Abstract
Cancer is a multifactorial, multi-stage disease, including complex cascades of signaling pathways—the cell growth governed by dysregulated and abrupt cell division. Due to the complexity and multi-regulatory cancer progression, cancer is still a challenging disease to treat and survive. The screening of extracts and fractions from plants and marine species might lead to the discovery of more effective compounds for cancer therapeutics. The isolated compounds and reformed analogs were known as future prospective contenders for anti-cancer chemotherapy. For example, Taxol, a potent mitotic inhibitor discovered from Taxus brevifolia, suppresses cell growth and arrest, induces apoptosis, and inhibits proliferation. Similarly, marine sponges show remarkable tumor chemo preventive and chemotherapeutic potential. However, there is limited research to date. Several plants and marine-derived anti-cancer compounds having the property to induce apoptosis have been approved for clinical trials. The anti-cancer activity kills the cell and slows the growth of cancer cells. Among cell death mechanisms, apoptosis induction is a more profound mechanism of cell death triggered by naturally isolated anti-cancer agents. Evading apoptosis is the major hurdle in killing cancer cells, a mechanism mainly regulated as intrinsic and extrinsic. However, it is possible to modify the apoptosis-resistant phenotype of the cell by altering many of these mechanisms. Various extracts and fractions successfully induce apoptosis, cell-cycle modulation, apoptosis, and anti-proliferative activity. Therefore, there is a pressing need to develop new anti-cancer drugs of natural origins to reduce the effects on normal cells. Here, we’ve emphasized the most critical elements: i) A better understanding of cancer progression and development and its origins, ii) Molecular strategies to inhibit the cell proliferation/Carcino-genesis, iii) Critical regulators of cancer cell proliferation and development, iv) Signaling Pathways in Apoptosis: Potential Targets for targeted therapeutics, v) Why Apoptosis induction is mandatory for effective chemotherapy, vi) Plants extracts/fractions as potential apoptotic inducers, vii) Marine extracts as Apoptotic inducers, viii) Marine isolated Targeted compounds as Apoptotic inducers (FDA Approved/treatment Phase). This study provides a potential therapeutic option for cancer, although more clinical studies are needed to verify its efficacy in cancer chemotherapy.
1 Cancer initiation and development: understanding cancer progression
Cancer is the uncontrolled growth of specific cells possessed mutated genes or dys-regulatory cell division and growth. The word cancer means carcinoma, derived from a Greek word—cancer well-defined by the unregulated and uncontrolled multiplication of the cell. The specific alteration in genotype classified the normal cells as cancer cells responsible for uncontrol growth, re-location, and cancer metastasis (Sudhakar, 2009; Nithya et al., 2014; Fouad and Aanei, 2017). The cancer cell has a tremendous ability to divide and re-divide and metastatic abilities. The unchecked and over multiplication of cells leads to cancer tissue development and tends to move to various locations, resulting in metastasis development. In cancer, the different proteins involved in cell-cell attachment and regulation play a crucial role in regulating cancer cell movement. The matrix metalloproteases regulate the initiation and development of metastasis and enhancement of tumor spreading. The dysregulation in the expression of cell-cell attachment receptors fails cancer cell attachment. It encourages the ability to circulate in the bloodstream and re-locate to other locations inside the body—the abnormality in cell division, growth, and development results in cancer formation. The process of cancer development mainly comprises two fundamental alterations; i) genetical alternation and ii) Cellular alteration. The dys-regulatory genetic or cellular mechanism detected in cancer development. Genetically, the essential genes involved in the onset of cancer mainly comprised oncogenes and tumor suppression genes. The activation and deactivation of various “tumor suppressor genes” regulate the activation of cancer initiation and development. Furthermore, the variation in established genetic mechanisms involves genetic mutation, chromosomal abrasion or alteration, i.e., addition or deletion, and changes in cell cycling. The cellular alteration involves the dysregulated signaling pathways involved in the regulatory mechanism of dividing cells (Fearon and Vogelstein, 1990; Vogelstein and Kinzler, 2004). However, the hallmarks of cancer cells include: Selective proliferative advantage. i) Vascularization, immune modulation. ii) Metabolic rewiring. iii) Abetting microenvironment. iv) Tissue invasion/metastasis. v) Altered stress response. The cancer development process or carcinogenesis is mainly composed of diversified steps. Due to its rapidly dividing ability, the cell forms several cells or tumors and translocates to another site, leading to metastasis. Apart from general genetic and cellular alterations, epigenetic variations include; i) hypomethylation of oncogenes, ii) hypermethylation of tumor suppressor genes, iii) depletion of hydroxymethylation, iv) changes of histone acetylation, and v) methylation patterns and vi) miRNA expression level variations, are known to be associated with many cancers. Apoptosis is the primary mechanism that plays an essential role in balancing cells growth and cell division to prevent uncontrol cancer (Hassan et al., 2014). Carcinogenesis occurs due to damage, insult, or induction of alteration via various modes, including; physical, chemical, genetic, and biological. The cancer development and establishment process comprises cancer initiation, promotion, and progression (Figure 1). Various carcinogens act as initiators of cancer, which results in cancer formation along with promotor molecules. As in cancer initiation, most carcinogens cause irreversible damage to DNA. This damage causes the mutation in a particular genetic code that could be transferred to daughter cells and rapidly dividing cells with a mutated pattern. The cancer initiation trigger point could be an internal route or external via direct interaction with receptors on the cell surface or via various unspecified means. Thus, cancer initiation is based on irreversible genetic changes via the different genetic modes, including; i) simple mutations, small deletions, transitions, and transversions in DNA molecules. In the second stage of cancer development, there are no changes in the structure of DNA. The promotion of cancer is a reversible stage of advancement but rather in the expression of the genome mediated through promoter-receptor interactions. Interestingly, cancer progression depends on the protein involved in the migration of cancer. The extracellular environment (ECM) of cells is a pool of substances that could act like inducers that target the particular site and activate the cancer development or progression mechanisms. The last stage is characterized by cancer progression and migration, and karyotypic instability is considered an irreversible stage. However, the previous stage provides in-depth knowledge of molecular alteration, genetic changes in various tumor suppression genes, oncogenes, protooncogenes, and chromosomal aberrations (Faya et al., 2014). A better understanding of cancer etiology, initiation, and development of cancer has thoughtful consequences for targeting cancer biomarkers in developing therapeutics and considering agents/chemicals as carcinogens. In the cell death context, the fundamental process controlling the number of cells in tissue or organ development is apoptosis; however, in most cancers, the activation of oncogenes and dys-regulatory tumor suppressor genes occurs. The absence of apoptosis found in primary cancers makes the rapidly dividing cells unable to undergo the fundamental process of cell death.
FIGURE 1
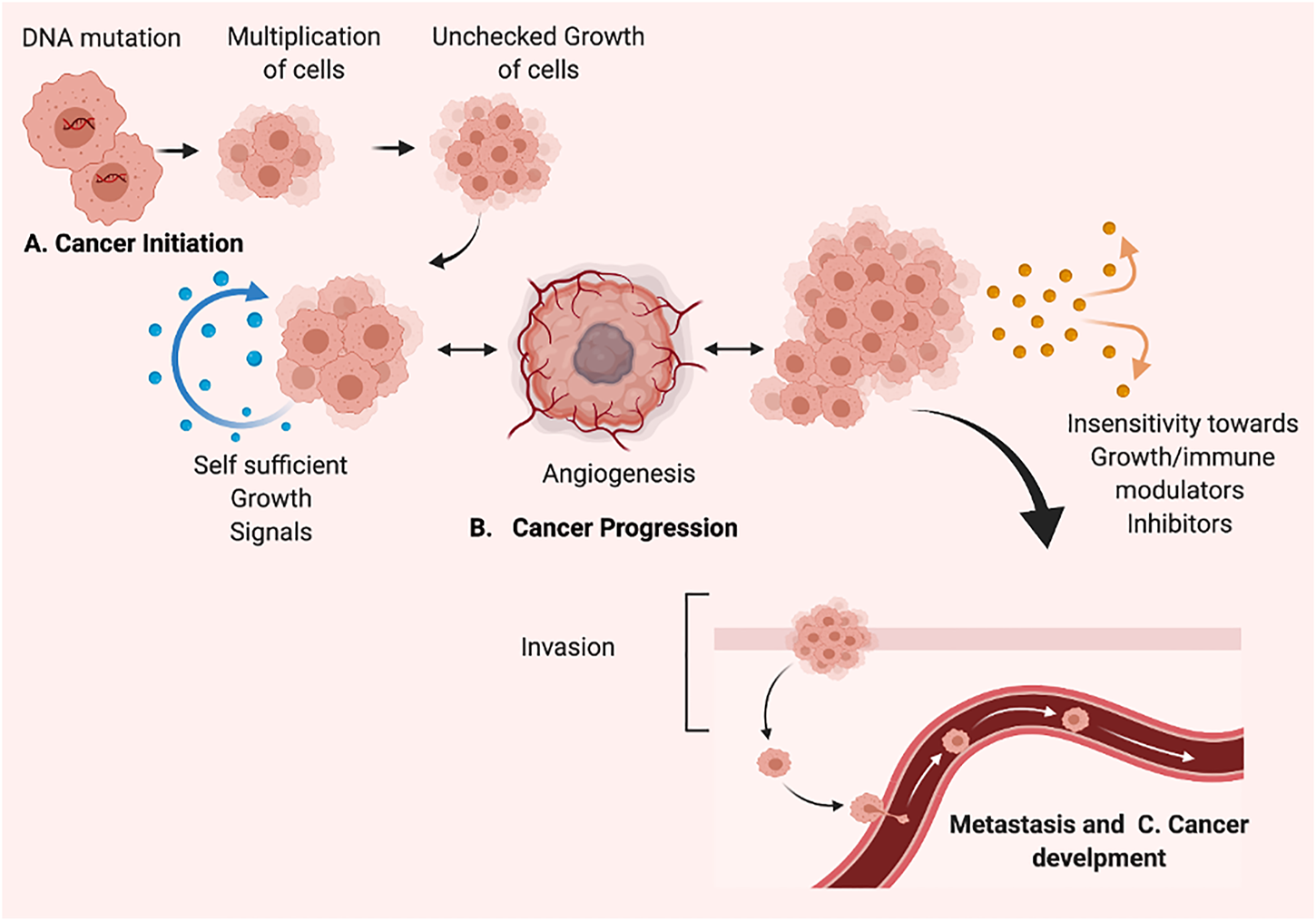
The process of cancer development/carcinogenesis, (A) Cancer initiation (B). Cancer Progression (C). Metastasis and Cancer development. Created with BioRender.com.
2 Molecular strategies to inhibit the cell proliferation/carcinogenesis
Various potential players play an active role in inhibiting cell proliferation. Here, we highlighted apoptosis regulating proteins/elements and other than apoptosis-inducing factors as a targeted strategy to regulate the process of carcinogenesis or cell proliferation. The apoptosis regulating strategy begins with the cell cycle—the regulators involved in cell division, cell arrest, and induction of apoptosis. The various genetic regulators of cell proliferation hold a remarkable role in cancer cell division and growth (as shown in Figure 2). The cell cycle plays a critical function in cellular genomic integrity and cell progression (Hanahan and Weinberg, 2000). It is important to know that the cell cycle comprises various stages such as the first one, G1 (gap 1), as well as the second, G2 (gap 2), and the last one, M (mitosis). For genetic information to be passed down across generations, DNA synthesis and genome replication must occur during the S phase. The M-phase is characterized by the separation of genetic information, the development of sister chromatids, and cell division. The G1 phase is the transition from M to S, while the G2 phase is the transition from S to M. These breaks (G1 and G2) are necessary to ensure that each phase is completed before moving on to the next. (Jacobson et al., 1997; Fulda et al., 2010). Cell cycle checkpoints are frequently activated in response to replication stress and DNA damage. Cycle-dependent kinase activation and inactivation are critical for cell growth and cell cycle regulation. (Hanahan and Weinberg, 2000; Fulda et al., 2010). In normal cell division, there is tight regulation at each checkpoint. Any dysregulation in those checkpoints results in the rapid division of cells without the hindering. The rapid division increases cell growth. Similarly, up-regulating the various pro-apoptosis proteins/elements and down-regulating the anti-apoptosis proteins might be a positive regulators of apoptosis. The signaling pathway is described in Section 3. The p53 role in induction apoptosis could be enhanced by up-regulating the transcription of mRNA and translated product. The use of inhibitors to suppress the activation of inhibitors of apoptosis (IAPs) also aids in the successful establishment of apoptosis in cancer cells. The other strategy involves various regulator proteins involved in cell progression and growth, such as MMPs (matrix metalloproteinases), play a vital role in cancer progression. MMP inhibition or blockade is a critical target for reducing metastatic potential. In addition to MMPs, metastasis suppressor genes such as MKK4 (mitogen-activated protein kinase 4), BRMS1 (breast cancer metastasis suppressor 1), and NM23 (non-metastasis gene 23) play important roles in metastasis inhibition. (Kerr et al., 1972; Neuman et al., 2002). Conventional therapy options are exceedingly tough, particularly in patients with metastatic disease. Invasion, intravasation, and extravasation are part of the metastatic pathway. The spread of cancer cells to distant places via the circulatory system marks the invasion phase. On the other hand, extravasation necessitates cancer cells penetrating the endothelium and the basement membrane. Cancer cells can develop in a secondary focus in extravasation. (Neuman et al., 2002; Fulda et al., 2010). Additionally, regulation of uPA and uPAR expression and TIMP expression are critical for metastasis prevention. (Kanduc et al., 2002). A complex net of signaling molecule cascades largely transduced several “pro-survival signaling pathways” that determined the fate of a cancer cell. Several cytokines and growth factors trigger pro-survival signaling cascades such as IP3K-PKB/Akt and MAPK. Nuclear factor-B (NFB) is a protein that plays a crucial function in controlling inflammation and immunological responses (Hakem and Harrington, 2005). The importance of blocking these pro-survival signaling pathways in various cancers has been thoroughly researched. Angiogenesis, autophagy, reduction of ER expression, intracellular ROS decrease production, and activation of “mitochondrial membrane potential” are among the signaling pathways that cause cytotoxicity in cancer cells.
FIGURE 2
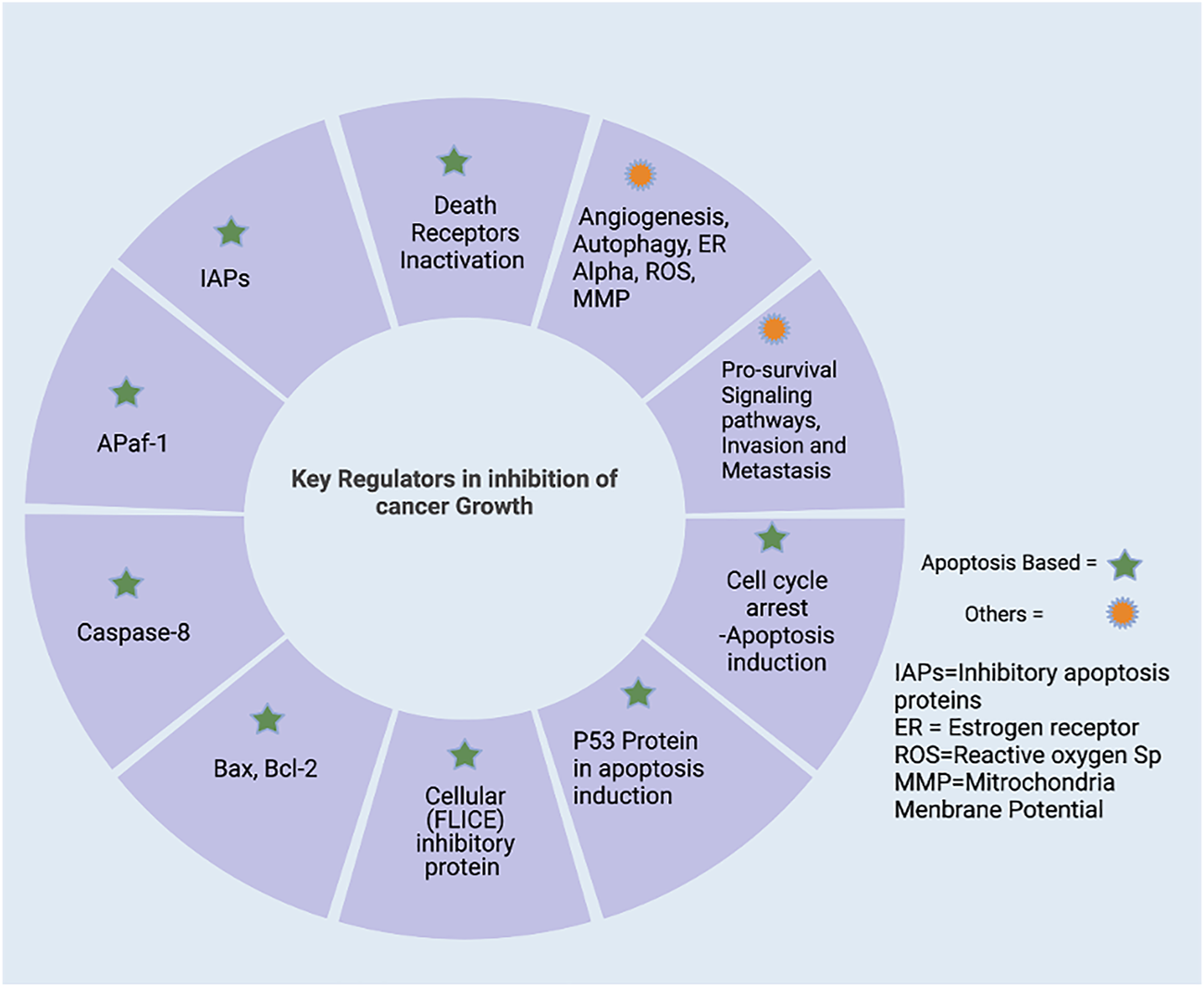
Targeting regulators in inhibition of cell progression. It is mainly divided into apoptosis regulators and others. Targeting Apoptosis includes i) up-regulation of pro-apoptotic (caspases protein, Bax, and pro-apoptotic member of Bcl-2 family) ii) down-regulation of anti-apoptotic proteins (IAPs). Also, by targeting the pro-survival signaling pathways, invasion and metastasis proteins dysregulated in cancer cells, created with BioRender.com.
