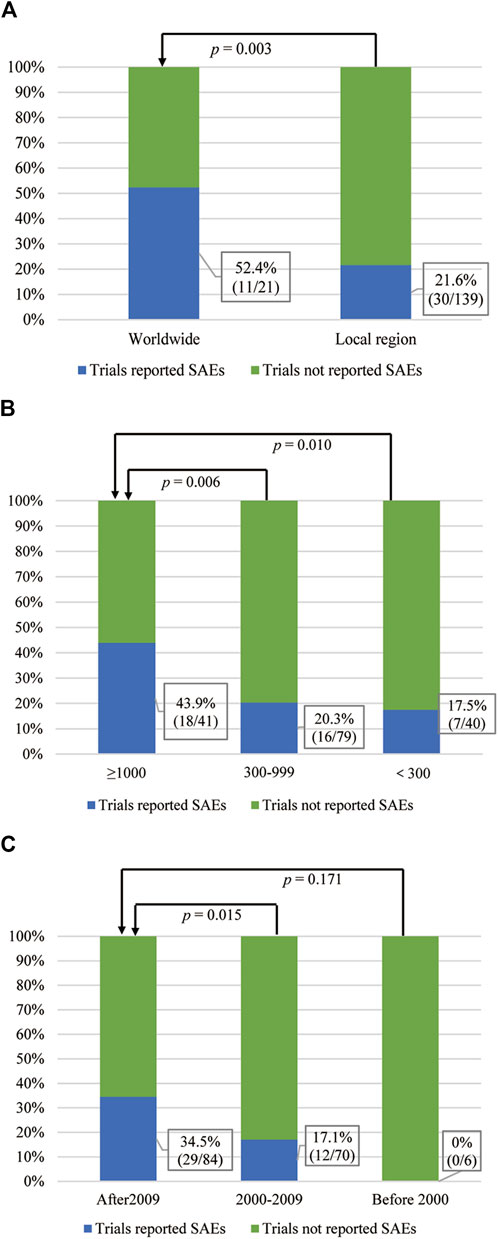
FIGURE 3. The correlation between the RP of SAEs and the influencing factors. (A) Region where clinical trials conducted and SAEs reported status. (B) Sample size and SAE reported status. (C) Publication time and SAEs reported status.
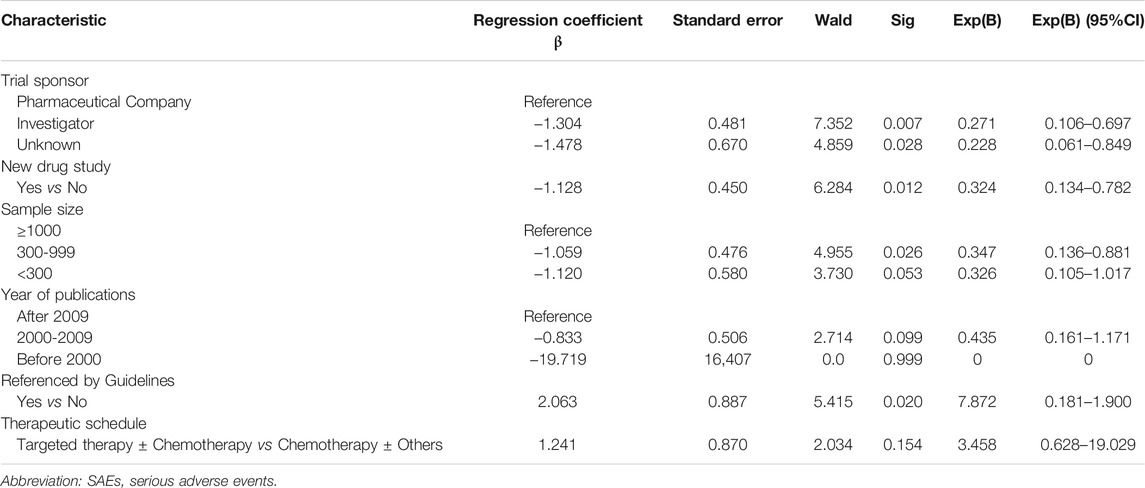
After adjusting for the nine factors, logistic regression analysis showed that pharmaceutical company sponsorship, new drug research and a sample size greater than 1000 were positive influencing factors for SAEs reporting (Table 5).
Discussion
The registration rate of oncology clinical trials has significantly increased since 2005 (Song and Kim, 2020), and the number of clinical trials for anticancer drugs has also increased in the past decade in China (Li et al., 2019). The China Food and Drug Administration (CFDA) has issued a series of innovations to accelerate new agent approvals in oncology (Wang, 2017). Randomized phase III clinical trials are considered to be the gold standard in clinical practice. Therefore, clinical trials and SAEs reports lay the foundation for selecting anticancer treatments and managing AEs in real-world practice. Chemotherapy and targeted therapy are still mainstream treatments in colorectal cancer, one of the most common malignancies worldwide. Safety is one of the leading factors in clinical decision-making, affecting patient quality of life and the benefit-risk ratio.
This article retrospectively analyzed 160 publications that met the inclusion criteria and showed that the RP of SAEs in phase III colorectal cancer clinical trials was only 25.5%, significantly lower than that of AEs, which was reported to be 96% in cancer clinical trials in a retrospective study (Sivendran et al., 2014). One of the reasons for the low RP of SAEs was insufficient attention to SAEs reports. Some researchers believed that systematic and complete SAEs reporting increased the workload and costs when the purpose of a clinical trial was only to verify drug efficacy (Wallace et al., 2016). Therefore, inadequate research funding was the other reason (Wallace et al., 2016).
Most publications included in this article did not report the type or prognosis of SAEs in detail. This was similar to a study examining the quality of SAEs reporting to sponsors by investigators from all clinical trials performed at Limoges University Hospital in 2012 (Crépin et al., 2016). In this study, 3.6% of the reports did not describe the seriousness of the SAEs, 9.3% were missing a causality assessment, and the date of SAEs onset was not mentioned in 5.7% of the reports. This phenomenon may be due to the lack of standard guidelines for SAEs reporting in clinical trials. On the other hand, the journal’s word count requirements may limit the author’s ability to provide a detailed SAEs description. The severity and duration of SAEs directly affect the prognosis and quality of life of patients, and both are essential factors for SAEs reports (Sartor, 2017). Detailed descriptions of the manifestation, severity, duration, and outcome of SAEs in phase III clinical trials, whose results have important reference value for clinical guidelines, have crucial guiding significance for real-world clinical practice. Therefore, in the future, journals about SAEs and SAEs case reports should be established for reporting SAEs in detail to better guide clinical practice and drug research and development, thereby improving cancer treatments and maximizing the benefits of patients.
