- 1Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Department of Breast Oncology, Peking University Cancer Hospital and Institute, Beijing, China
- 2Center for Reproductive Medicine, Beijing Key Laboratory of Reproductive Endocrinology and Assisted Reproductive Technology and Key Laboratory of Assisted Reproduction, Department of Obstetrics and Gynecology, Ministry of Education, Peking University Third Hospital, Beijing, China
- 3University of California, San Francisco Comprehensive Cancer Center, San Francisco, CA, United States
Purpose: To identify the optimal initial 5 years of adjuvant endocrine therapy for hormone receptor-positive postmenopausal early breast cancer (EBC) patients.
Methods: We conducted a systematic search of the PubMed, Web of Science, and EMBASE to obtain relevant studies published between January 2000 and January 2022. Randomized clinical trials assessing the efficacy and safety of initial 5 years of adjuvant endocrine therapy were included. The primary outcomes were disease-free survival and overall survival and the secondary outcome was severe adverse effects (SAEs). A Bayesian network meta-analysis was carried out to indirectly compare all regimens and the value of surface under the cumulative ranking curve (SUCRA) was used to obtain rankings.
Results: Eleven studies with 49,987 subjects were included. For DFS, exemestane (EXE) [hazard ratio (HR) 0.91, 95% confidence interval (95%CI) 0.87–0.96], anastrozole (ANA) (0.94, 0.90–0.97), letrozole (LET) (0.93, 0.89–0.97), tamoxifen (TAM) followed by EXE (0.91, 0.87–0.96), and TAM followed by ANA (0.92, 0.87–0.98) were more favorable than TAM, with TAM followed by EXE ranking as the first of SUCRA. For OS, only TAM followed by ANA showed significant superiority than TAM (HR 0.91, 95%CI 0.86–0.97) and ranked as the first of SUCRA. For SAEs, EXE (HR 1.72, 95%CI 1.04–2.98), ANA (1.58, 1.03–2.43), and LET (1.63, 1.02–2.57) showed greater associations with bone fracture than TAM. However, no significant difference in the incidences of cardiac events, thromboembolic events, and cerebrovascular events was found among all comparisons.
Conclusion: The sequential use of aromatase inhibitors, which has the best curative effects and relatively mild side effects, may be the optimal treatment mode for hormone receptor-positive postmenopausal EBC patients. In addition, the three kinds of aromatase inhibitors achieved roughly equal efficacy, but caused different types of SAEs.
Systematic Review Registration: [website], identifier [registration number].
Introduction
Female breast cancer represents the most commonly diagnosed malignancy all over the world, with an estimated 2.3 million new cancer cases diagnosed in 2020 (Sung et al., 2021). Hormone receptor-positive (estrogen receptor positive and/or progesterone receptor positive) breast cancer is the most common type of breast cancer, accounting for nearly 80% of all new cases (DeSantis et al., 2019). Endocrine therapy is available for patients with hormone receptor-positive breast cancer and has greatly improved the clinical outcomes. For postmenopausal women with hormone receptor-positive early breast cancer (EBC), tamoxifen (TAM) had been established as the gold standard of adjuvant endocrine therapy for nearly 30 years, which acted as an antagonist of estrogen by saturating the estrogen receptor (Baum, 1983; Osborne, 1998). After 5 years of treatment with TAM, the risk of breast cancer recurrence and the risk of death were reduced by 47 and 26%, respectively (Clarke et al., 1998). Nevertheless, close to half of patients eventually acquired resistance to TAM and relapsed. Moreover, lengthy use of TAM was associated with increased incidences of severe adverse events (SAEs), including gynecological complications and thromboembolic events (Abe et al., 2005).
The third-generation aromatase inhibitors (AIs) came out in the middle of 1990s, including two nonsteroidal agents (anastrozole, ANA and letrozole, LET) and one steroidal agent (exemestane, EXE). In contrast to the receptor binding capacity of TAM, AIs reduced the production of estrogen in tissue and plasma by preventing the conversion from androgen to estrogen in postmenopausal women (Smith and Dowsett, 2003). Oral administration of AIs could result in an inhibition rate of aromatase activity of more than 99% (Lønning, 2004). Based on this rationale, AIs were expected to induce greater efficacy for postmenopausal hormone receptor-positive patients than TAM. Indeed, in the ATAC trial, 5 years of ANA significantly prolonged the disease-free survival (DFS) of postmenopausal patients with hormone receptor-positive EBC than 5 years of TAM [hazard ratio (HR) 0.83, 95% confidence interval (95%CI) 0.71–0.96, p = 0.013], and this improvement was still significant after a median follow-up of 120 months (HR 0.86, 95%CI 0.78–0.95, p = 0.003) (Baum et al., 2002; Cuzick et al., 2010). The following BIG 1-98 study also revealed an 18% reduction in the risk of an event ending a period of DFS in patients receiving LET than TAM (HR 0.81, 95%CI 0.70–0.93, p = 0.003) (Thurlimann et al., 2005). With these results, it was recommended to incorporate AIs into the initial 5 years of endocrine therapy (Burstein et al., 2010).
Besides the upfront use of AIs, several large randomized controlled trials (RCTs) have explored the efficacy of switching to an AI after 2–3 years of TAM (Coombes et al., 2007; Kaufmann et al., 2007; Dubsky et al., 2012; Boccardo et al., 2013). Both the IES (TAM followed by EXE vs. TAM, HR 0.76, 95%CI 0.66–0.88, p = 0.0001) and the ITA trial (TAM followed by ANA vs. TAM, HR 0.71, 95%CI 0.52–0.97, p = 0.005) revealed significant improvements in DFS of switch strategy compared with TAM alone (Coombes et al., 2007; Boccardo et al., 2013). The combined results of the ABCSG-8 study and the ARNO trial 95 also indicated an improvement of 40% in DFS of switching from TAM to ANA (Jakesz et al., 2005; Kaufmann et al., 2007; Dubsky et al., 2012). As a result, the sequential use of TAM and an AI was another practical option for postmenopausal hormone receptor-positive EBC, especially for those who would early develop resistance to TAM or had relatively higher risk of endometrial cancer and deep venous thrombosis.
However, no significance in DFS was observed between 5 years of EXE and TAM followed by EXE for 5 years (HR 0.96, 95%CI 0.88–1.05, p = 0.39) (Derks et al., 2017). Furthermore, the head-to-head comparisons between two individual AIs could not conclude which AI was relatively better (Goss et al., 2013; Smith et al., 2017). Apparently, there was an unmet need to identify the potentially best regimen of initial adjuvant endocrine therapy. Therefore, in this network meta-analysis, we synthesized the latest evidence to indirectly compare the efficacy and safety among different 5 years of regimens of initial adjuvant endocrine therapy.
Methods
This study was carried out in accordance with the Cochrane Handbook (Cumpston et al., 2019) and reported based on the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement (Hutton et al., 2015).