3 Signaling pathways in apoptosis: Potential strategy for targeted therapeutics
Apoptosis is an essential physiological process of cell death that is meant to occur without the discharge of intracellular contents, and subsequent no activation of an inflammatory response as apoptosis is a crucial process in embryonic development, regulation of the immune system, and the response to DNA damage. However, dysregulation of apoptosis results in significant consequences in carcinogenesis. The imbalance between cell proliferation and cell death is considered the malignant tumor’s hallmark (Giorgi et al., 2008). Therefore, maintaining cellular homeostasis between proliferation and cell death is essential for normal physiological processes.
The signaling molecules of the apoptotic pathway played an essential role in the regulation of the apoptosis process. Therefore, these molecular proteins might be considered potential apoptotic biomarkers that can be targeted in advanced cancer treatment and therapeutics. Due to the development of potential biomarkers in cancer, targeted therapy research has emerged as an effective tool for cancer treatment compared to the conventional method. The standard chemotherapies act on rapidly dividing normal and cancerous cells, whereas targeted therapy plays an influential role as they act upon specific molecular targets related to specific cancer. The targeted apoptotic biomarkers in cancer are in clinical trials. However, the targeted therapies are designed so that they interact with their target, whereas many standard chemotherapies were identified because they kill cells. Furthermore, targeted therapies are often cytostatic as they block tumor cell proliferation, whereas standard chemotherapy agents are cytotoxic and kill tumor cells). Therefore, the apoptotic biomarkers and chemotherapy could combine targeted drug therapy to get the maximized results. Apoptosis has been extensively investigated over the last two decades. It is now a critical process of controlled death activated in response to cell injury and during normal “development and morphogenesis.” For instance, the apoptotic death of nearly half of newly produced peripheral neurotransmitters throughout formation forms the nervous system of vertebrates to regulate their quantity, so it matches the requirements of their target organs in the peripheral (Alberts et al., 2002). Induction of apoptosis is essential for complex organisms’ embryonic development and differentiation equilibrium. It occurs in a controlled manner, with characteristic morphologic markers such as “cell shrinkage, “chromatin condensation,” and “blabbing of the cytoplasmic membrane.” Apoptosis dysregulation has indeed been linked to several pathologies, notably tumor growth and the formation of cancerous cells’ chemoresistance (Gorski and Marra, 2002). Among mammals, apoptosis is triggered by two well-known pathways (Figure 3). Extrinsic signals, such as TNF “tumor necrosis factor,” Fas (CD95/APO1), and TRAIL (TNF-related apoptosis-inducing ligand receptors), might induce apoptosis. In contrast, internal stimuli, including mitochondrial transduction, also can trigger apoptosis. For example, the activation of cysteine aspartyl proteases (caspases) causes permeabilization of mitochondrial membranes, chromatin condensation, and DNA fragmentation, resulting in cell death (Zornig et al., 2001).
FIGURE 3
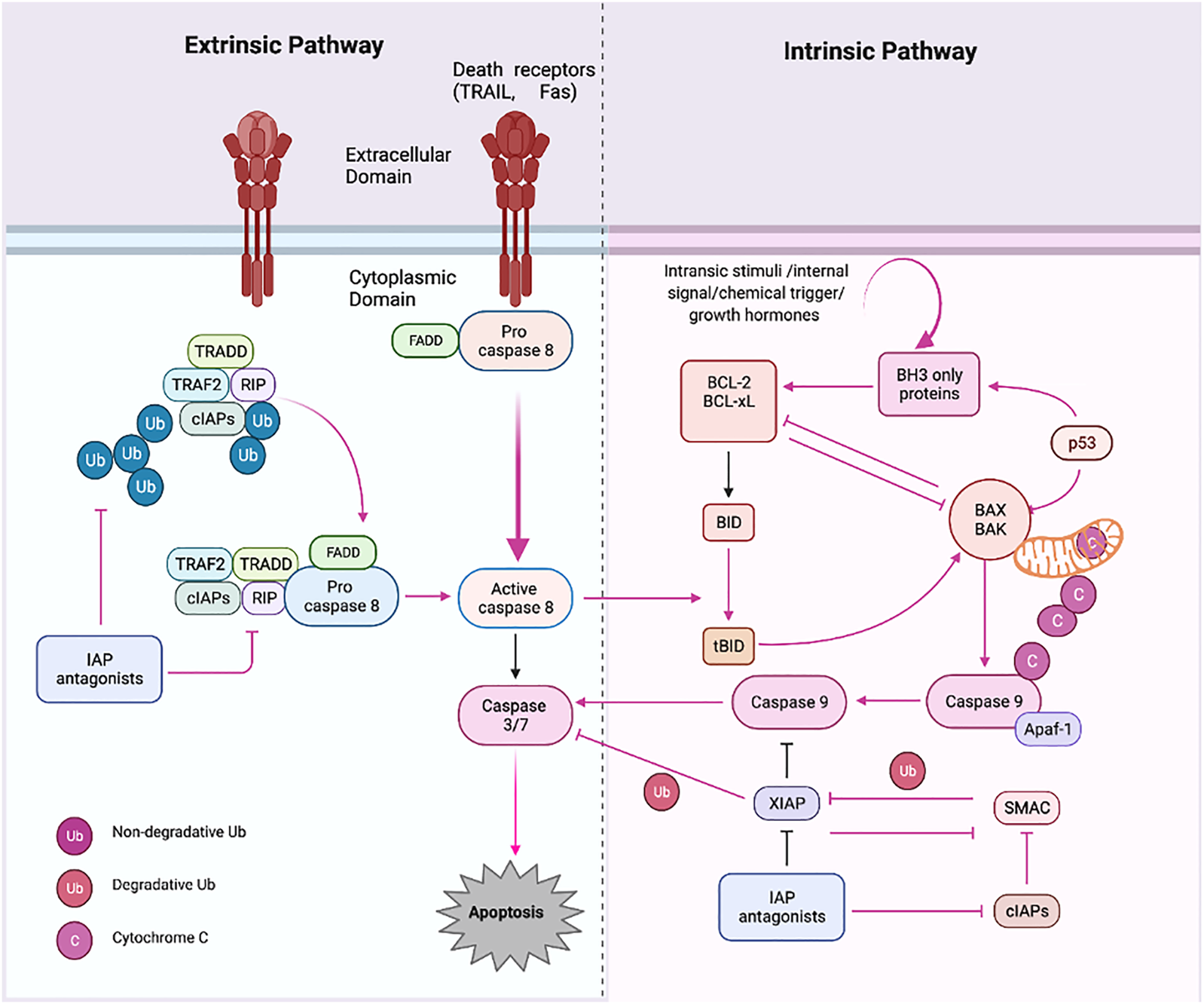
Apoptosis pathways. Apoptosis has two primary routes known as the i) extrinsic and ii) intrinsic pathway. First, external stimuli or ligand molecules activate the transduction, including death receptors (SDRs), leading to caspase 3/7 activation via/or activated caspase 8. Intrinsic pathway; via insertion of proapoptotic molecules BAX (protein) into the mitochondrial membrane results in the generation of cytochrome c, forming an apoptosome, which further triggers the apoptotic cascades beginning with proapoptotic activation caspase 9 and or then caspase 3. Created with BioRender.com.
The intrinsic route is triggered by physical and chemical inputs, including hypoxic conditions, growth factor shortages, cell detachment, and stress signals. The initiator caspases activation activates effector caspases; these are “aspartate-specific proteases” regulated post-translationally. Caspase inside as inactive proenzyme consists of a prodomain (a small and large subunit), which must be oligomerized and cleaved to activate. On the other hand, Caspases-independent apoptosis has been documented (LeBlanc, 2003; Bredesen et al., 2006). The death ligand binds the intracellular death domain of death receptors to begin the extrinsic pathway (Duckett et al., 1998; Venderova and Park, 2012). Many apoptotic signals are relayed to cell death machinery by p53, which interacts with other proteins like TNF, Fas, and TRAIL receptors, which are particular physiologic mediators of the extrinsic signaling route of apoptosis. Mitochondria have a role in several vital processes, including the caspase activators (cytochrome C), alterations in electron transport, mitochondrial membrane potential regulation, and the involvement of both pro-and antiapoptotic Bcl-2 family proteins (Bibel and Barde, 2000; Johnstone et al., 2002).
The proteins involved in the intrinsic route are “SMAC/DIABLO, Caspase-9, Bcl-2, Bcl-w, and Nox (Green, 2005). The intrinsic mechanism involves “Bax/Bak membrane insertion and cytochrome c release from the mitochondrial intermembrane gap into the cytosol (Anichini et al., 2006). The apoptosome is a multi-protein complex consisting of a “seven-spoke ring-shaped complex” that activates caspase 9 and then the caspase-3 signaling cascade, which leads to cell destruction and apoptosis (Hengartner, 2000). According to experimental findings, caspases have an overwhelming role in apoptosis (Ashkenazi and Dixit, 1998). There is a hopeful future for cancer therapy techniques that induce apoptosis in cancer cells by disrupting mitochondrial biogenesis, outflowing matrix calcium glutathione, or releasing membrane proteins due to mitochondrial failure. Anti-apoptotic proteins Bcl-2 and Bcl-xL block the cytochrome c release (Block et al., 1992). The development of a death-inducing signaling complex DISC which is made up of the adaptor molecule Fas-associated death domain (DD), procaspase-8, procaspase-10, and the cellular FLICE inhibitory proteins, is caused by the activation of SDRs by specific death ligands (DLs) (Heinrich et al., 1998). This Caspase 8 is activated in a real way that the “prodomain of caspase 8″ remains attached to the DISC. In contrast, the “active caspase 8 dissociates from the DISC” to initiate the cascade of caspase activation that occurs during the “execution phase of apoptosis (Kreuger et al., 2012). Traditionally, “caspases have been divided into i) initiator, ii) effector, and iii) executioner” types based on where they are located in an apoptotic signaling chain. At the same point, both extrinsic and intrinsic paths converge (execution phase). The execution phase is the last step in the apoptotic process (Kim, 2005; Elmore, 2007). Certain caspases have lengthy pro-domains that contain specific motif sequences, such as the death effector domain (DED) and caspase recruitment domains (CARD), which enable them to interact with other proteins and connect to signaling pathways. Caspases-1, caspase-2, caspase-4, caspase-5, caspase-9, caspase-11, and caspase-12 are involved in DED, whereas caspase-1, caspase-2, caspase-4, caspase-5, caspase-9, caspase-11, and caspase-12 are involved in CARD (Yuan and Akey, 2013). Caspases are critical apoptosis initiators and executors, and their function is strongly linked to their structure, which has distinct substrate preferences. The “Caspase-8 and 9 are initiator caspases, while Caspase-3, Caspase-6, Caspase-7, Caspase-10, CAD, and PARP” are effector or executioner caspases (Stergiou and Hengartner, 2004; Ghobrial et al., 2005). Caspase initiators are triggered by autocleavage, activating executioner caspases, which later proteolyze certain substrates, resulting in apoptosis. They have extended pro-domains that link big adaptor molecules, allowing them to multimerize and activate other caspases. However, effector caspases, on the other hand, have “short pro-domains” that execute apoptosis when activated by initiator caspases. As a result of the cytoplasmic endonuclease activated by executor caspases, chromatin condenses, cytoplasmic blebs develop, and apoptotic bodies are formed. Numerous target proteins are cleaved by caspases to regulate apoptotic cell death (Green, 2005). Caspases 3 and 4 are the essential executioner caspases, and an initiator caspase can activate them. Caspases-3 specifically activates endonuclease CAD, resulting in chromosomal DNA degradation and chromatin condensation inside nuclei. The cytoskeletal rearrangement and generation of cytoplasmic blebs and apoptotic bodies are both dependent on execution caspases (Medema et al., 1997). Execution caspases are activated first, followed by cytoplasmic endonuclease activation. Interestingly, nuclear material is degraded by cytoplasmic endonuclease, and proteases then degrade nuclear and cytoskeletal proteins.
4 Why apoptosis induction is mandatory for effective chemotherapy
An anti-cancer treatment mainly kills cancer cells and may or may not cause side effects to healthy cells. Currently accepted cancer therapy methods include a combination of medications, surgery, radiation, or a combination of all of these. Chemotherapeutic drugs can relieve symptoms, extend life, and even cure cancer in some cases. One of the best cancer drugs has a low risk of harming healthy cells in treating patients. Apoptosis substantially impacts both the lifespan of healthy cells and the prognosis of malignant cells. As a result, regulating default apoptosis could benefit cancer treatment and prevention. The aberrant response of malignant cells to apoptosis induction is caused by excessive cellular proliferation, which can also be described as overexpression of inhibitors or IAP family members. The Cell cycle-regulating genes are inactivated in cancer cells, and Bcl-2) adjusts their expression in tumors. Different malignancies, inflammatory illnesses, viral infections, and autoimmune diseases are caused by inhibiting or suppressing apoptosis.
Interestingly, apoptotic induction has emerged as a novel target for novel mechanism-based drug discovery (Ghavami et al., 2009; Sankari et al., 2012). Previously, it was thought that an increase in cellular proliferation was only linked to the rise in the number of cells accumulating in the body; however, it has now been discovered that a decrease in cell death is also responsible for cell proliferation. As a result, it is crucial to screen apoptotic inducers from natural products, either as crude extracts (plants or animal/marine organisms) or as separated components. Various studies have found evidence that compounds derived from natural sources effectively treat and prevent cancer. The isolated secondary metabolites from multiple natural sources, and mechanistic study (in-vitro), give better molecular fundamental knowledge of the future therapeutic agent. Various plants extracts, fractions, synthetic palladium, and other complexes are used to screen cytotoxicity and mode of cell death, which reduces the cost of isolation of phytochemicals and synthetic compounds and gives insight into a potential therapeutic agent. The ability to trigger apoptosis may serve as a unifying principle for many distinct types of chemo preventive drugs, each with a unique mode of action. Also, insight into the mechanisms of action of these chemicals could lead to new cancer-preventive and treatment strategies. Synthesis or modification of previously recognized medications in cancer treatment is still a significant research focus. Despite massive amounts of synthetic effort, the final products have improved slightly over a few prototypes. Thus, there is a constant demand for new chemotherapeutic agent prototypes–templates to develop more effective chemotherapy. Natural products (NPs) serve as models for new prototypes in the search for drug discovery. Apart from chemotherapy treatment, epidemiological studies suggest that eating lots of fruits and vegetables, rich in phytochemicals and other nutrients, may lower one’s risk of getting cancer (Jan and Chaudhry, 2019). In addition, it has been shown that natural product-derived tumor inhibitory chemicals have revealed a remarkable array of novel structural forms.
5 Plants extracts/fractions as potential regulator of pro-apoptotic and anti-apoptotic agents
Apoptosis is a cell death process that is conserved and well-controlled. It is essential to complex organisms’ development and homeostasis. Apoptosis inducers work very well in causing cytotoxicity in cancer cells. Due to their selective targeting of cancer cells, dys-regulatory apoptosis machinery only targets normal cells compared to cytotoxic agents. Therefore, they can work along with potential chemotherapy and reduce the risk of re-occurrence via killing the cell in a natural suicidal way. One of the essential factors in combination medication therapy is awareness of diverse phytochemicals and their synergistic approach. By indicating the presence of a potential agent, the extracts/fractions screening lowers the cost of phytochemical separation. Apoptosis’s external and intrinsic triggers include; radiation, growth factor withdrawal, ligands, tumor suppressor protein (p53), and oxidative stress (Hengartner, 2000; Hu et al., 2013). Additional pathological activities associated with the physiologic apoptosis pathway include cancer, inflammatory, and neurological diseases (Cairrao and Domingos, 2010). As shown in a substantial body of research, many cancerous tumors have a decreased capacity to die by apoptosis in reaction to physiological stimuli (Los et al., 1995). Therefore, it is critical to broadening the chemical repertory of agents that might induce or sensitize resistant cells to apoptosis to combat cancer. Furthermore, employing natural compounds as anti-carcinogenic or cytotoxicity-inducing compounds may be promising cancer prevention and therapy strategy. An in vitro investigation of secondary metabolites from various natural sources provides a better understanding of the molecular fundamentals of the therapeutic drug in the future (Israels and Israels, 2000).
5.1 Alkaloids based apoptosis induction
Alkaloids are chemical compounds found in nature that are predominantly made up of basic nitrogen atoms, neutral groups, and mildly acidic (Pucci et al., 2000; Khazaei et al., 2017a). Alkaloid Solamargine, located in the Chinese Plant Solanum incanum, has been demonstrated to trigger apoptosis in cancer cells (Hep-3B) and standard (“skin fibroblast cells”). Also, the level of TNF receptor I significantly elevated after solamargine treatment (Martin et al., 2000). Necrophilia guyanensis and Genipa Americana extract fractionation contain alkaloid cryptolepine, which appeared as the predominant active component. The cryptolepine compounds demonstrated substantial cytotoxic effects in human cells via the underlying mechanisms of apoptosis (Decock et al., 2011). Camptothecin (CPT), an inhibitor of topoisomerase I, causes triggered Oxygen radicals which cause DNA fragmentation and eventually induction and completion of apoptosis. (Cox et al., 1999).
