Abstract
In the past decade, immunotherapy has been the most promising treatment for gastrointestinal tumors. But the low response rate and drug resistance remain major concerns. It is therefore imperative to develop adjuvant therapies to increase the effectiveness of immunotherapy and prevent drug resistance. Ginseng has been used in Traditional Chinese medicine as a natural immune booster for thousands of years. The active components of ginseng, ginsenosides, have played an essential role in tumor treatment for decades and are candidates for anti-tumor adjuvant therapy. They are hypothesized to cooperate with immunotherapy drugs to improve the curative effect and reduce tumor resistance and adverse reactions. This review summarizes the research into the use of ginsenosides in immunotherapy of gastrointestinal tumors and discusses potential future applications.
Introduction
Gastrointestinal (GI) cancers are one of the most common malignant tumors globally, with a high incidence and mortality rate (Sung et al., 2021). Despite significant advances in disease diagnosis and drug development, GI cancers remain the leading cause of cancer death; thus, it is imperative to develop novel therapeutic approaches for patients affected by these cancers. The current treatment methods for GI cancers generally include adjuvant chemotherapy, chemoradiation, surgical resection, and perioperative chemotherapy. Among these, chemotherapy and radiotherapy are the first-line treatments for advanced GI tumors, and they can only prolong patients’ survival. Tumors easily develop strong resistance to chemotherapy drugs, with a high recurrence and metastasis rate, and patients have a poor overall prognosis (Baxter et al., 2021). Currently, immunotherapy is one of the most promising therapies to overcome these difficulties (Gourd, 2021).
Immunotherapy, which has recently emerged as a new beneficial therapy, relies on the immune system to attack and eliminate tumors. The first attempts to stimulate active host immunity were based on administration of cytokines, particularly interferon-γ, interleukin-2 (IL-2), IL-10, and GM-CSF. The use of immune checkpoint inhibitors has gradually gained the attention of the medical community (Kirkwood et al., 2012). The anti-CTLA-4 antibody ipilimumab and the anti-PD-1mAbs pembrolizumab and nivolumab were first approved by the United States FDA to treat metastatic melanoma in 2011 and 2014, respectively. Four years later, Dr. James P. Allison and Dr. Tasuku Honjo were awarded the 2018 Nobel Prize in Physiology or Medicine for their work on immune checkpoint therapy in cancer (Altmann, 2018). It is expected that if these antibodies are used to target the immune escape mechanisms, various cancers will be controlled or even cured. The FDA and EMA have also approved the use of immunotherapy for melanoma, lung cancer, head and neck squamous cell carcinoma, renal cell carcinoma, and other tumors (Gong et al., 2018). However, there are still many challenges to overcome. Immune checkpoint inhibitors are effective for treating tumors with high mismatch repair ability or microsatellite instability but are ineffective on microsatellite stable tumors. About 95% of colorectal cancers are microsatellite stable, and these patients cannot benefit from immunotherapy. Other challenges are low tumor mutation pressure and lack of immune cell infiltration, which are hypothesized to be the main mechanisms of immune resistance (Chan et al., 2019; Samstein et al., 2019). A variety of small molecules (Xu et al., 2021; Jiang et al., 2022) and targeted inhibitors (Li et al., 2019a; Lee and Konstantinopoulos, 2019; Wang et al., 2022; Zhang et al., 2022) can improve immunotherapy, and screening for boosters for immunotherapy has become a research hotspot.
Ginseng, which is the dried root or rhizome of Panax ginseng C.A. Mey., a plant of the Araliaceae family, is a potential source of such boosters. It is listed as “top grade” (i.e., a non-toxic medicinal grade) in Shen Nong’s Materia Medica and has been used in traditional medical clinics for thousands of years. It is believed to nourish the original Qi, replenish vital energy and body fluid, promote growth, soothe the nerves, and nourish the mind (Gao, 1990). Ginseng preparations have been widely used in medicine and health care in Asia and Europe (Rubio et al., 2018; Chen et al., 2020). Ginseng is rich in bioactive ingredients such as triterpene saponins and polysaccharides. Among these bioactive ingredients, the main components are ginsenosides, which have a wide range of pharmacological effects, such as anti-tumor (Linming et al., 2017), anti-oxidation (He et al., 2022), and anti-aging (Wang et al., 2021a) activities. They also protect the liver (Qu et al., 2021; Li et al., 2022a) and the cardiovascular and cerebrovascular systems (Zhang et al., 2019; Han et al., 2022a; Ni et al., 2022). This review describes the research progress in the use of ginsenosides in various tumor treatments with an emphasis on their use in immunotherapy of GI tumors.
Ginseng components are a candidate source of anti-tumor adjuvants
Ginseng is the most commonly used herbal medicine worldwide. Many scholars believe that ginseng can be used to identify drug candidates for adjuvant tumor treatment (Yun, 2001; Wong et al., 2015; Majeed et al., 2018). Recent studies have explored the anti-tumor activities of various components of ginseng and their synergistic anti-cancer effects.
Effects of ginseng components on immunity
Ginsenosides are the main components of ginseng, and they are categorized as protopanaxadiol-, protopanaxatriol, and oleanolic acid-type ginsenosides (Figure 1 and Table 1) based on the structure of the aglycon (Hou et al., 2021). To date, more than 100 types of ginsenosides have been isolated (Jia and Zhao, 2009; Wang et al., 2021b).
FIGURE 1
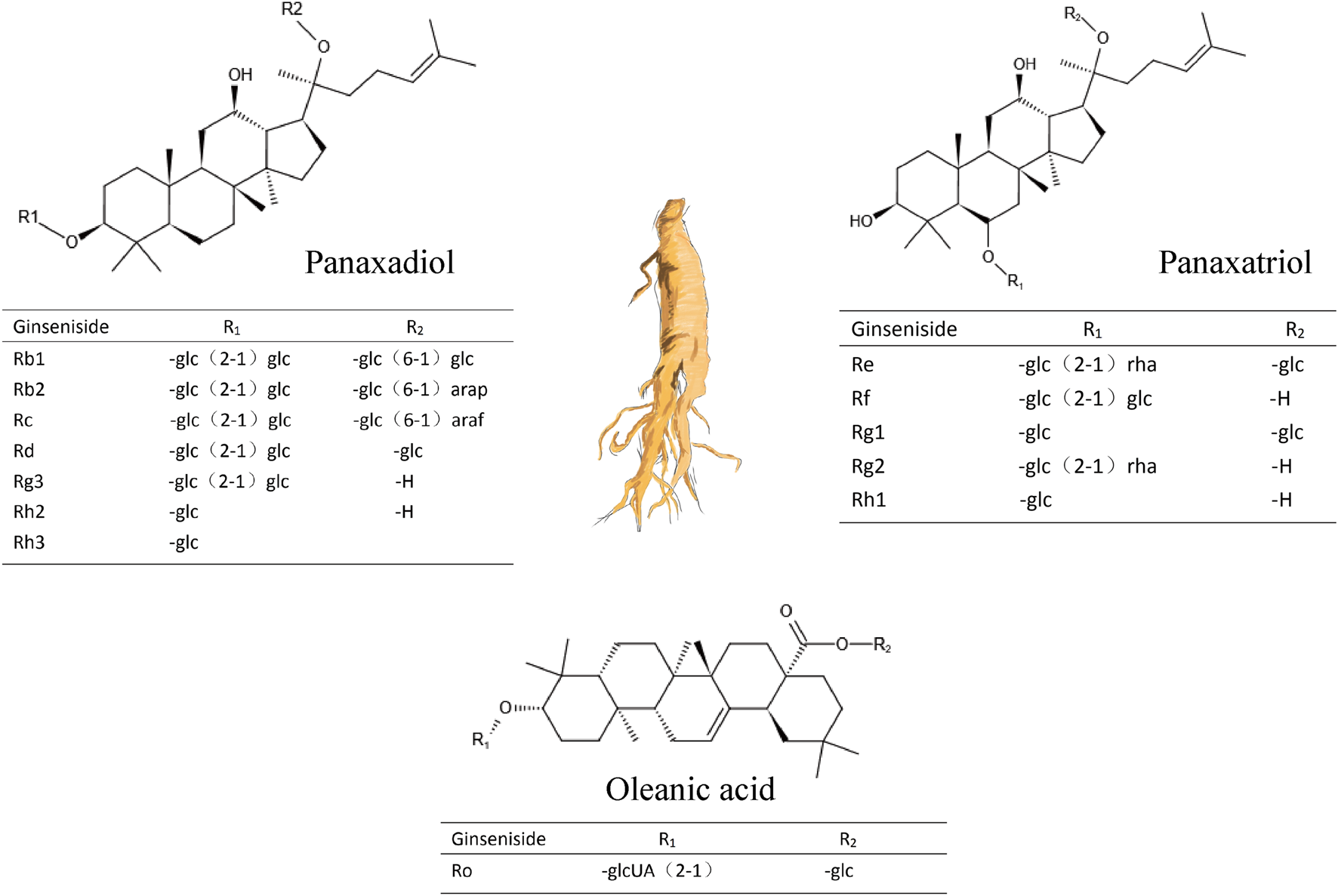
Three types of ginsenosides and their chemical structures.