Search Strategy
We searched bibliographic databases in PubMed, EMBASE, and Web of Science to obtain relevant studies published between January 2000 and January 2022. MeSH terms and free text were combined to search for concepts such as “Breast Neoplasms”, “Receptors, Estrogen”, “positive” and “endocrine therapy”. The full search strategy and detailed sources of records were available in Supplementary Appendix S1. In addition, we manually searched the reference lists of included studies to identify other potentially eligible papers.
Study Selection
Studies meeting the following criteria were included in the analysis: 1) RCTs; 2) postmenopausal adult female patients (≥18 years old) with histologically confirmed invasive breast cancer; 3) positive for estrogen receptors and/or progesterone receptors (≥1% of tumor nuclei positive in immunohistochemistry) (Allison et al., 2020); 4) local treatment with curative intent including surgery and radiation has been completed; 5) initial endocrine therapy with 5 years of regimens of TAM, AIs, or sequential TAM and an AI; 6) measurements of DFS, overall survival (OS), and SAEs; and 7) written in English. In particular, if there were multiple publications for one trial, only the most recently reported endpoints would be included. We excluded studies if they met one of the following terms: 1) non-RCT studies; 2) non-English publications; 3) patients in neoadjuvant or advanced settings; and 4) lacking control or inappropriate control group.
Data Extraction and Risk of Bias Assessment
Two independent reviewers (HaL and WP) extracted the data from included studies according to a pre-specified protocol. The following study characteristics were collected: study name, publication year, study design, patient population, treatment strategy, sample size, median follow-up, and main outcomes. Discrepancies were settled by discussion. The primary outcomes of this study included DFS and OS. The secondary outcome was SAEs. DFS was defined as the time from randomization to recurrence of tumor or death. OS was defined as the time between diagnosis and death for any cause. SAEs included four life-threatening events: bone fracture, cardiac events, thromboembolic events, and cerebrovascular events.
The same two reviewers independently assessed the risk of bias for each study using the Cochrane Risk of Bias tool and evaluated them as high, low, or unclear risk (Higgins et al., 2011). Differences in opinion between the two reviewers in particular studies were resolved by discussion.
Statistical Methodology
At first, we conducted traditional meta-analysis using Review Manager (version 5.3.5) to compare the efficacy of TAM, AIs, and TAM followed by an AI with a fixed-effects model. HRs with 95%CIs were calculated using the inverse variance method for time-to-event data. Pooled results were presented through forest plots. Both the Cochrane Q test and Higgins I2 index were used to assess heterogeneity across studies, with a p-value of <0.1 and an I2 value of >50% indicating significant heterogeneity (Lau et al., 1997). The potential publication bias was assessed by performing Egger’s and Begg’s test. Then, we employed a network meta-analysis to synthesize the therapeutic effects and safety of different regimens. We directly extracted the data from included studies to calculate the log HR for time-to-event data (DFS and OS) and the log odds ratio (OR) for dichotomous variable (rate of SAEs). For all three outcomes, the efficacy/safety of one regimen was superior than the other one if the corresponding HR/OR value was less than 1.
This network meta-analysis was conducted in the OpenBUGS 3.2.3 (www.openbugs.net) and GeMTC 0.14.3 (http://drugis.org/software/addis1/gemtc) for survival data (DFS and OS) and SAEs, respectively. A Bayesian fixed-effects model via Markov Chain Monte Carlo modeling (Stewart et al., 2014) was constructed to synthesize direct and indirect comparisons with the following parameters: number of chains, three; initial value, 0.5; number of simulation iterations, 30,000; number of adaptations, 3,000; and thinning factor, 10. In order to rank all regimens, we calculated the surface under the cumulative ranking curve (SUCRA). One regimen would be the best if its SUCRA value was 1, whereas one regimen would be the worst if its SUCRA value was 0 (Salanti et al., 2011). The model inconsistency in OpenBUGS was evaluated by Deviance Information Criterion (DIC), while random effects standard deviation and the value of inconsistency factor were used for the inconsistency assessment in GeMTC. Low DIC value, inconsistency factor approach to 0, and roughly equal random effects standard deviation between the consistency model and the inconsistency model indicated that the model was consistent (Dahabreh et al., 2012; Dias et al., 2013).
Results
Search Results and Study Characteristics
The flow diagram of detailed screening process was presented in Figure 1. A total of 2775 records were identified by searching electronic databases. After removing duplicates, we excluded 1734 irrelevant records by screening titles and abstracts. Through reviewing full-text articles, we excluded 61 records with the following reasons (Sung et al., 2021): non-RCT studies, n = 33 (DeSantis et al., 2019); non-English studies, n = 7 (Baum, 1983); patients in neoadjuvant or advanced settings were enrolled, n = 13; and (Osborne, 1998) lack of control or inappropriate control group, n = 8. Eventually, 11 studies with 49,987 patients were eligible for this network meta-analysis (Coombes et al., 2007; Kaufmann et al., 2007; Mouridsen et al., 2009; Cuzick et al., 2010; Dubsky et al., 2012; Boccardo et al., 2013; Goss et al., 2013; Aihara et al., 2014; Derks et al., 2017; Smith et al., 2017; De Placido et al., 2018). No extra studies were obtained by searching the reference lists of these articles. The study characteristics of included studies were shown in Table 1. All of included studies were phase III RCTs, of which eight were open-label and three were double-blind. In terms of the treatment strategy, two, five, two, and two trials compared the efficacy and safety between 5 years of an AI and 5 years of TAM, TAM followed by an AI for 5 and 5 years of TAM, TAM followed by an AI for 5 and 5 years of an AI, and 5 years of an AI and 5 years of another AI. The median follow-up ranged from 2.5 to 10 years (eight studies, ≥5 years; three studies, <5 years). All articles were published after 2000 years and reported the latest data of their own. In particular, for studies enrolling all kinds of subtypes of EBC, we only analyzed the data from hormone receptor-positive subset. The original data of DFS, OS, and SAEs were presented in Supplementary Appendix S2.
