- 1Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 3People’s Hospital of Nanjiang County, Bazhong, China
Background: Diabetic kidney disease (DKD) is an important public health problem worldwide that increases the mortality of patients and incurs high medical costs. Traditional Chinese Medicine injections (TCMIs) are widely used in clinical practice. However, their efficacy is unknown owing to a lack of definitive evidence. This study conducted a network meta-analysis (NMA) to evaluate the efficacy and safety of traditional Chinese medicine injections in the treatment of DKD to provide a reference for clinical treatment.
Methods: Total 7 databases had been searched, which included PubMed, Embase, Cochrane Library, Web of Science, China National Knowledge Infrastructure (CNKI), Chinese scientific journal database (VIP), WanFang, and SinoMed. Only randomised controlled trials (RCT) had been included for analysis. The retrieval time limit was from the establishment of the database until 20 July 2022. Cochrane Risk of Bias 2.0 tool was used to evaluate the quality of the studies. Network meta-analyses, and Trial Sequential Analyses (TSA) were used to analysis the effectiveness of the included RCTs for DKD. The Stata 15.1 and R 4.0.4 were used to perform the network meta-analysis. Sensitivity analysis was used to assess the robustness of the findings. The effect of the intervention evidence are summarized on the basis of the minimum background framework.
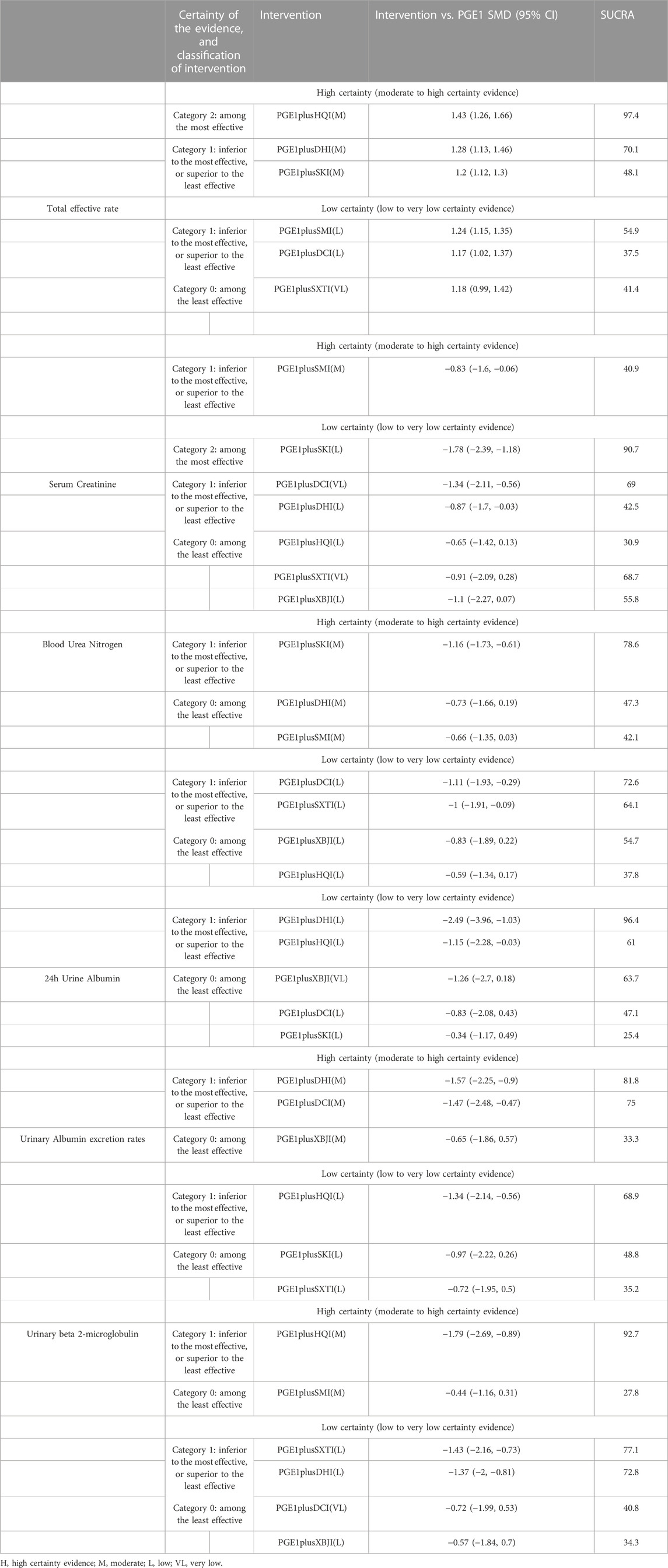
Results: NMA showed that the total effective rate of SMI, DCI, DHI, HQI, and SKI combined with alprostadil injection (PGE1) was better than PGE1 single used. Based on the surface under the cumulative ranking curve values, PGE1+DHI was the most effective for urinary albumin excretion rate and 24 h urinary albumin, PGE1+HQI was the most effective for the total response rate and β2-MG, and PGE1+SKI was the most effective for serum creatinine and blood urea nitrogen. Cluster analysis found that PGE1+HQI and PGE1+SKI could be the best treatments in terms of primary outcome measures. PGE1+SKI was found to be most effective on glomerular filtration function. PGE1+DHI was most effective for urinary protein-related indices.
Conclusion: The efficacy of TCMI combined with PGE1 was higher than PGE1 single used. PGE1+HQI and PGE1+SKI were the most effective treatments. The safety of TCMI treatment should be investigated further. This study needs to be validated using large-sample, double-blind, multicentre RCTs.
Systematic Review Registration: [https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=348333], identifier [CRD42022348333].
1 Introduction
Diabetic kidney disease (DKD) is one of the most serious microvascular complications of diabetes and has become a global public health challenge. 10.5% of adults have diabetes (Sun et al., 2022), and 40% of them developed into DKD (Alicic et al., 2017). This situation causing a heavy socioeconomic burden (de Boer et al., 2011; Afkarian et al., 2016; Kramer et al., 2018; Kume et al., 2019). DKD caused worse prognosis and increased risk of death in diabetic patients (Groop et al., 2009; Fox et al., 2012; Penno et al., 2018; Skupien et al., 2019). Preventing and delaying DKD progression is important in disease management for diabetes patients.
Currently, the main treatment methods for DKD are renin-angiotensin-aldosterone system (RAAS) blockers to regulate blood pressure, sodium-dependent glucose transporter 2 (SGLT-2) inhibitors, intensive insulin therapy to control blood glucose and intensive life management to improve obesity (Navaneethan et al., 2021). Urinary albumin is an important indicator for the evaluation and early diagnosis of DKD; a reduction in its levels can also alleviate DKD (Foundation, 2012). Recent studies have shown that Prostaglandin E1 (PGE1) can improve insulin resistance (Wei et al., 2018), reduce proximal tubular apoptosis (Mou et al., 2018; Zhang et al., 2020), and prevent vascular, glomerular, tubular, and interstitial changes(Bersani-Amado et al., 2020). A meta-analysis showed that PGE1 may positively affect DKD by reducing the urinary albumin excretion rate (UAER) and proteinuria (Wang et al., 2010).
Modern drugs mainly focus on delaying the disease process; hence, reversing DKD is a challenge and many new drugs are not approved for patients with an eGFR <30 mL/min. Traditional Chinese medicine (TCM) is widely used in the clinical prevention and treatment of DKD in China and has synergistic effects and safety advantages. The specific chemical mechanism of DKD protection by Chinese herbal medicine has been reviewed (Tang et al., 2021), which includes anti-inflammatory and antioxidant effects, inhibition of mesangial cell expansion, and reduction of podocyte injury (Xue et al., 2019; Zhong et al., 2019; Yang et al., 2022). Traditional Chinese medicine injection (TCMI) is a patented traditional Chinese drug registered by the National Medical Products Administration. In clinical practice, it is often combined with modern drug therapy to treat DKD. In recent years, several studies have demonstrated the efficacy of various TCMIs for the treatment of DKD (Yin et al., 2014; Liao et al., 2017; Wang et al., 2021; Xie et al., 2021).
The specific efficacies and therapeutic advantages of TCMIs are unclear, which causes clinical application problems. This study is the first article to systematically evaluate and compare the clinical efficacies, laboratory indicators, and safety of several commonly used TCMIs in combination with PGE1. The purpose of this study was to provide sufficient clinical evidence for TCM medicine and to provide a reference for the clinical use of TCMIs in the treatment of DKD.
2 Materials and methods
2.1 Standard evaluation of traditional Chinese medicine
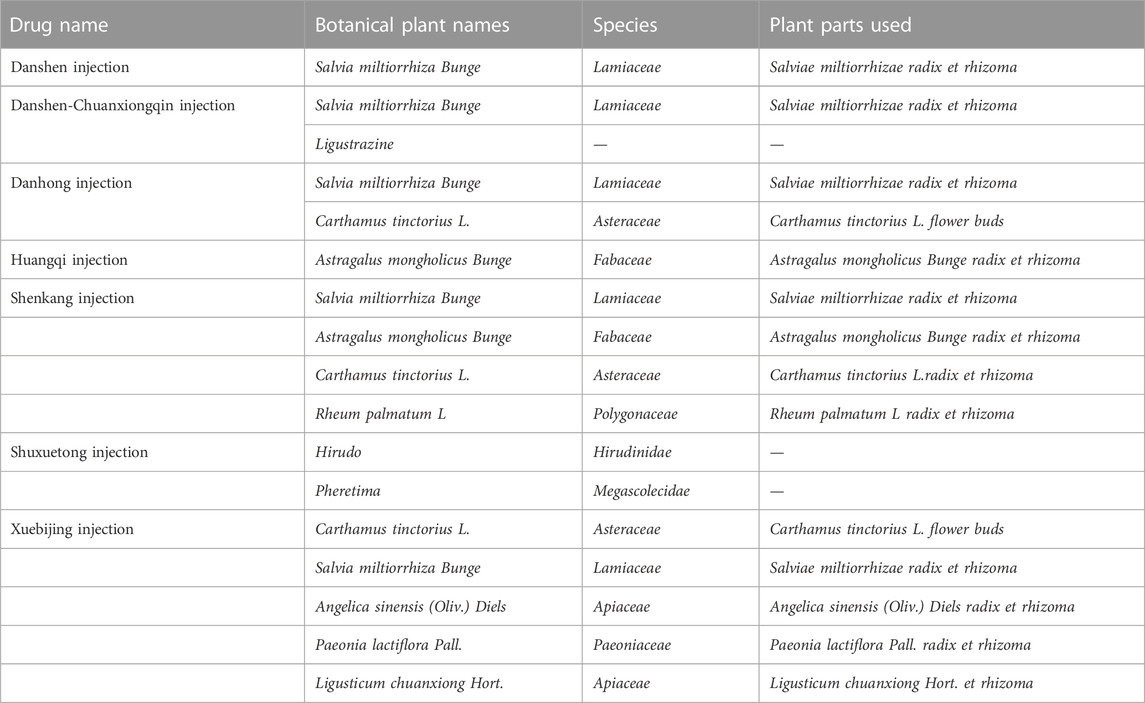
In order to make the study more accurate and reproducible, this study reported traditional Chinese medicine injections by referring to The ConPhyMP consensus (Heinrich et al., 2022). At the same time, we standardized the scientific names of botanical drug components with reference to Rivera et al. (2014). And validated in the databases of “Plant of the World Online” (http://www.plantsoftheworldonline.org) and “The World Flora Online” (WFO, http://www.worldfloraonline.org/). Summary tables describing the composition of agents and how they were reported in the original study were prepared in accordance with the principles described in the four pillars of ethnopharmacology. The composition and standard name of each injection are shown in Table 1. Other details are shown in Supplementary Tables S12, S13 (page 142–147).
