- 1The Second Clinical Medical College of Guangzhou University of Chinese Medicine, Guangzhou, China
- 2Guangdong Provincial Key Laboratory of Research on Emergency in Traditional Chinese Medicine, Clinical Research Team of Prevention and Treatment of Cardiac Emergencies with Traditional Chinese Medicine, Guangzhou, China
- 3Emergency Department of Guangdong Provincial Hospital of Traditional Chinese Medicine, Guangzhou, China
Background: Septic shock is associated with high morbidity and mortality. Studies have reported that Chinese herbal injections (CHIs) in combination with Western medicine (WM) were more favorable. However, the debate on optimal CHIs is ongoing. The objective of this study is to explore the comparative effectiveness of CHIs for septic shock.
Methods: We retrieved data from the English and Chinese databases with retrieval time from database inception to 30 September 2021. Network meta-analysis was performed, with evaluation of methodological quality among the included studies and assessment of strength of evidence among the outcomes.
Results: A total of 77 RCTs with 5,647 patients were included. All the studies were rated as some concerns. In terms of 28-days-mortality, Yiqifumai injection (YQFM)+WM, Shuxuetong injection (SXT)+WM, Xuebijing injection (XBJ)+WM, and Shenfu injection (SF)+WM were better than WM; YQFM + WM and SXT + WM were superior for Shenmai injection (SM)+WM; YQFM + WM was superior for SF + WM; YQFM + WM ranked first. Regarding ICU length of stay, SF + WM and XBJ + WM were better than WM; XBJ + WM was superior for SF + WM; XBJ + WM ranked first. Concerning hospital length of stay, Shenqifuzheng injection (SQFZ)+WM, Shengmai injection (SGM)+WM, and XBJ + WM had greater potential than WM and SF + WM; SQFZ + WM ranked first. As for SOFA score at 7-days, XBJ + WM and SF + WM were superior for WM; XBJ + WM was superior for SF + WM; XBJ + WM ranked first. Regarding procalcitonin level at 7-days, SF + WM, SM + WM, and Xiyanping injection (XYP)+WM were better than WM; XYP + WM was superior for SF + WM, SGM + WM, SM + WM, Danshen injection (DS)+WM, and XBJ + WM; XYP + WM ranked first. Concerning serum lactate level at 7-days, SF + WM and SM + WM were more effective than XBJ + WM and WM; SM + WM ranked first. The comparisons were rated as moderate (15.05%), low (40.86%), and very low quality (44.09%); the strength of evidence of ranking probability for hospital length of stay was low whereas the remaining outcomes were rated as very low.
Conclusions: CHIs combined with WM might have higher efficacies for septic shock than WM alone. YQFM, XBJ, SQFZ, XYP, SM, SGM, and SF may be the potential optimal CHIs for septic shock. More and better evidence is needed to validate the conclusions.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?, identifier CRD42021282958.
Introduction
As a common critical disease in emergency department and intensive care unit (ICU), septic shock is a critical syndrome caused by a dysregulated host response to infection, accompanied by circulatory failure that is difficult to correct (Shankar-Hari et al., 2016; Singer et al., 2016). Although western prevention plans or treatment programs for septic shock were being continually updated (Levy et al., 2018; Evans et al., 2021), worldwide, morbidity and mortality associated with septic shock are still high. In 2012, a global estimate for septic shock incidence was 11 per 100,000 people annually (Jawad et al., 2012), which increased to 19.3 per 1,00,000 person-years in 2016 (Shankar-Hari et al., 2017). In addition, the mortality of septic shock ranged from 20 to 50% based on the evaluations (Levy et al., 2012; Quenot et al., 2013; Kaukonen et al., 2014). More seriously, with the outbreak of COVID-19, the morbidity and mortality of septic shock may increase further (Karakike et al., 2021).
Septic shock therapy has conventionally been dominated by anti-infective drugs, intravenous fluid, and vasopressors, all of which, however, still have shortcomings in reducing mortality of septic shock (Bauer et al., 2020). To make up for the deficiency of conventional treatment approaches, adjuvant therapies for septic shock have been constantly explored, such as hydrocortisone, ascorbic acid, as well as thiamine (Marik et al., 2017; Annane et al., 2018), whereas the therapeutic effects of them were still far from satisfactory. Indeed, there is another promising complementary treatment, namely traditional Chinese medicine, going back over 1,000 years and being accepted as one of the main types of therapy for septic shock in China (Unschuld, 1999; Wang et al., 2020). Chinese herbal injections (CHIs), as one of the most common dosage forms of traditional Chinese medicine, have been proved to exert therapeutic effects on patients with septic shock (Zhou et al., 2016; Li, 2018; Ha et al., 2019).
However, there is a wide variety of CHIs used for septic shock; how to choose the most appropriate one remains a problem for clinicians when facing septic shock patients, especially patients in different disease states. There are no studies thus far comparing different categories of CHIs used in the treatment of septic shock. Consequently, we searched all the randomized controlled trials (RCTs) of CHIs used to treat septic shock and initiated this network meta-analysis (NMA) to compare the efficacy among them, hoping to provide some advice for clinical practice.
Methods
We have registered on the International Prospective Register of Systematic Reviews with registration number CRD42021282958. The study followed the PRISMA Extension Statement (Hutton et al., 2015). The full and detailed PRISMA checklist is provided in Supplementary File S1.
Search Strategy
We searched both English-language electronic databases (PubMed, embase, Web of Science, and Cochrane Library) and Chinese-language electronic databases (China National Knowledge Infrastructure, Wanfang database, Weipu Journal database, and Chinese Biomedical Literature database). RCTs published from database inception through 30 September 2021, were searched. The search strategies are detailed in Supplementary File S2. All the search results were downloaded and imported into EndNote X9 software.
Types of Studies
RCTs were the original studies we consented to the inclusion, in which randomized crossover trials were excluded if the effect size of the early phase was not available. We had no restrictions on language, country, date of publication, and stage of the RCTs.
Types of Participants
Patients aged 18 years or older, with the diagnosis of septic shock, were considered. The diagnostic criteria for septic shock were as follows:
(1) Sepsis 1.0: Sepsis-related hypotension persists despite adequate fluid resuscitation (systolic blood pressure <90 mmHg or decreases ≥40 mmHg from baseline in the absence of other causes for hypotension). Patients who appear to be normotensive after vasopressor therapy should also be considered (Bone et al., 1992).
(2) Sepsis 2.0: Sepsis-related hypotension persists despite adequate fluid resuscitation (systolic blood pressure <90 mmHg, mean arterial pressure <60 mmHg, or decreases ≥40 mmHg from baseline in the absence of other causes of hypotension) (Levy et al., 2003).
(3) Sepsis 3.0: Despite adequate fluid resuscitation, patients have serum lactate level >2 mmol/L (>18 mg/dl) regardless of the absence of hypovolemia and require vasopressor therapy to maintain mean arterial blood pressure ≥65 mmHg (Singer et al., 2016).
In addition, there were no restrictions on the gender, nationality, ethnicity, or race of the patients. However, studies targeting patients with concurrent septic shock and severe profiles of comorbidities (i.e., cardiac arrest and advanced cancer) that most likely impact prognosis were excluded.
Types of Interventions
We required the RCTs to have at least two interventions, i.e., CHIs plus Western medicine (WM) versus WM, or CHIs plus WM versus another type of CHIs plus WM. We requested that the CHIs should be used intravenously, but not restricted time of intervention, the configuration of the drug, frequency of medication, and treatment cycle.
Types of Outcomes
The primary outcome of this study was 28-days-mortality. Secondary outcomes included the following:
(1) ICU length of stay.
(2) Hospital length of stay.
(3) Sequential Organ Failure Assessment (SOFA)score at day 7 after interventions.
(4) Procalcitonin level at day 7 after interventions.
(5) Serum lactate level at day 7 after interventions.
(6) Adverse drug reactions (ADRs).
Data Extraction and Quality Assessment
According to the inclusion and exclusion criteria, two reviewers independently screened the original studies by reading titles, abstracts, and full texts. The titles, years of publication, first-authors, sample sizes, age and gender of participants, diagnostic criteria, interventions, trial duration, outcomes, ADRs, blinding, and random methods of the selected studies were extracted with a predesigned form and were further entered into Excel 356 software.
