- 1Graduate Institute of Biomedical Sciences, College of Medicine, China Medical University, Taichung, Taiwan
- 2Division of Nephrology and Kidney Institute, China Medical University Hospital, Taichung, Taiwan
- 3Management Office for Health Data, China Medical University Hospital, Taichung, Taiwan
- 4College of Medicine, China Medical University, Taichung, Taiwan
- 5Department of Family Medicine, China Medical University Hospital, Taichung, Taiwan
- 6Department of Chest Medicine, China Medical University Hospital, Taichung, Taiwan
- 7Department of Nuclear Medicine, PET Center, China Medical University Hospital, Taichung, Taiwan
- 8Department of Bioinformatics and Medical Engineering, Asia University, Taichung, Taiwan
- 9Center of Augmented Intelligence in Healthcare, China Medical University Hospital, Taichung, Taiwan
Background: Whether diabetes mellitus (DM) patients with chronic kidney disease (CKD) can glean individual renal benefit from dihydropyridine calcium channel blockers (DCCBs) remains to be determined. We conducted a nationwide, population-based, propensity score matching cohort study to examine the effect of DCCBs on CKD progression in DM patients with CKD.
Methods: One million individuals were randomly sampled from Taiwan’s National Health Insurance Research Database. The study cohort consisted of DM patients with CKD who used DCCBs. The comparison cohort was propensity-matched for demographic characteristics and comorbidities. The endpoint was advanced CKD or end-stage renal disease (ESRD). The Cox proportional hazards model was used to calculate the risks.
Results: In total, 9,761 DCCB users were compared with DCCB nonusers at a ratio of 1:1. DCCB users had lower risk of advanced CKD and ESRD than nonusers—with adjusted hazard ratio [aHR; 95% confidence interval (CI)] of 0.64 (0.53–0.78) and 0.59 (95% CI, 0.50–0.71) for advanced CKD and ESRD, respectively. DCCB users aged ≥65 years had the lowest incidence rates of advanced CKD and ESRD—with aHR (95% CI) of 0.47 (0.34–0.65) and 0.48 (0.35–0.65) for advanced CKD and ESRD, respectively. Finally, cumulative DCCB use for >1,100 days was associated with the lowest advanced CKD and ESRD risks [(aHR, 0.29 (95% CI, 0.19–0.44)].
Conclusion: DM patients with CKD who used DCCBs had lower risk of progression to advanced CKD and ESRD than nonusers did.
Introduction
Dihydropyridine calcium channel blockers (DCCBs), which bind to calcium channels located on vascular smooth muscles to interrupt calcium entry, are widely used in clinical practice to reduce blood pressure (Braunwald, 1982; Eisenberg et al., 2004). In addition, DCCBs can provide many cardiovascular (CV) benefits (Staessen et al., 1997; Nissen et al., 2004; Hasebe and Kikuchi, 2005; Haller, 2008). A meta-analysis of 175,634 patients demonstrated that DCCBs could reduce all-cause mortality and stroke risk and prevent heart failure (Costanzo et al., 2009). Systematic reviews have shown that DCCBs could reduce the risk of Parkinson disease (Lang et al., 2015), incidental dementia (Hussain et al., 2018), and sequelae of traumatic brain injury (Gurkoff et al., 2017). However, because of their vasodilatory effect, DCCBs have also been reported in association with increased gastrointestinal tract bleeding (Kaplan et al., 2000) and proischemic complication (Waters, 1991) risks.
Currently, whether DCCBs have individual benefits or harms on kidney function, particularly in diabetes mellitus (DM) patients with chronic kidney disease (CKD), who are at the highest risk for end-stage renal disease (ESRD), remains unclear (de Leeuw et al., 2004). This is partly because CKD and CV events share multiple risk factors and medications (Cai et al., 2013) and the other part due to intrinsic characteristic of DCCBs of vasodilatation of afferent arterioles and increased intraglomerular pressure (Carmines and Navar, 1989; Kimura et al., 1994). Although numerous randomized controlled trials have investigated the effects of DCCBs on renal function, most have compared angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin II receptor blockers (ARBs) with DCCBs (Chan et al., 1992; Bakris et al., 1996; Velussi et al., 1996; Campo et al., 1997; Estacio et al., 1998; Lewis et al., 2001; Rahman et al., 2012), combined DCCB–ACEI/ARB therapy with noncombination therapy (Hasebe and Kikuchi, 2005), DCCBs with diuretics (de Leeuw et al., 2004), or combined ACEIs/ARB–DCCB therapy with combined ACEI/ARB–diuretics therapy (Kaneshiro et al., 2009; Kohlmann et al., 2009; Bakris et al., 2010; Doi et al., 2010; Ishimitsu et al., 2011; Lee et al., 2012; Ogihara et al., 2014; Oshikawa et al., 2014). However, most of the aforementioned studies had a short follow-up duration (Campo et al., 1997; Doi et al., 2010; Ishimitsu et al., 2011; Lee et al., 2012), small sample size (Chan et al., 1992; Bakris et al., 1996; Campo et al., 1997; Doi et al., 2010; Ishimitsu et al., 2011), intrinsic heterogeneous population (CKD, DM, diabetic CKD, or diabetic non-CKD) (Fernández et al., 2001; Bakris et al., 2010; Ishimitsu et al., 2011), or did not consider possible effects of hypoglycemic agents on renal function in patients with DM (Chan et al., 1992; Bakris et al., 1996; Velussi et al., 1996; Fernández et al., 2001). Whether DCCBs have long-term “individual” effects on renal function remain to be determined. In their study involving patients with type 2 DM, Bakis et al. reported comparable effects of DCCBs and ACEIs in slowing renal disease progression; however, the sample size was only 52 patients (Bakris et al., 1996). We hypothesized that DCCBs have individual effects on delaying CKD progression, particularly in DM patients with CKD. We conducted a nationwide, population-based, propensity score matching study to examine the effects of DCCBs on CKD progression in DM patients with CKD.
