- 1Department of Cardiology, Fuwai Hospital, National Center for Cardiovascular Diseases, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, China
- 2Fuwai Hospital, Chinese Academy of Medical Sciences, Shenzhen, China
- 3Coronary Heart Disease Center, Fuwai Hospital, Chinese Academy of Medical Sciences, Beijing, China
Background: Despite the recommendations from mainstream guidelines, the use of angiotensin-converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARB) for acute coronary syndrome (ACS) patients without heart failure (HF) is controversial, as its evidence is lacking in the era of reperfusion and intensive secondary preventions. This study aimed to investigate the impacts of ACEI/ARB on outcomes of ACS patients without HF treated by percutaneous coronary intervention (PCI).
Methods: A total of 2,397 non-HF ACS patients treated by PCI were retrospectively recruited. Prognostic impacts of ACEI/ARB were assessed by unadjusted analysis, followed by propensity score matching (PSM) and propensity score matching weight (PSMW) analysis to control the between-group differences. The primary outcome was a composite of all-cause death and recurrent myocardial infarction (MI).
Results: Among the included patients, 1,805 (75.3%) were prescribed with ACEI/ARB at discharge. The median follow-up time was 727 (433–2016) days, with 129 (5.4%) primary endpoint events, consisting of 55 (2.3%) cases of all-cause death and 74 (3.1%) cases of recurrent MI. The use of ACEI/ARB was not associated with significant risk reduction of primary endpoint events in unadjusted analysis (hazard ratio [HR]: 0.95, 95% confidence interval [CI]: 0.64–1.39, p = 0.779), PSM analysis (HR: 0.94, 95% CI: 0.60–1.47, p = 0.784), and PSMW analysis (HR: 0.91, 95% CI: 0.55–1.49, p = 0.704). Similar results were observed for secondary outcomes of all-cause death, cardiac death, and recurrent MI.
Conclusion: For ACS patients without HF, the use of ACEI/ARB was not associated with lower risk of death or recurrent MI after PCI.
Introduction
According to most of the mainstream guidelines, angiotensin-converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARB) are generally recommended for all patients with acute coronary syndrome (ACS) unless contraindicated as they could effectively reduce the mortality and risk of recurrent myocardial infarction (MI), which has been demonstrated in many randomized clinical trials (Amsterdam et al., 2014; Ibanez et al., 2018; O'Gara et al., 2013; Collet et al., 2021; Bangalore et al., 2017). However, most of the evidence supporting these recommendations comes from studies of patients with substantially impaired cardiac function (i.e., ejection fraction [EF] < 35–40%) who were not treated by modernized secondary preventive measures, including percutaneous coronary intervention (PCI), dual antiplatelet therapy (DAPT), and lipid-lowering medications (Pfeffer et al., 1992; Yusuf et al., 1992; Ball et al., 1993; Rutherford et al., 1994). Accumulating evidence shows that ACEI/ARB is not effective for reducing mortality in ACS patients with a baseline EF > 40%, casting doubts on whether these agents should be routinely used as one of the long-term medications in every patient after the index coronary event (Parashar et al., 2015; Bangalore et al., 2017; Park et al., 2018; Cespón-Fernández et al., 2019; Raposeiras-Roubín et al., 2020). Meanwhile, over half of the patients acquire generally normal cardiac function with standard care after ACS, which could possibly attenuate the clinical significance for the blockade of renin-angiotensin system (RAS) with ACEI/ARB medications (Sutton et al., 2016; Chen et al., 2020). As the evidence is scarce regarding the routine use of ACEI/ARB in patients with normal left ventricular function in this context, the current study aimed to evaluate the impacts of ACEI/ARB on outcomes of ACS patients without heart failure (HF) after PCI.
Methods
Study Cohort
This observational study was conducted in a large-volume PCI center at a national tertiary care institute (Fuwai Hospital, Beijing) specializing in cardiovascular diseases, which has enrolled all patients undergoing emergent coronary angiography and PCI procedures from January 2010 to June 2017. ACS consisted of ST-segment elevation MI (STEMI) and non-ST-elevation ACS, while the diagnosis and classification of ACS is made according to guidelines and universal definitions up to date (Ibanez et al., 2018; Thygesen et al., 2019; Collet et al., 2021), including criteria of clinical presentations, typical characteristics on electrocardiogram, dynamical changes of cardiac enzymes, and imaging evidence. The current study included all patients diagnosed with ACS and subsequently undergoing emergent coronary angiography and PCI. The exclusion criteria were: 1) patients without available EF measurements, 2) patients who died during the index hospitalization, 3) patients with HF (definitions see below), and 4) patients having no follow-up records. The study was performed in accordance with principles set forth in the Declaration of Helsinki, and was approved by the ethics committee of the institute. All patients had signed the written informed consents during hospitalizations regarding the use of clinical data for the purpose of scientific research by the institute.
Definitions of Heart Failure and Examinations of Cardiac Function
Heart failure, in this study, was defined by the presence of any one of the following conditions: 1) a Killip II classification or above, 2) a measurement of EF < 50%, or 3) a presentation of HF symptoms in need of diuretics during the index hospitalization. After the emergent PCI procedure, patients were subsequently admitted to the coronary care unit. EF was measured by experienced technicians with transthoracic echocardiography on the first day after the index PCI procedure. The EF value, Killip classifications, clinical symptoms, and usage of diuretics were retrieved from the electronic medical record system to allow a comprehensive evaluation of patients’ cardiac function. A group of physicians (R.Z. Chen, J.Y. Zhou, and C. Liu) assessed the patient records and decided the classification of HF according to the definitions, and consensus was achieved through discussions in case of a dispute.
Outcomes and Follow-Up
The primary outcome for the current analysis was a composite endpoint of all-cause death and recurrent MI. The secondary outcomes included all-cause death, cardiac death, and recurrent MI. Patients were routinely followed up at 1, 6, and 12 months after discharge. The follow-up was completed independently by staffs of the information center at the institute using standardized questionnaires through phone-call interview, and the outcome data was then transferred to the research group on a monthly basis. Follow-up was also performed during rehospitalizations and outpatient visits at the institute due to adverse events or re-examinations. For those who survived more than a year, the subsequent follow-up would be made annually. A group of physicians (R.Z. Chen, J.Y. Zhou, and C. Liu) routinely assessed the reported adverse events. In case of a dispute, a consensus was reached through discussions.
Statistical Analysis
All the statistical analyses were performed using R 3.6.0 (R Core Team, Vienna, Austria) and Stata 15.0 (StataCorp, College Station, TX, United States). Multiple imputations were performed for missing values of lab test results using the mi command of Stata. The propensity score matching (PSM) was performed to control the between-group differences. Briefly, a logistic model was built to generate propensity score (PS) using all the collected baseline variables, indicating the probability of each patient being prescribed with ACEI/ARB before discharge (Austin, 2011). After that, patients not receiving ACEI/ARB were matched to those treated with ACEI/ARB by one-to-one matching using the nearest available pair matching method, with a caliper of 0.2×logit (PS). Receiver operating curve analysis was performed, and the area under the curve (AUC) was calculated to assess the performance of the PSM model. A matching weight was assigned to each patient based on the PS, and the propensity score matching weight (PSMW) analysis was performed to further balance the differences between two groups (Li and Greene, 2013). Density plots were drawn to compare the distribution of PS between ACEI/ARB users and non-users after matching and weighing. An absolute standardized mean difference (SMD) of 10% or less was considered to indicate an appropriate balance for between-group differences. Kaplan-Meier survival curve analysis was performed for the original dataset. Bivariable Cox regression was performed to assess the prognostic impacts of ACEI/ARB on various clinical outcomes in the original, PSM and PSMW datasets, respectively. Subgroup analysis for the primary outcome was performed across high-risk indications of ACEI/ARB (i.e., diabetes, hypertension, anterior infarction) and types of RAS inhibitors. Categorical variables are presented as numbers (%). Continuous variables are presented using mean ± SD if they follow the normal distribution. Otherwise, they are presented as medians with the 25th and 75th percentiles. A two-tailed p-value < 0.05 was considered statistically significant.
