- 1Guangdong Key Laboratory for Research and Development of Natural Drugs, School of Pharmacy, Guangdong Medical University, Zhanjiang, China
- 2Department of Pharmacy, Qingyuan People’s Hospital, The Sixth Affiliated Hospital of Guangzhou Medical University, Qingyuan, China
- 3Key Laboratory of Traditional Chinese Medicine and New Pharmacutical Development, Department of Pharmacology, Guangdong Medical University, Dongguan, China
- 4School of Nursing, Guangdong Medical University, Dongguan, China
Multidrug resistance (MDR) is thought to be one of the main reasons for the failure of chemotherapy in cancers. ATP-binding cassette subfamily B member 1 (ABCB1) or P-glycoprotein (P-gp) and ATP-binding cassette subfamily G member 2 (ABCG2) play indispensable roles in cancer cell MDR. Sigma-2 (σ2) receptor is considered to be a cancer biomarker and a potential therapeutic target due to its high expression in various proliferative tumors. Recently, σ2 receptor ligands have been shown to have promising cytotoxic effects against cancer cells and to modulate the activity of P-glycoprotein (ABCB1) in vitro experiments, but their specific effects and mechanisms remain to be elucidated. We found that A011, a σ2 receptor ligand with the structure of 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline, showed promising cytotoxicity against breast cancer MCF-7 and adriamycin-resistant MCF-7 (MCF-7/ADR), induced apoptosis, and reversed adriamycin (ADR) and paclitaxel resistance in MCF-7/ADR cells. Furthermore, we demonstrated that A011 increased the accumulation of rhodamine 123 and mitoxantrone in MCF-7/ADR cells. A011 significantly decreased the ATPase activity of the ABCB1 and down-regulated ABCG2 protein expression. In addition, A011, administered alone or in combination with ADR, significantly inhibited tumor growth in the MCF-7/ADR tumor-bearing nude mouse model. A011 may be a potential therapeutic agent for the treatment of tumor resistance.
Introduction
Female breast cancer is one of the most common malignancies in the world. Breast cancer surpassed lung cancer to become the most prevalent cancer in 2020, with an estimated 2.3 million new cases (Sung et al., 2021). Over the past decades, tremendous progress has been made in the development of chemotherapeutic drugs. However, tumor recurrence and metastasis remain one of the major challenges in cancer treatment during the long-term course of chemotherapy (Warren, 2009). Most anti-tumor drugs inevitably show reduced drug efficacy and tumors exhibit multidrug resistance (MDR) to chemotherapeutic drugs during long-term chemotherapy, leading to tumor recurrence in patients. It has been reported that over 90% deaths in the chemotherapy population are associated with MDR (Bukowski et al., 2020). Various mechanisms have been reported to be involved in the development of MDR in tumor cells, including ATP-binding cassette (ABC) transporter family-mediated drug efflux, apoptosis down-regulation and epigenetic regulation, etc., of which the ABC transporter family is the most widely studied (Abraham et al., 2009; Rocha et al., 2018; Assaraf et al., 2019; Bukowski et al., 2020).
The ABC transporter superfamily is one of the largest families of transmembrane proteins, consisting of 49 ABC transporter members (Asif et al., 2020). Structurally, ABC transporters are characterized by a common structure: two nucleotide-binding domains (NBDs), which can bind and hydrolyze ATP, and two transmembrane domains (TMDs), which utilize the energy provided by ATP hydrolysis to transport substrates outside the cell. Functionally, most ABC transporters can transport the substrates produced during cellular metabolism (e.g., sugars, lipids, ions, peptides, amino acids and toxic components) from the cytoplasm to outside the cell membrane, and thus play an important role in maintaining normal physiological and pathological processes in the organism (Borst and Elferink, 2002; Theodoulou and Kerr, 2015). Dysregulated ABC transporters are associated with tumor resistance (Dean et al., 2001; Amawi et al., 2019). More importantly, several ABC transporters have been frequently found to be overexpressed in a variety of tumor resistant cells, such as P-glycoprotein (P-gp, ABCB1) and breast cancer resistance protein (BCRP, ABCG2). It has been found that the ABC transporters could bind and transport chemotherapeutic drugs outside the cell by utilizing the energy provided by ATP hydrolysis, resulting in lowering the concentration of intracellular drug accumulation, reducing the efficacy of chemotherapeutic drugs and the failure of tumor treatment (Amawi et al., 2019; Liu, 2019; Eckenstaler and Benndorf, 2020). Therefore, there is an urgent need to seek small molecule inhibitors targeting ABC transporters to restore the efficacy of chemotherapeutic drugs.
A lot of studies have shown that σ2 receptor may be a potential therapeutic target for tumors (Zeng and Mach, 2017; Oyer et al., 2019; Schmidt and Kruse, 2019; Chen et al., 2021). The σ2 receptor was found to be overexpressed in rapidly proliferating tumors such as lung, breast and pancreatic cancers and was identified as an important biomarker of tumor cell proliferation (Zeng et al., 2020). And σ2 receptor ligands have been found to be potential agents for tumor therapy, with the capacity to inhibit proliferation and induce apoptosis in tumor cells (Nicholson et al., 2015; Cantonero et al., 2020). In addition, the σ2 receptor ligands exhibited promising anti-tumor proliferative activity against breast cancer MCF-7/ADR cells and could inhibit the activity of ABCB1, suggesting that σ2 receptor ligands may be potential therapeutic agent in anti-tumor MDR (Azzariti et al., 2006; Abate et al., 2011).
It was reported that σ2 receptor ligands with a 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline structure were capable of modulating the activity of ABCB1 (Pati et al., 2018). Recently, we synthesized a series of σ2 ligands with 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline structural derivatives, and found many of them showed high affinity for the σ2 receptor (Sun et al., 2018). Our previous study revealed that σ2 ligand A011 with a 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline structure had high affinity for σ2 receptor and showed good antitumor activity against a variety of tumor cells, including breast cancer MCF-7, MDA-MB-231 and lung cancer A549 cells (Li et al., 2022). However, its role and mechanism in tumor resistance remains to be further investigated.
Materials and methods
Chemicals and reagents
The σ2 receptor ligand A011 was prepared as previously reported (Feng et al., 2019). KO143 was purchased from MedChemExpress (Monmouth Junction, NJ, United States). Cisplatin (DDP), adriamycin (ADR), paclitaxel, verapamil, mitoxantrone and rhodamine 123 (Rh123) were acquired from Solarbio (Beijing, China). Cell Counting Kit-8 (CCK-8) was bought from Dojindo Laboratories (Japan). Pgp-Glo™ Assay Systems were purchased from Promega (Madison, USA). Anti-P Glycoprotein antibody, ABCG2, GAPDH, Goat Anti-Rabbit IgG H&L Secondary Antibody (Alexa Fluor 488), Goat Anti-Rabbit IgG H&L Secondary Antibody and Goat Anti-Mouse IgG H&L Secondary Antibody were purchased from Abcam (Cambridge, United Kingdom). SlowFadeTM Gold antifade reagent was purchased from Thermo Fisher (MA, United States).
Cell lines
ADR-resistant MCF-7 cells (MCF-7/ADR) and DDP-resistant A549 cells (A549/DDP) were purchased from Shanghai GuYan Biotech Co., Ltd. (Shanghai, China). DDP-resistant HepG2 cells (HepG2/DDP) were preserved in our laboratory. Their parental cells were originally imported from American Type Culture Collection (ATCC, Manassas, United States) and cultured with adriamycin or cisplatin to form MCF-7/ADR cells, A549/DDP and HepG2/DDP. Cells were grown in Minimum Eagle’s medium (for MCF-7/ADR), Dulbecco’s Modified Eagle Medium (for MCF-7) and RPMI 1640 (for A549, A549/DDP, HepG2, and HepG2/DDP), respectively, containing 10% fetal bovine serum (FBS), 100 U/ml penicillin, 100 μg/ml streptomycin at 37°C, 5% CO2. MCF-7/ADR cells were maintained using 2 µM ADR. A549/DDP and HepG2/DDP cells were maintained using 2 μg/ml cisplatin. All resistant cells were grown in drug-free medium 2 weeks before experiment.
Cytotoxicity assay
The CCK-8 kit was used to determine the cytotoxicity of chemotherapeutic agents in MCF-7/ADR, A549/DDP, HepG2/DDP and their parental cells. In brief, 5 × 103 cells per well were seeded into 96-well plates with 100 μl medium overnight. Subsequently, cells were cultured with different concentrations of A011, adriamycin, cisplatin, paclitaxel and combination, respectively. After 24, 48, and 72 h incubation, 10 μl CCK-8 was added into each well and incubated for 90 min at 37°C. The absorbance was then measured at 450 nm using a microplate reader. IC50 value was calculated as previously described. Survival rate = (ODtreatment—ODblank)/(ODcontrol—ODblank) × 100%, Resistant Fold (RF) = IC50 resistant cells/IC50 parental cells, Reversal Fold (FR) = IC50 Monotherapy/IC50 Combination therapy.