Methods
Data Source
In 1995, Taiwan launched a single-payer healthcare system from which the National Health Insurance (NHI) Research Database (NHIRD) was established. Data used in the present study were obtained from the Longitudinal Health Insurance Database, which contains information on 1 million individuals randomly sampled from NHIRD. The data were deidentified to protect privacy. The medical claims data contained information on diagnosis, outpatient visits, hospital admissions, drug prescriptions, and surgical procedures. The diagnostic classification was based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). The study was approved by the Research Ethics Committee at China Medical University and Hospital [CMUH104-REC2-115 (AR-4)].
Study Population
We enrolled DM patients who had subsequent new diagnosis of CKD (ICD-9 codes 585.xx). In this study, those who had the record of DM (ICD-9 codes 250.x0 or 250.x2) more than two times within 1 year were defined as DM patients. CKD was defined on the basis of ICD-9 codes 585.xx recorded in NHIRD for more than three times. The date of initial DCCB use was defined as the index date. Patients were followed up until date of first erythropoietin (EPO) prescription, onset of ESRD, withdrawal, death, or December 31, 2013. Diagnosis of ESRD was confirmed by ICD-9-CM codes and inclusion in the Registry for Catastrophic Illness Patient Database, a sub-classification of the NHIRD. Although NHIRD lacks information on CKD staging, the NHI program has stated that patients with estimated glomerular filtration rate (eGFR) < 15 ml/min/1.73 m2 and hemoglobin level <10 gm/dL may receive the official EPO prescription benefit package. Thus, in the present study, we defined patients with advanced CKD as those with CKD who started EPO prescriptions.
We excluded patients diagnosed as having advanced CKD or ESRD before the index date. A total of 9,761 DCCB users were compared with DCCB nonusers at a ratio of 1:1 using propensity scores after matching for demographic characteristics and baseline comorbidities. Baseline comorbidities included cancer, hyperlipidemia, stroke, chronic obstructive pulmonary disease, cirrhosis, arrhythmia, congestive heart failure, fibromyalgia, coronary artery disease, alcohol-related diseases, peripheral arterial occlusive disease, renal stone, and peptic ulcer disease (list of codes were presented in Supplementary Table S1). The covariates of baseline medications comprised statins, ACEIs/ARBs, loop diuretics, thiazides, potassium-sparing diuretics, non-DCCBs, alpha-blockers, beta-blockers, insulins, sulfonylureas, biguanides, miglitol, acarbose, thiazolidinedione, dipeptidyl peptidase-4 inhibitors, and other antidiabetics.
Statistical Analysis
Descriptive analyses of demographics, comorbidities, and medications of the cohorts are presented as frequencies and percentages for the categorical variables and as means and standard deviations for the continuous variables. The 1:N Case-Control Matching Macro (Parsons LS et al. SUGI 29) was used to match cases with controls (Parsons, 2012). DM patients with CKD who used DCCB were matched (1:1 ratio) with those who did not use DCCB according to their propensity score through nearest neighbor matching, initially to the eighth digit and then as required to the first digit. Therefore, matches were first made within a caliper width of 0.0000001, and then the caliper width was increased for unmatched cases to 0.1. We reconsidered the matching criteria and performed a rematch (greedy algorithm). For each DM patient on DCCB use, the corresponding comparisons were selected based on the nearest propensity score. A standardized mean difference of ≤0.1 indicates a negligible difference between the two cohorts. Absolute standardized mean difference per covariate before and after PSM was presented in Table 1 and Supplementary Table S2. Cumulative incidence rates of advanced CKD and ESRD were calculated based on the Kaplan-Meier method, and the between-cohort comparisons of the cumulative incidence curves were assessed by log-rank tests.
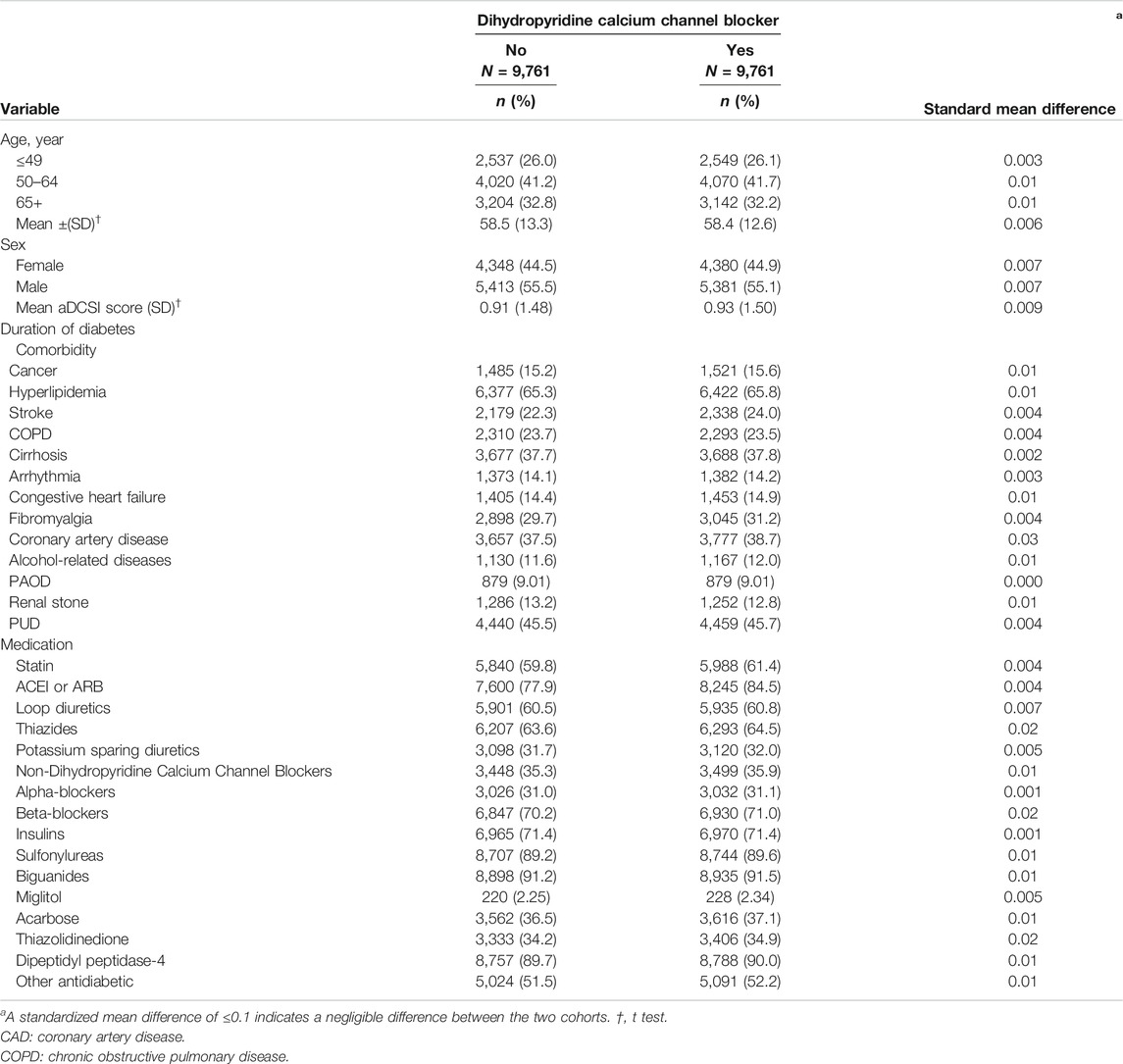
TABLE 1. Demographic characteristics and comorbidities in the propensity-score-matched cohorts with and without dihydropyridine Calcium Channel Blocker used among diabetes mellitus patients with chronic kidney disease.
