- Department of Pulmonary and Critical Care Medicine, Zhongshan Hospital, Fudan University, Shanghai, China
Background: Overweight and hyperglycemia might result in poor prognosis in patients with severe community-acquired pneumonia (SCAP). XueBiJing treatment could significantly improve the outcomes of patients with SCAP. We investigated the efficacy of XueBiJing injection in patients with SCAP stratified by body mass index (BMI) and fasting blood glucose (FBG).
Methods: This is a post hoc analysis of XueBiJing trial, a large prospective, randomized, controlled study conducted in 33 hospitals in China. We compared data from non-overweight (BMI <24 kg/m2, n = 425) vs. overweight (BMI ≥24 kg/m2, n = 250) patients as well as non-hyperglycemia (FBG <7 mmol/L, n = 315) vs. hyperglycemia (FBG ≥7 mmol/L, n = 360) patients with XueBiJing, 100 ml, q12 h, or a visually indistinguishable placebo treatment for 5–7 days.
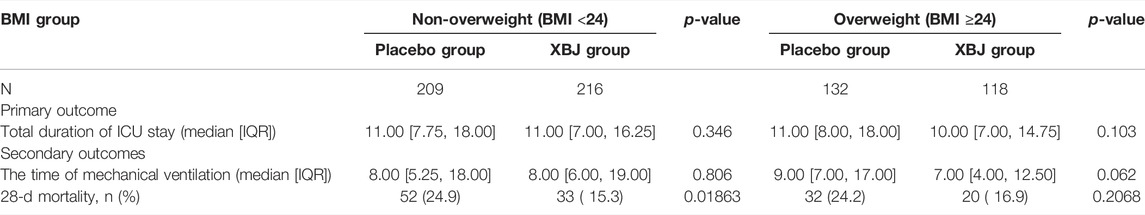
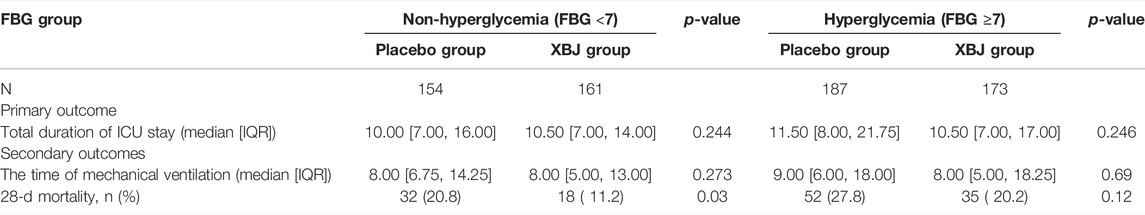
Results: Among patients with BMI <24 kg/m2 (n = 425), 33 (15.3%), XueBiJing recipients and 52 (24.9%) placebo recipients (p = 0.0186) died within 28 days. Among patients with BMI ≥24 kg/m2 (n = 250), XueBiJing recipients still had lower mortality (XueBiJing 16.9% vs. placebo 24.2%; p = 0.2068) but without significantly statistical difference. For the FBG group, patients with FBG <7 mmol/L (n = 315), 18 (11.2%) XueBiJing recipients and 32 (20.8%) placebo recipients (p = 0.030) died within 28 days. Among patients with FBG ≥7 mmol/L (n = 360), XueBiJing recipients still had lower mortality (XueBiJing 20.2% vs. placebo 27.8%; p = 0.120) but without significantly statistical difference. The total duration of the ICU stay and the duration of mechanical ventilation were similar in both groups (p > 0.05).
Conclusion: Overweight or hyperglycemia might weaken the efficacy of XueBiJing injection in the treatment of SCAP as indicated by the significant elevated risk of 28-day mortality. Additional studies are needed to validate our findings and to further understand the underlying mechanisms.
Background
Community-acquired pneumonia remains one of the leading infectious diseases despite advances in antibiotic treatment (Torres et al., 2015). The mortality rate among patients admitted to hospital with pneumonia is estimated at 8–15% (Fine et al., 1996). The main physiological function of the lung is gas exchange. At the same time, because it is directly exposed to the outside world, the lung is vulnerable to microbial invasion and environmental noxious particles (Hu et al., 2021). In healthy lungs, this defense function is mainly achieved through the production of epithelial barriers and surfactant proteins by alveolar cells (Ruaro et al., 2021). Obesity and hyperglycemia were reported as risk factors for poor prognosis of acquiring infections including community-acquired pneumonia (CAP). Obesity may influence either the risk of getting an infection or the outcome of an infection once it is established. Obese people are more likely than people of normal weight to develop serious complications of respiratory infections (Falagas and Kompoti, 2006; Kornum et al., 2010; Huttunen and Syrjänen, 2013). A recent large observational study suggested a stepwise increase in mortality and length of hospital stay in CAP patients with higher serum glucose levels (Lepper et al., 2012). Hyperglycemia was demonstrated to be a common occurrence during sepsis and independently associated with increased mortality in sepsis patients (van Vught et al., 2016). Clinical trials in patients with CAP in different nutritional and metabolic status are lacking. But, it is unclear whether obesity and hyperglycemia impact the choices of treatments and the effectiveness of drugs in CAP patients, not to mention patients with severe CAP (SCAP).
XueBiJing, an injectable prescription from traditional Chinese medicine, has been approved to treat severe infections (sepsis) in guidelines (China Food and Drug Administration, Beijing, China, Number Z20040033) (Qi et al., 2011; Li et al., 2020). Animal experiments and clinical research studies showed that XueBiJing combined with conventional therapy could improve the prognosis of patients with infectious diseases (Chen et al., 2018; Li et al., 2018; Li et al., 2021). More inspiringly, it was recently reported that XueBiJing injection led to a statistically significant improvement in the mortality, duration of mechanical ventilation, and duration of ICU stay in critically ill patients with SCAP (Song et al., 2019).