Results
Patient Cohort and Baseline Characteristics
From January 2010 to June 2017, a total of 4,151 patients underwent emergent coronary angiography and PCI due to ACS at the institute. Among these patients, five patients did not have available EF measurements, and 55 patients died during the index hospitalization. For the remaining 4,091 patients, 1,647 patients were excluded due to HF according to definitions, and 47 patients did not have follow-up records of any forms (i.e., phone-call interview, outpatient visits or re-hospitalizations at the institute). Finally, a total of 2,397 patients were included in the final analysis.
Baseline characteristics stratified by ACEI/ARB medication was shown in Table 1. Overall, ACEI/ARB was prescribed to 1805 (75.3%) patients, among whom 1,591 (88.1%) were prescribed with ACEI and 214 (11.9%) patients were prescribed with ARB (Supplementary Tables S1). Captopril (40.9%) was the most frequently prescribed ACEI, followed by imidapril (18.3%), ramipril (10.0%), perindopril (9.6%), and benazepril (7.1%), while fosinopril (1.7%) and enalapril (0.5%) were least frequently used. For ARB users, telmisartan (5.3%) and losartan (3.2%) were the major types being prescribed, while other types of ARB (i.e., candesartan, irbesartan, olmesartan and valsartan) were less frequently used. According to the equivalent dosages (Houston et al., 2017; Healthcare I, 2021), most of the ACEI/ARB users (91.6%) were on low-dose regimes. Compared with non-users, patients prescribed with ACEI/ARB at discharge had higher prevalence of hypertension, more stable hemodynamic status (i.e., lower heart rate, higher blood pressure), lower level of systemic inflammation, and less cardiac damage. Slight but significant difference in EF was observed between the two groups. Distributions of culprit lesions were also different, mainly due to more culprit lesions at left anterior descending arteries (32.6% vs. 24.0%), but less at right coronary arteries (RCA, 44.8% vs. 54.6%) in ACEI/ARB users compared with non-users. Moreover, patients not treated by ACEI/ARB medications acquired worse pre-intervention TIMI blood flow and longer door-to-balloon time. For discharge medications, ACEI/ARB users were more often prescribed with β-blockers, while small but significant difference in the use P2Y12 inhibitors was observed between two groups. Substantial between-group differences (SMD >0.1) were detected for many baseline variables, and therefore, analysis by PSM and PSMW was necessary.
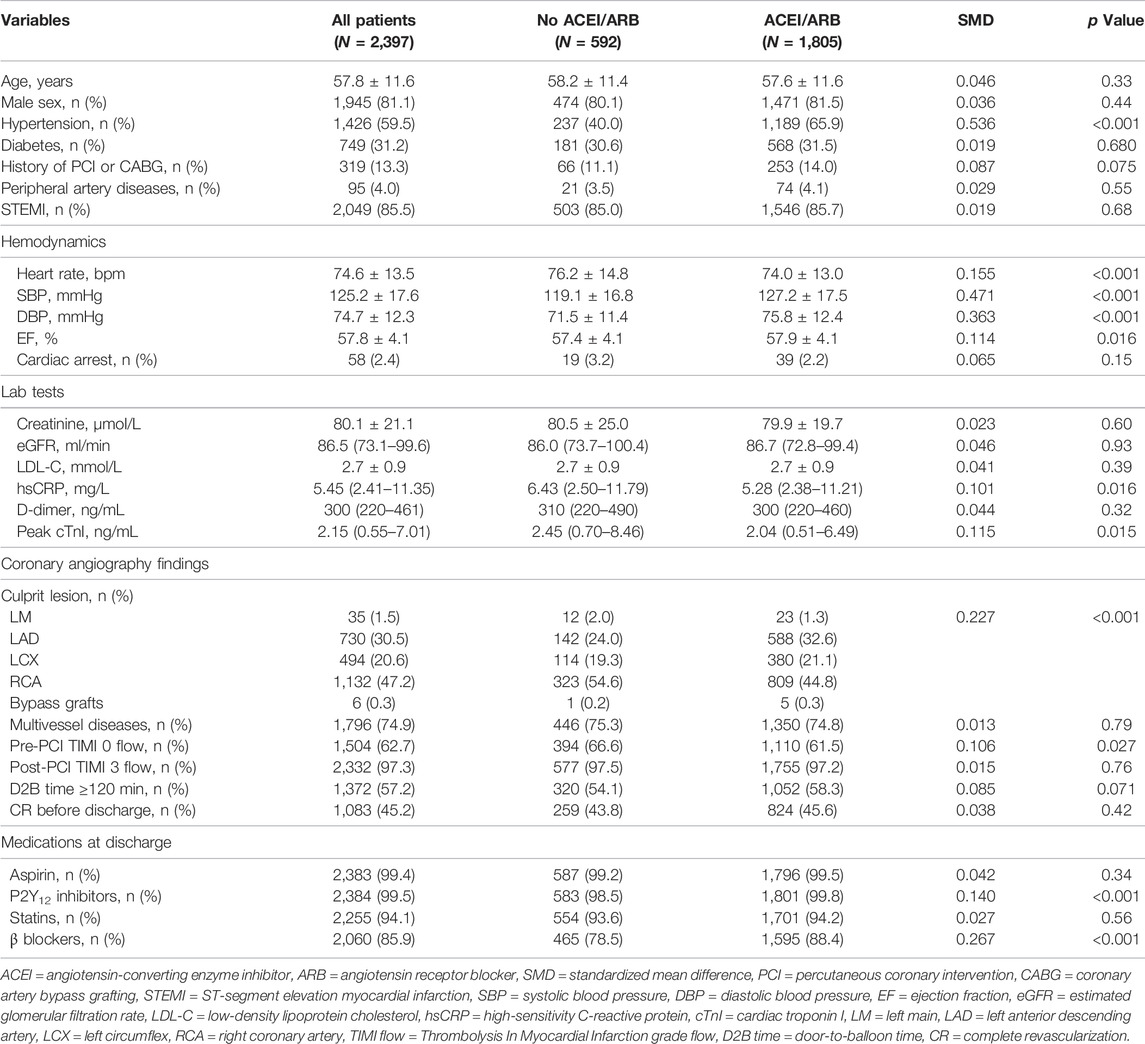
TABLE 1. Baseline characteristics of patients in the original dataset stratified by ACEI/ARB medication.
Post-Discharge Clinical Outcomes
During a median follow-up of 727 (433–2016) days, there were 129 (5.4%, incidence rate [IR]: 16.79/1000-person-year) primary endpoint events, composed of 55 (2.3%, IR: 7.16/1000-person-year [PY]) cases of all-cause death, 25 (1.0%, IR: 3.25/1000PY) cases of cardiac death, and 74 (3.1%, IR: 9.63/1000PY) cases of recurrent MI. Unadjusted Kaplan-Meier survival estimates did not demonstrate significant reduction in primary or secondary endpoint events for patients on ACEI/ARB treatments (Figure 1). Univariable Cox regression (Figure 2) also showed that the use of ACEI/ARB was not associated with lower risk of primary endpoint events (hazard ratio [HR]: 0.95, 95% confidence interval [CI]: 0.64–1.39, p = 0.779), with similar results for all-cause death (HR: 0.84, 95% CI: 0.47–1.48, p = 0.537), cardiac death (HR: 0.67, 95% CI: 0.30–1.53, p = 0.346), and recurrent MI (HR: 1.04, 95% CI: 0.62–1.76, p = 0.879).