5.2 Terpenes based apoptosis induction
The monoterpenes D-limonene and perillyl alcohol were found to have anti-carcinogenic properties in various cancer cell lines by exhibiting apoptosis. For example, perillyl alcohol was very effective in causing cell death of breast cancer cells (Klener et al., 2006). Similarly, Limonene inhibits stomach carcinogenesis caused by sodium chloride by enhancing apoptosis (Reddy et al., 1997). Taxol is a cytotoxic diterpene discovered in Taxus brevifolia that suppresses cell growth and proliferation. The mechanism of action is triggered via stabilizing power of Taxol during the spindle fiber formation in the cell division stage (mitosis). The polymerization of the Microtubule and improved stimulation of the formation of microtubule (MT) bundle impeded entry into the S phase, limiting cell division and apoptosis and also triggering necrosis at higher concentrations of Taxol (Fisher David, 1994). However, Paclitaxel-induced cell death also occurs via a signaling pathway not dependent on G2/M arrest (Pistritto et al., 2016).
5.3 Polyphenols based apoptotic inducers
Polyphenols obtained from Plant are abundant in the human diet and are deemed healthy at the recommended dose (one G/day) (Ren and Tang, 1999; Chaudhry et al., 2021a). A range of mutated cell lines (Schultze et al., 1991) show that gallic acid predominantly induces cell death. Similarly, in HL-60 cells, caffeine and tannic acids induce DNA breakage (Hajto et al., 1989). CAPE, the main ingredient in traditional medicinal propolis, has been shown to have particular cytotoxic activity against mutated cloned rat embryo fibroblast (CREF) cells by apoptotic cell death (Liu et al., 2000). CAPE, the main ingredient in traditional medicinal propolis, has been shown to have particular cytotoxic activity against mutated cloned rat embryo fibroblast (CREF) cells by apoptotic cell death (Liu et al., 2000). It has been established and demonstrated that curcumin, the primary color in turmeric, is a phenolic compound that causes apoptosis—a beneficial chemotherapeutic addition since it does not affect normal cells. The activation of apoptosis was boosted by curcumin’s ability to block cyclooxygenase (COX) products (Buhagiar et al., 1998; Bussing et al., 1999). Moreover, chilli pepper and hot red pepper contain capsaicin, triggering HL-60 cells to undergo apoptosis (Reed and Green, 2002).
5.4 Xanthones based apoptotic inducers
Xanthones are found in the naturally occurring substances in both plants and microbes. This new species from marine fungi contains anti-cancer xanthone compounds, which have been noticed recently. This is established that toxic compounds and anti-carcinogenic medication breakdown and elimination affect certain proteins. Xanthone compounds with anti-carcinogenic characteristics may be found in aquatic species and microalgae. In mangostana’s pericarp -mangostin, the xanthone induced apoptosis via up-regulation of caspase-3 (Thompson, 1995; Ashkenazi and Dixit, 1998).
5.5 Plants extracts regulate pro-apoptotic and anti-apoptosis proteins
The Table 1 shows the list of potential plant extracts as apoptotic inducers. Pepino, the extract was essential for the onset of DNA fragmentation and PARP cleavage (Ng and Bonavida, 2002). Similarly, Viscum album L. (Loranthaceae) extract in human lymphocytes causes cytotoxicity, more probably via apoptosis (Gorczyca et al., 1993; McNaught and Andrew, 1997). Salvia miltiorrhiza is often used to cure liver problems throughout Asia for centuries. Investigators found that sage salvia miltiorrhiza extracts showed significant cytotoxic activity and could have reduced the human hepatoma (HepG2) cell line (Manske Richard Helmuth, 1965). Apoptosis was shown to drive the cytotoxic activity of Conifer Tetraclinis articulate (essential oil) on several human cancers, including blood lymphocytes (Shu-Hui et al., 1996). Bussing et al. employed a seed extract of these and discovered that it produced oxygen radical mediators and induction of apoptosis. The “tumor necrosis factor” (alpha) and “interleukin-6" (IL-6) constitute pro-inflammatory factors, whereas “interleukin-5" (IL-5) and “interferon-gamma” (IFN-g) are T-cell associated cytokines (Shu-Wei, 1999). Genistein (a hydroxyisoflavone) was found to induce cell death in human promyelocytic (HL-60) leukemic cells according to flow cytometric analysis (Hiraoka et al., 1998). Vitex rotundifolia (leave) fraction remarkably induces apoptosis in MCF-7 & T47D via extrinsic and intrinsic pathways (Ariazi et al., 1999; Yano et al., 1999; Yeung et al., 1999). Squamous cell carcinoma was inhibited by an ethanolic extract of Azadirachta indica (neem) leaves in a hamster cancer model. The presence of extract increased the pro-apoptotic proteins (caspase-3 and -8, BIM, and Bcl-2), indicating that both internal and external mechanisms were significantly impacted. The Bax and Bcl-2 are up-regulated in breast cancer cell lines (MCF-7 and MDA-MB-231) by Phaseolus vulgaris (Fabaceae) extract treatment in cells, respectively (Bcl-2, Bcl-xL) (Weimin, 1999). In addition, a methanolic extract of Fragaria ananassa (Strawberry) increased Bax, Bid, and p73 expression in T-47D cells while decreasing Bcl-xL expression (King-Thom, 1997). According to apoptosis-inducing meisoindigo, the receptors on the cell surface and mitochondria are involved, according to the study. The colorectal cancer cell lines, methyl ferulate, a Tamarix aucheriana plant product, enhanced caspase-2, -3, -6, -7, -8, and -9 expression while decreasing the anti-apoptotic proteins Bcl2 and FLIP (Meyer et al., 2005). Inula racemosa (also known as pushkarmool) roots induced apoptosis and necrosis in human leukaemia cell line HL-60 when treated with an ethanolic extract of Inula racemosa roots. While cytochrome C (release) and Bax (translocation) dramatically reduced, this substance boosted the activity of Caspase-9, -3, and -8 (Makoto et al., 1994). The activation of intrinsic caspase-6, -8, and-9 by Oenocarpus bacaba aqueous extract promoted apoptosis in MCF-7 cells (Sakagami et al., 1994). Solanum lyratum (known as nightshades) chloroform extract is triggered by the extrinsic and intrinsic apoptotic mechanism in HSC-3, CAL-27 & SAS cancer cells. Among the cell lines studied, the extract decreased anti-apoptotic proteins (Bcl-2 and Bcl-xl) while elevating pro-apoptotic (Bax and Caspases) (Su et al., 1994). The cardiac glycoside (Ouabain) significantly decreased ROS and MMP levels while increasing Ca2+, activation of pro-apoptotic members, and reduced expression of Bcl-2 levels (Rao Chinthalapally et al., 1995). The cardiac glycoside (Ouabain) significantly decreased ROS and MMP levels while increasing Ca2+, activation of “pro-apoptotic” members, and reduced expression of Bcl-2 levels (Rao Chinthalapally et al., 1995). Cell cycle arrest and apoptosis were caused by “Rosamultic acid,” a triterpenoid, which cleaves PARP and activates caspase-3, -8, and -9 in SGC-7901 cells (Rao et al., 1995). A flavonoid produced from plants, Acacetin triggers an apoptotic cascade in stomach cancer cells (AGS cells) and kills them. “When ROS are produced, MMP collapses and “Bax and p53″ are increased “Bcl-2 is decreased”. Extrinsic activation of “caspase-8 and Bid proteins” is facilitated by an increase in the expressions of Fas and FasL (Wolvetang Ernst et al., 1996). Caspase-8 and Caspase-7 were activated in breast cancer (MCF-7) cells after exposure to extracts (methanol and & butanol) of “Oldenlandia diffusa” (Willd) Roxb. As a result, Bax expression was increased, and Bcl-2 levels were decreased (Matsumoto et al., 2003). Cell death was prevented in MCF-7 cells by Cucurbita ficifolia (chloroform) extract. There was an increase in the expression of FADD, BAK, BAX, and caspase-8, -9, and -3 (Nakagawa et al., 2007). Lycorine, an alkaloid molecule (from the Amaryllidaceae family), has been shown to enhance the “Bax/Bcl-2″ protein ratio and the activity of caspase-(8, -9, and -3) enzymes in cells compared to untreated cells (Chaudhry et al., 2019a). A mango peel ethanolic extract (Mangifera indica) has been shown to cause the death of HeLa cells. As a result of activating pro-apoptotic proteins (caspase-3, 7, 8 & 9) and the suppression of Bcl-2 (Chaudhry et al., 2019b). Celastrol, a pentacyclic triterpenoid, induces apoptosis via acting on receptor protein (death receptor) and internal pathways. Celastrol stimulated the signal transduction in mitochondria and increased Bax while decreasing Bcl-2 protein levels in the intrinsic route. To increase the expression of FAS and FASL, it induced FASL expression in the extrinsic pathway. Because celastrol activated caspases 9, 8, 3, and PARP, these enzymes began to break down proteins. Using a mouse model of cancer, Celastrol prevents the development of human glioblastoma xenografts by suppressing angiogenesis. It also induces apoptosis in osteosarcoma cells and increases the activities of pro-apoptotic caspase-3, -8, and 9 (Verdine, 1996; Chaudhry et al., 2020a). An ethanolic extract of Brucea javanica fruits was discovered to have apoptotic properties when tested on colon cancer (HT29) cells. The drug’s mode of action involves both receptor- and mitochondrial-mediated processes. The extract also increased TNF2 and DR6 in the extrinsic route. Caspase-8 and TRAIL-4 were also increased. Caspase-9 was also activated, which led to an increase in Bax, Bad, and cytochrome-c while decreasing Bcl-2 (Subapriya et al., 2005). Experiments on human cancer cell lines have shown that plant extracts can activate intrinsic and extrinsic pathways to cause cell death. They were also beneficial in vivo, killing cancer cells and delaying cancer progression. Because of this, even though these plant extracts have long been used in traditional cancer treatments, western science and medicine need proof of their anti-cancer properties using pure plant compounds isolated from plants. The intrinsic and external mechanisms in adult tumor cells can be affected by an N, N-dimethyl plant sphinganine (DMPS) derivative known as phytosphingosine (PS) (HL-60 cells). -Caspase-8 activation is a major element at the beginning of the intrinsic pathway, followed by the cytochrome C release and activation of pro-apoptotic proteins (caspase-9 and caspase-3, and inhibition of Bcl-2 (anti-apoptotic members of the family (Abadio Finco et al., 2016). Anthocyanins, a kind of flavonoid, are well-known for their medicinal qualities. Anthocyanin components produced from Vitis coignetiae destroy human leukaemia (U937) cells by apoptosis induction in these cells. Bax levels rise when Bid and caspases 3, 9, and 8 are activated, followed by a decrease in MMP, Bcl-2, and other MMPs, cIAP-1, and cIAP-2 levels well. Anthocyanins, which induce apoptosis, did not affect U937 cells with elevated Bcl-2 protein levels (Abaza et al., 2016). breast cancer (“MDA-MB-468″) cells treated with extracts (“Vatica diospyroides”) exhibit apoptosis as a result of Bax upregulation (Pal et al., 2010). An Anemone raddeana triterpenoid molecule, raddeanin A, is shown to activate apoptosis in stomach cancer cells. Raddeanin A upregulation pro-apoptotic protein (Bax) while decreasing the expressions of anti-apoptotic (BCL-xL) using molecular biology techniques. Additionally, in all three cell lines, this terpenoid increased the activity of caspase-3, -8, and -9, and the PARP enzyme (Orangi et al., 2016a). Non-small cell lung cancer cells” can be made to die by LA, which inhibits Bcl-2, FLIP, and XIAP while increasing “Bid” and caspase-3, -,8-9 (Chiu et al., 2015). LA is derived from the leaves of the Pinus koraiensis tree. The sBax and protein caspases up-regulated treated with Momordica cochinchinensis fruit extract, inducing apoptosis in MCF-7 (Chou et al., 2018). Using 34 HL-60 cells, ethyl acetate extract from Uncaria tomentoa (ethyl acetate (EA) extract) was found to activate the intrinsic pathway by compressing Matrix Metallo Protein and lowering anti-apoptotic (Bcl-XL protein), as well as by raising the membrane-bound Fas and activating caspase-8 (Sui et al., 2015). Protoberberine activated pro-apoptotic caspases, promoting cell death (apoptosis) in tongue cancer cells. Increased ratio (Bax/Bcl-2) and altered matrix protein were also observed (Pan et al., 2005). Two breast cancer cells were injected into athymic nude mice, and wogonin was found to have an anti-cancer effect in vivo. After 4 weeks of treatment, the xenograft burden was reduced by 88 percent (Chung et al., 2017a). Apoptosis in “breast cancer” (MCF-7) cells is caused by the methanolic fractions of Scrophularia oxystepala (Alshammari et al., 2020a). oleandrin, a carcinogenic glycoside, kills cancer cells in the U2OS and SaOS-2 lines by inducing cell apoptosis through the ROS Generation as well as the damage of Mitochondrial membrane potential, which discharges hydrolytic Enzymes into the cytosol and regulates the proteins (decreases the Bcl-2 level and increase Bax) along with caspases (caspases 9 and 8 and -3 (Liu et al., 2004).