The manifestations of AEs in patients with colorectal cancer were related to the drugs. The skin reactions reported in this article were more common for anti-EGFR antibodies, such as cetuximab and panitumumab, which was similar to previous reports. Some reviews and phase II clinical trials showed that the incidence of AEs and grade 3/4 AEs were 66.7% (Lynch et al., 2007) and 8%-16% (Soeda et al., 2014; Soda et al., 2015), respectively, for patients treated with cetuximab and 74.7% (Bouché et al., 2019) and 9%-15% (Nishi et al., 2016; Munemoto et al., 2018), respectively, for panitumumab. HFS was more common for regorafenib and capecitabine in this study. It has been reported that the incidence of HFS and grade 3/4 HFS were 65–69% and 15–16%, respectively, for regorafenib (Bekaii-Saab et al., 2019), and the incidence of grade 3/4 HFS for capecitabine was 8% (Soda et al., 2015) in non-phase III clinical trials. This study showed that regorafenib was related to hypertension and dyspnea, whose previously reported incidences were 62%–70% and 19%–23%, respectively, and the incidences above grade 3 were 7%–15% and 4%–6%, respectively (Bekaii-Saab et al., 2019). The incidences of hypertension, proteinuria, gastrointestinal perforation, and thrombosis were more common for bevacizumab, which was consistent with the results of many phase II clinical trials (Chen et al., 2006; Horita et al., 2011; Hong et al., 2012; Nakayama et al., 2012).
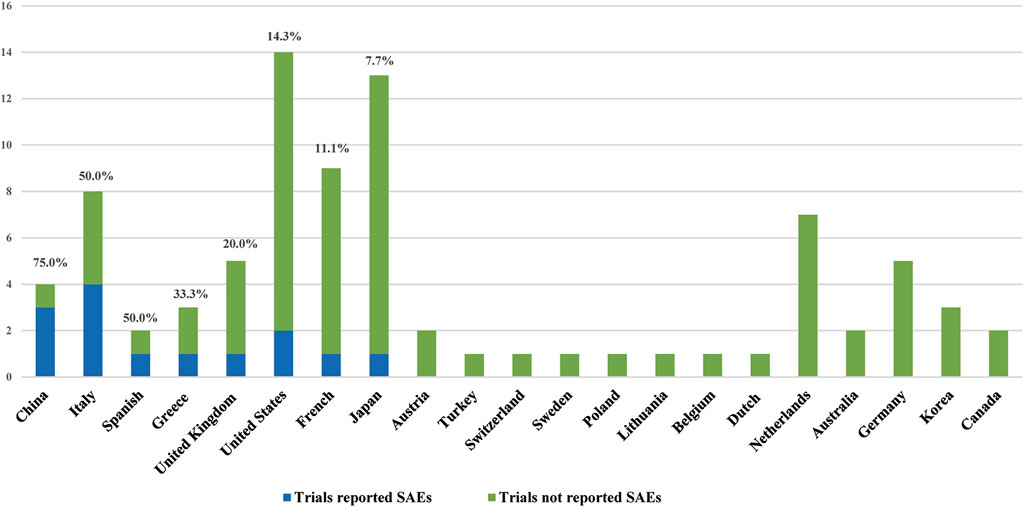
The chi-square analysis in this study showed that the RP of SAEs in clinical trials conducted worldwide (52.4%) was higher than that in those conducted in local region (21.6%). The worldwide clinical research is supervised and reviewed by an international ethics committee and global regulatory agencies. The management system is stricter, so the reporting of SAEs is more stringent. In addition, clinical studies conducted in only one country had various reports of SAEs. The RP of SAEs was higher in China, Spain, Italy, and Greece, at 75, 50, 50, and 33.3%, respectively (Figure 5). This may be related to the differences in supervision and management of clinical research in different regions and the differences in policies and regulations.
The RP of SAEs in new drug clinical research (45.5%) was significantly higher than that in non-new drug clinical studies (15.2%) (p < 0.001). In addition to the effectiveness of new drugs, the safety of new drugs was of paramount concern, so the RP of SAEs was higher. Non-new drug research mainly compared the efficacy of different treatment regimens and paid less attention to SAEs, and the RP of SAEs was lower. The SAEs reporting rate of clinical studies initiated by pharmaceutical companies (57.6%) was higher than that of investigators (20.7%) (p < 0.001). Among 33 clinical studies initiated by pharmaceutical companies, 69.7% (23) were new drug-related clinical studies, while only 39% (32 of 82) of clinical studies undertaken by investigators were new drug studies. The RP of SAEs in new drug clinical research was higher, so the RP of SAEs in clinical research initiated by pharmaceutical companies was higher. This was also the reason why the RP of SAEs was higher in clinical trials about second line and above treatment, and higher in targeted therapy based clinical trials.
This study showed that the RP of SAEs increased in the past 26 years, which may be attributed to the following. First, the National Health and Medical Research Council has provided increasingly rigorous regulations about how SAEs should be reported (Wallace et al., 2016). Second, the increasing attention paid to drug research safety has promoted the monitoring and management of data for clinical trials (Bhattacharyya et al., 2018). Pharmaceutical companies and journal editors have made recommendations on AEs (including SAE) reporting after a thorough discussion on how policies and guidelines were followed, what challenges existed, and how challenges should be addressed to improve AEs and SAEs reporting in clinical research publications to enhance the degree of authenticity and accuracy of clinical trial data (Lineberry et al., 2016). Third, the training recommendations in the Good Clinical Practice (GCP) guidelines require investigators and study coordinators executing a clinical trial to undergo training on GCP principles every 3 years (Shanley et al., 2017), enhancing investigators’ compliance with GCP (Kuusisto et al., 2011). Finally, SAEs reports are processed by an automated computer system instead of personal reports with the development of information technology, saving workforce resources and time and facilitating the analysis of reporting performance and the nature of SAEs reports (London et al., 2009; Pecoraro and Luzi, 2011). AEs capture and management systems for cancer clinical trials were set up to administer and manage clinical trials, improving the efficiency, accuracy, and safety of AEs reports (Lencioni et al., 2015).