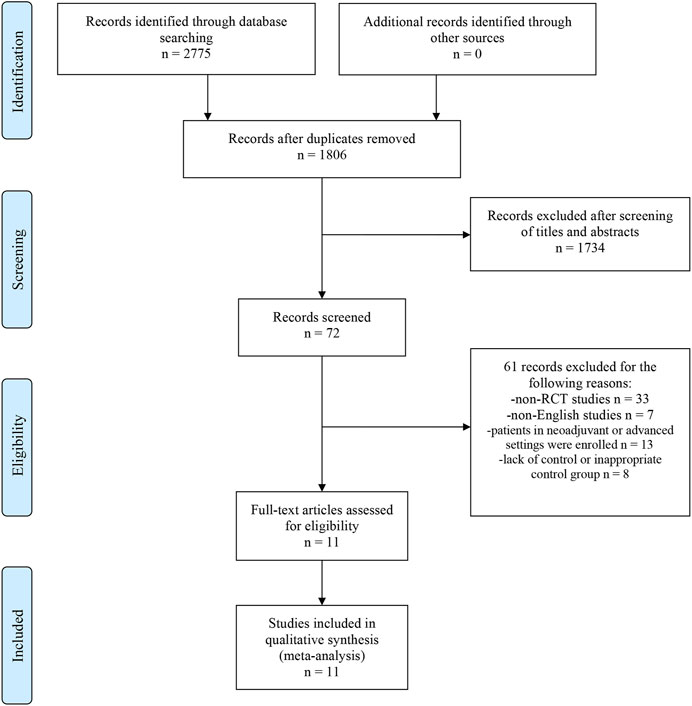
FIGURE 1. Flow Diagram of study selection process. Abbreviations: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analysis; RCT, randomized controlled trial.
Quality Assessment
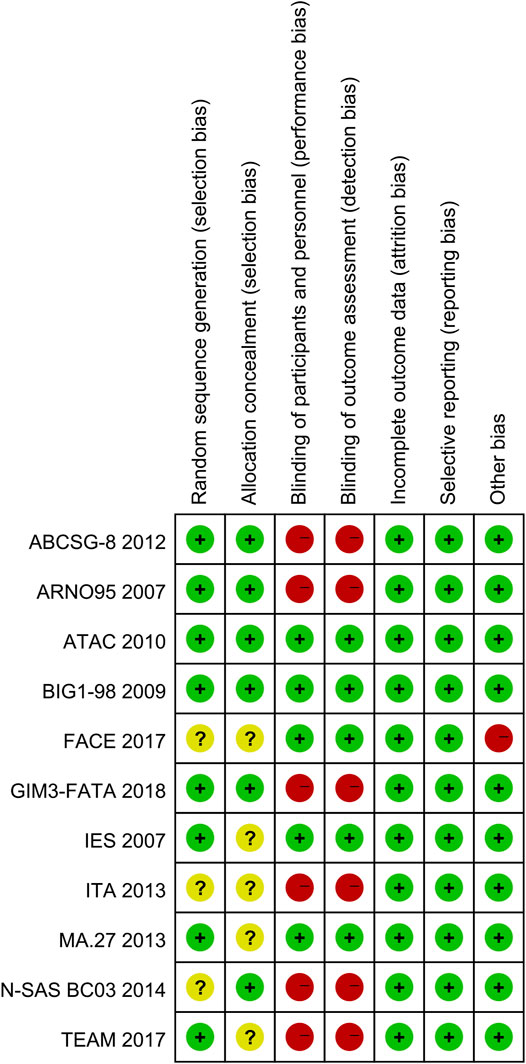
The risk of bias summary of included studies was as shown in Figure 2. Two studies were judged to be at low risk of bias (Cuzick et al., 2010; Aihara et al., 2014). Two studies were judged to be at unclear risk of bias by unclear methods of generating allocation sequences and setting allocation concealment (Boccardo et al., 2013; Goss et al., 2013). Seven studies were at high risk of bias (Coombes et al., 2007; Kaufmann et al., 2007; Mouridsen et al., 2009; Dubsky et al., 2012; Derks et al., 2017; Smith et al., 2017; De Placido et al., 2018). The major concern was that six studies with high risk of bias were open-label trials and no blinding of participants and personnel was performed. In addition, one study was judged to be at high risk in the domain of other bias because of early study termination (Smith et al., 2017).
Traditional Meta-Analysis
All 11 studies reported survival outcomes in postmenopausal patients with HR+ EBC treated with initial 5 years of adjuvant endocrine therapy. Among them, nine (Coombes et al., 2007; Kaufmann et al., 2007; Mouridsen et al., 2009; Cuzick et al., 2010; Dubsky et al., 2012; Boccardo et al., 2013; Aihara et al., 2014; Derks et al., 2017; De Placido et al., 2018) and eight (Coombes et al., 2007; Kaufmann et al., 2007; Cuzick et al., 2010; Dubsky et al., 2012; Boccardo et al., 2013; Aihara et al., 2014; Derks et al., 2017; De Placido et al., 2018) studies could be used for the direct meta-analysis of DFS and OS, respectively. For DFS, both AIs (HR 0.87, 95%CI 0.80–0.94, p = 0.0003) and TAM followed by an AI (0.79, 0.72–0.87, p < 0.00001) were significantly better than TAM (Figures 3A,B), with low heterogeneity (I2 = 0 and 9%, respectively). However, AIs did not significantly improve DFS than TAM followed by an AI (HR 0.95, 95%CI 0.88–1.03, p = 0.19) (Figure 3C). For OS, TAM followed by an AI was superior than TAM (HR 0.82, 95%CI 0.71–0.94, p = 0.005), with a low heterogeneity of I2 = 0% (Figure 3E). Nevertheless, there was no significance between AIs and TAM (HR 0.92, 95%CI 0.83–1.01, p = 0.07), and AIs and TAM followed by an AI (0.96, 0.87–1.05, p = 0.37) (Figures 3D,F). The assessment of publication bias can only be conducted in DFS between TAM and TAM followed by an AI, and OS between TAM and TAM followed by an AI, as the other four comparisons only included two studies. The Egger’s (p = 0.892 and 0.249) and Begg’s test (p = 1 and 0.308) revealed no publication bias and the Funnel plots were shown in Supplementary Appendix S3.
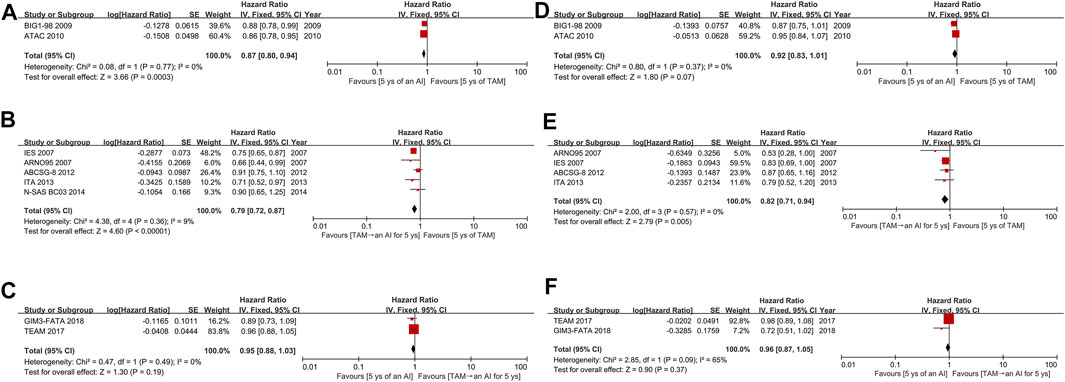
FIGURE 3. Forest plots for (A–C) DFS and (D–F) OS. Abbreviations: DFS, disease-free survival; OS, overall survival; AI, aromatase inhibitor; TAM, tamoxifen; ys, years.