Colony formation assay
MCF-7 and MCF-7/ADR cells (5 × 103 cells/well) were seeded into 6-well plates overnight. After 24 h incubation, cells were treated with A011 (0.3125, 0.625, 1.25, 2.5, 5 μM) for 24 h and cultured in drug-free medium for 10 days. During this period, cells were washed twice every other day with phosphate buffered saline (PBS) and cultured with fresh medium. Cells were then fixed with 4% paraformaldehyde for 15 min. Crystal violet was used to stain cells for 15 min. The numbers of colonies (cells >50) were counted.
Apoptosis assay
Annexin V-FITC and propidium iodide (PI) kits were used to determine the apoptotic cells induced by A011 in MCF-7/ADR and parental cells. In short, 2 × 105 cells per well were seeded into 6-well plates overnight. Different concentrations of A011 (5, 10, 20, 40 μM) was then added and incubated for 48 h. Subsequently, cells were harvested and washed twice with cold PBS before the addition of Annexin V-FITC and PI for 15 min at room temperature. Flow cytometry was used to detect the apoptotic cells.
Hoechst 33,258 fluorescent reagent was used to detect the effect of A011 on apoptosis in MCF-7/ADR and its parental cells. 2 × 105 cells per well were seeded into 6-well plates. After 24 h incubation, cells were treated with A011 (5, 10, 20 μM) for 48 h. The cells were then washed with PBS and fixed, Hoechst 33,258 was added to stain for 15 min, followed by washing with PBS and photographed under the fluorescence microscope.
Rh123 and mitoxantrone accumulation assay
Flow cytometry was used to detect the intracellular accumulation of Rh123 or mitoxantrone in MCF-7/ADR and its parental cells, with Rh123 and mitoxantrone being the fluorescent substrates for the ABCB1 and ABCG2 transporters, respectively. Briefly, 2 × 105 cells were seeded into 6-well plates. After 24 h incubation, different concentration of A011 (1.25, 2.5, 5, 10 μM), verapamil (10 μM) or KO143 (10 μM) was separately added to each well and incubated for 2 h at 37°C. Subsequently, each well was washed 3 times with PBS and incubated with Rh123 (5 μg/ml) or mitoxantrone (5 μmol/L) for 2 h at 37°C. Finally, all cells were collected and resuspended with cold PBS. Flow cytometry was applied to measure the fluorescence intensity of intracellular Rh123 and mitoxantrone. Verapamil and KO143 were used as positive agent of ABCB1 and ABCG2, respectively.
Rh123 and mitoxantrone efflux assay
Rh123 and mitoxantrone efflux were performed as previously described literature (Gao et al., 2020). In short, cells were seeded into 6-well plates (2 × 105 cells per well) overnight. Cells were then incubated with Rh123 (5 μg/ml) or mitoxantrone (5 μmol/L) for 2 h at 37°C. After 2 h incubation, cells were washed 3 times with PBS and cultured with different concentration of A011 (1.25, 2.5, 5, 10 μM), verapamil (10 μM) or KO143 (10 μM) for 0, 30, 60, 90, 120 min, respectively. Subsequently, cells were collected and washed 3 times with cold PBS. Flow cytometry was used to measure the fluorescence intensity of intracellular Rh123 and mitoxantrone, respectively.
ATPase activity of ABCB1 assay
The impact of A011 on ABCB1-mediated ATP hydrolysis was measured by the Pgp-Glo™ Assay kits. In brief, ABCB1 membranes were thawed and diluted at 4°C. Membranes were treated with various concentrations of A011 for 5 min at 37°C Mg2+ and ATP reagent was added into each well to trigger the reaction. After 40 min incubation, plates were removed to room temperature and ATP detection reagent was added to initiate luminescence for 20 min at room temperature. Subsequently, luminescence was read on a plate-reading luminometer.
Immunofluorescence assay
Immunofluorescence assay was performed to detect the influence of A011 on the intracellular localization of ABCB1 and ABCG2 in MCF-7/ADR cells. In brief, 2 × 104 cells per well were seed into 6-well plates overnight and treated with A011 (1.25, 2.5 μM). After 48 h incubation, cells were washed twice with cold PBS and fixed by 4% paraformaldehyde for 15 min. Bovine serum albumin (BSA) (2 mg/ml) was added to block proteins for 1 h, primary antibodies ABCB1 (1:200) or ABCG2 (1:200) were used to incubate proteins for 4 h at 4°C, which were subsequently blocked by second antibodies for 1 h. SlowFadeTM Gold antifade reagent was used to incubate proteins for 5 min. The fluorescence microscope was performed to collect the images.
Western blot assay
Western blot assays were applied to detect the protein expression of ABCB1 and ABCG2 after A011 treatment in MCF-7/ADR cells. Briefly, cells were cultured with or without A011 (1.25, 2.5, 5 μM) for 48 h. Radio-immune precipitation assay (RIPA) cell lysis buffer was then used to lysis cells for 30 min on the ice. The total protein concentrations were normalized using the BCA protein assay kit. Subsequently, proteins were load and separated by SDS-PAGE kits, following transferred into polyvinylidene difluoride (PVDF) membrane and blocked by non-fat milk. Primary antibodies (ABCB1 1:1,000, ABCG2 1:1,000) were incubated for overnight at 4°C. Horseradish peroxidase (HRP)-conjugated Mouse or Rabbit antibody was used to co-incubate with PVDF membrane for 1 h at room temperature. The protein bands were visualized by Immobilon Western HRP Substrate Kits and the relative protein expression level was analysis by ImageJ software.
Mouse xenograft assay
The MCF-7/ADR cells inoculated nude mice xenograft model was used for in vivo studies. The BALB/c female nude mice (4–6 weeks old, weighting 16–18 g) were bought from Guangdong Medical Laboratory Animal Centre (Guangdong, China). 1 × 107 cells were injected subcutaneously into the right flank of each nude mouse. When the tumor volume reached 100 mm3, nude mice were randomly divided into five groups (6 mice in each group):(1) Control (saline), 2) A011 (1 mg/kg), 3) ADR (5 mg/kg), 4) A011 (5 mg/kg), 5) A011 (1 mg/kg) plus ADR (5 mg/kg) (A011 + ADR). The drugs were administered intraperitoneally every 3 days for 21 days and the body weight and tumor volume (V) of nude mice were measured according to the following formula: V = 0.5a × b2 [“a” is the length (mm) and “b” is the width (mm)]. The nude mice were killed by spinal dislocation and the tumor tissues were dissected and weighed. The liver, kidney and tumor samples from nude mice were fixed and preserved. All animal experiments complied with the China Animal Welfare Guide. The protocol was reviewed and approved by the Experimental Animal Research Committee of Guangdong Medical University.
Histological analysis
The above tissue samples were dehydrated in ethanol, embedded in paraffin and sectioned with a microtome (4 μm). Sections were stained with hematoxylin and eosin for hematoxylin-eosin (HE) staining and terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labelling (TUNEL) staining, respectively. The immunohistochemistry (IHC) staining was performed with anti-ABCG2 or anti-ABCB1 antibody. The treated samples were observed under a microscope and photographed and the images were analyzed.
Statistical analysis
All experiments were conducted in at least three independent experiments. All data were analyzed using GraphPad Prism’s one-way ANOVA and shown as mean ± SD. Differences were considered statistically significant when the p value was less than 0.05.
Results
Cytotoxicity, resistance fold and reversal effect of A011
We determined the cytotoxicity of A011 and positive drugs ADR, cisplatin and paclitaxel on three drug-resistant cells MCF-7/ADR, A549/DDP and HepG2/DDP and their parental cells by CCK-8 kits. ADR, cisplatin and paclitaxel showed good antitumor effects on MCF-7, A549, and HepG2 cells, with significantly decreased cytotoxic effects in MCF-7/ADR, A549/DDP, and HepG2/DDP cells, with RF value mostly >5, indicating that the 3 cell lines were multidrug resistant. In contrast, the IC50 values of A011 were 3.79 ± 0.13 μM, 5.35 ± 0.42 μM, RF value 1.41, 4.59 ± 0.35 μM, 7.74 ± 0.32 μM, RF value 1.69, 4.07 ± 0.25 μM, 8.29 ± 0.04 μM, RF value 2.04 for MCF-7, MCF-7/ADR, A549, A549/DDP, HepG2 and HepG2/DDP cells, respectively (Table 1). A011 showed similar anti-proliferative effects on drug-resistant cells and their parental cells, indicating that A011 had excellent anti-tumor MDR effects (Figure 1A).
