- 1Department of Pharmacy, China-Japan Union Hospital of Jilin University, Jilin University, Changchun, Jilin, China
- 2Department of Immunology, College of Basic Medical Sciences, Jilin University, Changchun, Jilin, China
- 3Department of Nutrition and Food Hygiene, School of Public Health, Jilin University, Changchun, Jilin, China
- 4Department of Pharmacy, Siping Tumor Hospital, Siping, Jilin, China
Introduction: During the coronavirus disease 2019 (COVID-19) pandemic, a large number of critically ill and severe COVID-19 patients meet the diagnostic criteria for sepsis and even septic shock. The treatments for COVID-19 patients with sepsis are still very limited. For sepsis, improving ventilation is one of the main treatments. Nitric oxide (NO) and almitrine have been reported to improve oxygenation in patients with “classical” sepsis. Here, we conducted a systematic review and meta-analysis to evaluate the efficacy and safety of NO, almitrine, and the combination of both for COVID-19 (at the edge of sepsis) patients.
Method: A systematic search was performed on Embase, PubMed, the Cochrane Library, the Web of Science, Wanfang Data, and China National Knowledge Infrastructure. Randomized clinical trials, cohort studies, cross-sectional studies, case-control studies, case series, and case reports in COVID-19 patients with suspected or confirmed sepsis were performed. Study characteristics, patient demographics, interventions, and outcomes were extracted from eligible articles.
Results: A total of 35 studies representing 1,701 patients met eligibility criteria. Inhaled NO did not affect the mortality (OR 0.96, 95% CI 0.33–2.8, I2 = 81%, very low certainty), hospital length of stay (SMD 0.62, 95% CI 0.04–1.17, I2 = 83%, very low certainty), and intubation needs (OR 0.82, 95% CI 0.34–1.93, I2 = 56%, very low certainty) of patients with COVID-19 (at the edge of sepsis). Meanwhile, almitrine did not affect the mortality (OR 0.44, 95% CI 0.17–1.13, low certainty), hospital length of stay (SMD 0.00, 95% CI -0.29–0.29, low certainty), intubation needs (OR 0.94, 95% CI 0.5–1.79, low certainty), and SAEs (OR 1.16, 95% CI 0.63–2.15, low certainty). Compared with pre-administration, the PaO2/FiO2 of patients with NO (SMD-0.87, 95% CI -1.08–0.66, I2 = 0%, very low certainty), almitrine (SMD-0.73, 95% CI-1.06–0.4, I2 = 1%, very low certainty), and the combination of both (SMD-0.94, 95% CI-1.71–0.16, I2 = 47%, very low certainty) increased significantly.
Conclusion: Inhaled NO, almitrine, and the combination of the two drugs improved oxygenation significantly, but did not affect the patients’ mortality, hospitalization duration, and intubation needs. Almitrine did not significantly increase the patients’ SAEs. Well-designed high-quality studies are needed for establishing a stronger quality of evidence.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=367667, identifier CRD42022367667.
1 Introduction
Sepsis represents a syndrome characterized by pathological, physiological, and biochemical abnormalities instigated by infection (Singer et al., 2016). The clinical manifestations exhibited by a substantial number of critically ill and severely affected patients with coronavirus disease 2019 (COVID-19) meet the diagnostic criteria for sepsis and, in some instances, septic shock. Hypoxemia emerges as a characteristic symptom among individuals with severe COVID-19. The principal mechanism underlying hypoxemia involves the inflammatory-induced pulmonary shunt, along with the loss of surfactant due to alveolar congestion and alveolar collapse (Bersten et al., 1998). Hypoxic pulmonary vasoconstriction (HPV) denotes the inherent mechanism responsible for the automatic regulation of lung oxygen deficiency. In the presence of inadequate oxygen levels within the lung, HPV orchestrates the equilibrium of blood gas ratios, thereby mitigating the incidence of hypoxia. Simultaneously, pulmonary artery pressure serves as a strong negative prognostic indicator in acute respiratory distress syndrome (ARDS) (Squara et al., 1998). Consequently, for patients with severe COVID-19, particularly those with sepsis, augmenting ventilation status assumes paramount significance in addition to interventions such as fluid resuscitation, administration of vasoactive medications, anti-infective therapies, and other treatments.
Inhaled nitric oxide (NO), a specific pulmonary vasodilator initially employed in patients with pulmonary hypertension, exhibits limited systemic activity due to its rapid dissemination into the bloodstream. Consequently, the vasodilatory effects of NO primarily target the pulmonary circulation. By redistributing blood flow to well-ventilated regions, inhaled NO enhances the ventilator–perfusion ratio. In patients with ARDS, the administration of inhaled NO has shown improvements in gas exchange, alleviation of pulmonary hypertension, and mitigation of right ventricular failure (Frostell et al., 1991; Rossaint et al., 1993; Squara et al., 1998). In vitro studies have demonstrated that NO donors possess the ability to suppress the replication of certain viruses, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Lisi et al., 2021). In addition to these advantageous influences, NO exhibits immunomodulatory and anti-oxidant properties, potentially exerting a constructive impact on COVID-19 (Mir and Maurya, 2021; Mir and Maurya, 2022).
Inflammation has the potential to disrupt the intrinsic mechanism of HPV (Jolin and Bjertnaes, 1991), thereby contributing to ventilation/perfusion (V/Q) mismatch (Sylvester et al., 2012). Consequently, the exploration of selective pulmonary vasoconstrictors has emerged as a consideration. Almitrine, a specific pulmonary vasoconstrictor, has demonstrated the ability to enhance oxygenation in patients with ARDS by augmenting hypoxic pulmonary vasoconstriction (Mélot et al., 1989). Some researchers propose that by reinforcing hypoxic pulmonary vasoconstriction to improve the V/Q ratio, almitrine may attenuate the progression of hypoxemia, potentially obviating the need for mechanical ventilation and reducing the duration of ICU stay and mortality (Kalfon et al., 2022). Furthermore, reports have indicated the use of almitrine in combination with inhaled NO to enhance gas exchange in cases of ARDS, both with and without COVID-19 (Payen et al., 1993). However, NO and almitrine have played a certain role in the treatment of “classical” sepsis, but their efficacy and safety are also controversial.
Therefore, our study was designed to comprehensively assess the effectiveness and safety of almitrine, inhaled NO, and the combined use of inhaled NO in the treatment of patients with sepsis and COVID-19.
2 Methods
The systematic review was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guideline (Supplementary Appendix S1, appendix p1–8) (Page et al., 2021) and was registered with the National Institute for Health Research international prospective register of systematic reviews (PROSPERO registration number CRD42022367667) (Wang et al., 2022).
2.1 Search strategy and selection criteria
Electronic searches were carried out in Embase, PubMed, the Cochrane Library, the Web of Science, Wanfang Data, and China National Knowledge Infrastructure. The search terms we used were “SARS-CoV-2,” “Corona Virus Disease 2019,” “COVID-19,” “nitric oxide,” “NO,” “almitrine,” “iNO,” “NO and almitrine,” “nitric oxide and almitrine,” and relevant keywords for publications until 23.10.2023. The search strategies are available in Supplementary Appendix S1, appendix p9–11. Unpublished and ongoing studies were identified by searching pre-print servers including medRxiv. Searches were carried out by two reviewers (Y.W and K.Z) independently in a standardized manner, followed by screening through titles, abstracts, and full text. Disagreements were resolved by consensus with unresolved conflicts decided by a third reviewer (D.L).
