- 1Department of Gastroenterology, First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
- 2Ministry of Health and Family Welfare (OSD-DGHS), Dhaka, Bangladesh
- 3Department of Critical Care, M Abdur Rahim Medical College Hospital, Dinajpur, Bangladesh
- 4Department of Critical Care, Cox’s Bazar 250 Bed District Sadar Hospital, Cox’s Bazar, Bangladesh
- 5Department of Critical Care, 250 Bed Chattogram General Hospital, Chattogram, Bangladesh
- 6Hubei Key Laboratory of Embryonic Stem Cell Research, Institute of Neuroscience, Hubei University of Medicine, Shiyan, China
- 7Acute Medical Unit, University Hospital Limerick, Limerick, Ireland
- 8Department of Histology, Xi’an Jiaotong University, Xi’an, China
- 9Department of Internal Medicine, M Abdur Rahim Medical College Hospital, Dinajpur, Bangladesh
Objective: In this study, we investigated the efficacy and safety of remdesivir and tocilizumab combination therapy against dexamethasone for the management of severe COVID-19 patients.
Methods: This was a multicenter study. Cases were randomly chosen and divided into two groups using an odd–even ratio of 1:1 applied to the hospital registration number. Group A received remdesivir [5 mg/kg (<40 kg) or 200 mg (>40 kg) on day 1 and then 2.5 mg/kg (<40 kg) or 100 mg (>40 kg) daily] + tocilizumab [8 mg/kg up to 800 mg highest 12 h apart], and group B was the control and received dexamethasone 6 mg/day. In addition, a broad-spectrum antibiotic and other essential treatments were received by all patients. To evaluate the mortality risk, the sequential organ failure assessment (SOFA) score was calculated on day-1. Treatment outcomes were measured as time to clinical improvement; mortality rate; duration of ICU stay; total period of hospitalization; the rate of (Supplementary Material) oxygen use; time to clinical failure; National Early Warning Score-2 (NEWS), and the percentage of lung recovery on CT of chest on discharge. Clinical trial registration ID: NCT04678739.
Results: Remdesivir-Tocilizumab group had a lower mortality rate (25.49%) than the control (30.77%). The time to clinical improvement (Group A-9.41; B-14.21 days), NEWS-2 on discharge (Group A-0.89; B-1.2), duration of ICU stay (Group A-7.68; B-10.58), and duration of hospitalization (Group A-9.91; B-14.68) were less in the treatment group. Group A had a better percentage of lung recovery on chest CT than the control (Group A-22.13; B-11.74). All these differences were statistically significant (p= <0.05) in a t-test. However, no significant survival benefit was found among the study groups in Kaplan–Meier survival analysis, p = 0.739.
Conclusion: The remdesivir–tocilizumab combination had preferable outcomes compared to the dexamethasone therapy for the treatment of severe COVID-19 concerning mortality rate and clinical and pulmonary improvement, although it did not demonstrate a significant survival benefit.
Clinical Trial Registration: https://clinicaltrials.gov, NCT04678739.
Introduction
The coronavirus disease 2019 (COVID-19) was first reported in China and then quickly spread all over the world. (Velavan and Meyer, 2020) This was formerly recognized as the 2019‐nCoV. Tyrell and Bynoe were the first to isolate and describe coronaviruses in 1966. Coronavirus is an RNA virus and possesses the ability to infect humans and animals. (Tyrrell and Bynoe, 1966) SARS‐CoV‐2 is a member of the beta-corona virus family and contains a similar genome to a bat coronavirus. (Zhouyxwxea et al., 2020) COVID-19 has posed frequent challenges during its course, ranging from virus isolation to detection and prevention. It causes many human respiratory tract infections (RITs), varying from a mild cold to severe respiratory distress syndrome. (Heymann and Shindo, 2020) The COVID-19 treatment mostly depends on the patient’s clinical features and severity of the disease. This might include antivirals, corticosteroids, low molecular weight heparins (LMWHs), convalescence plasma, and immunoglobulins, though until now, no treatment can act specifically against COVID-19. (Stasi et al., 2020) SARS-CoV-2 is a highly infectious virus that causes injury to the pulmonary alveolar epithelium and results in acute respiratory distress syndrome or ARDS. Therefore, for the early identification and proper management, it is crucial to understand the features of COVID-19–induced ARDS. (Gibson et al., 2020) (Li and Ma, 2020) COVID-19 is a disease characterized by pneumonia of viral origin, a hyperimmune and hypercoagulation state, and till now there is no definitive therapy against this condition. All the international medical societies suggest supportive care and therapy, especially discouraging the use of corticosteroids and antibiotics for the management of COVID-19. All the initial studies of COVID-19 reported supportive care management and showed a high death rate with a long ventilator support duration (Kory et al., 2021). Though, mostly the antiviral agents remdesivir and favipiravir and immunomodulatory agents are in use, other drugs such as HCQ (hydroxychloroquine), chloroquine, and recently ivermectin have been prescribed for the mild-to-moderate type of COVID-19 infection. (Chowdhury, 2021) Though, the NIH (National Institute of Health) has discouraged the random use of antiviral drugs, COVID-19 management includes patient isolation, universal supportive care, respiratory, symptomatic, nutritional, and psychosomatic management (Liu and Liu, 2020a) (Panghzsea et al., 2020). According to the current state of knowledge and based on the literature reports, exploring possible antivirals and associated drugs that act against inflammatory conditions that can help fight against COVID-19–ARDS in severe COVID-19 patients is very important. But, till now, though outcomes had been reported separately regarding an antiviral such as remdesivir and an anti–interleukin-6 receptor monoclonal antibody, such as tocilizumab, no study has been performed regarding the combined efficacy of remdesivir and tocilizumab on severe COVID-19 patients. In this randomized controlled trial (RCT), we investigated the efficacy and outcome of the remdesivir and tocilizumab combination, in comparison to dexamethasone, for the treatment of severe COVID-19–ARDS. Our objective was to find and establish a better treatment option for severe COVID-19.