To consider the effect of the frequency variation in the use of DCCBs, DCCB use was considered as a time-dependent covariate in the Cox proportional hazards model. Hazard ratios (HRs) and the corresponding 95% confidence intervals (CIs) for advanced CKD and ESRD were estimated using the Cox proportional hazards models. A two‐tailed p value <0.05 was considered statistically significant. The data analysis was generated using SAS (version 9.4) of the SAS System for [Unix] (SAS Institute Inc., Cary, NC, United States), and the figures were created using R.
Results
Table 1 lists the demographic characteristics and comorbidities in the propensity score–matched cohorts. The average age of DCCB nonusers and DCCB users was 58.5 ± 13.3 and 58.4 ± 12.6 years, respectively; 44.5 and 44.9% of the DCCB nonusers and users were women, respectively. In the profiles of baseline comorbidities and medications, they did not significantly differ between the two cohorts according to standardized mean differences after PS matching in Table 1.
Table 2 presents the incidence values and HRs for advanced CKD or ESRD in the cohorts from Cox proportional hazards models with time-dependent exposure covariates. The incidence rates of advanced CKD and ESRD in DCCB nonusers were 3.27 and 3.64 per 1,000 person-years, respectively. The incidence rates of advanced CKD and ESRD in DCCB users were 2.28 and 2.59 per 1,000 person-year, respectively. The DCCB users had lower advanced CKD and ESRD risk than did nonusers [with HR (95% CI) of 0.71 (0.59–0.85) for advanced CKD and 0.72 (0.61–0.86) for ESRD].
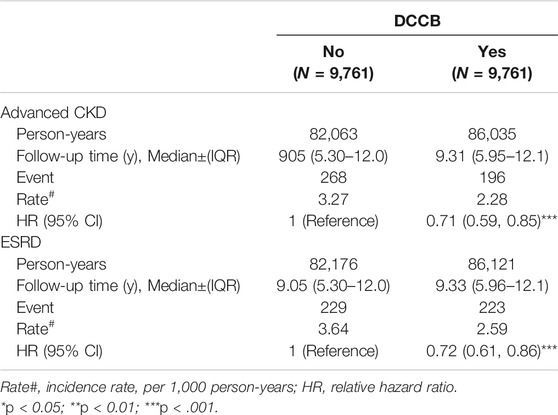
TABLE 2. Incidence and HRs of advanced CKD or ESRD in the dihydropyridine calcium channel blocker (DCCB) cohorts compared with those in the non-DCCB cohorts among diabetes mellitus patients with chronic kidney disease by Cox proportional hazard models with time-dependent exposure covariates.
Table 3 lists the incidence and HRs of advanced CKD or ESRD in the cohorts by age and sex from Cox proportional hazards models with time-dependent exposure covariates. Reduced incidence rates of advanced CKD and ESRD in DCCB users were observed in the ≥65-year age group compared with those in DCCB nonusers [with HR (95% CI) of 0.49 (0.36–0.68) in the ≥65-year age group for advanced CKD, respectively, and 0.76 (0.59–1.00) and 0.50 (0.38–0.67) in the 50–64- and ≥65-year age group for ESRD, respectively). Women using DCCBs were at lower advanced CKD and ESRD risks compared with women not using DCCBs [with HR (95% CI) of 0.67 (0.51–0.88) and 0.61 (0.47–0.78) for advanced CKD and ESRD, respectively].
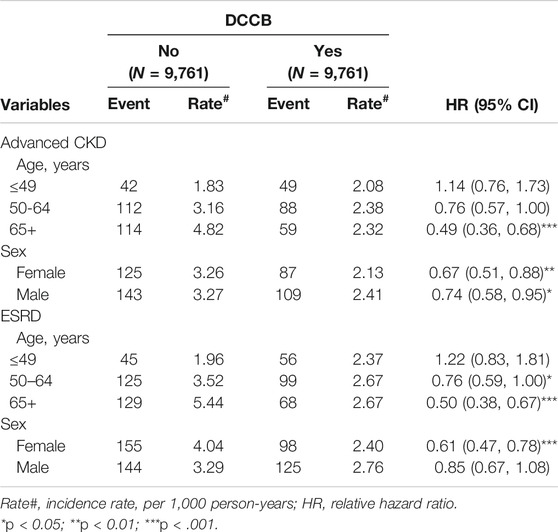
TABLE 3. Incidence and hazards ratio of advanced CKD or ESRD measured by age, sex, and comorbidity in the dihydropyridine calcium channel blocker (DCCB) cohorts among diabetes mellitus patients with chronic kidney disease compared with those in the non-DCCB cohorts by Cox proportional hazard models with time-dependent exposure covariates.