Network Meta-Analysis
Network structure diagrams for all analyses of DFS (Figure 4A), OS (Figure 4C), and SAEs (Supplementary Appendix S4) were plotted using the Network package in Stata 15.0 (Stata Corporation, College Station, TX, United States). The width of edge provided a measure of the number of direct comparisons between two regimens. The size of node was proportional to the number of randomized participants of each regimen. For example, both in DFS and OS, TAM ranked in the first among all regimens in the number of randomized participants and there were most direct comparisons between TAM and TAM followed by ANA.
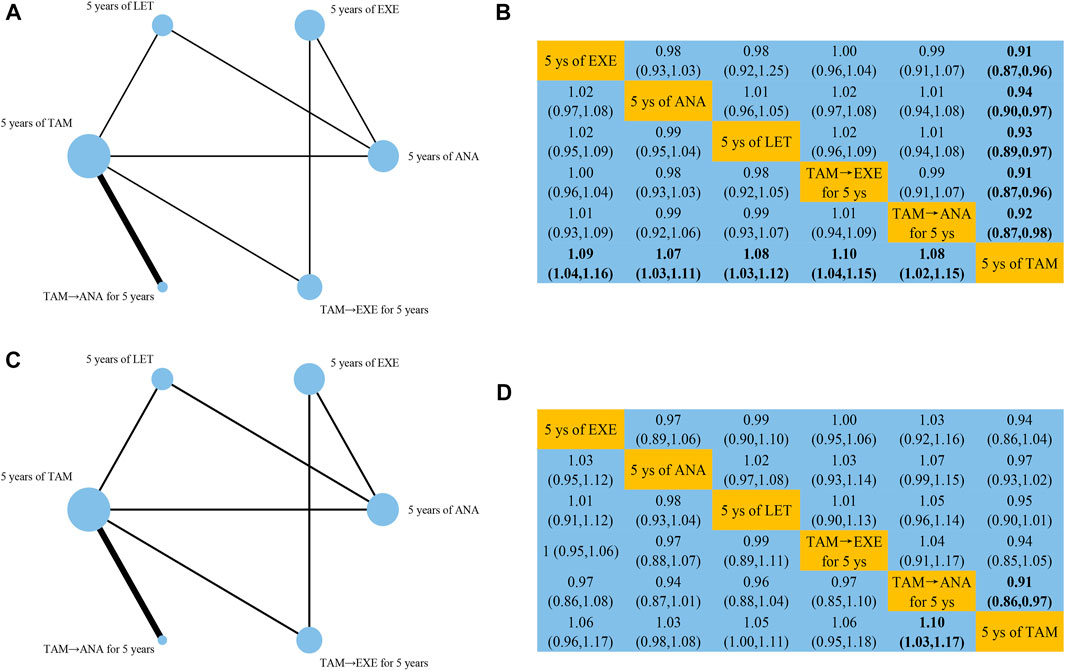
FIGURE 4. Network structure diagrams and league tables for (A,B) DFS and (C,D) OS. Abbreviations: EXE, exemestane; ANA, anastrozole; LET, letrozole.
Disease-Free Survival
10 studies reported data that could be used for the network meta-analysis of DFS (Coombes et al., 2007; Kaufmann et al., 2007; Mouridsen et al., 2009; Cuzick et al., 2010; Dubsky et al., 2012; Boccardo et al., 2013; Goss et al., 2013; Aihara et al., 2014; Derks et al., 2017; Smith et al., 2017). As shown in Figure 4B, EXE (HR 0.91, 95%CI 0.87–0.96), ANA (0.94, 0.90–0.97), LET (0.93, 0.89–0.97), TAM followed by EXE (0.91, 0.87–0.96), and TAM followed by ANA (0.92, 0.87–0.98) were significantly better than TAM. According to the cumulative SUCRA ranking curve (Figures 5A,B), TAM followed by EXE had the highest probability to be the best treatment (SUCRA 72.7, MeanRank 2.4), followed by EXE (72.5, 2.4), TAM followed by ANA (59, 3.1), LET (52.8, 3.4), ANA (43, 3.8), and TAM (0.1, 6).
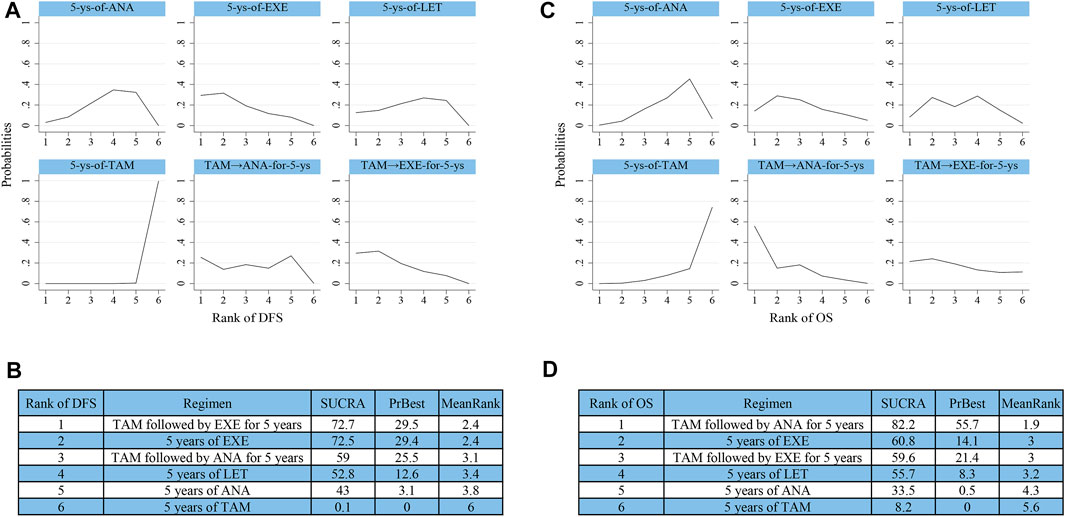
FIGURE 5. Ranking results of SUCRA for (A,B) DFS and (C,D) OS. Abbreviations: SUCRA, surface under the cumulative ranking curve.