TABLE 1
| Plant name | Extract/Fraction | Part used | Growth inhibition conc (μg/ml) | Target cell lines | Mechanism of cell death | References |
|---|---|---|---|---|---|---|
| Allium atroviolaceum | Methanolic Extract | Flower | 500 μg/ml (70% GI) | MCF-7, MDA-MB-231 | - Induces apoptosis | Ali et al. (2012) |
| - Modulating Cell Cycle Arrest | ||||||
| - Caspase-Dependent and p53-Independent Pathway | ||||||
| Phaseolus vulgaris (black turtle bean) | Extract | Seeds | 50 μg/ml | MCF-7 and MDA-MB231 | - Upregulation of Bax and downregulation of Bcl-2 and Bcl-xL | Mou et al. (2011) |
| - Activation of caspase -3/7 | ||||||
| Ganoderma lucidum | Ethanol extract | Chipped fruiting bodies | 234 μg/ml | MCF-7 | - Induces cell cycle arrest and apoptosis -Up‐regulation of p21/Waf1 and down‐regulation of cyclin D1 | Huang et al. (2008) |
| - Up‐regulation of pro‐apoptotic Bax protein | ||||||
| Echinophora Platyloba | Methanol Extract | Leaves | 25 μg/ml | MDA-MB-231 | - Induces Apoptosis and Cell Cycle Arrest at S-Phase | Bagheri et al. (2018) |
| - Up-regulation of bax and p27 | ||||||
| - Down-regulation of bcl-2 | ||||||
| Morinda Citrifolia | Ethyl-acetate extract | Fruit | 25 μg/ml | MCF-7, MDA-MB-231 | - Arrested the cell cycle in the G1/S phase in MCF-7 and G0/G1 phase in MDA-MB-231 cells | Kim et al. (2009) |
| - Downregulation of intracellular “ROS” and “mitochondrial membrane potential” | ||||||
| Fragaria ananassa Strawberry | Methanolic extract | Fruit | NA | T-47D | - Cleavage of MCL-1 | Lee et al. (2009) |
| - downregulation of BCL-xL | ||||||
| - Upregulation of expression of proapoptotic proteins such as BAX and BID | ||||||
| - Upregulation of p73 | ||||||
| - Activation of Caspase 3 and Caspase 9 | ||||||
| Vatica diospyroides | Acetone and methanolic extracts | Fruit | 1.60–17.45 μg/ml | MDA-MB-468 | - Induces “apoptosis” | Azzopardi et al. (2017a) |
| - “Up-regulation of Bax” | ||||||
| Averrhoa Bilimbi | Methanolic extract | Fruit, Leaves | NA | MCF-7 | - Anticancer Activity | Xue et al. (2013) |
| Carica papaya L | Aqueous Extract | Leaves | 1319.25 μg/ml | MCF-7 | - “Anti-proliferation” and “Apoptosis” Induction | Ahn et al. (2018) |
| Mimosa caesalpiniifolia | Ethanolic extract | Leaf | 5.0 μg/ml | MCF-7 | - Induces apoptosis | Somasagara et al. (2012) |
| - “DNA fragmentation” | ||||||
| Annona muricata | Aqueous extract | Leaves | NA | MCF-7, MDA-MB-231 | - Induces apoptosis | Cheng et al. (2007) |
| Acanthopanax sessiliflorus | Hexane fraction | Stem bark | 53.46 μg/ml 58.40 μg/ml | MDA-MB-231 and MCF-7 | - “Non-apoptotic cell death” via mitochondria associated with both ROS dependent and independent pathways | Ho et al. (2009) |
| Phaleria macrocarpa | Ethyl acetate fraction | Fruit | 18.10 μg/ml | MDA-MB-231 | - Induce G0/G1 and G2/M cell cycle arrest | Chung et al. (2008) |
| - Activation of caspase -8,9 and 3 | ||||||
| - Upregulation of Bax, Bid | ||||||
| - cytochrome c, p21, p27, p53 and SMAC | ||||||
| - Downregulation of Bcl-2, Bcl-w, XIAP and survivin | ||||||
| Stryphnodendron adstringens | Aqueous extract fraction | Leaves | 76.31 μg/ml 186.83 μg/ml | MCF-7, MDA-MB-435 | - Upregulation of Bax, caspase-9, active caspase-3, caspase-8, LC-3, and beclin-1 | Hajiaghaalipour et al. (2015) |
| - Downregulation of Bcl-2 | ||||||
| Avicennia Marina | Crude methanol extract and fraction | Leaves | 250 μg/ml | MDA-MB 231 | - DNA fragmentation. | Ma et al. (2016) |
| - Decreased mRNA expression level of Bcl-2 and increased p53 | ||||||
| Salvia chloroleuca | Hexane and methylene chloride fractions | Roots | 25.49–60.25 μg/ml | MCF-7 | - Induced a sub-G1 peak | Wang et al. (2008) |
| - DNA fragmentation | ||||||
| - ROS-mediated pathway | ||||||
| Scrophularia oxysepala | Methanolic subfractions | Aerial parts | 52.9–61.2 μg/ml | MCF-7 | - Activation of caspase-3 | Xu et al. (2019) |
| - Downregulation of Bcl-2 | ||||||
| Artocarpus altilis | Diethyl ether extract | Wood | 6.19 μg/ml | T-47D | - Induced apoptosis and sub-G1 phase formation | Ma et al. (2012) |
| Piper crocatum | Methanol extract | Leaves | 44.25 μg/ml | T-47D | - Inhibition of p44/p42 phosphorylation | Tang et al. (2013) |
| Pistacia atlanticasub kurdica | Methanol | Fruits skin | 1 mg/ml | T-47D | 1. Activation of caspase 3 | Zhao et al. (2017) |
| 2. Poly ADP ribose polymerase (PARP) cleavage | ||||||
| Vitex rotundifolia | Extract/fraction | leave | 6.30–63.09 μg/ml | MCF-7 | 1. Extrinsic and intrinsic pathway both activated | Khazaei et al. (2017b) |
| Vitex rotundifolia | fraction | leave | 10-79.43 μg/ml | T47D | 2. Extrinsic and intrinsic pathway both activated | Kumar et al. (2017) |
| Vitex negundo | Aqueous and Ethanolic extract | Leaves | 200–300 μg/ml | MCF-7 | 3. Induced apoptosis | Hu et al. (2002) |
| Jatropha curcas | Ethanol extract | Root bark | 36.55 μg/ml | MCF-7 | 4. Inducing anoikis | Birjandiana et al. (2018) |
| Vernonia amygdalina | Ethanol extract | Leaves | 46–56 μg/ml | MCF-7 and MDA-MB-231 | 5. Induced apoptosis | Sharma et al. (2016) |
| 6. G1/S phase cell cycle arrest | ||||||
| 7. Caspase-dependent | ||||||
| Strobilanthes crispa | Hexane extract | Stem | 42.5 μg/ml | MDA-MB-231 | 1. Induced apoptosis | Ranganatha et al. (2012) |
| Ixeris dentata | Methanol extract | - | 100–200 μg/ml | T-47D, MCF-7, SK-BR-3, and MDA-MB-231 | - Induced apoptosis | Azzopardi et al. (2017b) |
| - Via Akt-NF-κB signaling | ||||||
| Tinospora cordifolia | Chloroform fraction | Stems | 28.09–35.06 μg/ml | MCF-7 and MDA-MB-231 | 1. ROS mediated apoptosis | Nair et al. (2016) |
| Smilax china | Ethanol extract | Bark | NA | MDA-MB-231 | 2. Suppression of metastasis | Zuhrotun et al. (2017) |
| 3. “Modulation of uPA, uPAR and TIMP expression” | ||||||
| Bauhinia ungulata | Different fractions | Stem | 23.47 µg/mL | 4T1 | 4. Anti-tumor | Silva et al. (2014) |
| 5. Antimetastatic | ||||||
| 6. Decreasing the MMP-2 activity | ||||||
| 157Nicotiana glauca | Dichloromethane fraction | Stem | 17.98 μg/ml | MCF-7 | - Anti-Metastatic | Kim et al. (2018) |
| Euphorbia humifusa | Ethyl acetate fraction | Whole plant | 5 μg/ml | MDA-MB-231 | 7. Inhibition of NF-κB activity | Thamizhiniyan et al. (2015) |
| 8. Induced matrix metalloproteinase (MMP)-9 mRNA expression | ||||||
| Withania coagulans | Ethyl acetate | Aerial with fruit | NA | MCF-7, MDA-MB-231 | - Inhibited TNF-α induced NFκB activity | Kavitha et al. (2017) |
| Astragalus membranaceus | Water- ethanol extract | Roots | NA <100 μg/ml | MCF-7, SK-BR-3, and MDA-MB-231 | - Anti-proliferative | Sabino et al. (2018) |
| - Induced apoptosis | ||||||
| - Inhibition of PI3K/AKT/mTOR signaling pathway | ||||||
| Dillenia suffruticosa | Ethyl acetate extract | Roots | 36 μg/ml | MCF-7 | - “Induces apoptosis via inhibition of AKT and ERK”, and activation of JNK | Momtazi-borojeni et al. (2013) |
| Catharanthus roseus | Methanol extract | Leaves | > 200 μg/ml. | MDA-MB-231 | - Anti-invasive | Tayarani-Najarana et al. (2013) |
| - Suppressed the MMP-2 and MMP-9 activity | ||||||
| Forsythia koreana | Methanol extract | Fruit and leaves | NA | MDA-MB-231 | - “Suppressed invasion and MMPs activities” | Orangi et al. (2016b) |
| - Inhibited the receptor activator of nuclear factor kappa-B | ||||||
| Origanum majorana | Ethanolic extract | Leaves | NA | MDA-MB-231 | - “Anti-invasive and anti-metastatic | Arung et al. (2009) |
| - Downregulates the phosphorylation of IκB, nuclear level of NFκB and Nitric Oxide (NO) production” | ||||||
| Brassica oleracea | Extract | - | NA | MDA-MB-231 | - Anti-invasive | Wicaksono et al. (2009) |
| - Suppressed TPA-induced MMP-9 activity | ||||||
| Salvia triloba | Ethanolic crude extracts | Whole plant | NA | MCF 7 | - Antiangiogenesis | Rezaei et al. (2011) |
| - Inhibited the expression of VEGF at the mRNA and protein level | ||||||
| Eugenia jambolana | Ethyl acetate fractions | Seeds | 25 μg/ml | MCF-7, MDA-MB-231 | - Suppression of VEGF-induced angiogenesis | Chaudhry et al. (2019a) |
| Musa paradisiaca | Ethyl acetate fractions | Roots | 60 μg/ml | MCF-7,MDA-MB-231 | - Suppression of VEGF-induced angiogenesis | Chaudhry et al. (2019a) |
| Buxus sempervirens | Acetonic extract | Leaves and flowers | 7.74–12.5 μg/ml | MCF7, T47D, MCF10CA1a, and BT-20 | - Induces apoptosis | Chaudhry et al. (2019b) |
| - Cell cycle arrest, Autophagy | ||||||
| Cucurbita ficifolia | Chloroform | Fruit | 90 μg/ml | MCF-7 | - FADD; BAK; BAX; caspase-3, -9, and -8 Increased | Arulvasu et al. (2010) |
| Cyperus rotundus L. | Ethanol | Rhizome | 200 μg/ml | MDA-MB-231 | - Upregulation: Bax; DR5; activation of Bid; activation of caspase-3, -9, and -8 downregulation: Bcl-2; survivin; MMP | Engel et al. (2014) |
| Euphorbia hirta L. | Methanol | Whole plant | 25.26 μg/ml | MCF-7 | - Activation of caspase-2, -6, -8, -9, and -3 Increased | Wong et al. (2013) |
| Oldenlandia diffusa | Methanol and butanol | Whole plant | 0–20 μg/ml | MCF-7 | - Bax; activation of caspase-8 and -7 Increased and down regulation Bcl-2 | Koh et al. (2017) |
Plant extracts/fractions as apoptotic inducers for breast cancer (in vitro). updated and improved from (Israels and Israels, 2000).
6 Marine extracts/fractions as apoptosis regulator
Aquatic environments, especially marine species, contain abundant various natural compounds which may have therapeutic significance. However, recent marine biomaterials experience demonstrates that ocean life seems to be an untapped resource. Bioactive compounds found naturally are the most effective. Apart from a few exceptions, most compounds used in medicines originate through biological and chemical modifications rather than from the initial natural substance. Our previous studies confirm apoptosis induction in the cancer cell (in-vitro) by marine extracts and fractions. The Aaptos sp., marine (fraction) causes the DNA fragmentation in the MCF-7 cell line, which is a remarkable feature of apoptosis (late). Similarly, marine sponges (whole) extracts and fractions also induce the fragmentation in DNA in breast cancer (MCF-7) cell lines. The details information mention in Table 2. The “Stichopus chloronotus” and “Holothuria nobilis” fractions induced cytotoxicity via apoptosis induction in cervical cancer (HeLa) cells shows the remarkable potential of marine phytochemicals in the induction of apoptosis. The “Bruguiera gymnorrhiza” extracts potential phytochemicals that trigger DNA fragmentation, leading to the induction of apoptosis in MCF-7 cells. The other marine species, “Acanthaster planci” sp., and “Diadema setosum” sp., fractions Induction of apoptosis in HeLa cells. The “Xylocarpus moluccensis” induce the triggering of the Extrinsic pathway of apoptosis which causes the primary factor for cytotoxicity of fractions in human hepatocellular carcinoma (HepG2) and human cervical cancer cell line (HeLa). Some alkaloid chemicals discovered in traditional medicines, tetrandrine (TET) and cepharanthine (CEP), operate as apoptotic agents. There is reduction in the progression of malignant tumors. When viewing human leukemia cells treated with alkaloids, Xu et al. found that both initiator and effector caspases were dramatically increased (caspase-3 and& -6). Saikosaponin A, a triterpenoid saponin, was studied by Kim and colleagues to see if it could kill human colon cancer cell lines. According to the researchers’ findings, the activation of Bax and Bid proteins and the activation of caspase-2, -8, and -9 in saikosaponin A increased apoptosis in cells. Emodin (40 mg/kg/day) suppressed tumor weight in “nude mice xenografts” carrying “LS1034” colon cancer cells in vivo, according to in vitro findings. “Anti-apoptotic” proteins “Bcl-2” and Bcl-xL, Bax, XIAP and cFLIP are reduced in “HT-29”, “HCT-116” and “SW480” cells by the flavonoid compound Casticin. The “OVCAR-3” and “SKOV-3” (ovarian cancer) cell lines were exposed to kaempferol (kaempferol), a flavonoid, the levels of apoptosis-inducing factors like proteins (Bax, caspase-3, -8, & -9) were significantly increased. In contrast, apoptosis inhibiting proteins decreased in these cells. Similar outcomes were found when combined with the anti-inflammatory agent TRAIL.
TABLE 2
| Marine species | Cell line | Conc. Range IC50 (μM)/μg/mL) | Phosphatidylserine externalization (PS) (initial apoptosis) | DNA fragmentation (late Apoptosis) | Caspase activation | PARP cleavage | References |
|---|---|---|---|---|---|---|---|
| Aaptos sp., fractions | MCF-7 | 5.0–25.00 μg/ml | √ | √ | Ansari et al. (2017) | ||
| Stryphuous ponderosus AaptosTheonella Xestospongia | MCF-7 | 8.49 μg/ml | √ | √ | Nho et al. (2015) | ||
| 7.61 μg/ml | |||||||
| 21.88 μg/ml | |||||||
| 97.72 μg/ml | |||||||
| Stichopus chloronotus | HeLa | 6.71–8.54 μg/ml | √ | √ | Santos et al. (2017) | ||
| Holothuria nobilis | HeLa | 14.76–2.54 μg/ml | √ | √ | Santos et al. (2017) | ||
| Bruguiera gymnorrhiza | MCF-7 | 3.39–37.15 μg/ml | √ | √ | Yasser et al. (2015) | ||
| Acanthaster planci | HeLa | 0–18.93 μg/ml | √ | Shin et al. (2016) | |||
| Diadema setosum | HeLa | 0–30 μg/ml | √ | Shin et al. (2016) | |||
| Xylocarpus moluccensis | HeLa, HepG2 | 0.22–74.13 μg/ml | √ | Ihsan-ul- HaqMirza et al. (2013) | |||
| Aaptos sp. | THP-1 | 50–200 μM | √ | Zhou et al. (2018) | |||
| Aaptos sp. | THP-1 | 10–25 μM | √ | Zhou et al. (2018) | |||
| Aaptos sp. | THP-1 | 10–25 μM | √ | Zhou et al. (2018) | |||
| Amphibleptula sp. | AsPC-1 | 2.4 | μM | √ | 3 and 7 | Tor et al. (2015) | |
| BxPC-3 | 2.4 | μM | √ | 3 and 7 | |||
| PANC-1 | 2.4 μM | √ | 3 and 7 | ||||
| Aplysina aerophoba | Raji | 50 | √ | 3 and 7 | √ | Eltayeb et al., (2016) | |
| U937 | 50 μM | √ | √ | ||||
| Australian spongia sp. | AsPC-1 | 6.8 μM | √ | 3 and 7 | Kim et al. (2016) | ||
| Australian spongia sp. | PANC-1 | 6.8 μM | √ | 3 and 7 | Kim et al. (2016) | ||
| Australian spongia sp. | MIA PaCa-2 | 6.8 μM | Kim et al. (2016) | ||||
| Australian spongia sp. | BxPC-3 | 6.8 μM | Kim et al. (2016) | ||||
| Cacospongia mycofijiensis | MDA-MB-435 | 0.1 μM | 3 | Dhaheri et al. (2013) | |||
| Cacospongia scalaris | HeLa | 10 μg/ml | 3 | Rose et al. (2005) | |||
| T47D | |||||||
| Candidaspongia sp. | U251 HCT116 | 0.05–0.10 μM | √ | 3 and 12 | Zihlif et al. (2013) | ||
| Callyspongia sp. | HL-60 | 31.0–77.5 μM | √ | Harsha et al. (2017) | |||
| Crambe crambe | HepG2 | 0.5–2.5 μM | √ | 3 | Ait-Mohamed et al. (2011) | ||
| Crambe crambe | HepG2 | 0.5–2.5 μM | √ | 3 | Ait-Mohamed et al. (2011) | ||
| Crambe crambe | HepG2 | 0.5–2.5 μM | √ | 3 | Ait-Mohamed et al. (2011) | ||
| Dactylospongia elegans | U937 HL-60 | 5–15 μM | √ | Alshammari et al. (2020b) | |||
| Dinophysis fortii and | Hep3B | 0.01 μg/ml | √ | 3, 8, and 9 | Park et al. (2014) | ||
| Dinophysis acuminata | U937 | 0.008–0.010 μg/mL | √ | √ | 3 | √ | Kwan et al. (2016), Chung et al. (2017b), Kim et al. (2008), Moon et al. (2008) |
| Fascaplysinopsis sp. | K562 normoxic and hypoxic conditions | 0.01–0.2 μM | √ | 3 and 9 | Hudayah et al. (2017), Chaudhry et al. (2018) | ||
| Fasciospongia cavernosa | Hela | 10 μg/ml | √ | 3 | Hudayah et al. (2017) | ||
| T47D | 10 μg/ml | ||||||
| Forcepia sp. | CA46, Ramos, Daudi, HL-60, MDA-MD-231, MCF-7, HCT-116, HT-29 | 0.1 μM | √ | Chaudhry et al. (2020b) | |||
| Geodia japonica | HL-60 | 1.6–25 μg/ml | √ | √ | 3 | Chaudhry et al. (2020c), Chaudhry et al. (2021b) | |
| HT-29 | 5–30 μM | √ | |||||
| Geodia japonica | HL-60 | 4 μg/ml | 3 | Chaudhry et al. (2021c) | |||
| Hippospongia metachromia | HCT116 | 2.5–10 μM | √ | √ | 3 and 8 | Dyshlovoy et al. (2014), Calcabrini et al. (2017) | |
| PC-3 | 2–10 μM | √ | Guzman et al. (2015) | ||||
| Spirastrella spinispirulifera | MCF-7 | 0.0002–0.0005 μM | √ | 2,5,7,8, 9 | Florean et al. (2016) | ||
| Hyrtios erecta | Jurkat | 0.0002 μM | √ | 2, 3, 7, 8 ,and 9 | Guzman et al. (2013) | ||
| L3.6pL | 0.00001–0.01 μM | √ | Mooberry et al. (1999) | ||||
| Hyrtios sp. | K562 | 1.4–5.6 μM | √ | 3, 8, and 9 | |||
| DU145 | 0.01–1 μg/ml | √ | 3, 8, and 9 | De Stefano et al. (2012) | |||
| PC-3 | 0.01–1 μg/ml | √ | 3, 8, and 9 | Trisciuoglio et al. (2008) | |||
| LNCaP | 0.01 μg/ml | √ | |||||
| T24 | 0.1–0.8 μg/ml | √ | √ | 3 and 9 | Umeyama et al. (2010) | ||
| A498 | 0.5–3 μM | √ | 3, 8 and 9 | Roel et al. (2016) | |||
| lanthella sp. | HUVEC | 0.01–1 μM | √ | 3 and 7 | Kong et al. (2008) | ||
| Ircinia ramose, Psammocinia sp. | Jurkat | 0.01 μM | √ | 3, 8 and 9 | Shin et al. (2008) | ||
| Jaspis sp. | Bel-7402 | 0.5 μM | √ | Kim et al. (2008) | |||
| HepG2 | 10–20 μM | ||||||
| Jaspis stellifera | K562 | 0.012–0.054 μM | √ | 3 and 9 | √ | Moon et al. (2008), Ben-Califa et al. (2012), Del Poggetto et al. (2015) | |
| A549 | 0.02–1 μM | √ | 3 and 7 | √ | |||
| SF295 | 0.04–1 μM | √ | √ | ||||
| Jaspis sp. Pachastrissa sp. | B16 | 5 μM | √ | √ | 3 and 9 | √ | Cheung et al. (2010), Zhang et al. (2012) |
| HaCaT | 5 μg/ml | 3 | |||||
| Lanthella sp. | HL-60 | 19.9 μM | √ | Liu et al. (2005) | |||
| Lanthella sp. | HL-60 | 21.3 μM | √ | Liu et al. (2005) | |||
| Lanthella sp. | HL-60 | 21.5 μM | √ | Liu et al. (2005) | |||
| Leiodermatium sp. | AsPC-1 | 0.01 μM | √ | Liu et al. (2006) | |||
| BxPC-3 | 0.01 μM | √ | 3 | ||||
| MIA PaCa-2 | √ | 3 | |||||
| Leucetta chagosensis | √ | 3, 8 and 9 | Do et al. (2014) | ||||
| Monanchora pulchra | HeLa | 1.39–2.01 μM | √ | 3 and 7 | Lee et al. (2015) | ||
| Mycale sp. | 32D | 0.1 μM | Lu et al. (2007) | ||||
| Negombata magnifica | MKN45 NUGC-4 | − 10 | √ | 3 and 7 | Schneiders et al. (2009) | ||
| 0.01–10 μM | √ | ||||||
| Oceanapia sagittaria | MCF-7 | 0.5–2.5 μM | √ | Schyschka et al. (2008) | |||
| Petrosia sp. | SK-MEL-2 | 0.1–0.3 μg/ml | √ | 3 and 9 | Rothmeier et al. (2010) | ||
| Psammaplysilla sp. | Human endometrial Ishikawa | √ | Schumacher et al. (2010) | ||||
| Psammaplysilla | Ishikawa | 1 μM | √ | Schumacher et al. (2010) | |||
| ECC1 | 1 μM | ||||||
| Pseudoceratina sp. | K562 | 7.7–30.8 μM | √ | 3 and 9 | Wu et al. (2016) | ||
| Rhabdastrella globostellata | HUVEC | 1–10 μM | 3 and 7 | Huang et al. (2016) | |||
| Rhabdastrellav globostellata | HL-60 | √ | 3 | √ | Wu et al. (2015) | ||
| HL-60 | 10–25 μM | √ | 3, 8 and 9 | Aoki et al. (2006) | |||
| Rhizochalina incrustata | HT-29 | 1–6 μM | √ | 3 | Chinen et al. (2010) | ||
| THP-1 | 1–10 μM | √ | Wei et al. (2008) | ||||
| PC-3 | 0.5–4 μM | √ | 8 | ||||
| DU-145 | 0.5–4 μM | √ | 8 | ||||
| 22Rv1 | 0.5–4 μM | √ | 8 | Chen et al. (2017) | |||
| VCaP | 0.5–4 μM | √ | 8 | ||||
| Sarcotragus | SK-MEL-2 | 25–50 μM | √ | 3 and 9 | √ | Wang et al. (2016) | |
| Siphonochalina sp. | HepG2 | 17.18 μM | √ | 3 | Salma et al. (2009), Tang et al., (2014) | ||
| HCT-116 | 14.8 μM | √ | 3 | ||||
| Siphonochalina sp. | HepG2 | 24 μM | √ | 3 | Salma et al. (2009) | ||
| HCT-116 | 19.8 μM μM | √ | 3 | ||||
| Smenospongia aurea | Calu-1 | 0.05–0.1 μM | √ | √ | Yoo et al. (2012) | ||
| Stelletta sp. | U937 | 17.2–103.3 μM | √ | √ | 3, 8 and 9 | Nguyen et al. (2009) | |
| Xestospongia sp. | H460 | 5–40 μM | √ | 3 | √ | LaBarbera et al. (2009), Guzman et al. (2016) | |
| U373MG | 0.0031 μM |
Marine (sponges) extracts/fractions as apoptotic inducers for various cancer cell lines (in vitro). Improved and updated from (Shin et al., 2017) (Calcabrini et al., 2017).
