- 1Department of Pharmacy, Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital, Chengdu, China
- 2Personalized Drug Therapy Key Laboratory of Sichuan Province, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
- 3Key Laboratory of Molecular Target and Clinical Pharmacology and the State and NMPA Key Laboratory, School of Pharmaceutical Sciences and the Fifth Affiliated Hospital, Guangzhou Medical University, Guangzhou, China
Background: Ligustrazine injection has been widely used as adjunctive therapy in the treatment of acute cerebral infarction (ACI) during the past decades in China, but its clinical efficacy is not yet well confirmed. This study aims to evaluate the efficacy of ligustrazine injection as adjunctive therapy for ACI.
Methods: Databases including China National Knowledge Infrastructure (CNKI), China Science and Technology Journal Database (VIP), PubMed, Medline, Google Scholar, Chinese Biomedical Literature Database, Cochrane Library, Embase, Sino-Med, Wanfang Database, and Chinese Science Citation Database were systematically searched for the published randomized controlled trials (RCTs) on ligustrazine injection in the treatment of ACI until November 2020. Meta-analysis was performed on the primary outcome measure (i.e., clinical effective rate) and the secondary outcome measure [i.e., neurological deficit score (NDS), fibrinogen, low shear blood viscosity (LBV), and high shear blood viscosity (HBV)]. The quality of the included RCTs was assessed according to the M scoring system (the refined Jadad scale). Sensitivity analysis and subgroup analysis were conducted according to the methodological quality, years of publication, and sample size.
Results: Nineteen RCTs, containing 2022 patients, were included in this study. Meta-analysis indicated that ligustrazine injection combined with Western medicine could achieve a better effect in the treatment of ACI than using Western medicine alone in terms of clinical effective rate (RR = 1.24; 95% CI, 1.19–1.29), NDS (MD = −3.88; 95%CI, −4.51 to −3.61), fibrinogen (MD = −0.59; 95% CI, −0.76 to −0.42), LBV (MD = −2.11; 95% CI, −3.16 to −1.06), and HBV (MD = −0.88; 95% CI, −1.20 to −0.55).
Conclusions: This research indicated that ligustrazine injection as adjunctive therapy seemed to be more effective than using western medicine alone in treating ACI. However, more evidence is required to confirm the efficacy of ligustrazine injection due to the low methodological quality of the included RCTs.
Introduction
Acute cerebral infarction (ACI), a neurological deficit syndrome caused by circulatory dysfunction, is a major disease leading to disability or death. It was estimated that about 6.17 million people died from ACI in 2017 in the world and there is still no effective way to reduce the mortality (Inoue et al., 2006; Johnston et al., 2009; GBD 2016 Stroke Collaborators, 2019). At present, conventional therapy for treating ACI mainly contains anticoagulants, antithrombotics, and thrombolytics (Powers et al., 2018). As the most important therapy in the treatment of ACI, recombinant tissue plasminogen activator (rt-PA) and urokinase (United Kingdom) are the frequently used thrombolytic agents in China (The fourth session of the National Cerebrovascular Conference, 1996). However, the success of thrombolytic treatment is determined by the strict time window (within 4.5 h) and less than 3% of ACI patients can benefit from it (Asadi et al., 2015). Reasonable use of anticoagulation and antiplatelet agents can improve symptoms and reduce the recurrence rate for ACI patients in some degree, but the risk of intracranial hemorrhage may increase with their use [CAST (Chinese Acute Stroke Trial) Collaborative Group, 1997; International Stroke Trial Collaborative Group, 1997; Powers et al., 2018]. Although these Western medicines can accelerate a patient’s recovery to a certain extent, drug side effects and resistances are accompanied by their wide application in clinical practice [CAST (Chinese Acute Stroke Trial) Collaborative Group, 1997; International Stroke Trial Collaborative Group, 1997]. Traditional Chinese medicine (TCM) combined with conventional Western medicine could be a candidate to overcome the above shortcomings because of the remarkable effectiveness, high bioavailability, and rapid action (Yu et al., 2019).
As the dried rhizome of Conioselinum anthriscoides “Chuanxiong”, Chuanxiong (Conioselinum anthriscoides (H.Boissieu) Pimenov & Kljuykov cult.) was first recorded in Shennong’s Classic of Materia Medica (the earliest Pharmacopoeia of China) from the Warring States Period to the Han Dynasty (Zheng et al., 2018). In the past thousands of years, this herbal medicine has been widely used in treating cerebrovascular, cardiovascular, and renal diseases (Zhao et al., 2016). Ligustrazine (2, 3, 5, 6-tetramethylpyrazine, Supplementary Figure S1) is the active ingredient of Chuanxiong Rhizome and it was first isolated in 1957. Ligustrazine injection has been widely used for the treatment of ACI (Gao et al., 2015) and coronary heart disease (Guo et al., 2016) among the physicians in China during the past decades (Shao et al., 2015). Owing to the impressive effects of antithrombosis, the inhibition of platelet aggregation, and protection of endothelium, ligustrazine injection can improve the neurological function, blood flow, and attenuate cerebral ischemia reperfusion injury for ACI patients (Yu et al., 2016). To provide better efficacy in the treatment of ACI, ligustrazine injection is usually combined with Western medicine (Ozagrel sodium injection, edaravone injection, citicoline sodium injection, aspirin, alteplase, etc.) in China.
At present, a large number of clinical studies on ligustrazine injection as adjunctive therapy for ACI have been reported with potential positive results. However, neither systematic review nor meta-analysis on the efficacy of ligustrazine injection combined with Western medicine in the treatment of ACI is reported until now. Herein, this study aims to conduct a comprehensive and PRISMA-compliant systematic review with sensitivity and subgroup analysis to confirm the clinical efficacy of ligustrazine injection as adjunctive therapy in treating ACI (Moher et al., 2009).
Methods
Eligibility Criteria
Two authors independently searched and selected the eligible studies based on the following criteria: (1) Randomized controlled trials (RCTs) concerning the efficacy of ligustrazine injection as adjunctive therapy (ligustrazine injection combined with Western medicine vs. Western medicine alone) for the treatment of ACI were included in this study. The type of Western medicine used in the control group could be classified into antiplatelet drugs (ozagrel sodium injection, aspirin, clopidogrel, venorruton), anticoagulants (low-molecular-weight heparins, calcium injection), thrombolytic drugs (alteplase injection, lumbrokinase), fibrinolytic drugs (fibrinogenase injection), neuroprotectants (edaravone, citicoline sodium injection, nimodipine), statins (simvastatin, stabilizing atherosclerotic plaque), and dehydration drugs (mannitol injection, relieving cerebral edema); (2) duration of intervention was at least 1 week; (3) sample size of the included RCTs was at least 40 participants; (4) the ACI patients should meet the diagnostic criteria according to the standards revised by the fourth National Conference on Cerebrovascular Diseases in 1995 (Chinese society for Neuroscience, 1996); (5) primary outcome measure was the clinical effective rate, calculated with the following equation (Zhang et al., 2015): clinical effective rate (%) = (number of recovered patients + number of patients with significant improvement + number of patients with improvement)/total number of patients × 100%. The reduction of neurological deficit score (NDS) was determined as efficacy criteria. Recovery was determined that NDS was reduced from 91 to 100%. Significant improvement was determined that NDS was reduced from 46 to 90%. Improvement was determined that NDS was reduced from 18 to 45%. Ineffective was determined that NDS was reduced to less than 17%. Secondary outcome measures included NDS, fibrinogen, low shear blood viscosity (LBV), and high shear blood viscosity (HBV).
