- 1Department of Neurology, Shanghai Tenth People’s Hospital, Tongji University School of Medicine, Shanghai, China
- 2Tongji University School of Medicine, Shanghai, China
- 3Department of Traditional Chinese Medicine, Shanghai Tenth People’s Hospital, Tongji University School of Medicine, Shanghai, China
- 4School of Pharmacy, University of Nottingham, Nottingham, United Kingdom
- 5Clinical Research Center for Anesthesiology and Perioperative Medicine, Shanghai Fourth People’s Hospital, Tongji University School of Medicine, Shanghai, China
- 6Translational Research Institute of Brain and Brain-Like Intelligence, Shanghai Fourth People’s Hospital, Tongji University School of Medicine, Shanghai, China
Background: Although blood-activating Chinese medicine (BACM) has been reported as adjuvant therapy for intracranial hemorrhage (ICH) in China, high-quality evidence is still lacking. Our study aimed to collect the latest high-quality randomized controlled trials (RCTs) and to evaluate the efficacy and safety of BACM for ICH.
Methods: RCTs published between January 2015 and March 2022 were searched in databases, including China National Knowledge Infrastructure (CNKI), China Science and Technology Journal Database (VIP), Sino-Med, Wanfang, PubMed, Web of Science, Cochrane Library, and Embase without language restrictions. Eligible RCTs were included and both primary (clinical efficacy evidenced by decreased neurological deficit scores) and secondary outcomes (increased Barthel index, decreased NIHSS, hematoma volume, the volume of cerebral edema, the incidence of side effects, and mortality) were analyzed. The quality of included RCTs was assessed using the Cochrane risk of bias tool. In the meta-analysis, the pooled results were analyzed using Review Manager 5.3 and STATA14.0. Finally, The GRADEpro GDT software (Guideline Development Tool) was used to summarize the results. Sensitivity and subgroup analyses were conducted based on the follow-up time.
Results: Fifteen RCTs, involving 1,579 participants, were included for analysis in our study. The pooled outcomes indicated that BACM combined with western medicine treatment (WMT) was superior to WMT alone for patients with ICH, demonstrated by the improvements in efficacy (RR = 1.22 (95% CI, [1.13 to 1.32], p < 0.001), neurological functions (MDNIHSS = −2.75, 95% CI [−3.74 to −1.76], p < 0.001), and activities of daily living (MDBarthel index = 5.95, 95% CI [3.92 to 7.98], p < 0.001), as well as decreased cerebral hematoma, cerebral edema (MD cerebral hematoma = −2.94, 95% CI [−3.50 to −2.37, p < 0.001 and MDcerebral edema = −2.66, 95% CI [−2.95 to −2.37], p < 0.001), side effects and mortality (RR = 0.84 (95% CI [0.60 to 1.19], p = 0.330 and RR = 0.51 (95% CI, [0.16 to 1.65], p = 0.260). In addition, Conioselinum anthriscoides “Chuanxiong” [Apiaceae], Camellia reticulata Lindl. [Theaceae], and Bupleurum sibiricum var. jeholense (Nakai) C.D.Chu [Apiaceae]) were the most frequently used herbs in the treatment of ICH. Recently, there was a trend toward the extensive use of another two herbs, including Rheum palmatum L. [Polygonaceae], Astragalus mongholicus Bunge [Fabaceae]) for ICH.
Conclusion: BACM combined with WMT seems to be superior to WMT alone for patients with ICH. Further high-quality RCTs are warranted to confirm the efficacy and safety of BACM.
Introduction
Intracranial hemorrhage (ICH) is one of the most devastating types of stroke with an incidence of 24.6 per 100,000 individuals, imposing a heavy burden on the global health care system (Staykov et al., 2010; Poon et al., 2014). Recent evidence has shown that surgical treatment improves neurologic functions in neither short nor long terms after ICH (Hostettler et al., 2019). In addition, no pharmacological agents have been proven to improve the clinical outcome of patients with ICH (Hemphill et al., 2015; Ni et al., 2020).
According to the Traditional Chinese Medicine (TCM) theory, ICH is the result of specific injuries that accumulate in individuals, such as emotional irritability, improper diet, overwork, stagnation of Qi and Blood, endogenous Phlegm, imbalance of Yin and Yang, and other incentives. Therefore, ICH is related to “Wind,” “Extra heat,” “Phlegm,” “Blood stasis,” “Qi” and “Deficiency” (Liu and Guo, 2009; Zhang and Xing, 2019). Compared with other syndromes, “Blood stasis” is one of the most common syndromes in ICH (Yang et al., 2004; Zhang and Zhang, 2021) because “Wind,” “Extra heat,” “Phlegm,” “Qi” and “Deficiency” eventually evolve into “Blood stasis.” Therefore, “Blood stasis” is the leading cause of ICH and occurs throughout the whole process of ICH (Yang and Yan, 2014). In the “Blood Evidence,” Tang Rongchuan proposed that blood that leaves a blood vessel leads to “Blood stasis.” Modern Chinese medicine research suggests that “Blood stasis” obstructs the flow of new blood and impacts the recovery of hemorrhagic stroke patients (Zeng et al., 2022). TCM theory describes blood activation as promoting the body’s “Qi” and blood and restoring the body to a vigorous state. The purpose of activating blood circulation and removing blood stasis is to restore the normal blood flow and to improve the patient’s clinical symptoms. Therefore, use of blood-activating Chinese medicine (BACM) by hemorrhagic stroke patients is reasonable. An expert consensus from TCM also pointed out that BACM is the primary choice for ICH patients (Gao 2016). Effective removal of hematoma is essential for functional recovery in patients with ICH (Li et al., 2022).
Inflammatory response, cytotoxicity, and oxidative stress occur after ICH (Zhu et al., 2019). Research has shown close association of “Blood stasis” with blood viscosity, vascular endothelial permeability, and endothelial relaxation factors (Song, 2000). In TCM, Achyranthes bidentata Blume [Amaranthaceae], Camellia reticulata Lindl. [Theaceae], Conioselinum anthriscoides ‘Chuanxiong’ [Apiaceae], and Salvia miltiorrhiza Bunge [Lamiaceae] are termed blood-activating Chinese herbs (BACH), which are effective in harmonizing the blood and removing blood stasis (Liu et al., 2019). For example, Salvia miltiorrhiza Bunge [Lamiaceae] is widely used, and it has the potential to prevent cerebral edema (Zhang et al., 2003). Conioselinum anthriscoides ‘Chuanxiong’ [Apiaceae] can reduce the release of endothelin and inhibit cytotoxicity (Sun et al., 2008). Camellia reticulata Lindl. [Theaceae] acts as an antioxidant through its potent ability to scavenge free radicals (Pang et al., 2020). Furthermore, emerging evidence has shown that BACM increases concentrations of nerve growth factor and brain-derived neurotrophic factor in the plasma, reduces levels of homocysteine, tumor necrosis factor α, matrix metalloprotein 9, interleukin-6 (IL-6), and hypersensitive-c-reactive-protein in the serum (Wang et al., 2016a; Xi et al., 2018; Lin et al., 2022).
Recently, with the use of classical prescriptions, self-made prescriptions, or Chinese patent medicines, an array of randomized controlled trials (RCTs) on intracranial hemorrhage have been reported, focusing on the essence of activating blood circulation and removing blood stasis. Therefore, we aimed to analyze RCTs on BACM combined with western medicine treatments (WMT) such as Mannitol Injection, Sodium nitroprusside injection, and Symptomatic treatment prescribed for ICH patients to provide more reliable clinical evidence on BACM in managing ICH. Finally, reporting of this review is in strict accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) 2020 statement (Page et al., 2021).
Methods
Protocol registration
The Systematic Review and Meta-Analysis was registered in PROSPERO (Registration Number: CRD42022326159).
Information sources
RCTs published between January 2015 and March 2022 were searched in the following databases and registries: PubMed, Web of Science, Cochrane Library, Embase, Chinese Biomedical Literature Service System (SinoMed), China National Knowledge Infrastructure (CNKI), Chinese Scientific Journals Database (VIP), WanFang database, ClinicalTrials.gov, and Chinese Clinical Trial Register (ChiCTR).
Searching strategy
The following keywords were used in different combinations to search literatures published between January 2015 and March 2022: “Intracranial Hemorrhage,” “Cerebral Hemorrhage,” “SHU XUE,” “HUA YU,” “HUO XUE,” “blood activating agents,” “stasis removing agents,” and “activating blood.” The detailed searching strategies for all databases were presented in the Supplementary Text.
Inclusion criteria
Types of studies
All included articles were RCTs, including dissertations and journal articles.
Types of participants
According to the guidelines, we included participants of any age or gender with a primary clinical diagnosis of intracranial hemorrhage (Chinese Society of Neurology and Chinese Stroke Society, 2019).
Types of interventions
Intervention groups were treated with TCM alone or combined with WMT. In contrast, control groups were treated with one or more WMT, without the use of TCM herbs or other therapies. WMT in control groups included drugs for dehydration and intracranial pressure reduction, maintenance of water and electrolyte balance, control of blood pressure, blood sugar and lipids, and symptomatic treatment.
Types of outcomes
The primary outcomes was clinical efficacy, calculated with the following equation (The fourth session of the National Cerebrovascular Conference, 1996): effective clinical rate (%) (number of patients with recovery + number of patients with significant improvement + number of patients with improvement)/total number of patients × 100%. The reduction of neurological deficit score (NDS) was determined as an efficacy criterion. Recovery was defined as the reduction of 91–100% in NDS. Significant improvement was the reduction of 46–90% in NDS, and improvement was the decrease of 18–45% in NDS, while ineffectiveness was the drop of NDS to less than 17%.
Secondary outcomes included the reduction of the National Institute of Health Stroke Scale (NIHSS) and increased activities of daily living (Barthel Index). In addition, the volume of hematoma, the volume of cerebral edema, the incidence of side effects, and mortality were assessed as secondary outcomes.
Exclusion criteria
(1) Reviews, meta-analysis, basic medical research, or animal research;
(2) Secondary cerebral hemorrhage, including trauma, tumor, intracranial vascular malformation, subarachnoid hemorrhage, etc;
(3) Hemorrhagic transformation after ischemic stroke;
(4) Incomplete information or uncalculated outcomes;
(5) Both treatment groups and control groups were treated with traditional Chinese medicine combined with other therapies;
(6) No control group;
(7) BACM sequential therapy.
Study selection
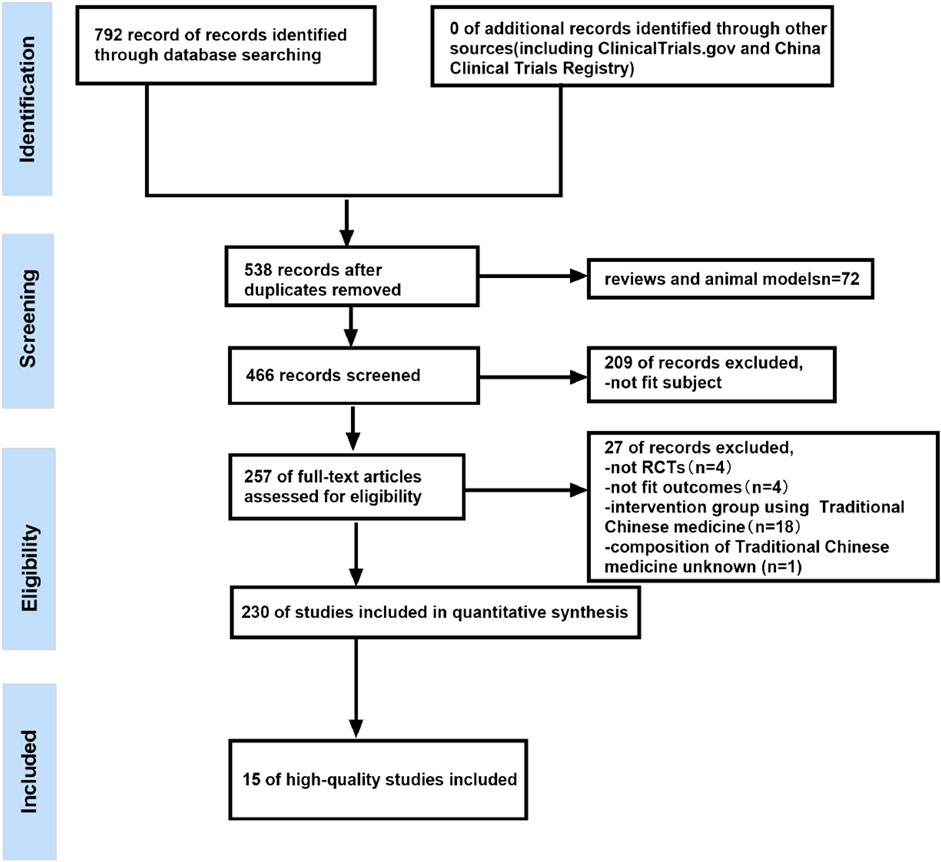
Two authors independently searched the databases for eligible RCTs based on the inclusion and exclusion criteria. After removing duplicate records and animal experiments, the records retrieved from all databases were imported into NoteExpress 3.5.0 and Endnote 20.3 and screened based on the title and abstract. Finally, some studies were excluded due to their failure to meet the inclusion criteria. Figure 1 showed details of the study selection process.