7 Marine isolated targeted compounds as apoptotic inducers (FDA approved/treatment phase)
Besides extracts/fractions, the various marine-based drug also show apoptosis-inducing potential. And over 50 years since cytarabine was authorized, four marine-derived drugs have been approved (Hu et al., 2013). Some other items have been included in the marine anticancer medicinal route in clinical studies in different phases of development as we focused on apoptotic inducers. Table 3 shows the list of respective drugs (approved or in clinical trials) having the potential for inducing apoptosis. These include; Brentuximab2 vedotin (AdcetrisTM), Brentuximab2 vedotin (AdcetrisTM), as “Antibody-drug3 conjugate in a mixture of anti-CD30 antibody and monomethyl auristatin E (MMAE), isolated from sea hare Dollabella auricularia/cyanobacteria” used for Blockage in cell cycle progression (G2 to M) induce induction of apoptosis phase III and I treatment of classical HL in a mixture using chemotherapy for curing HL and chronic large cell anaplastic lymphoma. Enfortumab vedotin, Marizomib, Polatuzumab Vedotin, use in phase III. Besides, GSK2857916, Aplidine, plitidepsin (Aplidin), Ladiratuzumab vedotin, Glembatumumab vedotin, Denintuzumab mafodotin, in phase II trials. However, Pinatuzumab vedotin, ASG-15ME, CEP-2563, Lifastuzumab vedotin, and Vandortuzumab vedotin were discontinued after phase I used for lymphoma, bladder, ovarian, as Peritoneal cancer. Vandortuzumab isolated from Cyanobacterium. (Caldora penicillata) acts as apoptotic inducer and mitotic inhibitor for Prostate cancer.
TABLE 3
| Drug | Source (marine) | Mechanism of action | Progress status | Treatment for | Ref |
|---|---|---|---|---|---|
| Brentuximab2 vedotin (AdcetrisTM) | “Antibody-drug3 conjugate in a mixture of anti-CD30 antibody and monomethyl auristatin E (MMAE), obtained from sea hare Dollabella auricularia/cyanobacteria” | Blockage in cell cycle progression (G2 to M) induce “induction of apoptosis” | Approved by FDA (United States 2018); Also, phase III and I treatment of classical HL in a mixture using chemotherapy | “It is utilized for curing HL and chronic large cell anaplastic lymphoma” | Dyshlovoy et al. (2016) |
| Enfortumab vedotin | Cyanobacterium Caldora penicillata | By interaction with the microtubular network Arrest the cell cycle, and eventually apoptosis | Phase III | For Urothelial cancers | Dyshlovoy et al. (2016) |
| Marizomib | Actinomycete | “Act as apoptosis | Phase III | Used for “multiple | Dyshlovoy et al. (2016) |
| Salinispora tropica | Stimulants” and | myeloma and | |||
| “inhibits proteasome” | Glioblastoma” | ||||
| Polatuzumab | Cyanobacterium | As a apoptosis stimulants | Phase III | For the treatment | Dyshlovoy et al. (2016) |
| vedotin | Caldora penicillata | arrest mitosis | of diffuse large B cell | ||
| also tubulin | lymphoma | ||||
| inhibition micro-tubulin | |||||
| Polymerization | |||||
| GSK2857916 | Cyanobacterium | Apoptosis stimulant via | Phase II | Multiple myeloma | Hood et al. (2001) |
| Caldora penicillata | “mitosis inhibitors tubulin | ||||
| polymerization inhibitors” | |||||
| Aplidine | Tunicate Aplidium | “Apoptosis stimulants”, cell | Phase II | “Multiple myeloma | Hood et al. (2001) |
| plitidepsin | alpicans | cycle inhibitors protein | precursor cell | ||
| (Aplidin) | “synthesis inhibitors” | Lymphoblastic” | |||
| “vascular endothelial | leukemia-lymphoma | ||||
| growth factor receptor-1 | |||||
| Antagonists” | |||||
| Ladiratuzumab | Cyanobacterium | Apoptosis stimulants | Phase II | For Breast cancer | Hood et al. (2001) |
| vedotin | Caldora penicillata | “mitosis inhibitors” | |||
| Glembatumumab | Cyanobacterium | Apoptosis stimulants | Phase-II | Brain cancer and Breast | Hood et al. (2001) |
| vedotin | Caldora penicillata | mitosis inhibitors tubulin | osteosarcoma | ||
| Denintuzumab | Cyanobacterium | Act as “Immunomodulators” | uveal melanoma | ||
| mafodotin | Caldora penicillata | mitosis inhibitors also inhibitors of tubulin polymerization | Phase II | Diffuse large B-cell | Konishi et al. (2009); Dyshlovoy et al. (2016) |
| (discontinued) | lymphoma | ||||
| Pinatuzumab | Cyanobacterium | Apoptosis stimulants | Phase I | Chronic lymphocytic | Hood et al. (2001); Dyshlovoy et al. (2016) |
| vedotin | Caldora penicillata | mitosis inhibitors tubulin | (discontinued) | leu emia diffuse large | |
| inhibitors tubulin | B cell lymphoma nHL | ||||
| polymerization inhibitors | |||||
| ASG-15ME | Cyanobacterium | Apoptosis stimulants | Phase I | Bladder cancer | Kijjoa et al. (2007); Dyshlovoy et al. (2016) |
| Caldora penicillata | mitosis inhibitors tubulin | (discontinued) | urogenital cancer | ||
| inhibitors tubulin | |||||
| polymerization inhibitors | |||||
| CEP-2563 | Protein tyrosine kinase | Phase I | Solid tumors | Kijjoa et al. (2007); Dyshlovoy et al. (2016) | |
| inhibitors | (discontinued) | ||||
| Lifastuzumab | Cyanobacterium | Apoptosis stimulants | Phase I | For various cancer Fallopian tube cancer | Dyshlovoy et al. (2016) |
| vedotin | Caldora penicillata | mitosis inhibitors tubulin | (discontinued) | non-small cell lung | |
| inhibitors tubulin | Cancer, ovarian cancer | ||||
| polymerization | Peritoneal cancer | ||||
| Vandortuzumab | Cyanobacterium | Apoptosis stimulants | Phase I | Prostate cancer | Dyshlovoy et al. (2016) |
| vedotin | Caldora penicillata | mitosis inhibitors | (discontinued) | ||
| polymerization inhibitors |
Marine base drugs (FDA Approved/treatment Phase) as Apoptotic inducers.
8 Conclusion
The potential use of natural substances as either a combination treatment with standard therapies or as an individual derivative molecule as anticancer medicine requires an understanding of cancer initiation and activation of cancer development, the role of signalling pathway in activation of cancer initiation and progression, pro-oncogenes, and oncogenes. Since apoptosis is a crucial regulating mechanism for normal cells, any disruption in this process might lead to unchecked cell growth. An in-depth mechanistic knowledge of apoptotic signalling pathways is necessary for the creation of efficient cancer therapies. Plant extracts, marine extracts/fractions, and the chemicals described in this research may represent a promising and efficient approach to cancer treatment. Researchers found that natural product derivatives may have an important role in the prevention and treatment of several different types of cancer. To verify their overall usefulness as potent chemotherapeutic drugs, however, more preclinical and clinical research are required. It is interesting to note that natural compounds produced from plant extracts/marine can be used in combination with possible anticancer treatment to enhance drug sensitivity in aggressive and resistant kind of cancer. Due to the promising results seen when using natural goods in conjunction with cancer medications, intensive study into the therapeutic uses of natural products is required to get the best possible outcomes in individualized, targeted cancer care. Similarly, the use of more than one compounds as multitargeted signaling pathway inhibitors or activator should be consider with one signaling target as apoptosis pathway. However, natural chemical derivatives that induce apoptosis, along with cutting-edge drug delivery methods like polymeric nanoparticles, may yield impressive results. Compounds with low solubility and bioavailability are less likely to be effective in the development of chemotherapy drugs. In conclusion, this study aims to bring to the notice of scientists and researchers the numerous beneficial impacts of natural products in the development of novel and safe pharmaceuticals or in combine delivery technique with anticancer treatments for probable sensitization of cancer therapy. It might also serve as a solid basis for further research into natural compounds in cancer therapeutics.
Statements
Author contributions
GC prepared the concept, wrote and reviewed the manuscript. MA, YS, and TS provided valuable input during the review process of this manuscript.
Acknowledgments
Authors would like to acknowledge the UMT for TAPERG/2021/UMT/807 grant provided to GC.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Abadio FincoF. D. B.KlossL.GraeveL. (2016). Bacaba (Oenocarpus bacaba) phenolic extract induces apoptosis in the MCF-7 breast cancer cell line via the mitochondria-dependent pathway. NFS J.5, 5–15. 10.1016/j.nfs.2016.11.001
2
AbazaM. S.AfzalM.Al-AttiyahR. J.GuleriR. (2016). Methylferulate from Tamarix aucheriana inhibits growth and enhances chemosensitivity of human colorectal cancer cells: Possible mechanism of action. BMC Complement. Altern. Med.16, 384. 10.1186/s12906-016-1358-8
3
AhnD. S.LeeH. J.HwangJ.HanH.KimB.ShimB.et al (2018). Lambertianic acid sensitizes non-small cell lung cancers to TRAIL-induced apoptosis via inhibition of XIAP/NF-κB and activation of caspases and death receptor 4. Int. J. Mol. Sci.19, 1476. 10.3390/ijms19051476
4
Ait-MohamedO.BattistiV.JoliotV.FritschL.PontisJ.MedjkaneS.et al (2011). Acetonic extract of buxus sempervirens induces cell cycle arrest, apoptosis and autophagy in breast cancer cells. PLoS One6 (9), e24537. 10.1371/journal.pone.0024537
5
AlbertsB.JohnsonA.LewisJ.RaffM.RobertsK.WalterP. (2002). Programmed cell death (apoptosis) - molecular biology of the cell. 4th ed. New York: Garland Science.
6
AliM. R.YongM. J.GyawaliR.MosaddikA.RyuY. C.ChoS. K.et al (2012). Mango (Mangifera indica L.) peel extracts inhibit proliferation of HeLa human cervical carcinoma cell via induction of apoptosis. J. Korean Soc. Appl. Biol. Chem.55, 397–405. 10.1007/s13765-012-1024-x
7
AlshammariG. M.BalakrishnanA.AlshatwiA. A.Al-KhalifaA. (2020). Cucurbita ficifolia fruit extract induces tp53/caspase- mediated apoptosis in MCF-7 breast cancer cells. Biomed. Res. Int.2020, 3712536. 10.1155/2020/3712536
8
AlshammariG. M.BalakrishnanA.AlshatwiA. A.Al-KhalifaA. (2020). Cucurbita ficifolia fruit extract induces tp53/caspase- mediated apoptosis in MCF-7 breast cancer cells. Biomed. Res. Int., 3712536. 10.1155/2020/3712536
9
AnichiniA.MortariniR.SensiM.ZanonM. (2006). APAF-1 signaling in human melanoma. Cancer Lett.238, 168–179. 10.1016/j.canlet.2005.06.034
10
AnsariJ. A.RastogiN.AhmadM. K.KhanA. R.ThakurR. (2017). ROS mediated pro-apoptotic effects of Tinospora cordifolia on breast cancer cells. Front. Biosci.9, 89–100. 10.2741/e788
11
AokiS.ChoS. H.OnoM.KuwanoT.NakaoS.KuwanoM.et al (2006). Bastadin 6, a spongean brominated tyrosine derivative, inhibits tumor angiogenesis by inducing selective apoptosis to endothelial cells. Anticancer. Drugs17, 269–278. 10.1097/00001813-200603000-00005
12
AriaziE. A.SatomiY.EllisM. J.HaagJ. D.ShiW.SattlerC. A.et al (1999). Activation of the transforming growth factor β signaling pathway and induction of cytostasis and apoptosis in mammary carcinomas treated with the anticancer agent perillyl alcohol. Cancer Res.59 (8), 1917–1928.
13
ArulvasuC.PrabhuD.ManikandanR.SrinivasanP. (2010). Induction of apoptosis by the aqueous and ethanolic leaf extract of Vitex negundo L. in MCF-7 human breast cancer cells. Int. J. Drug Disc.2 (1), 1–7. 10.9735/0975-4423.2.1.1-7
14
ArungE. T.WicaksonoB. D.HandokoY. A.KusumaI. W.YuliaD.SandraF. (2009). Anti-cancer properties of diethylether extract of wood from sukun (artocarpus altilis) in human breast cancer (T47D) cells. Trop. J. Pharm. Res.8.
15
AshkenaziA.DixitV. M. (1998). Death receptors: Signaling and modulation. Science281, 1305–1308. 10.1126/science.281.5381.1305
16
AzzopardiM.FarrugiaG.BalzanR. (2017). Cell-cycle involvement in autophagy and apoptosis in yeast. Mech. Ageing Dev.161 (2), 211–224. 10.1016/j.mad.2016.07.006
17
AzzopardiM.FarrugiaG.BalzanR. (2017). Cell-cycle involvement in autophagy and apoptosis in yeast. Mechanisms of Ageing and Development, 211–224.
18
BagheriE.HajiaghaalipourF.NyamathullaS.SalehenN. (2018). The apoptotic effects of Brucea javanica fruit extract against HT29 cells associated with p53 upregulation and inhibition of NF-κB translocation. Drug Des. devel. Ther.12, 657–671. 10.2147/DDDT.S155115
19
Ben-CalifaN.BisharaA.KashmanY.NeumannD. (2012). Salarin c, a member of the salarin superfamily of marine compounds, is a potent inducer of apoptosis. Investig. New Drugs30, 98–104.
20
BibelM.BardeY. A. (2000). Neurotrophins: Key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev.14, 2919–2937. 10.1101/gad.841400
21
BirjandianaE.MotamedaN.YassabN. (2018). Crude methanol extract of echinophora platyloba induces apoptosis and cell cycle arrest at S-phase in human breast cancer cells. Iran. J. Pharm. Res.17 (1), 307–316.
22
BlockG.PattersonB.SubarA. (1992). Fruit, vegetables, and cancer prevention: A review of the epidemiological evidence. Nutr. Cancer18, 1–29. 10.1080/01635589209514201
23
BredesenD. E.RaoR. V.MehlenP. (2006). Cell death in the nervous system. Nature443 (7113), 796–802. 10.1038/nature05293
24
BuhagiarJ. A.PodestaM. T.WilsonA. P.MicallefM. J.AliS. (1998). The induction of apoptosis in human melanoma, breast and ovarian cancer cell lines using an essential oil extract from the conifer Tetraclinis articulata. Anticancer Res.19.6B, 5435–5443.