Methodology
This was a multicenter study carried out in the COVID-19 Special Care and dependency unit of the M. Abdur Rahim Medical College Hospital, Cox’s Bazar 250-bed Sadar Hospital, and Chattogram General Hospital, Bangladesh. The estimated sample size was 384. This was calculated by using the following formula: n = z2pq/d2, where z = at 95% confidence limit the value of z is 1.96; n = required sample size, p = estimated prevalence = 0.5, q = 1-p, and d = margin of error at 5% (standard value of 0.05).
Inclusion and Exclusion Criteria
COVID-19 cases with positive RT-PCR for SARS-CoV-2 infection who required admission in the ICU with a National Early Warning Score-2 (NEWS-2) ≥5 were randomly chosen and included in the study. Then, they were divided into two study groups (A and B) by using an odd–even ratio of 1:1 applied to the hospital registration number. After enrollment, every case was further accessed and confirmed by the investigators or attending physician. Acute respiratory distress syndrome (ARDS) was identified according to the Berlin definition.
Patients who had a pre-existing uncontrolled chronic condition with major compromised organ function before COVID-19 (severe ischemic heart disease, epilepsy, advanced pulmonary/renal/hepatic disease, corpulmonale, long-standing severe uncontrolled DM, etc.), those who were immunocompromised, those with an advanced stage of carcinoma, in chemo or adjuvant therapy, pregnant patients, those with AIDS, pulmonary tuberculosis, those with contraindications/possible drug interactions to remdisivir/tocilizumab/dexamethasone, and those who were already hospitalized at the time of enrollment due to other causes were excluded from the study.
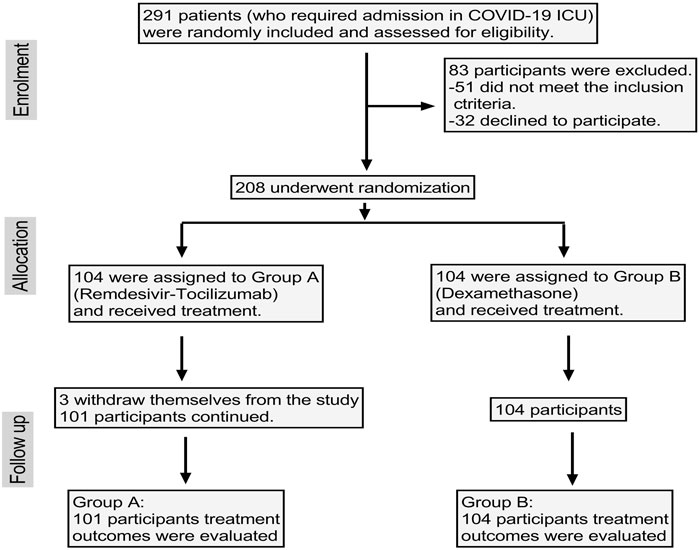
Initially, 291 patients were enrolled, among which 32 were unwilling to participate and 51 patients had pre-existing comorbid conditions or were hospitalized at that time due to other reasons and so were excluded. That left 208 patients enrolled, though three patients in group A withdrew themselves from the study. Finally, data of 205 patients were evaluated (Figure 1).
Study Groups and Treatment Outcome
Group A (n = 101) received remdesivir [5 mg/kg (<40 kg) or 200 mg (>40 kg) on day 1 and then 2.5 mg/kg (<40 kg) or 100 mg (>40 kg) daily]+ tocilizumab [8 mg/kg up to 800 mg highest 12 h apart]; and Group B (n = 104), the control group, received dexamethasone (6 mg/day in divided dose).
In addition, a broad-spectrum antibiotic (meropenem), PPI, ascorbic acid, cholecalciferol, zinc, and symptomatic treatments were received by all patients.
Treatment outcomes were measured as follows: time to clinical improvement, defined as the time from randomization to NEWS-2 score (National Early Warning Score 2) (Morgan, 1997) of ≤2 as maintained for 24 h; mortality rate; duration of ICU stay; total period of hospitalization; the rate of (Supplementary Material) oxygen use; time to clinical failure; NEWS-2 score on discharge; and the percentage of lung recovery on CT of the chest (the difference value of the CT involvement of lung pathology during admission and at the time of discharge). Sequential organ failure assessment (SOFA) scores were calculated to evaluate the mortality risk on the admission day. Routine follow-ups were obtained at an interval of every 24 h.
Ethics and Consent
The purpose of the study was properly explained to the patients, and written informed consent was obtained in every case. This study was approved by the ethical approval committee of M. Abdur Rahim Medical College, Approval No: M.A.R.M.C.D/2020/1985. Clinical trial registration: NCT04678739.
Statistical Analysis
Statistical analysis was carried out by SPSS version 27 and GraphPad prism 7. Each group was analyzed to calculate the mean ± SD and mean ± SEM. Frequency was calculated. T-tests, chi-square tests, and Kaplan–Meier survival analysis were performed among the groups to evaluate the statistical significance. Required groups were calculated in percentage and compared.
Results
General Demographics and the Treatment Outcome in the Study Groups
The total number of patients was 205 (M. Abdur Rahim Medical College Hospital- 95, Cox’s Bazar 250-bed Sadar Hospital- 63, and Chattogram General Hospital- 47); men 156 (76.09%) and women 49 (23.90%); group A 101 (25women, 76men, and mean age 56.64 years) and group B 104 subjects (24 women, 80men, and mean age 57.04 years) (Table 1). The highest affected group in our study was the 61–70 years age group, 23.41% (48), and the lowest was the 10–20 years group, 2.4% (Stasi et al., 2020). In the case of group A, this trend was 51–60 and 61–70 years; in group B, the age groups were 41–50 years and 61–70 years (Table 4).