Another two reviewers implemented quality assessment independently. We used Version 2 of the Cochrane risk-of-bias tool for randomized trials (RoB 2) (Sterne et al., 2019) to evaluate the quality of the included RCTs through the following aspects: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. Item “overall bias” summarized the overall assessment of the above five items. Each item contains “low risk”, “high risk”, and “some concerns”, and an RCT was rated as “low risk” overall only if all items of it were assessed as “low risk”. The current version (22 August 2019) of RoB 2 was used to produce a risk of bias graph.
When any disagreements occurred in the process of data extraction or quality assessment, the operators negotiated first. If the discrepancies were still unresolved, a third reviewer intervened in the arbitration.
Data Analysis
In this study, the program was analyzed by the Bayesian algorithm (Salanti, 2012). Pooled dichotomous-effect measures were expressed as pooled risk ratio (RR)with 95% confidence interval (CI) while pooled continuous variables were expressed as mean differences (MD) with 95%CI. The Markov Chain Monte Carlo methodology was adopted to construct the environment of analysis based on a random-effects model (Salanti et al., 2008; Mavridis and Salanti, 2013). Based on four Monte Carlo Markov Chains, we set the number of iterations as 200,000 and the first 10,000 were used for the annealing algorithm to eliminate the influence of the initial value. Brooks-Gelman-Rubin plots were used to evaluate the goodness-of-fit of the result (The median line and the 97.5% line tended to 1 after iteration, indicating that the model was stable) (Brooks and Gelman, 1998). If the goodness-of-fit of the result was still unsatisfactory, the number of iterations was further increased until the goodness-of-fit was satisfactory. Additionally, no closed loop was formed among the interventions, therefore, inconsistency in the NMA was unnecessary to be detected.
We produced league tables to express the comparisons between each pair of interventions and ranked the CHIs in each outcome by the ranking probabilities produced by surface under the cumulative ranking area curves (SUCRA)to find the most suitable CHIs (Dias et al., 2013). Moreover, the ranking probability of interventions shared by the primary outcome and each of the five secondary outcomes were aggregated into a comprehensive ranking respectively through cluster analyses.
Per-comparison I2 was used to measure the heterogeneity between each pair of interventions while global I2 was used to measure the overall heterogeneity. A higher value of the I2 denotes a greater degree of heterogeneity (Higgins and Thompson, 2002). Furthermore, sensitivity analysis and subgroup analysis were applied to identify the sources of the heterogeneity and evaluate the robustness of the pooled effects. In addition, funnel plots were used to explore publication bias in the outcomes with greater than or equal to 10 RCTs (Stuck et al., 1998).
All analyses were conducted using R 4.1.1 (gemtc package: NMA, assessment of heterogeneity, ranking probability of SUCRA, subgroup analysis; meta package: sensitivity analysis of pairwise interventions) and STATA 14.0 (publication bias and cluster analysis).
Grading the Quality of Evidence
We employed the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) to summarize the quality of evidence via two aspects, i.e., pairwise comparison and rank probability among the interventions in each outcome (Salanti et al., 2014). The GRADE has four grades: high, moderate, low, and very low. In this study, each item initially was rated as “high quality” and further downgraded through the following: study limitation, indirectness, heterogeneity/inconsistency, imprecision, and publication bias.
Results
Search Results
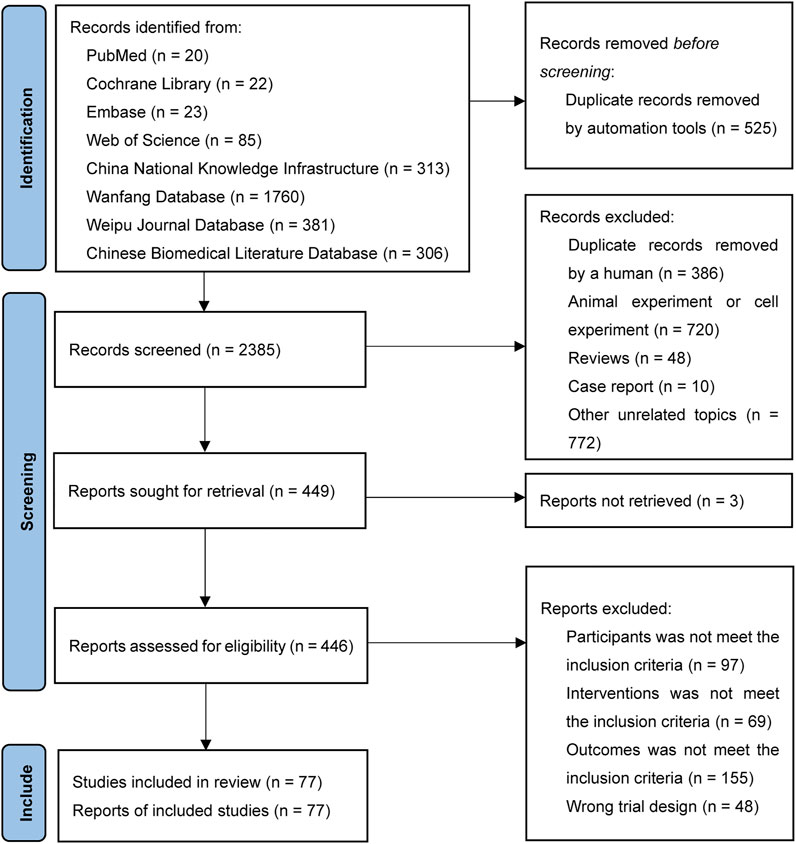
A total of 2,910 records were retrieved. After removing 911 duplicates, 1,550 records were removed in the first screening round by reading the titles and abstracts and 372 records were removed in the second screening round by reading the full text. Finally, 77 RCTs were included in our analysis with citations that are showed in Supplementary File S3. The flow chart of the literature search is provided in Figure 1.
Included Studies and Characteristics
The selected studies consisted of 2 English-language studies and 75 Chinese-language studies, including 5,647 patients and 3,209 male patients among them (58.92%, sex ratios of three studies were unavailable). All the RCTs were two-arm studies with the time of publication from 2009 to 2021, involving 10 kinds of CHIs: Shenfu injection (SF, 35 RCTs), Shenmai injection (SM, eight RCTs), Shengmai injection (SGM, five RCTs), Xuebijing injection (XBJ, 20 RCTs), Yiqifumai injection (YQFM, 2 RCTs), Danshen injection (DS, one RCT), Huangqi injection (HQ, one RCT), Shuxuetong (SXT, one RCT), Shenqifuzheng (SQFZ, three RCTs), and Xiyanping injection (XYP, one RCT). Thirty-seven (48.05%), 20 (25.97%), 10 (12.99%), 16 (20.78%), 19 (24.68%), and 20 (25.97%) studies, individually, contributed to six outcomes: 28-days-mortality, ICU length of stay, hospital length of stay, SOFA score at 7-days, procalcitonin level at 7-days and serum lactate level at 7-days. The details of the included CHIs are summarized in Supplementary File S4, and the details of the selected studies are shown in Table 1. The network graphs of the interventions with different outcomes are depicted in Figure 2.
