- 1Department of Neurology, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 2Lab of Neurodegenerative Disorders, Institute of Inflammation and Immunology (III), Frontiers Science Center for Disease-Related Molecular Network, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 3Centre for Rare Diseases, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 4Mental Health Center, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 5Department of Pathophysiology, West China College of Basic medical sciences and Forensic Medicine, Sichuan University, Chengdu, China
- 6Department of Geriatrics, Dazhou Central Hospital, Dazhou, Sichuan, China
- 7Department of Neurology, Xijing Hospital, Air Force Military Medical University, Xi’an, Shanxi, China
Background: Evidence from observational studies concerning the causal role of blood pressure (BP) and antihypertensive medications (AHM) on Parkinson’s disease (PD) remains inconclusive. A two-sample Mendelian randomization (MR) study was performed to evaluate the unconfounded association of genetic proxies for BP and first-line AHMs with PD.
Methods: Instrumental variables (IV) from the genome-wide association study (GWAS) for BP traits were used to proxy systolic BP (SBP), diastolic BP, and pulse pressure. SBP-associated variants either located within encoding regions or associated with the expression of AHM targets were selected and then scaled to proxy therapeutic inhibition of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, β-blockers, calcium channel blockers, and thiazides. Positive control analyses on coronary heart disease (CHD) and stroke were conducted to validate the IV selection. Summary data from GWAS for PD risk and PD age at onset (AAO) were used as outcomes.
Results: In positive control analyses, genetically determined BP traits and AHMs closely mimicked the observed causal effect on CHD and stroke, confirming the validity of IV selection methodology. In primary analyses, although genetic proxies identified by “encoding region-based method” for β-blockers were suggestively associated with a delayed PD AAO (Beta: 0.115; 95% CI: 0.021, 0.208; p = 1.63E-2; per 10-mmHg lower), sensitivity analyses failed to support this association. Additionally, MR analyses found little evidence that genetically predicted BP traits, overall AHM, or other AHMs affected PD risk or AAO.
Conclusion: Our data suggest that BP and commonly prescribed AHMs may not have a prominent role in PD etiology.
Introduction
Parkinson’s disease (PD) is one of the most prevalent neurodegenerative diseases lacking any neuroprotective treatments (Pringsheim et al., 2014), while hypertension ranks among the leading risk factors for all-cause death and disability-adjusted life-years worldwide (GBD 2017 Risk Factor Collaborators, 2018). Since the prevalence of PD and hypertension increases with age, their coexistence in the elderly is not uncommon. Therefore, understanding whether hypertension and antihypertensive medications (AHM) were causal for PD will make the medical decision more reasonable in clinical practice.
The role of hypertension and AHMs in PD has long been debated (Simon et al., 2007; Ton et al., 2007). Summary meta-analyses of epidemiologic studies indicated hypertension might increase the risk for PD (Hou et al., 2018; Chen et al., 2019). Meanwhile, some AHMs, such as calcium channel blockers (CCB), have emerged as prioritized repurposing options for PD prevention (Swart and Hurley, 2016; Katsi et al., 2021). However, considering the limited number of prospective cohort studies, these findings should be cautiously interpreted. Additionally, traditional observational studies are prone to residual confounding and reverse causation (Lawlor et al., 2008) and lack insights into the role of drug targets for specific AHMs.
Mendelian randomization (MR) is an analytical tool proposed to overcome some weaknesses associated with conventional observational studies on estimating the causal effects of risk factors (Davies et al., 2018). In this approach, genetic alleles are randomly assorted during meiosis and thus reducing bias resulting from conventional confounding factors or reverse causality. Meanwhile, analogous to a randomized controlled trial (RCT), the MR method has also been applied to develop a novel indication of the existing drugs by applying randomly assorted variants in the drug target gene (Storm et al., 2021).
Without preventive or disease-modifying interventions (Sardi et al., 2018), prevention strategies targeting modifiable risk factors and repurposing the existing drugs to novel indications are promising for PD. Hence, by using two-sample MR analyses, the aims of this study were to 1) investigate the direct causal link between blood pressure (BP) traits and PD risk and age at onset (AAO) and 2) examine the causal effect of different AHM classes on PD risk and AAO, all of which will benefit for PD in diagnosis, intervention, and prognostic assessment.
Methods
Data for exposure
Instrument selection for blood pressure
We extracted single nucleotide polymorphisms (SNP) of BP based on summary statistics in a genome-wide association study (GWAS) meta-analysis of 757,601 individuals from the International Consortium of Blood Pressure database and UK Biobank (Evangelou et al., 2018). In this study, BP traits incorporated systolic BP (SBP), diastolic BP (DBP), and pulse pressure (PP), which have been adjusted for AHM use by adding 15 and 10-mmHg to SBP and DBP, respectively. We restricted the set of SNPs to be significantly associated with the exposure with a p-value reaching genome-wide significance (p < 5 × 10–8), and the threshold of linkage disequilibrium (LD) was set at R2 = 0.001 using the 1,000 Genomes European reference panel. In addition, the above study adjusted effect estimates for body mass index (BMI), potentially introducing collider bias as BMI is causal for both elevated BP and PD, so a sensitivity analysis was performed using alternative UK Biobank GWAS summary statistics of SBP (N = 436419) and DBP (N = 436424) not adjusted for BMI(Mitchell et al., 2019).
Instrument selection for antihypertensive medications
Based on recent work by (Gill et al., 2019) and (Walker et al., 2019), we further chose genetic variants as proxies for the SBP lowering effects of first-line drugs for hypertension (Wright et al., 2018), including angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB), β-blockers (BB), CCB, and thiazide diuretic agents (Supplementary Table S1).