Table 4 shows incidence values and HRs for advanced CKD or ESRD stratified by duration of DCCB therapy. Compared with nonuse, cumulative DCCB use for >300 days reduced CKD risk (with HR [95% CI] of 0.63 [0.46–0.87] and 0.32 [0.21–0.48] for cumulative DCCB use for 301–1,100 and >1,100 days, respectively). Compared with nonuse, cumulative DCCB use for >300 days reduced ESRD risk [with aHR (95% CI) of 0.66 (0.49–0.89), and 0.28 (0.19–0.42) for cumulative DCCB use for 301–1,100, and >1,100 days, respectively].
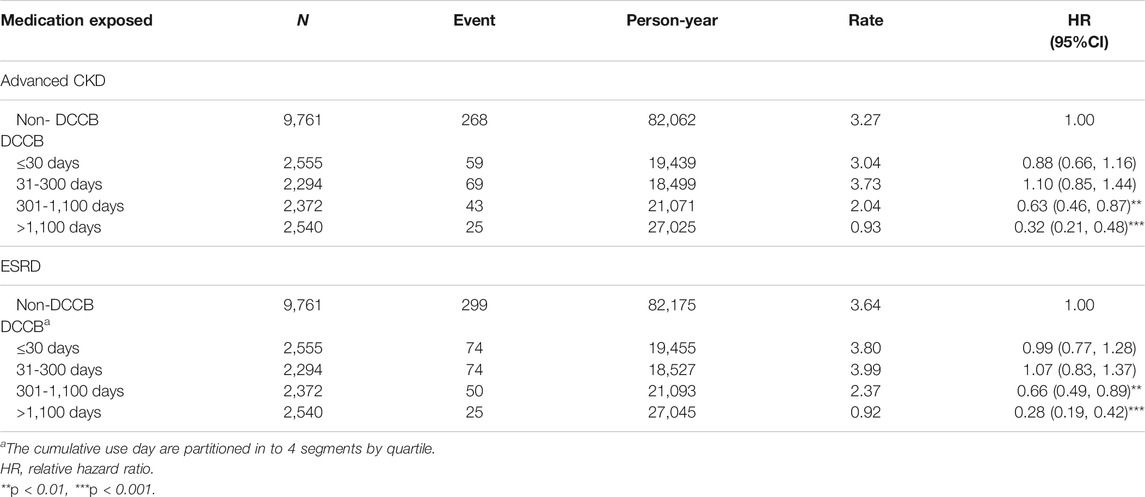
TABLE 4. Incidence and adjusted hazard ratio of advanced CKD or ESRD stratified by duration of dihydropyridine calcium channel blocker (DCCB) therapy among diabetes mellitus patients with chronic kidney disease.
Figure 1 depicts cumulative incidence curves of advanced CKD or ESRD for DCCB users and DCCB nonusers by propensity score matching. The p values from the log-rank tests for both cohorts were <0.001, and DCCB users were more likely to have lower advanced CKD and ESRD risks than were DCCB nonusers.
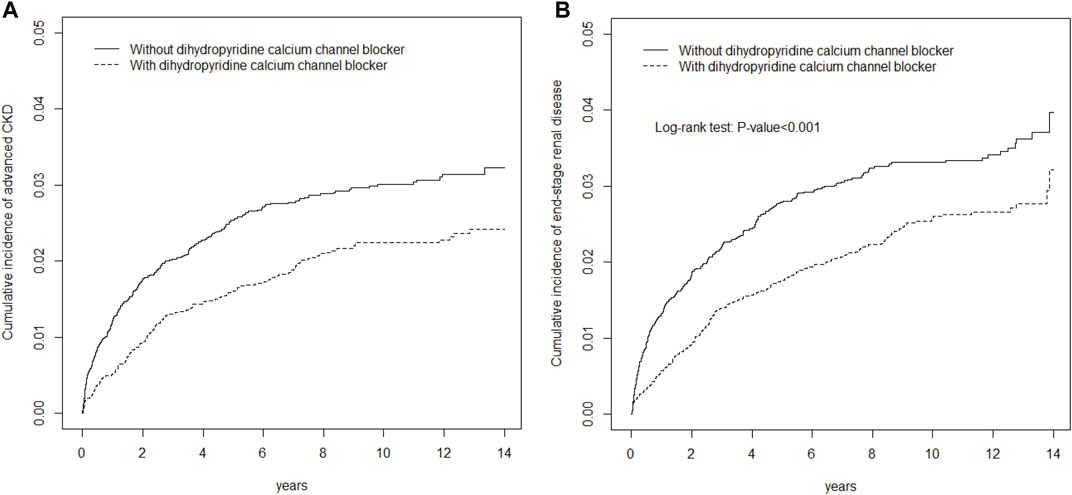
FIGURE 1. Cumulative incidence of EPO (A) or ESRD (B) curves for dihydropyridine calcium channel blocker (DCCB) users and dihydropyridine calcium channel blocker (DCCB) non-users by propensity score matched.