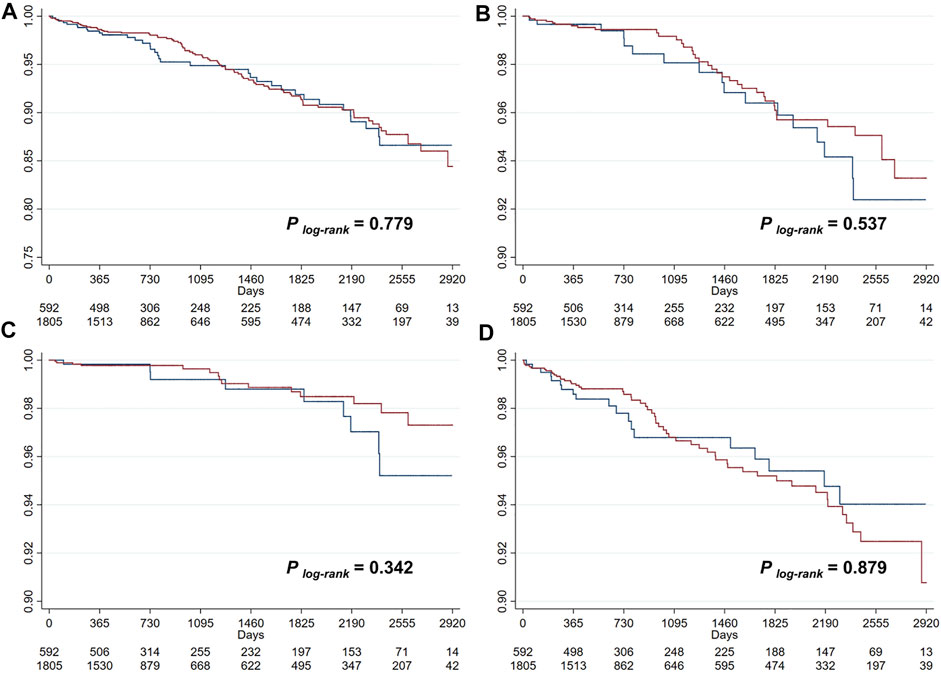
FIGURE 1. Kaplan-Meier survival curve analysis for the primary outcome (A), all-cause death (B), cardiac death (C), and recurrent myocardial infarction (D).
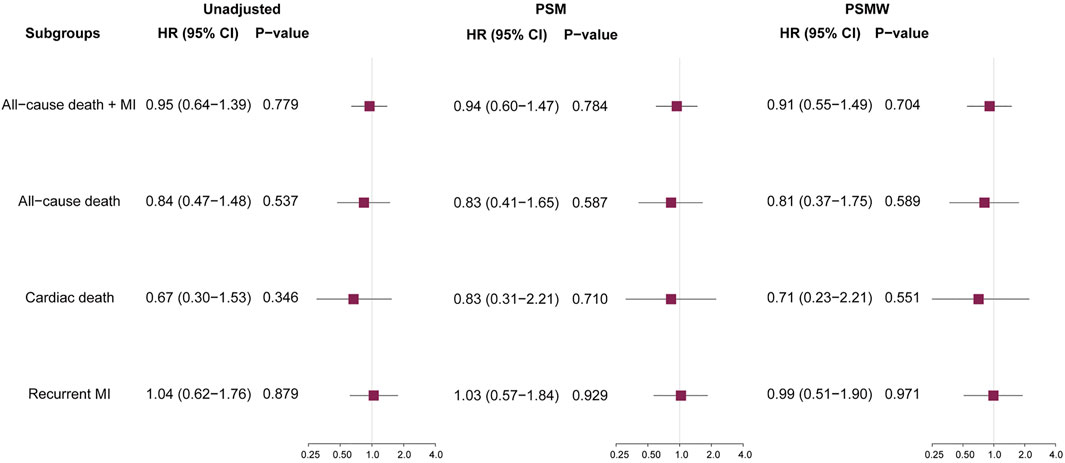
FIGURE 2. Prognostic impacts of ACEI/ARB on various outcomes. ACEI = angiotensin converting enzyme inhibitors, ARB = angiotensin receptor blockers, HR = hazard ratio, CI = confidence interval, MI = myocardial infarction, PSM = propensity score matching, PSMW = propensity score matching weight.
As significant differences (SMD >0.1) were detected for many baseline variables (Table 1), PSM was used to control for between-group imbalances. Before the PSM, the median propensity to be prescribed with ACEI/ARB was substantially higher for ACEI/ARB users (0.816 [0.717–0.882]) compared with non-users (0.676 [0.539–0.790], p < 0.001), and the distribution of PS was apparently different for two groups (Figure 3A). After the PSM procedure, a total of 543 pairs of ACEI/ARB users and non-users were matched. The between-group balances were achieved for most variables (Table 2), but residual differences remained for peripheral artery disease (SMD: 0.108), STEMI (SMD: 0.112), diastolic blood pressure (SMD: 0.142), EF (SMD: 0.110), estimated glomerular filtration rate (SMD: 0.112), high-sensitivity C-reactive protein (SMD: 0.158), and pre-PCI TIMI 0 flow (SMD: 0.131). No significant difference in propensity was detected for the two groups after PSM (0.698 [0.583–0.801] vs. 0.696 [0.578–0.801], p = 0.720), which was further confirmed by the similar distribution curves of propensity in the density plot (Figure 3B). The AUC for the PSM model was 0.72 (0.70–0.75), suggesting an adequate discrimination to differentiate ACEI/ARB users from non-users (Supplementary Figure S1). According to the PSM bivariable analysis (Figure 2), the use of ACEI/ARB was not associated with significant risk reduction in death or MI (HR: 0.94, 95% CI: 0.60–1.47, p = 0.784). Similar findings were observed for all-cause death (HR: 0.83, 95% CI: 0.41–1.65, p = 0.587), cardiac death (HR: 0.83, 95% CI: 0.31–2.21, p = 0.710), and recurrent MI (HR: 1.03, 95% CI: 0.57–1.84, p = 0.929).
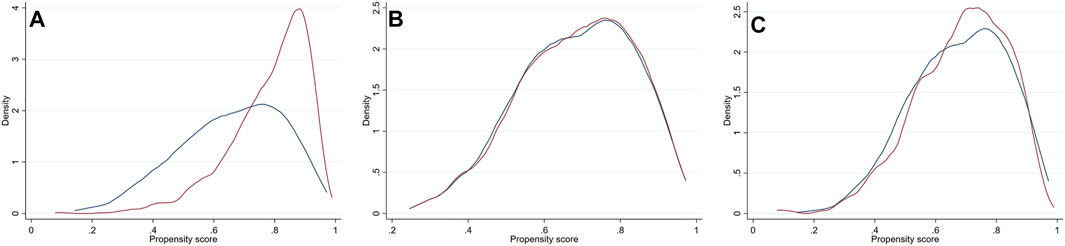
FIGURE 3. Density plots for the distribution of propensity score stratified by usage of ACEI/ARB in the original dataset (A), the PSM dataset (B) and PSMW dataset (C). Red line = ACEI/ARB users, blue line = ACEI/ARB non-users. ACEI = angiotensin converting enzyme inhibitors, ARB = angiotensin receptor blockers, PSM = propensity score matching, PSMW = propensity score matching weight.