In the main analyses (Gill et al., 2019), encoding or regulatory regions (promoters and enhancers) of pharmacologically active targets for these drugs were identified through the DrugBank database (Wishart et al., 2018) (http://www.drugbank.ca/; DrugBank V5.1.9) (67 target genes in total, Supplementary Table S1) and the GeneCards online platform (Fishilevich et al., 2017) (https://www.genecards.org/; GenHancer V5.9) (Supplementary Table S2). For all the identified variants in each gene, only variants significantly associated with SBP (p < 5 × 10–8) and clumped to an LD threshold of R2 < 0.4 were considered candidate proxies for each drug class (Burgess et al., 2018; Nowak and Arnlov, 2018; Georgakis et al., 2020). More stringent LD thresholds (R2 < 0.1 and R2 < 0.001, respectively) were conducted in sensitivity analyses.
In the additional analyses (Walker et al., 2019), cis-expression quantitative trait loci (eQTL) of AHM target genes identified above by DrugBank for each tissue were extracted from the latest GTEx dataset (GTEx Consortium et al., 2017) (https://www.gtexportal.org/home/datasets; release V8, dbGaP Accession phs000424.v8.p2) (67 target genes in 49 tissues, Supplementary Table S3). In the positive control and instrument validation step, we excluded SNPs with a null effect on SBP for each AHM target gene in two-sample MR analyses (p > 0.05). The selection strategy of LD thresholds was similar to the main analyses.
For all the selected instrumental variables (IV) in this study, F-statistics were above 10, indicating that weak instrumental bias is minimal (Bowden et al., 2016).
Positive control analysis
Positive control analyses were performed to validate the IV selection in our study. Firstly, for the IVs of blood traits and AHM targets identified by “encoding region-based method”, we examined the association of exposures of interest with coronary heart disease (CHD) and stroke because hypertension is an established risk factor for both CHD and stroke. In addition, for the IVs of AHM targets identified by “eQTL-based method,”, we examined the causative association of exposures of interest with SBP since the BP-lowering effect is the well-proven effect of AHMs.
Data for outcome
For the main outcome of PD risk, we used the largest and most comprehensive summary statistics data from a meta-analysis GWAS performed by the International Parkinson’s Disease Genomics Consortium, including 33,674 PD cases and 449,056 controls (Nalls et al., 2019). In addition, previous studies indicate that the genetic risk of PD is correlated with PD AAO (Escott-Price et al., 2015; Smolders et al., 2021), so PD AAO was used as the secondary outcome from a GWAS meta-analysis of 28,568 cases (Blauwendraat et al., 2019).
For the positive control outcomes, GWAS summary data for CHD were based on the CARDIoGRAMplusC4D Consortium, which conducted a meta-analysis of 60,801 CHD cases and 123,504 controls (Nikpay et al., 2015). Summary data of stroke were drawn from a recent large-scale meta-analysis of GWAS (MEGASTROKE) confined to European populations of 40,585 cases and 406,111 controls (Malik et al., 2018).
Primary analysis
Two-sample MR analyses were performed in this study. Three assumptions were established, including that the genetic instruments were associated with the exposure of interest, were independent of potential confounders, and could only affect the outcome through the exposure of interest and not through other pathways. Firstly, we examined the causal effect of BP traits on PD (Figure 1A). Second, we examined the causal effect of overall AHM and different AHMs on PD (Figure 1B). The Wald ratio test was used to calculate the causative effect when a single IV was available, while the multiplicative random effects inverse variance weighted (IVW) method was performed as the main analysis when multiple IVs were available (Lee, 2020). IVW generalized the Wald ratio through a meta-analysis process, and it is the most efficient analysis method with valid IVs because it accounts for heterogeneity in the variant-specific causal estimates (Burgess et al., 2019; Lee, 2020).

FIGURE 1. Flow diagram of the process for the two-sample MR analyses of blood pressure (A) and antihypertensives (B) with Parkinson’s disease. Abbreviations: IV, instrumental variable; SBP, systolic blood pressure; DBP, diastolic blood pressure; PP, pulse pressure; ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; BB, β-blockers; CCB, calcium channel blockers; CHD, coronary heart disease; MR, Mendelian randomization; IVW, inverse variance weighted; LD, linkage disequilibrium; PD, Parkinson’s disease; AAO, age at onset.
Sensitivity analysis
Sensitivity analyses accounting for certain violations of the MR assumptions due to different pleiotropy scenarios, including the MR-Egger regression, weighted median, simple mode, and weighted mode methods, were conducted to assess the robustness of the findings (Burgess et al., 2019). Furthermore, we used the MR Egger intercept and Cochran Q statistic to test the presence of directional pleiotropy and heterogeneity, respectively. If outlier IVs were detected using the MR pleiotropy residual sum and outlier (MR-PRESSO) test (Verbanck et al., 2018), the IVW MR analysis was performed again after removing outliers. The leave-one-out analysis was conducted within the IVW method to assess the influence of individual variants on the observed association. To identify specific drug targets driving the causal effect, we also examined the causal effect of each drug target within different AHM classes on PD.
Statistical analysis
The causal effect of SBP, DBP, and PP on outcomes was scaled to a 10-mmHg increment in BP levels. In contrast, associations of AHMs with outcomes were scaled to a 10-mmHg decrease in SBP to represent the therapeutic inhibition of different AHM classes. The association is considered to be significant after Bonferroni correction for BP traits [p < 0.016 (0.05/3)] and AHMs [p < 0.008 (0.05/6)]. A p-value above 0.016/0.008 but below 0.05 was considered suggestive of evidence for a potential association. False-discovery rate was used to correct for multiple testing when calculating the effect of a single drug target within different AHMs on PD, and an adjusted p-value of IVW or Wald ratio less than 0.05 is considered to be significant. The main statistical analyses were performed using ‘TwoSampleMR’ (V.0.5.6) in the R package (V.4.1.3) (Hemani et al., 2018).