Discussion
DM patients with CKD who used DCCBs had a lower risk of progression to advanced CKD or ESRD compared with those who did not use DCCBs. We suggested several mechanisms of DCCBs that could account for this finding: improved blood pressure reduction, improved maintenance of renal perfusion, and DCCBs’ antiatherosclerotic properties. Although the optimal blood pressure target for improving renal outcomes in patients with CKD remains unclear, the evidence supports blood pressure control through antihypertensives for patients with systolic blood pressure <130 mmHg (Chang et al., 2019). The Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hyperintensive Patients and the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation trial revealed that improved blood pressure control could lead to reduction in the risk of doubling of creatinine levels and progression to advanced CKD compared with the absence of blood pressure control (JATOS, 2005; Heerspink et al., 2010). By contrast, other studies have found that intensive blood pressure control does not improve renal outcomes (Ruggenenti et al., 2005; Appel et al., 2010; Ku et al., 2017). These findings suggest that improved renal outcomes require both improved blood pressure control and improved renal perfusion (Palmer, 2002; Ravera et al., 2006). DCCBs have been effective in lowering systolic blood pressure to <130 mmHg in different patient populations in combination with other antihypertensives (Haller, 2008; Matsui et al., 2009; Association, 2019). DCCBs have a beneficial effect on central systolic blood pressure and arterial stiffness (Matsui et al., 2009). In the past, a concern was raised regarding the effects of DCCBs on kidney function; DCCBs may cause vasodilatation of afferent renal arterioles, increase intraglomerular pressure, and promote proteinuria (Nosadini and Tonolo, 2002). In the present study, because individual information on baseline blood pressure, proteinuria, and eGFR were unavailable, the effects of DCCB on optimal blood pressure control, proteinuria, and eGFR were unknown for both cohorts. However, through propensity matching by antihypertensive classes and comorbidities, we demonstrated that compared with nonuse, DCCB use reduced the risk of progression to advanced CKD and ESRD, consistent with previous findings (Bakris et al., 1996; JATOS, 2005). DCCBs could both improve blood pressure control and preserve renal perfusion through the vasodilatory effect of systemic arterioles and renal arterioles, thus resulting in improved renal outcomes—reduced advanced CKD and ESRD risks. Further, Orekhov et al. have found DCCB could decrease the incorporation of [3H] thymidine, lower the intracellular cholesterol level, and inhibit proliferative activity of cultured cells, which exhibited antiatherosclerotic and atherogenic ability of DCCB in their in vitro models (Orekhov et al., 1988). A multicenter study found that DCCBs slow the progression of plaque volume in patients with hypertension (Kojima et al., 2011). Another study revealed that DCCBs could help slow the progression of minimal atherosclerotic lesions of coronary arteries (Waters et al., 1990). Because atherosclerosis and atherogenic factors could lead to early renal injuries and promote CKD progression (Chade et al., 2005; Kalra et al., 2005), DCCBs, with their antiatherosclerotic properties, would help reduce the risk of progression from early CKD to advanced CKD and ESRD.
Notably, in the present study, the DCCB users aged ≥65 years had the lowest advanced CKD and ESRD risks, suggesting DCCB safety and effectiveness in elderly DM patients with CKD. Elderly patients, particularly those with DM and CKD, may glean more benefits from DCCB use because advanced age is associated with greater arterial stiffness (Scuteri et al., 2018; Galvão et al., 2020). Future studies investigating the renal benefits of DCCBs for elderly patients with DM are warranted. Our study also found a dose–response relationship with DCCB use: of all patients, patients with the longest duration of DCCB use (i.e., >1,100 days) had the lowest advanced CKD and ESRD risks, which further strengthens the evidence of the benefits of DCCBs on renal outcomes.
This study has several limitations. First, participant information on systolic and diastolic blood pressure, pulse pressure, glycated hemoglobin level, glucose level, body mass index, low density lipoprotein level, triglyceride level, family history of kidney disease (including immunoglobulin A nephropathy or autosomal dominant polycystic kidney disease), and exercise habits was unavailable in NHIRD. Second, information on serum creatinine, eGFR, urine albumin-to-creatinine ratio, and urine protein-to-creatinine ratio was also lacking in NHIRD. The NHI program has stated that patients whose eGFR is < 15 ml/min/1.73 m2 and whose hemoglobin levels is < 10 gm/dL may receive the official EPO prescription benefit package. Because anemia is common in DM patients with CKD, using EPO prescription as a proxy for advanced CKD was reasonable. Third, medication compliance and blood pressure and glucose control among the patients could not be ensured. Also, those individuals on DCCBs may be inherently different from those not on the prescription hence the observed difference rather than the drug effect as the observed difference would be potential confounding by indication. Fourth, the dosage of the antihypertensives, as well as T-type or L-type DCCBs, was not considered. Fifth, ACEIs or ARBs were not completely matched; thus, their effects might not have been eliminated completely. We have used time-dependent model in this study, thus potential immortal time bias in studies for evaluating the effect of drug would be eliminated. Sixth, residual confounding might exist, and we did not run negative control outcome analysis to assess residual confounding should be announced here. Finally, response to antihypertensive treatment involves gene polymorphisms (Stavroulakis et al., 2000; Schelleman et al., 2004). Therefore, the results of this nationwide, population-based cohort study may not be directly applicable to other racial or ethnic populations.
In conclusion, DM patients with CKD who used DCCBs had lower risk of progression to advanced CKD and ESRD compared with those who did not use DCCBs. This study demonstrated the individual renal benefits of DCCBs in DM patients with CKD. Additional randomized controlled trials to confirm these findings are required.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by The NHIRD encrypts personal information of the patients to protect privacy and provides researchers with anonymous identification numbers associated with relevant claims information, including sex, date of birth, medical services received, and prescriptions. Therefore, patient’s consent is not required to access the NHIRD. This study was approved to fulfill the condition for exemption by the Institutional Review Board (IRB) of China Medical University (CMUH104-REC2-115-AR4). The IRB also specifically waived the consent requirement. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
Conception/Design: S-YL and C-HK; Provision of study materials: C-HK; Collection and/or assembly of data: S-YL, C-LL, and C-HK; Data analysis and interpretation: All authors; Manuscript writing: All authors; Final approval of manuscript: All authors.
Funding
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial Center (MOHW110-TDU-B-212-124004), China Medical University Hospital (DMR-109-231, DMR-110-089, DMR-111-090, DMR-111-091, DMR-111-105); Ministry of Science and Technology (MOST 110-2321-B-039-003).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.786203/full#supplementary-material
Supplementary Figure S1 | Distribution of covariates before propensity-scorematching.
Abbreviations
aHR, adjusted hazard ratio; CI, confidence interval; ICD-9-CM, International Classification of Diseases, Ninth revision, Clinical Modification; IQRs, interquartile ranges; LHID, Longitudinal Health Insurance Database; NHIRD, National Health Insurance Research Database.