25
BussingA.HerterIch-AkInpeluI.PfullerU. (1999). Apoptosis-associated generation of reactive oxygen intermediates and release of pro- inflammatory cytokines in human lymphocytes and granulocytes by extracts from the seeds of Acalypha wilkesiana. J. Ethnopharmacol.66 (3), 301–309. 10.1016/s0378-8741(98)00227-x
26
CairraoF.DomingosP. M. (2010). “Apoptosis: Molecular mechanisms,” in Encyclopedia of life sciences (ELS) (Chichester: John Wiley & Sons).
27
CalcabriniC.CatanzaroE.BishayeeA.TurriniE.FimognariC. (2017). Marine sponge natural products with anticancer potential: An updated review. Mar. Drugs15 (10), E310. 10.3390/md15100310
28
ChaudhryG. E.IslamiahM.ZafarM. N.BakarK.AzizN.SaidinJ.et al (2021). Induction of apoptosis by Acanthaster planci sp., and Diadema setosum sp., fractions in human cervical cancer cell line, HeLa. Asian pac. J. Cancer Prev.22 (5), 1365–1373. 10.31557/APJCP.2021.22.5.1365
29
ChaudhryG. E.JanR.MohammadH.MuhammadT. S. T. (2019a). Vitex rotundifolia fractions induce apoptosis in human breast cancer cell line, MCF-7, via extrinsic and intrinsic pathways. Res. Pharm. Sci.14 (3), 273–285. 10.4103/1735-5362.258496
30
ChaudhryG. E.JanR.ZafarM. N.MohammadH.MuhammadT. S. T. (2020). Phytochemistry and biological activity of Vitex rotundifolia L. Res. J Pharm Tech13 (11), 5534–5538.
31
ChaudhryG. E.JanR.ZafarM. N.MohammadH.MuhammadT. S. T. (2019b). Vitex rotundifolia fractions induced apoptosis in human breast cancer T-47D cell line via activation of extrinsic and intrinsic pathway. Asian pac. J. Cancer Prev.20 (12), 3555–3562. 10.31557/APJCP.2019.20.12.3555
32
ChaudhryG. E.KassimM. N. I.ZafarM. N.MohammadH.AndrianiY.IsmailN.et al (2020). Induction of apoptosis by Stichopus chloronotus and Holothuria nobilis fractions in human cervical cancer cell line, HeLa. Int. J. Res. Pharm. Sci.11 (1), 1238–1247.
33
ChaudhryG. E.SohimiN. K. A.MohamadH.ZafarM. N.AhmedA.SungY. Y.et al (2021). Xylocarpus moluccensis induces cytotoxicity in human hepatocellular carcinoma HepG2 cell line via activation of the extrinsic pathway. Asian pac. J. Cancer Prev.22 (S1), 17–24. 10.31557/APJCP.2021.22.S1.17
34
ChaudhryG.IslamiahM.IsmailN.MohamadH.SungY. Y.MuhammadT. S. T. (2018). Induction of apoptosis by aaptos sp., fractions in human breast cancer cell line, mcf-7. Int. J. Res. Pharm. Sci.9 (2), 328–337.
35
ChaudhryG. S.JanR.AkimA.ZafarM. N.MuhammadT. S. T.et al (2021). Breast cancer: A global concern, diagnostic and therapeutic perspectives, mechanistic targets in drug development. Adv. Pharm. Bull.11 (4), 580–594. 10.34172/apb.2021.068
36
ChaudhryG. S.RahmanN. H.VigneswariS.AzizA.HabsahM.et al (2020). Cytotoxicity effect and cell death mechanism of Bruguiera gymnorrhiza extracts on human breast cancer cell line (MCF-7). J. Adv. Pharm. Technol. Res.11 (4), 233–237.
37
ChenY.ZhouQ.ZhangL.ZhongY.FanG.ZhangZ.et al (2017). Stellettin b induces apoptosis in human chronic myeloid leukemia cells via targeting pi3k and stat5. Oncotarget8, 28906–28921. 10.18632/oncotarget.15957
38
ChengA. C.JianC. B.HuangY. T.LaiC. S.HsuP. C.PanM. H.et al (2007). Induction of apoptosis by Uncaria tomentosa through reactive oxygen species production, cytochrome c release, and caspases activation in human leukemia cells. Food Chem. Toxicol.45, 2206–2218. 10.1016/j.fct.2007.05.016
39
CheungF. W.LiC.CheC. T.LiuB. P.WangL.LiuW. K. (2010). Geoditin a induces oxidative stress and apoptosis on human colon HT29 cells. Mar. Drugs8, 80–90.
40
ChinenT.NagumoY.WatanabeT.ImaizumiT.ShibuyaM.KataokaT.et al (2010). Irciniastatin a induces jnk activation that is involved in caspase-8-dependent apoptosis via the mitochondrial pathway. Toxicol. Lett.199, 341–346.
41
ChiuC. H.ChouY. C.LinJ. P.KuoC. L.LuH. F.HuangY. P.et al (2015). Chloroform extract of Solanum lyratum induced G0/G1 arrest via p21/p16 and induced apoptosis via reactive oxygen species, caspases and mitochondrial pathways in human oral cancer cell lines. Am. J. Chin. Med.43, 1453–1469. 10.1142/S0192415X15500822
42
ChouW. H.LiuK. L.ShihY. L.ChuangY. Y.ChouJ.LuH. F.et al (2018). Ouabain induces apoptotic cell death through caspase- and mitochondria-dependent pathways in human osteosarcoma U-2 OS cells. Anticancer Res.38, 169–178. 10.21873/anticanres.12205
43
ChungH.JungY. M.ShinD. H.LeeJ. Y.OhM. Y.KimH. J.et al (2008). Anticancer effects of wogonin in both estrogen receptor-positive and -negative human breast cancer cell lines in vitro and in nude mice xenografts. Int. J. Cancer122, 816–822. 10.1002/ijc.23182
44
ChungT. W.ChoiH.LeeJ. M.HaS. H.KwakC. H.AbekuraF.et al (2017). Oldenlandia diffusa suppresses metastatic potential through inhibiting matrix metalloproteinase-9 and intercellular adhesion molecule-1 expression via p38 and ERK1/2 MAPK pathways and induces apoptosis in human breast cancer MCF-7 cells. J. Ethnopharmacol.195, 309–317.
45
ChungT. W.ChoiH.LeeJ. M.HaS. H.KwakC. H.AbekuraF.et al (2017). Oldenlandia diffusa suppresses metastatic potential through inhibiting matrix metalloproteinase-9 and intercellular adhesion molecule-1 expression via p38 and ERK1/2 MAPK pathways and induces apoptosis in human breast cancer MCF-7 cells. J. Ethnopharmacol.195, 309–317. 10.1016/j.jep.2016.11.036
46
CoxG.StewardW. P.O’ByrneK. J. (1999). The plasmin cascade and matrix metalloproteinases in non-small cell lung cancer. Thorax54 (2), 169–179. 10.1136/thx.54.2.169
47
De StefanoD.TommonaroG.MalikS. A.IodiceC.De RosaS.MaiuriM. C.et al (2012). Cacospongionolide and scalaradial, two marine sesterterpenoids as potent apoptosis-inducing factors in human carcinoma cell lines. PLoS One7, e33031. 10.1371/journal.pone.0033031
48
DecockJ.ThirkettleS.WagstaffL.EdwardsD. R. (2011). Matrix metalloproteinases: Protective roles in cancer. J. Cell. Mol. Med.15 (6), 1254–1265. 10.1111/j.1582-4934.2011.01302.x
49
Del PoggettoE.TanturliM.Ben-CalifaN.GozziniA.TusaI.CheloniG.et al (2015). Salarin C inhibits the maintenance of chronic myeloid leukemia progenitor cells. Cell Cycle14, 3146–3154. 10.1080/15384101.2015.1078029
50
DhaheriY. A.AttoubS.ArafatK.AbuQamarS.VialletJ. (2013). Anti-metastatic and anti-tumor growth effects of origanum majorana on highly metastatic human breast cancer cells: Inhibition of NFκB signaling and reduction of nitric oxide production. PLoS One8 (7), e68808. 10.1371/journal.pone.0068808
51
DoM. T.NaM.KimH. G.KhanalT.ChoiJ. H.JinS. W.et al (2014). Ilimaquinone induces death receptor expression and sensitizes human colon cancer cells to trail-induced apoptosis through activation of ros-erk/p38 mapk-chop signaling pathways. Food Chem. Toxicol.71, 51–59.
52
DuckettC. S.LiF.WangY.TomaselliK. J.ThompsonC. B.ArmstrongR. C.et al (1998). Human IAP-like protein regulates programmed cell death downstream of Bcl-xL and cytochrome c. Mol. Cell. Biol.18 (1), 608–615. 10.1128/mcb.18.1.608
53
DyshlovoyS. A.FedorovS. N.ShubinaL. K.KuzmichA. S.BokemeyerC.Keller-von AmsbergG.et al (2014). Aaptamines from the marine sponge Aaptos sp. Display anticancer activities in human cancer cell lines and modulate ap-1-, Nf-κB-, and p53-dependent transcriptional activity in mouse jb6 cl41 cells. Biomed. Res. Int.2014, 469309.
54
DyshlovoyS. A.OtteK.AlsdorfW. H.HauschildJ.LangeT.VenzS.et al (2016). Marine compound rhizochalinin shows high in vitro and in vivo efficacy in castration resistant prostate cancer. Oncotarget7, 69703–69717. 10.18632/oncotarget.11941
55
ElmoreS. (2007). Apoptosis: A review of programmed cell death. Toxicol. Pathol.35 (4), 495–516. 10.1080/01926230701320337
56
EltayebN. M.NgS. Y.IsmailZ.SalhimiS. M. (2016). Anti-invasive effect of catharanthus roseus extract on EFFECT OF Catharanthus roseus extract on highly metastatic human breast cancer MDA-MB-231 cell. Jurnal Teknologi Sci. Eng.78113, 35–40.
57
EngelN.FalodunA.KühnJ.KraglU.LangerP.NebeB.et al (2014). Pro-apoptotic and anti-adhesive effects of four African plant extracts on the breast cancer cell line MCF-7. BMC Complement. Altern. Med.14, 334. 10.1186/1472-6882-14-334
58
FayaM.MillimounoJ. D.LiuY.JiangLi.XiaomengLi. (2014). Targeting apoptosis pathways in cancer and perspectives with natural compounds from mother. Nat. Cancer Prev. Res. (Phila).7 (11), 1081–1107.
59
FearonE. R.VogelsteinB. (1990). A genetic model for colorectal tumorigenesis. Cell61, 759–767. 10.1016/0092-8674(90)90186-i
60
Fisher DavidE. (1994). Apoptosis in cancer therapy: Crossing the threshold. Cell78 (4), 539–542. 10.1016/0092-8674(94)90518-5
61
FloreanC.SchnekenburgerM.LeeJ. Y.KimK. R.MazumderA.SongS.et al (2016). Discovery and characterization of isofistularin-3, a marine brominated alkaloid, as a new DNA demethylating agent inducing cell cycle arrest and sensitization to trail in cancer cells. Oncotarget7, 24027–24049. 10.18632/oncotarget.8210
62
FouadY. A.AaneiC. (2017). Revisiting the hallmarks of cancer. Am. J. Cancer Res.7 (5), 1016–1036.
63
FuldaS.GalluzziL.KroemerG. (2010). Targeting mitochondria for cancer therapy. Nat. Rev. Drug Discov.9, 447–464. 10.1038/nrd3137
64
GhavamiS.HashemiM.AndeS. R.YeganehB.XiaoW.EshraghiM.et al (2009). Apoptosis and cancer: Mutations within caspase genes. J. Med. Genet.46 (8), 497–510. 10.1136/jmg.2009.066944
65
GhobrialI. M.WitzigT. E.AdjeiA. A. (2005). Targeting apoptosis pathways in cancer therapy. Ca. Cancer J. Clin.55 (3), 178–194. 10.3322/canjclin.55.3.178
66
GiorgiC.RomagnoliA.PintonP.RizzutoR. (2008). Ca2+ signaling, mitochondria and cell death. Curr. Mol. Med.8 (2), 119–130. 10.2174/156652408783769571
67
GorczycaW.GongJ.ArdeltB.TraganosF.DarZynkiewicZZ. (1993). The cell cycle related differences in susceptibility of HL-60 cells to apoptosis induced by various antitumor agents. Cancer Res.53 (13), 3186–3192.
68
GorskiS.MarraM. (2002). Programmed cell death takes flight: Genetic and genomic approaches to gene discovery in Drosophila. Physiol. Genomics9 (2), 59–69. 10.1152/physiolgenomics.00114.2001
69
GreenD. R. (2005). Apoptotic pathways: Ten minutes to dead. Cell121, 671–674. 10.1016/j.cell.2005.05.019
70
GuzmanE. A.MaersK.RobertsJ.Kemami-WangunH. V.HarmodyD.WrightA. E. (2015). The marine natural product microsclerodermin a is a novel inhibitor of the nuclear factor kappa B and induces apoptosis in pancreatic cancer cells. Investig. New Drugs33, 86–94.
71
GuzmanE. A.XuQ.PittsT. P.MitsuhashiK. O.BakerC.LinleyP. A.et al (2016). Leiodermatolide, a novel marine natural product, has potent cytotoxic and antimitotic activity against cancer cells, appears to affect microtubule dynamics, and exhibits antitumor activity. Int. J. Cancer139, 2116–2126.
72
GuzmanE.MaherM.TemkinA.PittsT.WrightA. (2013). Spongiatriol inhibits nuclear factor kappa B activation and induces apoptosis in pancreatic cancer cells. Mar. Drugs11, 1140–1151.
73
HajiaghaalipourF.KanthimathiM. S.SanusiJ.RajarajeswaranJ. (2015). White tea (Camellia sinensis) inhibits proliferation of the colon cancer cell line, HT-29, activates caspases and protects DNA of normal cells against oxidative damage. Food Chem.169, 401–410. 10.1016/j.foodchem.2014.07.005
74
HajtoT.KatarinaH.Hans-JoachimG. (1989). Modulatory potency of the β-galactoside specific lectin from mistletoe extract (Iscador) on the host defense system in vivo in rabbits and patients. Cancer Res.49 (17), 4803–4808.
75
HakemR.HarringtonL. (2005). The McGraw-hill companies inc. Cell death. 4th ed. New York: .
76
HanahanD.WeinbergR. A. (2000). The hallmarks of cancer. Cell100, 57–70. 10.1016/s0092-8674(00)81683-9
77
HarshaR. M.GhoshD.BanerjeeR.SalimathB. P. (2017). Suppression of VEGF-induced angiogenesis and tumor growth by Eugenia jambolana, Musa paradisiaca, and Coccinia indica extracts. Pharm. Biol.55, 1489–1499. 10.1080/13880209.2017.1307422
78
HassanM.WatariH.AbuAlmaatyA.OhbaY.SakuragiN. (2014). Apoptosis and molecular targeting therapy in cancer. Biomed. Res. Int.2014, 150845. 10.1155/2014/150845
79
HeinrichM.RoblesM.WestJ. E.Ortiz de MontellanoB. R.RodriguezE. (1998). Ethnopharmacology of Mexican asteraceae (compositae). Annu. Rev. Pharmacol. Toxicol.38, 539–565. 10.1146/annurev.pharmtox.38.1.539
80
HengartnerM. O. (2000). The biochemistry of apoptosis. Nature407, 770–776. 10.1038/35037710
81
HiraokaW.VazquezN.Nieves-NeiraW.ChanockS. J.PommierY. (1998). Role of oxygen radicals generated by NADPH oxidase in apoptosis induced in human leukemia cells. J. Clin. Invest.102 (11), 1961–1968. 10.1172/JCI3437
82
HoY. T.LuC. C.YangJ. S.ChiangJ. H.LiT. C.IpS. W.et al (2009). Berberine induced apoptosis via promoting the expression of caspase-8, -9 and -3, apoptosis-inducing factor and endonuclease G in SCC-4 human tongue squamous carcinoma cancer cells. Anticancer Res.29, 4063–4070.
83
HoodK. A.WestL. M.NorthcoteP. T.BerridgeM. V.MillerJ. H. (2001). Induction of apoptosis by the marine sponge (mycale) metabolites, mycalamide a and pateamine. Apoptosis6, 207–219. 10.1023/a:1011340827558
84
HuH.AhnN. S.YangX.LeeY. S.KangK. S. (2002). Ganoderma lucidum extract induces cell cycle arrest and apoptosis in MCF-7 human breast cancer cell. Int. J. Cancer102, 250–253. 10.1002/ijc.10707
85
HuQ.WuD.ChenW.YanZ.ShiY. (2013). Proteolytic processing of the caspase-9 zymogen is required for apoptosome-mediated activation of caspase-9. J. Biol. Chem.288 (21), 15142–15147. 10.1074/jbc.M112.441568
86
HuangH. H.KuoS. M.WuY. J.SuJ. H. (2016). Improvement and enhancement of antibladder carcinoma cell effects of heteronemin by the nanosized hyaluronan aggregation. Int. J. Nanomed.11, 1237–1251.
87
HuangY.ZhouY.FanY.ZhouD. (2008). Celastrol inhibits the growth of human glioma xenografts in nude mice through suppressing VEGFR expression. Cancer Lett.264, 101–106. 10.1016/j.canlet.2008.01.043
88
HudayahT.ChaudhryG.TaibM.IsmailN.MohammadT. S. T. (2017). Methanol extracts of four selected marine sponges induce apoptosis in human breast cancer cell line, MCF-7. Int. J. Res. Pharm. Sci.8 (4), 667–675.