Inclusion criteria were as follows: 1) Patients were confirmed COVID-19 and the SOFA score (absolute, median, and mean value) ≥2, or in accordance with the SOFA scoring tool, a certain system index (absolute, median, and mean value) should be within the scope of corresponding to the system score ≥2, such as the PaO2/FiO2 ratio (P/F) (absolute, median, and mean value) was less than 300 mmHg (Singer et al., 2016). According to the SpO2/FiO2 ratio (S/F) = 64 + 0.84*(P/F), the S/F of 315 was approximately equal to a P/F ratio of 300 mmHg (Rice et al., 2007). In this study, we defined that such COVID-19 patients were at the edge of sepsis. 2) The intervention of interest was inhaled NO, intravenous almitrine, or inhaled NO combined with intravenous almitrine with or without standard treatment. Comparator treatments included placebo, standard treatment, and no intervention. No control group studies were included. 3) Randomized clinical trials (RCTs), case-control studies, cohort studies, cross-sectional studies, case reports, case series, and grey literature were included. The language was limited to Chinese and English. Exclusion criteria were as follows: 1) Patients were not confirmed COVID-19. 2) The SOFA score (absolute, median, and mean value) ≤2 or any of the system indicators did not reach 2. 3) Data on SOFA score or certain indicators were not available in the text, Supplementary Materials, or relevant resources. 4) Studies without an available full text or incomplete or unavailable data, conference abstracts, posters, opinion articles, commentaries, animal experiments, and in vitro studies. The efficacy outcomes were 28–30 days mortality, in-hospital mortality, P/F, and intubation needs. The safety outcomes were serious adverse events (SAEs) such as acute kidney injury (AKI) (Al Sulaiman et al., 2022).
2.2 Search strategy and selection criteria
Two independent reviewers (Y.W and K.Z) extracted the eligible studies, and a third reviewer (D.L) validated them. The extracted information includes the published year, authors, country, study type, sample size, participant demographics, SOFA score, patients’ position, drug dosage, route of administration, control group, mortality outcome, safety outcome, and conclusion of authors.
Included studies were assessed for quality by three reviewers (RL.L, LP.L, and CJ.W) in a standardized process. The Risk of Bias 2.0 tool was used to assess the RCTs (Steudel et al., 1999; Sterne et al., 2019). The methodological quality of case-control and cohort studies was assessed based on the Newcastle–Ottawa Scale (NOS) (NOS, 2020). The methodological quality of the included case reports, case series, and cross-sectional studies was assessed based on JBI critical appraisal tools (JBI, 2020). The reviewers shared the quality assessment results and gained consensus through discussion. The quality of evidence was assessed by using the “Grading of Recommendations Assessment Development and Evaluation (GRADE)” tool (Granholm et al., 2019).
2.3 Data synthesis and analysis
The Review Manager v.5.4.1 software was used for statistical analysis. For dichotomous outcomes, the total number of participants and the number of events in each group were recorded. For continuous outcomes, the total number of participants, mean, and standard deviation were recorded. If the authors reported the median and interquartile range, we estimated the mean and standard deviation (Wan et al., 2014; Luo et al., 2018). We report odds ratio (OR) for dichotomous outcomes and standard mean differences (Std MDs) for continuous outcomes. The fixed-effect model was applied when the result of the Q test was not significant (p > 0.1) and I2<50%. The I2 statistic was used to measure heterogeneity (I2: 30%–60% was defined as moderate heterogeneity, and 80%–100% was defined as significant heterogeneity). Subgroup analyses would be conducted, if data are appropriate. If we could not implement a meta-analysis, we planned to comment based on the results of included studies.
3 Results
3.1 Search results
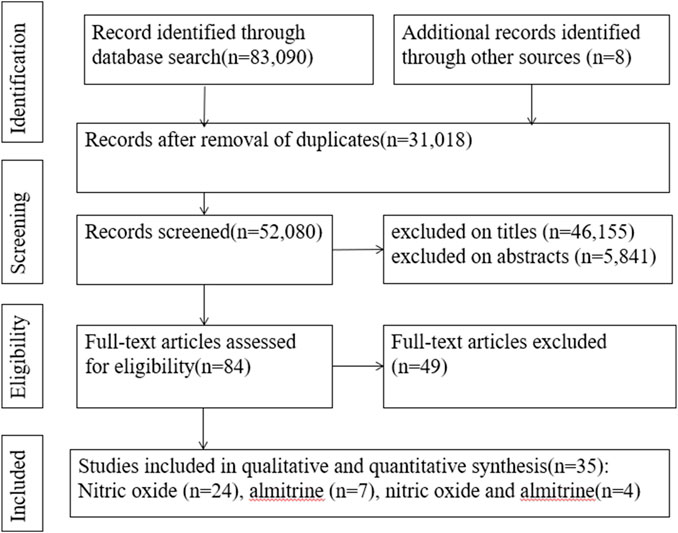
A search of the electronic databases on 23 October 2023 yielded 83,090 studies. After excluding duplicate articles and screening titles and abstracts, 84 articles were evaluated for full-text review. Among these, we found 35 relevant articles (2 RCTs, 19 cohort studies, 1 case-control study, 1 cross-sectional study, and 12 case reports) (Figure 1) (Abou-Arab et al., 2020; Bagate et al., 2020; Barthélémy et al., 2020; Cardinale et al., 2020; Ferrari et al., 2020; Huette et al., 2020; Losser et al., 2020; Safaee Fakhr et al., 2020; Tavazzi et al., 2020; Caplan et al., 2021; Chandel et al., 2021; Feng et al., 2021; Garfield et al., 2021; Giri et al., 2021; Herranz et al., 2021; Heuts et al., 2021; Huette et al., 2021; Laghlam et al., 2021; Longobardo et al., 2021; Lotz et al., 2021; Moni et al., 2021; Paramanathan et al., 2021; Ziehr et al., 2021; Al Sulaiman et al., 2022; Brown et al., 2022; Kalfon et al., 2022; Lubinsky et al., 2022; Poonam et al., 2022; Vives et al., 2022; Bicakcioglu et al., 2023; Blot et al., 2023; Di Fenza et al., 2023; Mekontso Dessap et al., 2023; Saccheri et al., 2023; van Zyl et al., 2023).
3.2 Study characteristics
In the 35 studies included, there were a total of 1,701 COVID-19 patients combined with sepsis, of whom 453 received mechanical ventilation. Ten studies reported SOFA scores of enrolled patients, of which three studies reported scores between 2 and 3 (2 for NO and 1 for almitrine), three studies reported scores between 4 and 5 (2 for NO and 1 for almitrine), and seven studies reported a score ≥ 6 (4 for NO and 3 for almitrine). The remaining 21 articles showed patients’ respiratory status, of which 19 studies included patients’ P/F ≤ 150 mmHg (15 for NO, 2 for almitrine, and 2 for NO combined with almitrine) and 2 studies reported patients’ P/F between 150 Hg and 300 mmHg(NO).
Patients usually received standard treatment (or standard of care) based on local guidelines. However, the majority patients were diagnosed with ARDS, and some of them used prone position to improve oxygenation. In addition, there is still no consensus on the dosage of NO and almitrine for such patients. The common dosage of NO is 10–80 parts per million (PPM) up to a maximum of 160–200 PPM, administered by inhalation. In some studies, patients’ dosage was adjusted according to PaO2 in arterial blood gas analysis. The usual dosage of almitrine is 2–16 μg/kg/min up to a maximum of 0.5 mg/kg/min, administered by injection. Characteristics of included studies and patients are presented in Table 1.
3.3 Assessment of study quality
The risk of bias of two RCTs was low to moderate (Supplementary Appendix S2, appendix p1–2). The methodological quality of 18 cohorts were moderate to high, and one case-control study was moderate (NOS assessment results are shown in Supplementary Appendix S2, appendix p3–22). The methodological quality of four case series was moderate, eight case reports was moderate to high, and one cross-sectional study was moderate (JBI assessment results are shown in Supplementary Appendix S2, appendix p23–35). Supplementary Appendix S2 (appendix p38–41) summarized the result of GRADE assessment for the certainty of evidence.