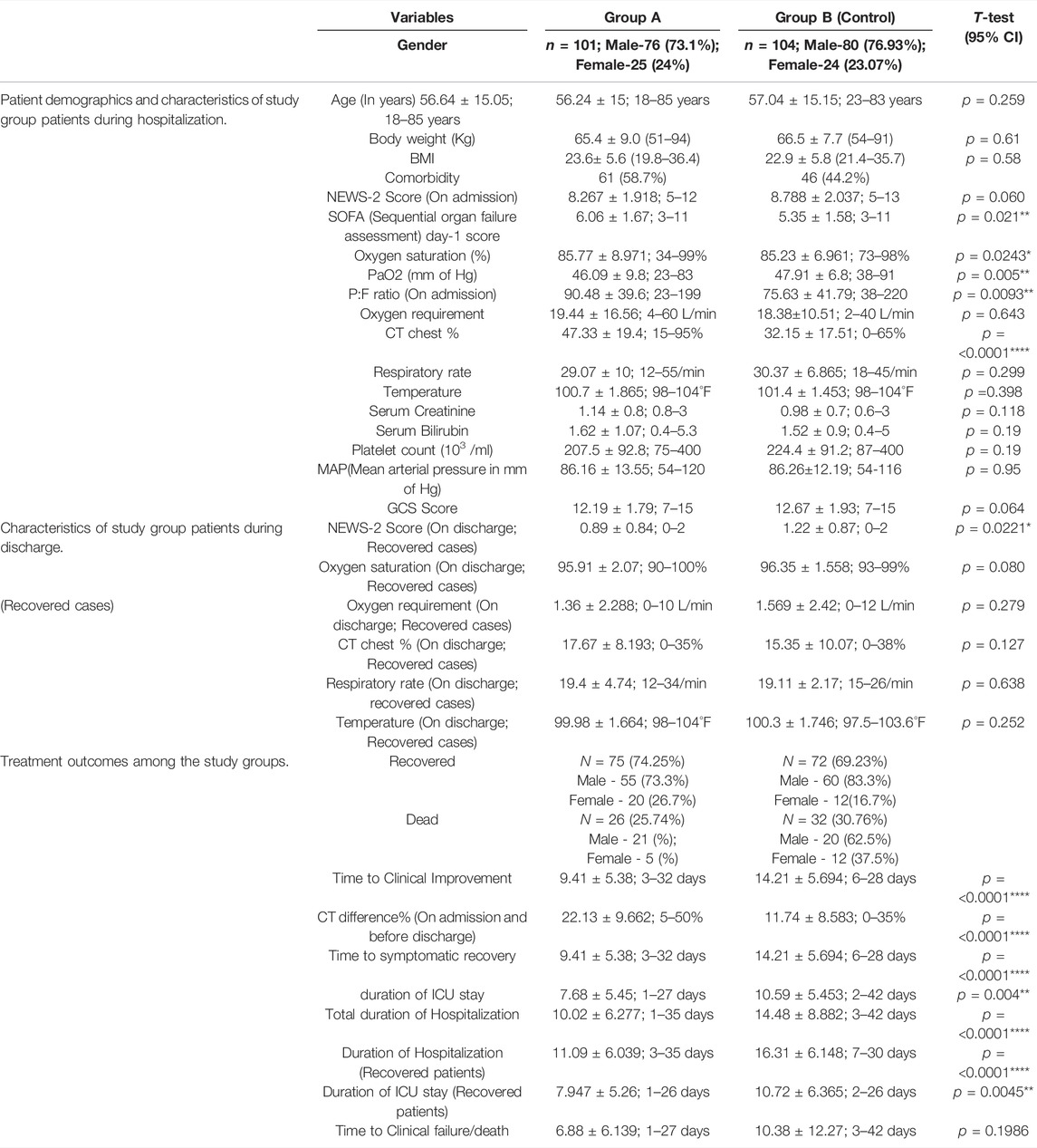
TABLE 1. Patient demographics, characteristics, and treatment outcomes among the study groups. Data presented as mean ± SD.
Group A showed a better recovery rate of 74.25% and a lower death rate of 25.74% than that of group B, 69.23%, and 30.76%, respectively. The mean value of the time to clinical improvement (Group A 9.41; Group B 14.21 days), National Early Warning Score-2 on discharge (Group A 0.89; Group B 1.2), duration of ICU stay (Group A-7.68; Group B-10.58), and the total hospitalization duration (Group A 9.91; Group B 14.68) were less in the remdesivir–tocilizumab treatment group. Among the survivors of our study population, the mean duration of ICU (Group A 7.947; Group B 10.72) stay and the duration of hospitalization (Group A-11.09; Group B-16.31) were found lower in group A. A superior improvement of pulmonary pathology in the CT chest findings was observed (difference of involvement between the admission and discharge) among the survivors (Group A-22.13; Group B-11.74) who received remdesivir–tocilizumab as a treatment. All these differences were statistically significant in a t-test p=<0.05. Among the study groups, higher CT chest involvement (group A-47.33%; group-B 32.15%), P:F ratio (group A-90.48; group B-75.63), SOFA day-1 score (group-A 6.06; group-B 5.35), and low PaO2 (group A-46.09; group B-47.91) were observed during admission in group A, which differs significantly with those of the control group B, p = <0.05. –Table 1.