Data extraction
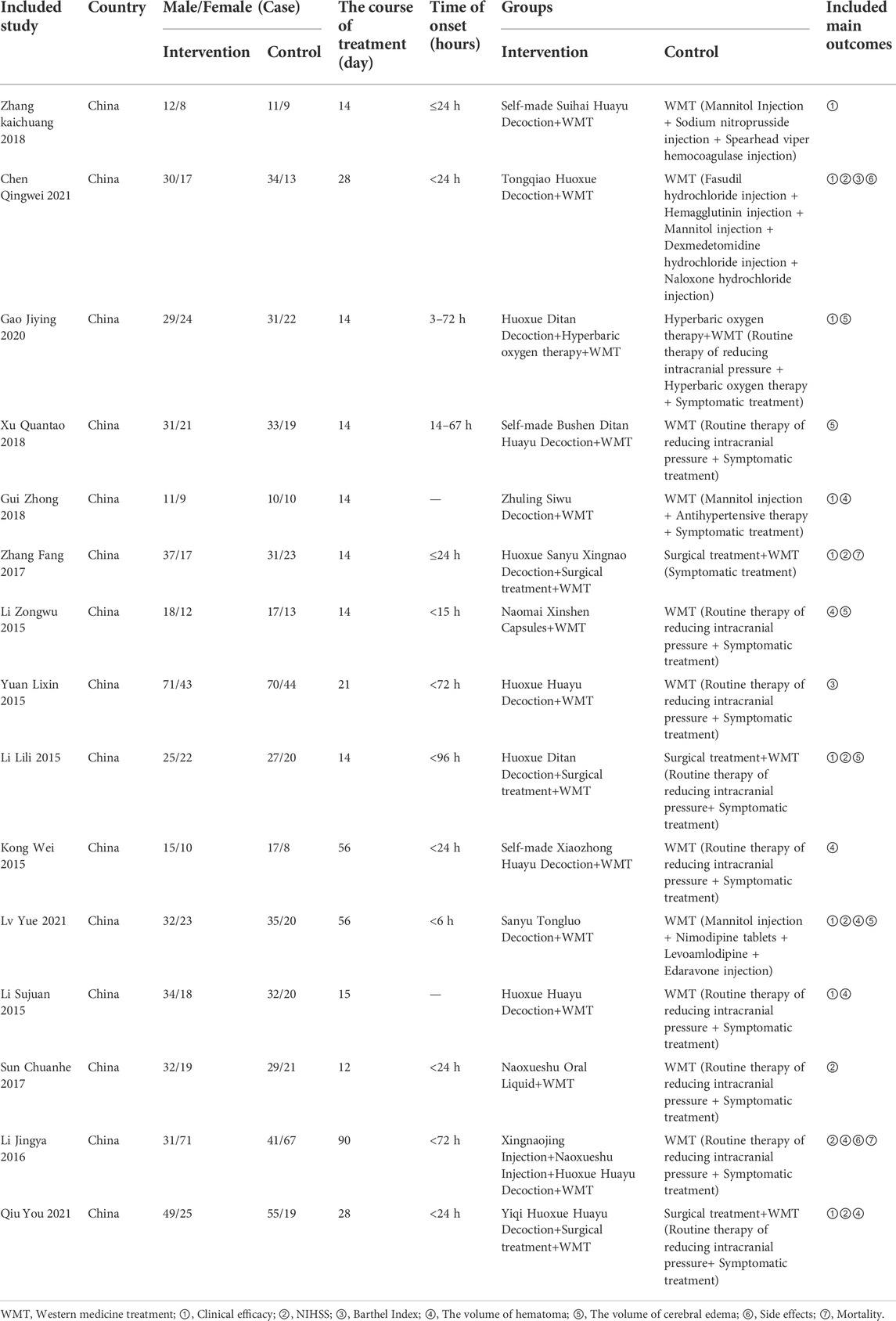
Two researchers independently used a pre-prepared extraction form to extract data from the included articles and cross-checked the data. Disagreement between researchers was resolved through consultation with a third reviewer until a consensus was reached. Data extracted from included studies were: 1) Name of the first author; 2) Year of publishing; 3) Country of study; 4) Mean age and mean volume of hematoma of patients; 5) The number of patients and sex ratio (male to female); 6) Course of treatment; 7) Time of onset; 8) Interventions in the BACM and the control groups; 9) Results of articles (If studies adopted different follow-up time points for patients, we would separately extract results).
Quality assessment
Selected articles were independently reviewed by two researchers based on seven aspects (including generation of random sequences, allocation concealment, blinding of subjects, investigators and outcome assessors, incomplete outcome reporting, selective outcome reporting, and other biases) using the Cochrane Risk of Bias Tool (Higgins et al., 2011). Articles with “low risk” in each domain were included for further analysis, whereas those with “unclear risk” or “high risk” in ≥3 domains were excluded (Chung et al., 2013). Conflicts were resolved by a third researcher or discussion until a consensus was reached.
Data analysis
Review Manager 5.3 was used for meta-analysis. The effect size was calculated using an odds ratio (OR) with 95% CI for dichotomous data. Continuous variables were expressed as the weighted mean difference (MD). Standardized mean difference (SMD) was considered when the measurement units differed. All variables were demonstrated with their effect sizes and their 95% confidence intervals (CIs). In addition, the fixed-effect model was used when heterogeneity was evaluated by Chi-square (Chi) test and I2 statistic (if p ≥ 0.05, or I2 ≤ 50%). Otherwise, the random-effect model was used. Furthermore, sensitivity analysis was conducted to evaluate the stability of outcomes and the overall efficacy of BACM combined with WMT for ICH treatment. Based on the patient’s follow-up time, subgroup analyses were conducted. Publication bias was evaluated through STATA14.0 using Begg’s test, Egger’s test, and funnel plot.
Assessment of evidence
The GRADE pro-GDT software (Guideline Development Tool) was used to evaluate the outcomes (Deng et al., 2019; Duan et al., 2021).
Results
Study selection
The detailed literature search and study selection process were shown in Figure 1. A total of 792 records were found using various searching strategies, inclusion and exclusion criteria. Among them, 254 records were duplicates, and 72 records were reviews or models. Furthermore, 209 articles were excluded because of inappropriate subjects. Then, 257 studies were manually screened according to the exclusion and inclusion criteria. Of these, four studies were not RCTs, four studies were unsuitable for outcomes analysis, one study did not state the composition of traditional Chinese medicine, and the control group in 18 studies also used BACM. Finally, A total of 15 high-quality studies were included after two researchers independently assessed 230 articles using the Cochrane Risk of Bias Tool.
Study characteristics
Characteristics of the included RCTs were provided in Table 1 and Supplementary Tables S1–S15. A total of 15 RCTs (Li, 2015a; Li, 2015b; Li, 2015c; Li et al., 2016a; Kong et al., 2015; Yuan et al., 2015; Sun, 2017; Zhang et al., 2017; Gui, 2018; Xu, 2018; Zhang, 2018; Gao et al., 2020; Chen et al., 2021; Lv et al., 2021; Qiu et al., 2021) involving 1,579 participants were included in this analysis, with 784 participants in the intervention group and 795 participants in the control group. The majority of patients had an onset to the presentation time of less than 24 h, with a maximum of 72 h, and the duration of treatment is mainly 2 weeks to 8 weeks, with a maximum of 90 days. The types of drugs in the intervention group were herbal prescriptions, classical decoctions, and Chinese patent medicines, including capsules and injections. The sample size of included studies ranged from 20 to 108, and all included studies were conducted in China with WMT used in the control group, including surgical treatment, blood pressure control, glycemic control, and reduction of intracranial pressure. BACM was used as an intervention strategy. Seven RCTs employed the effective clinical rate as an outcome measure, and 14 RCTs reported NIHSS as their outcome measures. In addition, hematoma volume in 10 articles and cerebral edema volume in eight articles were considered outcome measures, respectively. Additionally, two articles used the Barthel Index, the incidence of side effects, and mortality as outcome measures.
Assessment of risk of bias
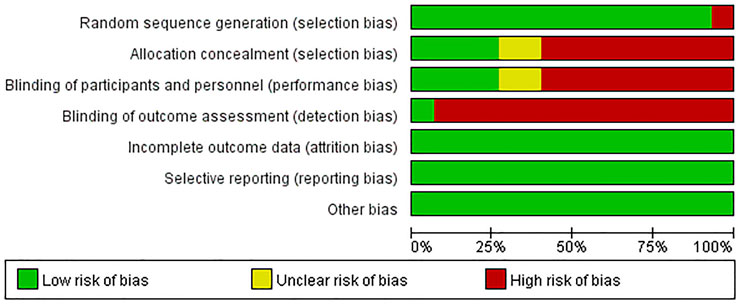
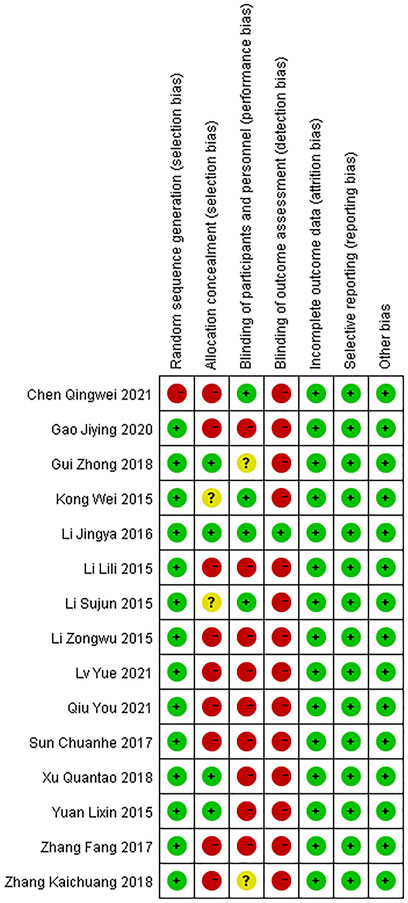
Results on the assessment of risk of bias of included studies were shown in Figures 2, 3. The Cochrane Risk of Bias Tool was used to assess this risk based on seven domains.
Domain 1: risk of bias arising from the randomization process
For the generation of random sequences, 14 trials were ranked “low risk” because the authors used a random number table (Li, 2015a; Li, 2015b; Li, 2015c; Kong et al., 2015; Yuan et al., 2015; Li et al., 2016b; Sun, 2017; Zhang et al., 2017; Gui, 2018; Xu, 2018; Zhang, 2018; Gao et al., 2020; Lv et al., 2021; Qiu et al., 2021); one trial was ranked “high risk” because patients were allocated to the control and treatment groups using the date of birth odd (Chen et al., 2021).