In consideration of the impact of overweight and hyperglycemia on the poor prognosis of CAP and the effects of XueBiJing on SCAP patients, the aim of this study was to investigate the impact of obesity and hyperglycemia on outcomes in patients with SCAP by post hoc analysis of the large, randomized, controlled XueBiJing trial in order to provide related data for physicians to make optimal clinical decision. We recommend XueBiJing for non-overweight and non-hyperglycemic patients due to the significant reduction in 28-day mortality.
Materials and Methods
Participants and Study Design
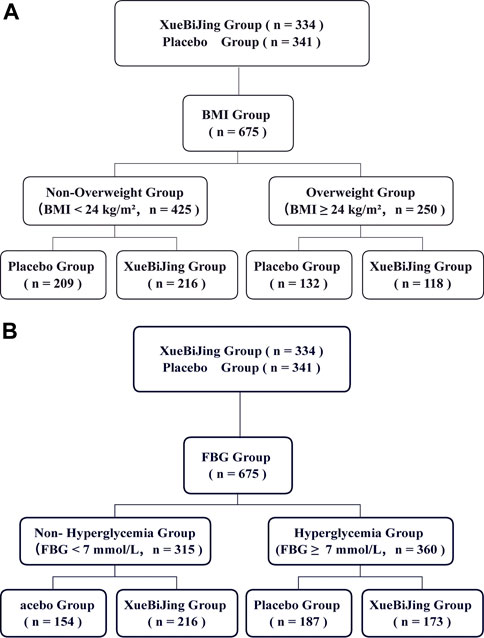
This study is a post hoc analysis of the main multicenter randomized controlled trial (RCT), “XueBiJing injection versus placebo for critically ill patients with severe community-acquired pneumonia: a randomized controlled trial.” The study design and results have been published in detail previously (Song et al., 2019). The study was approved by the Medical Ethics Committee of Zhongshan Hospital, Fudan University [2011–2038 (Hu et al., 2021)], and informed consents were obtained from all patients or their legal guardians before enrollment. Eligible 710 participants enrolled at 33 centers with SCAP were randomly administered with XueBiJing (n = 334) or placebo (n = 341) for 5–7 days. The participants were followed up for 28 days after randomization. The participants received the solvent only (normal saline, 200 ml, q12 h) in the placebo group and the solvent plus XueBiJing (normal saline 100 ml + XueBiJing 100 ml, q12 h) in the XueBiJing group. Both groups received a standard therapy (such as antibiotics) chosen by the attending physician according to the 2007 American Thoracic Society/Infectious Diseases Society of America guidelines (Mandell et al., 2007). Patients were categorized into two different BMI groups or FBG groups: non-overweight (BMI <24.0 kg/m2) and overweight (BMI ≥24.0 kg/m2) based on the Criteria of Weight for Adults released by the Ministry of Health of China and previous study (Liu et al., 2018; Dong et al., 2019; Wang et al., 2019) or non-hyperglycemia (FBG ≥7 mmol/L) and hyperglycemia (FBG ≥7 mmol/L) according to the international standard (Menke et al., 2015; American Diabetes Association, 2021).
Endpoints
In the XueBiJing study, an 8-day improvement in pneumonia severity index (PSI) risk rating was defined as the primary outcome. Secondary outcomes included 28-day mortality, the duration of mechanical ventilation, and the total duration of ICU stay. In this post hoc analysis, the primary outcome was 28-day mortality. The main secondary outcomes were the duration of mechanical ventilation and total duration of ICU stay.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation (SD) or median with interquartile range (IQR), while categorical variables were expressed as frequencies with percentages. Primary outcome analysis was a simple categorical frequency comparison by use of the chi-squared test. The secondary outcomes for the duration of mechanical ventilation and total duration of ICU stay were analyzed by the t-test or the Wilcoxon rank sum test as appropriate. Kaplan–Meier estimates were used for time-to-event variables, and differences among the groups were compared with a log rank test. HR and associated 95% CIs were estimated from the Cox proportional hazard model. All the analyses were performed on the modified intention-to-treat population, which comprised all the patients who were randomized to treatment. A two-tailed value of p < 0.05 was regarded as significant. All analyses were performed with R version 4.0.3.
Results
Of the 710 participants enrolled at 33 centers, 334 were assigned to the XueBiJing treatment group and 341 to the placebo treatment group; 35 participants were excluded from this study due to missing data. Figure 1 shows the study profile of our study. Demographic and basal clinical characteristics of all participants are listed in Tables 1, 2, which was classified according to BMI or FBG. The BMI of overweight patients (n = 250) was 26.28 ± 2.15 kg/m2, and 68.8% of them (n = 172) were male. The FBG of hyperglycemia patients (n = 360) was 10.63 ± 3.67 mmol/L, and 71.7% of them (n = 258) were male. Differences of baseline comorbidities, PSI score, or other biological parameters were not significantly different between high and not-high BMI or high and not-high FBG groups. In Tables 3, 4, the proportion of patients with ARDS and the baseline settings of mechanical ventilation was not significantly different from that of the compared group either.