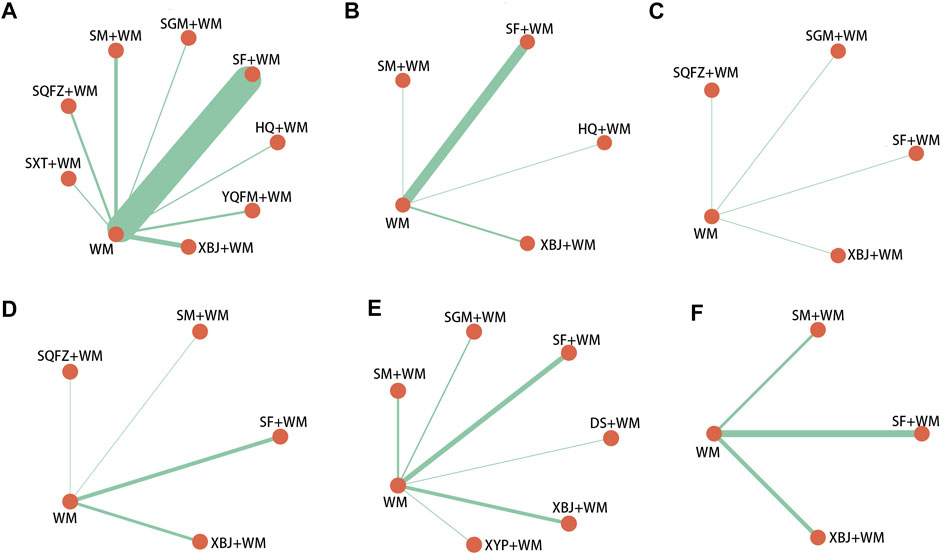
FIGURE 2. Network graph of different interventions (A) 28-days-motality (B) ICU length of stay (C) Hospital length of stay (D) SOFA score at day 7 after interventions (E) Procalcitonin level at day 7 after interventions (F) Serum lactate level at day 7 after interventions; WM, Western Medicine; SF, Shenfu injection; SM, Shenmai injection; SGM, Shengmai injection; XBJ, Xuebijing injection; YQFM, Yiqifumai injection; DS, Danshen injection; HQ, Huangqi injection; SXT, Shuxuetong injection; SQFZ, Shenqifuzheng injection; XYP, Xiyanping injection. The nodes were joined by different thickness lines which were generated to show whether there existed a direct relationship between treatments and the thickness was weighted according to the available direct evidence between them.
Methodological Quality
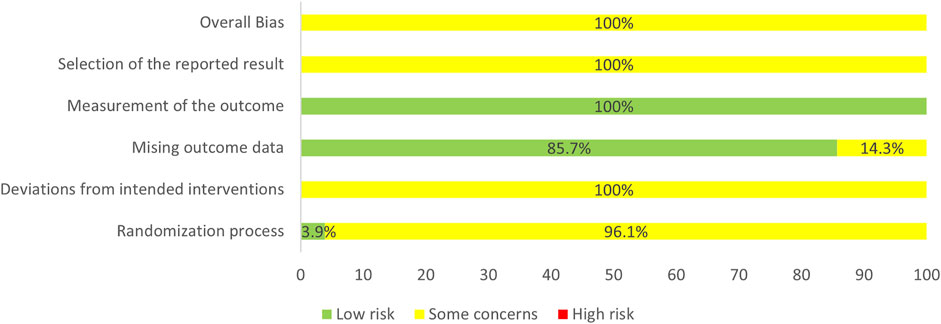
In the selected RCTs, 35 RCTs (45.45%) did not mention specific random methods, 2 RCTs (2.6%) performed central randomization, one RCT (1.3%) performed block randomization, one RCT (1.3%) performed simple randomization via coin toss method, and 38 RCTs (49.35%) performing simple randomization via the table of random digits. Seventy-four RCTs (96.1%) did not state the details of allocation concealment, which were evaluated as “some concerns” in “randomization process”. One RCT reported no blinding was used while the remaining RCTs did not report blinding. We guessed that the remaining included studies were difficult to implement blinding and probably did not use appropriate analyses (i.e., intention-to-treat analyses or modified intention-to-treat analyses). Thus, the item, “deviations from intended interventions”, was rated as “some concerns”. In addition, 66 RCTs reported the number of patients who participated in the assessment of each outcome measure while 11 RCTs did not report, which resulted in 14.3% of the studies being rated as “some concerns” in the “missing outcome data”. “Measurement of the outcome” assessment was generally a “low risk of bias” as all the outcomes were obtained from objective measures. “Selection of the reported result” of all the RCTs were rated as “some concerns” because pre-specified protocols of the selected RCTs were unavailable, which made it impossible to assess whether the results were selectively reported. In general, the risk of bias of the selected RCTs was rated as “some concerns”. The results of the assessment of the risk of bias are presented in Figure 3.
Network Meta-Analysis
In the Brooks-Gelman-Rubin plots, all the median lines and the 97.5% lines tended to 1, which indicated that all the model fits in the study were good. The Brooks-Gelman-Rubin plots are provided in Supplementary File S5.
28-Day Mortality
Eight CHIs (YQFM, SXT, SQFZ, SGM, XBJ, HQ, SF, and SM) were involved in assessing 28-days mortality. According to the RR and 95%CI between all the pairwise interventions, YQFM + WM, SXT + WM, XBJ + WM, and SF + WM were superior for WM; YQFM + WM and SXT + WM were superior for SM + WM; YQFM + WM was superior for SF + WM; no such evident effect was observed with other pairwise interventions. Moreover, YQFM + WM, with the highest-ranking probability of SUCRA (85%), had the best effectiveness in reducing 28-days mortality, followed by SXT + WM (79%) and SQFZ + WM (75%). More details about the between-intervention differences and the rank probability of SUCRA are shown in Figure 4E.
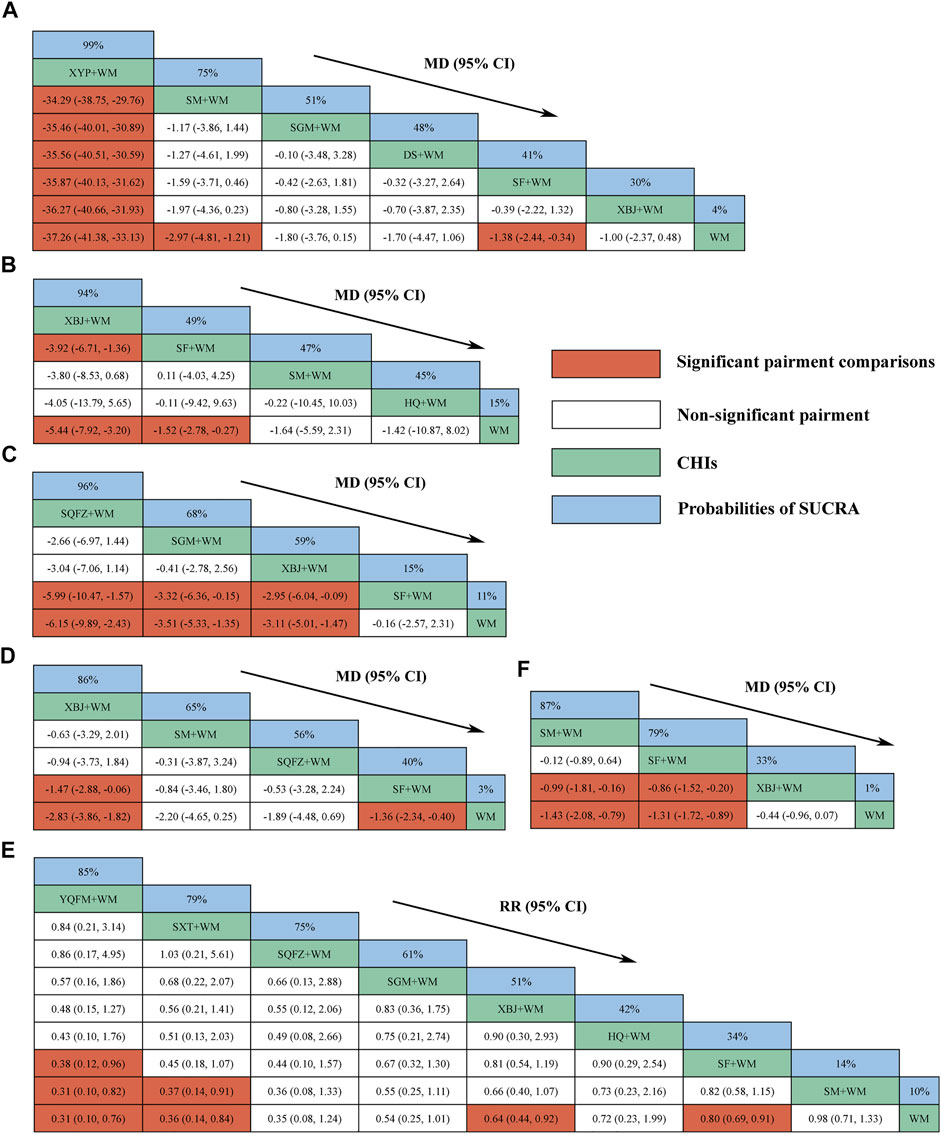
FIGURE 4. Relative effect sizes of efficacy at interventions in each outcome (A) Procalcitonin level at day 7 after interventions (B) ICU length of stay (C) Hospital length of stay (D) SOFA score at day 7 after interventions (E) 28-days-motality (F) Serum lactate level at day 7 after interventions; CHIs, Chinese herbal injections; WM, Western Medicine; SF, Shenfu injection; SM, Shenmai injection; SGM, Shengmai injection; XBJ, Xuebijing injection; YQFM, Yiqifumai injection; DS, Danshen injection; HQ, Huangqi injection; SXT, Shuxuetong injection; SQFZ, Shenqifuzheng injection; XYP, Xiyanping injection; SUCRA, surface under the cumulative ranking area curves. Highest probability of being the most efficient CHIs (With high SUCRA values) and Lowest probability of being the most efficient CHIs (With low SUCRA values).