Domain 2: risk of bias arising from the allocation concealment
For allocation concealment, four trials were considered“low risk” because they used opaque envelopes to randomize patients (Yuan et al., 2015; Li et al., 2016a; Gui, 2018; Xu, 2018). The allocation concealment was not mentioned in the rest of the trials. Nine trials were assessed as “high risk” because they conducted a random number table to assign patients (Li, 2015a; Li, 2015b; Sun, 2017; Zhang et al., 2017; Zhang, 2018; Gao et al., 2020; Chen et al., 2021; Lv et al., 2021; Qiu et al., 2021). Another two trials were regarded as “unclear risk” because they used a random method to assign patients and did not mention details of concealment (Li, 2015c; Kong et al., 2015).
Domain 3: risk of bias arising from blinding of participants and researchers
Four articles were regarded as “low risk” because these studies explicitly stated how participants and personnel were blinded (Kong et al., 2015; Li, 2015a; Li et al., 2016b; Chen et al., 2021). Two trials were regarded as “unclear risk” because these trials described the personnel who were blinded to the trial, and there was insufficient evidence to confirm participants were also blinded (Gui, 2018; Zhang, 2018). Nine articles were considered “high risk” because the authors did not provide sufficient information on whether personnel or participants were blinded to measures of treatment (Yuan et al., 2015; Li, 2015a; Li, 2015b; Sun, 2017; Zhang et al., 2017; Xu, 2018; Gao et al., 2020; Lv et al., 2021; Qiu et al., 2021).
Domain 4: risk of bias in the measurement of outcomes
One trial was considered “low risk” because the trial mentioned blinding of results (Li et al., 2016a). The rest of trials were judged as “high risk” because they did not state whether results were blinded or not.
Domain 5: risk of bias due to missing outcome data
Since complete clinical data of all patients were available, all trials were judged as “low risk of bias” in this domain.
Domain 6: risk of bias in the selection of the reported results
Outcomes in these trials were displayed without selection bias. We judged all trials as “low risk of bias.”
Domain 7: risk of bias from other ways
No other risk of bias was found in these 15 trials.
The use of the blood-activating Chinese herbs
Among the 230 RCTs, 15 herbs were used most frequently, including Conioselinum anthriscoides ‘Chuanxiong’ [Apiaceae] (53.28%), Camellia reticulata Lindl. [Theaceae] (49.34%), Bupleurum sibiricum var. jeholense (Nakai) C.D.Chu [Apiaceae] (48.47%), Prunus persica (L.) Batsch [Rosaceae] (47.60%), Rheum palmatum L. [Polygonaceae] (39.30%), Earthworm (38.86%), Astragalus mongholicus Bunge [Fabaceae] (34.50), Salvia miltiorrhiza Bunge [Lamiaceae] (34.50), Angelica sinensis (Oliv.) Diels [Apiaceae] (27.07), Achyranthes bidentata Blume [Amaranthaceae] (26.64%), Panax notoginseng (Burkill) F.H.Chen [Araliaceae] (26.64%), Leech (23.14%), Acorus calamus L. [Acoraceae] (17.90%), Rehmannia glutinosa (Gaertn.) DC. [Orobanchaceae] (14.85%), and Gastrodia elata Blume [Orchidaceae] (14.41%) in the forms of decoctions, injections, and oral granules/capsules/liquid. Among these 15 herbs, Rheum palmatum L. [Polygonaceae], Rehmannia glutinosa (Gaertn.) DC. [Orobanchaceae], Acorus calamus L. [Acoraceae], and Gastrodia elata Blume [Orchidaceae] were considered BACH to be used for patients with ICH in recent years. In addition, among the 15 RCTs, 15 Chinese herbs were commonly used, including Rheum palmatum L. [Polygonaceae] (80%), Conioselinum anthriscoides ‘Chuanxiong’ [Apiaceae] (80%), Camellia reticulata Lindl. [Theaceae] (60%), Astragalus mongholicus Bunge [Fabaceae] (53.3%), Prunus persica (L.) Batsch [Rosaceae] (46.67%), Panax notoginseng (Burkill) F.H.Chen [Araliaceae] (40%), Acorus calamus L. [Acoraceae] (40%), Angelica sinensis (Oliv.) Diels [Apiaceae] (33.33%), Earthworm (33.33%), Bupleurum sibiricum var. jeholense (Nakai) C.D.Chu [Apiaceae] (33.33%), Salvia miltiorrhiza Bunge [Lamiaceae] (33.33%), Leech (33.33%), Gastrodia elata Blume [Orchidaceae] (33.33%), Alisma plantago-aquatica L. [Alismataceae] (26.67%), Achyranthes bidentata Blume [Amaranthaceae] (26.67%), Wolfiporia cocos (F.A. Wolf) Ryvarden & Gilb (26.67%). Among15 included trials, the dosage of 15 BACH in treatment of ICH and the pharmacological mechanisms of these Chinese herbs were shown in Table 2.
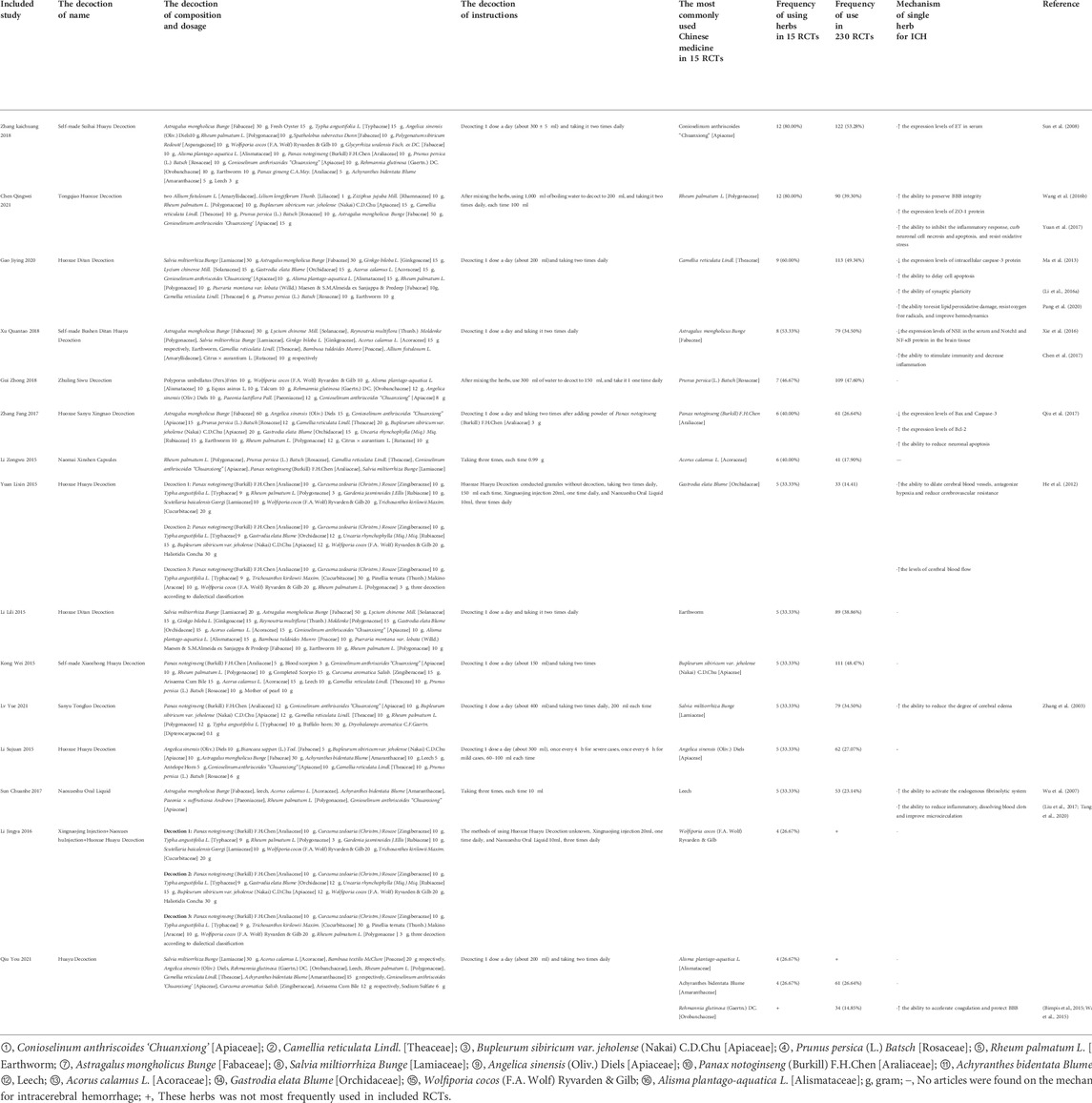
TABLE 2. Among 15 included trials, the dosage of 15 BACH in treatment of ICH and pharmacological mechanisms of these Chinese herbs.
Efficacy outcomes
Clinical effectiveness rate
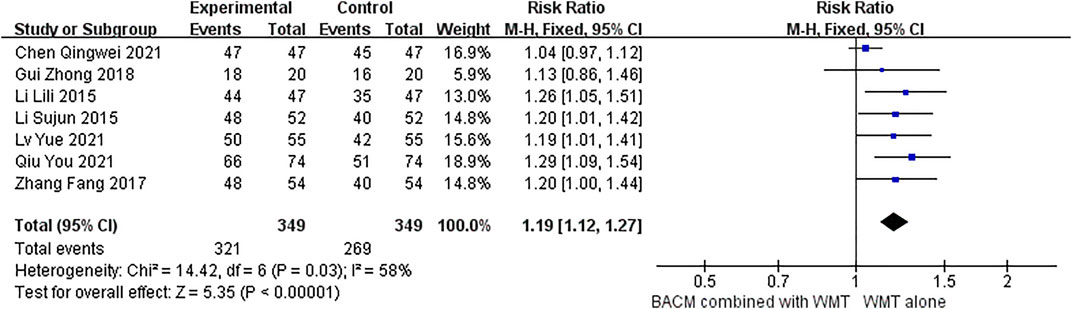
Seven studies (Zhang et al., 2017; Gui, 2018; Li, 2015a; Li, 2015b; Chen et al., 2021; Lv et al., 2021; Qiu et al., 2021) used the change of NIHSS as the marker of clinical effectiveness. As shown in Figure 4, we evaluated the efficacy of BACM plus WMT with the RR values. The Random-effects model was used due to the presence of strong heterogeneity (I2 = 58%; χ2 = 14.42; df = 6, p = 0.03). The difference was statistically significant, with the pooled RR of 1.19 (95% CI [1.12 to 1.27], p < 0.001). We omitted trials one-by-one and found that the RR was 1.22 (95% CI [1.13 to 1.32], p < 0.001) with little heterogeneity (I2 = 0%, p = 0.96) when the study (Chen et al., 2021) was deleted (Supplementary Figure S1). From the forest plot, the CI of this trial (Chen et al., 2021) had a smaller coverage than the other studies. We hypothesized that participants in this study (Chen et al., 2021) had milder clinical symptoms and better outcomes than patients in other studies because participants in Chen’s study (Chen et al., 2021) had the lowest mean NIHSS among the pooled trials. The funnel plot for publication bias (Supplementary Figure S2) did not show significant publication bias among the six RCTs, with Begg’s test (Z = 0.38, p = 0.707) and Egger’s test (T = −1.29, p = 0.2) showing insignificant difference.