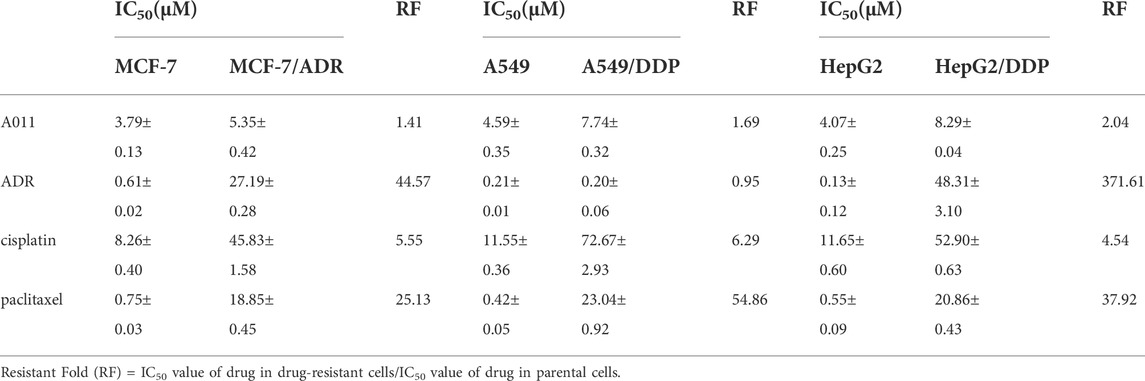
TABLE 1. IC50 values and resistant folds (RF) of A011, adriamycin (ADR), cisplatin (DDP) and paclitaxel on MCF-7/ADR, A549/DDP, HepG2/DDP and their parental cells after 48 h administration.
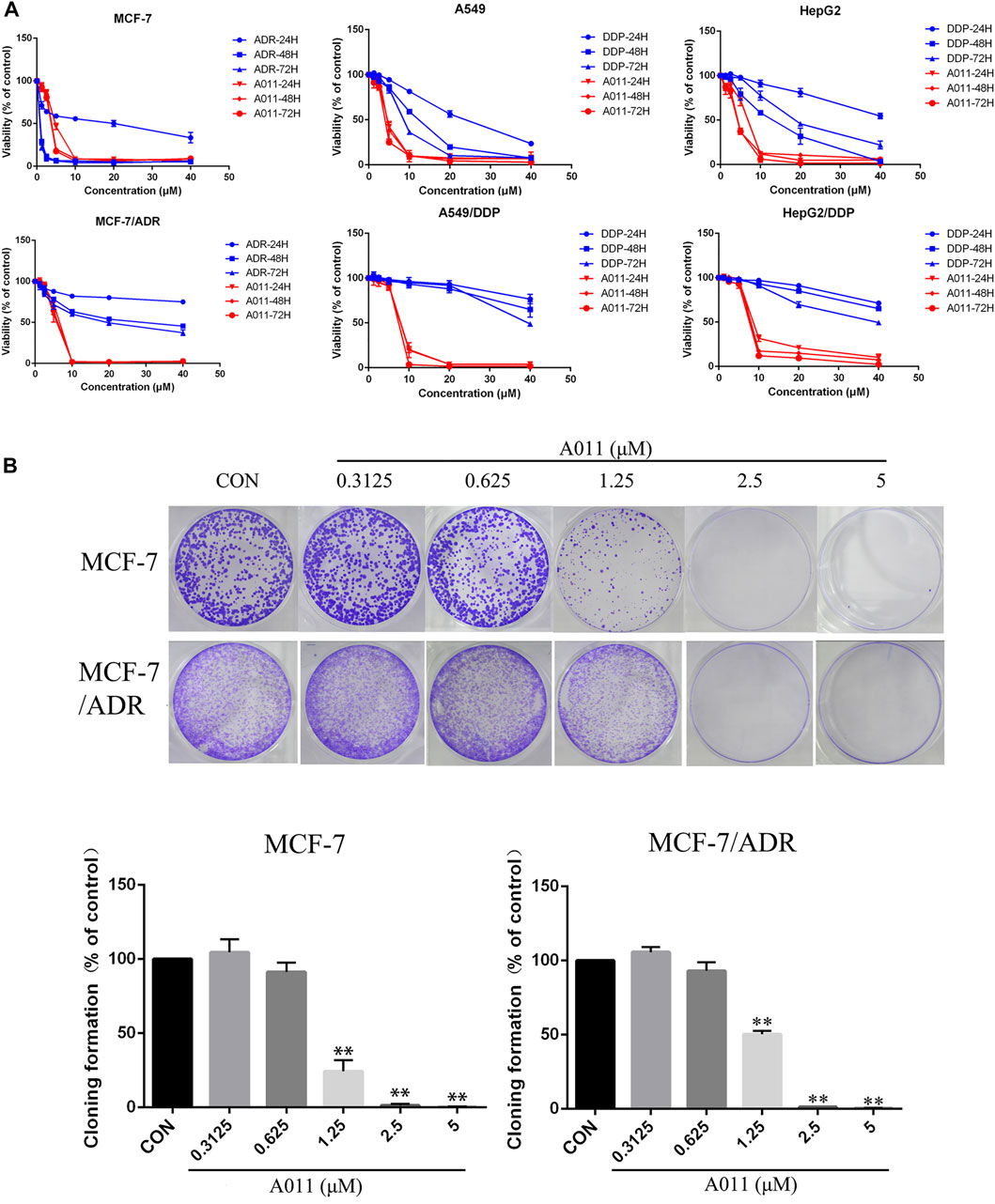
FIGURE 1. Effect of A011 on the viability of three drug-resistant cells MCF-7/ADR, A549/DDP, HepG2/DDP and their parental cells. (A) The cytotoxic effects of A011 on MCF-7/ADR, A549/DDP, HepG2/DDP and their parental cells, respectively. Cells were treated with a range of concentrations of A011, adriamycin (ADR) or cisplatin (DDP) for 24, 48 and 72 h. (B) Effect of A011 on the inhibition of clonogenic capacity of MCF-7 and MCF-7/ADR cells. Data represent mean ± SD of three different experiments. *p < 0.05, **p < 0.01 vs. Control.
Based on the results of the CCK-8 assay, A011 had a stronger anti-proliferative effect in MCF-7/ADR cells relative to A549/DDP and HepG2/DDP cells, and therefore MCF-7/ADR cells were used as the subsequent experimental cell line. Moreover, A011 showed >90% survival of MCF-7/ADR cells at 1.25 and 2.5 μM concentrations and were therefore selected as the concentration for combination treatment. The results showed that A011 at 1.25 and 2.5 μM concentrations significantly increased the cytotoxicity of ADR, cisplatin and paclitaxel in MCF-7/ADR cells. No sensitization was observed in parental cells. And the inhibition effect of A011 combined with ADR was superior compared with that of ABCB1 inhibitor verapamil and ABCG2 inhibitor KO143 combined with ADR (p < 0.05) (Table 2). In addition, A011 significantly inhibited the clonogenic ability of MCF-7/ADR cells and their parental cells (Figure 1B). These results suggested that A011 has excellent anti-tumor MDR activity and could enhance the sensitivity of MCF-7/ADR cells to ADR, cisplatin and paclitaxel.
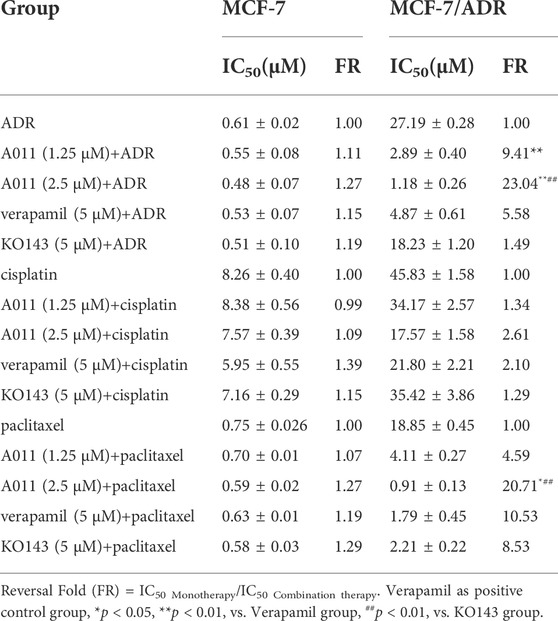
TABLE 2. Reversal of A011 on resistance of adriamycin (ADR), cisplatin and paclitaxel in MCF-7/ADR and MCF-7 cells.
A011 induced apoptosis in MCF-7/ADR and its parental cells.
Hoechst 33,258 staining showed that MCF-7/ADR and MCF-7 cells exhibited increased cytoplasmic density, nuclear consolidation, nuclear membrane nucleolus fragmentation and increased apoptotic vesicles in a dose-dependent manner after A011 treatment compared to cells from control group with intact cell structure (Figure 2A). Furthermore, the flow cytometry results showed that A011 (5, 10, 20, and 40 μM) significantly induced apoptosis in MCF-7/ADR and MCF-7 cells, with apoptosis rate of 5.20% ± 0.55%, 25.15% ± 9.99%, 95.6% ± 6.35%, 99.47% ± 0.15% for MCF-7/ADR cells and 9.47% ± 1.25%, 11.87% ± 1.84%, 43.19% ± 8.81% and 87.51% ± 2.70% for MCF-7 cells (Figure 2B). The results indicated A011 could inhibit tumor cells growth by inducing cell apoptosis in MCF-7 and MCF-7/ADR.