Overall Survival
Effects of initial 5 years of adjuvant endocrine therapy on OS were reported in nine studies (Coombes et al., 2007; Kaufmann et al., 2007; Cuzick et al., 2010; Dubsky et al., 2012; Boccardo et al., 2013; Goss et al., 2013; Aihara et al., 2014; Derks et al., 2017; Smith et al., 2017). The results of synthesized analysis indicated that only TAM followed by ANA showed significant superiority than TAM (HR 0.91, 95%CI 0.86–0.97) (Figure 4D). As shown in Figures 5C,D, TAM followed by ANA ranked as the best regimen (SUCRA 82.2, MeanRank 1.9), followed by EXE (60.8, 3), TAM followed by EXE (59.6, 3), LET (55.7, 3.2), ANA (33.5, 4.3), and TAM (8.2, 5.6). The DIC values of fixed-effects model were shown to be lower than that of random-effects model both in DFS (−29.98 vs. −29.11) and OS (−25.8 vs. −23.92). Thus, the fixed-effects model was chosen to reduce inconsistency in this network meta-analysis.
Severe Adverse Events
Ten (Coombes et al., 2007; Kaufmann et al., 2007; Mouridsen et al., 2009; Cuzick et al., 2010; Dubsky et al., 2012; Boccardo et al., 2013; Goss et al., 2013; Aihara et al., 2014; Derks et al., 2017; Smith et al., 2017), nine (Coombes et al., 2007; Kaufmann et al., 2007; Mouridsen et al., 2009; Cuzick et al., 2010; Boccardo et al., 2013; Goss et al., 2013; Aihara et al., 2014; Derks et al., 2017; Smith et al., 2017), seven (Kaufmann et al., 2007; Mouridsen et al., 2009; Cuzick et al., 2010; Boccardo et al., 2013; Aihara et al., 2014; Derks et al., 2017; Smith et al., 2017), and six (Kaufmann et al., 2007; Cuzick et al., 2010; Goss et al., 2013; Aihara et al., 2014; Derks et al., 2017; Smith et al., 2017) of included studies reported the incidences of bone fracture, cardiac events, thromboembolic events, and cerebrovascular events in each group. The network plots for the four SAEs were shown in sequence in Supplementary Appendix S4. We noticed that EXE (HR 1.72, 95%CI 1.04–2.98), ANA (1.58, 1.03–2.43), and LET (1.63, 1.02–2.57) showed greater associations with bone fracture than TAM (Supplementary Appendix S5A). However, there was no significant difference in the incidences of cardiac events, thromboembolic events, and cerebrovascular events among all regimens (Supplementary Appendix S5B–D). According to the ranking results of SUCRA (Supplementary Appendix S6), EXE and LET could result in more bone fracture and cardiac events, respectively. TAM and TAM followed by ANA were potentially associated with higher risk of thromboembolic events and cerebrovascular events, respectively. The inconsistency factors from the analyses of all four SAEs were close to 0. Moreover, the random effects standard deviations between consistency model and inconsistency model were shown to be roughly equal (data not shown). In short, the analysis model applied in this network meta-analysis was consistent.
Discussion
Hormone receptor-positive breast cancer patients tended to have a better prognosis compared with those with other subtypes due to the relatively low degree of malignancy and invasiveness. Stage I hormone receptor-positive breast cancers had a 5 years of breast cancer-specific survival of 99% and the median OS of stage IV diseases could reach 5 years (Waks and Winer, 2019). For postmenopausal women, the initial 5 years of adjuvant endocrine therapies primarily consisted of TAM, AIs, and TAM followed by an AI. Moreover, recent explorations of CDK4/6 inhibitors in adjuvant setting suggested that the addition of CDK4/6 inhibitors to standard endocrine therapy significantly improved the prognosis (Johnston et al., 2020; Gao et al., 2021). Therefore, in the era of CDK4/6 inhibitors, it seemed necessary to standardize the basic endocrine therapy and thereby reduce the discrepancy in outcomes resulted from drug differences. So far, the only two head-to-head studies (FACE and GIM3-FATA) comparing two AIs could not indicate which AI was better, though 5 years of an AI has been shown to be superior than TAM alone (Smith et al., 2017; De Placido et al., 2018). In order to identify the optimal initial endocrine therapy, we conducted this network meta-analysis using the results of the most recently updated studies.
Overall, this study included 11 phase III RCTs involving 49,987 postmenopausal women with hormone receptor-positive EBC, comparing the efficacy and safety of different initial 5 years of endocrine therapies. In traditional meta-analysis, we compared three treatment modes (TAM alone, the upfront use of AIs, and the sequential use of AIs). The results showed that both the upfront use of AIs and the sequential use of AIs were better than TAM alone in DFS. In terms of OS, only the sequential use of AIs was superior than TAM alone. These results were consistent with previous studies that regimens incorporating AIs could provide significant survival benefits (Thurlimann et al., 2005; Cuzick et al., 2010; Aromatase inhibitors versus tamoxifen in, 2015). Nevertheless, comparisons between the upfront use of AIs and the sequential use of AIs showed no significant difference in DFS and OS. This could be resulted from the differences in the nature of three AIs and make the following network analyses more interesting.
In the network model, a total of six regimens were included, which were EXE, ANA, LET, TAM followed by EXE, TAM followed by ANA, and TAM. For DFS, all five regimens that incorporated AIs were superior than TAM. This was consistent with the results of direct meta-analysis and the prevailing view that AIs were more active than TAM in treating hormone receptor-positive diseases in adjuvant setting (Thurlimann et al., 2005; Cuzick et al., 2010; Aromatase inhibitors versus tamoxifen in, 2015). The sequential use of EXE and the upfront use of EXE ranked as the top two of SUCRA for DFS. It was probably due to that EXE, as an irreversible suicide inhibitor, could maximize the benefits of DFS by its strongest inhibitory ability to estrogen (Boeddinghaus and Dowsett, 2001). Commonly, almost any form of anti-tumor therapies could cause side effects to some extent while eliminating tumor cells. In hormone receptor-positive diseases, the benefits in survival derived from endocrine therapy was achieved at the expense of damage to other aspects’ health (Coleman et al., 2007). In this study, only the sequential use of ANA was significantly better than TAM alone and ranked as the first of SUCRA for OS. We speculated that this was because ANA caused fewer SAEs such as bone fracture than EXE, which will be discussed in detail below.
As for SAEs, EXE, LET, and ANA had higher probabilities of causing bone fracture compared to TAM, ranking as the top three of SUCRA. These results could be explained by the major risk of AIs, namely accelerated bone resorption when estrogen conversion was almost completely inhibited (Chien and Goss, 2006). Although there was no significant difference in bone fracture rates among the three types of AIs, 5 years of EXE was the regimen most likely to cause bone fracture. This could be related to the stronger anti-estrogen effects of EXE compared to the other two AIs. However, the specific substudy on bone mineral density of MA.27 revealed no significant difference between hormone receptor-positive EBC patients receiving EXE and ANA regardless of the baseline bone mineral density T-score (Goss et al., 2014). Moreover, it was shown that EXE could exert mild androgenic effects as a steroidal agent and thereby reduce the extent of bone loss (Goss et al., 2004; Goss et al., 2007). These inconsistencies suggested the need for further research on the effects of AIs on bone-related events.