The top five journals for RPs of SAEs were N Engl J Med, Lancet, Lancet Oncol, JAMA, J Clin Oncol, with an average RP of SAEs 31.9%, which was significantly higher than that of other journals (16.7%, p = 0.030). This was affected by the journal’s requirements. For example, Lancet has provided readers with links to websites that published clinical trial protocols since 2009, and J Clin Oncol has disclosed agreements that were previously only open to journal editors and reviewers since 2011 (Song and Kim, 2020). The improvement of clinical trial transparency is beneficial to the authenticity of clinical research data.
Patients in some clinical trials completed electronic surveys regarding symptomatic AEs according to the Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) (Hagelstein et al., 2016) of the National Cancer Institute (NCI) during cancer treatment, which was demonstrated to be both feasible and informative (Chung et al., 2019). A pooled analysis showed that in oncology clinical trials, PRO and AEs reports had a different focus and were complementary (Atherton et al., 2015). Other systematic reviews showed that reported agreement between CTCAE and PRO ratings was poor to moderate in most trials (Atkinson et al., 2016). They provided evidence that PROs provided unique, valuable information that can complement CTCAE ratings, avoiding loss of AEs information because of a long interval between visits (Atkinson et al., 2016). The PRO-CTCAE included a rigorous method for capturing patient self-reports of symptomatic AEs in cancer clinical trials (Hagelstein et al., 2016) but has not been used worldwide. The effective combination of the PRO-CTCAE and clinician-reported CTCAE may be better for the management of AEs, especially SAEs, in cancer patients.
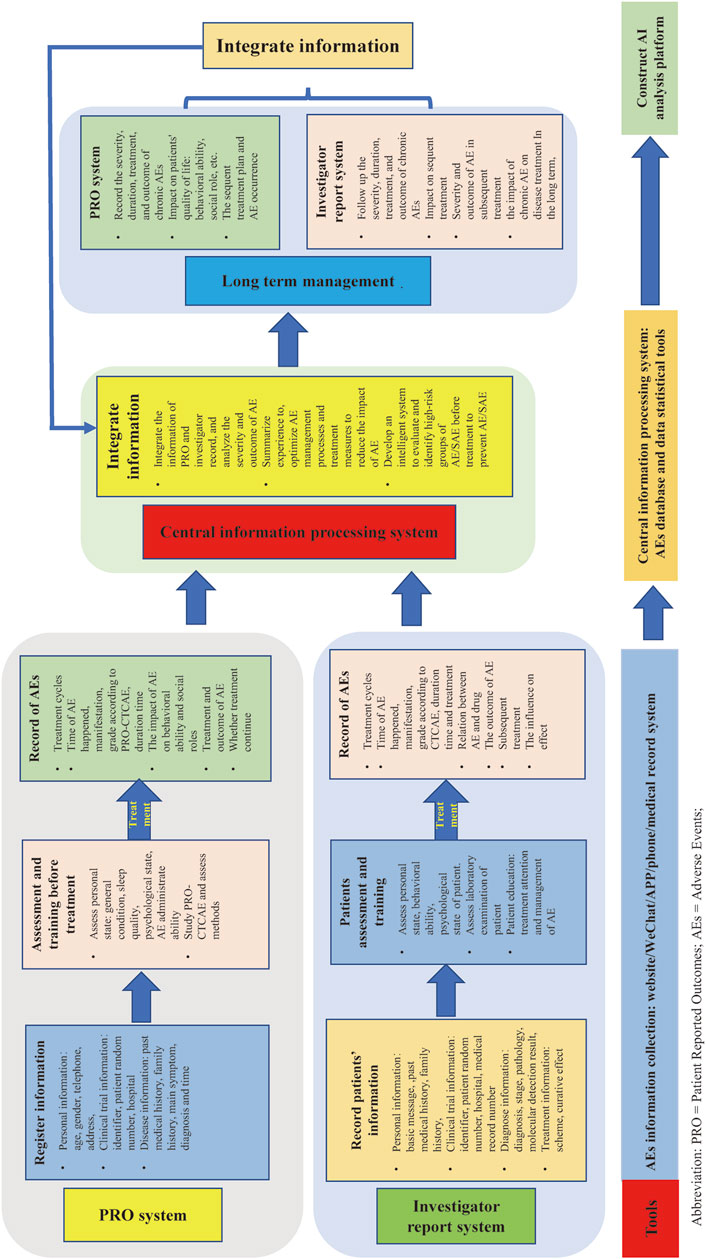
SAEs reports need more improvement. For example, improving the construction of SAEs reporting systems in electronic information platforms, establishing precise process and data collection methods, strengthening the training of medical staff, enhancing the safety ability assessment of patients via patient education, and improving the awareness and attention of SAEs have been reported. The authors believe that co-report of AEs/SAEs via PRO and researchers in clinical trials should be adopted in the future (Figure 6).
There were some shortcomings in this study. First, this was a retrospective study, and there may be omissions in data collection and selection bias. Second, the identification criteria for SAEs may vary because of the diverse designs of clinical trials and different judgment criteria of investigators. Third, bias existed in the data collection because the descriptions of SAEs in the publications were inconsistently attributed to the journal-specific publication requirements. Finally, this study’s included publications were all published clinical trials, and unpublished clinical trials, such as clinical trials with negative research results, were excluded. The reporting methods for SAEs have gradually improved as people pay increasing attention to SAEs. Independent reporting of SAEs by patients and researchers may better guide clinical practice and drug development in the future.