NIHSS
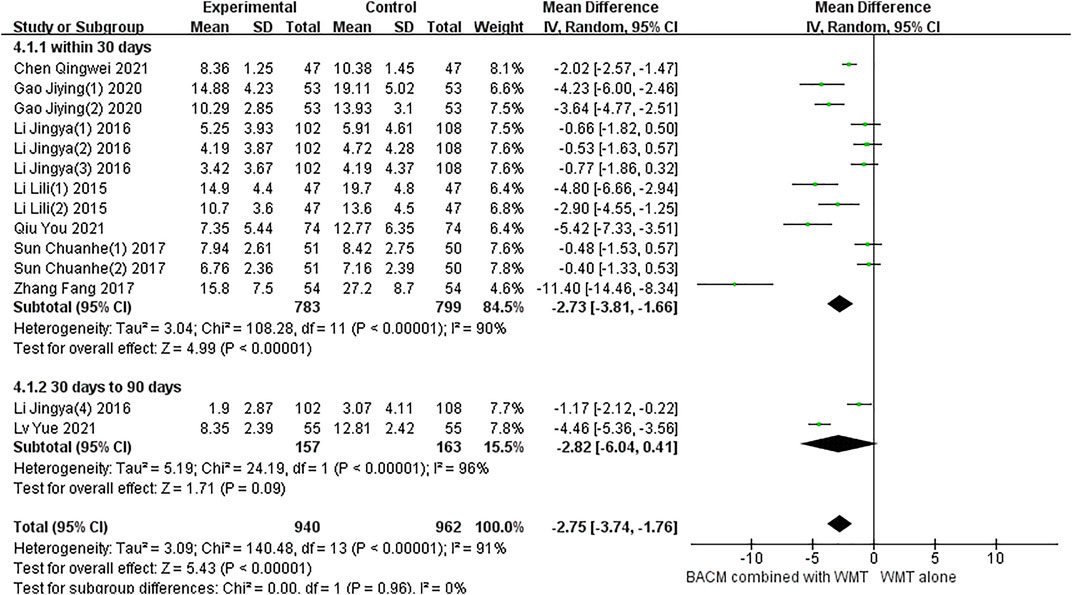
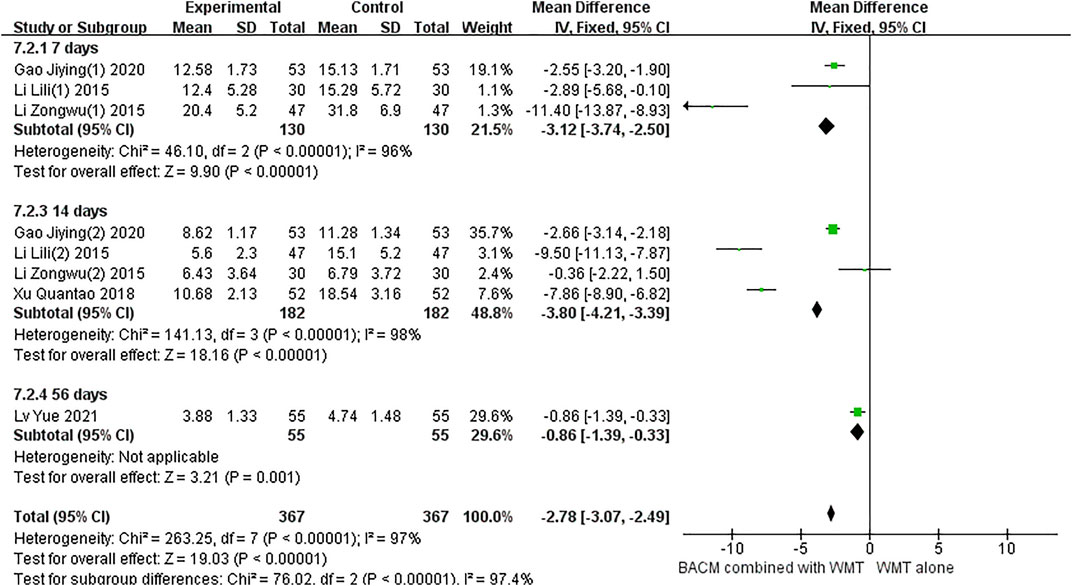
Eight RCTs with fourteen results reported the NIHSS in patients with ICH.and a total of 10 studies included multiple time points: day 5 (Sun, 2017), day 7 (Li et al., 2015; Li et al., 2016a; Gao et al., 2020), day 10 (Sun, 2017), day 14 (Gao et al., 2020; Zhang et al., 2017; Li, 2015a; Li et al., 2016a), day 21 (Li et al., 2016b), day 28 (Chen et al., 2021; Qiu et al., 2021), day 56 (Lv et al., 2021), day 90 (Li et al., 2016a). As shown in Figure 5, due to statistically significant heterogeneity (I2 = 91%; χ2 = 140.48; df = 13, p < 0.001), conclusions were obtained using a random-effects model (MD =−2.75, 95% CI [−3.74 to −1.76], p < 0.001) and subgroup analysis was performed according to time points. Twelve trials, including participants who received treatment for less than 30 days, were significantly heterogeneous (p < 0.001, I2 = 90%). The results of pooled twelve RCTs (Sun, 2017; Sun, 2017; Zhang et al., 2017; Li, 2015a; Li, 2015b; Li et al., 2016a; Li et al., 2016b; Li et al., 2016b; Gao et al., 2020; Gao et al., 2020; Chen et al., 2021; Qiu et al., 2021) showed that the difference was statistically significant (MD = −2.73, 95% CI [−3.81 to −1.66], p < 0.001). In addition, there was significant heterogeneity between the two RCTs (Li et al., 2016a; Lv et al., 2021) with a duration of 56 and 90 days, respectively (p < 0.01, I2 = 96%), and the results showed no statistically significant difference (MD = −2.82, 95% CI [−6.04 to 0.41], p = 0.09).
The significant heterogeneity in this study may be attributed to the fact that different types of BACM removing blood stasis had different therapeutic effects on patients with NDS. For example, participants in the study of Zhang et al. (Zhang et al., 2017) had more severe NDS, which may explain the presence of greater efficacy observed in this trial than in others. To examine publication bias, funnel plots were shown in Supplementary Figure S3, and the results of Begg’s test (Z = 2.08, p = 0.037) and Egger’s test (T = −1.82, p = 0.94) indicated that there was no significant publication bias in the 14 RCTs.
Barthel index
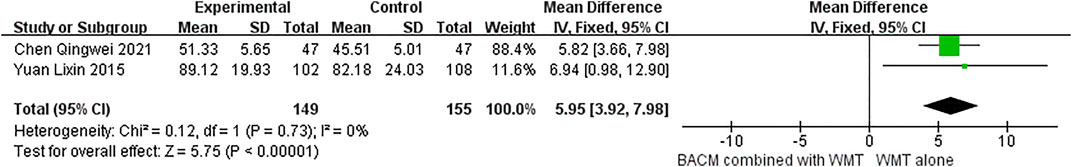
The Barthel index was reported in two RCTs (Yuan et al., 2015; Chen et al., 2021). As shown in Figure 6, BACM that promoted blood circulation and removed blood stasis combined with WMT was superior to WMT alone in improving patients’ quality of life (MD = 5.95,95% CI [3.92,7.98], p < 0.001). However, the result needs to be interpreted with caution due to the small sample size.
Hematoma volume
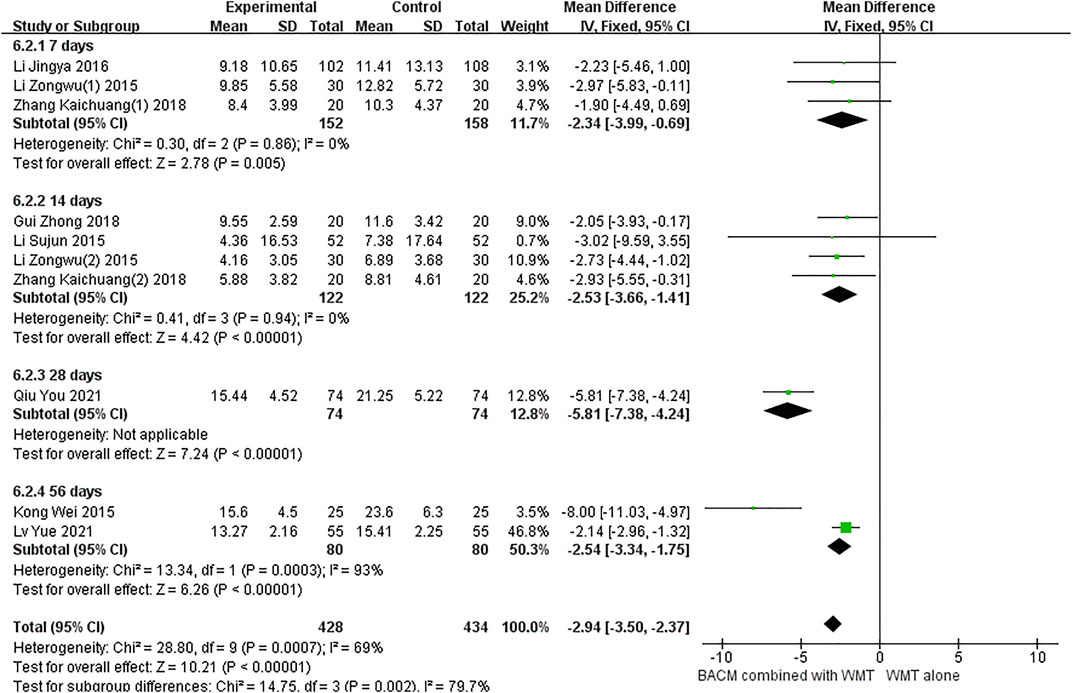
Of the fifteen trials, 10 (Li et al., 2016a; Li, 2015a; Li, 2015b; Li, 2015c; Zhang, 2018; Zhang, 2018; Gui, 2018; Qiu et al., 2021; Kong et al., 2015; Lv et al., 2021) used hematoma volume as a clinical outcome. As shown in Figure 7, we used a random-effects model (MD = −2.94, 95% CI [−3.50 to −2.37], p < 0.001) due to the presence of significant heterogeneity (p < 0.001, I2 = 69%). Clinical outcomes were measured with the change of hematoma volume after 1 week of onset in three RCTs (Li et al., 2016a; Li, 2015c; Zhang, 2018), 2 weeks in four RCTs (Zhang, 2018; Gui, 2018; Li, 2015a; Li, 2015b), 3 weeks in one RCT (Qiu et al., 2021), and 8 weeks in two RCTs (Kong et al., 2015; Lv et al., 2021). There was no heterogeneity between participants who underwent head CT scans after 1 and 2 weeks, respectively (p = 0.86, I2 = 0%; p = 0.94, I2 = 0%), and the results were statistically significant (MD1 week = −2.34, 95% CI [−3.99 to −0.69, p = 0. 005; MD2 week = −2.53, 95% CI [−3.66 to −1.41], p < 0.001). In addition, there was significant heterogeneity in the group of patients who underwent CT scan after 8 weeks (I2 = 93%, p = 0.0003). We speculated that the sample size of this study (Kong et al., 2015) was smaller than that of the other study (Lv et al., 2021), leading to an exaggerated absorption of cerebral hematoma in the group of BACM combined with WMT. As shown in Supplementary Figure S4, Begg’s test (Z = 1.25, p = 0.210) and Egger’s test (T = −0.93, p = 0.379) indicated the absence of significant publication bias in 10 trials.