3.4 Results of meta-analysis
3.4.1 Mortality outcomes
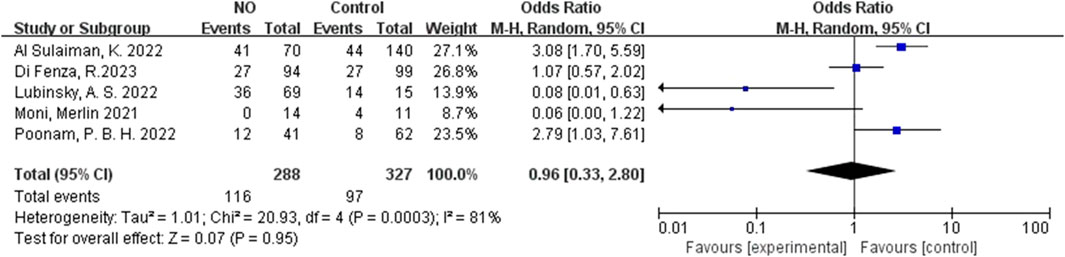
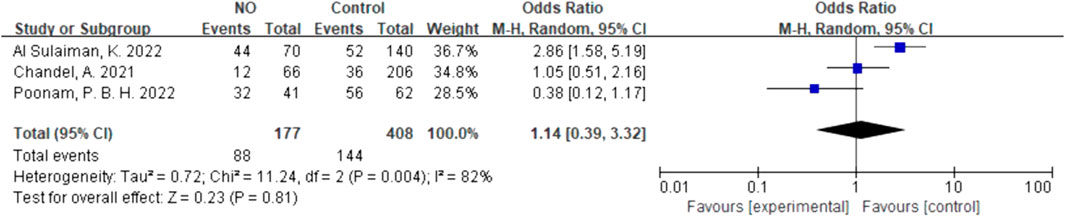
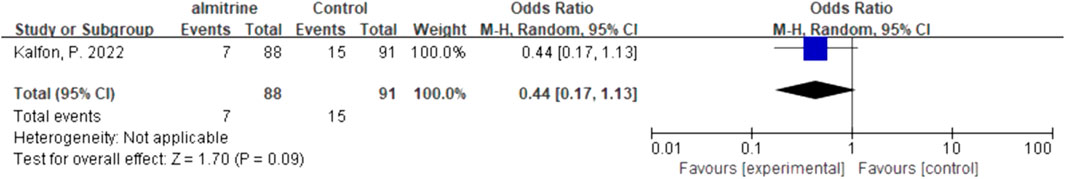
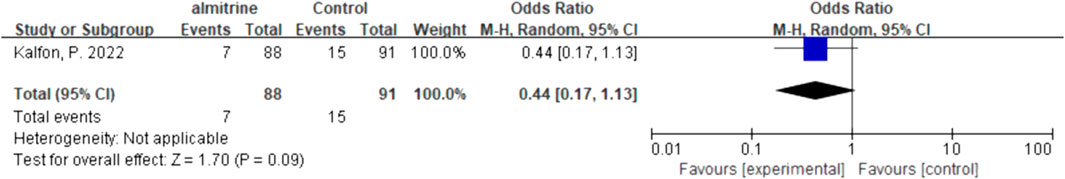
For inhalation NO, one RCT and four cohorts reported mortality at 28–30 days, and three cohorts reported hospital mortality. For almitrine, only one RCT reported mortality at 28–30 days and hospital mortality. Compared to the control group, inhaled NO might decrease mortality at 28–30 days (OR 0.96, 95% CI 0.33–2.8, I2 = 81%), but there was no significant difference between the two groups (Figure 2). In addition, inhaled NO might increase hospital mortality (OR 1.14, 95% CI 0.39–3.32, I2 = 82%), but there was no significant difference between the two groups (Figure 3). Compared to the control group, almitrine might decrease mortality at 28–30 days (OR 0.44, 95% CI 0.17–1.13), but there was no significant difference between the two groups (Figure 4). The result was similar to hospital mortality (OR 0.44, 95% CI 0.17–1.13) (Figure 5).
3.4.2 Synthesized data of P/F before and after administration
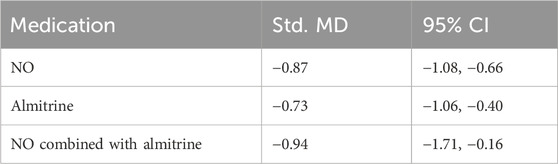
Due to the lack of inter-group data, we analyzed P/F before and after administration. Compared with pre-administration, the P/F of patients after the use of NO, almitrine, and NO–almitrine combination increased significantly (Table 2; Supplementary Appendix S2, Supplementary Figures S3–S5, appendix p36).
3.4.3 Hospital length of stay
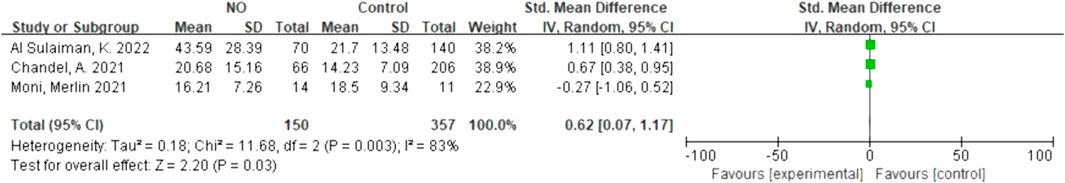
Due to the lack of data on the NO-almitrine combination, we used quantitative synthesis of the hospital length of stay of the patients treated with NO and almitrine alone. Compared to the control group, inhaled NO might shorten the hospital length of stay (SMD 0.62, 95% CI 0.07–1.17, I2 = 83%), but there was no significant difference between the two groups (Figure 6). For using almitrine, there was no difference in hospital length of stay between the intervention group and control group (SMD 0.00, 95% CI -0.29–0.29) (Figure 7).
3.4.4 Needs for intubation
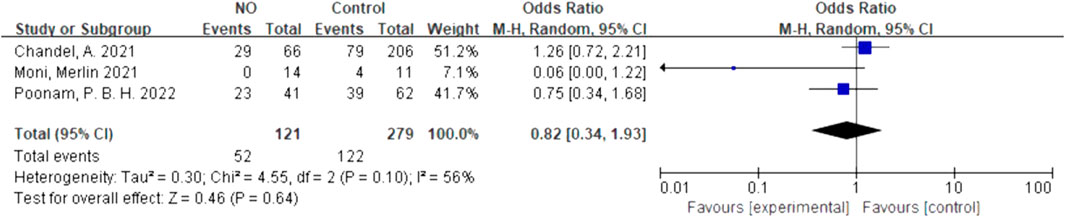
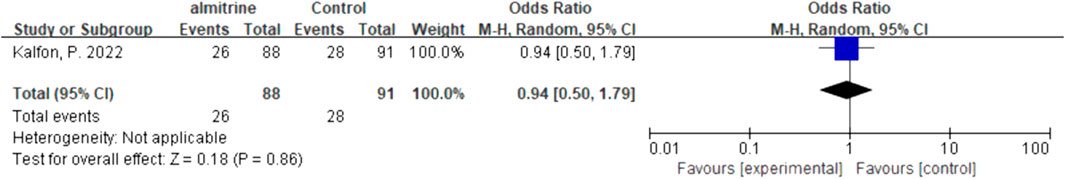
We quantitatively synthesized the intubation needs of the patients treated with NO and almitrine alone, due to a lack of data on NO combined with almitrine. For inhalation of NO, one RCT and two cohorts reported the need for intubation. For almitrine, only one RCT reported this outcome. Compared to the control group, inhaled NO might reduce the need for intubation, but there was no significant difference between the two groups (OR 0.82, 95% CI 0.34–1.93, I2 = 56%) (Figure 8). The RCT of almitrine showed similar trend (OR 0.94, 95% CI 0.5–1.79) (Figure 9).