2.2 Systematic review protocol and registration
The network meta-analysis was registered with the International Prospective Register of Systematic Reviews (PROSPERO) under the registration number CRD42022348333. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), its protocols, and the PRISMA-extension statement for network meta-analysis to report the current results (Shamseer et al., 2015; Page et al., 2021).
2.3 Literature search
This study searched PubMed, Embase, Cochrane Library, CNKI, VIP, Wanfang, and SinoMed databases, total 7 databases. The main search terms included “injection*,” “Diabetic Nephropathies,” “Nephropathies, Diabetic,” “Nephropathy, Diabetic,” “Diabetic Kidney Disease,” “Kidney Disease, Diabetic,” “Alprostadil,” “PGE1alpha,” “Prostaglandin E1alpha,” “PGE1,” “Lipo-PGE1” and others. References from previous systematic reviews and meta-analyses with similar topics were scanned for supplementation in the preliminary screening stage, references from eligible articles were scanned for supplementation in the full-text screening stage, and unpublished studies were not retrieved. The detailed search strategy is presented in Supplementary Tables S3–S10 (page 131–136). The retrieval time for each database was from database construction until 20 July 2022.
2.4 Inclusion and exclusion criteria
Inclusion criteria were determined based on PICO: (a) type of included studies: randomised controlled trials (RCTs); (b) patients: the subjects of the study were those who met the requirements of the DKD diagnostic criteria—no limitations existed in age, sex, or nationality; (c) interventions: in the treatment group, the intervention was TCMI + PGE1, which could be combined with conventional treatment (including the control of blood glucose, blood pressure, and blood lipids). The control group was treated with PGE1 in combination with conventional treatment; (d) outcome measures: the primary outcomes in this study were total effective rate (the calculation formula was as follows: total effective rate = marked effective rate + effective rate, markedly effective was defined as the main symptoms disappeared, and at least 50% reduction in the urine protein, or blood urea nitrogen (BUN) returned to normal, a decrease in at least 88.4 mmol L−1 of serum creatinine (Scr). Effective treatment showed that the main clinical symptoms were improved, the degree of urinary protein reduction was more than 33.3%, and BUN and Scr were decreased. The secondary outcomes included UAER, BUN, 24 h urinary albumin (24 h Alb), and urinary β2-microglobulin (β2-MG) levels. Studies that included only one outcome measure were eligible for inclusion. (e) The number of papers on the same TCMI should be greater than or equal to two.
The following exclusion criteria were used: (a) repeated articles; (b) incomplete or incorrect data; (c) non-conforming studies (including reviews, systematic reviews, meta-analyses, animal experiments, conference abstracts, reports, letters, case reports, etc.).
2.5 Study selection and data extraction
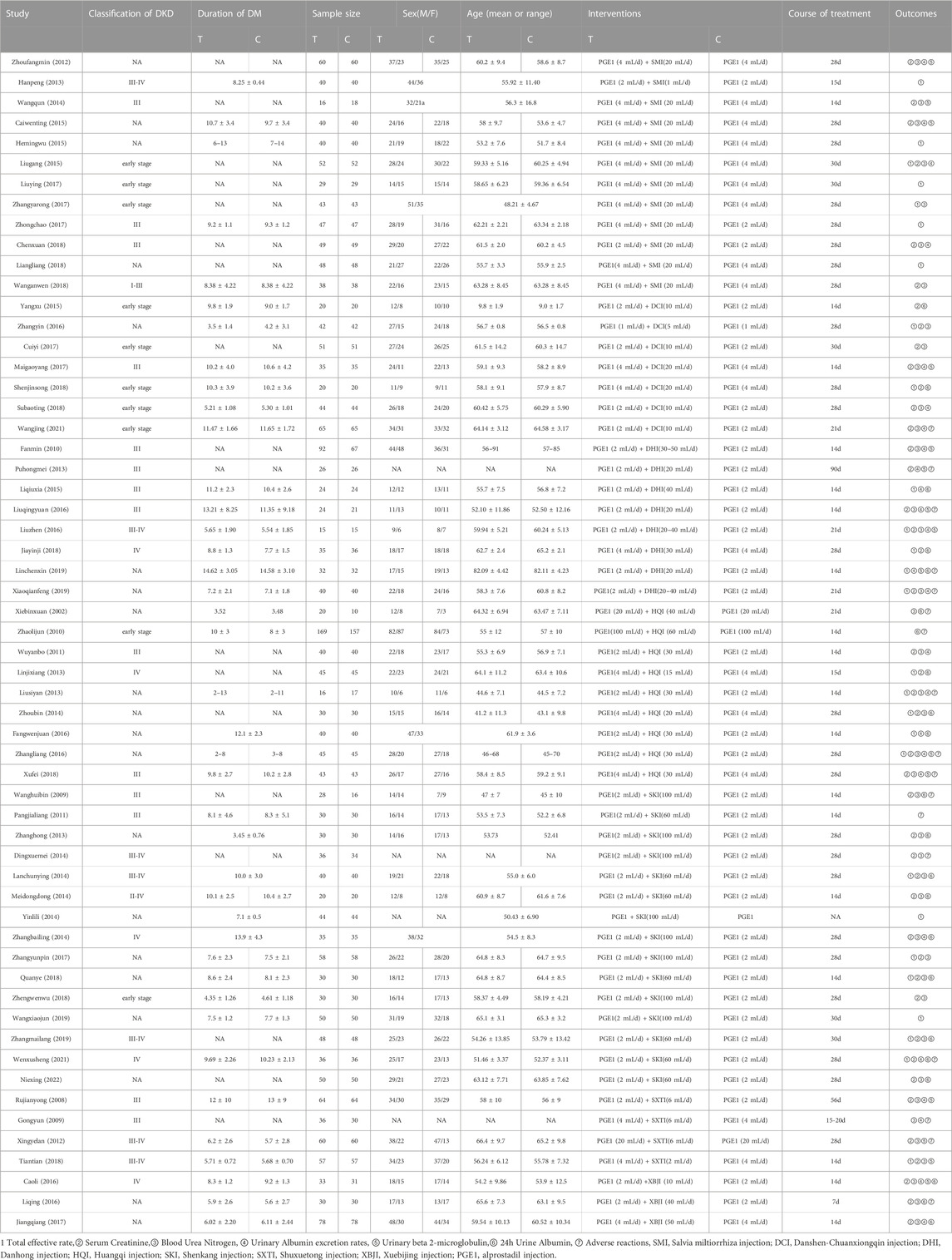
Two researchers (CYL and HYF) from related disciplines independently screened and crosschecked for inclusion. In the case of disagreement, a third researcher (RSY) can judge and provide a solution. Preliminary screening was carried out according to the title and abstract, and the included studies were then determined by reading the full text. Two researchers used uniform criteria for data extraction: the first author, year of publication, classification of DKD, duration of DM, sample size, male-to-female ratio, age, interventions, course of treatment, and outcomes.
2.6 Risk of bias assessment and quality assessment
The quality of the included studies was assessed by two investigators (CYL & HYF) using the Cochrane Risk of Bias 2.0 tool (Sterne et al., 2019) which included the randomisation process, deviations from intended interventions, missing outcome data, measurement of the outcome, selection of the reported result, selection of the reported result, and overall bias. The risk of bias was classified as “low risk,” “high risk,” and “some concerns.” We used the GRADE method for the entire network to provide a framework for the deterministic rating of each paired comparison evidence, divided into high, medium, low or very low (Puhan et al., 2014; Brignardello-Petersen et al., 2019).
2.7 Statistical analysis
This study used R4.0.4 and Stata15.1 software to calculate and draw graphs. For binary results, the combined results were calculated as odds ratio (OR). For continuous outcomes, this study used mean differences (MD), and standardised mean differences (SMD) were used when data units were inconsistent. All results are shown with 95% confidence intervals (95% CI). The league table was calculated using the Markov chain Monte Carlo method of the random-effects model through R4.0.4. The number of iterations was set to 200,000, and the first 100,000 iterations were used in the annealing algorithm to eliminate the influence of the initial values. Network diagrams were constructed using the Stata software to compare different interventions. Surface under the cumulative ranking curve (SUCRA) probability values were used to rank the detected treatments, with SUCRA values of 100% and 0% assigned to the best and worst treatments, respectively. Cluster analysis was used to compare the efficacy of TCMIs for different functions. The minimal contextualization framework was developed based on the results of SUCRA and GRADE assessments (Brignardello-Petersen et al., 2020). The bias information criterion was used to compare the fit of consistent and inconsistent models, and Cochran’s I2 statistic was used to assess statistical heterogeneity, with low, medium, and high I2 values of 25, 50, and 75%, respectively (Higgins et al., 2003). Funnel plots were used to detect publication bias in the primary outcome measures. Sensitivity analyses were carried out by excluding studies with a high-risk bias and those with courses of treatment that did not fall within 14–30 days. According to the information collected so far, TSA version 0.9 beta was used to calculate and draw the required information size and trial sequential monitoring boundaries.