Cerebral edema
Eight trials reported cerebral edema (Xu, 2018; Gao et al., 2020; Li, 2015a; Li, 2015b; Li, 2015c; Li, 2015; Gao et al., 2020; Lv et al., 2021). As shown in Figure 8, the heterogeneity was significant (I2 = 97%, p < 0.001), therefore, a random-effects model was used. We found that BACM combined with WMT was associated with a reduction in brain edema volume compared with WMT alone (MD = −2.74, 95% CI [−3.01 to −2.48], p < 0.001). Three RCTs (Gao et al., 2020; Li, 2015a; Li, 2015b) performed the second head CT scan after 1 week, four RCTs (Xu, 2018; Gao et al., 2020; Li, 2015a; Li, 2015b) performed the second head CT scan after 2 weeks, and one RCT (Lv et al., 2021) performed the second head CT scan after 8 weeks. Subgroup analysis showed that there was significant heterogeneity between the 1-week groups and the 2-week groups. In addition, we found that Li’s study (Li, 2015c) had significant heterogeneity. When this trial (Li, 2015a) was excluded, the heterogeneity was improved (I2 = 0, p = 0.82), and the difference was statistically significant (MD 1-week group = −2.57, 95%CI [−3.21 to −1.93], p < 0.001; MDtotal = −2.66, 95%CI [−2.95 to −2.37], p < 0.00001), as shown in Supplementary Figure S5). The small sample size and small mean edema volume in Li’s trial (Li, 2015a) brought about exaggerated effects and heterogeneity. Because absorption of cerebral edema was faster in Li’s study (Li L., 2015) than in the other two RCTs (Gao et al., 2020; Li, 2015a), the pooled value was larger. The effect of BACM combined with WMT was superior to that of WMT alone in the 1-week group (Gao et al., 2020; Li, 2015a) (MD = −2.57, 95% CI [−3.21 to −1,83], p < 0.001). In addition, there was significant heterogeneity in the pooled effect size (MD = −3.80, 95% CI [−4.21 to −3. 39]) for the four RCTs (Xu, 2018; Gao et al., 2020; Li, 2015a; Li, 2015b) at 2 weeks (I2 = 98%, p < 0.001). Apart from the baseline level of brain edema volume, no other factors contributed to this heterogeneity. To examine publication bias, funnel plots were shown in Supplementary Figure S6, and the results of Begg’s test (Z = 0.30, p = 0.764) and Egger’s test (T = −1.95, p = 0.109) did not show significant publication bias in seven RCTs.
Adverse reactions
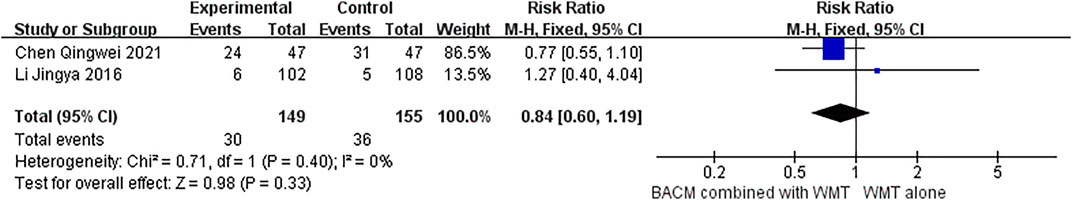
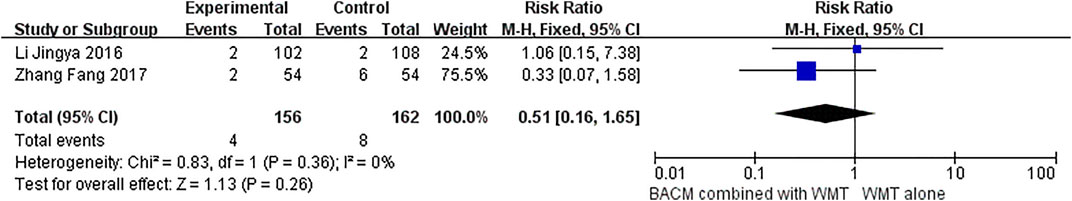
Two RCTs (Chen et al., 2021; Li et al., 2016a) demonstrated that BACM combined with WMT elicited adverse reactions in patients with ICH during the intervention. Chen et al. (Chen et al., 2021) showed that the BACM (Tongqiao Huoxue decoction) used by patients with ICH had side effects, including hepatic impairment in three patients, renal impairment in three patients, gastrointestinal reaction in nine patients, intracranial infection in four patients, pulmonary infections in four patients, and subcutaneous hemorrhage in one patient. However, some of these adverse reactions might be indirectly related to the intake of BACM. In addition, another study (Li et al., 2016b) reported adverse events in seven patients when BACM (Xingnaojing Injection/Naoxueshu Injection/Huoxue Huayu Decoction) was prescribed to patients with ICH, including pressure ulcer, diarrhea, deep venous thrombosis, allergy to BACM (Xingnaojing), renal function impairment, increased hematoma, and two deaths due to impaired renal function and increased hematoma. In the study by Zhang et al. (Zhang et al., 2017), two deaths were also reported. However, no statistical difference was found in the incidence of side effects and mortality between BACM and control groups (RR = 0.84 (95% CI [0.60 to 1.19], p = 0.33 and RR = 0.51 (95% CI, [0.16 to 1.65], p = 0.26), with forest plots shown in Figures 9, 10, respectively.
Sensitivity analysis
Sensitivity analysis was performed on the clinical effectiveness (Zhang et al., 2017; Gui, 2018; Li, 2015a; Li, 2015b; Chen et al., 2021; Lv et al., 2021; Qiu et al., 2021), hematoma volume (Li, 2015a; Li, 2015b; Li, 2015c; Li et al., 2016a; Zhang, 2018; Zhang, 2018; Gui, 2018; Qiu et al., 2021; Kong et al., 2015; Lv et al., 2021), and cerebral edema (Gao et al., 2020; Li, 2015a; Li, 2015b; Li, 2015c; Li, 2015; Gao et al., 2020; Xu, 2018; Lv et al., 2021). To reveal the stability of results, including clinical effectiveness, hematoma volume, and cerebral edema in the meta-analysis, we excluded RCTs one by one. None of these exclusions changed the statistical significance, suggesting that our results were robust. However, there was significant heterogeneity in cerebral hematoma, cerebral edema, and NIHSS.
Evaluation of quality
The GRADEpro Guideline Development Tool was used to assess the quality of evidence for nine outcomes, including the clinical effectiveness (Li, 2015a; Li, 2015b; Zhang et al., 2017; Gui, 2018; Chen et al., 2021; Lv et al., 2021; Qiu et al., 2021), NIHSS on day 30 (Li, 2015a; Li et al., 2016a; Li et al., 2016b; Li et al., 2016; Sun, 2017; Gao et al., 2021; Li et al., 2021; Sun, 2017; Gao et al., 2020; Zhang et al., 2017; Chen et al., 2021; Qiu et al., 2021), NIHSS from 30 to 90 days (Lv et al., 2021; Li et al., 2016a), Barthel index (Yuan et al., 2015; Chen et al., 2021), hematoma volume on day 7 (Li et al., 2016a; Li, 2015a; Zhang, 2018), hematoma volume on day 14 (Li, 2015a; Li, 2015b; Zhang, 2018; Gui, 2018), hematoma volume on day 56 (Kong et al., 2015; Lv et al., 2021), cerebral edema volume on day 7 (Li, 2015a; Li, 2015b; Gao et al., 2020), the volume of cerebral edema on day 14 (Li, 2015a; Li, 2015b; Xu, 2018; Gao et al., 2020). The levels of evidence ranged from very low, low, moderate, to high. Results were shown in Table 3.
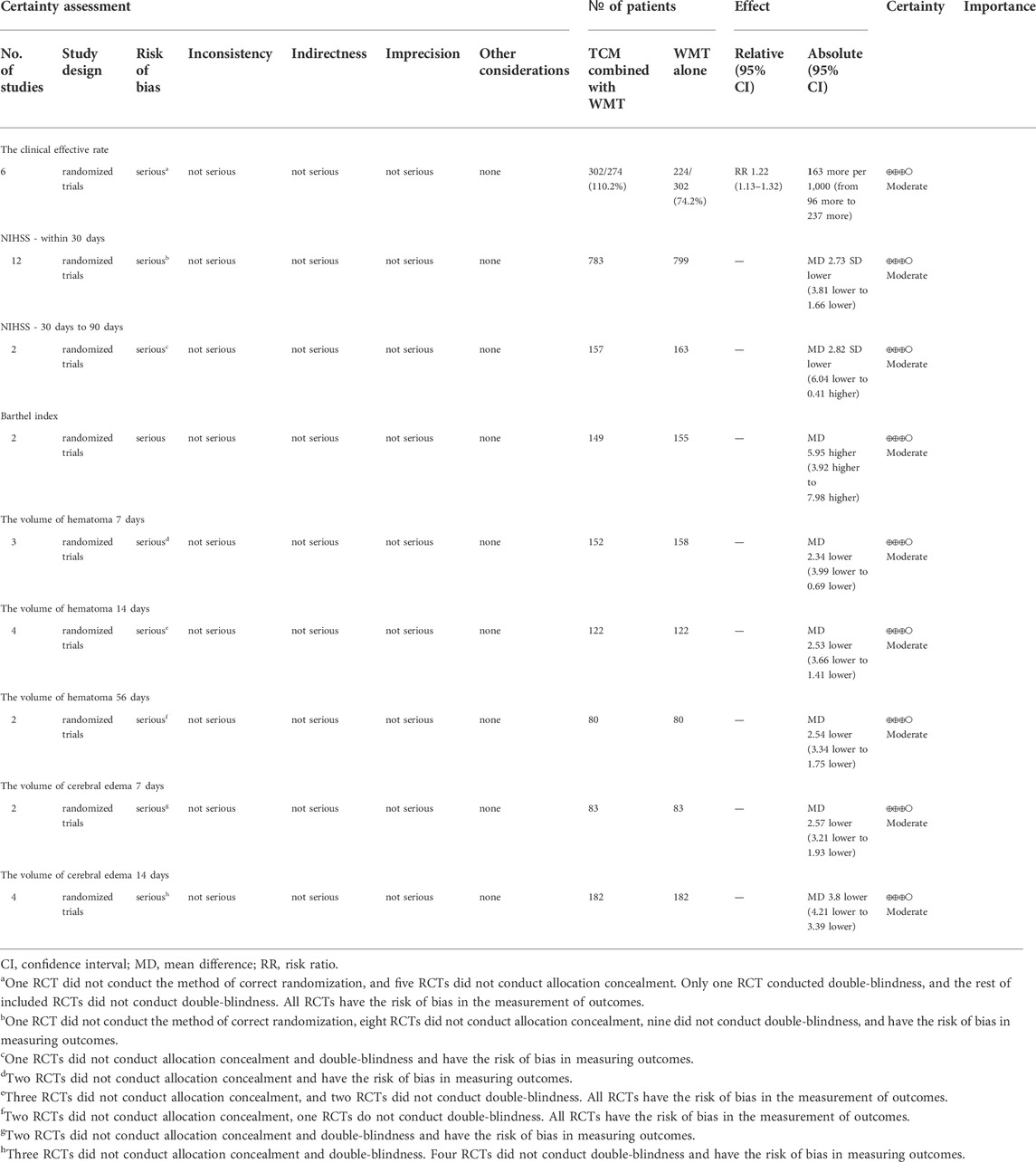
TABLE 3. GRADE summary of outcomes for blood-activating Chinese medicine to patients with intracerebral hemorrhage.
Discussion
Summary of findings
Increasing evidence indicates that BACM plays a role in neuroprotection and inhibition of inflammatory responses and has the potential to be used in hemorrhagic stroke treatment. In this meta-analysis, we evaluated 15 RCTs, and the result showed that BACM combined with WMT was more effective than WTM alone. These positive effects are mainly consistent with the systematic review of BACM combined with WMT in ICH treatment due to their efficacy in activating blood circulation and resolving blood stasis (Xu et al., 2022).