3.4.5 Safety outcomes
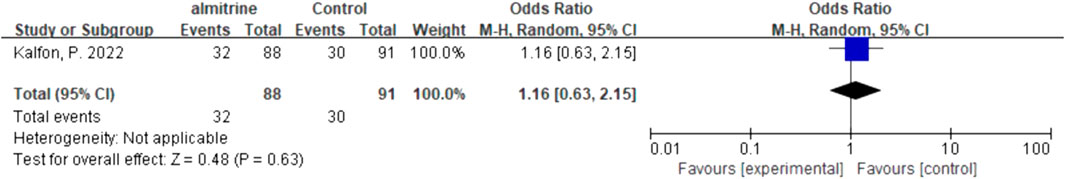
Due to significant heterogeneity (Supplementary Appendix S2, Supplementary Figure S6, appendix p37) and a lack of studies on safety outcomes, we only reported the result of quantitative synthesis for almitrine alone. Compared to the control group, almitrine might increase the SAEs, but there was no significant difference between the two groups (OR 1.16, 95% CI 0.63–2.15) (Figure 10).
4 Discussion
Critically ill and severe patients admitted to the ICU due to COVID-19 are at a higher risk of progressing to viral sepsis, and these patients would face more complex treatment. Data have shown that sepsis is one of the causes of death in patients with COVID-19 worldwide (Karakike et al., 2021). Among the many vulnerable organs in sepsis patients, the lung is the most vulnerable target organ, and patients often develop ARDS early, which is also one of the causes of death in sepsis patients (Tang and Tan, 2017). Therefore, sepsis and ARDS are not completely separate diseases in clinical treatment. At present, respiratory support is still the main treatment for sepsis and ARDS (Li et al., 2021), with active removal of pathogens and symptomatic support. In addition to ARDS, sepsis may also show other organ dysfunction in the clinic, such as coagulation function, liver and kidney function, or central nervous system dysfunction. Overall, whether sepsis, ARDS, or COVID-19, there is an urgent need for more effective drugs. Unfortunately, medications for sepsis are limited and their efficacy and safety are controversial. Despite significant clinical and basic research efforts in sepsis (especially virus-associated sepsis), there are few effective drugs for this disease worldwide, and no definitive treatment recommendations have been made in authoritative guidelines (Evans et al., 2021). Considering the urgency of sepsis treatment during the COVID-19 pandemic, people are trying to screen out drugs with a potential therapeutic value from previous treatments.
Higher incidences of pulmonary microthrombus and significant vascular endothelial injury were observed in critically ill patients with COVID-19 (Prakash et al., 2021), leading to poor oxygenation and pulmonary changes (Marini and Gattinoni, 2020). The vast majority of critically ill patients require mechanical ventilation owing to difficulties in maintaining oxygenation and ventilation, which remains a major challenge for critically ill patients of COVID-19 (Prakash et al., 2021). During the COVID-19 pandemic, it is essential to increase the number of days without ventilators for critically ill patients and minimize the need for respiratory support equipment. Therefore, dilating smooth muscle vessels and increasing alveolar blood flow to enhance oxygenation may be an option for treating critical illness (Longobardo et al., 2021). NO induces the relaxation of vascular smooth muscle and dilates pulmonary blood vessels, thereby increasing blood oxygenation and reducing the right-to-left shunt in the lung (Yu et al., 2019). Almitrine reduces intrapulmonary shunt by enhancing hypoxic pulmonary vasoconstriction (Reyes et al., 1988; Payen et al., 1993; Wysocki et al., 1994), which has been used for severe hypoxemia patients (Ranieri et al., 2012). NO and almitrine have been reported as a rescue strategy for “classical” ARDS in patients with severe hypoxemia. This treatment increased the P/F and reduced physiologic dead space fraction over 24 h (Reyes et al., 1988; Gebistorf et al., 2016). Almitrine and inhalation of NO were considered by some experts as a salvage treatment strategy for critically ill patients during the COVID-19 pandemic (Spieth and Zhang, 2014; Blot et al., 2023), including refractory hypoxemia. However, these treatments remain controversy. In this study, we found that NO, almitrine alone, and the combination of both significantly improved oxygenation in patients with COVID-19 (at the edge of sepsis), but did not affect the mortality, length of stay, or intubation needs of patients. In the face of the new medical challenge of COVID-19-induced sepsis, further research is still needed. In addition, the specific population and the specific circumstances in which these drugs are needed are to be studied and explored.
4.1 The combination of almitrine and NO
NO is a potent pulmonary vasodilator, while almitrine constricts pulmonary blood vessels. The combination of NO and almitrine appears to be contradictory, but some experts have utilized this combination as a rescue measure for critically ill patients (Laghlam et al., 2021). The rationale behind this combination lies in its potential to enhance the ventilation/perfusion ratio (V/Q) through selective vasoconstriction of pulmonary vessels in non-ventilated areas and selective vasodilation of pulmonary vessels in ventilated areas. Our findings indicate that this combination did not improve patient survival but did enhance oxygenation. It is crucial to exercise caution when employing this combination until further clarification regarding the mechanism, timing, and dosage is obtained. Additionally, almitrine is frequently used in conjunction with another drug called raubasine, which acts as a vasodilator, for the treatment of age-related cerebral disorders (Allain and Bentué-Ferrer, 1998) and certain pulmonary diseases. Notably, raubasine has demonstrated anti-SARS-CoV-2 effects in in vitro and animal studies, suggesting its therapeutic potential (Kumar et al., 2021; Mohseni et al., 2022).
4.2 Pregnant women
Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome coronavirus (SARS) have caused a large number of infectious deaths in pregnant women over the past 20 years. Because pregnant women have elevated levels of progesterone and estrogen, restricted lung expansion is more susceptible to pathogens (Selim et al., 2020). At present, there is still a lack of targeted respiratory interventions for pregnant women with hypoxic respiratory failure due to COVID-19 pneumonia, other than supplemental oxygen and mechanical ventilation. In another study of six pregnant women with severe or critical forms of COVID-19, 160–200 ppm of NO was found to be applied frequently, which appears to be well tolerated and may be beneficial to the people with hypoxic respiratory failure (Safaee Fakhr et al., 2020). Studies have shown that in patients with COVID-19, this innovative breathing intervention is feasible in pregnant women. At present, there have been many studies on the application of NO in the treatment of sepsis (non-COVID-19), but the experience of applying NO in the treatment of pregnant patients with COVID-19 remains very valuable.
4.3 Responders and non-responders
Most studies defined the responders as P/F increase >20% or 10 mmHg after administration (Ichinose et al., 2004; Charron et al., 2011). Whether patients with COVID-19 or without COVID-19 were “responders” to NO and almitrine and elements that predict potential responsiveness remain unclear. Manktelow C. et al. reported about 30%–40% of non-COVID-19 ARDS patients were non-respondence to inhaled NO to 67% of the patients with septic shock. They observed that septic shock was a significant predictor of NO inhalation responsiveness (Manktelow et al., 1997). Trachsel S. et al. reported endotoxin-exposed pigs that received inhaled NO responded by producing more endothelin-1, however, with higher levels in the responder group compared to the non-responder group (Trachsel et al., 2008).
As for COVID-19, among the included literature, only three studies compared the baseline characteristics of responders and non-responders, and the oxygenation of responders was lower than that of non-responders (Abou-Arab et al., 2020; Caplan et al., 2021; Garfield et al., 2021). Garfield B reported responders to inhaled NO also had higher baseline brain natriuretic peptides (Garfield et al., 2021). In addition, the prognosis of responders and non-responders is uncertain. Abou-Arab O. et al. stated that responders had a lower 28-day mortality rate (Caplan et al., 2021), and one study reported the ICU mortality of responders was similar to that of non-responders (p = 1.0) (Abou-Arab et al., 2020). During the pandemic, more in-depth research is needed on NO and almitrine responsiveness.