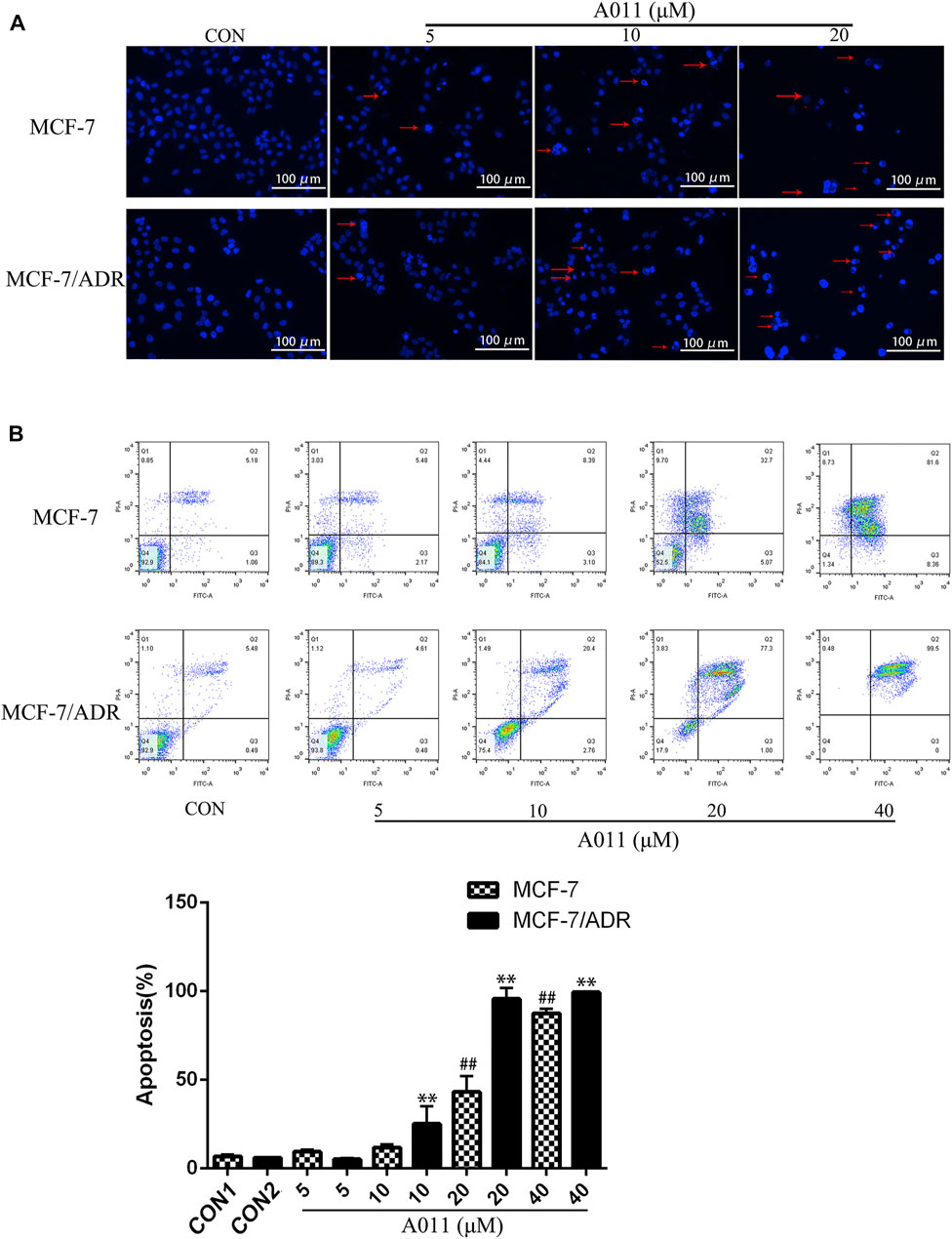
FIGURE 2. Effect of A011 on MCF-7/ADR and MCF-7 cell apoptosis. (A) Results of Hoechst 33,258 staining of MCF-7/ADR cells and their parental cells after A011 intervention. The red arrows indicated apoptotic vesicles. (B) Flow cytometry results of A011 on MCF-7/ADR cells and their parental cell apoptosis. Data represent mean ± SD of three different experiments. ##p < 0.01 vs. CON1; **p < 0.01 vs. CON2.
A011 significantly increased the accumulation and decreased the efflux of Rh123 and mitoxantrone in MCF-7/ADR cells
To investigate the mechanism by which A011 reversed drug resistance in tumor cells, we determined the effect of A011 on the function of ABCB1 and ABCG2 transporters. Verapamil and KO143 were inhibitors of the ABCB1 and ABCG2 transporters respectively, and were able to inhibit the transport of substrates outside the cell membrane by the ABCB1 and ABCG2 transporters, so they were used as positive control. In addition, Rh123 and mitoxantrone were fluorescent substrates for the ABCB1 and ABCG2 transporters respectively, and were able to be quantified by flow cytometry. The results showed that the accumulation of Rh123 or mitoxantrone in MCF-7/ADR cells was significantly lower than their accumulation in parental cells. A011 significantly increased the accumulation of Rh123 and mitoxantrone in MCF-7/ADR cells. Moreover, the intracellular accumulation of Rh123 was higher in the A011 group compared to the verapamil group (Figures 3A,B). In addition, efflux experiments showed that the amount of Rh123 or mitoxantrone in MCF-7/ADR cells was significantly reduced during the time course, whereas the addition of A011 significantly inhibited the efflux of Rh123 or mitoxantrone, suggesting that the increased accumulation of Rh123 and mitoxantrone in MCF-7/ADR cells was due to inhibition of their efflux (Figures 4A,B). These results indicated that A011 could inhibit the transport function of ABCB1 and ABCG2 transporters.
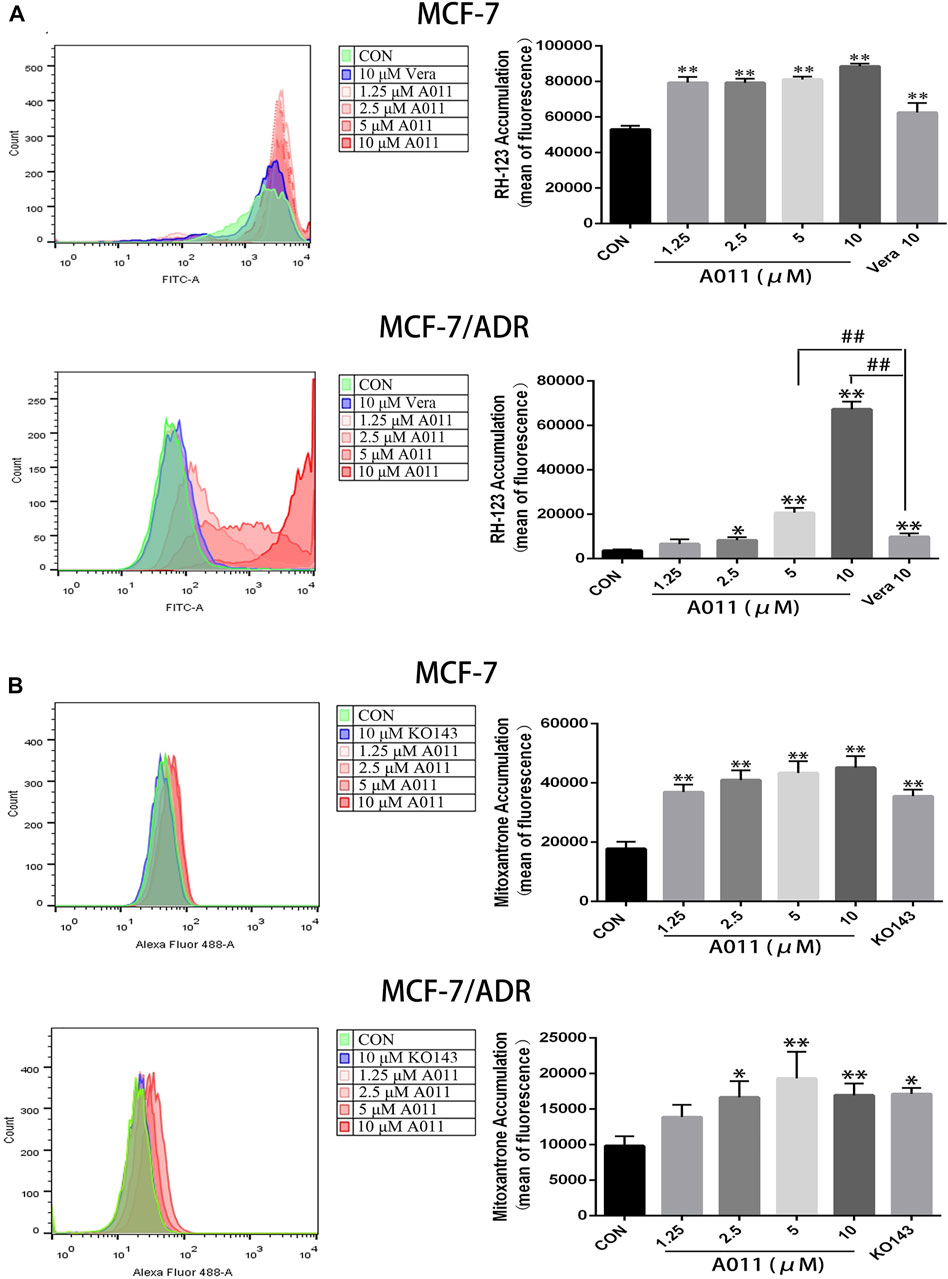
FIGURE 3. Effect of A011 on the transport function of ABCB1 and ABCG2 transporters. (A) A011 increased the accumulation of Rh123 in MCF-7/ADR and parental cells. (B) A011 increased the accumulation of mitoxantrone in MCF-7/ADR and parental cells. Verapamil (vera) and KO143 as a positive control group. Data represent mean ± SD of three different experiments. *p < 0.05, **p < 0.01 vs. CON.