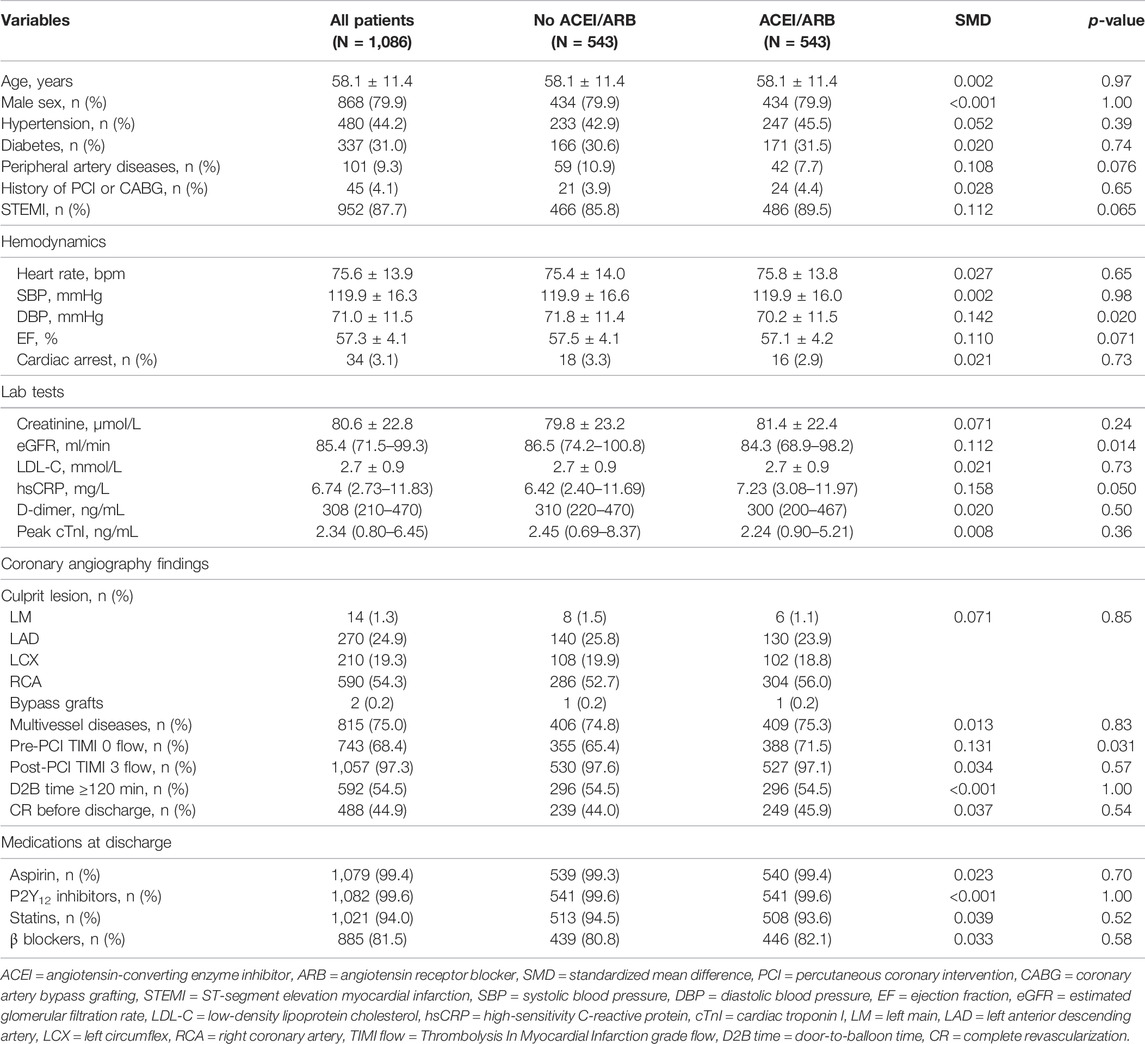
TABLE 2. Baseline characteristics of patients in the propensity score matching dataset stratified by ACEI/ARB medication.
As there were still residual between-group differences in the PSM dataset, we performed PSMW analysis to further reduce the systematic differences in baseline characteristics. Subsequently, all variables achieved an SMD below 0.1 (Table 3), and similar distribution of propensity for both groups was affirmed in the density plot (Figure 3C). Still, ACEI/ARB users did not acquire lower risk of death or MI (HR: 0.91, 95% CI: 0.55–1.49, p = 0.704), and the results remained similar for all-cause death (HR: 0.81, 95% CI: 0.37–1.75, p = 0.589, Figure 2), cardiac death (HR: 0.71, 95% CI: 0.23–2.21, p = 0.551) and recurrent MI (HR: 0.99, 95% CI: 0.51–1.90, p = 0.971).
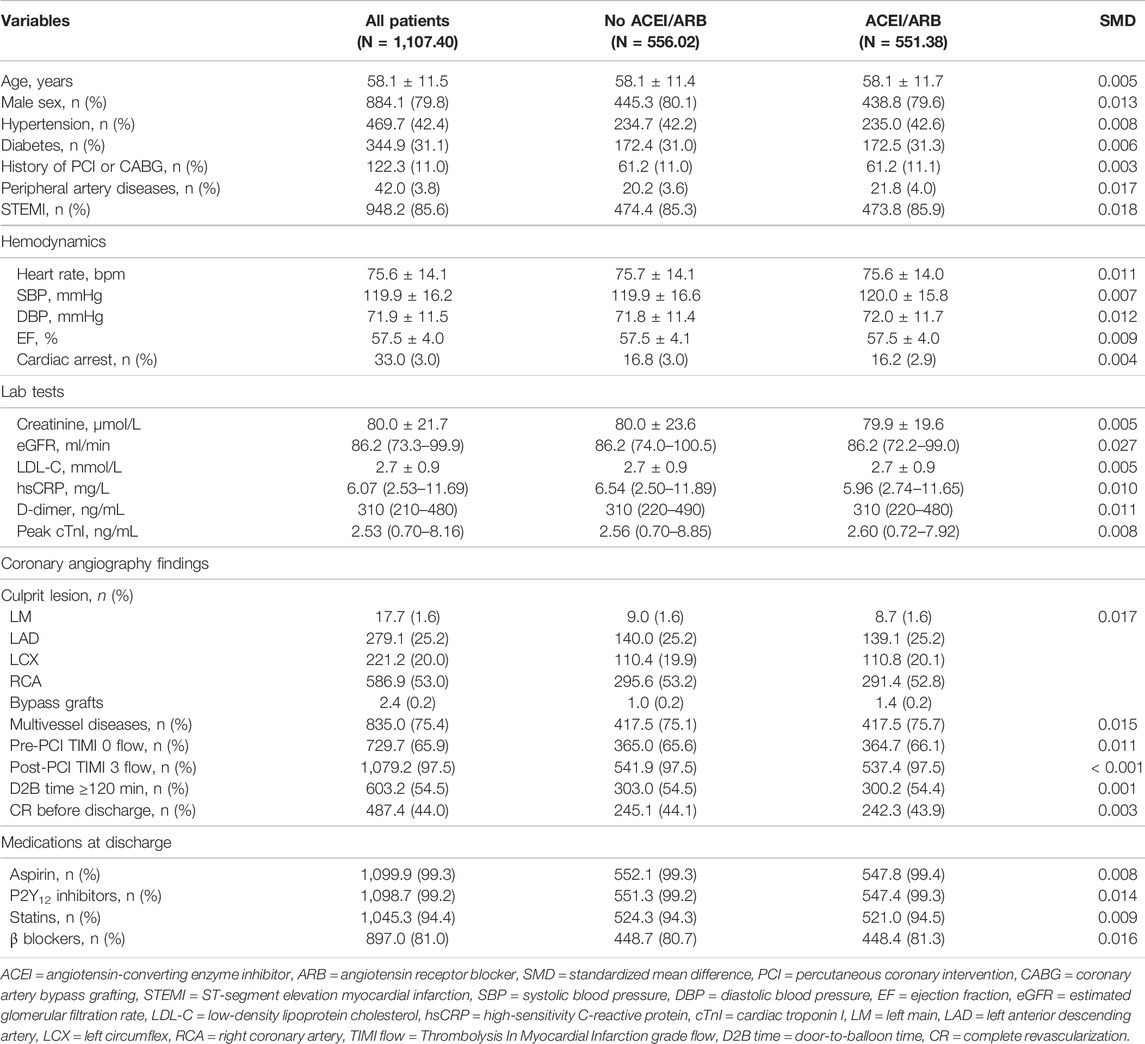
TABLE 3. Baseline characteristics of patients in the propensity matching weight dataset stratified by ACEI/ARB medication.