4.4 COVID-19-associated complications and NO
Infection by SARS-CoV-2 elicits a spectrum of complications, encompassing ARDS, AKI, and myocardial injury (Oxley et al., 2020; Ye et al., 2020). NO exerts selective dilation of pulmonary vessels within ventilated lung units, thereby improving ventilation/perfusion matching while averting systemic hypotension. Consequently, NO has been investigated as a potential treatment for COVID-19-associated ARDS. Moreover, NO may possess cardioprotective properties, as it can attenuate subclinical myocardial injuries normally observed during cardiopulmonary bypass procedures (Redaelli et al., 2022). Hence, this offers novel therapeutic avenues for managing COVID-19-related myocardial damage. Furthermore, inhaled NO could potentially yield favorable hemodynamic effects during cardiopulmonary bypass, thereby enhancing cardiac output and subsequently improving renal perfusion (Rezoagli et al., 2017). Utilizing NO may, thus, confer beneficial effects on cardiac output and provide renal function protection in patients with COVID-19 complicated by cardiovascular disorders.
4.5 Safety outcomes
A non-COVID-19 study showed that the plasma concentration and efficacy of almitrine increased in a dose-dependent manner, and perhaps its adverse events seemed to be also dose-dependent (Gallart et al., 1998). In this study, we found that adverse effects of 2 μg/kg/min almitrine were mild and infrequent, and the incidence was similar to that of the placebo groups (Kalfon et al., 2022). Meanwhile, no significant adverse events were observed by the investigators after the use of almitrine at a dose of 4-12 μg/kg/min (Losser et al., 2020; Caplan et al., 2021; Laghlam et al., 2021). However, considering the increase in the incidence rate of pulmonary thromboembolism in COVID-19 patients (Michard et al., 2001), some researchers recommended that almitrine should be used with caution, and the right ventricular loading conditions should be paid attention to after administration (Poissy et al., 2020).
A systematic review reported that inhaled NO might increase the risk of renal dysfunction, especially in patients with prolonged use and ARDS (non-COVID-19 (Ruan et al., 2015)). A multi-center cohort study included in this study showed that moderate-to-severe ARDS in critically ill patients of COVID-19 who received inhaled NO illustrated significantly higher odds of AKI (Al Sulaiman et al., 2022). A latest study on SAEs of NO showed that inhaled NO was associated with severe AKI and renal replacement therapy in critically ill patients of COVID-19 (Bobot et al., 2022). During the pandemic, some researchers tried to use NO for pregnant women with severe to critical COVID-19, and no acute adverse events related to NO were observed (Safaee Fakhr et al., 2020; Valsecchi et al., 2022). Based on the limited evidence, we suggest that doctors balance the benefit-to-risk ratio before prescribing NO for patients with COVID-19 (at the edge of sepsis) and pay attention to the renal function during administration.
4.6 Limitation
First, some results of this study have significant heterogeneity, but due to the lack of research, subgroup analysis and meta-regression cannot be carried out. However, despite the high heterogeneity of the research results, they still reflect the trend of the efficacy of NO and almitrine. Meanwhile, the SOFA score or related indicators of included patients were mean or median, so we speculated that not all patients confirmed sepsis, but the results of the patients still reflected a trend because some of these patients might or would develop sepsis. Second, a lack of high-quality clinical research studies limited our analyses. The majority of included studies were retrospective studies; these aspects could have introduced various confounders given the lack of risk adjustment or propensity score weighting. We included studies written only in English and Chinese, which also limits the scope of the review. Third, we found that the SOFA scores of included patients varied, and factors such as ethnic differences, the use of vasoactive drugs in many patients, and prone position had uncertain effects. Few studies analyzed the impact of these factors further and drew conclusions. Both ARDS and sepsis showed individual differences, which may also increase heterogeneity. In addition, the doses of NO and almitrine in the included studies were not uniform, and differences in management schemes for patients with sepsis in different countries and additional variability during the COVID-19 pandemic would increase the heterogeneity of the findings.
5 Conclusion
This systematic review demonstrated that both use of NO and almitrine alone, and the combination of the two drugs, could significantly improve oxygenation in patients. NO and almitrine might reduce the mortality, hospital length of stay, and intubation needs of patients, but there is no statistical significance, and almitrine did not significantly affect the SAEs. However, given the lack of clinical data, this conclusion needs more high-quality clinical evidence to verify. Moreover, there is no consensus on the dosage, applicable population, and respondent prediction of these drugs until now, which also increases the uncertainty of the conclusion of this study.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Author contributions
YW, KZ, and DL designed the study protocol. YW and KZ conducted the search strategy and screened and extracted data. LL, RL, and CW performed the risk of bias and quality of evidence assessments. JW and RL analyzed and interpreted the data. YW and KZ drafted the manuscript. DL reviewed and edited the manuscript. QY revised the manuscript. YT and SR reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1172447/full#supplementary-material
References
Abou-Arab, O., Huette, P., Debouvries, F., Dupont, H., Jounieaux, V., and Mahjoub, Y. (2020). Inhaled nitric oxide for critically ill Covid-19 patients: a prospective study. Crit. Care 24 (1), 645. doi:10.1186/s13054-020-03371-x
Allain, H., and Bentué-Ferrer, D. (1998). Clinical efficacy of almitrine-raubasine. An overview. Eur. Neurol. 39 (1), 39–44. doi:10.1159/000052069
Al Sulaiman, K., Korayem, G. B., Altebainawi, A. F., Al Harbi, S., Alissa, A., Alharthi, A., et al. (2022). Evaluation of inhaled nitric oxide (iNO) treatment for moderate-to-severe ARDS in critically ill patients with COVID-19: a multicenter cohort study. Crit. Care 26 (1), 304. doi:10.1186/s13054-022-04158-y
Bagate, F., Tuffet, S., Masi, P., Perier, F., Razazi, K., de Prost, N., et al. (2020). Rescue therapy with inhaled nitric oxide and almitrine in COVID-19 patients with severe acute respiratory distress syndrome. Ann. Intensive Care 10 (1), 151. doi:10.1186/s13613-020-00769-2