References
Appel, L. J., Wright, J. T., Greene, T., Agodoa, L. Y., Astor, B. C., Bakris, G. L., et al. (2010). Intensive Blood-Pressure Control in Hypertensive Chronic Kidney Disease. N. Engl. J. Med. 363, 918–929. doi:10.1056/NEJMoa0910975
Association, A. D. (2019). 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2019. Diabetes Care 42, S103–S123. doi:10.2337/dc19-S010
Bakris, G. L., Copley, J. B., Vicknair, N., Sadler, R., and Leurgans, S. (1996). Calcium Channel Blockers versus Other Antihypertensive Therapies on Progression of NIDDM Associated Nephropathy. Kidney Int. 50, 1641–1650. doi:10.1038/ki.1996.480
Bakris, G. L., Sarafidis, P. A., Weir, M. R., Dahlöf, B., Pitt, B., Jamerson, K., et al. (2010). Renal Outcomes with Different Fixed-Dose Combination Therapies in Patients with Hypertension at High Risk for Cardiovascular Events (ACCOMPLISH): a Prespecified Secondary Analysis of a Randomised Controlled Trial. Lancet 375, 1173–1181. doi:10.1016/S0140-6736(09)62100-0
Braunwald, E. (1982). Mechanism of Action of Calcium-Channel-Blocking Agents. N. Engl. J. Med. 307, 1618–1627. doi:10.1056/NEJM198212233072605
Cai, Q., Mukku, V. K., and Ahmad, M. (2013). Coronary Artery Disease in Patients with Chronic Kidney Disease: a Clinical Update. Curr. Cardiol. Rev. 9, 331–339. doi:10.2174/1573403x10666140214122234
Campo, C., Garcia-Vallejo, O., Barrios, V., Lahera, V., Manero, M., Esteban, E., et al. (1997). The Natriuretic Effect of Nifedipine Gastrointestinal Therapeutic System Remains Despite the Presence of Mild-To-Moderate Renal Failure. J. Hypertens. 15, 1803–1808. doi:10.1097/00004872-199715120-00093
Carmines, P. K., and Navar, L. G. (1989). Disparate Effects of Ca Channel Blockade on Afferent and Efferent Arteriolar Responses to ANG II. Am. J. Physiol. 256, F1015–F1020. doi:10.1152/ajprenal.1989.256.6.F1015
Chade, A. R., Lerman, A., and Lerman, L. O. (2005). Kidney in Early Atherosclerosis. Hypertension 45, 1042–1049. doi:10.1161/01.HYP.0000167121.14254.a0
Chan, J. C., Cockram, C. S., Nicholls, M. G., Cheung, C. K., and Swaminathan, R. (1992). Comparison of Enalapril and Nifedipine in Treating Non-insulin Dependent Diabetes Associated with Hypertension: One Year Analysis. BMJ 305, 981–985. doi:10.1136/bmj.305.6860.981
Chang, A. R., Lóser, M., Malhotra, R., and Appel, L. J. (2019). Blood Pressure Goals in Patients with CKD: A Review of Evidence and Guidelines. Clin. J. Am. Soc. Nephrol. 14, 161–169. doi:10.2215/cjn.07440618
Costanzo, P., Perrone-Filardi, P., Petretta, M., Marciano, C., Vassallo, E., Gargiulo, P., et al. (2009). Calcium Channel Blockers and Cardiovascular Outcomes: a Meta-Analysis of 175,634 Patients. J. Hypertens. 27, 1136–1151. doi:10.1097/HJH.0b013e3283281254
de Leeuw, P. W., Ruilope, L. M., Palmer, C. R., Brown, M. J., Castaigne, A., Mancia, G., et al. (2004). Clinical Significance of Renal Function in Hypertensive Patients at High Risk: Results from the INSIGHT Trial. Arch. Intern. Med. 164, 2459–2464. doi:10.1001/archinte.164.22.2459
Doi, M., Miyoshi, T., Hirohata, S., Kamikawa, S., Usui, S., Kaji, Y., et al. (2010). Combination Therapy of Calcium Channel Blocker and Angiotensin II Receptor Blocker Reduces Augmentation index in Hypertensive Patients. Am. J. Med. Sci. 339, 433–439. doi:10.1097/MAJ.0b013e3181d658c4
Eisenberg, M. J., Brox, A., and Bestawros, A. N. (2004). Calcium Channel Blockers: an Update. Am. J. Med. 116, 35–43. doi:10.1016/j.amjmed.2003.08.027
Estacio, R. O., Jeffers, B. W., Hiatt, W. R., Biggerstaff, S. L., Gifford, N., and Schrier, R. W. (1998). The Effect of Nisoldipine as Compared with Enalapril on Cardiovascular Outcomes in Patients with Non-insulin-dependent Diabetes and Hypertension. N. Engl. J. Med. 338, 645–652. doi:10.1056/NEJM199803053381003
Fernández, R., Puig, J. G., Rodríguez-Pérez, J. C., Garrido, J., and Redon, J. (2001). Effect of Two Antihypertensive Combinations on Metabolic Control in Type-2 Diabetic Hypertensive Patients with Albuminuria: a Randomised, Double-Blind Study. J. Hum. Hypertens. 15, 849–856. doi:10.1038/sj.jhh.1001279
Galvão, R. D. V., Pereira, C. S., Freitas, E. G. B., Lima, D. R. A. R. T., Santos, W. A. M., Souza, D. F., et al. (2020). Association between Diabetes Mellitus and central Arterial Stiffness in Elderly Patients with Systemic Arterial Hypertension. Clin. Exp. Hypertens. 42, 728–732. doi:10.1080/10641963.2020.1783547
Gurkoff, G. G., Shahlaie, K., Lyeth, B. G., and Berman, R. F. (2017). New Therapeutics For Traumatic Brain Injury 179-197. Elsevier.