3 Results
3.1 Study selection and characteristics
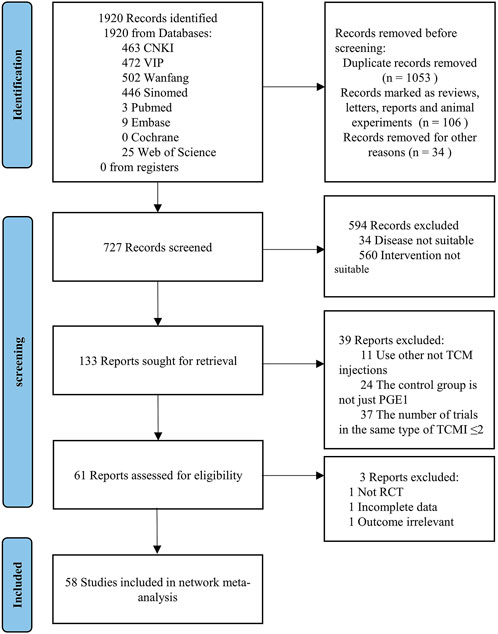
A total of 1920 studies were initially identified from the search, and 727 studies were retained after excluding duplicate literature, animal experiments, meetings, reports, and letters by screening titles and abstracts. After reading the titles and abstracts of the 727 studies, 34 studies in which disease was not suitable and 580 studies in which intervention was not suitable (including 184 studies with only PGE1 without comparison, 25 studies not combining PGE1, and 351 studies not combining TCMIs) were excluded, and 133 studies were retained. After reading the full text of the remaining literature, 11 studies without TCMI, 24 not only used PGE1 in the control group, and 37 studies in which the number of studies with the same type of TCMI ≤2 were excluded. Among the remaining 61 studies, one without an after-before control, one with incomplete data, and one with irrelevant outcome indicators from the input data were excluded. Finally, 58 studies from 2002 to 2022 were retained (Xie and Zhang, 2002; Ru et al., 2008b; Gong and Xie, 2009; Wang et al., 2009; Min et al., 2010; Zhao and Dong, 2010; Pang et al., 2011; Wu, 2011; Xing, 2012; Zhou and Lai, 2012a; b; Han and Zhang, 2013; Lin, 2013; Liu, 2013; Pu et al., 2013; Zhang et al., 2013; Ding, 2014; Lan, 2014; Mei et al., 2014; Wang et al., 2014; Yin, 2014; Zhang, 2014; Zhou et al., 2014; Cai et al., 2015; He, 2015; Li and Li, 2015; Liu and Guo, 2015; Yang et al., 2015; Cao et al., 2016; Fang et al., 2016; Li et al., 2016; Liu et al., 2016; Liu and Sun, 2016; Zhang, 2016; Zhang and Peng, 2016; Cui et al., 2017; Jiang and Qu, 2017; Liu, 2017; Mai et al., 2017; Zhang, 2017b; Zhang, 2017a; Zhong, 2017; Chen and Fu, 2018; Jia, 2018; Liang, 2018; Shen, 2018; Su, 2018; Tian et al., 2018; Wang, 2018; Xu and Liu, 2018; Ye, 2018; Zheng et al., 2018; Lin et al., 2019; Wang et al., 2019; Xiao, 2019; Zhang and Nan, 2019; Wang, 2021; Wen et al., 2021; Nie, 2022). The specific screening process is shown in Figure 1.
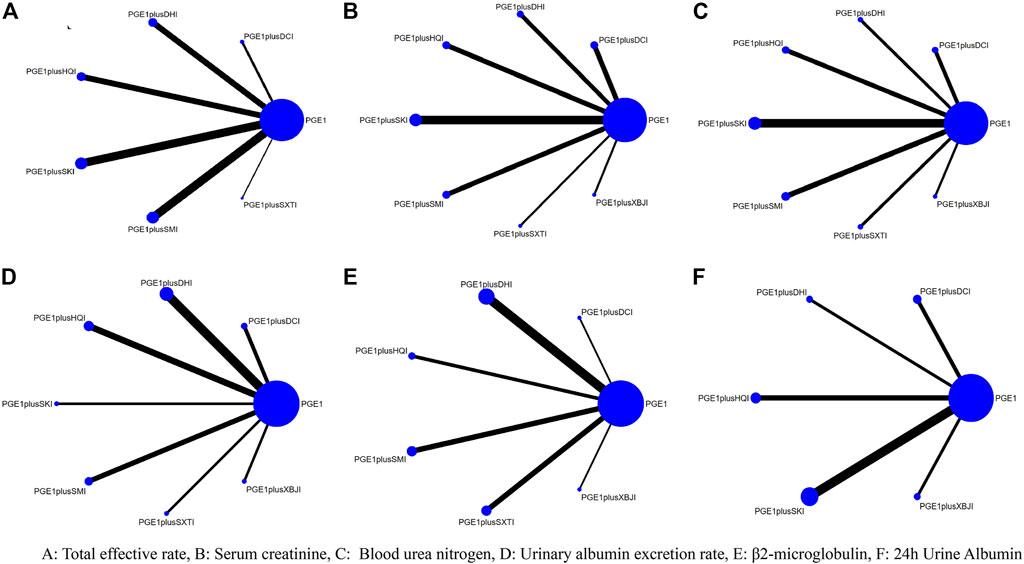
58 articles included in this study included a total of 4808 subjects and seven types of TCMIs, namely Salvia miltiorrhiza injection (SMI; 12 RCTS) (Zhou and Lai, 2012a; Han and Zhang, 2013; Wang et al., 2014; Cai et al., 2015; He, 2015; Liu and Guo, 2015; Zhang, 2017a; Liu, 2017; Zhong, 2017; Chen and Fu, 2018; Liang, 2018; Wang, 2018), Danshen-Chuanxiongqin injection (DCI; 7 RCTs) (Yang et al., 2015; Zhang and Peng, 2016; Cui et al., 2017; Mai et al., 2017; Shen, 2018; Su, 2018; Wang, 2021), Danhong injection (DHI; 8 RCTs) (Min et al., 2010; Pu et al., 2013; Li and Li, 2015; Liu et al., 2016; Liu and Sun, 2016; Jia, 2018; Lin et al., 2019; Xiao, 2019), Huangqi injection (HQI; 9 RCTs) (Xie and Zhang, 2002; Zhao and Dong, 2010; Wu, 2011; Lin, 2013; Liu, 2013; Zhou et al., 2014; Fang et al., 2016; Zhang, 2016; Xu and Liu, 2018), Shenkang injection (SKI;15 RCTs) (Wang et al., 2009; Pang et al., 2011; Zhang et al., 2013; Ding, 2014; Lan, 2014; Mei et al., 2014; Yin, 2014; Zhang, 2014; Zhang, 2017b; Ye, 2018; Zheng et al., 2018; Wang et al., 2019; Zhang and Nan, 2019; Wen et al., 2021; Nie, 2022), Shuxuetong injection (SXTI; 4 RCTs) (Ru et al., 2008a; Gong and Xie, 2009; Xing, 2012; Tian et al., 2018), and Xuebijing injection (XBJI; 3 RCTs) (Cao et al., 2016; Li et al., 2016; Jiang and Qu, 2017). The course of treatment ranged from 7 days to 3 months. The basic characteristics are shown in Table 2 and the comparative associations between each intervention and each outcome measure are shown in Figure 2. In addition, we collected the specific intervention method of each included study (Supplementary Table S14, page148–153).
3.2 Bias risk assessment and the grade of evidence
Among the 58 included studies, 15 studies described the methods used to generate the allocation sequence (Min et al., 2010; Zhou and Lai, 2012a; Pu et al., 2013; Yang et al., 2015; Cao et al., 2016; Liu et al., 2016; Zhang and Peng, 2016; Cui et al., 2017; Jiang and Qu, 2017; Liu, 2017; Mai et al., 2017; Zhong, 2017; Su, 2018; Tian et al., 2018; Wang, 2018), five studies were not random (Han and Zhang, 2013; Cai et al., 2015; Zhang, 2017a; Liang, 2018; Wen et al., 2021), the remaining studies did not explicitly address the random approach. None of the studies stated a pre-established research plan or analysis protocol. Overall, nine studies had a high risk (Han and Zhang, 2013; Lin, 2013; Yin, 2014; Cai et al., 2015; He, 2015; Zhang, 2017a; Zhang, 2017b; Liang, 2018; Wen et al., 2021), 49 studies had some concerns of bias(Xie and Zhang, 2002; Ru et al., 2008b; Gong and Xie, 2009; Wang et al., 2009; Min et al., 2010; Zhao and Dong, 2010; Pang et al., 2011; Wu, 2011; Zhou and Lai, 2012a; Xing, 2012; Liu, 2013; Pu et al., 2013; Zhang et al., 2013; Ding, 2014; Lan, 2014; Mei et al., 2014; Wang et al., 2014; Zhang, 2014; Zhou et al., 2014; Li and Li, 2015; Liu and Guo, 2015; Fang et al., 2016; Li et al., 2016; Liu et al., 2016; Liu and Sun, 2016; Zhang, 2016; Zhang and Peng, 2016; Cui et al., 2017; Jiang and Qu, 2017; Liu, 2017; Chen and Fu, 2018; Jia, 2018; Shen, 2018; Su, 2018; Tian et al., 2018; Xu and Liu, 2018; Ye, 2018; Zheng et al., 2018; Lin et al., 2019; Wang et al., 2019; Xiao, 2019; Zhang and Nan, 2019; Wang, 2021; Nie, 2022),. The results of the risk of bias assessment of the included studies are shown in Supplementary Figure S1 (page 3), Supplementary Table S1 (page 3–102). There are only indirect comparisons between TCMIs, which results in a very low-quality rating for pairwise comparisons, the details of evidence evaluation utilizing GRADE is available in the Supplementary Material (Supplementary Table S2, page 122–130).
3.3 Results of network meta-analysis
3.3.1 Primary outcome measures
3.3.1.1 Total effective rate
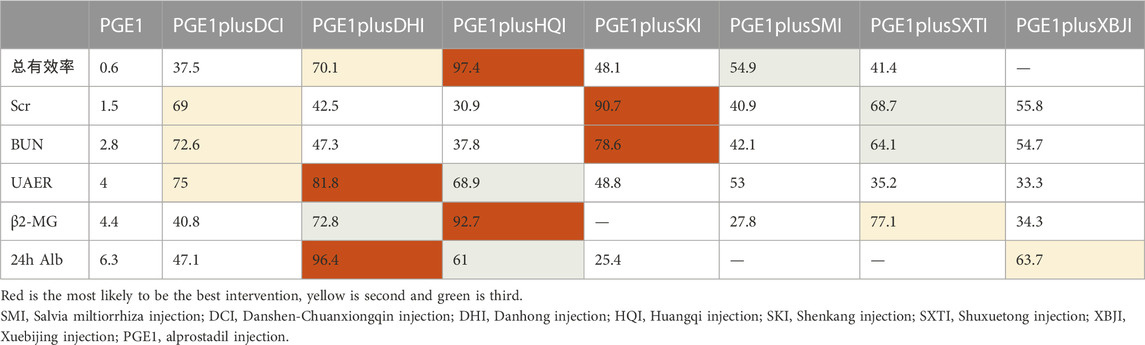
A total of 27 RCTs (Han and Zhang, 2013; Lin, 2013; Liu, 2013; Lan, 2014; Yin, 2014; Zhou et al., 2014; He, 2015; Li and Li, 2015; Liu and Guo, 2015; Fang et al., 2016; Liu and Sun, 2016; Zhang, 2016; Zhang and Peng, 2016; Zhang, 2017a; Zhang, 2017b; Liu, 2017; Zhong, 2017; Jia, 2018; Liang, 2018; Shen, 2018; Tian et al., 2018; Ye, 2018; Lin et al., 2019; Wang et al., 2019; Xiao, 2019; Zhang and Nan, 2019; Wen et al., 2021) reported the total effective rate, including six TCMIs and seven interventions. Five TCMIs combined with PGE1 were better than PGE1 alone, including PGE1+ DCI (RR: 1.17, CI: 1.02, 1.37), PGE1+DHI (RR: 1.28, CI: 1.13, 1.46), PGE1+HQI (RR: 1.43, CI: 1.26, 1.66), PGE1+SKI (RR: 1.2, CI: 1.12, 1.3), PGE1+SMI (RR: 1.24, CI: 1.15, 1.35), and PGE1+HQI were better than PGE1+DCI (RR: 1.23, CI: 1, 1.51), which suggested advantages in improving clinical symptoms (Table 3). According to the results of the SUCRA ranking (Table 4; Figure 3), PGE1+HQI (97.4%) was the best treatment, followed by PGE1+DHI (70.1%) and PGE1+SMI (54.9%).