RCTs were excluded when they did not satisfy the above criteria and: (1) Publication date of studies was not between the years 2007 and 2019; (2) formulation and dosages of intervention in the treatment group or control group were not specifically provided in the RCTs; (3) duplicate publication or incomplete data was found; (4) any other Chinese medicine was involved in the treatment group or control group; and (5) object of RCTs was animal or tissue cell.
Information Sources
The following databases were systematically searched by two authors for the published RCTs on the adjunctive therapy of ligustrazine injection for ACI during the years between 2007 and 2019, including China National Knowledge Infrastructure (CNKI), China Science and Technology Journal Database (VIP), PubMed, Medline, Google Scholar, Chinese Biomedical Literature Database, Cochrane Library, Embase, Sino-Med, Wanfang Database, and Chinese Science Citation Database. The latest search for the databases was performed on November 1, 2020.
Search Strategies
For English databases, the following keywords were searched in separate or joint ways: ligustrazine, tetramethylpyrazine, ligustrazine injection, acute cerebral infarction, cerebral infarction, stroke, and ischemic stroke. For Chinese databases, the following keywords were searched in separate or joint ways: Chuanxiongqin zhusheye (ligustrazine injection); Chuanxiongqin (ligustrazine, tetramethylpyrazine); Jixingnaogengsi (acute cerebral infarction); Naogengsi (cerebral infarction); Zhongfeng (stroke); and Quexuexingcuzhong (ischemic stroke). The detailed search strategy is shown in the Supplementary Text S1. Moreover, the references listed in the selected studies were searched to obtain the additional RCTs.
Study Selection
Basing on the inclusion and exclusion criteria, two authors independently searched the databases for eligible RCTs. Disagreements between the two authors were solved by discussion.
Data Collection Process
`One author screened the full text of the eligible RCTs and collected data. Another author carefully checked the accuracy and completeness of the collected data. Disagreements during this process were solved through discussion with the third author. Review Manager 5.2 (Cochrane Collaboration, Nordic Cochrane Centre, Copenhagen, Denmark) and STATA 12.0 (Stata Corp LLC, Texas, United States) were employed to evaluate the collected data in this meta-analysis.
Data Items
The following items in each study were collected: (1) First author, years of publication, and country of the included RCTs; (2) sample size of ACI patients; (3) therapeutic intervention (dosages and duration) in the treatment and control group; (4) outcome measures; and (5) adverse reactions.
Risk of Bias in Individual Studies
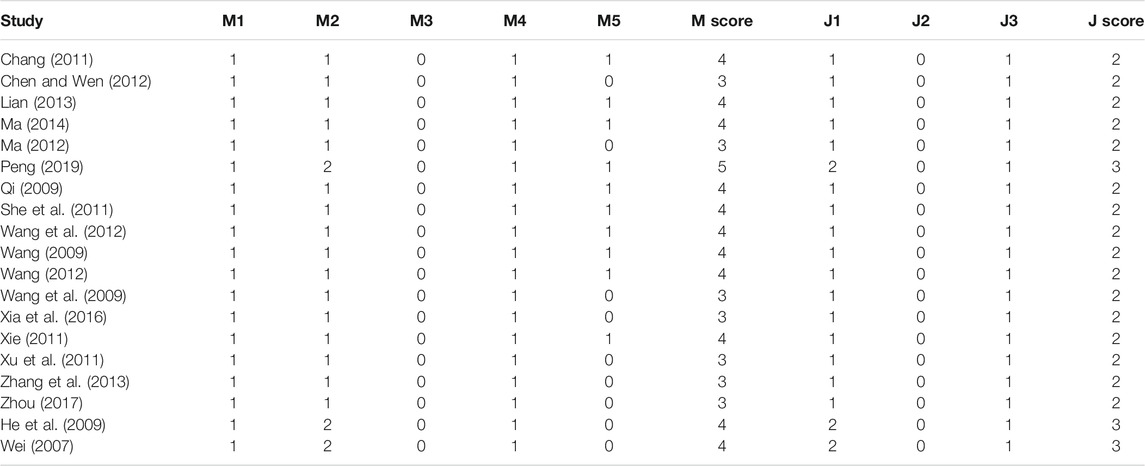
Two authors independently evaluated the quality of the included RCTs basing on the items of randomization methods, concealment of allocation, and blinding in the Jadad score (Supplementary Table S1) and M scoring system (Supplementary Table S2) (Jadad et al., 1996; Shao et al., 2018).
Quality Assessment
Jadad score was employed to assess the included RCTs basing on the description of randomization, blinding, and withdrawals (dropouts), and it ranges from 0 (poorest) to 5 (highest). Scores were obtained if randomization was described in the included RCTs, 1 score; appropriate randomization method, 1 score; double-blinding was described, 1 score; appropriate double-blinding method, 1 score; withdrawals and dropouts were described in the RCT, 1 score. RCTs scored 1 or 2 scores were regarded as low quality and 3 to 5 scores were regarded as high quality. Two authors independently evaluated the methodological quality of the included RCTs according to the Jadad score and M scoring system (range from −1 to 7 scores, RCTs with M score >3 were considered as high quality). Any controversy during the quality evaluation between the two authors was resolved by discussion.
Sensitivity and Subgroup Analysis
In this study, sensitivity analysis was conducted to evaluate the influence of low quality RCTs on the overall efficacy of ligustrazine injection combined with Western medicine in the treatment of ACI. Based on the sample size and years of publication of the included RCTs, subgroup analysis was conducted to evaluate if the overall effects were homogeneous in subgroups.
Publication Bias
In order to evaluate the potential publication bias, the funnel plots generated by STATA 12.0 were adopted in this meta-analysis. Begg’s test and Egger’s test were applied to determine the statistical significance of publication bias.
Statistical Analysis
Systematic review and meta-analysis were performed using RevMan 5.2. Dichotomous variables were expressed as pooled risk ratios (RR) with 95% confidence interval (CI) and continuous variables were expressed as the weighted mean difference (MD) with 95% CI. Heterogeneity across the RCTs was assessed by Chi-squared test and I2 statistic. If p < 0.05 or I2 > 50%, it suggested that there was significant statistical heterogeneity and the random-effect model was used to calculate the outcomes; otherwise, the fixed-effect model was considered. The Z-test was employed to verify the overall effects of ligustrazine injection as adjunctive therapy over using Western medicine alone in the treatment of ACI. Significant statistical difference was considered in this meta-analysis when p < 0.05.
Results
Study Selection
Study selection process and results are shown in Figure 1. A total of 4117 potential studies were initially identified from the databases based on the search strategies in the methods. Among them, 3302 studies were excluded because they were duplications, reviews, or animal experiments. Full texts of 189 studies were subjected to manual screening according to the eligibility criteria described in the methods. Finally, 19 RCTs were included for quality evaluation and meta-analysis.