89
Ihsan-ul- HaqMirzaB.TamaraP.KondratyukEun-JungP.BurnsB. E.MarlerL. E.et al (2013). Preliminary evaluation for cancer chemopreventive and cytotoxic potential of naturally growing ethnobotanically selected plants of Pakistan. Pharm. Biol.51 (3), 316–328. 10.3109/13880209.2012.728612
90
IsraelsE. D.IsraelsL. G. (2000). The cell cycle. Oncologist5 (6), 510–513. 10.1634/theoncologist.5-6-510
91
JacobsonM. D.WeilM.RaffM. C. (1997). Programmed cell death in animal development. Cell88, 347–354. 10.1016/s0092-8674(00)81873-5
92
JanR.ChaudhryG. E. (2019). Understanding apoptosis and apoptotic pathways targeted cancer therapeutics. Adv. Pharm. Bull.9 (2), 205–218. 10.15171/apb.2019.024
93
JohnstoneR. W.RuefliA. A.LoweS. W. (2002). Apoptosis: A link between cancer genetics and chemotherapy. Cell108, 153–164. 10.1016/s0092-8674(02)00625-6
94
KanducD.MittelmanA.SerpicoR.SinigagliaE.SinhaA. A.NataleC.et al (2002). Cell death: Apoptosis versus necrosis (review). Int. J. Oncol.21 (1), 165–170.
95
KavithaN.EinOonC.ChenY.KanwarJ. R.SasidharanPhaleriaS. (2017). Macrocarpa (Boerl.) fruit induce G0/G1 and G2/M cell cycle arrest and apoptosis through mitochondria-mediated pathway in MDA-MB-231 human breast cancer cell. J. Ethnopharmacol.201, 42–55.
96
KerrJ. F.WyllieA. H.CurrieA. R. (1972). Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer26 (4), 239–257. 10.1038/bjc.1972.33
97
KhazaeiS.Abdul HamidR.RamachandranV.Mohd EsaN.PanduranganA. K.DanazadehF.et al (2017). Cytotoxicity and proapoptotic effects of Allium atroviolaceum flower extract by modulating cell cycle arrest and caspase-dependent and p53-independent pathway in breast cancer cell lines. Evid. Based. Complement. Altern. Med.2017, 1468957. 10.1155/2017/1468957
98
KhazaeiS.HamidR. A.RamachandranV.EsaN. M.PanduranganA. K.DanazadehF.et al (2017). Cytotoxicity and proapoptotic effects of allium atroviolaceum flower extract by modulating cell cycle arrest and caspase-dependent and p53-independent pathway in breast cancer cell lines. Allium atroviolaceum flower extract by modulating cell cycle arrest and caspase-dependent and p53-independent pathway in breast cancer cell lines. Evid. Based. Complement. Altern. Med.2017, 1468957. 10.1155/2017/1468957
99
KijjoaA.WattanadilokR.CamposN.NascimentoM. S.PintoM.HerzW. (2007). Anticancer activity evaluation of kuanoniamines a and c isolated from the marine sponge oceanapia sagittaria, collected from the gulf of Thailand. Mar. Drugs5, 6–22.
100
KimB. M.ChoiY. J.HanY.YunY. S.HongS. H. N. (2009). N, N-dimethyl phytosphingosine induces caspase-8-dependent cytochrome c release and apoptosis through ROS generation in human leukemia cells. Toxicol. Appl. Pharmacol.239, 87–97. 10.1016/j.taap.2009.05.020
101
KimJ. Y.DaoT. T. P.SongK.ParkS. B.JangH.ParkM. K.et al (2018). Annona muricata leaf extract triggered intrinsic apoptotic pathway to attenuate cancerous features of triple negative breast cancer MDA-MB-231 cells. Evidence-Based Complementary Altern. Med.10, 1. 10.1155/2018/7972916
102
KimM. O.MoonD. O.HeoM. S.LeeJ. D.JungJ. H.KimS. K.et al (2008). Pectenotoxin-2 abolishes constitutively activated Nf-κB, leading to suppression of Nf-κB related gene products and potentiation of apoptosis. Cancer Lett.271, 25–33.
103
KimR. (2005). Recent advances in understanding the cell death pathways activated by anticancer therapy. Cancer103 (8), 1551–1560. 10.1002/cncr.20947
104
KimY. L.LeeS. K.ParkK. K.ChungW. Y. (2016). The inhibitory effects of forsythia koreana extracts on the metastatic ability of breast cancer cells and bone resorption by osteoclasts. J. Cancer Prev.21 (2), 88–94. 10.15430/JCP.2016.21.2.88
105
King-ThomC. (1997). Gastrointestinal toxicology of monogastrics", gastrointestinal microbiology. Springer US., 511–582.
106
KlenerP.AnderaL.KlenerP.NecasE.ZivnýJ. (2006). Cell death signalling pathways in the pathogenesis and therapy of haematologic malignancies: Overview of apoptotic pathways. Folia Biol.52 (1-2), 34–44.
107
KohR. Y.LimF. P.LingL. S. Y.LiewS. F.YewM. Y.et al (2017). Anticancer mechanisms of Strobilanthes crispa Blume hexane extract on liver and breast cancer cell lines. Oncol. Lett.14 (4), 4957–4964. 10.3892/ol.2017.6821
108
KongD.AokiS.SowaY.SakaiT.KobayashiM. (2008). Smenospongine, a sesquiterpene aminoquinone from a marine sponge, induces g1 arrest or apoptosis in different leukemia cells. Mar. Drugs6, 480–488.
109
KonishiH.KikuchiS.OchiaiT.IkomaH.KubotaT.IchikawaD.et al (2009). Latrunculin a has a strong anticancer effect in a peritoneal dissemination model of human gastric cancer in mice. Anticancer Res.29, 2091–2097.
110
KreugerM. R.GrootjansS.BiavattiM. W.VandenabeeleP.D'HerdeK. (2012). Sesquiterpene lactones as drugs with multiple targets in cancer treatment: Focus on parthenolide. Anticancer. Drugs23, 883–896. 10.1097/CAD.0b013e328356cad9
111
KumarS.SharmaV. K.YadavS.DeyS. (2017). Antiproliferative and apoptotic effects of black turtle bean extracts on human breast cancer cell line through extrinsic and intrinsic pathway. Chem. Cent. J.20 (111), 56. 10.1186/s13065-017-0281-5
112
KwanY. P.SaitoT.IbrahimD.Al-HassanF. M.Ein OonC.ChenY.et al (2016). Evaluation of the cytotoxicity, cell-cycle arrest, and apoptotic induction by Euphorbia hirta in MCF-7 breast cancer cells. Pharm. Biol.54, 1–14. 10.3109/13880209.2015.1064451
113
LaBarberaD. V.ModzelewskaK.GlazarA. I.GrayP. D.KaurM.LiuT.et al (2009). The marine alkaloid naamidine a promotes caspase-dependent apoptosis in tumor cells. Anticancer. Drugs20, 425–436. 10.1097/CAD.0b013e32832ae55f
114
LeBlancA. C. (2003). Natural cellular inhibitors of caspases. Prog. Neuropsychopharmacol. Biol. Psychiatry27 (2), 215–229. 10.1016/S0278-5846(03)00017-4
115
LeeH. Y.ChungK. J.HwangI. H.GwakJ.ParkS.JuB. G.et al (2015). Activation of p53 with ilimaquinone and ethylsmenoquinone, marine sponge metabolites, induces apoptosis and autophagy in colon cancer cells. Mar. Drugs13, 543–557.
116
LeeS. H.ParkS. M.ParkS. M.ParkJ. H.ShinD. Y.KimG. Y.et al (2009). Induction of apoptosis in human leukemia U937 cells by anthocyanins through down-regulation of Bcl-2 and activation of caspases. Int. J. Oncol.34, 1077–1083. 10.3892/ijo_00000234
117
LiuJ.ShenH. M.Choon-NamOngC. N. (2000). Salvia miltiorrhiza inhibits cell growth and induces apoptosis in human hepatoma HepG 2 cells. Cancer Lett.153 (1), 85–93. 10.1016/s0304-3835(00)00391-8
118
LiuJ.HuW. X.HeL. F.YeM.LiY. (2004). Effects of lycorine on HL 60 cells via arresting cell cycle and inducing apoptosis. FEBS Lett.578, 245–250. 10.1016/j.febslet.2004.10.095
119
LiuW. K.CheungF. W.CheC. T. (2006). Stellettin a induces oxidative stress and apoptosis in hl-60 human leukemia and lncap prostate cancer cell lines. J. Nat. Prod.69, 934–937.
120
LiuW. K.HoJ. C.CheC. T. (2005). Apoptotic activity of isomalabaricane triterpenes on human promyelocytic leukemia hl60 cells. Cancer Lett.230, 102–110. 10.1016/j.canlet.2004.12.034
121
LosM.Van de CraenM.PenningL. C.SchenkH.WestendorpM.BaeuerleP. A.et al (1995). Requirement of an ICE/CED-3 protease for Fas/APO-1-mediated apoptosis. Nature375 (6526), 81–83. 10.1038/375081a0
122
LuP. H.ChuehS. C.KungF. L.PanS. L.ShenY. C.GuhJ. H. (2007). Ilimaquinone, a marine sponge metabolite, displays anticancer activity via gadd153-mediated pathway. Eur. J. Pharmacol.556, 45–54.
123
MaY. S.WengS. W.LinM. W.LuC. C.ChiangJ. H.YangJ. S.et al (2012). Antitumor effects of emodin on LS1034 human colon cancer cells in vitro and in vivo: Roles of apoptotic cell death and LS1034 tumor xenografts model. Food Chem. Toxicol.50, 1271–1278. 10.1016/j.fct.2012.01.033
124
MaY.ZhuB.YongL.SongC.LiuX.YuH.et al (2016). Regulation of intrinsic and extrinsic apoptotic pathways in osteosarcoma cells following oleandrin treatment. Int. J. Mol. Sci.17, 1950. 10.3390/ijms17111950
125
MakotoI.SuzukiR.KoideT.SakaguchiN.OgiharaY.YabuY. (1994). Antioxidant, gallic acid, induces apoptosis in HL-60RG cells. Biochem. Biophys. Res. Commun.204 (2), 898–904. 10.1006/bbrc.1994.2544
126
Manske Richard HelmuthF. (1965). The alkaloids: Chemistry and physiology‖, 5. Academic Press.
127
MartinT. A.YeL.SandersA. J.LaneJ.JiangW. G. (2000). “Cancer invasion and metastasis: Molecular and cellular perspective,” in Madame curie bioscience database. Editors BlagosklonnyM. V.PardeeA. B. (Austin, TX: Landes Bioscience), 2013.
128
MatsumotoK.AkaoY.KobayashiE.OhguchiK.ItoT.TanakaT.et al (2003). ―Induction of apoptosis by xanthones from mangosteen in human leukemia cell lines. J. Nat. Prod.66, 1124–1127. 10.1021/np020546u
129
McNaughtA. D.AndrewW. (1997). Compendium of chemical terminology. Oxford Blackwell: IUPAC recommendations.
130
MedemaJ. P.ScaffidiC.KischkelF. C.ShevchenkoA.MannM.et al (1997). FLICE is activated by association with the CD95 death-inducing signaling complex (DISC). EMBO J.16 (10), 2794–2804. 10.1093/emboj/16.10.2794
131
MeyerM.SchreckR.PatrickA. B. (2005). H2O2 and antioxidants have opposite effects on activation of NF-kappa B and AP-1 in intact cells: AP-1 as secondary antioxidant-responsive factor. EMBO J.12 (5), 2005–2015. 10.1002/j.1460-2075.1993.tb05850.x
132
Momtazi-borojeniA. A.BehbahaniM.Sadeghi-aliabadiH. (2013). Antiproliferative activity and apoptosis induction of crude extract and fractions of Avicennia marina. Iran. J. Basic Med. Sci.16 (11), 1203–1208.
133
MooberryS. L.TienG.HernandezA. H.PlubrukarnA.DavidsonB. S. (1999). Laulimalide and isolaulimalide, new paclitaxel-like microtubule-stabilizing agents. Cancer Res.59, 653–660.
134
MoonD. O.KimM. O.KangS. H.LeeK. J.HeoM. S.ChoiK. S.et al (2008). Induction of G2/M arrest, endoreduplication, and apoptosis by actin depolymerization agent pextenotoxin-2 in human leukemia cells, involving activation of erk and jnk. Biochem. Pharmacol.76, 312–321.
135
MouH.ZhengY.ZhaoP.BaoH.FangW.XuN.et al (2011). Celastrol induces apoptosis in non-small-cell lung cancer A549 cells through activation of mitochondria- and Fas/FasL-mediated pathways. Toxicol. Vitro25, 1027–1032. 10.1016/j.tiv.2011.03.023
136
NairM. S.SorenK.SinghV.BoroB. (2016). Anticancer activity of fruit and leaf extracts of averrhoa bilimbi on MCF-7 human breast cancer cell lines: A preliminary study. Austin J. Pharmacol. Ther.4 (2), 1082.
137
NakagawaY.IinumaM.NaoeT.NozawaY.AkaoY. (2007). Characterized mechanism of alpha-mangostin-induced cell death: Caspase-independent apoptosis with release of endonuclease-G from mitochondria and increased miR-143 expression in human colorectal cancer DLD-1 cells. Bioorg. Med. Chem.15, 5620–5628. 10.1016/j.bmc.2007.04.071
138
NeumanM. G.KatzG. G.MalkiewiczI. M.MathurinP.TsukamotoH.AdachiM.et al (2002). Alcoholic liver injury and apoptosis--synopsis of the symposium held at ESBRA 2001: 8th congress of the European society for biomedical research on alcoholism, paris, september 16, 2001. Alcohol28 (2), 117–128. 10.1016/s0741-8329(02)00243-4
139
NgC. P.BonavidaB. (2002). A new challenge for successful immunotherapy by tumors that are resistant to apoptosis: Two complementary signals to overcome cross-resistance. Adv. Cancer Res.85, 145–174. 10.1016/s0065-230x(02)85005-9
140
NguyenH. T.ChauV. M.TranT. H.PhanV. K.HoangT. H.NguyenT. D.et al (2009). C29 sterols with a cyclopropane ring at C-25 and 26 from the Vietnamese marine sponge ianthella sp. in addition, their anticancer properties. Bioorg. Med. Chem. Lett.19, 4584–4588.
141
NhoK. J.ChunJ. M.KimH. K. (2015). Anti-metastatic effect of Smilax China L. extract on MDA-MB-231 cells. Mol. Med. Rep.11, 499–502. 10.3892/mmr.2014.2698
142
NithyaM.AmbikapathyV.PanneerselvamA.ThajuddinN. (2014). Anti-tumour activity of different extracts of ganoderma lucidum (curt.: Fr.) p. karst. World J. Pharm. Res.3 (4), 2204–2214.
143
OrangiM.ArdalanP.ShanehbandiD.KazemiT.YousefiB.Behnaz-AlsadatH.et al (2016). Cytotoxic and apoptotic activities of methanolic sub fractions of Scrophularia oxysepala against human breast cancer cell line. Evidence-based complementary and alternative medicine.
144
OrangiM.PasdaranA.ShanehbandiD.KazemiT.YousefiB.HosseiniB. A.et al (2016). Cytotoxic and apoptotic activities of methanolic subfractions of Scrophularia oxysepala against human breast cancer cell line. Evid. Based. Complement. Altern. Med.2016, 8540640. 10.1155/2016/8540640
145
PalH. C.SeharI.BhushanS.GuptaB. D.SaxenaA. K. (2010). Activation of caspases and poly (ADP-ribose) polymerase cleavage to induce apoptosis in leukemia HL-60 cells by Inula racemosa. Toxicol. Vitro24, 1599–1609. 10.1016/j.tiv.2010.06.007
146
PanM. H.LaiC. S.HsuP. C.WangY. J. (2005). Acacetin induces apoptosis in human gastric carcinoma cells accompanied by activation of caspase cascades and production of reactive oxygen species. J. Agric. Food Chem.53, 620–630. 10.1021/jf048430m
147
ParkS. E.ShinW. T.ParkC.HongS. H.KimG. Y.KimS. O.et al (2014). Induction of apoptosis in MDA-MB-231 human breast carcinoma cells with an ethanol extract of Cyperus rotundus L. by activating caspases. Oncol. Rep.32, 2461–2470.
148
PistrittoG.TrisciuoglioD.CeciC.GarufiA.D'OraziG. (2016). Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging8 (4), 603–619. 10.18632/aging.100934
149
PucciB.KastenM.GiordanoA. (2000). Cell cycle and apoptosis. Neoplasia2 (4), 291–299. 10.1038/sj.neo.7900101
150
RanganathaR. S.MaheshH.KishoreK. C.AnjaneyuluM.BibhaC.SatheesC. (2012). Raghavan extracts of strawberry fruits induce intrinsic pathway of apoptosis in breast cancer cells and inhibits tumor progression in mice. PLoS One7 (10), e47021.
151
RaoC. V.RivensonA.SimiB.ZangE.KelloffG.SteeleV.et al (1995). Chemoprevention of colon carcinogenesis by sulindac, a nonsteroidal anti- inflammatory agent. Cancer Res.55 (7), 1464–1472.
152
Rao ChinthalapallyV.RivensonA.SimiB.ReddyB. S. (1995). Chemoprevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolic compound. Cancer Res.55 (2), 259–266.
153
ReddyB. S.WangC. X.SamahaH.LubetR.SteeleV. E.KelloffG. J.et al (1997). Chemoprevention of colon carcinogenesis by dietary perillyl alcohol. Cancer Res.57, 420–425.
154
ReedJ. C.GreenD. R. (2002). Remodeling for demolition: Changes in mitochondrial ultrastructure during apoptosis. Mol. Cell9, 1–3. 10.1016/s1097-2765(02)00437-9
155
RenW. E. I. P. I. N. G.TangD. G. (1999). Extract of Solanum muricatum (Pepino/CSG) inhibits tumor growth by inducing apoptosis. Anticancer Res.191A, 403–408.