3.3.1.2 Scr
A total of 45 RCTs (Xie and Zhang, 2002; Ru et al., 2008b; Wang et al., 2009; Min et al., 2010; Wu, 2011; Zhou and Lai, 2012a; Xing, 2012; Lin, 2013; Liu, 2013; Pu et al., 2013; Zhang et al., 2013; Ding, 2014; Lan, 2014; Mei et al., 2014; Wang et al., 2014; Zhang, 2014; Zhou et al., 2014; Cai et al., 2015; Liu and Guo, 2015; Yang et al., 2015; Cao et al., 2016; Li et al., 2016; Liu et al., 2016; Liu and Sun, 2016; Zhang, 2016; Zhang and Peng, 2016; Zhang, 2017a; Zhang, 2017b; Cui et al., 2017; Jiang and Qu, 2017; Mai et al., 2017; Chen and Fu, 2018; Jia, 2018; Shen, 2018; Su, 2018; Tian et al., 2018; Wang, 2018; Xu and Liu, 2018; Ye, 2018; Zheng et al., 2018; Xiao, 2019; Zhang and Nan, 2019; Wang, 2021; Wen et al., 2021; Nie, 2022) reported the Scr, including seven TCMIs and 8 interventions. 4 TCMIs combined with PGE1 were better than single used PGE1, including PGE1+DCI (RR: −1.34, CI: −2.11, −0.56), PGE1+DHI (SMD: −0.87, CI: −1.7, −0.03), PGE1+SKI (SMD: −1.78, CI: −2.39, −1.18), and PGE1+SMI (SMD: −0.83, CI: −1.6, −0.06). PGE1+SKI was superior to PGE1+HQI (SMD: −1.13, CI: −2.12, −0.16), indicating its advantages in improving Scr (Table 3). According to the results of the SUCRA ranking (Table 4; Figure 3), PGE1+SKI (90.7%) was the best treatment, followed by PGE1+DCI (69%), and PGE1+ SXTI (68.7%).
3.3.2 Secondary outcome measures
3.3.2.1 BUN
A total of 40 RCTs (Xie and Zhang, 2002; Ru et al., 2008b; Gong and Xie, 2009; Wang et al., 2009; Min et al., 2010; Wu, 2011; Xing, 2012; Zhou and Lai, 2012a; Liu, 2013; Zhang et al., 2013; Ding, 2014; Lan, 2014; Mei et al., 2014; Wang et al., 2014; Zhang, 2014; Zhou et al., 2014; Cai et al., 2015; Liu and Guo, 2015; Cao et al., 2016; Li et al., 2016; Liu et al., 2016; Liu and Sun, 2016; Zhang, 2016; Zhang and Peng, 2016; Cui et al., 2017; Jiang and Qu, 2017; Mai et al., 2017; Zhang, 2017a; Zhang, 2017b; Chen and Fu, 2018; Su, 2018; Wang, 2018; Xu and Liu, 2018; Ye, 2018; Zheng et al., 2018; Xiao, 2019; Zhang and Nan, 2019; Wang, 2021; Nie, 2022) reported the BUN, including 7 TCMIs and 8 interventions. The results showed that PGE1+DCI (SMD: −1.11, CI: −1.93, −0.29), PGE1+SKI (SMD: −1.16, CI: −1.73, −0.61), and PGE1+SXTI (SMD: −1, CI: −1.91, −0.09) were better than single used PGE1 (Table 3). According to the results of the SUCRA ranking (Table 4; Figure 3), PGE1+SKI (78.6%) was the best treatment, followed by PGE1+DCI (72.6%) and PGE1+SXTI (64.1%).
3.3.2.2 UAER
A total of 25 RCTs (Ru et al., 2008b; Gong and Xie, 2009; Min et al., 2010; Wu, 2011; Zhou and Lai, 2012a; Liu, 2013; Pu et al., 2013; Zhang, 2014; Cai et al., 2015; Li and Li, 2015; Liu and Guo, 2015; Cao et al., 2016; Fang et al., 2016; Liu et al., 2016; Liu and Sun, 2016; Zhang, 2016; Jiang and Qu, 2017; Mai et al., 2017; Chen and Fu, 2018; Su, 2018; Xu and Liu, 2018; Lin et al., 2019; Xiao, 2019; Wang, 2021; Wen et al., 2021) reported UAER, including 7 TCMIs and 8 interventions. The results showed that using PGE1+DCI (SMD: −1.47, CI: −2.48, −0.47), PGE1+DHI (SMD: −1.57, CI: −2.25, −0.9), PGE1+HQI (SMD: −1.34, CI: −2.14, −0.56), and PGE1+SMI (SMD: −1.08, CI: −1.94, −0.22) were better than single used PGE1(Table 3). According to the results of the SUCRA ranking (Table 4; Figure 3), PGE1+DHI (81.8%) was the best treatment, followed by PGE1+DCI (75%) and PGE1+HQI (68.9%).
3.3.2.3 β2-MG
A total of 15 RCTs (Ru et al., 2008b; Min et al., 2010; Zhou and Lai, 2012a; Xing, 2012; Pu et al., 2013; Wang et al., 2014; Cai et al., 2015; Cao et al., 2016; Liu et al., 2016; Liu and Sun, 2016; Zhang, 2016; Mai et al., 2017; Tian et al., 2018; Xu and Liu, 2018; Lin et al., 2019) reported β2-MG, including 6 TCMIs and 7 interventions. On the one hand, using PGE1+DHI (SMD: −1.37, CI: −2, −0.81), PGE1+HQI (SMD: −1.79, CI: −2.69, −0.89), and PGE1+SXTI (SMD: −1.43, CI: −2.16, −0.73) was better than single used PGE1. On the other hand, PGE1+HQI (SMD:1.35, CI:0.21, 2.53) and PGE1+DHI (SMD:0.92, CI:0.04, 1.93) were better than PGE1+SMI, indicating excellent performance in improving β2-MG (Table 3). According to the results of the SUCRA ranking (Table 4; Figure 3), PGE1+HQI (92.7%) was the best treatment, followed by PGE1+SXTI (77.1%) and PGE1+DHI (72.8%).
3.3.2.4 24 h Alb
A total of 24 RCTs (Xie and Zhang, 2002; Wang et al., 2009; Zhao and Dong, 2010; Lin, 2013; Zhang et al., 2013; Lan, 2014; Mei et al., 2014; Zhang, 2014; Li and Li, 2015; Yang et al., 2015; Cao et al., 2016; Fang et al., 2016; Li et al., 2016; Zhang and Peng, 2016; Cui et al., 2017; Jiang and Qu, 2017; Jia, 2018; Shen, 2018; Ye, 2018; Lin et al., 2019; Zhang and Nan, 2019; Wen et al., 2021; Nie, 2022) reported 24 h Alb, including five TCMIs and six interventions. Using PGE1+DHI (SMD: −2.49, CI: −3.96, −1.03), PGE1+HQI (SMD: −1.15, CI: −2.28, −0.03) was better than using PGE1 alone. PGE1+ DHI (SMD: 2.15, CI: 0.48, 3.84) was better than PGE1+SKI, indicating that it may be better to improve 24 h Alb (Table 3). According to the results of the SUCRA ranking (Table 4; Figure 3), PGE1+DHI (96.4%) was the best treatment, followed by PGE1+XBJI (63.7%) and PGE1+HQI (61%).
3.4 Cluster analysis
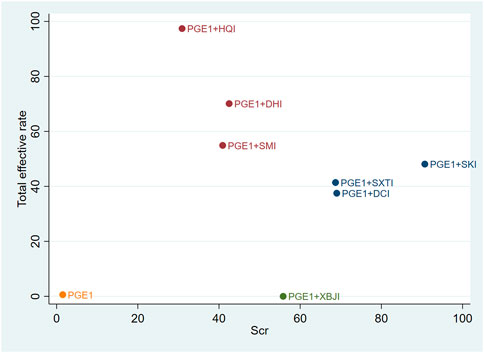
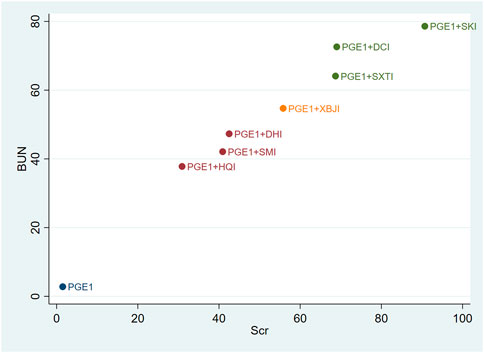
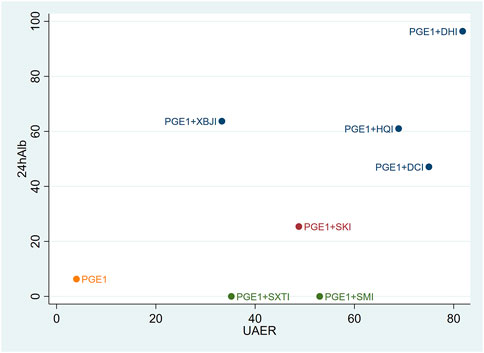
Cluster analysis was used to analyse the interventions for multi-dimensional outcomes and identify the best intervention measures under clustering of primary outcome indicators, glomerular filtration function, and urinary protein-related indicators. In terms of the primary outcome measure (total effective rate and Scr), PGE1+HQI and PGE1+SKI may be the best treatments (Figure 4). In terms of glomerular filtration function (Scr and BUN), PGE1+SKI was the best treatment (Figure 5). In terms of urinary protein-related indicators (24 h Alb & UAER), PGE1+DHI was the best treatment (Figure 6).