Conclusion
In conclusion, our findings showed that the RP of SAEs increased and aroused more researchers’ attention over time. However, more efforts should be made to improve the RP of SAEs and the quality of SAEs reporting. The patterns and outcomes of SAEs should be reported in detail and given more attention to better guide drug application by clinicians in the real world. In addition, independent reporting of SAEs by patients and researchers should be encouraged.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.754858/full#supplementary-material
References
André, T., Boni, C., Navarro, M., Tabernero, J., Hickish, T., Topham, C., et al. (2009). Improved Overall Survival with Oxaliplatin, Fluorouracil, and Leucovorin as Adjuvant Treatment in Stage II or III colon Cancer in the MOSAIC Trial. J. Clin. Oncol. 27 (19), 3109–3116. doi:10.1200/jco.2008.20.6771
Atherton, P. J., Watkins-Bruner, D. W., Gotay, C., Moinpour, C. M., Satele, D. V., Winter, K. A., et al. (2015). The Complementary Nature of Patient-Reported Outcomes and Adverse Event Reporting in Cooperative Group Oncology Clinical Trials: a Pooled Analysis (NCCTG N0591). J. Pain Symptom Manage. 50 (4), 470–e9. e479. doi:10.1016/j.jpainsymman.2015.04.016
Atkinson, T. M., Ryan, S. J., Bennett, A. V., Stover, A. M., Saracino, R. M., Rogak, L. J., et al. (2016). The Association between Clinician-Based Common Terminology Criteria for Adverse Events (CTCAE) and Patient-Reported Outcomes (PRO): a Systematic Review. Support Care Cancer 24 (8), 3669–3676. doi:10.1007/s00520-016-3297-9
Bennouna, J., Sastre, J., Arnold, D., sterlund, P., Greil, R., Van Cutsem, E., et al. (2013). Continuation of Bevacizumab After First Progression in Metastatic Colorectal Cancer (ML18147): A Randomised Phase 3 Trial. Lancet Oncol. 14, 29–37. doi:10.1016/S1470-2045(12)70477-1
Bekaii-Saab, T. S., Ou, F. S., Ahn, D. H., Boland, P. M., Ciombor, K. K., Heying, E. N., et al. (2019). Regorafenib Dose-Optimisation in Patients with Refractory Metastatic Colorectal Cancer (ReDOS): a Randomised, Multicentre, Open-Label, Phase 2 Study. Lancet Oncol. 20 (8), 1070–1082. doi:10.1016/s1470-2045(19)30272-4
Bhattacharyya, A., Gallo, P., Crisp, A., Lavange, L., Molenberghs, G., Pétavy, F., et al. (2018). The Changing Landscape of Data Monitoring Committees-Perspectives from Regulators, Members, and Sponsors. Biom. J. 61 (5), 1232–1241. doi:10.1002/bimj.201700307
Bouché, O., Ben Abdelghani, M., Labourey, J. L., Triby, S., Bensadoun, R. J., Jouary, T., et al. (2019). Management of Skin Toxicities during Panitumumab Treatment in Metastatic Colorectal Cancer. World J. Gastroenterol. 25 (29), 4007–4018. doi:10.3748/wjg.v25.i29.4007
Cascinu, S., Rosati, G., Nasti, G., Lonardi, S., Zaniboni, A., Marchetti, P., et al. (2017). Treatment Sequence with Either Irinotecan/Cetuximab Followed By FOLFOX-4 or the Reverse Strategy in Metastatic Colorectal Cancer Patients Progressing After First-Line FOLFIRI/Bevacizumab: An Italian Group For the Study of Gastrointestinal Cancer Phase III, Randomised Trial Comparing Two Sequences of Therapy in Colorectal Metastatic Patients. Eur. J. Cancer 83, 106e115. doi:10.1016/j.ejca.2017.06.029
Chen, H. X., Mooney, M., Boron, M., Vena, D., Mosby, K., Grochow, L., et al. (2006). Phase II Multicenter Trial of Bevacizumab Plus Fluorouracil and Leucovorin in Patients with Advanced Refractory Colorectal Cancer: an NCI Treatment Referral Center Trial TRC-0301. J. Clin. Oncol. 24 (21), 3354–3360. doi:10.1200/jco.2005.05.1573
Chung, A. E., Shoenbill, K., Mitchell, S. A., Dueck, A. C., Schrag, D., Bruner, D. W., et al. (2019). Patient Free Text Reporting of Symptomatic Adverse Events in Cancer Clinical Research Using the National Cancer Institute's Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). J. Am. Med. Inform. Assoc. 26 (4), 276–285. doi:10.1093/jamia/ocy169
Common Terminology Criteria for Adverse Events (CTCAE) v5.0 (2017). Common Terminology Criteria for Adverse Events (CTCAE) v5.0. U.S. Department of Health and Human Services National Institutes of Health National Cancer Institute. Available at https://www.meddra.org/ (Cited November 27, 2017).
Crépin, S., Villeneuve, C., and Merle, L. (2016). Quality of Serious Adverse Events Reporting to Academic Sponsors of Clinical Trials: Far from Optimal. Pharmacoepidemiol. Drug Saf. 25 (6), 719–724. doi:10.1002/pds.3982
Cunningham, D., Lang, I., Marcuello, E., Lorusso, V., Ocvirk, J., Shin, D.B., et al. (2013). Bevacizumab Plus Capecitabine Versus Capecitabine Alone in Elderly Patients with Previously Untreated Metastatic Colorectal Cancer (AVEX): An Open-Label, Randomised Phase 3 Trial. Lancet Oncol. 14, 1077–1085. doi:10.1016/S1470-2045(13)70154-2
De Gramont, A., Van Cutsem, E., Schmoll, H.-J., Tabernero, J., Clarke, S., Moore, M.J., et al. (2012). Bevacizumab Plus Oxaliplatin-Based Chemotherapy as Adjuvant Treatment For Colon Cancer (AVANT): A Phase 3 Randomised Controlled Trial. Lancet Oncol. 13 (12), 1225–1233. doi:10.1016/s1470-2045(12)70509-0