Results
Positive control analyses
As shown in Figure 2A, genetically elevated SBP, DBP, and PP were all positively associated with the risk of CHD and stroke (per 10-mmHg increment, all p values <0.016), consistent with previous evidence (Georgakis et al., 2020; Wan et al., 2021). When looking into AHMs (Figure 2B), overall AHM, BB, CCB, and Thiazides were inversely associated with the risk of CHD (Gill et al., 2019) (per 10-mmHg lower, all p values <0.008) except for ACEI. Besides, overall AHM, ACEI, BB, CCB, and Thiazides were associated with a reduced risk of stroke (per 10-mmHg lower, all p values <0.05). In summary, positive control analyses confirmed the validity of the predefined IV selection methodology.
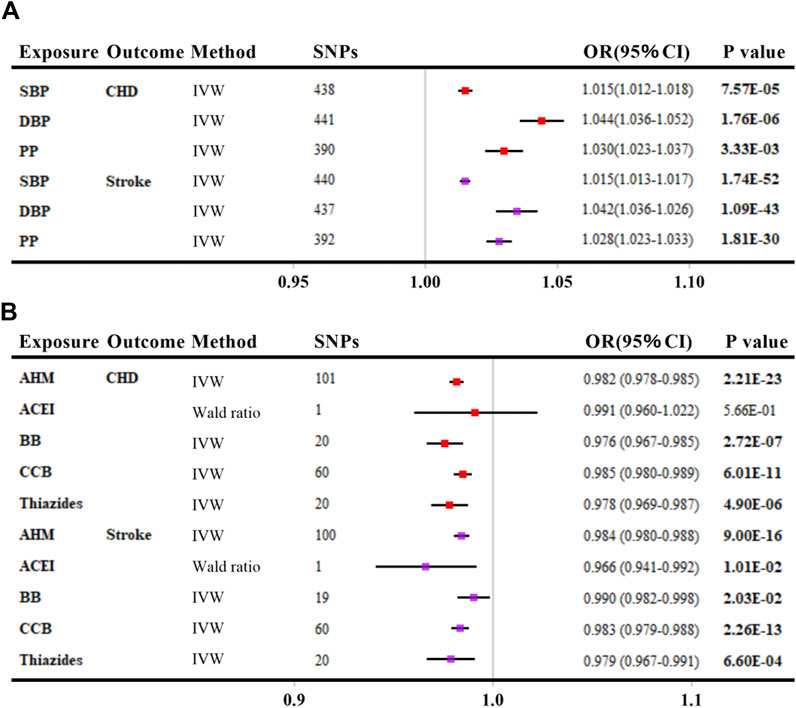
FIGURE 2. Positive control analyses investigating the effects of blood pressure (A) and antihypertensive medications (B) on coronary heart disease and stroke. The linkage disequilibrium thresholds of R2 were set as 0.001 for BP and 0.4 for AHMs. OR and 95% CIs were scaled to each 10-mmHg increment for BP traits and 10-mmHg lower in SBP for AHMs by ‘encoding region-based method’. p-value less than 0.05 was depicted in bold. Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; PP, pulse pressure; CHD, coronary heart disease; IVW, inverse variance weighted; AHM, antihypertensive medications; ACEI, angiotensin-converting enzyme inhibitors; BB, β-blockers; CCB, calcium channel blockers; SNP, single nucleotide polymorphism; OR, odds ratio; CI, confidence interval.
Genetically determined BP with PD risk (main outcome)
We found no association of genetically elevated SBP, DBP, or PP with PD risk, with p values greater than 0.05 in all MR analyses (per 10-mmHg increment) (Figure 3A and Supplementary Table S4). The effects of SBP and DBP on the risk of PD were also similar using alternative genetic instruments derived from the UK Biobank, which were not adjusted for BMI (Supplementary Table S5).
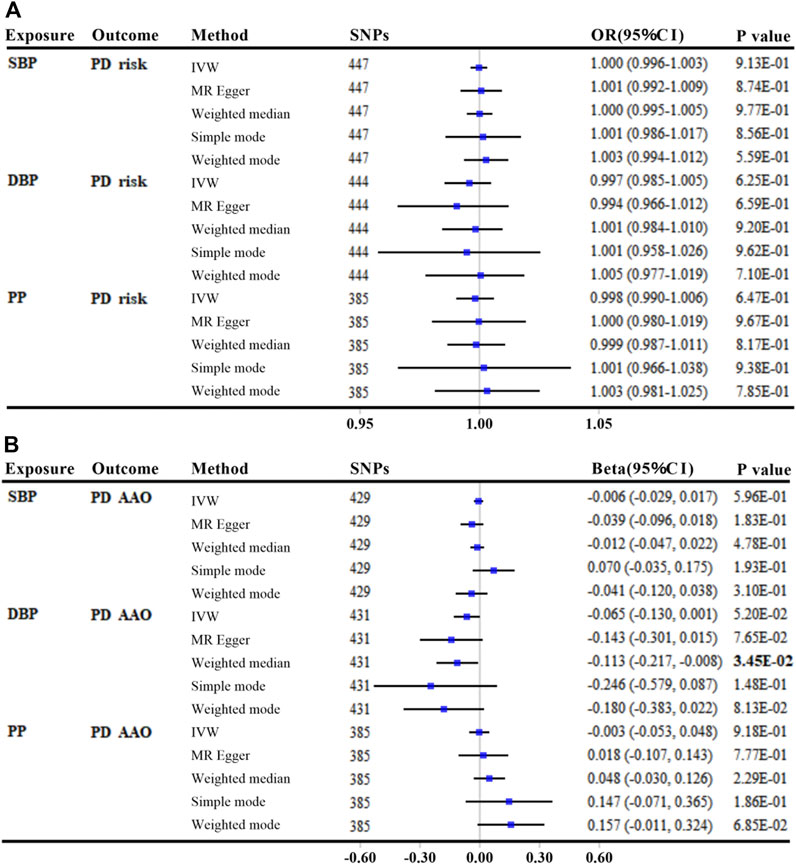
FIGURE 3. MR analyses between genetically predicted blood pressure and Parkinson’s disease risk (A) and age at onset (B). The linkage disequilibrium threshold of R2 was set as 0.001. OR/Beta & 95% CI was scaled to each 10-mmHg increment in BP traits. p-value less than 0.05 was depicted in bold. Abbreviations: MR, Mendelian randomization; SBP, systolic blood pressure; DBP, diastolic blood pressure; PP, pulse pressure; PD, Parkinson’s disease; IVW, inverse variance weighted; AAO, age at onset; SNP, single nucleotide polymorphism; OR, odds ratio; CI, confidence interval.