Haller, H. (2008). Effective Management of Hypertension with Dihydropyridine Calcium Channel Blocker-Based Combination Therapy in Patients at High Cardiovascular Risk. Int. J. Clin. Pract. 62, 781–790. doi:10.1111/j.1742-1241.2008.01713.x
Hasebe, N., and Kikuchi, K. (2005). Controlled-release Nifedipine and Candesartan Low-Dose Combination Therapy in Patients with Essential Hypertension: the NICE Combi (Nifedipine and Candesartan Combination) Study. J. Hypertens. 23, 445–453. doi:10.1097/00004872-200502000-00028
Heerspink, H. J., Ninomiya, T., Perkovic, V., Woodward, M., Zoungas, S., Cass, A., et al. (2010). Effects of a Fixed Combination of Perindopril and Indapamide in Patients with Type 2 Diabetes and Chronic Kidney Disease. Eur. Heart J. 31, 2888–2896. doi:10.1093/eurheartj/ehq139
Hussain, S., Singh, A., Rahman, S. O., Habib, A., and Najmi, A. K. (2018). Calcium Channel Blocker Use Reduces Incident Dementia Risk in Elderly Hypertensive Patients: A Meta-Analysis of Prospective Studies. Neurosci. Lett. 671, 120–127. doi:10.1016/j.neulet.2018.02.027
Ishimitsu, T., Ohno, E., Nakano, N., Furukata, S., Akashiba, A., Minami, J., et al. (2011). Combination of Angiotensin II Receptor Antagonist with Calcium Channel Blocker or Diuretic as Antihypertensive Therapy for Patients with Chronic Kidney Disease. Clin. Exp. Hypertens. 33, 366–372. doi:10.3109/10641963.2010.503299
JATOS, . (2005) The Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients (JATOS): Protocol, Patient Characteristics, and Blood Pressure during the First 12 Months. Hypertens. Res. 28, 513, doi:10.1291/hypres.28.513
Kalra, P. A., Guo, H., Kausz, A. T., Gilbertson, D. T., Liu, J., Chen, S. C., et al. (2005). Atherosclerotic Renovascular Disease in United States Patients Aged 67 Years or Older: Risk Factors, Revascularization, and Prognosis. Kidney Int. 68, 293–301. doi:10.1111/j.1523-1755.2005.00406.x
Kaneshiro, Y., Ichihara, A., Sakoda, M., Kurauchi-Mito, A., Kinouchi, K., and Itoh, H. (2009). Add-on Benefits of Amlodipine and Thiazide in Nondiabetic Chronic Kidney Disease Stage 1/2 Patients Treated with Valsartan. Kidney Blood Press. Res. 32, 51–58. doi:10.1159/000205521
Kaplan, R. C., Heckbert, S. R., Koepsell, T. D., Rosendaal, F. R., and Psaty, B. M. (2000). Use of Calcium Channel Blockers and Risk of Hospitalized Gastrointestinal Tract Bleeding. Arch. Intern. Med. 160, 1849–1855. doi:10.1001/archinte.160.12.1849
Kimura, K., Tojo, A., Hirata, Y., Hayakawa, H., Goto, A., and Omata, M. (1994). Effects of a Calcium Antagonist and an Angiotensin II Receptor Antagonist on Rat Renal Arterioles. Blood Press. Suppl. 5, 71
Kohlmann, O., Roca-Cusachs, A., Laurent, S., Schmieder, R. E., Wenzel, R. R., and Fogari, R. (2009). Fixed-dose Manidipine/delapril versus Losartan/hydrochlorothiazide in Hypertensive Patients with Type 2 Diabetes and Microalbuminuria. Adv. Ther. 26, 313–324. doi:10.1007/s12325-009-0015-8
Kojima, T., Miyauchi, K., Yokoyama, T., Yokoyama, K., Kurata, T., Suwa, S., et al. (2011). Azelnidipine and Amlodipine Anti-coronary Atherosclerosis Trial in Hypertensive Patients Undergoing Coronary Intervention by Serial Volumetric Intravascular Ultrasound Analysis in Juntendo University (ALPS-J). Circ. J. 75, 1071–1079. doi:10.1253/circj.cj-11-0141
Ku, E., Gassman, J., Appel, L. J., Smogorzewski, M., Sarnak, M. J., Glidden, D. V., et al. (2017). BP Control and Long-Term Risk of ESRD and Mortality. J. Am. Soc. Nephrol. 28, 671–677. doi:10.1681/ASN.2016030326
Lang, Y., Gong, D., and Fan, Y. (2015). Calcium Channel Blocker Use and Risk of Parkinson's Disease: a Meta-Analysis. Pharmacoepidemiol. Drug Saf. 24, 559–566. doi:10.1002/pds.3781
Lee, I. T., Hung, Y. J., Chen, J. F., Wang, C. Y., Lee, W. J., and Sheu, W. H. (2012). Comparison of the Efficacy and Safety Profiles of Two Fixed-Dose Combinations of Antihypertensive Agents, Amlodipine/benazepril versus Valsartan/hydrochlorothiazide, in Patients with Type 2 Diabetes Mellitus and Hypertension: a 16-week, Multicenter, Randomized, Double-Blind, Noninferiority Study. Clin. Ther. 34, 1735–1750. doi:10.1016/j.clinthera.2012.06.014
Lewis, E. J., Hunsicker, L. G., Clarke, W. R., Berl, T., Pohl, M. A., Lewis, J. B., et al. (2001). Renoprotective Effect of the Angiotensin-Receptor Antagonist Irbesartan in Patients with Nephropathy Due to Type 2 Diabetes. N. Engl. J. Med. 345, 851–860. doi:10.1056/NEJMoa011303
Matsui, Y., Eguchi, K., O'Rourke, M. F., Ishikawa, J., Miyashita, H., Shimada, K., et al. (2009). Differential Effects between a Calcium Channel Blocker and a Diuretic when Used in Combination with Angiotensin II Receptor Blocker on Central Aortic Pressure in Hypertensive Patients. Hypertension 54, 716. doi:10.1161/HYPERTENSIONAHA.109.131466
Nissen, S. E., Tuzcu, E. M., Libby, P., Thompson, P. D., Ghali, M., Garza, D., et al. (2004). Effect of Antihypertensive Agents on Cardiovascular Events in Patients with Coronary Disease and normal Blood Pressure: the CAMELOT Study: a Randomized Controlled Trial. JAMA 292, 2217–2225. doi:10.1001/jama.292.18.2217
Nosadini, R., and Tonolo, G. (2002). Cardiovascular and Renal protection in Type 2 Diabetes Mellitus: the Role of Calcium Channel Blockers. J. Am. Soc. Nephrol. 13 Suppl 3 (Suppl. 3), S216–S223. doi:10.1097/01.asn.0000034687.62568.9b
Ogihara, T., Saruta, T., Rakugi, H., Saito, I., Shimamoto, K., Matsuoka, H., et al. (2014). Combinations of Olmesartan and a Calcium Channel Blocker or a Diuretic in Elderly Hypertensive Patients: a Randomized, Controlled Trial. J. Hypertens. 32, 2054–2063. doi:10.1097/HJH.0000000000000281
Orekhov, A. N., Baldenkov, G. N., Tertov, V. V., Ryong, L. H., Kozlov, S. G., Lyakishev, A. A., et al. (1988). Cardiovascular Drugs and Atherosclerosis: Effects of Calcium Antagonists, Beta-Blockers, and Nitrates on Atherosclerotic Characteristics of Human Aortic Cells. J. Cardiovasc. Pharmacol. 12 Suppl 6 (Suppl. 6), S66–S68. doi:10.1097/00005344-198812006-00017
Oshikawa, J., Toya, Y., Morita, S., Taguri, M., Hanaoka, K., Hasegawa, T., et al. (2014). Angiotensin Receptor Blocker (ARB)-diuretic versus ARB-Calcium Channel Blocker Combination Therapy for Hypertension Uncontrolled by ARB Monotherapy. Clin. Exp. Hypertens. 36, 244–250. doi:10.3109/10641963.2013.810227
Palmer, B. F. (2002). Renal Dysfunction Complicating the Treatment of Hypertension. N. Engl. J. Med. 347, 1256–1261. doi:10.1056/NEJMra020676
Parsons, L. (2012). “Performing a 1: N Case-Control Match on Propensity Score,” in Proceedings of the 29th Annual SAS Users Group International Conference (Montreal, Canada: SAS Institute).