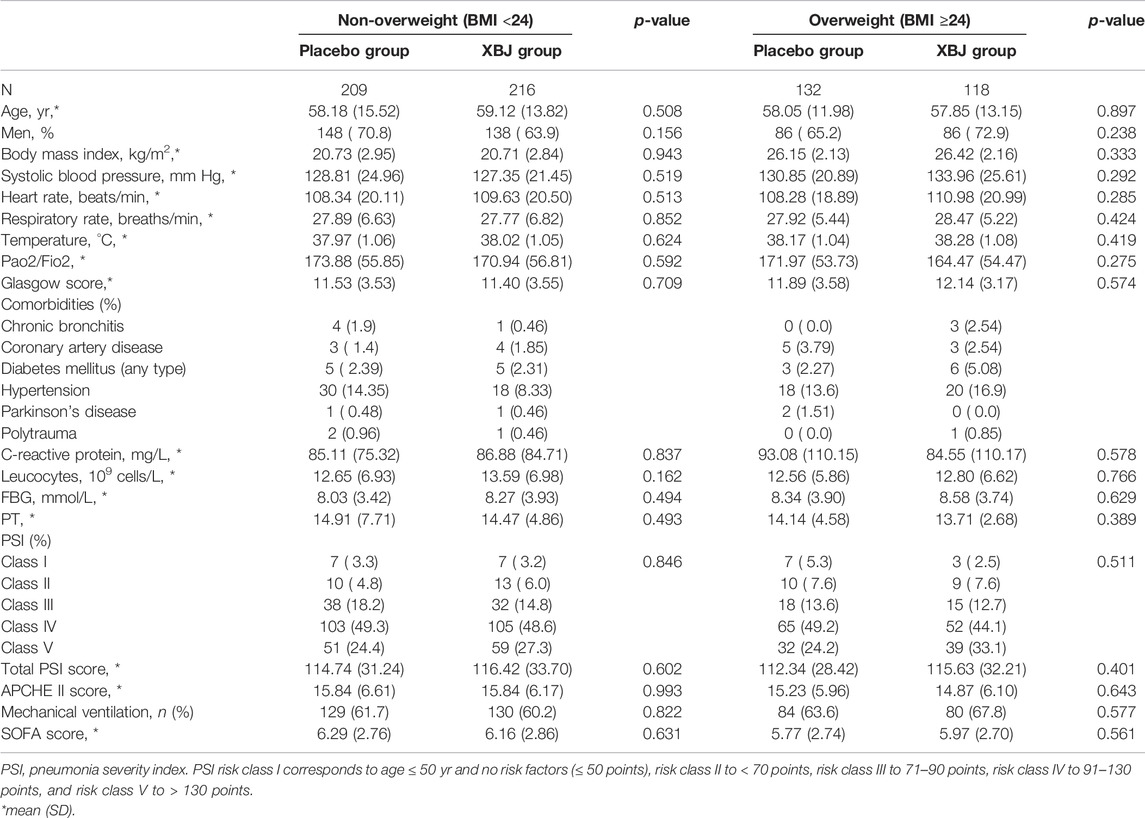
TABLE 1. Demographic and basal clinical characteristics of patients between XueBiJing and placebo groups in the BMI group.
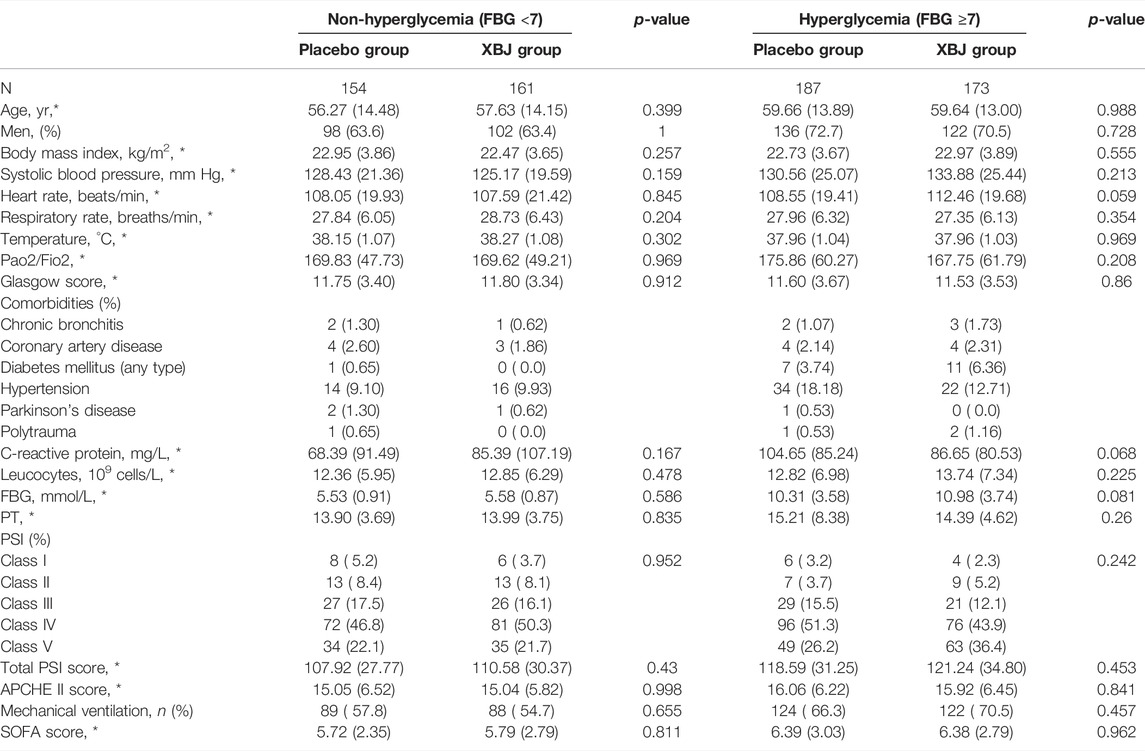
TABLE 2. Demographic and basal clinical characteristics of patients between XueBiJing and placebo groups in the FBG group.
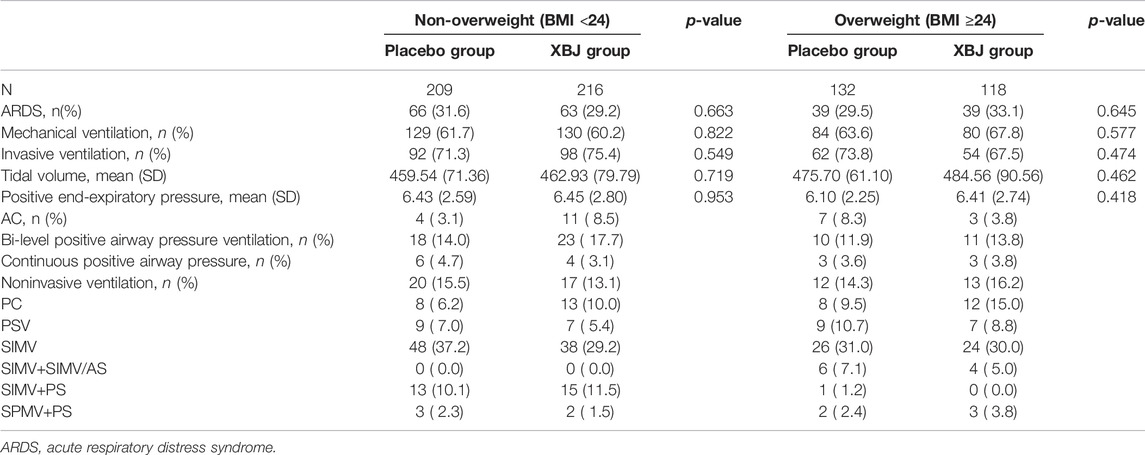
TABLE 3. Rate of patients with ARDS and the baseline settings of mechanical ventilation in the BMI group.