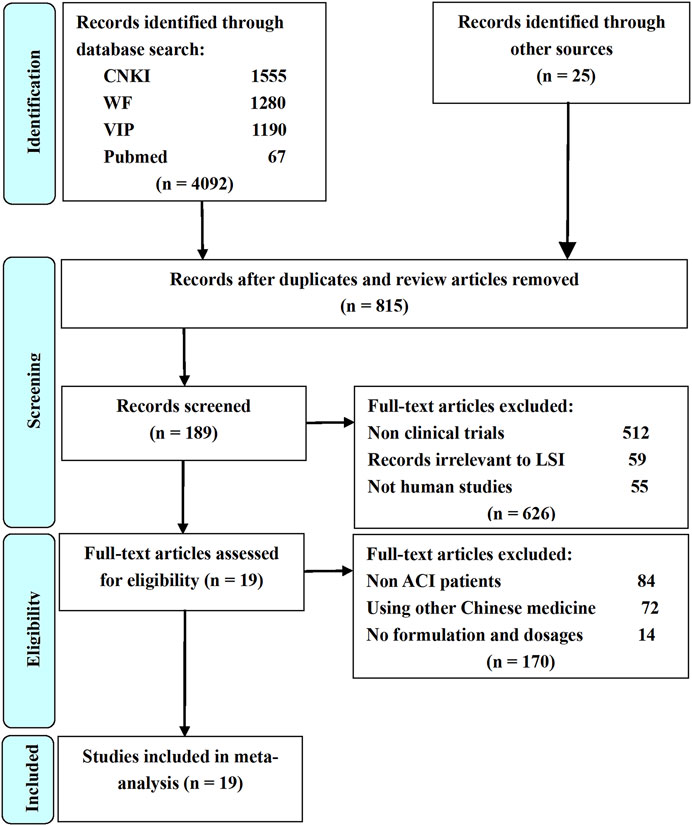
FIGURE 1. Flow diagram of study selection process. LSI is ligustrazine injection; CNKI is China National Knowledge Infrastructure; WF is Wangfang Data; VIP is Chinese Scientific Journal Database; Other sources are Medline, Chinese Biomedical Literature. Database, Cochrane Library, Embase, Google Scholar, Sino-Med, and Chinese Science Citation Database.
Study Characteristics
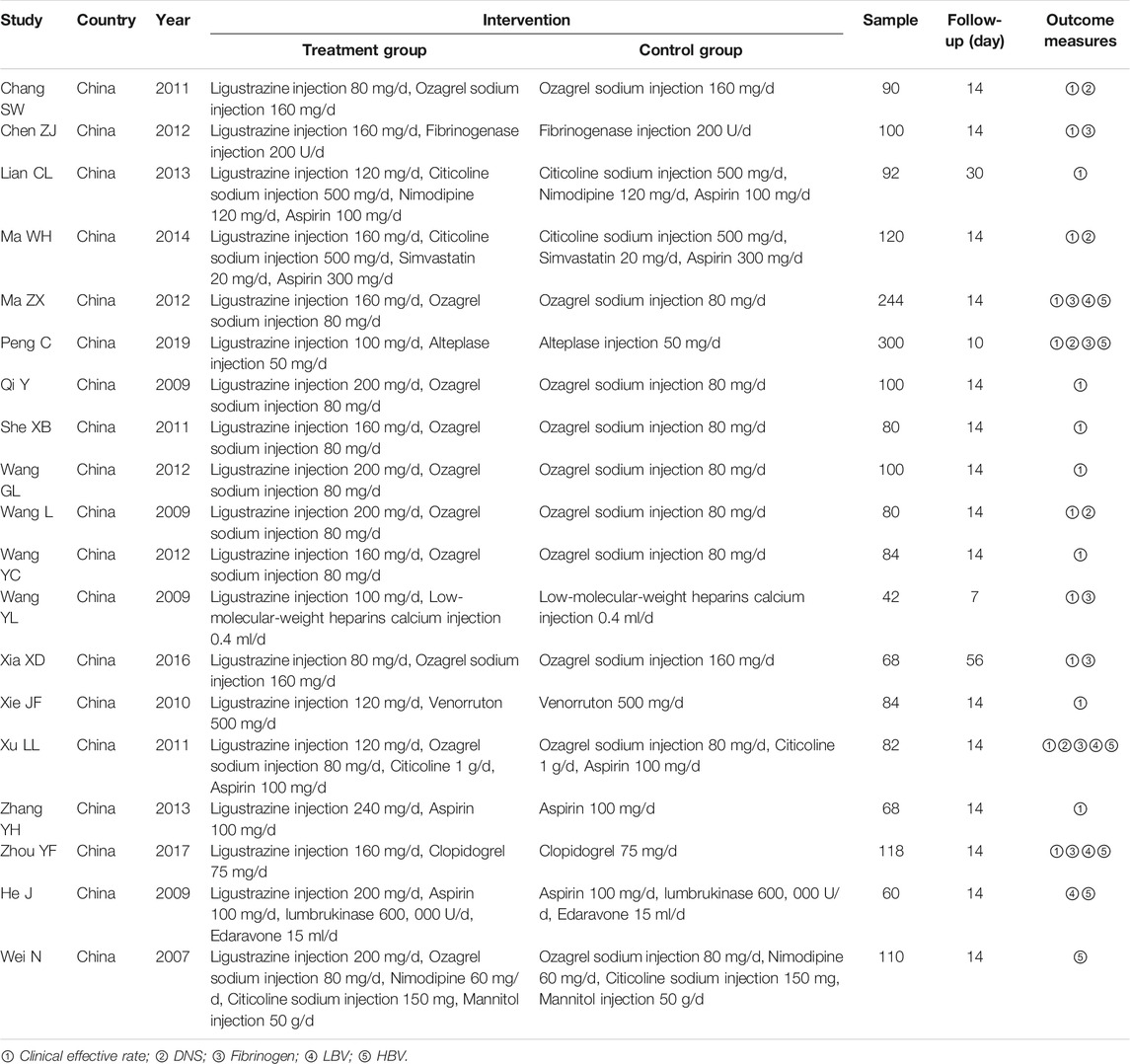
Characteristics of the included RCTs are provided in Table 1. All the 19 RCTs were published in Chinese journals (Wei, 2007; He et al., 2009; Qi, 2009; Wang, 2009; Wang et al., 2009; Chang, 2011; She et al., 2011; Xie, 2011; Xu et al., 2011; Chen and Wen, 2012; Ma, 2012; Wang, 2012; Wang et al., 2012; Lian, 2013; Zhang et al., 2013; Ma, 2014; Xia et al., 2016; Zhou, 2017; Peng, 2019). The participants in the 19 RCTs ranged from 42 to 300, with a total of 2022 ACI patients. The mean sample size of the included RCTs was 106.4 and the age of ACI patients was between 36 and 89 years old. The duration of intervention was at least 7 days, mostly 14 days. The Western medicine in the control group included ozagrel sodium injection, fibrinogenase injection, citicoline sodium injection, nimodipine, simvastatin, alteplase injection, low-molecular-weight heparins calcium injection, venorruton, clopidogrel, lumbrukinase, edaravone, mannitol injection, and aspirin. Combined with these Western medicines, the dosage of ligustrazine injection in the treatment group ranged from 80 to 240 mg per day. Seventeen RCTs employed clinical effective rate as the therapeutic indicator and five RCTs used NDS as the outcome measures. Seven RCTs reported the outcome measure fibrinogen as their therapeutic indicator. LBV was considered as the outcome measure in four RCTs and HBV was regarded as the outcome measure in six RCTs.