TABLE 1
| Ginseng component | Model | Pharmacology | Therapeutic dose | Pharmacological mechanism | References |
|---|---|---|---|---|---|
| KG-135 | HeLa cervical cancer cell line | Enhancement of HeLa cell apoptosis induced by etoposide | 0–75 μg/ml | Bax and p21Waf1/Cip1↑; G1 cyclin-dependent kinase↓; p53Ser−15↓; p-p53Ser−15↓ | Lee et al. (2010) |
| Rg3 | CTX-treated mice | Reversal of CTX-induced immunosuppression | 7.5/15 mg/kg | CD3+ ↑ CD4+↑ | Liu et al. (2019) |
| F2 | Alcoholic liver injury mouse model | Attenuates chronic-binge ethanol-induced liver injury | 50 mg/kg | IL-10↑; Treg cells↑; IL-17↓; Th17 cells↓ | Kim et al. (2020a) |
| 20S-protopanaxadiol | ER+ MCF-7 and ER− MDA-MB231 cell line | Estrogen-stimulated MCF-7 cell inhibition; enhancement of tamoxifen cytotoxicity | 5 μM/10 μM | estrogen-stimulated gene expression ↓ | Yu et al. (2007) |
| Rg5 | Nude mice bearing A549/T tumors | Preventing tumor drug resistance | 1–100 μM | p-Akt↓, Nrf2↓, | Feng et al. (2020) |
| GPs | K562, HL-60, KG1α cells | Mouse peritoneal macrophage-mediated cytotoxicity | 0, 25, 50, 100, and 200 mg/L | TNF-α↑, IL-1↑, IL-6↑, NO↑ | Wang et al. (2010) |
| Re3/Rk | Balb/c mice myelo-suppression model | Peripheral blood cells↑, bone marrow nucleated cell counts↑, thymus and spleen index↑ | 5/10 mg/kg | Bcl-2↑; bax↓caspase-3↓ | Han et al. (2019) |
The effects of ginseng components on immunity.
The immunomodulatory effects of ginseng components have been widely reported, and we review the pharmacological effects they have on the immune system. Ginseng KG-135 preparation, a mixture of three ginsenosides, Rk1, Rg,3, and Rg5, inhibits the phosphorylation of proteins in the p53 pathway, and thus enhances etoposide-induced HeLa cell apoptosis (Lee et al., 2010). Ginsenoside F2 regulates immunity by increasing IL-10 expression and the number of regulatory T cells while decreasing IL-17 expression and the number of Th17 cells (Kim et al., 2020a). Ginsenoside Rg5 targets the ABCB1 transporter and is combined with docetaxel to prevent drug resistance (Feng et al., 2020). Additionally, another active ingredient in ginseng, 20 S-protopanaxadiol, was found to inhibit the growth of ER-positive breast cancer cells and enhance tamoxifen cytotoxicity (Yu et al., 2007). Rg3 was shown to have a protective effect on immunosuppression induced by cyclophosphamide (CTX) (Liu et al., 2019), which is used as a broad-spectrum anti-tumor chemotherapy drug combined with immunotherapy to treat metastatic breast cancer in humanized mouse bone marrow (Roghanian et al., 2019). Rg3 was found to further reduce immune capacity after CTX injury. Another study showed that the ginsenosides Re and Rk3 can relieve symptoms of bone marrow suppression and improve bone marrow hematopoietic function, regulate bcl-2/bax balance, and inhibit the caspase-3 expression, preventing apoptosis of bone marrow nucleated cells (Han et al., 2019). When ginseng polysaccharides were used to treat mouse peritoneal macrophages, they were clearly cytotoxic to K562, HL-60, and KG1 cells (Wang et al., 2010).
Various components in ginseng often play different roles in immune regulation. In Traditional Chinese medicine (TCM) different combinations of herbs are chosen to prepare water decoctions that are prescribed to treat patients (Gao, 1990). In TCM, ginsenosides play a major role in T cell development, cytokine secretion, helper T cell function, and other putative signal pathways to enhance the total immune system. But in modern pharmacological studies, results of studies in which ginseng components are administered alone have disappointing. Even when used as immune adjuvants, they tend to work at higher concentrations than common small molecule drugs, which has led researchers to worry about their toxicity to the liver and kidney (Wang et al., 2010; Kim et al., 2020a). Based on the current reports, we believe that only a few components of ginseng have strong pharmacological activity and have the potential to be developed as small molecule drugs for chemotherapy. Instead, the vast majority of ginseng components have immune regulation functions and may be effective as immune adjuvants. Furthermore, ginseng components can affect to several known drug resistance mechanisms (De Vera et al., 2019; Bu et al., 2022; Shurin and Umansky, 2022). Their probable benefits to immunotherapy deserve our attention.
The effects of ginseng components on immune checkpoint blockade
Increasing evidence suggests that ginseng components may improve immunotherapy. In mice, ginseng polysaccharides were found to increase the anti-tumor responses to αPD-1 mAb by increasing the content of the microbial metabolite valeric acid and the Kynurenine/Tryptophan ratio. The therapeutic effect of ginseng polysaccharide contributes to the suppression of regulatory T cells and induction of effector T cells after this combination treatment (Han et al., 2022b). In an A549 xenograft mice model, ginsenosides Rk1 and Rg3 significantly inhibited tumor growth and reduced PD-L1 expression by blocking NF-κB signaling (Jiang et al., 2017; Hu et al., 2020), and they induced MDA-MB-231 cell apoptosis by blocking PI3K/Akt signaling (Hong and Fan, 2019). Because ginsenosides have high lipophilicity, researchers believe that they may be suitable for use as targeting vectors in addition to being used as drugs (Han et al., 2022b). The lipophilic structure of ginsenoside Rg3 is advantageous for packaging into liposomes for docetaxel delivery. Docetaxel delivered via Rg3 promoted tumor immunity by reversing the formation of an immunosuppressive microenvironment in triple-negative breast cancer (Xia et al., 2022). Ginseng-derived nanoparticles can reprogram tumor-associated macrophages in colon cancer to increase CCL5 and CXCL9 secretion and recruitment of CD8+ T cells into the tumor bed; these nanoparticles synergized with PD-1 mAb therapy with no detected systemic toxicity (Han et al., 2022b). STAT3 and PD-L1 are critically involved in cancer proliferation (Bu et al., 2017; Jiang et al., 2019; Zou et al., 2020), progression, and immunosuppression. The ginseng saponin metabolite, compound K, inhibits PD-L1 and STAT3 signaling in prostate cancer cells by activating miR193a-5p (Lee et al., 2021). The above attempts to use ginsenosides to increase the effectiveness of immunotherapy have achieved some success.
In summary, several studies have shown that ginseng components have great potential in immunotherapy, and the main components of ginseng, ginsenosides, remain a hot topic of research, with studies exploring their anti-tumor effects and mechanisms. However, through reviewing previous studies, we also found many common problems in these studies. In the past 20 years, traditional molecular biology methods have been used to study ginseng compounds, and researchers have stopped at verifying the signal pathways affected by ginsenosides and the pharmacological effects in vivo (Figure 2). However, how ginsenosides affect the tumor immune microenvironment remains unknown. This dilemma is due to the lack of identification of pharmacological targets and biomarkers in the study of ginsenosides. Technologies such as co-immunoprecipitation, surface plasmon resonance, and microscale thermophoresis are in the forefront of those being used to identify TCM targets (Xu et al., 2021b; Gu et al., 2022). The use of molecular interaction technology to identify the pharmacological targets of ginsenosides will help to deepen our understanding of the pharmacology of ginsenosides.