As for the analyses of the other three SAEs, there was no significant difference among the incidences of all regimens. As a selective estrogen receptor modulator, TAM’s roles varied among different organizations. For example, in breast tissue TAM acted as a potent estrogen antagonist by competitively binding to estrogen receptor, while in the heart TAM exerted estrogen-like protective actions (Christodoulakos et al., 2006). Moreover, it was found that TAM was associated with a protective effect to the arteries based on analyses of clinical data (Bradbury et al., 2005). In present study, indeed, TAM had the highest probability to be best regimen in terms of cardiac events. The cause of heart disease in LET monotherapy was unknown yet, which may be simply accidental or may be related to the lack of vascular protection of TAM (Thurlimann et al., 2005). For the incidence of thromboembolic events, TAM could be the worst regimen according to the results of SUCRA. This result was predictable to some extent because almost all included studies indicated significantly higher incidences of venous thrombosis in TAM alone than the other arm (ATAC, 3.5 vs. 2.1%; BIG1-98, 3.5 vs. 1.5%; IES, 2.3 vs. 1.2%; ARNO95, 1.3 vs. 0%). For the incidence of cerebrovascular events, TAM followed by ANA was shown to have the highest probability to be the worst regimen. This ranking result could be caused by a seemingly high ratio, since there were only three cases receiving the sequential use of ANA and one case receiving TAM alone who had cerebrovascular events in the entire analysis, respectively. Thus, this result should be interpreted with caution and further verified.
A previous network meta-analysis assessed the efficacy of adjuvant endocrine monotherapy (Yu et al., 2018). A total of 14 studies with 19,517 patients were included. The results indicated that LET and EXE might be the best agents for DFS and OS, respectively. This was consistent with one of our research conclusions that AIs were superior than TAM in terms of efficacy. However, they did not analyze the toxic effects due to incomplete and inconsistent data. Furthermore, there was no comparison about the sequential use of AIs in that study, which should be discussed emphatically since it has become an important part of adjuvant endocrine therapy. An earlier direct meta-analysis including seven RCTs compared the efficacy and safety among AIs, TAM, and the sequenced use of AIs (Rydén et al., 2016). Similar conclusion that AIs were better than TAM in efficacy was also obtained. Nevertheless, the improvement of the sequential use of AIs in OS was not significant compared with TAM alone. In our direct meta-analysis, the sequential use of AIs after TAM was shown to significantly improve OS, which may be resulted from a larger number of samples included in our study. This study also had several limitations. First, 10 out of 11 studies were carried out in western countries and regions, while only one study with 696 patients was conducted in Asia. This distribution bias of race may reduce the applicability of our findings. Second, seven out of 11 studies were evaluated as high risk of bias, which may lead to a decline in the quality of evidence. Third, some SAEs such as endometrial cancer and vaginal bleeding were not analyzed due to small numbers of studies.
Conclusion
In summary, any regimens involving AIs improved the DFS of postmenopausal hormone receptor-positive EBC patients compared with TAM alone. Only the sequential use of AIs especially ANA was superior than TAM alone in OS. No significant difference of survival was found in the direct comparison between the upfront use and the sequential use of AIs, or in the indirect comparisons among different AIs. In terms of safety, the sequential use of AIs was generally associated with less SAEs than the upfront use of AIs, with the categories of SAEs varying among different regimens. From a long-term perspective, the sequential use of AIs may be the best treatment mode for postmenopausal hormone receptor-positive EBC patients. Therefore, when making clinical decisions, physicians need to balance short-term and long-term benefits, and select suitable agents according to patients’ clinical characteristics and potential risk of side effects.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contricutions
HaL, WP, and JZ collected relevant literatures, extracted the data, and drafted the manuscript. BS, XL, YL, and JZ collected relevant literatures and conducted the statistical analysis. HR and HuL critically revised the manuscript for content. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.886954/full#supplementary-material
Abbreviations
AIs, aromatase inhibitors; ANA, anastrozole; CI, confidence interval; DFS, disease-free survival; EBC, early breast cancer; EXE, exemestane; DIC, Deviance Information Criterion; HR, hazard ratio; LET, letrozole; OR, odds ratio; OS, overall survival; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analysis; RCTs, randomized controlled trials; SAEs, severe adverse effects; SUCRA, surface under the cumulative ranking curve; TAM, tamoxifen.
References
Abe, O., Abe, R., Enomoto, K., Kikuchi, K., Koyama, H., Masuda, H., et al. (2005). Effects of Chemotherapy and Hormonal Therapy for Early Breast Cancer on Recurrence and 15-year Survival: an Overview of the Randomised Trials. Lancet 365 (9472), 1687–1717. doi:10.1016/S0140-6736(05)66544-0
Aihara, T., Yokota, I., Hozumi, Y., Aogi, K., Iwata, H., Tamura, M., et al. (2014). Anastrozole versus Tamoxifen as Adjuvant Therapy for Japanese Postmenopausal Patients with Hormone-Responsive Breast Cancer: Efficacy Results of Long-Term Follow-Up Data from the N-SAS BC 03 Trial. Breast Cancer Res. Treat. 148 (2), 337–343. doi:10.1007/s10549-014-3155-8
Allison, K. H., Hammond, M. E. H., Dowsett, M., McKernin, S. E., Carey, L. A., Fitzgibbons, P. L., et al. (2020). Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. J. Clin. Oncol. 38 (12), 1346–1366. doi:10.1200/jco.19.02309