Risk of Bias in Individual Studies
The quality of the 19 RCTs was assessed using the Jadad scoring system (ranged from 0 to 5 scores) and the M scoring system (ranged from 1 to 7 scores), respectively. Sixteen RCTs obtained 2 scores and three RCTs obtained 3 scores according to the Jadad scale. Seven RCTs obtained 3 and ten RCTs obtained above 3 scores based on the M scale (Table 2).
Clinical Effective Rate
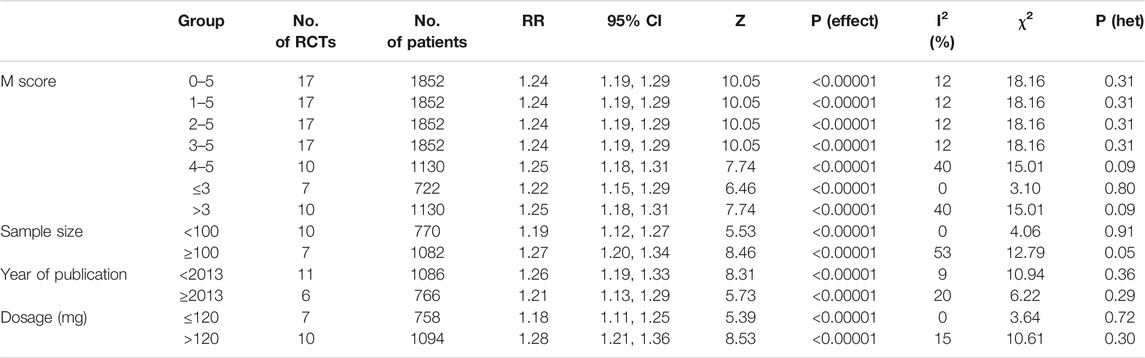
The pooled RR of clinical effective rate indicated that ligustrazine injection as adjunctive therapy was more effective than Western medicine alone in treating ACI. As shown in Figure 2, the pooled RR was 1.24 (95% CI, 1.19 to 1.29; Z = 10.05, p < 0.00001) with little heterogeneity (I2 = 12%; χ2 = 18.16; df = 16, p = 0.31) among the 17 RCTs using clinical effective rate as outcome measures. Publication bias of the primary outcome measure (clinical effective rate) was evaluated by funnel plots (Figure 3) and results showed that slight asymmetry was found in the plots. The results of Begg’s test (Z = 2.47, p = 0.013) and Egger’s test (t = 2.22, p = 0.042) suggested that publication bias was small. As shown in Table 3, sensitivity analysis was conducted to verify whether and how much the overall effect would be affected by the low quality RCTs. No significant difference was found during the gradual exclusion of lower quality of RCTs based on the M scoring system. There were slight changes (0.03 in magnitude) between the low quality (M scores ≤3) and high quality of RCTs (M scores >3). Subgroup analysis was conducted to evaluate whether the overall effect was affected by the publication date, sample size of RCTs, and dosages of ligustrazine injection. No significant difference was found in the pooled risk ratios of clinical effective rate (Table 3), which consistently demonstrated that ligustrazine injection as adjunctive therapy was apparently more effective in treating ACI.
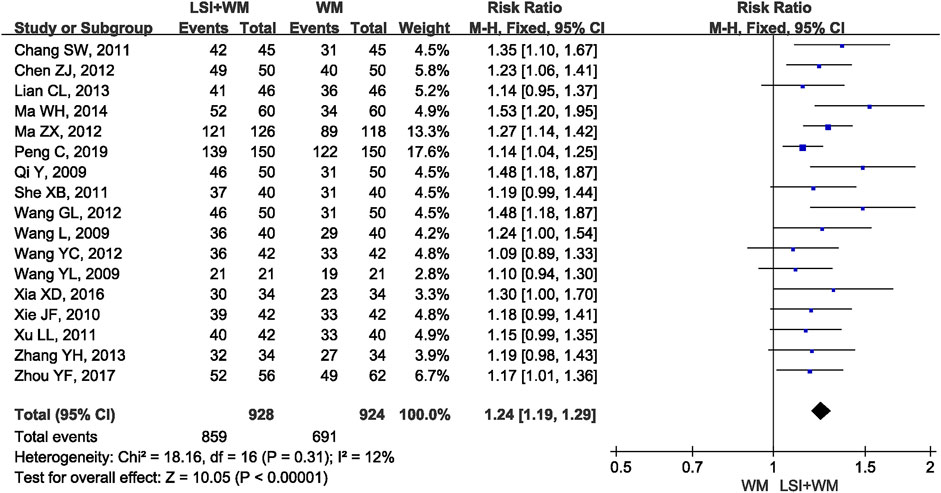
FIGURE 2. Forest plot of clinical effective rate of ligustrazine injection in treating ACI. LSI is ligustrazine injection and WM is Western medicine.
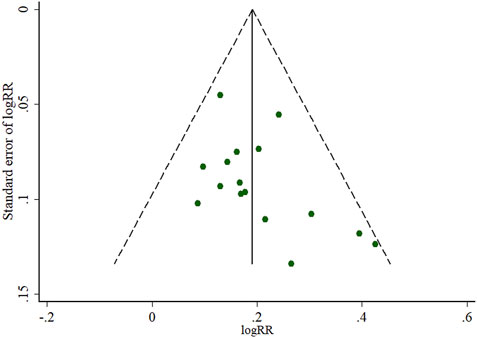
FIGURE 3. Funnel plots of the clinical effective rate of ligustrazine injection for ACI. LSI is ligustrazine injection and WM is Western medicine.
NDS
Five RCTs reported the NDS as their secondary outcome measure for ACI patients. As shown in Figure 4, ligustrazine injection as adjunctive therapy significantly reduced the NDS in ACI patients (MD = −3.88; 95% CI, −4.15 to −3.61; Z = 28.35, p < 0.00001) with moderate heterogeneity (I2 = 41%; χ2 = 6.80; df = 4, p = 0.15). As shown in Supplementary Table S3, results from the sensitivity analysis and subgroup analysis showed that no significant difference was found in the overall mean differences (MDs) of NDS. Results of Begg’s test (Z = −0.24, p = 1.00) and Egger’s test (t = 0.37, p = 0.739) suggested that there were no significant publication biases among the five RCTs.
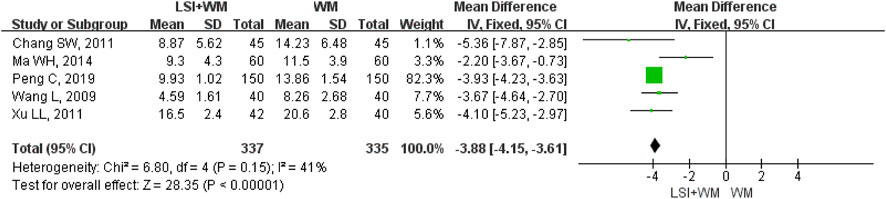
FIGURE 4. Forest plot of NDS of ligustrazine injection in treating ACI. LSI is ligustrazine injection and WM is Western medicine.