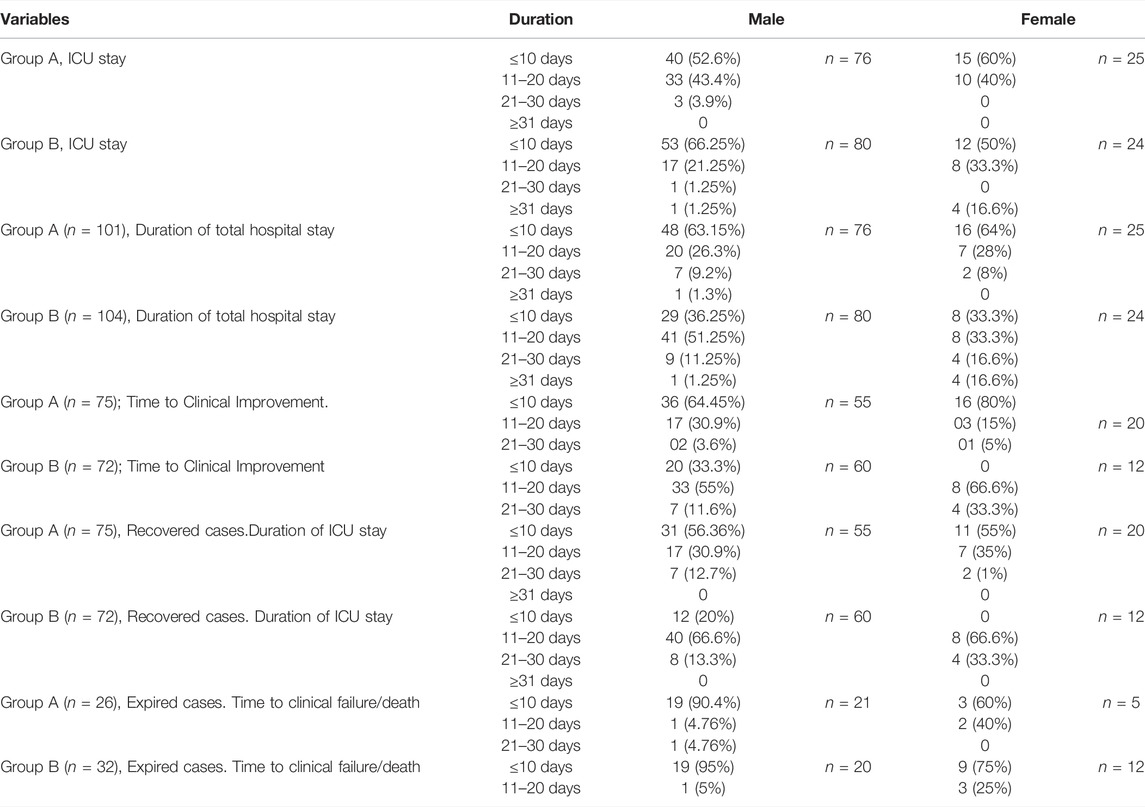
In all 60% of women in group A had a relatively shorter ICU stay (≤10 days) than that of the men. This was reversed in group B, where 66.25% of men recovered from the ICU within 10 days in comparison to 50% of women. In group A, both genders had almost similar duration of hospital stay, but men had a higher recovery period than women in group B, 4.8% (Stasi et al., 2020); patients in group B required >31 day of hospitalization. This was 0.09% (Velavan and Meyer, 2020) in group A. A large number of patients, 51.4%52), in Group A recovered within 10 days, compared to fewer, 19.2% (Roostaei Firozabad et al., 2021), in group B. In group A, 80% (Abbasajankea et al., 2021) of the females recovered within 10 days. This number was greater than that of the males, 64.45% (Annamaria et al., 2020). In group B, 33.3% (Roostaei Firozabad et al., 2021) of men achieved clinical improvement within 10 days, while no women recovered within this period. Among the survivors of group A, 41.5% (56.36% of men and 55% of thewomen) recovered within a period of 10 days. This was only 11.5% (20% of the men) in group B. Time to clinical failure/death was relatively fast in group B. –Table 2.
Survival Analysis Among the Treatment Groups
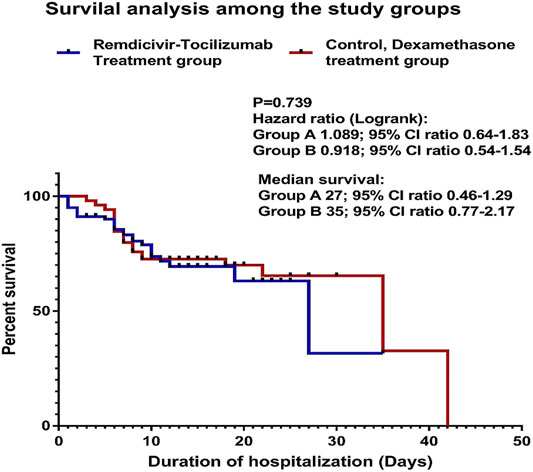
The Kaplan–Meier survival analysis was performed. The difference between the two study groups was not significant, p = 0.739. hazard ratio (log-rank): group A (remdesivir–tocilizumab) 1.089; 95% CI ratio 0.64–1.83; group B (control/dexamethasone) 0.918; 95% CI ratio 0.54–1.54. median survival: Group A 27; 95% CI ratio 0.46–1.29; group B 35; 95% CI ratio 0.77–2.17 (Figure 2).
Subgroup Analysis of the Total Duration of Hospitalization and ICU Stay Against the Age Group
Group A had a shorter duration of hospital stay and faster recovery. Of the total group A patients, 63.4% were discharged from the hospital within 10 days, and only 0.9% required >31 day; compared to 35.6 and 4.8% in group B. Regarding the ICU stay, Group B patients showed a faster recovery rate, 62.5%, within a period of 10 days than the 54.5% rate in group A. Though 8.6% of patients in group B required >31 days of ICU stay, no patients in group A required >31 days of ICU stay –Table 3.
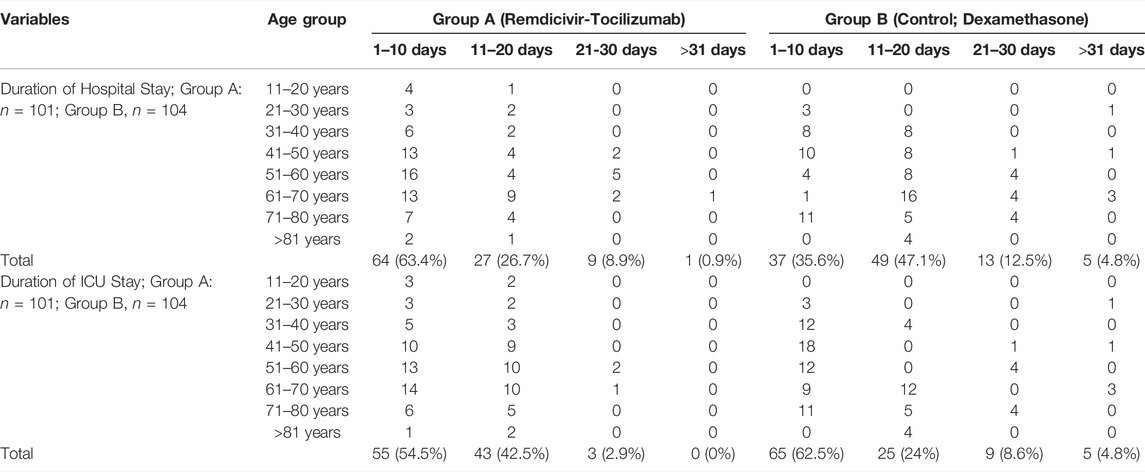
TABLE 3. Subgroup analysis of the total duration of hospitalization and ICU stay against the age group.