Aromatase Inhibitors versus Tamoxifen in Early Breast Cancer: Patient-Level Meta-Analysis of the Randomised Trials. Lancet (2015) 386(10001):1341–1352. doi: doi:10.1016/S0140-6736(15)61074-1
Baum, M., Budzar, A. U., Cuzick, J., Forbes, J., Houghton, J. H., Klijn, J. G., et al. (2002). Anastrozole Alone or in Combination with Tamoxifen versus Tamoxifen Alone for Adjuvant Treatment of Postmenopausal Women with Early Breast Cancer: First Results of the ATAC Randomised Trial. Lancet 359 (9324), 2131–2139. doi:10.1016/s0140-6736(02)09088-8
Baum, M. (1983). Controlled Trial of Tamoxifen as Adjuvant Agent in Management of Early Breast-Cancer. Lancet 1 (8319), 257–261. doi:10.1016/S0140-6736(85)92206-8
Boccardo, F., Guglielmini, P., Bordonaro, R., Fini, A., Massidda, B., Porpiglia, M., et al. (2013). Switching to Anastrozole versus Continued Tamoxifen Treatment of Early Breast Cancer: Long Term Results of the Italian Tamoxifen Anastrozole Trial. Eur. J. Cancer 49 (7), 1546–1554. doi:10.1016/j.ejca.2012.12.025
Boeddinghaus, I. M., and Dowsett, M. (2001). Comparative Clinical Pharmacology and Pharmacokinetic Interactions of Aromatase Inhibitors. J. Steroid Biochem. Mol. Biol. 79 (1-5), 85–91. doi:10.1016/s0960-0760(01)00126-1
Bradbury, B. D., Lash, T. L., Kaye, J. A., and Jick, S. S. (2005). Tamoxifen-treated Breast Carcinoma Patients and the Risk of Acute Myocardial Infarction and Newly-Diagnosed Angina. Cancer 103 (6), 1114–1121. doi:10.1002/cncr.20900
Burstein, H. J., Prestrud, A. A., Seidenfeld, J., Anderson, H., Buchholz, T. A., Davidson, N. E., et al. (2010). American Society of Clinical Oncology Clinical Practice Guideline: Update on Adjuvant Endocrine Therapy for Women with Hormone Receptor-Positive Breast Cancer. J. Clin. Oncol. 28 (23), 3784–3796. doi:10.1200/JCO.2009.26.3756
Chien, A. J., and Goss, P. E. (2006). Aromatase Inhibitors and Bone Health in Women with Breast Cancer. J. Clin. Oncol. 24 (33), 5305–5312. doi:10.1200/jco.2006.07.5382
Christodoulakos, G. E., Lambrinoudaki, I. V., and Botsis, D. C. (2006). The Cardiovascular Effects of Selective Estrogen Receptor Modulators. Ann. N. Y. Acad. Sci. 1092, 374–384. doi:10.1196/annals.1365.034
Clarke, M., Collins, R., Davies, C., Godwin, J., Gray, R., Peto, R., et al. (1998). Tamoxifen for Early Breast Cancer: an Overview of the Randomised Trials. Early Breast Cancer Trialists' Collaborative Group. Lancet 351 (9114), 1451–1467. doi:10.1016/S0140-6736(97)11423-4
Coleman, R. E., Banks, L. M., Girgis, S. I., Kilburn, L. S., Vrdoljak, E., Fox, J., et al. (2007). Skeletal Effects of Exemestane on Bone-Mineral Density, Bone Biomarkers, and Fracture Incidence in Postmenopausal Women with Early Breast Cancer Participating in the Intergroup Exemestane Study (IES): a Randomised Controlled Study. Lancet Oncol. 8 (2), 119–127. doi:10.1016/s1470-2045(07)70003-7
Coombes, R. C., Kilburn, L. S., Snowdon, C. F., Paridaens, R., Coleman, R. E., Jones, S. E., et al. (2007). Survival and Safety of Exemestane versus Tamoxifen after 2-3 Years' Tamoxifen Treatment (Intergroup Exemestane Study): a Randomised Controlled Trial. Lancet 369 (9561), 559–570. doi:10.1016/s0140-6736(07)60200-1
Cumpston, M., Li, T., Page, M. J., Chandler, J., Welch, V. A., Higgins, J. P., et al. (2019). Updated Guidance for Trusted Systematic Reviews: a New Edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 10, ED000142. doi:10.1002/14651858.Ed000142
Cuzick, J., Sestak, I., Baum, M., Buzdar, A., Howell, A., Dowsett, M., et al. (2010). Effect of Anastrozole and Tamoxifen as Adjuvant Treatment for Early-Stage Breast Cancer: 10-year Analysis of the ATAC Trial. Lancet Oncol. 11 (12), 1135–1141. doi:10.1016/s1470-2045(10)70257-6
Dahabreh, I. J., Trikalinos, T. A., Lau, J., Schmid, C., Trikalinos, T. A., Lau, J., et al. (2012). An Empirical Assessment of Bivariate Methods for Meta-Analysis of Test Accuracy. Rockville (MD): Agency for Healthcare Research and Quality US.
De Placido, S., Gallo, C., De Laurentiis, M., Bisagni, G., Arpino, G., Sarobba, M. G., et al. (2018). Adjuvant Anastrozole versus Exemestane versus Letrozole, Upfront or after 2 Years of Tamoxifen, in Endocrine-Sensitive Breast Cancer (FATA-GIM3): a Randomised, Phase 3 Trial. Lancet Oncol. 19 (4), 474–485. doi:10.1016/S1470-2045(18)30116-5
Derks, M. G. M., Blok, E. J., Seynaeve, C., Nortier, J. W. R., Kranenbarg, E. M., Liefers, G. J., et al. (2017). Adjuvant Tamoxifen and Exemestane in Women with Postmenopausal Early Breast Cancer (TEAM): 10-year Follow-Up of a Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 18 (9), 1211–1220. doi:10.1016/S1470-2045(17)30419-9
DeSantis, C. E., Ma, J., Gaudet, M. M., Newman, L. A., Miller, K. D., Goding Sauer, A., et al. (2019). Breast Cancer Statistics, 2019. CA Cancer J. Clin. 69 (6), 438–451. doi:10.3322/caac.21583
Dias, S., Welton, N. J., Sutton, A. J., Caldwell, D. M., Lu, G., and Ades, A. E. (2013). Evidence Synthesis for Decision Making 4: Inconsistency in Networks of Evidence Based on Randomized Controlled Trials. Med. Decis. Mak. 33 (5), 641–656. doi:10.1177/0272989x12455847
Dubsky, P. C., Jakesz, R., Mlineritsch, B., Pöstlberger, S., Samonigg, H., Kwasny, W., et al. (2012). Tamoxifen and Anastrozole as a Sequencing Strategy: a Randomized Controlled Trial in Postmenopausal Patients with Endocrine-Responsive Early Breast Cancer from the Austrian Breast and Colorectal Cancer Study Group. J. Clin. Oncol. 30 (7), 722–728. doi:10.1200/jco.2011.36.8993
Gao, H. F., Lin, Y. Y., Zhu, T., Ji, F., Zhang, L. L., Yang, C. Q., et al. (2021). Adjuvant CDK4/6 Inhibitors Combined with Endocrine Therapy in HR-Positive, HER2-Negative Early Breast Cancer: A Meta-Analysis of Randomized Clinical Trials. Breast 59, 165–175. doi:10.1016/j.breast.2021.07.002