Subgroup analysis was performed for the primary outcome across high-risk indications for ACEI/ARB (including diabetes, hypertension, and anterior infarction) and types of RAS inhibitors being prescribed. Systematic differences of baseline characteristics were common between ACEI/ARB users and non-users in various subgroups (Supplementary Tables S2–S9). PSM analysis was performed, and the AUC for PSM models in various subgroups were generally above 0.7, suggesting a good discrimination for patients with or without ACEI/ARB medications (Supplementary Figure S2). Although there were residual differences for some variables (Supplementary Tables S10–S17), the propensity was generally well-matched after the PSM procedure (Supplementary Figures S3–S10). With PSMW, both the propensity (Supplementary Figures S3–S10) and baseline differences were well controlled (Supplementary Table S18–S25). Still, the risk reduction by ACEI/ARB was not significant across the unadjusted, PSM and PSMW analyses, despite the complication of diabetes, hypertension, or anterior infarction (Figure 4). Moreover, neither the usage of ACEI nor ARB was associated with lower risk of all-cause death or recurrent MI as compared with patients without RAS blockade.
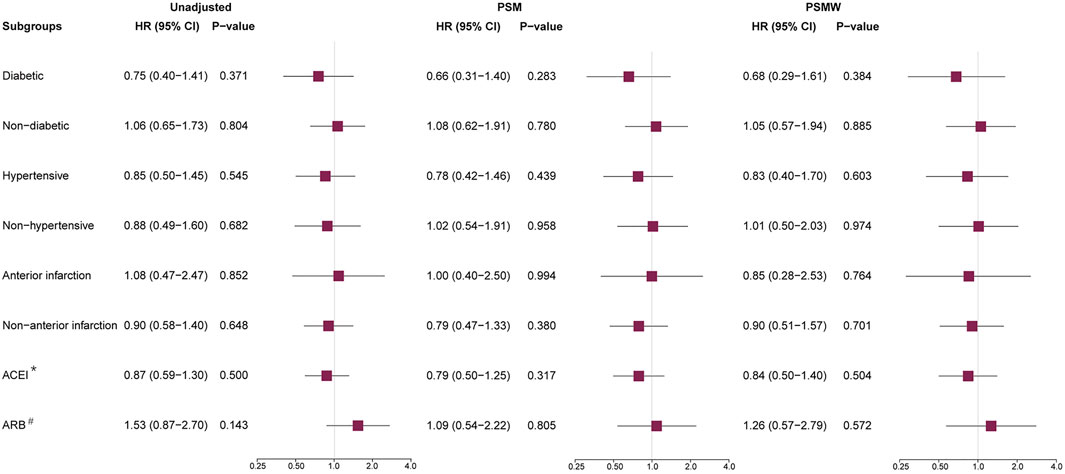
FIGURE 4. Subgroup analysis for the primary outcome. ACEI = angiotensin converting enzyme inhibitors, ARB = angiotensin receptor blockers, PSM = propensity score matching, PSMW = propensity score matching weight, HR = hazard ratio, CI = confidence interval. *: ACEI users vs. ACEI/ARB non-users. #: ARB users vs. ACEI/ARB non-users.
Discussions
In this observational study of ACS patients without HF treated by PCI, the use of ACEI/ARB was not associated with significant risk reduction of all-cause death, cardiac death, or recurrent MI. The neutral effect of ACEI/ARB was not altered in the PSM analysis, PSMW analysis, or subgroup analysis.
Gaps of Evidence for ACEI/ARB in Non-HF ACS Patients
Based on evidences from the era of thrombolysis (Pfeffer et al., 1992; Yusuf et al., 1992; Ball et al., 1993; Rutherford et al., 1994), ACEI/ARB is currently indicated for nearly all patients having experienced an acute coronary event (Amsterdam et al., 2014; Ibanez et al., 2018; O'Gara et al., 2013; Collet et al., 2021), with the expectation to control blood pressure, inhibit the left ventricular remodeling, suppress cardiovascular impacts of RAS system, and therefore bring about anti-atherosclerotic and cardioprotective effects (Thind, 1990; Lamas and Pfeffer, 1991; Nissen et al., 2004). However, the long-term benefit of ACEI/ARB has been questioned with the rapid development of revascularization techniques and the application of more intensive antiplatelet and lipid-lowering treatment (Braunwald et al., 2004; Raposeiras-Roubín et al., 2020). Meanwhile, the incidence of cardiac failure after ACS is also declining in recent years (Chen et al., 2020). Notably, studies in the field of HF suggest that traditionally recommended treatments (e.g., ACEI/ARB, β-blockers) might not be effective for HF patients with preserved or mid-range EF (Ponikowski et al., 2016; Ambrosy et al., 2018; Lund et al., 2018). It is therefore necessary to reassess the actual benefit for long-term use of ACEI/ARB after discharge, and reconsider the recommendations for its routine prescription among patients with generally normal cardiac function after ACS.
Previously, Roubin et al. demonstrated in the BleeMACS study that RAS blockers could not reduce 1-year mortality of ACS patients treated by PCI (HR: 0.84, 95% CI: 0.65–1.08) (Raposeiras-Roubín et al., 2020). Similarly, Parashar et al. suggest a limited reduction of mortality by ACEI/ARB for STEMI patients with EF > 40% (HR: 0.88, 95% CI: 0.57–1.36), with 714 patients needed to treat for preventing a case of death at 1 year (Parashar et al., 2015). Considering the new classification of HF, the current study used stricter inclusion criteria, which only incorporated patients with EF ≥ 50% and no clinical signs of HF, in order to minimize the chance of incorrect recruitment of HF patients with preserved (≥50%) or mid-range EF (40–49%). Nearly 60% of ACS-PCI patients in the original cohort were not complicated with clinical HF according to current definitions, among whom ACEI/ARB was not associated with the reduction in long-term risk of death and recurrent MI, which remained the same after matching and weighted analysis. The point estimates of HR for various outcomes in the current study generally fall within 0.8–1.0, which is in line with previous studies reporting ineffectiveness of ACEI/ARB (Parashar et al., 2015; Raposeiras-Roubín et al., 2020). Although the estimated HR values were lower in several high-risk subgroups (i.e., diabetic, hypertensive, anterior infarction), the wide confidence interval did not support a positive effect of ACEI/ARB for reducing mortality and MI. Moreover, neither the treatment with ACEI nor ARB was associated with fewer adverse events. Taken together, the routine use of ACEI/ARB medications in ACS patients with preserved or normal cardiac function result in limited risk reduction, for which the recommendation should be reconsidered in the era of reperfusion.