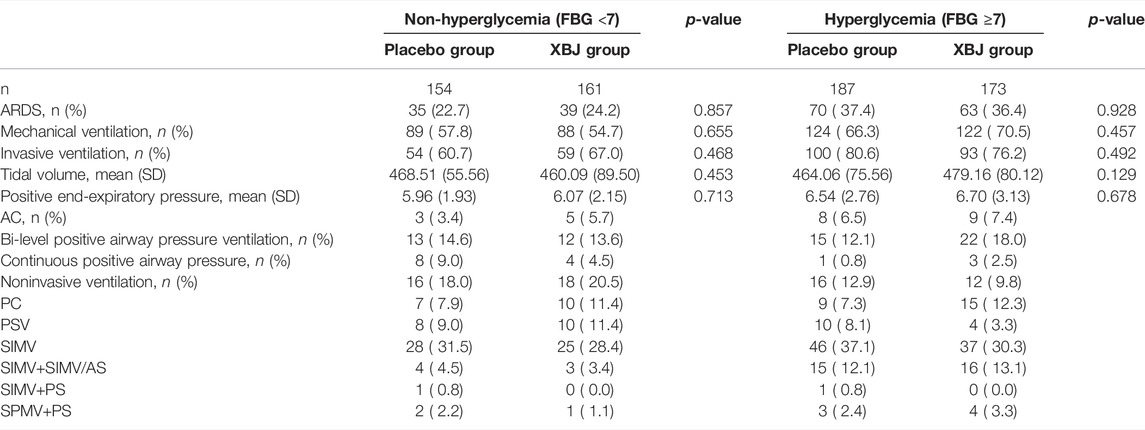
TABLE 4. Rate of patients with ARDS and the baseline settings of mechanical ventilation in the FBG group
In non-overweight and non-hyperglycemia groups, the XueBiJing injection group presented a significant lower 28-day mortality rate (15.3% or 11.2%) than that of the placebo treatment group (24.9% or 20.8%) (p = 0.0018 or p = 0.03). As for the secondary outcomes, both the duration of mechanical ventilation and total duration of ICU stay presented no statistical difference in non-overweight or non-hyperglycemia patients between XueBiJing injection and placebo treatment groups (Tables 5, 6).
In the non-overweight group (p = 0.018) and non-hyperglycemia group (p = 0.025), patients with XueBiJing injection had a significantly superior overall survival to those who were treated with placebo. In overweight patients (p = 0.18) and hyperglycemia patients (p = 0.11), no significant differences in mortality between XueBiJing injection and placebo treatment groups were seen (Figure 2). Some secondary outcomes are listed in Supplementary Tables S1, S2. Among non-overweight or non-hyperglycemia patients, the XueBiJing injection group presented a significantly lower PSI (94.84 ± 28.54 or 86.71 ± 26.81) than the placebo treatment group (102.11 ± 30.18 or 93.89 ± 25.78, p = 0.022 or p = 0.032) at day 4.
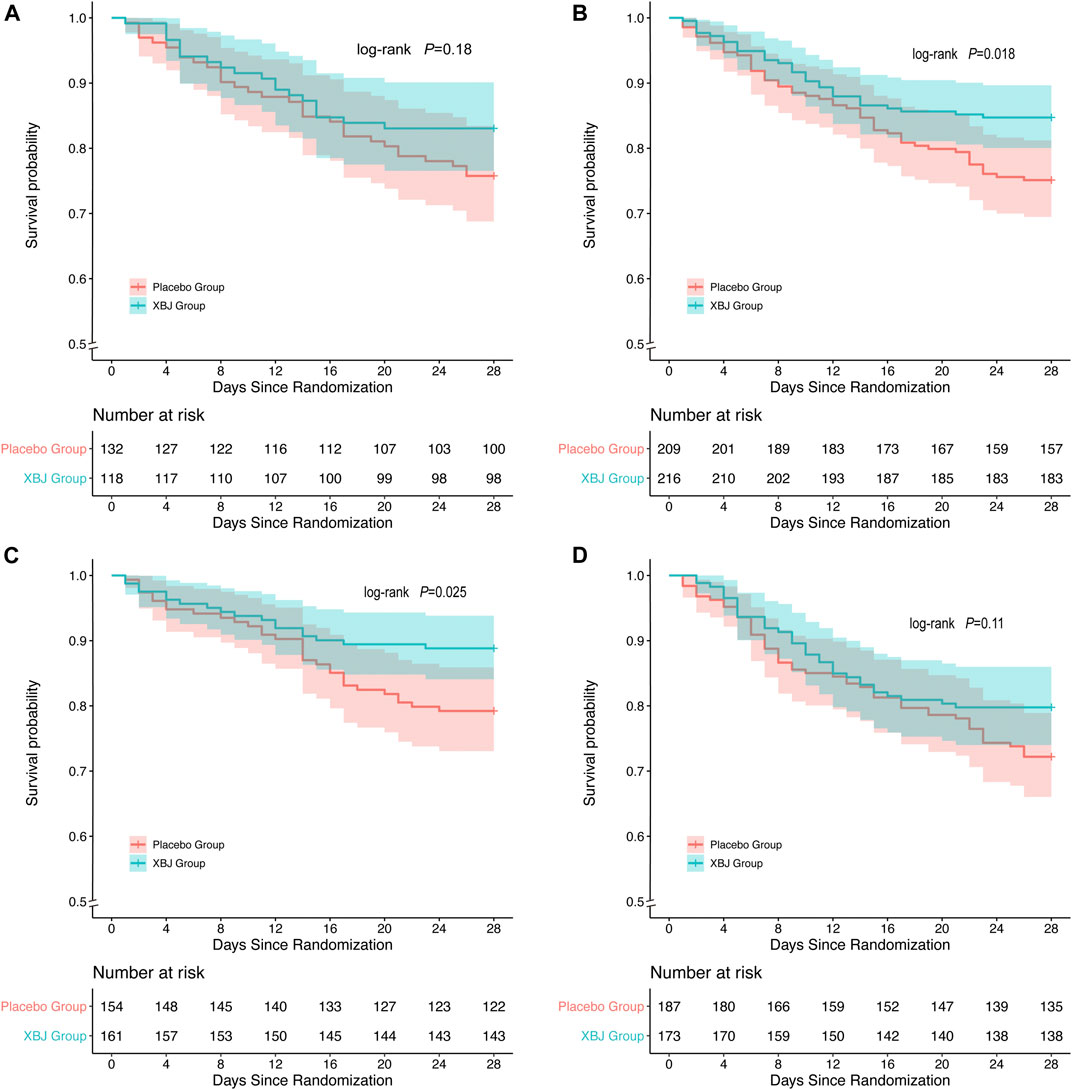
FIGURE 2. Kaplan–Meier survival curve of overall survival after XueBiJing (XBJ) and placebo for patients in (A,C) Patients with XBJ had a significantly inferior overall survival to those who with placebo: non-overweight group: (p = 0.018),non-hyperglycemia group (p = 0.025) (B,D) overweight group: p = 0.18, hyperglycemia group: p = 0.11
Adverse events (AEs) and clinically significant laboratory abnormalities are summarized in Supplementary Tables S3, S4. There were no significant differences in AEs among patients in the XueBiJing and placebo groups.