Genetically determined BP with PD AAO (secondary outcome)
There was little evidence of an association between either genetically predicted SBP or PP with PD AAO (all p values >0.05, per 10-mmHg increment) (Figure 3B and Supplementary Table S6). Using secondary GWAS statistics of SBP and DBP from the UK Biobank, we get similar results that each 10-mmHg increment in DBP was suggestively associated with a younger PD AAO only by MR Egger method (Beta: −3.734; 95% CI: −6.686, −0.783; p = 1.38E-02) (Supplementary Table S7).
Genetically therapeutic inhibition of AHM with PD risk (main outcome)
In the main analyses using ‘encoding region-based method’, there was no evidence that reducing SBP affected the risk of PD via the protein targets in overall AHM or different AHM classes (Figure 4 and Supplementary Table S8). When more stringent LD thresholds were set at R2 < 0.1 and R2 < 0.001, the results showed consistently null associations with the primary analyses for overall AHM and different AHMs (Supplementary Table S9).
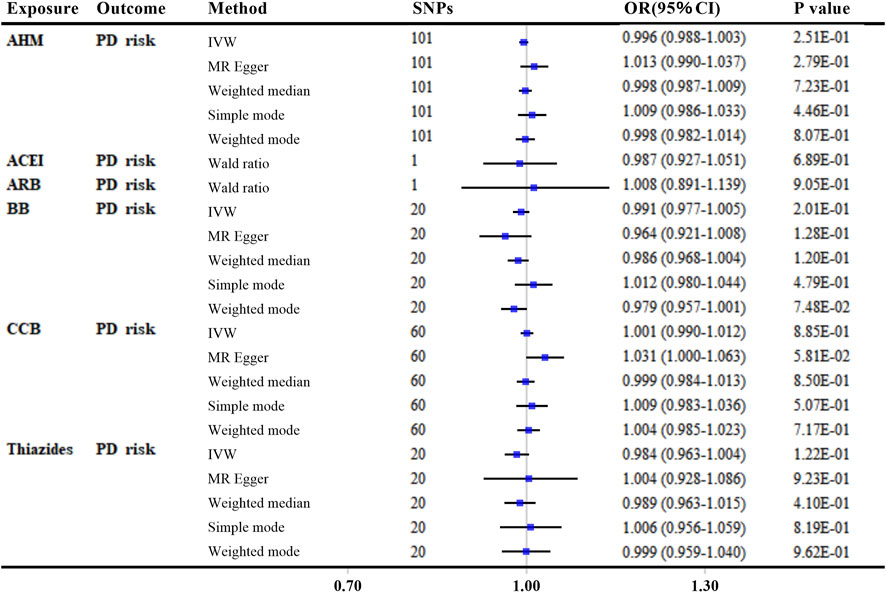
FIGURE 4. MR analyses of genetically predicted antihypertensive medications with Parkinson’s disease risk by “encoding region-based method”. Genetic proxies for AHMs were selected by “encoding region-based method”. The linkage disequilibrium threshold of R2 was set as 0.4. OR and 95% CI was scaled to each 10-mmHg lower in SBP. p-value less than 0.05 was depicted in bold. Abbreviations: MR, Mendelian randomization; AHM, antihypertensive medications; ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; BB, β-blockers; CCB, calcium channel blockers; PD, Parkinson’s disease; IVW, inverse variance weighted; SNP, single nucleotide polymorphism; OR, odds ratio; CI, confidence interval.
In the additional analyses using “eQTL-based method”, neither genetic proxies for overall AHM nor those of different AHMs were associated with PD risk (all p values >0.05, Supplementary Figure S1 and Supplementary Table S8). Additionally, MR analyses using more stringent LD thresholds (R2 < 0.1 and R2 < 0.001, respectively) remained non-significant for associations of overall AHM and different AHMs with PD risk (all p values >0.05, Supplementary Table S10).
Furthermore, individual target analyses only identified CACNA1H (OR: 1.431; 95% CI: 1.215–1.686; Adjusted p = 5.37E-04; per 10-mmHg lower) by “eQTL-based method” as a significant target that may drive the causal effect of CCB on PD risk (Supplementary Table S11).
Genetically therapeutic inhibition of AHM with PD AAO (secondary outcome)
In the primary analyses using “encoding region-based method”, although it was suggestive that genetic proxies for BB were associated with a delayed PD AAO (Beta: 0.115; 95% CI: 0.021,0.208; p = 1.63E-2; per 10-mmHg lower; R2 < 0.4) (Supplementary Figure S2 and Supplementary Table S12), more stringent LD thresholds (R2 < 0.1 and R2 < 0.001, respectively) failed to support this association in all MR methods (all p values >0.05, Supplementary Table S13).
In the secondary analyses using ‘eQTL-based method’, the results were suggestive of an association between thiazides and PD AAO only by IVW method (Beta: −0.287; 95% CI: −0.545, −0.030; p = 2.86E-02, Supplementary Figure S3 and Supplementary Table S12). However, restricting LD thresholds to R2 < 0.1 or R2 < 0.001 made the results null (Supplementary Table S14).