Barthélémy, R., Blot, P. L., Tiepolo, A., Le Gall, A., Mayeur, C., Gaugain, S., et al. (2020). Efficacy of almitrine in the treatment of hypoxemia in sars-cov-2 acute respiratory distress syndrome. Chest 158 (5), 2003–2006. doi:10.1016/j.chest.2020.05.573
Bersten, A. D., Davidson, K., Nicholas, T. E., and Doyle, I. R. (1998). Respiratory mechanics and surfactant in the acute respiratory distress syndrome. Clin. Exp. Pharmacol. Physiol. 25 (11), 955–963. doi:10.1111/j.1440-1681.1998.tb02352.x
Bicakcioglu, M., Kalkan, S., Duzenci, D., Yalcinsoy, M., Dogan, Z., and Ozer, A. B. (2023). Inhaled nitric oxide as rescue therapy in severe ARDS cases due to COVID-19 pneumonia: a single center experience. Eur. Rev. Med. Pharmacol. S. C. 27 (13), 6422–6428. doi:10.26355/eurrev_202307_33002
Blot, P. L., C, D. E. R., Deniau, B., Gaugain, S., Kindermans, M., Julian, N., et al. (2023). Efficacy of almitrine as a rescue therapy for refractory hypoxemia in COVID and non-COVID acute respiratory distress syndrome. A retrospective monocenter study. Minerva Anestesiol. 89 (3), 157–165. doi:10.23736/s0375-9393.22.16736-2
Bobot, M., Tonon, D., Peres, N., Guervilly, C., Lefèvre, F., Max, H., et al. (2022). Impact of dexamethasone and inhaled nitric oxide on severe acute kidney injury in critically ill patients with COVID-19. J. Clin. Med. 11 (20), 6130. doi:10.3390/jcm11206130
Brown, C. J., Rubel, N., Lai, J., Ward, C., McLean, J., Wheelock, M., et al. (2022). Initiation of inhaled nitric oxide by an air transport team in adult coronavirus disease 2019 respiratory failure. Air Med. J. 41 (4), 406–410. doi:10.1016/j.amj.2022.03.001
Caplan, M., Goutay, J., Bignon, A., Jaillette, E., Favory, R., Mathieu, D., et al. (2021). Almitrine infusion in severe acute respiratory syndrome coronavirus 2-induced acute respiratory distress syndrome: a single-center observational study. Crit. Care Med. 49 (2), e191–e198. doi:10.1097/ccm.0000000000004711
Cardinale, M., Esnault, P., Cotte, J., Cungi, P. J., and Goutorbe, P. (2020). Effect of almitrine bismesylate and inhaled nitric oxide on oxygenation in COVID-19 acute respiratory distress syndrome. Anaesth. Crit. Care Pain Med. 39 (4), 471–472. doi:10.1016/j.accpm.2020.05.014
Chandel, A., Patolia, S., Ahmad, K., Aryal, S., Brown, A. W., Sahjwani, D., et al. (2021). Inhaled nitric oxide via high-flow nasal cannula in patients with acute respiratory failure related to COVID-19. Clin. Med. Insights Circ. Respir. Pulm. Med. 15, 11795484211047065. doi:10.1177/11795484211047065
Charron, C., Repesse, X., Bouferrache, K., Bodson, L., Castro, S., Page, B., et al. (2011). PaCO2 and alveolar dead space are more relevant than PaO2/FiO2 ratio in monitoring the respiratory response to prone position in ARDS patients: a physiological study. Crit. Care 15 (4), R175. doi:10.1186/cc10324
Di Fenza, R., Shetty, N. S., Gianni, S., Parcha, V., Giammatteo, V., Safaee Fakhr, B., et al. (2023). High-dose inhaled nitric oxide in acute hypoxemic respiratory failure due to COVID-19: a multicenter phase 2 trial. Am. J. Respir. Crit. Care Med. 29, 1293–1304. doi:10.1164/rccm.202304-0637OC
Evans, L., Rhodes, A., Alhazzani, W., Antonelli, M., Coopersmith, C. M., French, C., et al. (2021). Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 47 (11), 1181–1247. doi:10.1007/s00134-021-06506-y
Feng, W. X., Yang, Y., Wen, J., Liu, Y. X., Liu, L., and Feng, C. (2021). Implication of inhaled nitric oxide for the treatment of critically ill COVID-19 patients with pulmonary hypertension. Esc. Heart Fail 8 (1), 714–718. doi:10.1002/ehf2.13023
Ferrari, M., Santini, A., Protti, A., Andreis, D. T., Iapichino, G., Castellani, G., et al. (2020). Inhaled nitric oxide in mechanically ventilated patients with COVID-19. J. Crit. Care 60, 159–160. doi:10.1016/j.jcrc.2020.08.007
Frostell, C., Fratacci, M. D., Wain, J. C., Jones, R., and Zapol, W. M. (1991). Inhaled nitric oxide. A selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstriction. Circulation 83 (6), 2038–2047. doi:10.1161/01.cir.83.6.2038
Gallart, L., Lu, Q., Puybasset, L., Umamaheswara Rao, G. S., Coriat, P., and Rouby, J. J. (1998). Intravenous almitrine combined with inhaled nitric oxide for acute respiratory distress syndrome. The NO Almitrine Study Group. Am. J. Respir. Crit. Care Med. 158 (6), 1770–1777. doi:10.1164/ajrccm.158.6.9804066
Garfield, B., McFadyen, C., Briar, C., Bleakley, C., Vlachou, A., Baldwin, M., et al. (2021). Potential for personalised application of inhaled nitric oxide in COVID-19 pneumonia. Br. J. Anaesth. 126 (2), e72–e75. doi:10.1016/j.bja.2020.11.006
Gebistorf, F., Karam, O., Wetterslev, J., and Afshari, A. (2016). Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) in children and adults. Cochrane Database Syst. Rev. 2016 (6), Cd002787. doi:10.1002/14651858.CD002787.pub3
Giri, A. R., Yarrarapu, S. N. S., Kaur, N., Hochwald, A., Crook, J., Helgeson, S., et al. (2021). Inhaled nitric oxide use in COVID19-induced hypoxemic respiratory failure. medRxiv. doi:10.1101/2021.08.19.21262314
Granholm, A., Alhazzani, W., and Møller, M. H. (2019). Use of the GRADE approach in systematic reviews and guidelines. Br. J. Anaesth. 123 (5), 554–559. doi:10.1016/j.bja.2019.08.015
Herranz, L., da Silveira, J. G., Trocado, L. F. L., Alvaraes, A. L., and Fittipaldi, J. (2021). Inhaled nitric oxide in patients with severe COVID-19 infection at intensive care unit - a cross sectional study. J. Crit. Care Med. (Targu Mures) 7 (4), 318–319. doi:10.2478/jccm-2021-0033
Heuts, S., Ubben, J. F., Banks-Gonzales, V., Sels, J. W., Lorusso, R., van Mook, W., et al. (2021). Nitric oxide ventilation improves recirculation and right ventricular function during veno-venous extracorporeal membrane oxygenation in a COVID-19 patient. J. Cardiothorac. Vasc. Anesth. 35 (9), 2763–2767. doi:10.1053/j.jvca.2020.09.137
Huette, P., Abou Arab, O., Jounieaux, V., Guilbart, M., Belhout, M., Haye, G., et al. (2021). Almitrine for COVID-19 critically ill patients - a vascular therapy for a pulmonary vascular disease: three case reports. World J. Clin. Cases 9 (14), 3385–3393. doi:10.12998/wjcc.v9.i14.3385
Huette, P., Beyls, C., Guilbart, M., Haye, G., Najid, F. Z., Mestan, B., et al. (2020). Acute cor pulmonale in COVID-19-related ARDS: improvement with almitrine infusion. JACC Case Rep. 2 (9), 1311–1314. doi:10.1016/j.jaccas.2020.06.011
Ichinose, F., Roberts, J. D., and Zapol, W. M. (2004). Inhaled nitric oxide: a selective pulmonary vasodilator: current uses and therapeutic potential. Circulation 109 (25), 3106–3111. doi:10.1161/01.Cir.0000134595.80170.62
JBI (2020). Critical appraisal tools. Available from: https://joannabriggs.org/critical-appraisaltools (Accessed December 1, 2022).