ICU Length of Stay
Four CHIs (XBJ, SF, SM, and HQ) were involved in reporting ICU length of stay. According to the MD and 95%CI between all the pairwise interventions, SF + WM and XBJ + WM were superior for WM; XBJ + WM was superior for SF + WM; no such evident effect was observed with other pairwise interventions. Moreover, XBJ + WM, with the highest-ranking probability of SUCRA (94%), had the best effectiveness in reducing ICU length of stay, followed by SF + WM (49%) and SM + WM (47%). More details about the between-intervention differences and the rank probability of SUCRA are shown in Figure 4B.
Hospital Length of Stay
Four CHIs (SQFZ, SGM, XBJ, and SF) were involved in reporting hospital length of stay. According to the MD and 95%CI between all the pairwise interventions, SQFZ + WM, SGM + WM, and XBJ + WM were superior for WM and SF + WM; no such evident effect was observed with other pairwise interventions. Moreover, SQFZ + WM, with the highest-ranking probability of SUCRA (96%), had the best effectiveness in reducing hospital length of stay, followed by SGM + WM (68%) and XBJ + WM (59%). More details about the between-intervention differences and the rank probability of SUCRA are shown in Figure 4C.
SOFA Score at Day 7 After Interventions
Four CHIs (XBJ, SM, SQFZ, and SF) were involved in reporting SOFA score at 7-days. According to the MD and 95%CI between all the pairwise interventions, XBJ + WM and SF + WM were superior for WM; XBJ + WM was superior for SF + WM; no such evident effect was observed with other pairwise interventions. Moreover, XBJ + WM, with the highest-ranking probability of SUCRA (86%), had the best effectiveness in reducing the SOFA score at 7-days, followed by SM + WM (65%) and SQFZ + WM (56%). More details about the between-intervention differences and the rank probability of SUCRA are shown in Figure 4D.
Procalcitonin Level at Day 7 After Interventions
Six CHIs (XYP, SM, SGM, DS, SF, and XBJ) were involved in reporting procalcitonin level at 7-days. According to the MD and 95%CI between all the pairwise interventions, SF + WM, SM + WM, and XYP + WM were superior for WM; XYP + WM was superior for SF + WM, SGM + WM, SM + WM, DS + WM, and XBJ + WM; no such evident effect was observed with other pairwise interventions. Moreover, XYP + WM, with the highest-ranking probability of SUCRA (99%), had the best effectiveness in reducing procalcitonin level at 7-days, followed by SM + WM (75%) and SGM + WM (51%). More details about the between-intervention differences and the rank probability of SUCRA are shown in Figure 4A.
Serum Lactate Level at Day 7 After Interventions
Three CHIs (SM, SF, and XBJ) were involved in reporting serum lactate level at 7-days. According to the MD and 95%CI between all the pairwise interventions, SF + WM and SM + WM were superior for XBJ + WM and WM; no such evident effect was observed with other pairwise interventions. Moreover, SM + WM, with the highest-ranking probability of SUCRA (87%), had the best effectiveness in reducing serum lactate level at 7-days, followed by SF + WM (79%) and XBJ + WM (33%). More details about the between-intervention differences and the rank probability of SUCRA are shown in Figure 4F.
Adverse Drug Reactions
Ten RCTs (12.99%) reported ADRs, in which only SGM had ADRs (ADRs rate of 6.66%). The ADRs of SGM encompassed: 2 allergic dermatitides, three nausea and vomiting, one bloating, one palpitation, and one headache. No patient withdrew from the studies because of the ADRs.
Cluster Analysis
Based on the SUCRA of the interventions shared by the pairwise outcomes, the cluster analysis was performed to integrate the effects of 28-days mortality with each of the first five secondary outcomes. As shown in Figure 5, XBJ + WM, SQFZ + WM, SGM + WM, SM + WM, and SF + WM achieved similarly superior effects over the others, and WM alone yielded the worst result.
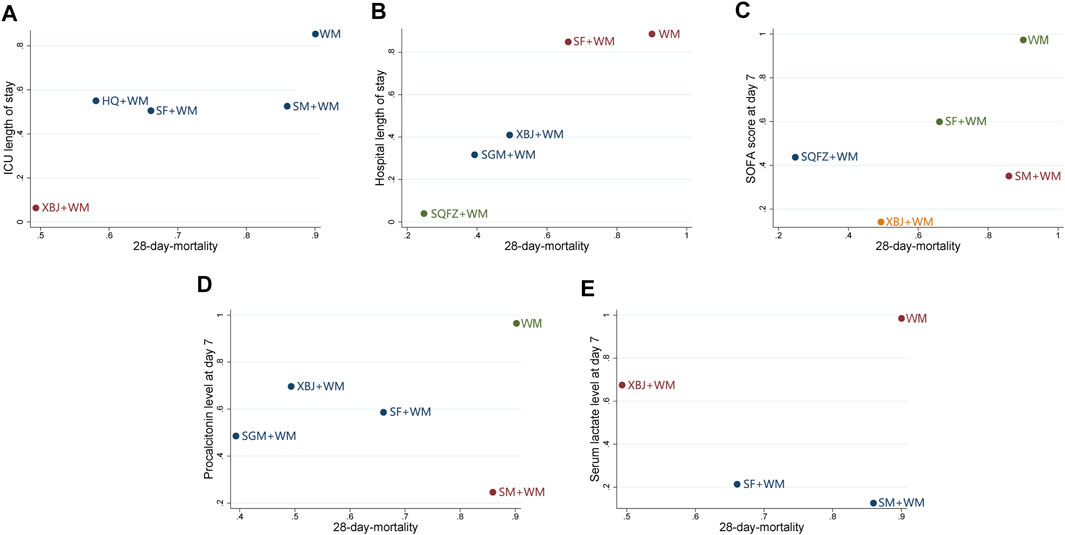
FIGURE 5. Cluster analysis plots (A) 28-days-motality (x-axis) and ICU length of stay (y-axis) (B) 28-days-motality (x-axis) and hospital length of stay (y-axis) (C) 28-days-motality (x-axis) and SOFA score at day 7 after interventions (y-axis) (D) 28-days-motality (x-axis) and procalcitonin level at day 7 after interventions (y-axis) (E) 28-days-motality (x-axis) and serum lactate level at day 7 after interventions (y-axis); WM, Western Medicine; SF, Shenfu injection; SM, Shenmai injection; SGM, Shengmai injection; XBJ, Xuebijing injection; HQ, Huangqi injection; SQFZ, Shenqifuzheng injection. Interventions with the same color belong to the same cluster, and interventions located in the lower-left corner indicate the optimal therapy for two different outcomes while located in the upper-right corner indicate the worst therapy.
Publication Bias
Regarding the funnel charts of SOFA score at 7-days and serum lactate level at 7-days, the angle between the correction guideline and the centerline was large, which suggested the existence of potential publication bias. By contrast, the funnel charts showed unremarkable asymmetry on both sides of the centerline in 28-days mortality, ICU length of stay, hospital length of stay, and procalcitonin level at 7-days suggesting that it had no publication bias. The funnel charts are shown in Figure 6.