Douillard, J.-Y., Siena, S., Cassidy, J., Tabernero, J., Burkes, R., Barugel, M., et al. (2010). Randomized, Phase III Trial of Panitumumab with Infusional Fluorouracil, Leucovorin, And Oxaliplatin (FOLFOX4) Versus FOLFOX4 Alone as First-Line Treatment in Patients with Previously Untreated Metastatic Colorectal Cancer: The PRIME Study. J. Clin. Oncol. 28 (31), 4697–4705. doi:10.1200/jco.2009.27.4860
Eduardo, D.-R., Auxiliadora, G.-E., Bartomeu, M., Javier, S., Albert, A., Manuel, V., et al. (2012). First-Line XELOX Plus Bevacizumab Followed By XELOX Plus Bevacizumab or Single-Agent Bevacizumab As Maintenance Therapy in Patients with Metastatic Colorectal Cancer: The Phase III MACRO TTD Study. Oncologist 17, 15–25. doi:10.1634/theoncologist.2011-0249
Fields, A.L.A., Keller, A., Schwartzberg, L., Bernard, S., Kardinal, C., Cohen, A., et al. (2009). Adjuvant Therapy with the Monoclonal Antibody Edrecolomab Plus Fluorouracil-Based Therapy Does Not Improve Overall Survival of Patients with Stage III Colon Cancer. J. Clin. Oncol. 27 (12), 1941–1947. doi:10.1200/jco.2008.18.5710
Fitzmaurice, C., Fitzmaurice, C., Allen, C., Barber, R. M., Barregard, L., Bhutta, Z. A., et al. (2017). Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-Years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 3 (4), 524–548. doi:10.1001/jamaoncol.2016.5688
Grothey, A., Van Cutsem, E., Sobrero, A., Siena, S., Falcone, A., Ychou, M., et al. (2013). Regorafenib Monotherapy for Previously Treated Metastatic Colorectal Cancer (CORRECT): an International, Multicentre, Randomised, Placebo-Controlled, Phase 3 Trial. Lancet 381 (26), 303–312. doi:10.1016/S0140-6736(12)61900-X
Group, Q. C., Gray, R., Barnwell, J., McConkey, C., Hills, R. K., Williams, N. S., et al. (2007). Adjuvant Chemotherapy versus Observation in Patients with Colorectal Cancer: a Randomised Study. Lancet 370 (9604), 2020–2029. doi:10.1016/s0140-6736(07)61866-2
Guan, Z.Z., Xu, J.M., Luo, R.C., Feng, F.Y., Wang, L.-W., Shen, L., et al. (2011). Efficacy And Safety of Bevacizumab Plus Chemotherapy in Chinese Patients with Metastatic Colorectal Cancer: A Randomized Phase III ARTIST Trial. Chin. J. Cancer. 30 (10), 682–689. doi:10.5732/cjc.011.10188
Hagelstein, V., Ortland, I., Wilmer, A., Mitchell, S. A., and Jaehde, U. (2016). Validation of the German Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE™). Ann. Oncol. 27 (12), 2294–2299. doi:10.1093/annonc/mdw422
Hecht, J.R., Mitchell, E., Chidiac, T., Scroggin, C., Hagenstad, C., Spigel, D., et al. (2009). A Randomized Phase IIIB Trial of Chemotherapy, Bevacizumab, And Panitumumab Compared with Chemotherapy And Bevacizumab Alone For Metastatic Colorectal Cancer. J. Clin. Oncol. 27 (5), 672–680. doi:10.1200/jco.2008.19.8135
Hecht, J.R., Trarbach, T., Hainsworth, J.D., Major, P., Jäger, E., Wolff, R.A., et al. (2011). Randomized, Placebocontrolled, Phase III Study of First-Line Oxaliplatin-Based Chemotherapy Plus PTK787/ZK 222584, An Oral Vascular Endothelial Growth Factor Receptor Inhibitor, in Patients with Metastatic Colorectal Adenocarcinoma. J. Clin. Oncol. 29 (15), 1997–2003. doi:10.1200/jco.2010.29.4496
Hoff, P.M., Hochhaus, A., Pestalozzi, B.C., Tebbutt, N.C., Li, J., Kim, T.W., et al. (2012). Cediranib Plus FOLFOX/CAPOX Versus Placebo Plus FOLFOX/CAPOX in Patients with Previously Untreated Metastatic Colorectal Cancer: A Randomized, Double-Blind, Phase III Study (HORIZON II). J. Clin. Oncol. 30 (29), 3596–3603. doi:10.1200/jco.2012.42.6031
Hong, Y. S., Lee, J., Kim, K. P., Lee, J. L., Park, Y. S., Park, J. O., et al. (2012). Multicenter Phase II Study of Second-Line Bevacizumab Plus Doublet Combination Chemotherapy in Patients with Metastatic Colorectal Cancer Progressed after Upfront Bevacizumab Plus Doublet Combination Chemotherapy. Invest. New Drugs 31 (1), 183–191. doi:10.1007/s10637-012-9853-3
Horita, Y., Yamada, Y., Kato, K., Hirashima, Y., Akiyoshi, K., Nagashima, K., et al. (2011). Phase II Clinical Trial of Second-Line FOLFIRI Plus Bevacizumab for Patients with Metastatic Colorectal Cancer: AVASIRI Trial. Int. J. Clin. Oncol. 17 (6), 604–609. doi:10.1007/s10147-011-0331-2
Iveson, T. J., Kerr, R. S., Saunders, M. P., Cassidy, J., Hollander, N. H., Tabernero, J., et al. (2018). 3 versus 6 Months of Adjuvant Oxaliplatin-Fluoropyrimidine Combination Therapy for Colorectal Cancer (SCOT): an International, Randomised, Phase 3, Non-inferiority Trial. Lancet Oncol. 19 (4), 562–578. doi:10.1016/s1470-2045(18)30093-7
Kerr, R.S., Love, S., Segelov, E., Johnstone, E.C., Falcon, B., Hewett, P., et al. (2016). Adjuvant Capecitabine Plus Bevacizumab Versus Capecitabine Alone in Patients with Colorectal Cancer (QUASAR 2): An Open-Label, Randomised Phase 3 Trial. Lancet Oncol. 17, 1543–1557. doi:10.1016/S1470-2045(16)30172-3
Köhne, C.-H., Bedenne, L., Carrato, A., Bouché, O., Popov, I., Gaspà, L., et al. (2013). A Randomised Phase III Intergroup Trial Comparing High-Dose Infusional 5-Fluorouracil with or Without Folinic Acid with Standard Bolus 5-Fluorouracil/Folinic Acid in the Adjuvant Treatment of Stage III Colon Cancer: The Pan-European Trial in Adjuvant Colon Cancer 2 Study. Eur. J. Cancer 49 (8), 1868–1875. doi:10.1016/j.ejca.2013.01.030