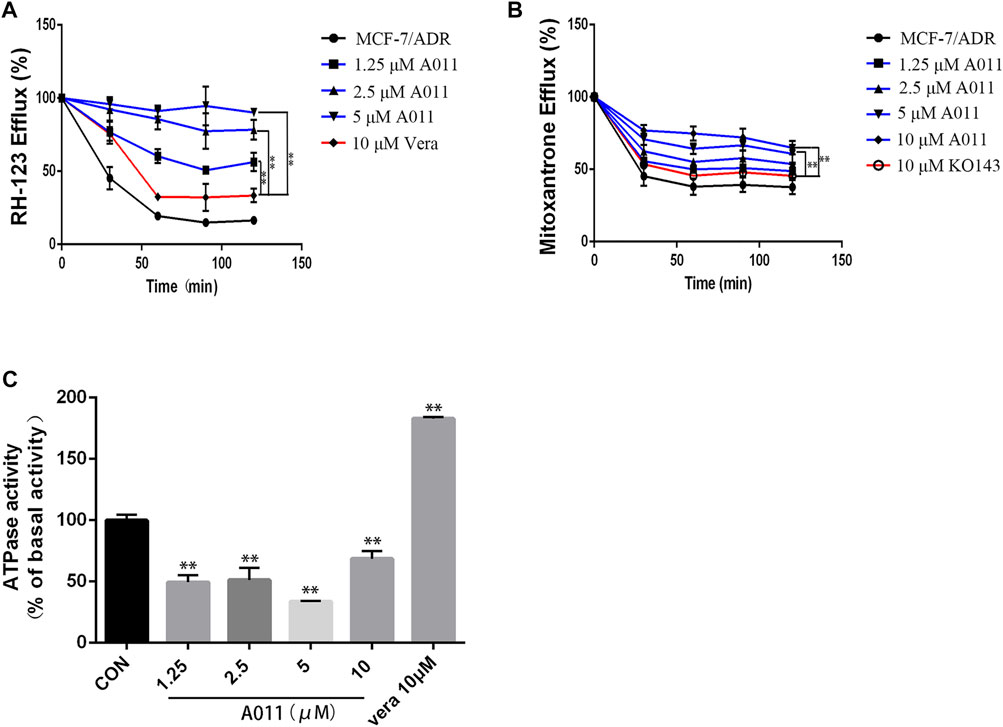
FIGURE 4. Effect of A011 on the transport function of ABCB1 and ABGC2 transporters. (A) A011 decreased the efflux of Rh123 in MCF-7/ADR cells. (B) A011 decreased the efflux of mitoxantrone in MCF-7/ADR cells. (C) A011 decreased the ATPase activity of the ABCB1 transporter. Verapamil (vera) and KO143 as a positive control group. Data represent mean ± SD of three different experiments. **p < 0.01 vs. CON.
A011 significantly decreased the ATPase activity of the ABCB1 transporter
To further investigate the role of A011 on the function of the ABCB1 transporter, we measured the effect of A011 on the ATPase activity of the ABCB1 transporter. The results showed that verapamil increased the activity of ABCB1 transporter ATPase, which was in agreement with the description of previous studies (Ledwitch et al., 2016). In contrast, A011 could significantly decrease the activity of ABCB1 transporter ATPase compared to the control group, indicating that A011 could inhibit the transport function of ABCB1 transporter by inhibiting the activity of ABCB1 transporter ATPase (Figure 4C).
A011 Decreased ABCG2 Protein Expression without Altering ABCB1 Protein Expression and Localization of ABCG2 and ABCB1 Proteins in MCF-7/ADR Cells
Given reducing the expression of transporter proteins and altering their localization in cells is one of the mechanisms to overcome MDR, we further investigated the effect of A011 on ABCB1 and ABCG2 proteins in MCF-7/ADR cells by immunofluorescence and western blot. Immunofluorescence assay showed that A011 did not alter the localization of ABCB1 and ABCG2 proteins in MCF-7/ADR cells (Figures 5A,B). And western blot showed that A011 down-regulated ABCG2 protein expression and did not alter ABCB1 protein expression (Figure 5C).
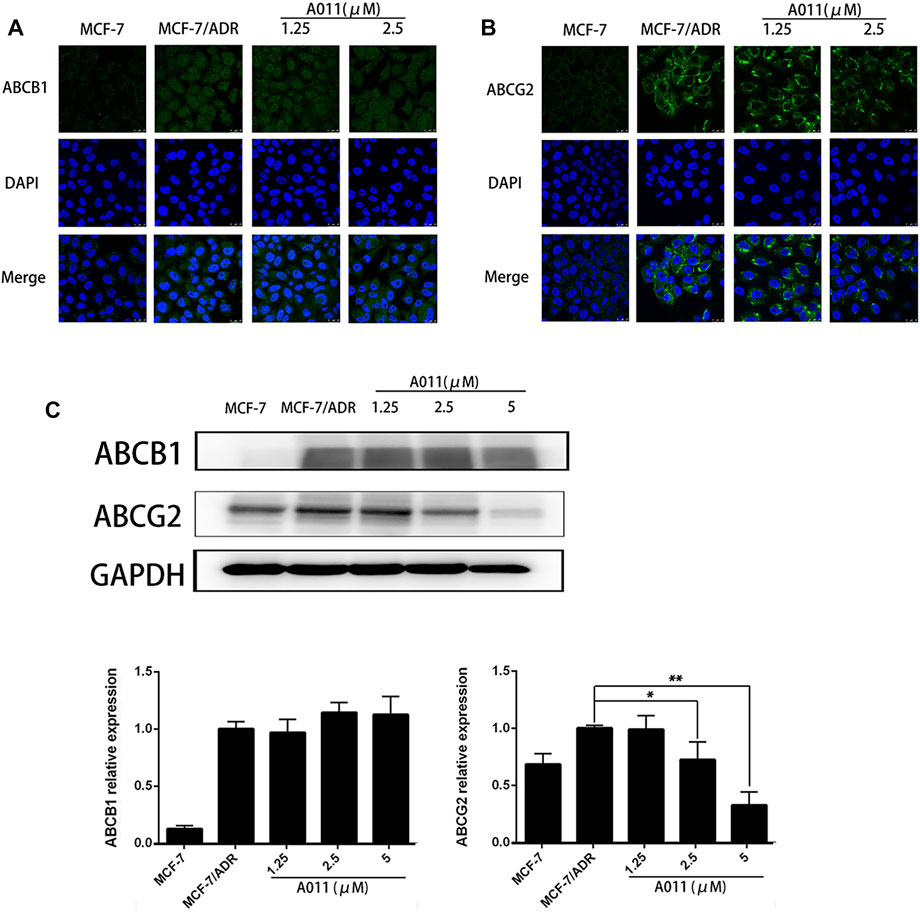
FIGURE 5. Effect of A011 on the expression and intracellular localization of ABCB1 and ABCG2 proteins. (A) A011 did not alter the localization of ABCB1 protein in MCF-7/ADR cells. Cells were treated with A011 for 48 h and then detected by immunofluorescence assay. (B) A011 did not alter the localization of ABCG2 protein in MCF-7/ADR cells. (C) A011 decreased ABCG2 protein expression in MCF-7/ADR cells without altering ABCB1 protein expression. Cells were treated with A011 for 48 h and then detected by western blot assay. Data represent mean ± SD of three different experiments. *p < 0.05, **p < 0.01 vs. Control. A011 Inhibited the Growth of MCF-7/ADR Xenograft Model in vivo.
To evaluate the anti-tumor MDR activity of A011 in vivo, we established a xenograft model with MCF-7/ADR cells in nude mice. A011 (1 mg/kg) or ADR (5 mg/kg) alone showed low growth inhibitory activity against MCF-7/ADR tumors, with growth inhibition rates of 6.94% and 22.58%, respectively. However, when ADR (5 mg/kg) was co-administered with A011 (1 mg/kg), the anti-tumor activity of ADR was significantly enhanced with the growth inhibition rate of 58.43%. A011 (5 mg/kg) alone showed good anti-tumor activity against MCF-7/ADR tumors with the growth inhibition rate of 43.13% (Figures 6A–C). In addition, there was no significant change in body weight in the A011 group compared to the saline group (Figure 6D). These results suggested that A011 alone or in combination had promising anti-tumor MDR activity and was well tolerated in vivo.