FIGURE 2
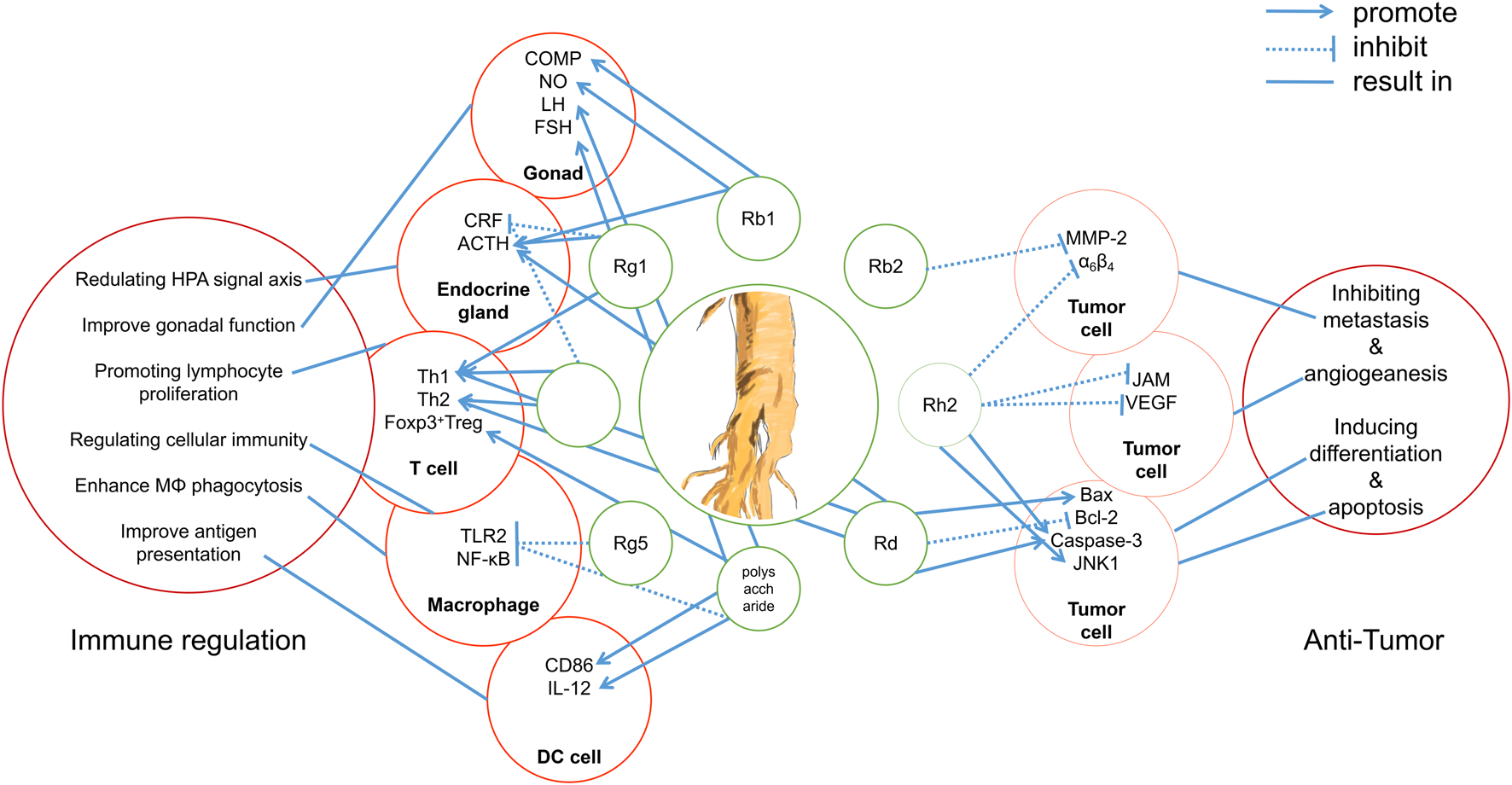
Integrated mechanisms of active components of ginseng.
Research progress in the use of ginsenosides in immunotherapy of digestive tract tumors
Research progress in the use of ginsenosides in liver cancer immunotherapy
Liver cancer is one of the six most common cancers in the world, and it is often the final outcome of all liver diseases (Gravitz and Pincock, 2014; Sung et al., 2021). The World Health Organization estimates that in 2030, more than 1 million patients will die of liver cancer per year. In the United States, the age-adjusted death rate from liver cancer increased by 43% between 2000 and 2016, from 10.5 per 100,000 U.S. standard population to 15.0 for men. The 5-year survival rate of liver cancer is 20%, making it the second most fatal tumor after pancreatic cancer (Siegel et al., 2022). Furthermore, the prevalence of liver cancer is still rising, and treatment methods are still lacking. Currently liver cancer is mainly treated using physical methods, such as chemotherapy, liver transplantation, and surgery, but researchers are actively exploring new drug targets and therapies (such as immunotherapy) (Centers for Disease Control and Pre-vention, 2018).
The earliest research on ginsenosides as tumor inhibitors for liver cancer can be traced back to a report published in 1997. Park et al. (1997) reported that ginsenoside Rh2 promotes apoptosis of human liver hepatoma SK-HEP-1 cells and subsequent PARP cleavage. PARP is responsible for mediating post-translational PARylation of substrate proteins involved in transcription and DNA damage repair (Noordermeer and van Attikum, 2019). PARP inhibitor (PARPi) inhibits the enzymatic activity of both PARP1 and PARP2, prevents PARylation, and causes the accumulation of DNA single-strand breaks. Therefore, PARPi is used to induce cell death in tumors with homologous recombination (HR) deficiency (Murai et al., 2012; Murai et al., 2014). Ginsenoside Rg1 was reported to inhibit the expression of a key DNA double-strand break repair factor and CtBP interacting protein, thereby impairing HR (Zhen et al., 2018). Chemical sensitivity analysis showed that the combination of Rg1 and olaparib, a first-line PARPi, improves the therapeutic effect of hepatoblastoma treatment. This indicates that Rg1 significantly enhances the sensitivity to DNA damage therapy and that it may be a sensitizer of PARPi.
NHE1 protein is an important therapeutic target for hepatocellular carcinoma (HCC) (Yang et al., 2010). Ginsenoside Rg3 was reported to downregulate NHE1 in vivo and in vitro, revealing it as an effective multi-target anti-tumor drug for the treatment of HCC. Epidermal growth factor (EGF) can significantly up-regulate the expression of NHE1 and increase the level of phosphorylated extracellular signal-regulated protein kinase (ERK1/2) and the expression of hypoxia-inducible factor 1α (HIF-1α). Rg3 was found to significantly reduce the expression of EGF, EGF receptor (EGFR), phosphorylated ERK1/2, and HIF-1α, indicating that it reduces the expression of NHE1 through overall inhibition of the EGF-EGFR-ERK1/2-HIF-α signaling pathway in HCC (Li et al., 2018).
A Korean study showed that ginsenosides Rb1, Rg3, and Rk1 reduce lipid accumulation and enhance the antioxidant function of the liver both in vitro and in vivo through the ER stress pathway (An et al., 2021). The source of these compounds, Korean Red Ginseng, could enhance the immune activity of NK cells via IL-33 by increasing the number of eosinophils (Kwon et al., 2021).
Research progress in the use of ginsenosides in immunotherapy of colorectal cancer
Colorectal cancer (CRC) is the leading cause of death after lung cancer, and one of the primary sources of cancer deaths worldwide (Siegel et al., 2022). Early screening has been shown to improve the 5-year survival rate of CRC patients, and the morbidity and mortality rates have shown a downward trend in recent years. Nevertheless, the prognosis of patients with metastatic CRC is still poor. In the United States, the median 5-year survival rate of these patients is only 12.5% (Siegel et al., 2014; Montminy et al., 2019). Therefore, there is a need to develop more treatments for CRC.
In the past 10 years, immunotherapy has successfully achieved long-term tumor suppression for refractory tumors (such as melanoma and non-small cell lung cancer). Hence, there are high hopes for the performance of these therapies in CRC.
Ginsenosides affect many factors responsible for the effects of immunotherapy. Ginsenoside Rh2 enhances the cytotoxicity of 5-fluorouracil (5-FU) in drug-resistant CRC cells (LoVo/5-FU and HCT-8/5-FU) and attenuates the expression of drug-resistance genes, such as MRP1, MDR1, LRP, and GST (Liu et al., 2018a). Ginsenoside Rh3 inhibits the proliferation of CRC cells in a dose- and time-dependent manner and induces cell apoptosis through upregulation of caspase-3 expression (Cong et al., 2020). Ginsenoside Rb1 decreases the key inflammatory cytokines TNF-α and IL-6 in a CT26 cancer cachexia mouse model (Lu et al., 2020), and ginsenoside Rb2 inhibits the expression of TGF-β1 in vitro and in vivo (Dai et al., 2019) though downregulation of Smad4 and phosphorylated Smad2/3 expression. Therefore, ginsenosides have the potential to enhance immunotherapy for CRC.
Research progress in the use of ginsenosides in immunotherapy of esophageal cancer and gastric cancer
The current gold standard treatment for esophageal cancer is adjuvant chemoradiotherapy followed by surgical resection (Vrána et al., 2018), but a low immune response rate and drug resistance are unsolved to PD-L1 blockade. Zhang et al. (2017) observed increased PD-L1 expression in esophageal cancer patients after radiotherapy and in irradiated esophageal squamous cells. High expression of PD-L1 usually suggests that such patients have higher sensitivity to immune checkpoint therapy and several trials are currently addressing the role of immunotherapy in adjuvant radiotherapy and chemotherapy for esophageal/gastroesophageal junction cancer (National Cancer Institute, 2022).