3.5 Minimally contextualized framework
PGE1 was selected as the reference group. Based on the comparison of whether the 95% confidence interval of the effect size of the reference group intersected the decision threshold for each intervention, the intervention measures were divided into “Category 0,” which had no difference compared with the intervention group, and “Category 1,” which was better than the intervention group. Then, a secondary classification was conducted based on the differences between the interventions. The intervention with the smallest effect size in Category 1 was taken as the reference, and the intervention with better effect was classified into Category 2. Interventions were then classified into high and low reliability categories based on GRADE classification, and the consistency of classification was checked by ranking results. In this study, those interventions with the highest ranking were ensured to be among the most effective (see Table 5).
3.6 Heterogeneity and consistency tests
This study found that most of the heterogeneity in the heterogeneity assessment was mild to moderate, in which the total effective rate was not substantially heterogeneous, and consistent models fit similarly or better than inconsistent models. See details in Supplementary Figures S2–S7 (page 103–108) and Supplementary Table S13 (page 103–108).
3.7 Safety
Seventeen RCTs (Xie and Zhang, 2002; Gong and Xie, 2009; Wang et al., 2009; Zhao and Dong, 2010; Pang et al., 2011; Xing, 2012; Liu, 2013; Pu et al., 2013; Ding, 2014; Li et al., 2016; Liu et al., 2016; Zhang, 2016; Xu and Liu, 2018; Lin et al., 2019; Xiao, 2019; Wang, 2021; Wen et al., 2021) reported the specific adverse reactions and safety of TCMIs, including pain, redness, and swelling at the injection site; dizziness; headache; vomiting; and diarrhoea. Only a descriptive analysis was performed because the description criteria of the various studies were not uniform. The specific information is given in Table 6.
3.8 Sensitivity analysis
Four studies had treatment durations that were not in the 14 days–30 days range (Ru et al., 2008b; Pu et al., 2013; Yin, 2014; Li et al., 2016). A study was included in the primary outcome, the total effective rate (Yin, 2014). The findings indicated that excluding this study did not significantly change the overall analysis. 3 studies were included in the primary outcome measure Scr (Ru et al., 2008b; Pu et al., 2013; Li et al., 2016). And the outcomes demonstrated that removing these studies did not significantly change the overall analysis.
Nine studies were identified as having a high risk of bias (Han and Zhang, 2013; Lin, 2013; Yin, 2014; Cai et al., 2015; He, 2015; Zhang, 2017a; Zhang, 2017b; Liang, 2018; Wen et al., 2021), 7 studies were included in the primary outcome measure total response rate (Han and Zhang, 2013; Lin, 2013; Yin, 2014; He, 2015; Zhang, 2017a; Zhang, 2017b; Wen et al., 2021), and 4 studies were included in the primary outcome measure Scr (Lin, 2013; Cai et al., 2015; Zhang, 2017b; Wen et al., 2021). The results revealed that removing these studies separately had no discernible impact on the overall analysis.
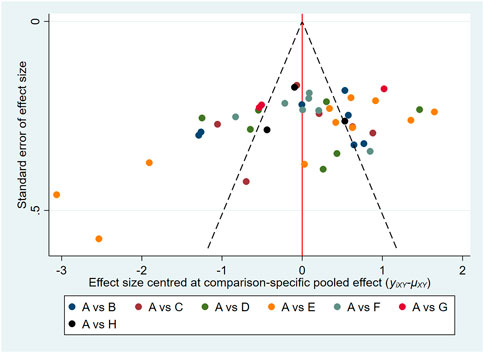
3.9 Publication bias
In this study, the funnel plots of the total effective rate (Figure 7) and Scr (Figure 8) were plotted. The results showed that the distribution of the total effective rate and Scr funnel plots were roughly symmetric, without obvious small-sample effect and publication bias.
3.10 Trial sequential analysis
For each of the groups of PGE1+SMI vs. PGE1, PGE1+DHI vs. PGE1, PGE1+SKI vs. PGE1, and PGE1+XBJI vs. PGE1, the cumulative Z-curve of Scr crosses the trial sequential monitoring and the required information size, indicating that SMI, DHI, SKI, and XBJI are effective for reducing Scr. Furthermore, the evidence of DCI, HQI, and SXTI was not sufficient. In terms of the total effective rate, the cumulative Z-curve crossed the trial sequential monitoring and the required information size in the comparisons of PGE1+HQI vs. PGE1 and PGE1+SKI vs. PGE1, which proved to be beneficial to the total effective rate, while the evidence of other injections was insufficient (Supplementary Figures S8–S20, page 109–121).
4 Discussion
4.1 Discussion of survey results
Our study found that SKI had obvious overall advantages in improving Scr and BUN levels. SKI is composed of Salvia miltiorrhiza Bunge, Astragalus mongholicus Bunge, Carthamus tinctorius L., Rheum palmatum L, and was approved to use on chronic kidney disease by China’s State Food and Drug Administration in 1999 (Licence No. YBZ08522004). The main components of SKI can reduce albuminuria, inhibit fibrosis, improve microcirculation, and regulate renal haemodynamic, and showed effects on glomerular and tubular lesions (Huang et al., 2012; Wang et al., 2012; Sun et al., 2016; Xu et al., 2020). A study showed that SKI can prevent renal tubular cell senescence under hyperglycaemia situation by reducing the expression of ageing markers P16INK4, cyclin D1, DcR2, and SA-β-Gal activity (Fu et al., 2019). It also can inhibit renal fibrosis and oxidative stress by downregulating the TGF-β/Smad3 signalling pathway. It brought significantly effects on improving Scr and BUN and alleviating renal injury (Wu et al., 2015; Wang et al., 2021). It also reduces glomerular hyperfiltration, hypertension, and hyperfusion situation (Zou et al., 2020).
DHI most effective on improving urinary protein levels. The main components in DHI are Salvia miltiorrhiza Bunge and Carthamus tinctorius L. Studies showed they can improve energy metabolism, oxidative stress, and autophagy and restore mitochondrial energy (Guo et al., 2021; Zeng et al., 2021). Study showed that DHI can inhibit glomerular hypertrophy, and markedly reduce urinary protein excretion in db/db mice (Liu et al., 2015). It also can delay the progression of renal injury by upregulating microRNA-30D-5P and targeting JAK1 (Deng et al., 2022).
HQI was the most effective for the total effective rate and β2-MG. The main component in HQI is Astragalus mongholicus Bunge. Main compounds of Astragalus mongholicus Bunge are polysaccharides, astragalus saponins, and flavonoids (Li et al., 2014), which inhibit oxidative stress (Ma et al., 2013), immune adjustment (Cho and Leung, 2007), anti-inflammatory (Zhang et al., 2003), and protect vascular endothelial cells (Wang et al., 2013; Zhu et al., 2013).
Studies have found that DKD occurs earlier than glomerular disease (Magri and Fava, 2009; Hasegawa et al., 2013). Therefore, the proximal renal tubules may be a new therapeutic target for the treatment of DKD. Astragalus mongholicus Bunge can ameliorate renal tubular injury and reduce the area, lumen, and wall to nearly normal (Sun et al., 2016), which is consistent with the results of this study.
4.2 Relationships and comparisons with other studies
To the best of our knowledge, this study is the first to compare the differences in the efficacy of TCMIs in the treatment of DKD through a network meta-analysis. Most of the previous studies (Zhang et al., 2022; Zhao et al., 2022), only conducted systematic reviews and network meta-analyses on TCM decoction. Those studies could not have stable quality control due to the diversity of ingredients and dose variability. The composition of TCMIs is more stable than TCM decoctions, which has quantitative significance. We comprehensively studied the RCTs of TCMIs combined with PGE1 in the treatment of DKD and ranked the advantage of each outcome index of different TCMIs to guide the clinical use.
4.3 Implications for clinical practice
This study found that SKI + PGE1 most effective on glomerular filtration function, DHI + PGE1 most effective on urinary protein, and HQI + PGE1 most effective on total effective rate and reduce clinical symptoms. TCMIs can effectively solve different problems of DKD. Non-study showed the effects of combination of multiple TCMIs in the treatment of DKD. This may be related to the complexity of the components, interactions, and other factors, which need to be further explored in subsequent studies.
Xie et al. found that the UAER of the 3 weeks treatment group decreased the fastest (Xie et al., 2021). A meta-analysis of the treatment of DKD with HQI found that the efficacy of a long course (>4 weeks) was better than that of a short course (<4 weeks) (Zhang and Kong, 2018). In this review, the duration of treatment in the 54 included studies focused on 1 month, 2 studies had longer treatment periods (Ru et al., 2008b; Pu et al., 2013), 1 study was in 7 days (Li et al., 2016) and 1 study was not mentioned (Yin, 2014).
Allergic reactions are the most common adverse events of using TCMIs (Wen et al., 2020). According to the studies included in this review, the adverse reactions of TCMIs are mild and can be relieved or eliminated by reducing the dosage, stopping medication, or symptomatic treatment (Xie and Zhang, 2002; Zhao and Dong, 2010; Pang et al., 2011; Zhang, 2016; Xu and Liu, 2018). The safety of TCMIs greatly improved by standardized the use in clinical application (Li et al., 2022). Li et al. improved the quality standard of solvent-enhancing polysorbate 80 in TCMI to reduce anaphylactic reactions (Li, 2018). However, the adverse reactions of patients still need to be concerned to avoid medical accidents.
TCMIs were widely used and effective in clinical practice. However, it was found that the specific extract components, complex pharmacological mechanisms and methodological descriptions of the botanical drugs were not clear in this included studies. In the future, relevant studies should follow the suggestions of consensus (Heinrich et al., 2020) and conduct more critical pharmacological studies on TCMIs.
4.4 Limitations
This study had the following limitations: (a) less reports on adverse reactions, and most of the studies had no clear safety assessment; (b) most of the literatures were “some concerns” in the risk assessment of bias and the quality of literatures was not high; (c) Have clinical heterogeneity due to the differences in botanical drug doses and treatment courses; (d) all included literatures were in China.
5 Conclusion
This study suggests that the combination of TCMIs and PGE1 provide additional benefits to patients with DKD. In terms of different outcome indicators, SKI had more effective on improving glomerular filtration function, DHI more effective on reducing urinary protein, and HQI more effective on improving renal tubular function. Despite the low incidence of adverse events, only a few studies have evaluated the safety of TCMIs. Further research on TCMIs treatment is needed for better understanding about TCMIs and guide the clinical application.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Author contributions
CL, HF, and RY designed the study and drafted the manuscript. CL and HF systems searched literature and extracted data. CL reviewed the included studies and performed statistical analyses. ZL, JL, ZHL and YJ provided useful suggestions and substantial revisions based on the content of the article. All listed authors made substantial, direct and intellectual contributions to the work and were approved for publication.