Analyzing individual targets identified ADRB1 (Beta: 0.192; 95% CI: 0.070, 0.314; adjusted p = 3.19E-02; per 10-mmHg lower) by ‘encoding region-based method’ and KCNH2 (Beta: −0.650; 95% CI: −0.983, −0.318; adjusted p = 3.64E-03; per 10-mmHg lower) by “eQTL-based method” as significant targets that may drive the causal effect of BB on PD AAO (Supplementary Table S15).
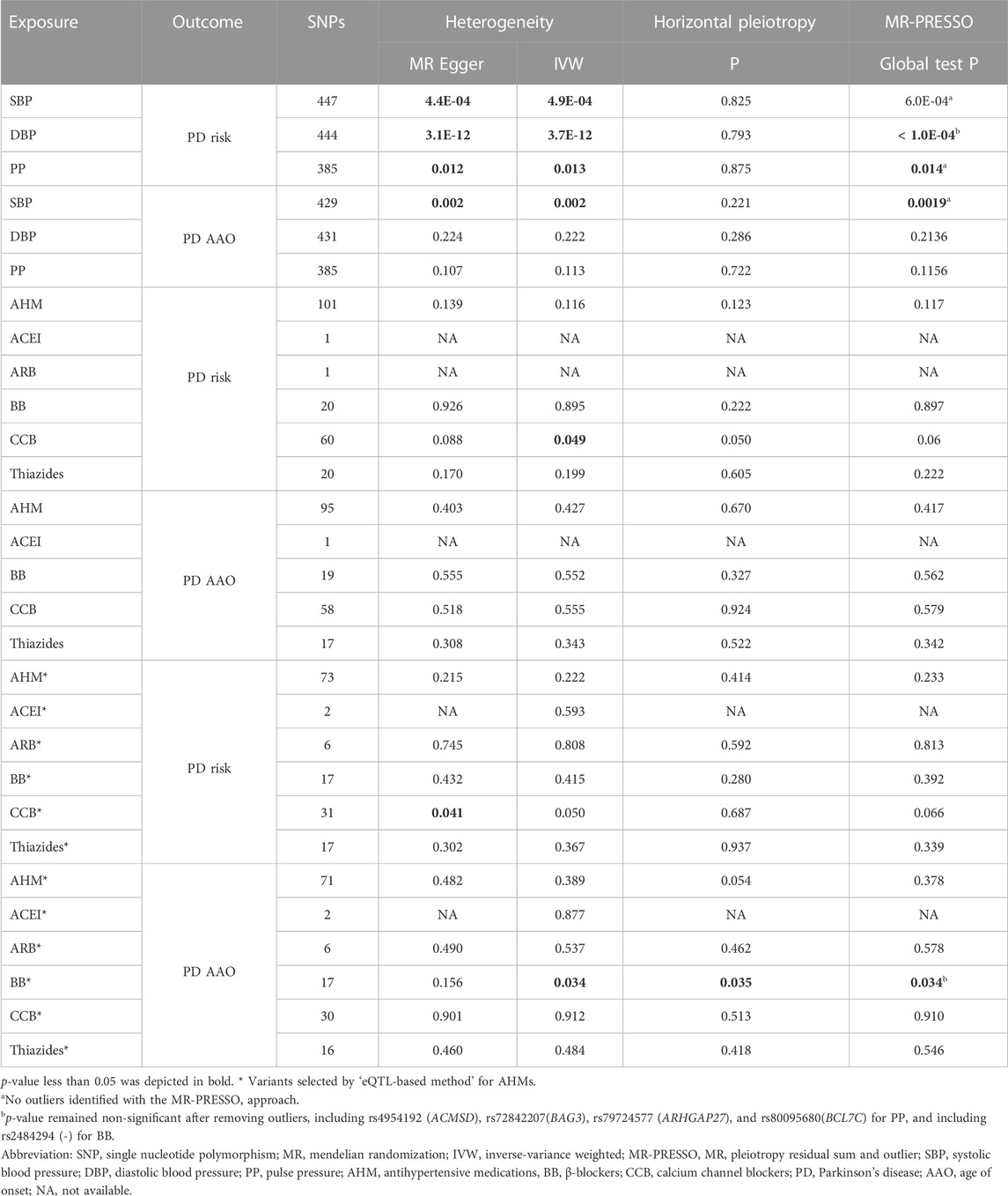
Heterogeneity and horizontal pleiotropy
Although there was limited evidence of heterogeneity and horizontal pleiotropy in MR analyses for BP traits and different AHMs, the MR-PRESSO approach and leave-one-out analyses indicated that our estimates were overall stable (Table 1 and Supplementary Figures S4–S7).
Discussion
To the best of our knowledge, the current study firstly assessed the causal effects of BP and antihypertensive medications on PD using two-sample MR analyses. However, our results failed to support that BP was causally associated with PD risk and AAO. There was also limited evidence that lowering SBP via the targets of first-line antihypertensive drugs affected PD. In summary, our study could help validate the role of BP in the pathogenesis of PD and avoid overestimating the repurposing role of antihypertensive drugs in PD prevention.