Rahman, M., Ford, C. E., Cutler, J. A., Davis, B. R., Piller, L. B., Whelton, P. K., et al. (2012). Long-term Renal and Cardiovascular Outcomes in Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Participants by Baseline Estimated GFR. Clin. J. Am. Soc. Nephrol. 7, 989–1002. doi:10.2215/cjn.07800811
Ravera, M., Re, M., Deferrari, L., Vettoretti, S., and Deferrari, G. (2006). Importance of Blood Pressure Control in Chronic Kidney Disease. J. Am. Soc. Nephrol. 17, S98–S103. doi:10.1681/ASN.2005121319
Ruggenenti, P., Perna, A., Loriga, G., Ganeva, M., Ene-Iordache, B., Turturro, M., et al. (2005). Blood-pressure Control for Renoprotection in Patients with Non-diabetic Chronic Renal Disease (REIN-2): Multicentre, Randomised Controlled Trial. Lancet 365, 939–946. doi:10.1016/s0140-6736(05)71082-5
Schelleman, H., Stricker, B. H., De Boer, A., Kroon, A. A., Verschuren, M. W., Van Duijn, C. M., et al. (2004). Drug-gene Interactions between Genetic Polymorphisms and Antihypertensive Therapy. Drugs 64, 1801–1816. doi:10.2165/00003495-200464160-00006
Scuteri, A., Rovella, V., Alunni Fegatelli, D., Tesauro, M., Gabriele, M., and Di Daniele, N. (2018). An Operational Definition of SHATS (Systemic Hemodynamic Atherosclerotic Syndrome): Role of Arterial Stiffness and Blood Pressure Variability in Elderly Hypertensive Subjects. Int. J. Cardiol. 263, 132–137. doi:10.1016/j.ijcard.2018.03.117
Staessen, J. A., Fagard, R., Thijs, L., Celis, H., Arabidze, G. G., Birkenhäger, W. H., et al. (1997). Randomised Double-Blind Comparison of Placebo and Active Treatment for Older Patients with Isolated Systolic Hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet 350, 757–764. doi:10.1016/s0140-6736(97)05381-6
Stavroulakis, G. A., Makris, T. K., Krespi, P. G., Hatzizacharias, A. N., Gialeraki, A. E., Anastasiadis, G., et al. (2000). Predicting Response to Chronic Antihypertensive Treatment with Fosinopril: the Role of Angiotensin-Converting Enzyme Gene Polymorphism. Cardiovasc. Drugs Ther. 14, 427–432. doi:10.1023/a:1007820401377
Velussi, M., Brocco, E., Frigato, F., Zolli, M., Muollo, B., Maioli, M., et al. (1996). Effects of Cilazapril and Amlodipine on Kidney Function in Hypertensive NIDDM Patients. Diabetes 45, 216–222. doi:10.2337/diab.45.2.216
Waters, D., Lespérance, J., Francetich, M., Causey, D., Théroux, P., Chiang, Y. K., et al. (1990). A Controlled Clinical Trial to Assess the Effect of a Calcium Channel Blocker on the Progression of Coronary Atherosclerosis. Circulation 82, 1940–1953. doi:10.1161/01.CIR.82.6.1940
Keywords: NHIRD: national health insurance, diabetes mellitus (DM), chronic kidney disease (CKD), dihydropyridine calcium channel blockers (DCCBs), end-stage renal disease (ESRD)
Citation: Lin S-Y, Lin C-L, Lin C-C, Hsu W-H, Hsu C-Y and Kao C-H (2022) Chronic Kidney Disease Progression Risk in Patients With Diabetes Mellitus Using Dihydropyridine Calcium Channel Blockers: A Nationwide, Population-Based, Propensity Score Matching Cohort Study. Front. Pharmacol. 13:786203. doi: 10.3389/fphar.2022.786203
Received: 29 September 2021; Accepted: 22 February 2022;
Published: 09 March 2022.
Edited by:
Carlos Alves, University of Coimbra, PortugalReviewed by:
Fu-Shun Yen, Dr. Yen’s Clinic, TaiwanDiogo Mendes, AIBILI—Association for Innovation and Biomedical Research on Light and Image, Portugal
Copyright © 2022 Lin, Lin, Lin, Hsu, Hsu and Kao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chia-Hung Kao, ZDEwMDQwQG1haWwuY211aC5vcmcudHc=
 Shih-Yi Lin
Shih-Yi Lin Cheng-Li Lin
Cheng-Li Lin Cheng-Chieh Lin
Cheng-Chieh Lin Wu-Huei Hsu
Wu-Huei Hsu Chung-Y. Hsu
Chung-Y. Hsu Chia-Hung Kao
Chia-Hung Kao