Funding
The study was supported by Sichuan Provincial Administration of Traditional Chinese Medicine (2020LC0153) and Sichuan Science and Technology Foundation (2021YFS0037).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1028257/full#supplementary-material
Abbreviations
24 h Alb, 24 h urinary albumin; 95% CI, 95% confidence intervals; β2-MG, urinary β2-microglobulin; BUN, blood urea nitrogen; CNKI, China National Knowledge Infrastructure; DCI, Danshen-Chuanxiongqin injection; DHI, Danhong injection; DKD, Diabetic kidney disease; HQI, Huangqi injection; MD, mean differences; NMA, network meta-analysis; PGE1, Prostaglandin E1; PROSPERO, International Prospective Register of Systematic Reviews; RAAS, reninangiotensin-aldosterone system; RCT, randomised controlled trial; RR, risk ratio; Scr, serum creatinine; SGLT-2, sodium-dependent glucose transporter 2; SKI, Shenkang injection; SMD, standardised mean differences; SMI, Salvia miltiorrhiza injection; SUCRA, Surface under the cumulative ranking curve; SXTI, Shuxuetong injection; TCM, Traditional Chinese medicine; TCMI, Traditional Chinese medicine injection; TSA, Trial Sequential Analyses; UAER, urinary albumin excretion rate; VIP, Chinese scientific journal database; XBJI, Xuebijing injection.
References
Afkarian, M., Zelnick, L. R., Hall, Y. N., Heagerty, P. J., Tuttle, K., Weiss, N. S., et al. (2016). Clinical manifestations of kidney disease among US adults with diabetes, 1988-2014. Jama 316 (6), 602–610. doi:10.1001/jama.2016.10924
Alicic, R. Z., Rooney, M. T., and Tuttle, K. R. (2017). Diabetic kidney disease: Challenges, progress, and possibilities. Clin. J. Am. Soc. Nephrol. 12 (12), 2032–2045. doi:10.2215/cjn.11491116
Bersani-Amado, L. E., da Rocha, B. A., Schneider, L. C. L., Ames, F. Q., do Nascimento, M. P., Bersani-Amado, C. A., et al. (2020). Prostaglandin E1 prevents histopathological changes improving renal function in experimental nephropathy induced by renal microembolism. Int. J. Clin. Exp. Pathol. 13 (7), 1624–1632.
Brignardello-Petersen, R., Florez, I. D., Izcovich, A., Santesso, N., Hazlewood, G., Alhazanni, W., et al. (2020). GRADE approach to drawing conclusions from a network meta-analysis using a minimally contextualised framework. Bmj 371, m3900. doi:10.1136/bmj.m3900
Brignardello-Petersen, R., Mustafa, R. A., Siemieniuk, R. A. C., Murad, M. H., Agoritsas, T., Izcovich, A., et al. (2019). GRADE approach to rate the certainty from a network meta-analysis: Addressing incoherence. J. Clin. Epidemiol. 108, 77–85. doi:10.1016/j.jclinepi.2018.11.025
Cai, W., Li, X., and Mao, C. (2015). “Effect analysis of Salvia miltiorrhiza injection combined with alprostadil injection in the treatment of diabetic nephropathy,” in A Collection of Contemporary medical articles.
Cao, L., Guo, Y., Liu, L., and Ren, B. (2016). Effect of Xuebijing injection combined with prostaglandin E1 in treatment of diabetic nephropathy. J. Mod. Integr. Traditional Chin. West. Med. 25 (24), 2645–2651. doi:10.3969/j.issn.1008-8849.2016.24.008
Chen, X., and Fu, M. (2018). Clinical observation of alprostadil injection combined with salvia miltiorrhiza injection in treatment of early diabetic nephropathy. Cap. Food Med. 25 (17).
Cho, W. C., and Leung, K. N. (2007). In vitro and in vivo immunomodulating and immunorestorative effects of Astragalus membranaceus. J. Ethnopharmacol. 113 (1), 132–141. doi:10.1016/j.jep.2007.05.020
Cui, Y., Zhang, M., Zhu, H., Cao, L., and He, W. (2017). Comparison of drug application in early treatment of diabetic nephropathy. Electron. J. Clin. Med. Literature 4 (55), 10806–10807. doi:10.16281/j.cnki.jocml.2017.55.090
de Boer, I. H., Rue, T. C., Hall, Y. N., Heagerty, P. J., Weiss, N. S., and Himmelfarb, J. (2011). Temporal trends in the prevalence of diabetic kidney disease in the United States. Jama 305 (24), 2532–2539. doi:10.1001/jama.2011.861
Deng, W., Huang, D., Xie, H., Wang, L., Shen, Q., Zeng, R., et al. (2022). Danhong injection represses diabetic retinopathy and nephropathy advancement in diabetic mice by upregulating microRNA-30d-5p and targeting JAK1. Bioengineered 13 (4), 8187–8200. doi:10.1080/21655979.2021.2006964
Ding, X. (2014). Effect of Shenkang injection combined with alprostadil on diabetic nephropathy. Tianjin Pharm. 26 (3).
Fang, W., Ma, J., Li, Q., Cai, X., and Su, H. (2016). Effect analysis of alprostadil combined with astragalus injection in the treatment of diabetic nephrotic edema. J. Guangxi Med. Univ. 33 (5).
Foundation, N. K. (2012). KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am. J. Kidney Dis. 60 (5), 850–886. doi:10.1053/j.ajkd.2012.07.005
Fox, C. S., Matsushita, K., Woodward, M., Bilo, H. J., Chalmers, J., Heerspink, H. J., et al. (2012). Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: A meta-analysis. Lancet 380 (9854), 1662–1673. doi:10.1016/s0140-6736(12)61350-6
Fu, B., Yang, J., Chen, J., Lin, L., Chen, K., Zhang, W., et al. (2019). Preventive effect of Shenkang injection against high glucose-induced senescence of renal tubular cells. Front. Med. 13 (2), 267–276. doi:10.1007/s11684-017-0586-8
Gong, Y., and Xie, Y. (2009). Clinical observation of Shuxuetong injection combined with Kaishi in the treatment of 36 cases of early diabetic nephropathy. Shandong Med. 49 (11).
Groop, P. H., Thomas, M. C., Moran, J. L., Wadèn, J., Thorn, L. M., Mäkinen, V. P., et al. (2009). The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes 58 (7), 1651–1658. doi:10.2337/db08-1543
Guo, Y., Yang, J. H., Cao, S. D., Gao, C. X., He, Y., Wang, Y., et al. (2021). Effect of main ingredients of Danhong Injection against oxidative stress induced autophagy injury via miR-19a/SIRT1 pathway in endothelial cells. Phytomedicine 83, 153480. doi:10.1016/j.phymed.2021.153480
Han, P., and Zhang, J. (2013). Analysis of Chinese medicine combined with prostaglandin E1 in the treatment of early type 2 diabetic nephropathy. China's Health Ind. 10 (33).
Hasegawa, K., Wakino, S., Simic, P., Sakamaki, Y., Minakuchi, H., Fujimura, K., et al. (2013). Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing Claudin-1 overexpression in podocytes. Nat. Med. 19 (11), 1496–1504. doi:10.1038/nm.3363
He, M. (2015). Effect of alprostadil injection combined with salvia miltiorrhiza injection on diabetic nephropathy. Chin. Pract. Med. (8).
Heinrich, M., Appendino, G., Efferth, T., Fürst, R., Izzo, A. A., Kayser, O., et al. (2020). Best practice in research – overcoming common challenges in phytopharmacological research. J. Ethnopharmacol. 246, 112230. doi:10.1016/j.jep.2019.112230
Heinrich, M., Jalil, B., Abdel-Tawab, M., Echeverria, J., Kulić, Ž., McGaw, L. J., et al. (2022). Best practice in the chemical characterisation of extracts used in pharmacological and toxicological research—the ConPhyMP—guidelines. Front. Pharmacol. 13, 953205. doi:10.3389/fphar.2022.953205
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. Bmj 327 (7414), 557–560. doi:10.1136/bmj.327.7414.557
Huang, B. Y., Cao, L. Y., and Fu, X. G. (2012). Effects of tanshinone IIA on Wnt/beta-catenin signaling pathway of high glucose induced renal tubular epithelial cell transdifferentiation. Zhongguo Zhong Xi Yi Jie He Za Zhi 32 (7), 965–969.
Jia, Y. (2018). Clinical study of prolidil combined with Danhong injection in the treatment of early diabetic nephropathy. World's latest Med. Inf. Dig. 18 (63), 165+170. doi:10.19613/j.cnki.1671-3141.2018.63.133
Jiang, Q., and Qu, D. (2017). Efficacy and safety of Xuebijing combined with alprostadil in patients with diabetic nephropathy. Laboratory Med. Clin. 14 (22), 3398–3400. doi:10.3969/j.issn.1672-9455.2017.22.047
Kramer, H., Boucher, R. E., Leehey, D., Fried, L., Wei, G., Greene, T., et al. (2018). Increasing mortality in adults with diabetes and low estimated glomerular filtration rate in the absence of albuminuria. Diabetes Care 41 (4), 775–781. doi:10.2337/dc17-1954
Kume, S., Araki, S. I., Ugi, S., Morino, K., Koya, D., Nishio, Y., et al. (2019). Secular changes in clinical manifestations of kidney disease among Japanese adults with type 2 diabetes from 1996 to 2014. J. Diabetes Investig. 10 (4), 1032–1040. doi:10.1111/jdi.12977
Lan, C. (2014). Effect of Shenkang combined with alprostadil on diabetic nephropathy. J. Chengde Med. Coll. 31 (4), 309–311. doi:10.15921/j.cnki.cyxb.2014.04.026
Li, H., Ma, S., Wang, L., Tian, B., and Sheng, J. (2022). Current situation and thinking on the safety of traditional Chinese medicine injection and its asepsis guarantee system. Chin. Tradit. Pat. Med. 44 (09), 2939–2943.
Li, Q., and Li, Q. (2015). Randomized controlled observation of Danhong injection combined with alprostadil in the treatment of early diabetic nephropathy. Clin. Res. Traditional Chin. Med. 7 (22).
Li, Q., Song, X., and Li, Z. (2016). ClilIical observation on thempeutic emct of xuebijing combined、=I,ith alprostadil for treatment of mabetic nephropathy. Chin. J. Integr. Traditional Chin. West. Med. First Aid 23 (3).
Li, W. (2018). Application actuality of polysorbate 80 (Twain 80) in traditional Chinese medicine injections. Chin. J. Ethnomedicine Ethnopharmacy 27 (23), 69–72.