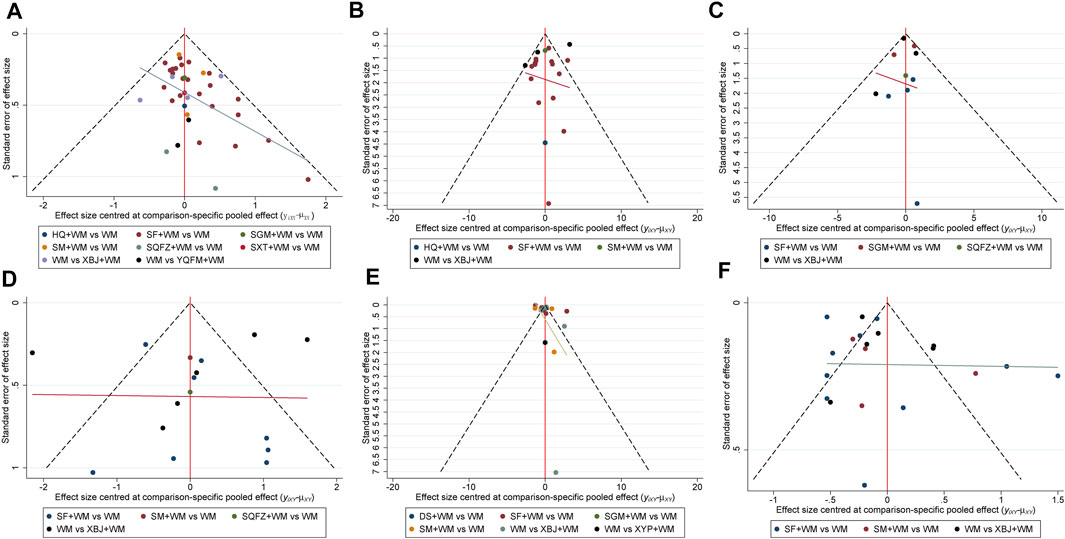
FIGURE 6. Funnel plots (A) 28-days-motality (B) ICU length of stay (C) Hospital length of stay (D) SOFA score at day 7 after interventions (E) Procalcitonin level at day 7 after interventions (F) Serum lactate level at day 7 after interventions; WM, Western Medicine; SF, Shenfu injection; SM, Shenmai injection; SGM, Shengmai injection; XBJ, Xuebijing injection; YQFM, Yiqifumai injection; DS, Danshen injection; HQ, Huangqi injection; SXT, Shuxuetong injection; SQFZ, Shenqifuzheng injection; XYP, Xiyanping injection.
Heterogeneity, Sensitivity Analysis, and Subgroup Analysis
As shown in Supplementary File S6, global I2 was 0.0, 77.8, 14.1, 91.1, 99.6, and 93.5% for 28-days-mortality, ICU length of stay, hospital length of stay, SOFA score at 7-days, procalcitonin level at 7-days, and serum lactate level at 7-days, respectively. The five secondary outcomes had significant heterogeneity in the pairwise comparisons: SF + WM versus WM in “procalcitonin level at 7-days” (I2 = 95.8%) and “serum lactate level at 7-days” (I2 = 93.6%), SM + WM versus WM in “procalcitonin level at 7-days” (I2 = 94.2%) and “serum lactate level at 7-days” (I2 = 83.9%), SGM + WM versus WM in “hospital length of stay” (I2 = 71.5%) and “procalcitonin level at 7-days” (I2 = 74.3%), XBJ + WM versus WM in “ICU length of stay” (I2 = 96.2%), “SOFA score at 7-days” (I2 = 92.9%), “procalcitonin level at 7-days” (I2 = 86.9%) and “serum lactate level at 7-days” (I2 = 84.8%). Sensitivity analysis suggested that no selected literature was the source of the heterogeneity, and the pooled outcomes were steady. The results of sensitivity analysis are shown in Supplementary File S7. Additionally, subgroup analysis was performed in the selected studies which adopted septic shock in Sepsis 2.0 as the diagnostic criteria (the number of studies that used diagnostic criteria from Sepsis 1.0 and Sepsis 3.0 was too small to execute subgroup analysis). The subgroup analysis indicated some dissimilarities from the overall results: In terms of 28-days-mortality, XBJ + WM were significantly more effective than SF + WM and SM + WM; regarding ICU length of stay, no differences were found between XBJ + WM versus SF + WM/SF + WM versus WM; concerning SOFA score at day 7 after interventions, no discrepancies were observed between XBJ + WM versus SF + WM. The results are shown in Supplementary File S8.
GRADE Evaluation of the Strength of Evidence
In the current study, the GRADE indicated the strength of evidence ranged from very low to moderate whereas most of the pairwise comparisons were rated as low (38, 40.86%) and very low (41, 44.09%), with only 14 (15.05%) comparisons being rated as moderate. In terms of ranking probability, the quality of evidence was low for hospital length of stay while the remaining outcomes were all rated as very low. The results of GRADE are detailed in Supplementary File S9.
Discussion
Different from a conventional pairwise meta-analysis which can only compare two treatment formats at a time, NMA, a mixed treatment comparison or multiple treatments comparison meta-analysis, can compare the effects of greater than or equal to two treatments and allow ranking of different treatments by combining direct and indirect evidence (Caldwell et al., 2005; Li et al., 2011; Mills et al., 2012). In the current study, a Bayesian framework was used to conduct the model fitting of NMA, comparing ten CHIs in the treatment of septic shock. As showed in the results, YQFM, XBJ, SQFZ, SM, XYP, and SGM combined with WM demonstrated better outcomes compared with other CHIs combined with WM or WM alone.
In traditional Chinese medicine, septic shock belongs to “collapse syndrome”, with clinical symptoms such as apathy or even coma, pale, cold extremities, respiratory weakness, sweat profusely, and weak pulse (Liang et al., 2021). Physicians of traditional Chinese medicine consider that the disease is caused by the pathogenic Qi assaulting the human body and the vital Qi of the human body losing rapidly (Wang, 2015). Therapeutic approaches principally encompassing restoring the Yang, supporting the Healthy Energy, and expulsing the pathogenic Qi are used to alleviate the condition (Liang et al., 2021). Based on the theoretical context, traditional formulations such as Shengmaisan Decoction, Sini Decoction, and Xuefuzhuyu Decoction are utilized in clinical practice (Liang et al., 2021), involving Panax ginseng C. A. Mey [Araliaceae; Ginseng Radix et Rhizoma Rubra], Aconitum carmichaeli Debeaux [Ranunculaceae; Aconiti Lateralis Radix Praeparata], Ophiopogon japonicus (Thunb.) Ker-Gawl [Asparagaceae; Radix Ophiopogonis], Schisandra chinensis (Turcz.) Baill [Schisandraceae; Schisandrae Chinensis Fructus], Salvia miltiorrhiza Bunge [Lamiaceae; Salviae Miltiorrhizae Radix et Rhizoma], Astragalus mongholicus Bunge [Fabaceae; Astragali Radix], and so on. However, the oral mode of administration was the main modality of drug administration in traditional Chinese medicine previously, which might associate with inadequate bioavailability and slow occurrence. CHIs, the injections made of active ingredients in Chinese medicine compounds or single Chinese medicine and are used intravenously, nonetheless, might have a faster onset of action and better utilization (Zhang, 2016). This means of drug administration is probably more suitable for septic shock treatment.