Ginsenoside Rd has been shown to increase the Bax/Bcl-2 ratio, caspase-3 and caspase-9 expression, the downregulation of Cyclin D1, and the inhibition of gastric cancer cell proliferation (Tian et al., 2020). Deng et al. (2020) found that ginsenoside Rh4 has an anti-esophageal cancer effect in vivo and in vitro. Rh4 significantly inhibits esophageal cancer cell growth by inducing G1 phase arrest. Mechanistically Rh4 may inhibit aerobic glycolysis-related protein expression by targeting Akt, and it also inhibits the expression of PD-L1 through the Akt/mTOR pathway.
Summary and outlook
There has been significant progress in the treatment of GI tumors through immunomodulation. Targeted drugs, such as immune checkpoint inhibitors and tumor suppressors, are one of the most promising types of therapeutic drugs. However, immune checkpoint inhibitors have only been shown to be efficacious for the treatment of a few types of tumors, such as malignant melanoma and non-small cell lung cancer. The application of these treatments to GI tumors still requires extensive clinical research. In addition, the current popular cancer treatments, including radiotherapy, chemotherapy, targeted inhibitors, and immune checkpoint therapy, still face challenges.
Ginsenosides and related preparations have been used in the clinic for a long time. They have played important roles in the improvement of postoperative fatigue after cancer (Kim et al., 2020b), reversing drug resistance (Jin et al., 2017; Wang et al., 2020a; Yuan et al., 2020), enhancing the efficacy of radiotherapy and chemotherapy (Liu et al., 2017; Liu et al., 2018b; Wang et al., 2020b), and inhibiting tumor cell proliferation and migration (Cai et al., 2013; Kee et al., 2019). Hence, they could potentially complement the first-line anti-GI tumor drugs, improving treatment effects and patient survival times and quality of life (Figure 3 and Table 2). Fuzheng is one of the mainstream anti-tumor strategies of TCM. Its main purpose is to enhance human health without drug toxicity, activate the immune system, and then suppress tumor progression by relying on the immune system (Liang et al., 2009; Zhang et al., 2010). The findings of TCM network pharmacology research in recent years also suggest the great potential of Fuzheng TCM in tumor treatment. The strategy of TCM in treating tumors is similar to immune checkpoint therapy (Zheng et al., 2018). In view of this, we recommend the use of TCM decoctions such as Sijunzi Decoction and Shenling Baizhu powder for the treatment of GI tumors (Zhang and Su, 2008; Xiao and Yang, 2011).
FIGURE 3
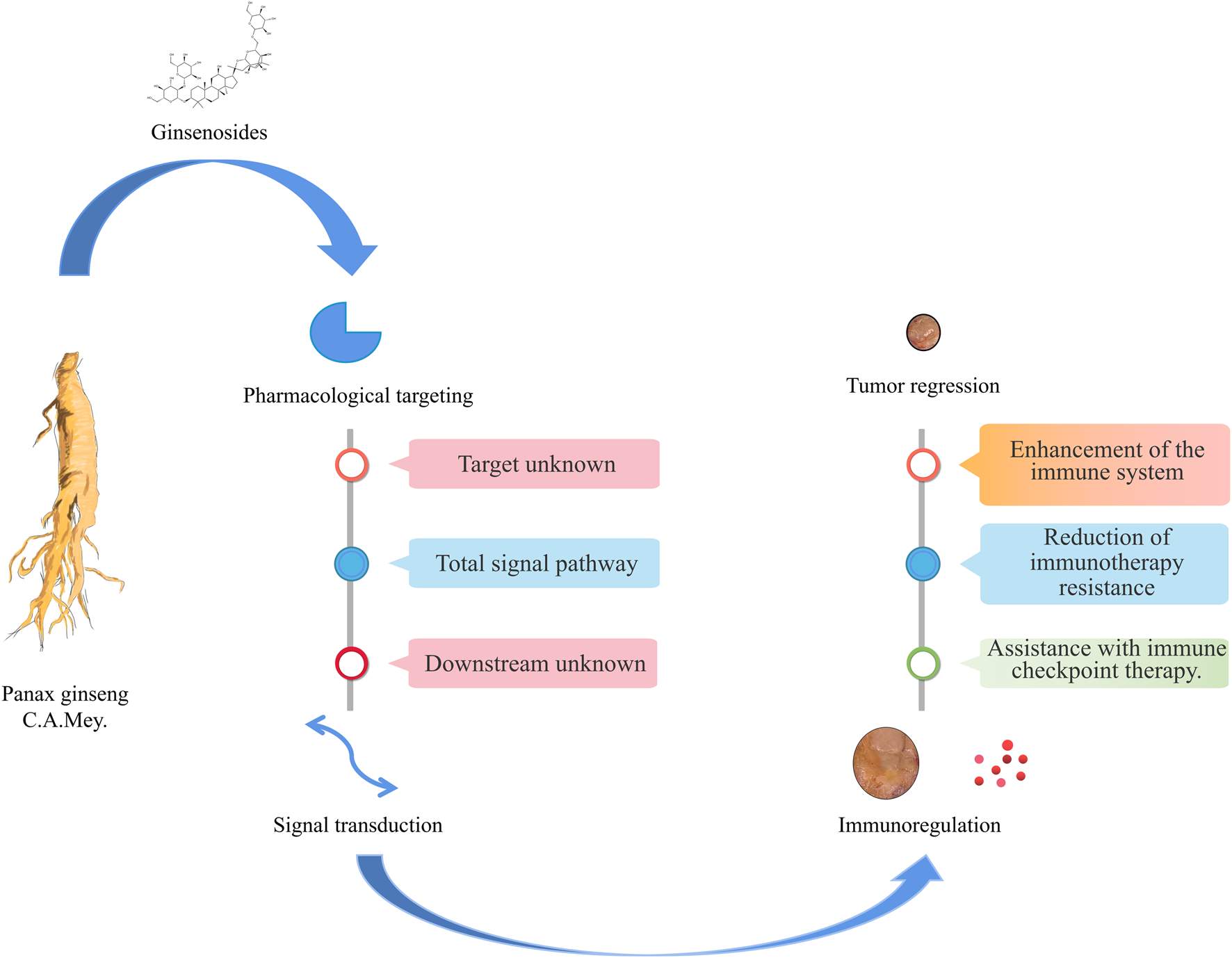
Ginsenosides in immunotherapy of gastrointestinal tumors.
TABLE 2
| Ginsenoside | Cancer type | Model | Pharmacology | Pharmacological mechanism | References |
|---|---|---|---|---|---|
| Rb1 | Hypopharyngeal Carcinoma | Xenograft rats | Inhibition of tumor growth with Apatinib treatment | Glycolysis↓; CD3+ and CD4+ T cells↑ CD4+/CD8+ T cell ratio↑ | Li et al. (2022b) |
| Colorectal cancer cachexia | CT-26 cell xenograft BALB/c mice | Recovery of organ weight; reduction of enlarged liver | TNF-α ↓; IL-6↓ | Cong et al. (2020) | |
| Rk1 | Non-small cell lung cancer | A549 cell | Cancer cell apoptosis | Akt↓, NF-κB↓, PD-L1↓ | Hu et al. (2020) |
| Rb2 | Colorectal cancer | HCT116 and SW620 cells | Inhibition of cell growth, adhesion, EMT, and metastasis | TGF-β1↓; Smad4↓; p-Smad2/3↓ | Lu et al. (2020) |
| Rg3 | Hepatoma carcinoma | SMMC-7721 and SK-Hep-1 cell | Inhibition of cell proliferation, migration, and invasion. | p-Akt↓, p-PI3K↓, MMP2↓ and MMP9↓, LncRNA HOTAIR↑ | Pu et al. (2021) |
| Colorectal carcinoma | SW48 and HCT15 cells | Inhibition of cell EMT, migration, and invasion. | IL-6↓, NICD and Hes1↓ | Li et al. (2021) | |
| Non-small cell lung cancer | A549 and A549/DDP cells | Restore the toxicity of T cells to cancer cells | Akt↓, NF-κB↓,PD-L1↓ | Jiang et al. (2017) | |
| Rh2 | Non-small cell lung cancer | C57BL/6J mice with non-small cell lung cancer | Synergistic antitumor effect with cyclophosphamide; upregulation of the immune deficiency caused by cyclophosphamide | FASN↓, glucocorticoid secretion↓,SREBP-1-FASN interaction ↓ | Qian et al. (2019) |
| Rh4 | Esophageal cancer | Eca109 and KYSE150 cells | Growth inhibition by inducing G1 phase arrest | AKT/mTOR↓, PD-L1↓, aerobic glycolysis ↓ | Deng et al. (2020) |
Immune-related pharmacological activities/mechanisms of ginsenosides.