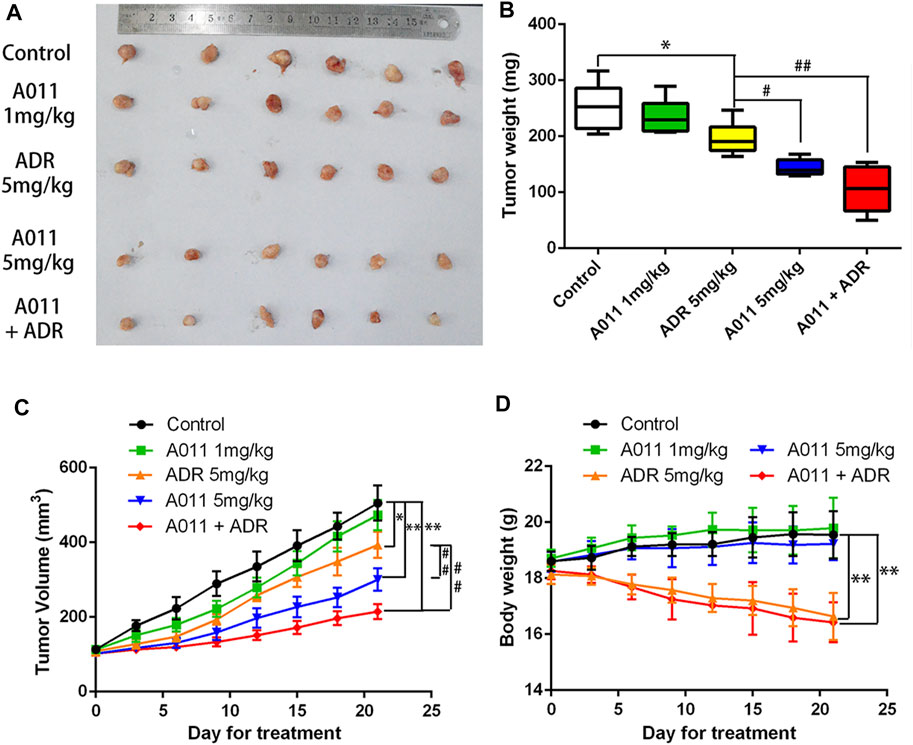
FIGURE 6. A011 inhibited the growth of MCF-7/ADR xenograft model in vivo. MCF-7/ADR cells were injected subcutaneously into the right flank of BALB/c-nu/nu nude mice. (A) Photographs of tumors. (B) Mean tumor weight was calculated for each group of tumors after dissection of nude mice. (C) Change in tumor volume over the 21 days treatment period. (D) Change in body weight of nude mice during the 21-days treatment period. Nude mice in Control group treated with saline. Nude mice in the A011 + ADR group were treated with A011 (1 mg/kg) in combination with ADR (adriamycin) (5 mg/kg), while the other groups were treated as depicted above. ADR: adriamycin. Data represent mean ± SD of three different experiments. *p < 0.05, **p < 0.01 vs. Control. #p < 0.05, ##p < 0.01 vs. ADR (5 mg/kg). A011 Induced Apoptosis and Downregulated ABCG2 Protein Expression in MCF-7/ADR Tumor Cells without Significant Toxicity to Liver and Kidney
To evaluate the effects of A011 in liver, kidney and tumor tissues in nude mice, we performed HE tissue staining, TUNEL staining and immunohistochemistry experiments respectively. HE staining of the liver and kidney showed that there was no difference between all treatment groups and control group, except for a small amount of inflammatory cell infiltration in the ADR (5 mg/kg) and A011 combined with ADR groups. In HE staining of tumor tissue, approximately 60% and 70% of tumor cells were necrotic in A011 (5 mg/kg) and A011 combined with ADR respectively, compared to 40% in the control group (Figure 7A). TUNEL staining showed that administration of A011 (5 mg/kg) alone or A011 (1 mg/kg) in combination with ADR (5 mg/kg) significantly induced an increase in MCF-7/ADR tumor apoptosis (Figure 7B). Immunohistochemistry of ABCG2 showed dark brown staining of ABCG2 protein in the saline and ADR (5 mg/kg) groups. As the concentration of A011 increased, the expression of ABCG2 was down-regulated (Figure 7C). A011 did not alter the expression of ABCB1 (Supplementary Figure S1). Above results suggest that A011 could induce apoptosis and down-regulate ABCG2 protein expression in MCF-/ADR tumor cells in vivo, without significant toxicity to liver and kidney tissues.
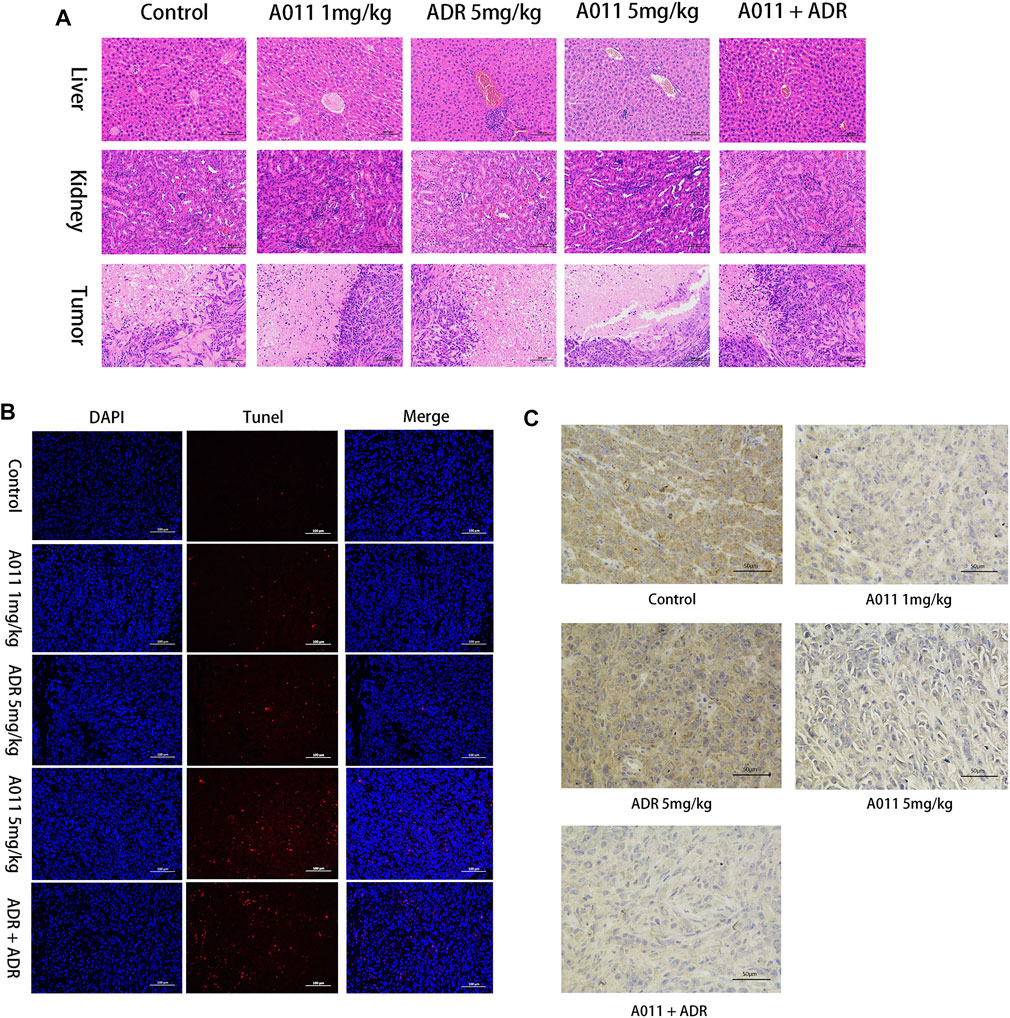
FIGURE 7. Histological analysis results. (A) HE staining of liver, kidney and tumor tissue (200×). (B) TUNEL staining of each group of tumor tissue. (C) Immunohistochemistry of ABCG2 protein in each group of tumor tissues. ADR: adriamycin. Scale bar = 100 μm or 50 μm.
Discussion
Intrinsic MDR and acquired MDR are two of the major barriers to tumor treatment, seriously threatening the survival and affecting the lives of cancer patients (Zheng, 2017; Al-Akra et al., 2019). Due to the narrow therapeutic window of most chemotherapeutic agents, the emergence of tumor MDR has greatly limited the clinical use of chemotherapeutic agents, therefore it is particularly important for cancer treatment to overcome tumor MDR.