Fibrinogen
Seven RCTs mentioned fibrinogen as the secondary outcome measure. As shown in Figure 5, results indicated that ligustrazine injection combined with Western medicine was superior to Western medicine alone in reducing the fibrinogen in ACI patients (MD = −0.59; 95% CI, −0.76 to −0.42; Z = 6.77, p < 0.00001) with high heterogeneity (I2 = 93%; χ2 = 86.28; df = 6, p < 0.00001). As shown in Supplementary Table S4, sensitivity analysis and subgroup analysis showed that there were little changes in the overall MDs of fibrinogen. Results from Begg’s test (Z = 0.30, p = 0.764) and Egger’s test (t = 1.24, p = 0.269) indicated that no significant publication biases were found among the included seven RCTs.
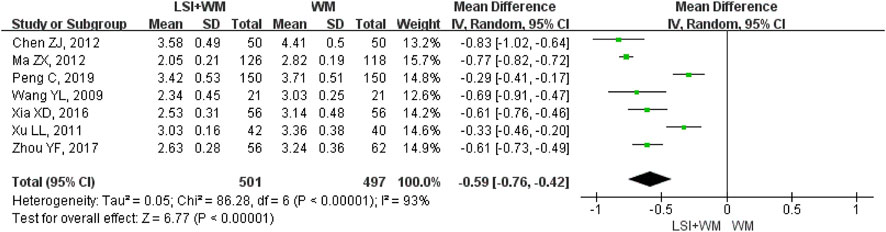
FIGURE 5. Forest plot of fibrinogen of ligustrazine injection in treating ACI. LSI is ligustrazine injection and WM is Western medicine.
LBV
LBV was reported as the outcome measure in four RCTs. As shown in Figure 6, results suggested that ligustrazine injection as adjunctive therapy was better than Western medicine alone in reducing LBV for ACI patients (MD = −2.11; 95% CI, −3.16 to −1.06; Z = 3.95, p < 0.0001) with high heterogeneity (I2 = 86%; χ2 = 21.17; df = 3, p < 0.0001). Sensitivity analysis and subgroup analysis were performed to evaluate the overall MDs influenced by the factors of low quality RCTs, sample size and publication date. Results showed that little differences were found in the overall MDs of LBV (Supplementary Table S5). Results of Begg’s test (Z = 0.34, p = 0.734) and Egger’s test (t = −0.64, p = 0.585) demonstrated that there were no significant publication biases among the four RCTs.
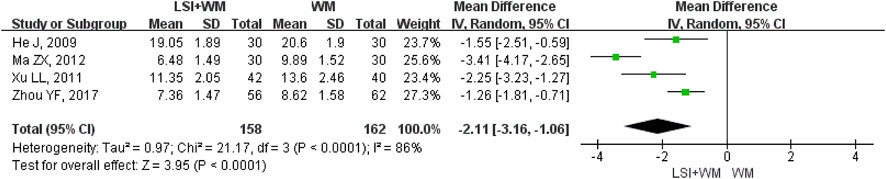
FIGURE 6. Forest plot of LBV of ligustrazine injection in treating ACI. LSI is ligustrazine injection and WM is Western medicine.
HBV
HBV was reported in six RCTs and it was selected for one of the outcome measures. As shown in Figure 7, results demonstrated that ligustrazine injection as adjunctive therapy was more effective than Western medicine alone to reduce HBV in the treatment of ACI patients (MD = −0.88; 95% CI, −1.20 to −0.55; Z = 5.27, p < 0.00001) with high heterogeneity (I2 = 98%; χ2 = 304.24; df = 5, p < 0.00001). In this meta-analysis, sensitivity analysis and subgroup analysis were conducted to verify if the overall MDs were affected by the low quality of RCTs, sample size and publication date and results suggested that little differences were found in the overall MDs of HBV (Supplementary Table S6). Results of Begg’s test (Z = 0, p = 1.00) and Egger’s test (t = −0.41, p = 0.70) indicated that no significant publication biases were found among the six RCTs.
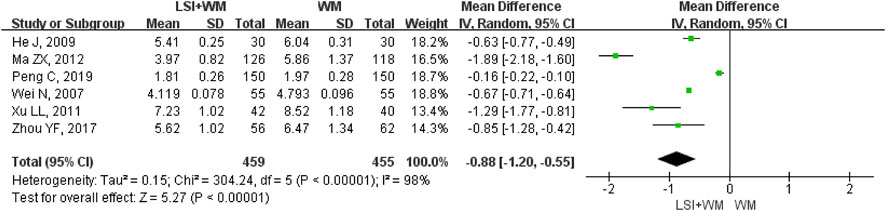
FIGURE 7. Forest plot of HBV of ligustrazine injection in treating ACI. LSI is ligustrazine injection and WM is Western medicine.
Adverse Reaction
Twelve RCTs indicated that the use of ligustrazine injection did not bring obvious adverse reactions for ACI patients during the intervention. The rest of the RCTs did not report adverse reactions.
Discussion
At present, conventional therapy with Western medicines mainly includes thrombolysis, controlling cerebral edema, improving microcirculation, preventing and treating complications, the applying of neuroprotective agents restoring, blood supply to ischemic area, reducing blood viscosity, and controlling hypertension. Although these Western medicines can accelerate a patient’s recovery to a certain extent, drug side effects and resistances are accompanied with their wide application in clinical practice (CAST (Chinese Acute Stroke Trial) Collaborative Group, 1997; International Stroke Trial Collaborative Group, 1997). Besides, frequent use of Western medicines might cause motor weakness on one or both sides of the body (Li et al., 2014). Therefore, more effective agents for ACI patients are highly demanded. In past years, the role of TCM in global health care has now been accepted by more and more physicians and researchers. The increasing number of systematic review and meta-analysis on the clinical efficacy of TCM as adjunctive therapy for treating various diseases can be the best evidence (Shi et al., 2021). Numerous studies have indicated that the combination of ligustrazine injection and Western medicines is beneficial for the treatment of ACI.
Recently, modern pharmacological experimental studies showed that ligustrazine plays important roles in antithrombosis in vivo and the mechanism could be attributed to the inhibition of platelet aggregation and protection of endothelium (Yang et al., 2012). As a new type of calcium ion antagonist and free radical scavenger, ligustrazine can pass through the blood brain barrier. High dose of ligustrazine can significantly increase the expression of Bcl-2 and reduce the expression of p53 (Zhang and Li, 2003). Besides, ligustrazine can alleviate cerebral ischemia reperfusion injury by regulating caspase-12 gene expression, thus reducing neuronal apoptosis (Tian et al., 2014). Although a large number of pharmacological experiments have demonstrated that ligustrazine injection is an effective agent for treating ACI, a comprehensive and systematic evaluation of ligustrazine injection for the treatment of ACI is rare, based on current rigorous international standards. Ligustrazine injection has been widely used to treat ACI in China over the past decades, this famous Chinese medicine is completely unknown to most Western physicians and researchers. Therefore, this study aims to provide an internationally accessible systematic review for evaluating the effectiveness and safety of ligustrazine injection in the treatment of ACI.