Goss, P. E., Qi, S., Josse, R. G., Pritzker, K. P., Mendes, M., Hu, H., et al. (2004). The Steroidal Aromatase Inhibitor Exemestane Prevents Bone Loss in Ovariectomized Rats. Bone 34 (3), 384–392. doi:10.1016/j.bone.2003.11.006
Goss, P. E., Hadji, P., Subar, M., Abreu, P., Thomsen, T., and Banke-Bochita, J. (2007). Effects of Steroidal and Nonsteroidal Aromatase Inhibitors on Markers of Bone Turnover in Healthy Postmenopausal Women. Breast Cancer Res. 9 (4), R52. doi:10.1186/bcr1757
Goss, P. E., Ingle, J. N., Pritchard, K. I., Ellis, M. J., Sledge, G. W., Budd, G. T., et al. (2013). Exemestane versus Anastrozole in Postmenopausal Women with Early Breast Cancer: NCIC CTG MA.27a Randomized Controlled Phase III Trial. J. Clin. Oncol. 31 (11), 1398–1404. doi:10.1200/jco.2012.44.7805
Goss, P. E., Hershman, D. L., Cheung, A. M., Ingle, J. N., Khosla, S., Stearns, V., et al. (2014). Effects of Adjuvant Exemestane versus Anastrozole on Bone Mineral Density for Women with Early Breast Cancer (MA.27B): a Companion Analysis of a Randomised Controlled Trial. Lancet Oncol. 15 (4), 474–482. doi:10.1016/S1470-2045(14)70035-X
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's Tool for Assessing Risk of Bias in Randomised Trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H., Cameron, C., et al. (2015). The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-Analyses of Health Care Interventions: Checklist and Explanations. Ann. Intern Med. 162 (11), 777–784. doi:10.7326/m14-2385
Jakesz, R., Jonat, W., Gnant, M., Mittlboeck, M., Greil, R., Tausch, C., et al. (2005). Switching of Postmenopausal Women with Endocrine-Responsive Early Breast Cancer to Anastrozole after 2 years' Adjuvant Tamoxifen: Combined Results of ABCSG Trial 8 and ARNO 95 Trial. Lancet 366 (9484), 455–462. doi:10.1016/s0140-6736(05)67059-6
Johnston, S. R. D., Harbeck, N., Hegg, R., Toi, M., Martin, M., Shao, Z. M., et al. (2020). Abemaciclib Combined with Endocrine Therapy for the Adjuvant Treatment of HR+, HER2-, Node-Positive, High-Risk, Early Breast Cancer (monarchE). J. Clin. Oncol. 38 (34), 3987–3998. doi:10.1200/jco.20.02514
Kaufmann, M., Jonat, W., Hilfrich, J., Eidtmann, H., Gademann, G., Zuna, I., et al. (2007). Improved Overall Survival in Postmenopausal Women with Early Breast Cancer after Anastrozole Initiated after Treatment with Tamoxifen Compared with Continued Tamoxifen: the ARNO 95 Study. J. Clin. Oncol. 25 (19), 2664–2670. doi:10.1200/jco.2006.08.8054
Lau, J., Ioannidis, J. P., and Schmid, C. H. (1997). Quantitative Synthesis in Systematic Reviews. Ann. Intern Med. 127 (9), 820–826. doi:10.7326/0003-4819-127-9-199711010-00008
Lønning, P. E. (2004). Aromatase Inhibitors in Breast Cancer. Endocr. Relat. Cancer 11 (2), 179–189. doi:10.1677/erc.0.0110179
Mouridsen, H., Mouridsen, H., Giobbie-Hurder, A., Goldhirsch, A., Thürlimann, B., Paridaens, R., et al. (2009). Letrozole Therapy Alone or in Sequence with Tamoxifen in Women with Breast Cancer. N. Engl. J. Med. 361 (8), 766–776. doi:10.1056/NEJMoa0810818
Osborne, C. K. (1998). Tamoxifen in the Treatment of Breast Cancer. N. Engl. J. Med. 339 (22), 1609–1618. doi:10.1056/NEJM199811263392207
Rydén, L., Heibert Arnlind, M., Vitols, S., Höistad, M., and Ahlgren, J. (2016). Aromatase Inhibitors Alone or Sequentially Combined with Tamoxifen in Postmenopausal Early Breast Cancer Compared with Tamoxifen or Placebo - Meta-Analyses on Efficacy and Adverse Events Based on Randomized Clinical Trials. Breast 26, 106–114. doi:10.1016/j.breast.2016.01.006
Salanti, G., Ades, A. E., and Ioannidis, J. P. (2011). Graphical Methods and Numerical Summaries for Presenting Results from Multiple-Treatment Meta-Analysis: an Overview and Tutorial. J. Clin. Epidemiol. 64 (2), 163–171. doi:10.1016/j.jclinepi.2010.03.016
Smith, I., Yardley, D., Burris, H., De Boer, R., Amadori, D., McIntyre, K., et al. (2017). Comparative Efficacy and Safety of Adjuvant Letrozole versus Anastrozole in Postmenopausal Patients with Hormone Receptor-Positive, Node-Positive Early Breast Cancer: Final Results of the Randomized Phase III Femara versus Anastrozole Clinical Evaluation (FACE) Trial. J. Clin. Oncol. 35 (10), 1041–1048. doi:10.1200/jco.2016.69.2871
Smith, I. E., and Dowsett, M. (2003). Aromatase Inhibitors in Breast Cancer. N. Engl. J. Med. 348 (24), 2431–2442. doi:10.1056/NEJMra023246
Stewart, G. B., Mengersen, K., and Meader, N. (2014). Potential Uses of Bayesian Networks as Tools for Synthesis of Systematic Reviews of Complex Interventions. Res. Synth. Methods 5 (1), 1–12. doi:10.1002/jrsm.1087
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Thurlimann, B., Thürlimann, B., Keshaviah, A., Coates, A. S., Mouridsen, H., Mauriac, L., et al. (2005). A Comparison of Letrozole and Tamoxifen in Postmenopausal Women with Early Breast Cancer. N. Engl. J. Med. 353 (26), 2747–2757. doi:10.1056/NEJMoa052258
Waks, A. G., and Winer, E. P. (2019). Breast Cancer Treatment: A Review. JAMA 321 (3), 288–300. doi:10.1001/jama.2018.19323
Keywords: adjuvant endocrine therapy, early breast cancer, network meta-analysis, tamoxifen, aromatase inhibitor
Citation: Liao H, Pei W, Zhong J, Shao B, Liu X, Liu Y, Zhang J, Rugo HS and Li H (2022) Efficacy and Safety of Initial 5 Years of Adjuvant Endocrine Therapy in Postmenopausal Hormone Receptor-Positive Breast Cancer: A Systematic Review and Network Meta-Analysis. Front. Pharmacol. 13:886954. doi: 10.3389/fphar.2022.886954
Received: 01 March 2022; Accepted: 13 May 2022;
Published: 30 May 2022.
Edited by:
Mustafizur Rahman, Khulna University, BangladeshReviewed by:
Mohammad Safiqul Islam, Noakhali Science and Technology University, BangladeshM. Tish Knobf, Yale University, United States
Copyright © 2022 Liao, Pei, Zhong, Shao, Liu, Liu, Zhang, Rugo and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huiping Li, aHVpcGluZ2xpMjAxMkBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Hao Liao
Hao Liao Wendi Pei
Wendi Pei Jianxin Zhong1
Jianxin Zhong1 Jiayang Zhang
Jiayang Zhang Huiping Li
Huiping Li