The pathophysiologic mechanisms of post-ICH injury are complex and encompass oxidative stress, inflammation, neurotoxicity, and thrombin formation, leading to brain edema (Shao et al., 2019). In addition, peri-hematomal edema (PHE) within the first 72 h after hemorrhage leads to tissue shifts, brain herniation, and risks of clinical deterioration (Leasure et al., 2016). Conservative medical treatments include reduction of intracranial pressure, control of blood pressure, inhibition of thrombin activation, and neuroprotective agents. Surgical treatment consists of mechanical thrombectomy, craniotomy for hematoma evacuation, minimally invasive surgery, and decompressive craniotomy (Chinese Society of Neurology and Chinese Stroke Society, 2019; Ni et al., 2020). Nowadays, practical pharmacological approaches for ICH are still lacking. In addition, surgical treatment does not improve the long-term prognosis for most patients with ICH (Ni et al., 2020).
Among the commonly used herbs for blood circulation, their efficacy in improving blood circulation in patients with ICH has been proven. Our study also showed that the herbs representing BACH were Conioselinum anthriscoides ‘Chuanxiong’ [Apiaceae], Camellia reticulata Lindl. [Theaceae], Bupleurum sibiricum var. jeholense (Nakai) C.D.Chu [Apiaceae], Prunus persica (L.) Batsch [Rosaceae], Rheum palmatum L. [Polygonaceae], Earthworm, Astragalus mongholicus Bunge [Fabaceae], Salvia miltiorrhiza Bunge [Lamiaceae], Angelica sinensis (Oliv.) Diels [Apiaceae], Panax notoginseng (Burkill) F.H.Chen [Araliaceae], Achyranthes bidentata Blume [Amaranthaceae], Leech, Acorus calamus L. [Acoraceae], Rehmannia glutinosa (Gaertn.) DC. [Orobanchaceae], and Gastrodia elata Blume [Orchidaceae]. In addition, some herbs, such as Rheum palmatum L. [Polygonaceae], Rehmannia glutinosa (Gaertn.) DC. [Orobanchaceae], Acorus calamus L. [Acoraceae], and Gastrodia elata Blume [Orchidaceae] were also considered adjuvant for ICH in the acute or postoperative recovery stage in recent studies (Li et al., 2015).
Molecular mechanisms underlying the therapeutic effect of commonly used BACM for ICH
(1) Rheum palmatum L. [Polygonaceae]: Rheum palmatum L. [Polygonaceae] is a traditional and famous Chinese medicinal herb. The expression of APQ4, tumor necrosis factor-α (TNF-α), and interleukin-1β (IL-1β) was increased in ICH, resulting in disruption of the blood-brain barrier (BBB) (Bimpis et al., 2015; Wang et al., 2015). Recently, modern pharmacological experimental studies showed that rhubarb significantly reduced levels of these inflammatory factors in the serum, alleviated a variety of inflammatory responses, and protected the BBB (Yuan et al., 2017). Moreover, a systematic review showed that rhubarb combined with WMT was proven to be efficacious in improving patients’ prognosis by accelerating the absorption of cerebral hematoma and ameliorating their neurological deficits (Liu et al., 2015). An upcoming randomized controlled clinical trial will investigate the safety and efficacy of Rheum palmatum L. [Polygonaceae] in managing acute ICH, which may again validate the importance of BACH in the treatment of ICH by promoting blood circulation and removing blood stasis. (ClinicalTrials. gov. https://clinicaltrials.gov/ [Accessed April 1, 2022]).
(2) Rehmannia glutinosa (Gaertn.) DC. [Orobanchaceae]: Catalpol, an iridoid compound extracted from Rehmannia glutinosa (Gaertn.) DC. [Orobanchaceae], is one of the active components of Rehmannia glutinosa. In animal experiments, it has been shown that Rehmannia glutinosa accelerated the procoagulant effects by shortening the thrombin time and prothrombin time, activated partial thromboplastin time, and reducing the content of fibrinogen, thereby modulating the intrinsic and extrinsic coagulation pathways (Jia et al., 2014; Yang, 2016). Additionally, the protective effect on the BBB was proven (Liu, 2018; Zhao et al., 2021). Furthermore, Rehmannia glutinosa decoction based on Rehmannia can mitigate neurological deficits and improve activities of daily living (Barthel index) for patients with ICH receiving surgery, which may be related to the increased expression of Notch1 and increased activation of Notch signaling pathway (Zhang et al., 2020; Li and Zhao, 2015).
(3) Gastrodia elata Blume [Orchidaceae]: it has antioxidant effects through up-regulating the PI3K signaling pathway and down-regulating the level of extracellular glutamate (Zhan et al., 2016). In animal models, it has been shown to accelerate absorption of cerebral hematoma, which is associated with up-regulating cellular immunity and proliferation of microvessels (Li and Wu, 2007). Besides, a study showed that compared with WMT alone, Gastrodia elata Blume [Orchidaceae] combined with WMT improved neurological functions and increased the clinical efficacy (He et al., 2012).
(4) Acorus calamus L. [Acoraceae]: Both volatile oil and β-asarone in Acorus calamus L. [Acoraceae] were the main active components (Irie and Keung, 2003). Research about Acorus calamus L. [Acoraceae] for ICH is uncommon, but the efficacy for cognitive dysfunction has been demonstrated (Ma et al., 2017). Two RCTs performed on patients with ICH after craniotomy have demonstrated that Changpu Yujin decoction, which consists primarily of calamus, reduces mortality and the degree of cerebral edema, relieves neurological deficits, promotes recovery, and improves prognosis (Fu et al., 2019; Li and Xiao, 2020).
At present, many trials have demonstrated the clinical efficacy of BACM (e.g: decoctions, injections, and oral forms etc) in the remediation of neurological deficits and improvement of prognosis. A representative is Buyang Huanwu decoction, which has been reported to significantly promote the recovery of ICH patients undergoing surgery (Jing et al., 2015; Yu et al., 2017). In addition, Xueshuantong Injection and Naoxueshu Oral Liquid also were used as an adjuvant therapy for ICH (Xu et al., 2014; Wu et al., 2017; Duan et al., 2021). Although BACM is significantly efficacious in the treatment of ICH, there is an increased risk of bleeding. It has been suggested that the risks of hematoma expansion and rebleeding must be evaluated when administering BACH to ICH patients (Ni et al., 2020). With the elucidation of the pharmacological mechanisms of herbal medicine for ICH, the mechanism of BACH will be further clarified in the future. More herbal preparations, decoctions, and injections will be considered new treatment options for patients with ICH.
Limitations
A number of limitations were found in this meta-analysis. First, the lack of rigorous design and whether the authors were blinded in some included trials may lead to publication bias and overestimation of the efficacy of BACM for ICH. Second, the small sample size in some trials may contribute to discrepant results. Third, the included RCTs did not differentiate between TCM syndromes, resulting in inaccurate results. Fourth, varied dosages of herbs and different decoction processes increased the heterogeneity of the results. Fifth, the specific timing of adding BACM was unclear. Sixth, since all participants were from China, there is not yet sufficient evidence to prove its efficacy in other ethnic groups. Therefore, more stringent, higher quality, larger sample sized clinical trials recruiting more patients from other countries are needed to elucidate the efficacy of BACM combined with WMT for ICH.
Implications for future research
① To clarify the design protocol and to reduce bias, studies need to register on ClinicalTrials.gov or the China Clinical Trial Registration Center before starting the trial;
② To improve research quality, it is advisable to conduct rigorous design of trials, with attention to randomization methods, allocation concealment, and blinding, according to the statement (Chan et al., 2013a; Chan et al., 2013b);
③ To conduct more high-quality clinical trials, increase the sample size, and include patients from other ethnic groups and regions;
④ To focus on “syndrome differentiation and treatment” in TCM;
⑤ Clearly record the time from the symptom onset to intake of BACM;
⑥ Extend the time of treatment and follow-up, paying attention to the long-term efficacy of BACM;
⑦ Complete the report of adverse reactions/events;
⑧ To improve the quality of the report, it is necessary to ensure that reports are complete, clear, and transparent as required (Schulz et al., 2010; Moher et al., 2012).
Conclusion
This meta-analysis suggests that BACM in combination with WTM is superior to WMT alone in ICH treatment. BACM combined with WMT has the potential to improve the overall efficacy, Barthel’s index, accelerate the absorption of hematoma, and decrease the edema volume without increasing the incidence of adverse events or mortality. However, our conclusion is inconclusive due to the high risk of bias and substantial heterogeneity. To further validate our conclusion, more high-quality RCTs are needed (Li and Zhao, 2015; Liu et al., 2015; Wu et al., 2017; Zhang et al., 2020).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
WL: Design, conception, searching articles, extracting data, evaluating quality, performing statistical analysis, interpreting data, drafting, and revising the article. JH and TH: Searching articles, extracting data, assessing quality, performing statistical analysis. LZ: Extracting data, evaluating quality, assessing evidence certainty. XZ and HL: Design, conception, proofreading, and revising the manuscript. All authors approved the final version of the article.
Funding
This work was supported by a grant from Shanghai Municipal Key Clinical Specialty (shslczdzk06102), and a grant from Shanghai Science and Technology Commission (19401972804).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.942657/full#supplementary-material
References
An, b J., Kim, T. J., and Yoon, B.-W. (2017). Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: An update. J. Stroke 19 (1), 3–10. doi:10.5853/jos.2016.00864
Bimpis, A., Papalois, A., Voumvourakis, K., Olah, O., Tiszlavicz, L., and Liapi, C. (2015). Neuronal tumour necrosis factor-α and interleukin-1β expression in a porcine model of intracerebral haemorrhage: Modulation by U-74389G. Brain Res. 1615, 98–105. doi:10.1016/j.brainres.2015.04.034
Chan, A. W., Tetzlaff, J. M., Altman, D. G., Laupacis, A., Gøtzsche, P. C., Krleža-Jerić, K., et al. (2013a). SPIRIT 2013 statement: Defining standard protocol items for clinical trials. Ann. Intern. Med. 158 (3), 200–207. doi:10.7326/0003-4819-158-3-201302050-00583
Chan, A. W., Tetzlaff, J. M., Gotzsche, P. C., Altman, D. G., Mann, H., Berlin, J. A., et al. (2013b). SPIRIT 2013 explanation and elaboration: Guidance for protocols of clinical trials. BMJ 346, e7586. doi:10.1136/bmj.e7586
Chen, C.-C., Chen, X., Li, T.-C., Lin, H.-L., Chu, Y.-T., Lee, H.-C., et al. (2017). PG2 for patients with acute spontaneous intracerebral hemorrhage: A double-blind, randomized, placebo-controlled study. Sci. Rep. 7 (1), 45628. doi:10.1038/srep45628
Chen, Q. W., Yao, Q. Y., Zhang, S. J., and Lu, L. M. (2021). Effect of tongqiao Huoxue decoction( 通窍活血汤) on nerve function and quality of life in patients with acute cerebral hemorrhage. Chin. Arch. Tradit. Chin. Med. 39 (05), 229–232. doi:10.13193/j.issn.1673-7717.2021.05.055
Chinese Society of Neurology, Chinese Stroke Society (2019). Chinese guidelines for diagnosis and treatment of acute intracerebral hemorrhage 2019. Chin. J. Neurol. (12), 994–1005. doi:10.5534/wjmh.2013.31.2.157
Chung, J. H., Lee, J. W., Jo, J. K., Kim, K. S., and Lee, S. W. (2013). A quality analysis of randomized controlled trials about erectile dysfunction. World J. Mens. Health 31 (2), 157–162. doi:10.5534/wjmh.2013.31.2.157
Deng, T., Wang, Y., W, Y. Y., Li, B. H., Jin, Y. H., Ren, X. Q., et al. (2019). Methodology for clinical practice guidelines--application of GRADEpro GDT in evidence grading of systematic reviews of interventional trial. Chin. J. Evid.-Bases Cardiovasc. Med. 11 (01), 1–5. doi: not available.