The σ2 receptor has been found to be highly expressed in rapidly proliferating tumors such as breast cancer and is considered to be one of the potential targets for the treatment of tumors (Huang et al., 2014). Most of the σ2 ligands not only show high affinity for the σ2 receptor but also exhibit excellent anti-tumor activity (Georgiadis et al., 2017; Sun et al., 2018; Liu et al., 2019), and those with a 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline structure could also interact with ABCB1 (Pati et al., 2015). Moreover, a few σ2 receptor agonists were found to be collateral sensitive and their anti-proliferative activity was stronger in cells with high P-gp expression than in P-gp negative cells, suggesting that σ2 receptor ligands may have promising activity in drug-resistant tumors (Niso et al., 2013; Abatematteo et al., 2021). In this study, we found that σ2 ligand A011 showed significant cytotoxic activity in three tumor cell lines MCF-7, A549 and HepG2 cells and generally showed no decrease in cytotoxic activity in drug-resistant cell lines MCF-7/ADR, A549/DDP and HepG2/DDP cells. In addition, A011 significantly increased the sensitivity of MCF-7/ADR cells to ADR at concentrations of 1.25 and 2.5 μM. And in vivo experiments, A011 (5 mg/kg) alone or A011 (1 mg/kg) co-administered with ADR showed promising anti-tumor activity, significantly inhibiting the growth of MCF-7/ADR tumors without significant toxicity, suggesting that A011 has the potential to overcome MDR.
Apoptosis is a form of programmed cell death that is co-regulated by multiple genes with important roles in maintaining the homeostasis of the internal environment and controlling cell proliferation (Goldar et al., 2015). Dysregulation of apoptosis is one of the hallmarks of cancer and is associated not only with tumorigenesis and progression, but also with tumor resistance to chemotherapeutic agents (Pistritto et al., 2016). We found that A011 could dose-dependently induce apoptosis in MCF-7/ADR and its parental cells, and the apoptosis induction of A011 was better in MCF-7/ADR than MCF-7 cells. In a MCF-7/ADR xenograft model, A011 (5 mg/kg) was able to induce an increased apoptosis relative to the control group, suggesting that A011 may also promote cell death by inducing MCF-7/ADR apoptosis.
ABC transporters have been found to be relatively highly expressed in drug-resistant tumors and able to transport intracellular chemotherapeutic agents to the extracellular compartment by relying on the energy provided by ATP hydrolysis, thereby mediating the resistance of tumor cells to chemotherapeutic agents (Choi and Yu, 2014; Beis, 2015). To elucidate the mechanism that A011 overcame MDR in MCF-7/ADR cells, we examined the activity and protein expression of the ABCB1 and ABCG2 transporters. Our results showed that A011 could inhibit ABCB1 transport function and ATPase activity, but had no effect on its protein expression. In addition, it was first discovered that A011 also inhibited the transport function and protein expression of ABCG2, which was further validated in ABCG2 protein immunohistochemical assay in vivo. Besides inhibiting ABC transporter activity and protein expression, ABC transporters as transmembrane proteins, altering their localization in cells is also part of the strategy to inhibit ABC transporter-mediated MDR (Zhao et al., 2019). However, A011 did not affect the localization of the ABCB1 and ABCG2 transporters in MCF-7/ADR cells. These results suggested that A011 could inhibit the function of ABCB1 and ABCG2 transporters and reduce the expression of ABCG2 protein, thereby overcoming MDR. However, the mechanisms of A011 inhibiting ATPase activity and expression of ABCG2 protein remain to be clarified.
A variety of σ2 ligands are currently being developed for clinical diagnosis and cancer treatment with PET imaging of tumors. Phase I clinical trial results for the σ2 radioligand [18F]ISO-1 showed that [18F]ISO-1 uptake values correlated with tumor Ki-67 (a gold standard proliferation biomarker) and are expected to be used for in vivo measurement of tumor proliferation status (Dehdashti et al., 2013). Studies have shown that a lot of selective σ2 receptor ligands displayed cytotoxic effects on a variety of human cancer cells, and inhibited tumor growth (Asong et al., 2019; Liu et al., 2019). We previously found that A011 was able to increase intracellular ROS and Ca2+ levels in MCF-7 cells and induced endoplasmic reticulum stress and autophagy (Li et al., 2022). In addition, the study showed that A011 was well tolerated and had no significant toxicity to liver and kidney tissues. These results provided further insight into the pharmacological role of the σ2 receptor and A011 may be a candidate for cancer treatment either alone or in combination with other anticancer agents.
Conclusion
In this study, we elucidated that the σ2 ligand A011, containing a 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline structure, exhibited excellent anti-breast cancer MDR activity both in vivo and in vitro, either alone or in combination with ADR. A011 demonstrated anti-MDR activity by inhibiting the transporting function of ABCB1 and ABCG2 transporters and thus was a potential therapeutic agent for the treatment of tumor resistance.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was reviewed and approved by the Experimental Animal Research Committee of Guangdong Medical University.
Author contributions
DX, ZZ, YH, and CZ designed the research. ZZ, SL, HZ, HL, CZ, and JL carried out the experiments and performed data analysis. DX, ZZ, YH, and CZ wrote and revised the manuscript. All of the authors have read and approved the final manuscript.
Funding
The work was supported by Discipline Construction Project of Guangdong Medical University (4SG22002G); Key Projects of Universities in Guangdong Province (2019KZDXM056).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.952980/full#supplementary-material
References
Abate, C., Niso, M., Contino, M., Colabufo, N. A., Ferorelli, S., Perrone, R., et al. (2011). 1-Cyclohexyl-4-(4-arylcyclohexyl)piperazines: Mixed σ and human Δ(8)-Δ(7) sterol isomerase ligands with antiproliferative and P-glycoprotein inhibitory activity. ChemMedChem 6, 73–80. doi:10.1002/cmdc.201000371
Abatematteo, F. S., Niso, M., Lacivita, E., and Abate, C. (2021). σ2 receptor and its role in cancer with focus on a MultiTarget directed ligand (MTDL) approach. Molecules 26, 3743. doi:10.3390/molecules26123743
Abraham, J., Edgerly, M., Wilson, R., Chen, C., Rutt, A., Bakke, S., et al. (2009). A phase I study of the P-glycoprotein antagonist tariquidar in combination with vinorelbine. Clin. Cancer Res. 15, 3574–3582. doi:10.1158/1078-0432.CCR-08-0938
Al-Akra, L., Bae, D. H., Leck, L. Y. W., Richardson, D. R., and Jansson, P. J. (2019). The biochemical and molecular mechanisms involved in the role of tumor micro-environment stress in development of drug resistance. Biochim. Biophys. Acta. Gen. Subj. 1863, 1390–1397. doi:10.1016/j.bbagen.2019.06.007
Amawi, H., Sim, H. M., Tiwari, A. K., Ambudkar, S. V., and Shukla, S. (2019). ABC transporter-mediated multidrug-resistant cancer. Adv. Exp. Med. Biol. 1141, 549–580. doi:10.1007/978-981-13-7647-4_12
Asif, M., Usman, M., Ayub, S., Farhat, S., Huma, Z., Ahmed, J., et al. (2020). Role of ATP-binding cassette transporter proteins in CNS tumors: Resistance- based perspectives and clinical updates. Curr. Pharm. Des. 26, 4747–4763. doi:10.2174/1381612826666200224112141
Asong, G., Zhu, X. Y., Bricker, B., Andey, T., Amissah, F., Lamango, N., et al. (2019). New analogs of SYA013 as sigma-2 ligands with anticancer activity. Bioorg. Med. Chem. 27, 2629–2636. doi:10.1016/j.bmc.2019.04.012
Assaraf, Y. G., Brozovic, A., Gonçalves, A. C., Jurkovicova, D., Line, A., Machuqueiro, M., et al. (2019). The multi-factorial nature of clinical multidrug resistance in cancer. Drug resist. updat. 46, 100645. doi:10.1016/j.drup.2019.100645
Azzariti, A., Colabufo, N. A., Berardi, F., Porcelli, L., Niso, M., Simone, G. M., et al. (2006). Cyclohexylpiperazine derivative PB28, a Sigma2 agonist and Sigma1 antagonist receptor, inhibits cell growth, modulates P-glycoprotein, and synergizes with anthracyclines in breast cancer. Mol. Cancer Ther. 5, 1807–1816. doi:10.1158/1535-7163.MCT-05-0402