Whether hypertension represents a risk factor for PD has not been fully elucidated for a long time, and observational studies in the literature that examined the risk for PD yielded conflicting results. Two studies reported that the risk of PD was not significantly related to high BP(Simon et al., 2007; Tan et al., 2008). In comparison, two other studies suggested that high BP slightly increased the risk of PD (Qiu et al., 2011; Lai et al., 2014), at least in women with arterial hypertension (Qiu et al., 2011). In comparison, another study instead reported that high BP exerted a protective role against the development of PD (Paganini-Hill, 2001). However, all these studies differed in sample size, geographic origin, and duration of the follow-up of the cohort population. Furthermore, concerning the role of antihypertensive drugs in the risk of developing PD, studies showed that among different classes of AHMs, dihydropyridine CCB, but not non-dihydropyridine CCB, may be associated with a reduced risk of PD (Tseng et al., 2021). For β-Adrenoceptor acting agents, a recent meta-analysis of epidemiologic studies suggests that the intake of β-adrenoceptor antagonists, including propranolol and metoprolol, may serve as a risk factor for PD development (Saengphatrachai et al., 2021).
However, PD may have a long prodromal stage up to decades before PD diagnosis, characterized by non-motor dysfunction such as SBP drop, sleep disturbances, and constipation (Postuma et al., 2013; Chen and Ritz, 2018). The prodromal population may be more susceptible to the diagnosis of abnormal BP and an altered tendency to use AHMs, resulting in the inability to accurately define the history of hypertension and AHMs before PD diagnosis in previous cohort studies. Apart from reverse causation, earlier epidemiologic studies could have been subject to unmeasured confounders, such as socioeconomic status, mood disorders, physical activity, and other drug use.
Due to the limitations of observational studies, MR analyses provide an attractive prospect to identify risk or protective factors. Although we provide robust results for the null association of BP and AHMs with PD using primary and secondary MR analyses, we should interpret these results cautiously. In this study, we assumed that the estimated effect of the targets of overall AHM or different AHMs on PD risk or AAO acted through SBP-lowering, but there is potentially an alternative mechanism by which the targets can affect PD. Hence, our null results for overall AHM and common AHM classes do not rule out possible benefits via competing mechanisms by using this drug for PD prevention. Future larger GWAS will be needed to verify that our results did not generate by accident. Furthermore, more precise mapping and mechanistic studies of targets for AHMs will also help elucidate the pathogenesis of PD.
The present study has some strengths. First, positive control analyses were performed to validate the IV selection strategies and confirmed that the approach was appropriate. Additionally, genetic proxies for AHMs were hypothesized to act through the SBP-lowering effect, which would help us understand the antihypertensive role in PD etiology. Thirdly, we included pharmacologically active targets with known biological functions to proxy each AHM class, contributing to better homogeneity of selected IVs. Last, we included AAO as a secondary outcome for PD risk, further supporting the findings from the main outcome.
This study has several limitations. First, MR analysis assumes that the SNPs selected as IVs for BP traits and AHMs influence PD only through the exposure of interest (no pleiotropic effects). Although there did exist some pleiotropic effects for genetic proxies of BP traits, BB, and CCB in our study, multiple methods confirmed the stability of our results. Second, considering the limited genetic proxies identified, the power of MR analyses for ACEI and ARB may be limited. Third, since all of the participants are mainly of European ancestry, the results of this study are not necessarily valid for other ethnic groups.
In conclusion, this study found little evidence that BP and antihypertensive drugs would affect PD risk and AAO. Future studies should consider our research, combined with other sources of evidence, to obtain a reliable answer about the role of hypertension and the potential repurposing of antihypertensives for PD prevention.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
ZJ, X-JG, BC, and Y-PC conceived and designed the study. ZJ, X-JG, W-MS, Q-QD, Y-LR, J-RL, and L-YC extracted the data. ZJ and X-JG contributed to the statistical analysis. ZJ wrote the first draft of the manuscript. ZJ, X-JG, YW, BC, and Y-PC revised and discussed the final edition.
Funding
This study was supported by the Science and Technology Bureau Fund of Sichuan Province (Grant No. 2021YFS0051 to YW and Grant No. 2023YFS0269 to Y-PC), the National Natural Science Fund of Sichuan (Grant No.2022NSFSC0749 to BC), and the National Natural Science Fund of China (Grant No. 81971188 to Y-PC).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1107248/full#supplementary-material
References
Blauwendraat, C., Heilbron, K., Vallerga, C. L., Bandres-Ciga, S., von Coelln, R., Pihlstrom, L., et al. (2019). Parkinson's disease age at onset genome-wide association study: Defining heritability, genetic loci, and alpha-synuclein mechanisms. Mov. Disord. 34 (6), 866–875. doi:10.1002/mds.27659