Li, X., Qu, L., Dong, Y., Han, L., Liu, E., Fang, S., et al. (2014). A review of recent research progress on the astragalus genus. Molecules 19 (11), 18850–18880. doi:10.3390/molecules191118850
Liang, L. (2018). Clinical effect of alprostadil injection combined with salvia miltiorrhiza injection on diabetic nephropathy. Shenzhen J. Integr. Traditional Chin. West. Med. 28 (21).
Liao, H., Hu, L., Cheng, X., Wang, X., Li, J., Banbury, L., et al. (2017). Are the therapeutic effects of Huangqi (Astragalus membranaceus) on diabetic nephropathy correlated with its regulation of macrophage iNOS activity? J. Immunol. Res. 2017, 3780572. doi:10.1155/2017/3780572
Lin, C., Li, G., and Fang, T. (2019). Clinical effect of Danhong injection on alprostadil in elderly patients with diabetic nephropathy. Chin. J. Traditional Chin. Med. (6).
Lin, J. (2013). “Effect of Astragalus injection combined with alprostadil on stage 4 diabetic nephropathy,” in Shanxi traditional Chinese medicine.7
Liu, G., and Guo, K. (2015). Effect analysis of alprostadil injection combined with salvia miltiorrhiza injection on 52 cases of early diabetic nephropathy. channel Pharm. 27 (2).
Liu, M., Pan, Q., Chen, Y., Yang, X., Zhao, B., Jia, L., et al. (2015). Administration of Danhong Injection to diabetic db/db mice inhibits the development of diabetic retinopathy and nephropathy. Sci. Rep. 5, 11219. doi:10.1038/srep11219
Liu, Q., Zhu, J., Li, D., and Jiang, F. (2016). Effect of alprostadil combined with Danhong injection on early diabetic nephropathy. Prim. Med. Forum 20 (29).
Liu, S. (2013). Effect of alprostadil combined with astragalus on 33 cases of diabetic nephropathy. Mod. diagnosis Treat. 24 (16).
Liu, Y. (2017). Clinical effect of alprostadil injection combined with salvia miltiorrhiza injection on early diabetic nephropathy. Wu Han, Hu Bei Province: Chinese and Foreign Women's Health Study.3
Liu, Z., and Sun, L. (2016). “Effect of Danhong injection combined with alprostadil on type 2 diabetic nephropathy,” in Evaluation and analysis of hospital drug use in China.10.
Ma, X., Zhang, K., Li, H., Han, S., Ma, Z., and Tu, P. (2013). Extracts from Astragalus membranaceus limit myocardial cell death and improve cardiac function in a rat model of myocardial ischemia. J. Ethnopharmacol. 149 (3), 720–728. doi:10.1016/j.jep.2013.07.036
Magri, C. J., and Fava, S. (2009). The role of tubular injury in diabetic nephropathy. Eur. J. Intern Med. 20 (6), 551–555. doi:10.1016/j.ejim.2008.12.012
Mai, G., Li, J., and Li, L. (2017). Clinical observation of alprostadil injection combined with salvia miltiorrhiza ligustrazine injection in the treatment of early diabetic nephropathy Modern Pharmaceutical applications in China 11 (10).
Mei, D., Li, H., Peng, Y., and Li, H. (2014). “Evaluation of Shenkang injection combined with alprostadil in the treatment of diabetic nephropathy,” in Chinese medical equipment, 11.B12
Min, F., Liu, F., Yang, Y., Duan, S., Ye, Y., and Huang, G. (2010). Clinical observation of Danhong injection combined with alprostadil injection in the treatment of early diabetic nephropathy. Cent. Pharm. 8 (3).
Mou, Y., Zhang, Y., Guo, C., Zhao, J., Zhang, Z., Zhou, X., et al. (2018). Integrated treatment of prostaglandin E1 and angiotensin-converting enzyme inhibitor in diabetic kidney disease rats: Possible role of antiapoptosis in renal tubular epithelial cells. DNA Cell Biol. 37 (2), 133–141. doi:10.1089/dna.2017.3690
Navaneethan, S. D., Zoungas, S., Caramori, M. L., Chan, J. C. N., Heerspink, H. J. L., Hurst, C., et al. (2021). Diabetes management in chronic kidney disease: Synopsis of the 2020 KDIGO clinical practice guideline. Ann. Intern Med. 174 (3), 385–394. doi:10.7326/m20-5938
Nie, X. (2022). Investigation on the effectiveness and safety of Shenkang injection combined with alprostadil in the treatment of diabetic nephropathy. Chin. Pract. Med. 17 (2).
Page, M. J., Moher, D., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. Bmj 372, n160. doi:10.1136/bmj.n160
Pang, J., Wei, C., and Yang, F. (2011). Effect of Shenkang injection combined with alprostadil on diabetic nephropathy. J. Clin. Ration. Drug Use 4.
Penno, G., Solini, A., Bonora, E., Orsi, E., Fondelli, C., Zerbini, G., et al. (2018). Defining the contribution of chronic kidney disease to all-cause mortality in patients with type 2 diabetes: The renal insufficiency and cardiovascular events (RIACE) Italian multicenter study. Acta Diabetol. 55 (6), 603–612. doi:10.1007/s00592-018-1133-z
Pu, H., Feng, J., and Ji, Q. (2013). Clinical study of Danhong injection combined with prostaglandin E_1 in the treatment of early diabetic nephropathy. J. Qiqihar Med. Coll. 34 (7), 951–952.
Puhan, M. A., Schünemann, H. J., Murad, M. H., Li, T., Brignardello-Petersen, R., Singh, J. A., et al. (2014). A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. Bmj 349, g5630. doi:10.1136/bmj.g5630
Rivera, D., Allkin, R., Obón, C., Alcaraz, F., Verpoorte, R., and Heinrich, M. (2014). What is in a name? The need for accurate scientific nomenclature for plants. J. Ethnopharmacol. 152 (3), 393–402. doi:10.1016/j.jep.2013.12.022
Ru, J., Mao, Y., and Guo, Z. (2008a). Clinical observation on Suxuetong injection with prostaglandin E1 for diabetic nephropathy. Chin. J. Hosp. Pharm. 28 (11), 905–907.
Ru, J., Mao, Y., and Guo, Z. (2008b). Effect of Shuxuetong combined with prostaglandin E1 on diabetic nephropathy. Chin. J. Hosp. Pharm. 28 (11).
Shamseer, L., Moher, D., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., et al. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. Bmj 350, g7647. doi:10.1136/bmj.g7647
Shen, J. (2018). Clinical analysis of alprostadil injection combined with salvia miltiorrhiza ligustrazine injection in the treatment of early diabetic nephropathy. Chin. Med. Guide 16 (18).
Skupien, J., Smiles, A. M., Valo, E., Ahluwalia, T. S., Gyorgy, B., Sandholm, N., et al. (2019). Variations in risk of end-stage renal disease and risk of mortality in an international study of patients with type 1 diabetes and advanced nephropathy. Diabetes Care 42 (1), 93–101. doi:10.2337/dc18-1369
Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: A revised tool for assessing risk of bias in randomised trials. Bmj 366, l4898. doi:10.1136/bmj.l4898
Su, B. (2018). Effect of salvia miltiorrhiza ligustrazine injection combined with alprostadil on inflammatory factors and urinary microalbumin in the treatment of early diabetic nephropathy. Jilin Med. 39 (3).
Sun, H., Saeedi, P., Karuranga, S., Pinkepank, M., Ogurtsova, K., Duncan, B. B., et al. (2022). IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 183, 109119. doi:10.1016/j.diabres.2021.109119
Sun, H., Wang, W., Han, P., Shao, M., Song, G., Du, H., et al. (2016). Astragaloside IV ameliorates renal injury in db/db mice. Sci. Rep. 6, 32545. doi:10.1038/srep32545
Tang, G., Li, S., Zhang, C., Chen, H., Wang, N., and Feng, Y. (2021). Clinical efficacies, underlying mechanisms and molecular targets of Chinese medicines for diabetic nephropathy treatment and management. Acta Pharm. Sin. B 11 (9), 2749–2767. doi:10.1016/j.apsb.2020.12.020
Tian, T., Pu, X., Jin, L., and Li, H. (2018). Effect of alprostadil injection combined with Shuxuetong injection on diabetic nephropathy and its influence on serum inflammatory factors, hemorheology and renal function. Traditional Chin. Med. Hebei 40 (6).
Wang, A. (2018). Effect of salvia miltiorrhiza injection combined with alprostadil on blood lipid and renal function in patients with early diabetic nephropathy. Chin. foreign Med. Res. 16 (21), 111–112. doi:10.14033/j.cnki.cfmr.2018.21.054
Wang, H., Deng, J. L., Yue, J., Li, J., and Hou, Y. B. (2010). Prostaglandin E1 for preventing the progression of diabetic kidney disease. Cochrane Database Syst. Rev. 5, Cd006872. doi:10.1002/14651858.CD006872.pub2
Wang, H., Fu, L., Sun, Y., and Sun, L. (2009). Effect of Shenkang injection combined with alprostadil on urinary protein in patients with early diabetic nephropathy. Chin. J. Hosp. Pharm. (9).
Wang, H., Song, H., Yue, J., Li, J., Hou, Y. B., and Deng, J. L. (2012). Rheum officinale (a traditional Chinese medicine) for chronic kidney disease. Cochrane Database Syst. Rev. 7, Cd008000. doi:10.1002/14651858.CD008000.pub2
Wang, J. (2021). Efficacy analysis of salvia miltiorrhiza ligustrazine injection combined with Kishi in the treatment of diabetic nephropathy. Inn. Mong. Med. J. 53 (1).
Wang, Q., Zai, G., Chen, R., and Liu, J. (2014). The value of alprostadil combined with salvia miltiorrhiza in the treatment of diabetic nephropathy with microproteinuria. Clin. Res. China 27 (12).
Wang, S. G., Xu, Y., Chen, J. D., Yang, C. H., and Chen, X. H. (2013). Astragaloside IV stimulates angiogenesis and increases nitric oxide accumulation via JAK2/STAT3 and ERK1/2 pathway. Molecules 18 (10), 12809–12819. doi:10.3390/molecules181012809
Wang, W. W., Liu, Y. L., Wang, M. Z., Li, H., Liu, B. H., Tu, Y., et al. (2021). Inhibition of renal tubular epithelial mesenchymal transition and endoplasmic reticulum stress-induced apoptosis with Shenkang injection attenuates diabetic tubulopathy. Front. Pharmacol. 12, 662706. doi:10.3389/fphar.2021.662706
Wang, X., Liu, W., and Xu, L. (2019). Clinical observation of alprostadil combined with Shenkang injection in the treatment of type 2 diabetic nephropathy. Chin. Med. Guide 17 (12).