The Subgroup Analysis of the Study Population Regarding Age Group
Full recovery was observed in the 11–20-years age group (5/5) in group A and the >81 years age group (4/4) in group B. The 21–30 years age group had a high mortality rate in both study groups, 60% in group A and 100% in group B. The 41–70 years age group had the highest number of hospital recoveries in group A, and the 31–70 years age group had the highest number of hospital recoveries in group B. Mortality was observed among the 51–60 years age group in group A and the 41–50 and 61–70 years age group in group B.–Table 4.
Subgroup Analysis of the Recovered and Death Cases Against Duration and Age
The early and the late age group of 11–40 years and 71->81 years, respectively, had a 100% hospital recovery within a 20 days period in group A. The same was observed among the 21–50 years and >81 years age groups in group B. Hospital recovery time was prolonged to >21 days among the 41–70 years age group in group A and 51–80 years age group in group B. Up to 80% hospital recovery was observed among all the age groups of group A within 10 days. On the other hand, most of the patients in group B were discharged from the hospital within 11–20 days. Most of the patients in all ages of group A recovered from the ICU within 10 days. On the contrary, the highest recoveries were seen within 11–20 days in group B. Group B had higher mortality (87.5%) than group A (61.5%) within 10 days –Table 5.
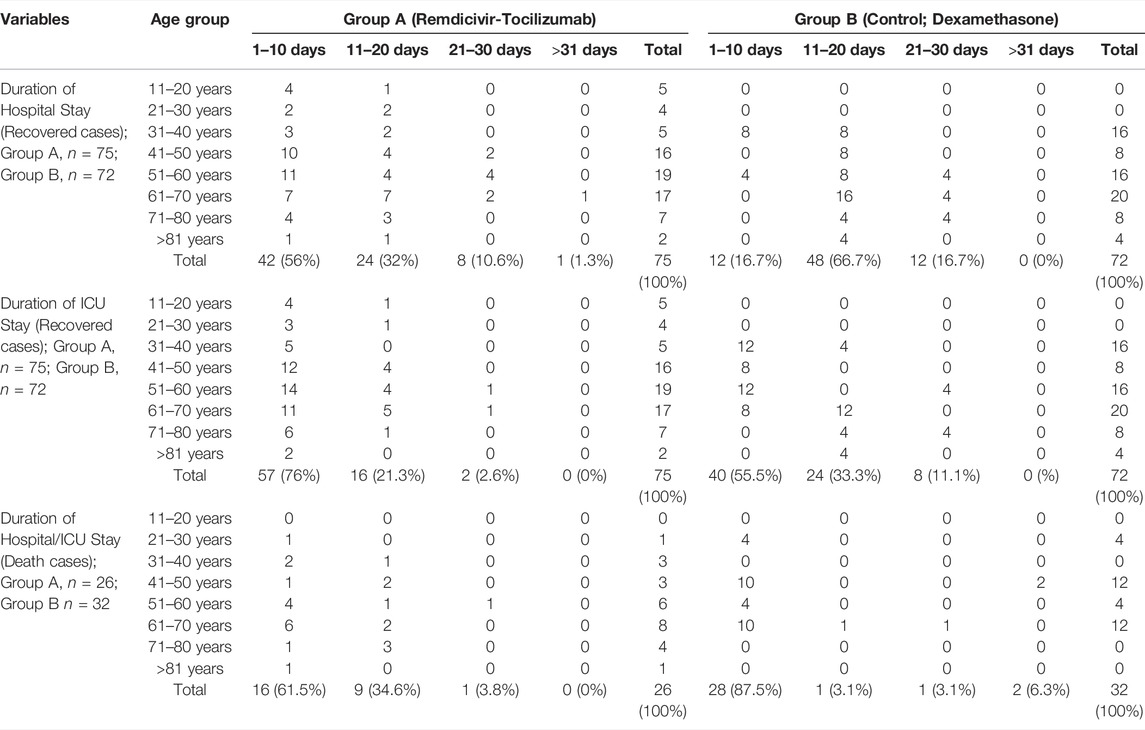
TABLE 5. Analysis of the recovered and expired COVID-19 cases against recovery duration /death and age.
Distribution and Subgroup Analysis of Comorbidity Among the Study Groups
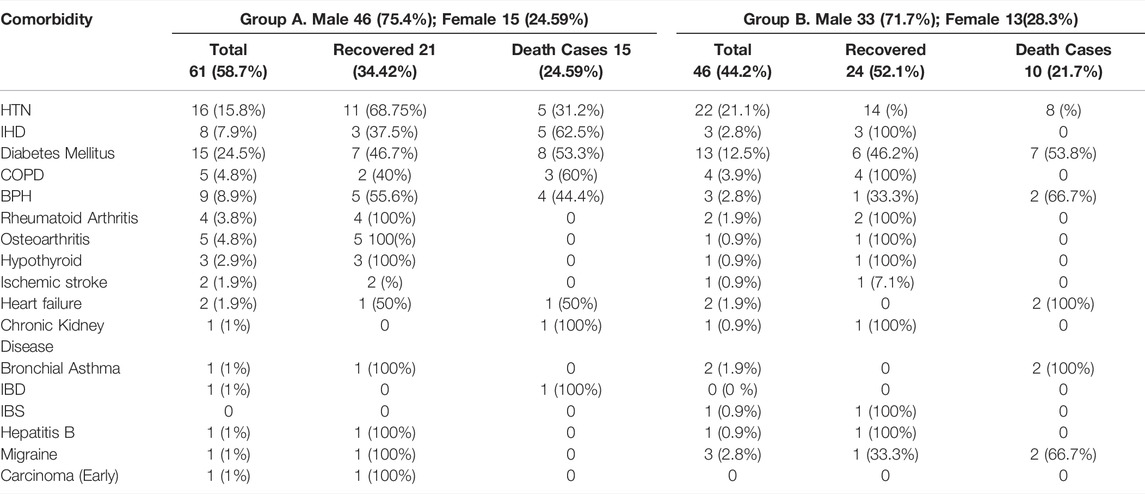
It was found that 58.7%61) patients in group A and 44.2%44) patients in group B had comorbid conditions. They were hypertension (HTN), diabetes mellitus (DM), ischemic heart disease (IHD), chronic obstructive pulmonary airway disease (COPD), rheumatoid arthritis (RA), benign prostatic hyperplasia (BPH), osteoarthritis, hypothyroid, ischemic stroke, heart failure, chronic kidney disease, bronchial asthma, inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), hepatitis B, migraine, and early carcinoma. Nearly similar numbers of comorbidities were noted in both study groups; 15.8% (Abbasajankea et al., 2021) and 21.1% (Cao et al., 2020) had HTN in groups A and B, respectively. A better percentage of recovery was achieved by the group A patients who had comorbidities with HTN than that of group B. Similarly, the percentage of death was high among those in group B who had HTN or DM and other comorbidities than in group A. In addition, in group A, diabetic patients with comorbidities experienced better recovery. –Table 6.