Köhne, C.H., Van Cutsem, E., Wils, J., Bokemeyer, C., El-Serafi, M., Lutz, M.P., et al. (2005). Phase III Study of Weekly High-Dose Infusional Fluorouracil Plus Folinic Acid with or Without Irinotecan in Patients with Metastatic Colorectal Cancer: European Organisation For Research And Treatment of Cancer Gastrointestinal Group Study 40986. J. Clin. Oncol. 23 (22), 4856–4865. doi:10.1200/jco.2005.05.546
Kuusisto, H., Virkki, M., Wuolijoki, E., and Keränen, T. (2011). Hospital Training Program Increases Awareness of Good Clinical Practice (GCP). Contemp. Clin. Trials 32 (3), 339–341. doi:10.1016/j.cct.2011.01.011
Lencioni, A., Hutchins, L., Annis, S., Chen, W., Ermisoglu, E., Feng, Z., et al. (2015). An Adverse Event Capture and Management System for Cancer Studies. BMC Bioinformatics 16 Suppl 13 (S13), S6. doi:10.1186/1471-2105-16-s13-s6
Li, J., Qin, S., Xu, R., Yau, T. C., Ma, B., Pan, H., et al. (2015). Regorafenib Plus Best Supportive Care versus Placebo Plus Best Supportive Care in Asian Patients with Previously Treated Metastatic Colorectal Cancer (CONCUR): a Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 16 (6), 619–629. doi:10.1016/s1470-2045(15)70156-7
Li, J., Qin, S., Xu, R. H., Shen, L., Xu, J., Bai, Y., et al. (2018). Effect of Fruquintinib vs Placebo on Overall Survival in Patients with Previously Treated Metastatic Colorectal Cancer: The FRESCO Randomized Clinical Trial. JAMA 319 (24), 2486–2496. doi:10.1001/jama.2018.7855
Li, J., Xu, R., Qin, S., Liu, T., Pan, H., Xu, J., et al. (2018). Aflibercept Plus FOLFIRI in Asian Patients with Pretreated Metastatic Colorectal Cancer: a Randomized Phase III Study. Future Oncol. 14 (20), 2031–2044. doi:10.2217/fon-2017-0669
Li, N., Huang, H. Y., Wu, D. W., Yang, Z. M., Wang, J., Wang, J. S., et al. (2019). Changes in Clinical Trials of Cancer Drugs in mainland China over the Decade 2009-18: a Systematic Review. Lancet Oncol. 20 (11), e619–e626. doi:10.1016/s1470-2045(19)30491-7
Lineberry, N., Berlin, J. A., Mansi, B., Glasser, S., Berkwits, M., Klem, C., et al. (2016). Recommendations to Improve Adverse Event Reporting in Clinical Trial Publications: a Joint Pharmaceutical Industry/journal Editor Perspective. BMJ 355, i5078. doi:10.1136/bmj.i5078
Lonardi, S., Sobrero, A., Rosati, G., Di Bartolomeo, M., Ronzoni, M., Aprile, G., et al. (2016). Phase III Trial Comparing 3–6 Months of Adjuvant FOLFOX4/XELOX in Stage II–III Colon Cancer: Safety And Compliance in the TOSCA Trial. Ann. Oncol. 27 (11), 2074–2081. doi:10.1093/annonc/mdw404
London, J. W., Smalley, K. J., Conner, K., and Smith, J. B. (2009). The Automation of Clinical Trial Serious Adverse Event Reporting Workflow. Clin. Trials 6 (5), 446–454. doi:10.1177/1740774509344778
Loupakis, F., Cremolini, C., Masi, G., Lonardi, S., Zagonel, V., Salvatore, L., et al. (2014). Initial Therapy with FOLFOXIRI And Bevacizumab For Metastatic Colorectal Cancer. N. Engl. J. Med. 371 (23), 1609–1618. doi:10.1056/NEJMoa1403108
Lynch, T. J., Kim, E. S., Eaby, B., Garey, J., West, D. P., and Lacouture, M. E. (2007). Epidermal Growth Factor Receptor Inhibitor-Associated Cutaneous Toxicities: an Evolving Paradigm in Clinical Management. Oncologist 12 (5), 610–621. doi:10.1634/theoncologist.12-5-610
Masi, G., Salvatore, L., Boni, L., Loupakis, F., Cremolini, C., Fornaro, L., et al. (2015). Continuation or Reintroduction of Bevacizumab Beyond Progression To First-Line Therapy in Metastatic Colorectal Cancer: Final Results of the Randomized BEBYP Trial. Ann. Oncol. 26, 724–730. doi:10.1093/annonc/mdv012
Moore, T. J., Cohen, M. R., and Furberg, C. D. (2007). Serious Adverse Drug Events Reported to the Food and Drug Administration, 1998-2005. Arch. Intern. Med. 167 (16), 1752–1759. doi:10.1001/archinte.167.16.1752
Munemoto, Y., Kanda, M., Oba, K., Kim, H. M., Takemoto, H., Denda, T., et al. (2018). A Phase II Trial to Evaluate the Efficacy of Panitumumab Combined with Fluorouracil-Based Chemotherapy for Metastatic Colorectal Cancer: the PF Trial. Cancer Chemother. Pharmacol. 81 (5), 829–838. doi:10.1007/s00280-018-3556-1
Nakayama, G., Uehara, K., Ishigure, K., Yokoyama, H., Ishiyama, A., Eguchi, T., et al. (2012). The Efficacy and Safety of Bevacizumab beyond First Progression in Patients Treated with First-Line mFOLFOX6 Followed by Second-Line FOLFIRI in Advanced Colorectal Cancer: a Multicenter, Single-Arm, Phase II Trial (CCOG-0801). Cancer Chemother. Pharmacol. 70 (4), 575–581. doi:10.1007/s00280-012-1948-1
Nishi, T., Hamamoto, Y., Nagase, M., Denda, T., Yamaguchi, K., Amagai, K., et al. (2016). Phase II Trial of Panitumumab with Irinotecan as Salvage Therapy for Patients with Advanced or Recurrent Colorectal Cancer (TOPIC Study). Oncol. Lett. 11 (6), 4049–4054. doi:10.3892/ol.2016.4532
Papadimitriou, C.A., Papakostas, P., Karina, M., Malettou, L., Dimopoulos, M.A., Pentheroudakis, G., et al. (2011). A Randomized Phase III Trial of Adjuvant Chemotherapy with Irinotecan, Leucovorin And Fluorouracil Versus Leucovorin And Fluorouracil For Stage II And III Colon Cancer: A Hellenic Cooperative Oncology Group Study. BMC Medicine 9 (1). doi:10.1186/1741-7015-9-10