Discussion
The results of the large multicenter trial showed that XueBiJing injection could improve the PSI of patients with SCAP and reduce the 28-day mortality rate compared with those of the placebo group. The 28-day mortality was reduced from 24.63% to 15.87% in patients treated with XueBiJing (p < 0.01), which confirmed the effectiveness of XueBiJing injection in the treatment of SCAP.
Our results showed that XueBiJing injection was significantly effective and could reduce the 28-day mortality rate for patients with BMI <24 kg/m2 or FBG <7 mmol/L. Even though the 28-day mortality rate for patients with BMI ≥24 kg/m2 or FBG ≥7.0 mmol/L presented a lower trend in the XueBiJing injection group, it was not significant. These results suggested that overweight as well as hyperglycemia might have an adverse impact on the efficacy of XueBiJing injection in the treatment of SCAP.
XueBiJing, an injectable prescription from traditional Chinese medicine (TCM), is extensively used in the conventional management of sepsis with a history of more than 10 years (Li et al., 2020). Previous studies have shown that XueBiJing could protect endothelial cells, improve microcirculation and coagulopathy, alleviate inflammatory reaction, and regulate anti-oxidative stress (Xu et al., 2015; Wang et al., 2016; Chen et al., 2017; Zuo et al., 2019). The protective effect appears to be mediated through downregulation of the TLR4 and NF-κB expressions (He et al., 2018). Previous gas chromatography/mass spectrometer (GC/MS)-based metabolomics study demonstrated that XueBiJing could increase the survival rate of the septic animal model by changing serum metabolites, which involved energy metabolism, glucose metabolism, and amino acid metabolism (Jiang et al., 2019).
Obesity is related to the occurrence and development of many diseases. Studies have shown that obesity is related to metabolic dysfunction (Hotamisligil, 2006; Shoelson et al., 2006). Excess adiposity and adipocyte dysfunction can lead to adverse outcomes by altering immune responses through releasing proinflammatory adipokines (Ouchi et al., 2011). Obesity affects lung function and high BMI reduces lung capacity, which are related to the adverse consequences of pneumonia (Rubinstein et al., 1990; Jones and Nzekwu, 2006). Consistent with the reports, our data also showed that obesity patients had a metabolic disorder, as indicated by the higher blood glucose level in the patients with BMI ≥24 kg/m2 (8.45 ± 3.82 mmol/L) than that in patients with BMI <24 kg/m2 (8.15 ± 3.68 mmol/L). On the other hand, it has been proven that overweight might affect the metabolism of the drug, thereby affecting the therapeutic effect (Hanley et al., 2010; Knibbe et al., 2015). Similarly, our data demonstrated that, not like patients with BMI <24 kg/m2, patients with BMI ≥24 kg/m2 only had the trend of a lower 28-day mortality rate by XueBiJing injection without significance. Hence, we asserted that BMI ≥24 kg/m2 might be one important risk factor for reducing the efficacy of XueBiJing. Therefore, a weight-based XueBiJing dosing strategy might be a better choice and need to be tested with future clinical research studies. Interestingly, there are still epidemiologic studies on general population or critical illness that showed beneficial effects of higher body mass index (BMI) on all-cause mortality (Flegal et al., 2013; Kahlon et al., 2013; Singanayagam et al., 2013). This “obesity survival paradox” and the underlying mechanisms at least need to be tested in SCAP.