TCMs are a treasure trove of screened natural drugs, and the exploration of these drugs will contribute to modern anti-tumor strategies. In the overall process of drug development, ginsenosides are still in the early stage. Many ginsenosides have not been optimized or undergone toxicological evaluation, and few clinical trials have been conducted. The research on ginsenoside regulation of immunity, like that on many TCM compounds widely used in clinical practice, has mostly focused on the development of small molecules and preliminary validation of efficacy. Our understanding of the synergistic effect and mechanism of TCM compounds is still relatively limited. Researchers have not found the biomarkers as a standard for the use of ginsenosides in treatment. In an alternative medicine clinical setting, doctors decide whether to use ginseng according to whether the patient has Qi deficiency (cancer-related fatigue) (Hsu et al., 2012; Yang et al., 2016; Kim et al., 2020b). The modernization of ginsenoside preparations is still in its early stage, and a few successes have been achieved. One example of such a success is Ginsenoside Rg3, named the “Shenyi capsule,” which has passed phase IV clinical trials and has been put into clinical use (Huang et al., 2009). Mechanism studies have shown that Rg3 inhibits the growth and angiogenesis of tumor endothelial cells and can significantly downregulate the expression of VEGF and endothelial marker CD31 (Liu et al., 2018b; Li and Qu, 2019). Therefore, the National Comprehensive Cancer Network clinical practice guidelines in oncology (Chinese Version) lists Shenyi capsule as a first-line drug for combined anti angiogenesis treatment and chemotherapy (National Comprehensive Cancer Network, 2006).
Pharmacology research into ginsenosides should be closely combined with frontier technology to interpret TCM from the latest perspective. The advantages of high-throughput technologies such as CyTOF (Sufi et al., 2021) and single-cell sequencing (Tang et al., 2009; Ramsköld et al., 2012; See et al., 2018; Sun et al., 2014; Paik et al., 2020) lie in the high resolution and sequencing depth. These methods can accurately assess cell heterogeneity, and even monitor the whole process of cell development and differentiation and detect protein levels on a single-cell scale. These technologies provide the possibility of obtaining a better understanding of the components in TCMs (Ai et al., 2019; Yang et al., 2022). For example, in a recent study, a non-targeted metabolomics strategy was used to explore the targets of ginsenoside Rg1 in Alzheimer’s disease. Fourteen potential metabolites involved in ten metabolic pathways including linoleic acid metabolism, arachidonic acid metabolism, tryptophan metabolism, and sphingolipid metabolism were affected by Rg1 (Li et al., 2019b). High-throughput virtual screening was used to explore ginsenoside Rb2 binding to TGF-β1 protein in colon cancer (Dai et al., 2019). High-throughput CRISPR screening identified PMAIP1 as resistant and WASH1 as sensitive to ginsenoside CK in a HeLa autophagic cell death model (Yang et al., 2020). These findings have greatly improved our understanding of the pharmacological mechanism of ginsenosides.
At present, research into the synergistic effects of ginsenosides and immunotherapy is still in its infancy. Our understanding of these synergistic effects and the mechanism of action of ginsenoside as a tumor suppressor is still relatively limited. There is an urgent need for in-depth exploration of targets such as CTLA-4 and PD-1/PD-L1 and related signaling pathways such as Akt (Deng et al., 2020; Yuan et al., 2020) and EGFR (Li et al., 2018). New clinical trials on immunotherapies targeting LAG3, TIM3, TIGIT, and BTLA are underway (Andrews et al., 2017; Zhang et al., 2018; Kraehenbuehl et al., 2022). Hopefully, with the development of clinical trials and combination medication research, ginsenosides may become a powerful ally in immunotherapy.
Statements
Author contributions
YF contributed the central idea and wrote the initial draft of the paper. JZ contributed to refining the ideas. FM, EW, and ZC carried out additional comment. JZ, LY, and ZW finalized this paper.
Funding
This work was supported by National Natural Science Foundation of China (82173106, 82130115); Shanghai Pujiang talent program (20PJ1413000).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
AiH. L.YeK.ZhangX.LvX.LiZ. H.ZhangS. D. (2019). The complete plastid genome of Iris domestica: A traditional Chinese medicine. Mitochondrial DNA. B Resour.4 (2), 4214–4215. 10.1080/23802359.2019.1693923
2
AltmannD. M . A. (2018). A Nobel prize-worthy pursuit: Cancer immunology and harnessing immunity to tumour neoantigens. Immunology155 (3), 283–284. 10.1111/imm.13008
3
AnM. Y.LeeS. R.HwangH. J.YoonJ. G.LeeH. J.ChoJ. A. (2021). Antioxidant and anti-inflammatory effects of Korean black ginseng extract through ER stress pathway. Antioxidants (Basel)10 (1), 62. 10.3390/antiox10010062
4
AndrewsL. P.MarciscanoA. E.DrakeC. G.VignaliD. A. (2017). LAG3 (CD223) as a cancer immunotherapy target. Immunol. Rev.276 (1), 80–96. 10.1111/imr.12519
5
BaxterN. N.KennedyE. B.BergslandE.BerlinJ.GeorgeT. J.GillS.et al (2021). Adjuvant therapy for stage.
6
BuL. L.YuG. T.WuL.MaoL.DengW. W.LiuJ. F.et al (2017). STAT3 induces immunosuppression by upregulating PD-1/PD-L1 in HNSCC. J. Dent. Res.96 (9), 1027–1034. 10.1177/0022034517712435
7
BuM. T.ChandrasekharP.DingL.HugoW. (2022). The roles of TGF-β and VEGF pathways in the suppression of antitumor immunity in melanoma and other solid tumors. Pharmacol. Ther.240, 108211. 10.1016/j.pharmthera.2022.108211
8
CaiJ. P.WuY. J.LiC.FengM. Y.ShiQ. T.LiR.et al (2013). Panax ginseng polysaccharide suppresses metastasis via modulating Twist expression in gastric cancer. Int. J. Biol. Macromol.57, 22–25. 10.1016/j.ijbiomac.2013.03.010
9
Centers for Disease Control and Pre-vention (2018). in Trends in liver cancer mortality among adults aged 25 and over in the United States, 2000–2016. Available at: https://www.cdc.gov/nchs/products/databriefs/db314.htm. National center for health sta-tistics
10
ChanT. A.YarchoanM.JaffeeE.SwantonC.QuezadaS. A.StenzingerA.et al (2019). Development of tumor mutation burden as an immunotherapy biomarker: Utility for the oncology clinic. Ann. Oncol.30, 44–56. 10.1093/annonc/mdy495
11
ChenW.YaoP.VongC. T.LiX.ChenZ.XiaoJ.et al (2020). Ginseng: A bibliometric analysis of 40-year journey of global clinical trials. J. Adv. Res.34, 187–197. 10.1016/j.jare.2020.07.016
12
CongZ.ZhaoQ.YangB.CongD.ZhouY.LeiX.et al (2020). Ginsenoside Rh3 inhibits proliferation and induces apoptosis of colorectal cancer cells. Pharmacology105 (5-6), 329–338. 10.1159/000503821
13
DaiG.SunB.GongT.PanZ.MengQ.JuW. (2019). Ginsenoside Rb2 inhibits epithelial-mesenchymal transition of colorectal cancer cells by suppressing TGF-β/Smad signaling. Phytomedicine56, 126–135. 10.1016/j.phymed.2018.10.025
14
De VeraA. A.GuptaP.LeiZ.LiaoD.NarayananS.TengQ.et al (2019). Immuno-oncology agent IPI-549 is a modulator of P-glycoprotein (P-gp, MDR1, abcb1)-mediated multidrug resistance (MDR) in cancer: In vitro and in vivo. Cancer Lett.442, 91–103. 10.1016/j.canlet.2018.10.020
15
DengX.ZhaoJ.QuL.DuanZ.FuR.ZhuC.et al (2020). Ginsenoside Rh4 suppresses aerobic glycolysis and the expression of PD-L1 via targeting AKT in esophageal cancer. Biochem. Pharmacol.178, 114038. 10.1016/j.bcp.2020.114038
16
FengS. L.LuoH. B.CaiL.ZhangJ.WangD.ChenY. J.et al (2020). Ginsenoside Rg5 overcomes chemotherapeutic multidrug resistance mediated by ABCB1 transporter: In vitro and in vivo study. J. Ginseng Res.44 (2), 247–257. 10.1016/j.jgr.2018.10.007
17
GaoX. M. (1990). Chinese medicine[M]. Beijing: China Medical Science and Technology Press.