Duan, J. Y., Liang, X., Jia, M., Du, W. Q., Wang, M., Lei, L., et al. (2021). Systematic review and Meta-analysis on efficacy and safety of Naoxueshu Oral Liquid in treatment of hypertensive intracerebral hemorrhage. China J. Chin. Mat. Med. 46 (12), 2984–2994. doi:10.19540/j.cnki.cjcmm.20210324.501
Feigin, V. L., Stark, B. A., Johnson, C. O., Roth, G. A., Bisignano, C., Abady, G. G., et al. (2021). Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the global burden of disease study 2019. Lancet Neurol. 20 (10), 795–820. doi:10.1016/S1474-4422(21)00252-0
Fu, Q. H., Yang, H. J., Xie, J., and Zhou, B. (2019). Observation on therapeutic effect of Changpu Yujin Decoction on cerebral edema after craniotomy for acute cerebral hemorrhage. Med. J. Natl. Defending Forces Southwest China 29 (5), 611–613. doi:10.3969/j.issn.1004-0188.2019.05.037
Gao, J. Y., Shi, D. L., Gao, X. L., Liu, T. F., Wang, L. W., Cao, B., et al. (2020). Effects of Huoxue ditan decoction combined with hyperbaric oxygen on recovery rate of neurologic function and hemodynamic level in patients with hypertensive intracerebral hemorrhage. Chin. Arch. Tradit. Chin. Med. 38 (09), 213–216. doi:10.13193/j.issn.1673-7717.2020.09.054
Gao, L. (2016). Expert consensus on hypertensive intracerebral hemorrhage in acute stage in diagnosis and treatment combining traditional Chinese medicine and Western medicine. Chin. General Pract. 19 (30), 3641–3648. doi:10.3969/j.issn.1007-9572.2016.30.001
Gui, Z. (2018). The clinical Observation on treating cerebral Edema after hypertensive cerebral Hemorrhage with activating Blood and water method. Master's degree. Guangzhou, China: Guangzhou University of Chinese Medicine.
He, F., Xiao, H., and Zhang, F. L. (2012). Clincal observation of gastrodin in the treatment of cerebral hemorrhage. Chin. J. Clin. Ration. Drug Use 5 (20), 12–13. doi:10.15887/j.cnki.13-1389/r.2012.20.117
Hemphill, J. C., Greenberg, S. M., Anderson, C. S., Becker, K., Bendok, B. R., Cushman, M., et al. (2015). Guidelines for the management of spontaneous intracerebral hemorrhage: A guideline for healthcare professionals from the American heart association/American stroke association. Stroke 46 (7), 2032–2060. doi:10.1161/STR.0000000000000069
Higgins, J. P. T., Altman, D. G., Gotzsche, P. C., Juni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Hostettler, I. C., Seiffge, D. J., and Werring, D. J. (2019). Intracerebral hemorrhage: An update on diagnosis and treatment. Expert Rev. Neurother. 19 (7), 679–694. doi:10.1080/14737175.2019.1623671
Irie, Y., and Keung, W. M. (2003). Rhizoma acori graminei and its active principles protect PC-12 cells from the toxic effect of amyloid-beta peptide. Brain Res. 963 (1-2), 282–289. doi:10.1016/s0006-8993(02)04050-7
Jia, X. M., Zhang, Z. L., and Wu, R. H. (2014). Effects of fresh rehmanniae radix and its processed products on blood of fevered and bleeding rats. Chin. J. Exp. Tradit. Med. Formulae 20 (6), 127–132. doi:10.11653/syfj2014060127
Jing, Z. J., Li, Y. L., Yang, J. Q., Liu, Y. N., Yang, W. Y., Zhou, R. T., et al. (2015). Observation on the curative effect of early application of Buyang Huanwu Decoction after hypertensive cerebral hemorrhage. Mod. J. Integr. Tradit. Chin. West. Med. 24 (31), 3463–3465. doi:10.3969/j.issn.1008-8849.2015.31.015
Kong, W., Yang, J. C., Zhang, P. Y., and Geng, S. L. (2015). Clinical effect of Xiaozhonghuayu decoction on hypertensive cerebral hemorrhage. Pharmacol. Clin. Chin. Mat. Med. 31 (01), 311–313. doi:10.13412/j.cnki.zyyl.2015.01.127
Leasure, A., Kimberly, W. T., Sansing, L. H., Kahle, K. T., Kronenberg, G., Kunte, H., et al. (2016). Treatment of edema associated with intracerebral hemorrhage. Curr. Treat. Options Neurol. 18 (2), 9. doi:10.1007/s11940-015-0392-z
Li, H. Q., Wei, J. J., Xia, W., Li, J. H., Liu, A. J., Yin, S. B., et al. (2015). Promoting blood circulation for removing blood stasis therapy for acute intracerebral hemorrhage: A systematic review and meta-analysis. Acta Pharmacol. Sin. 36 (6), 659–675. doi:10.1038/aps.2014.139
Li, J.-y., Yuan, L.-x., Zhang, G.-m., Zhou, L., Gao, Y., Li, Q.-b., et al. (2016a). Activating blood circulation to remove stasis treatment of hypertensive intracerebral hemorrhage: A multi-center prospective randomized open-label blinded-endpoint trial. Chin. J. Integr. Med. 22 (5), 328–334. doi:10.1007/s11655-016-2467-7
Li, W. W., Chen, L., and Liu, H. C. (2016b). Research progress on the pharmacological effects of safflower yellow pigment on cerebral vascular disease. World Latest Med. Inf. 16 (A0), 71–72+78. doi: not available.
Li, B., Huang, Y. X., Huang, Z. Q., Lin, L. Z., and Liang, Z. (2022). Breaking blood expelling stasis method accelerates hematoma resolution after hyperacute intracerebral hemorrhage and its potential mechanism. J. Chin. Physician 24 (01), 53–58. doi:10.3760/cma.j.cn431274-20201026-01449
Li, J., and Wu, S. (2007). Therapeutical effect of Salvia miltiorrhiza and gastrodin to rats after cerebral hemorrhage. J. Guizhou Med. Univ. 32 (2), 118–122. doi: not available.
Li, L. P., and Zhao, X. P. (2015). Interventional effect of dihuang yinzi on postoperative recovery of patients with spontaneous intracerebral hemorrhage. Chin. Med. Mod. Distance Educ. China 13 (8), 47–48. doi:10.3969/j.issn.1672-2779.2015.08.024
Li, Z. B., and Xiao, M. M. (2020). Observation on the effect of Changpu Yujin decoction in the treatment of acute cerebral hemorrhage after craniotomy. J. Pract. Tradit. Chin. Intern. Med. 36 (12), 1547–1548. doi: not available.
Li, L. (2015a). Influence of Huoxue ditan decoction on recovery of blood neural function in patients with hypertensive cerebral hemorrhage disease. Chin. J. Exp. Tradit. Med. Formulae 21 (04), 189–192. doi:10.13422/j.cnki.syfjx.2015040189
Li, S. J. (2015b). 52 cases of hemorrhagic apoplexy treated by activating blood and removing stasis. Henan Tradit. Chin. Med. 35 (11), 2644–2646. doi:10.16367/j.issn.1003-5028.2015.11.1135
Li, Z. W. (2015c). Clinical observation on CAIR capsule in the treatment of hydrocephalus at acute stage after cerebral hemorrhagic stroke. Master's degree. Guangzhou, China: Guangzhou University of Chinese Medicine.
Lin, X. W., Gu, Y., Wang, N., Wang, J. Y., and Liu, D. L. (2022). Therapeutic effect of xifeng Huayu tongluo prescription for acute cerebral hemorrhage patients with wind-phlegm-stasis obstruction syndrome and its influence on inflammatory factors, neural factors and blood-brain barrier. J. Guangzhou Univ. Tradit. Chin. Med. 39 (04), 756–763. doi:10.13359/j.cnki.gzxbtcm.2022.04.005
Liu, C. Y. (2018). Catalpol provides a protective effect on fibrillary Aβ1-42-induced barrier disruption in an in viro model of the blood-brain barrier. Master's degree. Nanjing, China: Nanjing University Of Chinese Medicine.
Liu, X. J., Zhang, Y. B., and Li, J. M. (2015). Traditional Chinese drugs for acute intracerebral hemorrhage: A meta-analysis of randomized controlled trials. Int. J. Tradit. Chin. Med. 37 (2), 169–177. doi:10.3760/cma.j.issn.1673-4246.2015.02.019
Liu, X. Y., Bai, L. T., Ji, P. H., Zhong, P. F., Wang, N. Y., and Ji, H. R. (2017). Research progress of hirudin. Asia-Pac. Tradit. Med. 13 (16), 50–52. doi: not available.
Liu, Y., Zhang, N., and Ai, M. (2019). Clinical effect of the method of promoting blood circulation and removing blood stasis in the treatment of hypertensive cerebral hemorrhage. Chin. J. Gerontology 39 (11), 2605–2607. doi:10.3969/j.issn.1005-9202.2019.11.011
Liu, Y. Z., and Guo, W. F. (2009). Research on the origin of the theory of stroke pathogenesis. Chin. J. Integr. Med. Cardiovasc. Cerebrovasc. Dis. 7 (6), 732–733. doi:10.3969/j.issn.1672-1349.2009.06.055
Lv, Y., Yu, J., Yu, X., Wu, C. H., and Li, Z. M. (2021). Effect of sanyu tongluo decoction( 散瘀通络汤) on IL - 17 and related factors of NF - κBp65 pathway in patients with hypertensive cerebral hemorrhage. Chin. Arch. Tradit. Chin. Med. 39 (07), 200–204. doi:10.13193/j.issn.1673-7717.2021.07.050
Ma, Q. R., Sun, J. P., Ma, J. B., Liu, G. H., Liang, l., Zhao, J., et al. (2013). Effects of safflower injection at different time points on cerebral hemorrhage in rats with peripheral nerve cells Caspase-3. China J. Pharm. Econ. (S3), 29–30. doi: not available.
Ma, Y. X., Li, G. H., Liu, J., Fan, Y. B., Huang, Y. L., Yang, D. H., et al. (2017). Effects of β-asarone on synaptic plasticity of hippocampal neurons in Alzheimer's disease rats. Guangdong Med. J. 38 (10), 1489–1492. doi:10.13820/j.cnki.gdyx.20170505.022
Moher, D., Hopewell, S., Schulz, K. F., Montori, V., Gøtzsche, P. C., Devereaux, P. J., et al. (2012). CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. Int. J. Surg. 10 (1), 28–55. doi:10.1016/j.ijsu.2011.10.001
Ni, X. J., Chen, Y. L., Cai, Y. F., Liao, W., Luo, X., Wu, D., et al. (2020). Evidence-based practice guideline on integrative medicine for stroke 2019. J. Evid. Based. Med. 20 (08), 137–152. doi:10.1111/jebm.12386
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372, n71. doi:10.1136/bmj.n71
Pang, J., Hou, J., Zhou, Z., Ren, M., Mo, Y., Yang, G., et al. (2020). Safflower yellow improves synaptic plasticity in APP/PS1 mice by regulating microglia activation phenotypes and BDNF/TrkB/ERK signaling pathway. Neuromolecular Med. 22 (3), 341–358. doi:10.1007/s12017-020-08591-6
Poon, M. T. C., Fonville, A. F., and Al-Shahi Salman, R. (2014). Long-term prognosis after intracerebral haemorrhage: Systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 85 (6), 660–667. doi:10.1136/jnnp-2013-306476
Qiu, S. S., Xue, L. F., Li, K. X., Tan, F. M., and Liu, B. (2017). Effects of panax notoginseng saponins on neuronal apoptosis and related genes in rats with intracerebral hemorrhage. J. Jinan Univ. Nat. Sci. Med. Ed. 38 (05), 387–392. doi: not available.
Qiu, Y., Li, X. B., and Gu, X. (2021). Clinical effects of Yiqi Huoxue Huayu Decoction combined with conventional treatment on patients with hypertensive intracerebral hemorrhage after minimally invasive surgery. Chin. Tradit. Pat. Med. 43 (12), 3339–3343. doi: not available.