Discussion
In this research, a multicenter study of 205 selected patients after exclusion was carried out in the COVID-19 special care and dependency unit of multiple health centers/hospitals in Bangladesh. Combination therapy of remdesivir with tocilizumab was carried out in the intervention group and was compared with that in the control group which was treated with dexamethasone. To evaluate equal treatment outcomes among the study groups, COVID-19 cases in ventilator support and with severe comorbidities were excluded. The post-intervention outcome was measured as described in the methodology. Kaplan-Meier survival analysis was performed, and no significant advantage of the remdesivir–tocilizumab treatment was observed against dexamethasone therapy, p = 0.739. However, the remdesivir–tocilizumab intervention group had a relatively lower mortality rate of 25.74% than that of the control group, which was 30.76%, in our study. There was significant reduction in the time to clinical improvement, NEWS-2 score on discharge, CT improvement (%), and duration of ICU and hospital stays in the remdesivir–tocilizumab group, p=<0.05 (Table 1). This reduction was equally observed among the patients with comorbidities. Patients with multiple comorbidities in Group A also had better prognosis and low mortality than those in the control group (Table 6). Therefore, the remdesivir–tocilizumab combination was found to have a favorable treatment outcome compared to dexamethasone therapy in case of severe COVID-19 concerning the mortality rate, time to clinical improvement, duration of ICU stay, and the total duration of hospitalization.
Currently, many trials are ongoing for the management of COVID-19, and several trials have been completed recently. (WHO Solidarity Trial Consortium PHPRea, 2021; Tabarsi et al., 2021; Abbasajankea et al., 2021; Les bujandai lajbfea et al., 2021; Dabbousesmeagea et al., 2021; Reis et al., 2021; Roostaei Firozabad et al., 2021; Granholm et al., 2021) Among these clinical trials, several discussed the repurposing of the approved drugs for the management of COVID-19. Possible therapeutic agents such as different antiviral drugs, existing drugs that have an antiviral effect, corticosteroids, and other possible agents are being investigated. A randomized controlled trial on the lopinavir/ritonavir therapy against the standard care in China has revealed no benefit against the standard management. (Cao et al., 2020) Corticosteroids, for example, dexamethasone and methylprednisolone have been suggested and used with patients of severe illness to prevent the overactive immune system and cytotoxic storm. Though, the improvement of COVID-19 patients mostly depends on disease severity, the age, existing comorbidity, and the physical status of the affected patient (Liu and Liu, 2020b), several studies have shown that remdesivir and tocilizumab clinically benefit patients with moderate-to-severe COVID-19, but none of these studies examined a combination of the two therapies (Wangzddgea et al., 2020; Singh et al., 2020; Cao et al., 2020; Greinonsdea et al., 2020; Xuhmltea et al., 2020; Stonefmsbnea et al., 2020; Lan et al., 2020). Thus, it was necessary to investigate these two drugs in combination for the treatment of severe COVID-19. As an antiviral agent, remdesivir was found to be beneficial for the management of COVID-19 (Wangzddgea et al., 2020; Singh et al., 2020). Similarly, the role of tocilizumab was also found preferable in multiple published studies (Xuhmltea et al., 2020; Stonefmsbnea et al., 2020; Lan et al., 2020). We also found comparable outcomes in our current study. Therefore, the remdesivir–tocilizumab combination might be a preferable choice of treatment for the management of severe COVID-19. Further study with a larger sample size is necessary to solidify the outcome of this combination.
Our study has limitations: the small sample size; exclusion of COVID-19 cases that required ventilator support, and COVID-19 patients with severe comorbidities are a concern. In addition, analysis depending on the severity of COVID-19–ARDS was not performed. But to the best of our effort, we selected severe COVID-19–ARDS cases devoid of serious or uncontrolled comorbidity to ensure proper comparison and outcome among the study groups without influence.