In our study, the efficacy of septic shock treatment could be further increased by combined use with CHIs, which was similar to other pairwise meta-analyses (Zhou et al., 2016; Li, 2018; Ha et al., 2019). Although the full mechanism of action of CHIs for septic shock remained unclear, partial potential mechanisms of action were elucidated presently. Animal experiments demonstrated that for lipopolysaccharide-induced shock rats, YQFM could decrease cerebral venule albumin leakage and cerebrovascular hyperpermeability (Li D. T. et al., 2019), reduce the content of inflammatory factors in the lungs which result in lung injury (Xia et al., 2018), and inhibit the exudation of mesenteric venules as well as their local inflammation (Yuan et al., 2011; Ayididaer et al., 2021), all of which, might be attributed to the ginsenoside Rb1 and Sch isandrin incorporating in YQFM. In addition, the main pharmacological actions of XBJ include inhibiting the expression level of TNF-α, IL-1, IL-6, IL-8, IL-17, NF-κB, ET-1, tissue factor, macrophage migration inhibitory factor, malondialdehyde, and myeloperoxidase, reducing the apoptosis rate of immune cells, promoting the expression level of IL-10, endothelial nitric oxide synthase, superoxide dismutase, and glutathione peroxidase, enhancing Treg apoptosis, polarizing the immune response from Th2 to Th1, downregulating the expression of the TLR4/NF-κB signaling pathway, restoring the balance of the matrix metalloproteinase/tissue inhibitors of metalloproteinase ratio (Li et al., 2021). Regarding SQFZ, animal experiments confirmed that the drug could alleviate the acute lung injury induced by lipopolysaccharide in shock rats, of which the mechanism might relate to reducing the level of TNF-α and down-regulating the expression of chemokines fractalkine mRNA in lung tissue or inducing the expression of heat shock protein-70 (Wang et al., 2007; Liang et al., 2016). As for SM, the drug protecting the cardiomyocytes and kidney cells of septic rats by up-regulating Bcl-2 protein and down-regulating Bax protein has been well established (Lin et al., 2013a; Lin et al., 2013b), and it has been confirmed that SM could inhibit the expression of inflammatory mediators (e.g., IL-6) and increase serum IgG level in an animal experiment (He et al., 2014). Additionally, as showed in this study, XYP exerted a meaningful reduction in procalcitonin level, which might inextricably link to its inhibiting ability of NF-κB, thus exerting functions of anti-inflammatory and immunomodulatory (Zhang and Cui, 2018). Besides, a mouse experiment observed a downward trend of INF-γ, TNF-α, and IL-2 in SGM treating mice, accompanied by increasing Occludin and decreasing MLCK protein compared with the model group (Lu et al., 2021), which indicated the relation between SGM, and septic shock might intimately relate to the proteins and inflammatory factors mentioned above.
In addition to the therapeutic effects of CHIs, ADRs should also be considered. From the descriptive results of our study, the ADRs only appeared in SGM and were non-fatal. Nevertheless, it was worth noting that the results might not be very persuasive as only ten studies in our study reported the events. Compared to other dosage forms of traditional Chinese medicine, CHIs have a higher ADRs rate; the drugs are more likely to have new or serious adverse effects than other types of injections (Li H. et al., 2019). Studies based on large sample sizes presented that the ADRs rate of YQFM, XBJ, SQFZ, SM, XYP and SGM were 0.176%–0.2%, 0.3%, 1.35%–1.53, 0.1, 2.1, and 0.8% separately (Zheng et al., 2006; Li et al., 2009; Hu, 2012; Ding et al., 2015; Chen and Zhu, 2018; Cao et al., 2019; Li K. et al., 2019; Zheng et al., 2019). The ADRs generally consisted of non-fatal events while anaphylactoid reaction or anaphylactic shock associated with a high risk of causing deaths (Li H. et al., 2019). A systematic review summarized previous studies and concluded the influencing factors of ADRs in CHIs: individual patient characteristics, characterizations of CHIs, pharmaceutical excipients, vehicles, and rational drug uses based on the theory of traditional Chinese medicine (Wang et al., 2018). Notably, risk factors for ADRs may be different in various CHIs. For example, drip rate exceeding 40 drops/min will increase the ADRs rate of YQFM (Cao et al., 2019); vehicle, dosage, patient age, drug combination, irrigating syringe, and fluid dripping were associated with ADRs of XBJ (Wang et al., 2019; Zheng et al., 2019); irrational compatibility and dosages may be potential risk factors for SM (Zhang et al., 2010). Anyhow, existing evidence showed that the ADRs of CHIs were mostly related to clinical irrational medicine use (Liu et al., 2018; Tan et al., 2019). It seems particularly important, therefore, to advocate the standard use of CHIs by clinicians (Xie et al., 2013).
Limitation
Although we have evaluated CHIs from many aspects, there are still some limitations. First, due to the limited number of RCTs involving the studies of YQFM (2 RCTs), HQ (1 RCT), SXT (1 RCT), DS (1 RCT), and XYP (1 RCT), the pooled outcomes might not be sufficiently convincing. Second, the promotion of the results may be restricted because all the included RCTs were conducted in China. Third, fewer selected studies 5) used the diagnostic criteria for septic shock in Sepsis 3.0 and this might influence the applicability of the results. Finally, the quality of evidence for most outcomes was low and need further evidence. However, our findings still have implications for the management of septic shock.
Conclusion
The results of our study showed that CHIs combined with WM were more effective than WM alone in the treatment of septic shock. YQFM, XBJ, SQFZ, XYP, SM, SGM, and SF deserve more attention when treating septic shock patients. The quality of evidence for most outcomes was low, therefore, high-quality RCTs are needed to confirm the conclusions.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
PH and BC done conception and design of the study. YF and SL performed the literature search, screening, and extraction. QW and HZ implemented quality assessment. PH, YC, and SZ performed the network meta-analysis. PH wrote the original draft. BC and SZ reviewed and edited the manuscript. All the authors approved the final version of the manuscript.
Funding
This work was sponsored by the National Natural Science Foundation of China (Grant NO.81273961 and NO.81303117), Science and Technology Foundation of Shenzhen City (Grant NO.JSGG20220226085800001), Science and Technology Foundation of Shenzhen City (Grant NO.JCYJ20190812164009243), and Guangdong Medical Research Foundation (Grant NO.B2020135).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.850221/full#supplementary-material
References
Annane, D., Renault, A., Brun-Buisson, C., Megarbane, B., Quenot, J. P., and Siami, S. (2018). Hydrocortisone Plus Fludrocortisone for Adults with Septic Shock. N. Engl. J. Med. 378 (9), 809–818. doi:10.1056/NEJMoa1705716
Ayididaer, A., Sun, K., Pan, C. S., Yan, L., Liu, Y. Y., Li, D. T., et al. (2021). Post‐treatment with Yiqifumai Injection and its Main Ingredients Attenuates Lipopolysaccharide‐induced Microvascular Disturbance in Mesentery and Ileum. Microcirculation 28 (4), e12680. doi:10.1111/micc.12680