18
GongJ.Chehrazi-RaffleA.ReddiS.SalgiaR. (2018). Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: A comprehensive review of registration trials and future considerations. J. Immunother. Cancer6 (1), 8. 10.1186/s40425-018-0316-z
19
GourdK. (2021). ESMO world congress on gastrointestinal cancer 2021. Lancet Oncol.22 (8), 1062. 10.1016/s1470-2045(21)00395-8
20
GravitzL.PincockS. (2014). Sickle-cell disease. Nature516 (7529), S1. 10.1038/515S1a
21
GuC.WangY.ZhangL.QiaoL.SunS.ShaoM.et al (2022). AHSA1 is a promising therapeutic target for cellular proliferation and proteasome inhibitor resistance in multiple myeloma. J. Exp. Clin. Cancer Res.41 (1), 11. 10.1186/s13046-021-02220-1
22
HanJ.XiaJ.ZhangL.CaiE.ZhaoY.FeiX.et al (2019). Studies of the effects and mechanisms of ginsenoside Re and Rk3 on myelosuppression induced by cyclophosphamide. J. Ginseng Res.43 (4), 618–624. 10.1016/j.jgr.2018.07.009
23
HanX.WeiQ.LvY.WengL.HuangH.WeiQ.et al (2022). Ginseng-derived nanoparticles potentiate immune checkpoint antibody efficacy by reprogramming the cold tumor microenvironment. Mol. Ther.30 (1), 327–340. 10.1016/j.ymthe.2021.08.028Y
24
HanY.LiX.YangL.ZhangD.LiL.DongX.et al (2022). Ginsenoside Rg1 attenuates cerebral ischemia-reperfusion injury due to inhibition of NOX2-mediated calcium homeostasis dysregulation in mice. J. Ginseng Res.46 (4), 515–525. 10.1016/j.jgr.2021.08.001
25
HeB.ChenD.ZhangX.YangR.YangY.ChenP.et al (2022). Oxidative stress and ginsenosides: An update on the molecular mechanisms. Oxid. Med. Cell.. Longev.2022, 20229299574. 10.1155/2022/9299574
26
HongY.FanD. (2019). Ginsenoside Rk1 induces cell cycle arrest and apoptosis in MDA-MB-231 triple negative breast cancer cells. Toxicology418, 22–31. 10.1016/j.tox.2019.02.010
27
HouM.WangR.ZhaoS.WangZ. (2021). Ginsenosides in Panax genus and their biosynthesis. Acta Pharm. Sin. B11 (7), 1813–1834. 10.1016/j.apsb.2020.12.017
28
HsuC. H.LeeC. J.ChienT. J.LinC. P.ChenC. H.YuenM. J.et al (2012). The relationship between Qi deficiency, cancer-related fatigue and quality of life in cancer patients. J. Tradit. Complement. Med.2 (2), 129–135. 10.1016/s2225-4110(16)30086-4
29
HuM.YangJ.QuL.DengX.DuanZ.FuR.et al (2020). Ginsenoside Rk1 induces apoptosis and downregulates the expression of PD-L1 by targeting the NF-κB pathway in lung adenocarcinoma. Food Funct.11 (1), 456–471. 10.1039/c9fo02166c
30
HuangJ. Y.SunY.FanQ. X.ZhangY. Q. (2009). Efficacy of Shenyi capsule combined with gemcitabine plus cisplatin in treatment of advanced esophageal cancer: A randomized controlled trial. Zhong Xi Yi Jie He Xue Bao7 (11), 1047–1051. Chinese. 10.3736/jcim20091105
31
JiaL.ZhaoY. (2009). Current evaluation of the millennium phytomedicine--ginseng (I): Etymology, pharmacognosy, phytochemistry, market and regulations. Curr. Med. Chem.16 (19), 2475–2484. 10.2174/092986709788682146
32
JiangP.GengL.MaoZ.WangQ.WangW.JiaoM.et al (2022). First-line chemotherapy plus immune checkpoint inhibitors or bevacizumab in advanced non-squamous non-small-cell lung cancer without EGFR mutations or ALK fusions. Immunotherapy14, 445–457. 10.2217/imt-2021-0112
33
JiangX.WangJ.DengX.XiongF.GeJ.XiangB.et al (2019). Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol. Cancer18, 10. 10.1186/s12943-018-0928-4
34
JiangZ.YangY.YangY.ZhangY.YueZ.PanZ.et al (2017). Ginsenoside Rg3 attenuates cisplatin resistance in lung cancer by downregulating PD-L1 and resuming immune. Biomed. Pharmacother.96, 378–383. 10.1016/j.biopha.2017.09.129
35
JinL.XuM.LuoX. H.ZhuX. F. (2017). Stephania tetrandra and ginseng-containing Chinese herbal formulation NSENL reverses cisplatin resistance in lung cancer xenografts. Am. J. Chin. Med.45 (2), 385–401. 10.1142/S0192415X17500240
36
KeeJ. Y.HanY. H.MunJ. G.ParkS. H.JeonH. D.HongS. H. (2019). Effect of Korean Red Ginseng extract on colorectal lung metastasis through inhibiting the epithelial-mesenchymal transition via transforming growth factor-β1/Smad-signaling-mediated Snail/E-cadherin expression. J. Ginseng Res.43 (1), 68–76. 10.1016/j.jgr.2017.08.007
37
KimJ. W.HanS. W.ChoJ. Y.ChungI. J.KimJ. G.LeeK. H.et al (2020). Korean red ginseng for cancer-related fatigue in colorectal cancer patients with chemotherapy: A randomised phase III trial. Eur. J. Cancer130, 51–62. 10.1016/j.ejca.2020.02.018
38
KimM. H.KimH. H.JeongJ. M.ShimY. R.LeeJ. H.KimY. E.et al (2020). Ginsenoside F2 attenuates chronic-binge ethanol-induced liver injury by increasing regulatory T cells and decreasing Th17 cells. J. Ginseng Res.44 (6), 815–822. 10.1016/j.jgr.2020.03.002
39
KirkwoodJ. M.ButterfieldL. H.TarhiniA. A.ZarourH.KalinskiP.FerroneS. (2012). Immunotherapy of cancer in 2012. Ca. Cancer J. Clin.62 (5), 309–335. 10.3322/caac.20132
40
KraehenbuehlL.WengC. H.EghbaliS.WolchokJ. D.MerghoubT. (2022). Enhancing immunotherapy in cancer by targeting emerging immunomodulatory pathways. Nat. Rev. Clin. Oncol.19, 37–50. 10.1038/s41571-021-00552-7
41
KwonH. J.LeeS.LeeH. H.ChoH.JungJ. (2021). Korean red ginseng enhances immunotherapeutic effects of NK cells via eosinophils in metastatic liver cancer model. Nutrients14 (1), 134. 10.3390/nu14010134
42
LeeE. K.KonstantinopoulosP. A. (2019). Combined PARP and immune checkpoint inhibition in ovarian cancer. Trends Cancer5 (9), 524–528. 10.1016/j.trecan.2019.06.004
43
LeeJ. H.LeeD. Y.LeeH. J.ImE.SimD. Y.ParkJ. E.et al (2021). Inhibition of STAT3/PD-L1 and activation of miR193a-5p are critically involved in apoptotic effect of compound K in prostate cancer cells. Cells10 (8), 2151. 10.3390/cells10082151
44
LeeW. H.ChoiJ. S.KimH. Y.ParkJ. H.ParkB. D.ChoS. J.et al (2010). Potentiation of etoposide-induced apoptosis in HeLa cells by co-treatment with KG-135, a quality-controlled standardized ginsenoside formulation. Cancer Lett.294 (1), 74–81. 10.1016/j.canlet.2010.01.024
45
LiA.YiM.QinS.ChuQ.LuoS.WuK. (2019). Prospects for combining immune checkpoint blockade with PARP inhibition. J. Hematol. Oncol.12 (1), 98. 10.1186/s13045-019-0784-8
46
LiB.QuG. (2019). Inhibition of the hypoxia-induced factor-1α and vascular endothelial growth factor expression through ginsenoside Rg3 in human gastric cancer cells. J. Cancer Res. Ther.15 (7), 1642–1646. 10.4103/jcrt.JCRT_77_17
47
LiG.ZhangN.GengF.LiuG.LiuB.LeiX.et al (2019). High-throughput metabolomics and ingenuity pathway approach reveals the pharmacological effect and targets of Ginsenoside Rg1 in Alzheimer's disease mice. Sci. Rep.9 (1), 7040. 10.1038/s41598-019-43537-4
48
LiX.LiuW.GengC.LiT.LiY.GuoY.et al (2021). Ginsenoside Rg3 suppresses epithelial-mesenchymal transition via downregulating notch-hes1 signaling in colon cancer cells. Am. J. Chin. Med.49 (1), 217–235. 10.1142/S0192415X21500129
49
LiX.TsauoJ.GengC.ZhaoH.LeiX.LiX. (2018). Ginsenoside Rg3 decreases NHE1 expression via inhibiting EGF-EGFR-ERK1/2-HIF-1 α pathway in hepatocellular carcinoma: A novel antitumor mechanism. Am. J. Chin. Med.46 (8), 1915–1931. 10.1142/S0192415X18500969
50
LiY.HeF.ZhangY.PanZ. (2022). Apatinib and ginsenoside-Rb1 synergetically Control the growth of hypopharyngeal carcinoma cells. Dis. Markers2022, 3833489. 10.1155/2022/3833489
51
LiY.ZhangS.ZhuZ.ZhouR.XuP.ZhouL.et al (2022). Upregulation of adiponectin by Ginsenoside Rb1 contributes to amelioration of hepatic steatosis induced by high fat diet. J. Ginseng Res.46 (4), 561–571. 10.1016/j.jgr.2021.10.005
52
LiangQ. L.PanD. C.XieJ. R. (2009). Effect of Shenqi Fuzheng injection combined with chemotherapy in treating advanced colorectal carcinoma. Zhongguo Zhong Xi Yi Jie He Za Zhi29 (5), 439–441. Chinese.