SCAP is a disease with significant biochemical and metabolic disturbance (Khardori and Castillo, 2012). In our analysis, we also noticed that certain proportion (50.7%) of patients had the problem of a higher glucose serum level without the present history of diabetes. The poorly regulated glucose metabolism in patients is often associated with increased levels of inflammatory markers (Chang and Yang, 2016). Compared with the non-hyperglycemia group, the hyperglycemia group had much intense inflammation (CRP 77.30 ± 100.20 vs. 96.12 ± 83.38 mg/L) (p = 0.016). Hyperglycemia influences a variety of host immune functions, such as chemotaxis, phagocytosis, and bactericidal activity of histiocytic cells (Akbar, 2001; Falguera et al., 2005; Eurich et al., 2010). A previous study already showed the mechanisms that glucose-induced alterations in endothelial cell function promote changes in the basement membrane structure and cause endothelial cell permeability; reactive oxygen species (ROS) have been suggested by others to be common mediators for hyperglycemic cell damage (Simon et al., 2000; Morss and Edelman, 2007). For CAP patients, high glucose serum levels lead to an increased length of hospital stay or higher mortality due to infections. Each 1mmol/L increase of glucose serum levels might result in the risk of in-hospital complications that increased to 3% (0.2–6%) (McAlister et al., 2005; Kornum et al., 2007). Our study also suggested that compared with the non-hyperglycemia group, the 28-day mortality rate in the hyperglycemia group is high, and the duration of ICU stay is long. In distribution studies, the increased level of glycosylation of plasma proteins could reduce the binding of a number of drugs in the blood (Gwilt et al., 1991; Mashayekhi-Sardoo et al., 2019). Hyperglycemia may lead to a decrease in the effect of XueBiJing, so dosage adjustment is required. Our results demonstrated a significantly lower 28-day mortality rate for patients with FBG <7 mmol/L, while only a decreased trend was observed for patients with FBG ≥7.0 mmol/L by XueBiJing injection. Our data suggested that hyperglycemia might have an adverse impact on the efficacy of XueBiJing injection in the treatment of SCAP, indicating that SCAP patients with hyperglycemia might needed to be reconsidered the XueBiJing dose or treatment plan. Better blood glucose level management is strongly recommended.
Limitations
The results of our study come with some limitations. Although our data come from a well-designed RCT, the analyses were planned post hoc, and the results cannot be considered confirmative. Second, the number of patients included in each group was relatively small and not suitable for multivariate or stratified analysis. A larger sample size would be necessary to validate our conclusions. Mortality is the most robust outcome of a noninferiority trial, but adding more outcomes will be more compelling.
Conclusion
In this post hoc analysis of a large randomized controlled trial, for overweight and hyperglycemia SCAP patients, in the non-overweight group (p = 0.018) and non-hyperglycemia group (p = 0.025), patients with XueBiJing injection had a significantly superior overall survival to those who were treated with placebo, and the addition of XueBiJing treatment only partially reduced the 28-day mortality but not significantly like the effect in non-overweight and non-hyperglycemia patients. Overweight and hyperglycemic SCAP patients therefore should be identified who are in need of the adjusted dose of XueBiJing injection and intensified care to reduce the risk of death.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This study was supported by the National Natural Science Foundation of China (project number 81970069), National Natural Science Foundation of China (project number 82170091), and Smart Healthcare Project of Zhongshan Hospital, Fudan University (project number 2020ZHZS18).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors wish to gratefully acknowledge all of the study centers and study participants who have devoted their time and effort to this trial.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.755536/full#supplementary-material
Abbreviations
APCHE, acute physiology and chronic health evaluation; ARDS, acute respiratory distress syndrome; BMI, body mass index; CAP, community-acquired pneumonia; CRP, C-reactive protein; FBG, fasting blood glucose; ICU, intensive care unit; PSI, pneumonia severity index; RCT, randomized controlled trial; SCAP, severe community-acquired pneumonia; ROS, reactive oxygen species; SOFA, Sequential Organ Failure Assessment; TCM, traditional Chinese medicine.
References
American Diabetes Association (2021). 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care, 44(Suppl. 1), S15-s33. doi:10.2337/dc21-S002
Akbar, D. H. (2001). Bacterial Pneumonia: Comparison between Diabetics and Non-diabetics. Acta Diabetol. 38 (2), 77–82. doi:10.1007/s005920170017
Chang, S. C., and Yang, W. V. (2016). Hyperglycemia, Tumorigenesis, and Chronic Inflammation. Crit. Rev. Oncol. Hematol. 108, 146–153. doi:10.1016/j.critrevonc.2016.11.003
Chen, G., Gao, Y., Jiang, Y., Yang, F., Li, S., Tan, D., et al. (2018). Efficacy and Safety of Xuebijing Injection Combined with Ulinastatin as Adjunctive Therapy on Sepsis: A Systematic Review and Meta-Analysis. Front. Pharmacol. 9, 743. doi:10.3389/fphar.2018.00743