Bauer, M., Gerlach, H., Vogelmann, T., Preissing, F., Stiefel, J., and Adam, D. (2020). Mortality in Sepsis and Septic Shock in Europe, North America and Australia between 2009 and 2019-results from a Systematic Review and Meta-Analysis. Crit. Care 24, 1–9. doi:10.1186/s13054-020-02950-2
Bone, R. C., Balk, R. A., Cerra, F. B., Dellinger, R. P., Fein, A. M., Knaus, W. A., et al. (1992). Definitions for Sepsis and Organ Failure and Guidelines for the Use of Innovative Therapies in Sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 101 (6), 1644–1655. doi:10.1378/chest.101.6.1644
Brooks, S. P., and Gelman, A. (1998). General Methods for Monitoring Convergence of Iterative Simulations. J. Comput. Graph. Stat. 7 (4), 434–455. doi:10.1080/10618600.1998.10474787
Caldwell, D. M., Ades, A., and Higgins, J. (2005). Simultaneous Comparison of Multiple Treatments: Combining Direct and Indirect Evidence. BMJ 331 (7521), 897–900. doi:10.1136/bmj.331.7521.897
Cao, H., Hao, C., Bi, J., Luo, R., Xie, A., Xie, W., et al. (2019). Clinical Safety Monitoring Study of Yiqi Fumai Lyophilized Injection. Drug Eval. Res. 42 (3), 467–471. doi:10.7501/j.issn.1674-6376.2019.03.015
Chen, C., and Zhu, S. (2018). Clinical Application Characteristics and Safety Evaluation of Shenqi Fuzheng Injection. Contemp. Med. Forum 16 (8), 164–166. doi:10.3969/j.issn.2095-7629.2018.08.119
Dias, S., Sutton, A. J., Ades, A., and Welton, N. J. (2013). Evidence Synthesis for Decision Making 2: a Generalized Linear Modeling Framework for Pairwise and Network Meta-Analysis of Randomized Controlled Trials. Med. Decis. Making 33 (5), 607–617. doi:10.1177/0272989X12458724
Ding, F., Shi, Q., Jiang, X., Liu, Y., Sang, R., Zhu, J., et al. (2015). Predictive Analysis on Shenmai Injection-Induced Adverse Reactions with Logistic Model and ROC Curve. Zhongguo Zhongyao Zazhi 40 (7), 1404–1409. doi:10.4268/cjcmm20150735
Evans, L., Rhodes, A., Alhazzani, W., Antonelli, M., Coopersmith, C. M., French, C., et al. (2021). Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Intensive Care Med. 47 (11), 1181–1247. doi:10.1007/s00134-021-06506-y
Ha, Y., Wang, X., Huang, P., Zhang, R., Xu, X., Li, B., et al. (2019). Effect of Shengmai Injection on Septic Shock,a Systematic Review and Meta-Analyse. J. Emerg. Traditional Chin. Med. 028 (011), 1893–1898. 1915. doi:10.3969/j.issn.1004-745X.2019.11.004
He, C., Shen, L., and Zhao, W. (2014). Effect of Shenmai Injection on Immunological Function in Sepsis Rats. Chin. J. Clin. Rational Drug Use 7 (1), 2. doi:10.15887/j.cnki.13-1389/r.2014.01.012
Higgins, J. P., and Thompson, S. G. (2002). Quantifying Heterogeneity in a Meta‐analysis. Stat. Med. 21 (11), 1539–1558. doi:10.1002/sim.1186
Hu, Y. (2012). Safety Evaluation of Shenqifuzheng Injection. China Pharmaceuticals 21 (21), 36–37. doi:10.3969/j.issn.1006-4931.2012.21.021
Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H., Cameron, C., et al. (2015). The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-Analyses of Health Care Interventions: Checklist and Explanations. Ann. Intern. Med. 162 (11), 777–784. doi:10.7326/M14-2385
Jawad, I., Lukšić, I., and Rafnsson, S. B. (2012). Assessing Available Information on the burden of Sepsis: Global Estimates of Incidence, Prevalence and Mortality. J. Glob. Health 2 (1), 010404. doi:10.7189/jogh.02.010404
Karakike, E., Giamarellos-Bourboulis, E. J., Kyprianou, M., Fleischmann-Struzek, C., Pletz, M. W., Netea, M. G., et al. (2021). Coronavirus Disease 2019 as Cause of Viral Sepsis: a Systematic Review and Meta-Analysis. Crit. Care Med. 49 (12), 2042. doi:10.1097/CCM.0000000000005195
Kaukonen, K. M., Bailey, M., Suzuki, S., Pilcher, D., and Bellomo, R. (2014). Mortality Related to Severe Sepsis and Septic Shock Among Critically Ill Patients in Australia and New Zealand, 2000-2012. Jama 311 (13), 1308–1316. doi:10.1001/jama.2014.2637
Levy, M. M., Artigas, A., Phillips, G. S., Rhodes, A., Beale, R., Osborn, T., et al. (2012). Outcomes of the Surviving Sepsis Campaign in Intensive Care Units in the USA and Europe: a Prospective Cohort Study. Lancet Infect. Dis. 12 (12), 919–924. doi:10.1016/S1473-3099(12)70239-6
Levy, M. M., Evans, L. E., and Rhodes, A. (2018). The Surviving Sepsis Campaign Bundle: 2018 Update. Intensive Care Med. 44 (6), 925–928. doi:10.1007/s00134-018-5085-0
Levy, M. M., Fink, M. P., Marshall, J. C., Abraham, E., Angus, D., Cook, D., et al. (2003). 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 29 (4), 530–538. doi:10.1007/s00134-003-1662-x
Li, C., Wang, P., Li, M., Zheng, R., Chen, S., Liu, S., et al. (2021). The Current Evidence for the Treatment of Sepsis with Xuebijing Injection: Bioactive Constituents, Findings of Clinical Studies and Potential Mechanisms. J. Ethnopharmacol. 265, 113301. doi:10.1016/j.jep.2020.113301
Li, D. T., Sun, K., Huang, P., Pan, C. S., Yan, L., Ayan, A., et al. (2019). Yiqifumai Injection and its Main Ingredients Attenuate Lipopolysaccharide‐induced Cerebrovascular Hyperpermeability through a Multi‐pathway Mode. Microcirculation 26 (7), e12553. doi:10.1111/micc.12553
Li, H., Deng, J., Deng, L., Ren, X., and Xia, J. (2019). Safety Profile of Traditional Chinese Herbal Injection: An Analysis of a Spontaneous Reporting System in China. Pharmacoepidemiol. Drug Saf. 28 (7), 1002–1013. doi:10.1002/pds.4805
Li, K., Liu, Y., Shi, B., Peng, L., and Tao, J. (2019). Analysis of Yiqi Fumai Lyophilized Injection Application and Adverse Reaction. Drug Eval. Res. 42 (10), 2075–2078. doi:10.7501/j.issn.1674-6376.2019.10.032
Li, T., Puhan, M. A., Vedula, S. S., Singh, S., and Dickersin, K. (2011). Network Meta-Analysis-Highly Attractive but More Methodological Research Is Needed. BMC Med. 9 (1), 1–5. doi:10.1186/1741-7015-9-79
Li, T. Q., Liu, X. M., Feng, M., Wang, L., Zhong, Y., Shi, D., et al. (2009). Systematic Review on the Application and Adverse Reactions of Shengmai Injection. Zhongguo Zhong Xi Yi Jie He Za Zhi 29 (11), 965–969. doi:10.3321/j.issn:1003-5370.2009.11.001
Li, X. (2018). Effectiveness and Safety of Shenmai Injection in Treatment of Shock:a Meta-Analysis. J. Tradit. Chin. Med. 38 (02), 155–166. doi:10.1016/j.jtcm.2017.05.001
Liang, J., Sun, X., and Wang, G. (2016). Effect of Shenqi Fuzheng Injection on Heat Shock Protein-70 in Lung Tissue of Rats with Endotoxin Induced Acute Lung Injury. J. HeBei United Univ. (Health Sciences) 18 (5), 4. doi:10.19539/j.cnki.2095-2694.2016.05.016
Liang, Q., Jiang, H., and Du, C. (2021). Research Progress in the Treatment of Septic Shock with Integrated Traditional Chinese and Western Medicine. J. Emerg. Traditional Chin. Med. 30 (2), 4. doi:10.3969/j.issn.1004-745X.2021.02.052
Lin, M., Ye, F., and Zhao, Z. (2013a). Effects of Shenmai Injection on Renal Apoptosis and Expressions of the Bcl-2/Bax Protein in Septic Rats. Zhejiang J. Integrated Traditional Chin. West. Med. 23 (9), 3. doi:10.3969/j.issn.1005-4561.2013.09.007
Lin, M., Zhang, Z., Ye, F., and Zhang, S. (2013b). Effects of Shenmai Iniection on Myocardial Apoptosis in Septic Rats. Zhonghua Zhongyiyao Xuekan 31 (7), 4. doi:10.13193/j.archtcm.2013.07.207.linms.085
Liu, Q., Tian, P., Wang, Y., Zhao, Q., Huang, L., Wang, J., et al. (2018). Analysis of Clinically Unreasonable Application in Compatibility of Traditional Chinese Medicine Injections. Chin. J. Clin. Rational Drug Use 11 (27), 2. doi:10.15887/j.cnki.13-1389/r.2018.27.052