53
LinmingL.YaningS. H.YinaJ.JihuaZ.LiQ.NaihongC. H. (2017). Research Progress on the effective components and mechanism of anti-tumor effect of ginseng[J]. Chin. Traditional Herb. Drugs48 (03), 582–596.
54
LiuG. W.LiuY. H.JiangG. S.RenW. D. (2018). The reversal effect of Ginsenoside Rh2 on drug resistance in human colorectal carcinoma cells and its mechanism. Hum. Cell.31 (3), 189–198. 10.1007/s13577-017-0189-3
55
LiuT.DuoL.DuanP. (2018). Ginsenoside Rg3 sensitizes colorectal cancer to radiotherapy through downregulation of proliferative and angiogenic biomarkers. Evid. Based. Complement. Altern. Med.2018, 1580427. 10.1155/2018/1580427
56
LiuW. Y.ZhangJ. W.YaoX. Q.JiangC.HeJ. C.NiP.et al (2017). Shenmai injection enhances the cytotoxicity of chemotherapeutic drugs against colorectal cancers via improving their subcellular distribution. Acta Pharmacol. Sin.38 (2), 264–276. 10.1038/aps.2016.99
57
LiuX.ZhangZ.LiuJ.WangY.ZhouQ.WangS.et al (2019). Ginsenoside Rg3 improves cyclophosphamide-induced immunocompetence in Balb/c mice. Int. Immunopharmacol.72, 98–111. 10.1016/j.intimp.2019.04.003
58
LuS.ZhangY.LiH.ZhangJ.CiY.HanM. (2020). Ginsenoside Rb1 can ameliorate the key inflammatory cytokines TNF-α and IL-6 in a cancer cachexia mouse model. BMC Complement. Med. Ther.20 (1), 11. 10.1186/s12906-019-2797-9
59
MajeedF.MalikF. Z.AhmedZ.AfreenA.AfzalM. N.KhalidN. (2018). Ginseng phytochemicals as therapeutics in oncology: Recent perspectives. Biomed. Pharmacother.100, 52–63. 10.1016/j.biopha.2018.01.155
60
MontminyE. M.KarlitzJ. J.LandreneauS. W. (2019). Progress of colorectal cancer screening in United States: Past achievements and future challenges. Prev. Med.120, 78–84. 10.1016/j.ypmed.2018.12.004
61
MuraiJ.HuangS. Y.DasB. B.RenaudA.ZhangY.DoroshowJ. H.et al (2012). Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res.72 (21), 5588–5599. 10.1158/0008-5472.CAN-12-2753
62
MuraiJ.HuangS. Y.RenaudA.ZhangY.JiJ.TakedaS.et al (2014). Stereospecific PARP trapping by BMN 673 and comparison with olaparib and rucaparib. Mol. Cancer Ther.13 (2), 433–443. 10.1158/1535-7163.MCT-13-0803
63
National Cancer Institute (2022). An investigational immuno-therapy study of nivolumab or placebo in patients with resected esophageal or gastroesophageal junction cancer (CheckMate 577). Available online: https://clinicaltrials.gov/ct2/show/NCT02743494 (accessed on November 13, 2018).
64
National Comprehensive Cancer Network (2006). The Complete Library of Nccn clinical practice guidelines in oncology (Chinese Version).
65
NiJ.LiuZ.JiangM.LiL.DengJ.WangX.et al (2022). Ginsenoside Rg3 ameliorates myocardial glucose metabolism and insulin resistance via activating the AMPK signaling pathway. J. Ginseng Res.46 (2), 235–247. 10.1016/j.jgr.2021.06.001
66
NoordermeerS. M.van AttikumH. (2019). PARP inhibitor resistance: A tug-of-war in BRCA-mutated cells. Trends Cell. Biol.29 (10), 820–834. 10.1016/j.tcb.2019.07.008
67
PaikD. T.ChoS.TianL.ChangH. Y.WuJ. C. (2020). Single-cell RNA sequencing in cardiovascular development, disease and medicine. Nat. Rev. Cardiol.17 (8), 457–473. 10.1038/s41569-020-0359-y
68
ParkJ. A.LeeK. Y.OhY. J.KimK. W.LeeS. K. (1997). Activation of caspase-3 protease via a Bcl-2-insensitive pathway during the process of ginsenoside Rh2-induced apoptosis. Cancer Lett.121 (1), 73–81. 10.1016/s0304-3835(97)00333-9
69
PuZ.GeF.WangY.JiangZ.ZhuS.QinS.et al (2021). Ginsenoside-Rg3 inhibits the proliferation and invasion of hepatoma carcinoma cells via regulating long non-coding RNA HOX antisense intergenic. Bioengineered12 (1), 2398–2409. 10.1080/21655979.2021.1932211
70
QianY.HuangR.LiS.XieR.QianB.ZhangZ.et al (2019). Ginsenoside Rh2 reverses cyclophosphamide-induced immune deficiency by regulating fatty acid metabolism. J. Leukoc. Biol.106 (5), 1089–1100. 10.1002/JLB.2A0419-117R
71
QuL.FuR.MaX.FanD. (2021). Hepatoprotective effects of ginsenoside Rk3 in acetaminophen-induced liver injury in mice by activation of autophagy. Food Funct.12 (19), 9128–9140. 10.1039/d1fo02081a
72
RamsköldD.LuoS.WangY. C.LiR.DengQ.FaridaniO. R.et al (2012). Full-length mRNA-Seq from single-cell levels of RNA and individual circulating tumor cells. Nat. Biotechnol.30 (8), 777–782. 10.1038/nbt.2282
73
RoghanianA.HuG.FraserC.SinghM.FoxallR. B.MeyerM. J.et al (2019). Cyclophosphamide enhances cancer antibody immunotherapy in the resistant bone marrow niche by modulating macrophage FcγR expression. Cancer Immunol. Res.7 (11), 1876–1890. 10.1158/2326-6066.CIR-18-0835
74
RubioC.PazS.TiusE.HardissonA.GutierrezA. J.Gonzalez-WellerD.et al (2018). Metal contents in the most widely consumed commercial preparations of four different medicinal plants (aloe, Senna, ginseng, and ginkgo) from Europe. Biol. Trace Elem. Res.186 (2), 562–567. 10.1007/s12011-018-1329-7
75
SamsteinRobert M.LeeC. H.ShoushtariA. N.HellmannM. D.ShenR.JanjigianY. Y.et al (2019). Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet.51, 202–206. 10.1038/s41588-018-0312-8
76
SeeP.LumJ.ChenJ.GinhouxF. (2018). Corrigendum: A single-cell sequencing guide for immunologists. Front. Immunol.9, 278. 10.3389/fimmu.2019.00278
77
ShurinM. R.UmanskyV. (2022). Cross-talk between HIF and PD-1/PD-L1 pathways in carcinogenesis and therapy. J. Clin. Investig.132 (9), e159473. 10.1172/JCI159473
78
SiegelR.DesantisC.JemalA. (2014). Colorectal cancer statistics. Ca. Cancer J. Clin.64 (2), 104–117. 10.3322/caac.21220
79
SiegelR. L.MillerK. D.FuchsH. E.JemalA. (2022). Cancer statistics, 2016. Ca. Cancer J. Clin.72 (1), 7–30. 10.3322/caac.21332
80
SufiJ.QinX.RodriguezF. C.BuY. J.VlckovaP.ZapateroM. R.et al (2021). Multiplexed single-cell analysis of organoid signaling networks. Nat. Protoc.16 (10), 4897–4918. 10.1038/s41596-021-00603-4
81
SunY.LiuQ.CaoZ.ChenY.GuanX.WuX.et al (2014). Tumor-stroma ratio is an independent predictor for survival in early cervical carcinoma. Gynecol. Oncol.12 (3), 81–86. 10.1016/j.ygyno.2013.11.003
82
SungH.FerlayJ.SiegelR. L.LaversanneM.SoerjomataramI.JemalA.et al (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca. A Cancer J. Clin.71 (3), 209–249. 10.3322/caac.21660
83
TangF.BarbacioruC.WangY.NordmanE.LeeC.XuN.et al (2009). mRNA-Seq whole-transcriptome analysis of a single cell. Nat. Methods6 (5), 377–382. 10.1038/nmeth.1315
84
TianY. Z.LiuY. P.TianS. C.GeS. Y.WuY. J.ZhangB. L. (2020). Antitumor activity of ginsenoside Rd in gastric cancer via up-regulation of Caspase-3 and Caspase-9. Pharmazie75 (4), 147–150. 10.1691/ph.2020.9931