Analysis of Effectiveness
Many RCTs on ligustrazine injection combined with Western medicine for the treatment of ACI. However, the clinical efficacy of ligustrazine injection is not yet well confirmed. Although a former meta-analysis published in 2016 had evaluated the efficacy and safety of ligustrazine in the treatment of cerebral infarction, the poor methodological quality (the Jadad score of all the studies was 1 point) prevented the author from making firm conclusions (Yu et al., 2016). The lack of a subgroup and sensitive analysis also resulted in its result being more unreliable. Moreover, the former meta-analysis was not conducted according to the PRISMA guidelines. This study aims to provide a PRISMA-compliant systematic review (PRISMA Checklist was provided in Supplementary Table S7) and meta-analysis for evaluating the efficacy of ligustrazine injection as adjunctive therapy in treating ACI. Meta-analysis results of the primary outcome measure (clinical effective rate) showed that ligustrazine injection combined with Western medicine appeared to be more effective than Western medicine alone (RR = 1.24; 95% CI, 1.19–1.29). Results of the secondary outcome measures including NDS (MD = −3.88; 95% CI, −4.15 to −3.61), fibrinogen (MD = −0.59; 95% CI, −0.76 to −0.42), LBV (MD = −2.11; 95% CI, −3.16 to −1.06), and HBV (MD = −0.88; 95% CI, −1.20 to −0.55) confirmed that the therapeutic effect of ligustrazine injection as adjunctive therapy surpassed the Western medicine alone in the treatment of ACI. Moreover, the combination of ligustrazine injection did not result in adverse reactions for ACI patients.
ACI is the ischemic necrosis or cerebral softening of local brain tissues caused by the obstruction of acute local blood supply in brain tissues, ischemia, and hypoxia (Regenhardt et al., 2018). The decrease or interruption of cerebral blood flow is the main reason of ACI, which seriously damages the function of the nervous system. Clinical efficacy of ligustrazine in the remediation of neurological deficits and reduction of the fibrinogen, LBV, and HBV has been demonstrated by many pharmacological experiments. Kong et al. found that ligustrazine could promote neural progenitor cells move to the damaged area by activating the phosphatidylinositol 3-kinase pathway to achieve the protective effect on the brain (Kong et al., 2016). Whole-blood viscosity and platelet aggregation are usually used as the prognostic indicators of ischemic cerebral and myocardial diseases. Cai et al. confirmed that ligustrazine could significantly decrease whole-blood viscosity and inhibit platelet aggregation through down-regulate the expression of CXCR4 in platelets, lymphocytes, and blood red cells (Cai et al., 2014). These available evidences also consistently supported the adjunctive use of ligustrazine injection in the treatment of ACI.
Limitations for This Review
Several potential limitations were found in this meta-analysis. First, all the included RCTs were collected from Chinese journals, restricting their worldwide attention. Second, almost all RCTs were not strictly designed based on the gold standard. Many RCTs did not clearly describe the allocation concealment and blinding, causing the potential for publication bias and overvaluation or undervaluation of the efficacy of ligustrazine injection. Third, various dosages of ligustrazine injection in the treatment groups might introduce heterogeneity for the results of the meta-analysis. Fourth, the sample size of some RCTs was relatively small and the duration of intervention was relatively short, which might cause inaccurate results for the meta-analysis. Hence, more strictly designed RCTs with higher quality, larger sample sizes, and longer duration of intervention are necessary to accurately confirm the efficacy of ligustrazine injection as adjunctive therapy for ACI.
Implications for Further Research
The methodological quality of many RCTs was poor. Hence, future studies on ligustrazine injection should be strictly performed in accordance with the standards of reporting trials.
At present, the efficacy of ligustrazine injection as adjunctive therapy in treating ACI has not been confirmed by a PRISMA-compliant systematic review and meta-analysis. Besides, adjunctive therapies of ligustrazine injection were rarely known by most Western physicians. Therefore, we conducted a PRISMA-compliant systematic review and meta-analysis for ligustrazine injection. This meta-analysis was superior to other studies on ligustrazine injection: (1) Other Chinese medicine involved in the control or treatment group was excluded during the study selection; (2) sensitivity analysis and subgroup analysis was conducted in this meta-analysis; (3) non-RCTs were excluded in this study; and (4) this meta-analysis was strictly performed according to the PRISMA requirements.
In general, the meta-analysis results based on the 19 RCTs suggested that ligustrazine injection as adjunctive therapy could provide a better efficacy in treating ACI than Western medicine alone. Because of the low quality of the 19 RCTs, further RCTs with higher methodological quality, larger sample size, and longer duration are still recommended to validate the efficacy of ligustrazine injection.
Conclusion
This meta-analysis suggested that ligustrazine injection as adjunctive therapy was more effective than Western medicine alone in the treatment of ACI. Ligustrazine injection combined with Western medicine was recommended to improve the total effective rate, remedy neurological deficits and reduce the fibrinogen, LBV, and HBV for ACI patients. There were no serious adverse reactions when using ligustrazine injection. However, more evidence is needed to accurately validate the efficacy of ligustrazine injection as the adjunctive therapy in treating ACI since the methodological quality of some RCTs was low.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author Contributions
HS: Design, conception, searching literatures, extracting data, evaluating quality, performing statistical analysis, interpreting data, drafting and revising article. XH: Searching literatures, extracting data, evaluating quality, performing statistical analysis, interpreting data, revising article. LZ: Searching literatures, extracting data, evaluating quality, revising article. SD: Performing statistical analysis, interpreting data. XY: Interpreting data, revising manuscript. XC: Interpreting data, revising article. XL: Design, conception, drafting article. SH: Design, conception, drafting and revising article. RT: Design, conception, revising article. All authors approved the final version of the article.
Funding
This work was supported by National Key Research and Development Program of China (2020YFC2005500), Key Research and Development Program of Science and Technology Department of Sichuan Province (2019YFS0514), China Postdoctoral Science Foundation (2021M690559) and Young Talents Project of Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital (Grant: 2021QN07).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.761722/full#supplementary-material
Abbreviations
ACI, acute cerebral infarction; CI, confidence interval; CNKI, China national knowledge infrastructure; HBV, high shear blood viscosity; LBV, low shear blood viscosity; LSI, ligustrazine injection; MD, mean difference; NDS, neurological deficit score; RCTs, randomized controlled trials; RR, relative risk; rt-PA, recombinant tissue plasminogen activator; TCM, traditional Chinese medicine; UK, urokinase; VIP, China Science and Technology Journal Database; WM, western medicine.
References
Asadi, H., Dowling, R., Yan, B., Wong, S., and Mitchell, P. (2015). Advances in Endovascular Treatment of Acute Ischaemic Stroke. Intern. Med. J. 45, 798–805. doi:10.1111/imj.12652
Cai, X., Chen, Z., Pan, X., Xia, L., Chen, P., Yang, Y., et al. (2014). Inhibition of Angiogenesis, Fibrosis and Thrombosis by Tetramethylpyrazine: Mechanisms Contributing to the SDF-1/CXCR4 Axis. Plos One 9, e88176. doi:10.1371/journal.pone.0088176
CAST (Chinese Acute Stroke Trial) Collaborative Group (1997). CAST: Randomised Placebo-Controlled Trial of Early Aspirin Use in 20,000 Patients with Acute Ischaemic Stroke. CAST (Chinese Acute Stroke Trial) Collaborative Group. Lancet 349, 1641–1649.