Beis, K. (2015). Structural basis for the mechanism of ABC transporters. Biochem. Soc. Trans. 43, 889–893. doi:10.1042/BST20150047
Borst, P., and Elferink, R. O. (2002). Mammalian ABC transporters in health and disease. Annu. Rev. Biochem. 71, 537–592. doi:10.1146/annurev.biochem.71.102301.093055
Bukowski, K., Kciuk, M., and Kontek, R. (2020). Mechanisms of multidrug resistance in cancer chemotherapy. Int. J. Mol. Sci. 21, 3233. doi:10.3390/ijms21093233
Cantonero, C., Camello, P. J., Abate, C., Berardi, F., Salido, G. M., Rosado, J. A., et al. (2020). NO1, a new Sigma 2 receptor/TMEM97 fluorescent ligand, downregulates SOCE and promotes apoptosis in the triple negative breast cancer cell lines. Cancers 12, 257. doi:10.3390/cancers12020257
Chen, A. F., Ma, W. H., Xie, X. Y., and Huang, Y. S. (2021). Sigma-2 receptor as a potential drug target. Curr. Med. Chem. 28, 4172–4189. doi:10.2174/0929867327666200902172615
Choi, Y. H., and Yu, A. M. (2014). ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr. Pharm. Des. 20, 793–807. doi:10.2174/138161282005140214165212
Dean, M., Hamon, Y., and Chimini, G. (2001). The human ATP-binding cassette (ABC) transporter superfamily. J. Lipid Res. 42, 1007–1017. doi:10.1016/s0022-2275(20)31588-1
Dehdashti, F., Laforest, R., Gao, F., Shoghi, K. I., Aft, R. L., Nussenbaum, B., et al. (2013). Assessment of cellular proliferation in tumors by PET using 18F-ISO-1. J. Nucl. Med. 54, 350–357. doi:10.2967/jnumed.112.111948
Eckenstaler, R., and Benndorf, R. A. (2020). 3D Structure of the Transporter ABCG2-What's new? Br. J. Pharmacol. 177, 1485–1496. doi:10.1111/bph.14991
Feng, Y., Xie, X. Y., Yang, Y. Q., Sun, Y. T., Ma, W. H., Zhou, P. J., et al. (2019). Synthesis and evaluation of pyrimidoindole analogs in umbilical cord blood ex vivo expansion. Eur. J. Med. Chem. 174, 181–197. doi:10.1016/j.ejmech.2019.04.042
Gao, L., Zhao, P., Li, Y., Yang, D., Hu, P., Li, L., et al. (2020). Reversal of P-Glycoprotein-Mediated multidrug resistance by novel curcumin analogues in paclitaxel-resistant human breast cancer cells. Biochem. Cell Biol. 98, 484–491. doi:10.1139/bcb-2019-0377
Georgiadis, M. O., Karoutzou, O., Foscolos, A. S., and Papanastasiou, I. (2017). Sigma receptor (σR) ligands with antiproliferative and anticancer activity. Molecules 22, 1408. doi:10.3390/molecules22091408
Goldar, S., Khaniani, M. S., Derakhshan, S. M., and Baradaran, B. (2015). Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian pac. J. Cancer Prev. 16, 2129–2144. doi:10.7314/apjcp.2015.16.6.2129
Huang, Y. S., Lu, H. L., Zhang, L. J., and Wu, Z. (2014). Sigma-2 receptor ligands and their perspectives in cancer diagnosis and therapy. Med. Res. Rev. 34, 532–566. doi:10.1002/med.21297
Ledwitch, K. V., Gibbs, M. E., Barnes, R. W., and Roberts, A. G. (2016). Cooperativity between verapamil and ATP bound to the efflux transporter P-glycoprotein. Biochem. Pharmacol. 118, 96–108. doi:10.1016/j.bcp.2016.08.013
Li, Y., Xie, X., Liao, S., Zeng, Z., Li, S., Xie, B., et al. (2022). A011, a novel small-molecule ligand of σ2 receptor, potently suppresses breast cancer progression via endoplasmic reticulum stress and autophagy. Biomed. Pharmacother. 152, 113232. doi:10.1016/j.biopha.2022.113232
Liu, C. C., Yu, C. F., Wang, S. C., Li, H. Y., Lin, C. M., Wang, H. H., et al. (2019). Sigma-2 receptor/TMEM97 agonist PB221 as an alternative drug for brain tumor. BMC Cancer 19, 473. doi:10.1186/s12885-019-5700-7
Liu, X. (2019). Transporter-mediated drug-drug interactions and their significance. Adv. Exp. Med. Biol. 1141, 241–291. doi:10.1007/978-981-13-7647-4_5
Nicholson, H., Comeau, A., Mesangeau, C., McCurdy, C. R., and Bowen, W. D. (2015). Characterization of CM572, a selective irreversible partial agonist of the sigma-2 receptor with antitumor activity. J. Pharmacol. Exp. Ther. 354, 203–212. doi:10.1124/jpet.115.224105
Niso, M., Abate, C., Contino, M., Ferorelli, S., Azzariti, A., Perrone, R., et al. (2013). Sigma-2 receptor agonists as possible antitumor agents in resistant tumors: Hints for collateral sensitivity. ChemMedChem 8, 2026–2035. doi:10.1002/cmdc.201300291
Oyer, H. M., Sanders, C. M., and Kim, F, J. (2019). Small-molecule modulators of Sigma1 and sigma2/TMEM97 in the context of cancer: Foundational concepts and emerging themes. Front. Pharmacol. 10, 1141. doi:10.3389/fphar.2019.01141
Pati, M. L., Abate, C., Contino, M., Ferorelli, S., Luisi, R., Carroccia, L., et al. (2015). Deconstruction of 6, 7-dimethoxy-1, 2, 3, 4-tetrahydroisoquinoline moiety to separate P-glycoprotein (P-gp) activity from σ2 receptor affinity in mixed P-gp/σ2 receptor agents. Eur. J. Med. Chem. 89, 691–700. doi:10.1016/j.ejmech.2014.11.001
Pati, M. L., Niso, M., Spitzer, D., Berardi, F., Contino, M., Riganti, C., et al. (2018). Multifunctional thiosemicarbazones and deconstructed analogues as a strategy to study the involvement of metal chelation, sigma-2 (σ2) receptor and P-gp protein in the cytotoxic action: In vitro and in vivo activity in pancreatic tumors. Eur. J. Med. Chem. 144, 359–371. doi:10.1016/j.ejmech.2017.12.024
Pistritto, G., Trisciuoglio, D., Ceci, C., Garufi, A., and D'Orazi, G. (2016). Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging (Albany NY) 8, 603–619. doi:10.18632/aging.100934
Rocha, C. R. R., Silva, M. M., Quinet, A., Cabral-Neto, J. B., and Menck, C. F. M. (2018). DNA repair pathways and cisplatin resistance: an intimate relationship. Clinics 73, e478s. doi:10.6061/clinics/2018/e478s
Schmidt, H. R., and Kruse, A. C. (2019). The molecular function of σ receptors: Past, present, and future. Trends Pharmacol. Sci. 40, 636–654. doi:10.1016/j.tips.2019.07.006
Sun, Y. T., Wang, G. F., Yang, Y. Q., Jin, F., Wang, Y., Xie, X. Y., et al. (2018). Synthesis and pharmacological evaluation of 6, 7-dimethoxy-1, 2, 3, 4-tetrahydroisoquinoline derivatives as sigma-2 receptor ligands. Eur. J. Med. Chem. 147, 227–237. doi:10.1016/j.ejmech.2017.11.016
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca. Cancer J. Clin. 71, 209–249. doi:10.3322/caac.21660
Theodoulou, F. L., and Kerr, I. D. (2015). ABC transporter research: Going strong 40 Years on. Biochem. Soc. Trans. 43, 1033–1040. doi:10.1042/bst20150139
Warren, M. (2009). Metastatic breast cancer recurrence: a literature review of themes and issues arising from diagnosis. Int. J. Palliat. Nurs. 15, 222–225. doi:10.12968/ijpn.2009.15.5.42347
Zeng, C., and Mach, R. H. (2017). The evolution of the sigma-2 (σ2) receptor from obscure binding site to bona fide therapeutic target. Adv. Exp. Med. Biol. 964, 49–61. doi:10.1007/978-3-319-50174-1_5
Zeng, C., Riad, A., and Mach, R. H. (2020). The biological function of sigma-2 receptor/TMEM97 and its utility in PET imaging studies in cancer. Cancers 12, 1877. doi:10.3390/cancers12071877
Zhao, R. Q., Wen, Y., Gupta, P., Lei, Z. N., Cai, C. Y., Liang, G., et al. (2019). Y(6), an epigallocatechin gallate derivative, reverses ABCG2-mediated mitoxantrone resistance. Front. Pharmacol. 9, 1545. doi:10.3389/fphar.2018.01545
Keywords: multidrug resistance, MCF-7/ADR, sigma-2 receptor, ABCB1, ABCG2
Citation: Zeng Z, Liao S, Zhou H, Liu H, Lin J, Huang Y, Zhou C and Xu D (2022) Novel Sigma-2 receptor ligand A011 overcomes MDR in adriamycin-resistant human breast cancer cells by modulating ABCB1 and ABCG2 transporter function. Front. Pharmacol. 13:952980. doi: 10.3389/fphar.2022.952980
Received: 25 May 2022; Accepted: 05 August 2022;
Published: 31 August 2022.
Edited by:
Takeo Tatsuta, Tohoku Medical and Pharmaceutical University, JapanReviewed by:
Zi-Ning Lei, Sun Yat-sen University, ChinaImran Shair Mohammad, University of North Carolina at Chapel Hill, United States
Marjana Novič, National Institute of Chemistry (Slovenia), Slovenia
Copyright © 2022 Zeng, Liao, Zhou, Liu, Lin, Huang, Zhou and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chenhui Zhou, Y2hlbmh1aXpoNkAxMjYuY29t; Daohua Xu, ZGFvaHVheDEwOEAxNjMuY29t
†These authors have contributed equally to this work
 Zhanwei Zeng
Zhanwei Zeng Shiyi Liao
Shiyi Liao Huan Zhou
Huan Zhou Hongyu Liu1,3
Hongyu Liu1,3 Jiantao Lin
Jiantao Lin Chenhui Zhou
Chenhui Zhou Daohua Xu
Daohua Xu