Impacts of More Intensive Secondary Preventions on the Efficacy of ACEI/ARB
Although the interpretation could be challenging, the observed ineffectiveness of ACEI/ARB could be due to the comprehensive advances of ACS treatment in the past decades. In studies demonstrating the reduction of MI by ACEI/ARB, most of patients have been treated noninvasively while not receiving DAPT or statins medications, suggesting an incomplete resolution of ischemia and higher risk of future thrombosis due to inadequate suppression of platelet function and progression of coronary plaques (Pfeffer et al., 1992; Yusuf et al., 1992; Ball et al., 1993; Rutherford et al., 1994; Fox, 2003). In this scenario, ACEI/ARB could have backed up the treatments through its anti-atherosclerotic effects secondary to the blood pressure control and the suppression of neural-hormonal system (Lamas and Pfeffer, 1991; Nissen et al., 2004). However, in the era of PCI, the coronary obstruction due to thrombus at the culprit lesion was instantly resolved by angioplasty or stenting, and the following risk of stent thrombosis, restenosis or progression of atherosclerotic plaques were comprehensively tackled by DAPT and lipid-lowering treatments (Amsterdam et al., 2014; Ibanez et al., 2018; O'Gara et al., 2013; Hoang et al., 2016; Robinson et al., 2014). In the PEACE trial, the addition of trandolapril did not reduce the risk of cardiovascular deaths, MI, or coronary revascularizations (HR: 0.96, 95% CI: 0.88–1.06) in a cohort of patients with stable coronary artery disease (CAD) (Braunwald et al., 2004). However, over 70% of the recruited patients have been treated by revascularizations and lipid-lowering medications. In the BleeMACS study (Raposeiras-Roubín et al., 2020), all the included ACS patients have undergone PCI, while over 90% of them receive DAPT and statins. A recent meta-analysis including 24 randomized trials in stable CAD patients without HF also shows that RAS blockade could effectively reduce mortality, MI, angina, HF, and revascularization when compared with placebos, but not with active controls (Bangalore et al., 2017). For the current cohort, over 90% of patients were on DAPT at discharge, while over 80% of them were prescribed with β-blockers, suggesting a prevalent use of intensive secondary preventions. Consequently, the vasoprotective effects of RAS inhibitors could be attenuated, as medications targeted for platelet inhibition, lipid lowering and plaque stabilization might have provided more specific and overwhelming protection than that of ACEI/ARB. Taken together, routine prescription of ACEI/ARB to ACS patients without HF might not be indispensable under the condition of active secondary preventions, and new evidence is needed to reassess the prognostic impacts of these medications.
Impacts of Low-Risk Profile on the Efficacy of ACEI/ARB
Aside from intensive secondary preventions, the baseline risk of the targeted patient group is also a decisive factor for the observed risk reduction with ACEI/ARB medications. In a recent meta-analysis, Sripal et al. have reported that the use of RAS inhibitors is only beneficial when the IR of all-cause death and cardiovascular death is higher than 14.10 and 7.65 per 1000-PY in the control group, respectively (Bangalore et al., 2017). Similar trends are observed in studies of non-HF ACS patients. In studies showing significant risk reduction for patients treated by ACEI/ARB, the 1-year mortality of control groups is generally over 10% (Milonas et al., 2010; Grall et al., 2015; Liu et al., 2019). On the contrary, the 1-year mortality of patients without RAS blockade could be as low as 1–3% in studies acquiring neutral findings (Pitt et al., 2001; Parashar et al., 2015; Park et al., 2018; Raposeiras-Roubín et al., 2020). In the current study, the all-cause mortality (2.1 vs. 2.8%) and cardiac mortality (0.9 vs. 1.5%) was both very low for patients with or without ACEI/ARB medication over a median follow-up of nearly 2 years. Besides, the IR of all-cause death (6.72 vs. 8.39 per 1000-PY) and cardiac death (2.83 vs. 4.44 per 1000-PY) was far below the aforementioned thresholds to detect the benefit of RAS inhibitors, which could be a major interpretation for the neutral findings in the current study. Several factors might have contributed the low-risk profile of the current cohort, including the generally preserved cardiac function, the exclusion of patients who failed to survive the hospitalizations, and the prevalent use of antiplatelet, lipid-lowering and anti-ischemic medications. Besides, the risk of left-ventricular remodeling was theoretically lower for the current cohort, since most of the patients had a culprit lesion at left circumflex or RCA (67.8%), while 97.3% of patients reached a post-PCI TIMI 3 grade flow. The median peak cardiac troponin I value was only 2.15 (0.55–7.01) ng/ml, suggesting an adequate resolution of emergent ischemia and limited myocardial damage. The favorable outcomes of reperfusion could have led to a lower risk of post-infarction left ventricular remodeling, for which the RAS blockade brings limited improvement to cardiac function and long-term outcomes even at very high dosages (Ganame et al., 2011; Masci et al., 2011; Park et al., 2018). In sum, non-HF ACS patients treated by PCI possessed an intrinsically lower risk profile, and ACEI/ARB might not be able to further improve patient outcomes in this occasion.
Impacts of ACEI/ARB Dosages for Outcome Improvements
According to previous research, differences in regimes and dosages of ACEI/ARB prescribed to patients could have affected the clinical efficacy being observed by researchers (Grall et al., 2015; Liu et al., 2019). With most of patients in the treatment group reaching high dosages, results from the HOPE trial (ramipril 10 mg daily) and EUROPA trial (perindopril 8 mg daily) have demonstrated that RAS blockade could reduce adverse events in patients with preserved cardiac function (Yusuf et al., 2000; Fox, 2003). Comparatively, trials assigning lower dosages of RAS inhibitors to the treatment group show limited clinical benefits (Pitt et al., 2001; Nissen et al., 2004; Al-Mallah et al., 2006), like the QUIET trial (quinapril 20 mg daily) and the CAMELOT trial (enalapril 20 mg daily). It seems patients need to be on high-dose regimes to maximize the benefits from ACEI/ARB treatment. In the current study, the majority of patients taking ACEI/ARB were on low-dose regime, which might have posed limited impacts on the outcomes. However, the PEACE trial fails to show benefits for stable CAD patients with preserved left ventricular function to take trandolapril 4 mg daily, a dosage previously demonstrated to improve the survival of hypertensive patients (Braunwald et al., 2004). In recent studies showing ineffectiveness of ACEI/ARB, details of daily regime are generally not available. It remained to be investigated whether improving dosages of ACEI/ARB could increase the benefit for patients on these medications. (Milonas et al., 2010; Parashar et al., 2015; Raposeiras-Roubín et al., 2020).
In sum, the current study called into questions regarding the necessity of routine use of ACEI/ARB for ACS patients without HF after PCI. Physicians could consider not prescribing ACEI/ARB for these low-risk patients if other risk factors could be well-controlled without the RAS blockade. Clinical guidelines should reconsider the recommendations of routine use of ACEI/ARB to all ACS patients, especially those patients with normal cardiac function.
Limitations
The major limitations of this study are as follow. Firstly, the current study was retrospective, and patients were not randomly assigned to receive ACEI/ARB treatment. Although matching methods of PSM and PSMW were used to control the between-group differences for all the collected baseline variables, unmeasured confounders could still affect the results. Moreover, the current study has no available data regarding the patient adherence or changes of ACEI/ARB medications after discharge. Discontinuation and modification of the treating regime could have attenuated the risk differences between ACEI/ARB users and non-users. Besides, the median 2-year follow-up might not be adequate to fully determine the prognostic impacts of ACEI/ARB in the long run. Finally, this study was accomplished in a single center. Although the sample size was large enough, only Chinese patients were included in this study. The extrapolation of current conclusions still requires further validation. Future multicenter randomized clinical trials are warranted to assess the efficacy of ACEI/ARB in non-HF ACS patients treated by PCI from different regions and populations.
Conclusion
For ACS patients without HF, the use of ACEI/ARB was not associated with lower risk of death or recurrent MI after PCI.
Data Availability Statement
The datasets presented in this article are not readily available because; The data used to support the findings of this study are available from the corresponding authors upon request. The institution (Fuwai Hospital) requires all requests for accessing any data of patients be applicated and processed in a case by case manner. Requests to access the datasets should be directed to;aGJ5YW5mdXdhaTIwMThAMTYzLmNvbQ==.