Bowden, J., Del Greco, M. F., Minelli, C., Davey Smith, G., Sheehan, N. A., and Thompson, J. R. (2016). Assessing the suitability of summary data for two-sample mendelian randomization analyses using MR-egger regression: the role of the I2 statistic. Int. J. Epidemiol. 45 (6), 1961–1974. doi:10.1093/ije/dyw220
Burgess, S., Ference, B. A., Staley, J. R., Freitag, D. F., Mason, A. M., Nielsen, S. F., et al. (2018). Association of LPA variants with risk of coronary disease and the implications for lipoprotein(a)-lowering therapies: A mendelian randomization analysis. JAMA Cardiol. 3 (7), 619–627. doi:10.1001/jamacardio.2018.1470
Burgess, S., Davey Smith, G., Davies, N. M., Dudbridge, F., Gill, D., Glymour, M. M., et al. (2019). Guidelines for performing Mendelian randomization investigations. Wellcome Open Res. 4, 186. doi:10.12688/wellcomeopenres.15555.2
Chen, H., and Ritz, B. (2018). The search for environmental causes of Parkinson's disease: Moving forward. J. Park. Dis. 8, S9–S17. doi:10.3233/JPD-181493
Chen, J., Zhang, C., Wu, Y., and Zhang, D. (2019). Association between hypertension and the risk of Parkinson's disease: A meta-analysis of analytical studies. Neuroepidemiology 52 (3-4), 181–192. doi:10.1159/000496977
Davies, N. M., Holmes, M. V., and Davey Smith, G. (2018). Reading mendelian randomisation studies: A guide, glossary, and checklist for clinicians. BMJ 362, k601. doi:10.1136/bmj.k601
Escott-Price, V., Nalls, M. A., Morris, H. R., Lubbe, S., Brice, A., et al. International Parkinson's Disease Genomics Consortium (2015). Polygenic risk of Parkinson disease is correlated with disease age at onset. Ann. Neurol. 77 (4), 582–591. doi:10.1002/ana.24335
Evangelou, E., Warren, H. R., Mosen-Ansorena, D., Mifsud, B., Pazoki, R., Gao, H., et al. (2018). Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat. Genet. 50 (10), 1412–1425. doi:10.1038/s41588-018-0205-x
Fishilevich, S., Nudel, R., Rappaport, N., Hadar, R., Plaschkes, I., Iny Stein, T., et al. (2017). GeneHancer: Genome-wide integration of enhancers and target genes in GeneCards. Database (Oxford) 2017, bax028. doi:10.1093/database/bax028
GBD 2017 Risk Factor Collaborators (2018). Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: A systematic analysis for the global burden of disease study 2017. Lancet 392 (10159), 1923–1994. doi:10.1016/S0140-6736(18)32225-6
Georgakis, M. K., Gill, D., Webb, A. J. S., Evangelou, E., Elliott, P., Sudlow, C. L. M., et al. (2020). Genetically determined blood pressure, antihypertensive drug classes, and risk of stroke subtypes. Neurology 95 (4), e353–e361. doi:10.1212/WNL.0000000000009814
Gill, D., Georgakis, M. K., Koskeridis, F., Jiang, L., Feng, Q., Wei, W. Q., et al. (2019). Use of genetic variants related to antihypertensive drugs to inform on efficacy and side effects. Circulation 140 (4), 270–279. doi:10.1161/CIRCULATIONAHA.118.038814
GTEx ConsortiumLaboratory, Data Analysis &Coordinating Center (LDACC)—Analysis Working GroupStatistical Methods groups—Analysis Working GroupEnhancing GTEx (eGTEx) groupsNIH Common FundNIH/NCIet al. (2017). Genetic effects on gene expression across human tissues. Nature 550 (7675), 204–213. doi:10.1038/nature24277
Hemani, G., Zheng, J., Elsworth, B., Wade, K. H., Haberland, V., Baird, D., et al. (2018). The MR-Base platform supports systematic causal inference across the human phenome. Elife 7, e34408. doi:10.7554/eLife.34408
Hou, L., Li, Q., Jiang, L., Qiu, H., Geng, C., Hong, J. S., et al. (2018). Hypertension and diagnosis of Parkinson's disease: A meta-analysis of cohort studies. Front. Neurol. 9, 162. doi:10.3389/fneur.2018.00162
Katsi, V., Papakonstantinou, I., Solomou, E., Antonopoulos, A. S., Vlachopoulos, C., and Tsioufis, K. (2021). Management of hypertension and blood pressure dysregulation in patients with Parkinson's disease-a systematic review. Curr. Hypertens. Rep. 23 (5), 26. doi:10.1007/s11906-021-01146-5
Lai, S. W., Liao, K. F., Lin, C. L., Lin, C. C., and Sung, F. C. (2014). Hearing loss may be a non-motor feature of Parkinson's disease in older people in Taiwan. Eur. J. Neurol. 21 (5), 752–757. doi:10.1111/ene.12378
Lawlor, D. A., Harbord, R. M., Sterne, J. A., Timpson, N., and Davey Smith, G. (2008). Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Stat. Med. 27 (8), 1133–1163. doi:10.1002/sim.3034
Lee, Y. H. (2020). Overview of mendelian randomization analysis. J. Rheum. Dis. 27 (4), 241–246. doi:10.4078/jrd.2020.27.4.241
Malik, R., Chauhan, G., Traylor, M., Sargurupremraj, M., Okada, Y., Mishra, A., et al. (2018). Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat. Genet. 50 (4), 524–537. doi:10.1038/s41588-018-0058-3
Mitchell, R., Elsworth, B., Mitchell, R., Raistrick, C., Paternoster, L., Hemani, G., et al. (2019). MRC IEU UK Biobank GWAS pipeline version 2. Bristol, United Kingdom: University of Bristol. doi:10.5523/bris.pnoat8cxo0u52p6ynfaekeigi
Nalls, M. A., Blauwendraat, C., Vallerga, C. L., Heilbron, K., Bandres-Ciga, S., Chang, D., et al. (2019). Identification of novel risk loci, causal insights, and heritable risk for Parkinson's disease: A meta-analysis of genome-wide association studies. Lancet Neurol. 18 (12), 1091–1102. doi:10.1016/S1474-4422(19)30320-5
Nikpay, M., Goel, A., Won, H. H., Hall, L. M., Willenborg, C., Kanoni, S., et al. (2015). A comprehensive 1,000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat. Genet. 47 (10), 1121–1130. doi:10.1038/ng.3396