Conclusion
According to this study, the remdesivir–tocilizumab combination had preferable outcomes compared to those of the dexamethasone therapy for the treatment of severe COVID-19 concerning the lower mortality rate, better clinical and pulmonary improvement, reduced duration of the ICU stay, and decreased hospitalization period, although the survival benefit of this combination therapy was not significant against dexamethasone alone.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethical approval committee of M. Abdur Rahim Medical College, Approval No: M.A.R.M.C.D/2020/1985. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
AM: research concept, project management, patient selection, treatment, data collection, statistical analysis, manuscript writing, and editing. AK, KA, and ST: patient selection, treatment, follow up, and data collection. MRK: manuscript writing and editing. MAA: statistical analysis. YL: interpreted the data of COVID-19 patients. SH: critical review of the manuscript, research supervision, and ensuring the quality of the research.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors are thankful for the support and cooperation of the First Affiliated Hospital of Xi’an Jiaotong University and the doctors and staff of the “COVID-19 critical care” of M Abdur Rahim Medical College Hospital, Cox’s Bazar Sadar Hospital, and Chattogram General Hospital.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.690726/full#supplementary-material
References
Abbasajankea, H. M., Al-Jumaili, A. A., Nassir, K. F., Al-Obaidy, M. W., Al Jubouri, A. M., Dakhil, B. D., et al. (2021). Assessment of COVID-19 Treatment Containing Both Hydroxychloroquine and Azithromycin: A Natural Clinical Trial. Int. J. Clin. Pract. 75 (4), e13856. doi:10.1111/ijcp.13856
Annamaria, P., Eugenia, Q., and Paolo, S. (2020). Anti-SARS-CoV-2 Hyperimmune Plasma Workflow. Transfus. Apher. Sci. 59 (5), 102850. doi:10.1016/j.transci.2020.102850
Cao, Y. C., Deng, Q. X., and Dai, S. X. (2020). Remdesivir for Severe Acute Respiratory Syndrome Coronavirus 2 Causing COVID-19: An Evaluation of the Evidence. Trav. Med Infect Dis 35, 101647. doi:10.1016/j.tmaid.2020.101647
Chowdhury, A. S. (2021). A Comparative Study on Ivermectin-Doxycycline and Hydroxychloroquine-Azithromycin Therapy on COVID-19 Patients. EJMO 5 (1), 63–70. doi:10.14744/ejmo.2021.16263
Dabbousesmeagea, H. M., El-Sayed, M. H., El Assal, G., Elghazaly, H., Ebeid, F. F. S., Sherief, A. F., et al. (2021). Safety and Efficacy of Favipiravir versus Hydroxychloroquine in Management of COVID-19: A Randomised Controlled Trial. Sci. Rep. 11 (1), 7282. doi:10.1038/s41598-021-85227-0
Généreux, M. S. (2019). Psychosocial Management before, during, and after Emergencies and Disasters-Results from the Kobe Expert Meeting. Int. J. Environ. Res. Public Health 16 (8), 1309.
Gibson, P. G., Qin, L., and Puah, S. H. (2020). COVID-19 Acute Respiratory Distress Syndrome (ARDS): Clinical Features and Differences from Typical Pre-COVID-19 ARDS. Med. J. Aust. 213 (2), 54–e1. doi:10.5694/mja2.50674
Granholm, A., Munch, M. W., Myatra, S. N., Vijayaraghavan, B. K. T., Cronhjort, M., Wahlin, R. R., et al. (2021). Higher vs Lower Doses of Dexamethasone in Patients with COVID-19 and Severe Hypoxia (COVID STEROID 2) Trial: Protocol for a Secondary Bayesian Analysis. Acta Anaesthesiol Scand. 65 (5), 702–710. doi:10.1111/aas.13793
Greinonsdea, J., Ohmagari, N., Shin, D., Diaz, G., Asperges, E., Castagna, A., et al. (2020). Compassionate Use of Remdesivir for Patients with Severe Covid-19. N. Engl. J. Med. 382 (24), 2327–2336. doi:10.1056/NEJMoa2007016
Gulati, S., Muddasani, R., Gustavo Bergerot, P., and Pal, S. K. (2021). Systemic Therapy and COVID19: Immunotherapy and Chemotherapy. Urol. Oncol. 39 (4), 213–220. doi:10.1016/j.urolonc.2020.12.022
Heymann, D. L., and Shindo, N. (2020). COVID-19: what Is Next for Public Health? Lancet 395 (10224), 542–545. doi:10.1016/S0140-6736(20)30374-3
Jin, Y.-H., Cai, L., Cheng, Z.-S., Cheng, H., Deng, T., Fan, Y.-P., et al. (2020). A Rapid Advice Guideline for the Diagnosis and Treatment of 2019 Novel Coronavirus (2019-nCoV) Infected Pneumonia (Standard Version). Mil. Med Res 7 (1), 4. doi:10.1186/s40779-020-0233-6
Kory, P., Meduri, G. U., Iglesias, J., Varon, J., and Marik, P. E. (2021). Clinical and Scientific Rationale for the "MATH+" Hospital Treatment Protocol for COVID-19. J. Intensive Care Med. 36 (2), 135–156. Epub 2020 Dec 15. PMID: 33317385. doi:10.1177/0885066620973585
Lan, S. H., Lai, C. C., Huang, H. T., Chang, S. P., Lu, L. C., and Hsueh, P. R. (2020). Tocilizumab for Severe COVID-19: a Systematic Review and Meta-Analysis. Int. J. Antimicrob. Agents 56 (3), 106103. doi:10.1016/j.ijantimicag.2020.106103
Les BujandaI Lajbfea, I., Loureiro-Amigo, J., Bastons, F. C., Guerra, I. E., Sánchez, J. A., Murgadella-Sancho, A., et al. (2021). Treatment of COVID-19 Pneumonia with Glucocorticoids (CORTIVID): a Structured Summary of a Study Protocol for a Randomised Controlled Trial. Trials 22 (1), 43. doi:10.1186/s13063-020-04999-4
Li, X., and Ma, X. (2020). Acute Respiratory Failure in COVID-19: Is it "typical" ARDS? Crit. Care 24 (1), 198. doi:10.1186/s13054-020-02911-9
Li, Z., Niu, S., Guo, B., Gao, T., Wang, L., Wang, Y., et al. (2020). Stem Cell Therapy for COVID-19, ARDS and Pulmonary Fibrosis. Cell Prolif 53 (12), e12939. doi:10.1111/cpr.12939
Liu, J., and Liu, S. (2020).The Management of Coronavirus Disease 2019 (COVID-19), J. Med. Virol. 92, 1484PMC7267323–1490. Epub 2020 May 22. PMID: 32369222. doi:10.1002/jmv.25965
Liu, J., and Liu, S. (2020). The Management of Coronavirus Disease 2019 (COVID-19). J. Med. Virol. 92 (9), 1484–1490. doi:10.1002/jmv.25965
Liu, J., and Liu, S. (2020). The Management of Coronavirus Disease 2019 (COVID-19). J. Med. Virol. 92 (9), 1484–1490. doi:10.1002/jmv.25965
Morgan, R. L. (1997). An Early Warning Scoring System for Detecting Developing Critical Illness. Available at: https://www.scienceopen.com/document?vid=28251d22-8476-40a6-916d-1a34796816e4.
National Early Warning Score (NEWS) 2 (2017). Standardising the Assessment of Acute-Illness Severity in the NHS. Royal College of Physicians.