Lu, J., Yu, Y., Wang, X. J., Chai, R. P., Lyu, X. K., Deng, M. H., et al. (2021). Mechanism of Shengmai Injection on Anti-sepsis and Protective Activities of Intestinal Mucosal Barrier in Mice. Chin. J. Integr. Med., 1–6. doi:10.1007/S11655-021-3292-Y
Marik, P. E., Khangoora, V., Rivera, R., Hooper, M. H., and Catravas, J. (2017). Hydrocortisone, Vitamin C, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: a Retrospective Before-After Study. Chest 151 (6), 1229–1238. doi:10.1016/j.chest.2016.11.036
Mavridis, D., and Salanti, G. (2013). A Practical Introduction to Multivariate Meta-Analysis. Stat. Methods Med. Res. 22 (2), 133–158. doi:10.1177/0962280211432219
Mills, E. J., Ioannidis, J. P., Thorlund, K., Schünemann, H. J., Puhan, M. A., and Guyatt, G. H. (2012). How to Use an Article Reporting a Multiple Treatment Comparison Meta-Analysis. Jama 308 (12), 1246–1253. doi:10.1001/2012.jama.11228
Quenot, J. P., Binquet, C., Kara, F., Martinet, O., Ganster, F., Navellou, J. C., et al. (2013). The Epidemiology of Septic Shock in French Intensive Care Units: the Prospective Multicenter Cohort EPISS Study. Crit. Care 17 (2), 1–10. doi:10.1186/cc12598
Salanti, G., Del Giovane, C., Chaimani, A., Caldwell, D. M., and Higgins, J. P. (2014). Evaluating the Quality of Evidence from a Network Meta-Analysis. PLoS One 9 (7), e99682. doi:10.1371/journal.pone.0099682
Salanti, G., Higgins, J. P., Ades, A., and Ioannidis, J. P. (2008). Evaluation of Networks of Randomized Trials. Stat. Methods Med. Res. 17 (3), 279–301. doi:10.1177/0962280207080643
Salanti, G. (2012). Indirect and Mixed‐treatment Comparison, Network, or Multiple‐treatments Meta‐analysis: many Names, many Benefits, many Concerns for the Next Generation Evidence Synthesis Tool. Res. Synth. Methods 3 (2), 80–97. doi:10.1002/jrsm.1037
Shankar-Hari, M., Harrison, D., Rubenfeld, G., and Rowan, K. (2017). Epidemiology of Sepsis and Septic Shock in Critical Care Units: Comparison between Sepsis-2 and Sepsis-3 Populations Using a National Critical Care Database. Bja: Br. J. Anaesth. 119 (4), 626–636. doi:10.1093/bja/aex234
Shankar-Hari, M., Phillips, G. S., Levy, M. L., Seymour, C. W., Liu, V. X., Deutschman, C. S., et al. (2016). Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). Jama 315 (8), 775–787. doi:10.1001/jama.2016.0289
Singer, M., Deutschman, C. S., Seymour, C. W., Shankar-Hari, M., Annane, D., Bauer, M., et al. (2016). The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). Jama 315 (8), 801–810. doi:10.1001/jama.2016.0287
Sterne, J. A., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: a Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 366. doi:10.1136/bmj.l4898
Stuck, A. E., Rubenstein, L. Z., and Wieland, D. (1998). Bias in Meta-Analysis Detected by a Simple, Graphical Test. Asymmetry Detected in Funnel Plot Was Probably Due to True Heterogeneity. Bmj: Br. Med. J. 316 (7129), 469. doi:10.1136/bmj.316.7129.469
Tan, J., Chen, G., Hu, J., and Tan, J. (2019). Impact of Clinical Pharmacist's Interventions in Rational Use of Traditional Chinese Medicine Injection. Shenzhen Zhongxiyi Jiehe Zazhi 29 (4), 2. doi:10.16458/j.cnki.1007-0893.2019.04.018
Unschuld, P. U. (1999). The Past 1000 Years of Chinese Medicine. Lancet 354, SIV9. doi:10.1016/s0140-6736(99)90352-5
Wang, C., Shi, Q. P., Ding, F., Jiang, X. D., Tang, W., Yu, M. L., et al. (2019). Reevaluation of the post-marketing Safety of Xuebijing Injection Based on Real-World and Evidence-Based Evaluations. Biomed. Pharmacother. 109, 1523–1531. doi:10.1016/j.biopha.2018.10.190
Wang, L., Wang, G., Li, T. Q., and Xiang, G. (2007). Effect of Shenqi Fuzheng Injection on Fractalkine Expression in Lung Tissue of Rats with Lipopolysaccharide-Induced Acute Lung Injury. Zhongguo Zhong Xi Yi Jie He Za Zhi 27 (1), 55–59. doi:10.3321/j.issn:1003-5370.2007.01.013
Wang, W. (2015). Analysis of Mechanism of Shenfu Injection in Treating Septic Shock. J. New Chin. Med. 47 (3), 3. doi:10.13457/j.cnki.jncm.2015.03.002
Wang, Y., Fan, L., Song, J., Cai, Y., Jiang, T., Wang, Y., et al. (2018). Retrospective Analysis and Discussion on 74 Cases of Adverse Reactions of Traditional Chinese Medicine Injection. Zhongguo Zhongyao Zazhi 43 (21), 4347–4351. doi:10.19540/j.cnki.cjcmm.20180815.004
Wang, Z., Wei, J., Zhu, H., Cao, Y., Yu, X., Chen, Y., et al. (2020). Consensus of Chinese Experts on Early Prevention and Blocking of Sepsis. J. Clin. Emerg. 21 (7), 517–529. doi:10.13201/j.issn.1009-5918.2020.07.001
Xia, Y., Dolgor, S., Wu, Y., Jiang, S., Xue, L., Zhang, Y., et al. (2018). Effect of Yiqi Fumai Lyophilized Injection on Lipopolysaccharide-Induced Acute Lung Injury in Mice. Drug Eval. Res. 41 (3), 8. doi:10.7501/j.issn.1674-6376.2018.03.004
Xie, Y., Li, M., Zhang, Y., Ma, R., Xian, S., Liu, J., et al. (2013). Technical Specifications for Rational Clinical Use of Parenterally Administered Chinese Medicine (Draft Version for Comments). Zhongguo Zhongyao Zazhi 38 (18), 3. doi:10.4268/cjcmm20131803
Yuan, Q., Wang, J., Fang, Q. H., Liu, Y. Y., Fan, J. Y., Zhang, S. W., et al. (2011). Attenuating Effect of Pretreatment with Yiqifumai on Lipopolysaccharide-Induced Intestine Injury and Survival Rate in Rat. J. Inflamm. 8 (1), 1–11. doi:10.1186/1476-9255-8-10
Zhang, B. (2016). Manual for the Rational Use of Traditional Chinese Medicine Injections Clinically. Beijing: China Press of Traditional Chinese Medicine.
Zhang, L., Hu, J., Xiao, L., Zhang, Y., Zhao, W., Zheng, W., et al. (2010). Adverse Drug Reactions of Shenmai Injection: a Systematic Review. J. Evidence-based Integr. Med. 3 (3), 177–182. doi:10.1111/j.1756-5391.2010.01089.x
Zhang, Q., and Cui, Q. (2018). Progress in the Study of In Vivo Distribution and Anti-inflammatory Mechanism of Andrographolide and its Derivatives. Chem. Life 38 (1), 7. doi:10.13488/j.smhx.20180102
Zheng, J., Yang, X., and Yang, W. (2006). Analysis of 1089 Cases of Clinical Adverse Reactions of Xiyanping Injection. China Med. Herald 3 (23), 107–108. doi:10.3969/j.issn.1673-7210.2006.23.073
Zheng, R., Wang, H., Liu, Z., Wang, X., Li, J., Lei, X., et al. (2019). A Real-World Study on Adverse Drug Reactions to Xuebijing Injection: Hospital Intensive Monitoring Based on 93 Hospitals (31,913 Cases). Ann. Transl. Med. 7 (6), 117. doi:10.21037/atm.2018.09.26
Keywords: Chinese herbal injections, Western medicine, septic shock, efficacy, Bayesian network meta-analysis
Citation: Huang P, Chen Y, Zhang H, Chen B, Zhao S, Feng Y, Lei S and Wu Q (2022) Comparative Efficacy of Chinese Herbal Injections for Septic Shock: A Bayesian Network Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 13:850221. doi: 10.3389/fphar.2022.850221
Received: 07 January 2022; Accepted: 14 March 2022;
Published: 07 April 2022.
Edited by:
George Qian Li, Western Sydney University, AustraliaReviewed by:
Chao Ren, First Affiliated Hospital of Chinese PLA General Hospital, ChinaJianping Liu, Beijing University of Chinese Medicine, China
Copyright © 2022 Huang, Chen, Zhang, Chen, Zhao, Feng, Lei and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bojun Chen, NzE5NTIzNDc2QHFxLmNvbQ==; Shuai Zhao, ZHJfbGlvc2Vybm9AZm94bWFpbC5jb20=
 Peiying Huang
Peiying Huang Yan Chen3
Yan Chen3 Sisi Lei
Sisi Lei