Pecoraro, F., and Luzi, D. (2011). Serious Adverse Event Reporting in a Medical Device Information System. Stud. Health Technol. Inform. 169, 834–838. doi:10.3233/978-1-60750-806-9-834
Peeters, M., Price, T.J., Cervantes, A., Sobrero, A.F., Ducreux, M., Hotko, Y., et al. (2010). Randomized Phase III Study of Panitumumab with Fluorouracil, Leucovorin, And Irinotecan (FOLFIRI) Compared with FOLFIRI Alone as Second-Line Treatment in Patients with Metastatic Colorectal Cancer. J Clin. Oncol. 28 (31), 4706–4713. doi:10.1200/jco.2009.27.6055
Péron, J., Maillet, D., Gan, H. K., Chen, E. X., and You, B. (2013). Adherence to CONSORT Adverse Event Reporting Guidelines in Randomized Clinical Trials Evaluating Systemic Cancer Therapy: a Systematic Review. J. Clin. Oncol. 31 (31), 3957–3963. doi:10.1200/jco.2013.49.3981
Popov, I., Carrato, A., Sobrero, A., Vincent, M., Kerr, D., Labianca, R., et al. (2008). Raltitrexed (Tomudex®) Versus Standard Leucovorin-Modulated Bolus 5-Fluorouracil: Results From the Randomised Phase III Pan-European Trial in Adjuvant Colon Cancer 01 (PETACC-1). Eur. J. Cancer. 44 (15), 2204–2211. doi:10.1016/j.ejca.2008.07.002
Porschen, R., Arkenau, H.-T., Kubicka, S., Greil, R., Seufferlein, T., Freier, W., et al. (2007). Phase III Study of Capecitabine Plus Oxaliplatin Compared with Fluorouracil And Leucovorin Plus Oxaliplatin in Metastatic Colorectal Cancer: A Final Report of the AIO Colorectal Study Group. J. Clin. Oncol. 25 (27), 4217–4223. doi:10.1200/jco.2006.09.2684
Qin, S., Li, J., Wang, L., Xu, J., Cheng, Y., Bai, Y., et al. (2018). Efficacy and Tolerability of First-Line Cetuximab Plus Leucovorin, Fluorouracil, and Oxaliplatin (FOLFOX-4) versus FOLFOX-4 in Patients with RAS Wild-type Metastatic Colorectal Cancer: the Open-Label, Randomized, Phase III TAILOR Trial. J. Clin. Oncol. 36 (30), 3031–3039. doi:10.1200/jco.201810.1200/JCO.2018.78.3183
Saini, A., Norman, A.R., Cunningham, D., Chau, I., Hill, M., Tait, D., et al. (2003). Twelve Weeks of Protracted Venous Infusion of Fluorouracil (5-FU) Is As Effective As 6 Months of Bolus 5-FU And Folinic Acid As Adjuvant Treatment in Colorectal Cancer. Brit. J. Cancer. 88 (12), 1859–1865. doi:10.1038/sj.bjc.6600995
Saltz, L. B., Clarke, S., Díaz-Rubio, E., Scheithauer, W., Figer, A., Wong, R., et al. (2008). Bevacizumab in Combination with Oxaliplatin-Based Chemotherapy as First-Line Therapy in Metastatic Colorectal Cancer: a Randomized Phase III Study. J. Clin. Oncol. 26 (12), 2013–2019. doi:10.1200/jco.2007.14.9930
Sartor, O. (2017). Adverse Event Reporting in Clinical Trials: Time to Include Duration as Well as Severity. Oncologist 23 (1), 1. doi:10.1634/theoncologist.2017-0437
Scharf, O., and Colevas, A. D. (2006). Adverse Event Reporting in Publications Compared with Sponsor Database for Cancer Clinical Trials. J. Clin. Oncol. 24 (24), 3933–3938. doi:10.1200/jco.2005.05.3959
Schmoll, H.-J., Cartwright, T., Tabernero, J., Nowacki, M.P., Figer, A., Maroun, J., et al. (2007). Phase III Trial of Capecitabine Plus Oxaliplatin As Adjuvant Therapy For Stage III Colon Cancer: A Planned Safety Analysis in 1,864 Patients. J. Clin. Oncol. 26 (1), 102–109. doi:10.1200/jco.2006.08.1075
Schmoll, H.-J., Cunningham, D., Sobrero, A., Karapetis, C.S., Rougier, P., Koski, S.L., et al. (2012). Cediranib with Mfolfox6 Versus Bevacizumab with Mfolfox6 as First-Line Treatment For Patients with Advanced Colorectal Cancer: A Doubleblind, Randomized Phase III Study (HORIZON III). J. Clin. Oncol. 30, 3588–3595. doi:10.1200/JCO.2012.42.5355
Shanley, T. P., Calvin-Naylor, N. A., Divecha, R., Wartak, M. M., Blackwell, K., Davis, J. M., et al. (2017). Enhancing Clinical Research Professionals' Training and Qualifications (ECRPTQ): Recommendations for Good Clinical Practice (GCP) Training for Investigators and Study Coordinators. J. Clin. Transl. Sci. 1 (1), 8–15. doi:10.1017/cts.2016.1
Sivendran, S., Latif, A., Mcbride, R. B., Stensland, K. D., Wisnivesky, J., Haines, L., et al. (2014). Adverse Event Reporting in Cancer Clinical Trial Publications. J. Clin. Oncol. 32 (2), 83–89. doi:10.1200/jco.2013.52.2219
Sobrero, A.F., Maurel, J., Fehrenbacher, L., Scheithauer, W., Abubakr, Y.A., Lutz, M.P., et al. (2008). EPIC: Phase III Trial of Cetuximab Plus Irinotecan After Fluoropyrimidine And Oxaliplatin Failure in Patients with Metastatic Colorectal Cancer. J. Clin. Oncol. 26 (14), 2311–2319. doi:10.1200/jco.2007.13.1193
Soda, H., Maeda, H., Hasegawa, J., Takahashi, T., Hazama, S., Fukunaga, M., et al. (2015). Multicenter Phase II Study of FOLFOX or Biweekly XELOX and Erbitux (Cetuximab) as First-Line Therapy in Patients with Wild-type KRAS/BRAF Metastatic Colorectal Cancer: The FLEET Study. BMC Cancer 15 (1), 695. doi:10.1186/s12885-015-1685-z
Soeda, H., Shimodaira, H., Gamoh, M., Ando, H., Isobe, H., Suto, T., et al. (2014). Phase II Trial of Cetuximab Plus Irinotecan for Oxaliplatin- and Irinotecan-Based Chemotherapy-Refractory Patients with Advanced And/or Metastatic Colorectal Cancer: Evaluation of Efficacy and Safety Based on KRAS Mutation Status (T-Core0801). Oncology 87 (1), 7–20. doi:10.1159/000360989