Jolin, A., and Bjertnaes, L. (1991). Hypoxic pulmonary vasoconstriction in the adult respiratory distress syndrome. Acta Anaesthesiol. Scand. Suppl. 95, 40–52. doi:10.1111/j.1399-6576.1991.tb03399.x
Kalfon, P., Payen, J. F., Rousseau, A., Chousterman, B., Cachanado, M., Tibi, A., et al. (2022). Effect of intravenous almitrine on intubation or mortality in patients with COVID-19 acute hypoxemic respiratory failure: a multicentre, randomised, double-blind, placebo-controlled trial. EClinicalMedicine 52, 101663. doi:10.1016/j.eclinm.2022.101663
Karakike, E., Giamarellos-Bourboulis, E. J., Kyprianou, M., Fleischmann-Struzek, C., Pletz, M. W., Netea, M. G., et al. (2021). Coronavirus disease 2019 as cause of viral sepsis: a systematic review and meta-analysis. Crit. Care Med. 49 (12), 2042–2057. doi:10.1097/ccm.0000000000005195
Kumar, S., Kashyap, P., Chowdhury, S., Kumar, S., Panwar, A., and Kumar, A. (2021). Identification of phytochemicals as potential therapeutic agents that binds to Nsp15 protein target of coronavirus (SARS-CoV-2) that are capable of inhibiting virus replication. Phytomedicine 85, 153317. doi:10.1016/j.phymed.2020.153317
Laghlam, D., Rahoual, G., Malvy, J., Estagnasié, P., Brusset, A., and Squara, P. (2021). Use of almitrine and inhaled nitric oxide in ARDS due to COVID-19. Front. Med. (Lausanne) 8, 655763. doi:10.3389/fmed.2021.655763
Li, M., Li, Q., Liu, J., and Zhao, H. (2021). Research progress in diagnosis and treatment of acute respiratory distress syndrome. Chin. J. Difficult Complicat. Cases 20 (03), 304–309. doi:10.3969/j.issn.1671-6450.2021.03.021
Lisi, F., Zelikin, A. N., and Chandrawati, R. (2021). Nitric oxide to fight viral infections. Adv. Sci. (Weinh) 8 (7), 2003895. doi:10.1002/advs.202003895
Longobardo, A., Montanari, C., Shulman, R., Benhalim, S., Singer, M., and Arulkumaran, N. (2021). Inhaled nitric oxide minimally improves oxygenation in COVID-19 related acute respiratory distress syndrome. Br. J. Anaesth. 126 (1), e44–e46. doi:10.1016/j.bja.2020.10.011
Losser, M. R., Lapoix, C., Delannoy, M., Champigneulle, B., and Payen, D. (2020). Almitrine as a non-ventilatory strategy to improve intrapulmonary shunt in COVID-19 patients. Anaesth. Crit. Care Pain Med. 39 (4), 467–469. doi:10.1016/j.accpm.2020.05.013
Lotz, C., Muellenbach, R. M., Meybohm, P., Mutlak, H., Lepper, P. M., Rolfes, C. B., et al. (2021). Effects of inhaled nitric oxide in COVID-19-induced ARDS - is it worthwhile? Acta Anaesthesiol. Scand. 65 (5), 629–632. doi:10.1111/aas.13757
Lubinsky, A. S., Brosnahan, S. B., Lehr, A., Elnadoury, O., Hagedorn, J., Garimella, B., et al. (2022). Inhaled pulmonary vasodilators are not associated with improved gas exchange in mechanically ventilated patients with COVID-19: a retrospective cohort study. J. Crit. Care 69, 153990. doi:10.1016/j.jcrc.2022.153990
Luo, D., Wan, X., Liu, J., and Tong, T. (2018). Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 27 (6), 1785–1805. doi:10.1177/0962280216669183
Manktelow, C., Bigatello, L. M., Hess, D., and Hurford, W. E. (1997). Physiologic determinants of the response to inhaled nitric oxide in patients with acute respiratory distress syndrome. Anesthesiology 87 (2), 297–307. doi:10.1097/00000542-199708000-00017
Marini, J. J., and Gattinoni, L. (2020). Management of COVID-19 respiratory distress. Jama 323 (22), 2329–2330. doi:10.1001/jama.2020.6825
Mekontso Dessap, A., Papazian, L., Schaller, M., Nseir, S., Megarbane, B., Haudebourg, L., et al. (2023). Inhaled nitric oxide in patients with acute respiratory distress syndrome caused by COVID-19: treatment modalities, clinical response, and outcomes. Ann. Intensive Car 13 (1), 57. doi:10.1186/s13613-023-01150-9
Mélot, C., Dechamps, P., Hallemans, R., Decroly, P., and Mols, P. (1989). Enhancement of hypoxic pulmonary vasoconstriction by low dose almitrine bismesylate in normal humans. Am. Rev. Respir. Dis. 139 (1), 111–119. doi:10.1164/ajrccm/139.1.111
Michard, F., Wolff, M. A., Herman, B., and Wysocki, M. (2001). Right ventricular response to high-dose almitrine infusion in patients with severe hypoxemia related to acute respiratory distress syndrome. Crit. Care Med. 29 (1), 32–36. doi:10.1097/00003246-200101000-00007
Mir, J. M., and Maurya, R. C. (2021). Nitric oxide as a therapeutic option for COVID-19 treatment: a concise perspective. New J. Chem. 45 (4), 1774–1784. doi:10.1039/d0nj03823g
Mir, J. M., and Maurya, R. C. (2022). Nitric oxide boosters as defensive agents against COVID-19 infection: an opinion. J. Biomol. Struct. Dyn. 40 (9), 4285–4291. doi:10.1080/07391102.2020.1852969
Mohseni, M., Bahrami, H., Farajmand, B., Hosseini, F. S., Amanlou, M., and Salehabadi, H. (2022). Indole alkaloids as potential candidates against COVID-19: an in silico study. J. Mol. Model 28 (6), 144. doi:10.1007/s00894-022-05137-4
Moni, M., Madathil, T., Sathyapalan, D. T., Menon, V., Gutjahr, G., Edathadathil, F., et al. (2021). A feasibility trial to evaluate the composite efficacy of inhaled nitric oxide in the treatment of covid 19 pneumonia: impact on viral load and clinical outcomes. medRxiv. doi:10.1101/2021.04.15.21255300
NOS (2020). Abbreviations.com. STANDS4 LLC. https://www.abbreviations.com/term/1418908 (Accessed December 1, 2022).
Oxley, T. J., Mocco, J., Majidi, S., Kellner, C. P., Shoirah, H., Singh, I. P., et al. (2020). Large-vessel stroke as a presenting feature of covid-19 in the young. N. Engl. J. Me 382 (20), e60. doi:10.1056/NEJMc2009787
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj 372, n71. doi:10.1136/bmj.n71
Paramanathan, S., Kyng, K. J., Laursen, A. L., Jensen, L. D., Grejs, A. M., and Jain, D. (2021). COVID-19 with severe acute respiratory distress in a pregnant woman leading to preterm caesarean section: a case report. Case Rep. Womens Health 30, e00304. doi:10.1016/j.crwh.2021.e00304
Payen, D. M., Gatecel, C., and Plaisance, P. (1993). Almitrine effect on nitric oxide inhalation in adult respiratory distress syndrome. Lancet 341 (8861), 1664. doi:10.1016/0140-6736(93)90801-m
Poissy, J., Goutay, J., Caplan, M., Parmentier, E., Duburcq, T., Lassalle, F., et al. (2020). Pulmonary embolism in patients with COVID-19: awareness of an increased prevalence. Circulation 142 (2), 184–186. doi:10.1161/circulationaha.120.047430
Poonam, P. B. H., Koscik, R., Nguyen, T., Rikhi, S., and Lin, H. M. (2022). Nitric oxide versus epoprostenol for refractory hypoxemia in Covid-19. PLoS One 17 (6), e0270646. doi:10.1371/journal.pone.0270646
Prakash, A., Kaur, S., Kaur, C., Prabha, P. K., Bhatacharya, A., Sarma, P., et al. (2021). Efficacy and safety of inhaled nitric oxide in the treatment of severe/critical COVID-19 patients: a systematic review. Indian J. Pharmacol. 53 (3), 236–243. doi:10.4103/ijp.ijp_382_21
Ranieri, V. M., Rubenfeld, G. D., Thompson, B. T., Ferguson, N. D., Caldwell, E., Fan, E., et al. (2012). Acute respiratory distress syndrome: the Berlin Definition. Jama 307 (23), 2526–2533. doi:10.1001/jama.2012.5669
Redaelli, S., Magliocca, A., Malhotra, R., Ristagno, G., Citerio, G., Bellani, G., et al. (2022). Nitric oxide: clinical applications in critically ill patients. Nitric Oxide 1 (121), 20–33. doi:10.1016/j.niox.2022.01.007