Ethics Statement
The studies involving human participants were reviewed and approved by Fuwai Hospital, National Center for Cardiovascular Diseases, Peking Union Medical College and Chinese Academy of Medical Sciences. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Conceptualization, RC and HY; Data curation, RC, CL, PZ, JL, JZ, XZ, YC, LS, SY, HZ and HY; Formal analysis, RC and YW; Funding acquisition, HY; Methodology, RC and SY; Resources, RC, CL, PZ, JL, JZ, YC, LS and HZ; Supervision, HZ and HY; Writing (original draft), RC and SY; Writing (review & editing), RC, CL, PZ, JL, JZ, YW, XZ, YC, SY, LS, HZ and HY. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (81970308), the Fund of “Sanming” Project of Medicine in Shenzhen (SZSM201911017), Shenzhen Key Medical Discipline Construction Fund (SZXK001), and the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2016-I2M-1-009).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We sincerely thank Dr. Yu Tan and Dr. Zhaoxue Sheng for their professional assistance in patient data acquisition, follow-up and statistical anlaysis.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.663811/full#supplementary-material
References
Al-Mallah, M. H., Tleyjeh, I. M., Abdel-Latif, A. A., and Weaver, W. D. (2006). Angiotensin-converting Enzyme Inhibitors in Coronary Artery Disease and Preserved Left Ventricular Systolic Function: a Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Coll. Cardiol. 47, 1576–1583. doi:10.1016/j.jacc.2005.11.073
Ambrosy, A. P., Mentz, R. J., Fiuzat, M., Cleland, J. G. F., Greene, S. J., O'Connor, C. M., et al. (2018). The Role of Angiotensin Receptor-Neprilysin Inhibitors in Cardiovascular Disease-Existing Evidence, Knowledge Gaps, and Future Directions. Eur. J. Heart Fail. 20, 963–972. doi:10.1002/ejhf.1159
Amsterdam, E. A., Wenger, N. K., Brindis, R. G., Casey, D. E., Ganiats, T. G., Holmes, D. R., et al. (2014). 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-elevation Acute Coronary Syndromes: Executive Summary: a Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 130, 2354–2394. doi:10.1161/CIR.0000000000000133
Austin, P. C. (2011). An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav. Res. 46, 399–424. doi:10.1080/00273171.2011.568786
Ball, S. G., Cowan, J. C., Winter, C., Mackintosh, A. F., Tan, L. B., Caldicott, L., et al. (1993). Effect of Ramipril on Mortality and Morbidity of Survivors of Acute Myocardial Infarction with Clinical Evidence of Heart Failure. The Acute Infarction Ramipril Efficacy (AIRE) Study Investigators. Lancet 342, 821–828.
Bangalore, S., Fakheri, R., Wandel, S., Toklu, B., Wandel, J., and Messerli, F. H. (2017). Renin Angiotensin System Inhibitors for Patients with Stable Coronary Artery Disease without Heart Failure: Systematic Review and Meta-Analysis of Randomized Trials. BMJ 356, j4. doi:10.1136/bmj.j4
Braunwald, E., Domanski, M. J., Fowler, S. E., Geller, N. L., Gersh, B. J., Hsia, J., et al. (2004). Angiotensin-converting-enzyme Inhibition in Stable Coronary Artery Disease. N. Engl. J. Med. 351, 2058–2068. doi:10.1056/NEJMoa042739
Cespón-Fernández, M., Raposeiras-Roubín, S., Abu-Assi, E., Manzano-Fernández, S., Flores-Blanco, P., Barreiro-Pardal, C., et al. (2019). Renin-angiotensin System Blockade and Risk of Heart Failure after Myocardial Infarction Based on Left Ventricular Ejection Fraction: a Retrospective Cohort Study. Am. J. Cardiovasc. Drugs 19, 487–495. doi:10.1007/s40256-019-00343-7
Chen, R., Liu, C., Zhou, P., Sheng, Z., Li, J., Zhou, J., et al. (2020). Associations between Cardiac Function and Long-Term Outcomes of Patients with Acute Myocardial Infarction Treated by Percutaneous Coronary Intervention. Chin. J. Heart Fail. Cardiomyopathy 04, 159–167. doi:10.3760/cma.j.cn101460-20200415-00037
Collet, J. P., Thiele, H., Barbato, E., Barthélémy, O., Bauersachs, J., Bhatt, D. L., et al. (2021). 2020 ESC Guidelines for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation. Eur. Heart J. 42, 1289–1367. doi:10.1093/eurheartj/ehaa575
Fox, K. M.EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators (2003). Efficacy of Perindopril in Reduction of Cardiovascular Events Among Patients with Stable Coronary Artery Disease: Randomised, Double-Blind, Placebo-Controlled, Multicentre Trial (The EUROPA Study). Lancet 362, 782–788. doi:10.1016/s0140-6736(03)14286-9
Ganame, J., Messalli, G., Masci, P. G., Dymarkowski, S., Abbasi, K., Van de Werf, F., et al. (2011). Time Course of Infarct Healing and Left Ventricular Remodelling in Patients with Reperfused ST Segment Elevation Myocardial Infarction Using Comprehensive Magnetic Resonance Imaging. Eur. Radiol. 21, 693–701. doi:10.1007/s00330-010-1963-8
Grall, S., Biere, L., Le Nezet, M., Bouvier, J. M., Lucas-Chauvelon, P., Richard, C., et al. (2015). Relationship between Beta-Blocker and Angiotensin-Converting Enzyme Inhibitor Dose and Clinical Outcome Following Acute Myocardial Infarction. Circ. J. 79, 632–640. doi:10.1253/circj.CJ-14-0633
Healthcare I. ACE Inhibitors (ACEIs) and ARBs: For Patients with Heart Failure. 2021. Available at: https://intermountainphysicianorg/provider-public/_layouts/Custom/KnowledgeRepository/KrDocumentFetchaspx?target=document&ncid=525935617&tfrm=default (Accessed June 23, 2021).