Sonawane, K. B., Cheng, N., and Hansen, R. A. (2018). Serious Adverse Drug Events Reported to the FDA: Analysis of the FDA Adverse Event Reporting System 2006-2014 Database. J. Manag. Care Spec. Pharm. 24 (7), 682–690. doi:10.18553/jmcp.2018.24.7.682
Song, S. Y., and Kim, E. (2020). The Clinical Trial Transparency in Oncology Significantly Increased over the Recent Years. J. Clin. Epidemiol. 119, 100–108. doi:10.1016/j.jclinepi.2019.11.018
Tournigand, C., André, T., Achille, E., Lledo, G., Flesh, M., Mery-Mignard, D., et al. (2004). FOLFIRI Followed by FOLFOX6 or the Reverse Sequence in Advanced Colorectal Cancer: a Randomized GERCOR Study. J. Clin. Oncol. 22 (2), 229–237. doi:10.1200/jco.2004.05.113
Van Cutsem, E., Bajetta, E., Valle, J., Köhne, C.-H., Randolph Hecht, J., Moore, M., et al. (2011). Randomized, Placebo-Controlled, Phase III Study of Oxaliplatin, Fluorouracil, And Leucovorin with or Without PTK787/ZK 222584 in Patients with Previously Treated Metastatic Colorectal Adenocarcinoma. J. Clin. Oncol. 29 (15), 2004–2010. doi:10.1200/jco.2010.29.5436
Van Cutsem, E., Köhne, C. H., Hitre, E., Zaluski, J., Chang Chien, C. R., Makhson, A., et al. (2009). Cetuximab and Chemotherapy as Initial Treatment for Metastatic Colorectal Cancer. N. Engl. J. Med. 360, 1408–1417. doi:10.1056/NEJMoa0805019
Van Cutsem, E., Yoshino, T., Lenz, H.J., Lonardi, S., Falcone, A., Limo´N, M.L., et al. (2018). Nintedanib For the Treatment of Patients with Refactory Metastatic Colorectal Cancer (LUME-Colon 1): A Phase III, International, Randomized, Placebo-Controlled Study. Ann. Oncol. 29, 1955–1963. doi:10.1093/annonc/mdy241
Van Hazel, G.A., Heinemann, V., Sharma, N.K., Findlay, M.P.N., Ricke, J., Peeters, M., et al. (2016). SIRFLOX: Randomized Phase III Trial Comparing First-Line Mfolfox6 (Plus or Minus Bevacizumab) Versus Mfolfox6 (Plus or Minus Bevacizumab) Plus Selective Internal Radiation Therapy in Patients with Metastatic Colorectal Cancer. J. Clin. Oncol. 34 (15), 1723–1731. doi:10.1200/jco.2015.66.1181
Wallace, S., Myles, P. S., Zeps, N., and Zalcberg, J. R. (2016). Serious Adverse Event Reporting in Investigator-Initiated Clinical Trials. Med. J. Aust. 204 (6), 231–233. doi:10.5694/mja15.01007
Wang, M. (2017). Clinical Trials and Drug Approvals Continue to Accelerate in China. Lancet Oncol. 18 (7), 855. doi:10.1016/s1470-2045(17)30406-0
Wasan, H.S., Gibbs, P., Sharma, N.K., Taieb, J., Heinemann, V., Ricke, J., et al. (2017). First-Line Selective Internal Radiotherapy Plus Chemotherapy Versus Chemotherapy Alone in Patients with Liver Metastases From Colorectal Cancer (FOXFIRE, SIRFLOX, And FOXFIRE-Global): A Combined Analysis of Three Multicentre, Randomised, Phase 3 Trials. Lancet Oncol. 18, 1159–1171. doi:10.1016/S1470-2045(17)30457-6
Xu, J., Kim, T.W., Shen, L., Sriuranpong, V., Pan, H., Xu, R., et al. (2018). Results of a Randomized, Double-Blind, Placebocontrolled, Phase III Trial of Trifluridine/Tipiracil (TAS-102) Monotherapy in Asian Patients with Previously Treated Metastatic Colorectal Cancer: The TERRA Study. J. Clin. Oncol. 36 (4), 350-358. doi:10.1200/jco.2017
Xu, RH., Muro, K., Morita, S., Iwasa, S., Han, S.W., Wang, W., et al. (2018). Modified XELIRI (Capecitabine Plus Irinotecan) Versus FOLFIRI (Leucovorin, Fluorouracil, And Irinotecan), Both Either with or Without Bevacizumab, As Second-Line Therapy For Metastatic Colorectal Cancer (AXEPT): A Multicentre, Open-Label, Randomised, Noninferiority, Phase 3 Trial. Lancet Oncol. 19 (5), 660–671. doi:10.1016/s1470-2045(18)30140-2
Ychou, M., Hohenberger, W., Thezenas, S., Navarro, M., Maurel, J., Bokemeyer, C., et al. (2009). A Randomized Phase III Study Comparing Adjuvant 5-Fluorouracil/Folinic Acid with FOLFIRI in Patientsfoll Owing Complete Resection of Liver Metastases From Colorectal Cancer. Ann. Oncol. 20, 1964–1970. doi:10.1093/annonc/mdp236
Keywords: colorectal cancer, phase III clinical trial, reported proportion, real world, SAEs
Citation: Yao Y, Liu Z, Zhang H, Li J, Peng Z, Yu J, Cao B and Shen L (2021) Serious Adverse Events Reporting in Phase III Randomized Clinical Trials of Colorectal Cancer Treatments: A Systematic Analysis. Front. Pharmacol. 12:754858. doi: 10.3389/fphar.2021.754858
Received: 07 August 2021; Accepted: 25 October 2021;
Published: 18 November 2021.
Edited by:
Jeff Guo, University of Cincinnati, United StatesReviewed by:
Adina Turcu-Stiolica, University of Medicine and Pharmacy of Craiova, RomaniaZuhair Abdulrahman Alqahtani, University of Cincinnati, United States
Copyright © 2021 Yao, Liu, Zhang, Li, Peng, Yu, Cao and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baoshan Cao, Y2FvYmFvc2hhbjA3MTFAYWxpeXVuLmNvbQ==; Lin Shen, c2hlbmxpbkBiam11LmVkdS5jbg==
†These authors have contributed equally to this work
 Yanhong Yao
Yanhong Yao Zhentao Liu1
Zhentao Liu1 Hua Zhang
Hua Zhang Jian Li
Jian Li Zhi Peng
Zhi Peng Jinyu Yu
Jinyu Yu Baoshan Cao
Baoshan Cao Lin Shen
Lin Shen