Wei, W., An, X. R., Jin, S. J., Li, X. X., and Xu, M. (2018). Inhibition of insulin resistance by PGE1 via autophagy-dependent FGF21 pathway in diabetic nephropathy. Sci. Rep. 8 (1), 9. doi:10.1038/s41598-017-18427-2
Wen, W., Liang, H., Yu, G., Zhao, J., Li, Y., Liu, W., et al. (2020). Research progress on anaphylaxis of traditional Chinese medicine injections. J. Hunan Univ. Traditional Chin. Med. 40 (01), 117–122.
Wen, X., Lv, J., and Li, W. (2021). Effect analysis of Shenkang injection combined with alprostadil in the treatment of diabetic nephropathy. Big Dr. 6 (12), 37–39.
Wu, X., Guan, Y., Yan, J., Liu, M., Yin, Y., Duan, J., et al. (2015). ShenKang injection suppresses kidney fibrosis and oxidative stress via transforming growth factor-β/Smad3 signalling pathway in vivo and in vitro. J. Pharm. Pharmacol. 67 (8), 1054–1065. doi:10.1111/jphp.12412
Wu, Y. (2011). Effect of Astragalus injection combined with alprostadil on 40 cases of early diabetic nephropathy. China Med. Sci. 1 (17).
Xiao, Q. (2019). Effect of alprostadil combined with Danhong injection on type 2 diabetic nephropathy. Mod. Chin. Dr. 57 (2).
Xie, B., and Zhang, X. (2002). Effect of astragalus injection combined with prostaglandin E1 on diabetic nephropathy. J. Hunan Coll. Traditional Chin. Med. 22 (3).
Xie, F., Zhang, B., Dai, S., Jin, B., Zhang, T., and Dong, F. (2021). Efficacy and safety of salvia miltiorrhiza (salvia miltiorrhiza Bunge) and ligustrazine injection in the adjuvant treatment of early-stage diabetic kidney disease: A systematic review and meta-analysis. J. Ethnopharmacol. 281, 114346. doi:10.1016/j.jep.2021.114346
Xing, Y. (2012). Clinical observation of Shuxuetong injection combined with alprostadil in the treatment of type 2 diabetic nephropathy. Chin. J. Coal Industry Med. 15 (6).
Xu, F., and Liu, Y. (2018). Clinical effect of Astragalus injection combined with alprostadil in the treatment of early diabetic nephropathy. J. Clin. Ration. Drug Use 11 (20).
Xu, S., He, L., Ding, K., Zhang, L., Xu, X., Wang, S., et al. (2020). Tanshinone IIA ameliorates streptozotocin-induced diabetic nephropathy, partly by attenuating PERK pathway-induced fibrosis. Drug Des. Devel Ther. 14, 5773–5782. doi:10.2147/dddt.S257734
Xue, H., Li, P., Luo, Y., Wu, C., Liu, Y., Qin, X., et al. (2019). Salidroside stimulates the Sirt1/PGC-1α axis and ameliorates diabetic nephropathy in mice. Phytomedicine 54, 240–247. doi:10.1016/j.phymed.2018.10.031
Yang, X., Qin, L., and Tang, X. (2015). Clinical study of alprostadil injection combined with salvia miltiorrhiza ligustrazine injection in the treatment of early diabetic nephropathy. Continuing Med. Educ. China 7 (23), 173–174. doi:10.3969/j,issn.1674-9308.2015.23.126
Yang, Y. Q., Tan, H. B., Zhang, X. Y., Zhang, Y. Z., Lin, Q. Y., Huang, M. Y., et al. (2022). The Chinese medicine Fufang Zhenzhu Tiaozhi capsule protects against renal injury and inflammation in mice with diabetic kidney disease. J. Ethnopharmacol. 292, 115165. doi:10.1016/j.jep.2022.115165
Ye, Q. (2018). Effect of alprostadil on diabetic nephropathy with Shenkang injection. China Health Nutr. 28 (25), 165–166. doi:10.3969/j.issn.1004-7484.2018.25.244
Yin, D., Yin, J., Yang, Y., Chen, S., and Gao, X. (2014). Renoprotection of Danshen Injection on streptozotocin-induced diabetic rats, associated with tubular function and structure. J. Ethnopharmacol. 151 (1), 667–674. doi:10.1016/j.jep.2013.11.025
Yin, L. (2014). Efficacy evaluation of Shenkang injection combined with lipid-soluble prostaglandin E_1 in the treatment of diabetic nephropathy. J Mod. drug Appl. China 8 (20).
Zeng, M., Zhou, H., He, Y., Wang, Z., Shao, C., Yin, J., et al. (2021). Danhong injection alleviates cerebral ischemia/reperfusion injury by improving intracellular energy metabolism coupling in the ischemic penumbra. Biomed. Pharmacother. 140, 111771. doi:10.1016/j.biopha.2021.111771
Zhang, B. (2014). Effect of Shenkang injection combined with alprostadil on diabetic nephropathy. Heilongjiang Med. Sci. 38 (4), 427–428. doi:10.3969/j.issn.1004-5775.2014.04.035
Zhang, H., He, B., Zhang, Y., and Li, P. (2013). Effect of Shenkang injection on 60 cases of diabetic nephropathy. Med. Front. (12), 243–244.
Zhang, L. (2016). Effect of alprostadil combined with astragalus injection on diabetic nephropathy. Chin. Disabil. Med. 24 (1), 112–114.
Zhang, L., Miao, R., Yu, T., Wei, R., Tian, F., Huang, Y., et al. (2022). Comparative effectiveness of traditional Chinese medicine and angiotensin converting enzyme inhibitors, angiotensin receptor blockers, and sodium glucose cotransporter inhibitors in patients with diabetic kidney disease: A systematic review and network meta-analysis. Pharmacol. Res. 177, 106111. doi:10.1016/j.phrs.2022.106111
Zhang, M., and Nan, J. (2019). “Effect of alprostadil combined with Shenkang injection on diabetic nephropathy,” in Chinese and foreign women's health study.8
Zhang, P., and Kong, W. (2018). Meta-analysis of Astragalus injection combined with alprostadil in the treatment of diabetic nephropathy. J. Guiyang Coll. Traditional Chin. Med. 40 (05), 41–46. doi:10.16588/j.cnki.issn1002-1108.2018.05.010
Zhang, W. J., Hufnagl, P., Binder, B. R., and Wojta, J. (2003). Antiinflammatory activity of astragaloside IV is mediated by inhibition of NF-kappaB activation and adhesion molecule expression. Thromb. Haemost. 90 (5), 904–914. doi:10.1160/th03-03-0136
Zhang, Y. (2017a). Curative effect and security analysis of alprostadil injection combined with salvia mittiorrhiza on early diabetic nephropathy. Clin. Med. Res. Pract. 2 (9), 25–26. doi:10.19347/j.cnki.2096-1413.201709012
Zhang, Y. (2017b). Effect of Shenkang injection combined with alprostadil on diabetic nephropathy. Chin. foreign Med. Res. 15 (8).
Zhang, Y. H., Zhang, Y. Q., Guo, C. C., Wang, L. K., Cui, Y. J., Dong, J. J., et al. (2020). Prostaglandin E1 attenuates high glucose-induced apoptosis in proximal renal tubular cells by inhibiting the JNK/Bim pathway. Acta Pharmacol. Sin. 41 (4), 561–571. doi:10.1038/s41401-019-0314-9
Zhang, Y., and Peng, Z. (2016). Curative effect of salvia miltiorrhiza ligustrazine combined with alprostadil on diabetic nephropathy. Chin. Med. 19 (2), 303–305. doi:10.3969/j.issn.1008-049X.2016.02.029
Zhao, J., Ai, J., Mo, C., Shi, W., and Meng, L. (2022). Comparative efficacy of seven Chinese patent medicines for early diabetic kidney disease: A bayesian network meta-analysis. Complement. Ther. Med. 67, 102831. doi:10.1016/j.ctim.2022.102831
Zhao, L., and Dong, H. (2010). Clinical study of Astragalus injection combined with alprostadil in the treatment of diabetic nephropathy. Chin. Contemp. Med. 17 (14).
Zheng, W., Yang, L., and Zhou, A. (2018). Effect of Shenkang injection on early diabetic nephropathy. J. Clin. Ration. Drug Use 11 (14).
Zhong, C. (2017). Clinical observation of alprostadil injection combined with salvia miltiorrhiza injection in treatment of early diabetic nephropathy. Health care Guide (33), 12–13. doi:10.3969/j.issn.1006-6845.2017.33.010
Zhong, Y., Lee, K., Deng, Y., Ma, Y., Chen, Y., Li, X., et al. (2019). Arctigenin attenuates diabetic kidney disease through the activation of PP2A in podocytes. Nat. Commun. 10 (1), 4523. doi:10.1038/s41467-019-12433-w
Zhou, B., Zhou, Y., Fu, Y., Shi, P., Lin, W., and Cao, J. (2014). Effect of alprostadil combined with Shenmai or astragalus on diabetic nephropathy. Clin. Assem. 29 (7).
Zhou, F., and Lai, F. (2012a). Clinical observation of alprostadil injection combined with salvia miltiorrhiza injection in treatment of early diabetic nephropathy. General Pract. China 15 (21).
Zhou, F., and Lai, F. (2012b). Effects of alprostadil injection combined with danshen injection on early diabetic nephropathy. Chin. General Pract. 15 (7C), 2436–2438.
Zhu, Y. P., Shen, T., Lin, Y. J., Chen, B. D., Ruan, Y., Cao, Y., et al. (2013). Astragalus polysaccharides suppress ICAM-1 and VCAM-1 expression in TNF-α-treated human vascular endothelial cells by blocking NF-κB activation. Acta Pharmacol. Sin. 34 (8), 1036–1042. doi:10.1038/aps.2013.46
Keywords: traditional medicine, diabetic kidney disease, randomized controlled trial, network meta-analysis, injections
Citation: Long C, Feng H, Liu Z, Li Z, Liu J, Jiang Y and Yue R (2023) Efficacy of traditional Chinese medicine injection for diabetic kidney disease: A network meta analysis and systematic review. Front. Pharmacol. 14:1028257. doi: 10.3389/fphar.2023.1028257
Received: 25 August 2022; Accepted: 06 February 2023;
Published: 17 February 2023.
Edited by:
Dan Tang, Guangdong Pharmaceutical University, ChinaReviewed by:
Mingsan Miao, Henan University of Traditional Chinese Medicine, ChinaYu Wu, Affiliated Hospital of Nanjing University of Chinese Medicine, China
Copyright © 2023 Long, Feng, Liu, Li, Liu, Jiang and Yue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rensong Yue, c29uZ3Jlbnl1ZUBjZHV0Y20uZWR1LmNu
†These authors have contributed equally to this work
 Caiyi Long
Caiyi Long Haoyue Feng1,2†
Haoyue Feng1,2† Zihan Li
Zihan Li Yayi Jiang
Yayi Jiang Rensong Yue
Rensong Yue