Reyes, A., Roca, J., Rodriguez-Roisin, R., Torres, A., Ussetti, P., and Wagner, P. D. (1988). Effect of almitrine on ventilation-perfusion distribution in adult respiratory distress syndrome. Am. Rev. Respir. Dis. 137 (5), 1062–1067. doi:10.1164/ajrccm/137.5.1062
Rezoagli, E., Ichinose, F., Strelow, S., Roy, N., Shelton, K., Matsumine, R., et al. (2017). Pulmonary and systemic vascular resistances after cardiopulmonary bypass: role of hemolysis. J. Cardiothorac. Vasc. Anesth. 31, 505–515. doi:10.1053/j.jvca.2016.06.009
Rice, T. W., Wheeler, A. P., Bernard, G. R., Hayden, D. L., Schoenfeld, D. A., Ware, L. B., et al. (2007). Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest 132 (2), 410–417. doi:10.1378/chest.07-0617
Rossaint, R., Falke, K. J., López, F., Slama, K., Pison, U., and Zapol, W. M. (1993). Inhaled nitric oxide for the adult respiratory distress syndrome. N. Engl. J. Med. 328 (6), 399–405. doi:10.1056/nejm199302113280605
Ruan, S. Y., Huang, T. M., Wu, H. Y., Wu, H. D., Yu, C. J., and Lai, M. S. (2015). Inhaled nitric oxide therapy and risk of renal dysfunction: a systematic review and meta-analysis of randomized trials. Crit. Care 19 (1), 137. doi:10.1186/s13054-015-0880-2
Saccheri, C., Morand, L., Juston, M., Doyen, D., Hyvernat, H., Lombardi, R., et al. (2023). Use of almitrine in spontaneously breathing patients with COVID-19 treated with high-flow nasal cannula oxygen therapy and with persistent hypoxemia. Respir. Res. 24 (1), 1. doi:10.1186/s12931-022-02308-y
Safaee Fakhr, B., Wiegand, S. B., Pinciroli, R., Gianni, S., Morais, C. C. A., Ikeda, T., et al. (2020). High concentrations of nitric oxide inhalation therapy in pregnant patients with severe coronavirus disease 2019 (COVID-19). Obstet. Gynecol. 136 (6), 1109–1113. doi:10.1097/aog.0000000000004128
Selim, M., Mohamed, S., Abdo, M., and Abdelhaffez, A. (2020). Is COVID-19 similar in pregnant and non-pregnant women? Cureus 12 (6), e8888. doi:10.7759/cureus.8888
Singer, M., Deutschman, C. S., Seymour, C. W., Shankar-Hari, M., Annane, D., Bauer, M., et al. (2016). The third international consensus definitions for sepsis and septic shock (Sepsis-3). Jama 315 (8), 801–810. doi:10.1001/jama.2016.0287
Spieth, P. M., and Zhang, H. (2014). Pharmacological therapies for acute respiratory distress syndrome. Curr. Opin. Crit. Care 20 (1), 113–121. doi:10.1097/mcc.0000000000000056
Squara, P., Dhainaut, J. F., Artigas, A., and Carlet, J. (1998). Hemodynamic profile in severe ARDS: results of the European Collaborative ARDS Study. Intensive Care Med. 24 (10), 1018–1028. doi:10.1007/s001340050710
Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. Bmj 366, l4898. doi:10.1136/bmj.l4898
Steudel, W., Hurford, W. E., and Zapol, W. M. (1999). Inhaled nitric oxide: basic biology and clinical applications. Anesthesiology 9, 1090–1121. doi:10.1097/00000542-199910000-00030
Sylvester, J. T., Shimoda, L. A., Aaronson, P. I., and Ward, J. P. (2012). Hypoxic pulmonary vasoconstriction. Physiol. Rev. 92 (1), 367–520. doi:10.1152/physrev.00041.2010
Tang, T., and Tan, L. (2017). The role of inflammatory response in the pathogenesis of septic ARDS. Chongqing Med. J. 46 (15), 2146–2149.
Tavazzi, G., Pozzi, M., Mongodi, S., Dammassa, V., Romito, G., and Mojoli, F. (2020). Inhaled nitric oxide in patients admitted to intensive care unit with COVID-19 pneumonia. Crit. Care 24 (1), 508. doi:10.1186/s13054-020-03222-9
Trachsel, S., Deby-Dupont, G., Maurenbrecher, E., Nys, M., Lamy, M., and Hedenstierna, G. (2008). Association between inflammatory mediators and response to inhaled nitric oxide in a model of endotoxin-induced lung injury. Crit. Care 12 (5), R131. doi:10.1186/cc7099
Valsecchi, C., Winterton, D., Safaee Fakhr, B., Collier, A. Y., Nozari, A., Ortoleva, J., et al. (2022). High-dose inhaled nitric oxide for the treatment of spontaneously breathing pregnant patients with severe coronavirus disease 2019 (COVID-19) pneumonia. Obstet. Gynecol. 140 (2), 195–203. doi:10.1097/aog.0000000000004847
van Zyl, A. G. P., Allwood, B. W., Koegelenberg, C. F. N., Lalla, U., and Retief, F. (2023). The effect of inhaled nitric oxide on shunt fraction in mechanically ventilated patients with COVID-19 pneumonia. Afr. J. Thorac. Crit. Care Me 29 (2), 64–66. doi:10.7196/AJTCCM.2023.v29i2.279
Vives, M., Gascó, I., Pla, G., Maciel, J. L., Ricart Hernandez, A., Regí Roman, K., et al. (2022). Inhaled nitric oxide in acute severe pulmonary hypertension and severe acute respiratory distress syndrome secondary to COVID-19 pneumonia: a case report. Am. J. Case Rep. 23, e937147. doi:10.12659/ajcr.937147
Wan, X., Wang, W., Liu, J., and Tong, T. (2014). Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 14, 135. doi:10.1186/1471-2288-14-135
Wang, Y., Zhu, K., Liu, L., Gao, J., Dong, L., and Liu, R. (2022). Efficacy and safety of inhaled nitric oxide in the treatment of COVID-19 (at the edge of sepsis) patients: a living systematic review. PROSPERO 2022 CRD42022367667 Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022367667.
Wysocki, M., Delclaux, C., Roupie, E., Langeron, O., Liu, N., Herman, B., et al. (1994). Additive effect on gas exchange of inhaled nitric oxide and intravenous almitrine bismesylate in the adult respiratory distress syndrome. Intensive Care Med. 20 (4), 254–259. doi:10.1007/bf01708960
Ye, Z., Zhang, Y., Wang, Y., Huang, Z., and Song, B. (2020). Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur. Radiol. 30 (8), 4381–4389. doi:10.1007/s00330-020-06801-0
Yu, B., Ichinose, F., Bloch, D. B., and Zapol, W. M. (2019). Inhaled nitric oxide. Br. J. Pharmacol. 176 (2), 246–255. doi:10.1111/bph.14512
Ziehr, D. R., Alladina, J., Wolf, M. E., Brait, K. L., Malhotra, A., La Vita, C., et al. (2021). Respiratory physiology of prone positioning with and without inhaled nitric oxide across the coronavirus disease 2019 acute respiratory distress syndrome severity spectrum. Crit. Care Explor 3 (6), e0471. doi:10.1097/cce.0000000000000471
Keywords: severe acute respiratory syndrome coronavirus 2, coronavirus disease 2019, sepsis, inhaled nitric oxide, almitrine
Citation: Wang Y, Yu Q, Tian Y, Ren S, Liu L, Wei C, Liu R, Wang J, Li D and Zhu K (2024) Unraveling the impact of nitric oxide, almitrine, and their combination in COVID-19 (at the edge of sepsis) patients: a systematic review. Front. Pharmacol. 14:1172447. doi: 10.3389/fphar.2023.1172447
Received: 23 February 2023; Accepted: 27 December 2023;
Published: 22 January 2024.
Edited by:
Syed A. Rizvi, Larkin University, United StatesReviewed by:
Santi M. Mandal, Indian Institute of Technology Kharagpur, IndiaJan Mohammad Mir, Islamic University of Science and Technology, India
Copyright © 2024 Wang, Yu, Tian, Ren, Liu, Wei, Liu, Wang, Li and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong Li, bGlkb25nMUBqbHUuZWR1LmNu; Kun Zhu, emh1a3VuQGpsdS5lZHUuY24=
†These authors have contributed equally to this work
 Ying Wang
Ying Wang Qian Yu1†
Qian Yu1† Liping Liu
Liping Liu Chaojie Wei
Chaojie Wei Renli Liu
Renli Liu Dong Li
Dong Li Kun Zhu
Kun Zhu