Qureshi, A. I., Tuhrim, S., Broderick, J. P., Batjer, H. H., Hondo, H., and Hanley, D. F. (2001). Spontaneous intracerebral hemorrhage. N. Engl. J. Med. 344 (19), 1450–1460. doi:10.1056/NEJM200105103441907
Qureshi, A. I., Mendelow, A. D., and Hanley, D. F. (2009). Intracerebral haemorrhage. Lancet 373 (9675), 1632–1644. doi:10.1016/S0140-6736(09)60371-8
Schulz, K. F., Altman, D. G., and Moher, D. (2010). CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. Trials 11, 32. doi:10.1186/1745-6215-11-32
Shao, Z., Tu, S., and Shao, A. (2019). Pathophysiological mechanisms and potential therapeutic targets in intracerebral hemorrhage. Front. Pharmacol. 10, 1079. doi:10.3389/fphar.2019.01079
Song, J. N. (2000). The relationship between phlegm and phlegm and blood stasis from the perspective of biochemistry. Chin. J. Basic Med. Traditional Chin. Med. 6 (03), 40–43. doi:10.3969/j.issn.1006-3250.2000.03.013
Staykov, D., Huttner, H. B., Köhrmann, M., Bardutzky, J., and Schellinger, P. D. (2010). Novel approaches to the treatment of intracerebral haemorrhage. Int. J. Stroke 5 (6), 457–465. doi:10.1111/j.1747-4949.2010.00487.x
Sun, C., H. (2017). Clinical observation of Naoxueshu oral liquid in preventing and treating secondary brain injury after hypertensive intracerebral hemorrhage. Master's degree. Shanghai, China: Shanghai University of Traditional Chinese Medicine.
Sun, Y. M., Lou, J. T., and Huang, G. Q. (2008). Clinical study on sodium ferulate for intracerebral hemorrhage in early stage. China J. Chin. Mat. Med. 33 (21), 2545–2548. doi: not available.
Tang, X. J., Feng, Y. J., Cui, Y. M., and Cui, J. B. (2020). Research progress on clinical application of hirudo in internal medicine. J. Pract. Tradit. Chin. Intern. Med. 34 (10), 86–89. doi:10.13729/j.issn.1671-7813.z20200300
The Fourth Session of the National Cerebrovascular Conference (1996). The norm of clinical neurologic deficit score (1995). Chin. J. Neurol. 29 (06), 62–64. doi: not available.
Wang, Y., Huang, J., Ma, Y., Tang, G., Liu, Y., Chen, X., et al. (2015). MicroRNA-29b is a therapeutic target in cerebral ischemia associated with aquaporin 4. J. Cereb. Blood Flow. Metab. 35 (12), 1977–1984. doi:10.1038/jcbfm.2015.156
Wang, M. Z., Zhang, L., Jiang, W. F., Liao, W. L., Dang, C. J., and Pan, W. D. (2016a). Clinical therapeutic effect and mechanism study in the prevention and treatment of the second brain injury after hypertensive cerebral hemorrhage by benefiting Qi, removing stasis and resolving phlegm. World J. Integr. Tradit. West. Med. 11 (11), 1544–1547. doi:10.13935/j.cnki.sjzx.161118
Wang, Y., Peng, F. A. N., Xie, G. U. I., Chen, Z.-Q., Li, H.-G., Tang, T. A. O., et al. (2016b). Rhubarb attenuates blood-brain barrier disruption via increased zonula occludens-1 expression in a rat model of intracerebral hemorrhage. Exp. Ther. Med. 12 (1), 250–256. doi:10.3892/etm.2016.3330
Wei, J. Q., and Ma, J. (2017). Effect of Naoxueshu oral liquid on perfusion of peripheral hematoma after hypertensive intracerebral hemorrhage. Chin. J. Integr. Med. Cardiovasc. Cerebrovasc. Dis. 15 (22), 2909–2912. doi: not available.
Wu, W., B., Hu, C., L., Yang, Y., S., Guo, F., Q., Sun, H., Xiao, J., et al. (2007). Effects of hirudo extract liquor on tPA and PAI-1 in experimental intracerebral peri-hematoma tissues of Wistar rats. Chin. J. Neuromed. 6 (10), 993–997. doi: not available.
Wu, L., Li, Y., Wang, X., Ren, X., Zhu, H., Sun, Y., et al. (2017). A systematic review and meta-analysis on the treatment of cerebral hemorrhage with NaoXueShu oral liquid. Biomed. Res. Int. 2017,1-11, 8542576. doi:10.1155/2017/8542576
Xi, Z. Z., Xu, L. Y., and Bao, L. M. (2018). Study on the efficacy of Buyang Huanwu Decoction in the treatment of cerebral hemorrhage and its effect on IL-6, hs-CRP, TNF-α, CD62P and CD42b. Shaanxi J. Tradit. Chin. Med. 39 (12), 1674–1676. doi: not available.
Xie, J., Gao, H. C., and Zheng, X. (2016). Effect of astragaloside IV on neurological function in acute cerebral hemorrhage rats. Chin. J. Pathophysiol. 32 (10), 1905–1908. doi: not available.
Xu, Q. T. (2018). Analysis of the effect of self-made Bushen Ditan Huayu Decoction in the treatment of hypertensive cerebral hemorrhage. Shenzhen J. Integr. Tradit. Chin. West. Med. 28 (05), 77–79. doi:10.16458/j.cnki.1007-0893.2018.05.035
Xu, D., Huang, P., Yu, Z., Xing, D. H., Ouyang, S., and Xing, G. (2014). Efficacy and safety of panax notoginseng saponin therapy for acute intracerebral hemorrhage, meta-analysis, and mini review of potential mechanisms of action. Front. Neurol. 5, 274. doi:10.3389/fneur.2014.00274
Xu, Y., Fang, R., Zhou, Y., Peng, X. W., Jiang, Q. L., Tang, J., et al. (2022). A meta-analysis of randomized controlled trials of traditional Chinese medicine for removing blood stasis and dredging orifices in the treatment of hypertensive cerebral hemorrhage. Mod. Tradit. Chin. Med. Materia Medica-World Sci. Technol. 23 (12), 4389–4397. doi:10.11842/wst.20210602007
Yang, B. C. (2016). A comparative study on the dynamic change law of catalpol in different time and decoction liquid during the decoction of Rehmannia glutinosa and its effect on hemostasis. China Health Stand. Manag. 7 (10), 134–136. doi:10.3969/j.issn.1674-9316.2016.10.095
Yang, F., and Yan, Y. M. (2014). A brief discussion on stroke and phlegm stasis. Heilongjiang J. Traditional Chin. Med. 43 (03), 2–3. doi: not available.
Yang, L., Huang, Y., Cai, Y. F., Du, B. X., Chen, H. X., Lu, M., et al. (2004). Analysis on the distribution and evolution law of phlegm and blood stasis syndrome in 1418 stroke patients. Liaoning J. Traditional Chin. Med. 31 (06), 459–460. doi:10.13192/j.ljtcm.2004.06.19.yangl.012
Yu, C. X., Sun, S. A., Zhu, Y. J., and Zhang, H. J. (2017). Effect of Buyang Huanwu Decoction combined with low-dose mannitol on patients with cerebral edema after hypertensive intracerebral hemorrhage. Mod. J. Integr. Tradit. Chin. West. Med. 26 (16), 1789–1791. doi:10.3969/j.issn.1008-8849.2017.16.028
Yuan, L. X., Chen, C., Zhang, G. M., Zhou, L., Chen, Y., Cui, Y. F., et al. (2015). Clinical observation on 114 cases of cerebral hemorrhage treated by activating blood and removing stasis. Hebei J. Tradit. Chin. Med. 37 (03), 357–359. doi: not available.
Yuan, M. G., Li, J. X., Gu, H., and Guo, W. F. (2017). Protective effect and related mechanisms of rhubarb in intracerebral hemorrhage. Chin. Arch. Tradit. Chin. Med. 35 (7), 1766–1768. doi:10.13193/j.issn.1673-7717.2017.07.035
Zeng, Z. Y., Wu, H. J., Yang, H., and Zhang, B. (2022). Research progress of the theory of promoting blood circulation and removing blood stasis in the treatment of hemorrhagic stroke. J. Guizhaou Univ. Traditional Chin. Med. 44 (01), 69–73. doi:10.16588/j.cnki.issn2096-8426.2022.01.015
Zhan, H. D., Zhou, H. Y., Sui, Y. P., Du, X. L., Wang, W. H., Dai, L., et al. (2016). The rhizome of Gastrodia elata Blume - an ethnopharmacological review. J. Ethnopharmacol. 189, 361–385. doi:10.1016/j.jep.2016.06.057
Zhang, Z. Z., Zhang, B. H., and Chen, M. (2003). Experimental study of Danshen injection in preventing and treating cerebral edema in acute stage of cerebral hemorrhage. Zhejiang J. Integr. Tradit. Chin. West. Med. 13 (06), 29–31. doi: not available.
Zhang, F., Li, W. J., and Hou, T. T. (2017). Influence of activating blood to remove stasis and refreshment decoction for plasma MMP - 9 and neural function of cerebral hemorrhage patients with microinvasive evacuation of hematoma. Chin. Arch. Tradit. Chin. Med. 35 (05), 1254–1256. doi:10.13193/j.issn.1673-7717.2017.05.055
Zhang, Y., Xu, K., Hou, W., Wei, X. W., Zhang, Z., Chang, T., et al. (2020). Effects of Dihuang Yinzi on regeneration of endogenous neural stem cells and expression of Notch1 protein in rats with intracerebral hemorrhage. China J. Tradit. Chin. Med. Pharm. 35 (6), 3105–3108. doi: not available.
Zhang, K. C. (2018). Observation on the clinical curative effect of self-made Suihaihuayu Decoction in the treatment of hypertensive intracerebral hemorrhage(HICH). Master's degree. Haerbin, China: Heilongjiang University of Chinese Medicine.
Zhang, L. T., and Zhang, G. M. (2021). Study on distribution of TCM syndrome elements in hemorrhagic stroke patients. Jilin J. Tradit. Chin. Med. 41 (02), 205–208. doi:10.13463/j.cnki.jlzyy.2021.02.020
Zhang, X. Y., and Xing, Y. D. (2019). Theory of etiology and pathogenesis of apoplexy and its hierarchy. Shandong J. Tradit. Chin. Med. 38 (05), 418–421. doi:10.16295/j.cnki.0257-358x.2019.05.005
Zhao, D., Lu, Y., and Yu, G. (2021). Effects of on behavior and blood-brain barrier in Alzheimer's disease mice. J. Zhejiang Univ. Med. Sci. 50 (5), 553–560. doi:10.3724/zdxbyxb-2021-0056
Keywords: intracranial hemorrhage, traditional Chinese medicine, blood stasis, systematic review, meta-analysis
Citation: Lin W, Hou J, Han T, Zheng L, Liang H and Zhou X (2022) Efficacy and safety of traditional Chinese medicine for intracranial hemorrhage by promoting blood circulation and removing blood stasis: A systematic review and meta-analysis of randomized controlled trials. Front. Pharmacol. 13:942657. doi: 10.3389/fphar.2022.942657
Received: 12 May 2022; Accepted: 24 August 2022;
Published: 28 September 2022.
Edited by:
Jirong Yue, West China Hospital, Sichuan University, ChinaReviewed by:
Bin Yu, Nanjing University of Chinese Medicine, ChinaMonika E. Czerwińska, Medical University of Warsaw, Poland
Copyright © 2022 Lin, Hou, Han, Zheng, Liang and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huazheng Liang, aHVhemhlbmdfbGlhbmdAdG9uZ2ppLmVkdS5jbg==; Xiaoyu Zhou, eGlhb3l1emhvdTE5NzlAMTYzLmNvbQ==
 Wenjian Lin
Wenjian Lin Jingjing Hou2
Jingjing Hou2 Huazheng Liang
Huazheng Liang Xiaoyu Zhou
Xiaoyu Zhou