Hoang, V., Alam, M., Addison, D., Macedo, F., Virani, S., and Birnbaum, Y. (2016). Efficacy of Angiotensin-Converting Enzyme Inhibitors and Angiotensin-Receptor Blockers in Coronary Artery Disease without Heart Failure in the Modern Statin Era: a Meta-Analysis of Randomized-Controlled Trials. Cardiovasc. Drugs Ther. 30, 189–198. doi:10.1007/s10557-016-6652-7
Houston, B. A., Schneider, A. L., Vaishnav, J., Cromwell, D. M., Miller, P. E., Faridi, K. F., et al. (2017). Angiotensin II Antagonism Is Associated with Reduced Risk for Gastrointestinal Bleeding Caused by Arteriovenous Malformations in Patients with Left Ventricular Assist Devices. J. Heart Lung Transpl. 36, 380–385. doi:10.1016/j.healun.2016.12.016
Ibanez, B., James, S., Agewall, S., Antunes, M. J., Bucciarelli-Ducci, C., Bueno, H., et al. (2018). 2017 ESC Guidelines for the Management of Acute Myocardial Infarction in Patients Presenting with ST-Segment Elevation: The Task Force for the Management of Acute Myocardial Infarction in Patients Presenting with ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 39, 119–177. doi:10.1093/eurheartj/ehx393
Lamas, G. A., and Pfeffer, M. A. (1991). Left Ventricular Remodeling after Acute Myocardial Infarction: Clinical Course and Beneficial Effects of Angiotensin-Converting Enzyme Inhibition. Am. Heart J. 121, 1194–1202. doi:10.1016/0002-8703(91)90682-8
Li, L., and Greene, T. (2013). A Weighting Analogue to Pair Matching in Propensity Score Analysis. Int. J. Biostat 9, 215–234. doi:10.1515/ijb-2012-0030
Liu, P. Y., Chen, C. L., Yu, M. C., Ko, Y. L., Hsu, S. Y., Chou, H. H., et al. (2019). Doses of Renin-Angiotensin System Inhibitors but Not Beta-Blockers Predict Outcome after ST-Elevation Myocardial Infarction. Acta Clin. Belg. 74, 334–341. doi:10.1080/17843286.2018.1528708
Lund, L. H., Claggett, B., Liu, J., Lam, C. S., Jhund, P. S., Rosano, G. M., et al. (2018). Heart Failure with Mid-range Ejection Fraction in CHARM: Characteristics, Outcomes and Effect of Candesartan across the Entire Ejection Fraction Spectrum. Eur. J. Heart Fail. 20, 1230–1239. doi:10.1002/ejhf.1149
Masci, P. G., Ganame, J., Francone, M., Desmet, W., Lorenzoni, V., Iacucci, I., et al. (2011). Relationship between Location and Size of Myocardial Infarction and Their Reciprocal Influences on post-infarction Left Ventricular Remodelling. Eur. Heart J. 32, 1640–1648. doi:10.1093/eurheartj/ehr064
Milonas, C., Jernberg, T., Lindbäck, J., Agewall, S., Wallentin, L., Stenestrand, U., et al. (2010). Effect of Angiotensin-Converting Enzyme Inhibition on One-Year Mortality and Frequency of Repeat Acute Myocardial Infarction in Patients with Acute Myocardial Infarction. Am. J. Cardiol. 105, 1229–1234. doi:10.1016/j.amjcard.2009.12.032
Nissen, S. E., Tuzcu, E. M., Libby, P., Thompson, P. D., Ghali, M., Garza, D., et al. (2004). Effect of Antihypertensive Agents on Cardiovascular Events in Patients with Coronary Disease and normal Blood Pressure: the CAMELOT Study: a Randomized Controlled Trial. JAMA 292, 2217–2225. doi:10.1001/jama.292.18.2217
O'Gara, P. T., Kushner, F. G., Ascheim, D. D., Casey, D. E., Chung, M. K., de Lemos, J. A., et al. (2013). 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: Executive Summary: a Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: Developed in Collaboration with the American College of Emergency Physicians and Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc. Interv. 82, E1–E27. doi:10.1002/ccd.24776
Parashar, A., Agarwal, S., Krishnaswamy, A., Garg, A., Poddar, K. L., Sud, K., et al. (2015). Renin-angiotensin System Antagonists in Patients without Left Ventricular Dysfunction after Percutaneous Intervention for ST-Segment Elevation Myocardial Infarction. Am. J. Cardiol. 116, 508–514. doi:10.1016/j.amjcard.2015.05.007
Park, K., Kim, Y. D., Kim, K. S., Lee, S. H., Park, T. H., Lee, S. G., et al. (2018). The Impact of a Dose of the Angiotensin Receptor Blocker Valsartan on post-myocardial Infarction Ventricular Remodelling. ESC Heart Fail. 5, 354–363. doi:10.1002/ehf2.12249
Pfeffer, M. A., Braunwald, E., Moyé, L. A., Basta, L., Brown, E. J., Cuddy, T. E., et al. (1992). Effect of Captopril on Mortality and Morbidity in Patients with Left Ventricular Dysfunction after Myocardial Infarction. Results of the Survival and Ventricular Enlargement Trial. The SAVE Investigators. N. Engl. J. Med. 327, 669–677. doi:10.1056/NEJM199209033271001
Pitt, B., O'Neill, B., Feldman, R., Ferrari, R., Schwartz, L., Mudra, H., et al. (2001). The QUinapril Ischemic Event Trial (QUIET): Evaluation of Chronic ACE Inhibitor Therapy in Patients with Ischemic Heart Disease and Preserved Left Ventricular Function. Am. J. Cardiol. 87, 1058–1063. doi:10.1016/s0002-9149(01)01461-8
Ponikowski, P., Voors, A. A., Anker, S. D., Bueno, H., Cleland, J. G., Coats, A. J., et al. (2016). 2016 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Developed with the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 18, 891–975. doi:10.1002/ejhf.592
Raposeiras-Roubín, S., Abu-Assi, E., Cespón-Fernández, M., Ibáñez, B., García-Ruiz, J. M., D'Ascenzo, F., et al. (2020). Impact of Renin-Angiotensin System Blockade on the Prognosis of Acute Coronary Syndrome Based on Left Ventricular Ejection Fraction. Rev. Esp Cardiol. (Engl Ed. 73, 114–122. doi:10.1016/j.rec.2019.02.012
Robinson, J. G., Nedergaard, B. S., Rogers, W. J., Fialkow, J., Neutel, J. M., Ramstad, D., et al. (2014). Effect of Evolocumab or Ezetimibe Added to Moderate- or High-Intensity Statin Therapy on LDL-C Lowering in Patients with Hypercholesterolemia: the LAPLACE-2 Randomized Clinical Trial. JAMA 311, 1870–1882. doi:10.1001/jama.2014.4030
Rutherford, J. D., Pfeffer, M. A., Moyé, L. A., Davis, B. R., Flaker, G. C., Kowey, P. R., et al. (1994). Effects of Captopril on Ischemic Events after Myocardial Infarction. Results of the Survival and Ventricular Enlargement Trial. SAVE Investigators. Circulation 90, 1731–1738. doi:10.1161/01.cir.90.4.1731
Sutton, N. R., Li, S., Thomas, L., Wang, T. Y., de Lemos, J. A., Enriquez, J. R., et al. (2016). The Association of Left Ventricular Ejection Fraction with Clinical Outcomes after Myocardial Infarction: Findings from the Acute Coronary Treatment and Intervention Outcomes Network (ACTION) Registry-Get with the Guidelines (GWTG) Medicare-Linked Database. Am. Heart J. 178, 65–73. doi:10.1016/j.ahj.2016.05.003
Thind, G. S. (1990). Angiotensin Converting Enzyme Inhibitors: Comparative Structure, Pharmacokinetics, and Pharmacodynamics. Cardiovasc. Drugs Ther. 4, 199–206. doi:10.1007/BF01857634
Thygesen, K., Alpert, J. S., Jaffe, A. S., Chaitman, B. R., Bax, J. J., Morrow, D. A., et al. (2019). Fourth Universal Definition of Myocardial Infarction (2018). Rev. Esp Cardiol. (Engl Ed. 72, 72–69. doi:10.1016/j.rec.2018.11.011
Yusuf, S., Pepine, C. J., Garces, C., Pouleur, H., Salem, D., Kostis, J., et al. (1992). Effect of Enalapril on Myocardial Infarction and Unstable Angina in Patients with Low Ejection Fractions. Lancet 340, 1173–1178. doi:10.1016/0140-6736(92)92889-n
Keywords: acute coronary syndrome (ACS), ACEI (angiotensin-converting enzyme inhibitor), ARB (angiotensin II receptor blocker), heart failure, percutaneous coroanry intervention (PCI)
Citation: Chen R, Liu C, Zhou P, Li J, Zhou J, Wang Y, Zhao X, Chen Y, Yan S, Song L, Zhao H and Yan H (2022) Prognostic Impacts of Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers in Acute Coronary Syndrome Patients Without Heart Failure. Front. Pharmacol. 13:663811. doi: 10.3389/fphar.2022.663811
Received: 04 February 2021; Accepted: 03 March 2022;
Published: 05 April 2022.
Edited by:
Antonella De Angelis, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Francesca Giordana, Azienda Sanitaria Ospedaliera S. Croce e Carle Cuneo, ItalyAndrea Mastinu, University of Brescia, Italy
Fernanda Priviero, University of South Carolina, United States
Copyright © 2022 Chen, Liu, Zhou, Li, Zhou, Wang, Zhao, Chen, Yan, Song, Zhao and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hanjun Zhao, MTUyMTAwMjA4MDhAMTYzLmNvbQ==; Hongbing Yan, aGJ5YW5mdXdhaTIwMThAMTYzLmNvbQ==, aGJ5YW5mdXdhaUBhbGl5dW4uY29t
 Runzhen Chen
Runzhen Chen Chen Liu1,2
Chen Liu1,2 Peng Zhou
Peng Zhou Ying Wang
Ying Wang Hongbing Yan
Hongbing Yan