85
VránaD.MatzenauerM.NeoralČ.AujeskýR.VrbaR.MelicharB.et al (2018). From tumor immunology to immunotherapy in gastric and esophageal cancer. Int. J. Mol. Sci.20 (1), 13. 10.3390/ijms20010013
86
WangC. Z.HouL.WanJ. Y.YaoH.YuanJ.ZengJ.et al (2020). Ginseng berry polysaccharides on inflammation-associated colon cancer: Inhibiting T-cell differentiation, promoting apoptosis, and enhancing the effects of 5-fluorouracil. J. Ginseng Res.44 (2), 282–290. 10.1016/j.jgr.2018.12.010
87
WangH.RongX.ZhaoG.ZhouY.XiaoY.MaD.et al (2022). The microbial metabolite trimethylamine N-oxide promotes antitumor immunity in triple-negative breast cancer. Cell. Metab.34 (4), 581–594.e8. 10.1016/j.cmet.2022.02.010
88
WangH.ZhengY.SunQ.ZhangZ.ZhaoM.PengC.et al (2021). Ginsenosides emerging as both bifunctional drugs and nanocarriers for enhanced antitumor therapies. J. Nanobiotechnology19, 322. 10.1186/s12951-021-01062-5
89
WangJ.ZuoG.LiJ.GuanT.LiC.JiangR.et al (2010). Induction of tumoricidal activity in mouse peritoneal macrophages by ginseng polysaccharide. Int. J. Biol. Macromol.46, 389–395. 10.1016/j.ijbiomac.2010.02.007
90
WangP.SongD.WanD.LiL.MeiW.LiX.et al (2020). Ginsenoside panaxatriol reverses TNBC paclitaxel resistance by inhibiting the IRAK1/NF-κB and ERK pathways. PeerJ8, e9281. 10.7717/peerj.9281
91
WangZ.WangL.JiangR.LiC.ChenX.XiaoH.et al (2021). Ginsenoside Rg1 prevents bone marrow mesenchymal stem cell senescence via NRF2 and PI3K/Akt signaling. Free Radic. Biol. Med.174, 182–194. 10.1016/j.freeradbiomed.2021.08.007
92
WongA. S.CheC. M.LeungK. W. (2015). Recent advances in ginseng as cancer therapeutics: A functional and mechanistic overview. Nat. Prod. Rep.32 (2), 256–272. 10.1039/c4np00080c
93
XiaJ.MaS.ZhuX.ChenC.ZhangR.CaoZ.et al (2022). Versatile ginsenoside Rg3 liposomes inhibit tumor metastasis by capturing circulating tumor cells and destroying metastatic niches. Sci. Adv.8 (6), eabj1262. 10.1126/sciadv.abj1262
94
XiaoH.YangJ. (2011). Immune enhancing effect of modified Sijunzi decoction on patients with colorectal cancer undergoing chemotherapy. Chin. J. Integr. Tradit. West. Med.31, 164–167.
95
XuH.Van der JeughtK.ZhouZ.ZhangL.YuT.SunY.et al (2021). Atractylenolide I enhances responsiveness to immune checkpoint blockade therapy by activating tumor antigen presentation. J. Clin. Investig.131 (10), e146832. 10.1172/JCI146832
96
YangL.LiT. T.ChuY. T.ChenK.TianS. D.ChenX. Y.et al (2016). Traditional Chinese medical comprehensive therapy for cancer-related fatigue. Chin. J. Integr. Med.22 (1), 67–72. 10.1007/s11655-015-2105-6
97
YangX.WangD.DongW.SongZ.DouK. (2010). Over-expression of Na+/H+ exchanger 1 and its clinicopathologic significance in hepatocellular carcinoma. Med. Oncol.27 (4), 1109–1113. 10.1007/s12032-009-9343-4
98
YangY.LiJ.ZhouQ. (2022). The complete chloroplast genome sequence of the Trichosanthes Kirilowii Maxim. (Cucurbitaceae)[J].
99
YangY.LiuX.LiS.ChenY.ZhaoY.WeiY.et al (2020). Genome-scale CRISPR screening for potential targets of ginsenoside compound K. Cell. Death Dis.11 (1), 39. 10.1038/s41419-020-2234-5
100
YuY.ZhouQ.HangY.BuX.JiaW. (2007). Antiestrogenic effect of 20S-protopanaxadiol and its synergy with tamoxifen on breast cancer cells. Cancer109 (11), 2374–2382. 10.1002/cncr.22659
101
YuanZ.LiangX.ZhanY.WangZ.XuJ.QiuY.et al (2020). Targeting CD133 reverses drug-resistance via the AKT/NF-κB/MDR1 pathway in colorectal cancer. Br. J. Cancer122 (9), 1342–1353. 10.1038/s41416-020-0783-0
102
YunT. K. (2001). Panax ginseng--a non-organ-specific cancer preventive [J]. Lancet. Oncol.2, 49–55. 10.1016/S1470-2045(00)00196-0
103
ZhangH.DongW.ZhaoH.HuY.YouX.SunT.et al (2022). Case report: Benefits of a NSCLC patient with EGFR A289G/F287_G288insHA cis mutations from immunotherapy in combination with antiangiogenesis and chemotherapy and sequential treatment of EGFR-TKI. Front. Oncol.12, 826938. 10.3389/fonc.2022.826938
104
ZhangH.SuZ. X. (2008). Curative effect of Shenling Baizhu powder on chemotherapy-induced toxicity in advanced gastric cancer[J]. Changsha, Hunan, China: Journal of Traditional Chinese Medicine University of Hunan.
105
ZhangJ.LiuM.HuangM.ChenM.ZhangD.LuoL.et al (2019). Ginsenoside F1 promotes angiogenesis by activating the IGF-1/IGF1R pathway. Pharmacol. Res.144, 292–305. 10.1016/j.phrs.2019.04.021
106
ZhangQ.BiJ.ZhengX.ChenY.WangH.WuW.et al (2018). Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat. Immunol.19 (7), 723–732. 10.1038/s41590-018-0132-0
107
ZhangW.PangQ.ZhangX.YanC.WangQ.YangJ.et al (2017). Programmed death-ligand 1 is prognostic factor in esophageal squamous cell carcinoma and is associated with epidermal growth factor receptor. Cancer Sci.108 (4), 590–597. 10.1111/cas.13197
108
ZhangY.GuoL. L.ZhaoS. P. (2010). Effect of shenqi fuzheng injection combined with chemotherapy in treating colorectal cancer. Zhongguo Zhong Xi Yi Jie He Za Zhi30 (3), 280–282. Chinese.
109
ZhenN.JinL.MaJ.ZhuJ.GuS.WangJ.et al (2018). Ginsenoside Rg1 impairs homologous recombination repair by targeting CtBP-interacting protein and sensitizes hepatoblastoma cells to DNA damage. Anticancer. Drugs29 (8), 756–766. 10.1097/CAD.0000000000000646
110
ZhengJ.WuM.WangH.LiS.WangX.LiY.et al (2018). Network pharmacology to unveil the biological basis of health-strengthening herbal medicine in cancer treatment. Cancers10 (11), 461. 10.3390/cancers10110461
111
ZouS.TongQ.LiuB.HuangW.TianY.FuX. (2020). Targeting STAT3 in cancer immunotherapy. Mol. Cancer19, 145. 10.1186/s12943-020-01258-7
Summary
Keywords
traditional Chinese medicine, ginseng, ginsenosides, gastrointestinal tumors, immunotherapy
Citation
Feng Y, Ma F, Wu E, Cheng Z, Wang Z, Yang L and Zhang J (2022) Ginsenosides: Allies of gastrointestinal tumor immunotherapy. Front. Pharmacol. 13:922029. doi: 10.3389/fphar.2022.922029
Received
17 April 2022
Accepted
26 September 2022
Published
31 October 2022
Volume
13 - 2022
Edited by
Young-Su Yi, Kyonggi University, South Korea
Reviewed by
Tanya Biswas Sardana, University of Delhi, India
Yoshiharu Motoo, Komatsu Sophia Hospital, Japan
Updates
Copyright
© 2022 Feng, Ma, Wu, Cheng, Wang, Yang and Zhang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiwei Zhang, jovzhang@shutmc.edu.cn
This article was submitted to Ethnopharmacology, a section of the journal Frontiers in Pharmacology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.