Paganini, M., Bosco, G., Perozzo, F. A. G., Kohlscheen, E., Sonda, R., Bassetto, F., et al. (2021). The Role of Hyperbaric Oxygen Treatment for COVID-19: A Review. Adv. Exp. Med. Biol. 1289, 27–35. doi:10.1007/5584_2020_568
Panghzsea, Y., Guan, H., Zhou, S., Wang, Y., Li, Q., Zhu, T., et al. (2020). Initial CT Findings and Temporal Changes in Patients with the Novel Coronavirus Pneumonia (2019-nCoV): a Study of 63 Patients in Wuhan, China. Eur. Radiol. 30 (6), 3306–3309. doi:10.1007/s00330-020-06731-x
Pollard, C. A., Morran, M. P., and Nestor-Kalinoski, A. L. (2020). The COVID-19 Pandemic: a Global Health Crisis. Physiol. Genomics 52 (11), 549–557. doi:10.1152/physiolgenomics.00089.2020
Reis, G., Moreira Silva, E. A. D. S., Medeiros Silva, D. C., Thabane, L., Singh, G., Park, J. J. H., et al. (2021). Effect of Early Treatment with Hydroxychloroquine or Lopinavir and Ritonavir on Risk of Hospitalization Among Patients with COVID-19: The TOGETHER Randomized Clinical Trial. JAMA Netw. Open 4 (4), e216468. doi:10.1001/jamanetworkopen.2021.6468
Roostaei Firozabad, A., Meybodi, Z. A., Mousavinasab, S. R., Sahebnasagh, A., Jelodar, M. G., Karimzadeh, I., et al. (2021). Efficacy and Safety of Levamisole Treatment in Clinical Presentations of Non-hospitalized Patients with COVID-19: a Double-Blind, Randomized, Controlled Trial. BMC Infect. Dis. 21 (1), 297. Published 2021 Mar 24. doi:10.1186/s12879-021-05983-2
Singh, A. K., Singh, A., Singh, R., and Misra, A. (2020). Remdesivir in COVID-19: A Critical Review of Pharmacology, Pre-clinical and Clinical Studies. Diabetes Metab. Syndr. 14 (4), 641–648. doi:10.1016/j.dsx.2020.05.018
Stasi, C., Fallani, S., Voller, F., and Silvestri, C. (2020). Treatment for COVID-19: An Overview. Eur. J. Pharmacol. 889, 173644. doi:10.1016/j.ejphar.2020.173644
Stonefmsbnea, J. H., Frigault, M. J., Serling-Boyd, N. J., Fernandes, A. D., Harvey, L., Foulkes, A. S., et al. (2020). Efficacy of Tocilizumab in Patients Hospitalized with Covid-19. N. Engl. J. Med. 383 (24), 2333–2344. doi:10.1056/NEJMoa2028836
Sun, T., Guo, L., Tian, F., Dai, T., Xing, X., Zhao, J., et al. (2020). Rehabilitation of Patients with COVID-19. Expert Rev. Respir. Med. 14 (12), 1249–1256. Epub 2020 Oct 12. PMID: 32799694. doi:10.1080/17476348.2020.1811687
Tabarsi, P., Barati, S., Jamaati, H., Haseli, S., Marjani, M., Moniri, A., et al. (2021). Evaluating the Effects of Intravenous Immunoglobulin (IVIg) on the Management of Severe COVID-19 Cases: A Randomized Controlled Trial. Int. Immunopharmacol 90, 107205. doi:10.1016/j.intimp.2020.107205
Tyrrell, D. A., and Bynoe, M. L. (1966). Cultivation of Viruses from a High Proportion of Patients with Colds. Lancet 1 (7428), 76–77. doi:10.1016/s0140-6736(66)92364-6
Velavan, T. P., and Meyer, C. G. (2020). The COVID-19 Epidemic. Trop. Med. Int. Health 25, 278–280. doi:10.1111/tmi.13383
Wangzddgea, Y., Zhang, D., Du, G., Du, R., Zhao, J., Jin, Y., et al. (2020). Remdesivir in Adults with Severe COVID-19: a Randomised, Double-Blind, Placebo-Controlled, Multicentre Trial. Lancet 395 (10236), 1569–1578. doi:10.1016/S0140-6736(20)31022-9
WHO Solidarity Trial Consortium PHPRea (2021). Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results. N. Engl. J. Med. 384 (6), 497–511. doi:10.1056/NEJMoa2023184
Xuhmltea, X., Han, M., Li, T., Sun, W., Wang, D., Fu, B., et al. (2020). Effective Treatment of Severe COVID-19 Patients with Tocilizumab. Proc. Natl. Acad. Sci. U S A. 117 (20), 10970–10975. doi:10.1073/pnas.2005615117
Keywords: COVID-19, SARS-Cov-2, remdecivir, tocilizumab, dexamethasone, COVID-19 ARDS, Bangladesh
Citation: Mohiuddin Chowdhury ATM, Kamal A, Abbas KU, Talukder S, Karim MR, Ali MA, Nuruzzaman M, Li Y and He S (2022) Efficacy and Outcome of Remdesivir and Tocilizumab Combination Against Dexamethasone for the Treatment of Severe COVID-19: A Randomized Controlled Trial. Front. Pharmacol. 13:690726. doi: 10.3389/fphar.2022.690726
Received: 04 April 2021; Accepted: 22 February 2022;
Published: 05 April 2022.
Edited by:
Giuseppa Pistritto, Italian Medicines Agency (AIFA), ItalyReviewed by:
Rahul Kashyap, Mayo Clinic, United StatesTakayuki Shiomi, International University of Health and Welfare, Japan
Copyright © 2022 Mohiuddin Chowdhury, Kamal, Abbas, Talukder, Karim, Ali, Nuruzzaman, Li and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abu Taiub Mohammed Mohiuddin Chowdhury, ZHJfbW9oaXVkZGluY2h5QHlhaG9vLmNvbQ==; Shuixiang He, ZHl5eWp4a0B4anR1LmVkdS5jbg==
 Abu Taiub Mohammed Mohiuddin Chowdhury
Abu Taiub Mohammed Mohiuddin Chowdhury Aktar Kamal3
Aktar Kamal3 Md Rezaul Karim
Md Rezaul Karim Yarui Li
Yarui Li