Chang, S. W. (2011). Clinical Observation of Ozagrel Sodium Injection Combined with Ligustrazine Injection in the Treatment of Acute Cerebral Infarction. Chin. J. Pract. Nerv. Dis. 14, 46–47. (In Chinese). doi:10.3969/j.issn.1673-5110.2011.17.022
Chen, Z. J., and Wen, X. (2012). Study on the Effect of Fibrinogenase Injection Combined with Ligustrazine Injection in Treating Acute Cerebral Infarction Patients. Guide China Med. 10, 294–295. (In Chinese). doi:10.3969/j.issn.1671-8194.2012.19.211
Chinese society for Neuroscience (1996). Chinese Society for Neurosurgery. Diagnosis of Various Types of Cerebrovascular Disease. Chin. J. Neurol. 29, 379.
Gao, H. J., Liu, P. F., Li, P. W., Huang, Z. Y., Yu, F. B., Lei, T., et al. (2015). Ligustrazine Monomer against Cerebral Ischemia/reperfusion Injury. Neural Regen. Res. 10, 832–840. doi:10.4103/1673-5374.156991
GBD 2016 Stroke Collaborators (2019). Global, Regional, and National burden of Stroke, 1990-2016: a Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 18, 439–458. doi:10.1016/S1474-4422(19)30034-1
Guo, M., Liu, Y., and Shi, D. (2016). Cardiovascular Actions and Therapeutic Potential of Tetramethylpyrazine (Active Component Isolated from Rhizoma Chuanxiong): Roles and Mechanisms. Biomed. Res. Int. 2016, 2430329. doi:10.1155/2016/2430329
He, J., Nie, J. H., and Li, Y. G. (2009). Effect of Ligustrazine on Serum Rheology and Serum ICAM-1 in Patients with Acute Cerebral Infarction. China Foreign Med. Treat. 13, 63–64. (In Chinese). doi:10.3969/j.issn.1674-0742.2009.13.057
Inoue, T., Kobayashi, M., Uetsuka, Y., and Uchiyama, S. (2006). Pharmacoeconomic Analysis of Cilostazol for the Secondary Prevention of Cerebral Infarction. Circ. J. 70 (4), 453–458. doi:10.1253/circj.70.453
International Stroke Trial Collaborative Group (1997). A Randomized Trial of Aspirin, Subcutaneous Heparin, Both, or Neither Among 19,435 Patients with Acute Ischaemic Stroke. Lancet 349, 1569–1581.
Jadad, A. R., Moore, R. A., Carroll, D., Jenkinson, C., Reynolds, D. J., Gavaghan, D. J., et al. (1996). Assessing the Quality of Reports of Randomized Clinical Trials: Is Blinding Necessary? Control. Clin. Trials. 17, 1–12. doi:10.1016/0197-2456(95)00134-4
Johnston, S. C., Mendis, S., and Mathers, C. D. (2009). Global Variation in Stroke burden and Mortality: Estimates from Monitoring, Surveillance, and Modelling. Lancet Neurol. 8, 345–354. doi:10.1016/S1474-4422(09)70023-7
Kong, X., Zhong, M., Su, X., Qin, Q., Su, H., Wan, H., et al. (2016). Tetramethylpyrazine Promotes Migration of Neural Precursor Cells via Activating the Phosphatidylinositol 3-kinase Pathway. Mol. Neurobiol. 53, 6526–6539. doi:10.1007/s12035-015-9551-1
Li, L., Zhang, H., Meng, S. Q., and Qian, H. Z. (2014). An Updated Meta-Analysis of the Efficacy and Safety of Acupuncture Treatment for Cerebral Infarction. PLoS ONE 9, e114057. doi:10.1371/journal.pone.0114057
Lian, C. L. (2013). Observation of 46 Cases of Acute Cerebral Infarction Treated by the Combination of Traditional Chinese and Western Medicine. J. Pract. Tradit. Chin. Med. 29, 456. (In Chinese). doi:10.3969/j.issn.1004-2814.2013.06.028
Ma, Z. X. (2012). Therapeutic Effect of Ligustrazine Injection Combined with Ozagrel Sodium Injection on 126 Patients with Acute Cerebral Infarction. Med. Innov. China. 9, 28–29. (In Chinese). doi:10.3969/j.issn.1674-4985.2012.09.015
Ma, W. H. (2014). Efficacy of Ligustrazine Injection on Acute Cerebral Infarction and its Effect on Serum CRP. Zhejiang Clin. Med. J. 16, 789–790. (In Chinese).
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement. Open Med. 6, e1000097. doi:10.1371/journal.pmed.1000097
Peng, C. (2019). Clinical Study on Ligustrazine Phosphate Injection Combined with Alteplase in Treatment of Acute Cerebral Infarction. Drugs Clin. 34, 308–312. (In Chinese). doi:10.7501/j.issn.1674-5515.2019.02.008
Powers, W. J., Rabinstein, A. A., Ackerson, T., Adeoye, O. M., Bambakidis, N. C., Becker, K., et al. (2018). 2018 Guidelines for the Early Management of Patients with Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 49, e46–99. doi:10.1161/STR.0000000000000158
Qi, Y. (2009). Observation on the Clinical Efficacy of Ozagrel Sodium Injection Combined with Ligustrazine Injection in the Treatment of Acute Cerebral Infarction. Chin. Foreign Health Dig. 6, 160–161. (In Chinese).
Regenhardt, R. W., Das, A. S., Lo, E. H., and Caplan, L. R. (2018). Advances in Understanding the Pathophysiology of Lacunar Stroke: a Review. JAMA Neurol. 75, 1273–1281. doi:10.1001/jamaneurol.2018.1073
Shao, H., Zhao, L., Chen, F., Zeng, S., Liu, S., and Li, J. (2015). Efficacy of Ligustrazine Injection as Adjunctive Therapy for Angina Pectoris: A Systematic Review and Meta-Analysis. Med. Sci. Monit. 21, 3704–3715. doi:10.12659/msm.895362
Shao, H., Li, M., Chen, F., Chen, L., Jiang, Z., and Zhao, L. (2018). The Efficacy of Danshen Injection as Adjunctive Therapy in Treating Angina Pectoris: a Systematic Review and Meta-Analysis. Heart Lung Circ. 27, 433–442. doi:10.1016/j.hlc.2017.10.016
She, X. B., Jing, H. W., and Zhang, X. X. (2011). Clinical Observation of Ozagrel Sodium Injection Combined with Ligustrazine Injection in Treatment of Acute Cerebral Infarction. Zhejiang Clin. Med. J. 13, 861–862. (In Chinese). doi:10.3969/j.issn.1008-7664.2011.08.009
Shi, X. J., Fan, F. C., Liu, H., Ai, Y. W., Liu, Q. S., Jiao, Y. G., et al. (2021). Traditional Chinese Medicine Decoction Combined with Antipsychotic for Chronic Schizophrenia Treatment: a Systematic Review and Meta-Analysis. Front. Pharmacol. 11, 616088. doi:10.3389/fphar.2020.616088
The fourth session of the National Cerebrovascular Conference (1996). The Norm of Clinical Neurologic Deficit Score. Chin. J. Neurol. 29, 381–383.