Chen, Y., Tong, H., Pan, Z., Jiang, D., Zhang, X., Qiu, J., et al. (2017). Xuebijing Injection Attenuates Pulmonary Injury by Reducing Oxidative Stress and Proinflammatory Damage in Rats with Heat Stroke. Exp. Ther. Med. 13 (6), 3408–3416. doi:10.3892/etm.2017.4444
Dong, Z., Tan, Z., and Chen, Z. (2019). Association of BMI and Lipid Profiles with Axillary Osmidrosis: a Retrospective Case-Control Study. J. Dermatolog Treat. 2019, 1–4. doi:10.1080/09546634.2019.1688232
Eurich, D. T., Gamble, J. M., Marrie, T. J., and Majumdar, S. R. (2010). Dysglycaemia and 90 Day and 1 Year Risks of Death or Readmission in Patients Hospitalised for Community-Acquired Pneumonia. Diabetologia 53 (3), 497–503. doi:10.1007/s00125-009-1598-y
Falagas, M. E., and Kompoti, M. (2006). Obesity and Infection. Lancet Infect. Dis. 6 (7), 438–446. doi:10.1016/S1473-3099(06)70523-0
Falguera, M., Pifarre, R., Martin, A., Sheikh, A., and Moreno, A. (2005). Etiology and Outcome of Community-Acquired Pneumonia in Patients with Diabetes Mellitus. Chest 128 (5), 3233–3239. doi:10.1378/chest.128.5.3233
Fine, M. J., Smith, M. A., Carson, C. A., Mutha, S. S., Sankey, S. S., Weissfeld, L. A., et al. (1996). Prognosis and Outcomes of Patients with Community-Acquired Pneumonia. A Meta-Analysis. Jama 275 (2), 134–141. doi:10.1001/jama.1996.03530260048030
Flegal, K. M., Kit, B. K., Orpana, H., and Graubard, B. I. (2013). Association of All-Cause Mortality with Overweight and Obesity Using Standard Body Mass index Categories: a Systematic Review and Meta-Analysis. Jama 309 (1), 71–82. doi:10.1001/jama.2012.113905
Gwilt, P. R., Nahhas, R. R., and Tracewell, W. G. (1991). The Effects of Diabetes Mellitus on Pharmacokinetics and Pharmacodynamics in Humans. Clin. Pharmacokinet. 20 (6), 477–490. doi:10.2165/00003088-199120060-00004
Hanley, M. J., Abernethy, D. R., and Greenblatt, D. J. (2010). Effect of Obesity on the Pharmacokinetics of Drugs in Humans. Clin. Pharmacokinet. 49 (2), 71–87. doi:10.2165/11318100-000000000-00000
He, F., Wang, J., Liu, Y., Wang, X., Cai, N., Wu, C., et al. (2018). Xuebijing Injection Induces Anti-inflammatory-like Effects and Downregulates the Expression of TLR4 and NF-Κb in Lung Injury Caused by Dichlorvos Poisoning. Biomed. Pharmacother. 106, 1404–1411. doi:10.1016/j.biopha.2018.07.111
Hotamisligil, G. S. (2006). Inflammation and Metabolic Disorders. Nature 444 (7121), 860–867. doi:10.1038/nature05485
Hu, Y., Ciminieri, C., Hu, Q., Lehmann, M., Königshoff, M., and Gosens, R. (2021). WNT Signalling in Lung Physiology and Pathology. Handb Exp. Pharmacol. 269, 305–336. doi:10.1007/164_2021_521
Huttunen, R., and Syrjänen, J. (2013). Obesity and the Risk and Outcome of Infection. Int. J. Obes. (Lond) 37 (3), 333–340. doi:10.1038/ijo.2012.62
Jiang, Y., Zou, L., Liu, S., Liu, X., Chen, F., Liu, X., et al. (2019). GC/MS-based Metabonomics Approach Reveals Effects of Xuebijing Injection in CLP Induced Septic Rats. Biomed. Pharmacother. 117, 109163. doi:10.1016/j.biopha.2019.109163
Jones, R. L., and Nzekwu, M. M. (2006). The Effects of Body Mass index on Lung Volumes. Chest 130 (3), 827–833. doi:10.1378/chest.130.3.827
Kahlon, S., Eurich, D. T., Padwal, R. S., Malhotra, A., Minhas-Sandhu, J. K., Marrie, T. J., et al. (2013). Obesity and Outcomes in Patients Hospitalized with Pneumonia. Clin. Microbiol. Infect. 19 (8), 709–716. doi:10.1111/j.1469-0691.2012.04003.x
Khardori, R., and Castillo, D. (2012). Endocrine and Metabolic Changes during Sepsis: an Update. Med. Clin. North. Am. 96 (6), 1095–1105. doi:10.1016/j.mcna.2012.09.005
Knibbe, C. A., Brill, M. J., van Rongen, A., Diepstraten, J., van der Graaf, P. H., and Danhof, M. (2015). Drug Disposition in Obesity: toward Evidence-Based Dosing. Annu. Rev. Pharmacol. Toxicol. 55, 149–167. doi:10.1146/annurev-pharmtox-010814-124354
Kornum, J. B., Nørgaard, M., Dethlefsen, C., Due, K. M., Thomsen, R. W., Tjønneland, A., et al. (2010). Obesity and Risk of Subsequent Hospitalisation with Pneumonia. Eur. Respir. J. 36 (6), 1330–1336. doi:10.1183/09031936.00184209
Kornum, J. B., Thomsen, R. W., Riis, A., Lervang, H. H., Schønheyder, H. C., and Sørensen, H. T. (2007). Type 2 Diabetes and Pneumonia Outcomes: a Population-Based Cohort Study. Diabetes Care 30 (9), 2251–2257. doi:10.2337/dc06-2417
Lepper, P. M., Ott, S., Nüesch, E., von Eynatten, M., Schumann, C., Pletz, M. W., et al. (2012). Serum Glucose Levels for Predicting Death in Patients Admitted to Hospital for Community Acquired Pneumonia: Prospective Cohort Study. Bmj 344, e3397. doi:10.1136/bmj.e3397
Li, C., Wang, P., Li, M., Zheng, R., Chen, S., Liu, S., et al. (2021). The Current Evidence for the Treatment of Sepsis with Xuebijing Injection: Bioactive Constituents, Findings of Clinical Studies and Potential Mechanisms. J. Ethnopharmacol 265, 113301. doi:10.1016/j.jep.2020.113301
Li, C., Wang, P., Zhang, L., Li, M., Lei, X., Liu, S., et al. (2018). Efficacy and Safety of Xuebijing Injection (A Chinese Patent) for Sepsis: A Meta-Analysis of Randomized Controlled Trials. J. Ethnopharmacol 224, 512–521. doi:10.1016/j.jep.2018.05.043
Li, T., Qian, Y., Miao, Z., Zheng, P., Shi, T., Jiang, X., et al. (2020). Xuebijing Injection Alleviates Pam3CSK4-Induced Inflammatory Response and Protects Mice from Sepsis Caused by Methicillin-Resistant Staphylococcus aureus. Front. Pharmacol. 11, 104. doi:10.3389/fphar.2020.00104