Nowak, C., and Arnlov, J. (2018). A Mendelian randomization study of the effects of blood lipids on breast cancer risk. Nat. Commun. 9 (1), 3957. doi:10.1038/s41467-018-06467-9
Paganini-Hill, A. (2001). Risk factors for Parkinson's disease: the leisure world cohort study. Neuroepidemiology 20 (2), 118–124. doi:10.1159/000054770
Postuma, R. B., Gagnon, J. F., Pelletier, A., and Montplaisir, J. (2013). Prodromal autonomic symptoms and signs in Parkinson's disease and dementia with Lewy bodies. Mov. Disord. 28 (5), 597–604. doi:10.1002/mds.25445
Pringsheim, T., Jette, N., Frolkis, A., and Steeves, T. D. (2014). The prevalence of Parkinson's disease: A systematic review and meta-analysis. Mov. Disord. 29 (13), 1583–1590. doi:10.1002/mds.25945
Qiu, C., Hu, G., Kivipelto, M., Laatikainen, T., Antikainen, R., Fratiglioni, L., et al. (2011). Association of blood pressure and hypertension with the risk of Parkinson disease: the national FINRISK study. Hypertension 57 (6), 1094–1100. doi:10.1161/HYPERTENSIONAHA.111.171249
Saengphatrachai, W., Praditukrit, K., Owattanapanich, W., Pitakpatapee, Y., and Srivanitchapoom, P. (2021). The association between developing Parkinson's disease and beta-adrenoceptor acting agents use: A systematic review and meta-analysis. J. Neurol. Sci. 430, 120009. doi:10.1016/j.jns.2021.120009
Sardi, S. P., Cedarbaum, J. M., and Brundin, P. (2018). Targeted therapies for Parkinson's disease: From genetics to the clinic. Mov. Disord. 33 (5), 684–696. doi:10.1002/mds.27414
Simon, K. C., Chen, H., Schwarzschild, M., and Ascherio, A. (2007). Hypertension, hypercholesterolemia, diabetes, and risk of Parkinson disease. Neurology 69 (17), 1688–1695. doi:10.1212/01.wnl.0000271883.45010.8a
Smolders, S., Philtjens, S., Crosiers, D., Sieben, A., Hens, E., Heeman, B., et al. (2021). Contribution of rare homozygous and compound heterozygous VPS13C missense mutations to dementia with Lewy bodies and Parkinson's disease. Acta Neuropathol. Commun. 9 (1), 25. doi:10.1186/s40478-021-01121-w
Storm, C. S., Kia, D. A., Almramhi, M. M., Bandres-Ciga, S., Finan, C., International Parkinson's Disease Genomics, C., et al. (2021). Finding genetically-supported drug targets for Parkinson's disease using Mendelian randomization of the druggable genome. Nat. Commun. 12 (1), 7342. doi:10.1038/s41467-021-26280-1
Swart, T., and Hurley, M. J. (2016). Calcium Channel antagonists as disease-modifying therapy for Parkinson's disease: Therapeutic rationale and current status. CNS Drugs 30 (12), 1127–1135. doi:10.1007/s40263-016-0393-9
Tan, L. C., Koh, W. P., Yuan, J. M., Wang, R., Au, W. L., Tan, J. H., et al. (2008). Differential effects of black versus green tea on risk of Parkinson's disease in the Singapore Chinese Health Study. Am. J. Epidemiol. 167 (5), 553–560. doi:10.1093/aje/kwm338
Ton, T. G., Heckbert, S. R., Longstreth, W. T., Rossing, M. A., Kukull, W. A., Franklin, G. M., et al. (2007). Calcium channel blockers and beta-blockers in relation to Parkinson's disease. Park. Relat. Disord. 13 (3), 165–169. doi:10.1016/j.parkreldis.2006.08.011
Tseng, Y. F., Lin, H. C., Chao, J. C., Hsu, C. Y., and Lin, H. L. (2021). Calcium Channel blockers are associated with reduced risk of Parkinson's disease in patients with hypertension: A population-based retrospective cohort study. J. Neurol. Sci. 424, 117412. doi:10.1016/j.jns.2021.117412
Verbanck, M., Chen, C. Y., Neale, B., and Do, R. (2018). Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 50 (5), 693–698. doi:10.1038/s41588-018-0099-7
Walker, V. M., Kehoe, P. G., Martin, R. M., and Davies, N. M. (2019). Repurposing antihypertensive drugs for the prevention of alzheimer's disease: A mendelian randomization study. Int. J. Epidemiol. 49, 1132–1140. doi:10.1093/ije/dyz155
Wan, E. Y. F., Fung, W. T., Schooling, C. M., Au Yeung, S. L., Kwok, M. K., Yu, E. Y. T., et al. (2021). Blood pressure and risk of cardiovascular disease in UK Biobank: A mendelian randomization study. Hypertens. (dallas, tex) 77 (2), 367–375. doi:10.1161/HYPERTENSIONAHA.120.16138
Wishart, D. S., Feunang, Y. D., Guo, A. C., Lo, E. J., Marcu, A., Grant, J. R., et al. (2018). DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 46 (D1), D1074–D1082. doi:10.1093/nar/gkx1037
Keywords: Parkinson’s disease, age at onset, Mendelian randomization, blood pressure, antihypertensive medications
Citation: Jiang Z, Gu X-J, Su W-M, Duan Q-Q, Ren Y-L, Li J-R, Chi L-Y, Wang Y, Cao B and Chen Y-P (2023) Protective effect of antihypertensive drugs on the risk of Parkinson’s disease lacks causal evidence from mendelian randomization. Front. Pharmacol. 14:1107248. doi: 10.3389/fphar.2023.1107248
Received: 24 November 2022; Accepted: 13 February 2023;
Published: 23 February 2023.
Edited by:
Dubravka Svob Strac, Rudjer Boskovic Institute, CroatiaReviewed by:
Félix Javier Jiménez-Jiménez, Hospital Universitario del Sureste, SpainJiguang Wang, Shanghai Jiao Tong University, China
Copyright © 2023 Jiang, Gu, Su, Duan, Ren, Li, Chi, Wang, Cao and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bei Cao, Y2FvYmVpLm5ldXJvbG9neUBmb3htYWlsLmNvbQ==; Yong-Ping Chen, eW9uZ3BpbmcuY2hlbkB3Y2hzY3UuY24=
†These authors have contributed equally to this work
 Zheng Jiang
Zheng Jiang Xiao-Jing Gu4†
Xiao-Jing Gu4† Wei-Ming Su
Wei-Ming Su Bei Cao
Bei Cao Yong-Ping Chen
Yong-Ping Chen