Tian, Y., Li, Y. P., and Han, R. D. (2014). Effects of Tetramethylpyrazine on Expression of Caspase 12 and Apoptosis in Rats with Focal Cerebral Ischemia and Reperfusion. J. Clin. Exp. Med. 13, 16–19. doi:10.13638/j.issn.1671-4040.2019.02.050
Wang, Y. L., Yang, X. R., and Jiang, Y. Z. (2009). The Clinical Observation of Treating Acute Cerebral Infarction with Low-Molecular-Weight Heparin Calcium and Ligustrazine Injection. China Med. Her. 6, 67–68. (In Chinese). doi:10.3969/j.issn.1673-7210.2009.08.042
Wang, G. L., Zhang, W., Dong, C. X., and Zhang, H. (2012). Therapeutic Effect of Ozagrel Sodium Injection Combined with Ligustrazine Injection in Treating Acute Cerebral Infarction. Chin. Foreign Health Dig. 9, 197–198. (In Chinese). doi:10.3969/j.issn.1672-5085.2012.02.158
Wang, L. (2009). Observation on the Efficacy of Ozagrel Sodium Injection Combined with Ligustrazine Injection in the Treatment of Acute Cerebral Infarction. Chin. Med. Mod. Distance Educ. 7, 27–28. (In Chinese).
Wang, Y. C. (2012). Clinical Observation of Ozagrel Sodium Injection Combined with Ligustrazine Injection in the Treatment of Acute Cerebral Infarction. Med. Front. 32, 223, (In Chinese). doi:10.3969/j.issn.2095-1752.2012.32.234
Wei, N. (2007). Effect of Ligustrazine on Blood Viscosity and Erythrocyte Aggregation index in Patients with Cerebral Infarction. J. Xinxiang Med. Coll. 24 (6), 588–590. (In Chinese). doi:10.3969/j.issn.1004-7239.2007.06.018
Xia, X. D., Shen, W., Chen, X., Ye, Z., and Cui, J. (2016). Effects of Ozagre1 Sodium Combined with Ligustrazine Injection on Serum Levels of HCY, CRP and Fibrinogen in Patients with Acute Cerebral Infarction. Prog. Mod. Biomed. 16, 5502–5504. (In Chinese). doi:10.13241/j.cnki.pmb.2016.28.026
Xie, J. F. (2011). Clinical Observation of 42 Cases of Acute Cerebral Infarction Treated by Ligustrazine Injection Combined with Venoruton. J. China Tradit. Chin. Med. Inform. 2, 73. (In Chinese).
Xu, L. L., Meng, Q. Y., Tian, L., Liu, X. H., and Liu, T. (2011). Curative Effect of Tetramethylpyrazine on Acute Cerebral Infarction. J. Pract. Tradit. Chin. Intern. Med. 25, 57–58. (In Chinese). doi:10.3969/j.issn.1671-7813.2011.09.32
Yang, W. H., Gong, G. Q., Zhou, Y., Zhang, Z. X., and Li, J. (2012). Effect and Mechanism of Tetramethylpyrazine on Antithrombotic. Acta Metall. Sin. 17, 241–245.
Yu, T., Guo, X., Zhang, Z., Liu, R., Zou, L., Fu, J., et al. (2016). Meta-Analysis of the Clinical Effectiveness and Safety of Ligustrazine in Cerebral Infarction. Evid. Based Complement. Alternat. Med. 2016, 3595946. doi:10.1155/2016/3595946
Yu, D., Liao, X., Robinson, N., Cui, R., Zhao, J., and Zhao, H. (2019). 12 Kinds of Chinese Medicine Injections for Acute Cerebral Infarction: Protocol for a Systematic Review and Network Meta-Analysis. Eur. J. Integr. Med. 27, 75–80. doi:10.1016/j.eujim.2019.02.012
Zhang, M., and Li, P. (2003). Experimental Study on the Effect of Ligustrazine on the Expression of Apoptosis Related Genes after Cerebral Ischemia. Chin. Tradit. Pat. Med. 25, 78–79. doi:10.1016/j.eujim.2019.02.012
Zhang, Y. H., Cai, X. Z., and Guo, M. H. (2013). Effects of Ligustrazine Injection on Serum High-Sensitivity C-Reactive Protein, Interleukin-6 and Matrix Metalloproteinases-9 in Treating Acute Cerebral Infarction. J. Clin. Med. Pract. 17, 78–80. (In Chinese). doi:10.7619/jcmp.201317026
Zhang, X., Wu, J., and Zhang, B. (2015). Xuesaitong Injection as One Adjuvant Treatment of Acute Cerebral Infarction: a Systematic Review and Meta-Analysis. BMC Complement. Altern. Med. 15, 36. doi:10.1186/s12906-015-0560-4
Zhao, Y., Liu, Y., and Chen, K. (2016). Mechanisms and Clinical Application of Tetramethylpyrazine (An Interesting Natural Compound Isolated from Ligusticum Wallichii): Current Status and Perspective. Oxid. Med. Cel. Longev. 2016, 2124638. doi:10.1155/2016/2124638
Zheng, Q., Huang, Y. Y., Zhu, P. C., Tong, Q., Bao, X. Y., Zhang, Q. H., et al. (2018). Ligustrazine Exerts Cardioprotection in Animal Models of Myocardial Ischemia/Reperfusion Injury: Preclinical Evidence and Possible Mechanisms. Front. Pharmacol. 9, 729. doi:10.3389/fphar.2018.00729
Keywords: ligustrazine injection, adjunctive therapy, acute cerebral infarction, systematic review, meta-analysis
Citation: Shao H, He X, Zhang L, Du S, Yi X, Cui X, Liu X, Huang S and Tong R (2021) Efficacy of Ligustrazine Injection as Adjunctive Therapy in Treating Acute Cerebral Infarction: A Systematic Review and Meta-Analysis. Front. Pharmacol. 12:761722. doi: 10.3389/fphar.2021.761722
Received: 20 August 2021; Accepted: 28 October 2021;
Published: 22 November 2021.
Edited by:
Karl Tsim, Hong Kong University of Science and Technology, Hong Kong SAR, ChinaReviewed by:
Jiarui Wu, Beijing University of Chinese Medicine, ChinaWantong Zhang, China Academy of Chinese Medical Sciences, China
Copyright © 2021 Shao, He, Zhang, Du, Yi, Cui, Liu, Huang and Tong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinxia Liu, Y3VwZmx5c2VhQDE2My5jb20=; Shengfeng Huang, aHNmQGd6aG11LmVkdS5jbg==; Rongsheng Tong, dG9uZ3JzQDEyNi5jb20=
†These authors contributed equally to this work
 Huikai Shao
Huikai Shao Xia He
Xia He Lijuan Zhang
Lijuan Zhang Shan Du
Shan Du Xiaoqing Yi
Xiaoqing Yi Xiaojiao Cui
Xiaojiao Cui Xinxia Liu
Xinxia Liu Shengfeng Huang
Shengfeng Huang Rongsheng Tong
Rongsheng Tong