Liu, F., Wang, W., Ma, J., Sa, R., and Zhuang, G. (2018). Different Associations of Sufficient and Vigorous Physical Activity with BMI in Northwest China. Sci. Rep. 8 (1), 13120. doi:10.1038/s41598-018-31227-6
Mandell, L. A., Wunderink, R. G., Anzueto, A., Bartlett, J. G., Campbell, G. D., Dean, N. C., et al. (2007). Infectious Diseases Society of America/American Thoracic Society Consensus Guidelines on the Management of Community-Acquired Pneumonia in Adults. Clin. Infect. Dis. 44 (Suppl. 2), S27–S72. doi:10.1086/511159
Mashayekhi-Sardoo, H., Mohammadpour, A. H., Nomani, H., and Sahebkar, A. (2019). The Effect of Diabetes Mellitus on Pharmacokinetics, Pharmacodynamics and Adverse Drug Reactions of Anticancer Drugs. J. Cel Physiol 234 (11), 19339–19351. doi:10.1002/jcp.28644
McAlister, F. A., Majumdar, S. R., Blitz, S., Rowe, B. H., Romney, J., and Marrie, T. J. (2005). The Relation between Hyperglycemia and Outcomes in 2,471 Patients Admitted to the Hospital with Community-Acquired Pneumonia. Diabetes Care 28 (4), 810–815. doi:10.2337/diacare.28.4.810
Menke, A., Casagrande, S., Geiss, L., and Cowie, C. C. (2015). Prevalence of and Trends in Diabetes Among Adults in the United States, 1988-2012. Jama 314 (10), 1021–1029. doi:10.1001/jama.2015.10029
Morss, A. S., and Edelman, E. R. (2007). Glucose Modulates Basement Membrane Fibroblast Growth Factor-2 via Alterations in Endothelial Cell Permeability. J. Biol. Chem. 282 (19), 14635–14644. doi:10.1074/jbc.M608565200
Ouchi, N., Parker, J. L., Lugus, J. J., and Walsh, K. (2011). Adipokines in Inflammation and Metabolic Disease. Nat. Rev. Immunol. 11 (2), 85–97. doi:10.1038/nri2921
Qi, F., Liang, Z. X., She, D. Y., Yan, G. T., and Chen, L. A. (2011). A Clinical Study on the Effects and Mechanism of Xuebijing Injection in Severe Pneumonia Patients. J. Tradit Chin. Med. 31 (1), 46–49. doi:10.1016/s0254-6272(11)60011-3
Ruaro, B., Salton, F., Braga, L., Wade, B., Confalonieri, P., Volpe, M. C., et al. (2021). The History and Mystery of Alveolar Epithelial Type II Cells: Focus on Their Physiologic and Pathologic Role in Lung. Int. J. Mol. Sci. 22 (5), 2566. doi:10.3390/ijms22052566
Rubinstein, I., Zamel, N., DuBarry, L., and Hoffstein, V. (1990). Airflow Limitation in Morbidly Obese, Nonsmoking Men. Ann. Intern. Med. 112 (11), 828–832. doi:10.7326/0003-4819-112-11-828
Shoelson, S. E., Lee, J., and Goldfine, A. B. (2006). Inflammation and Insulin Resistance. J. Clin. Invest. 116 (7), 1793–1801. doi:10.1172/JCI29069
Simon, H. U., Haj-Yehia, A., and Levi-Schaffer, F. (2000). Role of Reactive Oxygen Species (ROS) in Apoptosis Induction. Apoptosis 5 (5), 415–418. doi:10.1023/a:1009616228304
Singanayagam, A., Singanayagam, A., and Chalmers, J. D. (2013). Obesity Is Associated with Improved Survival in Community-Acquired Pneumonia. Eur. Respir. J. 42 (1), 180–187. doi:10.1183/09031936.00115312
Song, Y., Yao, C., Yao, Y., Han, H., Zhao, X., Yu, K., et al. (2019). XueBiJing Injection versus Placebo for Critically Ill Patients with Severe Community-Acquired Pneumonia: A Randomized Controlled Trial. Crit. Care Med. 47 (9), e735–e743. doi:10.1097/CCM.0000000000003842
Torres, A., Sibila, O., Ferrer, M., Polverino, E., Menendez, R., Mensa, J., et al. (2015). Effect of Corticosteroids on Treatment Failure Among Hospitalized Patients with Severe Community-Acquired Pneumonia and High Inflammatory Response: a Randomized Clinical Trial. Jama 313 (7), 677–686. doi:10.1001/jama.2015.88
van Vught, L. A., Wiewel, M. A., Klein Klouwenberg, P. M., Hoogendijk, A. J., Scicluna, B. P., Ong, D. S., et al. (2016). Admission Hyperglycemia in Critically Ill Sepsis Patients: Association with Outcome and Host Response. Crit. Care Med. 44 (7), 1338–1346. doi:10.1097/CCM.0000000000001650
Wang, L., Du, X., Dong, J. Z., Liu, W. N., Zhou, Y. C., Li, S. N., et al. (2019). Body Mass index and All-Cause Mortality in Patients with Atrial Fibrillation: Insights from the China Atrial Fibrillation Registry Study. Clin. Res. Cardiol. 108 (12), 1371–1380. doi:10.1007/s00392-019-01473-3
Wang, L., Liu, Z., Dong, Z., Pan, J., and Ma, X. (2016). Effects of Xuebijing Injection on Microcirculation in Septic Shock. J. Surg. Res. 202 (1), 147–154. doi:10.1016/j.jss.2015.12.041
Xu, Q., Liu, J., Guo, X., Tang, Y., Zhou, G., Liu, Y., et al. (2015). Xuebijing Injection Reduces Organ Injuries and Improves Survival by Attenuating Inflammatory Responses and Endothelial Injury in Heatstroke Mice. BMC Complement. Altern. Med. 15, 4. doi:10.1186/s12906-015-0519-5
Keywords: severe community-acquired pneumonia, post hoc analysis, randomized controlled trial, 28-day mortality rate, XueBiJing injection
Citation: Song Y, Wang X, Chen C, Wei T, Lang K, Yang D and Song Y (2022) Weaker Response to XueBiJing Treatment in Severe Community-Acquired Pneumonia Patients With Higher Body Mass Index or Hyperglycemia: A Post Hoc Analysis of a Randomized Controlled Trial. Front. Pharmacol. 13:755536. doi: 10.3389/fphar.2022.755536
Received: 07 November 2021; Accepted: 12 April 2022;
Published: 03 June 2022.
Edited by:
Daniela Rigano, University of Naples Federico II, ItalyCopyright © 2022 Song, Wang, Chen, Wei, Lang, Yang and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong Yang, eWFuZy5kb25nQHpzLWhvc3BpdGFsLnNoLmNu
†These authors have contributed equally to this work
 Yansha Song
Yansha Song Xiaocen Wang
Xiaocen Wang Cuicui Chen†
Cuicui Chen† Tingting Wei
Tingting Wei Ke Lang
Ke Lang Dong Yang
Dong Yang Yuanlin Song
Yuanlin Song