156
RezaeiP. F.FouladdelSh.CristofanonS.GhaffariS. M.AminG. R.AziziE. (2011). Comparative cellular and molecular analysis of cytotoxicity and apoptosis induction by doxorubicin and Baneh in human breast cancer T47D cells. Cytotechnology63 (5), 503–512.
157
RoelM.RubioloJ. A.Guerra-VarelaJ.SilvaS. B.ThomasO. P.Cabezas-SainzP.et al (2016). Marine guanidine alkaloids crambescidins inhibit tumor growth and activate intrinsic apoptotic signaling inducing tumor regression in a colorectal carcinoma zebrafish xenograft model. Oncotarget7, 83071–83087. 10.18632/oncotarget.13068
158
RoseP.HuangQ.OngC. N.WhitemanM. (2005). Broccoli and watercress suppress matrix metalloproteinase-9 activity and invasiveness of human MDA-MB-231 breast cancer cells. Toxicol. Appl. Pharmacol.209 (2), 105–113. 10.1016/j.taap.2005.04.010
159
RothmeierA. S.SchneidersU. M.WiedmannR. M.IschenkoI.BrunsC. J.RudyA.et al (2010). The marine compound spongistatin 1 targets pancreatic tumor progression and metastasis. Int. J. Cancer127, 1096–1105.
160
SabinoA. P. L.EustáquioL. M. S.MirandaA. C. F.BiojoneC.MariosaT. N.GouvêaC. M. C. P.et al (2018). Stryphnodendron adstringens ("Barbatimão") leaf fraction: Chemical characterization, antioxidant activity, and cytotoxicity towards human breast cancer cell lines. Appl. Biochem. Biotechnol.184 (4), 1375–1389. 10.1007/s12010-017-2632-z
161
SakagamiH.KuribayashiN.IidaM.SakagamiT.TakedaM.FuKuchiK.et al (1994). Induction of DNA fragmentation by tannin-and lignin-related substances. Anticancer Res.15 (5B), 2121–2128.
162
SalmaY.LafontE.ThervilleN.CarpentierS.BonnafeM. J.LevadeT.et al (2009). The natural marine anhydrophytosphingosine, jaspine b, induces apoptosis in melanoma cells by interfering with ceramide metabolism. Biochem. Pharmacol.78, 477–485.
163
SankariS. L.MasthanK. M.BabuN. A.BhattacharjeeT.ElumalaiM. (2012). Apoptosis in cancer-an update. Asian pac. J. Cancer Prev.13 (10), 4873–4878. 10.7314/apjcp.2012.13.10.4873
164
SantosK. M.GomesI. N. F.RomaoW.RibeiroR. I. M. A.Silva-OliveiraR. J.PintoF. E.et al (2017). Bauhinia stem extracts as a possible new treatment for breast cancer and metastasis: Inhibition of migration, invasion and of the activity of matrix metalloproteinases. J. Biotechnol. Biomater.7, 6.
165
SchneidersU. M.SchyschkaL.RudyA.VollmarA. M. (2009). Bh3-only proteins mcl-1 and bim as well as endonuclease g are targeted in spongistatin 1-induced apoptosis in breast cancer cells. Mol. Cancer Ther.8, 2914–2925.
166
SchultzeJ. L.StettinA.BergP. A. (1991). Demonstration of specifically sensitized lymphocytes in patients trea ted with an aqueous mistletoe extract (Viscum album L.). Klin. Wochenschr.69 (9), 397–403. 10.1007/BF01647413
167
SchumacherM.CerellaC.EifesS.ChateauvieuxS.MorceauF.JasparsM.et al (2010). Heteronemin, a spongean sesterterpene, inhibits tnf alpha-induced NF-κB activation through proteasome inhibition and induces apoptotic cell death. Biochem. Pharmacol.79, 610–622.
168
SchyschkaL.RudyA.JeremiasI.BarthN.PettitG. R.VollmarA. M. (2008). Spongistatin 1: A new chemosensitizing marine compound that degrades xiap. Leukemia22, 1737–1745. 10.1038/leu.2008.146
169
SharmaK.PachauriS. D.KhandelwalK.AhmadH.AryAA.BialaP.et al (2016). Anticancer effects of extracts from the fruit of Morinda citrifolia (noni) in breast cancer cell lines. Drug Res.66 (3), 141–147. 10.1055/s-0035-1555804
170
ShinD. Y.KimG. Y.KimN. D.JungJ. H.KimS. K.KangH. S.et al (2008). Induction of apoptosis by pectenotoxin-2 is mediated with the induction of DR4/DR5, EGR-1 and NAG-1, activation of caspases and modulation of the Bcl-2 family in p53-deficient Hep3B hepatocellular carcinoma cells. Oncol. Rep.19, 517–526.
171
ShinS. A.LeeH. N.ChooG. S.KimH. J.CheJ. H.JungJ. Y.et al (2017). Ixeris dentata (thunb. Ex thunb.) nakai extract inhibits proliferation and induces apoptosis in breast cancer cells through akt/NF-κB pathways. Int. J. Mol. Sci.18 (2), E275. 10.3390/ijms18020275
172
ShinS. Y.KimC. G.JungY. J.JungY.JungH.ImJ.et al (2016). Euphorbia humifusa Willd exerts inhibition of breast cancer cell invasion and metastasis through inhibition of TNFα-induced MMP-9 expression. BMC Complement. Altern. Med.16 (1), 413. 10.1186/s12906-016-1404-6
173
Shu-HuiH.TsaiT. R.LinC. N.YenM. H.KuoK. W. (1996). Solamargine purified from Solanum incanum Chinese herb triggers gene expression of human TNFR I which may lead to cell apoptosis. Biochem. Biophys. Res. Commun.229, 1–5. 10.1006/bbrc.1996.1748
174
Shu-WeiY. (1999). Synthesis and biological evaluation of analogues of cryptolepine, an alkaloid isolated from the Suriname rainforest 1. J. Nat. Prod.62 (7), 976–983.
175
SilvaM. J. D.CarvalhoA. J. S.RochaC. Q.VilegasW.SilvaM. A.GouvêaC. M. C. P. (2014). Ethanolic extract of Mimosa caesalpiniifolia leaves: Chemical characterization and cytotoxic effect on human breast cancer MCF-7 cell line. South Afr. J. Bot.93, 64–69.
176
SomasagaraR. R.HegdeM.ChiruvellaK. K.MusiniA.ChoudharyB.RaghavanS. C.et al (2012). Extracts of strawberry fruits induce intrinsic pathway of apoptosis in breast cancer cells and inhibits tumor progression in mice. PLoS One7 (10), e47021. 10.1371/journal.pone.0047021
177
StergiouL.HengartnerM. O. (2004). Death and more: DNA damage response pathways in the nematode C. elegans. Cell Death Differ.11 (1), 21–28. 10.1038/sj.cdd.4401340
178
SuZ. Z.LinJ.PrewettM.GoldsteinN. I.FisherP. B. (1994). Apoptosis mediates the selective toxicity of caffeic acid phenethyl ester (CAPE) toward oncogene-transformed rat embryo fibroblast cells. Anticancer Res.15 (5), 1841–1848.
179
SubapriyaR.BhuvaneswariV.NaginiS. (2005). Ethanolic neem (Azadirachta indica) leaf extract induces apoptosis in the hamster buccal pouch carcinogenesis model by modulation of Bcl-2, Bim, caspase 8 and caspase 3. Asian pac. J. Cancer Prev.6, 515–520.
180
SudhakarA. (2009). History of cancer, ancient and modern treatment methods. J. Cancer Sci. Ther.1 (2), 1–4. 10.4172/1948-5956.100000e2
181
SuiC. G.MengF. D.LiY.JiangY. H. (2015). Antiproliferative activity of rosamulticacidis associated with induction of apoptosis, cell cycle arrest, inhibition of cell migration and caspase activation in human gastric cancer (SGC-7901) cells. Phytomedicine22, 796–806. 10.1016/j.phymed.2015.05.004
182
TangS. A.ZhouQ.GuoW. Z.QiuY.WangR.JinM.et al (2014). In vitro antitumor activity of stellettin b, a triterpene from marine sponge jaspis stellifera, on human glioblastoma cancer sf295 cells. Mar. Drugs12, 4200–4213.
183
TangS. Y.ZhongM. Z.YuanG. J.HouS. P.YinL. L.JiangH.et al (2013). Casticin, a flavonoid, potentiates TRAIL-induced apoptosis through modulation of anti-apoptotic proteins and death receptor 5 in colon cancer cells. Oncol. Rep.29, 474–480. 10.3892/or.2012.2127
184
Tayarani-NajaranaZ.AsilibJ.AioubibE.EmamiS. A. (2013). Growth inhibition and apoptosis induction of salvia chloroleuca on MCF-7 breast cancer cell line. Iran. J. Pharm. Res.12 (4), 789–799.
185
ThamizhiniyanV.ChoiY. W.KimY. K. (2015). The cytotoxic nature of Acanthopanax sessiliflorus stem bark extracts in human breast cancer cells. Saudi J. Biol. Sci.22 (6), 752–759.
186
ThompsonC. B. (1995). Apoptosis in the pathogenesis and treatment of disease. Science267, 1456–1462. 10.1126/science.7878464
187
TorY. S.YazanL. S.FooJ. B.WibowoA.IsmailN.CheahY. K.et al (2015). Induction of apoptosis in MCF-7 cells via oxidative stress generation, mitochondria-dependent and caspase-independent pathway by ethyl acetate extract of Dillenia suffruticosa and its chemical profile. PLoS One10 (6), e0127441. 10.1371/journal.pone.0127441
188
TrisciuoglioD.UranchimegB.CardellinaJ. H.MeragelmanT. L.MatsunagaS.FusetaniN.et al (2008). Induction of apoptosis in human cancer cells by candidaspongiolide, a novel sponge polyketide. J. Natl. Cancer Inst.100, 1233–1246.
189
UmeyamaA.MatsuokaN.MineR.NakataA.ArimotoE.MatsuiM.et al (2010). Polyacetylene diols with antiproliferative and driving Th1 polarization effects from the marine sponge Callyspongia sp. J. Nat. Med.64, 93–97.
190
VenderovaK.ParkD. S. (2012). Programmed cell death in Parkinson’s disease. Cold Spring Harb. Perspect. Med.2 (8), a009365. 10.1101/cshperspect.a009365
191
VerdineG. L. (1996). The combinatorial chemistry of nature. Nature384 (1), 11–13. 10.1038/384011a0
192
VogelsteinB.KinzlerK. W. (2004). Cancer genes and the pathways they control. Nat. Med.10, 789–799. 10.1038/nm1087
193
WangM. K.DingL. S.WuF. E. (2008). Antitumor effects of raddeanin A on S180, H22 and U14 cell xenografts in mice. Ai Zheng27, 910–913.
194
WangR.ZhangQ.PengX.ZhouC.ZhongY.ChenX.et al (2016). Stellettin b induces g1 arrest, apoptosis and autophagy in human non-small cell lung cancer A549 cells via blocking PI3K/Akt/mTOR pathway. Sci. Rep.6, 27071.
195
WeiS. Y.LiM.TangS. A.SunW.XuB.CuiJ. R.et al (2008). Induction of apoptosis accompanying with g(1) phase arrest and microtubule disassembly in human hepatoma cells by jaspolide b, a new isomalabaricane-type triterpene. Cancer Lett.262, 114–122. 10.1016/j.canlet.2007.11.039
196
WeiminF. (1999). Possible mechanisms of paclitaxel-induced apoptosis. Biochem. Pharmacol.57 (11), 1215–1221. 10.1016/s0006-2952(99)00006-4
197
WicaksonoB. D.HandokoY. A.ArungE. T.KusumaI. W.YuliaD.PancaputraA. N.et al (2009). Antiproliferative effect of the methanol extract of piper crocatum ruiz & pav leaves on human breast (T47D) cells in-vitro. Trop. J. Pharm. Res.8 (4).
198
Wolvetang ErnstJ.LarmJ. A.MoutsoulasP.LAwenA. (1996). Apoptosis induced by inhibitors of the plasma membrane NADH-oxidase involves Bcl-2 and calcineurin. Cell Growth Differ.7 (10), 1315–1325.
199
WongF. C.WooC. C.HsuA.KwongB.TanH. (2013). The anti-cancer activities of vernonia amygdalina extract in human breast cancer cell lines are mediated through caspase-dependent and p53-independent pathways. PLoS One8 (10), e78021. 10.1371/journal.pone.0078021
200
WuJ. C.WangC. T.HungH. C.WuW. J.WuD. C.ChangM. C.et al (2016). Heteronemin is a novel c-Met/STAT3 inhibitor against advanced prostate cancer cells. Prostate76, 1469–1483. 10.1002/pros.23230
201
WuS. Y.SungP. J.ChangY. L.PanS. L.TengC. M. (2015). Heteronemin, a spongean sesterterpene, induces cell apoptosis and autophagy in human renal carcinoma cells. Biomed. Res. Int.2015, 738241.
202
XuW.WangX.TuY.MasakiH.TanakaS.OndaK.et al (2019). Tetrandrine and cepharanthine induce apoptosis through caspase cascade regulation, cell cycle arrest, MAPK activation and PI3K/Akt/mTOR signal modification in glucocorticoid resistant human leukemia Jurkat T cells. Chem. Biol. Interact.310, 108726. 10.1016/j.cbi.2019.108726
203
XueG.ZouX.ZhouJ. Y.SunW.WuJ.XuJ. L.et al (2013). Raddeanin A induces human gastric cancer cells apoptosis and inhibits their invasion in vitro. Biochem. Biophys. Res. Commun.439, 196–202. 10.1016/j.bbrc.2013.08.060
204
YanoH.TatsutaM.IisHiH.BabaM.SakaiN.UedoN. (1999). Attenuation by d‐limonene of sodium chloride‐enhanced gastric carcinogenesis induced by N‐methyl‐N′‐nitro‐N‐nitrosoguanidine in Wistar rats. Int. J. Cancer82 (5), 665–668. 10.1002/(sici)1097-0215(19990827)82:5<665::aid-ijc8>3.0.co;2-e
205
YasserM. T.SaadS. D.LoiyE.AhmedH.Majed AhmedAl-M.MohamadT. A.et al (2015). Vitro anti-metastatic and antioxidant activity of nicotiana glauca fraction against breast cancer cells. Adv. Biol. Res.9 (2), 95–102.
206
YeungT. K.GermondC.ChenX.WangZ. (1999). The mode of action of taxol: Apoptosis at low concentration and necrosis at high concentration. Biochem. Biophys. Res. Commun.263 (2), 398–404. 10.1006/bbrc.1999.1375
207
YooH.LeeY. S.LeeS.KimS.KimT. Y. (2012). Pachastrissamine from Pachastrissa sp. Inhibits melanoma cell growth by dual inhibition of Cdk2 and erk-mediated foxo3 downregulation. Phytother. Res.26, 1927–1933.
208
YuanS.AkeyC. W. (2013). Apoptosome structure, assembly, and procaspase activation. Structure21 (4), 501–515. 10.1016/j.str.2013.02.024
209
ZhangY. W.GhoshA. K.PommierY. (2012). Lasonolide a, a potent and reversible inducer of chromosome condensation. Cell Cycle11, 4424–4435. 10.4161/cc.22768
210
ZhaoY.TianB.WangY.DingH. (2017). Kaempferol sensitizes human ovarian cancer cells-OVCAR-3 and SKOV-3 to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-Induced apoptosis via JNK/ERK-CHOP pathway and up-regulation of death receptors 4 and 5. Med. Sci. Monit.23, 5096–5105. 10.12659/msm.903552
211
ZhouR.ChenH.ChenJ.ChenX.WenY.XuL.et al (2018). Extract from Astragalus membranaceus inhibit breast cancer cells proliferation via PI3K/AKT/mTOR signaling pathway. BMC Complement. Altern. Med.18 (1), 83. 10.1186/s12906-018-2148-2
212
ZihlifM.AfifiF.Abu-DahabR.MajidA. M. S. A.SomrainH.et al (2013). The antiangiogenic activities of ethanolic crude extracts of four Salvia species.Salvia species. BMC Complement. Altern. Med.13, 358. 10.1186/1472-6882-13-358
213
ZornigM.HueberA.BaumW.EvanG. (2001). Apoptosis regulators and their role in tumorigenesis. Biochim. Biophys. Acta1551, 1–37. 10.1016/s0304-419x(01)00031-2
214
ZuhrotunN. F.AstutiM.MurdiatiA.Mubarika HaryanaS. (2017). Anti-proliferation and apoptosis induction of aqueous leaf extract of carica papaya L. On human breast cancer cells MCF-7. Pak. J. Biol. Sci.20 (1), 36–41. 10.3923/pjbs.2017.36.41
Summary
Keywords
apoptosis, marine drug, cancer, natural product, targeted therapeutic, apoptotic inducers
Citation
Chaudhry G-e-S, Md Akim A, Sung YY and Sifzizul TMT (2022) Cancer and apoptosis: The apoptotic activity of plant and marine natural products and their potential as targeted cancer therapeutics. Front. Pharmacol. 13:842376. doi: 10.3389/fphar.2022.842376
Received
23 December 2021
Accepted
13 July 2022
Published
10 August 2022
Volume
13 - 2022
Edited by
Nehad M. Ayoub, Jordan University of Science and Technology, Jordan
Reviewed by
Pamela Ovadje, Innovate Calgary, Canada
Mrutyunjay Jena, Berhampur University, India
Huidi Liu, Harbin Medical University, China
Updates
Copyright
© 2022 Chaudhry, Md Akim, Sung and Sifzizul.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gul-e-Saba Chaudhry, sababiochem@gmail.com, gul.saba@umt.edu.my
This article was submitted to Pharmacology of Anti-Cancer Drugs, a section of the journal Frontiers in Pharmacology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.